Abstract
Context
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that originates from central nervous system lesions or recessions. Current estimates suggest that this disease affects over 35 million people worldwide. However, lacking effective drugs is the biggest handicap in treating AD. In traditional Chinese medicine (TCM), Polygala tenuifolia Willd. (Polygalaceae) is generally used to treat insomnia, memory dysfunction and neurasthenia.
Objective
This review article explores the role of P. tenuifolia and its active components in anti-Alzheimer’s disease.
Methods
Literature for the last ten years was obtained through a search on PubMed, SciFinder, CNKI, Google Scholar, Web of Science, Science Direct and China Knowledge Resource Integrated with the following keywords: Polygala tenuifolia, polygalasaponin XXXII (PGS 32), tenuifolin, polygalacic acid, senegenin, tenuigenin, Alzheimer’s disease.
Results
Polygala tenuifolia and its active components have multiplex neuroprotective potential associated with AD, such as anti-Aβ aggregation, anti-Tau protein, anti-inflammation, antioxidant, anti-neuronal apoptosis, enhancing central cholinergic system and promote neuronal proliferation.
Conclusions
Polygala tenuifolia and its active components exhibit multiple neuroprotective effects. Hence, P. tenuifolia is a potential drug against Alzheimer’s disease, especially in terms of prevention.
Keywords: Polygalasaponin XXXII, tenuifolin, polygalacic acid, senegenin, tenuigenin, neuroprotective effects, multitarge
Introduction
Alzheimer’s disease (AD), also called senile dementia, is a chronic neurodegenerative disease that originates from central nervous system lesions or recessions. The number of people with AD globally is predicted to exceed one billion in 2050 (Anand et al. 2014; Khoury et al. 2017). According to the ‘World Alzheimer Report 2015’, the number of patients with AD in China is the highest in the world, and it is equivalent to approximately 1 out of every 20 elderly people (Prince et al. 2015). AD has affected not only the elderly population but also the middle-aged group (Castellani et al. 2010). An increase in the number of patients is accompanied by a remarkable social pressure. In the United States alone, more than $170 billion is used to treat AD per year (Reitz and Mayeux 2014). Although AD is a major health problem worldwide, the pathogenesis of AD has only begun to be explored gradually in the future.
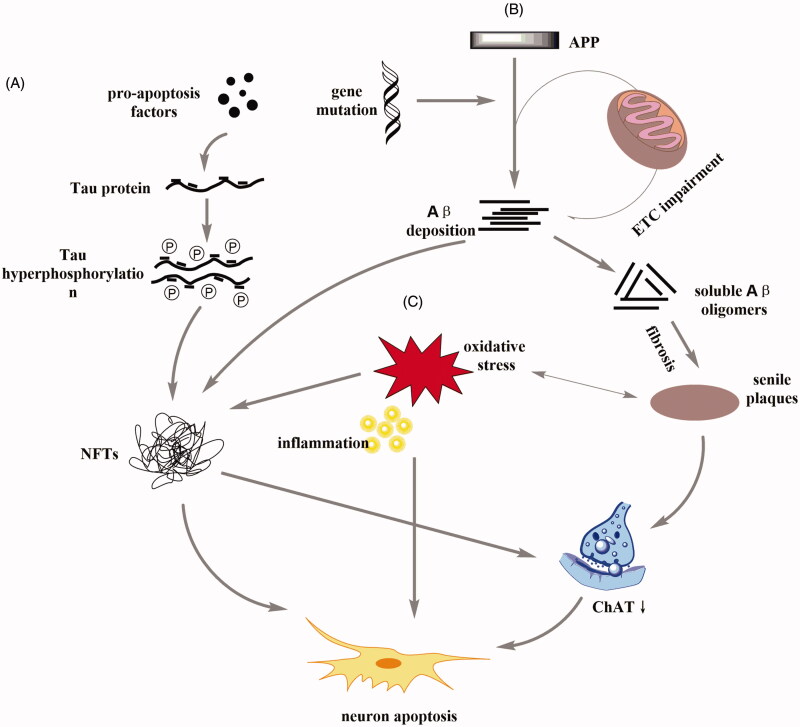
Pathological hallmarks of AD include senile plaque, neurofibrillary tangles (NFTs), synaptic loss and neuronal dysfunction (Hong et al. 2016; Das and Yan 2017; Ishizuka and Hanamura 2017). Abnormal aggregation of Aβ protein in senile plaques was previously considered to triggers the pathological cascade of AD, suggesting that the accumulation of Aβ in insoluble Aβ fibrils promotes the development of AD (Gouras et al. 2015). The NFTs in AD composed of paired helical filaments aggregated by hyperphosphorylated tau (p-tau) (Liu et al. 2010). Tau, a microtubule associated protein, is a major component of the paired helical filaments observed in the brains of AD patients, which normally binds to tubulin to promote microtubule stability (Amemori et al. 2015). p-Tau is positively correlated with AD development in clinical experiments (Zhang et al. 2017). Furthermore, oxidative stress interferes with AD development by inducing plaque and NFTs formation, subsequent synaptic and neuronal loss (Chen and Zhong 2014; Friedemann et al. 2015). The role of central cholinergic damage in AD development has also been widely investigated. The loss of cholinergic neurons, the synthesis and release of choline acetyltransferase (ChAT), and decreased sensitivity to acetylcholine receptors cause a cognitive dysfunction in the brains of patients with AD (Pu and Wang 2010; Ferreira-Vieira et al. 2016). The above pathogenesis of AD is shown in Figure 1.
