Abstract
Essentially all cells contain a variety of spatially restricted regions that are important for carrying out specialized functions. Often, these regions contain specialized transcriptomes that facilitate these functions by providing transcripts for localized translation. These transcripts play a functional role in maintaining cell physiology by enabling quick response to changes in cellular environment. Here, we review how RNA molecules are trafficked within cells, with a focus on the subcellular locations to which they are trafficked, mechanisms that regulate their transport, and clinical disorders associated with misregulation of the process.
Keywords: RNA binding protein, zipcode, RNA localization, RNA cis-element, RNA transport
Introduction
Gene expression is regulated through a complex coordination of events that occur at distinct steps during the lifetime of a mRNA. Whereas control of RNA synthesis by RNA polymerases, transcription factors, and chromatin states is widely studied, less well-characterized post-transcriptional mechanisms are also essential in defining gene expression landscapes in individual cell types. These cell-type specific gene expression profiles define the protein composition of cells and ultimately cell-type specific functions. One poorly understood aspect of post-transcriptional regulation is RNA localization.
Much of the work on RNA localization has been performed in neuronal systems because early studies recognized the importance of RNA localization in these polarized cell types, and they are amenable to study due to their size and morphology. Neurons are highly polarized, with long distances between the soma, where RNAs are synthesized in the nucleus, and projections (axons/dendrites/neurites), where cells must quickly respond to stimuli (Figure 1A). These cell types have therefore been a critical model system to understand how RNA localization controls gene expression using imaging-based approaches as reviewed previously1–5. A number of neurological diseases, including amyotrophic lateral sclerosis (ALS) and fragile X syndrome (FXS), have been associated with misregulated RNA localization in neurons. Recent advances in high-throughput sequencing and microscopy techniques have also revealed that RNAs are localized to subcellular compartments (e.g. endoplasmic reticulum and mitochondria) in many nonpolarized cell types6–9. This observation has led to some key questions. Do neurons and other cell types use a common set of mechanisms to achieve RNA localization? In other words, does an ‘RNA localization code’ exist that transcends cell types? What are the biological roles of creating localized transcriptomes that are different across subcellular compartments and cell types? What are the consequences of misregulated RNA localization? Despite the realization that local transcriptomes contribute to cell function across diverse cell types, many gaps in our understanding of how these processes are regulated still exist.
Figure 1: Mechanisms of RNA localization.
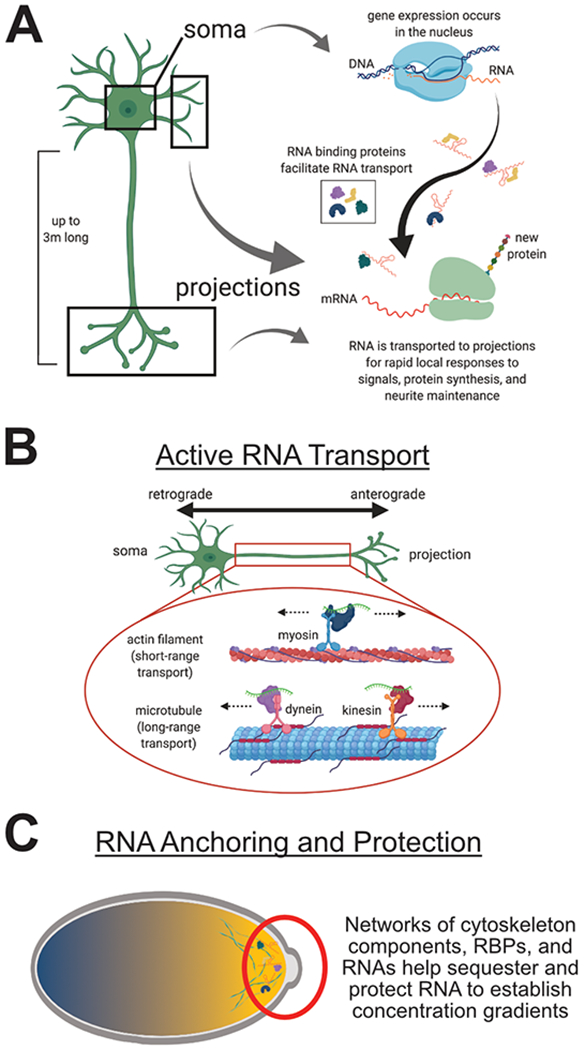
A) Local RNA transcriptomes are established in neurons to facilitate local protein production in response to stimuli. RNA binding proteins are essential for transport of these RNAs. B) RNAs are transported along cytoskeletal networks in various cell types. Short range transport is typically mediated by actin filaments and myosins, which can facilitate retrograde and anterograde movement. Long range transport is achieved with microtubules in both retrograde and anterograde movement using dynein and kinesin, respectively. C) Concentration gradients of RNAs are established using anchors composed of proteins that sequester RNA.
How does RNA get there?
Several RNA localization mechanisms have been observed in various cell types. Active transport of RNA as components of ribonucleoprotein (RNP) granules along cytoskeleton scaffolds is the most widely observed mechanism10. Long-range localization of RNA is often accomplished via active transport in neurons (Figure 1B), but cytoskeletal-associated RNA localization has also been observed in spherical cells such as yeast and Drosophila embryos where sometimes shorter distances are traversed10–13. Transcripts can also achieve localization with a combination of active transport followed by anchoring once they have arrived at a specific location. This mechanism has been characterized in multiple cell types including Aplysia sensory neurons14 and yeast11,15. Additionally, in Drosophila oocytes passive diffusion followed by anchoring of nanos mRNA helps establish a concentration gradient across the cell16,17 (Figure 1C). Lastly, local transcriptomes can be established by selective RNA degradation or local protection. This occurs when an RNA is unstable or susceptible to decay unless it is stabilized or protected within a specific location18. Even though this mechanism seems energetically wasteful, there is evidence that this process is utilized by Drosophila embryos19. These mechanisms of RNA localization are not mutually exclusive, and a single RNA transcript may be influenced by a combination of mechanisms to achieve finely tuned regulation of its localization20. Additionally, all of these localization mechanisms require additional protein factors to interact with the RNA. These RNA binding proteins (RBPs) are critical for understanding how RNAs are localized. Below we will discuss in detail how RNAs are localized through different mechanisms in different cell types.
Active transport of RNAs within cells
There is abundant evidence for active RNA transport along cytoskeleton scaffolds. This mechanism was characterized by inhibition of RNA transport following treatment with cytoskeletal depolymerizing drugs including nocodazole and cytochalasin21,22. Long range and short range cytoskeleton-mediated transport are thought to use different machinery. Long range transport is mostly mediated by dyneins and kinesins on microtubules whereas shorter transport is mediated by myosins on actin filaments, as reviewed elsewhere1,10 (Figure 1B).
The cytoskeleton is composed of two major structures, microtubules and microfilaments. RNA transport mechanisms have been described using both of these structures. Microtubules are polymers of the protein tubulin. In neurons, transport of many mRNAs [e.g. beta-actin (ActB)23, Activity-regulated cytoskeleton-associated protein (Arc)24, and calcium/calmodulin-dependent protein kinase II (CaMKIIɑ)25] to axons and dendrites often occurs along microtubules26. Microtubules are also implicated in RNA transport within Drosophila embryos (bicoid (bcd) mRNA26 and oskar (osk) mRNA18) as well as myelin basic protein (MBP) mRNA in oligodendrocytes27. Cardiomyocytes also require microtubules for transport of transcripts to sarcomeres28.
Transport along actin microfilaments by myosin motors is responsible for the bulk of RNA transport in yeast10, where ASH1 mRNA is localized into dividing daughter cells and accumulates at the bud tip to regulate mating type switching11. ASH1 mRNA is exclusively localized along actin filaments yet there is also evidence for local sequestration activity after reaching the bud tips12. In this case, transport along actin is responsible for both getting transcripts to the correct region and refining their localization at the bud tip.
Both microtubules and actin filaments are used to actively localize RNAs in many cell types. RNA movements are facilitated by larger RNP complexes which contain both protein and RNA. Interactions between these components and adapter and motor proteins associated with the cytoskeleton facilitate active transport of sometimes multiple RNAs at once29. For example, CaMKIIα, Neurogranin, and Arc mRNAs are targeted to dendrites within the same RNP granule30. The exact composition of cytoskeleton-associated RNPs can also regulate the speed and direction of transport31,32. However, combinatorial effects of each factor make it difficult to discern exactly how an RNA achieves its specific destination. Further research into what factors are included in RNP complexes and how they impact trafficking is necessary to understand how RNAs are actively transported along the cytoskeleton, and RNP composition and dynamics are extensively reviewed here33.
Anchoring of RNAs creates local RNA enrichment
RNAs that arrive near their destination via either active or passive mechanisms can be anchored into position through interactions with proteins and the cytoskeleton. In addition to facilitating active transport, actin also plays a role in refining and stabilizing transcripts at a destination. Both active transport as well as anchoring to a specific region sometimes requires the cytoskeleton to achieve stable localization17,21.
Active transport followed by specific anchoring of RNA is a common mechanism in Drosophila and Xenopus; however, both active and passive mechanisms prior to anchoring are used. For vg1 mRNA, microtubules are responsible for transport to the vegetal hemisphere, whereas microfilaments are important for anchoring at the cortex22. However, gurken mRNA requires dynein for both transport to and retention at the anterodorsal corner, indicating that anchoring can occur via microtubules18,34. Nos mRNA does not associate with microtubules in Drosophila and is thought to localize solely through diffusion and cytoplasmic streaming16. It is trapped by an unknown protein complex containing Rumpelstiltskin at the posterior end of the embryo17,35. Even in a large Drosophila embryo, RNA anchoring alone is sufficient for maintenance of RNA localization.
Similar mechanisms are used in budding yeast to generate local concentrations of Ash1 mRNA to control mate type switching. Active transport of ASH1 mRNA into the daughter cells is dependent on interaction with myosin motor protein She1. Without She1, ASH1 mRNA does not localize correctly11,15. However, if She1 is available but Bni111 or Bud636 is mutated, specific localization to the bud tip is unstable. This strongly indicates that RNA is generally localized by active transport but specifically retained by anchoring factors like Bni1 and Bud6.
RNA anchoring is observed in other eukaryotes as well. Beta-actin mRNA is anchored to protrusions in chicken embryo fibroblasts as well as the leading edge of rat adenocarcinoma cells by EF1ɑ37. This protein is capable of simultaneously binding mRNA and F-actin. Loss of its actin binding domain results in diffuse beta-actin mRNA that is no longer localized to the leading edge. Other anchoring factors include the adenomatous polyposis coli complex (APC) which is also important for RNA localization in fibroblast protrusions. It associates with RNP granules, and without APC expression, RNAs no longer localize to protrusions38. Therefore, anchoring of RNAs to the cytoskeleton is critical for defining the precise localization of transcripts in several cell types.
Local RNA transcriptomes are regulated by selective degradation
Control of local transcriptomes via selective degradation or protection in subcellular regions is less studied and has been observed in only a few cell types (Figure 1C). For example, Drosophila embryos localize Heat Shock Protein 83 (Hsp83) mRNAs to the posterior pole by protecting transcripts from degradation in that region39,40. These transcripts are widely expressed throughout the embryo until they are selectively degraded following fertilization of the embryo. This is largely regulated by sequence in the 3’UTR of Hsp83 that can be deleted or exchanged for other UTRs to disrupt its spatial stability phenotype39. This sequence in the 3’ UTR is bound by Smaug, resulting in degradation of the transcript19.
