Abstract
Background:
In the intensive care unit (ICU), inactivity is common, contributing to ICU-acquired weakness and poor outcomes. Actigraphy may be useful for measuring activity in the ICU.
Objectives:
To use actigraphy to characterize inactivity and activity in critically ill patients.
Methods:
This prospective observational study involved 48-hour wrist actigraphy in medical ICU (MICU) patients, with activity data captured across 30-second epochs. Inactivity (zero-activity epochs) and activity (levels of non-zero activity) were summarized across key patient (e.g., age) and clinical (e.g., mechanical ventilation status) variables, and compared using multivariable regression.
Results:
Overall, 189,595 30-second epochs were collected in 34 MICU patients. Zero-activity comprised 122,865 (65%) of epochs. Inactivity was 24% and 13% more prevalent, respectively, in patients receiving mechanical ventilation (versus none, p<0.001) and in the highest (versus lowest) organ failure score tertile (p=0.03). Ambulatory (versus non-ambulatory) patients exhibited more activity (26 more movements per epoch, p<0.001), while those in the highest (versus lowest) organ failure score tertile exhibited less activity (19 fewer movements per epoch, p=0.03). Significant inactivity/activity differences were not observed when evaluated based on age, sedation, or restraint status.
Conclusions:
Actigraphy demonstrated that MICU patients are profoundly inactive, including those who are young, non-sedated and non-restrained. Hence, ICU-specific, non-patient-related factors may contribute to inactivity, an issue requiring further investigation.
Keywords: actigraphy, activity, inactivity, critical illness, ICU, mobilization
INTRODUCTION
Critically ill patients often experience prolonged bedrest and inactivity, placing them at risk for adverse short- and long-term outcomes including intensive care unit (ICU)-acquired weakness (ICU-AW).1 It is estimated ICU-AW is associated with 30% higher in-hospital and 13% higher 1-year mortality.2 Many factors contribute to ICU-AW, including sedative infusions, neuromuscular blockade, glucocorticoids, and critical illness itself.2,3 Efforts to mobilize patients early may help prevent ICU-AW, reduce length of stay, and improve functional status after discharge.4,5 Despite this literature, many barriers impede mobilization efforts, including incomplete knowledge of its benefits, a lack of champions, and inexperience.1,3,6,7 Nevertheless, early mobilization efforts are gaining traction, and are recommended in ICU clinical practice guidelines.8
Despite guideline-supported efforts, tools to measure activity in the ICU are limited. Measurement of activity is important, as it can inform mobility practices and identify inactive patients who may benefit from mobilization. Most ICU mobility tools involve direct observation by trained observers, which involves activity measurement over discrete, rather than continuous time periods, and is infeasible on a large scale.9
As an alternative measurement tool, actigraphy involves use of an accelerometer to log patient activity, usually with a wristwatch-like device. Actigraphy is generally well tolerated, low cost, and, most importantly, provides continuous and objective activity data not provided by other tools.10–14 While actigraphy has been used for decades to evaluate activity and estimate sleep,15–18 prior studies involving actigraphy in critically ill patients mostly involved small convenience samples, utilized manufacturer-provided software for sleep-wake estimation, or presented broad descriptions of patient activity.13,19–21 Though is it commonly believed that critically ill patients are generally inactive, to build knowledge in this area, we performed an exploratory analysis of data from a feasibility study of actigraphy in MICU patients.22 The original study was performed to assess the feasibility of continuous monitoring by actigraph over a prolonged period of time in a continuous sample of MICU patients. By examining zero- and non-zero activity levels across patient- and ICU-specific variables, our objective is to utilize actigraphy to identify factors associated with inactivity and low activity, and to highlight critically ill patient subpopulations who would most benefit from mobility interventions. The overarching goal of this work is to advance research on methods to design, motivate, evaluate, and sustain interventions aimed at promoting mobility in critically ill patients.
THEORY AND CALCULATIONS
We hypothesized that actigraphy would demonstrate that MICU patients are inactive and have low non-zero activity levels, in particular those who are older, with higher organ failure scores, or requiring sedation, restraints, or mechanical ventilation.
