Abstract
Brain aging is a complex process that includes atrophy, vascular injury, and a variety of age-associated neurodegenerative pathologies, together determining an individual’s course of cognitive decline. While Alzheimer’s disease and related neurodegenerative dementias (ADRD) contribute to the heterogeneity of brain aging, these conditions themselves are also heterogeneous in their clinical presentation, progression and pattern of neural injury. We reviewed studies that leveraged data-driven approaches to examining heterogeneity in ADRD, with a principal focus on neuroimaging studies exploring subtypes of regional neurodegeneration patterns. Over the past decade, the steadily increasing wealth of clinical, neuroimaging, and molecular biomarker information collected within large-scale observational cohort studies has allowed for a richer understanding of the variability of disease expression within the aging and ADRD continuum. Moreover, the availability of these large-scale data sets has supported the development and increasing application of clustering techniques for studying disease heterogeneity in a data-driven manner. In particular, data-driven studies have led to new discoveries of previously unappreciated disease subtypes characterized by distinct neuroimaging patterns of regional neurodegeneration, which are paralleled by heterogeneous profiles of pathological, clinical, and molecular biomarker characteristics. Incorporating these findings into novel frameworks for more differentiated disease stratification holds great promise for improving individualized diagnosis, prognosis of expected clinical progression, and opportunities for development of precision medicine approaches for therapeutic intervention. We conclude by providing an account of the principal challenges associated with data-driven heterogeneity analyses and outline avenues for future developments in the field.
Keywords: Alzheimer’s disease, heterogeneity, clustering, neuroimaging, machine learning, frontotemporal dementia, Lewy body dementias, brain aging, MRI, PET
1. Understanding heterogeneity from brain aging to Alzheimer’s disease and related dementias
Brain aging is a complicated heterogeneous process that is associated with brain atrophy, cognitive decline, vascular brain injury, and a variety of age-associated pathologies (1–3). Recently, neuroimaging-based machine learning methods were developed to predict individual brain age from magnetic resonance (MR) images, which enabled exploration of the heterogeneity through deviation from normative brain aging trajectories (4–12). Currently, considerable attention is being given to understanding heterogeneity in brain aging and dementia using data-driven clustering methods combined with neuroimaging. A recent report in a healthy aging cohort discovered five distinct subtypes of advanced brain aging using a data-driven clustering approach (12). One subtype resembled Alzheimer’s disease (AD)-like changes, but the other subtypes were characterized by atrophy patterns suggestive of other neurodegenerative diseases, demonstrating the differential contribution of AD and related dementias (ADRD) to the deviation from healthy brain aging.
While comorbid ADRD contribute to the heterogeneity of brain aging, these conditions themselves are also heterogeneous in their progression and pattern of neural injury. To date, most of the existing research on studying heterogeneity in ADRD departs from ‘a priori’ categorizations of recognized clinical or pathologic disease variants and aims to understand clinical and neurobiological differences between these variants (see(13–22) for extensive reviews of this literature). By contrast, the increasing availability of large-scale data sets over the last years has supported the development and application of clustering techniques for exploring disease heterogeneity in a less biased, data-driven manner. Such data-driven discovery of previously unappreciated disease subtypes may lead to novel frameworks for more differentiated disease stratification that holds great promise for improving individualized diagnosis and prognosis.
In the present article we give an up-to-date overview of studies that leveraged data-driven approaches to studying heterogeneity in ADRD, with a principal focus on exploring subtypes of regional neurodegeneration patterns (Table 1; see supplementary materials for information on the systematic bibliometric approach). While most of these studies have focused on AD dementia and its clinical at-risk populations such as Mild cognitive impairment (MCI), we also review the emerging literature on data-driven neuroimaging subtypes in frontotemporal (FTD) and Lewy-body dementia (LBD) for a comprehensive coverage of the principle age-related neurodegenerative dementias. We put the neuroimaging-based subtyping findings into context of data-driven subtyping studies using other phenotyping data, particularly neuropsychological and molecular biomarker data.
Table 1.
Studies using data-driven clustering techniques in neuroimaging for characterizing heterogeneity in brain aging, AD and related dementias
| Studies | Modality and sample | Method | Main findings |
|---|---|---|---|
| AD Dementia | |||
| Noh et al., 2014 (33), Na et al., 2016 (117) |
Structural MRI N = 152 AD dementia patients from the Samsung Medical Center (SMC) cohort. |
Ward’s clustering applied on vertex-wise cortical thickness features. | Three distinct cortical thinning subtypes, including medial temporal-dominant (34%), parietal-dominant (18%), and diffuse atrophy (48%). The parietal-dominant subtype was younger and exhibited faster cognitive decline compared to the other subtypes. |
| Hwang et al. 2016 (34) | Structural MRI N = 77 AD dementia patients from ADNI cohort. |
Ward’s clustering applied on vertex-wise cortical thickness features. | Three cortical thinning subtypes: medial temporal (19%), diffuse (56%), and parietal dominant (25%). |
| Dong et al., 2016 (37) | Structural MRI Pooled sample of amnestic MCI (N = 530) and AD dementia (N = 314) patients from ADNI. |
Semi-supervised clustering technique applied on 153 brain ROIs. | Four subtypes: one with minimal atrophy and elevated tau (13%), one with cortical atrophy and proportionately greater executive dysfunction (28%), and two with typical AD atrophy and contribution of vascular disease (59%). |
| Zhang et al., 2016 (39) | Structural MRI N = 188 AD dementia patients from ADNI for model derivation; Factor compositions were inferred in separate amyloid-positive MCI (N = 147) and CN (N = 43) samples. | Bayesian model derived latent atrophy factors from voxel-wise gray matter volume. | Three main factors: temporal atrophy associated with memory decline, neocortical atrophy associated with executive function decline, and subcortical atrophy including striatum, thalamus, and cerebellum, that was associated with the slowest cognitive decline across domains. Subjects were assigned to the different factors in a probabilistic manner |
| Park et al., 2017 (35) | Structural MRI N = 225 AD dementia patients from the SMC cohort; Validation dataset of N = 131 AD dementia patients from ADNI. |
Graph-theory-based clustering approach (Louvain method) applied on vertex-wise cortical thickness features. | Three subtypes with high reproducibility (>90%): parietal-predominant (P; 35%), medial temporal-predominant (MT, 36%), and diffuse (D, 29%) atrophy. The P subtype showed the worst clinical presentation across cognitive domains. |
| Poulakis et al., 2018 (38) | Structural MRI N = 299 AD dementia patients, pooled from the multicentric AddNeuroMed and ADNI cohorts. |
Unsupervised random forest-based clustering of volume measures from 162 atlas-defined cortical and subcortical regions. | Two distinct ‘typical’ subtypes (72%), as well as three different atypical subtypes (29%), including limbic predominant, minimal atrophy, and hippocampal sparing. |
| Ten Kate et al., 2018 (36) | Structural MRI N = 299 amyloid-positive AD dementia patients in discovery cohort, and two independent validation datasets (N = 181; N = 227). Subtype classification in N = 603 amyloid-positive MCI patients. |
Nonnegative matrix factorization of gray matter volumes from 1024 equally sized cortical and subcortical area parcellations. | Four atrophy subtypes: (i) medial-temporal predominant with worst memory and highest vascular lesion burden (19%); (ii) parieto-occipital atrophy with poor executive and visuospatial functioning (28%); (iii) mild atrophy with best cognitive performance, but highest CSF tau levels (35%); (iv) diffuse cortical atrophy (18%). Subtype classifications in amyloid-positive MCI patients (‘prodromal AD’) showed similar biomarker characteristics as the AD dementia subtypes. |
| Young et al., 2018 (41) | Structural MRI Two separate samples of pooled AD dementia, MCI, and CN subjects from ADNI (N = 793 and N = 576, respectively)1. |
Combined clustering and event based modeling of 13 atlas-defined subcortical and cortical volumes. Algorithm derives subtypes together with their estimated stage. | Three distinct spatiotemporal atrophy patterns defined by differential atrophy start: (i) typical, starting in the medial temporal lobe; (ii) cortical, starting in temporo-frontal areas; (iii) subcortical, starting in the basal ganglia. |
| Sui et al., 2018 (46) | Diffusion Tensor MRI N = 48 AD dementia patients from ADNI for model derivation; Factor compositions were inferred in separate samples of MCI (N = 134) and CN subjects (N = 50). |
Latent Dirichlet Allocation of voxel-wise fractional anisotropy values within the brain’s white matter. | Three latent factors of microstructural white matter impairment categorized subjects in a probabilistic fashion: (i) temporo-frontal; (ii) parietal; and (iii) long fiber bundle factor (corpus callosum, superior longitudinal fasciculus). Latent factors had differential associations with memory and executive function decline. |