In addition to regulation of RNA abundance through canonical RNA degradation pathways (e.g. via the TRAMP complex), non-canonical roles of nonsense-mediated decay (NMD) have also been characterized. NMD was initially described to degrade mRNAs containing premature termination codons (PTCs); however, this mechanism also regulates the abundance of non-PTC containing RNAs in dendrites41 and axons42, which in turn influences synaptic plasticity and growth cone formation, respectively. While the degradation strategies that control RNA localization come at an energetic cost, it is effective at creating specific localized transcriptomes in a regulated way.
An RNA has arrived. Now what?
Local RNA transcriptomes may serve a variety of roles in establishing cell type specific functions by creating temporally and spatially regulated proteomes to guide differentiation and responses to environmental stimuli. The predominant theory on how RNA localization accomplishes this is through enhancing local protein production efficiency by having the mRNA translated where the protein ultimately exerts its effect. Transporting a single mRNA is energetically efficient since a single mRNA molecule can be used to synthesize multiple copies of proteins43, reviewed more extensively here2,18,44. Similarly, colocalization of mRNAs encoding members of a protein complex could produce high concentrations of multi-subunit complex components in a specific location, thus increasing complex formation efficiency and decreasing the formation of off-target, nonfunctional complexes45–48. mRNAs can also be localized in a translationaly silenced form. Local signals are therefore needed before initiating protein synthesis49. Finally, mRNAs can be localized to prevent protein toxicity in specific subcellular compartments, as is the case for MBP in oligodendrocytes27. However, there could be many additional reasons for RNA localization that are not yet clear. For example, the RNAs themselves might have a function as is the case for many non-coding RNAs (e.g. miRNAs and lncRNAs) and at least one mRNA50.
Translation of localized RNAs
In many cell types, mRNA co-localizes with its encoded protein. This has been observed in neurons51, Drosophila embryos52, and HEK293 cells7. This phenomenon is suggestive of local translation which has been shown across species and cell types. Due to the difficult nature of quantifying local transcriptomes and proteomes, most studies focus on a single mRNA and its encoded protein. Below we discuss multiple specific examples that support the importance of mRNA localization in producing higher local protein concentrations (Figure 1A). In these examples, local mRNA translation can be important to the function of the synthesized protein and overall cell function.
In yeast, ASH1 mRNA is locally translated in the daughter bud to control mate type switching. Normally, ASH1 mRNA is not translated until it arrives in the bud tip53. Depletion of Puf6p, a translational repressor of ASH1 mRNA, causes ASH1 mRNA translation before it arrives at the bud tip, resulting in both cells having the same mating type53. Thus, ASH1 mRNA must be properly localized, and only translated in this location to function correctly.
Local translation has been demonstrated to occur at neuronal dendritic spines, which are substantial distances from the cell nucleus. It has long been suggested that ribosomes locally translate mRNAs, but we now know that ribosomes are localized to dendritic spines along with many translation factors54 and engage in active translation in many neuronal cell types (reviewed here3). One of the best examples is beta-actin mRNA, which is localized to dendritic spines in neurons55. Reducing beta-actin mRNA translation with morpholinos provided evidence that local translation of this transcript in retinal neurons is required for axonal branching and stabilization56.
The majority of protrusion-enriched transcripts in fibroblasts are locally translated to produce localized proteins57. This is especially true for actin-associated proteins which help structure the protrusions. Furthermore, mRNAs encoding ribosome subunits have been shown to localize to protrusions of migrating cells in a LARP6 dependent manner. Local translation of ribosomal proteins at these protrusions enhances ribosome biogenesis and local protein production58. In each of these cases, the localized mRNA helps determine localization of the resulting proteins which aid critical cell functions.
Assembly of multi-subunit protein complexes
Formation of multi-subunit complexes can depend on chaperones, subunit stoichiometry, and local concentrations of each subunit. Local translation of the protein subunits can increase their local concentrations, thereby facilitating complex assembly by reducing the physical distance between components59. Additionally, spatially isolating complex members could help reduce unwanted protein interactions that might result in non-canonical complexes or other unfavorable interactions.
Local transcriptome mapping using proximity labeling has revealed that RNAs encoding complex members co-localize to the ER and mitochondria in yeast8. These results support previous observations that mRNAs encoding several mitochondrial associated complex members are found near the mitochondria (reviewed here60). Presumably, the mRNAs that encode ER and mitochondrial protein complexes localize to the organelle to facilitate local translation and assembly.
This phenomenon also occurs in less defined subcellular locations. mRNAs encoding the seven subunits of the Actin stabilizing complex Arp2/3 localize to fibroblast protrusions where they are required for stable growth46. Localization of these seven separate transcripts to protrusions aid in Arp2/3 assembly through local translation and assembly. In some cases, local translation of protein complex members can result in co-translational assembly as observed for heteromeric ion channels47 and histone acetyltransferase48.
RNA localization in response to environmental stimuli
mRNAs can also be localized in response to a specific signal, such as following glutamate stimulation in neurons, as reviewed here49. It is suggested that mRNAs are localized to produce local proteins that help remodel or stabilize activated synapses.
Intestinal absorptive enterocytes also localize RNAs across their apicobasal axis in response to refeeding to initiate recovery after starvation61. This reorganization changes the translational status of many mRNAs and may contribute to the cellular response to the refeeding stimulus, although exactly how this happens is unknown.
Control of cell size and projection length might also use active mRNA transport followed by local protein translation62. This model suggests that localization of mRNA to projections, local translation to produce protein, followed by retrograde protein diffusion back to the nucleus allows the cell to sense projection length. This mechanism has been suggested to regulate cilia, flagella63,64, and neurite length65,66. However, it could be broadly applicable to other subcellular components such as organelle size.
Role of cis and trans factors in RNA localization
The RNA localization mechanisms discussed above rely on multiple components, specifically RNA and their protein binding partners. First, cis-elements within the RNAs themselves, often referred to as zipcodes, ‘mark’ the RNA for a destination. Information can be stored in the RNA in the form of linear sequences or in RNA structure. These RNA features facilitate interactions between the RNA and specific trans-acting RBPs, which typically associate with RNAs through an RNA binding domain (RBD)31,67. Further interactions between RBPs and other proteins, such as those associated with the cytoskeleton68, create a network of interactions that regulate subcellular localization patterns of RNA. RNP granules can vary in their composition. This produces a heterogenous pool of RNPs inside of cells, further complicating study of their protein and RNA components. For example, Arc and CaMKIIα mRNAs associate selectively with Barentsz (Btz)- and Staufen 2 (Stau2)- containing RNPs, respectively whereas RNAs such as Lysophospholipase 1 (Lypla1) and Cap-Binding Protein (CBP80) are found in both of them69. A handful of RBPs and their cognate zipcodes are known (Table 1). However, these complex multivalent interactions have been difficult to dissect. For example, a single RNA can interact with multiple RBPs at once, and likewise a single RBP can have the capacity to recognize different zipcodes and therefore multiple RNAs. Below, we will focus on the role and identification of these cis- and trans-acting factors.
Table 1: Known RNA/RBP pairs that drive RNA localization.
Many additional RBPs and mRNAs are known to facilitate localization and be localized; however, we did not include those in this table since either the RBP component or the mRNA zipcode is unknown. abbreviations used: cytoplasmic polyadenlyation element (CPE), spliced oskar localization element (SOLE), exon junction complex (EJC), hnRNP A2 response element (A2RE), untranslated region (UTR), open reading frame (ORF), pumilio homology domain (PHD), RNA recognition motif (RRM), K homology (KH), double stranded RNA binding domain (dsRBD), arginine-glycine-glycine (RGG).
| RBP; RBDs (if known) | mRNA (species) | zipcode | transport mechanism | cellular function | references |
|---|---|---|---|---|---|
| STAU; 4 conserved dsRBDs |
oskar (Drosophila) bicoid (Drosophila) vg1 (Xenopus) CamKlla (mammalian) |
3′ UTR (SOLE; EJC deposition) 3′ UTR and 5′ stem loops 3′ UTR (366nt) 3′ UTR (30nt) |
active transport (kinesin) with biased random walk active transport (dynein) and anchoring active transport (kinesin) and anchoring active transport |
germ line differentiation embryonic patterning embryonic development memory formation; dendrites |
183–186 88–90, 187 22, 188, 189 190–192 |
| ZBP1, VERA, IMP1; 2 RRM and 4 KH |
ActB (mammalian) vg1 and vegT (Xenopus) Tau (rat) |
3′ UTR, 5′-GGACU-3′ (4-8) and 5′-ACA-3′ (22-24) 3′ UTR (element X4) U-rich sequence |
active transport, diffusion, and anchoring active transport (kinesin) and anchoring active transport (kinesin) |
fibroblast movement and axon guidance embryonic endoderm differentiation axonal polarity maintenance |
23, 193–196 77, 197–200 201–203 |
| hnRNPA/B, Squid, Hrp48; RRM, KH, and RGG |
MBP (mammalian) gurken (Drosophila) oskar (Drosophila) |
3′ UTR (21nt A2RE) 5′ UTR 3′ UTR (SOLE; EJC deposition) |
active transport active transport (dynein) and anchoring active transport (kinesin) |
myelin sheath formation embryonic development germ line differentiation |
204, 205 34, 206, 207 208, 209 |
| CPEB | MAP2 (rat) ZO-1 (mouse) cyclinB1 and Xbub3 (Xenopus) | 3′ UTR CPE 3′ UTR (5 conserved CPEs) 3′ UTR CPE |
active transport unknown unknown |
microtubule assembly epithelial tight junction assembly and polarity mitotic spindle and division |
84 85 210 |
| Rump | nanos (Drosophila) | 3′ UTR (4 partly redundant regions) | diffusion, streaming, and anchoring | embryonic polarity | 16, 35 |
| ARC1 | Arc1 (mammalian) | 3′ UTR | active transport and exovesicles | synaptic plasticity | 179, 215–215 |
| She2/She3 | ASH1 (yeast) | ORFs and 3′ UTR | active transport (myosin) and anchoring | mate type switching | 11, 12, 211, 212 |
| Sec27 | OXA1 (yeast) | 3′ UTR and ORF | unknown | mitochondrial inner membrane biogenesis | 216, 217 |
| Puf3p; PHD |
COX17 (yeast) | 3′ UTR (UGUR motif) | unknown | mitochondrial biogenesis and motility | 218–220 |
| RBP-L/RBP-P; 3 RRM |
glutelin (rice) prolamine (rice) |
ORF (repeated motifs) and 3′ UTR (U-rich) ORF (repeated motifs) and 3′ UTR (U-rich) |
active transport active transport |
grain development grain development |
221, 222 221, 222 |
| FMRP; KH and RGG |
MAP1b and CamKlla (mammalian) | G-quadruplex | active transport | neurogenesis and memory formation | 140, 141, 223, 224 |
| SMAUG | hsp83 (Drosophila) | 3′ UTR | degradation and local protection | development; maternal transcript elimination | 19, 40 |
| LARP6 | ribosomal protein mRNAs (mammalian) | 5′ TOP | unknown | fibroblast protrusion formation | 58 |
| TDP-43; RRM and Gly-rich C-term |
NEFL and RAC1 (mammalian) | 3′ UTR | active transport | neuronal development and plasticity | 109, 225 |
| NOVA | girk2 (mouse) | intronic and 3′ UTR YCAY | unknown | spinal motor neuron dendrite activity | 226 |
Cis-elements or zipcodes
Zipcode elements serve as the signal to target an RNA for delivery to a specific subcellular location. They can vary from a few bases to a kilobase in their length. Although hundreds to thousands of RNAs are known to be trafficked to a variety of subcellular locations, the sequence elements within those transcripts that regulate transport have been identified for only a few dozen. Most of the known localization regulatory elements are located in 3′ untranslated regions (UTRs) of mRNA, although some localization elements have been identified in 5′ UTRs14,70, coding regions11,12,71–73, and intronic sequences74. Additionally, localization elements are often repetitive and redundant. This repetition might allow many low affinity sequences to act in union to achieve specificity. Most of the zipcodes identified are thought to function independently and be modular in nature. Mutation and truncation experiments using reporter transcripts have led to the discovery of a small number of necessary and sufficient zipcode sequences. Combined with additional biochemical methods, these approaches have uncovered zipcodes that depend on primary sequence and/or RNA structures for their function and have been reviewed here75,76 (Table 1).
