Abstract
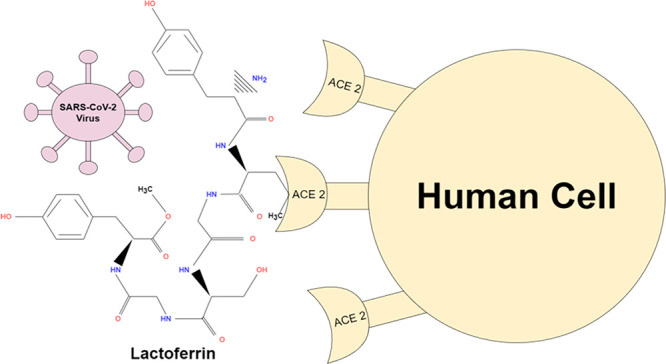
COVID-19 is currently considered as a life-threatening pandemic viral infection. Finding an antiviral drug or a vaccine is the only route for humans’ survival against it. To date, no specific antiviral treatment has been confirmed. Antimicrobial peptides (AMPs) have been widely regarded as a promising solution to combat harmful microorganisms. They are biologically active molecules produced by different organisms as an essential component of their innate immune response against invading pathogens. Lactoferrin (LF), one of the AMPs, is an iron-binding glycoprotein that is present in several mucosal secretions. The antiviral activity of LF exists against a wide range of human and animal viruses (DNA and RNA). LF was proven to increase the host immunity against viral infection. Since LF is one of the constituents of breast milk and significantly located at the mucosal layers of the human body, it is considered the first line of defense against microbial infection. LF was reported to have antiviral activity against SARS-CoV infection. The significant antiviral activity of LF makes it a potential option as an immunity enhancer, a drug or a drug conjugate with conventional antivirals. The affordability, environmental safety, and efficiency of LFs will make them superior to all other control strategies.
Keywords: AMPs, antiviral, COVID-19, lactoferrin
Coronaviruses are zoonotic, enveloped, positive, single-stranded RNA viruses that can infect animals and humans, causing respiratory, gastrointestinal, hepatic, and neurologic diseases.1 The family of Coronaviruses is classified into four genera: alpha, beta, gamma, and delta.2 Coronaviruses infecting humans are categorized within alpha and beta, among which originated two major epidemics: severe acute respiratory syndrome coronaviruses (SARS-CoVs)1 and Middle East respiratory syndrome (MERS).1 Animals are the natural reservoir hosts that play a crucial role in transmitting Coronaviruses.3 The present outbreak of coronavirus, known as Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) or (COVID-19), is considered the third documented transmitted animal-to-human-infection, and was declared as pandemic by the World Health Organization (WHO).4 COVID-19 is currently considered as one of the most life-threatening pandemic viral infections that humanity has witnessed in the past decades. Since SARS-CoV-2 shares 96% similarity to the whole genome of coronavirus infecting bats, this indicates that bats are most likely the hosts that transmitted COVID 19 to humans.5 Furthermore, there were some reports mentioning snakes, pangolins, and minks as other possible virus reservoirs for SARS-CoV-2 human infection6,7
Phelan et al.8 proposed human-to-human transmission of SARS-CoV-2 which is the main source of infection. Moreover, routes of transmission were confirmed to take place through respiratory droplets, contact, and aerosol. In addition, Gu et al.9 suggested the digestive system as an alternative transmission route for its infection. The virus was detected in feces of several infected cases, which may indicate the possibility of virus replication in the digestive system.9 However, it is still not confirmed that eating virus-contaminated food may be a source of infection and transmission. The virus stability in vitro varies depending on the subjected surfaces: plastic (72 h), stainless steel (48 h), cardboard (24 h), copper (4 h) and 3 h in air.10
The viral genome of SARS-Cov-2 showed approximately 80% similarity with SARS-CoV. However, it was nearly 95% identical with seven conserved replicase domains, indicating that both viruses belong to the same species. However, phylogenetic analysis showed that RaTG13 and the spike gene (S) in bats are the closest relative to SARS-CoV-2, thus forming a distinct lineage from other SARS-CoVs.5
The basic reproduction number of infection (R0) was estimated to be 2.0–2.5 by the WHO,11 indicating a higher transmission infection rate than SARS-CoV. Confirmed cases and deaths are elevating daily and the short-term solution, so far, is home quarantine to lessen the infection rates. This is affecting the world economy and will later cause severe implications on human welfare. Accordingly, finding an antiviral drug or a vaccine is the only route for survival of humans against COVID-19 infection. Several routes of antiviral drugs are still under study and to date, no specific antiviral treatment has been confirmed.