| Lowe et al., 2018 (45) | Tau-PET Pooled sample of N=86 amyloid-positive amnestic MCI (N = 35) and AD dementia (N = 51) patients from the Mayo Clinic cohorts. |
Ward’s hierarchical clustering applied to 47 regional Tau-PET (flortaucipir) values. | Three clusters mainly reflecting incremental involvement of medial temporal (57%) to temporo-parietal (36%) to frontal lobe regions (7%). Cluster with highest and most widespread Tau-PET signal had younger age at onset. |
| Whitwell et al., 2018 (47) | Tau-PET N = 62 amyloid-positive AD dementia patients. |
K-median clustering of Tau-PET (flortaucipir) values from entorhinal and neocortical composite ROIs. | Three clusters: (i) low entorhinal and cortical (34%), (ii) low entorhinal but high cortical uptake (34%), and (iii) high cortical and entorhinal uptake (32%). Cluster (ii) had lowest prevalence of APOE4 carriers and highest percentage of atypical AD presentations. |
| Varol et al 2017 (118) | Structural MRI N = 123 AD dementia patients from ADNI. |
Non-linear learning algorithm for classification and subtyping applied on 153 brain ROIs. | Three subtypes: (i) diffuse (24%), (ii) precuneus and temporal lobe atrophy with some prefrontal involvement (51%), and (iii) predominant atrophy in the hippocampus and medial temporal lobe (25%). |
| Sun et al. 2019 (116) | Combined structural MRI and neuropsychologic testing. Two separate samples of AD dementia patients from ADNI used as discovery (N = 149) and replication (N = 170) cohorts. |
Hierarchical Bayesian clustering applied simultaneously on voxel-wise gray matter volumes and neuropsychological test scores. | Three atrophy-cognitive factors categorized subjects in a probabilistic fashion: (i) medial temporal atrophy with episodic memory deficits; (ii) lateral temporal atrophy with language deficits; (iii) posterior cortical atrophy with visuospatial and executive function deficits |
| Jeon et al. 2019 (48) | Combined structural MRI, Tau-PET, and Amyloid-PET. N = 83 AD dementia patients. | Hierarchical clustering applied on fused features from vertex-wise cortical thickness, Tau-PET (THK5351), and Amyloid-PET (flutemetamol). | Three distinct subtypes of overlapping Tau-PET signal and structural atrophy: (i) medial temporal-dominant (53%), (ii) parietal-dominant (23%), and (iii) diffuse subtype (24%). Regional amyloid pattern did not differ across subtypes. |
| MCI and other at risk populations | |||
| Jung et al. 2016 (80) | Structural MRI N = 613 older individuals with subjective memory impairment from SMC cohort. |
Ward’s hierarchical clustering of vertex-wise cortical thickness values. | Three cortical thickness subtypes: (i) no/minimal atrophy (52%), (ii) diffuse atrophy (35%), and (iii) AD-like temporal atrophy (13%). No/minimal atrophy subtype had higher prevalence of depression; AD-like atrophy subtype had highest age, more vascular risk factors, and lowest cognitive performance. |
| Eavani et al.,2018 (12) | Combined structural MRI and resting state fMRI. N = 400 cognitively normal elderly subjects from the Baltimore Longitudinal Study of Aging (BLSA) cohort. |
“Mixture of Experts” clustering applied on combined voxel-wise gray matter volume and functional connectivity features for subjects who deviated from normative brain aging trajectories. | Five subtypes of accelerated brain aging: (i) AD-like changes with contribution of vascular brain injury (27%); (ii) fronto-orbital atrophy but increased functional measures (15%); (iii) high brain tissue reserve counterbalancing brain loss in early AD (24%); (iv) pattern similar to those found in patients with Lewy body pathology (20%); (v) reduced function in motor network (14%). |
| Habes et al., 2018 (44) | Combined FLAIR and T1-MRI. N = 1836 participants across adult age range from population-based cohort study (SHIP); Replication sample of N = 307 CN and MCI participants from BLSA cohort. |
Nonnegative matrix factorization applied on white matter hyperintensity maps. | Four distinct regional distribution patterns of vascular lesions: (i) Frontal periventricular; (ii) posterior periventricular; (iii) dorsal periventricular; (iv) deep lesions. Frontal periventricular lesions showed strongest association with distributed gray matter atrophy, dorsal periventricular lesions with AD polygenic risk score. |
| Kim et al. 2019 (82) | Structural MRI N = 662 amnestic MCI patients from SMC cohort. |
Graph-theoretical clustering method (Louvain method) applied on vertex-wise cortical thickness features. | Three subtypes: (i) no/minimal (39%), (ii) medial-temporal (31%), and (iii) parieto-temporal (30%). Parieto-temporal subtype had highest frequency of APOE4 carriers and amyloid PET positivity, and elevated risk of dementia conversion. |
| Ezzati et al. 2019 (81) | Structural MRI N = 696 amnestic MCI patients from ADNI. |
Latent class analysis (LCA) applied on atlas-based regional volumes, decomposed into the 10 most informative ROIs using principal component analysis. |
Four aMCI subgroups were found: (i) most similar to normal controls in brain structure and function (58%), (ii) with characteristics similar to early AD (33%), (iii) with highest global and medial temporal atrophy and worst overall cognitive performance (5%), and (iv) minimal atrophy but poor executive function performance (4%). |
| Frontotemporal Dementia | |||
| Whitwell et al., 2009 (86) Whitwell et al., 2013 (87) |
Structural MRI N = 66 bvFTD patients. |
Ward’s clustering applied on gray matter volumes from 26 cortical and subcortical ROIs. | Four distinct atrophy subtypes: two were characterized by predominant temporal lobe atrophy (‘temporal-dominant’ (9%) and ‘temporofrontoparietal’ (41%)), the other two by predominant frontal lobe atrophy (‘frontal-dominant’ (32%) and ‘frontotemporal’ (18%)). |
| Cerami et al. 2016 (88) | FDG-PET N = 52 bvFTD patients. |
Ward’s clustering applied on FDG-PET measurements from 16 cortical and subcortical ROIs. | Two major hypometabolism variants: a “frontal” variant with predominant executive and language deficits (48%), and a “temporo-limbic” variant with poor performance on long-term memory tasks (52%). |
| Ranasinghe et al., 2016 (89) | Structural MRI N = 104 bvFTD patients. |
Euclidian distance-based clustering applied on gray matter volumes from 18 preselected temporo-frontal network ROIs. | Four clusters of network-specific atrophy: two involved the fronto-insular salience network (‘frontal’ (31%) and ‘frontotemporal’ (25%)), and another two involved either subcortical-predominant (35%) or anterior temporal semantic-appraisal network features (9%). |
| Matias-Guiu et al., 2018(90); Matias-Guiu et al., 2019 (91) |
FDG-PET N = 91 PPA patients, including non-fluent, semantic, and logopenic variants. |
Ward’s clustering applied on FDG-PET measurements from 116 atlas-defined brain regions. | Differential hypometabolic patterns separated the three clinical PPA variants, but suggested a further splitting of non-fluent and logopenic variants into two subtypes each, which also differed in their specific language deficits. |
| Young et al., 2018 (41) | Structural MRI N=1722 presymptomatic and symptomatic carriers of autosomal-dominant FTD mutations from the multicentric GENFI study. |
Combined clustering and event based modeling of 13 atlas-defined subcortical and cortical volumes. Algorithm derives subtypes together with their estimated stage. | Four distinct spatiotemporal patterns of atrophy progression in familial FTD, which had high correspondence with genetic subtypes. |
| Caminiti et al. 2019 (119) | FDG-PET N=72 DLB patients |
Ward’s clustering applied on FDG-PET measurements from 14 occipital, parietal, and temporal ROIs known to be involved in DLB. | Two clusters characterized by slightly different occipital hypometabolism. Cluster with more severe occipital involvement (43%) had worse global cognition and higher risk for developing visual hallucinations. |
| Lewy body dementia | |||
| Uribe et al. 2016 (106); Uribe et al. 2019 (108) |
Structural MRI N=88 nondemented PD patients |
Ward’s clustering applied on vertex-wise cortical thickness features. | Three distinct clusters: (i) non-atrophic (33%), (ii) parieto-temporal (34%), and (iii) occipital-frontal (33%) cortical atrophy. The parieto-temporal atrophy subtype resembled an AD-typical pattern and showed lowest cognitive performance. |
| Uribe et al. 2018 (107) | Structural MRI N=77 de novo PD patients from PPMI |
Ward’s clustering applied on 360 atlas-based cortical thickness features. | Two distinct patient subgroups in this early disease stage: one with mainly anterior temporal-frontal atrophy (43%), and the other with predominant posterior parieto-occipital atrophy (57%) that also showed lower memory scores. |
Number refers to initial sample size before additional quality control procedures
Number refers to sample size before additional quality control procedures
2. Alzheimer’s disease dementia
2.1. Heterogeneity in neuroimaging patterns: from hypothesis-driven to data-driven approaches
AD is commonly defined based on a characteristic amnestic-predominant dementia syndrome that is reflected by a stereotypic pattern of regional pathology progression that begins in the medial temporal lobe and extends into the temoroparietal neocortex (23). However, there are also well-recognized “atypical” clinical presentations that do not fit into this scheme, including a visuospatial variant, a language variant, and a ‘frontal’ variant with predominant dysexecutive deficits (24–26). Similarly, neuropathological studies have reported atypical patterns of neurofibrillary tangle distribution in subsets of patients, including a “limbic-predominant” subtype with relatively lower neocortical over medial temporal involvement, and a “hippocampal-sparing” subtype with the opposite pattern(27).
