The cardiovascular community faces unprecedented challenges during the coronavirus disease 2019 (COVID-19) pandemic. For heart transplant (HT) clinicians, the global pandemic has unique implications for patients, including those on the waiting list and transplant recipients. These populations are at increased risk for both acquisition of COVID-19 infection and progression to severe disease given multiple healthcare contacts, underlying health conditions, and immunosuppression; targeted prevention and treatment strategies are needed.
Past experience with previous coronavirus epidemics such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) demonstrated that transplant patients had similar presentations to the general population.1 Data are rapidly accumulating on the impact of COVID-19 within the transplant community. COVID-19 cases have already been reported in 2 HT recipients in China. Both patients survived, one with a mild form of illness allowing for treatment and recovery at home, the other with progressive respiratory failure requiring inpatient admission, intravenous immunoglobulin, and methylprednisolone.2 Mild-moderate presentations with uncomplicated courses have also been reported after kidney and liver transplant. Since these initial reports, a growing number of solid organ transplant recipients have been hospitalized for COVID-19, particularly in New York City. In-hospital disease transmission is increasing, with hospitals increasingly cohorting patients with COVID-19 in separate intensive care units and wards, irrespective of transplant status. Specific to HT recipients, manifestations of myocarditis—with high troponin levels, ECG changes, and new left ventricular dysfunction—may be mistaken for rejection. Yet endomyocardial biopsies are being restricted because of risks of exposure. The scope and scale of the current epidemic are unmatched, and online reports raise concerns about rapidly progressive disease leading to morbidity and mortality.3
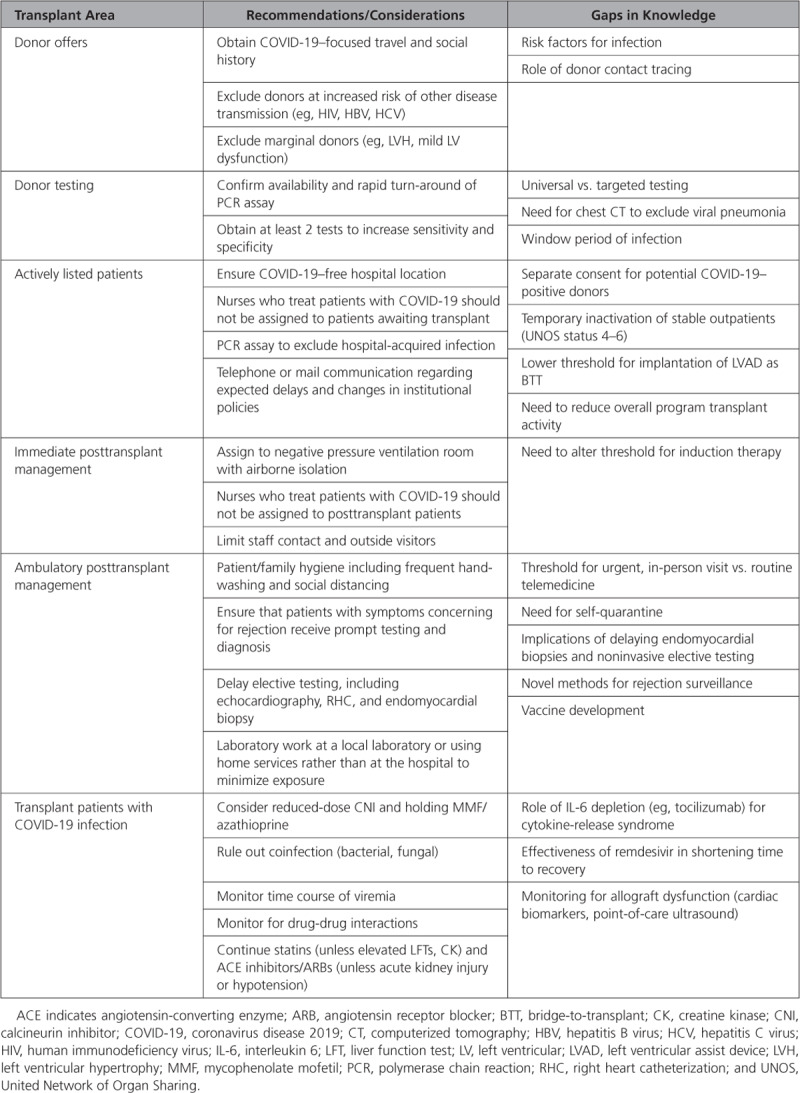
Important decisions have already arisen about actively listed patients. At any given time, a significant portion of patients are waiting in-hospital for HT. These patients are at higher risk for contracting the virus compared with others waiting at home. If they are subsequently infected with COVID-19, they are at risk for more severe infection (or coinfection) because of their underlying health conditions, and risk delisting. If possible, these patients should not be cared for by nurses who also have COVID-19–positive patients. However, as cases continue to rise, this may not be feasible. For all listed patients, no matter whether they are hospitalized or at home, transplant centers should highlight the wait list mortality risk–benefit ratio and provide institutional updates by direct telephone communication or a letter followed by a telephone communication.