MATERIALS AND METHODS
This exploratory analysis was performed as a part of a prospective observational study evaluating the feasibility of 48-hour wrist actigraphy in consecutively enrolled patients in a Medical Intensive Care Unit (MICU).22 The original study was performed to assess the feasibility of continuous monitoring by actigraph over a prolonged period of time in a consecutive sample of MICU patients. All enrolled patients or surrogates provided oral informed consent. The UCLA institutional review board approved the study.
Study Setting and Participants
This study occurred in an academic MICU with 24 private rooms and a nurse-to-patient ratio of 1:2. Potentially eligible patients were identified from the daily MICU census. All patients aged 18 and older were considered eligible for enrollment. Patients who were moribund, awaiting transfer out of the MICU, awaiting procedures involving the wrist, with no available wrist (e.g., due to lines placed in the hand or arm), or unable to provide informed consent in English were excluded.
Actigraphy and Patient Data
As part of the feasibility study, each enrolled patient underwent 48-hour wrist actigraphy recording using the Phillips Respironics Actiwatch Spectrum Pro (Andover, Maryland, USA). Actigraphy recording began at 12:00 on the day of enrollment, or soon thereafter, with activity levels recorded by the device every 30 seconds (one epoch). We chose the 12:00 start time and 48-hour duration to balance the desire to record one complete day and night with the fact that some participants would be the ICU for a short period of time.
After consent was obtained, actigraphs were placed on each patient’s right wrist, or if unavailable (i.e., due to arterial line), the left wrist. Each day, trained research personnel confirmed appropriate positioning of the actigraph devices on each enrolled subject. After up to 48 hours, actigraphs were removed and data were uploaded onto a computer for analysis. To identify time-based trends in inactivity and activity, during both the day and night, we divided actigraph data into 4-hour time blocks, as follows: 06:00–09:59, 10:00–13:59, 14:00–17:59, 18:00–21:59, 22:00–01:59, and 02:00–05:59.
Patient baseline (pre-ICU) and ICU variables of potential interest were collected during this study, based on prior research and our own clinical experiences. From the electronic medical record, these variables included age, gender, body mass index, and admission diagnosis. Over the 48-hour recording period, we collected daily Sequential Organ Failure Assessment (SOFA) organ failure scores, along with sedation, restraint, and mechanical ventilation status. Patients and/or surrogates also reported patients’ ambulatory status prior to admission. Sedation, restraint, and mechanical ventilation status were dichotomized into ever vs. never during the 48-hour actigraph recording period. Only patients providing wrist actigraph data during the allotted 48-hour period were included in this analysis; for these patients, there were no gaps in actigraph recordings or missing demographic or clinical data. Duplicate data entry was performed and abstracted data were audited by a third independent clinician to verify accuracy. Due to the design of original feasibility study, information on patient behaviors and patient care activities were not collected.
Statistical Analysis
Baseline and ICU data were evaluated using median and interquartile range for continuous variables and proportions for categorical variables. As a method to characterize inactivity and activity, we evaluated the proportion of epochs equaling zero activity (representing inactivity) and mean levels of non-zero activity (representing activity). Inactivity and non-zero activity levels were stratified by baseline and ICU variables. Subsequently, we estimated between-strata differences in inactivity and activity using univariable and multivariable logistic (to evaluate zero versus non-zero epochs) and Poisson regression models (to evaluate non-zero activity levels per epoch). Generalized estimating equations were used to account for repeated measures across subjects. Variance inflation factors were used to confirm the lack of multicollinearity in the multivariable model. Finally, we displayed zero-activity epochs using bar plots and non-zero activity levels using linear fit plots. For the initial feasibility study, a sample size of 35 was calculated for a feasibility proportion of 90%, with a 95% CI plus or minus 10%. All analyses were performed using STATA version 14.0 (College Station, TX).