Several previous studies on understanding heterogeneity in neuroimaging patterns of AD have used a hypothesis-driven design(28–31), in which individual MRI scans are classified into predefined subtypes based on neocortical vs medial temporal involvement. This approach could provide important information on differential clinical, biomarker, and prognostic characteristics of these “a priori” atrophy subtypes, highlighting the clinical usefulness of MRI-based subtype stratification. However, these hypothesis-driven studies were per design limited to prior definitions of neuropathological subtypes and cannot provide any information on whether these patterns represent a comprehensive description of the atrophy subtypes that are present in the larger population of patients. On the other hand, more novel data-driven neuroimaging investigations consistently revealed subtypes characterized by atrophy patterns that visually resembled the predefined neuropathological subtypes (Fig.1), but also pointed to the existence of more diverse atrophy patterns that go beyond a broad characterization of differential medial temporal vs neocortical involvement.
Figure 1:
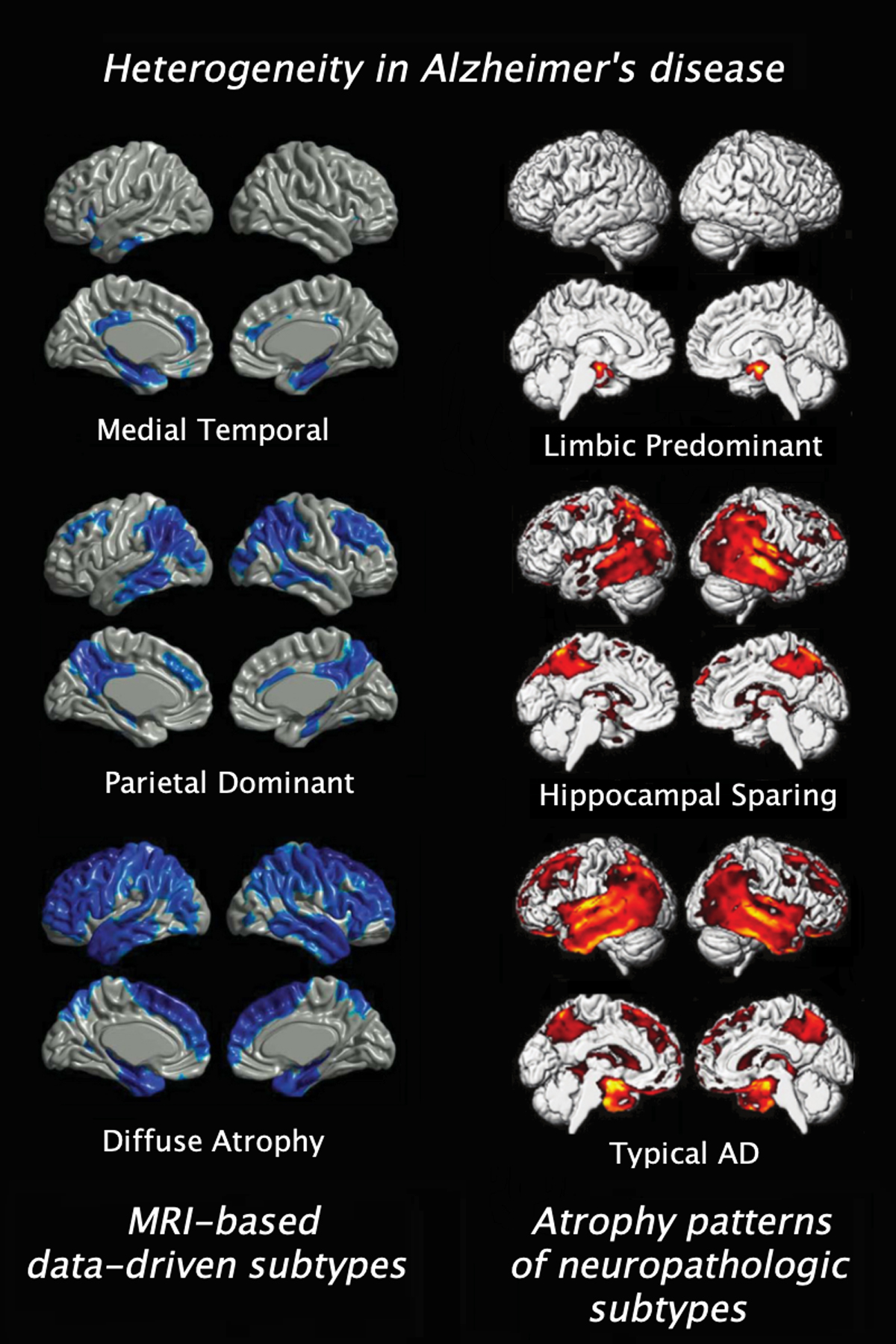
Resemblance between gray matter loss associated with the neuropathological subtypes of differentially distributed neurofibrillary tangles (56) and data-driven subtypes captured with clustering MRI measures (33)
The most common way of subtyping AD neuroimaging patterns in a data-driven manner is using unsupervised clustering approaches on regional neuroimaging features(32). Clustering, in this context, aims to find subgroups of patients with similar spatial patterns of neuroimaging abnormalities. From a methodological perspective, approaches mainly differ with respect to (i) clustering techniques, (ii) validation methods, and (iii) features used for clustering. One of the first of these data-driven studies used Ward’s clustering of cortical thickness features to categorize mild AD into distinct atrophy subtypes, including medial temporal-dominant (34%), parietal-dominant (18%), and diffuse atrophy (48%)(33). Later investigations showed that regional hypometabolism on FDG-PET could further dissociate those subtypes(34), and that they were reproducible using a different methodology, namely an unsupervised graph-theory-based clustering approach(35). Replication of results within an independent sample is indeed crucial to demonstrate that the identified subtypes reflect a true characteristic of the disease, rather than corresponding to a specific variation observed within a single sample. However, one limitation of the approaches employed in(33,35) is that these clustering techniques force each patient to be associated with a single subtype, which implies that all patients assigned to a subtype would be characterized by the same mean atrophy pattern.
This issue can be addressed using clustering techniques that allow patients to be associated with multiple subtypes to varying degrees. For example, non-negative matrix factorization, as applied to voxel-wise gray matter volumes in(36), identifies distinct atrophy patterns that act as a basis set to generate all possible atrophy profiles observed in patients. This model simultaneously learns “loadings” (assignments) of patients to these atrophy patterns as well, allowing patients to be assigned to multiple patterns. Interestingly, the study found four AD atrophy subtypes and reproduced them across three different datasets (one discovery, and two validation datasets). Despite the reproducibility of those subtypes, other studies have identified even five distinct atrophy subtypes(37,38). Unsupervised random forest-based clustering applied on regional gray matter volumes in two independent AD cohorts found two distinct “typical” subtypes (~70%), as well as three different atypical subtypes (~30%) termed “limbic predominant”, “minimal atrophy”, and “hippocampal sparing”(38). Notably, the distinct minimal atrophy subtype had also been reported in hypothesis-driven studies(28,29) as well as by two other data-driven studies(36,37).
An alternative approach to model a one-to-many mapping between patients and disease subtypes is to use probabilistic models that assign patients to subtypes in a probabilistic (soft) fashion. Such a model was employed in(39), where latent Dirichlet allocation(40) was used as the clustering technique to identify distinct latent factors of overlapping atrophy patterns from structural MRI data of AD patients. The method does not explicitly cluster patients into subtypes, but instead identifies distinct atrophy factors corresponding to latent subtypes, and patients are probabilistically assigned to these subtypes. The study confirmed the reported temporal and neocortical atrophy subtypes, but also found a more unusual subcortical atrophy subtype covering striatum, thalamus, and cerebellum. Indeed, most patients, both in(36) and(39), were found to express multiple latent atrophy factors rather than predominantly expressing a single atrophy factor. While being biologically plausible, this may also relate to the general limitation of clustering methods to not properly disentangle heterogeneity caused by disease stage and disease subtype.
Thus, one potential issue with subtype analysis, in general, is that the variance captured by putative subtypes may correspond to different disease stages rather than different disease expression patterns (subtypes), or a mixture of both. This limitation was explicitly addressed in(41) using a computational technique that combines clustering and disease progression modelling to group patients with common phenotypes across the range of disease stages. Like the approaches in(36,39), this model assigns patients to subtypes with varying probabilities, and it could be demonstrated that this assignment was stronger when additionally considering the stage information. While this emphasizes the importance of accounting for heterogeneity in disease stage, it must be noted that the method only infers the stage information from cross-sectional data. Including actual longitudinal neuroimaging data into the clustering methods is a promising approach that remains to be explored in more detail(42).
