Abstract
Photoactivation of bioactive molecules allows manipulation of cellular processes with high spatiotemporal precision. The recent emergence of visible-light excitable photoprotecting groups has the potential to further expand the established utility of the photoactivation strategy in biological applications by offering higher tissue penetration, diminished phototoxicity, and compatibility with other light-dependent techniques. Nevertheless, a critical barrier to such applications remains the significant hydrophobicity of most visible-light excitable photocaging groups. Here, we find that applying the conventional 2,6-sulfonation to meso-methyl BODIPY photocages is incompatible with their photoreaction due to an increase in the excited state barrier for photorelease. We present a simple, remote sulfonation solution to BODIPY photocages that imparts water solubility and provides control over cellular permeability while retaining their favorable spectroscopic and photoreaction properties. Peripherally disulfonated BODIPY photocages are cell impermeable, making them useful for modulation of cell-surface receptors, while monosulfonated BODIPY retains the ability to cross the cellular membrane and can modulate intracellular targets. This new approach is generalizable for controlling BODIPY localization and was validated by sensitization of mammalian cells and neurons by visible-light photoactivation of signaling molecules.
Photoactivation of small bioactive molecules is a powerful approach to manipulate and study cellular events with high spatiotemporal resolution.1,2 Photoprotecting groups (PPGs) covalently attached to bioactive molecules mask their biological activity and allow subsequent removal upon exposure to light. Established PPGs, including those based on nitrobenzyl,3 ruthenium4 (RuBi), coumarin5 and others,6 have been used instrumentally in a wide variety of biological7,8 and materials9,10 applications. The recent emergence of visible-light excitable photocages, spanning a range of structural classes,11−15 has the potential to further expand the already significant utility of photocaging in these fields. For example, extension of the excitation wavelength beyond the traditional UV-region6 expands the operational window in which uncaging light can be delivered, enabling photoactivation of multiple cues through orthogonally caged molecules.16,17 Furthermore, longer wavelength light can penetrate deeper into tissue and is less harmful to biological matter, opening the door to new applications, such as in drug delivery.18−20
We recently introduced meso-methyl BODIPYs as photoprotecting groups in the visible range.21,22 The narrow excitation band, adaptable synthetic chemistry, and overall biocompatibility of BODIPYs23 make them promising candidates for visible-light photocaging. Meso-methyl BODIPY PPGs effectively release cargo in living cells, in part because of their large extinction coefficients.21,22 Further chemical modification to methyl-BODIPY cages can improve photorelease quantum efficiency,24 extend excitation wavelength into the far-red,25 or allow postsynthetic functionalization for targeting to subcellular locations,26 making them a versatile platform for photorelease.
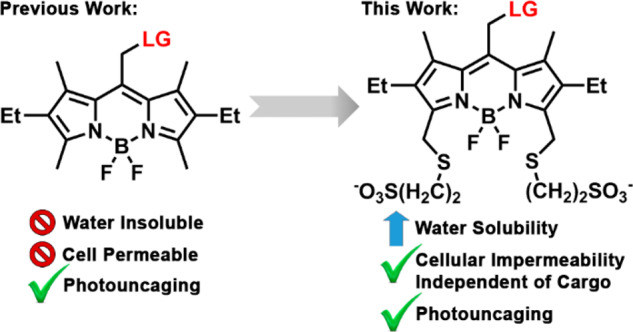
Nevertheless, all BODIPY PPGs reported to date are inherently highly hydrophobic, which severely limits their potential concentration and thus utility in water-based solutions. Moreover, the hydrophobic nature of BODIPYs makes them highly cell permeable (Scheme 1). While advantageous when specifically pursuing intracellular interventions, this property makes BODIPY PPGs less effective when targeting extracellular proteins or plasma membrane-residing receptors and complicates analysis when the released molecule can act both intra- and extracellularly.
