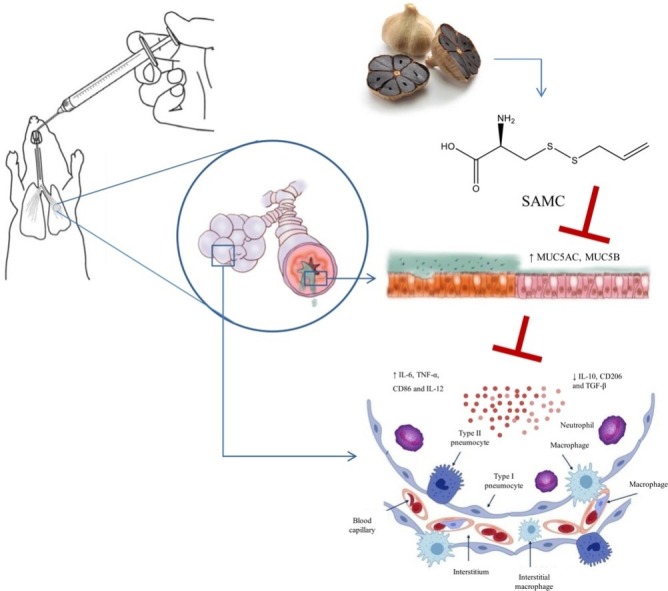
Graphical abstract
Keywords: SAMC, Mucin overproduction, Inflammation, MAPKs, PI3K-Akt, Acute respiratory distress syndrome
Abstract
Cytokine storm is an important cause of acute respiratory distress syndrome and multiple organ failure. Excessive secretion and accumulation of mucins on the surface of airway cause airway obstruction and exacerbate lung infections. MUC5AC and MUC5B are the main secreted mucins and overexpressed in various inflammatory responses. S-allylmercaptocysteine, a water-soluble organic sulfur compound extracted from garlic, has anti-inflammatory and anti-oxidative effects for various pulmonary diseases. The aim of this work was to investigate the therapeutic effects of SAMC on mucin overproduction and inflammation in 16HBE cells and LPS-induced ARDS mice. Results show that SAMC treatment ameliorated inflammatory cell infiltration and lung histopathological changes in the LPS-induced ARDS mice. SAMC also inhibited the expressions of MUC5AC and MUC5B, decreased the production of pro-inflammatory markers (IL-6, TNF-α, CD86 and IL-12) and increased the production of anti-inflammatory markers (IL-10, CD206 and TGF-β). These results confirm that SAMC had potential beneficial effects on suppressed hyperinflammation and mucin overexpression. Furthermore, SAMC exerted the therapeutic effects through the inhibition of phosphorylation of MAPKs and PI3K-Akt signaling pathways in the 16HBE cells and mice. Overall, our results demonstrate the effects of SAMC on the LPS-induced mucin overproduction and inflammation both in the 16HBE cells and mice.
1. Introduction
Acute respiratory distress syndrome (ARDS) is a clinical manifestation of the lung injury caused by infectious, non-infectious, and other damaging events, which is characterized by hypoxic respiratory failure and often accompanied by severe pulmonary inflammation [1,2]. Lung inflammation imbalance can induce cytokine storm, leading to worsening of ARDS. When severe acute respiratory syndrome (SARS) caused by coronavirus (SARS-CoV) swept China in 2003, the cytokine storm was the direct cause of deaths for many patients [3]. In 2009, hyperinflammation was observed in patients with ARDS caused by H1N1 virus infection [4]. In coronavirus disease 2019 (COVID-19), the cytokine storm syndrome was also an important cause of respiratory failure in many patients, especially young patients [5,6]. There are currently no effective drugs to cure the cytokine storm in patients with ARDS. Therefore, there is an urgent need to explore alternative and complementary medicines.
In normal physiology, mucins play a critical role in defending the respiratory tract against pathogens and toxins [7]. However, the excessive production of bronchial mucins blocks the airway, restricts airflow, and accelerates the decline of lung functions [8]. In addition, it also endangers mucociliary functions and decreases mucus clearance, leading to aggravated lung infections [9]. MUC5AC and MUC5B, two major secreted mucins, are secreted by goblet/mucous cells including many specialized epithelial cells, which are closely related to the viscoelasticity of sputum [10,11]. Mei-Juan Liu et al. demonstrate that lipopolysaccharides (LPS) could induce the high expression of MUC5AC and epidermal growth factor receptor (EGFR) in 16HBE cells [12].
After the intratracheal administration, LPS activates the EGF receptor and subsequently triggers downstream signaling cascades such as mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase-Akt (PI3K-Akt) signaling pathways [13]. MAPK members are the critical signal transduction molecules that involve in inflammatory responses and regulate the synthesis of MUC5AC, including p38 MAP kinases, c-jun N-terminal kinases (JNK), extracellular signal-related kinases 1/2 (ERK1/2) [14]. Besides, Akt-mTOR-STAT3, as a downstream of PI3K, also regulates inflammation and the MUC5AC expression [15]. Therefore, drugs regulating MAPKs and PI3K-Akt signaling pathways may have the therapeutic potential for mucus hypersecretion and pulmonary cytokine storm.
Aged garlic extract (AGE) is an odorless product created through prolonged extraction of fresh garlic, which has a strong anti-oxidative, free radical scavenging, anti-inflammatory and other activities [[16], [17], [18]]. S-allylmercaptocysteine (SAMC), as the major water-soluble garlic component of AGE, has shown the prospect in the treatment of mucus hypersecretion and anti-inflammation in an in-vitro COPD model [19]. However, the therapeutic effects of SAMC on LPS-induced mucin overexpression and cytokine storm in the ARDS model have not yet evaluated.