A potentially bigger issue in identifying subtypes using unsupervised clustering approaches is that subtypes are not even guaranteed to relate directly to the disease. That is, beyond capturing variance of different stages or expression patterns, some clusters may also be related to different age groups, sex, or any other unaccounted confounding variables, unless samples are carefully designed to mitigate the effects of such variables. One possible solution is to harness power of clustering and supervised learning techniques (e.g., classification, regression) together. An example solution to address common confounding effects (e.g., age, sex) in the identification of distinct disease paths differentiating patients from healthy controls was proposed in(43). The application of this method to the ADNI data-set showed a minimal atrophy subtype (13%), a subtype with distributed atrophy (28%), and two subtypes with prominent medial temporal lobe involvement resembling purer AD pathology (59%)(37). One disadvantage of the technique is its high computational demand when applied to high dimensional data (e.g. voxel-wise maps).
While the large majority of data-driven neuroimaging studies on AD subtyping and related methodological development has focused on neurodegeneration patterns in structural MRI data, these methods are now also beginning to be used for exploring heterogeneity in neuropathological patterns evidenced by other MRI and PET-imaging modalities ((44–48); Table 1). Initial exploration of clustering in tau-PET imaging supported the broad differential subtypes of medial temporal- vs. neocortical-predominant tau distribution in-vivo(47). However, data-driven explorations of detailed tau-PET patterns are still limited by low sample sizes of well-defined patient populations examined using this novel imaging technique.
2.2. Data-driven subtyping studies using other phenotyping modalities
In addition to data-driven neuroimaging studies, considerable heterogeneity among AD dementia patients has also been revealed by applying clustering methods to comprehensive neuropsychological test data (reviewed in(49)). Across different studies, clustering approaches have identified two broad cognitive subtypes of AD dementia patients, corresponding to those with a typical memory-impaired cognitive profile and those with relatively spared memory compared to non-memory domains(50). However, at a higher clustering resolution more detailed cognitive subtypes could also be revealed depending on the relative degree of memory impairment and the specific non-memory domains that were most affected(49,50). According to a recent neuropsychological profiling study in late-onset AD dementia patients, the majority (48%) had a relatively homogeneous impairment across memory and other cognitive domains, whereas smaller subsets of patients had isolated relative impairments in memory (18%), visuospatial functioning (14%), language (9%), or executive functioning (8%)(51). Other data-driven subtyping studies using dimensional clustering of CSF biomarker data in clinically diagnosed AD dementia (52–54) found different subtypes characterized by differing tau biomarker levels. These included a relatively small subgroup with extremely high levels of tau pathology (including both phosphorylated and total tau) that showed a more aggressive disease course over time, and a distinct subgroup with clearly abnormal amyloid levels but relatively low levels of both tau markers, which did not differ in global disease severity from the other clusters.
The degree to which the subtyping findings derived from different phenotyping modalities correspond to overlapping patient subgroups is currently not clear. Based on overlapping cognitive and neuroimaging characteristics reported across the different subtyping studies(27,33,37,47,50,55,56) one may infer a possible correspondence between the memory-predominant cognitive subtype, the medial temporal-dominant atrophy subtype, and the limbic-predominant neuropathologic subtype on one hand, and the memory-spared cognitive, neocortical-dominant atrophy, and hippocampal-sparing neuropathologic subtypes on the other hand. Similarly, the distinct CSF subgroups with extremely high and low tau levels may also potentially map onto this broad distinction between memory/medial temporal-dominant and non-memory/cortical-dominant presentations, respectively, given that these have also been linked to corresponding differences in overall tau levels and pace of cognitive decline(27,45,47,57,58).
However, due to the current lack of cross-modal subtyping studies the actual correspondence between the different reported subtypes remains unknown, and there are obvious differences in the subtyping granularities reported for the different modalities and even across individual studies for a given modality. Only a concurrent subtype classification of the same patient sample, which applies analogous clustering approaches to the different modalities, will allow assessing to which degree the different modality-specific subtypes map to overlapping patient subgroups.
2.3. Possible mechanisms underlying heterogeneity
Relatively little is known about the pathophysiologic mechanisms that underlie the development of distinct atrophy patterns and clinical presentations in the face of common core pathologic features of AD. Recently, spatially comprehensive analyses of brain-wide regional gene expression profiles in the human brain have indicated that the typical regional patterns of AD pathology accumulation might be explained by specific molecular characteristics of the affected brain regions(59,60). However, gene expression profiles associated with the pathologic distribution patterns characterizing atypical AD subtypes have not yet been investigated. With respect to subject-specific risk factors, the APOE4 genotype has consistently been found to be more strongly associated with a typical memory-predominant presentation and related atrophy profile than with any other AD subtype(49–51,55,61). Recent exploratory genetic studies could also provide initial evidence for genetic factors that may be specifically associated with other cognitively defined subtypes of AD(62), although these still require further replication in independent studies. Intriguingly, specific neurodevelopmental learning disabilities have been linked to atypical clinical variants of AD, raising the possibility that developmental disturbances might render the implicated neurofunctional networks more vulnerable to AD-related neurodegeneration in these patients(63,64). It remains to be determined whether specific neurodevelopmental characteristics may also bias to any of the data-driven neurodegeneration subtypes described above.
Several other age-related pathologies that are often comorbid with AD are well-known to influence the regional neurodegeneration pattern and clinical phenotype of the disease(65–70). However, limited data exists on the relation between comorbid pathologies and specific AD subtypes. For example, comorbid TDP-43 and cerebrovascular disease appear both to be considerably more frequent in limbic-predominant and typical neuropathologic subtypes of AD compared to the hippocampal-sparing subtype(27,71,72).
Post-mortem neuropathologic characteristics of neuroimaging-defined AD atrophy subtypes have not yet been studied. However, several of these neuroimaging studies reported a higher cerebrovascular burden, as measured by white matter hyperintensities (WMH), in medial temporal-predominant subtypes(36,37). Investigations of further MRI markers of small vessel disease concluded that limbic-predominant and typical AD atrophy subtypes may indeed more often present with hypertensive arteriopathy, whereas hippocampal-sparing and minimal atrophy AD subtypes may have a higher prevalence of cerebral amyloid angiopathy (30). Interestingly, a data-driven structural covariance analysis of WMH in a large community-based sample identified distinct regional distribution patterns of WMH that also showed differential relations to age, polygenic AD risk, and cortical atrophy(44). Together this data points to differential age- and disease-dependent pathophysiologic mechanisms underlying the appearance of regionally specific vascular lesions and related cortical atrophy patterns, which may intersect in patients with comorbid AD and cerebrovascular disease.
3. Mild cognitive impairment
MCI is a clinical syndrome defined by impairment of cognitive function, often amnestic, but without sufficient functional impairment required for a diagnosis of dementia. While the condition often represents prodromal AD, patients may also progress to other dementia types, remain stable, or even revert to normal cognition(73,74). From a neuropathologic point of view, it has been reported that less than 25% of MCI patients have pure AD pathology. On the other hand, mixed pathologies were most common, and another 20–30% had primarily non-AD pathologies, including cerebrovascular disease and other age-related pathologies primarily targeting the medial temporal lobe (75–79).
Recent data-driven studies on AD atrophy subtypes have demonstrated that the identified patterns can already be detected in prodromal disease stages as represented by amyloid-positive MCI patients(36,39). However, only few studies have employed data-driven clustering approaches to explore heterogeneous neuroimaging patterns in unselected MCI cohorts, which potentially also encompass non-AD related atrophy subtypes ((80–82); Table 1). One study applied an established graph-theory-based clustering approach to cortical thickness features from structural MRI scans of a large monocentric memory clinic cohort of amnestic MCI patients(82). The analysis identified one cluster without any evidence of neurodegeneration (39%), as well as two different “medial-temporal” (31%) and “parieto-temporal” (30%) cortical atrophy patterns that resembled the AD atrophy subtypes captured with the same method(35). However, only the parieto-temporal subtype had increased amyloid-PET positivity, APOE4 prevalence, and risk of AD dementia conversion, suggesting that the medial temporal subtype also contained a high proportion of non-AD pathophysiology accounting for this atrophy pattern.
Biologically distinct MCI subgroups have also been studied using clustering applied to diverse multimodal CSF and neuroimaging-derived features(83–85). For example, one study applied Ward’s clustering to such data of amnestic MCI patients from the ADNI cohort. Similar to the subtyping based on regional atrophy patterns(81,82), this analysis identified a minimal pathologic subtype (15%), and two other subgroups with biomarker profiles similar to early (43%) and more advanced prodromal AD (5%) (83). Furthermore, a fourth cluster (37%) showed severe medial temporal atrophy, together with low tau levels, only slightly abnormal amyloid load, and high WMH burden, corroborating the common contribution of non-AD pathophysiology to medial temporal degeneration in amnestic MCI patients (75–79).
