Abstract
1H–15N HMBC spectra of norditerpenoid alkaloids and their synthetic azabicyclic analogues were obtained to investigate the impacts of the through-space effect of steric compression, protonation, and formation of intramolecular hydrogen bonding on the 15N NMR spectroscopy of these natural products and their piperidine-containing analogues. A rare 15N NMR effect of steric compression is demonstrated in half-cage A/E-rings of norditerpenoid alkaloid free bases and their synthetic azabicyclic analogues, in which the distribution of the lone pair of electrons of the tertiary amine N-atom is sterically restricted by bridged cycloalkanes, e.g., cyclopentane, cyclohexane, and cycloheptane rings. This results in significant changes in the 15N chemical shift, typically by at least ∼10 ppm. The lone pair of electrons of the N-atom in the piperidine ring are sterically compressed whether the bridged cyclohexane ring adopts a chair or boat conformation. The 15N chemical shifts of 1α-OMe norditerpenoid alkaloid free bases significantly increase (ΔδN ≥ 15.6 ppm) on alkaloid protonation and thence the formation of an intramolecular hydrogen bond between N+-H and 1α-OMe. The intramolecular hydrogen bonds between the N-atom and 1α-OH of 1α-OH norditerpenoid alkaloid free bases, karacoline, condelphine, and neoline stabilize their A-rings, adopting an unusual twisted-boat conformation, and they also significantly increase δN of the tertiary amine N-atom.
Introduction
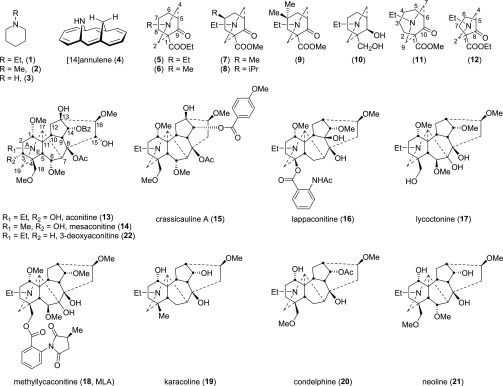
For nonisotopically enriched samples, the sensitivity of 15N NMR is limited by the low natural abundance of 15N nuclei (0.36%, I = 1/2) and its intrinsic low receptivity. Typically, compared with the 1H signals in a sample, the signal-to-noise ratio will be of the order of 104 smaller. This essentially precludes direct measurement without recourse to extended acquisition times or extremely concentrated samples. These limitations can be overcome through judicious use of inverse-detected 15N NMR experiments, e.g., 1H–15N heteronuclear multiple-bond correlation (1H–15N HMBC) spectroscopy.1,2 Chemical shifts of 15N nuclei (δN) in simple aliphatic amines, e.g., N-Et-piperidine (1), N-Me-piperidine (2), and N-H-piperidine (3), have been reported,3−6 and steric and electronic effects of Nα- and Nβ-substituents on δN have also been investigated.6,7 A rare, indeed possibly the only case that has demonstrated the through-space 1H NMR effect of steric compression caused by a secondary amine deshielding a proton that is close to the N-atom in space has been reported for imino[14]annulene (4, Figure 1).8 We are investigating the effects of such a steric compression in the synthetic azabicycles (5–12) and their related norditerpenoid alkaloids (13–21)9,10 and how such steric interactions impact the tertiary amine using 1H–15N HMBC spectroscopy to report on the environment of the N-atom.
Figure 1.
[14]Annulene (4), synthetic azabicycles (5–12), and norditerpenoid alkaloids (13–22).
