The “Moore swab” is a classic environmental surveillance tool whereby a gauze pad tied with string is suspended in flowing water or wastewater contaminated with human feces and harboring enteric pathogens that pose a human health threat. In contrast to single volume “grab” samples, Moore swabs act as continuous filters to “trap” microorganisms, which are subsequently isolated and confirmed using appropriate laboratory methods. Continuous filtration is valuable for the isolation of transiently present pathogens such as human-restricted Salmonella enterica serovars Typhi and Paratyphi A and B.
KEYWORDS: Moore swab, environmental surveillance, typhoidal Salmonella, waterborne pathogens, environmental bacteriology, filtration
ABSTRACT
The “Moore swab” is a classic environmental surveillance tool whereby a gauze pad tied with string is suspended in flowing water or wastewater contaminated with human feces and harboring enteric pathogens that pose a human health threat. In contrast to single volume “grab” samples, Moore swabs act as continuous filters to “trap” microorganisms, which are subsequently isolated and confirmed using appropriate laboratory methods. Continuous filtration is valuable for the isolation of transiently present pathogens such as human-restricted Salmonella enterica serovars Typhi and Paratyphi A and B. The technique was first proposed (1948) to trace Salmonella Paratyphi B systematically through sewers to pinpoint the residence of a chronic carrier responsible for sporadic outbreaks of paratyphoid fever. From 1948 to 1986, Moore swabs proved instrumental to identify long-term human reservoirs (chronic carriers) and long-cycle environmental transmission pathways of S. Typhi and Paratyphi, for example, to decipher endemic transmission in Santiago, Chile, during the 1980s. Despite limitations such as intermittent shedding of typhoidal Salmonella by humans and the effects of dilution, S. Typhi and S. Paratyphi have been recovered from sewers, surface waters, irrigation canals, storm drains, flush toilets, and septic tanks by using Moore swabs. Driven by the emergence of multiple antibiotic-resistant S. Typhi and S. Paratyphi A strains that limit treatment options, several countries are embarking on accelerated typhoid control programs using vaccines and environmental interventions. Moore swabs, which are regaining appreciation as important components of the public health/environmental microbiology toolbox, can enhance environmental surveillance for typhoidal Salmonella, thereby contributing to the control of typhoid fever.
INTRODUCTION
Looking at the fact in all its bearings, the only rational explanation of it seems to be, that the sewer does not propagate intestinal fever because it exhales foul gases, but solely because it is the actual recipient and vehicle of the fever poison.
—William Budd (1)
William Budd’s incrimination of sewage effluents as a major contaminant and cause of enteric illness (1) preceded Gaffky’s isolation of Salmonella enterica subspecies enterica serovar Typhi as the pathogenic cause of typhoid fever in 1886 (2) by 25 years. Recovery of S. Typhi from sewage became routine after the introduction of highly selective Wilson and Blair’s bismuth sulfite agar in 1927 (3), which allowed isolation of S. Typhi from complex mixed sewage and fecal samples (4–9). Indeed, since these discoveries, wastewater has been repeatedly subject to systematic surveillance, and outbreak responses to enteric illnesses have relied upon practical methods to detect pathogens in sewage and contaminated water.
In 1946, Brendan Moore (Fig. 1) of the Public Health Laboratory of Exeter was called upon to identify the sources of infection responsible for clusters of paratyphoid fever cases in North Devon, England, between 1943 and 1945 and for a larger outbreak of 25 cases in 1946 (10). Moore’s epidemiologic investigation linked the cases to a swimming area likely contaminated with sewage carrying Salmonella enterica subspecies enterica serovar Paratyphi B, the agent of paratyphoid B fever, and with consumption of ice cream from a truck vendor (10). Previously, environmental sources that had been shown to be contaminated with paratyphoid bacilli included untreated sewage (9) and feces-contaminated drinking/cooking water (11). For decades, public health officers had utilized sewage surveillance to detect shedders of these bacilli by inoculating sewage samples into enrichment broths, subculturing the broth onto selective media, identifying suspicious colonies, further subculturing to confirm characteristic biochemical profiles, serotyping by agglutination with specific antisera to surface antigens, and, when relevant and available, further typing with bacteriophages (12).
FIG 1.

Portrait of Brendan Moore. (Reprinted with permission from the Report and Transactions of the Devonshire Association for the Advancement of Science [133].)
To identify the specific foci of infection behind these North Devon outbreaks, Moore and colleagues applied systematic “sewage tracing” (10, 13). Moore’s predecessors would have expended enormous human and material resources to collect directly a volume of sewage at single time points, i.e., “catch” or “grab” sampling, across the town and then to check each sample for evidence of paratyphoid bacilli, while hoping that a sampling event would coincide with fecal excretion of paratyphoid bacilli into one of the sewers. Moreover, fecal excretion of S. Typhi and S. Paratyphi by short-term or chronic carriers may be intermittent (14–17), rendering even aggregates (composites) of several grab samples over an interval inadequate. To preserve resources and overcome the challenge posed by intermittent shedding, Moore sought to lay “traps” in the municipal sewers that could filter the microbial contents of flowing sewage continuously over extended periods of time.
A simple technique was therefore used of taking a piece of gauze about four feet in length and six inches wide, folding it into a pad of eight thicknesses and attaching it firmly by one end to a long piece of stout string. The gauze was then immersed in the flowing sewage, the string attached suitably just under the manhole cover, and the gauze left in position for 48 hours (10).
Moore applied the same classical bacteriology techniques of his day to enrich, select, isolate, and confirm S. Paratyphi B from these integrated sewer samples. He traced excretion of paratyphoid B bacilli to the single-family home of the local ice cream truck vendor and his wife. Fecal cultures from the ice cream truck owner’s wife confirmed that she was a chronic (putative gallbladder) carrier of paratyphoid bacilli and the likely source of infection for the 1946 outbreak. In North Devon, and in Sidmouth, Moore refined his technique for tracing excretion of S. Paratyphi and S. Typhi to single blocks and housing units (13, 18, 19).
This humble innovation to traditional sewage sampling paved the way for dozens of identifications of residual excreters of Salmonella Typhi and Paratyphi over the subsequent decades. Named after its dutiful inventor, this device became known as the “Moore swab” or generically as the “sewer swab,” “gauze pad,” or “sewer pad” method of trap sampling for enteric pathogens in sewage. Over the past 75 years, Moore’s classical technique for the detection of typhoidal Salmonella in sewage has been adapted to many fecal borne pathogens, including Vibrio cholerae O1 (20–26), Aeromonas spp. (27, 28), nontyphoidal Salmonella serovars (29), Escherichia coli O157:H7 (30), Campylobacter spp. (31), and poliovirus (32–38), among others (39). The utility of the method has expanded beyond the tracing of active pathogen excretion in sewage and rivers to include investigations of ongoing outbreaks, systematic environmental surveillance, bacterial enumeration in surface waters, and septic tank surveillance. In the ensuing sections, we first provide brief instructions on the Moore swab technique and its construction and then highlight several of these expanded uses, focusing on the detection of typhoidal Salmonella and Vibrio cholerae O1 as archetypal examples. We aim to stimulate new public health expertise and revitalize this versatile technique in both academic and public health laboratories where enteric disease surveillance and outbreak response to fecally transmitted human pathogens are integral to control efforts.
MOORE SWAB CONSTRUCTION AND PARAMETERS
Moore swabs are crafted using strips of cotton gauze cut into 6-inch by 48-inch lengths and folded eight times until an 8-ply square pad is formed (Fig. 2). The 6-inch by 6-inch square-pad is tied by a string, twine, wire, or fishing line around the center (10, 18, 40) and often sterilized in an autoclave. For a defined and controllable mesh size, cheese cloth may be substituted in place of cotton gauze (41–44). Long slits may also be cut into the square pad (41) or the uncut pad may simply be tied at a corner. Variations of the Moore swab have rendered it suitable for sampling typhoidal Salmonella from water closets (45), poliomyelitis virus from sewers (38), and other enteric pathogens from surface waters (46).
FIG 2.
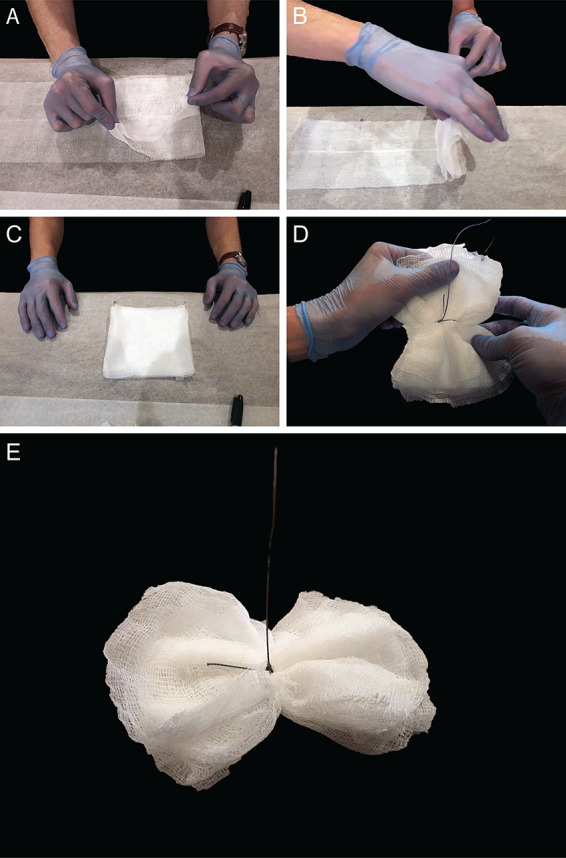
Constructing a Moore swab. (A and B) A length of gauze, 6 inches by 48 inches, is folded onto itself in a pleated pattern to form a pad. (C and D) The gauze pad is tied at the center with high-test fishing line. (E) The Moore swab may be suspended in flowing sewers or surface waters.
After its introduction, the method was expanded upon with variations on the setting, swab parameters, and postsampling enrichment and selection methods (Table 1). In recognition of differences in preference and available resources among enteric laboratories globally, the parameters and bacteriological steps in Table 1 represent examples drawn from the literature and author experience.
TABLE 1.
