Abstract
Vitamin A is an essential nutrient, critical for proper embryonic development in mammals. Both embryonic vitamin A-deficiency or -excess lead to congenital malformations or lethality in mammals, including humans. This is due to the defective transcriptional action of retinoic acid, the active form of vitamin A, that regulates in a spatial- and temporal-dependent manner the expression of genes essential for organogenesis. Thus, an adequate supply of vitamin A from the maternal circulation is vital for normal mammalian fetal development. Provitamin A carotenoids circulate in the maternal bloodstream and are available to the embryo. Of all the dietary carotenoids, β-carotene is the main vitamin A precursor, contributing at least 30% of the vitamin A intake in the industrialized countries and often constituting the sole source of retinoids (vitamin A and its derivatives) in the developing world. In humans, up to 40% of the absorbed dietary β-carotene is incorporated in its intact form in chylomicrons for distribution to other organs within the body, including the developing tissues. Here, it can serve as a source of vitamin A upon conversion into apocarotenoids by its cleavage enzymes. Given that β-carotene is carried in the bloodstream by lipoproteins, and that the placenta acquires, assembles and secretes lipoproteins, it is becoming evident that the maternal-fetal transfer of β-carotene relies on lipoprotein metabolism. Here, we will explore the current knowledge about this important biological process, the cross-talk between carotenoid and lipid metabolism in the context of the maternal-fetal transfer of this provitamin A precursor, and the mechanisms whereby β-carotene is metabolized by the developing tissues.
Keywords: β-carotene, β-carotene-15, 15′-oxygenase (BCO1), β-carotene-9’, 10′-oxygenase (BCO2), retinoids, microsomal triglyceride transfer protein (MTP), lipoproteins
1. Dietary sources, metabolism and factors affecting circulating levels of β-carotene
β-carotene, like other hundreds of carotenoids existing in nature, is a C40 isoprenoid compound synthesized predominantly in plants to confer the bright yellow, red and orange color to fruits and flowers and to function in support of photosynthesis and pollination or as a precursor of phytohormones [1, 2]. Only a handful of carotenoids, such as α- and β-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin, are plentiful in the human diet and can be detected in significant amounts in the human plasma [3]. Humans obtain β-carotene exclusively from yellow, red and orange fruits and vegetables, as well as dark green leafy vegetables or from the meat of animals that have ingested them [3]. β-carotene is the most abundant carotenoid in the human diet, with the highest concentration found in leaf, fruit, and root tissues of many vegetable crops [4]. The root crop carrot (Daucus carrota L.) has some of the highest concentrations of β-carotene, with levels ranging from 3.2 to 6.1 mg/100 g fresh weight [4]. However, cruciferous leafy vegetable crops can have concentrations equal to, or higher than carrot. Values in kale and collards, for instance, range from 3.8 to 10.0 mg β-carotene/100 g fresh weight [4].
β-carotene is known for its antioxidant capacity [5, 6], however, in mammalian tissues β-carotene also serves as a precursor of vitamin A, an essential micronutrient that is vital to many critical biological functions throughout the life-cycle [7], including embryonic development [8]. β-carotene accounts for at least 30% of the dietary vitamin A intake in most of the world populations, and for some populations it represents the sole source of the vitamin [6, 9, 10]. β-carotene yields retinoids (vitamin A and its derivatives) upon its enzymatic cleavage mediated predominantly by the cytoplasmic enzyme β-carotene-15,15’-oxygenase (BCO1) [11]. The BCO1-mediated cleavage results in two molecules of retinaldehyde which can be oxidized by the action of the retinaldehyde dehydrogenase family of enzymes (RALDH or ALDH1 family) to generate all-trans retinoic acid, the active form of vitamin A (Figure 1). Retinoic acid functions as a ligand for specific nuclear receptors, retinoic acid receptor (RAR) or retinoid X receptor (RXR) that, by homo- or hetero-dimerization, regulate the transcription of several hundred target genes [12]. The clearance of retinoic acid is catalyzed by the family of the CYP26 enzymes, cytochrome P450 enzymes that oxidize retinoic acid into more polar compounds, like 4-hydroxy or 4-oxo retinoic acid, believed to be transcriptionally inactive [13]. Alternatively, retinaldehyde generated from the β-carotene cleavage can be reduced to retinol, the alcohol form of vitamin A, by the membrane-associated retinol dehydrogenase (RDH) and short-chain dehydrogenase/reductase (DHRS) family of enzymes [14]. This enzymatic conversion is reversible and it is considered the rate-limiting step of retinoic acid synthesis [14]. Retinol can then be esterified, mainly by the action of lecithin:retinol acyltransferase (LRAT), into retinyl esters (Figure 1), the storage form of vitamin A in various tissues, predominantly in liver, but also lung, adipose tissues, heart and kidney [15].
Figure 1. Symmetric and asymmetric cleavage of β-carotene.
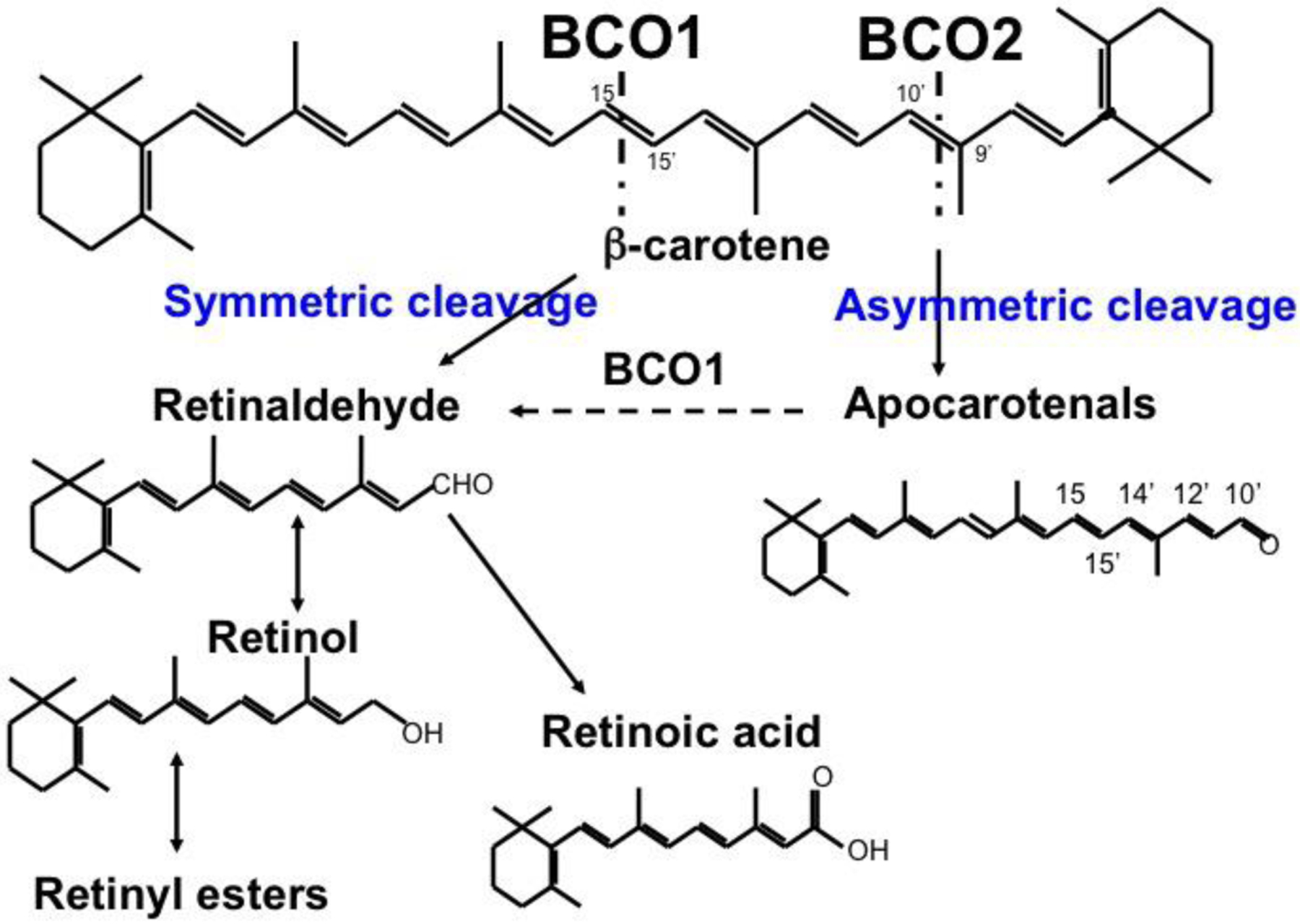
Central cleavage of β-carotene at the 15,15’ double bond is catalyzed by BCO1 to yield two molecules of retinal. Asymmetric cleavage at the 9’,10’ double bond is catalyzed by BCO2, and yields β-apo-10’-carotenal (depicted) and β-ionone (not shown). Retinaldehyde can be oxidized to retinoic acid or reduced to retinol, which can then be esterified to form retinyl esters.
A second cleavage enzyme, β-carotene-9’,10’-oxygenase (BCO2), is localized in the mitochondria and can cleave β-carotene asymmetrically, even though it has ten-fold lower catalytic activity compared to BCO1 [16]. BCO2 displays a broader substrate specificity than BCO1, being more efficient at cleaving other carotenoids with 3-OH-β-ionone ring sites as a substrate, including β-cryptoxanthin, another provitamin A carotenoid. BCO2 cleaves β-carotene specifically at the 9′−10′ double bond to produce a β-ionone and β-apo-10′-carotenal [16,17]. The latter, as well as other β-apocarotenoids of various chain lengths generated from oxidative breakdown of β-carotene in food and animal tissues [18], can be converted into one molecule of retinaldehyde. This conversion was originally proposed to be mediated by a mechanism similar to β-oxidation [19,20]. Subsequently, Von Lintig and colleagues proposed a BCO1-dependent “stepwise cleavage” to generate retinoid from β-apocarotenoids [21] (Figure 1). Note that, in reality, apocarotenoids, cleavage products of parent carotenoids [2], fit the definition of retinoids as proposed by Sporn [22], i.e. natural and synthetic chemical species that bear a structural resemblance to all-trans-retinol, with or without the biological activity of vitamin A. As mice lacking Bco1 accumulated β-carotene in tissues when fed a diet containing β-carotene as the only source of vitamin A, despite the expression of BCO2, it was suggested that β-carotene is a poor substrate for BCO2 in vivo [23, 24]. Therefore, it was proposed that, through the “stepwise cleavage”, BCO2 could be critical for the conversion of asymmetric provitamin A carotenoids, such as β-cryptoxanthin, into retinoids [25]. Moreover, the Von Lintig group also demonstrated that by cleaving carotenoids, including β-carotene, BCO2 protects the mitochondria from the toxic effects of excess of carotenoids, which otherwise would lead to oxidative stress [26].