An RNA zipcode alone is not always enough to facilitate localization. Some elements are necessary but not sufficient, suggesting that neighboring RNA sequences can be an important context for the zipcode. For example, xcat2 mRNA in Xenopus oocytes localizes to the vegetal pole using six repeats of a short motif, UGCAC, present within the 3′ UTR of xcat2. However, insertion of this motif into the 3′ UTR of another mRNA, vg1, did not result in its localization to the vegetal pole77. Additionally, nuclear processing also plays an important role in mRNA localization. For example, oskar localization at the posterior pole of the Drosophila oocyte requires both spliced oskar localization element (SOLE, which comprises nucleotides of both exon 1 and exon 2) and the exon junction complex (EJC) deposited during splicing for efficient transport along the microtubules by kinesin71,78.
Many bacterial RNAs are also differentially distributed at locations such as in the cytoplasm in a helical like pattern, at membranes, and at polar or septal sites79,80. In E. coli, the bglG–bglF operon, which codes for proteins necessary for aryl-β-glucoside metabolism, localize to the cell membrane. The bglG–bglF zipcode is located within the sequence encoding the first two transmembrane helices of bglF and is uracil-rich, a common feature observed in transmembrane proteins across species81,82.
Another example of an element that hints at conservation across species and cell types is the cytoplasmic polyadenylation element (CPE). CPE is a nucleotide sequence (UUUUUAU) within the 3′ UTR of most mRNAs which binds to the CPE-binding protein (CPEB). Although this interaction has been most studied for its effects on translation upon synaptic activation83 studies have shown that CPEB can also act as a transport protein for dendritic mRNAs such as MAP2 and intestinal apical RNA such as ZO-1 responsible for maintaining tight junction assembly and cell polarity84,85.
Lastly, many zipcode elements depend on RNA secondary and/or tertiary structures, e.g. stem loops, for recognition by trans-factors31,86. For example, bicoid mRNA 3’UTR stem loops87,88 are recognized by the RBP Staufen (STAU)89,90. The complex roles RNA structure plays in defining zipcodes contributes to the difficulty of finding them since structural elements are much more difficult to define and identify computationally than linear sequence motifs.
Cis-elements in non-coding RNAs
Non-coding RNA such as miRNAs and lncRNAs also use sequence elements to direct localization patterns (Table 2). For example, miR-29b contains a hexanucleotide 3′motif (AGUGUU) responsible for nuclear localization91. This is in contrast to the cytoplasmic enrichment observed commonly for miRNAs. Additionally, the lncRNA MALAT1 uses sequences present at both the 5′ and 3′ ends to target its localization to paraspeckles within the nucleus92. Recent studies using massively parallel reporter assays (MPRAs) have also discovered C-rich motifs present in several lncRNAs that lead to their nuclear enrichment93,94. lncRNA BC1 and it’s human analog BC200 are enriched in dendrites and synapses, where it represses translation locally in nerve cells by inhibiting eIF4a-mediated unwinding of RNA duplexes95–97. The lack of evidence for the role of lncRNAs is primarily due to the fact that techniques developed to study complex RNA-RNA interactions are still in their infancy. Further, there has been some evidence that RNA modifications might also play a role in localization. For example, mitochondrial localization of tRNAGlu in the RNA virus Leishmania is driven by a specific modification of the tRNA98.
Table 2: Localized noncoding RNA and their RBP partners.
abbreviations used: K homology (KH), arginine-glycine-glycine (RGG).
| RBP; RBDs (if known) | ncRNA (species) | zipcode | transport mechanism | cellular function | references |
|---|---|---|---|---|---|
| FMRP; KH and RGG |
BC1 (mouse) BC200 (human) |
unknown unknown |
unknown unknown |
dendritic mRNA translation regulation dendritic mRNA translation regulation |
95, 227 95, 227 |
| ANT2 | mir29-b (human) | unknown | unknown | cell division | 91, 228 |
| RNPS1, SRm160, and IBP160 |
NEAT2/MALAT1 (human) | region E and region M | unknown | nuclear speckle function | 92 |
Identification of potential cis-ating elements using computational methods (e.g. MEME99, HOMER100, SeAMotE101, and others dependent on structure102–105 have had limited success. Despite our increasing understanding of principles governing cis-localization elements using specific examples, we still lack a systematic charaterization of general rules. One of the challenges to overcome is the implementation of strategies that combine sequence motifs and structural features while keeping in mind the redundancy observed in many known zipcode examples. In the future, focusing on transcriptome-wide studies coupled with high-throughput functional assays along with advanced in silico analyses will further efforts to uncover novel zipcodes.
Trans-factors that regulate localization
RBPs regulate all aspects of RNA metabolism, including transcription, splicing, translation, and decay. RNA localization is also subject to regulation by RBPs, which bind and recognize RNA elements to form RNP complexes. RBPs generally contain a variety of RBDs that impart specificity. Some of the common RBDs are the RNA recognition motif (RRM), K homology (KH) domain, DEAD-box motif, and zinc finger domains106. These domains allow for sequence specific binding to short single stranded RNA (ssRNA) motifs. For example, RBPs including heterogeneous nuclear ribonucleoproteins (hnRNPs) recognize specific elements such as a 21-nt sequence known as hnRNP A2 response element (A2RE) present in many dendritic mRNAs107. Additionally, some RBPs recognize RNA secondary structure, sequence elements present within structural elements, or a combination of the two. Many RBPs also contain intrinsically disordered regions (IDRs) that could regulate the assembly and dynamics of RNP complexes108. Most of the RBPs involved in localization have been identified through genetic screens and/or affinity purification of proteins that bind the localized transcript. Using these approaches, conserved roles in localization have been identified for a few RBPs across various species67 (Table 1).
The complex combinatorial regulation of RNA localization by multiple RBPs was demonstrated in a recent study109. The use of genetic manipulations and high-resolution imaging showed that TDP-43 binds to Rac1 mRNA. Additional interactions occur between the mRNA and either FMRP or Staufen. These interactions further regulate Rac1 mRNA transport to mouse dendrites by facilitating anterograde or retrograde movement, respectively. This illustrates that a number of different RBPs in cooperation with each other might dictate the appropriate location of the target mRNA.109
Protein capture using oligo-dT to purify polyA mRNA followed by mass spectrometry has found that over 1500 proteins directly bind polyA containing RNA110. Further experiments then identified the sequence binding preferences of many of these RBPs. Two techniques, RNAcompete111 and RNA Bind-n-Seq (RBNS)112, were developed to provide an inventory of RBP motif preferences and their specificity. To complement these in vitro approaches and identify RNA sequences bound by RBPs in vivo, a number of techniques involving crosslinking combined with RBP immunoprecipitation such as RIP-seq113, CLIP-seq114, HITS-CLIP115, eCLIP116, and PAR-CLIP117 were developed. These methods, in conjunction with further variations (reviewed in118), have enabled high resolution profiling of transcriptome wide RBP-RNA binding interactions. It still remains to be seen if these techniques and databases can be routinely successfully applied to the study of RNA localization on a transcriptomic scale to derive regulatory principles that govern the process. However, given their utility in the study of other RNA-based processes, the possibility is intriguing.
Methods that allow the detection of RNA-RNA interactions may also provide insight into RNA localization mechanisms. These include techniques which combine the use of cross-linking agents, proximity ligation, and RNA-seq to profile RNA interactomes (CLASH, SPLASH, PARIS, LIGR-seq and MARIO)119. Combining these techniques with RBP-based approaches may have the potential to yield rich datasets that detail the RBP-RNA and RNA-RNA interactions that regulate RNA localization.
Clinical Relevance
RNA localization is critical for proper development from yeast to humans11,120–122. These initial observations, coupled with recent technological advances, have linked RNA localization to clinical disease (Figure 2). To date, RNA localization defects contribute to neuromuscular/skeletal diseases, neuronal disorders such as Alzheimer’s, depression, embryonal disorders, and cancer initiation123. But which RNAs are mislocalized, what binding partners regulate their localization, and what causes their mislocalization remain unknown. Furthermore, the lack of functional studies have stalled our understanding of the direct significance of RNA mislocalization during the establishment of disease phenotypes.
Figure 2: Misregulation of RNA localization is relevant to disease phenotypes.