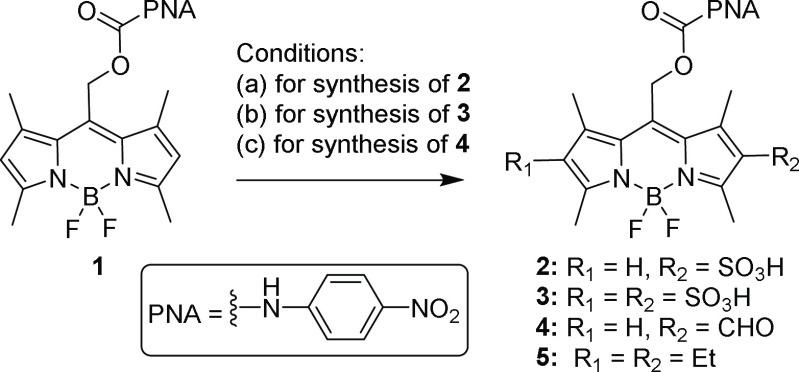
Scheme 1. Water-Soluble BODIPY Photocages.
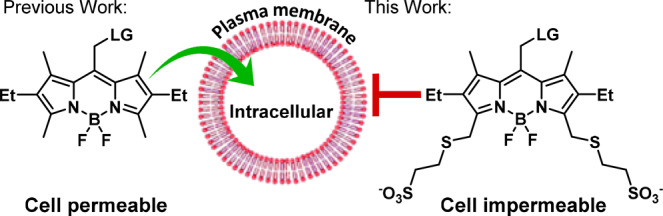
We therefore sought to develop water-soluble BODIPY PPGs with controlled cellular localization, while retaining their favorable spectroscopic and photoreaction properties. We initially opted for 2,6-sulfonation27 as a minimal structural modification28 that also provides improved photobleaching resistance.29 2- and 2,6-sulfo BODIPYs 2 and 3, bearing p-nitroaniline (PNA) as a model leaving group, were synthesized by sulfonation of 1 using a sulfur trioxide–pyridine complex (Scheme 2).30 Surprisingly, both 2 and 3 did not release PNA when irradiated with green light (545/30 nm, 49 mW/cm2). In contrast, 1 is an effective photocage, with ε × Φrel = 3 and t1/2 = 3.8 min (Figure 1a and Table S1). We hypothesized that the electron-withdrawing effect of sulfonate is responsible for the diminished photoreaction. Indeed, a comparable BODIPY with 2-aldehyde (4) also failed to release the leaving group, while 2,6-diethyl BODIPY 5 photolyzed with ε × Φrel = 19 and t1/2 = 31 s, better than 1 (Table S1).
Scheme 2. Synthesis/Structures of BODIPYs 1–5.
(a) SO3–Pyridine, DMF, 60 °C, 24 h; (b) SO3–Pyridine, DMF, 60 °C, 48 h; (c) DMF, POCl3, DCM, 0 °C to rt, 3 h.
Figure 1.
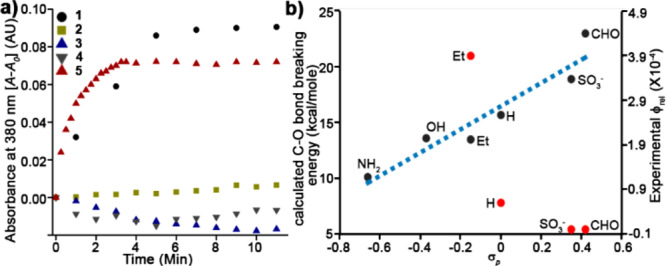
Light-induced release from meso-methyl BODIPY PPGs. (a) PNA release from 1–5 (10 μM in CH3CN/water 7/3) irradiated with 545/30 nm (49 mW/cm2) light for the indicated times. (b) Observed Φrel and a DFT calculated C–O bond breaking energy for derivatives 1–5 as plotted versus σp Hammett constants.
The observed trend in Φrel (5 > 1 > 4 ≈ 2 ≈ 3) suggests a strong effect of the 2,6-positions on the photoreaction with a positive influence of electron donation. We therefore modeled the excited state geometries of 1–5 to explore the consequences of 2,6-substitution on photouncaging efficiency (B3LYP/6-31+G(d,p), SMD = H2O). No fundamental change in the nature of the excited state exists between 1–5. Neither chromophore planarity nor position of the LUMO (meso carbon) differs across compounds 1–5 (Computation S1–S7). However, a relaxed potential energy scan of the C–O bond breaking coordinates (Computation S8–S13) reveals that electron-withdrawing groups (EWGs) at the 2,6-positions substantially raise the barrier for C–O bond heterolysis on the triplet surface compared to electron-donating groups (Figure 1b), consistent with a previous report of these positions’ effect on BODIPY photostability.31 These computations further support our previous hypothesis regarding photorelease in meso-methyl BODIPYs, i.e. formation of a meso carbocation diradical intermediate during the photoreaction.24 Thus, electron-donating groups (EDGs) at the 2,6-positions stabilize the resulting carbocation, lowering the barrier for its formation, and conversely, EWGs increase the barrier to photoreaction.