Method considerations and parameters for the isolation and identification of S. Typhi using the Moore swab technique
| Step | Options | Details or examples (references) |
|---|---|---|
| Site/source selection | Untreated effluent | Public sewer network (10, 18, 40, 56, 60, 61, 63); sewage leaving a building, residence, or hospital (55, 62, 64, 92); repurposed wastewater, e.g., for irrigation (69, 76) |
| Treatment plant influent/effluent | Wastewater entering/exiting a treatment plant (134, 135) | |
| Environmental | Surface water, e.g., canals, rivers, streams, and storm drains (55, 57, 58, 76) | |
| Flush toilets | Toilet prior to sewer (45) or septic tank (126) | |
| Parameters | Swab construction | Gauze type (fiber composition, mesh size, absorbency), gauze measurements (by size or by mass), string or wire attachment |
| Sampling schedule | Frequency, duration, replicates | |
| Sample handling and processing | Transport conditions and time | |
| Dilution schema | Direct inoculation, 5- or 10-fold dilution schema | |
| Enrichment | Preenrichment | Universal preenrichment broth, nutrient broth, sterile saline |
| Selective enrichment | Varies by species, e.g., selenite-F broth for Salmonella | |
| Differentiation/selection | Differential solid media | For enteric bacteria, e.g., Salmonella-Shigella agar, deoxycholate-citrate agar, xylose-lysine-deoxycholate agar |
| Highly selective solid media | Varies by species, e.g., Wilson and Blair (bismuth sulfite) agar for S. Typhi | |
| Identification (classical) | Biochemical reactions | Triple sugar iron agar, lysine iron agar, enzyme activity, urea broth, fermentation, commercial kits/panels |
| Serotyping | For Salmonella, agglutination with anti-O, anti-H, and anti-Vi antisera | |
| Identification (modern) | Pulse-field gel electrophoresis | |
| PCR | PCR or quantitative real-time PCR | |
| Genomics | Whole-genome, 16S ribosomal, or shotgun sequencing |
SALMONELLA TYPHI AND PARATYPHI
Typhoid and paratyphoid fevers, caused by ingestion of S. Typhi and S. Paratyphi A or B, respectively, are enteric illnesses that emerged as ideal candidates for environmental surveillance based on their epidemiology. These human host-restricted infections (47) by definition have no animal reservoir and are spread from person to person via the fecal-oral route by the consumption of food or water vehicles contaminated with the pathogen, either directly through short-cycle or indirectly through long-cycle transmission. The former usually takes place through a food vehicle contaminated by a temporary or chronically (≥12 months) infected individual who is shedding bacteria in their feces. Asymptomatic chronic carriers can harbor S. Typhi or S. Paratyphi in their gallbladders and intermittently excrete bacteria for decades. In long-cycle transmission, humans become infected from the consumption of vehicles derived from environmental contamination such as untreated sewage/septic system effluents reaching untreated water supplies or being used to irrigate crops that are consumed uncooked (48, 49). Viable S. Typhi bacilli have been recovered from septic tanks after 24 to 27 days (50, 51), vegetables after 1 to 2 months, and soil after 2 to 3 months (52, 53). S. Typhi can also travel considerable distances through soil, groundwater, and surface water (54). Thus, surveillance for these pathways is key to interrupt amplified transmission in high-incidence populations and to detect and eliminate residual reservoirs (i.e., temporary and chronic carriers).
Historically, it had been difficult to grow typhoidal Salmonella serovars from environmental sources; however, 20th century milestones in the formulation of enrichment broths, selective media, and both serotyping and bacteriophage typing markedly expanded the toolbox of the public health and environmental officer (12). After Moore’s classical publications, the technique was rapidly adopted across the United Kingdom and the United States to solve similar challenges—sporadic but persistent outbreaks of typhoid fever in small towns—epidemiologically akin to Moore’s experiences in North Devon and Sidmouth. Each group expanded upon Moore’s initial methodology to suit the needs of their surveillance program and to compare bacteriological methods.
Tracing a chronic carrier through sewers and rivers.
The classical use of the Moore swab, as described, was to trace an unknown but suspected chronic carrier through sewer or river sampling. The dilemma was typical: a small outbreak of a few cases of enteric fever would occur every few years or so, oftentimes of a single phage type, and a public health official or medical officer would eventually suspect an asymptomatic chronic gallbladder carrier of typhoidal Salmonella in the town. Moore swabs were systematically deployed in the jurisdiction at select sewage access sites and rivers or streams epidemiologically linked to the cases. Moore’s initial approach as described in 1950:
The infected swab, collected after 48 hours from the manhole under examination, is delivered to the laboratory in a sterile container. It is washed with 20 ml of nutrient broth and the washings pipetted off into a sterile tube. Three 5-fold dilutions of the infected material are plated in 0.1 ml volumes on Wilson and Blair’s medium, the latter plates being carefully dried before incubation. Five milliliters of washings are inoculated into 200 ml of selenite medium in a liter flask. After overnight incubation, the selenite medium is subcultured (a) to Wilson and Blair’s medium for typhoid colonies, and (b) to ‘mannitol-lead-acetate’ medium for other organisms of the Salmonella group (13).
Moore confirmed suspicious colonies by biochemical and agglutination methods.
When typhoidal Salmonella-positive points were discovered and mapped, additional swabs were deployed upstream of sewage flow of the positive point. This was repeated systematically until results showed that the swabs were positive downstream and negative upstream of an identifiable point of possible contamination, such as a block of houses sharing a common sewer (55). This general approach was also used in sampling rivers and streams until a pipe carrying wastewater with human fecal material was identified (56, 57) or septic tanks were observed to be located nearby (58). In all cases, a team of public health workers was then required to interview the people living at or near the suspected source and to obtain stool samples to confirm bacteriologically the silent excretion. Confirmation of the typhoidal Salmonella serovar and bacteriophage type further supported links between clinical isolates and isolates recovered from the environment. Frequently, chronic carriers discovered by this method gave no past medical history of enteric illness, emphasizing the importance of unbiased surveillance approaches. Finally, the utmost care for confidentiality was maintained during these investigations to avoid the social stigmatization of someone being labeled as a chronic typhoid carrier (59).
In the town of Purbrook, United Kingdom (population, ∼6,000), five pediatric cases of typhoid that occurred over 4 years led public health authorities to collect grab samples of water from the local river downstream of the town. One grab sample in 1949 finally yielded S. Typhi. Lendon and Mackenzie (56) observed that an overflow sewage pipe discharged into the river where the grab sample had been positive for S. Typhi. Accessing manholes, they systematically placed Moore swabs at sewer junctions upflow along the sewage pipe, documenting positives until they reached a point where the swabs became negative. They found the sewage pipe from a single household to be positive and this proved to be the home of a 70-year-old chronic carrier of S. Typhi.
In Farnham, United Kingdom, Hobbs (60) reported on a case of typhoid in a 7-year-old child who had exposure to a sewage-contaminated river and the use of Moore swabs to trace and pinpoint the house of a carrier. Greenberg et al. (40) and Shearer et al. (61) described detection of a single carrier in the isolated town of Portola, CA (population, ∼2,200), via use of Moore swabs in sewers; that carrier had been responsible for cases of typhoid occurring intermittently over ∼5 years. Robinson (62) used the sewer of a mental health facility housing known carriers of S. Typhi and S. Paratyphi as a site to compare methods: gauze versus alginate wool swabs, five different liquid enrichment media, four solid selective media, and dilution techniques. His conclusions were that selenite-F broth was most effective for enrichment of S. Typhi from sewage, that Wilson and Blair agar was the most selective medium, outperforming MacConkey agar, and that dilutions offered variable results likely dependent on sewage composition but should be included. In British Columbia, Bowmer et al. (58) were first to trace a chronic typhoid carrier household via storm drains and septic tank outflows. Interestingly, two phage types were present in the carrier, but only one was recovered from the infected children who had played downstream in the contaminated water. Pilsworth (63) traced a typhoid carrier through sewage in West Mersea, United Kingdom, and affirmed that 37°C was ideal for incubations. In 1964, Bokkenheuser (14) authored a comprehensive review of methodology to detect typhoid carriers. These classic investigations are listed in Table 2, with notable variations and unique perspectives discussed in ensuing sections.
TABLE 2.
A chronological review of the classical uses of Moore swabs and methods thereof for the filtration of typhoidal Salmonella from environmental samplesa
| Author(s) | Publication yr | Organism(s) | Prompting circumstances | Location(s) | Sampling site(s) | Date(s) | Time in situ | Bacteriological methodsb | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Moore | 1948 | S. Paratyphi B | A paratyphoid outbreak in a small coast town from June–August 1946 yielded 25 diagnosed cases of paratyphoid fever and preceded by sporadic cases: two in 1943, three in 1944, and six in 1945. Sewer samples remained positive for paratyphoid B bacilli for 18 mo after | North Devon, UK | Municipal sewers (25 manholes) | 1946–1948 | 48 h | Selenite-F broth, desoxycholate-citrate medium, Vi-bacteriophage typing | 10 |
| Moore | 1950 | S. Typhi | Modification of bacteriological methods to detect Salmonella Typhi in sewers and a description of various comparative experiments on the relative efficiency of standard bacteriology methods | North Devon, UK | Municipal sewers | Not reported | 48 h | Nutrient broth, Wilson and Blair agar, selenite enrichment broth, mannitol-lead-acetate medium, lactose-sucrose medium, agglutination, biochemical and serological testing, Vi-bacteriophage typing | 13 |
| Cruickshank | 1950 | Review of Vi antibody tests, culture media, systematic sewer tracing, and Vi-bacteriophage typing for their utility and importance | 19 | ||||||
| Jones | 1951 | S. Typhi | A sporadic case of typhoid fever in a hospital engineer who cleaned the hospital sewer was diagnosed 14 mo after a massive hospital outbreak involving 125 cases | Oswestry, UK | Hospital sewer: Main and six traps | 28 July 1949 to 13 March 1950 | 48 h | Selenite-F broth, deoxycholate citrate agar, Wilson and Blair agar | 64 |
| Lendon and Mackenzie | 1951 | S. Typhi | Two cases of typhoid fever in 1944 and three cases in 1948 appeared independently associated with a particular river located next to a sewer overflow | Purbrook, UK | Municipal sewers | 1948–1949 | 5–7 days | Selenite-F broth, deoxycholate citrate agar, Wilson and Blair agar | 56 |
| Moore, Perry, and Chard | 1952 | S. Typhi, Paratyphi | 1–4 cases occurring every 1–4 years since 1930, suggestive of a local human reservoir of infection | Sidmouth, UK | Municipal sewers | 1946–1949 | 48 h | Nutrient broth, selenite-F broth, Wilson and Blair agar, Hynes’ deoxycholate-citrate medium, agglutination, biochemical and serological testing, Vi-bacteriophage typing | 18 |
| Kwantes and Speedy | 1955 | S. Paratyphi | Outbreaks of paratyphoid fever occurred in 17 people in 1952 and 5 people in 1953, prompting suspicions of a paratyphoid carrier | Goodwick, Wales, UK | Water Closet (Direct toilet) | 1954 | 3–4 days | Selenite-F broth, Wilson and Blair agar | 45 |
| Kelly, Clark, and Coleman | 1955 | Coxsackie, Mycobacterium tuberculosis, S. Typhi | Five cases of typhoid fever occurred over 3 years in families living within 1 mile of a creek downstream of a sewage plant | New York State, USA | Sewage plants, sewers | Not reported | 4 days | Buffered 30% glycerol, tetrathionate medium, bismuth sulfite agar, modified Endo's agar, Vi-bacteriophage typing | 134 |
| Murdock and Lawson | 1955 | S. Typhi | Several cases of typhoid fever in adolescents—three in 1951, three in 1953, and two in 1954—were of the same Vi-phage type C and seemed linked to a pair of streams | Belfast, Northern Ireland | Stream, fishpond | 1954 | 72 h | Selenite-F broth, bismuth sulfite medium, Vi-bacteriophage typing | 57 |
| Hobbs | 1956 | S. Typhi | A 7-year-old boy diagnosed with typhoid fever from unknown source reportedly played near sewage overflow from heavy rains. Five historical cases of typhoid had occurred over the past 28 years | Farnham, UK | Municipal sewers | 1955 | 3 days | Not reported | 60 |
| Greenberg, Wickenden, and Lee | 1957 | S. Typhi | A carrier was suspected in four cases of typhoid fever occurring over 5 years | Portola, CA | Municipal sewers | 20 February to 1 April 1957 | 48 h | Selenite-F, bismuth sulfite agar, TSI, urea agar, Vi-bacteriophage typing | 40 |
| Shearer, Browne, Gordon, and Hollister | 1959 | S. Typhi | 61 | ||||||
| Bloom, Mack, and Mallmann | 1958 | Salmonella spp. | Methods were compared to isolate Salmonella from sewage before, after, and downstream from sewage plants and pumping stations | Lansing and East Lansing, MI | Sewage plants and pumping stations | October 1956 to December 1957 | 24–72 h | Tetrathionate broth, selenite brilliant green medium, bismuth sulfite agar, biochemical and serological testing | 135 |
| Robinson | 1958 | S. Typhi | Surveillance in a local mental health hospital where known carriers of S. Typhi and Paratyphi resided | Not reported | Hospital sewer | Not reported | 48 h | 5 different liquid media, 4 different solid media | 62 |
| Bowmer, Hudson, and Sunderland | 1959 | S. Typhi | Four cases of typhoid fever of the same phage type were diagnosed in 1953, 1955–1957 | Burnaby, British Columbia, Canada | Storm drains | 18 February to 1 April 1957 | 48 h | Nutrient broth, bismuth sulfite agar, biochemical and serological testing | 58 |
| Pilsworth | 1960 | S. Typhi | 20 cases of typhoid fever between August 1950 and December 1956, some of similar phage types | West Mersea, UK | Municipal sewers: 11 drainage areas and 1 pump | November 1952 to May 1954 | 3 days | Sterile saline, selenite broth, selenite brilliant green broth, deoxycholate citrate agar, Wilson and Blair agar, biochemical and serological testing, Vi-bacteriophage typing | 63 |
| Bokkenheuser | 1964 | Review of Vi antibody tests, chronic carrier state, specimen transportation, enrichment media, isolation media, sewage, and bacteriophage typing for the detection of typhoid carriers | 14 | ||||||
| Callaghan and Brodie | 1968 | S. Typhi | Laboratory aspects of a large postoutbreak surveillance program | Aberdeen, Scotland | Large-diameter municipal sewers | July 1964 to September 1966 | 7 days | Ringer solution, mannitol selenite broth, modified bismuth sulfite agar, modified Salmonella-Shigella agar, biochemical and serological tests, Vi-bacteriophage typing | 136 |
| Moore | 1971 | Review of history of sanitation and bacteriology practices important in typhoid investigations and control, and discussion of challenges ahead | 12 | ||||||
| Conn, Heymann, Jamieson, McWilliam, and Scott | 1972 | S. Typhi | Eight cases in Edinburgh from 1963–1970; in summer 1970, three of these directly connected with drinking from the Water of Leith river | Edinburgh, UK | River, draining surface water, household sewage | 27 July to 27 October 2019 | 7 days | Selenite-F broth, deoxycholate citrate agar, MacConkey medium, Kohn's two-tube medium, biochemical and serological tests, phage typing | 55 |
| McGinnis and DeWalle | 1983 | Review of the data available on the movement and survival of typhoid bacilli under various environmental conditions, including soil, septic tanks, sewers, and rivers | 54 | ||||||
| Sears, Ferreccio, Levine, Cordano, Monreal, Black, Ottone, Rowe, and Chilean Typhoid Committee | 1984 | S. Typhi | Urban endemicity in a modern industrialized metropolitan area where a majority of household sewage is left untreated prior to its use in crop irrigation | Santiago, Chile | Rivers and irrigation canals | January to March 1983 | 48–72 h | Selenite-F broth, Salmonella-Shigella agar, bismuth sulfite agar, deoxycholate-citrate agar, triple sugar iron agar slants | 76 |
Gray shading indicates review articles.