Figure 1.
Schematic diagram of common pathogenesis of AD. (A) The presence of pro-apoptotic factors can promote the phosphorylation of tau protein to form neurofibrillary tangles (NFTs). NFTs can directly promote neuronal apoptosis, or indirectly lead to neuronal apoptosis by affecting the activity of ChAT. (B) The electron transport chain (ETC) is a key step in the energy release of the mitochondria. ETC dysfunction and gene mutations trigger metabolic disorders of amyloid precursor protein (APP) and promote Aβ deposition. Aβ deposition can both promote NFTs and affect the content of ChAT through the formation of senile plaques. (C) Inflammation and oxidative stress can directly stimulate neurons and participate in the development of NFTs and senile plaques.
In response to this disease, drugs such as galantamine, tacrine, memantine, donepezil and rivastigmine have been used in clinical settings (Piemontese 2017). Although these medicines can alleviate AD-associated symptoms, some adverse events, such as liver toxicity, diarrhoea and vomiting also appear (Grutzendler and Morris 2001; Lane et al. 2018). Unfortunately, several promising drugs have failed in phase II/III clinical trials. Therefore, the selection of multi-target drugs will be a potentially effective strategy for AD treatment. Some active extracts or components from TCM have properties of neurological disorders treatment because of its multicomponent and multitarget properties and low toxicity (Wu et al. 2011; Wang et al. 2013).
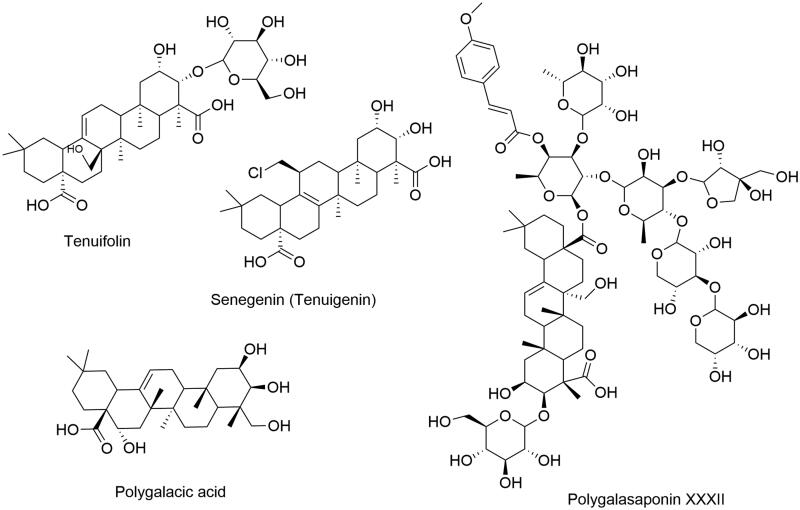
Polygala tenuifolia Willd. (Polygalaceae), also known as Yuan Zhi, has been reported to be enriched with triterpene saponins, onjisaponins and polygalasaponins (Jin et al. 2014), and it also exhibits protective effects on the central nervous system and is frequently used to treat memory dysfunction, insomnia and neurasthenia (Zhang et al. 2016). As the main pharmacologically active components of P. tenuifolia, polygalasaponins, including polygalasaponin XXXII (PGS 32), tenuifolin, polygalacic acid and senegenin (tenuigenin), have been shown to possess multiplex neuroprotective potential associated with AD, such as anti-β-amyloid (Aβ) aggregation (Jia et al. 2004; Park et al. 2019), anti-Tau (Xu et al. 2012), anti-inflammation (Cheong et al. 2011; Wang et al. 2017), antioxidant (Zhang et al. 2011), enhancing central cholinergic system, anti-neuronal apoptosis (Li et al. 2015), promote neuronal proliferation (Park et al. 2008; Zhu et al. 2016). The chemical structures are presented in Figure 2.
Figure 2.
Chemical structures of active compounds from Polygala tenuifolia.
Neuroprotective effects of Polygala tenuifolia
Accumulated evidence suggests that P. tenuifolia extract and many active components [such as PGS 32, tenuifolin, senegenin (tenuigenin) and polygalacic acid] exert neuroprotective effects (Table 1), including anti-Aβ aggregation, anti-Tau, anti-inflammation, antioxidant, enhancing central cholinergic system, anti-neuronal apoptosis and promote neuronal proliferation.
Table 1.