In this study, we evaluated the effect of SAMC on mucin overproduction and inflammatory responses in 16HBE cells and mice. LPS was used to induce MUC5AC overexpression and inflammation. MAPKs and PI3K-Akt signaling pathways involved in the protective effect of SAMC were also examined both in vitro and in vivo.
2. Materials and methods
2.1. Regents
RPMI-1640 and fetal bovine serum (FBS) were purchased from Biological Industries (Beit Haemek Ltd., Israel). 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT), penicillin and streptomycin were purchased from Solarbio Biotechnology (Beijing, China). Lipopolysaccharides from Escherichia coli O55:B5 (LPS) were purchased from Sigma-Aldrich Co. (St Louis, MO, U.S.A.). Mouse mucin-5 subtype AC (MUC5AC) ELISA kit was purchased from CUSABIO (Wuhan, China). IL-6, IL-12, IL-10, TGF-β enzyme-linked immunosorbent assay kits were purchased from Shanghai MultiSciences (Lianke) Biotech Co., Ltd. (Shanghai, China). Anti-MUC5AC, AKT, mTOR, p-mTOR, STAT3, p-STAT3, GAPDH and β-ACTIN antibodies were purchased from Abcam (Cambridge, MA, U.S.A.). Anti-p-AKT antibody was obtained from Proteintech Group, Inc. (Chicago, IL, U.S.A.). Anti-JNK, p-JNK, p38 MAPK, p-p38 MAPK antibodies were purchased from Cell Signaling Technology (Danvers, MA, U.S.A.). Horseradish peroxidase (HRP)-conjugated antibodies were bought from Jackson Immuno Research Laboratories, Inc. (West Grove, PA, U.S.A.). Other reagents used in the experiment were all of analytical grade.
2.2. Cell culture and treatment
The human bronchial epithelial cell line (16HBE) was a generous gift from the Department of Pulmonary Disease, Qilu Hospital, Shandong University (Shandong, China). 16HBE cells were cultured in the RPMI-1640 medium supplemented with 10 % (v/v) FBS, 100 U/mL of penicillin G and 100 μg/mL of streptomycin, at 37 °C in a humidified, 5% CO2 incubator. Before experiments, 16HBE cells were cultured in serum-free RPMI-1640 for 12 h to maintain a low basal level of cytokines and MUC5AC expression. Then, 16HBE cells were stimulated with various concentrations of LPS for 12 h to study the effect of LPS on the MUC5AC expression and cytokine secretion. The cells were treated with SAMC for another 11 h after 1 h treatment with LPS.
2.3. MTT assay
The MTT assay was conducted to assess the cell viability. Briefly, 16HBE cells (8000 cells/well) were seeded in 96-well plates and treated with SAMC and LPS. After the treatment, 20 μl of MTT solution (5 mg/mL) was added into each well and incubated at 37 °C for another 4 h. After the medium with MTT was removed, 100 μl/well DMSO was added to dissolve the formatted crystal formazan. Cell viability was assessed by recording the absorbance at 490 nm using Infinite® M200 PRO NanoQuant Reader (TECAN, Switzerland).
2.4. Animal experiment
The 6−8-week old BALB/c mice were purchased from Jinan Pengyue Experimental Animal Breeding Co. LTD (Certificate No. SCXK 20190003, Shandong, China). The animals were fed with standard commercial diets and water and housed in independent ventilated cages in a temperature-controlled animal house (25 ± 2 °C) with a 12-h day/night cycle. The animals were allowed to acclimatize for 1 week. The experiment was carried out according to the Declaration of Helsinki, and all animal protocols and procedures were approved by the Animal Care and Use Committee of Shandong University (No. 2016020, Jinan, Shandong, China). The mice were randomly divided into 5 groups (n = 5): control group (C), model group (M), LPS + SAMC 20 group (20 mg/kg, L), LPS + SAMC 40 group (40 mg/kg, H), LPS + NAC group (500 mg/kg, P). After anesthesia, LPS (10 mg/kg) was instilled into the lungs of mice through a high-pressure syringe (Penn-Century, PA, USA). Then, mice were orally administrated with SAMC, NAC or the vehicle after 6 and 18 h, respectively. Control mice received the same volume of saline. All mice were sacrificed at 24 h.
2.5. Hematoxylin and eosin
The lung tissues were fixed in 4% paraformaldehyde for 24 h and then embedded in paraffin wax and sectioned (at 4-μm thickness). The tissue sections were stained with hematoxylin and eosin (HE) following the standard protocols. The slides were then examined with a morphometric microscope (Olympus Corporation, Tokyo, Japan). Lung injury scoring criteria of the ARDS mouse model were as following: alveolar and interstitial hemorrhage, pulmonary edema, alveolar or interstitial inflammatory cells infiltration or aggregation, and thickness of alveolar wall/hyaline membrane formation. Lung injury was scored according to the severity of the damage range of 0–4 with 0 for no damage and 4 for the maximum damage.
2.6. AB-PAS and immunohistochemistry staining
The lung tissues were fixed in 4% paraformaldehyde for 24 h and then embedded in paraffin wax and sectioned (at 4-μm thickness). The tissue sections were stained with Alcian blue-periodic acid Schiff (AB-PAS) following the standard protocols. The slides were observed under a morphometric microscope at 400× to reflect the localization of mucins in mouse airways.
