Abstract
Soil salinity is a major factor affecting rice growth and productivity worldwide especially at seedling stage. Many genes for salt tolerance have been identified and applied to rice breeding, but the actual mechanism of salt tolerance remains unclear. In this study, seedlings of 664 cultivated rice varieties from the 3000 Rice Genome Project (3K-RG) were cultivated by hydroponic culture with 0.9% salt solution for trait identification. A genome-wide association study (GWAS) of salt tolerance was performed using different models of analysis. Twenty-one QTLs were identified and two candidate genes named OsSTL1 (Oryza sativa salt tolerance level 1) and OsSTL2 (Oryza sativa salt tolerance level 2) were confirmed using sequence analysis. Haplotype and sequence analysis revealed that gene OsSTL1 was a homolog of salt tolerance gene SRP1 (Stress associated RNA-binding protein 1) in Arabidopsis. The hap1 of OsSTL1 was identified as the superior haplotype and a non-synonymous SNP was most likely to be the functional site. We also determined that the level of salt tolerance was improved by combining haplotypes of different genes. Our study provides a foundation for molecular breeding and functional analysis of salt tolerance in rice seedlings.
Subject terms: Genome informatics, Genome-wide association studies, Plant genetics
Introduction
Rice (Oryza sativa L.) is an important food crop1 and soil salinity is a major factor affecting its growth and productivity worldwide2. Approximately one-third of the land area on which rice is grown is affected by salinity3–5. Rice seedlings are particularly sensitive to biotic and abiotic stresses6. High salinity impedes water and nutrient absorption from the soil. This inhibits seedling growth and ultimately decreases yield7,8. Therefore, development of salt-tolerant varieties in rice is a very critical target of rice breeding programs in coastal areas9,10.
An understanding of the genetic basis of salinity tolerance is required to facilitate development of varieties with salinity tolerance using marker-assisted selection11. Salt tolerance in rice is controlled by multiple genes. Many QTLs for salt tolerance at the seedling stage have been reported2,3,5,6,12,13. However, only two genes have been cloned, namely, SKC1, and qSE35,14. These genes have been applied to rice breeding6, but the actual mechanism of salt tolerance remains unclear15. Therefore, identification of further QTLs/genes related to salt tolerance will help in characterizing unknown salt tolerance mechanisms and facilitate breeding applications.
Genome-wide association studies have been widely used to identify genetic variants affecting complex traits, either by comparative analysis or correlation analysis, and have identified many SNPs associated with target traits13,16–18. For example, thirty-three candidate genes in a protein interaction network were associated with salt tolerance in rice seedlings in a genome-wide association study (GWAS) using 295 accessions13. Because molecular marker-assisted selection (MAS) demands explicit genetic architecture of agronomic traits19 identification of new QTLs/genes related to salt tolerance in rice and confirmation of elite alleles is necessary for their utilization in modern molecular breeding.
In the present study, we performed a GWAS using 664 cultivated rice accessions from the 3,000 Rice Genome (3K-RG)1. Integrated gene annotation, genetic variation, homology and haplotype analysis were performed to identify candidate genes and possible causal polymorphisms for salt tolerance traits. Our results provide insight into the genetic architecture of salt tolerance and markers derived from the newly identified genes will be useful in improving salinity stress tolerance in rice seedlings.
Materials and Methods
Plant materials and experiments
The rice diversity panel comprising 664 cultivated rice accessions from the 3K Rice Genome (3K-RG)1,20 included 226 genotypes from the mini-core collection that were selected from an original core set of 4,310 accessions21, and 438 lines from the International Rice Molecular Breeding Network22. Both collections have accessions from 44 countries representing major rice-growing regions of the world (Table S1). In order to analyze evolutionary aspects we studied an additional publicly available set of 446 wild rice accessions from a previous report23. The public transcriptomic data of IR29 was download from NCBI (https://www.ncbi.nlm.nih.gov/gds), series accession ID: GSE119720.
Phenotypic data for salt tolerance
After treatment with salt solution, the symptoms of salt damage in the roots and leaves of seedlings were assessed visually24. From July 5 to September 10 in 2015, the diversity panel was cultivated in hydroponic culture with open-air environment that the average temperature from 14 °C to 32 °C at Xinjiang academy of agricultural sciences with 0.9% salt solution for two months for trait identification. First, the rice seeds were surface-sterilized and soaked in water to promote germination for 2 days at 30 °C. For each variety, seven seeds with normal budding and the same growth potential were transplanted to a gauze-based foam board (30 cm × 42 cm). Each foam board containing 10 varieties was placed in a plastic box containing a 0.9% salt solution. The salt solution was replaced once every two weeks for about 2 months (Fig. S1). Phenotyping was based on the International Rice Research Institute (IRRI) salt tolerance/alkali identification criteria (i.e., salt tolerance/alkali standard growth score and salt/alkali standard dead leaf percentage)25–27. After salt stress, the symptoms of plant leaves and roots were observed and recorded on the basis of seven criteria (Shoot length (SL), Root length (RL), Shoot fresh weight (SFW), Shoot dry weight (SDW), Root fresh weight (RFW), Root dry weight (RDW) and Salt tolerance level (STL)). Salt tolerance level (STL) was based on the percentage of dead leaf blade tissue.