RESULTS
Baseline and ICU Characteristics
Overall, 135 consecutive patients were screened from November 2014 to January 2015; 48 (36%) met eligibility criteria and 35 (73% of eligible patients) consented to participation (Figure 1). Thirty-four enrolled patients contributed actigraph data: 33 (97%) completed 48 hours, 1 (3%) completed 34.2 hours, 28 (82%) wore actigraphs on the right wrist and 3 (9%) on the left wrist (unknown wrist for 3 patients). Median (IQR) patient age was 60 (44, 69) years old, 17 (50%) were female, 21 (64%) non-Hispanic white race only, and 32 (91%) were ambulatory before ICU admission (Table 1). In the ICU, 14 of 34 (41%) patients were admitted with respiratory failure, and 11 (32%), 7 (21%), and 3 (9%) ever received mechanical ventilation, continuous sedative infusions, and wrist restraints, respectively. Median (IQR) average daily SOFA score in the ICU was 5 (3, 9). No patients died during the recording period.
Figure 1.
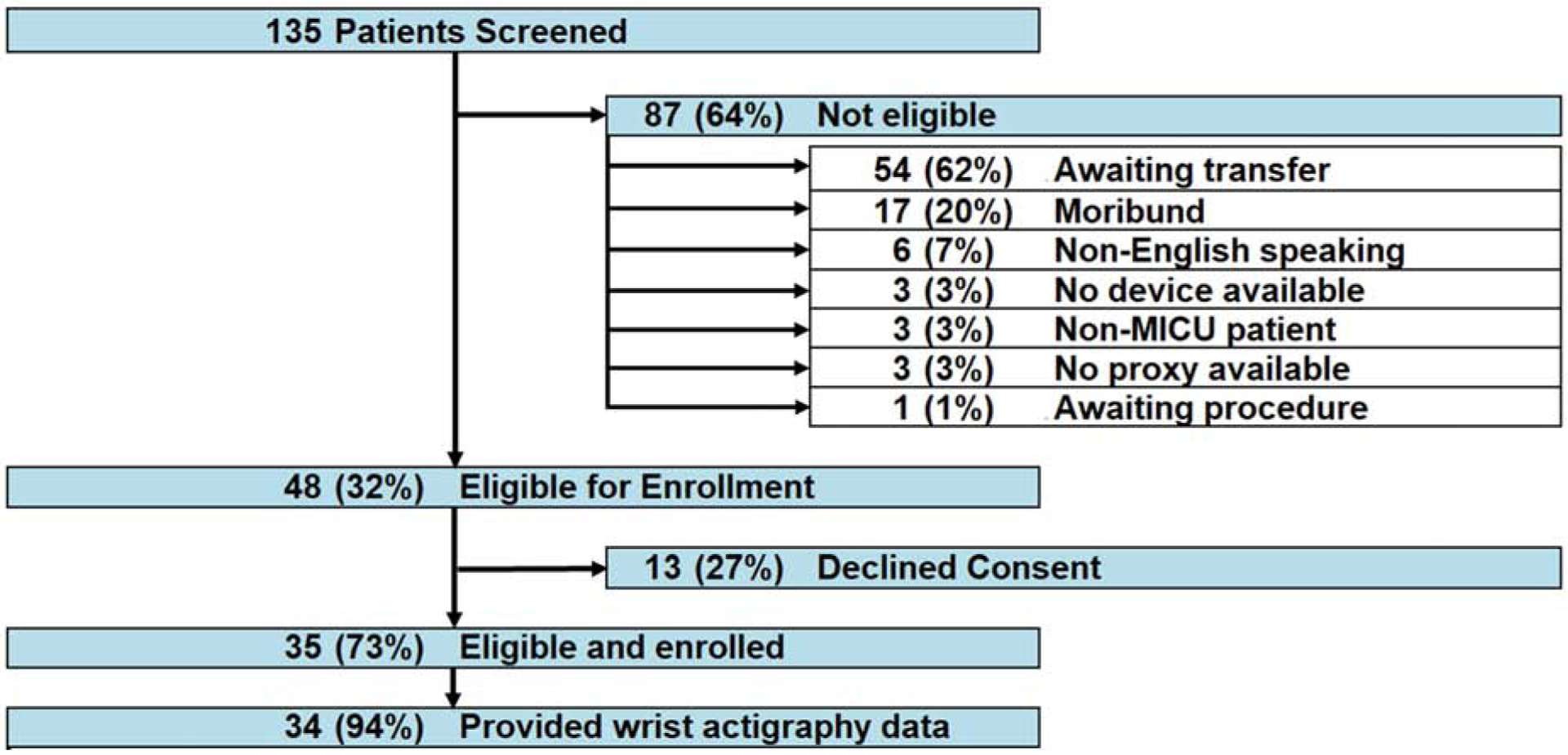
Patient flow diagram
Table 1.
