Abstract
Heart failure (HF) is a quintessential geriatric cardiovascular condition, with more than 50% of hospitalizations occurring in adults age 75 years or older. In older patients, HF is closely linked to processes inherent to aging, which include cellular and structural changes to the myocardium, vasculature, and skeletal muscle. In addition, HF cannot be considered in isolation of physical functioning, or without the social, psychological, and behavioral dimensions of illness. The role of frailty, depression, cognitive impairment, nutrition, and goals of care are each uniquely relevant to the implementation and success of medical therapy. In this paper, we discuss a model of caring for older adults with HF through a 4-domain framework that can address the unique multidimensional needs and vulnerabilities of this population. We believe that clinicians who embrace this approach can improve health outcomes for older adults with HF.
Keywords: domain management, geriatric patient, heart failure
Heart failure (HF) is predominantly a condition of aging, doubling in prevalence from 6% in those age 60 to 79 years to approximately 14% in those age ≥80 years; the mean age of adults with HF exceeds 70 years (1). Annual rates of the initial episode of acute decompensated HF per 1,000 person-years more than triples between the 55 to 64 years and ≥75 years age groups, regardless of sex and race (1). The high prevalence and incidence of HF likely relates to a constellation of HF risk factors, such as coronary artery disease and hypertension, whose prevalence rise with age, as well as age-related maladaptive changes that directly affect the cardiovascular system. HF rarely occurs in isolation and represents just 1 maladaptive process that affects older adults. The complex interplay of medical factors, physiological aging, cognitive and physical function, and social and environmental context all contribute to health outcomes of older adults with HF independent of ejection fraction. The intricacy of multiple factors at work underscores the importance of a comprehensive and multidimensional assessment when considering the best approach to HF management in this population.
The biopsychosocial construct of disease management provides a solid framework to approach caring for a complex older adult with HF. In Dr. George Engel’s 1977 description of the biopsychosocial construct, “The Need for a New Medical Model: A Challenge for Biomedicine,” he argued that the medical domain, as measured by biological variables, was the dominant model of disease; this approach, he believed, was overly reductionist (2). The necessary context is provided when one considers the social, psychological, and behavioral dimensions of illness; this biopsychosocial model of disease, proposed nearly 50 years ago, can provide a holistic and multidimensional approach to caring for older HF patients. Furthermore, the relevance of using this biopsychosocial model for approaching a complex geriatric population with HF is supported by recent data. This review presents evidence supporting a domain management approach to assessing older adults with HF, our effort to formalize Engel’s philosophy of clinical care to a practical contemporary framework, and highlights areas for future work. The approach includes a 4-dimensional framework: medical, mind and emotions, physical function, and social environment (Central Illustration). Clinical tools (Table 1, Online Appendix) and management strategies are proposed to aid clinicians in the management of the vulnerable and complex older HF population.
CENTRAL ILLUSTRATION. Domain Management Approach to HF in the Geriatric Patient.
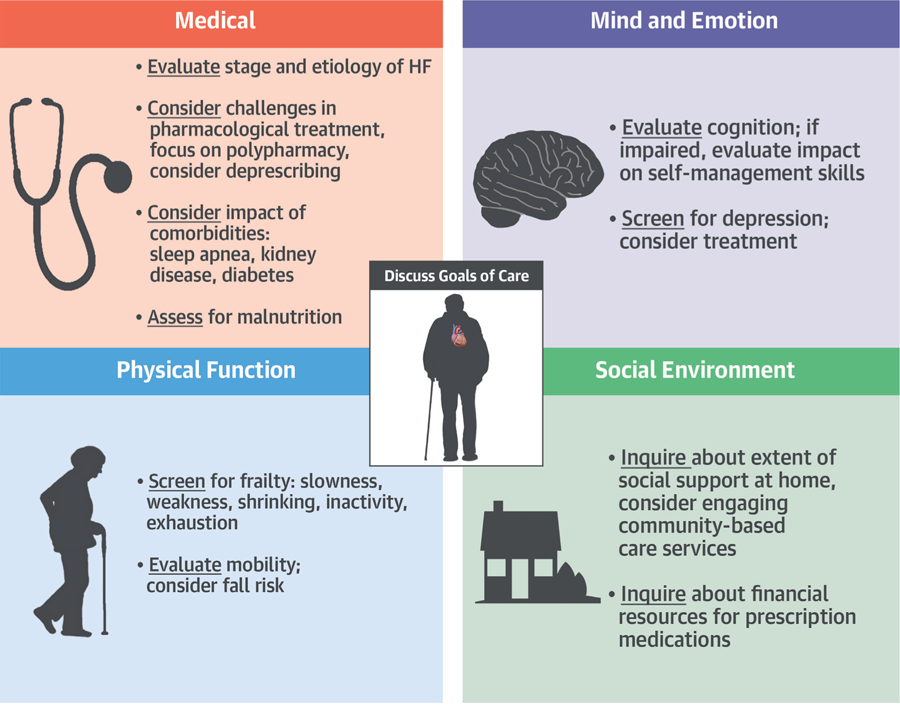
Clinicians caring for older adults with HF should consider using this 4-domain framework in their routine clinical work. When utilized, this holistic model can address the unique multidimensional needs and vulnerabilities of this population. HF = heart failure.
TABLE 1.
Checklist for Care of the Geriatric Patient With HF
| Medical Issues | Mind and Emotions |
|---|---|
| Standard HF evaluation Comorbidity screen Medication intake/reconciliation Nutritional assessment (MNA SF) |
The Mini-Cog PHQ-2 |
| Physical Function | Social Environment |
| ADLs/IADLs Gait speed over 5 meters Timed Up and Go test FRAIL questionnaire |
Social support Adaptable/safe environment plan Access to nutrition Access to transportation Medication management |
ADL = activity of daily living; FRAIL = Fatigue, Resistance, Ambulation, Illnesses, and Loss of Weight; HF = heart failure; IADL = instrumental activity of daily living; MNA SF = Mini Nutritional Assessment short-form; PHQ-2 = first 2 questions of the Patient Health Questionnaire.
MEDICAL DOMAIN
The medical domain encompasses conditions and syndromes frequently encountered in older adults with HF. In addition to evaluation of the etiology, stage, and chronicity of HF, clinicians caring for older adults with HF must consider multimorbidity, polypharmacy, and nutritional status. These conditions are not only common, but essential aspects of HF in older patients with important implications on management and prognosis.
MULTIMORBIDITY.
Multimorbidity is defined as the concurrence of multiple chronic conditions (3), and is common among older adults with HF; 90% have at least 3 comorbid conditions and 50% have at least 5 comorbid conditions (4). For heart failure with preserved ejection fraction (HFpEF), comorbid conditions, including lung disease, diabetes, obesity, and chronic kidney disease, have been implicated in its pathophysiology through the promotion of inflammation and microvascular dysfunction (5). Although not directly related to its pathophysiology per se, age-related structural changes in other organ systems and comorbid conditions are also common in heart failure with reduced ejection fraction (HFrEF), with important implications on prognosis (6). Both hospitalizations and mortality are frequently driven by noncardiovascular causes in both HFpEF and HFrEF (7). Consequently, to optimize HF management, the effect of therapeutic strategies on the management of concurrent conditions (and vice versa) must be considered irrespective of ejection fraction. For example, renal insufficiency, which influences drug clearance, and conditions like chronic obstructive pulmonary disease, which may have drug–disease interactions and can lead to therapeutic competition, have important implications in HF management. Managing HF in the context of multimorbidity is further complicated by the gap in knowledge that has resulted from the exclusion of older adults with multimorbidity from major HF randomized controlled trials (8,9). Given the observation that comorbidity burden is strongly associated with adverse outcomes, it is critically important to consider the potential effect of multimorbidity on prognosis when making management decisions, as the risks of such therapeutic interventions (medications and procedures) may be immediate and significant, whereas potential benefits, usually realized over years, may be attenuated, especially in the setting of a limited life expectancy.
POLYPHARMACY.