Results and Discussion
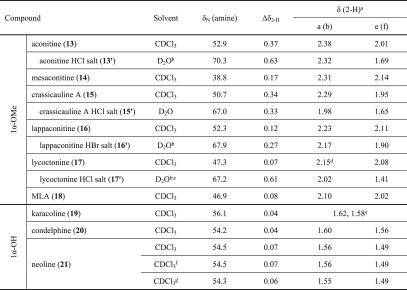
1H–15N HMBC spectroscopic experiments were carried out on three sample piperidines (1–3, Table 1), synthetic azabicycles (5–12, Table 2), and their related norditerpenoid alkaloids (Table 3), aconitine (13), mesaconitine (14), crassicauline A (15), lappaconitine (16), lycoctonine (17), methyllycaconitine (18, MLA), karacoline (19), condelphine (20), and neoline (21). In addition, several protonated forms of these compounds (1′–3′, 13′, and 15′–17′) have been studied. All of the 1H–15N HMBC spectra were externally calibrated with a MeNO2 solution (50% in CDCl3, v/v) following the IUPAC guidelines.11,12
Table 1. δN of Piperidines (δ in ppm).
With additional two drops of DMSO-d6.
With additional drops of NaOD solution (30% in D2O, w/w), pD ∼ 13.
Table 2. Key NMR Data of Synthetic [3.3.1]Azabicycles (δ in ppm).
Orientation label: a = axial, e = equatorial, a′ = pseudo-axial, and e′ = pseudo-equatorial. Chemical shifts of overlapping signals were extracted from HSQC.
With additional two drops of DMSO-d6.
Table 3. Key NMR Data of the Selected Norditerpenoid Alkaloids (δ in ppm).
Orientation label: a = axial, e = equatorial in a chair conformation, b = bowsprit, and f = flagpole in a boat conformation. Chemical shifts of overlapping signals were extracted from HSQC.
With additional two drops of DMSO-d6.
With additional drops of DCl (35% in D2O), pD ∼2.
Chemical shift of this overlapping signal was extracted from COSY.
No evidence was obtained to identify the orientation of these protons unambiguously.
With additional two drops of pyridine-d5.
With additional two drops of pyridine-d5 and two drops of NaOD solution (30% in D2O, w/w).
Steric Compression
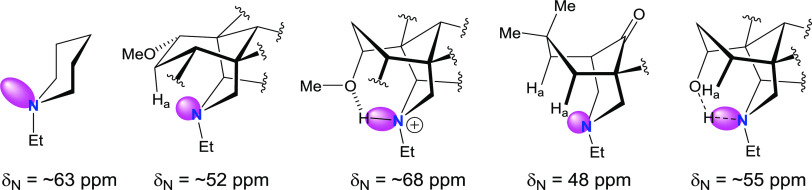
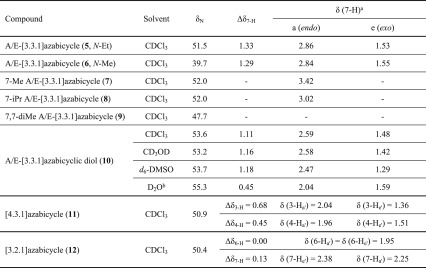
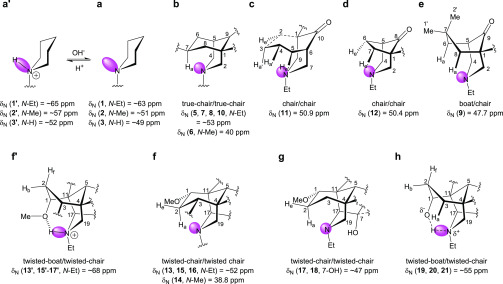
In CDCl3, synthetic A/E-[3.3.1]azabicyclic free bases (5–8 and 10) and their relative 1α-OMe norditerpenoid alkaloid free bases (13–18) show the rare NMR effect of steric compression on 7-Ha (2-Ha) as the chemical shifts of 7-Ha (2-Ha) are larger than those of 7-He (2-He). The chemical shift of an axial proton (∼1.1 ppm) attached to cyclohexane is normally at a higher field than that of its geminal equatorial proton (1.6 ppm) by ∼0.5 ppm due to the magnetic anisotropic effect,13,14 and the reversed order of chemical shifts that are observed in [3.3.1]azabicyclic compounds (5–8, 10, and 13–18) is indicative of the electron cloud of 7-Ha (2-Ha) being repulsed by the lone pair of electrons of the tertiary amine N-atoms in such half-cage [3.3.1]azabicycles. Therefore, these 7-Ha (2-Ha) are deshielded and their chemical shifts increase.15 Similar effects are displayed in [4.3.1]- and [3.2.1]azabicycles (11 and 12). The values of Δδ7-H of synthetic [3.3.1]azabicyclic free bases (5–8 and 10, ≥1.11 ppm) are significantly larger