The expression of MUC5AC in lung tissues was detected by an immunohistochemical analysis. In brief, the sections were dewaxed, rehydrated, and subjected to microwave-based antigen retrieval in the citrate buffer. After blocking with the goat serum, the lung tissues were incubated overnight with an anti-MUC5AC antibody. 3,3-diaminobenzidine (DAB, ZSGB-BIO) was used to make the immunostaining visible. These sections were photographed using the microscope at 400×.
2.7. Analysis of bronchoalveolar lavage fluid (BALF)
After the 24-h LPS challenge, the mice were sacrificed and subjected to bronchoalveolar lavage (BAL) using 0.8 mL PBS three times. The recovery rate of the fluid was approximately 80 %. The collected bronchoalveolar lavage fluid was used to measure the expressions of MUC5AC protein, pro-inflammatory cytokines (IL-6, IL-12) and anti-inflammatory cytokines (IL-10, TGF-β) by the ELISA assay. All protocols were conducted according to the specified manufacturer’s instructions.
2.8. Western blot analysis
16HBE cells and lung tissues were lysed in Radio-Immunoprecipitation Assay (RIPA) lysis buffers (Solarbio, Beijing, China). Cell lysates and lung homogenates were centrifuged at 12,000×g for 30 min. Equal quantities of protein extracts were loaded on a SDS-PAGE and transferred to PVDF membranes (Millipore Corp., Bedford, MA, U.S.A.). The membranes were blocked with 5% (w/v) non-fat milk for 2 h at room temperature, and then incubated with diluted primary antibodies overnight at 4 °C. After washed three times for 10 min with 0.1 % Tween-20 in TBST, the membranes were incubated with a horseradish peroxidase conjugated secondary antibody for 1 h at room temperature. The protein bands were detected using enhanced chemiluminescence detection reagents (Millipore Corp., Bedford, MA, U.S.A.) under visualization in a Minichemi™Chemiluminescence Imaging System (Sagecreation, Beijing, China).
2.9. RT-PCR analysis
Total RNA was isolated from 16HBE cells and lung tissues using a TRIzol Reagent (CWBIO, Beijing, China), following the manufacturer’s instructions. The cDNA was synthesized from 2 μg RNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, U.S.A.), and the levels of gene mRNA transcripts were analyzed by using the specific primers and Ultra SYBR Mixture (CWBIO, Beijing, China) on CFX96M Touch Real-Time PCR Detection System (Bio Rad, Hercules, California, U.S.A.). The PCR primer sequences for tested genes were shown in Table 1 . Relative expressions of the different genes were determined with the 2−ΔΔCT method using GAPDH as the endogenous control.
Table 1.
Primer sequences used for RT-PCR analysis.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| Mouse β-ACTIN | TCCATCATGAAGTGTGACGT | GAGCAATGATCTTGATCTTCAT |
| Mouse Muc5ac | CTACTGACTGCACCAACACAT | GTGCAGTCCCCATGTACTGT |
| Mouse Muc5b | TAGCATGAGCGCCTTACACC | CACGACGCAGTTGGATGTTG |
| Mouse IL-6 | TGTGCAATGGCAATTCTGAT | CTCTGAAGGACTCTGGCTTTG |
| Mouse TNF-α | GGCGGTGCCTATGTCTCA | TCCACTTGGTGGTTTGTGA |
| Mouse CD86 | TCTGCCGTGCCCATTTACAA | TGTGCCCAAATAGTGCTCGT |
| Mouse IL-12 | GGGGTGTAACCAGAAAG | CATGAGGAATTGTAATAGCG |
| Mouse IL-10 | ACCTGGTAGAAGTGATGCC | CACCTTGGTCTTGGAGCT |
| Mouse CD206 | AGGCTGATTACGAGCAGTGG | CCATCACTCCAGGTGAACCC |
| Mouse TGF-β | AAGTGGGCCGAGAACTGG | CCTCCTTCACGCTGACAATC |
| Mouse Egfr | GAAGAAGTGCCCCCGAAACT | TCGTAGTAGTCAGGCCCACA |
| Human β-ACTIN | CCAACCGCGAGAAGATGA | CCAGAGGCGTACAGGGATAG |
| Human MUC5AC | AGCCGGGAACCTACTACTCG | TGGTCATAGGCTTCGTGCAG |
| Human MUC5B | CAAGTCCGCATCAACACGAC | TGCTGAGTACTTGGACGCTC |
| Human IL-6 | CCTGAACCTTCCAAAGATGGC | TTCACCAGGCAAGTCTCCTCA |
| Human TNF-α | AGCCCATGTTGTAGCAAACC | TGAGGTACAGGCCCTCTGAT |
| Human CD86 | CGACGTTTCCATCAGCTTGTC | CGCGTCTTGTCAGTTTCCAG |
| Human IL-12 | CACAAAGGAGGCGAGGTTCT | TTTGGGTTCTTTCTGGTCCTT |
| Human IL-10 | CCAGACATCAAGGCGCATGT | GATGCCTTTCTCTTGGAGCTTATT |
| Human CD206 | GATTGCAGGGGGCTTATGGG | CGGACATTTGGGTTCGGGAG |
| Human TGF-β | GCAACAATTCCTGGCGATACC | ATTTCCCCTCCACGGCTCAA |
| Human EGFR | CCGCAAAGTGTGTAACGGAA | CCACTGATGGAGGTGCAGTT |
2.10. Statistical analysis
All experimental data are presented as mean ± SD of at least three experiments. The one-way analysis of variance (ANOVA) was used to determine whether there were any statistically significant differences between the means of independent groups with a post hoc test for between-group comparisons using GraphPad Prism 5.0. P < 0.05 was considered to be statistically significant.