Wilson and Blair agar is bismuth sulfite agar and is sometimes modified in modern preparations. The terminology above follows the citation.
A residual carrier after a major hospital outbreak.
In 1951, an outbreak of 135 known cases of typhoid fever occurred in a hospital in Oswestry, United Kingdom (64). After meticulous collection of serial stool cultures and Vi-agglutination serology tests of 435 staff, 19 asymptomatic excreters of S. Typhi were detected along with 15 incubatory carriers who subsequently became clinical cases. Fourteen months after the outbreak was addressed, yet another case occurred in a hospital employee working at the hospital sewage treatment site. By placing Moore swabs in the main sewer and in sewers draining each of six wards, S. Typhi was pinpointed to a single ward. The responsible chronic carrier of S. Typhi was detected and linked epidemiologically using Vi-phage typing. A. C. Jones, the public health official behind this work, noted, “this type of sewage examination might well be employed as a supplementary postepidemic method of ensuring that an institution has been cleared of chronic carriers” (64).
The water-closet (toilet) swab.
Kwantes and Speedy reconfigured Moore swabs into string-wound cylinders to allow their placement directly into water closets (i.e., toilets) to detect a paratyphoid carrier (45). In Goodwick, Wales, this modification was necessary because wastewater exiting numerous houses entered a common drain accessed by a single manhole. This sewer configuration precluded the systematic tracing upstream from the manhole to individual houses. By extending toilet swabbing schedules to accommodate the occupants’ weekend bathroom habits, the individual toilet method proved successful in identifying an unknown carrier of S. Paratyphi B (45).
Sewage-contaminated water.
Three young boys in Belfast, Northern Ireland, who had been playing near a polluted stream became acutely ill with typhoid fever in 1951 (57). Six weeks later, a workman near the stream also fell ill with typhoid having the same Vi-phage type as the boys’ isolates. Murdock and Lawson were unable to link these initial cases epidemiologically nor isolate S. Typhi from the stream using Moore swabs (57). Three years passed until two more cases occurred that were associated with exposure to the same stream and another nearby stream. S. Typhi of the same Vi-phage type was detected using Moore swabs in water of the second stream and from a septic pipe draining sewage from several houses. Upon inquiry and testing fecal specimens from the residents, Murdock and Lawson discovered a single chronic S. Typhi carrier (57). A fluorescein color dye test confirmed the connection between the carrier’s septic effluents and the second stream (57).
Bowmer et al. (58) fittingly entitled their 1959 investigation of four sporadic cases of bacteriophage-matched typhoid fever in schoolchildren from 1953 to 1957 as “Typhoid fever: where there’s a case, there’s a carrier.” Indeed, a single carrier was discovered by employing the Moore swab technique in stormwater drains of a municipality that lacked a piped sewerage system and relied exclusively upon household septic tanks. Bowmer’s team traced the contamination with typhoid bacilli to a roadside stormwater drain that serviced three houses containing seven persons. A 59-year-old woman’s coproculture yielded S. Typhi of the same bacteriophage type as the four cases. The carrier subsequently underwent cholecystectomy that revealed S. Typhi in her bile, gallstones, and gallbladder wall tissue (58), consistent with typhoidal persistence (65–67).
THE SANTIAGO, CHILE, EXPERIENCE
During the late 19th and early 20th century, treatment of municipal water supplies by chlorination, sand filtration, or both resulted in a precipitous decline in typhoid fever incidence in the major cities of Europe and North America (68). Installation and maintenance of piped sewerage networks also contributed importantly to control typhoid fever. A major exception to this trend was the metropolitan region of Santiago, Chile, where high annual incidence rates of endemic typhoid fever persisted through the 1980s, despite 96% of households having access to treated, bacteriologically monitored, potable water and 70% to 80% having flush toilets to remove human fecal waste (69). In Santiago during that era, typhoid incidence showed a marked summer seasonality and affected all socioeconomic strata (69). Two hypotheses were posed to explain this enigmatic high-incidence endemicity: (i) an unusually high prevalence of chronic biliary carriers of S. Typhi who not only served as a large long-term reservoir of infection but also maintained high-incidence short-cycle transmission through contamination of food vehicles in homes, food kiosks, and restaurants, or (ii) an unconventional irrigation practice in which untreated sewage, after it had coursed through the city, was used to irrigate crops grown in fields in the periphery.
In support of the first hypothesis, which was by far the most widely accepted in the 1970s, Santiago was shown to have an exceptionally high prevalence of cholelithiasis among adults (70). Of 1,000 consecutive Santiago residents (796 females and 204 males) undergoing cholecystectomy in seven hospitals in Santiago from July to October 1980 and who had their bile cultured, 38 (3.8%) bile cultures yielded S. Typhi and 35 (3.5%) grew S. Paratyphi (30 of 35 were Paratyphi B) (71). Levine et al. used those data, along with the prevalence of cholelithiasis in Santiago by age and sex, to estimate the prevalence of chronic typhoid carriers by age and sex (70). The overall carrier prevalence was 694/105 persons >10 years of age. However, among women aged ≥40 years, the prevalence of S. Typhi biliary carriers exceeded 2,000/105 (i.e., >2% of the women). This very high estimated prevalence of typhoid carriers, corroborated subsequently by prospective data from case/control studies (72) and prevalence surveys of food handlers that included stool cultures (73, 74), gave credibility to those who argued that chronic carriers were playing the key role in maintaining the high typhoid incidence in Santiago.
Evidence incriminating the role of environmental contamination being responsible for maintaining the high incidence of typhoid in Santiago required active investigation. Careful inspection of the manner in which raw sewage was handled in Santiago and a series of painstaking environmental bacteriology studies utilizing Moore swabs incriminated the use of untreated sewage in the dry summer months to irrigate extensive fields of vegetable crops that were eaten uncooked as the practice that was responsible for maintaining amplified long-cycle transmission. This could not have been achieved without Moore swabs.
In the 1970s and 1980s, neighborhood sewers in Santiago deposited raw untreated sewage into two waterways, including a giant open sewage canal (Zanjon de la Aguada) that traversed the metropolitan region from east to west and to a lesser extent into the Mapocho River that coursed the city from northeast to southwest in a gentle S-like trajectory. In the southwestern part of the city, the Zanjon emptied its sewage into the Mapocho River, which then coursed for several kilometers without additional sewage entering the river in an attempt to allow self-cleansing of the contaminated water. In the western and southwestern parts of the metropolitan region, these waters, which remained untreated, were subsequently used to irrigate lettuce, cabbage, and celery crops in the rainless summers, i.e., vegetables that were consumed uncooked. These vegetables, which were trucked back to the markets and supermarkets of the city for sale, ultimately contaminated food preparation areas of kitchens in households and restaurants (72, 75).
Moore swabs proved to be a critical tool in elucidating the previously enigmatic mode of transmission responsible for maintaining amplified high-incidence endemic S. Typhi disease in Santiago. From January to March, 1983, i.e., during the Chilean summer, Sears and colleagues deployed 133 Moore swabs into irrigation canals (Fig. 3) (76). Of the 93 swabs recovered and cultured, 4 of 45 (8.9%) from the Mapocho River and 4 of 31 (12.9%) from the Zanjon de la Aguada yielded S. Typhi. Prior to this, Chilean microbiologists at the Instituto de Salud Pública (Institute of Public Health [ISP]) had used traditional “grab” sampling in their environmental bacteriologic attempts to determine if S. Typhi was present in these wastewaters. Notably, only once among 100 grab samples collected over several years was S. Typhi isolated (77).
FIG 3.
Placement of Moore swabs in the irrigation canals outside Santiago, Chile, in January of 1983.
In collaboration with the ISP, Sears et al. used virtually identical bacteriological methods as the ISP had previously employed but substituted the Moore swab method for “grab” sampling (76). This change in collection technique led to repeated recovery of S. Typhi from these irrigation waters in 1983, thereby supporting the hypothesis that vegetables irrigated with untreated sewage water were underlying Santiago’s unusual pervasiveness of typhoid endemicity (76). This was also the first demonstration of the efficacy and utility of the Moore swab in recovering typhoid bacilli from large-diameter irrigation canals in a highly endemic urban setting. This observation is highly relevant in sub-Saharan African cities today where urban watercourses function as open sewers.