Among older adults with HF, there often exists a tension among the benefits of guideline-directed medical therapy, adherence to therapy, and polypharmacy risks. Polypharmacy is defined as the use of ≥5 medications, and is nearly universal in patients with HF, partially as a consequence of guideline-based care, and partially because of multimorbidity (10). Even among those with a low comorbidity burden, up to 6 medications are currently recommended for HF alone (11). Despite the fact that the majority of clinical trials have excluded older adults (8), guidelines regarding medications have recommended their use, regardless of age or comorbidity (12). Older adults with HF are often prescribed more than 10 medications, and some are even prescribed as many as 20 (10,13,14). Studies across a variety of conditions have demonstrated that polypharmacy is associated with a myriad of adverse outcomes including falls (15), disability (16), and hospitalization (17,18). As the number of medications an individual takes increases, the risk for adverse events rises exponentially (17); this results from drug–drug interactions (warfarin and aspirin), drug–disease interactions (nonsteroidal anti-inflammatory drugs in HF), and drug–person interactions (digoxin use in older adults). Older adults with HF are at high risk for these interactions, due to common age-related processes that alter pharmacokinetics and pharmacodynamics, changes in cardiovascular structure and function (19), and the coexistence of geriatric conditions such as frailty and cognitive impairment.
Consequently, it is important to identify the presence of polypharmacy and consider its potentially detrimental effects when deciding to initiate or up-titrate medications in older adults with HF. Medication reconciliation is an important first step in optimizing prescribing practices, which may include the discontinuation of agents with limited benefit and/or potential for harm. Tools like the medication appropriateness index (20) and the STOPP/START (Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions/Screening Tool to Alert Doctors to the Right Treatment) criteria (21) may be helpful to identify potentially inappropriate medications that may be discontinued. Pharmacist-based interventions have also demonstrated some promise for identifying and eliminating agents with minimal benefit and risk for harm.
For scenarios in which pharmacological agents commonly used in HF have side-effect profiles that negatively affect quality of life, it may be reasonable to consider deprescribing such agents after explicitly discussing the risks and benefits of such an approach. Deprescribing has been defined as the process of drug discontinuation under supervision of a health care professional, with the goals of reducing undesirable effects, managing polypharmacy, and/or improving outcomes (22,23). In a study of older adults with a broad range of diagnoses, approximately 40% desired to stop 1 or more of their medications (24), suggesting that patients may be amenable to (or even prefer) deprescribing in some circumstances. To date, data on the deprescribing of commonly-used agents like beta-blockers and renin-angiotensin-aldosterone system inhibitors in HF have been limited to small observational studies. Moreover, these studies have specifically examined deprescribing in the setting of improved left ventricular function in individuals with prior moderate-to-severe HF (25) and among individuals with stable chronic HF (26). Given the observation among these studies that discontinuing agents that provide neurohormonal inhibition may lead to worsening HF symptoms and/or deterioration in left ventricular function, there is a need for more rigorous evaluation of deprescribing, especially in the setting of geriatric conditions like multimorbidity, frailty, cognitive decline, and polypharmacy, which are likely to affect the risk-benefit ratio of such a practice. There is also a need to explore whether patients with HFpEF would benefit from the deprescribing of agents such as neurohormonal inhibitors, including beta-blockers, which have yet to consistently demonstrate a positive effect on outcomes. For now, until more robust data is available, decisions on deprescribing should be driven by patients’ preferences and clinicians’ judgment (23).
BODY COMPOSITION AND NUTRITION.
Obesity in middle age is a strong risk factor for developing HF as an older adult (27), and weight loss during middle age protects against incident HF (28). Nevertheless, in older patients with HF, weight loss is associated with increased long-term mortality (29,30), whereas obesity appears to be protective of mortality (31); the responsible mechanisms for this “obesity paradox” have not yet been fully defined, but explain why current guidelines carry no definitive recommendation for weight loss (or maintenance) in older patients with comorbid obesity and HF. Several recent studies suggest that weight loss through bariatric surgery (32) or hypocaloric diet (33) can benefit highly selected patients with HF. Body composition and nutritional status are understudied, but are likely important mediating factors of the benefits and risks of weight loss in HF.
The development of HF in older adults is associated with more rapid loss of lean body mass and skeletal muscle than typical age-related decrements (34). Cachexia, defined as body weight loss of >5% to 10% over 12 months in conjunction with findings such as decreased muscle strength, fatigue, poor appetite, and/or disproportionate loss of lean body mass, is a particularly worrisome sign in HF, with a 1-year mortality of nearly 40% (35). The mechanisms of cardiac cachexia are often attributed to chronic inflammation, and cachectic patients with HF also have evidence of increased neurohormonal activity (36). Few interventions targeted at cachexia have been studied in patients with HF, although protein supplementation may hold promise (37). More common than overt cachexia in older patients with HF is sarcopenic obesity, defined using dual-energy x-ray absorptiometry as the combination of skeletal muscle mass 2 or more SDs below the mean of young, healthy sex-matched individuals and fat mass >60% of age- and sex-matched norms (38). Sarcopenic obesity is associated with incident disability in older adults (38), and may contribute directly to impairments in oxygen utilization and to exercise limitations (39).
Although not extensively studied, the available information indicates that poor nutritional status is associated with sarcopenia independent of HF severity (40). Depending on which population is studied and which measure is used, the prevalence of malnutrition in HF is at least 15%, and is up to 90% in patients with advanced HF. No single method is universally accepted to assess nutritional status in HF. The Mini Nutritional Assessment (MNA) provides a good balance between ease of administration and assessment of multiple domains in older patients. The short-form MNA (6 questions; 0 to 14 points) assigns scores to decreased food intake, weight loss, mobility, disease acuity, cognitive or psychological state, and body mass index; patients are assigned into categories of normal nutrition, at-risk of malnutrition, and malnourished. In at-risk or malnourished patients, additional information on food intake, living environment, perceived nutritional and health status, and anthropometric measures can clarify the diagnosis. This information is prognostically important; in hospitalized patients with HF, malnourished status by the MNA quadruples the long-term risk of death (41).
Dietary counseling.
Although HF dietary recommendations have traditionally focused on sodium restriction, this strategy may not always be beneficial. In addition to the potential for excess neurohormonal activation (42), studies to date demonstrate that advice to reduce sodium intake can be associated with parallel decreases in overall calorie and micronutrient intake (43). Perhaps relatedly, several studies suggest that a low-sodium diet intervention can worsen clinical outcomes in patients with HF, including hospitalization and mortality (44–46). The ongoing SODIUM-HF (Study of Dietary Intervention Under 100 Mmol in Heart Failure) trial will provide additional guidance on appropriate sodium intake; however, data from the study pilot indicate that dietary counseling for patients with HF should broadly cover topics beyond sodium (47). The DASH (Dietary Approaches to Stop Hypertension) and Mediterranean eating patterns provide sufficient protein and micronutrient intake, therefore representing reasonable choices (48). Counseling should incorporate family or other caregivers and should consider patient-specific barriers to healthy eating habits. In addition to reduced smell and taste sensation commonly experienced by older adults, patients with HF may eat poorly due to depression, anxiety, and symptoms of HF such as dyspnea or nausea (49). Moreover, patients with HF frequently have additional dietary restrictions posed by medications (e.g., warfarin) or comorbid conditions such as diabetes mellitus and chronic kidney disease. All of these aspects require careful consideration when optimizing nutritional status, and ongoing consultation with a dietitian is often appropriate.
The value of individualized nutritional counseling in patients with HF was recently demonstrated in the PICNIC (Nutritional Intervention Program in Hospitalized Patients with Heart Failure who are Malnourished) study (50). In PICNIC, 120 patients hospitalized with HF who were malnourished according to the MNA were randomly assigned to usual care or a 6-month comprehensive nutritional support strategy. The intervention was delivered by a physician-led dietitian team and provided patient-specific recommendations accounting for comorbid conditions and symptom-related or logistical challenges. Compared with the usual care group, the PICNIC study intervention group patients had markedly lower 1-year mortality (20% vs. 48%; hazard ratio: 0.37; 95% confidence interval: 0.19 to 0.72; p = 0.003) and readmission for HF (10% vs. 36%; hazard ratio: 0.21; 95% confidence interval: 0.09 to 0.52; p = 0.001).
MIND AND EMOTION DOMAIN
This domain encompasses the intersection between cognition and emotional brain function with the ability to manage HF. Although cognitive impairment and/or depression may be subtle, failure to recognize these conditions may lead to downstream impairments in communication, as well as self-care. Accordingly, cognitive impairment and depression represent important conditions to consider among older adults with HF.
COGNITIVE IMPAIRMENT.