3. Results
3.1. SAMC and LPS have no cytotoxicity on 16HBE cells in the test concentrations
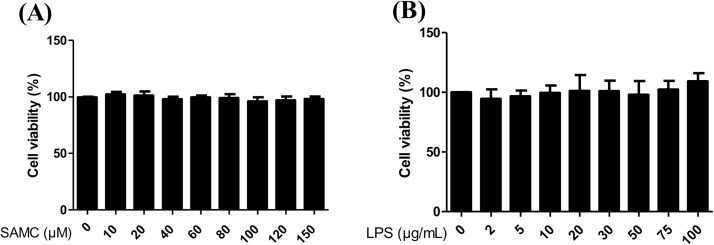
Cell cytotoxicity was detected by the MTT assay. 16HBE cells were treated with various doses of SAMC or LPS for 12 h. As shown in Fig. 1 A and B, no cell cytotoxicity was found under the tested concentrations of SAMC and LPS on 16HBE cells.
Fig. 1.
Cell viability of 16HBE cells following the exposure to SAMC or LPS. The 16HBE cells were exposed to 0-150 μM SAMC (A) or 0-100 μg/mL LPS (B) for 12 h, and cell viability was detected by the MTT assay.
3.2. SAMC inhibits the expression of mucins in LPS-induced 16HBE cells and mice
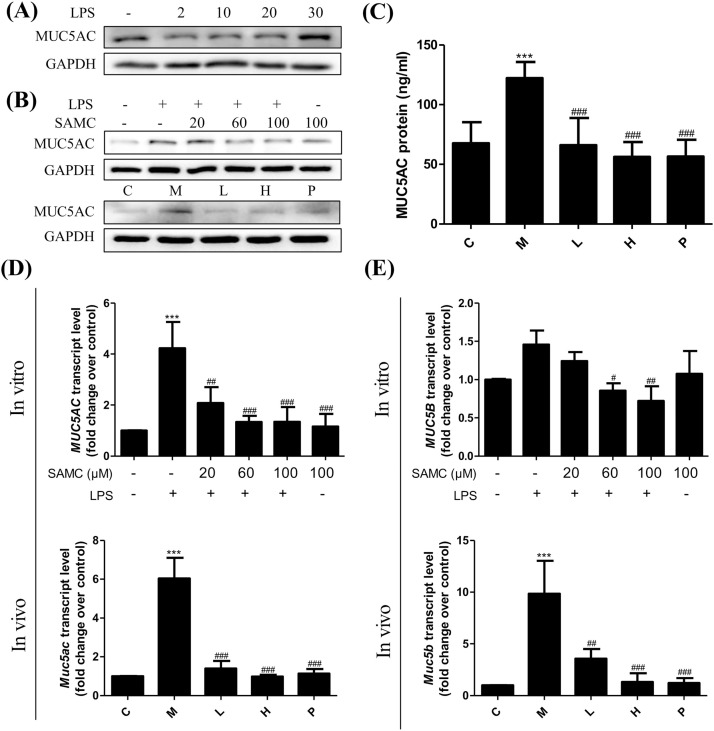
MUC5AC and MUC5B are mainly secreted by the large-airway submucosal glands, and their overexpression probably can be greatly caused by cough, airway obstruction and infection. As shown in Fig. 2 A, the level of MUC5AC was significantly increased when the concentration of LPS was up to 30 μg/mL. Thus, 30 μg/mL LPS was selected and used for the further experiments. Strikingly, the administration with SAMC significantly decreased the production of MUC5AC after 1 h treatment of LPS in 16HBE cells and in mice via the western blot (Fig. 2B), ELISA (Fig. 2C) and PCR analyses (Fig. 2D). The MUC5B content was determined by a RT-PCR analysis both in vitro and in vivo. Results demonstrate that the MUC5B gene level was significantly reduced after the treatment with SAMC (Fig. 2E).
Fig. 2.
Effects of SAMC on mucus hypersecretion induced by LPS in 16HBE cells and mice. (A) MUC5AC protein expression induced by LPS in 16HBE cells stimulated by different concentrations of LPS. (B) SAMC decreased the expression of MUC5AC protein both in vitro and in vivo. (C) SAMC reduced MUC5AC protein in mouse BALF determined by ELISA. (D) SAMC inhibited MUC5AC/ Muc5ac mRNA both in vitro and in vivo. (E) SAMC inhibited MUC5B/ Muc5b mRNA both in vitro and in vivo. Study groups: C: control group, orally treated with the vehicle only; M: model group, LPS was instilled into the lungs of mice through a high-pressure syringe; L and H: orally treated with SAMC 20 and 40 mg/kg after the LPS challenge, respectively; P: positive control, NAC dosed at 500 mg/kg; n = 5 per group. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control group; # p < 0.05, ## p < 0.01, ### p < 0.001 compared with the model group. The data are represented as means ± SD. All the experiments were repeated three times.