Patient Characteristics (N = 34)
| Baseline Variables | |
| Age, median (IQR)a | 60 (44,69) |
| Female, n (%) | 17 (50%) |
| Non-Hispanic White Race, n (%) | 21 (64%) |
| Ambulatory prior to ICU admission | 32 (91 %) |
| BMI Classificationb | |
| Underweight (BMI <18) | 3 (9%) |
| Normal (BMI 18–24.9) | 13 (38%) |
| Overweight (BMI 25–29.9) | 9 (26%) |
| Obese (BMI > 30) | 9 (26%) |
| ICU Variables | |
| Admission Diagnosis Category | |
| Respiratory Failure | 14 (41%) |
| Gastrointestinal | 3 (9%) |
| Sepsis | 7 (21%) |
| Cardiovascular | 4 (12%) |
| Otherc | 6 (18%) |
| Average Daily SOFA Organ Failure Score, median (IQR)a,c | 5 (3, 9) |
| Ever Mechanically Ventilatedc | 11 (32%) |
| Ever Received Continuous Sedative Infusionc | 7 (21%) |
| Ever Restrainedc | 3 (9%) |
Abbreviations: IQR = Interquartile Range; ICU = Intensive Care Unit; BMI = Body Mass Index; SOFA = Sequential Organ Failure Assessment
Stratifed by tertile in univariable and multivariable analyses
Includes monitoring for procedures (2 of 34, 6%), renal (1 of 34, 6%), endocrine (1 of 34, 3%), and other (2 of 34, 6%)
During 48-hour actigraphy recording period only
Inactivity (Zero-Activity Epochs)
Across 34 patients and 101 patient-days, we collected 189,595 30-second epochs of actigraphy-based activity data. Overall, 122,865 (65%) epochs had zero activity, with 61% zeroes (56,022 epochs out of 189,595) during normal waking hours (06:00 to 21:59).
Inactivity (zero-activity epochs), stratified by time-of-day and baseline and ICU characteristics, are depicted in Figure 2 and Table 2. Compared to 06:00–09:59, the mean proportion of zero-activity epochs was significantly higher from 22:00 to 01:59 and 02:00 to 05:59 (mean [95% CI] proportion zero-activity epochs 62% [55–70%] versus 72% [64–78%] and 76% [70–80%], respectively, adjusted differences [95% CI] = 9% [3–15%] and 14% [9–19%], multivariable p=0.002 and p<0.001) (Table 2). Additionally, patients in the highest (versus lowest) average daily SOFA score tertile were significantly more inactive (mean [95% CI] proportion zero-activity epochs = 77% [65–86%] versus 58% [48–52%], adjusted difference = 13% [2–24%], multivariable p=0.03), along with patients who were ever (versus never) mechanically ventilated (79% [65–89%] versus 58% [51–64%], adjusted difference = 24% [11–38%], multivariable p<0.001) (Table 2). Notably, there were no significant differences in inactivity by age, ambulatory status prior to ICU admission, or admission diagnosis category, or in patients who ever received continuous sedative infusions or restraints.
Figure 2.