The effect of active agents in Polygala tenuifolia against Alzheimer's disease.
| Active agents | Molecular formula | Method | Model | Dose | Effect | Reference |
|---|---|---|---|---|---|---|
| Polygalasaponin XXXII | C79H118O38 | In vitro | Primary cortical neurons, PC12 cells | 1–100 µg/ml | Heighten the survival rate of neurons | (Zhou et al. 2016) |
| In vivo | Kunming mice (18–20 g, male), C57BL/6J mice (18–20 g), Wistar rats (230–260 g) | 7.5–30 mg/kg | Significant improvement scopolamine-induced memory impairment by upregulated TrκB and p-TrκB level | (Zhou et al. 2016) | ||
| Senegenin | C30H45ClO6 | In vitro | Primary cortical neurons from Neonatal SD rats | 2 µM | Activate PI3K/Akt signalling pathway to elevate neuron survival | (Pi et al. 2016) |
| In vivo | SD rats (280 ± 30 g) | 18.5–74 mg/kg | Reduces accumulation of Aβ1–40 and abnormal tau phosphorylation in the brain by modulating the PI3K/Akt pathway | (Chen et al. 2015) | ||
| Polygalacic acid | C30H48O6 | In vitro | – | – | – | – |
| In vivo | Kunming mice (18–22 g, male) | 3–12 mg/kg | Improves the cholinergic system anti-oxidative stress and anti-neuritis, and enhances cognitive function by reducing AChE activity while increasing ChAT activity | (Guo et al. 2016) | ||
| Tenuifolin | C36H56O12 | In vitro | PC12 cells | 1–40 µg/ml | Reduces Aβ25–35-induced cytotoxicity in PC12 cells | (Liu et al. 2015) |
| In vivo | C57BL/6J mice (26–30 g, male) | 3–9 mg/kg | Improve the memory dysfunction caused by Aβ25–35 | (Liu et al. 2015) |
‘–’ means no relevant literature found
Anti-Aβ
Senile plaque, abnormal aggregation of Aβ, is an important pathological feature of AD, and it is the foremost origin during the development of neurocytotoxicity. The formation and clearance of Aβ in the brain of normal people are in equilibrium, and this balance is broken in the AD patient’s brain due to excessive Aβ. Aβ has two forms of existence, dissolution and deposition. Aβ in the dissolved state promotes the growth of neurites and the survival of neurons. On the contrary, it causes axonal degeneration and neuronal degeneration (Pike et al. 1992).
Polygala tenuifolia extract and several components derived from P. tenuifolia have exhibited anti-Aβ effects. For example, tenuifolin which was separated from the crude extract of P. tenuifolia inhibits Aβ secretion without changing the ratio of Aβ1–40 and Aβ1–42 in African green monkey COS-7 cells which transfected with either APP695 cDNA and the Swedish mutation, the effect may be related to inhibition of the β-site APP cleaving enzyme (Lv et al. 2009). Meanwhile, tenuigenin can reduce the level of Aβ in human neuroblastoma SH-SY5Y APP695 cells via inhibiting the activity of BACE1 (Jia et al. 2004). In the AD rat model, tenuigenin inhibits Aβ1–40 aggregation, decrease phosphorylation level of TauSer396 by down-regulating of ubiquitin expression and up-regulating activity of ubiquitin ligase E3 and 26S proteasome at doses of 37.0 and 74.0 mg/kg. This result indicates that the role of tenuigenin in combating AD may be related to the ubiquitin–proteasome pathway (Chen et al. 2015).
In addition to reducing the aggregation of Aβ, P. tenuifolia also has a therapeutic effect on Aβ-induced neurotoxicity. Senegenin protect PC12 cells from Aβ25–35-induced cytotoxicity by increasing neurite number, average length and maximum length as well as the expression of MAP2 and Gap-43 (Jesky and Chen 2016). PSM-04, a crude extract from the root of P. tenuifolia, reduces Aβ aggregation and neurotoxicity caused by oligomeric Aβ, inhibits hydrogen peroxide (H2O2)-induced oxidative stress and Aβ-induced neuronal apoptosis, increases the expression of superoxide dismutase-2 protein in brain tissues of 5xFAD (Tg) mice (Park et al. 2019).
Anti-Tau
Another hallmark of AD is NFTs, and highly phosphorylated tau proteins are the major content of NFTs. Tau protein kinases, such as glycogen synthase kinase-3, cyclin-dependent protein kinase-5 and mitogen-activated protein kinases (MAPK), play a key role in the development of phosphorylation of tau protein (Martin et al. 2013). Studies have shown that P. tenuifolia can regulate the imbalance between tau protein kinases and phosphatases. In AD rat model induced by Aβ1–42, tenuigenin significantly decreased the expression of GSK-3β and CDK-5 while up-regulated the activity of PP-1 and PP-2A and inhibited the abnormal phosphorylation of tau in hippocampus (Chen et al. 2012a). Another study indicates that the tenuigenin protects neurons against Aβ1–40-induced tau phosphorylation by down-regulating PKA protein expression, increasing PP-2A activity and inhibiting hyperphosphorylation of the MAPT Ser396 in rat brain neurons (Xu et al. 2012). O-Linked N-acetylglucosamine (O-GlcNAc) glycosylation is a dynamic post-translational modification of nucleocytoplasmic proteins which regulate by glycosyltransferase O-GlcNAc transferase (OGT) and glycoside hydrolase O-GlcNAcase (OGA). In the presence of OGT, O-GlcNAc is transferred from the UDP-Glc NAc donor via the O-glycosidic bond and ligated to the hydroxyl group of serine or threonine. In contrast, OGA can eliminate the protein modification of O-GlcNAc (Zhu et al. 2014). Previous studies have shown that O-GlcNAc can inhibit the phosphorylation of tau (Lefebvre et al. 2003). In P12 cells, tenuigenin can increase the level of O-GlcNAc glycosylation in a dose-dependent manner, restrain phosphorylation levels of tau proteins at Ser396/404, Ser202, Thr205, Thr212 and Thr217 (Chen et al. 2012b).