Genotypic data of 664 materials and GWAS for salt tolerance
Genetic variation (single nucleotide polymorphism (SNP)) data for the 664 accessions were obtained from the 3K-RG publicly available database, which included approximately 17 million highly credible SNPs and 2.4 million indels aligned to the cv. Nipponbare IRGSP 1.0 reference genome. Publicly available SNP set for the 446 wild rice accessions were downloaded from http://www.ncgr.ac.cn/RiceHap323.
We identified 3,513,863, 2,280,242 and 1,855,669 SNPs as a credible SNP set after removing SNPs with missing rates >30% and minor allele frequencies <5% in full, indica and japonica populations. Principal component (PC) and kinship matrix analyses were performed in order to account for population structure. A neighbor-joining tree was constructed using 68,376 SNPs evenly distributed throughout the genome. The first three PC were used to construct the PC matrix. We performed a GWAS of salt tolerance traits of the 664 accessions using a compressed mixed linear model (CMLM) and general linear model (GLM) using the SNP set and default settings in GAPIT software. We also performed FaST-LMM using FaST-LMM software28 in order to compare three different models. Due to the non-independence of SNPs caused by strong LD, the thresholds derived from the total number of markers were usually too rigorous for detection of significant associations. Suggestive thresholds were calculated using the formula “-log10(1 / effective number of independent SNPs)” as described previously28–30 and effective numbers of independent SNPs were determined by PLINK31 (window size 50, step size 50, r2 ≥ 0.2) to be 117,880, 94,282 and 42,831 in the full population as well as in indica and japonica subpopulations, respectively. We divided the STL (Y) of each accession into the original genotypic effect (G) and the fixed effect of population structure (P). Through regression analysis of each PC on Y, we calculated the average effect of each PC in the PC matrix on each individual (P); G was excluding P from Y. Then G was randomly reshuffled as G-new, and the P + G-new was reconstructed to the novel phenotype of each accession. The conditional permutation test of total of 1,000 sets of P + Gr was performed using GLM with the same parameters and PC matrix. The threshold was therefore set at -log (P) = 4 to identify significant associations. LD heatmaps surrounding peaks in the GWAS were constructed using “LD heatmaps” in the R package32.
Data analysis
Differences in phenotypic values between alleles of each non-synonymous SNP were assessed by Student’s t-tests. The sequence alignment of each gene was determined using non-synonymous SNPs associated with STL, and differences in phenotypic value between haplotypes of each gene were calculated by one-way ANOVA or Student’s t-tests. Duncan’s multiple range tests were conducted to make comparisons if the results of the one-way ANOVA were significant (P < 0.01). Nucleotide diversity (π)33,34 and Tajima’s D35 were calculated using an in-house Perl script.
Results
Population structure and phenotypic variation in ST traits among 664 cultivated rice accessions
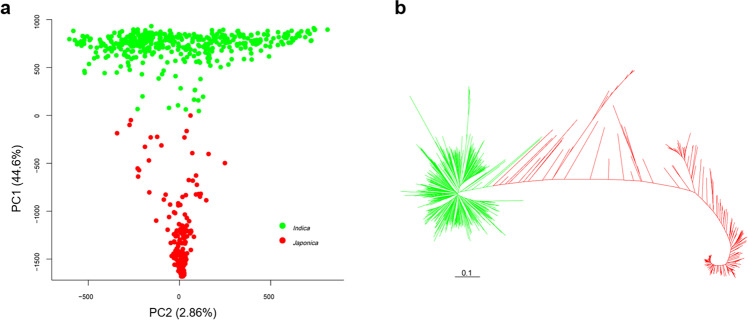
Prior to GWAS we performed a PC and kinship matrix analysis based on 3.5 million SNPs to characterize the population structure (Fig. 1). There was a distinct subpopulation structure; PC1 separated the accessions into indica (428 accessions) and japonica (236 accessions) subpopulations explaining 44.6% of the total genetic variation. The kinship matrix and neighbor-joining tree also showed similar results (Figs. 1 and S2).
Figure 1.
Population structure of 664 rice accessions. (a) Principal component analysis and (b) neighbor-joining tree for all accessions; green lines represent indica, and red lines represent japonica. PC analysis was performed using 3.5 million SNPs with missing data rates ≤ 30% and minor allele frequency ≥ 5%. Neighbor-joining tree was constructed using 68,376 SNPs evenly distributed throughout the genome.
In order to confirm differences in phenotype between the indica and japonica subpopulations we performed statistical analyses of all seven salt tolerance-related traits. There were significant differences between indica and japonica for RFW, RDW and SFW but no differences in RL, SL, SDW and STL (Fig. S3). The descriptive statistics for the two sub-populations are provided in (Table 1) and the distribution for each trait is shown in Fig. S4. The mean STL were 4.91 and 5.14 in indica and japonica, respectively. These results indicated the presence of genetic variation in salt tolerance in both indica and japonica.
Table 1.