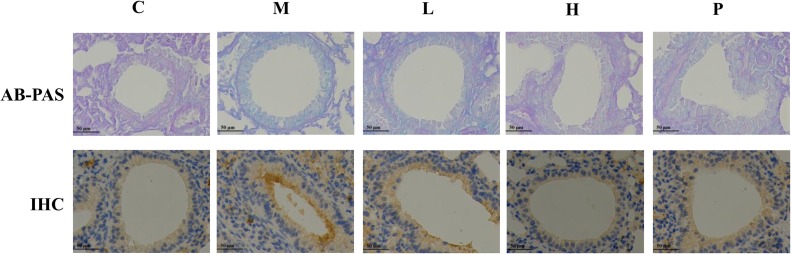
To examine the airway morphologic changes, we performed the AB-PAS staining. As seen in Fig. 3 , the airway surface mucin secretion was increased, small airways were thickened and alveolar space was collapsed after exposure to LPS. SAMC administration notably alleviated these histopathological changes. Immunohistochemical experiments showed that the MUC5AC positive staining in the airway epithelium was increased in the lung tissues of the LPS-induced mice, and this augmentation was decreased in mice treated with SAMC (Fig. 3).
Fig. 3.
Effects of SAMC on murine airway mucins. Sections show staining with AB-PAS (400×) and immunohistochemical staining (400×) of MUC5AC. The airway surface mucins were shown as the blue staining for the AB-PAS positive and brown staining for MUC5AC. Study groups: C: control group, orally treated with the vehicle only; M: model group, LPS was instilled into the lungs of mice through a high-pressure syringe; L and H: orally treated with SAMC at 20 and 40 mg/kg after the LPS challenge, respectively; P: positive control, NAC dosed at 500 mg/kg. n = 5 per group.
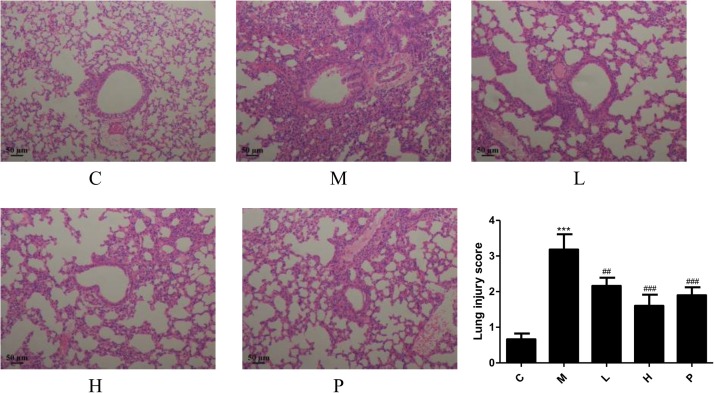
3.3. SAMC ameliorates histopathological changes of lung tissues
The hematoxylin and eosin staining was performed to evaluate the effect of SAMC on the LPS-induced murine lung damage in the ARDS model. As shown in Fig. 4 , the control group had the complete lung tissue structure, uniform alveolar septum, no edema or inflammatory cell aggregation. The lung tissues of the LPS group were notably injured, including alveolar hemorrhage, interstitial and alveolar edema, thickening of alveolar septum and inflammatory cell infiltration. These histopathological changes were effectively alleviated after the administration of SAMC (20 and 40 mg/kg) and NAC as shown in the morphological changes and lung injury scores in Fig. 4.
Fig. 4.
Effects of SAMC on lung morphometric changes in the LPS-induced ARDS mice. Sections are shown for the HE staining with the magnification of 100. Study groups: C: control group, orally treated with the vehicle only; M: model group, LPS was instilled into the lungs of mice through a high-pressure syringe; L and H: orally treated with SAMC 20 and 40 mg/kg after the LPS challenge, respectively; P: positive control, NAC dosed at 500 mg/kg; n = 5 per group. *** p < 0.001 compared with the control group; ## p < 0.01, ### p < 0.001 compared with the model group. The data are represented as means ± SD.
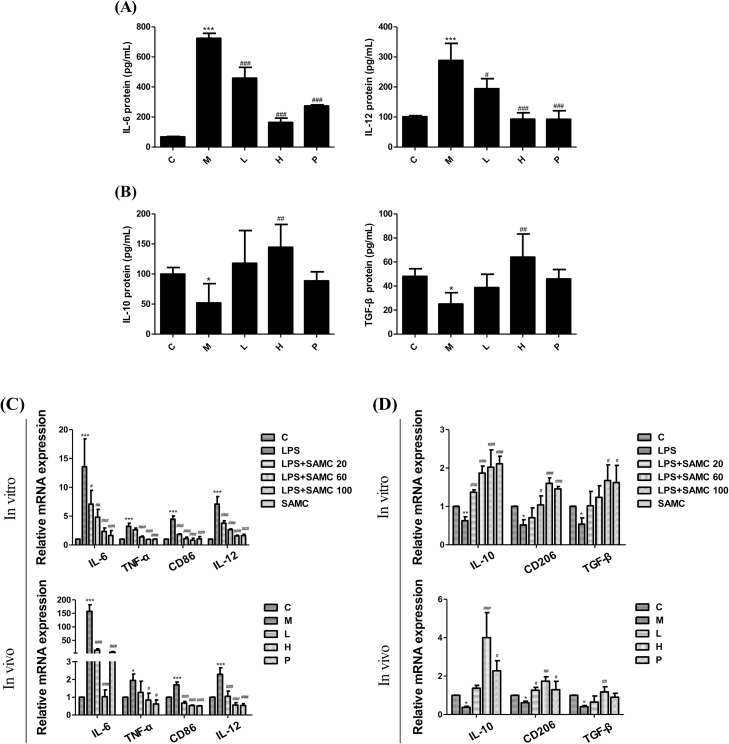
3.4. SAMC suppresses inflammatory responses in 16HBE cells and mice
To determine the anti-inflammatory activity of SAMC, we detected typical inflammatory cytokines in the bronchoalveolar lavage fluid (BALF) by ELISA kits. Results show that SAMC treatment suppressed the expressions of pro-inflammatory cytokines IL-6 (Fig. 5 A) and IL-12 (Fig. 5B), and elevated expressions of anti-inflammatory cytokines IL-10 (Fig. 5C) and TGF-β (Fig. 5D). We also evaluated mRNA expression changes in the pro-inflammatory markers (IL-6, TNF-α, CD86 and IL-12) and anti-inflammatory markers (IL-10, CD206 and TGF-β) in 16HBE cells and lung tissues by the RT-PCR analysis (Fig. 5E and F). These results consistently suggest that SAMC inhibited the cytokines, and helped to reconstitute inflammatory microenvironments.