As the pandemic continues to evolve, a center’s transplant volume may require staged reduction to meet intensive care unit bed, staffing, and medical equipment needs of the majority nontransplant population.4 Many centers in the United States, particularly in New York, have inactivated most of their HT waiting list, reserving active transplant status for only those patients with a presumed wait list mortality of 1 to 2 weeks, thus limiting transplant to patients in tiers 1 or 2 of the new heart allocation policy. Restrictions are also in effect for highly sensitized patients requiring intensive perioperative care and prolonged immunosuppression and with the need for frequent biopsy surveillance. It is uncertain whether women may be disproportionally affected in access to transplant, given their high sensitivity rates. For listed patients who are hospitalized without a strict contraindication to durable left ventricular assist device implantation, left ventricular assist device as a bridge to transplant may be a viable strategy to get at-risk patients home and out of the hospital, minimizing their exposure to COVID-19. Left ventricular assist device implants should not be performed in elective cases because of resource constraints and potential for nosocomial infection.
These issues of feasibility and safety extend not only to recipients but also to organ donors, organ procurement organizations, and recovery teams. As COVID-19 continues to spread, we must be vigilant in choosing uninfected donors, recognizing that many may be asymptomatic carriers and that current testing has limitations. Chest computed tomography may also be necessary to exclude radiographic pneumonia. In discussing donor offers with listed patients, expanded consent should include the potential risks of a COVID-19–positive donor, despite procedures in place to mitigate risk. It is equally important to protect procurement teams, who are asked to perform time-sensitive, invasive procedures in unfamiliar facilities that may lack appropriate personal protective equipment or experienced operating room staff. Important organ procurement organization measures in the United States include expansion of the Uniform Donor Risk Assessment Interview, which now includes targeted questions on exposure to COVID-19. Mandatory COVID-19 testing for all donors is the goal, which is now occurring with increasing frequency in most regions of the United States. If donors test positive for COVID-19, their organs should not be used for transplant, although they could be considered for scientific purposes to better understand the effects of the virus on the myocardium. COVID-19–specific codes have been used to inform the United Network of Organ Sharing, the federally contracted organization that keeps track of all solid organ transplantation, of the circumstances under which an organ was not accepted.3
For patients who require HT during this pandemic, rapid polymerase chain reaction testing to exclude community- or hospital-acquired COVID-19 infection is required, and extra precautions should be taken to mitigate the risk of postoperative exposure. This extends to visitors (many of whom are restricted at various hospitals across the country), nurses, respiratory therapists, and other intensive care unit staff. Negative pressure rooms in a COVID-19–free zone should be standard of care. Recipients should be retested before hospital discharge and receive focused education around disease prevention at home.
The changing landscape also affects the post-HT ambulatory population. To reduce hospital volumes, most outpatient practices have shifted toward telemedicine. Virtual medicine minimizes in-person contact with the healthcare system, reducing patient risk of exposure. However, patients still must go to laboratories for serum drug levels of immunosuppressive agents. One question that arises is how we can or should monitor for rejection in stable outpatients if right heart catheterization and endomyocardial biopsy are deemed nonurgent. Perhaps institutions will shift to increased use of noninvasive monitoring with echocardiography, gene profiling (for acute cellular rejection), or donor-derived cell free DNA (for antibody-mediated rejection). What is considered “medically necessary” for these patients? What will be the implications of postponing some of this routine care on the rates of rejection or cardiac allograft vasculopathy in the longer term?
Multidisciplinary HT teams must counsel patients and families on appropriate preventative measures. In 1 epidemiological study in China of 87 HT recipients, 97% adopted prevention and quarantine measures with a low rate of infection.5 We should recognize that given their immunosuppressed states, these patients may have both typical (respiratory) and atypical (gastrointestinal) COVID-19 illness. For HT patients admitted with COVID-19 infection, some have advocated for reduced immunosuppression and moderate-dose IV steroids.
The role of novel therapy (eg, remdesivir, tocilizumab) should be tested in clinical trials before treatment algorithms are promulgated widely, with particular attention to drug–drug interactions and QT prolongation.
Many questions remain, and the available knowledge on the virus and its impact on special populations is rapidly evolving (Table). As a community, we have a responsibility to share information in real time, gather more research, and provide guidance to other transplant providers, our multidisciplinary teams, and patients. Transparency with patients and other centers is key for the transplant community to come through this pandemic stronger.
Table.
Areas for Consideration During COVID-19 Pandemic
Disclosures
None.
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 2.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020 Mar 17; doi: 10.1016/j.healun.2020.03.006. doi: 10.1016/j.healun.2020.03.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Network for Organ Sharing. COVID-19 and solid organ transplant. https://unos.org/covid/. Accessed March 25, 2020.
- 4.Kumar D, Manuel O, Natori Y, Egawa H, Grossi P, Han SH, Fernández-Ruiz M, Humar A. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020 Mar 23; doi: 10.1111/ajt.15876. doi: 10.1111/ajt.15876. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren ZL, Hu R, Wang ZW, Zhang M, Ruan YL, Wu ZY, Wu HB, Hu XP, Hu ZP, Ren W, et al. Epidemiologic and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China: a descriptive survey report. J Heart Lung Transplant. 2020;39:412–417. doi: 10.1016/j.healun.2020.03.008. doi: 10.1016/j.healun.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