4. Frontotemporal degeneration
FTD refers to a spectrum of clinical, pathological, and genetic heterogeneous disorders. From a clinical perspective, FTD is comprised of two predominant presentations, including the behavioral variant frontotemporal degeneration (bvFTD), characterized by progressive social and executive dysfunction, and the primary progressive aphasias (PPA), which encompass recognized semantic, nonfluent-agrammatic, and logopenic variants.
To date, only a handful of neuroimaging studies have aimed to characterize the neurodegenerative heterogeneity among these broad clinical FTD syndromes in a data-driven manner((41,86–91); Table 1). A first study in bvFTD used Ward’s clustering of regional gray matter volumes from structural MRI and identified a total of four distinct atrophy subtypes, of which two were characterized by predominant temporal lobe atrophy (‘temporal-dominant’ (9%) and ‘temporofrontoparietal’ (41%)), and the other two by predominant frontal lobe atrophy (‘frontal-dominant’ (32%) and frontotemporal’ (18%)(86)). This broad distinction into temporal-predominant and frontal-predominant atrophy subtypes of bvFTD could later be reproduced in independent clustering studies using measures of regional network degeneration on MRI(89) or region-specific hypometabolism as evidenced by FDG-PET(88).
Clustering of regional FDG-PET measurements was also used in a data-driven neuroimaging study of a clinically diverse sample of PPA patients. Here, the identified hypometabolic patterns separated the three canonical PPA variants, but suggested a further splitting of the non-fluent and logopenic variants into two subtypes each (90,91). Finally, application of an algorithm combining clustering and disease progression modelling on structural MRI data from a large cohort of familial FTD patients identified four distinct spatiotemporal patterns of atrophy progression, and these had a high correspondence with the main genetic subgroups (GRN, C9orf72, MAPT)(41). Moreover, early stages of the distinct atrophy subtypes could already be detected in presymptomatic mutation carriers, highlighting the potential of this approach for early detection and classification of neurodegeneration subtypes at presymptomatic disease stages of FTD.
5. Lewy body dementias
LBD, including Parkinson’s disease dementia (PDD) and dementia with Lewy bodies (DLB), is characterized by a distinct dementia syndrome with predominant attention, executive, and visuospatial impairments, which may fluctuate over time and are typically accompanied by distinct neuropsychiatric symptoms (92). A typical neuroimaging pattern of LBD has been described that involves predominant posterior cortical neurodegeneration with notable occipital involvement and relative sparing of the medial temporal lobe (93–95). However, like other broadly-defined dementia categories, individual clinical presentations within this characteristic LBD syndrome are heterogeneous (96–100).
Several neuroimaging studies could demonstrate that inter-individual differences in the core symptom dimensions of LBD associate with regionally-specific neurodegeneration as measured by structural MRI or FDG-PET (101–103). While individual atrophy patterns of DLB patients were recently also shown to associate differentially with specific predefined AD atrophy subtypes (105), we only identified a single data-driven neuroimaging study on neurodegeneration subtypes in LBD. The study applied Ward’s clustering on regional FDG-PET measurements in a sample of DLB patients and described two clusters that were only differentiated by slightly different occipital hypometabolism (104). The cluster with more severe occipital involvement (43%) had worse global cognition and higher risk for developing visual hallucinations over clinical follow-up. While this suggests that hypometabolic patterns may be relatively homogeneous among DLB patients, one has to note that the sample size was rather small for a data-driven exploration
While to our knowledge no data-driven neuroimaging studies have yet been conducted to explore regional neurodegeneration subtypes in PDD, data-driven studies applying Ward’s clustering to MRI-derived cortical thickness features have described heterogeneous atrophy patterns among nondemented PD patients(106–108). It remains to be determined whether the distinct cortical atrophy patterns in these patients show pattern-specific associations with progression to PDD or may represent early forms of distinct PDD subtypes.
6. Advantages, drawbacks and future directions
The recent advances in neuroimaging and machine learning offer sophisticated approaches to characterizing heterogeneity in ADRD. Through facilitating the discovery of different neurodegeneration subtypes, these approaches can capture granular individual differences that are mostly obscured in traditional group-level analyses. This discovery is highly critical to elucidate biological mechanisms that need to be understood for the development of drugs that are specifically tailored for specific disease variants or even for individuals.
On the other hand, the results of such novel approaches must be interpreted carefully (for a recent review on limitations of data-driven subtyping(109)). First, due to lack of ground truth labels, the use of clustering techniques for subtype discovery needs external validation steps using independent data modalities and cohorts, to ensure that the discovered subtypes reflect reproducible and biologically meaningful entities. Second, from a methodological perspective, methods to disentangle heterogeneity may also have stability issues, since results may change with slight changes in input features or model parameters. Thus, assessment of the stability of results, through subsampling, out-of-sample replication, and parameter perturbations, should be a part of the standard use(110–112).
In the case of AD dementia data-driven studies across multiple independent cohorts could provide converging evidence for the existence of at least three distinct atrophy subtypes that broadly resemble the neocortical vs medial temporal patterns characterizing the neuropathologically-defined subtypes (Fig.1). However, at a more granular level the findings of more detailed subtypes characterized by differentiated cortical atrophy patterns are more variable across the existing studies. Consensus-based summaries or repositories of the voxel-wise maps characterizing the different subtypes reported across data-driven studies would allow coordinate-based meta-analyses to reveal the most robust subtype-defining spatial patterns(113–115). In this context, more standardization of protocols across prospective cohort studies would be an important future step to reduce variance in subject recruitments and phenotyping variables, thus facilitating replication and synthesis between studies.
Moreover, the computational subtyping approaches also critically depend on the overall quality of the phenotyping variables used to describe the individual patients, and increasing the sensitivity and specificity of these variables to the underlying disease process may represent an important venue for future research to move the field forward. Furthermore, longitudinal neuroimaging assessments, while not yet well explored in subtyping studies, may be better suited for deciphering disease subtypes based on actual neurodegeneration trajectories instead of cross-sectional measures(42). In addition, most of the neuroimaging-based subtyping work so far has focused on anatomical MRI data, which may be less sensitive to early neurodegenerative processes compared to microstructural or functional imaging modalities. While these latter modalities are also beginning to be used for studying neurodegenerative heterogeneity in ADRD(46,88,90,104), more attention should be given to pathology-specific PET-imaging data in data-driven work due to the distinct advantages it offers for understanding the regional distribution of molecular disease processes(45,47,48).
Finally, current subtyping approaches typically make use of either relatively simplistic metrics from multiple biomarker modalities (e.g., hippocampal volume and CSF biomarker status) (83–85) or of high-dimensional data from one single modality, such as spatial atrophy patterns (see Table1) or differentiated cognitive profiles (50), and the degree to which the resulting subtypes correspond to overlapping patient subgroups is currently unknown. More systematic cross-modal subtyping comparisons and the direct combination of high-dimensional information from multiple modalities(48,116) in future clustering approaches may achieve a more comprehensive definition of distinct disease subtypes in AD and related dementias (Fig.2). Such a comprehensive multimodal definition will strongly benefit from the increasing availability of large-scale standardized datasets from well-phenotyped patient cohorts in combination with current advances in methods and power for computational analytics.