Four large-scale field trials of live oral typhoid vaccine in ∼500,000 Chilean school children carried out in the 1980s markedly decreased the incidence of typhoid in that high-risk age group (78–81). Based on the evidence generated by Sears and team (76) in collaboration with the ISP, the Ministry of Health recommended to the government of Chile that the use of untreated sewage water to irrigate crops should be prohibited, but this recommendation was not heeded. In 1991, after an absence of a century, cholera returned to South America, resulting in massive outbreaks in Peru (82), Ecuador (83), Argentina (84), and Colombia (85), before spreading more widely through the continent (86). A small outbreak of 41 cases of El Tor cholera hit the metropolitan region of Santiago in April 1991 (87). Facing the threat of cholera, the Ministry of Health and other arms of the government of Chile mobilized rapidly and effectively and finally outlawed the use of untreated sewage water to irrigate crops (75, 88). This ban was strictly policed. Despite the unpopularity of this ban among farmers and agricultural workers whose livelihoods were affected, this intervention abruptly interrupted the cholera outbreak and also led to a precipitous fall in the transmission of typhoid, resulting in the end of amplified endemic transmission (75). Within a few years, only sporadic low-level transmission due to chronic carriers contaminating food vehicles continued, and that residual incidence progressively declined over the following decade. These data confirm the key role that use of contaminated irrigation waters had previously been playing in maintaining amplified transmission and perpetuating endemic typhoid in Santiago (75, 89).
CHOLERA
Cholera, the diarrheal illness caused by cholera toxin-producing Vibrio cholerae of serogroup O1, in its severe clinical form, cholera gravis, can rapidly dehydrate and kill even healthy adults if water and electrolyte losses are not expeditiously replaced. The current 7th pandemic of cholera due to the El Tor biotype began in 1961 in Southeast Asia, disseminated to south and east Asia during the 1960s, reached Africa by 1970 and South America in 1991. In newly affected areas, cholera tends to occur in explosive epidemics, and in endemic foci, it exhibits marked seasonality. Since the early 1970s, Moore swabs have played an important role in cholera surveillance in threatened as well as cholera-stricken communities and in tracing infected persons with clinical and asymptomatic infections. Examples are described below from low- and middle-income and from high-income countries.
Anticipatory sentinel sewage surveillance for healthy carriers.
In 1973, widespread occurrence of cholera in neighboring Malawi, Mozambique, and Angola prompted South Africa to initiate surveillance to detect cholera in presumed high-risk populations (20, 21). It was assumed that the many migrant laborers who came from the above-mentioned countries to work long term in South Africa’s coal and gold mines would be likely introducers of V. cholerae O1. Isaäcson et al. undertook a three-pronged surveillance system among 20,000 miners that included (i) systematic Moore swab surveillance of sewers serving the mines and an acclimatization center (21), (ii) expeditious collection of rectal swabs from miners served by sewers that yielded V. cholerae O1 from surveillance Moore swabs and (iii) physical isolation and rectal swab cultures from cases of diarrheal illness to confirm cholera cases.
Although sewer surveillance using Moore swabs began in November 1973, there were no isolations of V. cholerae O1 until late March 1974. Rectal swabs from miners whose toilets were served by the culture-positive sewer line revealed two miners with subclinical infection. One week later, as sewer swabs remained positive, there were six subclinical carriers and four clinical cholera cases. A full-blown outbreak ensued that lasted through early May. During that period, Moore swabs in sewers were positive while increasing numbers of confirmed clinical cases and asymptomatic infections were detected. The acclimatization center was identified as the key source of infection of new workers (21). Positive Moore sewer swabs continued for 2 weeks after there were no further clinical or subclinical infections.
Outbreaks of cholera in unexpected venues.
In the 1970s, sporadic cases of cholera gravis were confirmed in U.S. citizens who had not traveled. These U.S. cholera cases, which yielded an unusual highly hemolytic V. cholerae O1 El Tor Inaba strain, led to extensive use of Moore swabs to detect V. cholerae O1 in sewage, septic tanks, and environmental waters. Early in the 7th pandemic, El Tor biotype isolates typically were highly hemolytic, but by the 1970s, El Tor strains were uniformly nonhemolytic. Weissman et al. (22) isolated the same hemolytic V. cholerae O1 El Tor Inaba from a Moore swab placed in the septic tank draining the house of a 1973 Port La Vaca, Texas, patient as from the patient himself.
In 1978, 11 cases of cholera occurred along southwest Louisiana’s Gulf Coast, all due to hemolytic V. cholerae O1 El Tor Inaba. An epidemiologic investigation incriminated consumption of cooked crabs as the vehicle of transmission (90). Isolation of V. cholerae O1 from the municipal sewer system serving the town of the index case in Louisiana in 1978 indicated that there might be other cases (24). During September and October 1978, V. cholerae O1 El Tor Inaba was recovered from sewerage systems of six municipalities, while epidemiological investigations (including stool cultures) of persons with diarrheal illness in three of these towns confirmed cholera cases. Additionally, 11 of 21 Moore swabs from sewer systems serving communities where no cases of cholera were identified also grew V. cholerae O1.
V. cholerae O1 was detected in cultures of 7 of 10 (70%) Moore swabs placed in sewer lines serving homes of known infected persons prior to their receiving antibiotics, while the pathogen was isolated from only 1 of 16 (6%) swabs removed from sewers serving infected persons 1 to 7 days after they had commenced therapy with tetracycline (24). V. cholerae O1 was also recovered using Moore swabs from 2 of 4 septic tanks serving households of infected individuals. In one culture-negative septic tank, the Moore swabs had been placed 25 days after the patient was hospitalized; the other negative septic tank had “special treatment equipment.”
In one Louisiana town, Barrett et al. (24) used sewer swabs to track V. cholerae O1 in a manner reminiscent of Moore’s tracing the source of S. Paratyphi B. They recovered V. cholerae O1 from a Moore swab cultured on 9 September 1978 from the intake line of a sewage treatment plant. Four days thereafter, a second Moore swab was placed into the sewage treatment intake line, and swabs were also inserted into lines of 17 pumping stations draining different sections of town. Swabs from two sites yielded V. cholerae O1: one from the treatment plant intake and the second from a pumping station serving ∼50 blocks of dwellings. They then placed swabs in the tributary lines and, in 4 days, traced the source of infection to a 2-block area amenable to a Moore-like house-to-house search for the infected person(s). Meanwhile, a resident from this small area was admitted to the hospital with cholera gravis, and the stool culture grew V. cholerae O1. The patient’s daughter, who had subclinical V. cholerae O1 infection and remained at home, was the presumed source of V. cholerae O1 in the sewage line following her parent’s hospitalization (24).
With cholera, as with typhoidal Salmonella, Moore swabs can detect subclinical or mild infections of infected persons who do not seek medical care. Barrett et al. noted that in Louisiana (and most other jurisdictions), sewer swab surveillance was under the control of the local health department and did not require obtaining individual consent from the household members served by the sewers (24). Whereas sewer swab surveillance cannot detect infected persons not served by a sewerage system, septic tank surveillance is also effective, albeit more labor intensive (24). The CDC continues to recommend Moore swabs as a routine environmental surveillance method for the detection of V. cholerae O1 because of its practicality and effectiveness (91).
Two more isolated cases of cholera were reported in Texas in 1981. Moore swabs were placed in sewers of the three Texas cities harboring cases, and sewers draining houses of the cases yielded V. cholerae O1. These 1981 isolates were the same hemolytic El Tor Inaba strain exhibiting an identical restriction endonuclease pattern as the 1973 and 1978 Gulf of Mexico isolates, leading to the conclusion that this strain is indigenous to the environment of the Gulf of Mexico (25). It is now well established that V. cholerae O1 constitutes the autochthonous flora of brackish water environments where they adhere to chitinous zooplankton, whether in the Gangetic delta or the Gulf of Mexico.
Epidemiologic investigation of an outbreak in a middle-income setting.
In July 1974, a small cluster of six cases of cholera due to V. cholerae O1 El Tor Ogawa occurred on the island of Guam, the U.S. Territory in the Mariana Islands (23). The cases occurred among construction workers who consumed an epidemiologically incriminated vehicle of transmission, a raw salted fish dish prepared from fish caught in Agana Bay. Moore swabs placed in the septic tank of one case and in the sewer downstream of the house of two other cases grew V. cholerae O1 El Tor Ogawa (23). V. cholerae O1 El Tor Ogawa was also isolated from two sewer lines that were not draining houses of known cholera patients and from water of the mouth of the Fonte River.
Detection of live oral cholera vaccine in sewage.
In 1991, Simanjuntak et al. (26) investigated the safety, immunogenicity, and transmissibility of live oral cholera vaccine CVD 103-HgR compared to that of placebo in 200 children aged 2 to 5 years old in North Jakarta, Indonesia. After the administration of vaccine or placebo to the children, Moore swabs were placed in the open sewers or privies draining the latrines of 97 households of study participants to assess the environmental persistence of the vaccine strain. While non-O1 V. cholerae was recovered from human wastewater in 46 of the 97 sites, the CVD 103-HgR vaccine strain was not recovered from any specimens. These results support the sensitivity of the Moore swab method for detecting Vibrio species while establishing low transmissibility of the vaccine strain itself (26).
DISCUSSION AND FUTURE DIRECTIONS
There are several fundamental challenges to sampling an urban environment for a pathogen that is transiently present (14, 18). Variables include unequivocal presence of a source of contamination, intermittent excretion, variability in pathogen load within the environmental source, diameter and flow rate of the sampling site, size of the population contributing fecal matter (household, block, neighborhood, district, and city), presence of disinfectants or antibiotics deleterious to the organism, variations in the materials used to prepare the swab and its dimensions, and variations in bacteriologic methods (Table 1).
In Santiago, Chile, in 1983, Sears et al. (92) determined the sensitivity of the Moore swab under conditions designed to minimize these limitations: pipes draining the homes of bacteriologically confirmed asymptomatic chronic typhoid carriers (n = 10) were tested; each sewer was sampled for 48 to 72 h on 2 to 3 separate occasions during periods when the carriers were known to be home, usually Friday to Monday; small-diameter open sewer drains located on the household property that were shared with one or two other homes were tested (Fig. 4); and no carrier was taking antibiotics. Finally, standardized bacteriological methods were used to isolate S. Typhi from sewage (selenite-F broth enrichment; Salmonella-Shigella agar, Wilson and Blair [bismuth sulfite] agar, and deoxycholate citrate lactose sucrose agar to detect suspicious colonies; inoculation of suspect colonies into triple sugar iron agar slants; serotyping with specific antisera to confirm Typhi serovar; and phage typing of confirmed S. Typhi isolates). In total, 24 swabs were placed in sewers draining the homes of 10 known chronic carriers (minimum 2 swabs at different times per home). Six positive swabs correctly identified homes of 5 of the 10 carriers, indicating the method, as used, was 50% sensitive in identifying carrier households (92). Sears et al. (92) may have underestimated the sensitivity, since the period of sampling may not have in fact coincided with deposition of S. Typhi into the sewer by the carrier, particularly if shedding was intermittent or if the carrier did not use the connected toilet during the sampling period. In cases of institutional sewage surveillance (e.g., hospitals and prisons), disinfectants may diminish sensitivity of the culture-based bacteriology (93).
FIG 4.

Sewer swab placed in the small diameter sewer draining three households in Santiago, Chile, in January 1983.
Efficacy of the Moore swab method to yield a viable pathogen also depends on the bacteriologic methods employed and the survival of the target organism. As pathogenic typhoidal Salmonella evolved into host-restricted serovars, traits that support enhanced survival in the environment were lost (94). Thus, organisms that have adapted to occupy environmental niches may out compete the human host-restricted pathogen in environmental samples.
S. Typhi may possibly exist in a “viable but nonculturable” (VBNC) state in water during adverse environmental conditions (95–97), as does V. cholerae O1 in brackish waters of the Gangetic delta. Under conditions of salinity, temperature, and pH of water during noncholera seasons, V. cholerae O1 remains alive but cannot be cultured directly; yet, it revives at the onset of cholera season when it becomes readily cultivable from environmental samples (98). Reversion to the cultivable state also occurs in vivo when VBNC V. cholerae O1 passes through the human intestine. This was documented in a clinical study where attenuated Vibrio cholerae O1 live oral vaccine strain CVD 101, when subjected to appropriate conditions, became noncultivable. However, an inoculum fed to healthy adult U.S. volunteers resulted in recovery of viable vaccine organisms from coprocultures from the volunteers (99). That VBNC nontyphoidal Salmonella does not regain infectivity in susceptible animals (100, 101) argues against the notion that VBNC typhoidal Salmonella regains infectivity when ingested by humans.