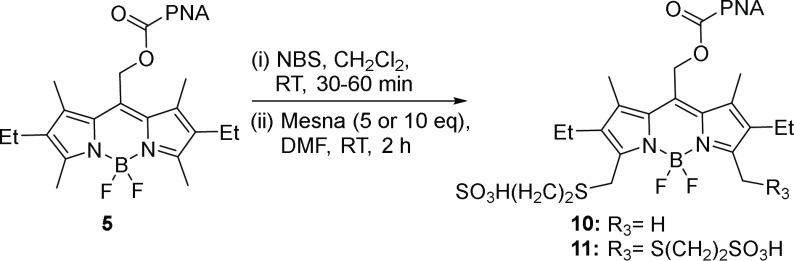
To circumvent deleterious electronic effects on the BODIPY core when introducing sulfonates, we harnessed our recently reported functionalization method; a one-pot, two-step protocol, to install an unprotected functional group on an in situ activated α-methyl.26 We synthesized (Figure S1a) tertiary amine (6), ether (7), and thioether (8–9) containing BODIPY PPGs. Although amine substitution gave the highest synthetic yields, tertiary amines could no longer undergo photorelease (6, Figure S1d), likely because of a competing electron transfer (PeT) mechanism.32−34 Instead, we find thiol nucleophiles best combine high chemical yield with efficient photorelease in BODIPY thioether (Figure S1). Thus, 2-mercaptoethanesulfonic acid sodium salt (MESNA) was used to introduce sulfonic acid groups, affording BODIPYs 10 and 11 in 42% and 33% yield, respectively (Scheme 3).
Scheme 3. Synthesis of Sulfonated BODIPYs 10 and 11.
As expected, both 10 and 11 show improved water solubility compared to 5. In mixtures of CH3CN/water, 5 features absorbance λmax at 545 nm, a shoulder at 511 nm, and an ∼2.1 peak/shoulder ratio. But, in water, the peak red shifts and broadens (556 nm), and the ratio collapses to 1.1, a nearly 1.9-fold reduction and characteristic of aggregation35 (Figure 2a). In contrast, the absorption spectra of 10 and 11 are nearly identical in either pure water or a mixture of CH3CN/water, establishing their high water solubility (Figures 2a, S2). Critically, both 10 and 11 retain photoreleasing ability, in stark contrast to core-sulfonated 2 or 3. Both 10 and 11 possess comparable quantum yield to 5 (Φrel = (3.6–5.1) × 10–4) (Figures 2b, S3–S5 and Tables S1, S2), but afford higher photochemical yields (59–60% vs 46%), probably due to their increased solubility. These results establish peripheral MESNA as a small, readily implemented modification to meso-methyl BODIPYs that improve solubility while maintaining high photorelease efficiency.
Figure 2.
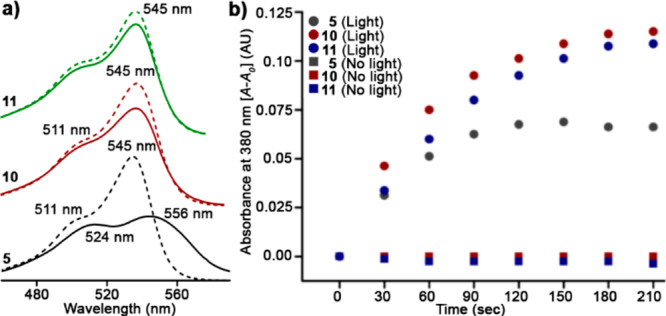
MESNA-BODIPY PPGs. (a) Absorbance spectra of BODIPYs 5, 10, and 11 (20 μM) directly dissolved in CH3CN/water 7/3 (dashed line) or water (solid line). (b) Light-induced release of PNA from 5, 10, and 11 (10 μM, CH3CN/water 7/3) following, or not, irradiation with 545/30 nm light (49 mW/cm2) for the indicated times.
