Abstract
Background
The objective of this study was to identify a subgroup of patients with head and neck squamous cell carcinoma (HNSCC) who might be suitable for hypofractionated radiotherapy (RT‐hypo) during the COVID‐19 pandemic.
Methods
HNSCC cases (oropharynx/larynx/hypopharynx) treated with definitive RT‐hypo (60 Gy in 25 fractions over 5 weeks), moderately accelerated radiotherapy (RT‐acc) alone (70 Gy in 35 fractions over 6 weeks), or concurrent chemoradiotherapy (CCRT) during 2005‐2017 were included. Locoregional control (LRC) and distant control (DC) after RT‐hypo, RT‐acc, and CCRT were compared for various subgroups.
Results
The study identified 994 human papillomavirus–positive (HPV+) oropharyngeal squamous cell carcinoma cases (with 61, 254, and 679 receiving RT‐hypo, RT‐acc, and CCRT, respectively) and 1045 HPV– HNSCC cases (with 263, 451, and 331 receiving RT‐hypo, RT‐acc, and CCRT, respectively). The CCRT cohort had higher T/N categories, whereas the radiotherapy‐alone patients were older. The median follow‐up was 4.6 years. RT‐hypo, RT‐acc, and CCRT produced comparable 3‐year LRC and DC for HPV+ T1‐2N0‐N2a disease (seventh edition of the TNM system [TNM‐7]; LRC, 94%, 100%, and 94%; P = .769; DC, 94%, 100%, and 94%; P = .272), T1‐T2N2b disease (LRC, 90%, 94%, and 97%; P = .445; DC, 100%, 96%, and 95%; P = .697), and T1‐2N2c/T3N0‐N2c disease (LRC, 89%, 93%, and 95%; P = .494; DC, 89%, 90%, and 87%; P = .838). Although LRC was also similar for T4/N3 disease (78%, 84%, and 88%; P = .677), DC was significantly lower with RT‐hypo or RT‐acc versus CCRT (67%, 65%, and 87%; P = .005). For HPV– HNSCC, 3‐year LRC and DC were similar with RT‐hypo, RT‐acc, and CCRT in stages I and II (LRC, 85%, 89%, and 100%; P = .320; DC, 99%, 98%, and 100%; P = .446); however, RT‐hypo and RT‐acc had significantly lower LRC in stage III (76%, 69%, and 91%; P = .006), whereas DC rates were similar (92%, 85%, and 90%; P = .410). Lower LRC in stage III predominated in patients with laryngeal squamous cell carcinoma receiving RT‐acc (62%) but not RT‐hypo (80%) or CCRT (92%; RT‐hypo vs CCRT: P = .270; RT‐acc vs CCRT: P = .004). CCRT had numerically higher LRC in comparison with RT‐hypo or RT‐acc in stage IV (73%, 65%, and 66%; P = .336).
Conclusions
It is proposed that RT‐hypo be considered in place of CCRT for HPV+ T1‐T3N0‐N2c (TNM‐7) HNSCCs, HPV– T1‐T2N0 HNSCCs, and select stage III HNSCCs during the COVID‐19 outbreak.
Keywords: altered fractionation, chemoradiotherapy, COVID‐19, head and neck cancer, hypofractionation, outcome, radiotherapy
Short abstract
Hypo‐fractionated radiotherapy has disease control comparable to that of chemoradiotherapy in select head and neck cancers, and it is a potential alternative for this subgroup during the COVID‐19 pandemic.
Introduction
The emergence of the COVID‐19 novel coronavirus pandemic in late 2019 to early 2020 has necessitated a rapid societal response that is particularly critical within the health care sector. This has resulted in the necessary diversion of resources to care for infected patients and has put significant strain on cancer care systems. The management of patients presenting with head and neck cancer (HNC) involves multidisciplinary, resource‐intensive approaches and is especially vulnerable to resource constraints associated with the COVID‐19 pandemic. Nonsurgical treatment for head and neck squamous cell carcinoma (HNSCC) often requires a prolonged course of radiotherapy (RT) plus or minus the addition of concurrent chemoradiotherapy (CCRT) and significant supportive care measures. Recent data suggest that patients with cancer are at higher risk of contracting the SARS‐CoV‐2 virus in comparison with the general public, 1 , 2 and if infected, they often experience poorer outcomes from COVID‐19. 3 Patients with HNC are at higher risk of becoming infected when multiple trips to the treatment center over many weeks are required (hindering protective self‐isolation and social distancing), because of movement through multiple departments within the hospital, and because of the immunosuppressive effects of both radiation and chemotherapy. These risks may be mitigated by the adoption of treatment strategies that would minimize the need for systemic chemotherapy and/or reduce the number of RT fractions delivered. Shorter treatment courses may also be needed operationally if RT capacity is compromised because of potential staffing shortages and/or an increased demand for RT occurs in place of compromised surgical resources. In such instances, waiting times to commence treatment will inevitably increase with a well‐recognized adverse effect on cancer control. 4
Minimizing ambulatory visits with shorter RT courses, avoiding twice daily RT, and balancing the risk and benefit of chemotherapy are strategies being adopted by colleagues in Europe. 5 , 6 However, robust contemporary data for hypofractionation RT are lacking, especially for once daily treatments. Hypofractionation using once daily RT is a form of accelerated RT with shorter courses involving moderately increased doses per fraction and delivery over 3 to 5 weeks, which is briefer in comparison with a conventional course over 6 to 7 weeks. This is a traditional RT approach 7 in recent historical practice in the United Kingdom and Canada. 8 , 9 , 10 However, these approaches were not addressed in the Meta‐Analysis of Radiotherapy in squamous cell Carcinomas of Head and neck of altered‐fractionation regimens, nor are there other randomized trial data from which to draw the usual inferences about efficacy. 11 Moreover, hypofractionated radiotherapy (RT‐hypo) schedules have typically not been included in phase 3 randomized trials other than rarely as a control arm. 11 , 12 , 13 , 14 Thus, contemporary data are needed to evaluate the outcomes of RT‐hypo alone in comparison with other altered‐fractionation regimens or standard‐fractionation CCRT. We reasoned that this analysis would identify HNSCC subgroups that could obtain similar rates of locoregional control (LRC) and distant control (DC) when managed with RT‐hypo alone in comparison with CCRT and thus facilitate evidence‐based HNC care in response to the COVID‐19 pandemic.
Our academic cancer center exists in a metropolitan area within a universal health care system and delivers primary RT to more than 400 patients with mucosal HNC annually. RT alone has been one of the treatment options for patients with locally advanced head and neck squamous cell carcinoma (LAHNSCC) with contraindications to chemotherapy, such as cardiac risk, renal or hepatic impairment, frailty or advanced age, and patient choice. Patients with LAHNSCCs with minimal nodal burden are also often offered altered‐fractionation RT alone as an alternative to CCRT because outcomes for many small–tumor burden cases despite an advanced stage can be favorable after RT alone. 15 We reviewed our experience with altered‐fractionation RT alone for human papillomavirus–positive (HPV+) patients with oropharyngeal squamous cell carcinoma (OPSCC) and HPV– patients with HNSCC by using contemporaneously collected data from an institutional, prospective HNC database in which outcomes are recorded at the point of care. 16 The aim of this study was to identify subgroups of patients with comparatively high LRC and a low risk of distant metastasis when treated with RT alone.