Figure 2:
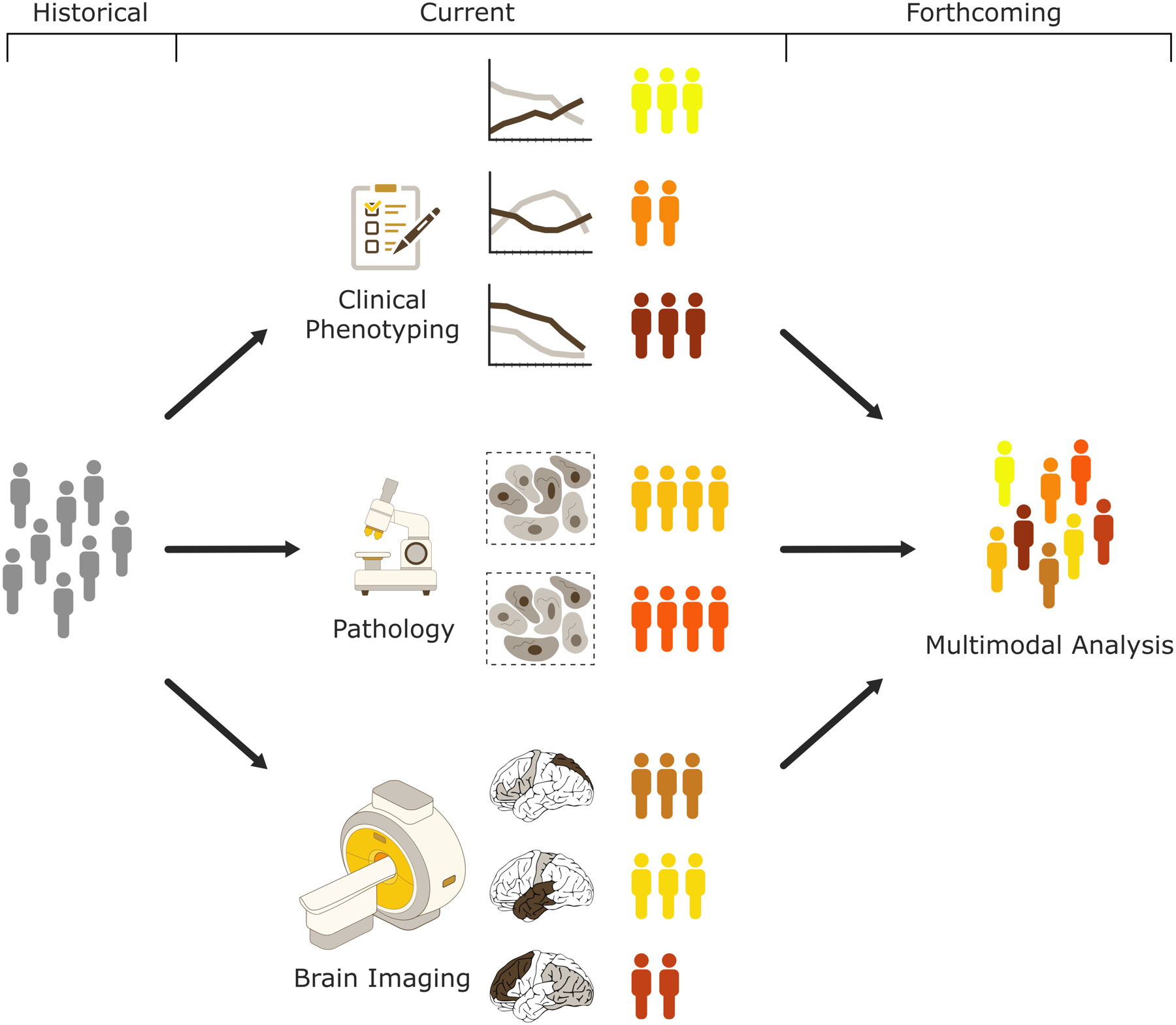
Over the past decade data-driven clustering approaches have helped in making new discoveries of previously unappreciated subtypes for the ADRD conditions, offering novel frameworks for improving individualized diagnosis and prognosis. Often these approaches made use of either simplistic metrics from multiple modalities or high-dimensional data from one single modality. As more phenotypic information is being collected within large-scale observational cohort studies together with improvements in computational power and algorithmic solutions, future work will incorporate combination of high-dimensional information from multiple modalities (i.e., clinical, pathological, and imaging) that may achieve a more comprehensive definition of distinct disease subtypes in AD and related dementia. Towards that standardization in data acquisition and harmonization between various cohorts is key element for future success for such frameworks implementing data synthesis across the different domains.
Supplementary Material
Acknowledgements
This study was supported in part by The Allen H. and Selma W. Berkman Charitable Trust (Accelerating Research on Vascular Dementia) and NIH grant no. (1RF1AG054409, R01 HL127659-04S1, AG057832, AG054519, NS107027, NS092091, AG055005, P30-AG010124 and R01-AG055005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
Dr. McMillan has received compensation for consulting services from Axon Advisors. Dr. Wolk reports grants from Eli Lilly/Avid, grants from Merck, grants from Biogen, personal fees from GE Healthcare, personal fees from Neuronix Ltd, outside the current work. Dr. Habes, Grothe, Tunc and Davatzikos report no biomedical financial interests or potential conflicts of interest.
References
- 1.Habes M, Erus G, Toledo JB, Zhang T, Bryan N, Laune LJ, et al. (2016): White matter hyperintensities and imaging patterns of brain aging in the general population. Brain 139(Pt 4): 1164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA (2018): Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 83: 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U (2010): Trajectories of brain aging in middle-aged and older adults: regional and individual differences. NeuroImage 51: 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habes M, Janowitz D, Erus G, Toledo J, Resnick SM, Doshi J, et al. (2016): Advanced Brain Aging: relationship with epidemiologic and genetic risk factors, and overlap with Alzheimer disease atrophy patterns. Transl Psychiatry 6: e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franke K, Ziegler G, Klöppel S, Gaser C (2010): Estimating the age of healthy subjects from T1-weighted {MRI} scans using kernel methods: Exploring the influence of various parameters. NeuroImage 50: 883–892. [DOI] [PubMed] [Google Scholar]
- 6.Cole JH, Franke K (2017): Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci 40: 681–690. [DOI] [PubMed] [Google Scholar]
- 7.Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H, Initiative ADN (2013): BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer’s Disease. PLoS ONE 8: e67346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufmann T, van der Meer D, Doan NT, Schwarz E, Lund MJ, Agartz I, et al. (2019): Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci 22: 1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Knol MJ, Tiulpin A, Dubost F, de Bruijne M, Vernooij MW, et al. (2019): Gray Matter Age Prediction as a Biomarker for Risk of Dementia. Proc Natl Acad Sci U S A 116: 21213–21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherubini A, Caligiuri ME, Peran P, Sabatini U, Cosentino C, Amato F (2016): Importance of Multimodal MRI in Characterizing Brain Tissue and its Potential Application for Individual Age Prediction. IEEE J Biomed Health Inform. 10.1109/JBHI.2016.2559938 [DOI] [PubMed] [Google Scholar]
- 11.Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, et al. (2017): Predicting brain-age from multimodal imaging data captures cognitive impairment. NeuroImage 148: 179–188. [DOI] [PubMed] [Google Scholar]
- 12.Eavani H, Habes M, Satterthwaite TD, An Y, Hsieh M-K, Honnorat N, et al. (2018): Heterogeneity of structural and functional imaging patterns of advanced brain aging revealed via machine learning methods. Neurobiol Aging 71: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorm AF (1985): Subtypes of Alzheimer’s dementia: a conceptual analysis and critical review. Psychol Med 15: 543–553. [DOI] [PubMed] [Google Scholar]
- 14.Ritchie K, Touchon J (1992): Heterogeneity in senile dementia of the Alzheimer type: individual differences, progressive deterioration or clinical sub-types? J Clin Epidemiol 45: 1391–1398. [DOI] [PubMed] [Google Scholar]
- 15.Wallin A, Blennow K (1996): Clinical subgroups of the Alzheimer syndrome. Acta Neurol Scand Suppl 165: 51–57. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC (1998): Clinical subtypes of Alzheimer’s disease. Dement Geriatr Cogn Disord 9 Suppl 3: 16–24. [DOI] [PubMed] [Google Scholar]
- 17.Cummings JL (2000): Cognitive and behavioral heterogeneity in Alzheimer’s disease: seeking the neurobiological basis. Neurobiol Aging 21: 845–861. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong RA (2012): On the “classification” of neurodegenerative disorders: discrete entities, overlap or continuum? Folia Neuropathol 50: 201–208. [DOI] [PubMed] [Google Scholar]
- 19.Stanley K, Walker Z (2014): Do patients with young onset Alzheimer’s disease deteriorate faster than those with late onset Alzheimer’s disease? A review of the literature. Int Psychogeriatr 26: 1945–1953. [DOI] [PubMed] [Google Scholar]
- 20.Bergeron D, Bensaidane R, Laforce R (2016): Untangling Alzheimer’s Disease Clinicoanatomical Heterogeneity Through Selective Network Vulnerability - An Effort to Understand a Complex Disease. Curr Alzheimer Res 13: 589–596. [DOI] [PubMed] [Google Scholar]
- 21.Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, et al. (2016): Brain atrophy in Alzheimer’s Disease and aging. Ageing Res Rev 30: 25–48. [DOI] [PubMed] [Google Scholar]
- 22.Taipa R, Sousa AL, Melo Pires M, Sousa N (2016): Does the Interplay Between Aging and Neuroinflammation Modulate Alzheimer’s Disease Clinical Phenotypes? A Clinico-Pathological Perspective. J Alzheimers Dis JAD 53: 403–417. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Braak E (1991): Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82: 239–259. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann M, Ghosh PM, Madison C, Laforce RJ, Corbetta-Rastelli C, Weiner MW, et al. (2013): Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain J Neurol 136: 844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Moller C, Lehmann M, et al. (2015): Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp 36: 4421–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips JS, Da Re F, Dratch L, Xie SX, Irwin DJ, McMillan CT, et al. (2018): Neocortical origin and progression of gray matter atrophy in nonamnestic Alzheimer’s disease. Neurobiol Aging 63: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011): Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byun MS, Kim SE, Park J, Yi D, Choe YM, Sohn BK, et al. (2015): Heterogeneity of Regional Brain Atrophy Patterns Associated with Distinct Progression Rates in Alzheimer’s Disease. PloS One 10: e0142756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira D, Verhagen C, Hernandez-Cabrera JA, Cavallin L, Guo C-J, Ekman U, et al. (2017): Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications. Sci Rep 7: 46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira D, Shams S, Cavallin L, Viitanen M, Martola J, Granberg T, et al. (2018): The contribution of small vessel disease to subtypes of Alzheimer’s disease: a study on cerebrospinal fluid and imaging biomarkers. Neurobiol Aging 70: 18–29. [DOI] [PubMed] [Google Scholar]
- 31.Risacher SL, Anderson WH, Charil A, Castelluccio PF, Shcherbinin S, Saykin AJ, Schwarz AJ (2017): Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurology 89: 2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hastie T, Tibshirani R, Friedman J (2009): Ensemble Learning The Elements of Statistical Learning. New York, NY: Springer New York. [Google Scholar]