In humans, bioaccessibility and bioavailability of β-carotene are dependent upon numerous factors, including the dietary regimen, food matrix, micelle formation, mechanical and chemical changes during digestion, etc. [3]. Moreover, the intestinal absorption of β-carotene via the apical membrane of the enterocytes of the small intestine is highly regulated depending on the vitamin A status. A key player in this process is the intestine-specific homeobox (ISX) protein that functions as a transcriptional repressor of Bco1 and Scarb1 [27–30]. The Scarb1 gene encodes the scavenger receptor B1 (SR-B1) that has been implicated in the uptake of dietary β-carotene by enterocytes from the intestinal lumen [31].
Interestingly, Isx is transcriptionally up-regulated by retinoic acid by virtue of a retinoic acid responsive element (RARE) in its promoter [27]. Thus, in vitamin A sufficiency, retinoic acid increases the expression of Isx, reducing the expression of Scarb1 and Bco1, which in turn attenuate uptake and cleavage of dietary β-carotene, respectively [27–30]. In contrast, under limiting tissue vitamin A availability (i.e., reduced tissue retinoic acid levels) the corresponding lower levels of Isx result in enhanced β-carotene absorption (via SR-B1) and vitamin A production (via BCO1) [27–30]. Thus, ISX is considered a master regulator of intestinal β-carotene uptake and catabolism.
In addition to this ISX-dependent mechanism, polymorphisms in the genes encoding BCO1 and SR-B1 have also been shown to affect intestinal absorption of β-carotene and its conversion to retinoid, thus subsequently influencing distribution of intact β-carotene to various tissues [32, 33]. Upon ingestion and uptake by the enterocytes, β-carotene is cleaved into retinoids to be incorporated as retinyl esters within chylomicrons and, together with other dietary lipids, secreted into the lymphatic system, as detailed below. The efficiency of intestinal β-carotene conversion into retinoids also varies among species [34]. Importantly, unlike mice, in humans up to 40% of the dietary ingested β-carotene is not converted into vitamin A [3], likely due to polymorphisms in the BCO1 gene that affect the activity of the enzyme [35]. This reduced cleavage efficiency results in increased incorporation of intact β-carotene into chylomicrons for delivery to other sites of the body. Due to all the above-mentioned factors, as well as others not discussed here (reviewed by [3, 36]), the concentration of β-carotene in the human bloodstream is variable, normally ranging from 0.2 to 0.7 μM [37].
2. Intestinal absorption of β-carotene and its distribution throughout the body: the role of lipoproteins
Dietary retinoids and carotenoids share similar mechanisms of intestinal absorption. Upon ingestion, they are emulsified in the intestinal lumen with mixed micelles consisting of fatty acids, monoacylglycerols, lysophospholipids, phospholipids, cholesterol and bile salts [15]. Dietary retinyl esters are hydrolyzed by pancreatic triglyceride lipase (PTL), pancreatic lipase related protein 2 (PLRP2) or intestinal brush border retinyl ester hydrolase (REH) enzymes resulting in free retinol that is taken up by the enterocytes [38–40]. Studies in Caco-2 cells, a widely used cell culture model for enterocytes [41], showed that these cells rapidly take up retinol, independent of the presence of free fatty acids [42]. This uptake also seemed to be a saturable, carrier-mediated process at physiological doses, whereas it occurred by passive diffusion at pharmacological doses [42, 43]. Despite these and other findings suggesting the existence of a specific receptor-mediating retinol absorption from the luminal side of the enterocytes, the identity of this hypothetical receptor has not been confirmed to date. Differentiated Caco-2 cells also take up β-carotene in a concentration-dependent saturable process, supporting the existence of a protein-mediated facilitated absorption mechanism [44]. Indeed, SR-B1 has been shown to mediate the uptake of β-carotene in intestinal cells [31]. In this study, the investigators measured uptake of β-carotene in vivo in wild-type and SR-B1 knockout mice, and in vitro by using brush border membranes isolated from these same mouse strains [31]. High fat and high cholesterol diet-fed SR-B1 knockout mice absorbed significantly less β-carotene compared to wild-type mice [31]. Similarly, reduced uptake of β-carotene in brush border membrane vesicles from SR-B1 mutant mice compared to wild-type controls was observed. Furthermore, brush border vesicle from rabbit intestine took up less β-carotene from different donor micelles after treatment with proteinase K as well as apoA1 and SR-B1 antibodies [31]. Interestingly, β-carotene uptake was also shown to be increased in Cos cells overexpressing either SR-B1 or the fatty acid translocase cluster of differentiation 36 (CD36) [31]. Recent data suggest that CD36 may indirectly regulate intestinal absorption of β-carotene by modulating the synthesis rate of chylomicrons [45] in which β-carotene is incorporated. Another cell surface lipid transporter, Niemann-Pick C1–like 1 (NPC1L1), may also be involved in the uptake of β-carotene, based on the reduced intracellular carotenoid concentration observed upon its inhibition by ezetimibe, at least in Caco-2 cells [46]. Thus, multiple studies support the hypothesis that SR-B1 is a mediator of dietary β-carotene absorption from the intestinal lumen. In addition, other lipid cell surface receptors, such as CD36 and NPC1L1, may also participate in this process.
Once within the enterocytes, retinol is esterified by the endoplasmic reticulum resident enzymes LRAT [47] and potentially diacyl-glycerol acyl transferase 1 (DGAT1), at least under certain circumstances, such as supraphysiological intake of vitamin A [48, 49]. The synthesized retinyl esters are then stored in lipid droplets or packaged with intestinal apolipoprotein B (apoB) containing triglyceride-rich lipoproteins, i.e., chylomicrons [50]. Chylomicron assembly is critically dependent on the structural protein apoB and the chaperone microsomal triglyceride transfer protein (MTP) [51, 52]. MTP transfers several lipids from donor vesicles to acceptor vesicles in vitro [53]. Evidence that this lipid transfer activity is important in the assembly and secretion of apoB-containing lipoproteins comes from studies using MTP inhibitors and from the identification of specific mutations in MTP that resulted in the loss of lipid transfer activity [54, 55]. MTP is predominanly regulated at the level of transcription, with a robust correlation between mRNA levels and enzymatic activity [56]. In addition to transferring lipids, MTP also interacts with apoB to promote assembly and secretion of apoB-containing lipoproteins [57]. Specifically, it interacts and lipidates apoB co-translationally; in the absence of MTP, apoB is synthesized and degraded [57]. Morevoer, mutations in MTTP result in a disease called aβ-lipoproteinemia. Among other clinical symptoms, human subjects affected by these mutations manifest signs of fat-soluble vitamin deficiencies [58, 59]. Thus, MTP potentially plays a critical role in the absorption of fat-soluble vitamins.
Studies investigating the secretion of retinol by Caco-2 cells revealed that these cells mainly secrete retinyl ester associated with chylomicrons and not with smaller size apoB-containing lipoproteins [42]. The secretion of retinyl ester was shown to be dependent on the assembly and secretion of larger chylomicrons and was independent of the retinol uptake and intracellular retinyl ester levels. This highlights that retinyl esters only associate with larger chylomicrons and serve as a good marker to study the metabolism of larger chylomicron particles, despite constituting a small fraction of these particles [60]. It is unclear where, when and how retinyl esters are transferred to chylomicrons within cells prior to their secretion by enterocytes. It is likely that MTP transfers retinyl esters to chylomicrons, but - surprisingly - this has not yet been experimentally demonstrated. Identification of these mechanisms may yield novel information such as additional signals for chylomicron assembly and secretion as well as the deposition of retinyl esters into these particles.
The β-carotene absorbed by the enterocyte undergoes cleavage, predominantly by BCO1, to yield retinladehyde first and subsequently retinol which, upon conversion into retinyl esters, is incorporated into chylomicrons for secretion in the bloodstream, as discussed above [11]. However, as mentioned earlier, in humans up to 40% of ingested β-carotene is not converted into retinoids [6, 61]. Thus, intact β-carotene can also be directly incorporated into chylomicrons. Indeed, secretion studies in Caco-2 cells also proved that, like retinyl esters, β-carotene was mainly secreted with chylomicrons [44]. Studies in humans also established the association of β-carotene with chylomicrons upon a bolus dose of β-carotene. In male subjects, administered a single oral dose of β-carotene, the provitamin A carotenoid was detected in chylomicrons with a peak at 6 hours post-consumption that dropped afterwards, due to chylomicron clearance from the circulation [62]. A similar study also showed the first appearance of the provitamin A carotenoid in chylomicrons upon a single oral β-carotene administration in 9 human subjects [63]. The mechanisms that enable incorporation of β-carotene within chylomicrons have not been elucidated. However, novel studies from our laboratories suggest that MTP can transfer β-carotene between donor and acceptor vesicles in vitro (Figure 2). Specifically, purified MTP transfers β-carotene, although less efficiently than triglycerides (Figure 2A). Similar to triglycerides (Figure 2B), the transfer of β-carotene was significantly reduced in MTP-deficient liver homogenates compared to wild-type mouse liver controls (Figure 2C). Therefore, these findings suggest that MTP may transfer β-carotene onto chylomicrons for secretion.
Figure 2. MTP transfers β-carotene.