Materials and Methods
Study Population
With institutional research ethics board approval, we assembled a cohort of patients with newly diagnosed oropharyngeal and laryngohypopharyngeal squamous cell carcinoma (SCC) treated with definitive altered‐fractionation RT or concurrent chemoradiotherapy (CCRT) between 2005 and 2017. The time cohort was chosen to coincide with the routine use of intensity‐modulated RT and subsequently daily image‐guided, intensity‐modulated RT. OPSCCs without HPV testing were excluded, as were HPV+ laryngohypopharyngeal SCCs because of the uncertain significance of HPV positivity at these disease sites. Cases treated with conventional‐fractionation or nonstandard‐fractionation RT‐alone schemas were excluded because of insufficient sample sizes. Patients receiving other systemic agents, such as epidermal growth factor receptor inhibitors or experimental immunotherapy agents, were also excluded because of the insufficient sample sizes over the study period.
Clinical characteristics, including treatment failures and Radiation Therapy Oncology Group grade 3/4 late toxicities, were collected prospectively. Patients were staged and treated according to the seventh edition of the TNM system (TNM‐7). Survival outcomes were supplemented by linkage to the Ontario provincial cancer registry. The tumor HPV status was ascertained by p16 staining (diffuse staining in >70% of tumor cells) supplemented by polymerase chain reaction for equivocal p16 staining cases. 17 , 18
Treatment and Outcome Assessment
All patients were managed with multidisciplinary input according to institutional protocols as previously described. 19 Treatment decisions were based on patient wishes and multidisciplinary team discussions including at least a radiation oncologist, a medical oncologist, and a surgical oncologist. Challenging cases or deviations of care from standard institutional protocols were discussed in weekly multidisciplinary tumor boards. RT target volumes were peer‐reviewed in a weekly RT quality assurance round. All patients were treated with daily image guidance before the delivery of RT.
Generally, stages I and II were treated with RT alone. Stage III was treated with either altered‐fractionation RT alone or CCRT. Stage IV was treated with CCRT, whereas altered‐fractionation RT alone was reserved for patients who had declined or had contraindications to chemotherapy. CCRT comprised high‐dose (100 mg/m2) cisplatin delivered every 3 weeks (days 1, 22, and 43)—or occasionally weekly (40 mg/m2) when concerns about chemotherapy tolerance existed—concurrently with RT (70 Gy in 35 fractions over 7 weeks). The most commonly used altered‐fractionation RT‐alone regimens were RT‐hypo with 60 Gy in 25 fractions over 5 weeks (60 Gy/25f/5w; 2.4 Gy per fraction, 5 fractions per week) 8 and moderately accelerated radiotherapy (RT‐acc) delivering 70 Gy in 35 fractions over 6 weeks (70 Gy/35f/6w; 2.0 Gy per fraction; requiring an additional weekly fraction delivered twice daily 6 hours apart on 1 day per week). 20 The biologically effective dose (BED) when α/β equals 10 for a tumor 21 , 22 , 23 and dose levels to gross target volumes and elective target volumes are summarized in Table 1. We estimated the average gains in tumor control from adding chemotherapy to radiation as equivalent to 8.8 Gy as per Fowler. 24 Hyperfractionated accelerated radiotherapy delivered with integrated neck surgery (HARDWINS; 64 Gy in 40 fractions over 4 weeks, 1.6 Gy per fraction, 10 fractions per week) 25 was also used for patients with bulky laryngeal tumors who were unable to receive chemotherapy. These patients have been excluded from this analysis because HARDWINS exposes patients to 20 days of twice daily fractionation, which would be inappropriate in the COVID‐19 pandemic environment and should be avoided in elderly patients, who are unlikely to experience a benefit from intense‐dose fractionation 11 and are also the most vulnerable to COVID‐19.
TABLE 1.
Dose Targets for Different Radiation Fractionation Regimens for Intensity‐Modulated Radiation Therapy With Simultaneously Integrated Boost
| RT‐hypo a | RT‐acc b | With Chemotherapy c | |
|---|---|---|---|
| BED10 (for tumor) d | 71.76 Gy | 76.74 Gy | 72.12 + 8.80 = 80.92 Gy e |
| Dose to gross targets | 60.0 Gy | 70.0 Gy | 70.0 Gy |
| Dose to intermediate targets | 56.0 Gy | 63.0 Gy | 63.0 Gy |
| Dose to elective targets | 50.0 Gy | 56.0 Gy | 56.0 Gy |
Abbreviations: BED10, biologically effective dose when the α/β ratio equals 10; RT‐acc, moderately accelerated radiotherapy; RT‐hypo, hypofractionated radiotherapy.
Sixty Gy in 25 fractions over 5 weeks (5 fractions per week, every day).
Seventy Gy in 35 fractions over 6 weeks (6 fractions per week, every day, twice a day once per week, 6 hours apart).
Seventy Gy in 35 fractions over 7 weeks (5 fractions per week, every day).
BED10 was calculated with the following formula:
BED10 = D × [1 + d/(α/β)] – [(0.693/α) × (T – Tk )/Tp ]
where D is the total radiation dose, d is the radiation dose per fraction, α/β is equal to 10 Gy for tumors, α is equal to 0.35G–1, T is the overall treatment time (the first treatment is assumed to occur on day 0 when the overall treatment time is calculated), Tk is the onset time for accelerated repopulation (estimated to be 28 days), and Tp is the average doubling time during accelerated repopulation (3 days).
Chemotherapy added 8.8 Gy to the BED10 for the regimen with 70 Gy in 35 fractions over 7 weeks.
Patients were assessed for a treatment response by clinical/endoscopic examination and head and neck computed tomography or magnetic resonance imaging 3 months after the completion of RT. Salvage surgery was undertaken, if feasible, for any residual disease. Follow‐up surveillance was conducted at 3‐month intervals for the first 2 years, at 4‐month intervals during the third year, at 6‐month intervals in the fourth and fifth years, and annually afterward. Locoregional failure (LRF) was confirmed with tissue biopsy. Distant metastasis determination was based on imaging reports with or without biopsy.
Statistical Analysis
Disease control outcomes (LRC and DC) of RT‐hypo (60 Gy/25f/5w) were compared with those of RT‐acc (70 Gy/35f/6w) and standard CCRT and were stratified by TNM‐7 stages for HPV+ OPSCC and HPV– HNSCC cohorts separately. Because laryngohypopharyngeal SCCs are rarely caused by HPV infection, 26 we excluded those HPV+ cases, and we analyzed HPV‐untested and HPV– laryngohypopharyngeal SCC together with HPV– OPSCC as an HPV– HNSCC cohort. LRC, DC, and Radiation Therapy Oncology Group grade 3/4 late toxicities were calculated via competing risk methods with Gray's test for comparison, where deaths without the event of interest were considered as competing risks.
Multivariable analyses, adjusted for smoking and age, were performed if the actuarial rates were significantly different among treatment regimens. All times to events were calculated from the date of RT completion. All tests were 2‐tailed, with a probability <.05 considered statistically significant. Statistical analyses were performed with R version 3.1.2 and SAS 9.4.
Results
Of the 2900 consecutive patients with HNSCC treated with definitive image‐guided, intensity‐modulated RT, 861 were excluded for the following reasons: OPSCC with an unascertained HPV status (n = 135), HPV+ laryngohypopharyngeal SCC (n = 32), targeted therapy or immunotherapy (mostly due to trial enrollment; n = 384), and other RT regimens (n = 310). The remaining 2039 patients (70%) were eligible for analysis; they included 994 HPV+ patients with OPSCC, 308 HPV– patients with OPSCC, 602 patients with laryngeal SCC, and 135 patients with hypopharyngeal SCC (see the Consolidated Standards of Reporting Trials diagram in the Supporting Information). The clinical characteristics of these 2039 patients appear in Table 2. Of all cases, 1010 (49.5%) were managed with CCRT, and 1029 (50.5%) were managed with RT alone. Not surprisingly, RT‐hypo patients (n = 324) and RT‐acc patients (n = 705) were generally older with a less favorable performance status in comparison with the CCRT cohort, but CCRT cases had higher T and N categories among both HPV+ and HPV– patients.