The degree of sulfonation influences the cellular uptake of BODIPY PPGs. HEK 293T cells display intracellular fluorescence, along with bright fluorescent puncta (presumably aggregates), when treated with non-MESNA BODIPY 5 (Figure 3a). In contrast, mono-MESNA 10 shows a higher degree of cellular fluorescence (2-fold higher than 5, Figure S6), but without any observable puncta (Figure 3b). The higher intracellular fluorescence may be a result of better water solubility of 10 compared to 5, leading to higher effective concentration in the buffer. Di-MESNA 11, with two sulfonates, was completely cell impermeable and showed no intracellular or membrane-associated fluorescence (Figure 3c). These results are in line with previous observations of sulfonated coumarin photocages.36 No toxicity or phototoxicity was observed for compound 5, 10, or 11 (Figure S7).
Figure 3.
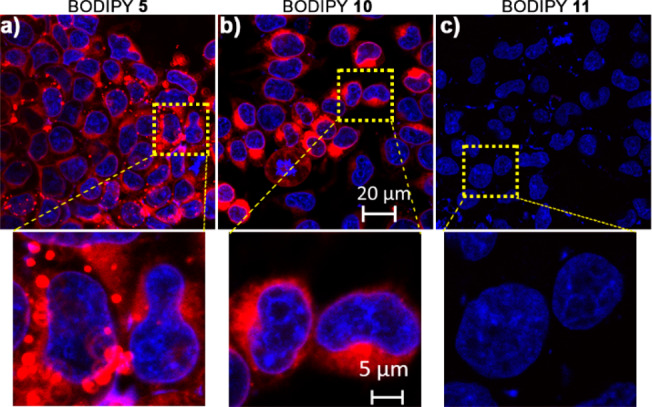
Confocal fluorescence microscopy of HEK 293T cells treated with BODIPY 5, 10, or 11 (2 μM) for 30 min and costained with Hoechst dye. Cells were washed thrice and imaged.
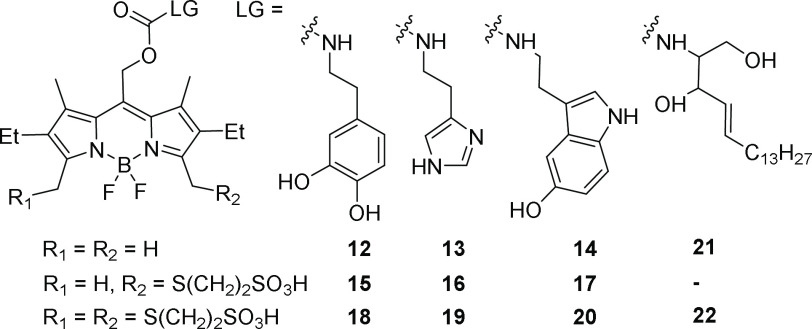
To evaluate the dependence of sulfonated BODIPY PPG cellular permeability on the nature of the leaving group, we compared sets of non-, mono-, and di-MESNA BODIPYs bearing three caged biogenic amines: serotonin, dopamine, and histamine (compounds 12–20, Scheme 4), forming a series of leaving groups with decreased hydrophobicity. The cellular permeability of non- and mono-MESNA-BODIPYs was highly dependent on the polarity of the leaving group while di-MESNA-BODIPY was completely cell impermeable, irrespective of the leaving group polarity (Figure S6).
Scheme 4. Structures of BODIPYs 12–20.
Collectively, these data establish that the solubility of BODIPY photocages can be significantly improved by sulfonation without compromising photoreaction properties and that their cellular permeability can be predetermined by tuning the number of sulfonates.