Descriptive statistics for seven phenotypes in subpopulations.
| Phenotype | Range | Mean ± SD | Skewness | Kurtosis | CV (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ind | Jap | Ind | Jap | Ind | Jap | Ind | Jap | Ind | Jap | |
| SL (mm) | 4.00~ 34.20 | 16.30~ 34.00 | 23.11 ± 3.78 | 23.69 ± 3.39 | 0.12 | 0.40 | 1.57 | 0.03 | 0.16 | 0.14 |
| SFW (g) | 0.18~ 1.23 | 0.23~ 0.92 | 0.48 ± 0.14 | 0.45 ± 0.12 | 0.89 | 0.97 | 3.06 | 1.22 | 0.29 | 0.27 |
| SDW (g) | 0.03~ 0.28 | 0.02~ 0.25 | 0.15 ± 0.04 | 0.14 ± 0.03 | 0.14 | 0.22 | 0.26 | 0.57 | 0.27 | 0.21 |
| RL (mm) | 8.80~ 24.60 | 9.60~ 26.30 | 15.41 ± 2.85 | 15.13 ± 2.80 | 0.41 | 0.84 | 0.17 | 1.72 | 0.18 | 0.19 |
| RFW (g) | 0.15~ 1.28 | 0.18~ 1.05 | 0.49 ± 0.15 | 0.46 ± 0.14 | 0.92 | 0.91 | 2.20 | 1.20 | 0.31 | 0.30 |
| RDW (g) | 0.02~ 0.14 | 0.02~ 0.12 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.88 | 1.01 | 2.21 | 1.57 | 0.33 | 0.33 |
| STL | 1~ 9 | 1~ 9 | 4.91 ± 1.80 | 5.14 ± 1.46 | 0.48 | 0.33 | 0.03 | 0.77 | 0.37 | 0.28 |
CV: coefficient of variation; Ind, Indica; Jap, Japonica.
Correlation analysis showed that STL was significantly correlated with most aboveground and below ground traits. RFW and RDW were highly correlated (0.872), whereas the STL was not significantly correlated with RL (0.002) (Table S2). The STL of most varieties was about 5 and a very small proportion (11 accessions) had a low STL indicative of high salt tolerance. We therefore adopted STL as a meaningful indicator of salt tolerance and focused on salt tolerance traits in subsequent analyses.
Identification of QTLs for STL by GWAS
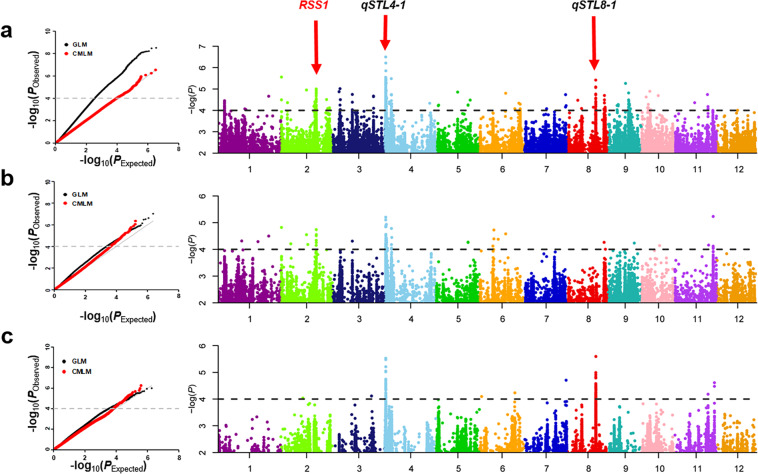
The general linear model (GLM), compressed mixed linear model (CMLM) and Factored Spectrally Transformed Linear Mixed Models (FaST-LMM) were performed using GAPIT and FaST-LMM to identify association signals in the indica, japonica and full populations, using the STL data and 3.5 million SNPs. Manhattan plots of STL show that detection using all three models was consist, especially CMLM and FaST-LMM (Figs. 2 and S5–6).
Figure 2.
GWAS for salt tolerance in rice seedlings. Quantile–quantile plots and Manhattan plots for the GWAS in the full (a), indica (b) and japonica (c) populations using CMLM. In quantile-quantile plots, black dots are for GLM, and red points are for CMLM. In Manhattan plots, the gene in red was previously cloned, and QTLs in black were considered to be important. Dashed horizontal line for each population indicates the suggestive threshold (P = 1.0 × 10−4).
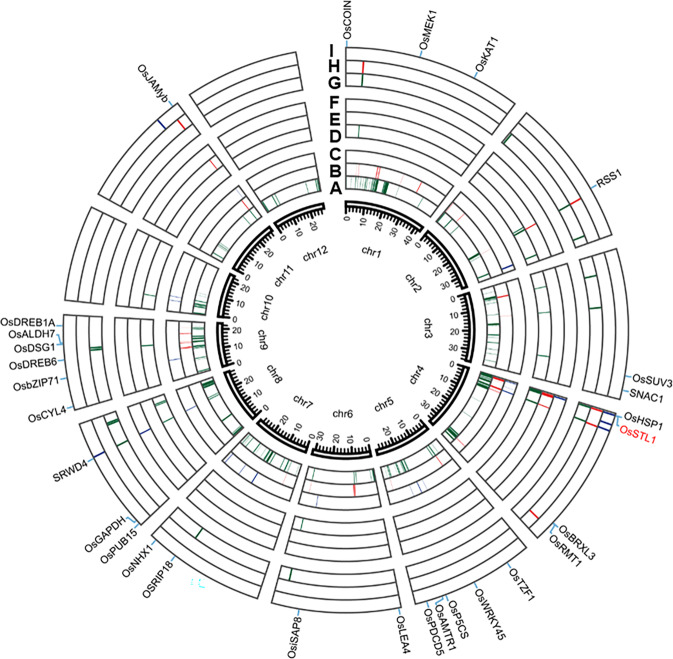
We identified 321, 76, and 103 SNPs for STL by GWAS at -log (P) significance levels of 4 in the full population, indica and japonica subpopulations, respectively, when using CMLM (Fig. 2). With reported genome-wide linkage disequilibrium (LD) decay rates of up to 167 kb16,23 adjacent significant SNPs with distances less than 170 kb were merged into single association signals. The SNPs with the minimum P value in a signal region was considered the lead SNP. Fifteen, 4 and 2 QTLs were identified by GWAS using CMLM (Table S3), 124, 32 and 16 QTLs were identified using GLM model (Table S4), and 15, 6 and 5 QTLs were identified using FaST-LMM (Table S5) in the full population, indica and japonica, respectively (Fig. 3). Most of the QTLs detected in CMLM (12/15, 4/4, 2/2) were also detected by FaST-LMM, and all QTLs identified by CMLM and FaST-LMM were included among those detected by GLM.