The prevalence of cognitive impairment in HF is approximately 40% (51), in contrast to the general population where prevalence estimates range from 16% to 20% (52). Older adults with HF may be at risk not only for age-related cognitive decline, including Alzheimer’s disease and other etiologies of dementia, but also for HF-related cognitive impairment (recently termed “cardio cerebral syndrome”) (53). The most common domain abnormalities in HF, in decreasing order, are learning and memory, executive function, and complex attention (54). Cognitive impairment may range in severity from mild cognitive impairment (objective memory impairment, but preserved function in daily life), to dementia (representing a significant decline from prior level of function), and to more severe dementia (marked by significant impairment that interferes with activities of daily living). Potential mechanisms for cognitive impairment are complex, and may include reduced cardiac output; a high burden of cardiovascular risk factors; and the involvement of other neurohormonal, nutritional, and inflammatory mechanisms (53). Sleep apnea, which is highly coexistent with HF, is associated with progressive memory loss and executive dysfunction (55). Worsened volume status is also associated with worsened executive function and memory that improves, but does not normalize, after better compensation of HF status (56).
Cognitive impairment in patients with HF has been linked to poor outcomes: worse health-related quality of life, increased spousal/caregiver distress, increased disability, increased hospital readmission risk, worse cardiovascular outcomes at 180 days, and increased mortality risk (57). Because patients with HF and cognitive impairment experience greater difficulty with self-care (58), identification of cognitive impairment through screening may help clinicians with decision making about recommending community-based services. Clinicians who care for older adults with HF should integrate screening for cognitive impairment into their routine workflow (59,60). Screening cognitive tools can be administered by trained nonphysicians. HF guidelines recommend screening for cognitive impairment, but do not identify how. Because clinicians are usually pressed for time, the Mini-Cog, which is an ultrashort cognitive “vital signs” measure, should be considered for screening. The Mini-Cog is a clock drawing and word recall test, which takes (on average) 3 min to complete. Mini-Cog use has been validated in a variety of populations of older adults with HF (54,61). Alternate tools such as the Montreal Cognitive Assessment can be used (57), but take longer to deploy. If cognitive dysfunction is identified, then steps should be taken to investigate and treat underlying causes. Consideration should be given for referral to a memory specialist (e.g., neurologist or geriatrician), as well as neuropsychological testing and brain imaging for more definitive diagnoses. Patients with cognitive impairment are more likely to make medication errors, so steps should be taken to simplify the medication regimen and engage the patient’s social support structure to help with this component of care.
Treatment of concurrent cognitive impairment is challenging. Studies have shown that decompensated HF and volume overload are associated with declines in memory and executive function, and that optimizing HF may help (56). Treating sleep apnea, which is associated with volume loss in the hippocampus and frontal lobes, has also been shown to improve short-term memory and executive function (62).
DELIRIUM.
Delirium, defined as an acute disturbance in attention and awareness that develops over a short period of time, is highly prevalent among older adults with acute HF. Approximately 15% of older adults presenting to the emergency department for management of acute HF have delirium (63), with approximately 17% to 23% experiencing delirium during the hospitalization (64,65). Those with delirium are more likely to have dementia and functional dependence. Their outcomes are poor, with increased risk for placement in a nursing home after discharge, hospital readmission, in-patient mortality, and 30-day post-discharge mortality (63–65). Management of delirium in older adults with acute HF in the hospital setting may be challenging, but its cornerstone is a multicomponent nonpharmacological approach that includes reorientation, early mobilization, therapeutic activities, nutrition, sleep strategies, and hearing and vision adaptations (66).
DEPRESSION.
Depression is highly prevalent among patients with HF. A recent meta-analysis of more than 80,000 HF patients in a variety of settings reported a depression prevalence of approximately 29% (67). The specific prevalence of depression among older adults with HF is less well described. In 1 study of older patients with HF managed in primary care settings, the prevalence of major depression and/or dysthymia was 9%, and the prevalence of minor depression was 10% (68). When assessing cognition, it is important to assess for depression as well, given their shared common features. The Patient Health Questionnaire (PHQ) is a validated 9-item instrument that can be used to assess for depressive symptoms (69). Asking the first 2 questions of the PHQ (PHQ-2) offers a convenient screen for depressive symptoms, and the negative predictive value is 99% for those who answer no to both questions (70). An alternative that may be more appropriate for geriatric populations is the 15-item Geriatric Depression Scale (71), which has been tested and used extensively in older adults.
The hippocampus, which is a set of bilateral components of the limbic system in the center of the brain, is thought to be the center for emotion, memory, and the autonomic nervous system. In older adults with dementia who do not have HF, abnormalities in the hippocampus are associated with depressive symptoms and memory impairment (72). The hippocampus is vulnerable to cerebral hypoxia, and may be affected by poor cerebral blood flow that can occur in HF. Brain imaging studies to assess cerebral blood flow have demonstrated that patients with HF who have poor flow to the hippocampus do worse on memory testing and have a higher rate of depression (73). Therefore, cognitive impairment and depression in older adults with HF may be manifestations of similar underlying processes (74,75). These observations require further investigation.
Depression is associated with poor outcomes in patients with HF, including impaired self-care, diminished quality of life, and increased use of health care resources. These patients additionally have an increased risk of hospitalizations, and an approximate 40% increased risk-adjusted hazard of all-cause mortality (67,76). Management of depression in older adults with HF is challenging. Symptoms of HF and depression may overlap, and it may be difficult for clinicians to tease out which to treat, and to what degree these symptoms should be remedied. Interventions for depression that have shown benefit in randomized clinical trials in this population include exercise (77) and cognitive behavioral therapy (78). Several randomized clinical trials of selective serotonin reuptake inhibitors in mostly older adults with HF failed to show a benefit in either depression or other clinical outcomes (79,80), suggesting differing pathophysiological mechanisms for mood disorders in patients with HF compared with those without. Reduced cerebral blood flow to the hypothalamus, as discussed in the previous text, may play a role. Increasing cerebral blood flow using a mechanical cardiac support agent, such as continuous-flow left ventricular assist device (LVAD) therapy, may lead to improvements in both depression and anxiety (81).
PHYSICAL FUNCTION DOMAIN
The physical function domain encompasses a person’s ability to perform a variety of physical activities of increasing complexity, ranging from basic (bathing and dressing) to advanced (preparing meals, managing medications, and driving), and includes the risk for falls. Loss of aerobic function, strength, and balance are a continuum that includes frailty. Clinicians caring for older adults with HF should know their patients’ baseline functional status, as any change from baseline is important when evaluating new symptoms and changes in condition.
PHYSICAL FUNCTIONING AND FRAILTY.
Functional decline is common in this population, and may be related to aging, progression of HF, or both. Aerobic decline is a hallmark of HF (independent of frailty) with increased dyspnea and fatigability, but assessments of strength and balance are also important considerations, especially because they often determine the capacity for aerobic activities in patients who are severely weakened (82). Frailty, a biological syndrome reflecting impaired physiological reserve and heightened vulnerability to stressors (83), is also extremely common in older adults with HF (83–85). Both HF and frailty, as well as associated conditions of cachexia and sarcopenia, share common biological mechanisms (86). The up-regulation of inflammatory biomarkers lead to increased levels of hormones, such as cortisol and growth hormone, which contribute to downstream effects leading to a catabolic state and muscle wasting (83). Development of HF is associated with an increased loss of lean muscle mass, with abnormalities similar to sarcopenia of aging (87,88). Among 2,815 participants in the Health, Aging, and Body Composition study (mean age 73.6 ± 2.9 years), 111 developed incident HF over the 6-year study period. Upon entry into the study, higher body mass predicted development of HF; however, loss of lean body mass was greater after developing HF (34). Patients with HFpEF who reported greater levels of physical activity had lower risks of hospitalization and mortality in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) trial (89). Skeletal muscle abnormalities are common in patients with HF, with or without systolic dysfunction, and play a large role in exercise intolerance (90). Inactivity is in part responsible, leading to muscle atrophy. In addition, skeletal muscle utilizes high-energy phosphates in an inefficient manner; as a result, lactic acid accumulates more rapidly, contributing to limited exercise capacity. Skeletal muscle dysfunction can also involve respiratory muscles, which contributes to fatigue and dyspnea on exertion. The importance of skeletal muscle dysfunction provides part of the rationale for using cardiac rehabilitation in patients with HF. Therefore, HFpEF and HFrEF are inextricably connected to physical function, frailty, and body composition in ways that are just beginning to be appreciated.