Fig. 5.
Effects of SAMC on the inflammatory factors in the LPS-induced 16HBE cells and mice. The IL-6 (A), IL-12 (B), IL-10 (C) and TGF-β (D) levels in the BALF determined by the ELISA kits. The mRNA expressions of pro-inflammatory markers (IL-6, TNF-α, CD86 and IL-12) (E) and anti-inflammatory markers (IL-10, CD206 and TGF-β) (F) in 16HBE cells and mice were assayed by RT-PCR. Study groups: C: control group, orally treated with the vehicle only; M: model group, LPS was instilled into the lungs of mice through a high-pressure syringe; L and H: orally treated with SAMC 20 and 40 mg/kg after the LPS challenge, respectively; P: positive control, NAC dosed at 500 mg/kg. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control group; # p < 0.05, ## p < 0.01, ### p < 0.001 compared with the model group. The data are represented as means ± SD. All the experiments were repeated three times.
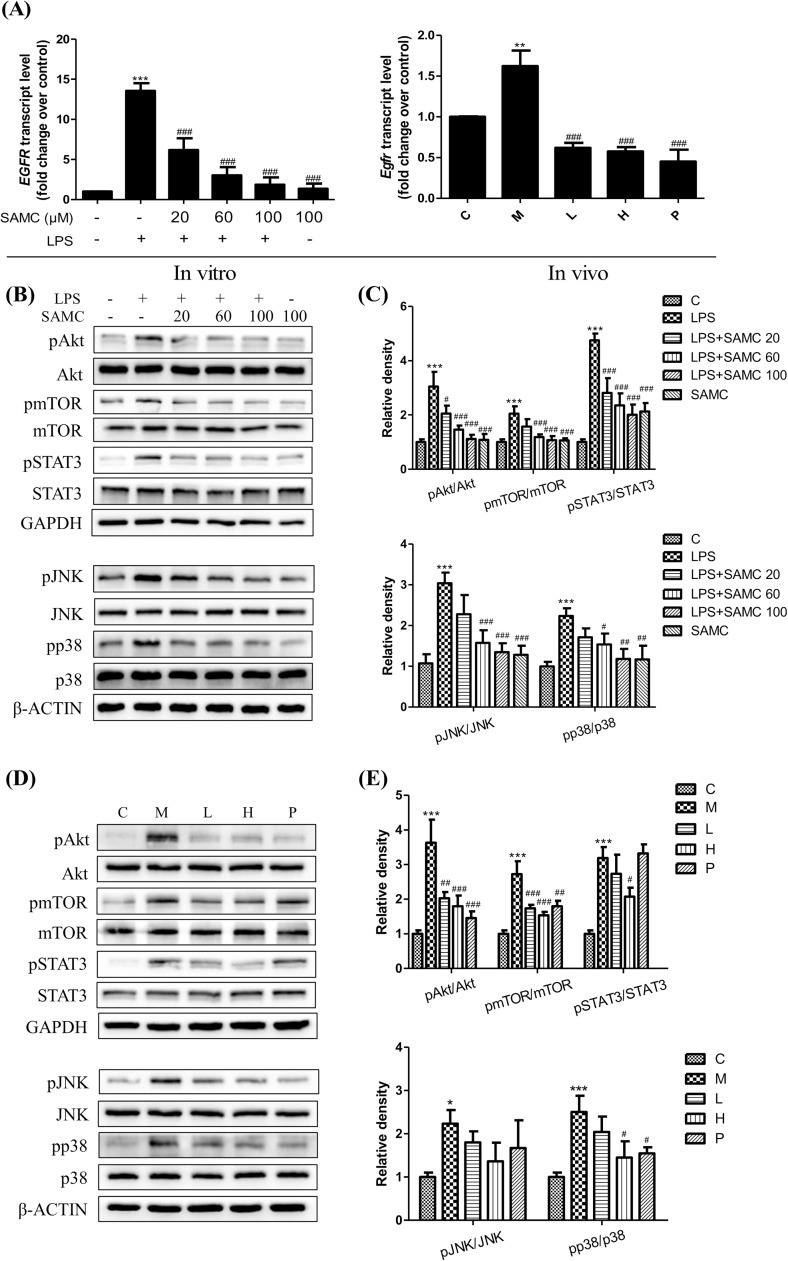
3.5. SAMC inhibits EGFR activation and phosphorylation of downstream MAPKs and Akt-mTOR-STAT3 signaling pathways in 16HBE cells and mice
Previous studies reveal that the EGFR activation plays a key role in mediating mucin synthesis and anti-inflammation. In this study, the RT-PCR analysis was used to detect the gene expression of EGFR in 16HBE cells and mice after the treatment with or without SAMC. As presented in Fig. 6 A, the treatment with SAMC reduced EGFR levels compared to the LPS stimulated group both in 16HBE cells and in mice. In order to examine MAPKs and Akt-mTOR-STAT3 pathways in the downstream targeting of the EGFR signaling, the phosphorylation status of p38, JNK, Akt, mTOR and STAT3 were determined both in 16HBE cells and mice after the treatment with SAMC and LPS by the western blot analysis. Our results clearly show that the SMAC treatment reduced the phosphorylation of these proteins compared with the LPS groups in vitro (Fig. 6B and C) and in vivo (Fig. 6D and E).