- 33.Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H, et al. (2014): Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology 83: 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang J, Kim CM, Jeon S, Lee JM, Hong YJ, Roh JH, et al. (2016): Prediction of Alzheimer’s disease pathophysiology based on cortical thickness patterns. Alzheimers Dement Amst Neth 2: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J-Y, Na HK, Kim S, Kim H, Kim HJ, Seo SW, et al. (2017): Robust Identification of Alzheimer’s Disease subtypes based on cortical atrophy patterns. Sci Rep 7: 43270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ten Kate M, Dicks E, Visser PJ, van der Flier WM, Teunissen CE, Barkhof F, et al. (2018): Atrophy subtypes in prodromal Alzheimer’s disease are associated with cognitive decline. Brain J Neurol 141: 3443–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong A, Toledo JB, Honnorat N, Doshi J, Varol E, Sotiras A, et al. (2017): Heterogeneity of neuroanatomical patterns in prodromal Alzheimer’s disease: links to cognition, progression and biomarkers. Brain J Neurol 140: 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulakis K, Pereira JB, Mecocci P, Vellas B, Tsolaki M, Kloszewska I, et al. (2018): Heterogeneous patterns of brain atrophy in Alzheimer’s disease. Neurobiol Aging 65: 98–108. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Mormino EC, Sun N, Sperling RA, Sabuncu MR, Yeo BTT (2016): Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer’s disease. Proc Natl Acad Sci U S A 113: E6535–E6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blei DM, Ng AY, Jordan MI (2003): Latent dirichlet allocation. J Mach Learn Res 3: 993–1022. [Google Scholar]
- 41.Young AL, Marinescu RV, Oxtoby NP, Bocchetta M, Yong K, Firth NC, et al. (2018): Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun 9: 4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marinescu RV, Eshaghi A, Lorenzi M, Young AL, Oxtoby NP, Garbarino S, et al. (2019): DIVE: A spatiotemporal progression model of brain pathology in neurodegenerative disorders. NeuroImage 192: 166–177. [DOI] [PubMed] [Google Scholar]
- 43.Dong A, Honnorat N, Gaonkar B, Davatzikos C (2015): CHIMERA: Clustering of heterogeneous disease effects via distribution matching of imaging patterns. [DOI] [PMC free article] [PubMed]
- 44.Habes M, Sotiras A, Erus G, Toledo JB, Janowitz D, Wolk DA, et al. (2018): White matter lesions: Spatial heterogeneity, links to risk factors, cognition, genetics, and atrophy. Neurology 91: e964–e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowe VJ, Wiste HJ, Senjem ML, Weigand SD, Therneau TM, Boeve BF, et al. (2018): Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain J Neurol 141: 271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sui X, Rajapakse JC (2018): Profiling heterogeneity of Alzheimer’s disease using white-matter impairment factors. NeuroImage Clin 20: 1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitwell JL, Graff-Radford J, Tosakulwong N, Weigand SD, Machulda M, Senjem ML, et al. (2018): [(18) F]AV-1451 clustering of entorhinal and cortical uptake in Alzheimer’s disease. Ann Neurol 83: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon S, Kang JM, Seo S, Jeong HJ, Funck T, Lee S-Y, et al. (2019): Topographical Heterogeneity of Alzheimer’s Disease Based on MR Imaging, Tau PET, and Amyloid PET. Front Aging Neurosci 11: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martorelli M, Sudo FK, Charchat-Fichman H (2018): This is not only about memory: A systematic review on neuropsychological heterogeneity in Alzheimer’s disease. Psychol Neurosci. [Google Scholar]
- 50.Scheltens NME, Tijms BM, Koene T, Barkhof F, Teunissen CE, Wolfsgruber S, et al. (2017): Cognitive subtypes of probable Alzheimer’s disease robustly identified in four cohorts. Alzheimers Dement J Alzheimers Assoc 13: 1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crane PK, Trittschuh E, Mukherjee S, Saykin AJ, Sanders RE, Larson EB, et al. (2017): Incidence of cognitively defined late-onset Alzheimer’s dementia subgroups from a prospective cohort study. Alzheimers Dement J Alzheimers Assoc 13: 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iqbal K, Flory M, Khatoon S, Soininen H, Pirttila T, Lehtovirta M, et al. (2005): Subgroups of Alzheimer’s disease based on cerebrospinal fluid molecular markers. Ann Neurol 58: 748–757. [DOI] [PubMed] [Google Scholar]
- 53.van der Vlies AE, Verwey NA, Bouwman FH, Blankenstein MA, Klein M, Scheltens P, van der Flier WM (2009): CSF biomarkers in relationship to cognitive profiles in Alzheimer disease. Neurology 72: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 54.Wallin AK, Blennow K, Zetterberg H, Londos E, Minthon L, Hansson O (2010): CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology 74: 1531–1537. [DOI] [PubMed] [Google Scholar]
- 55.Dickerson BC, Wolk DA (2011): Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry 82: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML, et al. (2012): Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol 11: 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholl M, Ossenkoppele R, Strandberg O, Palmqvist S, Jogi J, Ohlsson T, et al. (2017): Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain J Neurol 140: 2286–2294. [DOI] [PubMed] [Google Scholar]
- 58.Ossenkoppele R, Schonhaut DR, Scholl M, Lockhart SN, Ayakta N, Baker SL, et al. (2016): Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain J Neurol 139: 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grothe MJ, Sepulcre J, Gonzalez-Escamilla G, Jelistratova I, Scholl M, Hansson O, Teipel SJ (2018): Molecular properties underlying regional vulnerability to Alzheimer’s disease pathology. Brain J Neurol 141: 2755–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sepulcre J, Grothe MJ, d’Oleire Uquillas F, Ortiz-Teran L, Diez I, Yang H-S, et al. (2018): Neurogenetic contributions to amyloid beta and tau spreading in the human cortex. Nat Med 24: 1910–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolk DA, Dickerson BC (2010): Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci U S A 107: 10256–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukherjee S, Mez J, Trittschuh EH, Saykin AJ, Gibbons LE, Fardo DW, et al. (2018): Genetic data and cognitively defined late-onset Alzheimer’s disease subgroups. Mol Psychiatry. 10.1038/s41380-018-0298-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller ZA, Mandelli ML, Rankin KP, Henry ML, Babiak MC, Frazier DT, et al. (2013): Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain J Neurol 136: 3461–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller ZA, Rosenberg L, Santos-Santos MA, Stephens M, Allen IE, Hubbard HI, et al. (2018): Prevalence of Mathematical and Visuospatial Learning Disabilities in Patients With Posterior Cortical Atrophy. JAMA Neurol 75: 728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. (2013): Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136: 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. (2013): Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun 1: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, et al. (2014): TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol (Berl) 127: 811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Josephs KA, Dickson DW, Tosakulwong N, Weigand SD, Murray ME, Petrucelli L, et al. (2017): Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: a longitudinal retrospective study. Lancet Neurol 16: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA (2016): TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain J Neurol 139: 2983–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. (2018): Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain J Neurol 141: 2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jellinger KA (2012): Neuropathological subtypes of Alzheimer’s disease. Acta Neuropathol (Berl) 123: 153–154. [DOI] [PubMed] [Google Scholar]
- 72.Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, et al. (2015): TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol 78: 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell AJ, Shiri-Feshki M (2009): Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 119: 252–265. [DOI] [PubMed] [Google Scholar]
- 74.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. (2006): Mild cognitive impairment. Lancet Lond Engl 367: 1262–1270. [DOI] [PubMed] [Google Scholar]
- 75.Jicha GA, Petersen RC, Knopman DS, Boeve BF, Smith GE, Geda YE, et al. (2006): Argyrophilic grain disease in demented subjects presenting initially with amnestic mild cognitive impairment. J Neuropathol Exp Neurol 65: 602–609. [DOI] [PubMed] [Google Scholar]
- 76.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. (2014): Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol (Berl) 128: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abner EL, Kryscio RJ, Schmitt FA, Fardo DW, Moga DC, Ighodaro ET, et al. (2017): Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol 81: 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jicha GA, Nelson PT (2019): Hippocampal Sclerosis, Argyrophilic Grain Disease, and Primary Age-Related Tauopathy. Contin Minneap Minn 25: 208–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. (2019): Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain J Neurol. 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jung N-Y, Seo SW, Yoo H, Yang J-J, Park S, Kim YJ, et al. (2016): Classifying anatomical subtypes of subjective memory impairment. Neurobiol Aging 48: 53–60. [DOI] [PubMed] [Google Scholar]