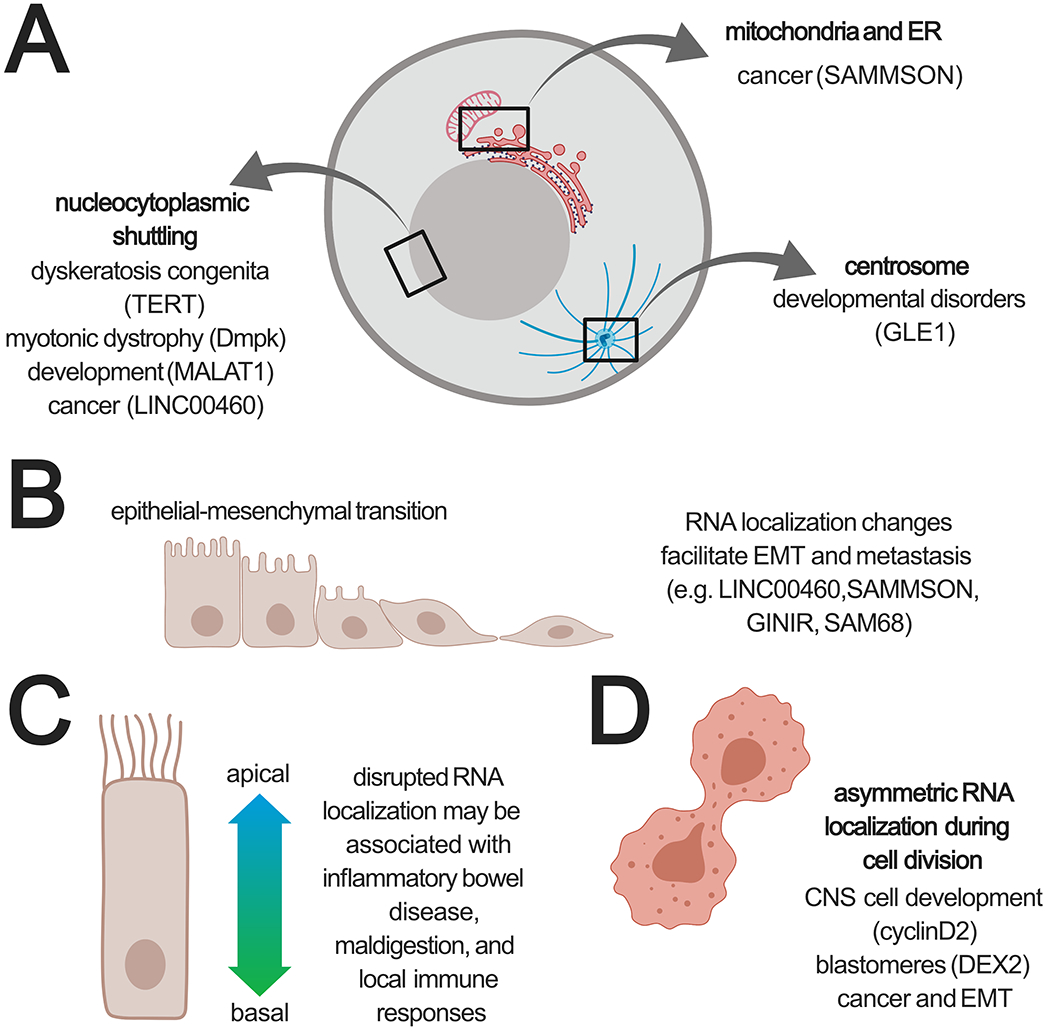
A) RNA localization patterns to various subcellular compartments are disrupted in various diseases. B) Establishment of EMT is associated with RNA localization changes. C) Gradients of RNA concentrations are observed between the apical and basal axis of epithelial cells; however, it is not clear if disruption of these patterns contributes to disease phenotypes. D) Asymmetric distribution of RNAs helps establish developmental states and disruption of these patterns contributes to establishment of cancer.
Neuronal and Neuromuscular Diseases
A handful of neurological disorders have been attributed to defects in RNA localization, and have previously been reviewed extensively here123–126. Below we highlight a handful of these key findings for amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), fragile X syndrome (FXS), and myotonic dystrophy (DM). RNA localization has also been implicated in other neurological disorders such as depression and Alzheimer’s, though many more studies are needed to find a definite link127,128. Taken together, these associations suggest that small perturbations in RNA localization can manifest in a variety of neuronal diseases, though few have been thoroughly explored.
ALS is a neurodegenerative disease characterized by the loss of motor neurons129. Mutations in a number of genes found in ALS patients have been implicated to cause defects in RNA metabolism, including alternative splicing, stabilization, and localization. These include SOD1, TARDBP (TDP-43), FUS, and C9orf72, all of them covered extensively in other reviews130. Mutations in the RBP TDP-43 are strongly linked to ALS131. TDP-43 has also been shown to regulate RNA localization132, but how the potential mislocalization of RNA in ALS neurons relates to disease phenotype is still unknown.
SMA is another neurodegenerative disease associated with a loss of motor neurons133. SMA results from insufficient levels of the protein SMN. SMN, like TDP-43, facilitates RNA localization to neurites133. Reduced levels of SMN results in mislocalization of various transcripts, such as ActB, growth-associated protein 43 (GAP43), and neuritin/cpg15123,134. Overexpression of RNP granule assembly and transport regulating RBPs, HuD and IMP1/ZBP1, can restore localization of transcripts and partially rescue the phenotype. These findings also implicate SMN as a regulator of RNA transport135,136. However, as with ALS, it is unknown whether and how RNA mislocalization in SMA neurons contributes to phenotypes.
FXS is a neurological disorder that results from the loss of FMRP expression137. Loss of FMRP results in dysregulated RNA localization in neurons, resulting in slower RNA granule localization as well as mislocalized mRNAs, such as Kif26a in dendrites138,139. The role of FMRP in regulating neuronal RNA localization is well established and covered in other reviews139–141. Again, whether mislocalization of RNAs within FXS neurons is related to disease phenotypes is unknown.
Misregulated sequestration of RNA can have dramatic impacts on cellular function. In DM, mutant Dmpk mRNA contains expanded CUG repeats that trap transcripts in the nucleus. The retention of these transcripts in the nucleus is sufficient to generate a DM phenotype in mice142. This example illustrates the importance of exploring mechanisms behind transcript localization and mislocalization to better understand its impact on human disease.
Stem Cells and Development
Development relies on strict temporal and spatial control of gene expression17,39,143,144. Control of RNA localization is one method cells use to regulate cell fate and function. For example, spermatid maturation and non-genomic inheritance of traits is influenced by localization of specific RNA transcripts (mainly small RNAs) as the sperm develops to various compartments, such as the head, mid-piece, or tail. It is becoming increasingly clear that RNAs are transferred to sperm as they mature. Irregularities in the amount of RNA in spermatid have been associated with sperm maturation, affecting human male infertility, and others have implicated these RNAs in seeding non-genetic inheritance in zygotes145,146. While future studies are needed to investigate the implication of mislocalized transcripts, it is clear local RNAs play a key role during sperm maturation and inheritance147–149.
Similarly, defined RNA localization patterns also govern temporal control of translation in oocytes and cell polarity in Drosophila embryos120. These observations suggest that RNA localization might be a conserved phenomenon necessary for cell differentiation during development. Indeed, localization of RNA is important in the developing mammalian embryo as well. Discovered more than 100 years ago, the balbiano body is an organelle consisting of endoplasmic reticulum, golgi, proteins, and RNA150. It is known to be asymmetrically positioned across species and is involved in fate determination through RNA sequestration, though its importance in mammals remains unknown.
Localization of individual RNA transcripts affects mammalian cell differentiation. For example, NEAT2/MALAT1 displays differential localization patterns during development. Regulation of its localization is important for RNA shuttling, splicing, and differential gene expression92,151. NEAT2/MALAT1 is localized to the nucleus in the oocyte, but becomes cytoplasmic in the developing embryo, only to return to its nuclear localization in differentiated cell states152. CDX2 mRNA localization is also polarized in blastomeres to establish cell polarity in early mammalian embryonic development144. The consequence of mislocalizing these RNAs has yet to be reported.
RNA localization patterns are also particularly important during asymmetric cell division. Certain cell types, e.g. B and T cells, hematopoietic cells, and ganglions in the nervous system153–155, utilize asymmetric cell division for fate determination. For example, localization of CYCLIN D2 mRNA in neural progenitor cells of the developing neocortex allows them to maintain their pluripotency whereas the daughter cell differentiates156,157. These observations suggest that RNA localization might be a conserved phenomenon that regulates cell differentiation during development.
Developmental disorders attributed to disruption of RNA localization patterns are continuing to be discovered. Loss of the RBP Gle1 disrupts centrosomal RNA localization and centrosome function in human cells, leading to lethal motor neuron disease and fetal hydrops158–160. Dyskeratosis Congenita is a stem cell disorder caused by mislocalization of TERT transcripts from Cajal bodies to nucleoli, resulting in telomerase shortening in patient stem cells161 and predisposing affected individuals to aplastic anemia.
Mature cell function also relies on proper RNA localization. As discussed above, intestinal epithelial cell RNA localization helps regulate local protein production along the apicobasal axis61 (Figure 2C). Consequences of disrupting RNA localization in these cell types may underlie disease states such as inflammatory bowel disease, maldigestion, or local immune responses162,163.
Cancer
Asymmetric RNA localization to daughter cells may contribute to cancer initiation and progression164,165 (Figure 2D). Recently, hundreds of RBPs involved in post-transcriptional RNA modifications, alternative splicing, and localization have been implicated in numerous cancers166,167. These studies have led to speculations that RNA mislocalization may play a role in cancer progression. Whether these observations are a cause, consequence, or correlation is unclear. In Melanoma, cytoplasmic and mitochondrial localization of long noncoding RNA SAMMSON has been shown to increase clonogenic potential, and knock-down of SAMMSON decreases melanoma viability168. Additionally, the noncoding RNA GINIR associates with centrosomal proteins, with over-expression resulting in disruption of Brca1 protein activity and more aggressive tumors169.
A link between RNA localization and epithelial mesenchymal transition (EMT) is also emerging (Figure 2B), supported by the observation that altered localization of certain transcripts promotes cell adhesion and migration170. The long noncoding RNA LINC00460 promotes EMT in head and neck squamous cell carcinoma by localizing to the nucleus171. SAM68 transcripts are normally nuclear, but become cytoplasmic during cell adhesion processes, a key factor in promoting cancer metastasis172,173. Local protein translation at the tips of filopodia also increases Rho and Rho Kinase (ROCK) activation in the pseudopodia of tumor cells86,170. The local transcriptome found there includes mRNAs encoding for proteins such as M-Ras, whose overexpression is known to be sufficient for EMT transition174.
The observations described above implicate misregulation of RNA localization during disease establishment, but very few cases have established a direct link. This is due to the technical challenges associated with functional studies of RNA and RBP localization, particularly in disease contexts. These challenges are reflected by the paucity of data we have describing direct links between individual RNAs, their RBP regulators, mechanisms that drive localization, and ultimately the dependence of cellular functions on establishment of local transcriptomes. Fundamental insights have been gained from manipulatable model systems, but the relevance of mechanisms observed in these systems to disease phenotypes is unclear. Further studies utilizing advanced cell culture models such as iPSCs and organoids, advancing RNA visualization tools, and developing more sophisticated approaches to analyze large data sets may help to address these challenges.
Discussion
Many transcriptome-scale sequencing51,57,175–177 and imaging178 experiments have illuminated how pervasive the phenomenon of RNA localization is. However, we are still left without a mechanistic understanding of how the vast majority of these RNAs end up at their destination and why their transport is regulated. Specific examples of mislocalized RNAs leading to specific phenotypes are rare, even though there are many connections between RNA localization and cellular function or human diseases. For thousands of localized RNAs, further work is needed to understand the cis-elements and trans-factors that regulate their transport as well as the effect that their localization has on cell function and physiology. These studies are required before we can begin to formulate a more thorough understanding of general mechanisms that govern RNA localization, some of which might transcend cell types and species.
In addition to the RNA localization mechanisms described above, it is likely that additional RNA localization patterns exist, possibly even some that are trans-cellular. Recent studies have shown transport of Arc mRNA across synapses between motor neurons and muscle cells179,180. This process is mediated through interactions between the retroviral-like Arc1 protein and retrotransposon-like sequences within the 3′ UTR of its own mRNA. Disruption of trans-synaptic Arc1 mRNA transfer leads to defective synaptic plasticity. Furthermore, genetic mutations in the human Arc protein are linked to autism181 and schizophrenia182. These findings are intriguing, and suggest that we have much yet to learn about modes of RNA localization, cell types that use them, and consequences of its disruption.
Synopsis.
Here we review what is currently known about mechanisms of RNA localization in a variety of cell types. Many reviews have previously focused on mechanisms, consequences, and diseases associated with defective RNA localization in the central nervous system. However, here we have compiled what is known about mechanisms and consequences of RNA localization across other cell types and organisms, including yeast, oocytes, epithelial cells, and in diseases such as cancer and those affecting development.