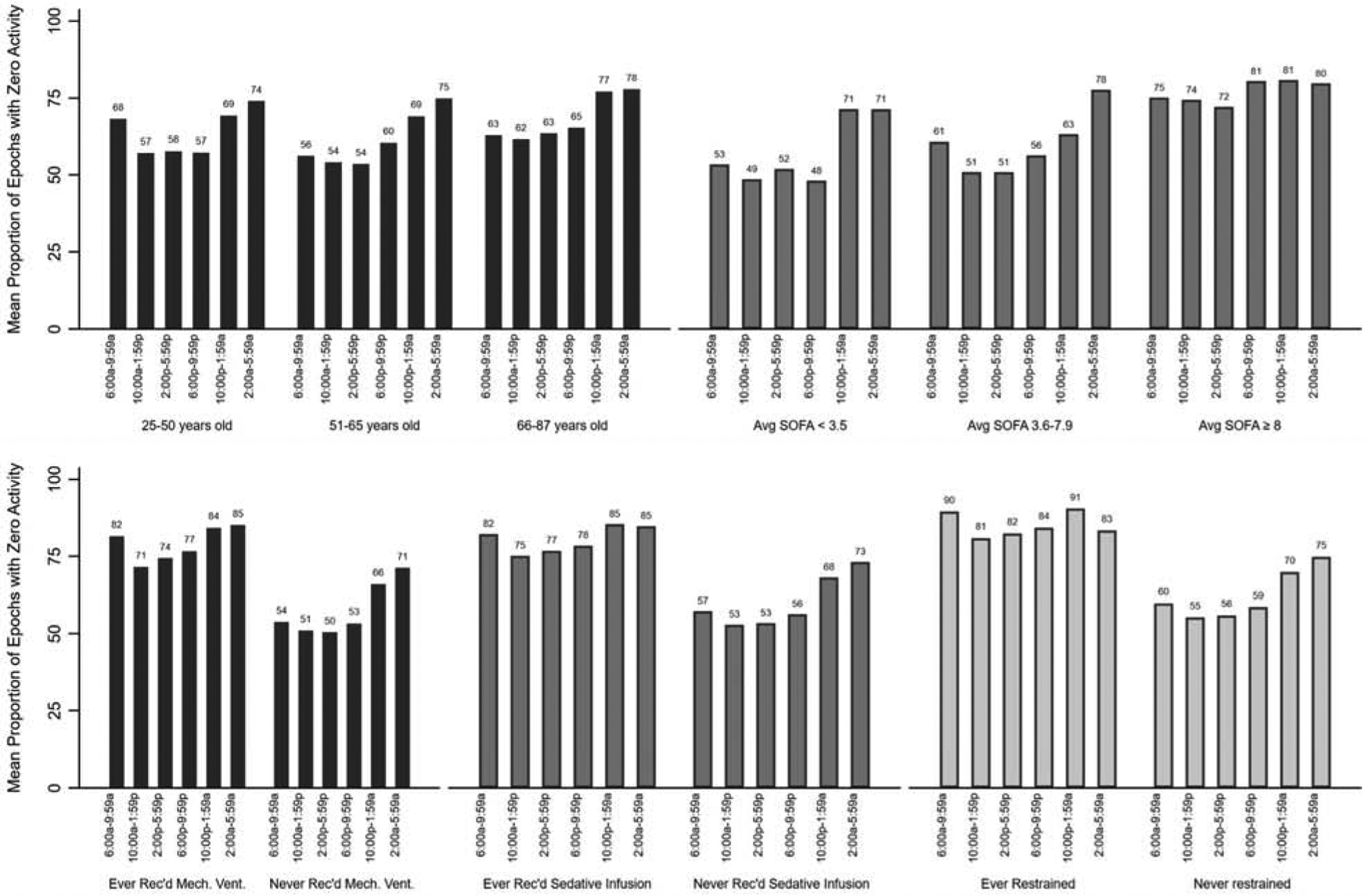
Proportion of zero-activity epochs
Table 2.