TABLE 2.
Clinical Characteristics of HNSCC by Treatment Regimen
| Variable | HPV+ OPSCC (n = 994) | HPV– HNSCC (n = 1045) | ||||||
|---|---|---|---|---|---|---|---|---|
| CCRT | RT‐hypo a | RT‐acc b | P | CCRT | RT‐hypo a | RT‐acc b | P | |
| Cases, No. (%) | 679 (68) | 61 (6) | 254 (26) | 331 (32) | 263 (25) | 451 (43) | ||
| Age, median (range), y | 57.4 (22.7‐80.9) | 61 (41.7‐92.2) | 66.8 (33.2‐86.7) | <.001 | 59.8 (31.9‐77.3) | 70.5 (22.3‐91.5) | 69.4 (27.4‐91.3) | <.001 |
| Age, No. (%) | <.001 | <.001 | ||||||
| ≤70 y | 661 (97) | 42 (69) | 162 (64) | 315 (95) | 128 (49) | 243 (54) | ||
| >70 y | 18 (3) | 19 (31) | 92 (36) | 16 (5) | 135 (51) | 208 (46) | ||
| Sex, No. (%) | <.001 | .050 | ||||||
| Female | 104 (15) | 25 (41) | 45 (18) | 69 (21) | 58 (22) | 70 (16) | ||
| Male | 575 (85) | 36 (59) | 209 (82) | 262 (79) | 205 (78) | 381 (84) | ||
| Zubrod PS, No. (%) | <.001 | .290 | ||||||
| 0 | 501 (74) | 40 (66) | 122 (48) | 171 (52) | 133 (51) | 226 (50) | ||
| 1 | 157 (23) | 15 (25) | 110 (43) | 136 (41) | 96 (37) | 177 (39) | ||
| 2‐4 | 21 (3) | 6 (10) | 22 (9) | 22 (7) | 32 (12) | 46 (10) | ||
| Smoking PYs, median (range) | 8 (0‐108) | 20 (0‐135) | 12.2 (0‐80) | .004 | 35 (0‐150) | 40 (0‐120) | 40 (0‐150) | .062 |
| Smoking status, No. (%) | .220 | <.001 | ||||||
| Current | 175 (26) | 20 (33) | 56 (22) | 200 (61) | 110 (42) | 227 (51) | ||
| Former | 261 (38) | 26 (43) | 110 (44) | 107 (32) | 126 (48) | 166 (37) | ||
| None | 243 (36) | 15 (25) | 86 (34) | 23 (7) | 26 (10) | 56 (12) | ||
| Unknown | 0 | 0 | 2 | 1 | 1 | 2 | ||
| Excessive alcohol, No. (%) | .180 | <.001 | ||||||
| Yes | 217 (33) | 21 (36) | 76 (31) | 215 (65) | 110 (44) | 211 (49) | ||
| No | 446 (67) | 38 (64) | 171 (69) | 112 (35) | 138 (56) | 223 (51) | ||
| Unknown | 16 | 2 | 7 | 4 | 15 | 17 | ||
| TNM‐7 T category, No. (%) | <.001 | <.001 | ||||||
| T1‐T2 | 380 (56) | 47 (77) | 162 (64) | 85 (26) | 210 (80) | 267 (59) | ||
| T3‐T4 | 299 (44) | 14 (23) | 92 (36) | 246 (75) | 53 (20) | 284 (41) | ||
| TNM‐7 N category, No. (%) | <.001 | <.001 | ||||||
| N0‐N2a | 72 (11) | 40 (66) | 93 (36) | 83 (25) | 225 (85) | 334 (74) | ||
| N2b | 341 (50) | 16 (26) | 104 (41) | 104 (31) | 18 (7) | 57 (13) | ||
| N2c | 204 (30) | 5 (8) | 47 (19) | 113 (34) | 16 (6) | 48 (10) | ||
| N3 | 62 (9) | 0 (0) | 10 (4) | 31 (9) | 4 (2) | 12 (3) | ||
| Cycles of chemotherapy, No. (%) c | NA | NA | NA | NA | ||||
| 1 or 2 | 679 (72) | NA | NA | 225 (68) | NA | NA | ||
| 3 | 193 (28) | NA | NA | 106 (32) | NA | NA | ||
| RT completion, No. (%) | .710 | .270 | ||||||
| No | 16 (2) | 0 (0) | 6 (2) | 9 (2) | 3 (1) | 14 (3) | ||
| Yes | 663 (98) | 61 (100) | 248 (98) | 322 (98) | 260 (99) | 437 (97) | ||
| RT break, No. (%) | .230 | .011 | ||||||
| No | 561 (83) | 47 (77) | 216 (86) | 254 (77) | 227 (86) | 364 (81) | ||
| Yes | 117 (17) | 14 (23) | 36 (14) | 77 (23) | 36 (14) | 85 (19) | ||
| 3‐y outcomes, % (95% CI) | ||||||||
| Overall survival | 91 (88‐93) | 73 (63‐86) | 82 (77‐87) | <.001 | 63 (57‐68) | 71 (66‐77) | 68 (64‐73) | .500 |
| Local control | 98 (96‐99) | 95 (85‐98) | 95 (92‐97) | .097 | 85 (80‐88) | 84 (79‐88) | 82 (78‐85) | .698 |
| Regional control | 95 (94‐97) | 95 (85‐98) | 96 (92‐98) | .994 | 86 (81‐89) | 94 (90‐96) | 91 (88‐93) | .003 |
| Distant control | 90 (87‐92) | 90 (79‐95) | 90 (85‐93) | .876 | 79 (74‐83) | 95 (92‐97) | 88 (84‐90) | <.001 |
| Grade 3/4 LT | 16 (14‐19) | 9 (4‐20) | 12 (8‐17) | .034 | 21 (17‐26) | 4 (2‐8) | 11 (8‐14) | <.001 |
| Locoregional control | 94 (92‐95) | 92 (81‐96) | 94 (90‐96) | .901 | 76 (71‐80) | 80 (75‐85) | 77 (73‐81) | .442 |
Abbreviations: CCRT, concurrent chemoradiotherapy;HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; LT, late toxicity; NA, not applicable; OPSCC, oropharyngeal squamous cell carcinoma; PS, performance status scale; PY, pack‐year; RT, radiotherapy; RT‐acc, moderately accelerated radiotherapy; RT‐hypo, hypofractionated radiotherapy; TNM‐7, seventh edition of the TNM system. 95% CI: 95% confidence interval. Bold values denote statistical significance at the p <0.05 level.
Sixty Gy in 25 fractions over 5 weeks.
Seventy Gy in 35 fractions over 6 weeks.
Weekly cisplatin was converted to cycles: 1 to 3 doses were considered 1 cycle, 4 to 5 doses were considered 2 cycles, and 6 to 7 doses were considered 3 cycles.
Outcome of the HPV+ OPSCC Group
The median follow‐up was 4.8 years. No significant differences in LRC or DC were found among the T1‐T2N0, T1‐T2N1‐N2a, T1‐2N2b, or T1‐2N2c/T3N0‐N2c subgroups by treatment regimens. However, the CCRT cohort had significantly higher DC for the T4 or N3 subgroup in comparison with the RT‐hypo or RT‐acc regimens: the 3‐year DC rates were 87% (95% CI, 80%‐91%), 67% (95% CI, 7%‐88%), and 65% (95% CI, 46%‐77%), respectively (P = .005; Table 3 and Fig. 1). Multivariable analysis confirmed the effect of CCRT in distant metastasis risk reduction (P = .03; Table 4).
TABLE 3.