Frailty in HF populations is not related to age or functional class (91). In a recent meta-analysis of 26 studies of frailty in HF patients, the prevalence of frailty varied by how it was measured: slightly higher with multidimensional frailty measures (47%), and slightly lower for physical frailty measures (43%). Multiple approaches for assessment of frailty in cardiovascular disease have been proposed, but a universally accepted definition is lacking (92,93). Frailty can be assessed by gait speed <0.8 m/s captured over 5 m. Cutpoints for gait speed vary in different populations, and are likely lower in older, frail patients with HF. Other assessments of physical performance include short physical performance battery, grip strength, chair stands, and the Timed Up and Go Test (85,94). As assessed by the Fried frailty criteria, frailty is associated with increased risk of a number of adverse outcomes including mortality (95), functional decline (95), and hospitalization (95–97). More refined approaches to frailty assessment may be needed in populations with advanced HF, where frailty is essentially universal. For example, in a recent study of patients with advanced HF undergoing implantation of an LVAD, the standard Fried frailty criteria failed to discriminate between those at highest risk for inpatient mortality or prolonged hospital length of stay (98). Nevertheless, a small study found frailty improved in nearly one-half of older adults with advanced HF 6 months after LVAD placement (99).
One of the more promising interventions in HF is exercise training. The HF-ACTION (HF: A Controlled Trial Investigating Outcomes of Exercise Training) study showed a reduction in the adjusted risk for the combined endpoint of all-cause mortality or hospitalization. Quality of life and mental depression also improved and were sustained over time (77,100,101). Although vastly underutilized, cardiac rehabilitation is currently approved for HFrEF, and offers an excellent venue to preserve and improve physical function. Nonetheless, applying cardiac rehabilitation to older adults is often limited by issues of access and the specific needs of vulnerable or frail patients (102). Although options for home care and telehealth are often touted for their potential to reach older patients at home, their safety and efficacy in frail HF patients remains to be proven.
Exercise has demonstrated benefit in HFpEF, as well; in a recent randomized controlled trial of 100 obese patients with HFpEF, aerobic exercise training improved exercise capacity, which is a major correlate of health-related quality of life in this population (33). Exercise training is particularly well-suited to treat HFpEF given its anti-inflammatory effects; its positive effect on the myocardium, vascular function, and skeletal muscle (103); and its ability to mitigate the consequences of frailty (104). Given the promising findings to date, the first randomized trial of a physical function intervention in older patients hospitalized with decompensated HF is underway. The REHAB-HF (Trial of Rehabilitation Therapy in Older Acute HF Patients) is designed to determine if addressing deficits in balance, mobility, strength, and endurance improves physical function and reduces rehospitalizations. REHAB-HF will address key evidence gaps concerning the role of physical rehabilitation in the care of older acute HF patients with both HFrEF and HFpEF (105).
FALLS AND SYNCOPE.
Older adults with HF represent an especially high-risk population for falls, given a constellation of risk factors including age, frailty, and cognitive impairment. In addition, age-related changes in cardiovascular structure and function predispose older adults with HF to falls. Attenuated baroreceptor and autonomic reflexes, impaired adrenergic responsiveness, and impaired maintenance of intravascular volume related to decreased salt/water handling increase the risk of syncope (106). Multimorbidity and polypharmacy, which are nearly universal in HF, represent 2 additional risk factors for falls (107–109). The prevalence of falls specifically among older adults with HF has been shown to be as high as 43% over a 2-year period (110,111). Given the observation that the risk for falls rises as the number of risk factors accumulates (112), older adults with HF likely represent a population whose risk for falls is matched by few other disease states. Falls are a common cause of health care utilization among older adults, and are associated with significant costs and substantial morbidity and mortality (109,113). This high risk for falls makes social support and safe environments very important. In a recently published meta-analysis examining interventions for preventing falls in older adults, a combination of exercise, vision assessment, and vision treatment was the intervention most associated with reduction in injurious falls in this population (114).
SOCIAL ENVIRONMENT DOMAIN
The social environment domain encompasses a variety of factors affecting an individual’s life outside of the clinic or hospital setting. Clinicians caring for older adults with HF benefit from insights into patients’ physical and social living environments, including the extent of family support, as well as an understanding of the financial barriers (health insurance, ability to afford medications, and so on) patients may have. A unique challenge in this population is social isolation. Insights about psychological and financial burdens faced by the patient’s caregivers are important. Overburdened caregivers face burnout and risk of personal harm.
SOCIAL ISOLATION.
Social isolation occurs when an individual’s social network size is reduced, resulting in a paucity of social contacts (115). Social isolation is common in older adults due to increased disability, reduced economic resources, loss of spouses and contemporaries, and dispersion of family. This type of isolation is closely related to loneliness (116), which is the psychological embodiment of social isolation. Social isolation is associated with increased incidence of chronic and infectious illnesses, cognitive and functional decline, worse health-related quality of life, and increased mortality risk (115,117). These findings have been described in both hospitalized and ambulatory patients with HF (118–120). In particular, living with HF is characterized by feelings of powerlessness, hopelessness, and social dysfunction (121). Older patients with HF perceive their disease as debilitating, progressively compromising their functional status, and often disrupting social functioning leading to social isolation and loneliness (121). Although the mechanisms linking social isolation and poor outcomes are not entirely clear, they may be related to a heightened inflammatory state, as patients with social isolation have elevated C-reactive protein, fibrinogen, and systemic blood pressure (115).
Identification of social isolation is important because interventions are possible. Engagement of the patient’s existing social circle or family, if existent, is critically important. Other approaches that may ease the burden of social isolation are utilization of informal caregivers, community lay workers, or volunteers, as well as transitional care models for those patients being discharged after hospitalization. Referral for group activities with an educational or support input (122) has shown some benefit.
SOCIAL SUPPORT AND BURDEN ON CAREGIVERS.
The rigors of HF management are often overwhelming to patients of any age, but may be especially so for older adults. Social support, often provided on a daily basis from a spouse, partner, extended family, or friends, is a critical component of success for most patients. Being married has been associated with improved outcomes in patients with HF and ischemic heart disease (123,124). Spouses of patients with HF are on average healthier than those of patients with fewer chronic conditions and better mobility (125), but are still often older (mean age 71 years) and have their own medical conditions (125). Clinicians caring for older adults with HF should not only proactively identify and communicate with the key persons supporting the patient, but also seek a basic understanding of the patient’s caregivers’ health and functional status.
Clinicians should recognize that caregiving for older adults with HF often comes at a cost. Caregiving for patients with chronic illnesses is associated with worse psychological and physical health (126), including an increased risk of mortality for the caregiver (127). Identification of caregivers who are experiencing higher strain and burnout may be important, because this has been associated with greater patient symptoms, lower patient quality of life, and higher patient clinical event risk in those with HF (128).
LOSS OF INDEPENDENCE.
With increasing age, the ability of individuals to live independently diminishes, and even those living in their own home may begin to lose the ability to manage aspects of their care due to physical, cognitive, or social limitations. Cardiovascular clinicians may be the first to recognize that a patient with HF is not managing his or her complex medication regimen, or weighing themselves daily and adjusting diuretic doses accordingly, which may reveal cognitive impairment. Due to a loss of social contacts and loneliness, older adults may prefer to move to a setting with other seniors and activities (129). Other factors that can necessitate a move include physical inability to manage stairs or care for a large home, or the death or illness of a partner who managed the cooking and shopping. Functional decline following acute illness often results in transfer to post-acute skilled nursing facilities, and failure to recover to baseline may ultimately require use of paid caregivers upon return home, or a transition to an alternative living arrangement (130).
Some individuals are able to remain in their own home with family caregivers (often adult children) or paid care providers. People requiring ongoing skilled care may live in nursing homes long-term, and those who are unable to transfer independently may be limited to intermediate-care nursing facilities or adult foster homes. Assisted living facilities require the individual to be ambulatory (i.e., to be able to exit the facility on their own in event of an emergency).
Living situations can pose challenges with self-management. For example, access to low- or no-added sodium diets may not be possible, many individuals will not perform daily weights, and self-titration of diuretics may be overly burdensome. Medicare home health services to assess cardiopulmonary status and titrate medications may be ordered for people in assisted living facilities, adult foster homes, or their own homes.
INTEGRATION WITH GOALS OF CARE
Evaluating older adults with HF using a 4-domain framework that includes medical, mind, physical function, and social environment provides the nuanced and holistic understanding of older adults with HF that is necessary to optimize care of this vulnerable population. Because patient preferences and priorities may have implications on decision making, findings from this 4-domain framework assessment should be integrated with a goals of care discussion.
GOALS OF CARE DISCUSSION.