than those of Δδ2-H of natural alkaloid free bases (13–18, ≤0.37 ppm), as the synthetic [3.3.1]azabicycles (5–8 and 10) adopt true-chair/true-chair conformations, and the A/E-[3.3.1]azabicycles of the natural norditerpenoid alkaloid free bases (13–18) are in twisted-chair/twisted-chair conformations due to through-space repulsion between the 12-He′/O-atom of 1α-OMe acting on the A-rings and 19-Ha/6α-He′ (6α-OMe) acting on the E-rings.10,16
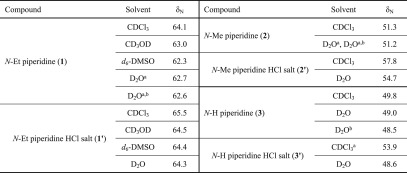
It is notable that the chemical shifts δN for N-Et, N-Me, and N-H piperidine (1–3) occur at 64.1, 51.3, and 49.8 ppm (in CDCl3, Table 1), respectively. Likewise, similar differences for δN between N-Et (5, 7, 8, and 10) and N-Me (6) bicyclic piperidine analogues were obtained. Shifts of synthetic N-Et [3.3.1]azabicyclic free bases (5, 7, 8, and 10) (in CDCl3, Table 2) and N-Et 1α-OMe norditerpenoid alkaloid free bases (13 and 15–18) are found at ∼50 ppm (in CDCl3, Table 3), and those of N-Me [3.3.1]azabicyclic free bases (6) and N-Me mesaconitine (14) resonate at ∼39 ppm (in CDCl3). Thus, the N-alkyl substituents (Et, Me, H) sensitively influence δN in simple piperidine. In the half-cage molecules, the typical 15N shift is reduced by ∼12 ppm when compared with such piperidines. This could be a result of the deformation in size and hybridization of the orbitals for the lone pair of electrons of the tertiary amine N-atoms.3
To investigate solvent effects on chemical shift, the 15N shifts for N-Et piperidine (1) were recorded in CDCl3, CD3OD, DMSO-d6, D2O, and NaOD solutions (in D2O, pD ∼13) resulting in a value of ∼63 ppm, thus showing negligible differences. Similarly, the 15N shifts of diol (10) were obtained from solutions in CDCl3, CD3OD, DMSO-d6, and D2O and were typically ∼54 ppm. Therefore, the difference of δN between simple piperidines (1–3) and [3.3.1]azabicycles (5–8, 10, and 13–18) is not caused by solvent effects.
The distribution of the N-atom lone pair of electrons in piperidines (1–3) is not restricted by the steric hindrance caused by bridged cyclohexane rings (Figure 2a), but those in the half-cage [3.3.1]azabicycles adopting chair/chair conformations are restricted by 7-Ha (2-Ha) (Figures 2b,f); therefore, the distribution of the N-atom lone pair of electrons are concentrated around the N-atom increasing the electron density and decreasing the δN by ∼10 ppm.
Figure 2.
A/E-[3.3.1]azabicyclic frames of norditerpenoid alkaloids and their analogues. Pink: lone pair of electrons of the N-atom.
Similar results of δN were observed in synthetic [4.3.1]- and [3.2.1]azabicycles (11 and 12), which resonate at 50.9 and 50.4 ppm (Table 2), respectively; these results show that the amine N-atoms are shielded by endo protons attached to C3 and C4 of [4.3.1]azabicycles (11, Figure 2c) and endo protons attached to C6 and C7 of [3.2.1]azabicycles (12, Figure 2d).