Zero activity epochs in the ICU, as measured using actigraphy
| n (N=34) | Total Epochs (N= 189,595) | Zero Activity Epochs, Proportion (95% CI)a | Unadjusted Difference (95% CI)b | P value | Adjusted Difference (95% CI)b | P value | |
|---|---|---|---|---|---|---|---|
| Baseline Variables | |||||||
| Age Tertile | |||||||
| 25–50 years old | 12 | 66,196 | 64 (49, 77) | REF | REF | REF | REF |
| 51–65 years old | 11 | 61,471 | 62 (53, 70) | −2 (−17, 13) | 0.79 | −9 (−20, 2) | 0.13 |
| 66–87 years old | 11 | 61,928 | 68 (54, 80) | 4 (−12, 21) | 0.61 | 3 (−9, 15) | 0.67 |
| Gender | |||||||
| Female | 17 | 94,607 | 68 (57, 77) | REF | REF | REF | REF |
| Male | 17 | 94,988 | 61 (52, 70) | −7 (−19, 6) | 0.28 | −2 (−14, 11) | 0.78 |
| Race | |||||||
| White | 21 | 117,884 | 62 (53, 71) | REF | REF | REF | REF |
| Non-white | 13 | 71,711 | 69 (58, 78) | 7 (−5, 19) | 0.28 | 6 (−4, 15) | 0.26 |
| Ambulatory Prior to ICU | |||||||
| No | 2 | 9,822 | 70 (0, 100) | REF | REF | REF | REF |
| Yes | 32 | 179,773 | 65 (57, 71) | −5 (−24, 14) | 0.60 | 2 (−20, 23) | 0.89 |
| BMI Classification | |||||||
| Underweight (BMI <18) | 3 | 16,905 | 47 (18, 78) | −14 (−31, 2) | 0.09 | −12 (−36, 11) | 0.30 |
| Normal (BMI 18–24.9) | 13 | 71,580 | 61 (49, 72) | REF | REF | REF | REF |
| Overweight (BMI 25–29.9) | 9 | 50,496 | 74 (57, 86) | 13 (−3, 28) | 0.11 | 16 (0, 31) | 0.05 |
| Obese (BMI >30) | 9 | 50,614 | 66 (54, 77) | 5 (−9, 19) | 0.50 | 12 (3, 22) | 0.009 |
| Admission Diagnosis Category | |||||||
| Respiratory Failure | 14 | 77,045 | 67 (56, 77) | REF | REF | REF | REF |
| Gastrointestinal | 3 | 16,858 | 65 (8, 98) | −2 (−31, 26) | 0.87 | −2 (−28, 24) | 0.90 |
| Sepsis | 7 | 39,739 | 67 (45, 84) | 0 (−18, 18) | 1.00 | 2 (−14, 17) | 0.85 |
| Cardiovascular | 4 | 22,653 | 53 (27, 77) | 14 (−3, 32) | 0.10 | −8 (−20, 4) | 0.17 |
| Other | 6 | 33,300 | 64 (48, 78) | 3 (−11, 17) | 0.66 | 6 (−5, 18) | 0.29 |
| ICU Variables | |||||||
| Time of day | |||||||
| 06:00–09:59 | - | 32,121 | 62 (55, 70) | REF | REF | REF | REF |
| 10:00–13:59 | - | 27,813 | 60 (51, 68) | −3 (−9, 3) | 0.36 | −4 (−10, 2) | 0.22 |
| 14:00–17:59 | - | 32,640 | 58 (50, 66) | −4 (−10, 2) | 0.16 | −5 (−11, 1) | 0.11 |
| 18:00–21:59 | - | 32,640 | 61 (52, 69) | −2 (−8, 5) | 0.62 | −2 (−8, 4) | 0.51 |
| 22:00–01:59 | - | 32,221 | 72 (64, 78) | 9 (3, 15) | 0.003 | 9 (3, 15) | 0.002 |
| 02:00–05:59 | - | 32,160 | 76 (70, 80) | 13 (7, 19) | <0.001 | 14 (9, 19) | <0.001 |
| Average Daily SOFA Tertile | |||||||
| 0.0 – 3.5 | 13 | 72,683 | 58 (48, 52) | REF | REF | REF | REF |
| 3.6 – 7.9 | 11 | 57,409 | 60 (46, 73) | 2 (−12, 17) | 0.74 | −4 (−19, 12) | 0.65 |
| 8.0 – 18.3 | 10 | 56,802 | 77 (65, 86) | 19 (7, 32) | 0.002 | 13 (2, 24) | 0.03 |
| Mechanically Ventilated | |||||||
| Never | 23 | 129,014 | 58 (51, 64) | REF | REF | REF | REF |
| Ever | 11 | 60,581 | 79 (65, 89) | 21 (9, 33) | 0.003 | 24 (11, 38) | <0.001 |
| Received Continuous Sedation | |||||||
| Never | 27 | 150,004 | 61 (54, 67) | REF | REF | REF | REF |
| Ever | 7 | 39,591 | 81 (9, 38) | 20 (7, 33) | 0.01 | −10 (−31, 10) | 0.32 |
| Restrained | |||||||
| Never | 31 | 172,632 | 63 (56, 69) | REF | REF | REF | REF |
| Ever | 3 | 16,963 | 85 (63, 95) | 22 (14, 31) | <0.001 | −6 (−31, 19) | 0.65 |
| All Subjectsc | 34 | 189,595 | 65 (58, 71) | ||||
Abbreviations: ICU = Intensive Care Unit; BMI = Body Mass Index; SOFA = Sequential Organ Failure Assessment
Within-patient 95% confidence intervals
Derived using predictive margins of a logistic regression of zero versus nonzero activity epochs, using clustering to account for within-patient correlation of activity levels. Multivariable model involved all variables reported in this table. Expressed as marginal differences in proportion of zero activity levels.