To highlight the ability to tune the cellular accessibility of caged biomolecules with BODIPY compounds, we synthesized two sphingosine-caged BODIPY derivatives, 21 and 22 (Scheme 4). 21 is based on the traditional, nonsulfonated BODIPY photocage, while 22 utilizes the di-MESNA-BODIPY scaffold. Our hypothesis was that 21 could pass through plasma membrane to effect localized uncaging of sphingosine intracellularly, triggering Ca2+ release, while 22 would be retained externally and would be incompetent to trigger internal Ca2+ release.37 Consistent with this hypothesis, treatment of HeLa cells with 21, followed by green uncaging light, results in large Ca2+ transients detected by the fluorescent Ca2+ indicator, fura-2 (Figure S8a–c). Induction of Ca2+ transients requires uncaging light: we observed no Ca2+ oscillations in the absence of light (Figure S8d). In contrast, the uncaging of extracellularly targeted 22 results in no Ca2+-associated transient (Figure S8e). Together, these data show that di-MESNA-BODIPY cages can retain even lipophilic bioactive molecules in the extracellular space.
The enhanced solubility of MESNA-BODIPYs makes them promising candidates to modulate cell surface receptors. We utilized them to control the availability of the neuromodulator dopamine. We examined the localization of BODIPY-caged dopamine compounds in cultured neurons. While BODIPY-dopamine 12 shows significant cytosolic accumulation (Figure S9a,d), both mono- (15) and di-MESNA-BODIPY-dopamine 18 display little to no cellular uptake (Figure S9b,e and S9c,f), consistent with the localization of di-MESNA-BODIPY cages in HEK cells (Figures 3, S6).
Di-MESNA BODIPY-dopamine 18 delivers dopamine in a light-dependent fashion to neurons. Ca2+ imaging in hippocampal neurons treated with dopamine (5 μM) results in fluorescence oscillations (Figure 4a,b).22,38 Hippocampal neurons treated with di-MESNA-BODIPY-dopamine 18 (5 μM) and irradiated with green light also show Ca2+ oscillations (Figure 4c), with 34% of neurons responding compared to 46% with dopamine alone (Figure S10). In the absence of green light, 18 has little effect on the activity of hippocampal neurons (Figure 4d), and green light alone, when 18 is not present, does not evoke a similar Ca2+ response (Figure 4e). Finally, preincubation with the dopamine receptor antagonist butaclamol (100 μM) prior to green light uncaging in the presence of 18 results in a substantial reduction in the number of Ca2+ transients (Figures 4f, S10).
Figure 4.
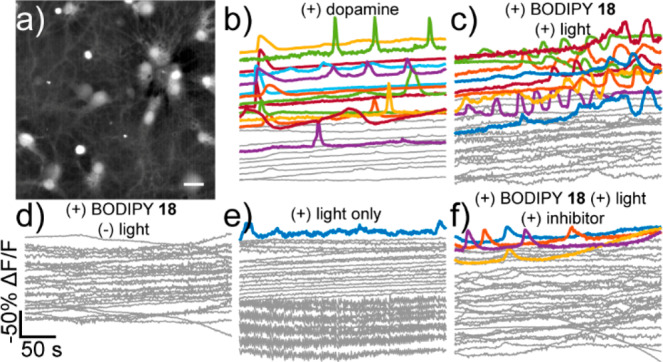
Dopamine uncaging in neurons. (a) Widefield fluorescence image from neurons stained with fura-2. Scale bar is 20 μm. (b–f) Uncaging of dopamine from 18 causes Ca2+ oscillations in cultured rat hippocampal neurons. Ca2+ imaging in neurons treated with (b) dopamine (5 μM), (c) 18 (5 μM) and uncaging light, (d) 18 (5 μM) without light, or (e) only with uncaging light and (f) 18 (5 μM) and light in the presence of the dopamine receptor antagonist butaclamol (100 μM). Plots represent ΔF/Fmax for representative cells vs time and are inverted. A decrease in fluorescence represents a rise in cellular Ca2+. Uncaging light was provided for 10 s at 90 mW/mm2. Gray traces are neurons which did not show < −20% ΔF/F. Colored traces did show < −20% ΔF/F response.
Complementary experiments using the cell-impermeable mono-MESNA-BODIPY caged histamine 16 reveal that this caged compound can also effectively modulate neuronal physiology and Ca2+ signaling in a light-dependent fashion (Figure S11). We further show that the spatial resolution of uncaging can be controlled (Figure S12).