Figure 3.
Circos map of all association signals for salt tolerance in the full population, indica and japonica using GLM, CMLM and FaST-LMM. The colored blocks in each layer from inner to outer represent QTLs detected from in the full population (a), indica subpopulation (b), japonica subpopulation (c) using GLM, and in the same populations (d,e,f) using CMLM and (g,h,i) using FaST-LMM, respectively. Twenty-eight known genes are labeled with black script at the outermost layer; red color represents candidate genes detected in this study.
By minimizing false positives caused by population structure we determined that CMLM was the most suitable of the three models for detecting STL QTL in our populations by comparing the quantile-quantile plots from the three models in each population (Figs. 2 and S5–6). We also optimized the parameters in the CMLM for STL in each GWAS panel. Detailed comparisons identified qSTL4-1 all three populations with Chr4_487087 as its lead SNP. Three of four QTLs in indica and both QTLs in japonica were also identified in the full population, indicating that these detected QTLs were stable in both sub-populations. The fact that almost half of the QTLs (10/21) overlapped with those found previously36 supported the reliability of the present results (Table S3).
One of the QTLs detected by CMLM probably corresponded to the cloned salt tolerance gene RSS1 (Fig. 3, Table S3). The RSS1 protein has a putative role in maintenance and viability of meristematic cells in rice plants under salt stress37. There were as many as 28 and 1 cloned genes related to salt tolerance that corresponded to present QTLs detected by the GLM and FaST-LMM, respectively (Fig. 3, Tables S4-5).
Determination of candidate genes within selected QTLs
We chose two QTLs for detailed study; qSTL8-1 was identified in the full population and japonica subpopulation, and was also identified in a previous report36 whereas qSTL4-1 was detected in all three populations implying that they were worthy of pursuing to the candidate gene level (Figs. 4a and 5a).
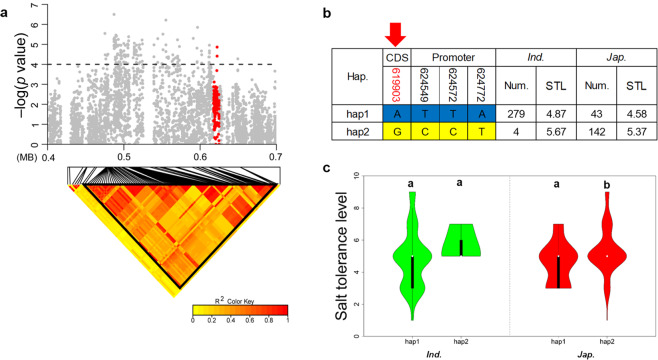
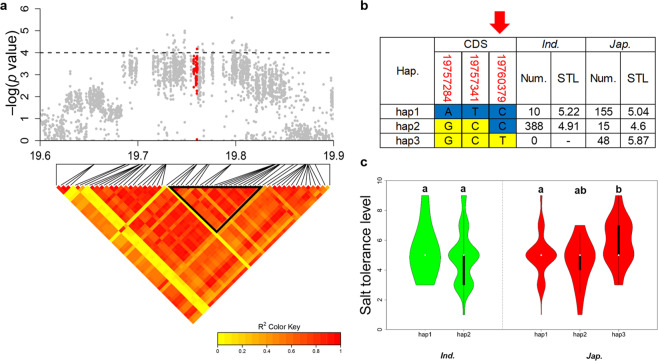
Figure 4.
Dissection of OsSTL1 for salt tolerance on chromosome 4. (a) Local Manhattan plot (upper) and LD heatmap (lower) surrounding the lead SNP for STL on chromosome 4. Red dots represent all SNPs within OsSTL1. (b) Different haplotypes of OsSTL1 in indica and japonica. The red number identifies the nonsynonymous mutation; red arrow shows the functional site. (c) Comparison of the STL trait for salt tolerance among OsSTL1 haplotypes in indica and japonica using one-way ANOVA. Green violins represent indica and red violins represent japonica. Different letters indicate significant differences (p < 0.01) detected by one-way ANOVA.
Figure 5.