Acknowledgements
This work was supported by funding from the RNA Bioscience Initiative at the University of Colorado Anschutz Medical Campus.
References:
- 1.Das S, Singer RH, Yoon YJ. The travels of mRNAs in neurons: do they know where they are going? Curr Opin Neurobiol. 2019;57:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryder PV, Lerit DA. RNA localization regulates diverse and dynamic cellular processes. Traffic. 2018;19(7):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt CE, Martin KC, Schuman EM. Local translation in neurons: visualization and function. Nat Struct Mol Biol. 2019;26(7):557–566. [DOI] [PubMed] [Google Scholar]
- 4.Lee BH, Bae S-W, Shim JJ, Park SY, Park HY. Imaging Single-mRNA Localization and Translation in Live Neurons. Mol Cells. 2016;39(12):841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taliaferro JM. Classical and emerging techniques to identify and quantify localized RNAs. Wiley Interdiscip Rev RNA. 2019;10(5):e1542. [DOI] [PubMed] [Google Scholar]
- 6.Xia C, Babcock HP, Moffitt JR, Zhuang X. Multiplexed detection of RNA using MERFISH and branched DNA amplification. Sci Rep. 2019;9(1):7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fazal FM, Han S, Parker KR, et al. Atlas of Subcellular RNA Localization Revealed by APEX-Seq. Cell. 2019;178(2):473–490.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Tang W, Li Z, et al. Mapping spatial transcriptome with light-activated proximity-dependent RNA labeling. Nat Chem Biol. October 2019. doi: 10.1038/s41589-019-0368-5 [DOI] [PubMed] [Google Scholar]
- 9.Nicchitta CV. A platform for compartmentalized protein synthesis: protein translation and translocation in the ER. Curr Opin Cell Biol. 2002;14(4):412–416. [DOI] [PubMed] [Google Scholar]
- 10.López de Heredia M, Jansen R-P. mRNA localization and the cytoskeleton. Curr Opin Cell Biol. 2004;16(1):80–85. [DOI] [PubMed] [Google Scholar]
- 11.Long RM, Singer RH, Meng X, Gonzalez I, Nasmyth K, Jansen RP. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science. 1997;277(5324):383–387. [DOI] [PubMed] [Google Scholar]
- 12.Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol Cell. 1998;2(4):437–445. [DOI] [PubMed] [Google Scholar]
- 13.Lantz VA, Clemens SE, Miller KG. The actin cytoskeleton is required for maintenance of posterior pole plasm components in the Drosophila embryo. Mech Dev. 1999;85(1–2):111–122. [DOI] [PubMed] [Google Scholar]
- 14.Meer EJ, Wang DO, Kim S, Barr I, Guo F, Martin KC. Identification of a cis-acting element that localizes mRNA to synapses. Proc Natl Acad Sci U S A. 2012;109(12):4639–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Münchow S, Sauter C, Jansen RP. Association of the class V myosin Myo4p with a localised messenger RNA in budding yeast depends on She proteins. J Cell Sci. 1999;112 ( Pt 10):1511–1518. [DOI] [PubMed] [Google Scholar]
- 16.Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol. 2003;13(14):1159–1168. [DOI] [PubMed] [Google Scholar]
- 17.Kugler J-M, Lasko P. Localization, anchoring and translational control of oskar, gurken, bicoid and nanos mRNA during Drosophila oogenesis. Fly . 2009;3(1):15–28. [DOI] [PubMed] [Google Scholar]
- 18.Lipshitz HD, Smibert CA. Mechanisms of RNA localization and translational regulation. Curr Opin Genet Dev. 2000;10(5):476–488. [DOI] [PubMed] [Google Scholar]
- 19.Semotok JL, Luo H, Cooperstock RL, et al. Drosophila maternal Hsp83 mRNA destabilization is directed by multiple SMAUG recognition elements in the open reading frame. Mol Cell Biol. 2008;28(22):6757–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farris S, Lewandowski G, Cox CD, Steward O. Selective localization of arc mRNA in dendrites involves activity- and translation-dependent mRNA degradation. J Neurosci. 2014;34(13):4481–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom K, Beach DL. mRNA localization: motile RNA, asymmetric anchors. Curr Opin Microbiol. 1999;2(6):604–609. [DOI] [PubMed] [Google Scholar]
- 22.Yisraeli JK, Sokol S, Melton DA. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development. 1990;108(2):289–298. [DOI] [PubMed] [Google Scholar]
- 23.Tiruchinapalli DM, Oleynikov Y, Kelic S, et al. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci. 2003;23(8):3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyford GL, Yamagata K, Kaufmann WE, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14(2):433–445. [DOI] [PubMed] [Google Scholar]
- 25.Mikl M, Vendra G, Kiebler MA. Independent localization of MAP2, CaMKIIα and β-actin RNAs in low copy numbers. EMBO Rep. 2011;12(10):1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eliscovich C, Buxbaum AR, Katz ZB, Singer RH. mRNA on the move: the road to its biological destiny. J Biol Chem. 2013;288(28):20361–20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbarese E, Barry C, Chou CH, et al. Expression and localization of myelin basic protein in oligodendrocytes and transfected fibroblasts. J Neurochem. 1988;51(6):1737–1745. [DOI] [PubMed] [Google Scholar]
- 28.Lewis YE, Moskovitz A, Mutlak M, Heineke J, Caspi LH, Kehat I. Localization of transcripts, translation, and degradation for spatiotemporal sarcomere maintenance. J Mol Cell Cardiol. 2018;116:16–28. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm JE, Vale RD. RNA on the move: the mRNA localization pathway. J Cell Biol. 1993;123(2):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Tatavarty V, Korza G, Levin MK, Carson JH. Multiplexed dendritic targeting of alpha calcium calmodulin-dependent protein kinase II, neurogranin, and activity-regulated cytoskeleton-associated protein RNAs by the A2 pathway. Mol Biol Cell. 2008;19(5):2311–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buxbaum AR, Haimovich G, Singer RH. In the right place at the right time: visualizing and understanding mRNA localization. Nat Rev Mol Cell Biol. 2015;16(2):95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsumori K, Takei Y, Hirokawa N. Components of RNA granules affect their localization and dynamics in neuronal dendrites. Mol Biol Cell. 2017;28(11):1412–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51(6):685–690. [DOI] [PubMed] [Google Scholar]
- 34.Delanoue R, Herpers B, Soetaert J, Davis I, Rabouille C. Drosophila Squid/hnRNP helps Dynein switch from a gurken mRNA transport motor to an ultrastructural static anchor in sponge bodies. Dev Cell. 2007;13(4):523–538. [DOI] [PubMed] [Google Scholar]
- 35.Jain RA, Gavis ER. The Drosophila hnRNP M homolog Rumpelstiltskin regulates nanos mRNA localization. Development. 2008;135(5):973–982. [DOI] [PubMed] [Google Scholar]
- 36.Beach DL, Salmon ED, Bloom K. Localization and anchoring of mRNA in budding yeast. Curr Biol. 1999;9(11):569–578. [DOI] [PubMed] [Google Scholar]
- 37.Liu G, Grant WM, Persky D, Latham VM Jr, Singer RH, Condeelis J. Interactions of elongation factor 1alpha with F-actin and beta-actin mRNA: implications for anchoring mRNA in cell protrusions. Mol Biol Cell. 2002;13(2):579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mili S, Moissoglu K, Macara IG. Genome-wide screen reveals APC-associated RNAs enriched in cell protrusions. Nature. 2008;453(7191):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bashirullah A, Cooperstock RL, Lipshitz HD. Spatial and temporal control of RNA stability. Proc Natl Acad Sci U S A. 2001;98(13):7025–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bashirullah A, Halsell SR, Cooperstock RL, et al. Joint action of two RNA degradation pathways controls the timing of maternal transcript elimination at the midblastula transition in Drosophila melanogaster. EMBO J. 1999;18(9):2610–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Notaras M, Allen M, Longo F, et al. UPF2 leads to degradation of dendritically targeted mRNAs to regulate synaptic plasticity and cognitive function. Mol Psychiatry. October 2019. doi: 10.1038/s41380-019-0547-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colak D, Ji S-J, Porse BT, Jaffrey SR. Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell. 2013;153(6):1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C, Han B, Zhou R, Zhuang X. Real-Time Imaging of Translation on Single mRNA Transcripts in Live Cells. Cell. 2016;165(4):990–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cody NAL, Iampietro C, Lécuyer E. The many functions of mRNA localization during normal development and disease: from pillar to post: mRNA localization in development and disease. WIREs Dev Biol. 2013;2(6):781–796. [DOI] [PubMed] [Google Scholar]
- 45.Williams NK, Dichtl B. Co-translational control of protein complex formation: a fundamental pathway of cellular organization? Biochem Soc Trans. 2018;46(1):197–206. [DOI] [PubMed] [Google Scholar]
- 46.Mingle LA, Okuhama NN, Shi J, Singer RH, Condeelis J, Liu G. Localization of all seven messenger RNAs for the actin-polymerization nucleator Arp2/3 complex in the protrusions of fibroblasts. J Cell Sci. 2005;118(Pt 11):2425–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu F, Jones DK, de Lange WJ, Robertson GA. Cotranslational association of mRNA encoding subunits of heteromeric ion channels. Proc Natl Acad Sci U S A. 2016;113(17):4859–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kassem S, Villanyi Z, Collart MA. Not5-dependent co-translational assembly of Ada2 and Spt20 is essential for functional integrity of SAGA. Nucleic Acids Res. 2017;45(12):7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Formicola N, Vijayakumar J, Besse F. Neuronal ribonucleoprotein granules: Dynamic sensors of localized signals. Traffic. 2019;20(9):639–649. [DOI] [PubMed] [Google Scholar]
- 50.Crerar H, Scott-Solomon E, Bodkin-Clarke C, et al. Regulation of NGF Signaling by an Axonal Untranslated mRNA. Neuron. 2019;102(3):553–563.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zappulo A, van den Bruck D, Ciolli Mattioli C, et al. RNA localization is a key determinant of neurite-enriched proteome. Nat Commun. 2017;8(1):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lécuyer E, Yoshida H, Parthasarathy N, et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131(1):174–187. [DOI] [PubMed] [Google Scholar]
- 53.Gu W, Deng Y, Zenklusen D, Singer RH. A new yeast PUF family protein, Puf6p, represses ASH1 mRNA translation and is required for its localization. Genes Dev. 2004;18(12):1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35(3):535–545. [DOI] [PubMed] [Google Scholar]
- 55.Paquin N, Chartrand P. Local regulation of mRNA translation: new insights from the bud. Trends Cell Biol. 2008;18(3):105–111. [DOI] [PubMed] [Google Scholar]
- 56.Wong HH-W, Lin JQ, Ströhl F, et al. RNA Docking and Local Translation Regulate Site-Specific Axon Remodeling In Vivo. Neuron. 2017;95(4):852–868.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mardakheh FK, Paul A, Kümper S, et al. Global Analysis of mRNA, Translation, and Protein Localization: Local Translation Is a Key Regulator of Cell Protrusions. Dev Cell. 2015;35(3):344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dermit M, Dodel M, Lee FCY, et al. Subcellular mRNA localization regulates ribosome biogenesis in migrating cells. doi: 10.1101/829739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Batada NN, Shepp LA, Siegmund DO. Stochastic model of protein-protein interaction: why signaling proteins need to be colocalized. Proc Natl Acad Sci U S A. 2004;101(17):6445–6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Margeot A, Garcia M, Wang W, Tetaud E, di Rago JP, Jacq C. Why are many mRNAs translated to the vicinity of mitochondria: a role in protein complex assembly? Gene. 2005;354:64–71. [DOI] [PubMed] [Google Scholar]