In summary, we introduce biocompatible BODIPY PPGs with substantially improved water-solubility, user-designated control over cellular localization and high photorelease efficiency. Initial efforts to directly sulfonate the BODIPY core improved solubility but abolished photorelease. A combination of computation and in vitro characterization suggests that EWGs at the 2,6-positions destabilize the carbocation formed during the photoreaction. We circumvented this barrier by introducing remote sulfonation, resulting in an increase in water solubility and the ability to regulate cellular localization through the degree of sulfonation. The cellular impermeability of peripherally disulfonated BODIPYs makes them promising candidates for use in modulation of extracellular proteins and cell-surface receptors, while monosulfonated BODIPYs retain the ability to cross the cellular membrane and can modulate intracellular targets. Moreover, the peripheral sulfonation strategy presented herein should be applicable to BODIPY fluorophores at large, providing a convenient route to confer water solubility and control cellular permeability.
Acknowledgments
R.W. and E.W.M. acknowledge generous support from the Binational Science Foundation (2016060) and from the Rosalinde and Arthur Gilbert Foundation. R.W. acknowledges support from the European Research Council (GAtransport). A.H.W. acknowledges support from the National Science Foundation (CHE-1464956). D.F.M. acknowledges support from the Israel Science Foundation (1310/15). P.L. was supported by an A*STAR graduate fellowship, and M.X.N. was supported in part by the NIH (T32GM066698). D.K. was supported in part by the Planning and Budgeting Committee (PBC) of the Israeli Council for Higher Education.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b13219.
The authors declare no competing financial interest.
Supplementary Material
References
- Mayer G.; Heckel A. Biologically active molecules with a ″light switch″. Angew. Chem., Int. Ed. 2006, 45 (30), 4900–21. 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- Lee H. M.; Larson D. R.; Lawrence D. S. Illuminating the chemistry of life: design, synthesis, and applications of ″caged″ and related photoresponsive compounds. ACS Chem. Biol. 2009, 4 (6), 409–27. 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchornik A.; Amit B.; Woodward R. B. Photosensitive protecting groups. J. Am. Chem. Soc. 1970, 92 (21), 6333–6335. 10.1021/ja00724a041. [DOI] [Google Scholar]
- Zayat L.; Calero C.; Alborés P.; Baraldo L.; Etchenique R. A New Strategy for Neurochemical Photodelivery: Metal–Ligand Heterolytic Cleavage. J. Am. Chem. Soc. 2003, 125 (4), 882–883. 10.1021/ja0278943. [DOI] [PubMed] [Google Scholar]
- Givens R. S.; Matuszewski B. Photochemistry of phosphate esters: an efficient method for the generation of electrophiles. J. Am. Chem. Soc. 1984, 106 (22), 6860–6861. 10.1021/ja00334a075. [DOI] [Google Scholar]
- Klan P.; Solomek T.; Bochet C. G.; Blanc A.; Givens R.; Rubina M.; Popik V.; Kostikov A.; Wirz J. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 2013, 113 (1), 119–91. 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbruck N.; Courtney T.; Naro Y.; Deiters A. Optochemical Control of Biological Processes in Cells and Animals. Angew. Chem., Int. Ed. 2018, 57 (11), 2768–2798. 10.1002/anie.201700171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q.; Xing B. Photoactive molecules for applications in molecular imaging and cell biology. Chem. Soc. Rev. 2010, 39 (8), 2835–2846. 10.1039/b915574k. [DOI] [PubMed] [Google Scholar]
- Ruskowitz E. R.; DeForest C. A. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nature Reviews Materials 2018, 3 (2), 17087. 10.1038/natrevmats.2017.87. [DOI] [Google Scholar]