Nontyphoidal Salmonella enterica serovars survive and replicate inside intracellular vacuoles of waterborne free-living amoeba (Acanthamoeba) (102, 103). S. Typhi shows enhanced survival when incubated in the presence of Acanthamoeba castellanii (104), which may suggest that a similar symbiosis occurs in the environment. Such alternative environmental states of typhoidal Salmonella, if real, could be missed by the culture-based Moore swab.
Arguably, a true positive can also be defined as the presence of S. Typhi DNA in an environmental sample. Molecular methods, such as quantitative real-time PCR (qPCR), offer the potential to monitor drinking water for contamination with typhoidal Salmonella (105, 106) or to detect S. Typhi DNA in sewage samples from households of typhoid cases compared with those of controls (107). Ideally, a multitargeted PCR (108) and culture-based techniques should be combined to permit the isolation of viable organisms. Salmonella DNA persists (109), and molecular detection of bacteria from complex environmental matrices has notable challenges with both sensitivity and specificity at all stages of the process (108).
Bisha et al. (46) have adapted the filtration concept behind Moore’s sewer swab for a pump-based filtration device coined the “modified Moore swab (MMS),” in which cheese cloth or gauze is packed into a polyvinyl chloride pipe and a volume of water is pumped through the cloth. MMS is typically used to enumerate various fecal indicators and pathogens from surface water sources, including Salmonella spp., Escherichia coli, and Listeria monocytogenes, among others (42, 44, 46, 110–114). While quantitative, this modification of the Moore swab method has not yet been adapted to accommodate the intermittent excretion of S. Typhi and S. Paratyphi (115). MMS devices could prove useful for the filtration of typhoidal Salmonella if reconfigured to remain in situ for several days.
Recovery of a viable culturable organism is increasingly critical as disease surveillance and control efforts for typhoidal Salmonella shift toward using data from whole-genome sequencing (WGS), which requires a live organism (116). In these analyses, geospatial, geographical, and temporal data are integrated with WGS data derived from S. Typhi isolated from positive blood or stool cultures to monitor genotype circulation and antimicrobial resistance (117–120) and to assess S. Typhi population structures before and after a typhoid vaccination intervention (121). Few genomic epidemiology analyses apply WGS to environmentally derived typhoidal Salmonella isolates alongside clinical isolates. However, in one example, a genetic comparison completed in the mid-1990s using pulse-field gel electrophoresis (PFGE) to characterize clinical and environmental (sewage and river water) isolates from Santiago, Chile, from 1983 demonstrated overlap between the human and environmental samples (122). While the genomic resolution of PFGE is insufficient to decipher microcirculation patterns compared with that of WGS (123, 124), these data further supported the conclusion that irrigation of vegetable crops with untreated sewage was responsible for amplified long-cycle transmission that maintained typhoid endemicity in Santiago in the 1980s (69, 75, 76). Within this gap in typhoidal genomic epidemiology, the Moore swab may provide live culturable environmental isolates that, compared against human-derived isolates, will directly contribute to new models of transmission mechanics supporting either short-cycle or long-cycle transmission.
CONCLUSION
This review details the practical utility of the Moore swab in outbreak and endemic settings for the isolation of viable S. Typhi, S. Paratyphi, and Vibrio cholerae O1 from various environmental sources, including sewage and surface waters. This tool may initially appear unappealing to the academic research community; however, when paired with quality-ensured diagnostic bacteriology, Moore’s technique has repeatedly exposed human reservoirs and environmental amplification mechanisms of these pathogens under varied circumstances. In several examples presented, the method’s flexibility proved critical to its public health utility. However, the challenges faced in sampling sewage and wastewater cannot be understated, and overcoming these should be a priority for funding agencies.
Modern advances in filtration technologies, molecular techniques, and next-generation sequencing have expanded and improved the precision of the toolkit available to public health laboratories, especially those located in high-income countries. Nevertheless, for low- and middle-income countries and for global academic researchers with limited funding, sustainable routine surveillance and bacteriology for enteric pathogens are essentially limited to affordable culture-based techniques. Indeed, Moore swabs in their classical applications have resurfaced in recent conference proceedings describing environmental surveillance programs for S. Typhi in Kolkata, India (125), and Samoa (126) and have been part of standardization efforts in the field (127, 128).
Given its simplicity and affordability, the Moore swab is well suited in resource-limited settings where typhoidal Salmonella is broadly endemic and sewage monitoring is feasible either through a municipal sewage network or household toilets connected to septic tanks. The identification of hot spots in a community or at certain public facilities (e.g., bus stations, rural hospitals, or ports and terminals) may inform epidemiological investigations, target vaccination campaigns, and help differentiate short-cycle versus long-cycle transmission patterns. PCR or advanced separation methods can improve the sensitivity of pathogen detection but should be combined with culture-based methods to recover viable organisms for further characterization. Subsequent in vitro studies may be performed, and next-generation genome sequencing can pave the way for genomic epidemiology and phylogeographical analyses connecting isolates from cases, contacts, and the environment.
Driven by the emergence and spread of S. Typhi harboring multiple antibiotic resistances (129) and availability of an efficacious new typhoid vaccine that is prequalified by the World Health Organization (130), the public health community is accelerating control of typhoid in many areas of endemicity (131, 132). Improved typhoidal disease surveillance and environmental microbiology can play important roles in guiding typhoid control, identifying human reservoirs, and incriminating specific modes of transmission that involve environmental contamination amenable to intervention. Where typhoid cases are rare, or in countries such as Fiji and Samoa, where mass vaccination efforts with typhoid vaccines are planned, sporadic and residual cases likely associated with human reservoirs of chronic biliary infection will become proportionately more important in the coming decade. These reservoirs and any associated environmental transmission pathways can be traced and identified through sewage networks, septic systems, and surface waters using Moore’s original application of the sewer swab.
ACKNOWLEDGMENTS
The motivation behind the manuscript was driven by our collaboration with the Centro para Vacunas en Desarollo (CVD-Chile) located in Santiago, Chile, where M.M.L. and colleagues carried out studies with the Moore swab in the 1980s as described above, and the enormous efforts by the Ministry of Health of Samoa for the accelerated control of typhoid fever, which includes an environmental sampling protocol adapted by M.J.S. and colleagues using Moore swabs to sample septic tanks, hospital effluents, and river water for S. Typhi.
M.J.S. and M.M.L. are funded by the Bill & Melinda Gates Foundation grants entitled “Current prevalence of chronic typhoid carriers and residual transmission of typhoid fever in Santiago, Chile” (OPP1161058) and “Strengthening typhoid surveillance and microbiological lab capacity in Samoa” (OPP1194582). M.J.S. received support to initiate Moore swab environmental surveillance in Samoa through a Benjamin H. Kean Travel Fellowship in Tropical Medicine by the American Society of Tropical Medicine & Hygiene (ASTMH) and a Medical Scholars award from the Infectious Diseases Society of America (IDSA) Foundation. Expanded environmental surveillance by the Ministry of Health of Samoa is currently supported by the Green Climate Fund.
Biographies

Michael J. Sikorski, BS, is an MD-PhD candidate in Microbiology and Immunology through the Medical Scientist Training Program at the University of Maryland School of Medicine. Under the comentorship of Dr. Myron M. Levine (Center for Vaccine Development and Global Health) and Dr. David A. Rasko (Institute for Genome Sciences), Michael is studying the genomics and genomic epidemiology of endemic typhoidal Salmonella circulating in the island nation of Samoa. In 2018 to 2019, Michael introduced the Moore swab technique to collaborators at the Ministry of Health of Samoa for sewage, septic tank, and surface water surveillance of typhoidal Salmonella. From 2017 to 2019, Michael worked with the Centro para Vacunas en Desarollo-Chile on a prevalence study of residual chronic gallbladder carriers of Salmonella Typhi decades after hyperendemic typhoid transmission was halted. Michael earned his degree in Bioengineering from the University of Maryland, College Park, where he worked in the laboratory of Peter Kofinas, PhD.

Myron M. Levine, MD, DTPH, is the Simon and Bessie Grollman Distinguished Professor at the University of Maryland School of Medicine, Associate Dean for Global Health, Vaccinology and Infectious Diseases, and Founder and Former Director of the Center for Vaccine Development and Global Health (CVD). Since 1970, Dr. Levine has worked on vaccine research and is widely recognized as a pioneer of the discipline of “vaccinology.” After founding CVD in 1974, he served as Director until 2015, developing vaccines to prevent certain infections—such as cholera, Shigella dysentery, typhoid fever, and nontyphoidal Salmonella—that have enormous disease burdens in impoverished populations and developing countries. Dr. Levine served as external advisor to the Chilean Typhoid Fever Control Program (1979 to 1992) and serves in a similar capacity for the nascent Samoa Typhoid Fever Control Program. In 1995, Dr. Levine was elected to the National Academy of Medicine and the American Academy of Microbiology.