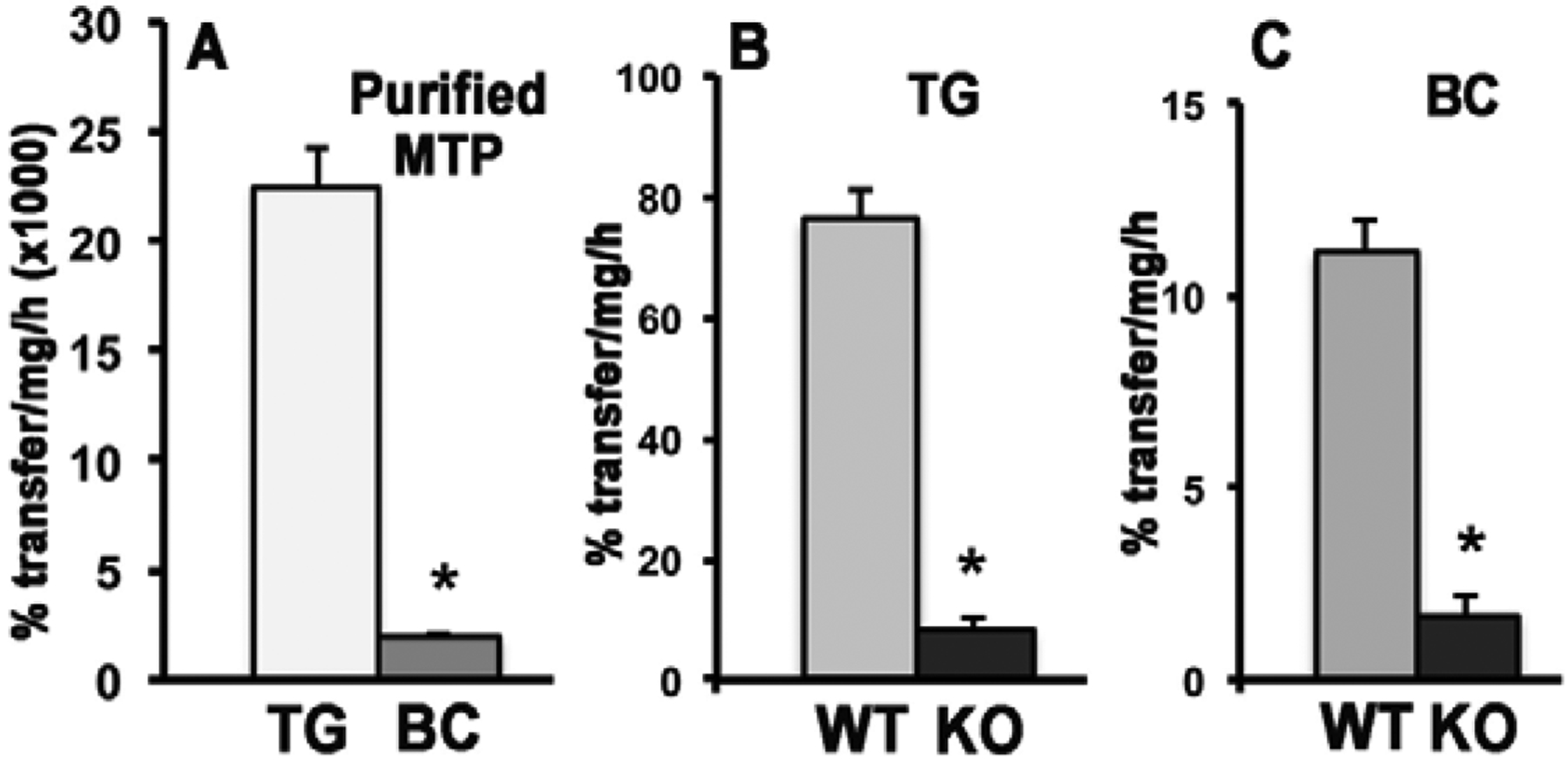
β-carotene-containing donor vesicles were made by mixing 100 μl of β-carotene (stock 10 mg/mL in chloroform) with 70 μl of phosphatidylcholine (stock 20 mg/mL in chloroform), evaporated under nitrogen and then resuspended and sonicated in 4.5 mL of 15 mM Tris-HCl buffer, pH 7.4, 1 mM EDTA, 150 mM NaCl, 0.02% NaN3. For the assay, 20 μL of β-carotene-containing donor vesicles (DV) and 5 μL of acceptor vesicles (AV) containing only phosphatidylcholine were used, along with 50 μL BSA (10 mg/mL).. 1 μg purified bovine liver MTP protein (A) or 200 μg mouse liver homogenate protein (B and C) were incubated for 18 h (for BC) or 1 h (for TG) in water bath at 37 C. Excitation at 355 nm and emission at 535 nm were used to measure β-carotene. TG transfer was measured as reported [53]. Data are means ± SD; n=3 replicates/condition; WT, wild-type C57Bl/6J liver homogenates; KO, MTP-deficient liver homogenates (wild type C57Bl/6J background). TG, triglycerides; BC, β-carotene. Statistical analysis within each panel by t-test, *, p<0.05.
Retinyl esters and intact β-carotene as part of chylomicrons are released into systemic circulation at the thoracic duct. In the circulation, triglycerides in chylomicrons are hydrolyzed by endothelial cell-bound lipoprotein lipase, resulting in smaller chylomicron remnants. There is evidence that lipoprotein lipase can also hydrolyze retinyl esters and retinol released after the hydrolysis is taken up by peripheral tissues [64].
Chylomicron remnants still contain significant amounts of retinyl esters that are taken up by hepatocytes through LDL receptor (LDLR) and other cell-surface proteins that have high affinity for apo-E rich chylomicron remnants [51, 65]. Whereas the majority of the retinyl ester within chylomicron remnants is cleared by the liver, about 25% of them is taken up by the peripheral tissues. In the hepatocytes, retinyl esters are hydrolyzed to retinol by retinyl ester hydrolase (REH), carboxylesterase and/or lipases, followed by transfer of the majority of retinol to hepatic stellate cells (HSCs) for storage [66]. The mechanisms of uptake of β-carotene as part of remnants (or other lipoproteins) by the liver and extrahepatic tissues has been poorly studied. Presumably, β-carotene follows the same uptake pathways of the retinoids within the lipoproteins. Shete and colleageus [67] provided some evidence for a role of hepatic LDLR in this process. Indeed, these authors showed a significant 50% reduction of hepatic β-carotene uptake in Ldlr−/− dams upon a single β-carotene administration.
It is established that β-carotene can circulate in the bloodstream not only in association with chylomicrons but also with the other classes of lipoproteins to a varying degree and with a dynamic exchange of the provitamin A carotenoid among various lipoproteins [62, 63, 68, 69]. About 60–70% of intact β-carotene is transported in LDL in the human circulation [70]. In the fasting circulation, β-carotene is mainly associated with VLDL and LDL, the lipoproteins containing apoE and apoB moieties [69], and in the postprandial circulation β-carotene in the triglyceride-rich fraction is considered a marker of intestinal β-carotene absorption [71]. Despite the mouse having a higher prevalence of HDL in the bloodstream, Wassef and colleagues [72] demonstrated that 24 hours after a single administration of β-carotene, the provitamin A carotenoid was distributed among all the circulating lipoproteins with the highest concentration in LDL+ IDL fraction, as in humans. These findings suggest that the mouse could be a good experimental model to study the mechanisms of tissue uptake of β-carotene from lipoproteins.
3. The transport of β-carotene across the placenta: implication for a critical role of lipoprotein metabolism
It is well established that both vitamin A deficiency and excess result in congenital malformations most of which are not compatible with life [8, 73]. These dramatic effects are due to the transcriptional regulatory activity of retinoic acid that controls in a spatial- and temporal- dependent manner the expression of a plethora of genes that enable proper development of essentially all organs and tissues [8, 73]. Since there is no de novo fetal synthesis of vitamin A, to meet its requirement for retinoids, the developing mammalian embryo relies on circulating maternal vitamin A that reaches the embryo through the maternal-fetal barrier [8]. This barrier comprises the placenta and the yolk sac, both assisting in nutrient transfer to the developing tissues. However, the yolk sac is considered a vestigial organ in many vertebrate species, it is mostly a transient developmental organ, and it shows considerable species variation [74]. Moreover, the yolk sac membranes are not in contact with the maternal bloodstream [74]. Thus, this review will focus exclusively on the placental transfer and metabolism of β-carotene. In the maternal bloodstream, two major retinoid forms can be identified. First, retinol bound to retinol-binding protein (RBP or RBP4) is the major form in the fasting state, when most retinol is secreted into the circulation from the liver stores bound to RBP, its sole specific carrier in the circulation [75]. Second, retinyl ester packaged in chylomicrons and their remnants may account for the majority of circulating retinoids upon dietary vitamin A intake [66]. Although at lower concentrations, other forms of vitamin A circulate in the bloodstream, including provitamin A carotenoids incorporated in lipoprotein particles [8]. As mentioned above, provitamin A carotenoids include β-carotene, α-carotene and β-cryptoxanthin, which can all be converted into vitamin A by enzymatic cleavage [16, 25]. However, virtually nothing is known about the potential impact that α-carotene and β-cryptoxanthin might have on embryogenesis. Thus, the following sections of this review will focus exclusively on the functions of β-carotene.
As the most abundant carotenoid present in the human diet and the major dietary vitamin A precursor, β-carotene is an important source of retinoids for the developing tissues [8]. The molecular mechanisms of the transplacental transfer of intact β-carotene from mother to fetus still remain to be fully elucidated, but critical knowledge has begun to emerge from in vitro and in vivo studies. Since β-carotene travels in the maternal bloodstream in association with lipoproteins, it was expected that key players of the lipoprotein metabolic pathway would be implicated in its maternal-fetal transfer. In human term placenta LDLR and LDLR-related protein 1 (LRP1) have been localized in the syncytiotrophoblast facing the maternal blood and in stromal cells [76]. The apoB receptor LRP2 was found restricted to the cytotrophoblast cells, precursor of the syncytiotrophoblasts [77]. Immunohistochemical analysis of human placenta also localized the very low-density lipoprotein/apolipoprotein E receptor (VLDLR) in the syncytiotrophoblast and intermediate trophoblast cells [78]. VLDLR messenger RNA was found in villous core endothelial cells and in Hofbauer cells, which are of mesenchymal origin, in the mesoderm of the chorionic villus [78]. Northern blot analysis also showed that VLDLR expression was 2.6 times higher at term compared to the first trimester, thus suggesting an important role for the VLDLR in placental lipid uptake towards the end of pregnancy [78]. Similarly, apoB and MTP, with its smaller subunit protein disulphide-isomerase (PDI) required for secretion of the lipoprotein particles [51, 52], were found in the syncytiotrophoblasts facing maternal blood and other placental cell types [77]. Importantly, it was recently reported that placental trophoblast BeWo cells can secrete apolipoprotein B-100 (apoB-100) containing lipoproteins [76, 77]. A recent study from Kallol et al. [79] also provided novel insights on maternal-fetal cholesterol transfer through the ATP-binding cassette transporter A1/apolipoprotein A1 (ABCA1/apoA1) pathway. By using human primary villous trophoblasts from term placentas, Kallol and colleagues evaluated cholesterol uptake and efflux rates in the cytotrophoblasts and the syncytiotrophobast layer, in relation to their differentiation state. They discovered that at term, when the development of the fetus is completed, the ABCA1/apoA1 pathway is highly activated in order to deliver excess cholesterol back to the maternal circulation, rather than to the fetus, pointing to a potential protective role of ABCA1/apoA1-mediated cholesterol efflux that might avoid harmful cholesterol accumulation in placenta and consequentially in the fetus. Overall, these data indicate that key receptors and mechanisms involved in lipoprotein metabolism and functions exist at the placenta-fetal barrier.