Dissection of OsSTL2 for salt tolerance on chromosome 8. (a) Local Manhattan plot (upper) and LD heatmap (lower) surrounding the lead SNP for STL on chromosome 8. Red dots represent all SNPs within OsOsSTL2. (b) Different haplotypes of OsSTL2 in indica and japonica. The red number identifies the nonsynonymous mutation; red arrow shows the functional site. (c) Comparison of the STL trait for salt tolerance among OsSTL2 haplotypes in indica and japonica using one-way ANOVA. Green violins represent indica and red violins represent japonica. Different letters indicate significant differences (p < 0.01) detected by one-way ANOVA.
qSTL4-1 contained 154 SNPs at suggestive thresholds of four. The candidate region of 0.49 Mb to 0.62 Mb in qSTL4-1 was evaluated using pairwise LD correlations with a threshold r2 > 0.638 (Fig. 4a). The candidate region in qSTL4-1 contained 12 genes of which three were retrotransposons. The other 9 genes were possible candidate genes. Haplotype analysis was performed for each gene. One candidate gene had variable numbers of non-synonymous SNPs with a -log (P) value greater than two and significant SNPs within the promoter. There was no non-synonymous SNP with a -log (P) value greater than 2 in two genes (Fig. S7c & e) and the other 7 genes showed very significant differences between haplotypes in indica or japonica rice (Figs. 4, S7a-b,d,f-g).
Two of the genes differing between indica and japonica were LOC_Os04g01780 and LOC_Os04g01920. According to the gene annotations LOC_Os04g01780 is an uncharacterized expressed ACR (Act Domain Repeat), COG1399 (Clusters of Orthologous Groups 1399) family gene, and LOC_Os04g01920 is an expressed protein gene. Many genes for salt tolerance have been annotated as zinc finger family members. The third gene in the group, LOC_Os04g02000, was annotated as a zinc finger family protein gene and therefore was more likely to be the candidate functional gene for salt tolerance. According to the public transcriptomic data (The public transcriptomic data of IR29 was download from NCBI (https://www.ncbi.nlm.nih.gov/gds), series accession ID: GSE119720), the RPKMRSalt/RPKMControls (RPKM, Reads Per Kilobase per Million mapped reads) of LOC_Os04g02000 expression in IR29 was greater than 1.4, indicating differential gene expression between salt and controls. This candidate gene was finally named as OsSTL1.
qSTL8-1 contained total of 14 significant SNPs. The candidate region of 19.74 Mb to 19.85 Mb in qSTL8-1 was evaluated using pairwise LD correlations (at threshold r2 > 0.6) (Fig. 5a). Three genes were located in the candidate region and were therefore considered more likely to be candidate genes. Haplotype analysis was performed for each gene as above. LOC_Os08g31850 and LOC_Os08g31860 showed no significant difference between the indica and japonica subpopulations (Fig. S8a) whereas LOC_Os08g31870 was annotated as a cell division cycle protein gene, and showed significant differences within the japonica subpopulation, but not in indica (Figs. 5 and S8b).
LOC_Os08g31870 is putatively expressed cell division cycle protein 48 and this led us to hypothesize that LOC_Os08g31870 was more likely to be the candidate functional gene. According to the public transcriptomic data (The public transcriptomic data of IR29 was download from NCBI (https://www.ncbi.nlm.nih.gov/gds), series accession ID: GSE119720), the RPKMRSalt/RPKMControls (RPKM, Reads Per Kilobase per Million mapped reads) of LOC_Os08g31870 expression in IR29 was greater than 2, indicating differential gene expression between salt and controls. This candidate gene was finally named as OsSTL2.
Elite alleles and the origin of the OsSTL1
We focused on OsSTL1 with one non-synonymous SNP in the coding region (Chr4_619903, base G-A, amino acid P-S) located on RanBP2-type domain and 3 SNPs in the promoter region; two haplotypes (hap1 and hap2) were identified (Fig. 4b). There was a significant difference in STL between hap1 and hap2 of OsSTL1 in japonica; the mean STL of hap1 (4.58, 43 accessions) was lower than that for hap2 (5.37, 142 accessions). Therefore, hap1 of OsSTL1 was a superior genotype and its frequency in japonica could be increased in that subpopulation in order to increase its overall salt tolerance (Fig. 4). On the other hand, this haplotype was almost fixed in indica. Comparing the two haplotypes, the non-synonymous mutation Chr4_619903 was more likely to be the functional site in OsSTL1.
We compared OsSTL1 with homologs in other crops using BLAST and found that the homolog SRP1 (AT2G17975) in Arabidopsis was involved in salt tolerance39. Stress associated RNA-binding protein 1 (SRP1) encodes a C2C2-type zinc finger protein in Arabidopsis. A knock-out mutation in the srp1 allele reduced sensitivity to ABA and salt stress at all growth stages whereas SRP1-overexpressing seedlings were more sensitive to ABA and salt than wild type.
For confirmation of the superior haplotype and functional site of OsSTL1 we investigated 446 wild rice (O. rufipogon) accessions using publicly available sequence data23 to determine the origin of OsSTL1. We compared the nucleotide diversity (π)33,34 and Tajima’s D35 values for wild rice with those for the indica and japonica subpopulations. The π value for OsSTL1 increased from wild rice to japonica, πjaponica/ πwild > 2, D = 4.67, and deviated significantly from zero. It was possible that balanced selection occurred in japonica during its domestication (Table S6). The π value for OsSTL1 in indica was less than that for wild rice, πwild/πindica > 2, D = −1.33 indicating positive selection in indica. Although the overall diversity in cultivated rice was slightly increased it was clear that indica and japonica had undergone different selection directions in adapting to different environments during domestication.