REFERENCES
- 1.Budd W. 1859. Intestinal fever no. III. Part 2 of 2. Lancet 74:458–459. doi: 10.1016/S0140-6736(02)72953-X. [DOI] [Google Scholar]
- 2.Gaffky G. 1886. On the etiology of enteric fever, p 205–257. Recent essays by various authors on bacteria in relation to disease. New Sydenham Society, London, United Kingdom. [Google Scholar]
- 3.Wilson WJ, Blair EV. 1927. Use of a glucose bismuth sulphite iron medium for the isolation of B. typhosus and B. proteus. J Hyg (Lond) 26:374–391. doi: 10.1017/s0022172400009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson WJ. 1928. Isolation of B. typhosus from sewage and shellfish. Br Med J 1:1061–1062. doi: 10.1136/bmj.1.3520.1061-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray J. 1929. The isolation of B. paratyphosus B from sewage. Br Med J 1:142–144. doi: 10.1136/bmj.1.3551.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Begbie RS, Gibson HJ. 1930. Occurrence of typhoid-paratyphoid bacilli in sewage: some further observations. Br Med J 2:55–56. doi: 10.1136/bmj.2.3627.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gell P, Hobbs BC, Allison VD. 1945. An outbreak of water-borne typhoid investigated by bacteriophage typing and “selective” sewage examination. J Hyg (Lond) 44:120–128. doi: 10.1017/s0022172400035944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming G. 1933. Typhoid-paratyphoid in institution sewage. Br Med J 1:412–413. doi: 10.1136/bmj.1.3766.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson WJ, Blair EM. 1931. Further experience of the bismuth sulphite media in the isolation of Bacillus typhosus and B. paratyphosus B from faeces, sewage and water. J Hyg (Lond) 31:138–161. doi: 10.1017/S0022172400010743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore B. 1948. The detection of paratyphoid carriers in towns by means of sewage examination. Mon Bull Minist Health Public Health Lab Serv 7:241–248. [Google Scholar]
- 11.Jones DJ, Gell PGH, Knox R. 1942. A water-borne outbreak of paratyphoid B fever. Lancet 240:362–363. doi: 10.1016/S0140-6736(00)46163-5. [DOI] [Google Scholar]
- 12.Moore B. 1971. Typhoid: epidemiological investigation and control measures. Public Health 85:152–158. doi: 10.1016/s0033-3506(71)80054-9. [DOI] [PubMed] [Google Scholar]
- 13.Moore B. 1950. The detection of typhoid carriers in towns by means of sewage examination. Mon Bull Minist Health Public Health Lab Serv 9:72–78. [Google Scholar]
- 14.Bokkenheuser V. 1964. Detection of typhoid carriers. Am J Public Health Nations Health 54:477–486. doi: 10.2105/ajph.54.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leavitt JW. 1992. “Typhoid Mary” strikes back: bacteriological theory and practice in early twentieth-century public health. Isis 83:608–629. doi: 10.1086/356292. [DOI] [PubMed] [Google Scholar]
- 16.Thomson S. 1954. The number of bacilli harboured by enteric carriers. J Hyg (Lond) 52:67–70. doi: 10.1017/s0022172400027261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie AB. 1964. Treatment of typhoid carriers with ampicillin. Br Med J 1:1609–1611. doi: 10.1136/bmj.1.5398.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore B, Perry EL, Chard ST. 1952. A survey by the sewage swab method of latent enteric infection in an urban area. J Hyg 50:137–156. doi: 10.1017/S0022172400019501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruickshank JC. 1950. The newer methods in the investigation of outbreaks of enteric fever. J R Sanit Inst 70:34–38. doi: 10.1177/146642405007000104. [DOI] [PubMed] [Google Scholar]
- 20.Isaäcson M, Clarke KR, Ellacombe GH, Smit WA, Smit P, Koornhof HJ, Smith LS, Kriel LJ. 1974. The recent cholera outbreak in the South African gold mining industry. A preliminary report. S Afr Med J 48:2557–2560. [PubMed] [Google Scholar]
- 21.Isaäcson M. 1975. Practical aspects of a cholera surveillance programme. South African Med J 49:1699–1702. [PubMed] [Google Scholar]
- 22.Weissman JB, Dewitt WE, Thompson J, Muchnick CN, Portnoy BL, Feeley JC, Gangarosa EJ, DeWitt J, Thompson WE, Muchnick CN, Portnoy JC, Feeley BL, Gangarosa EJ. 1974. A case of cholera in Texas, 1973. Am J Epidemiol 100:487–498. doi: 10.1093/oxfordjournals.aje.a112061. [DOI] [PubMed] [Google Scholar]
- 23.Merson MH, Martin WT, Craig JP, Morris GK, Blake PA, Craun GF, Feeley JC, Camacho JC, Gangarosa EJ. 1977. Cholera on Guam, 1974: epidemiologic findings and isolation of non-toxinogenic strains. Am J Epidemiol 105:349–361. doi: 10.1093/oxfordjournals.aje.a112393. [DOI] [PubMed] [Google Scholar]
- 24.Barrett TJ, Blake PA, Morris GK, Puhr ND, Bradford HB, Wells JG. 1980. Use of Moore swabs for isolating Vibrio cholerae from sewage. J Clin Microbiol 11:385–388. doi: 10.1128/JCM.11.4.385-388.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shandera WX, Hafkin B, Martin DL, Taylor JP, Maserang DL, Wells JG, Kelly M, Ghandi K, Kaper JB, Lee JV, Blake PA. 1983. Persistence of cholera in the United States. Am J Trop Med Hyg 32:812–817. doi: 10.4269/ajtmh.1983.32.812. [DOI] [PubMed] [Google Scholar]
- 26.Simanjuntak CH, O’Hanley P, Punjabi NH, Noriega F, Pazzaglia G, Dykstra P, Kay B, Suharyono, Budiarso A, Rifai AR, Wasserman SS, Losonsky G, Kaper J, Cryz S, Levine MM. 1993. Safety, immunogenicity, and transmissibility of single-dose live oral cholera vaccine strain CVD 103-HgR in 24- to 59-month-old Indonesian children. J Infect Dis 168:1169–1176. doi: 10.1093/infdis/168.5.1169. [DOI] [PubMed] [Google Scholar]
- 27.Bravo Fariñas L, Monte Boada RJ, Zorrilla Aguila C, Padilla Ramírez M. 1989. Application of the Moore swab method to the isolation of Aeromonas spp. from residual waters. Rev Cuba Med Trop 41:413–418. (In Spanish.) [PubMed] [Google Scholar]
- 28.Villarruel-López A, Fernández-Rendón E, Mota-de-la-Garza L, Ortigoza-Ferado J. 2005. Presence of Aeromonas spp. in water from drinking-water- and wastewater-treatment plants in Mexico City. Water Environ Res 77:3074–3079. doi: 10.2175/106143005x73974. [DOI] [PubMed] [Google Scholar]
- 29.Kinde H, Adelson M, Ardans A, Little EH, Willoughby DD, Berchtold D, Read DH, Breitmeyer R, Kerr D, Tarbell R, Hughes E, Adelson AM, Willoughby DD, Berchtold ED, Read BDH, Breitmeyer AR, Kerr ED, Tarbell DR, Hughe E. 1997. Prevalence of Salmonella in municipal sewage treatment plant effluents in Southern California. Avian Dis 41:392–398. doi: 10.2307/1592195. [DOI] [PubMed] [Google Scholar]
- 30.Cooley M, Carychao D, Crawford-Miksza L, Jay MT, Myers C, Rose C, Keys C, Farrar J, Mandrell RE. 2007. Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS One 2:e1159. doi: 10.1371/journal.pone.0001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez H, Otth L, Wilson M. 2003. Isolation of thermotolerant species of Campylobacter from river water using two collection methods. Arch Med Vet 35:95–97. [Google Scholar]
- 32.Maccallum FO, Cockburn WC, Smithard EHR, Wright SL. 1952. The use of gauze swabs for the detection of poliomyelitis virus in sewers, p 484–487. Proceedings of the second international poliomyelitis conference, Copenhagen, Denmark. J. B. Lippincott Co, Philadelphia, PA. [Google Scholar]
- 33.MacCallum FO, Goffe AP, Beveridge J, Phillips AH, Macrea AD, Cockburn W. 1953. Investigation of poliomyelitis virus in sewage in England and Wales in 1951 using sewer swab technique. VI Congr Inter Microbiol, abst 4482.
- 34.Kelly S, Winsser J, Winkelstein W. 1957. Poliomyelitis and other enteric viruses in sewage. Am J Public Health Nations Health 47:72–77. doi: 10.2105/ajph.47.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sattar SA, Westwood J. 1977. Isolation of apparently wild strains of poliovirus type 1 from sewage in the Ottawa area. Can Med Assoc J 116:25–27. [PMC free article] [PubMed] [Google Scholar]
- 36.Zdrazilek J, Sramova H, Hoffmanova V. 1977. Comparison of poliovirus detection in sewage and stool samples; a study in a crèche in the third week after vaccination. Int J Epidemiol 6:169–172. doi: 10.1093/ije/6.2.169. [DOI] [PubMed] [Google Scholar]
- 37.Matrajt G, Naughton B, Bandyopadhyay AS, Meschke JS. 2018. A review of the most commonly used methods for sample collection in environmental surveillance of poliovirus. Clin Infect Dis 67:S90–S97. doi: 10.1093/cid/ciy638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiley JS, Chin TDY, Gravelle CR, Robinson S. 1962. Enterovirus in sewage during a poliomyelitis epidemic. J Water Pollut Control Fed 34:168–178. [Google Scholar]
- 39.Cooley MB, Quiñones B, Oryang D, Mandrell RE, Gorski L. 2014. Prevalence of Shiga toxin producing Escherichia coli, Salmonella enterica, and Listeria monocytogenes at public access watershed sites in a California Central Coast agricultural region. Front Cell Infect Microbiol 4:30. doi: 10.3389/fcimb.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberg AE, Wickenden RW, Lee TW. 1957. Tracing typhoid carriers by means of sewage. Sewage Ind Waste 29:1237–1242. [Google Scholar]
- 41.Azizoglu RO, Gorski L, Kathariou S. 2014. Isolation of Listeria monocytogenes from food and water: official and experimental protocols. Curr Protoc Microbiol 33:1–19. doi: 10.1002/9780471729259.mc09b05s33. [DOI] [PubMed] [Google Scholar]
- 42.Haymaker J, Sharma M, Parveen S, Hashem F, May EB, Handy ET, White C, East C, Bradshaw R, Micallef SA, Callahan MT, Allard S, Anderson B, Craighead S, Gartley S, Vanore A, Kniel KE, Solaiman S, Bui A, Murray R, Craddock HA, Kulkarni P, Foust D, Duncan R, Taabodi M, Sapkota AR. 2019. Prevalence of Shiga-toxigenic and atypical enteropathogenic Escherichia coli in untreated surface water and reclaimed water in the Mid-Atlantic U.S. Environ Res 172:630–636. doi: 10.1016/j.envres.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 43.Mack WN, Mallmann WL, Bloom HH, Krueger BJ. 1958. Isolation of enteric viruses and salmonellae from sewage: I. Comparison of coliform and enterococci incidence to the isolation of viruses. Sewage Ind Waste 30:957–962. [Google Scholar]
- 44.Zhu L, Torres M, Betancourt WQ, Sharma M, Micallef SA, Gerba C, Sapkota AR, Sapkota A, Parveen S, Hashem F, May E, Kniel K, Pop M, Ravishankar S. 2019. Incidence of fecal indicator and pathogenic bacteria in reclaimed and return flow waters in Arizona, USA. Environ Res 170:122–127. doi: 10.1016/j.envres.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 45.Kwantes W, Speedy W. 1955. Detection of a paratyphoid carrier by sewer and water-closet swabs. Mon Bull Minist Health Public Health Lab Serv 14:120–123. [PubMed] [Google Scholar]
- 46.Bisha B, Perez-Mendez A, Danyluk MD, Goodridge LD. 2011. Evaluation of modified moore swabs and continuous flow centrifugation for concentration of Salmonella and Escherichia coli O157:H7 from large volumes of water. J Food Prot 74:1934–1937. doi: 10.4315/0362-028X.JFP-11-133. [DOI] [PubMed] [Google Scholar]
- 47.Wain J, House D, Parkhill J, Parry C, Dougan G. 2002. Unlocking the genome of the human typhoid bacillus. Lancet Infect Dis 2:163–170. doi: 10.1016/s1473-3099(02)00225-6. [DOI] [PubMed] [Google Scholar]
- 48.González-Guzmán J. 1989. An epidemiological model for direct and indirect transmission of typhoid fever. Math Biosci 96:33–46. doi: 10.1016/0025-5564(89)90081-3. [DOI] [PubMed] [Google Scholar]