Kim and colleagues [80] demonstrated that intact β-carotene supplemented to pregnant female mice can be taken up by the placenta and from here secreted towards the embryo where it serves as a source for in situ synthesis of retinoids upon its BCO1-mediated cleavage. Studies in wild-type dams maintained on a regular chow diet and supplemented with one dose of β-carotene via intraperitoneal injection, showed a transcriptional downregulation of Lrp1 and Vldlr in the placenta [81]. Although the protein levels were not measured in this study, these findings suggested that placental β-carotene uptake might be attenuated under conditions of maternal vitamin A-sufficiency. In contrast, placental uptake of β-carotene was increased, as well as Lpl mRNA expression, in marginally deficient dams lacking both LRAT and RBP (Lrat−/−Rbp−/−) [82]. In addition, retinol levels in the placenta of these double knockout dams were found to be higher compared to WT placentas, suggesting that under a marginal vitamin A deficient status the conversion of β-carotene into retinoids is maximized in order to provide an adequate amount of retinoids to support embryogenesis [82]. Importantly, when the Lrat−/−Rbp−/− dams were on a vitamin A deficient diet (a model of severe vitamin A deficiency [80]), and then supplemented with β-carotene at an early stage of gestation during the window of major organogenesis (6.5–9.5 days post coitum, dpc), the embryos displayed a less severe phenotype and almost 40% of them appeared grossly normal compared to embryos from non-supplemented mothers [82]. These data further reinforce the notion that β-carotene can attenuate the detrimental effects of vitamin A deficiency specifically during the early stages of development, when the retinoid requirements need to be critically met to ensure embryonic survival. Under excessive vitamin A intake during pregnancy (66 μg of retinol/g of diet) a single maternal dose of β-carotene at mid gestation in wild-type mice did not result in changes in retinoid levels in maternal serum, liver, placentas and embryo [72]. However, the levels of β-carotene in the embryos were dramatically reduced and placental Bco1 expression was significantly downregulated, suggesting that an excessive intake of preformed vitamin A may activate mechanisms that protect the developing tissues from retinoid toxicity [72]. These studies demonstrated that the maternal status is critical to modulate the mechanisms that control how much β-carotene is taken up from the maternal circulation and metabolized by the placenta. These observations confirmed some earlier investigation in humans showing that in women with vitamin A deficiency (serum retinol ≤ 0.7 μmol/L), maternal serum β-carotene and placenta and cord serum retinol levels were significantly inversely correlated, suggesting that the conversion of β-carotene into retinol might be more effective in a sub-adequate vitamin A status [83]. The role of the LDLR was investigated in wild type and Ldlr−/− pregnant female mice administered a single dose of β-carotene, but this study failed to prove the involvement of this receptor in β-carotene uptake by the placenta or the embryo [67]. In fact, no difference in the levels of the provitamin A and retinoids were observed in the placental/fetal unit between Ldlr−/− mice and the wild-type control strain.
We have also provided evidence about the implication of lipoprotein biosynthesis in the transfer of β-carotene from the placenta towards the fetus [84]. Our studies showed that the transfer of β-carotene circulating in the maternal bloodstream from the placenta to the fetus is mediated by lipoproteins and regulated by the availability of the provitamin A precursor with a feed-forward mechanism (Figure 3). Specifically, we showed that β-apo-10’-carotenal, generated in placenta upon asymmetric cleavage of β-carotene by BCO2, up-regulates the expression and activity of MTP and hence lipoprotein biosynthesis. These findings also supported the emerging notion that β-apo-10’-carotenal, and other β-apocarotenoids, acts as nuclear receptor antagonists of retinoic acid [85–87]. Unlike β-apo-10’-carotenal, retinoic acid attenuated both the transcription and the activity of MTP [84]. We also provided evidence that this mechanism involves the regulation of the transcription of Hnf4α, an activator of MTP [88]. However, these findings did not unequivocally identify the direct target(s) of the β-apo-10’-carotenal action. The late transcriptional response of Mttp and Hnf4α (24 h after the maternal administration of β-carotene) suggested that neither of these genes is likely such target, even though they both contain a RARE in their promoter, which seem to be a requirement for the regulatory mechanism described in this report. It is plausible that the direct target(s) of the β-apo-10’-carotenal transcriptional control of placental lipoprotein biogenesis may be another factor(s) upstream of HNF4α. However, its identity has remained unclear to date. This model also pointed to a temporal shift in the generation of β-carotene metabolites, retinoic acid vs. β-apo-10’-carotenal, by BCO1 and BCO2 as the key factor that optimizes placental lipoprotein biosynthesis and, ultimately, transfer of intact β-carotene to the embryo [84]. Placental basal mRNA expression of Bco1 seems to be comparable to Bco2, at least at 14.5 dpc [89]. However, a further analysis of the transcriptional regulation of these genes, as well as of the activity of the corresponding enzymes in placenta, in the presence or absence of maternal β-carotene supplementation, may be needed to fully validate the proposed model.
Figure 3. Proposed model for maternal fetal transfer of intact β-carotene (β-car) upon a single maternal administration of β-carotene.
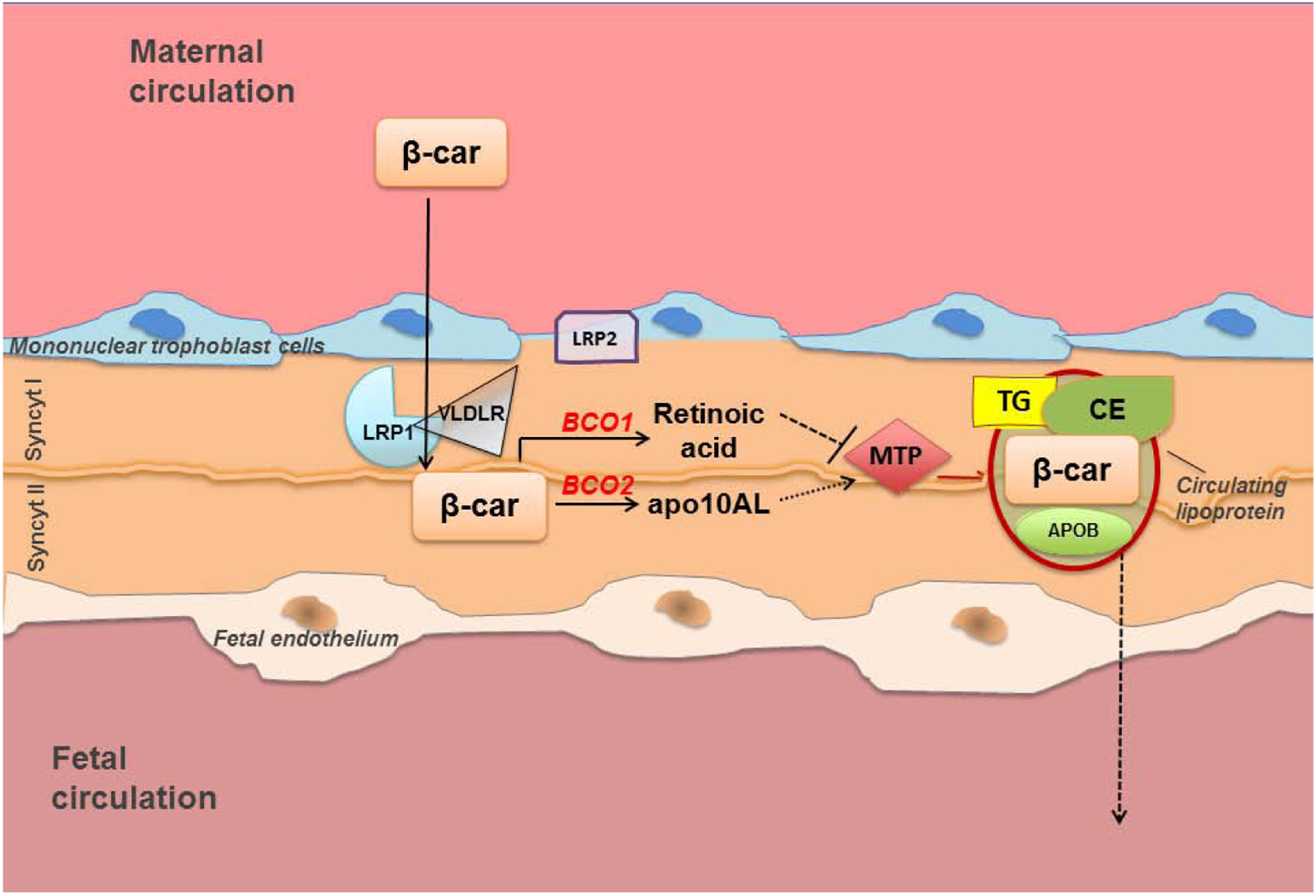
Intact β-carotene from the maternal bloodstream is transported toward the fetal circulation in association with lipoproteins assembled within the placenta syncytiotrophoblast cells. Under condition of vitamin A sufficiency, the lipoprotein receptors LRP1 and VLDLR presumably play a role in the uptake. Placental BCO1 cleaves the provitamin A carotenoid symmetrically to yield retinaldehyde (not shown), which in turn is oxidized to retinoic acid. Through this pathway, retinoic acid levels increase in placenta early after β-carotene administration (4h), thus initially suppressing MTP transcription and activity. Asymmetric cleavage of β-carotene by BCO2 also occurs, generating one β-ionone ring and β-apo-10’carotenal (apo10AL). Apo10AL levels rise as retinoic acid declines. At 24h post maternal administration of β-carotene apo10AL then increases the transcription and activity of placental MTP, which in turn stimulates lipoprotein biosynthesis and ultimately the transfer of β-carotene toward the fetal circulation. This temporally-regulated generation of apo10AL vs. retinoic acid from β-carotene in placenta and their antagonistic transcriptional activity on MTP are proposed as the key factors that optimize placental lipoprotein biogenesis and lipoprotein-mediated transfer of β-carotene to the fetus. apoB, apolipoprotein B-100; FA, fatty acids; CE, cholesteryl esters; Syncyt I, syncytiotrophoblast layer I; Syncyt II, syncytiotrophoblast layer II; TG, triglycerides.