Antimicrobial peptides (AMPs) have been widely regarded as a promising solution to combat harmful microorganisms.12 They are biologically active molecules produced by a wide variety of organisms as an essential component of their innate immune response against invading pathogens. AMPs are short sequence peptides polymer ranging from 10 to 100 amino acids, positively charged, amphiphilic. They have been isolated from organisms, belonging to six kingdoms, including humans. The Antimicrobial Peptide Database (APD) contains more than 3000 antimicrobial peptides, among which are 189 AMPs with antiviral activities.12
The antimicrobial effect of AMPs was reported to either act on the membrane of different pathogenic microorganisms including bacteria, fungus, and virus or modulate the innate immune response in higher organisms. Depending on the kind of pathogen, the AMPs may be expressed either constitutively or inducibly.13
The antiviral activity of the AMPs was shown against enveloped RNA and DNA viruses, except some nonenveloped viruses.14,15 The antiviral activity of AMPs takes place according to its action due to adsorption on the viral surface and entry process or its direct effect on the viral envelope.16
The AMPs positively charged residues enable them to interact electrostatically with negatively charged cell surface molecules such as heparan sulfate. Heparan sulfate consists of glycosaminoglycan molecules that are strongly related to viral attachment.17 It was proven that AMPs that block heparan sulfate should be able to reduce the viral infection.18 Also, the AMPs antiviral effect is related to their ability to inhibit the spread of a virus from a cell to another cell across tight junctions (cell-to-cell spread) or inhibit the formation of giant cells (syncytium).13
Lactoferrin (LF), one of the AMPs, is an iron-binding glycoprotein that is present in several mucosal secretions which have a significant activity in the innate immune system. LF is known to have a wide spectrum of antimicrobial activity against bacteria, fungi, and several viruses.19 The antiviral activity of LF exists against a wide range of human and animal viruses (both DNA and RNA) due to its ability to inhibit replication of those viruses. Moreover, LF was proven to increase the host immunity against viral infection, rather than acting against the virus after infection, by preventing virus entry to the host cell, through blocking cellular receptors, or direct binding to the virus particles. Since LF is one of the constituents of breast milk, studies have shown that at least part of the antiviral properties of breast milk can be attributed to a direct antiviral activity of LF.20 Many studies have proven that LF has a significant role against viruses, among which are HCV, HSV, HIV, polio-, and the rotavirus. However, the LF antiviral mechanisms vary among viruses, where it may bind either directly to the virus particle or to the host cell receptor or coreceptor.21,22 LF is significantly located at the mucosal layers of the human body, which is considered as the first defense line against microbial infection,20 so LF is likely to have an efficient antiviral activity against Coronaviruses.
The significant antiviral activity of LF makes it a potential option as a drug or a drug conjugate with conventional antivirals. This combination will lessen or eliminate the side effects caused by the antiviral drugs and may prevent drug resistance. Many studies reported synergistic activities of the combinations of conventional drugs with LF in vitro.20
In 2011, Lang et al.,23 reported the antiviral activity of LF against SARS-CoV infection through an increased host immune response. LF interfered at the stage of the virus adsorption by blocking the binding of the spike protein to host cell receptors.
The COVID-19 virus outbreak in late 2019, which spread from China, led to a pandemic infection. So far, current approaches have not yet proven to be efficient. Many alternatives are proposed nowadays, and antiviral peptides are among them. This article may reveal more facts about the feasibility of using the AMPs in general and LF in particular, as an antiviral treatment. As a result, it can be a corner stone for more future research on the LFs as antiviral therapy in general and against COVID-19 in particular. The affordability, environmental safety, and efficiency of LFs will make them superior to all other control strategies, especially since LF is a safe natural protein that is a commercially already produced drug at an affordable price. Therefore, the objectives of this article are short-term and long-term objectives. For the short-term objectives, the first is to ensure efficient and instant treatment and protection of humans from the viral infections such as that caused by COVID-19 and as a result, decrease the humans’ mortality rate that has been rising at an exponential rate since the abrupt appearance of this viral infection. The second is to prevent the transmission of this disease among humans by increasing their immunity. A long-term objective is protection of humans from this viral infection, which will bring back normal life and which will have a huge impact on the economy worldwide after the huge financial loss that was caused due to the spread of that infection. The second-long-term objective is to ensure the healthiness of people and to protect them not only against COVID-19 but also against any emerging viral infection, and accordingly, this will maximize the social welfare of humans.
Author Contributions
S.E. and M.A. conceived the idea, drafted the manuscript, revised the first draft and read and approved the final version of the manuscript.
The authors declare no competing financial interest.