Three year Outcomes of HPV+ Oropharyngeal Squamous Cell Carcinoma and HPV– Head and Neck Squamous Cell Carcinoma
| Subgroup by TNM‐7 | Treatment Regimen | Cases, No. | LRC, % (95% CI) | DC, % (95% CI) | Grade 3/4 LT, % (95% CI) |
|---|---|---|---|---|---|
| HPV+ oropharyngeal squamous cell carcinoma | |||||
| T1‐T2N0 | CCRT | 0 | NA | NA | NA |
| RT‐hypo | 15 | 100 | 93 (52‐99) | 0 | |
| RT‐acc | 24 | 100 | 96 (69‐99) | 6 (1‐39) | |
| P | 39 | .524 | .757 | .428 | |
| T1‐T2N1‐N2a | CCRT | 36 | 94 (78‐99) | 94 (78‐99) | 8 (3‐25) |
| RT‐hypo | 18 | 94 (61‐99) | 94 (60‐99) | 0 | |
| RT‐acc | 48 | 100 | 100 | 2 (0‐17) | |
| P | 102 | .769 | .272 | .034 | |
| T1‐2N0‐2a | CCRT | 36 | 94 (78‐99) | 94 (78‐99) | 8 (3‐25) |
| RT‐hypo | 33 | 97 (78‐100) | 94 (76‐98) | 0 | |
| RT‐acc | 72 | 100 | 99 (90‐100) | 4 (1‐15) | |
| P | 141 | .527 | .742 | .015 | |
| T1‐2N2b | CCRT | 227 | 97 (94‐99) | 95 (91‐97) | 12 (8‐18) |
| RT‐hypo | 10 | 90 (29‐99) | 100 | 34 (12‐96) | |
| RT‐acc | 74 | 94 (84‐98) | 96 (87‐99) | 14 (7‐26) | |
| P | 311 | .445 | .697 | .056 | |
| T1‐2N2c/T3N0‐2c | CCRT | 241 | 95 (92‐97) | 87 (82‐91) | 16 (12‐21) |
| RT‐hypo | 9 | 89 (20‐98) | 89 (20‐98) | 11 (1‐92) | |
| RT‐acc | 62 | 93 (82‐97) | 90 (78‐95) | 14 (7‐28) | |
| P | 312 | .494 | .838 | .622 | |
| T4 or N3 | CCRT | 175 | 88 (82‐92) | 87 (80‐91) | 24 (18‐31) |
| RT‐hypo | 9 | 78 (16‐94) | 67 (7‐88) | 14 (2‐101) | |
| RT‐acc | 46 | 84 (68‐92) | 65 (46‐77) | 17 (9‐34) | |
| P | 230 | .677 | .005 | .510 | |
| HPV– head and neck squamous cell carcinoma | |||||
| Stage I/II | CCRT | 2 a | 100 | 100 | 0 |
| RT‐hypo | 179 | 85 (79‐90) | 99 (96‐100) | 5 (2‐9) | |
| RT‐acc | 209 | 89 (84‐93) | 98 (94‐99) | 6 (3‐10) | |
| P | 390 | .320 | .446 | .891 | |
| Stage III | CCRT | 48 | 91 (76‐96) | 90 (76‐95) | 20 (11‐36) |
| RT‐hypo | 39 | 76 (58‐87) | 92 (77‐97) | 3 (0‐20) | |
| RT‐acc | 98 | 69 (58‐77) | 85 (76‐91) | 9 (5‐17) | |
| P | 185 | .006 | .410 | .031 | |
| Stage IV | CCRT | 281 | 73 (68‐78) | 77 (71‐81) | 21 (17‐27) |
| RT‐hypo | 45 | 65 (46‐77) | 82 (66‐90) | 3 (0‐22) | |
| RT‐acc | 144 | 66 (57‐73) | 75 (66‐81) | 20 (14‐28) | |
| P | 470 | .336 | .715 | .008 | |
| HPV– oropharyngeal squamous cell carcinoma | |||||
| Stage I/II/III | CCRT | 14 | 85 (43‐96) | 93 (49‐99) | 29 (12‐68) |
| RT‐hypo | 42 | 88 (72‐95) | 100 | 5 (1‐21) | |
| RT‐acc | 38 | 87 (70‐94) | 97 (80‐100) | 8 (3‐24) | |
| P | 94 | .946 | .254 | .102 | |
| Stage IV | CCRT | 125 | 76 (67‐83) | 80 (72‐86) | 29 (21‐38) |
| RT‐hypo | 17 | 57 (21‐76) | 76 (43‐90) | 7 (1‐63) | |
| RT‐acc | 72 | 62 (49‐72) | 76 (63‐84) | 13 (7‐26) | |
| P | 147 | .100 | .655 | .058 | |
| HPV– laryngeal squamous cell carcinoma | |||||
| Stage I/II | CCRT | 2 a | 100 | 100 | 0 |
| RT‐hypo | 141 | 83 (76‐89) | 99 (95‐100) | 4 (2‐10) | |
| RT‐acc | 194 | 89 (83‐93) | 98 (94‐99) | 5 (3‐10) | |
| P | 337 | .146 | .542 | .792 | |
| Stage III | CCRT | 28 | 92 (69‐98) | 89 (68‐96) | 12 (4‐35) |
| RT‐hypo | 26 | 80 (55‐91) | 88 (66‐96) | 4 (1‐31) | |
| RT‐acc | 67 | 62 (47‐72) | 81 (68‐89) | 10 (5‐21) | |
| P | 121 | .004 | .444 | .413 | |
| Stage IV | CCRT | 86 | 71 (60‐80) | 77 (66‐85) | 12 (7‐22) |
| RT‐hypo | 18 | 71 (37‐87) | 82 (49‐94) | 0 | |
| RT‐acc | 40 | 74 (54‐85) | 78 (59‐88) | 15 (6‐33) | |
| P | 144 | .886 | .982 | .279 | |
| HPV– hypopharyngeal squamous cell carcinoma | |||||
| Stage I/II/III | CCRT | 6 | 100 | 83 0‐98) | 33 (10‐100) |
| RT‐hypo | 9 | 75 (7‐93) | 100 | 0 | |
| RT‐acc | 8 | 75 (9‐93) | 88 (11‐98) | 12 (2‐93) | |
| P | 23 | .424 | .545 | .187 | |
| Stage IV | CCRT | 70 | 71 (58‐80) | 71 (58‐80) | 19 (11‐32) |
| RT‐hypo | 10 | 70 (18‐89) | 90 (28‐99) | 0 | |
| RT‐acc | 32 | 66 (42‐80) | 67 (44‐81) | 40 (25‐65) | |
| P | 112 | .962 | .325 | .022 |
Abbreviations: CCRT, concurrent chemoradiotherapy;DC, distant control; HPV, human papillomavirus; LRC, locoregional control; LT, late toxicity; NA, not applicable; RT‐acc, moderately accelerated radiotherapy; RT‐hypo, hypofractionated radiotherapy; TNM‐7, seventh edition of the TNM system. Bold values denote statistical significance at the p <0.05 level.
The 2 stage II cases were T2N0 glottic squamous cell carcinoma with a bulky primary and suspicious T3 disease (suspicious thyroid cartilage invasion on computed tomography). Hence, these were treated as T3 tumors with CCRT.
FIGURE 1.