Overall, the mechanisms of transport of intact β-carotene from the placenta to the fetal bloodstream are still largely unknown. The current literature strongly suggest that this process is mediated by lipoproteins and point to β-apocarotenoids as critical β-carotene metabolites modulating the placental-fetal transfer of the vitamin A precursor. Such mechanism could be instrumental for the placenta to fine tune the flux of provitamin A carotenoid towards the fetus depending upon the maternal vitamin A status and the fetal demand for retinoids. Novel results from our laboratories investigated this mechanism under a maternal regimen of high vitamin A intake during gestation (220 IU of vitamin A/g of diet vs. 14 IU of vitamin A/g of diet, in the case of dams on the vitamin A sufficient regimen). Under this excessive, but non toxic, dietary regimen the levels of supplemented β-carotene in wild-type embryos were undetectable, despite similar concentrations of the vitamin A precursor in the maternal liver and placentas compared to wild-type dams on the vitamin A sufficient diet [72]. Interestingly, maternal β-carotene supplementation did not upregulate the transcription of placental Mttp, apoB and Hnf4a (Figure 4A), as previously reported in the case of wild-type dams on the vitamin A sufficient diet [72]. Consequently, MTP activity was significantly lower in the placenta from mothers on the vitamin A excess diet when compared to those of dams on the vitamin A sufficient diet (Figure 4B). Finally, the transfer of β-carotene from the placenta towards the embryo was dramatically attenuated in the case of the dams maintained on the high vitamin A regimen, similar to instances in which MTP was pharmacologically inhibited (Figure 4C).
Figure 4. β-carotene suppresses placental lipoprotein biosynthesis under a maternal regimen of high vitamin A intake during pregnancy.
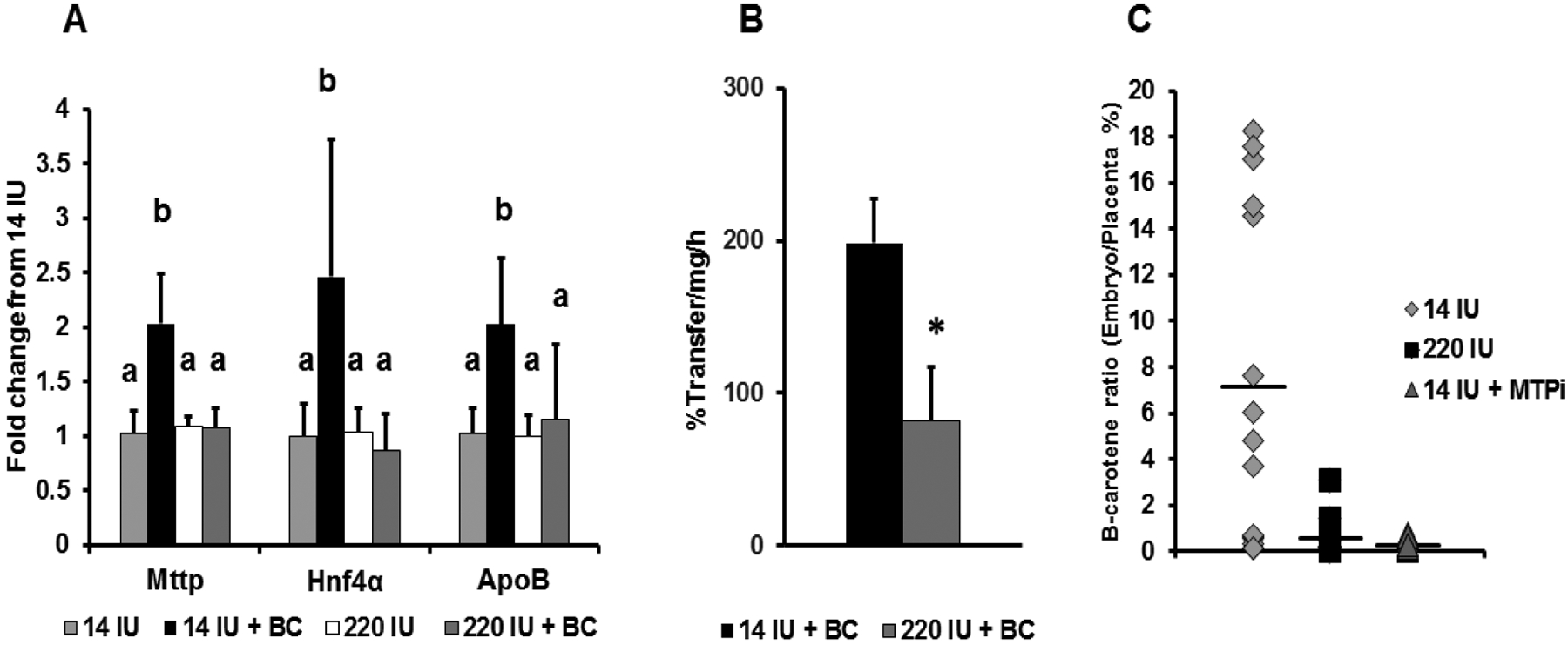
Tissues from wild-type dams on the vitamin A excess (220 IU of vitamin A/g diet) or sufficient (14 IU of vitamin A/g diet) diet during pregnancy with or without β-carotene (BC) supplementation by intraperiotenal injection (or vehicle, Veh) are from previously published studies from our laboratories [72,84]. (A) Placental qPCR analysis of Mttp, its transcriptional activator Hnf4a, and apoB in WT dams on a vitamin A sufficient or excess diet administered vehicle (Veh) or β-carotene at mid-gestation and sacrificed after 24 h. Data are presented as mean ± S.D. of duplicate determinations and are representative of three independent determinations. n= 1–2 placentas/dam from four to six wild-type dams per group. Statistical analysis by ANOVA. *, p < 0.05. Data for the vitamin A sufficient diet group where previously published (78). (B) Placental MTP activity expressed as percent transfer of lipids/mg/h (n = 1–3 placentas/dam from 3–4 β-carotene-treated dams). Dams as in panel A. (C) Ratio of β-carotene content in each placenta and its corresponding embryo expressed as percent. β-carotene was directly injected into the placentas of wild-type dams at 14.5 dpc, as previously described [84].. Data from dams on the vitamin A sufficient diet pretreated with the MTP inhibitor lomitapide (14 IU MTPi) or not (14 IU) were previously published [84].. β-carotene levels in placenta and embryos were measured by HPLC [80] Individual values are shown. n = 1–7 placentas/dam from 4 wild-type dams per group.
All together, these data suggest that under a condition of excessive maternal intake of vitamin A, and hence elevated tissue retinoid levels, β-carotene may suppress placental lipoprotein biosynthesis to ultimately protect the embryo from the potential harmful effects of vitamin A toxicity. Further studies are needed to unequivocally prove this model. Importantly, these studies revealed the discovery that a fundamental biological process such as lipoprotein biosynthesis is one of the targets of β-apocarotenoids action highlights the existence of a specific cross-talk between macro- and micro-nutrient metabolisms previously unforeseen. In addition, it is likely that similar mechanisms exist to regulate the assimilation of other essential micronutrients during development, such as, for example, lutein and zeaxanthin, non-provitamin A carotenoids that are emerging as important modulators of infant and child visual and cognitive development [90].
4. β-carotene metabolism in the developing tissues
Our laboratory first demonstrated that hat the maternal circulating intact β-carotene can serve as an alternative source of vitamin A for embryonic synthesis of retinoic acid [80]. Kim and colleagues generated a mutant mouse strain lacking Bco1 in the Rbp knockout genetic background, which is susceptible to diet-induced vitamin A deficiency [91]. The Bco1−/−Rbp−/− dams had severely malformed Bco1+/−Rbp−/− embryos (carrying one copy of the wild-type Bco1 allele). However, β-carotene supplementation rescued 61% of these offspring from developmental defects, since the embryos could cleave β-carotene via BCO1 [80]. These latter findings demonstrated in vivo the ability of intact β-carotene circulating in the maternal bloodstream to cross the placenta toward the fetus, as well as the ability of embryonic BCO1 to produce vitamin A from β-carotene in the developing tissues. This model also provided additional important insights into the role of BCO1 during embryogenesis. Interestingly, the deletion of Bco1 in the embryo worsened the severity of vitamin A deficiency and thus the embryonic malformations of the Rbp−/− mice. Indeed, in addition to the Rbp−/− like phenotype (i.e. eye malformations and peripheral edema), the double-knockout embryos (Bco1−/−Rbp−/−) from Bco1−/−Rbp−/− dams deprived of dietary vitamin A during pregnancy displayed cleft face and palate or exencephaly [80]. The severe congenital defects were accompanied by reduced levels of retinoids in the embryo, and were due to the lack of BCO1 in the developing tissues, rather than to the more severe maternal vitamin A-deficient status. This study also showed that BCO1 deficiency manifests itself in an autosomal dominant fashion, but with different degrees of penetrance depending upon the gene copy number. The reasons for this latter phenotype remain unclear to date. Kim and colleagues also demonstrated that, in addition to cleaving β-carotene, embryonic BCO1 also modulates the esterification of retinol, cholesterol and diacylglycerols, impacting not only retinoid but also lipid metabolism in the embryo [92, 93]. Although it has not been elucidated whether this is a carotenoid-independent action of BCO1, this work further supported the notion of a cross-talk between carotenoid and lipid metabolism [80, 94, 95]. Such cross-talk could be particularly relevant during embryogenesis, as lipids are critical regulators of proper development, especially of the central nervous system [96–98].