- 49.Crump JA. 2019. Progress in typhoid fever epidemiology. Clin Infect Dis 68:S4–S9. doi: 10.1093/cid/ciy846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson JW, Minear RA, Nedved TK. 1971. Septic tanks and the environment. Institute for Environmental Quality, Chicago, IL. [Google Scholar]
- 51.Green CE, Beard PJ. 1938. Survival of E. typhi in sewage treatment plant processes. Am J Public Health Nations Health 28:762–770. doi: 10.2105/ajph.28.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melick CO. 1917. The possibility of typhoid infection through vegetables. J Infect Dis 21:28–38. doi: 10.1093/infdis/21.1.28. [DOI] [Google Scholar]
- 53.Creel RH. 1912. Vegetables as possible factor in the dissemination of typhoid fever. Public Health Rep 27:187–193. doi: 10.2307/4567429.19314289 [DOI] [Google Scholar]
- 54.McGinnis JA, DeWalle F. 1983. The movement of typhoid organisms in saturated, permeable soil. J Am Water Works Assoc 75:266–271. doi: 10.1002/j.1551-8833.1983.tb05137.x. [DOI] [Google Scholar]
- 55.Conn NK, Heymann CS, Jamieson A, Mcwilliam JM, Scott TG. 1972. Water-borne typhoid fever caused by an unusual Vi-phage type in Edinburgh. J Hyg (Lond) 70:245–253. doi: 10.1017/s0022172400022300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lendon NC, Mackenzie RD. 1951. Tracing a typhoid carrier by sewage examination. Mon Bull Minist Health Public Health Lab Serv 10:23–27. [Google Scholar]
- 57.Murdock CR, Lawson G. 1955. The application of modern techniques to the detection of a typhoid carrier. Ulster Med J 24:139–142. [PMC free article] [PubMed] [Google Scholar]
- 58.Bowmer EJ, Hudson VG, Sunderland WF. 1959. Typhoid fever: where there’s a case, there’s a carrier. Can Med Assoc J 80:179–186. [PMC free article] [PubMed] [Google Scholar]
- 59.Marineli F, Tsoucalas G, Karamanou M, Androutsos G. 2013. Mary Mallon (1869–1938) and the history of typhoid fever. Ann Gastroenterol 26:132–134. [PMC free article] [PubMed] [Google Scholar]
- 60.Hobbs FB. 1956. Tracing a typhoid carrier by means of sewer swabs. Lancet 270:855–856. doi: 10.1016/s0140-6736(56)91319-8. [DOI] [PubMed] [Google Scholar]
- 61.Shearer LA, Browne AS, Gordon RB, Hollister AC. 1959. Discovery of typhoid carrier by sewage sampling. JAMA 169:1051–1055. doi: 10.1001/jama.1959.03000270033008. [DOI] [PubMed] [Google Scholar]
- 62.Robinson RG. 1958. The isolation of enteric organisms from sewage and the development of the “sewage pad” technique. J Med Lab Technol 15:79–95. [PubMed] [Google Scholar]
- 63.Pilsworth R. 1960. Detection of a carrier of Salmonella typhi by means of sewer swabs. Mon Bull Minist Health Public Health Lab Serv 19:201–207. [PubMed] [Google Scholar]
- 64.Jones AC. 1951. A hospital outbreak of typhoid fever. Bacteriological and serological investigations. J Hyg (Lond) 49:335–348. doi: 10.1017/s002217240004420x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gal-Mor O. 2018. Persistent infection and long-term carriage of typhoidal and nontyphoidal salmonellae. Clin Microbiol Rev 32:e00088-18. doi: 10.1128/CMR.00088-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez-Escobedo G, Marshall JM, Gunn JS. 2011. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol 9:9–14. doi: 10.1038/nrmicro2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crawford RW, Rosales-Reyes R, Ramirez-Aguilar MDLL, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. 2010. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A 107:4353–4358. doi: 10.1073/pnas.1000862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanderslott S, Phillips MT, Pitzer VE, Kirchhelle C. 2019. Water and filth: reevaluating the first era of sanitary typhoid intervention (1840–1940). Clin Infect Dis 69:S377–S384. doi: 10.1093/cid/ciz610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levine MM, Black RE, Clements ML, Sears S, Morris JG, Lanata C. 1987. Studies to control endemic typhoid fever in Chile. US Army Medical Research and Development Command, Fort Detrick, Frederick, MD. [Google Scholar]
- 70.Levine MM, Black RE, Lanata C. 1982. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. J Infect Dis 146:724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 71.Ristori C, Rodríguez H, Vicent P, Lobos H, D'Ottone K, García J, Pinto ME, Nercelles P, Cisneros L. 1982. Investigation of the Salmonella typhi-paratyphi carrier state in cases of surgical intervention for gallbladder disease. Bull Pan Am Health Organ 16:161–171. [PubMed] [Google Scholar]
- 72.Black RE, Cisneros L, Levine MM, Banfi A, Lobos H, Rodriguez H. 1985. Case-control study to identify risk factors for paediatric endemic typhoid fever in Santiago, Chile. Bull World Health Organ 63:899–904. [PMC free article] [PubMed] [Google Scholar]
- 73.Ferreccio C, Berrios G, Levine M, Garcia J, Silva W, Rodriguez H. 1988. Fiebre tifoidea en Santiago: rol de los munipuladores de alimentos en escuelas primarias. Rev Méd Chile 116:952–956. [PubMed] [Google Scholar]
- 74.Ferreccio C, Levine MM, Astroza L, Berrios G, Solari V, Misraji A, Pefaur C. 1990. Detection of chronic S. Typhi carriers: a practical method applied to food handlers. Rev Med Chil 118:33–37. [PubMed] [Google Scholar]
- 75.Gauld JS, Hu H, Klein D, Levine MM. 2018. Typhoid fever in Santiago, Chile: insights from a mathematical model utilizing venerable archived data from a successful disease control program. PLoS Negl Trop Dis 12:e0006759. doi: 10.1371/journal.pntd.0006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sears SD, Ferreccio C, Levine MM, Cordano AM, Monreal J, Black RE, Ottone KD, Rowe B, Committee T. 1984. The use of Moore swabs for isolation of Salmonella Typhi from irrigation water in Santiago, Chile. J Infect Dis 149:640–642. doi: 10.1093/infdis/149.4.640. [DOI] [PubMed] [Google Scholar]
- 77.Lobos JH, Grieve R, Quijada ML, Brandt H. 1974. Investigation of the genus Vibrio in sewage. Boletín Del Inst Bacteriológico Chile 16:40–42. [Google Scholar]
- 78.Levine MM, Black RE, Ferreccio C, Germanier R. 1987. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet 329:1049–1052. doi: 10.1016/S0140-6736(87)90480-6. [DOI] [PubMed] [Google Scholar]
- 79.Black RE, Levine MM, Ferreccio C, Lou Clements M, Lanata C, Rooney J, Germanier R, Chilean Typhoid Committee. 1990. Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Vaccine 8:81–84. doi: 10.1016/0264-410X(90)90183-M. [DOI] [PubMed] [Google Scholar]
- 80.Levine MM, Ferreccio C, Cryz S, Ortiz E. 1990. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet 336:891–894. doi: 10.1016/0140-6736(90)92266-K. [DOI] [PubMed] [Google Scholar]
- 81.Ferreccio C, Levine MM, Rodriguez H, Contreras R. 1989. Comparative efficacy of two, three, or four doses of TY21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. J Infect Dis 159:766–769. doi: 10.1093/infdis/159.4.766. [DOI] [PubMed] [Google Scholar]
- 82.Swerdlow DL, Mintz ED, Rodriguez M, Tejada E, Ocampo C, Espejo L, Greene KD, Saldana W, Seminario L, Tauxe RV. 1992. Waterborne transmission of epidemic cholera in Trujillo, Peru: lessons for a continent at risk. Lancet 340:28–33. doi: 10.1016/0140-6736(92)92432-f. [DOI] [PubMed] [Google Scholar]
- 83.Weber JT, Mintz ED, Cañizares R, Semiglia A, Gomez I, Sempértegui R, Dávila A, Greene KD, Puhr ND, Cameron DN, Tenover FC, Barrett TJ, Bean NH, Ivey C, Tauxe RV, Blake PA. 1994. Epidemic cholera in Ecuador: multidrug-resistance and transmission by water and seafood. Epidemiol Infect 112:1–11. doi: 10.1017/s0950268800057368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cookson ST, Stamboulian D, Demonte J, Quero L, Martinez de Arquiza C, Aleman A, Lepetic A, Levine MM. 1997. A cost-benefit analysis of programmatic use of CVD 103-HgR live oral cholera vaccine in a high-risk population. Int J Epidemiol 26:212–219. doi: 10.1093/ije/26.1.212. [DOI] [PubMed] [Google Scholar]
- 85.Cárdenas V, Saad C, Varona M, Linero M. 1993. Waterborne cholera in Riohacha, Colombia, 1992. Bull Pan Am Health Organ 27:313–330. [PubMed] [Google Scholar]
- 86.Tauxe RV, Blake PA. 1992. Epidemic cholera in Latin America. JAMA 267:1388. doi: 10.1001/jama.1992.03480100098039. [DOI] [PubMed] [Google Scholar]
- 87.Levine M. 1991. South America: the return of cholera. Lancet 338:45–46. doi: 10.1016/0140-6736(91)90024-J. [DOI] [Google Scholar]
- 88.Ferreccio C, Marco C, Vargas C, Bhutta ZA, Delgado I, Muñoz X, Vargas C, Muñoz X, Bhutta ZA, Ferreccio C. 2018. Typhoid fever in Chile 1969–2012: analysis of an epidemic and its control. Am J Trop Med Hyg 99:26–33. doi: 10.4269/ajtmh.18-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shuval HI. 1993. Investigation of typhoid fever and cholera transmission by raw wastewater irrigation in Santiago, Chile. Water Sci Technol 27:167–174. doi: 10.2166/wst.1993.0341. [DOI] [Google Scholar]
- 90.Blake PA, Allegra DT, Snyder JD, Barrett TJ, McFarland L, Caraway CT, Feeley JC, Craig JP, Lee JV, Puhr ND, Feldman RA. 1980. Cholera—a possible endemic focus in the United States. N Engl J Med 302:305–309. doi: 10.1056/NEJM198002073020601. [DOI] [PubMed] [Google Scholar]
- 91.Centers for Disease Control and Prevention. Examination of food and environmental samples, p 27–37. Laboratory methods for the diagnosis of Vibrio cholerae. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 92.Sears SD, Ferreccio C, Levine MM. 1986. Sensitivity of moore sewer swabs for isolating Salmonella typhi. Appl Environ Microbiol 51:425–426. doi: 10.1128/AEM.51.2.425-426.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goh KT, Teo SH, Tay L, Monteiro E. 1992. Epidemiology and control of an outbreak of typhoid in a psychiatric institution. Epidemiol Infect 108:221–229. doi: 10.1017/s0950268800049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacKenzie KD, Wang Y, Musicha P, Hansen EG, Palmer MB, Herman DJ, Feasey NA, White AP. 2019. Parallel evolution leading to impaired biofilm formation in invasive Salmonella strains. PLoS Genet 15:e1008233. doi: 10.1371/journal.pgen.1008233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cho JC, Kim SJ. 1999. Viable, but non-culturable, state of a green fluorescence protein-tagged environmental isolate of Salmonella typhi in groundwater and pond water. FEMS Microbiol Lett 170:257–264. doi: 10.1111/j.1574-6968.1999.tb13382.x. [DOI] [PubMed] [Google Scholar]
- 96.Zeng B, Zhao G, Cao X, Yang Z, Wang C, Hou L. 2013. Formation and resuscitation of viable but nonculturable Salmonella typhi. Biomed Res Int 2013:907170. doi: 10.1155/2013/907170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho J-C, Kim S-J. 1999. Green fluorescent protein-based direct viable count to verify a viable but non-culturable state of Salmonella typhi in environmental samples. J Microbiol Methods 36:227–235. doi: 10.1016/S0167-7012(99)00038-X. [DOI] [PubMed] [Google Scholar]
- 98.Sultana M, Nusrin S, Hasan NA, Sadique A, Ahmed KU, Islam A, Hossain A, Longini I, Nizam A, Huq A, Siddique AK, Sack DA, Sack RB, Colwell RR, Alam M. 2018. Biofilms comprise a component of the annual cycle of Vibrio cholerae in the Bay of Bengal estuary. mBio 9:e00483-18. doi: 10.1128/mBio.00483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colwell RR, Brayton P, Herrington D, Tall B, Huq A, Levine MM. 1996. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J Microbiol Biotechnol 12:28–31. doi: 10.1007/BF00327795. [DOI] [PubMed] [Google Scholar]