References
- Wu Z.; McGoogan J. M. (2020) Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323, 1239. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang D.; Leibowitz J. L. (2015) The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res. 206, 120–33. 10.1016/j.virusres.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S. (2020) Another Decade, Another Coronavirus. N. Engl. J. Med. 382, 760–2. 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Committee on Taxonomy of Viruses (ICTV); . The species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2 Coronaviridae. Nat. Microbiol 2020. [DOI] [PMC free article] [PubMed]
- Zhou P.; Yang X.-L.; Wang X.-G.; Hu B.; Zhang L.; Zhang W.; Si H.-R.; Zhu Y.; Li B.; Huang C.-L.; Chen H.-D.; Chen J.; Luo Y.; Guo H.; Jiang R.-D.; Liu M.-Q.; Chen Y.; Shen X.-R.; Wang X.; Zheng X.-S.; Zhao K.; Chen Q.-J.; Deng F.; Liu L.-L.; Yan B.; Zhan F.-X.; Wang Y.-Y.; Xiao G.-F.; Shi Z.-L. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273. 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W.; Wang W.; Zhao X.; Zai J.; Li X. (2020) Homologous recombination within the spike glycoprotein of the newly identified coronavirus my boost cross-species transmission from snake to human. J. Med. Virol. 92, 433–40. 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T. T. Y., Shum M. H. H., Zhu H. C., Tong Y., Ni X., Liao Y., Wei W., Cheung W. Y., Li W., Li L., Leung G. M., Holmes E. C., Hu Y., and Guan Y. (2020) Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. bioRxiv preprint, 10.1101/2020.02.13.945485. [DOI] [Google Scholar]
- Phelan A. L.; Katz R.; Gostin L. O. (2020) The novel Coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 323 (8), 709–710. 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- Gu J.; Han B.; Wang J. (2020) COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 158, 1518. 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N.; Bushmaker T.; Morris D. H.; Holbrook M. G.; Gamble A.; Williamson B. N.; Tamin A.; Harcourt J. L.; Thornburg N. J.; Gerber S. I.; Lloyd-Smith J. O.; de Wit E.; Munster V. J. (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382, 1564. 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2020) WHO Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-mission-briefing-on-covid-19---13-march-2020.
- Wang G.; Li X.; Wang Z. (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 44, D1087–D1093. 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen H.; Hamill P.; Hancock R. E. (2006) Peptide antimicrobial agents. Clin. Microbiol. Rev. 19, 491–511. 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann K. B.; Lee A.; Wan J.; Roginski H.; Coventry M. J. (2003) The effect of bovine lactoferrin and lactoferricin B on the ability of feline calicivirus (a norovirus surrogate) and poliovirus to infect cell cultures. J. Appl. Microbiol. 95, 1026–1033. 10.1046/j.1365-2672.2003.02071.x. [DOI] [PubMed] [Google Scholar]
- Pietrantoni a; Ammendolia M; Tinari a; Siciliano R; Valenti P; Superti F (2006) Bovine lactoferrin peptidic fragments involved in inhibition of Echovirus 6 in vitro infection. Antiviral Res. 69, 98–106. 10.1016/j.antiviral.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Belaid A.; Aouni M.; Khelifa R.; Trabelsi A.; Jemmali M.; Hani K. (2002) In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J. Med. Virol. 66, 229–234. 10.1002/jmv.2134. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C. (2001) Brief overview on cellular virus receptors. Virus Res. 82, 3–8. 10.1016/S0168-1702(01)00380-X. [DOI] [PubMed] [Google Scholar]
- WuDunn D.; Spear P. G. (1989) Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63, 52–58. 10.1128/JVI.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorland L. H. (1999) Lactoferrin: a multifunctional glycoprotein. Apmis 107 (11), 971–981. 10.1111/j.1699-0463.1999.tb01499.x. [DOI] [PubMed] [Google Scholar]
- van der Strate B. W.; Beljaars L.; Molema G.; Harmsen M. C.; Meijer D. K. (2001) Antiviral activities of lactoferrin. Antiviral Res. 52, 225–239. 10.1016/S0166-3542(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Superti F.; Ammendolia M. G.; Valenti P.; Seganti L. (1997) Antirotaviral activity of milk proteins: lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29. Med. Microbiol. Immunol. 186 (2–3), 83–91. 10.1007/s004300050049. [DOI] [PubMed] [Google Scholar]
- Ikeda M.; Nozaki A.; Sugiyama K.; Tanaka T.; Naganuma A.; Tanaka K.; Sekihara H.; Shimotohno K.; Saito M.; Kato N. (2000) Characterization of antiviral activity of lactoferrin against hepatitis C virus infection in human cultured cells. Virus Res. 66, 51–63. 10.1016/S0168-1702(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Lang J.; Yang N.; Deng J.; Liu K.; Yang P.; Zhang G.; Jiang C. (2011) Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One 6 (8), e23710. 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]