The asymmetric cleavage of β-carotene generates β-ionone and β-apo-10′-carotenal which can potentially be cleaved by BCO2, yielding retinaldehyde and its downstream retinoid derivatives, retinol and retinoic acid [11]. As mentioned earlier, the BCO2-mediated cleavage pathway is not considered a major source of retinoids, but rather a mechanism to reduce oxidative stress resulting from the toxic accumulation of carotenoids in mitochondria, at least in adult mammalian tissues [26]. Recently, though, the work of Spiegler and colleagues [99] shed light on the potential role of BCO2 and its apocarotenoid cleavage product(s) during mammalian embryogenesis. The authors demonstrated that, like in the case of BCO1, BCO2 deficiency exacerbated embryonic vitamin A deficiency. Indeed, the deletion of Bco2 on the vitamin A deficiency-susceptible Rbp−/− background reduced embryonic retinol levels, regardless of the vitamin A content of the diet. Moreover, the lack of Bco2 resulted in a more severe phenotype of the Bco2−/−Rbp−/− embryos, compared to the Rbp−/− embryos, when the dams were fed a vitamin A deficient diet throughout gestation. Interestingly, maternal β-carotene supplementation impaired fertility and did not restore normal embryonic development in the Bco2−/−Rbp−/− mice, despite the expression of BCO1, demonstrating that BCO2 prevents β-carotene toxicity during embryogenesis under severe vitamin A deficiency. Thus, the ability to scavenge β-carotene or its spontaneous oxidative cleavage products by BCO2 seems as important for embryonic development as the ability to cleave β-carotene by BCO1, at least when β-carotene is the only available vitamin A source. Likely, during vitamin A deficiency, when oxidative stress is already high [100, 101], the relative importance of BCO2 during embryogenesis may be greater than it is under more normal circumstances. The exact mechanisms whereby excessive carotenoids induce toxicity have not been clearly elucidated, although a potential role has been ascribed to carotenoid derivatives generated upon spontaneous carotenoid oxidation, at least in adult tissues [26]. Interestingly, a few earlier reports indicated that apocarotenoids can restore growth in vitamin A-deprived rats, chickens and quails [102–104], however, it remained unclear whether apocarotenoids performed this action per se or upon conversion to retinoids. In agreement with this notion, Spiegler and colleagues [99] showed that β-apo-10′-carotenal can restore normal development in this model of severe vitamin A deficiency in a dose-dependent manner. Their data suggested that β-apo-10′-carotenal is a potent retinoid precursor that strongly supports survival and development; even if it does not fully rule out that this metabolite might also have other retinoid-independent functions. Importantly, β-apocarotenoids are not solely generated upon enzymatic cleavage of β-carotene, but are also formed upon the spontaneous oxidation of carotenoids in mammalian tissues and in foods, where they can be relatively abundant [105–107]. β-apo-10′-carotenal, and presumably other β-apocarotenoids, cross the placental-fetal barrier [84]. Thus, the effects of these β-carotene metabolites from foods or other maternal tissues (e.g., liver) on mammalian embryogenesis might have been underestimated and need to be evaluated. Overall, the data from these studies pointed to an important role of BCO2 during embryogenesis and indicated that β-apo-10′-carotenal could be more effective than β-carotene in promoting normal development in a mouse model of embryonic vitamin A deficiency. Perhaps, considerations should be given to the possibility of including β-apo-10′-carotenal in nutritional interventions for vitamin A deficient pregnant women to support normal development of the fetus.
Clearly, these studies [80, 99] and other [81, 82] indicated that β-carotene can be used as a source of retinoids by the developing embryo under condition of vitamin A deficiency. What about the embryonic metabolism of the provitamin A carotenoid under sufficient or even excessive maternal intake of vitamin A? This question was addressed by Wassef and colleagues [72] who investigated retinoid and β-carotene metabolism in developing mouse tissues after a single β-carotene administration to pregnant wild-type mice fed purified diets with either sufficient or excess vitamin A concentrations. Interestingly, the high vitamin A maternal diet (which corresponded to elevated concentration of retinoids in the developing tissues) prevented embryonic accumulation of β-carotene and increased retinoic acid degradation via CYP26A1. However, based on the authors’ findings, it seemed unlikely that the low levels of β-carotene in the embryonic tissues were the result of enhanced β-carotene cleavage or attenuated β-carotene uptake by the embryo. Rather, the authors postulated a key regulatory role of the transfer of β-carotene from the placenta into the fetal-circulation via placental-synthesized lipoprotein. Such potential mechanism - discussed earlier - was indeed later described by Costabile and colleagues [84] and further corroborated by our recent results shown in Figure 4.
5. Future research and conclusions
The importance of proper vitamin A supply during pregnancy is well appreciated, but our understanding of maternal-fetal transfer of β-carotene, the most important dietary vitamin A precursor, is still very limited. Mouse models with conditional deletion of genes in different cells of the placenta or different organs/tissue of the developing embryo would be critical to unequivocally demonstrate the role of different enzymes and transporters. However, these studies are hindered by the lack of adequate information in regards to the cellular localization of the β-carotene cleavage enzymes, as well as of other key players of retinoid and lipid metabolism, within the placenta and the embryonic tissues/organs throughout gestation. Often, appropriate reagents, such as IHC-grade antibodies against BCO1 and BCO2, for example, are not available, representing another challenge to carry on these studies. Many fundamental questions remain to be answered. For example, in regards to the type of lipoprotein delivered to the placenta, it is not know what is the relative contribution of β-carotene from chylomicron remnants vs. other lipoproteins. If a lipoprotein-specific uptake exists at the placental barrier, perhaps it is also dependent on the maternal vitamin A status. Furthermore, our understanding of all the steps necessary for the developing tissues to acquire β-carotene and metabolize it into retinoids is not complete. For example, which lipoprotein receptors are mainly involved in the uptake of β-carotene at the maternal barrier? How does intact β-carotene cross the multicellular layers of the placenta before being secreted into the fetal circulation? How do these particles are cleared from the fetal circulation to supply the embryonic tissues? In this respect, it would be important to decipher the role played by the fetal liver vs, the other developing organs in acquiring and utilizing β-carotene from these particles. Which dehydrogenase/reductase enables utilization of retinoid from β-carotene during embryogenesis? Comparative studies in humans are also needed and require reagents that are often unavailable or not validated. Identifying the role of specific β-carotene metabolites that are transported across placenta and/or utilized by the fetus, and unraveling the cross-talk between micro- and macro-nutrients that govern the maternal-fetal transport of the most abundant dietary vitamin A precursor, have the potential to result in novel appropriate dietary practice and/or interventions during pregnancy. Understanding these biological processes will ultimately help reducing the risks of birth defects or of long-term diseases of the offspring linked to an abnormal maternal intake of micronutrients.
Highlights.
Beta-carotene can serve as a “local” source of retinoids for the developing tissues.
The maternal-fetal transfer of beta-carotene is mediated by lipoproteins.
Beta-carotene metabolites regulate the placental-fetal transfer of beta-carotene with a feed-forward mechanism.
Placental microsomal triglyceride transfer protein (MTP) is the downstream target of the transcriptional regulation by retinoic acid and beta-apo10-carotenoids.
The maternal vitamin A status influences the efficiency of transfer of intact beta-carotene and its utilization by the embryo.
BCO1 and BCO2 contribute to embryonic development under conditions of maternal vitamin A deficiency.
Acknowledgements
The work form the author’s laboratory presented in this review was supported by grants from the U.S. National Institute of Health (NIH): R01HD057493, R01HD057493-02S1 and R01HD083331 to LQ; and R01HL137202, R01DK121490, and VA Merit Award BX4113 to MMH. The grant NIH R01HD094778 to LQ and MMH supported the previously unpublished data described here and the writing of this review. The work from the lab of LQ described in this review was also supported by USDA National Institute of Food and Agriculture, Hatch project, accession number 1018402. YKK was partially supported by grant NIH R01EY027405.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Hashimoto H, Uragami C, Cogdell RJ, Carotenoids and Photosynthesis, Subcell. Biochem 79 (2016) 111–139. [DOI] [PubMed] [Google Scholar]
- [2].Harrison EH, Quadro L, Apocarotenoids: Emerging Roles in Mammals, Annu. Rev. Nutr 38 (2018) 153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moran NE, Mohn ES, Hason N, Erdman JW Jr., Johnson EJ, Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids, Adv. Nutr 9 (2018) 465–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kopsell DA, Kopsell DE, Carotenoids in Vegetables: Biosynthesis, Occurrence, Impacts on Human Health, and Potential for Manipulation, in: Watson RR, Preedy VR (Eds.) Bioactive Foods in Promoting Health Fruits and Vegetables, Elsevier, Inc., Place Published, 2010, pp. 645–662. [Google Scholar]
- [5].Bohm F, Edge R, Truscott TG, Interactions of dietary carotenoids with singlet oxygen and free radicals: potential effects for human health, Acta Biochim. Pol 59 (2012) 27–30. [PubMed] [Google Scholar]
- [6].Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK, β-carotene is an important vitamin A source for humans, J. Nutr 140 (2010) 2268S–2285S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].The Biochemistry of Retinoid Signaling II, Asson-Batres MA, Rochette-Egly C, Eds., Springer, Dordrecht, 2016. [Google Scholar]
- [8].Spiegler E, Kim YK, Wassef L, Shete V, Quadro L, Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues, Biochim. Biophys. Acta, 1821 (2012) 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weber D, Grune T, The contribution of β-carotene to vitamin A supply of humans, Mol. Nutr. Food Res 56 (2012) 251–258. [DOI] [PubMed] [Google Scholar]