Elite alleles and the origin of OsSTL2
We also evaluated OsSTL2 in which there were three non-synonymous SNPs (Chr8_19757284, base A-G, amino acid L-P, Chr8_19757341, base T-C, amino acid H-R and Chr8_19760379, base C-T, amino acid E-K) and three haplotypes (hap1, hap2 and hap3) were identified (Fig. 5b). The performance of hap3 of OsSTL2 differed significantly from hap1 and hap2 in japonica. There was no significant difference in STL between hap1 and hap2 of OsSTL2 in both subpopulations. The hap1 and hap2 of OsSTL2 was the superior genotype in both indica and japonica (Fig. 5). By comparing the two haplotypes, the SNP of Chr8_19760379 was most likely to the functional site. The identified superior genotype and functional site of the candidate gene will help to improve salinity tolerance in rice by breeding.
Nucleotide diversity (π) and Tajima’s D were calculated for OsSTL2. The four populations had the same level of diversity (Table S7) suggesting no past selection of OsSTL2 in the different populations.
Haplotype combinations of OsSTL1 and OsSTL2
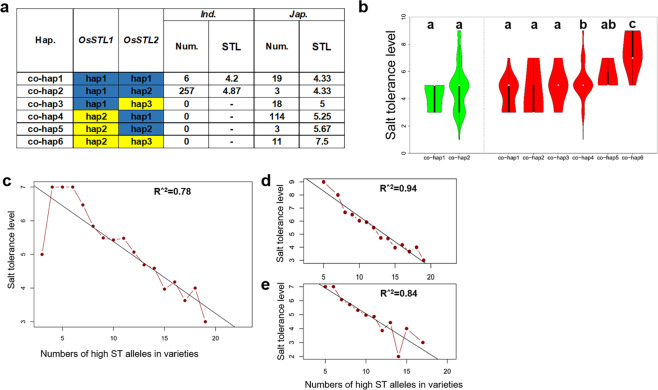
In order to improve breeding of salt tolerant genotypes for efficient utilization of OsSTL1 and OsSTL2, we performed combination haplotype analysis of the two genes. The co-hap1 (combination with hap1 of OsSTL1 and hap1 of OsSTL2) and co-hap2 (combination with hap1 of OsSTL1 and hap2 of OsSTL2) represented the superior allele; the co-hap3(combination with hap1 of OsSTL1 and hap3 of OsSTL2), co-hap4 (combination with hap2 of OsSTL1 and hap1 of OsSTL2) and co-hap5 (combination with hap2 of OsSTL1 and hap2 of OsSTL2) represented the intermediate allele, and co-hap6 (combination with hap2 of OsSTL1 and hap3 of OsSTL2) represented the inferior allele. The mean STL of accessions with co-hap1 in japonica had significant lower values (p < 0.05) than those with co-hap4 and co-hap6, whereas, the mean STL of accessions with co-hap6 in japonica were significantly higher (p < 0.05) than those with other haplotypes as determined by one-way ANOVA (Fig. 6a,b). Based on these results, we concluded that the haplotypes of the two candidate genes were correctly identified and that pyramiding of the individually superior haplotypes improved the level of salt tolerance.
Figure 6.
Functional validation of haplotype combinations of OsSTL1 and OsSTL2. (a) STL for different haplotype combinations of OsSTL1 and OsSTL2. Blue color represents superior alleles and yellow color represents inferior alleles. (b) Comparison of the STL trait for salt tolerance among haplotype combinations of OsSTL1 and OsSTL2 in indica and japonica using one-way ANOVA. Green violins represent indica and red violins represent japonica. Different letters indicate significant differences (p < 0.05) detected by one-way ANOVA. Pyramiding of lead SNP of high salt tolerance alleles in the full population (c), indica (d) and japonica (e).
We screened 11 varieties with STL = 1 from the salt tolerance assays. Two were japonica and nine were indica. Ten of these varieties were co-hap2 (Table S8). Clearly, the overall salinity tolerance level in breeding populations can be achieved by increasing the frequency of co-hap2.
Discussion
In recent years, more than 170 genes for salt stress response have been cloned by forward or reverse genetic strategies in rice5,14,15,40,41. However, only a few reported genes for salt tolerance have been applied in rice breeding5,6,14. Gene pyramiding has become a powerful strategy to address complex traits6. Based on those studies, the salt tolerant genes will be used for new variety improvement by molecular assisted selection. However, molecular design breeding has not been made a great of progress because there have been no enough accurate genetic loci of the important agronomic traits and precise superior haplotypes dissected so far.
The resequencing based genotype provides vast natural variations, and the functional genes and elite alleles (superior haplotype) in natural populations could be explored by GWAS13,42. We detected the previously known salt stress tolerance gene RSS1 in our QTL analysis using CMLM. Many previously salt stress response genes identified using mutants and gene differential expression with no mutations or few variations in our populations were not detected in this model. Therefore, different populations may be required to detect different functional genes.
Among 664 diverse rice accessions, we identified functional candidate genes OsSTL1 and OsSTL2. We confirmed that hap1 of OsSTL1 was superior haplotype, while hap1 and hap2 of OsSTL2 were superior haplotypes. In addition, we identified the functional sites of the two candidate genes as the SNP Chr4_619903 and the Chr8_19760379, in which bases A and C caused the respective superior alleles.