Anti-inflammation
Activated microglia play a crucial role in neuroinflammation. Aβ can induces microglia to release inflammatory cytokines by binding to receptors on the plasma membrane of microglia (Yu and Ye 2015). The water extract of P. tenuifolia roots (2, 4 and 8 μg/mL) inhibited the protein expression of nitric oxide (NO), nitric oxide synthase (iNOS), prostaglandin E2 (PGE2), interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α) and cyclooxygenase-2 (COX-2) in lipopolysaccharide (LPS)-treated BV2 microglia. And the water extract of P. tenuifolia roots also blocked the translocation and transcriptional activity of NF-κB by impeding IκB-α degradation and inhibiting TLR4 and MyD88 expression (Cheong et al. 2011). Tenuigenin inhibits LPS-induced PGE2 and NO production and decrease iNOS and COX-2 gene expression in RAW 264.7 macrophages by inhibiting MAPK/NF-kB and activating the Nrf2/HO-1 signalling pathways (Lv et al. 2016). Tenuigenin also inhibits inflammatory cytokines (IL-1β, IL-6 and TNF-α) and PGE2 expression via activation of Nrf2-mediated HO-1 signalling pathway in LPS-activated murine BV2 microglia cells (Wang et al. 2017).
Antioxidant
Oxidative stress is one of the most important factors in the development of AD. When the body is subjected to harmful stimuli, excessive production of high active molecules (Reactive Oxygen Species, ROS) leads to imbalance between oxidation and antioxidant defences which results in oxidative stress (Yan et al. 2013). Excessive ROS can cause protein damage, induce oxidative stress and ultimately lead to cell death (Perry et al. 1998; Stringfellow et al. 2014). Evidence has shown that H2O2 can also accelerate apoptosis (Pierce et al. 1991). As a catalytic enzyme, superoxide dismutases (SOD) can reduce O2− to H2O2 (Arteel 2003). Glutathione peroxidase (GSH-Px) is another antioxidant enzyme that protects cells by catalysing the reduction of peroxides (Beutler 1972). Tenuigenin has been found to protect rat hippocampal neurons against streptozotocin-induced oxidative stress, neuronal damage and cognitive dysfunction by increasing the SOD and GSH-Px activities, down-regulating 4-hydroxy-2-nonenal adducts levels and inhibiting the phosphorylation of tau proteins (Huang et al. 2018). PSM-04, an extract from the root of P. tenuifolia, has neuroprotective effects via inhibiting ROS generation induced by H2O2, increasing the expression of SOD-2 and brain derived neurotrophic factor (BDNF), reducing the neurotoxicity induced by oligomeric Aβ1–42 and suppressing amyloid plaques in the hippocampus (Park et al. 2019). Tenuigenin (10 μM) protects SH-SY5Y cells against 6-hydroxydopamine-induced damage and apoptosis by increasing SOD and GSH, MMP levels, inhibiting caspase-3 activation, and stimulating TH expression (Liang et al. 2011). Tenuigenin also inhibited the formation of reactive oxygen species promoted by methylglyoxal, in rat hippocampal neurons in primary culture (Chen et al. 2010). These evidences suggest that P. tenuifolia may be effective in treating AD associated with oxidative stress.
Enhancing central cholinergic system
Acetylcholinesterase (AChE) is an essential enzyme in nerve conduction that terminates the signal transmission by catalyses the hydrolysis of the neurotransmitter acetylcholine (ACh). AChE also participates in cell development and maturation, promotes neuronal development and regeneration (Soreq and Seidman 2001). AChE has become a considerable biomarker for diagnosing AD, because of reduction in AChE activity in brains of AD patients (Meena et al. 2019). And reducing acetylcholinesterase while increasing the availability of ACh in central cholinergic synapses could be beneficial to alleviating the symptoms of AD (Wu et al. 2018). Thus, AChE inhibitors are most promising for treatment of AD (Davidsson et al. 2001). BT-11 (extract of dried root of P. tenuifolia) restrained AChE activity in a dose-dependent and non-competitive manner (IC50 value: 263.7 μg/mL) in Aβ1–42-induced SD rats (Park et al. 2002). The results of the Y-maze task show that tenuigenin can improve the learning and memory ability of mice in the model group, and it can also reduce the AChE activity and lower malondialdehyde level, while increasing SOD activities (Huang et al. 2013). Polygallic acid is a hydrolysate of triterpenoid saponin which has the function of regulating cholinergic activity. It can significantly increase ACh and ChAT expression, decrease AChE actility in the hippocampus and frontal cortex (Guo et al. 2016).