- [10].West CE, Meeting requirements for vitamin A, Nutr. Rev 58 (2000) 341–345. [DOI] [PubMed] [Google Scholar]
- [11].von Lintig J, Provitamin A metabolism and functions in mammalian biology, Am. J. Clin. Nutr 96 (2012) 1234S–1244S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iskakova M, Karbyshev M, Piskunov A, Rochette-Egly C, Nuclear and extranuclear effects of vitamin A, Can. J. Physiol. Pharmacol 93 (2015) 1065–1075. [DOI] [PubMed] [Google Scholar]
- [13].Isoherranen N, Zhong G, Biochemical and physiological importance of the CYP26 retinoic acid hydroxylases, Pharmacol. Ther (2019) 107400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kedishvili NY, Retinoic Acid Synthesis and Degradation, Subcell. Biochem 81 (2016) 127–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blaner WS, Li Y, Brun PJ, Yuen JJ, Lee SA, Clugston RD, Vitamin A Absorption, Storage and Mobilization, Subcell. Biochem 81 (2016) 95–125. [DOI] [PubMed] [Google Scholar]
- [16].Dela Sena C, Sun J, Narayanasamy S, Riedl KM, Yuan Y, Curley RW Jr., Schwartz SJ, Harrison EH, Substrate Specificity of Purified Recombinant Chicken β-Carotene 9’,10’-Oxygenase (BCO2), J. Biol. Chem 291 (2016) 14609–14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kiefer C, Sumser E, Wernet MF, Von Lintig J, A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila, Proc. Natl. Acad. Sci. USA, 99 (2002) 10581–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schaub P, Wust F, Koschmieder J, Yu Q, Virk P, Tohme J, Beyer P, Nonenzymatic β-Carotene Degradation in Provitamin A-Biofortified Crop Plants, J. Agric. Food Chem 65 (2017) 6588–6598. [DOI] [PubMed] [Google Scholar]
- [19].Wang XD, Russell RM, Liu C, Stickel F, Smith DE, Krinsky NI, Beta-oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from beta-apocarotenoic acids, J. Biol. Chem 271 (1996) 26490–26498. [PubMed] [Google Scholar]
- [20].Krinsky NI, Wang XD, Tang G, Russell RM, Mechanism of carotenoid cleavage to retinoids, Ann. N Y. Acad. Sci 691 (1993) 167–176. [DOI] [PubMed] [Google Scholar]
- [21].Amengual J, Widjaja-Adhi MA, Rodriguez-Santiago S, Hessel S, Golczak M, Palczewski K, von Lintig J, Two carotenoid oxygenases contribute to mammalian provitamin A metabolism, J. Biol. Chem 288 (2013) 34081–34096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sporn MB, Dunlop NM, Newton DL, Smith JM, Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids), Fed. Proc 35 (1976) 1332–1338. [PubMed] [Google Scholar]
- [23].Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A, CMO1 Deficiency Abolishes Vitamin A Production from β-Carotene and Alters Lipid Metabolism in Mice, J. Biol. Chem 282 (2007) 33553–33561. [DOI] [PubMed] [Google Scholar]
- [24].Palczewski G, Amengual J, Hoppel CL, von Lintig J, Evidence for compartmentalization of mammalian carotenoid metabolism, FASEB J. 28 (2014) 4457–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kelly ME, Ramkumar S, Sun W, Colon Ortiz C, Kiser PD, Golczak M, von Lintig J, The Biochemical Basis of Vitamin A Production from the Asymmetric Carotenoid β-Cryptoxanthin, ACS Chem. Biol 13 (2018) 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J, A mitochondrial enzyme degrades carotenoids and protects against oxidative stress, FASEB J. 25 (2011) 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J, Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX, J. Biol. Chem 288 (2013) 9017–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J, ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β,β-carotene absorption and vitamin A production, FASEB J. 24 (2010) 1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Widjaja-Adhi MA, Lobo GP, Golczak M, Von Lintig J, A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption, Hum. Mol. Genet, 24 (2015) 3206–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Widjaja-Adhi MAK, Palczewski G, Dale K, Knauss EA, Kelly ME, Golczak M, Levine AD, von Lintig J, Transcription factor ISX mediates the cross talk between diet and immunity, Proc. Natl. Acad. Sci. U. S. A, 114 (2017) 11530–11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H, Class B scavenger receptor-mediated intestinal absorption of dietary β-carotene and cholesterol, Biochemistry, 44 (2005) 4517–4525. [DOI] [PubMed] [Google Scholar]
- [32].Borel P, Lietz G, Goncalves A, Szabo de Edelenyi F, Lecompte S, Curtis P, Goumidi L, Caslake MJ, Miles EA, Packard C, Calder PC, Mathers JC, Minihane AM, Tourniaire F, Kesse-Guyot E, Galan P, Hercberg S, Breidenassel C, Gonzalez Gross M, Moussa M, Meirhaeghe A, Reboul E, CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans, J. Nutr 143 (2013) 448–456. [DOI] [PubMed] [Google Scholar]
- [33].Borel P, Desmarchelier C, Bioavailability of Fat-Soluble Vitamins and Phytochemicals in Humans: Effects of Genetic Variation, Annu. Rev. Nutr 38 (2018) 69–96. [DOI] [PubMed] [Google Scholar]
- [34].Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, Erdman JW Jr., Review of animal models in carotenoid research, J. Nutr 129 (1999) 2271–2277. [DOI] [PubMed] [Google Scholar]
- [35].Borel P, Desmarchelier C, Nowicki M, Bott R, A Combination of Single-Nucleotide Polymorphisms Is Associated with Interindividual Variability in Dietary β-Carotene Bioavailability in Healthy Men, J. Nutr 145 (2015) 1740–1747. [DOI] [PubMed] [Google Scholar]
- [36].Bohn T, Provitamin A Carotenoids: Occurrence, Intake and Bioavailability, Vitamin A and Carotenoids: Chemistry, Analysis, Function and Effects, Royal Society of Chemistry (2012) 142–161. [Google Scholar]
- [37].Biesalski HK, Nohr D, The Importance of β-Carotene in the Context of Vitamin A, Vitamin A and Carotenoids: Chemistry, Analysis, Function and Effects, Royal Society of Chemistry, (2012) 39–54. [Google Scholar]
- [38].van Bennekum AM, Fisher EA, Blaner WS, Harrison EH, Hydrolysis of retinyl esters by pancreatic triglyceride lipase, Biochemistry, 39 (2000) 4900–4906. [DOI] [PubMed] [Google Scholar]
- [39].Reboul E, Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins, Nutrients, 5 (2013) 3563–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Reboul E, Berton A, Moussa M, Kreuzer C, Crenon I, Borel P, Pancreatic lipase and pancreatic lipase-related protein 2, but not pancreatic lipase-related protein 1, hydrolyze retinyl palmitate in physiological conditions, Biochim. Biophys. Acta 1761 (2006) 4–10. [DOI] [PubMed] [Google Scholar]
- [41].Hussain MM, Glick JM, Rothblat GH, In vitro model systems: Cell cultures used in lipid and lipoprotein research, Curr. Opin. Lipidol 33 (1992) 173–178 [Google Scholar]
- [42].Nayak N, Harrison EH, Hussain MM, Retinyl ester secretion by intestinal cells: a specific and regulated process dependent on assembly and secretion of chylomicrons, J. Lipid Res 42 (2001) 272–280. [PubMed] [Google Scholar]
- [43].Quick TC, Ong DE, Vitamin A metabolism in the human intestinal Caco-2 cell line, Biochemistry. 29 (1990) 11116–11123. [DOI] [PubMed] [Google Scholar]
- [44].During A, Hussain MM, Morel DW, Harrison EH, Carotenoid uptake and secretion by CaCo-2 cells: β-carotene isomer selectivity and carotenoid interactions, J. Lipid Res 43 (2002) 1086–1095. [DOI] [PubMed] [Google Scholar]
- [45].Buttet M, Traynard V, Tran TT, Besnard P, Poirier H, Niot I, From fatty-acid sensing to chylomicron synthesis: role of intestinal lipid-binding proteins, Biochimie, 96 (2014) 37–47. [DOI] [PubMed] [Google Scholar]
- [46].During A, Dawson HD, Harrison EH, Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe, J. Nutr 135 (2005) 2305–2312. [DOI] [PubMed] [Google Scholar]
- [47].O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS, Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT), J. Biol. Chem 280 (2005) 35647–35657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cheng D, Iqbal J, Devenny J, Chu CH, Chen L, Dong J, Seethala R, Keim WJ, Azzara AV, Lawrence RM, Pelleymounter MA, Hussain MM, Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption, J. Biol. Chem 283 (2008) 29802–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wongsiriroj N, Jiang H, Piantedosi R, Yang KJ, Kluwe J, Schwabe RF, Ginsberg H, Goldberg IJ, Blaner WS, Genetic dissection of retinoid esterification and accumulation in the liver and adipose tissue, J. Lipid Res 55 (2014) 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].O’Byrne SM, Blaner WS, Retinol and retinyl esters: biochemistry and physiology, J. Lipid Res 547 (2013) 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hussain MM, Intestinal lipid absorption and lipoprotein formation, Curr. Opin. Lipidol 25 (2014) 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sirwi A, Hussain MM, Lipid transfer proteins in the assembly of apoB-containing lipoproteins, J. Lipid Res 59 (2018) 1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Iqbal J, Walsh MT, Hammad SM, Cuchel M, Tarugi P, Hegele RA, Davidson NO, Rader DJ, Klein RL, Hussain MM, Microsomal Triglyceride Transfer Protein Transfers and Determines Plasma Concentrations of Ceramide and Sphingomyelin but Not Glycosylceramide, J. Biol. Chem 290 (2015) 25863–25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hussain MM, Iqbal J, Anwar K, Rava P, Dai K, Microsomal triglyceride transfer protein: a multifunctional protein, Front. Biosci 8 (2003): s500–s506. [DOI] [PubMed] [Google Scholar]
- [55].Walsh MT, Hussain MM, Targeting microsomal triglyceride transfer protein and lipoprotein assembly to treat homozygous familial hypercholesterolemia, Crit. Rev. Clin. Lab Sci 54 (2017) 26–48. [DOI] [PubMed] [Google Scholar]
- [56].Hussain MM, Nijstad N, Franceschini L, Regulation of microsomal triglyceride transfer protein, Clin. Lipidol 6 (2011) 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bakillah A, Nayak N, Saxena U, Medford RM, Hussain MM, Decreased secretion of ApoB follows inhibition of ApoB-MTP binding by a novel antagonist, Biochemistry, 39 (2000) 4892–4899. [DOI] [PubMed] [Google Scholar]
- [58].Rader DJ, Brewer HB Jr., Aβlipoproteinemia. New insights into lipoprotein assembly and vitamin E metabolism from a rare genetic disease, JAMA, 270 (1993) 865–869. [DOI] [PubMed] [Google Scholar]
- [59].Ferreira F, Patel V, Matts S, A successful spontaneous pregnancy in aβlipoproteinemia: Amsterdam or the art of vitamin replacement? BMJ Case Rep. (2014) bcr2014206754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hussain MM, Kedees MH, Singh K, Athar H, Jamali NZ, Signposts in the assembly of chylomicrons, Front. Biosc 6 (2001) D320–331. [DOI] [PubMed] [Google Scholar]
- [61].Bohn T, Desmarchelier C, El SN, Keijer J, van Schothorst E, Ruhl R, Borel P, β-Carotene in the human body: metabolic bioactivation pathways - from digestion to tissue distribution and excretion, Proc. Nutr. Soc 78 (2019) 68–87. [DOI] [PubMed] [Google Scholar]