Goals of care and advance care planning are complicated for patients with HF who are living longer, are experiencing more comorbidities, and are faced with decisions about complex therapies and interventions. Even compensated advanced HF treated with guideline-directed therapies can be characterized by unpredictable patterns of decompensation and improvement, making it difficult for providers to prognosticate and for patients to weigh the risks and benefits of interventions (131,132). Older adults with multimorbidity often experience a decline in functional status, cognitive impairment, depression, increased frailty, reduced quality of life, and frequent hospitalizations (5,133–135). When asked, HF patients often do not identify HF as their main health problem (136,137). This difficulty in prognostication, variability in symptom course, and increasing array of therapeutic and diagnostic interventions underscores the importance of having goals of care discussions with all older adults with HF. Initiation of a goals of care discussion with patients and families may be aided by utilizing a value-based approach, where medical management decisions are viewed as value propositions (health outcome/cost) (138). Goals of care discussions need not be exhaustive or complicated, but may involve simply asking patients to reflect on what is of value to them and how decisions about therapies interface with the bigger picture of their goals and preferences. Several organizations now advocate for early goals of care discussions, which are vital to facilitating shared decision making in HF (139).
PALLIATIVE CARE.
Published HF guidelines (140) offer a Class I, Level of Evidence: B recommendation that palliative and supportive care is effective for patients with symptomatic advanced HF to improve quality of life. The guideline document states that palliative care should address symptom control, psychosocial distress, health-related quality of life, preferences about end-of-life care, caregiver support, and assurance of access to evidence-based disease-modifying interventions. Palliative care embraces a domain management framework. Although classically considered as a strategy to optimize the management of symptoms, palliative care also involves advance care planning, guided discussion of care goals, promoting an understanding of disease progression, and provision of information about hospice, when appropriate (141). Although there remains a historical bias that palliative care is applicable only in the terminal phase of life when survival is no longer the goal, the goals of palliative care are applicable across the disease spectrum starting as early as the time of diagnosis.
Several studies have demonstrated that palliative care services have generated improvements in symptoms and class of HF, as well as spiritual wellbeing and social support. Palliative care services also ameliorate anxiety, depression, and social isolation (141–144). Despite these benefits in all 4 domains, palliative care services remain underused in the management of older adults with HF (144,145).
PRACTICAL CONSIDERATIONS
Even among patients with similar HF phenotypes, important differences across the 4-domain framework could have important implications for management and, therefore, should be incorporated into the routine care of older adults with HF. Table 2 depicts 2 patients with ischemic cardiomyopathy and symptomatic HFrEF, with key differences in multimorbidity and polypharmacy, cognitive impairment and depression, frailty, and social support. The prognosis of these 2 patients, as well as the risk-benefit ratio for various therapeutic strategies, are likely to differ, underscoring the relevance of the 4-domain framework. The following practical approach applies and incorporates the 4-domain framework into routine management of older adults with HF.
TABLE 2.
Domain Management Approach and Cardiac Decision-Making
| An 82-year-old woman with a distant history of myocardial infarction is referred to your outpatient clinic several months after a first hospitalization for acute decompensated HF. In the hospital, she was diagnosed with an ischemic cardiomyopathy and HFrEF. Her LV ejection fraction is 30%. She has been experiencing fatigue for the past several years. On examination, she appears euvolemic. Her current medication regimen includes metoprolol XL 100 mg daily, lisinopril 5 mg daily, and spironolactone 25 mg daily. She does not have a defibrillator. How should she be treated next? | |
| Patient A | Patient B |
 |
 |
| Medical/Surgical Domain: In addition to cardiac history noted above, patient has diabetes, hypertension, and stage 2 CKD. Her weight has been stable over the last year with normal appetite. | Medical/Surgical Domain: In addition to cardiac history noted above, patient has diabetes, hypertension, stage 3 CKD, COPD, peptic ulcer disease, and a history of stroke with minimal residual neurological deficits. She has lost 10 pounds over the last year and has poor appetite. |
| Mental Status/Emotions/Coping Domain: No history of depression. The Mini-Cog is normal. | Mental Status/Emotions/Coping Domain: Reports feeling sad and lonely most days of the week. The Mini-Cog is positive suggesting cognitive impairment. |
| Physical Function Domain: She is able to get dressed on her own with some dyspnea. Her measured gait speed in your clinic is 1.1 m/s. She has a firm handshake. | Physical Function Domain: She is able to get dressed on her own with some dyspnea. Her measured gait speed in your clinic is 0.6 m/s. She has a weak handshake, and reports fatigue with limitations in taking the stairs and walking. |
| Living Environment Domain: Living alone in own home and manages her own medications. Adult children live in same city within driving distance. | Living Environment Domain: Living in own home. Her daughter sets up medications for her. Adult children live in same city within driving distance. |
| Considerations: Focus on optimizing ACC/AHA/HFSA guideline-directed medical therapy, including increasing ACEI or switching from ACEI to ARNI. Consider a “trial” of reduced dose of BB in the setting of excessive fatigue. Consider more intensive screening for depression as a further contributor. Spironolactone was introduced before ACEI has been maximized, adding to polypharmacy and risk of hyperkalemia, and requiring more frequent laboratory monitoring. Consider temporarily eliminating spironolactone and reintroducing it once ACEI or ARNI have been maximized if patient remains symptomatic. Discuss preferences for care when her heart or breathing stop and if she would want an attempt at resuscitation. Discuss whether she is willing to have an implantable device, then refer for ICD evaluation for prevention of sudden cardiac death. Discuss decision-making and identify preferred surrogate decision-maker. Encourage patient to fill out advance directive forms, including health care power of attorney and living will. | Considerations: Patient is frail, likely malnourished, and has cognitive impairment. Consider reducing dose of BB in setting of fatigue, and avoid hypotension that may exacerbate risk of falls at home. Spironolactone was introduced before ACEI has been maximized, adding to polypharmacy and risk of hyperkalemia, and requiring more frequent laboratory monitoring. Consider temporarily eliminating spironolactone and reintroducing it once ACEI has been maximized if patient remains symptomatic. Convene family meeting to discuss goals of care, and preferences regarding ICD implantation in setting of the patient’s guarded prognosis. Emphasize that cognitive impairment is associated with medication self-management errors, and family members should oversee distribution of medications into pill box. Consider referral for home care skilled nursing visit or house calls physician or advanced practice practitioner visit (team care), and consultation with a dietitian. See patient in clinic more frequently if possible, or via more frequent telephone or telemedicine/video connection. Encourage engagement of community family and friend network. Patient is not ready for hospice referral, but consider a 1-time outpatient consultation with a palliative medicine clinician for introductory purposes. |
ACC = American College of Cardiology; ACEI = angiotensin-converting enzyme inhibitor; AHA = American Heart Association; ARNI = angiotensin receptor neprilysin inhibitor; BB = beta-blocker; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HFSA = Heart Failure Society of America; ICD = implantable cardioverter-defibrillator; LV = left ventricular.
BE AWARE.
Given the high prevalence of multimorbidity, cognitive impairment, and frailty among older adults with HF, together with their potential effect on outcomes, clinicians should be aware of these conditions. Understanding the definitions of these conditions is important, as they are no longer exclusively found in the Geriatrics literature. As the population ages and the median age of patients with HF rises, these conditions are becoming increasingly recognized and discussed in the Cardiovascular literature. Consequently, any cardiovascular clinician caring for older adults with HF should be familiar with conditions such as frailty and cognitive impairment, even if they fall outside of the traditional cardiovascular scope of practice.
SCREEN.
Screening for geriatric conditions across the 4-domain framework is relatively simple and can be conveniently performed in either the outpatient or bedside inpatient setting. Screening can be done by physicians and advanced practice providers, or by nurses and medical assistants as part of a “team” effort. Table 1 provides a checklist that may be used to screen for conditions across the 4-domain framework. Tools such as the Mini-Cog and PHQ-2 (Online Appendix) are designed to serve as screening tools that can be performed quickly and conveniently to assess for conditions that may require further attention. A smartphone app called the “Frailty Tool,” featuring the Essential Frailty Toolset (146) derived from older adults with cardiovascular disease, is now available for both iPhone and Android devices. Although not well-studied specifically in HF, the routine use of such tools to screen for geriatric conditions, which can be subtle, are likely to be beneficial given their high prevalence and potential effect on outcomes.
REFER.
Older adults with HF frequently contend with a number of concurrent conditions across the 4-domain framework and, as a result, may benefit from a team-based approach to care. Referring patients who screen positive for conditions such as polypharmacy, nutritional deficiencies, depression, frailty, and/or limited social support can enhance the attention and care that vulnerable older adults often require. Incorporating pharmacists, dieticians, physical therapists, and/or social workers into the management of older adults with HF may be beneficial. Similarly, collaborating with primary care physicians, geriatricians, and/or neurologists to provide comprehensive care in a coordinated fashion that optimizes prioritized outcomes and minimizes competing recommendations can add value to care.