Anti-neuronal apoptosis
Neuronal apoptosis plays a vital role in central nervous system development and many neurodegenerative diseases. Moreover, caspase-mediated pathway was closely related to the progression of apoptosis. When this pathway is activated, caspase mobilizes the death programme via wrecking key agents of the cellular infrastructure and triggering factors that mediate cell injure. Cytochrome c (Cyt c), an electron mediator in mitochondria, also activates caspase-mediated pathways when it is transferred from damaged mitochondria to cytoplasm (Friedlander 2003). The Bcl-2 family is also involved in apoptosis. Bax and Bcl-2 are two major regulators of apoptosis. Bax can promote apoptosis, whereas Bcl-2 restrains apoptosis. The higher ratio of Bax/Bcl-2 implicates that apoptosis occurs (Yang and Korsmeyer 1996). Tenuigenin protects PC12 cells against Aβ25–35-induced apoptosis. After tenuigenin (50, 100, 200 μmol/L) for 24 h, the survival rate of PC12 cells was significantly increased, the apoptosis rate and Cyt c expression level were lower than those in the model group (p < 0.01). And the ratio of Bcl-2/Bax increased to 0.64, 1.29 and 1.84, respectively (Yang et al. 2013). In primary rat hippocampal neuronal cultures, tenuigenin protected neurons from methylglyoxal-induced excitotoxicities in a dose-dependent manner (1–4 μg/mL). Moreover, western blot assays indicate that tenuigenin can increase the expression of Bcl-2 and down-regulate the expression of Bax and caspase-3 (Chen et al. 2010).
Promote neuronal proliferation
The therapeutic strategy to promote neuron regeneration and inhibiting neuron apoptosis may be promising in treatment of AD. BDNF is a considerable neurotrophic factor involved in neuron plasticity, development, survival and differentiation of the neurons via activating tropomyosin-related kinase (Trk) receptors (Binder and Scharfman 2004). At a dose of 5 μg/mL, senegenin promoted neuritogenesis with a notable increase in the number of neurites, mean length, and maximum length by up-regulating MAP2 and Gap-43 expression or inhibition of ASK1 and JNK signalling pathways (Jesky and Chen 2016). Another research suggests that senegenin exerts neuroprotective effects may be associated with the PI3K/Akt signalling pathway (Pi et al. 2016). In addition, P. tenuifolia extract can also promote the nerve growth factor release in astroglial cells (Yabe et al. 2003). PGS32 significantly increased BDNF content by up-regulation the phosphorylation of ERK, CREB and synapsin I (Xue et al. 2009). PGS32 can also ameliorate against scopolamine-induced memory impairments in AD mice model via up-regulating the level of the p-TrkB, enhanced high frequency stimulation-induced long-term potentiation in the dentate gyrus of rats, and protecting of neurons from damage caused by glutamate and ROS (Zhou et al. 2016).
Conclusions
AD is a chronic neurodegenerative disease with a high incidence rate and a large number of patients, which seriously impact on the patients’ quality of life. However, no effective drugs have been found to prevent and treat this disease (Li et al. 2016). Polygala tenuifolia and its active components have exhibited a wide range of pharmacologic effects in vitro and in vivo, such as neuroprotective, immunomodulatory, anti-inflammatory, hepatoprotection, antioxidative, antibacterial and antitumor effects. However, despite many promising pre-clinical reports, there are no toxicological studies and clinical trials have been reported to date. Further studies should focus on the toxicological and pharmacokinetics aspects of the potential anti-AD agents, and that will help researchers determine the safe dose and range of medication, provide a basis for the structural transformation of new drugs, and ensure the safety of clinical medications. The quality control is another major challenge in P. tenuifolia application. Due to the differences in habitat, harvest time and processing methods, the treatment effect of P. tenuifolia is also uneven (Su et al. 2018). Therefore, it is considerably important to formulate drug quality control standards and clarify processing conditions and provide uniform and reliable medicinal materials for clinical use.
Funding Statement
This study was supported by the National Natural Science Foundation of China [grant number. 81860755, 21606137], the Key Research and Development Programme of Ningxia [grant number: 2018BFH02001 and 2018BFH03023], Ningxia University’s First-Class Subject (Traditional Chinese Medicine) Construction Project [grant number: NXYLXK2017A06].
Disclosure statement
The authors declare that they have no conflict of interest.
Author contributions
All authors designed the study. Xinxin Deng, Xinqi Liu, Ruizhou Wang, Yanna Jiao and Lu Han conducted the literature survey and data analysis; Xinxin Deng and Shipeng Zhao drafted the manuscript; Huifeng Hao provided writing ideas; Shuyan Han and Changcai Bai supervised the preparation of the final version of the paper.