N-Et 7,7-diMe [3.3.1]azabicycle (9) adopts a boat/chair conformation, and its δN is smaller than those of N-Et [3.3.1]azabicycles (5, 7, 8, and 10) which adopt chair/chair conformations. The lone pair of electrons of 7,7-diMe [3.3.1]azabicycle (9) are restricted by 6-Ha and 8-Ha (Figure 2e),17 thus the electron density of the N-atom of 7,7-diMe [3.3.1]azabicycle (9) is more concentrated in comparison with those of [3.3.1]azabicycles (5, 7, 8, and 10). The fact that such 15N shift effects can be larger has been previously reported by Roberts and co-workers.3 From our studies, we conclude that the 15N shift effects make them useful reporter nuclei of the substitution pattern. Indeed, a possible reason is a difference in size and hybridization of the orbitals for the unshared electrons in such tertiary amines,4 and whether the substituents adopt axial or equatorial positions.3
Due to the proximity in space between 7-OH and the N-atom (Figure 2g), the amine δN of norditerpenoid alkaloid free bases with N-Et and 7-OH (17 and 18) resonate at ∼47 ppm, slightly lower than those of alkaloid free bases with N-Et and 7-H (13, 15, and 16), which resonate at ∼52 ppm.
Protonation
δN of N-Et piperidine (1) resonates at ∼63 ppm (in CDCl3, CD3OD, DMSO-d6, and D2O), showing only a small difference4 from its HCl salt (1′) resonating at ∼65 ppm (in CDCl3, CD3OD, DMSO-d6, and D2O). δN of N-Et (1), N-Me (2), and N-H free bases (3) were also measured in NaOD solutions (in D2O, pD ∼13), and these δN have no significant difference from those of their corresponding free bases (1–3) obtained in D2O.
The A/E-[3.3.1]azabicyclic rings of protonated 1α-OMe norditerpenoid alkaloid salts (13′ and 15′–17′) adopt twisted-boat/twisted-chair conformations, which are stabilized by hydrogen bonds between N+-H and 1α-OMe (Figure 2f′).10,16 The δN of norditerpenoid alkaloid free bases (13 and 15–17; resonating at 52.9, 50.7, 52.3, and 47.3 ppm, respectively; in CDCl3) are at a higher field than those of their protonated forms (13′ and 15′–17′; 70.3, 67.0, 67.9, and 67.2 ppm, respectively; in D2O), and the δN of these natural products (13 and 15–17) increase by 17.4, 16.3, 15.6, and 19.9 ppm, respectively, on protonation. The ΔδN (≥15.6 ppm) between norditerpenoid alkaloids (13 and 15–17) and their salts (13′ and 15′–17′) are further supported by the recently reported ΔδN = 18.0 ppm between 3-deoxyaconitine (22) (δN = 40.7 ppm, in acetone-d6) and its trifluoroacetate salt (δN = 58.7 ppm, in acetone-d6).18
The space that the lone pair of electrons of the N-atoms in piperidines (1–3) occupy (Figure 2a) is similar to that of their HCl salts (1′–3′) (fixed in the N+–H-bonds; Figure 2a′); hence, the δN of simple piperidines (1–3) show only a small change on protonation. The distribution of the lone pair of electrons of the N-atoms in norditerpenoid alkaloid free bases (13 and 15–17) are restricted by 2-Ha through space (Figure 2f), which is near the N-atoms, and these electrons are fixed by N+–H-bonds and are away from the N-atom (Figure 2f′), leading to a decrease in the electron density when the alkaloids (13 and 15–17) are protonated.