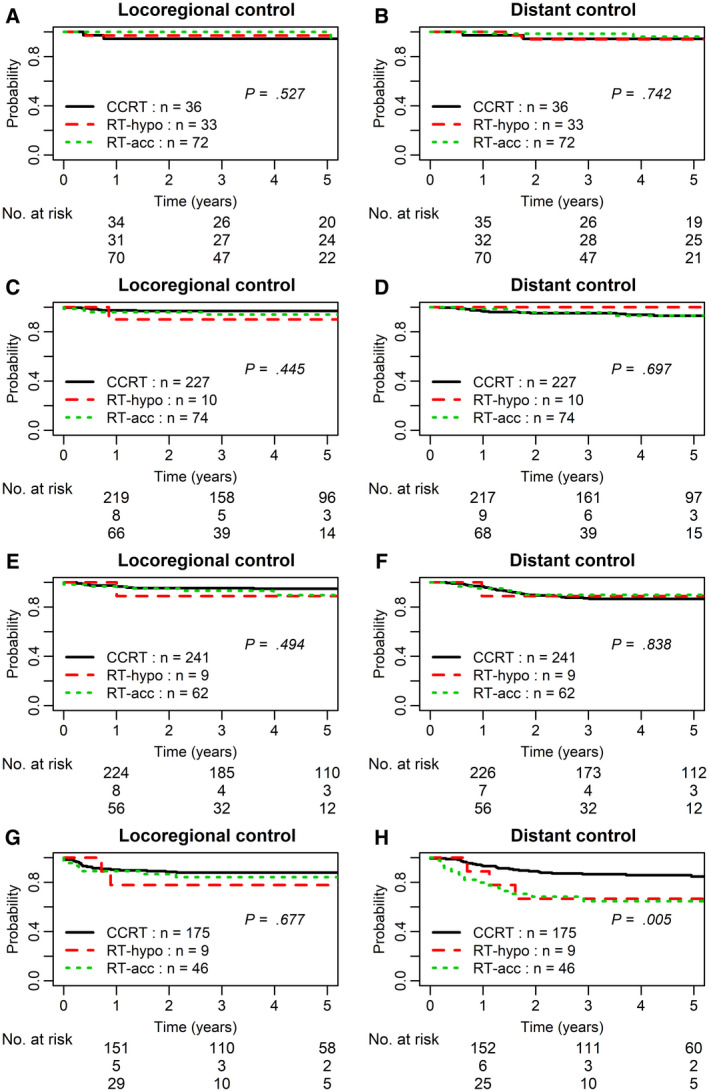
Locoregional control and distant control of human papillomavirus–positive oropharyngeal squamous cell carcinoma by treatment regimen: (A,B) T1‐2N0‐N2a subset, (C,D) T1‐2N2b subset, (E,F) T1‐2N2c/T3N0‐N2c subset, and (G,H) T4 or N3 subset. RT‐hypo refers to hypofractionated radiotherapy alone with 60 Gy in 25 fractions over 5 weeks (2.4 Gy per fraction, 5 fractions per week); RT‐acc refers to moderately accelerated radiotherapy alone with 70 Gy in 35 fractions delivered over 6 weeks (6 fractions per week), usually with additional twice daily treatment 6 hours apart on 1 day per week; CCRT, concurrent chemoradiotherapy.
TABLE 4.
Multivariable Analysis for Subgroups With Significant Differences Among Treatment Regimens
| T4 or N3 HPV+ OPSCC | HR (95% CI) | P | Global P |
|---|---|---|---|
| Distant Metastasis | |||
| RT regimen | .030 | ||
| CCRT | Reference | ||
| RT‐hypo | 1.67 (0.44‐6.30) | .450 | |
| RT‐acc | 2.61 (1.28‐5.31) | .008 | |
| Age | .440 | ||
| ≤70 y | Reference | ||
| >70 y | 1.36 (0.62‐3.01) | ||
| Smoking status | .100 | ||
| None/ex‐smoker | Reference | ||
| Current smoker | 1.70 (0.90‐3.21) | ||
| Stage III HPV– HNSCC | HR (95% CI) | P | Global P |
|---|---|---|---|
| Locoregional Failure | |||
| RT regimen | .011 | ||
| CCRT | Reference | ||
| RT‐hypo | 3.40 (1.05‐10.99) | .041 | |
| RT‐acc | 4.89 (1.69‐14.14) | .003 | |
| Age | .690 | ||
| ≤70 y | Reference | ||
| >70 y | 0.88 (0.45‐1.69) | ||
| Smoking status | .180 | ||
| None/ex‐smoker | Reference | ||
| Current smoker | 0.65 (0.35‐1.23) | ||
| Stage III Laryngeal SCC | HR (95% CI) | P | Global P |
|---|---|---|---|
| Locoregional Failure | |||
| RT regimen | .004 | ||
| CCRT | Reference | ||
| RT‐hypo | 3.90 (0.76‐19.98) | .100 | |
| RT‐acc | 8.95 (2.15‐37.26) | .003 | |
| Age | .230 | ||
| ≤70 y | Reference | ||
| >70 y | 0.60 (0.26‐1.37) | ||
| Smoking status | .710 | ||
| None/ex‐smoker | Reference | ||
| Current smoker | 0.86 (0.39‐1.91) | ||
Abbreviations: CCRT, concurrent chemoradiotherapy; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; HR, hazard ratio; OPSCC, oropharyngeal squamous cell carcinoma; RT, radiotherapy; RT‐acc, moderately accelerated radiotherapy; RT‐hypo, hypofractionated radiotherapy; SCC, squamous cell carcinoma. Bold values denote statistical significance at the p <0.05 level.
Outcome of the HPV– HNSCC Group
The median follow‐up was 4.5 years. The CCRT, RT‐hypo, and RT‐acc regimens all had high 3‐year LRC (100%, 85%, and 89%; P = .320) and DC (100%, 99%, and 98%; P = .446) in the stage I/II patients. Notably, two T2N0 stage II glottic SCC cases received CCRT because of bulky primaries with suspicious but not definitive thyroid cartilage invasion on computed tomography, and they were downstaged to stage II according to TNM staging rules 27 , 28 but were treated as stage III cancers. For stage III HPV– HNSCC, both RT‐hypo and RT‐acc regimens had reduced LRC in comparison with CCRT with 3‐year LRC rates of 76% (95% CI, 58%‐87%), 69% (95% CI, 58%‐77%), and 91% (95% CI, 76%‐96%), respectively (P = .006; Table 3 and Fig. 2). Multivariable analysis confirmed the effect of chemotherapy on the reduction of LRF risks (P = .011; Table 4). The significantly higher risk of LRF mainly occurred in stage III laryngeal SCC treated with RT‐acc (92% [95% CI, 69%‐98%] vs 62% [95% CI, 47%‐72%]; P = .004) but not with RT‐hypo (92% [95% CI, 69%‐98%] vs 80% [95% CI, 55%‐91%]; P = .270; Table 3).
FIGURE 2.