- 61.Moor AE, Golan M, Massasa EE, et al. Global mRNA polarization regulates translation efficiency in the intestinal epithelium. Science. 2017;357(6357):1299–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rishal I, Fainzilber M. Cell size sensing-a one-dimensional solution for a three-dimensional problem? BMC Biol. 2019;17(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lechtreck KF, Van De Weghe JC, Harris JA, Liu P. Protein transport in growing and steady-state cilia. Traffic. 2017;18(5):277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hendel NL, Thomson M, Marshall WF. Diffusion as a Ruler: Modeling Kinesin Diffusion as a Length Sensor for Intraflagellar Transport. Biophys J. 2018;114(3):663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toriyama M, Sakumura Y, Shimada T, Ishii S, Inagaki N. A diffusion-based neurite length-sensing mechanism involved in neuronal symmetry breaking. Mol Syst Biol. 2010;6(1). https://www.embopress.org/doi/abs/10.1038/msb.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perry RB-T, Rishal I, Doron-Mandel E, et al. Nucleolin-Mediated RNA Localization Regulates Neuron Growth and Cycling Cell Size. Cell Rep. 2016;16(6):1664–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Besse F, Ephrussi A. Translational control of localized mRNAs: restricting protein synthesis in space and time. Nat Rev Mol Cell Biol. 2008;9(12):971–980. [DOI] [PubMed] [Google Scholar]
- 68.Gagnon JA, Mowry KL. Molecular motors: directing traffic during RNA localization. Crit Rev Biochem Mol Biol. 2011;46(3):229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fritzsche R, Karra D, Bennett KL, et al. Interactome of two diverse RNA granules links mRNA localization to translational repression in neurons. Cell Rep. 2013;5(6):1749–1762. [DOI] [PubMed] [Google Scholar]
- 70.Bi J, Tsai N-P, Lin Y-P, Loh HH, Wei L-N. Axonal mRNA transport and localized translational regulation of kappa-opioid receptor in primary neurons of dorsal root ganglia. Proc Natl Acad Sci U S A. 2006;103(52):19919–19924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghosh S, Marchand V, Gáspár I, Ephrussi A. Control of RNP motility and localization by a splicing-dependent structure in oskar mRNA. Nat Struct Mol Biol. 2012;19(4):441–449. [DOI] [PubMed] [Google Scholar]
- 72.Serano J, Rubin GM. The Drosophila synaptotagmin-like protein bitesize is required for growth and has mRNA localization sequences within its open reading frame. Proc Natl Acad Sci U S A. 2003;100(23):13368–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van De Bor V, Hartswood E, Jones C, Finnegan D, Davis I. gurken and the I factor retrotransposon RNAs share common localization signals and machinery. Dev Cell. 2005;9(1):51–62. [DOI] [PubMed] [Google Scholar]
- 74.Buckley PT, Lee MT, Sul J-Y, et al. Cytoplasmic intron sequence-retaining transcripts can be dendritically targeted via ID element retrotransposons. Neuron. 2011;69(5):877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jambhekar A, Derisi JL. Cis-acting determinants of asymmetric, cytoplasmic RNA transport. RNA. 2007;13(5):625–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bovaird S, Patel D, Padilla J-CA, Lécuyer E. Biological functions, regulatory mechanisms, and disease relevance of RNA localization pathways. FEBS Lett. 2018;592(17):2948–2972. [DOI] [PubMed] [Google Scholar]
- 77.Chang P, Torres J, Lewis RA, Mowry KL, Houliston E, King ML. Localization of RNAs to the mitochondrial cloud in Xenopus oocytes through entrapment and association with endoplasmic reticulum. Mol Biol Cell. 2004;15(10):4669–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hachet O, Ephrussi A. Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature. 2004;428(6986):959–963. [DOI] [PubMed] [Google Scholar]
- 79.Russell JH, Keiler KC. Subcellular localization of a bacterial regulatory RNA. Proc Natl Acad Sci U S A. 2009;106(38):16405–16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fei J, Sharma CM. RNA Localization in Bacteria. Microbiol Spectr. 2018;6(5). doi: 10.1128/microbiolspec.RWR-0024-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nevo-Dinur K, Nussbaum-Shochat A, Ben-Yehuda S, Amster-Choder O. Translation-independent localization of mRNA in E. coli. Science. 2011;331(6020):1081–1084. [DOI] [PubMed] [Google Scholar]
- 82.Prilusky J, Bibi E. Studying membrane proteins through the eyes of the genetic code revealed a strong uracil bias in their coding mRNAs. Proc Natl Acad Sci U S A. 2009;106(16):6662–6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu L, Wells D, Tay J, et al. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron. 1998;21(5):1129–1139. [DOI] [PubMed] [Google Scholar]
- 84.Huang Y-S, Carson JH, Barbarese E, Richter JD. Facilitation of dendritic mRNA transport by CPEB. Genes Dev. 2003;17(5):638–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagaoka K, Udagawa T, Richter JD. CPEB-mediated ZO-1 mRNA localization is required for epithelial tight-junction assembly and cell polarity. Nat Commun. 2012;3:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.St Johnston D Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol. 2005;6(5):363–375. [DOI] [PubMed] [Google Scholar]
- 87.Macdonald PM, Kerr K. Redundant RNA recognition events in bicoid mRNA localization. RNA. 1997;3(12):1413–1420. [PMC free article] [PubMed] [Google Scholar]
- 88.Weil TT, Forrest KM, Gavis ER. Localization of bicoid mRNA in late oocytes is maintained by continual active transport. Dev Cell. 2006;11(2):251–262. [DOI] [PubMed] [Google Scholar]
- 89.Ferrandon D, Elphick L, Nüsslein-Volhard C, St Johnston D. Staufen protein associates with the 3’UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell. 1994;79(7):1221–1232. [DOI] [PubMed] [Google Scholar]
- 90.Ferrandon D, Koch I, Westhof E, Nüsslein-Volhard C. RNA-RNA interaction is required for the formation of specific bicoid mRNA 3’ UTR-STAUFEN ribonucleoprotein particles. EMBO J. 1997;16(7):1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hwang H-W, Wentzel EA, Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science. 2007;315(5808):97–100. [DOI] [PubMed] [Google Scholar]
- 92.Miyagawa R, Tano K, Mizuno R, et al. Identification of cis- and trans-acting factors involved in the localization of MALAT-1 noncoding RNA to nuclear speckles. RNA. 2012;18(4):738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shukla CJ, McCorkindale AL, Gerhardinger C, et al. High-throughput identification of RNA nuclear enrichment sequences. EMBO J. 2018;37(6). doi: 10.15252/embj.201798452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lubelsky Y, Ulitsky I. Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature. 2018;555(7694):107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muslimov IA, Santi E, Homel P, Perini S, Higgins D, Tiedge H. RNA transport in dendrites: a cis-acting targeting element is contained within neuronal BC1 RNA. J Neurosci. 1997;17(12):4722–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin D, Pestova TV, Hellen CUT, Tiedge H. Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Mol Cell Biol. 2008;28(9):3008–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tiedge H, Chen W, Brosius J. Primary structure, neural-specific expression, and dendritic location of human BC200 RNA. J Neurosci. 1993;13(6):2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaneko T, Suzuki T, Kapushoc ST, et al. Wobble modification differences and subcellular localization of tRNAs in Leishmania tarentolae: implication for tRNA sorting mechanism. EMBO J. 2003;22(3):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bailey TL, Johnson J, Grant CE, Noble WS. The MEME Suite. Nucleic Acids Res. 2015;43(W1):W39–W49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duttke SH, Chang MW, Heinz S, Benner C. Identification and dynamic quantification of regulatory elements using total RNA. Genome Res. 2019;29(11):1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agostini F, Cirillo D, Ponti RD, Tartaglia GG. SeAMotE: a method for high-throughput motif discovery in nucleic acid sequences. BMC Genomics. 2014;15:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Ponty Y, Blanchette M, Lecuyer E, Waldispühl J. SPARCS: a web server to analyze (un) structured regions in coding RNA sequences. Nucleic Acids Res. 2013;41(W1):W480–W485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lorenz R, Bernhart SH, Höner Zu Siederdissen C, et al. ViennaRNA Package 2.0. Algorithms Mol Biol. 2011;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao J, Reinharz V, Major F, Waldispühl J. RNA-MoIP: prediction of RNA secondary structure and local 3D motifs from sequence data. Nucleic Acids Res. 2017;45(W1):W440–W444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kazan H, Ray D, Chan ET, Hughes TR, Morris Q. RNAcontext: a new method for learning the sequence and structure binding preferences of RNA-binding proteins. PLoS Comput Biol. 2010;6:e1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cléry A, Allain FH-T. FROM STRUCTURE TO FUNCTION OF RNA BINDING DOMAINS. Landes Bioscience; 2013. [Google Scholar]
- 107.Shan J, Munro TP, Barbarese E, Carson JH, Smith R. A molecular mechanism for mRNA trafficking in neuronal dendrites. J Neurosci. 2003;23(26):8859–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davey NE. The functional importance of structure in unstructured protein regions. Curr Opin Struct Biol. 2019;56:155–163. [DOI] [PubMed] [Google Scholar]
- 109.Chu J-F, Majumder P, Chatterjee B, Huang S-L, Shen C-KJ. TDP-43 Regulates Coupled Dendritic mRNA Transport-Translation Processes in Co-operation with FMRP and Staufen1. Cell Rep. 2019;29(10):3118–3133.e6. [DOI] [PubMed] [Google Scholar]
- 110.Castello A, Fischer B, Eichelbaum K, et al. Insights into RNA Biology from an Atlas of Mammalian mRNA-Binding Proteins. Cell. 2012;149(6):1393–1406. [DOI] [PubMed] [Google Scholar]
- 111.Ray D, Kazan H, Chan ET, et al. Rapid and systematic analysis of the RNA recognition specificities of RNA-binding proteins. Nat Biotechnol. 2009;27(7):667–670. [DOI] [PubMed] [Google Scholar]
- 112.Lambert N, Robertson A, Jangi M, McGeary S, Sharp PA, Burge CB. RNA Bind-n-Seq: quantitative assessment of the sequence and structural binding specificity of RNA binding proteins. Mol Cell. 2014;54(5):887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wheeler EC, Van Nostrand EL, Yeo GW. Advances and challenges in the detection of transcriptome-wide protein-RNA interactions: Advances and challenges in detection of transcriptome-wide protein-RNA interactions. WIREs RNA. 2018;9(1):e1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, Darnell RB. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302(5648):1212–1215. [DOI] [PubMed] [Google Scholar]