The frequency of the A base allele in Chr4_619903 of OsSTL1 in japonica was 23% (43 of 185 accessions), indicating that the utilization level of this allele was relatively low. Hence, the frequency of this allele can be increased to improve overall salt tolerance in japonica. By contrast the frequency of the same allele in indica was almost 98.5% and overall improvement in salt tolerance in this subspecies would require exploitation of the different genes. The frequency of the C base allele in Chr8_19760379 of OsSTL2 was high in both indica (99.3%) and japonica (78.0%) indicating far less gains in overall salt tolerance being obtained by selection of alleles at this locus (Fig. 6a,b). These results suggested that OsSTL1 in indica and japonica might have experienced different levels of selection in adaptation to different environments during domestication, however there was no evidence of differential selection of OsSTL2 in the two subpopulations. Currently, the OsSTL1 is most important genetic resource for molecular breeding to improve salt tolerance in japonica. The two candidate genes were only identified by GWAS and haplotype analysis, further functional tests will be needed.
Many major genes that significantly contribute to important agronomic traits can be pyramided to develop elite rice varieties19,43. The greatest gains are to be made by identification of the most superior functional allele (lead SNP) at each locus38. We identified the lead SNP in each of 21 QTLs and confirmed its superiority by phenotype. We then examined the correlation of the number of superior SNPs with STL values and found that STL increased with number of superior SNPs; the R2 values for the full population, indica and japonica were 0.78, 0.94 and 0.84, respectively (Fig. 6c–e, Table S9). These results indicate that as for many other traits, pyramiding of superior alleles of important genes for salinity stress will lead to new varieties with improved tolerance. Obviously the precise identification of superior haplotypes will have a critical bearing on the outcomes of molecular breeding.
Supplementary information
Acknowledgements
The research was supported by The Projects of Ministry of Science and Technologies in China (2015BAD02B01) and The National Key Research and Development Program of China (2017YFD010050504).
Author contributions
J.Y., F.W. and Z.L. designed the experiments. J.Y., Z.Z., Y.Z., X.W. and F.T. performed most of experiments. X.W.,Y.Z. and J.Y. analyzed the data. F.W. and Z.L. conceived and supervised the project. X.W., Y.Z., N.K. and Z.L. wrotethe manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jie Yuan, Xueqiang Wang and Yan Zhao.
Contributor Information
Fengbin Wang, Email: xjnkywfb@163.com.
Zichao Li, Email: lizichao@cau.edu.cn.
Supplementary information
is available for this paper at 10.1038/s41598-020-66604-7.
References
- 1.Wang W, et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature. 2018;557:43–49. doi: 10.1038/s41586-018-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, et al. Identification of QTLs with main, epistatic and QTL x environment interaction effects for salt tolerance in rice seedlings under different salinity conditions. Theor Appl Genet. 2012;125:807–815. doi: 10.1007/s00122-012-1873-z. [DOI] [PubMed] [Google Scholar]
- 3.Prasad SR, Bagali PG, Hittalmani S, Shashidhar HE. Molecular mapping of quantitative trait loci associated with seedling tolerance to salt stress in rice (Oryza sativa L.) Current Science. 2000;78:162–164. [Google Scholar]
- 4.Takehisa H, et al. Identification of quantitative trait loci for plant growth of rice in paddy field flooded with salt water. Field Crops Research. 2004;89:85–95. doi: 10.1016/j.fcr.2004.01.026. [DOI] [Google Scholar]
- 5.He Y, et al. A quantitative trait locus, qSE3, promotes seed germination and seedling establishment under salinity stress in rice. Plant J. 2019;97:1089–1104. doi: 10.1111/tpj.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang Y, et al. Simultaneous Improvement and Genetic Dissection of Salt Tolerance of Rice (Oryza sativa L.) by Designed QTL Pyramiding. Front Plant Sci. 2017;8:1275. doi: 10.3389/fpls.2017.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan SL, et al. Proteomic identification of OsCYP2, a rice cyclophilin that confers salt tolerance in rice (Oryza sativa L.) seedlings when overexpressed. BMC Plant Biol. 2011;11:34. doi: 10.1186/1471-2229-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismail A, Takeda S, Nick P. Life and death under salt stress: same players, different timing? J Exp Bot. 2014;65:2963–2979. doi: 10.1093/jxb/eru159. [DOI] [PubMed] [Google Scholar]
- 9.Lin HX, et al. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor Appl Genet. 2004;108:253–260. doi: 10.1007/s00122-003-1421-y. [DOI] [PubMed] [Google Scholar]
- 10.Ali AJ, et al. Hidden diversity for abiotic and biotic stress tolerances in the primary gene pool of rice revealed by a large backcross breeding program. Field Crops Research. 2006;97:66–76. doi: 10.1016/j.fcr.2005.08.016. [DOI] [Google Scholar]
- 11.Molla KA, Debnath AB, Ganie SA, Mondal TK. Identification and analysis of novel salt responsive candidate gene based SSRs (cgSSRs) from rice (Oryza sativa L.) BMC Plant Biol. 2015;15:122. doi: 10.1186/s12870-015-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng, H. et al. Genetic structure, linkage disequilibrium and association mapping of salt tolerance in japonica rice germplasm at the seedling stage. Molecular Breeding35, 10.1007/s11032-015-0342-1 (2015).