Fig. 6.
Effects of SAMC on the expressions of EGFR (A) and pp38, pJNK, pAkt, pmTOR and pSTAT3 in 16HBE cells (B) and mice (D). (C) and (E) are the corresponding quantification histograms of (B) and (D), respectively. Study groups: C: control group, orally treated with the vehicle only; M: model group, LPS was instilled into the lungs of mice through a high-pressure syringe; L and H: orally treated with SAMC 20 and 40 mg/kg after the LPS challenge, respectively; P: positive control, NAC dosed at 500 mg/kg. ** p < 0.01, *** p < 0.001 compared with the control group; ## p < 0.01, ### p < 0.001 compared with the model group. The data are represented as means ± SD. All the experiments were repeated three times.
4. Discussion
ARDS is characterized by severe pulmonary gas exchange and pulmonary dynamic injury [1]. The most common causes are local or systemic inflammation, including pneumonia and sepsis [20]. Cytokine storm that rapidly produces a variety of cytokines such as IL-6, IL-12, TNF-α, IFN-γ, MCP-1 and IL-8 results in cell death, disruption of the epithelial and endothelial barriers, and edema [21] which is an important cause of acute respiratory distress syndrome and multiple organ failure [22]. Pseudomonas aeruginosa LPS is an immunocompromised opportunistic pathogen, which generally causes infectious diseases of the lung such as ARDS, pneumonia and sepsis. Yanagihara et al. report that a single instillation of LPS (P. aeruginosa lipopolysaccharide) into the lungs of mice induces hyperinflammation and leads to the hyperplasia and metaplasia of secretory cells [23]. Therefore, the LPS-induced 16HBE cell and ARDS mouse models were used in the present study. It has reported that aged garlic extract (AGE) has an immunoregulatory ability, which can improve the inflammation-related processes [24,25]. In this study, we demonstrate that SAMC extracted from AGE alleviated pulmonary morphological changes, including alveolar congestion, infiltration of inflammatory cells, incrassation in alveolar walls and interstitial edema. Moreover, SAMC exerted an important regulatory effect in the inflammatory response through the suppression of the production of pro-inflammatory markers such as IL-6, TNF-α, CD86 and IL-12 and the elevation of anti-inflammatory markers such as IL-10, CD206 and TGF-β. Those results indicate that SAMC reduced the LPS-induced ARDS damage, ameliorated inflammatory responses, and was beneficial for the balance of body immune system.
In recent years, the issue of mucin hypersecretion increasingly attracts people's attention. The airway mucus layer is composed of water, ions, mucins, proteins, and lipids, and its major function is to capture foreign allergens, pathogens and pollutant, and to clear these stimulus from the lung by ciliary transport or coughing [26]. Mucins are the major components of the mucus layer and characterized by the presence of a large number of O-glycans and an extensive number of tandem repeats rich in serine and threonine residues [27]. In general, the presence of mucin production can protect the airway surface, however, in the case of sustained overproduction of mucins caused by secretory cell hyperplasia, it will block the airway, reduce lung function, and even threaten life [28]. Several reports have shown that SAMC, a water-soluble organic sulfur compound, has beneficial effects in mucin overproduction and inflammatory disorders [29]. In the present study, we confirmed that the SMAC treatment ameliorated the bronchial mucus hypersecretion as indicated by the AB-PAS and IHC staining, and decreased the expressions of MUC5AC and MUC5B both in vitro and in vivo.
EGFR is a member of the ErbB family of receptor tyrosine kinases, and whose activation can cause the activation of multiple downstream signaling pathways [30]. Activation of EGFR induced by LPS can promote the release of inflammatory cytokines and upregulate the expression of the MUC5AC gene and protein in vitro and in vivo [31]. MAPKs and Akt-STAT3 are the major downstream pathways of EGFR, which subsequently upregulate the MUC5AC expression after being phosphorylated. Our present results reveal that the SAMC treatment suppressed the expression of EGFR and reduced the phosphorylation levels of p38, JNK, Akt, mTOR and STAT3 compared with the LPS stimulated model group. These results suggest that SAMC probably inhibited inflammation and mucin overproduction through the co-regulated the phosphorylation of MAPKs and Akt-STAT3 signaling pathways.
In summary, our study suggested that SAMC ameliorated the inflammation and mucus hypersecretion via MAPKs and PI3K-Akt signaling pathways in the LPS-induced 16HBE cells and mice. These data show that SAMC could be considered as a potential therapeutic candidate for mucin overproduction and various inflammatory diseases.
Declaration of Competing Interest
The authors declare that there are no competing interests associated with the manuscript.