Intramolecular Hydrogen Bonding
The A-ring of 1α-OH norditerpenoid alkaloid free bases adopts twisted-boat conformations10 stabilized by H-bonds between tertiary amine N-atom and 1α-OH (Figure 2h).19,20 Compared with δN (47.7 ppm, in CDCl3) of 7,7-diMe [3.3.1]azabicycle (9), which adopts a boat/chair conformation, δN of 1α-OH norditerpenoid alkaloid free bases (19–21) are higher field, resonating at ∼55 ppm for all three. The lone pair of electrons of the N-atom in 7,7-diMe [3.3.1]azabicycle (9) are significantly compressed by 6-Ha and 8-Ha through space (Figure 2e). The lone pair of electrons of the N-atom in 1α-OH natural alkaloids (19–21) are shared and fixed by intramolecular H-bond to 1α-OH. Thus, the electrons are distributed away from the N-atom, leading to an increase of δN (Figure 2h), typically of 6–8 ppm compared with δN of boat/chair analogue (9). Neoline (21) in CDCl3 was treated with additional pyridine-d5 and NaOD solution (30% in D2O, w/w) successively for cleaving the intramolecular H-bond. In comparison with those of neoline (21) in CDCl3, no notable change was observed in the 1H NMR and 1H–15N HMBC spectra of neoline (21) in basified CDCl3, demonstrating that this intramolecular H-bond is stable, and it holds the A-ring in a twisted-boat conformation.
Conclusions
A rare 15N NMR spectroscopic effect of steric compression has been demonstrated in the A/E-rings of several norditerpenoid alkaloid free bases and their synthetic azabicyclic analogues using 1H–15N HMBC spectroscopy. The distribution of the tertiary amine N-atom lone pair of electrons is restricted in the half-cage azabicycles; therefore, the electron density of the N-atom increases and its δN decreases. δN of norditerpenoid alkaloids bearing 1α-OMe significantly increase on protonation. The intramolecular hydrogen bonds between the N-atom and 1α-OH of 1α-OH norditerpenoid alkaloid free bases not only stabilize the A-rings, adopting twisted-boat conformation, but they also increase the δN of the tertiary amine N-atom. Thus, 1H–15N HMBC spectroscopy has been demonstrated to be an excellent reporter for the analysis of the electron density of substituted piperidine alkaloids. It is particularly useful for certain norditerpenoids with complex substitution patterns and half-cage skeleta.
Experimental Section
General Methods
Chemicals and Materials
Condelphine (98%) was donated by Carbosynth Ltd. (U.K.). Mesaconitine was extracted and then purified by sulfuric acid acid-base cycling from the ground roots of Aconitum napellus. After column chromatography to homogeneity, it was indistinguishable from a commercial sample (Sigma-Aldrich, U.K.). Aconitine (95%) and crassicauline A (98%) were purchased from Sigma-Aldrich (U.K.); lappaconitine hydrobromide (98%) and neoline (98%) were purchased from Carbosynth (U.K.); and lycoctonine (99%), methyllycaconitine perchlorate (99%), and karacoline (98%) were purchased from Latoxan (France). All other chemicals were purchased from Sigma-Aldrich (U.K.) and used as received.
Deuterated solvents, chloroform-d (CDCl3), methanol-d4 (CD3OD), dimethyl sulfoxide-d6 (DMSO-d6), deuterium oxide (D2O), pyridine-d5, and NaOD solution (30% in D2O, w/w) were used for NMR experiments (99.8% D atom, Cambridge Isotope Laboratories, USA). All other solvents were of HPLC grade, ≥99.9% purity (FisherScientific, U.K. and VWR, U.K.). Petroleum ether (FisherScientific, U.K.) specifically refers to the 40–60 °C distillate.
Instrumentation
1H NMR spectra were recorded on a Bruker Avance III (1H Larmor precession frequency 500 MHz) spectrometer at 25 °C. Chemical shifts were expressed in parts per million (ppm) downfield shift from tetramethylsilane (TMS) or 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TMSP) as internal or external standards, and residual (protio) solvent peaks were also used as internal standards if required. Chemical shifts (δH) were reported as position (accurate δH of overlapping signals were extracted from two-dimensional (2D) NMR spectra, e.g., HSQC, COSY, and NOESY), relative integral, multiplicity, and assignment. Multiplicity was abbreviated: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet; br = broad. Coupling constants (J) are line separations (absolute values expressed in hertz, Hz), rounded and rationalized to 0.1 Hz.
13C NMR spectra were recorded with complete proton decoupling on a Bruker Avance III (13C Larmor precession frequency 125 MHz) spectrometer at 25 °C as well as 2D NMR experiments including HSQC, HMBC, and H2BC. Chemical shifts are expressed in ppm downfield shift from TMS or TMSP as internal or external standards, and solvent peaks were also used as internal standards if required, and they were reported as position (δC), number of attached protons (CH3, CH2, CH, quat = quaternary), and assignment.