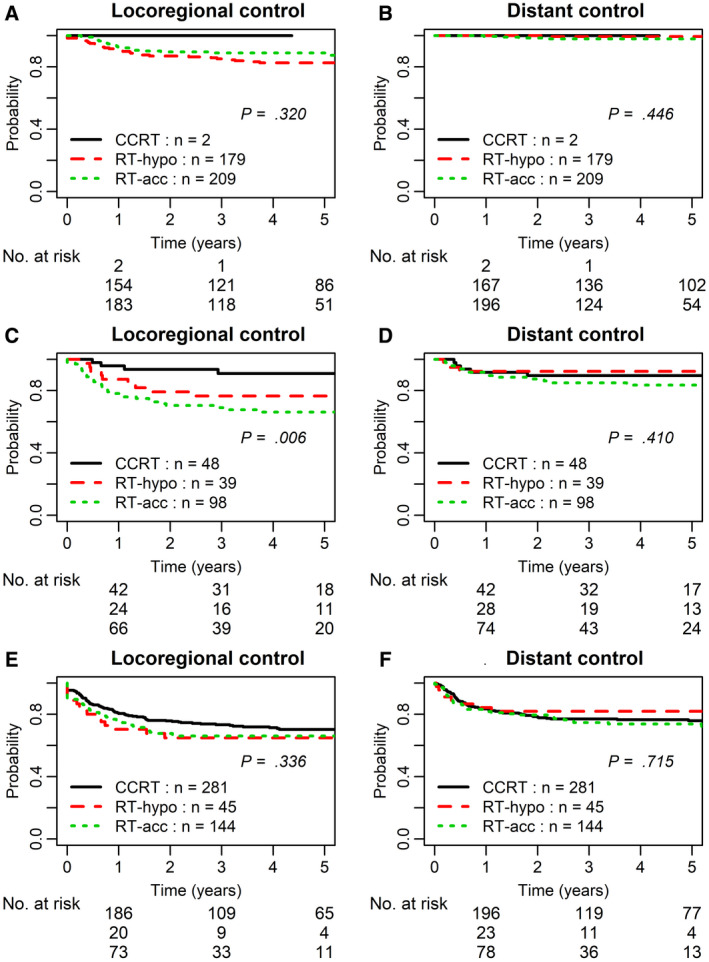
Locoregional control and distant control of human papillomavirus–negative head and neck squamous cell carcinoma by treatment modality: (A,B) stage I/II subset, (C,D) stage III subset, and (E,F) stage IV subset. RT‐hypo refers to hypofractionated radiotherapy alone with 60 Gy in 25 fractions over 5 weeks (2.4 Gy per fraction, 5 fractions per week); RT‐acc refers to moderately accelerated radiotherapy alone with 70 Gy in 35 fractions delivered over 6 weeks (6 fractions per week), usually with additional twice daily treatment 6 hours apart on 1 day per week; CCRT, concurrent chemoradiotherapy.
Late Toxicity
Grade 3 and 4 late toxicities were observed in all cohorts but were more frequent in the CCRT cohorts for both HPV+ OPSCC (16% [95% CI, 14%‐19%], 9% [95% CI, 4%‐20%], and 12% [95% CI, 8%‐17%]; P = .034) and HPV‐HNSCC (21% [95% CI, 17%‐26%], 4% [95% CI, 2%‐8%], and 11% [95% CI, 8%‐14%]; P < .001) with CCRT, RT‐hypo, and RT‐acc, respectively (Table 2).
Discussion
The COVID‐19 pandemic necessitates the need to significantly reassess usual‐care processes and may mandate pragmatic decision making, including minimizing ambulatory RT visits to the cancer center by using shorter RT courses, avoiding twice daily RT, and balancing the risk and benefit of chemotherapy. However, robust contemporary data for shorter course RT‐hypo are lacking, especially for once daily treatments.
This single‐institution cohort study assigned treatment nonrandomly, but we cautiously suggest that T1‐T3N0‐N2c HPV+ OPSCC and T1‐T2N0 HPV– HNSCC have comparable LRC and DC with either RT‐hypo (60 Gy/25f/5w) or RT‐acc (70 Gy/35f/6w) in comparison with CCRT; we acknowledge small sample sizes in some subgroups and a potential selection bias. The benefit of CCRT is evident in T4 or N3 HPV+ OPSCC. For stage III HPV– HNSCC, the benefit of chemotherapy is most evident, mainly in stage III laryngeal SCC in comparison with RT‐acc, but the benefit (if any) is less obvious in comparison with RT‐hypo. For stage IV HPV– HNSCC, LRC with CCRT is numerically but nonsignificantly higher, and this is likely related to a selection bias. The results were not predicted by BED calculations, which are subject to variations in their inherent assumptions. 22 , 23
In the absence of robust level I evidence from randomized controlled trials, this analysis provides benchmarks for LRC and DC rates with RT alone in a contemporaneously treated cohort of different subgroups of HPV+ and HPV– HNSCC. The results are relevant to COVID‐19 pandemic strategic planning for patients with HNSCC when taking shorter courses without twice daily treatment and avoiding immunosuppression by chemotherapy may become necessary. We have identified patient cohorts for whom LRC and DC are similar or minimally compromised in the absence of a prolonged course of radiation and concurrent chemotherapy. In addition, updated outcome data with hypofractionation or accelerated RT alone will facilitate discussions with patients who are unfit for chemotherapy.
The outcomes of RT alone in this study require cautious interpretation because of the nonrandom nature of treatment assignment, which is subject to a selection bias and other unquantifiable factors. On the one hand, CCRT is often offered for more advanced‐stage and bulky tumors; on the other hand, RT alone is often offered to frail, elderly patients. In addition, some subgroups represent very small sample sizes. Nonetheless, this study is not intended to suggest replacing CCRT with RT alone as the standard of care for LAHNSCC but rather provides an alternative locoregional treatment strategy for those with relatively low risks of distant metastasis. Although patients with T4 and N2c to N3 HNSCC clearly need chemotherapy for optimal disease control, stage III disease and some stage IV disease with relatively small nodal volumes and nonbulky primary tumors may be considered for altered‐fractionation RT approaches, especially in a crisis‐planning setting where risk and benefit need to be balanced. It must be emphasized that this report reflects a quaternary cancer center experience where multidisciplinary cancer conferences and radiation oncology peer‐review quality assurance routinely guide practice.
Several altered‐fractionation RT regimens have been reported in the literature. 29 , 30 , 31 , 32 Large fraction sizes may help to overcome radioresistance; however, late toxicity is a concern. Hypofractionation with 60 Gy/25f/5w with 2.4 Gy per fraction appears to be a well‐tolerated regimen and is a potentially attractive approach during COVID‐19 planning because of its relatively short treatment timeframe and avoidance of twice daily treatments. This regimen was reported by Jackson et al 8 in 2001 as a dose‐escalation strategy for T3 glottic cancer, and it is minimally less intense in comparison with the protocol of 62.5 Gy in 25 fractions recently described by Thomson et al 29 for intermediate‐stage HNSCC in a phase 2 trial, albeit with the inclusion of cetuximab as a potential radiosensitizer. Early and late BED calculations (not shown in the current article) for the slight fraction size increase in Thomson et al's schedule are closer to the more traditional schedule of 2.0 Gy × 35 daily fractions and, therefore, could provide a similar reasonable alternative. We have used the 2.4 Gy per fraction schedule since 1999 for T2 to T3 glottic cancer 33 and have expanded it to other patients with LAHNSCC unsuitable for chemotherapy, 34 and we have found it generally well tolerated. Further intensification by adding an additional 4.8 Gy (2.4 Gy × 2 fractions) for a total of 64.8 Gy in 27 fractions was explored in the recently reported Japan Clinical Oncology Group 0701 randomized trial, 35 although our group has no direct experience with this schedule. A major concern for RT‐hypo is potential late toxicity, (eg, chondronecrosis or severe fibrosis), especially consequential injuries resulting from incomplete resolution of acute mucosal toxicity as well as supraglottis edema, which could result in an airway obstruction leading to temporary or rarely permanent tracheostomy. We did not observe increased late effects in the cohorts managed with hypo‐RT or RT‐acc regimens, although this endpoint would have been influenced by the fact that CCRT was delivered to more patients with advanced‐stage disease. If an additional “boost” dose is advocated beyond the 60 Gy in 25 fractions used in this report, we recommend that it should be delivered to a carefully defined small volume with clear spatial separation from critical structures, including bone and major neurovascular structures (eg, the carotid artery).