- 115.Licatalosi DD, Mele A, Fak JJ, et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456(7221):464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Nostrand EL, Pratt GA, Shishkin AA, et al. Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nat Methods. 2016;13(6):508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hafner M, Landthaler M, Burger L, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141(1):129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin C, Miles WO. Beyond CLIP: advances and opportunities to measure RBP-RNA and RNA-RNA interactions. Nucleic Acids Res. 2019;47(11):5490–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gong J, Ju Y, Shao D, Zhang QC. Advances and challenges towards the study of RNA-RNA interactions in a transcriptome-wide scale. Quantitative Biology. 2018;6(3):239–252. [Google Scholar]
- 120.St Johnston D, Driever W, Berleth T, Richstein S, Nüsslein-Volhard C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development. 1989;107 Suppl:13–19. [DOI] [PubMed] [Google Scholar]
- 121.Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol Cell. 2005;97(1):97–110. [DOI] [PubMed] [Google Scholar]
- 122.Arn EA, Cha BJ, Theurkauf WE, Macdonald PM. Recognition of a bicoid mRNA localization signal by a protein complex containing Swallow, Nod, and RNA binding proteins. Dev Cell. 2003;4(1):41–51. [DOI] [PubMed] [Google Scholar]
- 123.Wang ET, Taliaferro JM, Lee J-A, et al. Dysregulation of mRNA Localization and Translation in Genetic Disease. J Neurosci. 2016;36(45):11418–11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dahm R, Macchi P. Human pathologies associated with defective RNA transport and localization in the nervous system. Biol Cell. 2007;99(11):649–661. [DOI] [PubMed] [Google Scholar]
- 125.Chin A, Lécuyer E. RNA localization: Making its way to the center stage. Biochim Biophys Acta Gen Subj. 2017;1861(11 Pt B):2956–2970. [DOI] [PubMed] [Google Scholar]
- 126.Wang W, van Niekerk E, Willis DE, Twiss JL. RNA transport and localized protein synthesis in neurological disorders and neural repair. Dev Neurobiol. 2007;67(9):1166–1182. [DOI] [PubMed] [Google Scholar]
- 127.Baj G, D’Alessandro V, Musazzi L, et al. Physical exercise and antidepressants enhance BDNF targeting in hippocampal CA3 dendrites: further evidence of a spatial code for BDNF splice variants. Neuropsychopharmacology. 2012;37(7):1600–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baleriola J, Walker CA, Jean YY, et al. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell. 2014;158(5):1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zarei S, Carr K, Reiley L, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fiesel FC, Kahle PJ. TDP-43 and FUS/TLS: cellular functions and implications for neurodegeneration. FEBS J. 2011;278(19):3550–3568. [DOI] [PubMed] [Google Scholar]
- 131.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19(R1):R46–R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alami NH, Smith RB, Carrasco MA, et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81(3):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fallini C, Bassell GJ, Rossoll W. Spinal muscular atrophy: the role of SMN in axonal mRNA regulation. Brain Res. 2012;1462:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Donlin-Asp PG, Bassell GJ, Rossoll W. A role for the survival of motor neuron protein in mRNP assembly and transport. Curr Opin Neurobiol. 2016;39:53–61. [DOI] [PubMed] [Google Scholar]
- 135.Akten B, Kye MJ, Hao LT, et al. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc Natl Acad Sci U S A. 2011;108(25):10337–10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fallini C, Donlin-Asp PG, Rouanet JP, Bassell GJ, Rossoll W. Deficiency of the Survival of Motor Neuron Protein Impairs mRNA Localization and Local Translation in the Growth Cone of Motor Neurons. J Neurosci. 2016;36(13):3811–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. [DOI] [PubMed] [Google Scholar]
- 138.Kao D-I, Aldridge GM, Weiler IJ, Greenough WT. Altered mRNA transport, docking, and protein translation in neurons lacking fragile X mental retardation protein. Proc Natl Acad Sci U S A. 2010;107(35):15601–15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pilaz L-J, Lennox AL, Rouanet JP, Silver DL. Dynamic mRNA Transport and Local Translation in Radial Glial Progenitors of the Developing Brain. Curr Biol. 2016;26(24):3383–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goering R, Hudish LI, Guzman BB, et al. FMRP promotes RNA localization to neuronal projections through interactions between its RGG domain and G-quadruplex RNA sequences. bioRxiv. September 2019:784728. doi: 10.1101/784728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mankodi A, Logigian E, Callahan L, et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289(5485):1769–1773. [DOI] [PubMed] [Google Scholar]
- 143.Yi H, Xue L, Guo M-X, et al. Gene expression atlas for human embryogenesis. FASEB J. 2010;24(9):3341–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jedrusik A, Parfitt D-E, Guo G, et al. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev. 2008;22(19):2692–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Platts AE, Dix DJ, Chemes HE, et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet. 2007;16(7):763–773. [DOI] [PubMed] [Google Scholar]
- 146.Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet. 2016;17(12):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schuster A, Tang C, Xie Y, Ortogero N, Yuan S, Yan W. SpermBase: A Database for Sperm-Borne RNA Contents. Biol Reprod. 2016;95(5):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sharma U, Sun F, Conine CC, et al. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev Cell. 2018;46(4):481–494.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peng H, Shi J, Zhang Y, et al. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 2012;22(11):1609–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hadzhinesheva V, Nikolova V, Chakarova I, Delimitreva S, Markova M, Zhivkova R. Mammalian Balbiani body as a sign of ancestral oocyte asymmetry. Acta Morphol Anthropol. 2015;22:159–162. [Google Scholar]
- 151.Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30(1):34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jansova D, Tetkova A, Koncicka M, Kubelka M, Susor A. Localization of RNA and translation in the mammalian oocyte and embryo. PLoS One. 2018;13(3):e0192544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yen B Asymmetric Cell Division in the Generation of Immunity and Tolerance. 2018. doi: 10.7916/D8NP3GB7 [DOI] [Google Scholar]
- 154.Gómez-López S, Lerner RG, Petritsch C. Asymmetric cell division of stem and progenitor cells during homeostasis and cancer. Cell Mol Life Sci. 2014;71(4):575–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583–597. [DOI] [PubMed] [Google Scholar]
- 156.Tsunekawa Y, Britto JM, Takahashi M, Polleux F, Tan S-S, Osumi N. Cyclin D2 in the basal process of neural progenitors is linked to non-equivalent cell fates. The EMBO Journal. 2012;31(8):1879–1892. doi: 10.1038/emboj.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsunekawa Y, Kikkawa T, Osumi N. Asymmetric inheritance of Cyclin D2 maintains proliferative neural stem/progenitor cells: a critical event in brain development and evolution. Dev Growth Differ. 2014;56(5):349–357. [DOI] [PubMed] [Google Scholar]
- 158.Nousiainen HO, Kestilä M, Pakkasjärvi N, et al. Mutations in mRNA export mediator GLE1 result in a fetal motoneuron disease. Nat Genet. 2008;40(2):155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jao L-E, Akef A, Wente SR. A role for Gle1, a regulator of DEAD-box RNA helicases, at centrosomes and basal bodies. Mol Biol Cell. 2017;28(1):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Okamura M, Yamanaka Y, Shigemoto M, et al. Depletion of mRNA export regulator DBP5/DDX19, GLE1 or IPPK that is a key enzyme for the production of IP6, resulting in differentially altered cytoplasmic mRNA expression and specific cell defect. PLOS ONE. 2018;13(5):e0197165. doi: 10.1371/journal.pone.0197165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Batista LFZ, Pech MF, Zhong FL, et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature. 2011;474(7351):399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yarani R, Mirza AH, Kaur S, Pociot F. The emerging role of lncRNAs in inflammatory bowel disease. Exp Mol Med. 2018;50(12):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carow B, Hauling T, Qian X, Kramnik I, Nilsson M, Rottenberg ME. Spatial and temporal localization of immune transcripts defines hallmarks and diversity in the tuberculosis granuloma. Nat Commun. 2019;10(1):1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yao Y, Dai W. Genomic Instability and Cancer. J Carcinog Mutagen. 2014;5. doi: 10.4172/2157-2518.1000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zeraati M, Moye AL, Wong JWH, et al. Cancer-associated noncoding mutations affect RNA G-quadruplex-mediated regulation of gene expression. Sci Rep. 2017;7(1):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neelamraju Y, Gonzalez-Perez A, Bhat-Nakshatri P, Nakshatri H, Janga SC. Mutational landscape of RNA-binding proteins in human cancers. RNA Biol. 2018;15(1):115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang B, Babu KR, Lim CY, et al. A comprehensive expression landscape of RNA-binding proteins (RBPs) across 16 human cancer types. RNA Biol. October 2019:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leucci E, Vendramin R, Spinazzi M, et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature. 2016;531(7595):518–522. doi: 10.1038/nature17161 [DOI] [PubMed] [Google Scholar]
- 169.Panda S, Setia M, Kaur N, et al. Noncoding RNA Ginir functions as an oncogene by associating with centrosomal proteins. PLOS Biology. 2018;16(10):e2004204. doi: 10.1371/journal.pbio.2004204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stuart HC, Jia Z, Messenberg A, et al. Localized Rho GTPase Activation Regulates RNA Dynamics and Compartmentalization in Tumor Cell Protrusions. Journal of Biological Chemistry. 2008;283(50):34785–34795. doi: 10.1074/jbc.m804014200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jiang Y, Cao W, Wu K, et al. LncRNA LINC00460 promotes EMT in head and neck squamous cell carcinoma by facilitating peroxiredoxin-1 into the nucleus. Journal of Experimental & Clinical Cancer Research. 2019;38(1). doi: 10.1186/s13046-019-1364-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huot M-E E, Huot M, Brown CM, Lamarche-Vane N, Richard S. An Adaptor Role for Cytoplasmic Sam68 in Modulating Src Activity during Cell Polarization. Molecular and Cellular Biology. 2009;29(7):1933–1943. doi: 10.1128/mcb.01707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bergeman J, Caillier A, Houle F, Gagné LM, Huot M-É. Localized translation regulates cell adhesion and transendothelial migration. Journal of Cell Science. 2016:jcs.191320. doi: 10.1242/jcs.191320 [DOI] [PubMed] [Google Scholar]
- 174.Ward KR, Zhang K-X, Somasiri AM, Roskelley CD, Schrader JW. Expression of activated M-Ras in a murine mammary epithelial cell line induces epithelial-mesenchymal transition and tumorigenesis. Oncogene. 2004;23(6):1187–1196. [DOI] [PubMed] [Google Scholar]
- 175.Taliaferro JM, Vidaki M, Oliveira R, et al. Distal Alternative Last Exons Localize mRNAs to Neural Projections. Mol Cell. 2016;61(6):821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cajigas IJ, Tushev G, Will TJ, tom Dieck S, Fuerst N, Schuman EM. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron. 2012;74(3):453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tushev G, Glock C, Heumüller M, Biever A, Jovanovic M, Schuman EM. Alternative 3’ UTRs Modify the Localization, Regulatory Potential, Stability, and Plasticity of mRNAs in Neuronal Compartments. Neuron. 2018;98(3):495–511.e6. [DOI] [PubMed] [Google Scholar]