1H–15N HMBC spectra were recorded on a Bruker Avance III (15N Larmor precession frequency 51 MHz) spectrometer at 25 °C. The spectra were externally calibrated with a MeNO2 solution (50% in CDCl3, v/v), and chemical shifts (δN) are expressed in ppm downfield shift from TMS or TMSP as internal or external standards.
Positive-ion [M + H]+ mode mass spectrometry was performed, on samples dissolved in methanol, on an Agilent ESI-Q-TOF mass spectrometer. High-resolution mass spectrometry (HR-MS) was within 5 ppm error unless otherwise stated.
A PerkinElmer 65 spectrum FT-IR spectrometer was used to obtain the IR spectra.
Removal of solvents by evaporation in the procedures specifically refers to the use of a Buchi R-114 rotary evaporator with warming samples to 40 °C on a Buchi B-480 water bath and in vacuo (50–500 millibar). Residual solvents were removed under high vacuum for ∼14 h and the NMR data of the products were recorded.
Chromatography
Flash chromatography was performed using silica gel 60A 35–70 μm (Fluorochem Ltd, U.K. and Sigma-Aldrich, U.K.) with the indicated solvents. Thin-layer chromatography (TLC) was performed using 0.2 mm thick precoated silica gel plates (Merck KGaA 60 F254). Compounds were visualized by ultraviolet radiation (UV, λ = 254 nm) and by staining with different reagent(s) including iodine vapor, potassium permanganate aq. solution (0.05 M), p-anisaldehyde solution (p-anisaldehyde: conc. aq. H2SO4/H2O/acetic acid = 3:2:50:40, v/v), ninhydrin solution (0.2% w/v ninhydrin in EtOH), or Dragendorff’s reagent: bismuth subnitrate (1.7 g), acetic acid (20 mL), H2O (80 mL), and 50% w/v solution of potassium iodide in H2O (100 mL) were mixed and stored as a stock solution. Stock solution (10 mL) and acetic acid (20 mL) were mixed and made up to 100 mL with H2O to give Dragendorff’s reagent.
Synthesis and Structural Evaluation
Details of preparation, ESI-MS and NMR spectroscopy (1H, 13C, COSY, HSQC, HMBC, NOESY), structural analysis, and crystallographic data were reported in our previous works.9,10
Synthesis of Ethyl 3-Methyl-9-oxo-3-azabicyclo[3.3.1]nonane-1-carboxylate (6)21
A solution of ethyl cyclohexanone-2-carboxylate (4.44 mmol, 0.748 mL, 95%), 2.2 equiv formaldehyde (9.768 mmol, 0.713 mL, 38% aq v/v), and 1.1 equiv of methylamine (4.88 mmol, 0.608 mL, 33% in EtOH) in EtOH (25 mL) was stirred at 40 °C for 2 days under an atmosphere of anhydrous nitrogen. After the solvents were removed by evaporation, the crude product was purified by chromatography over silica gel (12.5% EtOAc in petroleum ether) to yield the title compound (280 mg, 28%) as a yellow oil. Rf = 0.36 (12.5% EtOAc in petroleum ether). HR-ESI-MS: m/z calcd for C12H20NO3: 226.1443, found: 226.1443 [M + H]+ and m/z calcd for C12H19NO3Na: 248.1263, found: 248.1262 [M + Na]+. IR: νmax (NaCl)/cm–1 1733 (ester, C=O), 1717 (ketone, C=O). δH (500 MHz, CDCl3): 1.26 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.49–1.57 (m, 1H, 7-He), 2.00–2.09 (m, 1H, 6-He), 2.10–2.17 (m, 1H, 6-Ha), 2.15–2.29 (m, 1H, 8-He), 2.25 (s, 3H, NCH3), 2.40–2.45 (m, 1H, 5-He), 2.50 (dddd, J = 14.2, 12.3, 6.3, 2.0 Hz, 1H, 8-Ha), 2.59 (dd, J = 11.2, 3.8 Hz, 1H, 4-Ha), 2.76–2.89 (m, 1H, 7-Ha), 2.96 (dd, J = 11.3, 1.9 Hz, 1H, 2-Ha), 3.04 (dt, J = 11.2, 2.3 Hz, 1H, 4-He), 3.11 (dd, J = 11.3, 2.2 Hz, 1H, 2-He), 4.19 (q, J = 7.1 Hz, 2H, OCH2CH3). δH (125 MHz, CDCl3): 13.81 (OCH2CH3), 20.17 (C7), 34.01 (C6), 36.76 (C8), 44.76 (NCH3), 47.06 (C5), 58.54 (C1), 60.90 (OCH2CH3), 62.27 (C4), 63.98 (C2), 170.88 (ester), 212.33 (C9).