- Zhao H.; Sterner E. S.; Coughlin E. B.; Theato P. o-Nitrobenzyl Alcohol Derivatives: Opportunities in Polymer and Materials Science. Macromolecules 2012, 45 (4), 1723–1736. 10.1021/ma201924h. [DOI] [Google Scholar]
- Chaudhuri A.; Venkatesh Y.; Behara K. K.; Singh N. D. Bimane: A Visible Light Induced Fluorescent Photoremovable Protecting Group for the Single and Dual Release of Carboxylic and Amino Acids. Org. Lett. 2017, 19 (7), 1598–1601. 10.1021/acs.orglett.7b00416. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Steinmetz M. G. Photochemical cyclization with release of carboxylic acids and phenol from pyrrolidino-substituted 1,4-benzoquinones using visible light. Org. Lett. 2005, 7 (17), 3729–32. 10.1021/ol051362k. [DOI] [PubMed] [Google Scholar]
- Gorka A. P.; Nani R. R.; Zhu J.; Mackem S.; Schnermann M. J. A near-IR uncaging strategy based on cyanine photochemistry. J. Am. Chem. Soc. 2014, 136 (40), 14153–9. 10.1021/ja5065203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebej P.; Wintner J.; Muller P.; Slanina T.; Al Anshori J.; Antony L. A.; Klan P.; Wirz J. Fluorescein analogues as photoremovable protecting groups absorbing at approximately 520 nm. J. Org. Chem. 2013, 78 (5), 1833–43. 10.1021/jo301455n. [DOI] [PubMed] [Google Scholar]
- Umeda N.; Takahashi H.; Kamiya M.; Ueno T.; Komatsu T.; Terai T.; Hanaoka K.; Nagano T.; Urano Y. Boron dipyrromethene as a fluorescent caging group for single-photon uncaging with long-wavelength visible light. ACS Chem. Biol. 2014, 9 (10), 2242–6. 10.1021/cb500525p. [DOI] [PubMed] [Google Scholar]
- Hansen M. J.; Velema W. A.; Lerch M. M.; Szymanski W.; Feringa B. L. Wavelength-selective cleavage of photoprotecting groups: strategies and applications in dynamic systems. Chem. Soc. Rev. 2015, 44 (11), 3358–77. 10.1039/C5CS00118H. [DOI] [PubMed] [Google Scholar]
- Olson J. P.; Banghart M. R.; Sabatini B. L.; Ellis-Davies G. C. Spectral evolution of a photochemical protecting group for orthogonal two-color uncaging with visible light. J. Am. Chem. Soc. 2013, 135 (42), 15948–54. 10.1021/ja408225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. M.; Silva E.; Reis R. L. Light-triggered release of photocaged therapeutics - Where are we now?. J. Controlled Release 2019, 298, 154–176. 10.1016/j.jconrel.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Vorobev A. Y.; Moskalensky A. E. Long-wavelength photoremovable protecting groups: On the way to in vivo application. Comput. Struct. Biotechnol. J. 2020, 18, 27–34. 10.1016/j.csbj.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani R. R.; Gorka A. P.; Nagaya T.; Yamamoto T.; Ivanic J.; Kobayashi H.; Schnermann M. J. In Vivo Activation of Duocarmycin–Antibody Conjugates by Near-Infrared Light. ACS Cent. Sci. 2017, 3 (4), 329–337. 10.1021/acscentsci.7b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami P. P.; Syed A.; Beck C. L.; Albright T. R.; Mahoney K. M.; Unash R.; Smith E. A.; Winter A. H. BODIPY-derived photoremovable protecting groups unmasked with green light. J. Am. Chem. Soc. 2015, 137 (11), 3783–6. 10.1021/jacs.5b01297. [DOI] [PubMed] [Google Scholar]
- Rubinstein N.; Liu P.; Miller E. W.; Weinstain R. meso-Methylhydroxy BODIPY: a scaffold for photo-labile protecting groups. Chem. Commun. (Cambridge, U. K.) 2015, 51 (29), 6369–72. 10.1039/C5CC00550G. [DOI] [PubMed] [Google Scholar]
- Loudet A.; Burgess K. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem. Rev. 2007, 107 (11), 4891–932. 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- Slanina T.; Shrestha P.; Palao E.; Kand D.; Peterson J. A.; Dutton A. S.; Rubinstein N.; Weinstain R.; Winter A. H.; Klan P. Search of the Perfect Photocage: Structure-Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups. J. Am. Chem. Soc. 2017, 139 (42), 15168–15175. 10.1021/jacs.7b08532. [DOI] [PubMed] [Google Scholar]