For reference, a healthy adult undergoing 24-hour actigraphy exhibits 49% zeroes, with 19% from 06:00–09:59, 41% from 10:00–13:59, 35% from 14:00–17:59, 31% from 18:00–21:59, 85% from 22:00–01:59, and 83% from 02:00–05:59.
Non-Zero Activity Levels
Overall, 66,721 epochs did not equal zero, with a mean±SD non-zero activity level of 55±70 (healthy adult = 132±141) (Figure 3, Table 3). Non-zero activity levels were significantly higher in patients who were ambulatory prior to ICU admission (mean±SD non-zero activity level per epoch = 57±71 versus 31±35, adjusted difference [95% CI] = 35 [21 to 51], multivariable p<0.001), and in the highest (versus lowest) average daily SOFA score tertile (39±57 versus 58±71, adjusted difference [95% CI] = − 22 [−43 to −2], multivariable p=0.03). Notably, non-zero activity levels did not differ significantly by age, BMI category, admission diagnosis category, time-of-day, or in patients who ever received mechanical ventilation, continuous sedative infusions, or restraints.
Figure 3.
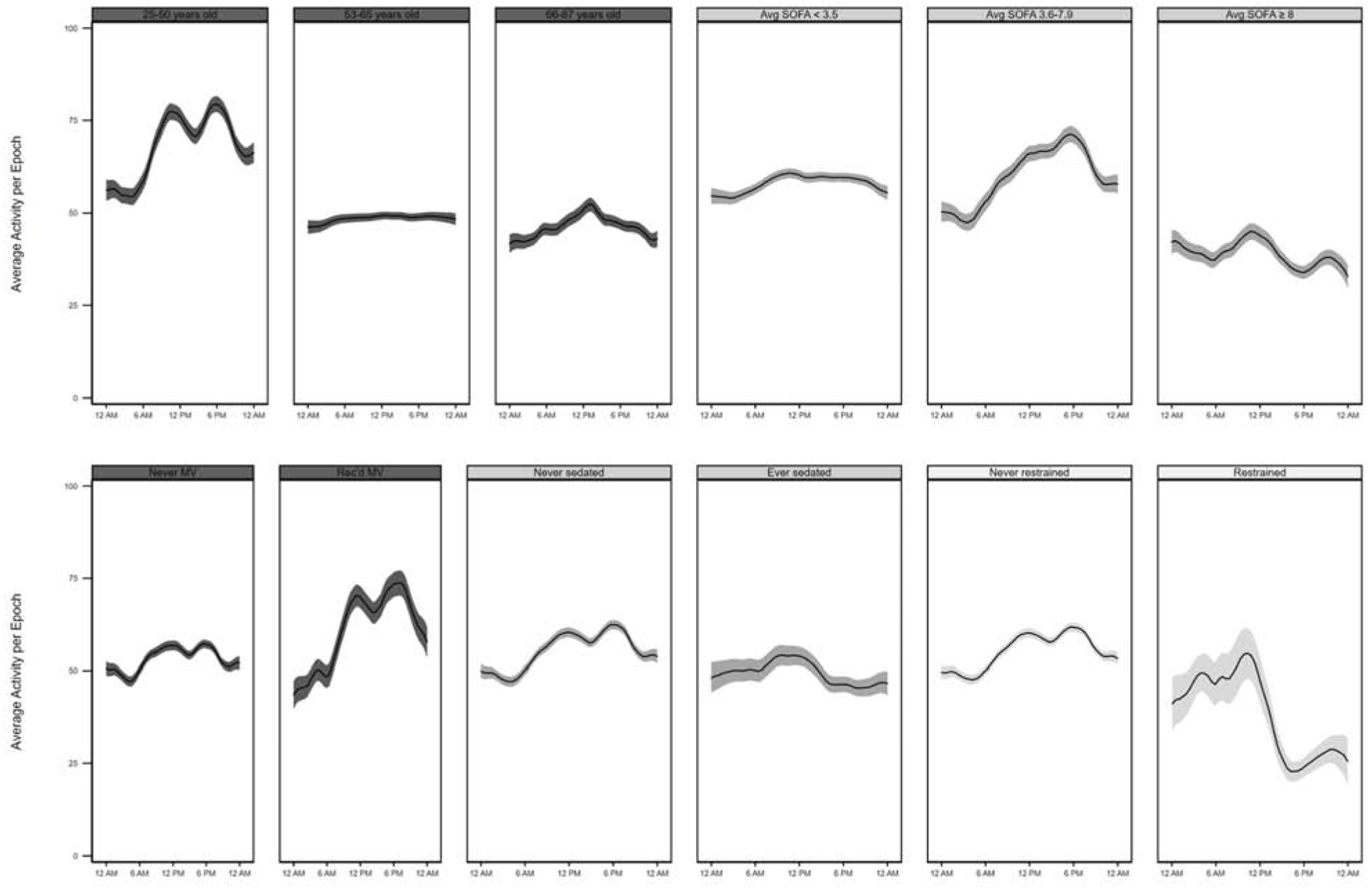
Actigraphy-based non-zero activity levels over the 24-hour day, expressed as a linear prediction plot with 95% confidence interval
Table 3.
Non-zero activity counts in the ICU, as measured using actigraphy