Preparation of Piperidinium HCl Salts (1′–3′) Crassicauline A HCl Salt (15′), and Lycoctonine HCl Salt (17′)
The free base (15 or 17, ∼20 mg) in MeOH (3 mL) was acidified with conc. aq. HCl (∼37%) to pH = 2; then, all of the solvents and HCl residue were removed by evaporation to afford the HCl salts as white solids.
Characterization data and related spectra (Figures S1–S121) are all presented in the Supporting Information.
Acknowledgments
We thank Carbosynth Ltd. (U.K.) for the donated condelphine (20). We thank Zarqa University, Jordan, for the Studentship to A.M.A.Q. We thank the University of Bath for the partial support of Dr. Z.Z.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01648.
Characterization data and related spectra (Figures S1–S121) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mueller L. Sensitivity enhanced detection of weak nuclei using heteronuclear multiple quantum coherence. J. Am. Chem. Soc. 1979, 101, 4481–4484. 10.1021/ja00510a007. [DOI] [Google Scholar]
- Köck M.; Junker J.; Lindel T. Impact of the 1H, 15N-HMBC experiment on the constitutional analysis of alkaloids. Org. Lett. 1999, 1, 2041–2044. 10.1021/ol991009c. [DOI] [PubMed] [Google Scholar]
- Duthaler R. O.; Williamson K. L.; Giannini D. D.; Bearden W. H.; Roberts J. D. Natural-abundance nitrogen-15 nuclear magnetic resonance spectroscopy. Steric and electronic effects on nitrogen-15 chemical shifts of piperidines and decahydroquinolines. J. Am. Chem. Soc. 1977, 99, 8406–8412. 10.1021/ja00468a006. [DOI] [Google Scholar]
- Duthaler R. O.; Roberts J. D. Steric and electronic effects on 15N chemical shifts of piperidine and decahydroquinoline hydrochlorides. J. Am. Chem. Soc. 1978, 100, 3882–3889. 10.1021/ja00480a037. [DOI] [Google Scholar]
- Duthaler R. O.; Roberts J. D. Steric and electronic effects on 15N chemical shifts of saturated aliphatic amines and their hydrochlorides. J. Am. Chem. Soc. 1978, 100, 3889–3895. 10.1021/ja00480a038. [DOI] [Google Scholar]
- Wong T. C.; Collazo L. R.; Guziec F. S. Jr. 14N and 15N NMR studies of highly sterically hindered tertiary amines. Tetrahedron 1995, 51, 649–656. 10.1016/0040-4020(94)00971-V. [DOI] [Google Scholar]
- Pace V.; Holzer W.; Ielo L.; Shi S.; Meng G.; Hanna M.; Szostak R.; Szostak M. 17O NMR and 15N NMR chemical shifts of sterically-hindered amides: ground-state destabilization in amide electrophilicity. Chem. Commun. 2019, 55, 4423–4426. 10.1039/C9CC01402K. [DOI] [PubMed] [Google Scholar]
- Vogel E.; Brocker U.; Junglas H. syn-1,6-Imino-8,13-methano[14]annulene. Angew. Chem., Int. Ed. 1980, 19, 1015–1016. 10.1002/anie.198010151. [DOI] [Google Scholar]
- Hardick D. J.; Blagbrough I. S.; Cooper G.; Potter B. V. L.; Critchley T.; Wonnacott S. Nudicauline and elatine as potent norditerpenoid ligands at rat neuronal α-bungarotoxin binding sites: Importance of the 2-(methylsuccinimido)benzoyl moiety for neuronal nicotinic acetylcholine receptor binding. J. Med. Chem. 1996, 39, 4860–4866. 10.1021/jm9604991. [DOI] [PubMed] [Google Scholar]