- 13.Yu J, Zao W, He Q, Kim TS, Park YJ. Genome-wide association study and gene set analysis for understanding candidate genes involved in salt tolerance at the rice seedling stage. Mol Genet Genomics. 2017;292:1391–1403. doi: 10.1007/s00438-017-1354-9. [DOI] [PubMed] [Google Scholar]
- 14.Ren ZH, et al. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Guo Y. Unraveling salt stress signaling in plants. J Integr Plant Biol. 2018;60:796–804. doi: 10.1111/jipb.12689. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet. 2010;42:961–967. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, et al. Genomic architecture of heterosis for yield traits in rice. Nature. 2016;537:629–633. doi: 10.1038/nature19760. [DOI] [PubMed] [Google Scholar]
- 18.Liu HJ, Yan J. Crop genome-wide association study: a harvest of biological relevance. Plant J. 2019;97:8–18. doi: 10.1111/tpj.14139. [DOI] [PubMed] [Google Scholar]
- 19.Zeng D, et al. Rational design of high-yield and superior-quality rice. Nat Plants. 2017;3:17031. doi: 10.1038/nplants.2017.31. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, et al. The 3,000 rice genomes project. GigaScience. 2014;3:7. doi: 10.1186/2047-217X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, et al. A core collection and mini core collection of Oryza sativa L. in China. Theor Appl Genet. 2011;122:49–61. doi: 10.1007/s00122-010-1421-7. [DOI] [PubMed] [Google Scholar]
- 22.Yu SB, et al. Molecular diversity and multilocus organization of the parental lines used in the International Rice Molecular Breeding Program. Theor Appl Genet. 2003;108:131–140. doi: 10.1007/s00122-003-1400-3. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, et al. A map of rice genome variation reveals the origin of cultivated rice. Nature. 2012;490:497–501. doi: 10.1038/nature11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu XY, Mei MT, Yan XL, Zheng SL, Lu YG. Preliminary Detection of Quantitative Trait Loci for Salt Tolerance in Rice. Chinese Journal of Rice Science. 2000;14:65–70. [Google Scholar]
- 25.Lee, K. S. Variability and genetics of salt tolerance in japonica rice (Oryza sativa L.). J International Rice Research Institute Repository (1995).
- 26.Qi D-L, Han LZ, Zhang SY. Methods of Characterization and Evaluation of Salt or Alkaline Tolerance in Rice. Journal of Plant Genetic Resources. 2005;6:226–231. [Google Scholar]
- 27.Lee SY, et al. Mapping of quantitative trait loci for salt tolerance at the seedling stage in rice. Molecules and Cells. 2006;21:192. [PubMed] [Google Scholar]
- 28.Lippert C, et al. FaST linear mixed models for genome-wide association studies. Nat Methods. 2011;8:833–835. doi: 10.1038/nmeth.1681. [DOI] [PubMed] [Google Scholar]
- 29.Yang W, et al. Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat Commun. 2014;5:5087. doi: 10.1038/ncomms6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, et al. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat Genet. 2016;48:1233–1241. doi: 10.1038/ng.3636. [DOI] [PubMed] [Google Scholar]
- 31.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin, J. H., Blay, S., Mcneney, B. & Graham, J. LDheatmap: An R Function for Graphical Display of Pairwise Linkage Disequilibria between Single. Journal of Statistical Software16 (2006).
- 33.Watterson GA. On the number of segregating sites in genetical models without recombination. Theoretical Population Biology. 1975;7:256–276. doi: 10.1016/0040-5809(75)90020-9. [DOI] [PubMed] [Google Scholar]
- 34.Tajima F. Evolutionary relationship of DNA sequences in finite populations. Genetics. 1983;105:437–460. doi: 10.1093/genetics/105.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Leon TB, Linscombe S, Subudhi PK. Molecular Dissection of Seedling Salinity Tolerance in Rice (Oryza sativa L.) Using a High-Density GBS-Based SNP Linkage Map. Rice. 2016;9:52. doi: 10.1186/s12284-016-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa D, Morita H, Hattori T, Takeda S. Molecular characterization of the rice protein RSS1 required for meristematic activity under stressful conditions. Plant Physiol Biochem. 2012;61:54–60. doi: 10.1016/j.plaphy.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Yano K, et al. Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat Genet. 2016;48:927–934. doi: 10.1038/ng.3596. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, et al. A Novel RNA-Binding Protein Involves ABA Signaling by Post-transcriptionally Repressing ABI2. Front Plant Sci. 2017;8:24. doi: 10.3389/fpls.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan P, et al. SENSITIVE TO SALT1, An Endoplasmic Reticulum-Localized Chaperone, Positively Regulates Salt Resistance. Plant Physiol. 2018;178:1390–1405. doi: 10.1104/pp.18.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakaminami K, et al. AtPep3 is a hormone-like peptide that plays a role in the salinity stress tolerance of plants. Proc Natl Acad Sci USA. 2018;115:5810–5815. doi: 10.1073/pnas.1719491115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, et al. Loci and natural alleles underlying robust roots and adaptive domestication of upland ecotype rice in aerobic conditions. PLoS Genet. 2018;14:e1007521. doi: 10.1371/journal.pgen.1007521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Li Y, Zhu JK. Developing naturally stress-resistant crops for a sustainable agriculture. Nat Plants. 2018;4:989–996. doi: 10.1038/s41477-018-0309-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.