INCORPORATE INTO DECISION MAKING.
To appropriately and effectively apply clinical practice guidelines, it is important to appreciate when such guidelines may not be applicable, or when recommendations need to be individualized to best fit the clinical context. Assessing older adults with HF using the 4-domain framework can help identify patients who warrant the latter, due to limited prognosis and/or concurrent conditions that affect the risk-benefit ratio of guideline-concordant therapies. Similarly, the 4-domain framework can also identify those without major deficits who have longer life expectancy and may benefit from more intensive therapies. For example, decisions regarding advanced therapies, including the use of LVADs and cardiac transplantation, palliative inotropes, device implantation, and even medication management, would likely be better informed with the incorporation of the 4-domain framework, compared with decisions based on age alone. Goals of care discussions and efforts to better understand patient priorities, which in some cases may include quality of life over longevity, remain paramount for decision making in this population.
FUTURE DIRECTIONS
Clinical care of older HF patients based on the domain management model needs additional evidence and research related to implementation (147). The majority of recommendations are derived by experts, because few controlled trial data exist for this population—a fact that should be viewed as a challenge to investigators to pursue studies that will address these issues. Whereas much is known about the prevalence of multimorbidity among older adults with HF, less is known about the way multimorbidity may alter the utility of therapeutic strategies in HF. Deprescribing is an early concept, so more study is needed on attitudes toward deprescribing and its effect on outcomes to ensure it is patient-centered. Dietary interventions that can combat cachexia and malnutrition and assess the role of weight loss in older populations with HF are also needed, but cost, delivery, and cultural issues are important barriers. The prevalence of cognitive impairment and depression, and association with outcomes among older adults with HF are well-known, but strategies to prevent and/or treat these are limited. Future research would benefit from prospectively studying the effect of screening for these conditions and developing novel therapeutic interventions to reverse or mitigate their effect on outcomes. The development and/or implementation of physical activity interventions to prevent, reverse, and/or mitigate frailty remains a potentially important arena for future research. The benefit of cardiac rehabilitation services for older adults with acute HF in the ongoing REHAB-HF study will hopefully provide additional insights on the benefits of physical activity interventions. Further research is needed on interventions for social isolation and loneliness in older adults with HF where novel community-based programs or informal aides may improve outcomes for those patients who lack social support. Furthermore, more study is needed on novel technologies designed to help older adults live more safely at home, as well as connect to family members and health care providers.
CONCLUSIONS
Optimizing the care of older adults with HF is a challenging and important priority in the current health care system. The aging United States population, ongoing epidemic of chronic diseases with HF at the helm, and shifts in health care from volume to value suggest that care of this population by cardiologists and other cardiovascular providers will become increasingly commonplace. Cardiologists and other cardiovascular specialists are more frequently faced with the need to provide comprehensive care, which is slowly becoming an expectation as payment systems, publicly-available quality metrics, and patient and family expectations evolve. Consequently, there is an emerging need for cardiovascular providers to develop requisite knowledge and skills that fall outside of most providers’ comfort zone and the traditional paradigm of cardiovascular training. This domain management approach and future evidence along its domains can guide clinicians in a variety of health care settings as they care for this highly complex and vulnerable patient population.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Ms. Erin Campbell for her excellent editorial assistance.
Dr. Gorodeski is supported by The Hunnell Fund. Dr. Goyal is supported by National Institute on Aging grant R03AG056446. Dr. Hummel has received research funding from PurFoods, LLC. Dr. Hart has served on the Speakers Bureau of Zoll and Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LVAD
left ventricular assist device
- MNA
The Mini Nutritional Assessment
- PHQ
Patient Health Questionnaire
Footnotes
The views expressed in this paper by the American College of Cardiology’s (ACC’s) Geriatric Cardiology Member Section Council do not necessarily reflect the views of the Journal of the American College of Cardiology or the ACC.
APPENDIX For supplemental information, please see the online version of this paper.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196: 129–36. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain AM, St Sauver JL, Gerber Y, et al. Multimorbidity in heart failure: a community perspective. Am J Med 2015;128:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 5.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 6.Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014;64:2281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol 2017;70:2476–86. [DOI] [PubMed] [Google Scholar]
- 8.Cherubini A, Oristrell J, Pla X, et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med 2011;171:550–6. [DOI] [PubMed] [Google Scholar]
- 9.Arnett DK, Goodman RA, Halperin JL, Anderson JL, Parekh AK, Zoghbi WA. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and U.S. Department of Health and Human Services. J Am Coll Cardiol 2014;64: 1851–6. [DOI] [PubMed] [Google Scholar]
- 10.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011; 124:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70: 776–803. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 13.Dunlay SM, Eveleth JM, Shah ND, McNallan SM, Roger VL. Medication adherence among community-dwelling patients with heart failure. Mayo Clin Proc 2011;86:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gastelurrutia P, Benrimoj SI, Espejo J, Tuneu L, Mangues MA, Bayes-Genis A. Negative clinical outcomes associated with drug-related problems in heart failure (HF) outpatients: impact of a pharmacist in a multidisciplinary HF clinic. J Card Fail 2011;17:217–23. [DOI] [PubMed] [Google Scholar]
- 15.Freeland KN, Thompson AN, Zhao Y, Leal JE, Mauldin PD, Moran WP. Medication use and associated risk of falling in a geriatric outpatient population. Ann Pharmacother 2012;46:1188–92. [DOI] [PubMed] [Google Scholar]
- 16.Jyrkkä J, Enlund H, Lavikainen P, Sulkava R, Hartikainen S. Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf 2011;20: 514–22. [DOI] [PubMed] [Google Scholar]
- 17.Marcum ZA, Amuan ME, Hanlon JT, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc 2012;60:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res 2015;15:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai X, Hummel SL, Salazar JB, Taffet GE, Zieman S, Schwartz JB. Cardiovascular physiology in the older adults. J Geriatr Cardiol 2015;12: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samsa GP, Hanlon JT, Schmader KE, et al. A summated score for the medication appropriateness index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol 1994;47:891–6. [DOI] [PubMed] [Google Scholar]
- 21.O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44: 213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol 2015;80:1254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossello X, Pocock SJ, Julian DG. Long-term use of cardiovascular drugs: challenges for research and for patient care. J Am Coll Cardiol 2015;66:1273–85. [DOI] [PubMed] [Google Scholar]
- 24.Kalogianis MJ, Wimmer BC, Turner JP, et al. Are residents of aged care facilities willing to have their medications deprescribed? Res Social Adm Pharm 2016;12:784–8. [DOI] [PubMed] [Google Scholar]
- 25.Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail 2011;17:527–32. [DOI] [PubMed] [Google Scholar]
- 26.Hopper I, Samuel R, Hayward C, Tonkin A, Krum H. Can medications be safely withdrawn in patients with stable chronic heart failure? Systematic review and meta-analysis. J Card Fail 2014;20:522–32. [DOI] [PubMed] [Google Scholar]
- 27.Eaton CB, Pettinger M, Rossouw J, et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail 2016;9:e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundström J, Bruze G, Ottosson J, Marcus C, Näslund I, Neovius M. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation 2017;135:1577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossignol P, Masson S, Barlera S, et al. Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: insights from the GISSI-HF and Val-HeFT trials. Eur J Heart Fail 2015;17:424–33. [DOI] [PubMed] [Google Scholar]
- 30.Zamora E, Díez-López C, Lupón J, et al. Weight loss in obese patients with heart failure. J Am Heart Assoc 2016;5:e002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah R, Gayat E, Januzzi JL Jr., et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol 2014;63: 778–85. [DOI] [PubMed] [Google Scholar]