Concurrent cisplatin chemotherapy for more advanced‐stage disease, regardless of the tumor HPV status, remains the current standard of care and should be continued as long as center resources permit. Our study shows that cisplatin enhances LRC in HPV– HNSCC, especially stage III disease, as well as DC in T4 or N3 HPV+ OPSCC. Stage IV HPV– HNSCC had numerically better LRC and DC with the addition of cisplatin, although this did not reach statistical significance. We presume that this is related to a selection bias as well as the small sample sizes of the RT‐alone regimens. LAHNSCCs with bulkier tumors or with very adverse nodal burdens with extranodal extension are most likely to receive CCRT. Such tumors may intrinsically possess more aggressive tumor biology. In addition, approximately two‐thirds of HPV– patients with LAHNSCC receiving CCRT in this study were unable to complete 3 cycles of chemotherapy because of acute toxicities and poor tolerance, as reported previously, 36 and this may contribute to lower LRC in stage IV HPV– LAHNSCC (only 70%). In addition, many HPV– patients with LAHNSCC are unable to receive chemotherapy because of an elderly age or comorbidities closely related to their lifestyle (smoking or alcohol). Alternative approaches are needed.
The decision to adapt HNSCC treatment approaches in the COVID‐19 environment is not simple. Balances must be achieved between risks and benefits that are highly contingent on a rapidly changing pandemic environment, with consideration given to each individual patient in terms of both his or her cancer and the consequences of a COVID‐19 infection. Thomson et al 37 have recently published American Society of Radiation Oncology–European Society for Radiotherapy and Oncology consensus recommendations for risk‐adapted RT of HNSCC in the COVID‐19 environment. There was a strong agreement to continue concomitant chemoradiotherapy when indicated as a standard of care in early phases of the pandemic, whereas with progression into a later scenario of severe resource constraints, there was a similarly strong agreement to transition to RT‐hypo schedules.
As COVID‐19 infection becomes more prevalent in the community, the risk of contracting infection will increase for patients with cancer and health care workers, and this will be a precursor to the reduction of treatment resources. Hopefully, societal responses such as testing, contact tracing, isolation, and social distancing will mitigate or even prevent a severe reduction in the capacity to treat. The incremental introduction of RT‐hypo to replace CCRT in appropriately selected patients with HNSCC may be beneficial.
In conclusion, albeit with obvious limitations, this large cohort study shows that once daily RT‐hypo (60 Gy/25f/5w, 2.4 Gy per fraction, 5 fractions per week) has LRC and DC comparable to those with RT‐acc (70 Gy/35f/6w, 2.0 Gy per fraction, 6 fractions per week) administered over 6 weeks, which also requires additional twice daily RT once weekly, and standard CCRT delivered over 7 weeks for T1‐3N0‐N2c HPV+ OPSCC and T1‐T2N0 HPV– HNSCC. We propose that the uneasy trade‐off from omitting chemotherapy can be considered by using shorter course RT during the COVID‐19 outbreak for 1) HPV+ TNM‐7 T1‐T3/N0‐N2c disease and 2) HPV– T1‐T2N0 disease. RT‐hypo may also be considered for stage III (T1‐2N1/T3N1) HPV– HNSCC with less bulky primaries or low‐volume nodal disease. It represents an attractive approach for the treatment of such patients during the COVID‐19 outbreak when shorter and fewer hospital visits and avoidance of immunosuppressive chemotherapy are needed to reduce the risk of contracting the SARS‐CoV‐2 virus. It also provides an alternative intensification strategy for patients who are unable to receive chemotherapy. Careful patient selection that balances the risks and benefits of treatment is paramount for optimizing HNC care in this setting. During the COVID‐19 pandemic, shorter courses of fractionated RT without potentially immunosuppressive chemotherapy have re‐emerged as a strategy for treating patients effectively while protecting them from undue or prolonged exposure within the health care environment.
Funding Support
The authors acknowledge the Bartley‐Smith/Wharton Fund, the Gordon Tozer Fund, the Wharton Head and Neck Translational Fund, the Discovery Fund, the Dr. Mariano Elia Fund, the Petersen‐Turofsky Fund, and the Joe and Cara Finley Center for Head and Neck Cancer Research at the Princess Margaret Cancer Foundation for supporting the academic activities of Shao Hui Huang, John Waldron, Jie Su, Wei Xu, Li Tong, and Brian O’Sullivan. They also acknowledge the O. Harold Warwick Prize of the Canadian Cancer Society for supporting the academic activities of Brian O’Sullivan.
Conflict of Interest Disclosures
John Kim reports equity in ImmVue Therapeutics, leadership in Ontario Health (Cancer Care Ontario), and leadership in the Radiation Medicine Program of the Princess Margaret Cancer Program outside the submitted work. Meredith Giuliani reports work on advisory boards for Elekta, AstraZeneca, and Bristol‐Myers Squibb and grants from Eli Lilly outside the submitted work. Andrew Hope reports nonfinancial support from Elekta outside the submitted work. Anna Spreafico reports consultancy work for Merck (compensated), Bristol‐Myers Squibb (compensated), Novartis (compensated), Oncorus (compensated), and Janssen (compensated) and grant/research support (clinical trials) from Novartis, Bristol‐Myers Squibb, Symphogen, AstraZeneca/Medimmune, Merck, Bayer, Surface Oncology, Northern Biologics, Janssen Oncology/Johnson & Johnson, Roche, Regeneron, Alkermes, and Array Biopharma outside the submitted work. Aaron R. Hansen reports advisory/consulting/research work for Genentech/Roche, Merck, GlaxoSmithKline, Bristol‐Myers Squibb, Novartis, Boston Biomedical, Boehringer‐Ingelheim, AstraZeneca, and Medimmune outside the submitted work. The other authors made no disclosures.
Author Contributions
Shao Hui Huang: Conceptualization, formal analysis, data curation, data interpretation, manuscript drafting, and manuscript editing. Brian O’Sullivan: Conceptualization, formal analysis, data curation, data interpretation, manuscript drafting, and manuscript editing. Jie Su: Formal analysis, data curation, data interpretation, and manuscript editing. Jolie Ringash: Data curation, data interpretation, and manuscript editing. Scott V. Bratman: Data curation, data interpretation, and manuscript editing. John Kim: Data curation, data interpretation, and manuscript editing. Ali Hosni: Data curation, data interpretation, and manuscript editing. Andrew Bayley: Data curation, data interpretation, and manuscript editing. John Cho: Data curation, data interpretation, and manuscript editing. Meredith Giuliani: Data curation, data interpretation, and manuscript editing. Andrew Hope: Data curation, data interpretation, and manuscript editing. Anna Spreafico: Data curation, data interpretation, and manuscript editing. Aaron R. Hansen: Data curation, data interpretation, and manuscript editing. Lillian L. Siu: Data curation, data interpretation, and manuscript editing. Ralph Gilbert: Data curation, data interpretation, and manuscript editing. Jonathan C. Irish: Data curation, data interpretation, and manuscript editing. David Goldstein: Data curation, data interpretation, and manuscript editing. John de Almeida: Data curation, data interpretation, and manuscript editing. Li Tong: Data curation, data interpretation, and manuscript editing. Wei Xu: Formal analysis, data curation, data interpretation, and manuscript editing. John Waldron: Conceptualization, formal analysis, data curation, data interpretation, manuscript drafting, and manuscript editing.