References
- Amemori T, Jendelova P, Ruzicka J, Urdzikova LM, Sykova E.. 2015. Alzheimer’s disease: mechanism and approach to cell therapy. Int J Mol Sci. 16:26417–26451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, Gill KD, Mahdi AA.. 2014. Therapeutics of Alzheimer's disease: past, present and future. Neuropharmacology. 76 Pt A:27–50. [DOI] [PubMed] [Google Scholar]
- Arteel GE. 2003. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 124:778–790. [DOI] [PubMed] [Google Scholar]
- Beutler E. 1972. Disorders due to enzyme defects in the red blood cell. Adva Metab Disord. 60:131–160. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE.. 2004. Brain-derived neurotrophic factor. Growth Factors (Chur, Switzerland). 22:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Rolston RK, Smith MA.. 2010. Alzheimer disease. Dis Mon. 56:484–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Huang XB, Chen WQ, Wang Q.. 2012a. Experimental study of tenuigenin regulating phosphorylation of Tau protein. Tianjin J Trad Chinese Med. 19:45–47. [Google Scholar]
- Chen Q, Chen YQ, Ye HY, Yu JQ, Shi QQ, Huang Y.. 2015. The mechanism of tenuigenin for eliminating waste product accumulation in cerebral neurons of Alzheimer’s disease rats via ubiquitin-proteasome pathway. Chinese J Integrated Trad Western Med. 35:327–332. [PubMed] [Google Scholar]
- Chen Y, Huang X, Chen W, Wang N, Li L.. 2012b. Tenuigenin promotes proliferation and differentiation of hippocampal neural stem cells. Neurochem Res. 37:771–777. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Huang XB, Li ZX, Yin LL, Chen WQ, Li L.. 2010. Tenuigenin protects cultured hippocampal neurons against methylglyoxal-induced neurotoxicity. Eur J Pharmacol. 645:1–8. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhong C.. 2014. Oxidative stress in Alzheimer’s disease. Neurosci Bull. 30:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong MH, Lee SR, Yoo HS, Jeong JW, Kim GY, Kim WJ, Jung IC, Choi YH.. 2011. Anti-inflammatory effects of Polygala tenuifolia root through inhibition of NF-kappaB activation in lipopolysaccharide-induced BV2 microglial cells. J Ethnopharmacol. 137:1402–1408. [DOI] [PubMed] [Google Scholar]
- Das B, Yan R.. 2017. Role of BACE1 in Alzheimer’s synaptic function. Transl Neurodegener. 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsson P, Blennow K, Andreasen N, Eriksson B, Minthon L, Hesse C.. 2001. Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with Alzheimer’s disease. Neurosci Lett. 300:157–160. [DOI] [PubMed] [Google Scholar]
- Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM.. 2016. Alzheimer's disease: targeting the cholinergic system. Curr Neuropharmacol. 14:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedemann M, Helk E, Tiiman A, Zovo K, Palumaa P, Tougu V.. 2015. Effect of methionine-35 oxidation on the aggregation of amyloid-beta peptide. Biochem Biophys Rep. 3:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander RM. 2003. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 348:1365–1375. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Olsson TT, Hansson O.. 2015. Beta-amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics. 12:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Morris JC.. 2001. Cholinesterase inhibitors for Alzheimer's disease. Drugs. 61:41–52. [DOI] [PubMed] [Google Scholar]
- Guo C, Shen J, Meng Z, Yang X, Li F.. 2016. Neuroprotective effects of polygalacic acid on scopolamine-induced memory deficits in mice. Phytomedicine. 23:149–155. [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. 2016. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 352:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JN, Wang CY, Wang XL, Wu BZ, Gu XY, Liu WX, Gong LW, Xiao P, Li CH.. 2013. Tenuigenin treatment improves behavioral Y-maze learning by enhancing synaptic plasticity in mice. Behav Brain Res. 246:111–115. [DOI] [PubMed] [Google Scholar]
- Huang XB, Chen YJ, Chen WQ, Wang NQ, Wu XL, Liu Y.. 2018. Neuroprotective effects of tenuigenin on neurobehavior, oxidative stress, and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Brain Circ. 4:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka Y, Hanamura K.. 2017. Drebrin in Alzheimer’s disease. Adv Exp Med Biol. 1006:203–223. [DOI] [PubMed] [Google Scholar]
- Jesky R, Chen H.. 2016. The neuritogenic and neuroprotective potential of senegenin against Abeta-induced neurotoxicity in PC 12 cells. BMC Complement Altern Med. 16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Jiang Y, Ruan Y, Zhang Y, Ma X, Zhang J, Beyreuther K, Tu P, Zhang D.. 2004. Tenuigenin treatment decreases secretion of the Alzheimer’s disease amyloid beta-protein in cultured cells. Neurosci Lett. 367:123–128. [DOI] [PubMed] [Google Scholar]