- 32.Shimada YJ, Tsugawa Y, Brown DF, Hasegawa K. Bariatric surgery and emergency department visits and hospitalizations for heart failure exacerbation: population-based, self-controlled series. J Am Coll Cardiol 2016;67: 895–903. [DOI] [PubMed] [Google Scholar]
- 33.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forman DE, Santanasto AJ, Boudreau R, et al. Impact of incident heart failure on body composition over time in the Health, Aging, and Body Composition study population. Circ Heart Fail 2017;10:e003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997;349:1050–3. [DOI] [PubMed] [Google Scholar]
- 36.Gaggin HK, Belcher AM, Gandhi PU, Ibrahim NE, Januzzi JL Jr. Serial echocardiographic characteristics, novel biomarkers and cachexia development in patients with stable chronic heart failure. J Cardiovasc Transl Res 2016;9: 429–31. [DOI] [PubMed] [Google Scholar]
- 37.Rozentryt P, von Haehling S, Lainscak M, et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle 2010;1:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12: 1995–2004. [DOI] [PubMed] [Google Scholar]
- 39.Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr Heart Fail Rep 2015;12:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitoh M, Dos Santos MR, Ebner N, et al. Nutritional status and its effects on muscle wasting in patients with chronic heart failure: insights from Studies Investigating Co-morbidities Aggravating Heart Failure. Wien Klin Wochenschr 2016;128:497–504. [DOI] [PubMed] [Google Scholar]
- 41.Lin H, Zhang H, Lin Z, Li X, Kong X, Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev 2016;21:549–65. [DOI] [PubMed] [Google Scholar]
- 42.Gupta D, Georgiopoulou VV, Kalogeropoulos AP, et al. Dietary sodium intake in heart failure. Circulation 2012;126:479–85. [DOI] [PubMed] [Google Scholar]
- 43.Jefferson K, Ahmed M, Choleva M, et al. Effect of a sodium-restricted diet on intake of other nutrients in heart failure: implications for research and clinical practice. J Card Fail 2015;21:959–62. [DOI] [PubMed] [Google Scholar]
- 44.Paterna S, Fasullo S, Parrinello G, et al. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am J Med Sci 2011;342:27–37. [DOI] [PubMed] [Google Scholar]
- 45.Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond) 2008;114:221–30. [DOI] [PubMed] [Google Scholar]
- 46.Doukky R, Avery E, Mangla A, et al. Impact of dietary sodium restriction on heart failure outcomes. J Am Coll Cardiol HF 2016;4:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colin-Ramirez E, McAlister FA, Zheng Y, Sharma S, Ezekowitz JA. Changes in dietary intake and nutritional status associated with a significant reduction in sodium intake in patients with heart failure. A sub-analysis of the SODIUM-HF pilot study. Clin Nutr ESPEN 2016;11:e26–32. [DOI] [PubMed] [Google Scholar]
- 48.Levitan EB, Lewis CE, Tinker LF, et al. Mediterranean and DASH diet scores and mortality in women with heart failure: The Women’s Health Initiative. Circ Heart Fail 2013;6:1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lennie TA, Moser DK, Heo S, Chung ML, Zambroski CH. Factors influencing food intake in patients with heart failure: a comparison with healthy elders. J Cardiovasc Nurs 2006;21:123–9. [DOI] [PubMed] [Google Scholar]
- 50.Bonilla-Palomas JL, Gámez-López AL, Castillo-Domínguez JC, et al. Nutritional intervention in malnourished hospitalized patients with heart failure. Arch Med Res 2016;47:535–40. [DOI] [PubMed] [Google Scholar]
- 51.Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail 2017;23: 464–75. [DOI] [PubMed] [Google Scholar]
- 52.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med 2013;29: 753–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Havakuk O, King KS, Grazette L, et al. Heart failure-induced brain injury. J Am Coll Cardiol 2017;69:1609–16. [DOI] [PubMed] [Google Scholar]
- 54.Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini-cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail 2015;8:8–16. [DOI] [PubMed] [Google Scholar]
- 55.Malhotra A, Orr JE, Owens RL. On the cutting edge of obstructive sleep apnoea: where next? Lancet Respir Med 2015;3:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kindermann I, Fischer D, Karbach J, et al. Cognitive function in patients with decompensated heart failure: the Cognitive Impairment in Heart Failure (CogImpair-HF) study. Eur J Heart Fail 2012;14:404–13. [DOI] [PubMed] [Google Scholar]
- 57.Yzeiraj E, Tam DM, Gorodeski EZ. Management of cognitive impairment in heart failure. Curr Treat Options Cardiovasc Med 2016;18:4. [DOI] [PubMed] [Google Scholar]
- 58.Currie K, Rideout A, Lindsay G, Harkness K. The association between mild cognitive impairment and self-care in adults with chronic heart failure: a systematic review and narrative synthesis. J Cardiovasc Nurs 2015;30:382–93. [DOI] [PubMed] [Google Scholar]
- 59.Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail 2015;8:384–409. [DOI] [PubMed] [Google Scholar]
- 60.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal KS, Kazim R, Xu J, Borson S, Taffet GE. Unrecognized cognitive impairment and its effect on heart failure readmissions of elderly adults. J Am Geriatr Soc 2016;64:2296–301. [DOI] [PubMed] [Google Scholar]
- 62.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med 2011;183: 1419–26. [DOI] [PubMed] [Google Scholar]
- 63.Rizzi MA, Torres Bonafonte OH, Alquezar A, et al. Prognostic value and risk factors of delirium in emergency patients with decompensated heart failure. J Am Med Dir Assoc 2015;16:799.e1–6. [DOI] [PubMed] [Google Scholar]
- 64.Uthamalingam S, Gurm GS, Daley M, Flynn J, Capodilupo R. Usefulness of acute delirium as a predictor of adverse outcomes in patients >65 years of age with acute decompensated heart failure. Am J Cardiol 2011;108:402–8. [DOI] [PubMed] [Google Scholar]
- 65.Honda S, Nagai T, Sugano Y, et al. Prevalence, determinants, and prognostic significance of delirium in patients with acute heart failure. Int J Cardiol 2016;222:521–7. [DOI] [PubMed] [Google Scholar]
- 66.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA 2017;318:1161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sokoreli I, de Vries JJ, Pauws SC, Steyerberg EW. Depression and anxiety as predictors of mortality among heart failure patients: systematic review and meta-analysis. Heart Fail Rev 2016;21:49–63. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan MD, Newton K, Hecht J, Russo JE, Spertus JA. Depression and health status in elderly patients with heart failure: a 6-month prospective study in primary care. Am J Geriatr Cardiol 2004; 13:252–60. [DOI] [PubMed] [Google Scholar]
- 69.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med 2010;8:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yesavage JA. Geriatric depression scale. Psychopharmacol Bull 1988;24:709–11. [PubMed] [Google Scholar]
- 72.Alves TC, Busatto GF. Regional cerebral blood flow reductions, heart failure and Alzheimer’s disease. Neurol Res 2006;28:579–87. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki H, Matsumoto Y, Ota H, et al. Hippocampal blood flow abnormality associated with depressive symptoms and cognitive impairment in patients with chronic heart failure. Circ J 2016;80: 1773–80. [DOI] [PubMed] [Google Scholar]
- 74.Garcia S, Spitznagel MB, Cohen R, et al. Depression is associated with cognitive dysfunction in older adults with heart failure. Cardiovasc Psychiatry Neurol 2011;2011:368324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alosco ML, Spitznagel MB, Raz N, et al. The interactive effects of cerebral perfusion and depression on cognitive function in older adults with heart failure. Psychosom Med 2013;75:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macchia A, Monte S, Pellegrini F, et al. Depression worsens outcomes in elderly patients with heart failure: an analysis of 48,117 patients in a community setting. Eur J Heart Fail 2008;10: 714–21. [DOI] [PubMed] [Google Scholar]
- 77.Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA 2012;308: 465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med 2015;175:1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol 2010;56:692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Angermann CE, Gelbrich G, Stork S, et al. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA 2016;315:2683–93. [DOI] [PubMed] [Google Scholar]
- 81.Reynard AK, Butler RS, McKee MG, Starling RC, Gorodeski EZ. Frequency of depression and anxiety before and after insertion of a continuous flow left ventricular assist device. Am J Cardiol 2014; 114:433–40. [DOI] [PubMed] [Google Scholar]
- 82.Forman DE, Arena R, Boxer R, et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2017;135:e894–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joyce E Frailty in advanced heart failure. Heart Fail Clin 2016;12:363–74. [DOI] [PubMed] [Google Scholar]
- 84.Murad K, Kitzman DW. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Fail Rev 2012;17:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joseph SM, Rich MW. Targeting frailty in heart failure. Curr Treat Options Cardiovasc Med 2017; 19:31. [DOI] [PubMed] [Google Scholar]
- 87.Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options—a mini-review. Gerontology 2014;60: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Georgiadou P, Adamopoulos S. Skeletal muscle abnormalities in chronic heart failure. Curr Heart Fail Rep 2012;9:128–32. [DOI] [PubMed] [Google Scholar]