Acknowledgments
This work was supported by the funds from the Major Project of Science and Technology of Shandong Province (Grants #2015ZDJS04001 and 2018CXGC1411).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2020.105032.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Matuschak G.M., Lechner A.J. Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment. Missouri Med. 2010;107(4):252–258. [PMC free article] [PubMed] [Google Scholar]
- 2.Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. J. Immunol. 2015;194(3):855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baas T., Taubenberger J.K., Chong P.Y., Chui P., Katze M.G. SARS-CoV virus-host interactions and comparative etiologies of acute respiratory distress syndrome as determined by transcriptional and cytokine profiling of formalin-fixed paraffin-embedded tissues. J. Interferon Cytokine Res. 2006;26(5):309–317. doi: 10.1089/jir.2006.26.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng X.W., Lu J., Wu C.L., Yi L.N., Xie X., Shi X.D., Fang S.S., Zan H., Kung H.F., He M.L. Three fatal cases of pandemic 2009 influenza A virus infection in Shenzhen are associated with cytokine storm. Respir. Physiol. Neurobiol. 2011;175(1):185–187. doi: 10.1016/j.resp.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J., Rubin B.K., Voynow J.A. Mucins, mucus, and goblet cells. Chest. 2018;154(1):169–176. doi: 10.1016/j.chest.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Rogers D.F., Barnes P.J. Treatment of airway mucus hypersecretion. Ann. Med. 2006;38(2):116–125. doi: 10.1080/07853890600585795. [DOI] [PubMed] [Google Scholar]
- 9.Mall M.A. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J. Aerosol Med. Pulm. Drug Deliv. 2008;21(1):13–24. doi: 10.1089/jamp.2007.0659. [DOI] [PubMed] [Google Scholar]
- 10.Bansil R., Turner B.S. The biology of mucus: composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018;124:3–15. doi: 10.1016/j.addr.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Button B., Goodell H.P., Atieh E., Chen Y.C., Williams R., Shenoy S., Lackey E., Shenkute N.T., Cai L.H., Dennis R.G., Boucher R.C., Rubinstein M. Roles of mucus adhesion and cohesion in cough clearance. Proc. Natl. Acad. Sci. U. S. A. 2018;115(49):12501–12506. doi: 10.1073/pnas.1811787115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M.-J., Gao F.-S., Liu Y.-Q., Liu Y. EGFR plays an essential role in lipopolysaccharide (LPS)-induced MUC5AC hypersecretion in human bronchial epithelial cells. Biomed. Res. 2018;29(11):2337–2341. [Google Scholar]
- 13.Yang J., Li Q., Zhou X.D., Kolosov V.P., Perelman J.M. Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-κB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol. Cell. Biochem. 2011;351(1–2):29–40. doi: 10.1007/s11010-010-0708-y. [DOI] [PubMed] [Google Scholar]
- 14.Garrington T.P., Johnson G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 1999;11(2):211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 15.Abdelhamed S., Ogura K., Yokoyama S., Saiki I., Hayakawa Y. AKT-STAT3 pathway as a downstream target of EGFR signaling to regulate PD-L1 expression on NSCLC cells. J. Cancer. 2016;7(12):1579–1586. doi: 10.7150/jca.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai J., Ide N., Nagae S., Moriguchi T., Matsuura H., Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994;60(5):417–420. doi: 10.1055/s-2006-959522. [DOI] [PubMed] [Google Scholar]
- 17.Jeong Y.Y., Ryu J.H., Shin J.H., Kang M.J., Kang J.R., Han J., Kang D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules. 2016;21(4):430. doi: 10.3390/molecules21040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D.Y., Li H., Lim H.J., Lee H.J., Jeon R., Ryu J.H. Anti-inflammatory activity of sulfur-containing compounds from garlic. J. Med. Food. 2012;15(11):992–999. doi: 10.1089/jmf.2012.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M., Wang Y., Zhang Y., Zhang F., Zhao Z., Li S., Zhang J., Cao X., Zhang D. S-allylmercapto-l-cysteine modulates MUC5AC and AQP5 secretions in a COPD model via NF-кB signaling pathway. Int. Immunopharmacol. 2016;39:307–313. doi: 10.1016/j.intimp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Goodman R.B., Pugin J., Lee J.S., Matthay M.A. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14(6):523–535. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 21.Gerlach H. Agents to reduce cytokine storm. F1000Research. 2016;5:2909. doi: 10.12688/f1000research.9092.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am. J. Emerg. Med. 2008;26(6):711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Yanagihara K., Seki M., Cheng P.W. Lipopolysaccharide induces mucus cell metaplasia in mouse lung. Am. J. Respir. Cell Mol. Biol. 2001;24(1):66–73. doi: 10.1165/ajrcmb.24.1.4122. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues C., Percival S.S. Immunomodulatory effects of glutathione, garlic derivatives, and hydrogen sulfide. Nutrients. 2019;11(2):295. doi: 10.3390/nu11020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Balasubramanian N., Quinn M.T. Neutrophil immunomodulatory activity of natural organosulfur compounds. Molecules. 2019;24(9):1809. doi: 10.3390/molecules24091809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knowles M.R., Boucher R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109(5):571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton D.J., Rousseau K., McGuckin M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 28.Vestbo J., Prescott E., Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am. J. Respir. Crit. Care Med. 1996;153(5):1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 29.Schäfer G., Kaschula C.H. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anticancer Agents Med. Chem. 2014;14(2):233–240. doi: 10.2174/18715206113136660370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D., Jin M., Bai C., Zhou J., Shen Y. Peroxiredoxin 6 suppresses Muc5ac overproduction in LPS-induced airway inflammation through H(2)O(2)-EGFR-MAPK signaling pathway. Respir. Physiol. Neurobiol. 2017;236:84–90. doi: 10.1016/j.resp.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Deshmukh H.S., Case L.M., Wesselkamper S.C., Borchers M.T., Martin L.D., Shertzer H.G., Nadel J.A., Leikauf G.D. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am. J. Respir. Crit. Care Med. 2005;171(4):305–314. doi: 10.1164/rccm.200408-1003OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.