- [62].Johnson EJ, Russell RM, Distribution of orally administered β-carotene among lipoproteins in healthy men, Am. J. Clin. Nutr 56 (1992) 128–135. [DOI] [PubMed] [Google Scholar]
- [63].Traber MG, Diamond SR, Lane JC, Brody RI, Kayden HJ, β-Carotene transport in human lipoproteins. Comparisons with a-tocopherol, Lipids, 29 (1994) 665–669. [DOI] [PubMed] [Google Scholar]
- [64].Blaner WS, Obunike JC, Kurlandsky SB, Al-Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ, Lipoprotein lipase hydrolysis of retinyl esters, J. Biol. Chem 269 (1994) 16559–16565. [PubMed] [Google Scholar]
- [65].Ishibashi S, Perrey S, Chen Z, Osuga J, Shimada M, Ohashi K, Harada K, Yazaki Y, Yamada N, Role of the low density lipoprotein (LDL) receptor pathway in the metabolism of chylomicron remnants. A quantitative study in knockout mice lacking the LDL receptor, apolipoprotein E, or both, J. Biol. Chem 271 (1996) 22422–22427. [DOI] [PubMed] [Google Scholar]
- [66].D’Ambrosio DN, Clugston RD, Blaner WS, Vitamin A metabolism: an update, Nutrients, 3 (2011) 63–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shete V, Costabile BK, Kim YK, Quadro L, Low-Density Lipoprotein Receptor Contributes to β-Carotene Uptake in the Maternal Liver, Nutrients, 812 (2016) 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Erdman JW Jr., Bierer TL, and Gugger ET, Absorption and transport of carotenoids, Ann. N.Y. Acad. Sci 691 (1993) 76–85. [DOI] [PubMed] [Google Scholar]
- [69].Parker RS, Absorption, metabolism, and transport of carotenoids, FASEB J. 10 (1996) 542–551. [PubMed] [Google Scholar]
- [70].Bjornson LK, Kayden HJ, Miller E, Moshell AN, The transport of alpha-tocopherol and β-carotene in human blood, J. Lipid Res 17 (1976) 343–352. [PubMed] [Google Scholar]
- [71].van Vliet T, van Vlissingen MF, van Schaik F, van den Berg H, β-Carotene absorption and cleavage in rats is affected by the vitamin A concentration of the diet, J. Nutr 126 (1996) 499–508. [DOI] [PubMed] [Google Scholar]
- [72].Wassef L, Shete V, Rodas CB,R, Quadro L, High preformed vitamin A intake during pregnancy prevents embryonic accumulation of intact β-carotene from the maternal circulation in mice, J. Nutr 145 (2015) 1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Clagett-Dame M, Knutson D, Vitamin A in reproduction and development, Nutrients, 3 (2011) 385–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cindrova-Davies T, Jauniaux E, Elliot MG, Gong S, Burton GJ, Charnock-Jones DS, RNA-seq reveals conservation of function among the yolk sacs of human, mouse, and chicken, Proc. Natl. Acad. Sci. U S A, 114 (2017) E4753–E4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME, Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein, EMBO J. 17 (1999) 4633–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kamper M, Mittermayer F, Cabuk R, Gelles K, Ellinger I, Hermann M, Estrogen-enhanced apical and basolateral secretion of apolipoprotein B-100 by polarized trophoblast-derived BeWo cells, Biochimie, 138 (2017) 116–123. [DOI] [PubMed] [Google Scholar]
- [77].Kamper M, Manns CC, Plieschnig JA, Schneider WJ, Ivessa NE, Hermann M, Estrogen enhances secretion of apolipoprotein B-100 containing lipoproteins by BeWo cells, Biochimie, 112 (2015) 121–128. [DOI] [PubMed] [Google Scholar]
- [78].Wittmaack FM, Gafvels ME, Bronner M, Matsuo H, McCrae KR, Tomaszewski JE, Robinson SL, Strickland DK, Strauss JF 3rd, Localization and regulation of the human very low density lipoprotein/apolipoprotein-E receptor: trophoblast expression predicts a role for the receptor in placental lipid transport, Endocrinology, 136 (1995) 340–348. [DOI] [PubMed] [Google Scholar]
- [79].Kallol S, Huang X, Muller S, Ontsouka CE, Albrecht C, Novel Insights into Concepts and Directionality of Maternal(−)Fetal Cholesterol Transfer across the Human Placenta, Int. J. Mol. Sci 198 (2018) 2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L, β-Carotene and its cleavage enzyme β-carotene-15,15`-oxygenase (CMOI) affect retinoid metabolism in developing tissues, FASEB J. 25 (2011) 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wassef L, Shete V, Hong A, Spiegler E, Quadro L, β-Carotene supplementation decreases placental transcription of LDL receptor-related protein 1 in wild-type mice and stimulates placental β-carotene uptake in marginally vitamin A-deficient mice, J. Nutr 142 (2012) 1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wassef L, Spiegler E, Quadro L, Embryonic phenotype, β-carotene and retinoid metabolism upon maternal supplementation of β-carotene in a mouse model of severe vitamin A deficiency, Arch. Biochem. Biophys 539 (2013) 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dimenstein R, Trugo NM, Donangelo CM, Trugo LC, Anastacio AS, Effect of subadquate maternal vitamin A status on placental transfer of retinol and b-carotene to the human fetus, Biol. Neonate, 69 (1996) 230–234. [DOI] [PubMed] [Google Scholar]
- [84].Costabile BK, Kim YK, Iqbal J, Zuccaro MV, Wassef L, Narayanasamy S, Curley RW Jr., Harrison EH, Hussain MM, Quadro L, β-Apo-10’-carotenoids Modulate Placental Microsomal Triglyceride Transfer Protein Expression and Function to Optimize Transport of Intact β-Carotene to the Embryo, J. Biol. Chem 291 (2016) 18525–18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Eroglu A, Hruszkewycz DP, Dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW Jr., Harrison EH, Naturally Occurring Eccentric Cleavage Products of Provitamin A β-Carotene Function as Antagonists of Retinoic Acid Receptors, J. Biol. Chem 287 (2012) 15886–15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sun J, Narayanasamy S, Curley RW Jr., Harrison EH, β-Apo-13-carotenone regulates retinoid X receptor transcriptional activity through tetramerization of the receptor, J. Biol. Chem 289 (2014) 33118–33124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Eroglu A, Hruszkewycz DP, Curley RW Jr., Harrison EH, The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRalpha, Arch. Biochem. Biophys 504 (2010) 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dai K, Hussain MM, NR2F1 disrupts synergistic activation of the MTTP gene transcription by HNF-4alpha and HNF-1alpha, J. Lipid Res 53 (2012) 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L, β-Carotene and its cleavage enzyme β-carotene-15,15`-oxygenase (CMOI) affect retinoid metabolism in developing tissues, FASEB J. 255 (2011) 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Giordano E, Quadro L, Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health, Arch. Biochem. Biophys 647 (2018) 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL, Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency, Endocrinology, 146 (2005) 4479–4490. [DOI] [PubMed] [Google Scholar]
- [92].Kim YK, Zuccaro MV, Costabile BK, Rodas R, Quadro L, Tissue- and sex-specific effects of β-carotene 15,15’ oxygenase (BCO1) on retinoid and lipid metabolism in adult and developing mice, Arch. Biochem. Biophys 572 (2015) 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dixon JL, Kim YK, Brinker A, Quadro L, Loss of β-carotene 15,15’-oxygenase in developing mouse tissues alters esterification of retinol, cholesterol and diacylglycerols, Biochim. Biophy.s Acta, 1841 (2014) 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lee SA, Jiang H, Trent CM, Yuen JJ, Narayanasamy S, Curley RW Jr., Harrison EH, Goldberg IJ, Maurer MS, Blaner WS, Cardiac dysfunction in β-carotene-15,15’-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism, Am. J. Physiol. Heart Circ. Physiol 307 (2014) H1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Palczewski G, Widjaja-Adhi MA, Amengual J, Golczak M, von Lintig J, Genetic dissection in a mouse model reveals interactions between carotenoids and lipid metabolism, J. Lipid Res 57 (2016) 1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Haas D, Muenke M, Abnormal sterol metabolism in holoprosencephaly, Am. J. Med. Genet. C. Semin. Med. Genet 154C (2010) 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Farese RV Jr., Cases S, Ruland SL, Kayden HJ, Wong JS, Young SG, Hamilton RL, A novel function for apolipoprotein B: lipoprotein synthesis in the yolk sac is critical for maternal-fetal lipid transport in mice, J. Lipid Res 37 (1996) 347–360. [PubMed] [Google Scholar]
- [98].Farese RV Jr., Ruland SL, Flynn LM, Stokowski RP, Young SG, Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes, Proc. Natl. Acad. Sci. U. S. A 92 (1995) 1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Spiegler E, Kim YK, Hoyos B, Narayanasamy S, Jiang H, Savio N, Curley RW Jr., Harrison EH, Hammerling U, Quadro L, β-apo-10’-carotenoids support normal embryonic development during vitamin A deficiency, Sci. Rep 8 (2018) 8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Arruda SF, Siqueira EM, de Valencia FF, Vitamin A deficiency increases hepcidin expression and oxidative stress in rat, Nutrition, 25 (2009) 472–478. [DOI] [PubMed] [Google Scholar]
- [101].Sohlenius-Sternbeck AK, Appelkvist EL, DePierre JW, Effects of vitamin A deficiency on selected xenobiotic-metabolizing enzymes and defenses against oxidative stress in mouse liver, Biochem. Pharmacol 59 (2000) 377–383. [DOI] [PubMed] [Google Scholar]
- [102].Sharma RV, Mathur SN, Ganguly J, Studies on the relative biopotencies and intestinal absorption of different apo-β-carotenoids in rats and chickens, Biochem. J 158 (1976) 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Al-Hasani SM, Vitamin A activity of β-apo-carotenals in Coturnix coturnix japonica, J. Nutr 94 (1968) 402–406. [DOI] [PubMed] [Google Scholar]
- [104].al-Hasani SM, Parrish DB, Forms of vitamin A and of carotenoids in tissues, blood serum and yolk of eggs from Cotournix coturnix japonica fed -apo-carotenals, J. Nutr 102 (1972) 1437–1440. [DOI] [PubMed] [Google Scholar]
- [105].Fleshman MK, Lester GE, Riedl KM, Kopec RE, Narayanasamy S, Curley RW Jr., Schwartz SJ, Harrison EH, Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo melons: determinations of β-carotene bioaccessibility and bioavailability, J. Agric. Food Chem 59 (2011) 4448–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kopec RE, Riedl KM, Harrison EH, Curley RW Jr., Hruszkewycz DP, Clinton SK, Schwartz SJ, Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma, J. Agric. Food Chem 58 (2010) 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Shmarakov I, Fleshman MK, D’Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW Jr., von Lintig J, Rubin LP, Harrison EH, Blaner WS, Hepatic stellate cells are an important cellular site for β-carotene conversion to retinoid, Arch. Biochem. Biophys 504 (2010) 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