- Peterson J. A.; Wijesooriya C.; Gehrmann E. J.; Mahoney K. M.; Goswami P. P.; Albright T. R.; Syed A.; Dutton A. S.; Smith E. A.; Winter A. H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140 (23), 7343–7346. 10.1021/jacs.8b04040. [DOI] [PubMed] [Google Scholar]
- Kand D.; Pizarro L.; Angel I.; Avni A.; Friedmann-Morvinski D.; Weinstain R. Organelle-Targeted BODIPY Photocages: Visible-Light-Mediated Subcellular Photorelease. Angew. Chem., Int. Ed. 2019, 58 (14), 4659–4663. 10.1002/anie.201900850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wories H. J.; Koek J. H.; Lodder G.; Lugtenburg J.; Fokkens R.; Driessen O.; Mohn G. R. A Novel Water-Soluble Fluorescent-Probe - Synthesis, Luminescence and Biological Properties of the Sodium-Salt of the 4-Sulfonato-3,3′,5,5′-Tetramethyl-2,2’-Pyrromethen-1,1’-Bf2 Complex. Recl Trav Chim Pay B 1985, 104 (11), 288–291. 10.1002/recl.19851041104. [DOI] [Google Scholar]
- Fan G.; Yang L.; Chen Z. J. Water-soluble BODIPY and aza-BODIPY dyes: synthetic progress and applications. Front. Chem. Sci. Eng. 2014, 8 (4), 405–417. 10.1007/s11705-014-1445-7. [DOI] [Google Scholar]
- Komatsu T.; Oushiki D.; Takeda A.; Miyamura M.; Ueno T.; Terai T.; Hanaoka K.; Urano Y.; Mineno T.; Nagano T. Rational design of boron dipyrromethene (BODIPY)-based photobleaching-resistant fluorophores applicable to a protein dynamics study. Chem. Commun. 2011, 47 (36), 10055–10057. 10.1039/c1cc13367e. [DOI] [PubMed] [Google Scholar]
- Kim J.; Kim Y. A water-soluble sulfonate-BODIPY based fluorescent probe for selective detection of HOCl/OCl– in aqueous media. Analyst 2014, 139 (12), 2986–2989. 10.1039/C4AN00466C. [DOI] [PubMed] [Google Scholar]
- Kawatani M.; Kamiya M.; Takahashi H.; Urano Y. Factors affecting the uncaging efficiency of 500nm light-activatable BODIPY caging group. Bioorg. Med. Chem. Lett. 2018, 28 (1), 1–5. 10.1016/j.bmcl.2017.11.030. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Yang M.; Li C.; Dorh N.; Xie F.; Luo F.-T.; Tiwari A.; Liu H. Near-infrared fluorescent probes based on piperazine-functionalized BODIPY dyes for sensitive detection of lysosomal pH. J. Mater. Chem. B 2015, 3 (10), 2173–2184. 10.1039/C4TB01878H. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Yang M.; Mazi W.; Adhikari K.; Fang M.; Xie F.; Valenzano L.; Tiwari A.; Luo F.-T.; Liu H. Unusual Fluorescent Responses of Morpholine-Functionalized Fluorescent Probes to pH via Manipulation of BODIPY’s HOMO and LUMO Energy Orbitals for Intracellular pH Detection. ACS Sensors 2016, 1 (2), 158–165. 10.1021/acssensors.5b00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Ji G.; Jung J.-M.; Son Y.-A. A BODIPY based highly selective fluorescence turn-on sensor toward VIB and IIB metal ions. Mol. Cryst. Liq. Cryst. 2016, 636 (1), 159–167. 10.1080/15421406.2016.1201406. [DOI] [Google Scholar]
- Niu S. L.; Ulrich G.; Ziessel R.; Kiss A.; Renard P. Y.; Romieu A. Water-soluble BODIPY derivatives. Org. Lett. 2009, 11 (10), 2049–52. 10.1021/ol900302n. [DOI] [PubMed] [Google Scholar]
- Nadler A.; Yushchenko D. A.; Muller R.; Stein F.; Feng S.; Mulle C.; Carta M.; Schultz C. Exclusive photorelease of signalling lipids at the plasma membrane. Nat. Commun. 2015, 6, 10056. 10.1038/ncomms10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N.; Stephan M.; Höglinger D.; Nadler A. A Click Cage: Organelle-Specific Uncaging of Lipid Messengers. Angew. Chem., Int. Ed. 2018, 57 (40), 13339–13343. 10.1002/anie.201807497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezcano N.; Bergson C. D1/D5 Dopamine Receptors Stimulate Intracellular Calcium Release in Primary Cultures of Neocortical and Hippocampal Neurons. J. Neurophysiol. 2002, 87 (4), 2167–2175. 10.1152/jn.00541.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.