- 178.Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348(6233):aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ashley J, Cordy B, Lucia D, Fradkin LG, Budnik V, Thomson T. Retrovirus-like Gag Protein Arc1 Binds RNA and Traffics across Synaptic Boutons. Cell. 2018;172(1-2):262–274.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pastuzyn ED, Day CE, Kearns RB, et al. The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell. 2018;173(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alhowikan AM. Activity-Regulated Cytoskeleton-Associated Protein Dysfunction May Contribute to Memory Disorder and Earlier Detection of Autism Spectrum Disorders. Med Princ Pract. 2016;25(4):350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fromer M, Pocklington AJ, Kavanagh DH, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zimyanin VL, Belaya K, Pecreaux J, et al. In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell. 2008;134(5):843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Brendza RP, Serbus LR, Duffy JB, Saxton WM. A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science. 2000;289(5487):2120–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66(1):23–35. [DOI] [PubMed] [Google Scholar]
- 186.St Johnston D, Beuchle D, Nüsslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66(1):51–63. [DOI] [PubMed] [Google Scholar]
- 187.Trovisco V, Belaya K, Nashchekin D, et al. bicoid mRNA localises to the Drosophila oocyte anterior by random Dynein-mediated transport and anchoring. Elife. 2016;5. doi: 10.7554/eLife.17537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Betley JN, Heinrich B, Vernos I, Sardet C, Prodon F, Deshler JO. Kinesin II mediates Vg1 mRNA transport in Xenopus oocytes. Curr Biol. 2004;14(3):219–224. [DOI] [PubMed] [Google Scholar]
- 189.Yoon YJ, Mowry KL. Xenopus Staufen is a component of a ribonucleoprotein complex containing Vg1 RNA and kinesin. Development. 2004;131(13):3035–3045. [DOI] [PubMed] [Google Scholar]
- 190.Mori Y, Imaizumi K, Katayama T, Yoneda T, Tohyama M. Two cis-acting elements in the 3’ untranslated region of alpha-CaMKII regulate its dendritic targeting. Nat Neurosci. 2000;3(11):1079–1084. [DOI] [PubMed] [Google Scholar]
- 191.Mallardo M, Deitinghoff A, Müller J, et al. Isolation and characterization of Staufen-containing ribonucleoprotein particles from rat brain. Proc Natl Acad Sci U S A. 2003;100(4):2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kohrmann M, Luo M, Kaether C, DesGroseillers L, Dotti CG, Kiebler MA. Microtubule-dependent recruitment of Staufen-green fluorescent protein into large RNA-containing granules and subsequent dendritic transport in living hippocampal neurons. Mol Biol Cell. 1999;10(9):2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yamagishi M, Ishihama Y, Shirasaki Y, Kurama H, Funatsu T. Single-molecule imaging of β-actin mRNAs in the cytoplasm of a living cell. Exp Cell Res. 2009;315(7):1142–1147. [DOI] [PubMed] [Google Scholar]
- 194.Lifland AW, Zurla C, Yu J, Santangelo PJ. Dynamics of Native β-actin mRNA Transport in the Cytoplasm. Traffic. 2011;12(8):1000–1011. doi: 10.1111/j.1600-0854.2011.01209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang HL, Eom T, Oleynikov Y, et al. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31(2):261–275. [DOI] [PubMed] [Google Scholar]
- 196.Chao JA, Patskovsky Y, Patel V, Levy M, Almo SC, Singer RH. ZBP1 recognition of β-actin zipcode induces RNA looping. Genes Dev. 2010;24(2):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Deshler JO, Highett MI, Schnapp BJ. Localization of Xenopus Vg1 mRNA by Vera protein and the endoplasmic reticulum. Science. 1997;276(5315):1128–1131. [DOI] [PubMed] [Google Scholar]
- 198.Deshler JO, Highett MI, Abramson T, Schnapp BJ. A highly conserved RNA-binding protein for cytoplasmic mRNA localization in vertebrates. Curr Biol. 1998;8(9):489–496. [DOI] [PubMed] [Google Scholar]
- 199.Havin L, Git A, Elisha Z, et al. RNA-binding protein conserved in both microtubule- and microfilament-based RNA localization. Genes & Development. 1998;12(11):1593–1598. doi: 10.1101/gad.12.11.1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kwon S, Abramson T, Munro TP, John CM, Köhrmann M, Schnapp BJ. UUCAC- and Vera-Dependent Localization of VegT RNA in Xenopus Oocytes. Current Biology. 2002;12(7):558–564. doi: 10.1016/s0960-9822(02)00740-6 [DOI] [PubMed] [Google Scholar]
- 201.Aronov S, Aranda G, Behar L, Ginzburg I. Visualization of translated tau protein in the axons of neuronal P19 cells and characterization of tau RNP granules. J Cell Sci. 2002;115(Pt 19):3817–3827. [DOI] [PubMed] [Google Scholar]
- 202.Litman P, Barg J, Rindzoonski L, Ginzburg I. Subcellular localization of tau mRNA in differentiating neuronal cell culture: implications for neuronal polarity. Neuron. 1993;10(4):627–638. [DOI] [PubMed] [Google Scholar]
- 203.Atlas R, Behar L, Elliott E, Ginzburg I. The insulin-like growth factor mRNA binding-protein IMP-1 and the Ras-regulatory protein G3BP associate with tau mRNA and HuD protein in differentiated P19 neuronal cells. J Neurochem. 2004;89(3):613–626. [DOI] [PubMed] [Google Scholar]
- 204.Torvund-Jensen J, Steengaard J, Reimer L, Fihl LB, Laursen LS. Transport and translation of MBP mRNA is regulated differently by distinct hnRNP proteins. J Cell Sci. 2014;127(Pt 7):1550–1564. [DOI] [PubMed] [Google Scholar]
- 205.Hoek KS, Kidd GJ, Carson JH, Smith R. hnRNP A2 selectively binds the cytoplasmic transport sequence of myelin basic protein mRNA. Biochemistry. 1998;37(19):7021–7029. [DOI] [PubMed] [Google Scholar]
- 206.Norvell A, Kelley RL, Wehr K, Schüpbach T. Specific isoforms of squid, a Drosophila hnRNP, perform distinct roles in Gurken localization during oogenesis. Genes Dev. 1999;13(7):864–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dienstbier M, Boehl F, Li X, Bullock SL. Egalitarian is a selective RNA-binding protein linking mRNA localization signals to the dynein motor. Genes Dev. 2009;23(13):1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huynh J-R, Munro TP, Smith-Litière K, Lepesant J-A, St Johnston D. The Drosophila hnRNPA/B homolog, Hrp48, is specifically required for a distinct step in osk mRNA localization. Dev Cell. 2004;6(5):625–635. [DOI] [PubMed] [Google Scholar]
- 209.Yano T, López de Quinto S, Matsui Y, Shevchenko A, Shevchenko A, Ephrussi A. Hrp48, a Drosophila hnRNPA/B homolog, binds and regulates translation of oskar mRNA. Dev Cell. 2004;6(5):637–648. [DOI] [PubMed] [Google Scholar]
- 210.Groisman I, Huang Y-S, Mendez R, Cao Q, Theurkauf W, Richter JD. CPEB, Maskin, and Cyclin B1 mRNA at the Mitotic Apparatus: Implications for Local Translational Control of Cell Division. Cell. 2000;103(3):435–447. [DOI] [PubMed] [Google Scholar]
- 211.Olivier C, Poirier G, Gendron P, Boisgontier A, Major F, Chartrand P. Identification of a conserved RNA motif essential for She2p recognition and mRNA localization to the yeast bud. Mol Cell Biol. 2005;25(11):4752–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Long RM. She2p is a novel RNA-binding protein that recruits the Myo4p-She3p complex to ASH1 mRNA. The EMBO Journal. 2000;19(23):6592–6601. doi: 10.1093/emboj/19.23.6592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dynes JL, Steward O. Dynamics of bidirectional transport ofArc mRNA in neuronal dendrites. The Journal of Comparative Neurology. 2007;500(3):433–447. doi: 10.1002/cne.21189 [DOI] [PubMed] [Google Scholar]
- 214.Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21(4):741–751. [DOI] [PubMed] [Google Scholar]
- 215.Huang F, Chotiner JK, Steward O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci. 2007;27(34):9054–9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sylvestre J, Margeot A, Jacq C, Dujardin G, Corral-Debrinski M. The Role of the 3′ Untranslated Region in mRNA Sorting to the Vicinity of Mitochondria Is Conserved from Yeast to Human Cells. MBoC. 2003;14(9):3848–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zabezhinsky D, Slobodin B, Rapaport D, Gerst JE. An Essential Role for COPI in mRNA Localization to Mitochondria and Mitochondrial Function. Cell Rep. 2016;15(3):540–549. [DOI] [PubMed] [Google Scholar]
- 218.Saint-Georges Y, Garcia M, Delaveau T, et al. Yeast mitochondrial biogenesis: a role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS One. 2008;3(6):e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.García-Rodríguez LJ, Gay AC, Pon LA. Puf3p, a Pumilio family RNA binding protein, localizes to mitochondria and regulates mitochondrial biogenesis and motility in budding yeast. J Cell Biol. 2007;176(2):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Olivas W, Parker R. The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J. 2000;19(23):6602–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tian L, Chou H-L, Zhang L, Okita TW. Targeted Endoplasmic Reticulum Localization of Storage Protein mRNAs Requires the RNA-Binding Protein RBP-L. Plant Physiol. 2019;179(3):1111–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tian L, Chou H-L, Zhang L, et al. RNA-Binding Protein RBP-P Is Required for Glutelin and Prolamine mRNA Localization in Rice Endosperm Cells. Plant Cell. 2018;30(10):2529–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Banerjee A, Ifrim MF, Valdez AN, Raj N, Bassell GJ. Aberrant RNA translation in fragile X syndrome: From FMRP mechanisms to emerging therapeutic strategies. Brain Res. 2018;1693(Pt A):24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14(6):926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Majumder P, Chu J-F, Chatterjee B, Swamy KBS, Shen C-KJ. Co-regulation of mRNA translation by TDP-43 and Fragile X Syndrome protein FMRP. Acta Neuropathol. 2016;132(5):721–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Racca C, Gardiol A, Eom T, Ule J, Triller A, Darnell RB. The Neuronal Splicing Factor Nova Co-Localizes with Target RNAs in the Dendrite. Front Neural Circuits. 2010;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zalfa F, Adinolfi S, Napoli I, et al. Fragile X mental retardation protein (FMRP) binds specifically to the brain cytoplasmic RNAs BC1/BC200 via a novel RNA-binding motif. J Biol Chem. 2005;280(39):33403–33410. [DOI] [PubMed] [Google Scholar]
- 228.Kriegel AJ, Terhune SS, Greene AS, Noon KR, Pereckas MS, Liang M. Isomer-specific effect of microRNA miR-29b on nuclear morphology. J Biol Chem. 2018;293(36):14080–14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Saunders C, Cohen RS. The role of oocyte transcription, the 5′ UTR, and translation repression and derepression in Drosophila gurken mRNA and protein localization. Mol Cell. 1999;3(1):43–54. [DOI] [PubMed] [Google Scholar]
- 230.Groisman I, Huang Y-S, Mendez R, Cao Q, Theurkauf W, Richter JD. CPEB, Maskin, and Cyclin B1 mRNA at the Mitotic Apparatus. Cell. 2000;103(3):435–447. doi: 10.1016/s0092-8674(00)00135-5 [DOI] [PubMed] [Google Scholar]
- 231.Olivas W The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. The EMBO Journal. 2000;19(23):6602–6611. doi: 10.1093/emboj/19.23.6602 [DOI] [PMC free article] [PubMed] [Google Scholar]