- Zeng Z.; Qasem A. M. A.; Kociok-Köhn G.; Rowan M. G.; Blagbrough I. S. The 1α-hydroxy-A-rings of norditerpenoid alkaloids are twisted-boat conformers. RSC Adv. 2020, 10, 18797–18805. 10.1039/D0RA03811C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witanowski M. Nitrogen n.m.r. spectroscopy. Pure Appl. Chem. 1974, 37, 225–233. 10.1351/pac197437010225. [DOI] [Google Scholar]
- Harris R. K.; Becker E. D.; De Menezes S. M. C.; Goodfellow R.; Granger P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. 10.1351/pac200173111795. [DOI] [PubMed] [Google Scholar]
- Garbisch E. W.; Griffith M. G. Proton couplings in cyclohexane. J. Am. Chem. Soc. 1968, 90, 6543–6544. 10.1021/ja01025a069. [DOI] [Google Scholar]
- Williams D. H.; Fleming I.. Spectroscopic Methods in Organic Chemistry, 6th ed.; McGraw-Hill: London, 2008; p 165. [Google Scholar]
- Winstein S.; Carter P.; Anet F. A. L.; Bourn A. J. R. The effects of steric compression on chemical shifts in half-cage and related molecules. J. Am. Chem. Soc. 1965, 87, 5247–5249. 10.1021/ja00950a046. [DOI] [Google Scholar]
- Pelletier S. W.; Djarmati Z. Carbon-13 nuclear magnetic resonance: aconitine-type diterpenoid alkaloids from Aconitum and Delphinium species. J. Am. Chem. Soc. 1976, 98, 2626–2636. 10.1021/ja00425a036. [DOI] [Google Scholar]
- Malpass J. R.; Belkacemi D.; Russell D. R. Studies of stereoselectivity in cycloaddition of cyclic dienes to 2-azabicyclo[2.2.2]octene derivatives; through-space effects on 15N NMR shifts of bicyclic amines and lactams. Tetrahedron 2002, 58, 197–204. 10.1016/S0040-4020(01)01125-5. [DOI] [Google Scholar]
- Wang F.-P.; Chen D.-L.; Deng H.-Y.; Chen Q.-H.; Liu X.-Y.; Jian X. X. Further revisions on the diterpenoid alkaloids reported in a JNP paper (2012, 75, 1145-1159). Tetrahedron 2014, 70, 2582–2590. 10.1016/j.tet.2014.01.066. [DOI] [Google Scholar]
- Pelletier S. W.; Djarmati Z.; Lajsic S.; De Camp W. H. Alkaloids of Delphinium staphisagria. The structure and stereochemistry of delphisine, neoline, chasmanine, and homochasmanine. J. Am. Chem. Soc. 1976, 98, 2617–2625. 10.1021/ja00425a035. [DOI] [Google Scholar]
- Mu Z.-Q.; Gao H.; Huang Z.-Y.; Feng X.-L.; Yao X.-S. Puberunine and puberudine, two new C18-diterpenoid alkaloids from Aconitum barbatum var. puberulum. Org. Lett. 2012, 14, 2758–2761. 10.1021/ol3008217. [DOI] [PubMed] [Google Scholar]
- Grangier G.; Trigg W. J.; Lewis T.; Rowan M. G.; Potter B. V. L.; Blagbrough I. S. Synthesis of C5-substituted AE-bicyclic analogues of lycoctonine, inuline and methyllycaconitine. Tetrahedron Lett. 1998, 39, 889–892. 10.1016/S0040-4039(97)10648-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.