- Jin ML, Lee DY, Um Y, Lee JH, Park CG, Jetter R, Kim OT.. 2014. Isolation and characterization of an oxidosqualene cyclase gene encoding a β-amyrin synthase involved in Polygala tenuifolia Willd. saponin biosynthesis. Plant Cell Rep. 33:511–519. [DOI] [PubMed] [Google Scholar]
- Khoury R, Patel K, Gold J, Hinds S, Grossberg GT.. 2017. Recent progress in the pharmacotherapy of Alzheimer’s disease. Drugs Aging. 34:811–820. [DOI] [PubMed] [Google Scholar]
- Lane CA, Hardy J, Schott JM.. 2018. Alzheimer’s disease. Eur J Neurol. 25:59–70. [DOI] [PubMed] [Google Scholar]
- Lefebvre T, Ferreira S, Dupont-Wallois L, Bussière T, Dupire MJ, Delacourte A, Michalski JC, Caillet-Boudin ML.. 2003. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins-a role in nuclear localization. Biochim Biophys Acta. 1619:167–176. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang L, Yang CC.. 2016. Multi-target strategy and experimental studies of traditional Chinese medicine for Alzheimer’s disease therapy. Curr Top Med Chem. 16:537–548. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao Y, Liu P, Zhu X, Chen M, Wang H, Lu D, Qi R.. 2015. Senegenin inhibits hypoxia/reoxygenation-induced neuronal apoptosis by upregulating RhoGDIalpha. Mol Neurobiol. 52:1561–1571. [DOI] [PubMed] [Google Scholar]
- Liang Z, Shi F, Wang Y, Lu L, Zhang Z, Wang X, Wang X.. 2011. Neuroprotective effects of tenuigenin in a SH-SY5Y cell model with 6-OHDA-induced injury. Neurosci Lett. 497:104–109. [DOI] [PubMed] [Google Scholar]
- Liu P, Zhao YF, Li YM.. 2010. Advances in Tau-mediated neurodegeneration and Tau-focused drug for Alzhehimer’s disease. Sci Sin Chim. 40:906–913. [Google Scholar]
- Liu YM, Li ZY, Hu H, Xu SP, Chang Q, Liao YH, Pan RL, Liu XM.. 2015. Tenuifolin, a secondary saponin from hydrolysates of polygalasaponins, counteracts the neurotoxicity induced by Aβ25-35 peptides in vitro and in vivo. Pharmacol Biochem Behav. 128:14–22. [DOI] [PubMed] [Google Scholar]
- Lv H, Ren W, Zheng Y, Wang L, Lu G, Yi P, Ci X.. 2016. Tenuigenin exhibits anti-inflammatory activity via inhibiting MAPK and NF-kappaB and inducing Nrf2/HO-1 signaling in macrophages. Food Funct. 7:355–363. [DOI] [PubMed] [Google Scholar]
- Lv J, Jia H, Jiang Y, Ruan Y, Liu Z, Yue W, Beyreuther K, Tu P, Zhang D.. 2009. Tenuifolin, an extract derived from tenuigenin, inhibits amyloid-beta secretion in vitro. Acta Physiol (Oxf)). 196:419–425. [DOI] [PubMed] [Google Scholar]
- Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Yardin C, Terro F.. 2013. Tau protein kinases: involvement in Alzheimer’s disease. Ageing Res Rev. 12:289–309. [DOI] [PubMed] [Google Scholar]
- Meena VK, Chaturvedi S, Sharma RK, Mishra AK, Hazari PP.. 2019. Potent acetylcholinesterase selective and reversible homodimeric agent based on tacrine for theranostics. Mol Pharm. 16:2296–2308. [DOI] [PubMed] [Google Scholar]
- Park CH, Choi SH, Koo JW, Seo JH, Kim HS, Jeong SJ, Suh YH.. 2002. Novel cognitive improving and neuroprotective activities of Polygala tenuifolia Willd extract, BT-11. J Neurosci Res. 70:484–492. [DOI] [PubMed] [Google Scholar]
- Park H, Kang S, Nam E, Suh YH, Chang KA.. 2019. The protective effects of PSM-04 against beta amyloid-induced neurotoxicity in primary cortical neurons and an animal model of Alzheimer’s disease. Front Pharmacol. 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lee K, Heo H, Lee M, Kim JW, Whang WW, Kwon YK, Kwon H.. 2008. Effects of Polygala tenuifolia root extract on proliferation of neural stem cells in the hippocampal CA1 region. Phytother Res. 22:1324–1329. [DOI] [PubMed] [Google Scholar]
- Perry G, Castellani RJ, Hirai K, Smith MA.. 1998. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimers Dis. 1:45–55. [DOI] [PubMed] [Google Scholar]
- Pi T, Zhou XW, Cai L, Zhang W, Su CF, Wu WT, Ren XM, Luo HM.. 2016. PI3K/Akt signaling pathway is involved in the neurotrophic effect of senegenin. Mol Med Rep. 13:1257–1262. [DOI] [PubMed] [Google Scholar]
- Piemontese L. 2017. New approaches for prevention and treatment of Alzheimer’s disease: a fascinating challenge. Neural Regen Res. 12:405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GB, Parchment RE, Lewellyn AL.. 1991. Hydrogen peroxide as a mediator of programmed cell death in the blastocyst. Differentiation. 46:181–186. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Cummings BJ, Cotman CW.. 1992. beta-Amyloid induces neuritic dystrophy in vitro: similarities with Alzheimer pathology. Neuroreport. 3:769–772. [DOI] [PubMed] [Google Scholar]
- Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M.. 2015. World Alzheimer Report 2015: the global impact of dementia – an analysis of prevalence, incidence, cost and trends. London (UK): ADI. [Google Scholar]
- Pu YL, Wang YH.. 2010. Damage and mechanism of central cholinergic system in Alzheimer’s disease. Chinese J Gerontol. 24:3840–3842. [Google Scholar]
- Reitz C, Mayeux R.. 2014. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 88:640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H, Seidman S.. 2001. Acetylcholinesterase–new roles for an old actor. Nat Rev Neurosci. 2:294–302. [DOI] [PubMed] [Google Scholar]
- Stringfellow HM, Jones MR, Green MC, Wilson AK, Francisco JS.. 2014. Selectivity in ROS-induced peptide backbone bond cleavage. J Phys Chem A. 118:11399–11404. [DOI] [PubMed] [Google Scholar]
- Su J, Tao MK, Chen XH, Li Q.. 2018. On quality problems of polygalae radix and primary processing of traditional Chinese medicinal materials. Chinese Pharm Aff. 32:23–28. [Google Scholar]
- Wang X, Li M, Cao Y, Wang J, Zhang H, Zhou X, Li Q, Wang L.. 2017. Tenuigenin inhibits LPS-induced inflammatory responses in microglia via activating the Nrf2-mediated HO-1 signaling pathway. Eur J Pharmacol. 809:196–202. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wan H, Li J, Zhang H, Tian M.. 2013. Molecular imaging in traditional Chinese medicine therapy for neurological diseases. Biomed Res Int. 2013:608430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Gao Y, Kang D, Huang B, Huo Z, Liu H, Poongavanam V, Zhan P, Liu X.. 2018. Design, synthesis and biological evaluation of tacrine-1,2,3-triazole derivatives as potent cholinesterase inhibitors. Med Chem Commun. 9:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TY, Chen CP, Jinn TR.. 2011. Traditional Chinese medicines and Alzheimer’s disease. Taiwan J Obstet Gyne. 50:131–135. [DOI] [PubMed] [Google Scholar]
- Xu KL, Chen Q, Liu W, Yao YY, Xia XX, Zhang BL, Feng C, Li YF.. 2012. Effect of tenuigenin on tau protein phosphorylation at Ser396 site in neurons of AD rats induced by Aβ1-40. Chinese J Pathophysiol. 34:3–1609. [Google Scholar]
- Xue W, Hu JF, Yuan YH, Sun JD, Li BY, Zhang DM, Li CJ, Chen NH.. 2009. Polygalasaponin XXXII from Polygala tenuifolia root improves hippocampal-dependent learning and memory. Acta Pharmacol Sin. 30:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Tuchida H, Kiyohara H, Takeda T, Yamada H.. 2003. Induction of NGF synthesis in astrocytes by onjisaponins of Polygala tenuifolia, constituents of kampo (Japanese herbal) medicine, Ninjin-yoei-to. Phytomedicine. 10:106–114. [DOI] [PubMed] [Google Scholar]
- Yan MH, Wang X, Zhu X.. 2013. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radical Bio Med. 62:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Korsmeyer SJ.. 1996. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 88:386–401. [PubMed] [Google Scholar]
- Yang XZ, Chen Q, Chen QL, Bb J, Ye HY.. 2013. Protection of tenuigenin against apoptosis of PC12 cells induced by amyloid beta-protein fragment 1-40. Chin J Pharmacol Toxicity. 27:379–384. [Google Scholar]
- Yu Y, Ye RD.. 2015. Microglial Abeta receptors in Alzheimer’s disease. Cell Mol Neurobiol. 35:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Qi RB, Wang ZG, Zhao YR, Wang HD, Lu DX.. 2011. Effect of senegenin on H2O2-induced damage in hippocampal neurons of SD rats. Chinese J Pathophysiol. 27:1059–1065. [Google Scholar]
- Zhang LL, Song WS, Wang K, Sun WM, Wang YF.. 2017. Progress in mechanism of pathogenesis and medical treatment of Alzheimer’s disease. Wor Chin Med. 12:1200–1208. [Google Scholar]
- Zhang TZ, Rong WW, Li Q, Bi KS.. 2016. Research progress on Polygalae Radix. Chin Tradit Herbal Drugs. 47:2381–2389. [Google Scholar]
- Zhou H, Xue W, Chu SF, Wang ZZ, Li CJ, Jiang YN, Luo LM, Luo P, Li G, Zhang DM, et al. 2016. Polygalasaponin XXXII, a triterpenoid saponin from polygalae radix, attenuates scopolamine-induced cognitive impairments in mice. Acta Pharmacol Sin. 37:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XQ, Li XM, Zhao YD, Ji XL, Wang YP, Fu YM, Wang HD, Lu DX, Qi RB.. 2016. Effects of senegenin against hypoxia/reoxygenation-induced injury in PC12 cells. Chin J Integr Med. 22:353–361. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Shan X, Yuzwa SA, Vocadlo DJ.. 2014. The emerging link between O-GlcNAc and Alzheimer disease. J Biol Chem. 289:34472–34481. [DOI] [PMC free article] [PubMed] [Google Scholar]