Supporting information
Supplementary Material
Huang SH, O’Sullivan B, Su J, Ringash J, Bratman SV, Kim J, Hosni A, Bayley A, Cho J, Giuliani M, Hope A, Spreafico A, Hansen AR, Siu LL, Gilbert R, Irish JC, Goldstein D, de Almeida J, Tong L, Xu W, Waldron J. Hypofractionated radiotherapy alone with 2.4 Gy per fraction for head and neck cancer during the COVID‐19 pandemic: The Princess Margaret experience and proposal. Cancer. 2020:126:3426‐3437. 10.1002/cncr.32968
References
- 1. Yu J, Ouyang W, Chua MLK, et al. SARS‐CoV‐2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. Published online March 25, 2020. doi: 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID‐19–infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84:5‐10. [DOI] [PubMed] [Google Scholar]
- 5. You B, Ravaud A, Canivet A, et al. The official French guidelines to protect patients with cancer against SARS‐CoV‐2 infection. Lancet Oncol. Published online March 25, 2020. doi: 10.1016/S1470-2045(20)30204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Felice F, Polimeni A, Valentini V. The impact of coronavirus (COVID‐19) on head and neck cancer patients' care. Radiother Oncol. 2020;147:84‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Withers HR, Peters LJ, Taylor JM, et al. Local control of carcinoma of the tonsil by radiation therapy: an analysis of patterns of fractionation in nine institutions. Int J Radiat Oncol Biol Phys. 1995;33:549‐562. [DOI] [PubMed] [Google Scholar]
- 8. Jackson SM, Hay JH, Flores AD. Local control of T3N0 glottic carcinoma by 60 Gy given over five weeks in 2.4 Gy daily fractions. One more point on the biological effective dose (BED) curve. Radiother Oncol. 2001;59:219‐220. [DOI] [PubMed] [Google Scholar]
- 9. Wylie JP, Sen M, Swindell R, et al. Definitive radiotherapy for 114 cases of T3N0 glottic carcinoma: influence of dose‐volume parameters on outcome. Radiother Oncol. 1999;53:15‐21. [DOI] [PubMed] [Google Scholar]
- 10. Harwood AR, Bryce DP, Rider WD. Management of T3 glottic cancer. Arch Otolaryngol. 1980;106:697‐699. [DOI] [PubMed] [Google Scholar]
- 11. Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta‐analysis. Lancet. 2006;368:843‐854. [DOI] [PubMed] [Google Scholar]
- 12. O’Sullivan B, Hui Huang S, Keane T, et al. Durable therapeutic gain despite competing mortality in long‐term follow‐up of a randomized hyperfractionated radiotherapy trial for locally advanced head and neck cancer. Clin Transl Radiat Oncol. 2020;21:69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous‐cell carcinoma: three meta‐analyses of updated individual data. MACH‐NC Collaborative Group. Meta‐Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949‐955. [PubMed] [Google Scholar]
- 14. Pignon JP, le Maitre A, Maillard E, et al. Meta‐Analysis of Chemotherapy in Head and Neck Cancer (MACH‐NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4‐14. [DOI] [PubMed] [Google Scholar]
- 15. Garden AS, Kies MS, Morrison WH, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol. 2013;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong K, Huang SH, O’Sullivan B, et al. Point‐of‐care outcome assessment in the cancer clinic: audit of data quality. Radiother Oncol. 2010;95:339‐343. [DOI] [PubMed] [Google Scholar]
- 17. Lewis JS Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018;142:559‐597. [DOI] [PubMed] [Google Scholar]
- 18. Fakhry C, Lacchetti C, Rooper LM, et al. Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement of the College of American Pathologists guideline. J Clin Oncol. 2018;36:3152‐3161. [DOI] [PubMed] [Google Scholar]
- 19. Huang SH, O’Sullivan B, Su J, et al. Prognostic importance of radiologic extranodal extension in HPV‐positive oropharyngeal carcinoma and its potential role in refining TNM‐8 cN‐classification. Radiother Oncol. 2020;144:13‐22. [DOI] [PubMed] [Google Scholar]
- 20. Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous‐cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933‐940. [DOI] [PubMed] [Google Scholar]
- 21. Fowler JF. Biological factors influencing optimum fractionation in radiation therapy. Acta Oncol. 2001;40:712‐717. [DOI] [PubMed] [Google Scholar]
- 22. Fowler JF, Harari PM, Leborgne F, et al. Acute radiation reactions in oral and pharyngeal mucosa: tolerable levels in altered fractionation schedules. Radiother Oncol. 2003;69:161‐168. [DOI] [PubMed] [Google Scholar]
- 23. Ho KF, Fowler JF, Sykes AJ, et al. IMRT dose fractionation for head and neck cancer: variation in current approaches will make standardisation difficult. Acta Oncol. 2009;48:431‐439. [DOI] [PubMed] [Google Scholar]
- 24. Fowler JF. Correction to Kasibhatla et al. How much radiation is the chemotherapy worth in advanced head and neck cancer? (Int J Radiat Oncol Biol Phys 2007;68:1491‐1495). Int J Radiat Oncol Biol Phys. 2008;71:326‐329. [DOI] [PubMed] [Google Scholar]
- 25. Waldron J, Warde P, Irish J, et al. A dose escalation study of hyperfractionated accelerated radiation delivered with integrated neck surgery (HARDWINS) for the management of advanced head and neck cancer. Radiother Oncol. 2008;87:173‐180. [DOI] [PubMed] [Google Scholar]
- 26. Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108:djv403. [DOI] [PubMed] [Google Scholar]
- 27. Sobin LH, Brierley J, Gospodarowicz M, et al. Principles of cancer staging. In: O’Sullivan B, Brierley J, D’Cruz A, et al, eds. UICC Manual of Clinical Oncology. 9th ed. John Wiley & Sons Ltd; 2015:34‐39. [Google Scholar]
- 28. Gress D, Edge S, Greene F, et al. Principles of cancer staging. In: Amin M, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:3‐30. [Google Scholar]
- 29. Thomson DJ, Ho KF, Ashcroft L, et al. Dose intensified hypofractionated intensity‐modulated radiotherapy with synchronous cetuximab for intermediate stage head and neck squamous cell carcinoma. Acta Oncol. 2015;54:88‐98. [DOI] [PubMed] [Google Scholar]
- 30. Franzese C, Fogliata A, Franceschini D, et al. Impact of hypofractionated schemes in radiotherapy for locally advanced head and neck cancer patients. Laryngoscope. 2020;130:E163‐E170. [DOI] [PubMed] [Google Scholar]
- 31. Eisbruch A, Harris J, Garden AS, et al. Multi‐institutional trial of accelerated hypofractionated intensity‐modulated radiation therapy for early‐stage oropharyngeal cancer (RTOG 00‐22). Int J Radiat Oncol Biol Phys. 2010;76:1333‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meade S, Gaunt P, Hartley A, et al. Feasibility of dose‐escalated hypofractionated chemoradiation in human papilloma virus–negative or smoking‐associated oropharyngeal cancer. Clin Oncol (R Coll Radiol). 2018;30:366‐374. [DOI] [PubMed] [Google Scholar]
- 33. Rock K, Huang SH, Tiong A, et al. Partial laryngeal IMRT for T2N0 glottic cancer: impact of image guidance and radiation therapy intensification. Int J Radiat Oncol Biol Phys. 2018;102:941‐949. [DOI] [PubMed] [Google Scholar]
- 34. O’Sullivan B, Huang SH, Perez‐Ordonez B, et al. Outcomes of HPV‐related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother Oncol. 2012;103:49‐56. [DOI] [PubMed] [Google Scholar]
- 35. Kodaira T, Kagami Y, Shibata T, et al. Results of a multi‐institutional, randomized, non‐inferiority, phase III trial of accelerated fractionation versus standard fractionation in radiation therapy for T1‐2N0M0 glottic cancer: Japan Clinical Oncology Group Study (JCOG0701). Ann Oncol. 2018;29:992‐997. [DOI] [PubMed] [Google Scholar]
- 36. Spreafico A, Huang SH, Xu W, et al. Impact of cisplatin dose intensity on human papillomavirus–related and –unrelated locally advanced head and neck squamous cell carcinoma. Eur J Cancer. 2016;67:174‐182. [DOI] [PubMed] [Google Scholar]
- 37. Thomson DJ, Palma D, Guckenberger M, et al. Practice recommendations for risk‐adapted head and neck cancer radiotherapy during the COVID‐19 pandemic: an ASTRO‐ESTRO consensus statement. Int J Radiat Oncol Biol Phys. Published online April 14, 2020. doi: 10.1016/j.ijrobp.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