- 100.Lesn J, Berthet S, Binard S, Rouxel A, Humbert F. 2000. Changes in culturability and virulence of Salmonella typhimurium during long-term starvation under desiccating conditions. Int J Food Microbiol 60:195–203. doi: 10.1016/S0168-1605(00)00311-1. [DOI] [PubMed] [Google Scholar]
- 101.Smith RJ, Newton AT, Harwood CR, Barer MR. 2002. Active but nonculturable cells of Salmonella enterica serovar Typhimurium do not infect or colonize mice. Microbiology 148:2717–2726. doi: 10.1099/00221287-148-9-2717. [DOI] [PubMed] [Google Scholar]
- 102.Tezcan-Merdol D, Ljungström M, Winiecka-Krusnell J, Linder E, Engstrand L, Rhen M. 2004. Uptake and replication of Salmonella enterica in Acanthamoeba rhysodes. Appl Environ Microbiol 70:3706–3714. doi: 10.1128/AEM.70.6.3706-3714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bleasdale B, Lott PJ, Jagannathan A, Stevens MP, Birtles RJ, Wigley P. 2009. The Salmonella pathogenicity island 2-encoded type III secretion system is essential for the survival of Salmonella enterica serovar Typhimurium in free-living amoebae. Appl Environ Microbiol 75:1793–1795. doi: 10.1128/AEM.02033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Douesnard-Malo F, Daigle F. 2011. Increased persistence of Salmonella enterica serovar Typhi in the presence of Acanthamoeba castellanii. Appl Environ Microbiol 77:7640–7646. doi: 10.1128/AEM.00699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karkey A, Jombart T, Walker AW, Thompson CN, Torres A, Dongol S, Tran Vu Thieu N, Pham Thanh D, Tran Thi Ngoc D, Voong Vinh P, Singer AC, Parkhill J, Thwaites G, Basnyat B, Ferguson N, Baker S. 2016. The ecological dynamics of fecal contamination and Salmonella typhi and Salmonella paratyphi A in municipal Kathmandu drinking water. PLoS Negl Trop Dis 10:e0004346. doi: 10.1371/journal.pntd.0004346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saha SSK, Tanmoy AM, Andrews JR, Sajib MSI, Yu AT, Baker S, Luby SP, Saha S. 2019. Evaluating PCR-based detection of Salmonella typhi and paratyphi A in the environment as an enteric fever surveillance tool. Am J Trop Med Hyg 100:43–46. doi: 10.4269/ajtmh.18-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giri S. 2019. Environmental surveillance for Salmonella and AMR genes in Tamil Nadu, India 11th International Conference on Typhoid and Other Invasive Salmonelloses. Sabin Vaccine Institute, Hanoi, Vietnam. [Google Scholar]
- 108.Nair S, Patel V, Hickey T, Maguire C, Greig DR, Lee W, Godbole G, Grant K, Chattaway MA. 2019. Real-time PCR assay for differentiation of typhoidal and nontyphoidal Salmonella. J Clin Microbiol 57:e00167-19. doi: 10.1128/JCM.00167-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou Z, Lundstrøm I, Tran-Dien A, Duchêne S, Alikhan N-F, Sergeant MJ, Langridge G, Fotakis AK, Nair S, Stenøien HK, Hamre SS, Casjens S, Christophersen A, Quince C, Thomson NR, Weill F-X, Ho SYW, Gilbert MTP, Achtman M. 2018. Pan-genome analysis of ancient and modern Salmonella enterica demonstrates genomic stability of the invasive para C lineage for millennia. Curr Biol 28:2420.e10–2428.e10. doi: 10.1016/j.cub.2018.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sbodio A, Maeda S, Lopez-Velasco G, Suslow TV. 2013. Modified Moore swab optimization and validation in capturing E. coli O157:H7 and Salmonella enterica in large volume field samples of irrigation water. Food Res Int 51:654–622. doi: 10.1016/j.foodres.2013.01.011. [DOI] [Google Scholar]
- 111.McEgan R, Rodrigues CAP, Sbodio A, Suslow TV, Goodridge LD, Danyluk MD. 2013. Detection of Salmonella spp. from large volumes of water by modified Moore swabs and tangential flow filtration. Lett Appl Microbiol 56:88–94. doi: 10.1111/lam.12016. [DOI] [PubMed] [Google Scholar]
- 112.Callahan MT, Van Kessel JA, Micallef SA. 2019. Salmonella enterica recovery from river waters of the Maryland Eastern Shore reveals high serotype diversity and some multidrug resistance. Environ Res 168:7–13. doi: 10.1016/j.envres.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 113.Bisha B, Adkins JA, Jokerst JC, Chandler JC, Pérez-Méndez A, Coleman SM, Sbodio AO, Suslow TV, Danyluk MD, Henry CS, Goodridge LD. 2014. Colorimetric paper-based detection of Escherichia coli, Salmonella spp., and Listeria monocytogenes from large volumes of agricultural water. J Vis Exp 88:e51414. doi: 10.3791/51414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Truchado P, Hernandez N, Gil MI, Ivanek R, Allende A. 2018. Correlation between E. coli levels and the presence of foodborne pathogens in surface irrigation water: establishment of a sampling program. Water Res 128:226–233. doi: 10.1016/j.watres.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 115.Gopinath S, Carden S, Monack D. 2012. Shedding light on Salmonella carriers. Trends Microbiol 20:320–327. doi: 10.1016/j.tim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 116.Yap K-P, Thong KL. 2017. Salmonella Typhi genomics: envisaging the future of typhoid eradication. Trop Med Int Health 22:918–925. doi: 10.1111/tmi.12899. [DOI] [PubMed] [Google Scholar]
- 117.Tanmoy AM, Westeel E, De Bruyne K, Goris J, Rajoharison A, Sajib MSI, Van Belkum A, Saha SK, Komurian-Pradel F, Endtz HP, De Bruyne K, Goris J, Rajoharison A, Sajib MSI, van Belkum A, Saha SK, Komurian-Pradel F, Endtz HP. 2018. Salmonella enterica serovar Typhi in Bangladesh: exploration of genomic diversity and antimicrobial resistance. mBio 9:e02112-18. doi: 10.1128/mBio.02112-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liaquat S, Sarwar Y, Ali A, Haque A, Farooq M, Martinez-Ballesteros I, Laorden L, Garaizar J, Bikandi J. 2018. Virulotyping of Salmonella enterica serovar Typhi isolates from Pakistan: absence of complete SPI-10 in Vi negative isolates. PLoS Negl Trop Dis 12:e0006839. doi: 10.1371/journal.pntd.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hendriksen RS, Leekitcharoenphon P, Lukjancenko O, Lukwesa-Musyani C, Tambatamba B, Mwaba J, Kalonda A, Nakazwe R, Kwenda G, Jensen JD, Svendsen CA, Dittmann KK, Kaas RS, Cavaco LM, Aarestrup FM, Hasman H, Mwansa J. 2015. Genomic signature of multidrug-resistant Salmonella enterica serovar Typhi isolates related to a massive outbreak in Zambia between 2010 and 2012. J Clin Microbiol 53:262–272. doi: 10.1128/JCM.02026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ingle DJ, Nair S, Hartman H, Ashton PM, Dyson ZA, Day M, Freedman J, Chattaway MA, Holt KE, Dallman TJ. 2019. Informal genomic surveillance of regional distribution of Salmonella Typhi genotypes and antimicrobial resistance via returning travellers. PLoS Negl Trop Dis 13:e0007620. doi: 10.1371/journal.pntd.0007620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dyson ZA, Thanh DP, Bodhidatta L, Mason CJ, Srijan A, Rabaa MA, Vinh PV, Thanh TH, Thwaites GE, Baker S, Holt KE. 2017. Whole genome sequence analysis of Salmonella Typhi isolated in Thailand before and after the introduction of a national immunization program. PLoS Negl Trop Dis 11:e0005274. doi: 10.1371/journal.pntd.0005274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thong KL, Cordano AM, Yassin RM, Pang T. 1996. Molecular analysis of environmental and human isolates of Salmonella typhi. Appl Environ Microbiol 62:271–274. doi: 10.1128/AEM.62.1.271-274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baker S, Holt KE, Clements ACA, Karkey A, Arjyal A, Boni MF, Dongol S, Hammond N, Koirala S, Duy PT, Nga TVT, Campbell JI, Dolecek C, Basnyat B, Dougan G, Farrar JJ. 2011. Combined high-resolution genotyping and geospatial analysis reveals modes of endemic urban typhoid fever transmission. Open Biol 1:110008. doi: 10.1098/rsob.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baker S, Hanage WP, Holt KE. 2010. Navigating the future of bacterial molecular epidemiology. Curr Opin Microbiol 13:640–645. doi: 10.1016/j.mib.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moe CL. 2019. Development and application of pilot Salmonella Typhi environmental surveillance program in Kolkata, India 11th International Conference on Typhoid and Other Invasive Salmonelloses. Sabin Vaccine Institute, Hanoi, Vietnam. [Google Scholar]
- 126.Sikorski MJ, Pemita P, Tupua S, Ballard S, Thomsen R, Naseri T, Tennant S, Levine MM. 2019. Sampling to detect Salmonella Typhi within Samoan concrete septic tanks serving households of typhoid fever cases—a new use for Moore swabs, p 183 11th International Conference on Typhoid and Other Invasive Salmonelloses. Sabin Vaccine Institute, Hanoi, Vietnam. [Google Scholar]
- 127.Meschke JS. 2019. Approach for harmonization and quality control of environmental surveillance methods for typhoid 11th International Conference on Typhoid and Other Invasive Salmonelloses. Sabin Vaccine Institute, Hanoi, Vietnam. [Google Scholar]
- 128.Meschke JS. 2019. A standardized approach for the performance assessment of different environmental surveillance methods for S. Typhi. Am J Trop Med Hyg 101:184. [Google Scholar]
- 129.Levine MM, Simon R. 2018. The gathering storm: is untreatable typhoid fever on the way? mBio 9:e00482-18. doi: 10.1128/mBio.00482-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mohan VK, Varanasi V, Singh A, Pasetti MF, Levine MM, Venkatesan R, Ella KM. 2015. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis 61:393–402. doi: 10.1093/cid/civ295. [DOI] [PubMed] [Google Scholar]
- 131.Meiring JE, Laurens MB, Patel P, Patel P, Misiri T, Simiyu K, Mwakiseghile F, Tracy JK, Masesa C, Liang Y, Henrion M, Rotrosen E, Gmeiner M, Heyderman R, Kotloff K, Gordon MA, Neuzil KM. 2019. Typhoid Vaccine Acceleration Consortium Malawi: a phase III, randomized, double-blind, controlled trial of the clinical efficacy of typhoid conjugate vaccine among children in Blantyre, Malawi. Clin Infect Dis 68:S50–S58. doi: 10.1093/cid/ciy1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shakya M, Colin-Jones R, Theiss-Nyland K, Voysey M, Pant D, Smith N, Liu X, Tonks S, Mazur O, Farooq YG, Clarke J, Hill J, Adhikari A, Dongol S, Karkey A, Bajracharya B, Kelly S, Gurung M, Baker S, Neuzil KM, Shrestha S, Basnyat B, Pollard AJ. 2019. Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N Engl J Med 381:2209–2218. doi: 10.1056/NEJMoa1905047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laver F. 1991. Brendan Moore, MD, BSc, FRCPath. 1912–1990. Rep Trans Devonshire Assoc Adv Sci 123:317–318. [Google Scholar]
- 134.Kelly SM, Clark ME, Coleman MB. 1955. Demonstration of infectious agents in sewage. Am J Public Health Nations Health 45:1438–1446. doi: 10.2105/ajph.45.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bloom HH, Mack WN, Mallmann WL. 1958. Enteric viruses and salmonellae isolation: II. media comparison for salmonellae. Sewage Ind Waste 30:1455–1460. [Google Scholar]
- 136.Callaghan P, Brodie J. 1968. Laboratory investigation of sewer swabs following the Aberdeen typhoid outbreak of 1964. J Hyg (Lond) 66:489–497. doi: 10.1017/s0022172400028230. [DOI] [PMC free article] [PubMed] [Google Scholar]