- 89.Hegde SM, Claggett B, Shah AM, et al. Physical activity and prognosis in the TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). Circulation 2017;136:982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014;113: 1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol 2017;236:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joyce E Frailty and cardiovascular disease: a two-way street? Cleve Clin J Med 2018;85:65–8. [DOI] [PubMed] [Google Scholar]
- 93.Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 2008;12: 29–37. [DOI] [PubMed] [Google Scholar]
- 94.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez-Pascual C, Paredes-Galan E, Ferrero-Martinez AI, et al. The frailty syndrome is associated with adverse health outcomes in very old patients with stable heart failure: a prospective study in six Spanish hospitals. Int J Cardiol 2017;236:296–303. [DOI] [PubMed] [Google Scholar]
- 96.Chaudhry SI, McAvay G, Chen S, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the Cardiovascular Health Study. J Am Coll Cardiol 2013;61:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. J Am Coll Cardiol HF 2013;1:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joseph SM, Manghelli JL, Vader JM, et al. Prospective assessment of frailty using the fried criteria in patients undergoing left ventricular assist device therapy. Am J Cardiol 2017;120: 1349–54. [DOI] [PubMed] [Google Scholar]
- 99.Maurer MS, Horn E, Reyentovich A, et al. Can a left ventricular assist device in individuals with advanced systolic heart failure improve or reverse frailty? J Am Geriatr Soc 2017;65:2383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schopfer DW, Forman DE. Growing relevance of cardiac rehabilitation for an older population with heart failure. J Card Fail 2016;22:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tucker WJ, Nelson MD, Beaudry RI, et al. Impact of exercise training on peak oxygen uptake and its determinants in heart failure with preserved ejection fraction. Card Fail Rev 2016;2: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011;2011:569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reeves GR, Whellan DJ, Duncan P, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: design and rationale. Am Heart J 2017;185:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Forman DE, Lipsitz LA. Syncope in the elderly. Cardiol Clin 1997;15:295–311. [DOI] [PubMed] [Google Scholar]
- 107.Sibley KM, Voth J, Munce SE, Straus SE, Jaglal SB. Chronic disease and falls in community-dwelling Canadians over 65 years old: a population-based study exploring associations with number and pattern of chronic conditions. BMC Geriatr 2014;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tchalla AE, Dufour AB, Travison TG, et al. Patterns, predictors, and outcomes of falls trajectories in older adults: the MOBILIZE Boston Study with 5 years of follow-up. PLoS One 2014;9: e106363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tinetti ME, Kumar C. The patient who falls: “It’s always a trade-off”. JAMA 2010;303:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee PG, Cigolle C, Blaum C. The cooccurrence of chronic diseases and geriatric syndromes: the health and retirement study. J Am Geriatr Soc 2009;57:511–6. [DOI] [PubMed] [Google Scholar]
- 111.Lee K, Pressler SJ, Titler M. Falls in patients with heart failure: a systematic review. J Cardiovasc Nurs 2016;31:555–61. [DOI] [PubMed] [Google Scholar]
- 112.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701–7. [DOI] [PubMed] [Google Scholar]
- 113.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 1997;337:1279–84. [DOI] [PubMed] [Google Scholar]
- 114.Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA 2017;318:1687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A 2013;110:5797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Molloy GJ, McGee HM, O’Neill D, Conroy RM. Loneliness and emergency and planned hospitalizations in a community sample of older adults. J Am Geriatr Soc 2010;58:1538–41. [DOI] [PubMed] [Google Scholar]
- 117.Shankar A, McMunn A, Demakakos P, Hamer M, Steptoe A. Social isolation and loneliness: prospective associations with functional status in older adults. Health Psychol 2017;36: 179–87. [DOI] [PubMed] [Google Scholar]
- 118.Krumholz HM, Butler J, Miller J, et al. Prognostic importance of emotional support for elderly patients hospitalized with heart failure. Circulation 1998;97:958–64. [DOI] [PubMed] [Google Scholar]
- 119.Mard S, Nielsen FE. Single living predicts a higher mortality in both women and men with chronic heart failure. Dan Med J 2016;63. [PubMed] [Google Scholar]
- 120.Arestedt K, Saveman BI, Johansson P, Blomqvist K. Social support and its association with health-related quality of life among older patients with chronic heart failure. Eur J Cardiovasc Nurs 2013;12:69–77. [DOI] [PubMed] [Google Scholar]
- 121.Yu DS, Lee DT, Kwong AN, Thompson DR, Woo J. Living with chronic heart failure: a review of qualitative studies of older people. J Adv Nurs 2008;61:474–83. [DOI] [PubMed] [Google Scholar]
- 122.Cattan M, White M, Bond J, Learmouth A. Preventing social isolation and loneliness among older people: a systematic review of health promotion interventions. Ageing Soc 2005;25:41–67. [DOI] [PubMed] [Google Scholar]
- 123.Luttik ML, Jaarsma T, Veeger N, van Veldhuisen DJ. Marital status, quality of life, and clinical outcome in patients with heart failure. Heart Lung 2006;35:3–8. [DOI] [PubMed] [Google Scholar]
- 124.Quinones PA, Kirchberger I, Heier M, et al. Marital status shows a strong protective effect on long-term mortality among first acute myocardial infarction-survivors with diagnosed hyperlipidemia–findings from the MONICA/KORA myocardial infarction registry. BMC Public Health 2014;14:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dunlay SM, Roger VL, Weston SA, Bangerter LR, Killian JM, Griffin JM. Patient and spousal health and outcomes in heart failure. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA 2014;311:1052–60. [DOI] [PubMed] [Google Scholar]
- 127.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA 1999;282:2215–9. [DOI] [PubMed] [Google Scholar]
- 128.Bidwell JT, Lyons KS, Lee CS. Caregiver wellbeing and patient outcomes in heart failure: a meta-analysis. J Cardiovasc Nurs 2017;32:372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomeer MB, Mudrazija S, Angel JL. Relationship status and long-term care facility use in later life. J Gerontol B Psychol Sci Soc Sci 2016;71: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boland L, Légaré F, Perez MM, et al. Impact of home care versus alternative locations of care on elder health outcomes: an overview of systematic reviews. BMC Geriatr 2017;17:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adler ED, Goldfinger JZ, Kalman J, Park ME, Meier DE. Palliative care in the treatment of advanced heart failure. Circulation 2009;120: 2597–606. [DOI] [PubMed] [Google Scholar]
- 132.McDonagh TA, Blue L, Clark AL, et al. European Society of Cardiology Heart Failure Association standards for delivering heart failure care. Eur J Heart Fail 2011;13:235–41. [DOI] [PubMed] [Google Scholar]
- 133.Lewis EF. Assessing the impact of heart failure therapeutics on quality of life and functional capacity. Curr Treat Options Cardiovasc Med 2013; 15:425–36. [DOI] [PubMed] [Google Scholar]
- 134.Selman L, Beynon T, Higginson IJ, Harding R. Psychological, social and spiritual distress at the end of life in heart failure patients. Curr Opin Support Palliat Care 2007;1:260–6. [DOI] [PubMed] [Google Scholar]
- 135.Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail 2007;13:643–8. [DOI] [PubMed] [Google Scholar]
- 136.Joyce E, Chung C, Badloe S, et al. Variable contribution of heart failure to quality of life in ambulatory heart failure with reduced, better, or preserved ejection fraction. J Am Coll Cardiol Hf 2016;4:184–93. [DOI] [PubMed] [Google Scholar]
- 137.Lum HD, Carey EP, Fairclough D, et al. Burdensome physical and depressive symptoms predict heart failure-specific health status over one year. J Pain Symptom Manage 2016;51:963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol 2016;1:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation 2012;125:1928–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 141.Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med 2015;18:134–42. [DOI] [PubMed] [Google Scholar]
- 142.Brannstrom M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail 2014;16: 1142–51. [DOI] [PubMed] [Google Scholar]
- 143.Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol 2017;70: 331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sahlen KG, Boman K, Brannstrom M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: based on a randomized controlled trial. Palliat Med 2016;30: 296–302. [DOI] [PubMed] [Google Scholar]
- 145.Szekendi MK, Vaughn J, Lal A, Ouchi K, Williams MV. The prevalence of inpatients at 33 U.S. hospitals appropriate for and receiving referral to palliative care. J Palliat Med 2016;19: 360–72. [DOI] [PubMed] [Google Scholar]
- 146.Afilalo J, Lauck S, Kim DH, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol 2017; 70:689–700. [DOI] [PubMed] [Google Scholar]
- 147.Tinetti M, Huang A, Molnar F. The Geriatrics 5M’s: a new way of communicating what we do. J Am Geriatr Soc 2017;65:2115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