- 81.Ezzati A, Zammit AR, Habeck C, Hall CB, Lipton RB (2019): Detecting biological heterogeneity patterns in ADNI amnestic mild cognitive impairment based on volumetric MRI. Brain Imaging Behav. 10.1007/s11682-019-00115-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim HJ, Park J-Y, Seo SW, Jung YH, Kim Y, Jang H, et al. (2019): Cortical atrophy pattern-based subtyping predicts prognosis of amnestic MCI: an individual-level analysis. Neurobiol Aging 74: 38–45. [DOI] [PubMed] [Google Scholar]
- 83.Nettiksimmons J, DeCarli C, Landau S, Beckett L, Initiative ADN, others (2014): Biological heterogeneity in ADNI amnestic mild cognitive impairment. Alzheimers Dement 10: 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gamberger D, Lavrac N, Srivatsa S, Tanzi RE, Doraiswamy PM (2017): Identification of clusters of rapid and slow decliners among subjects at risk for Alzheimer’s disease. Sci Rep 7: 6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ota K, Oishi N, Ito K, Fukuyama H (2016): Prediction of Alzheimer’s Disease in Amnestic Mild Cognitive Impairment Subtypes: Stratification Based on Imaging Biomarkers. J Alzheimers Dis JAD 52: 1385–1401. [DOI] [PubMed] [Google Scholar]
- 86.Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. (2009): Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain J Neurol 132: 2932–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitwell JL, Xu J, Mandrekar J, Boeve BF, Knopman DS, Parisi JE, et al. (2013): Frontal asymmetry in behavioral variant frontotemporal dementia: clinicoimaging and pathogenetic correlates. Neurobiol Aging 34: 636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cerami C, Dodich A, Lettieri G, Iannaccone S, Magnani G, Marcone A, et al. (2016): Different FDG-PET metabolic patterns at single-subject level in the behavioral variant of fronto-temporal dementia. Cortex J Devoted Study Nerv Syst Behav 83: 101–112. [DOI] [PubMed] [Google Scholar]
- 89.Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW, et al. (2016): Distinct Subtypes of Behavioral Variant Frontotemporal Dementia Based on Patterns of Network Degeneration. JAMA Neurol 73: 1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matias-Guiu JA, Diaz-Alvarez J, Ayala JL, Risco-Martin JL, Moreno-Ramos T, Pytel V, et al. (2018): Clustering Analysis of FDG-PET Imaging in Primary Progressive Aphasia. Front Aging Neurosci 10: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matias-Guiu JA, Diaz-Alvarez J, Cuetos F, Cabrera-Martin MN, Segovia-Rios I, Pytel V, et al. (2019): Machine learning in the clinical and language characterisation of primary progressive aphasia variants. Cortex J Devoted Study Nerv Syst Behav 119: 312–323. [DOI] [PubMed] [Google Scholar]
- 92.Gomperts SN (2016): Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia. Contin Minneap Minn 22: 435–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT (2004): Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain J Neurol 127: 791–800. [DOI] [PubMed] [Google Scholar]
- 94.Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. (2010): Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 74: 885–892. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez-Redondo R, Garcia-Garcia D, Clavero P, Gasca-Salas C, Garcia-Eulate R, Zubieta JL, et al. (2014): Grey matter hypometabolism and atrophy in Parkinson’s disease with cognitive impairment: a two-step process. Brain J Neurol 137: 2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Janvin CC, Larsen JP, Salmon DP, Galasko D, Hugdahl K, Aarsland D (2006): Cognitive profiles of individual patients with Parkinson’s disease and dementia: comparison with dementia with lewy bodies and Alzheimer’s disease. Mov Disord Off J Mov Disord Soc 21: 337–342. [DOI] [PubMed] [Google Scholar]
- 97.Aarsland D, Bronnick K, Ehrt U, De Deyn PP, Tekin S, Emre M, Cummings JL (2007): Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry 78: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rongve A, Bronnick K, Ballard C, Aarsland D (2010): Core and suggestive symptoms of dementia with lewy bodies cluster in persons with mild dementia. Dement Geriatr Cogn Disord 29: 317–324. [DOI] [PubMed] [Google Scholar]
- 99.Varanese S, Perfetti B, Monaco D, Thomas A, Bonanni L, Tiraboschi P, Onofrj M (2010): Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol 257: 1004–1011. [DOI] [PubMed] [Google Scholar]
- 100.Morenas-Rodriguez E, Sala I, Subirana A, Pascual-Goni E, Sanchez-Saudinos MB, Alcolea D, et al. (2018): Clinical Subtypes of Dementia with Lewy Bodies Based on the Initial Clinical Presentation. J Alzheimers Dis JAD 64: 505–513. [DOI] [PubMed] [Google Scholar]
- 101.Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, et al. (2009): Correlations between gray matter reductions and cognitive deficits in dementia with Lewy Bodies and Parkinson’s disease with dementia. Mov Disord Off J Mov Disord Soc 24: 1740–1746. [DOI] [PubMed] [Google Scholar]
- 102.Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, et al. (2010): Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord Off J Mov Disord Soc 25: 615–622. [DOI] [PubMed] [Google Scholar]
- 103.Morbelli S, Chincarini A, Brendel M, Rominger A, Bruffaerts R, Vandenberghe R, et al. (2019): Metabolic patterns across core features in dementia with lewy bodies. Ann Neurol 85: 715–725. [DOI] [PubMed] [Google Scholar]
- 104.Caminiti SP, Sala A, Iaccarino L, Beretta L, Pilotto A, Gianolli L, et al. (2019): Brain glucose metabolism in Lewy body dementia: implications for diagnostic criteria. Alzheimers Res Ther 11: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oppedal K, Ferreira D, Cavallin L, Lemstra AW, Ten Kate M, Padovani A, et al. (2019): A signature pattern of cortical atrophy in dementia with Lewy bodies: A study on 333 patients from the European DLB consortium. Alzheimers Dement J Alzheimers Assoc 15: 400–409. [DOI] [PubMed] [Google Scholar]
- 106.Uribe C, Segura B, Baggio HC, Abos A, Marti MJ, Valldeoriola F, et al. (2016): Patterns of cortical thinning in nondemented Parkinson’s disease patients: Cortical Thickness Cluster Analysis in PD. Mov Disord 31: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Uribe C, Segura B, Baggio HC, Abos A, Garcia-Diaz AI, Campabadal A, et al. (2018): Cortical atrophy patterns in early Parkinson’s disease patients using hierarchical cluster analysis. Parkinsonism Relat Disord 50: 3–9. [DOI] [PubMed] [Google Scholar]
- 108.Uribe C, Segura B, Baggio HC, Abos A, Garcia-Diaz AI, Campabadal A, et al. (2019): Progression of Parkinson’s disease patients’ subtypes based on cortical thinning: Parkinsonism Relat Disord 64: 286–292. [DOI] [PubMed] [Google Scholar]
- 109.Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, Fair DA (2019): The Heterogeneity Problem: Approaches to Identify Psychiatric Subtypes. Trends Cogn Sci 23: 584–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Halkidi M, Batistakis Y, Vazirgiannis M (2001): On Clustering Validation Techniques. J Intell Inf Syst 17: 107–145. [Google Scholar]
- 111.Rousseeuw PJ (1987): Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 20: 53–65. [Google Scholar]
- 112.Davies DL, Bouldin DW (1979): A Cluster Separation Measure. IEEE Trans Pattern Anal Mach Intell PAMI-1: 224–227. [PubMed] [Google Scholar]
- 113.Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJJ, et al. (2011): The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes 4: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gorgolewski KJ, Varoquaux G, Rivera G, Schwartz Y, Sochat VV, Ghosh SS, et al. (2016): NeuroVault.org: A repository for sharing unthresholded statistical maps, parcellations, and atlases of the human brain. NeuroImage 124: 1242–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reid AT, Bzdok D, Genon S, Langner R, Muller VI, Eickhoff CR, et al. (2016): ANIMA: A data-sharing initiative for neuroimaging meta-analyses. NeuroImage 124: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 116.Sun N, Mormino EC, Chen J, Sabuncu MR, Yeo BTT (2019): Multi-modal latent factor exploration of atrophy, cognitive and tau heterogeneity in Alzheimer’s disease. NeuroImage 201: 116043. [DOI] [PubMed] [Google Scholar]
- 117.Na HK, Kang DR, Kim S, Seo SW, Heilman KM, Noh Y, Na DL (2016): Malignant progression in parietal-dominant atrophy subtype of Alzheimer’s disease occurs independent of onset age. Neurobiol Aging 47: 149–156. [DOI] [PubMed] [Google Scholar]
- 118.Varol E, Sotiras A, Davatzikos C (2017): HYDRA: Revealing heterogeneity of imaging and genetic patterns through a multiple max-margin discriminative analysis framework. NeuroImage 145: 346–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caminiti SP, Ballarini T, Sala A, Cerami C, Presotto L, Santangelo R, et al. (2018): FDG-PET and CSF biomarker accuracy in prediction of conversion to different dementias in a large multicentre MCI cohort. NeuroImage Clin 18: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