| n (N=34) | Non-Zero Epochs (N=66,721) | Mean (SD) Non-Zero Activity Levela | Unadjusted Difference (95% CI)b | P value | Adjusted Difference (95% CI)b | P value | |
|---|---|---|---|---|---|---|---|
| Baseline Variables | |||||||
| Age Tertile | |||||||
| 25–50 years old | 12 | 23,811 | 69 (83) | REF | REF | REF | REF |
| 51–65 years old | 11 | 23,338 | 49 (61) | −21 (−40, −2) | 0.01 | −1 (−17, 15) | 0.91 |
| 66–87 years old | 11 | 19,572 | 47 (61) | −23 (−46, 1) | 0.07 | −1 (−24, 21) | 0.90 |
| Gender | |||||||
| Female | 17 | 30,085 | 62 (78) | REF | REF | REF | REF |
| Male | 17 | 36,636 | 50 (62) | −13 (−31, 6) | 0.16 | −15 (−30, 0) | 0.05 |
| Race | |||||||
| White | 21 | 44,500 | 59 (72) | REF | REF | REF | REF |
| Non-white | 13 | 22,221 | 49 (66) | −9 (−27, 9) | 0.32 | −12 (−25, 1) | 0.06 |
| Ambulatory Prior to ICU | |||||||
| No | 2 | 2,985 | 31 (35) | REF | REF | REF | REF |
| Yes | 32 | 63,736 | 57 (71) | 25 (14, 36) | <0.001 | 35 (20, 51) | <0.001 |
| BMI Category | |||||||
| Underweight (BMI <18) | 3 | 8,949 | 85 (97) | 28 (−9, 65) | 0.13 | 6 (−27, 39) | 0.74 |
| Normal (BMI 18–24.9) | 13 | 27,576 | 57 (67) | REF | REF | REF | REF |
| Overweight (BMI 25–29.9) | 9 | 13,123 | 42 (55) | −14 (−30, 1) | 0.07 | −12 (−30, 5) | 0.16 |
| Obese (BMI > 30) | 9 | 17,073 | 48 (64) | −9 (−24, 7) | 0.29 | −4 (−24, 16) | 0.67 |
| Admission Diagnosis Category | |||||||
| Respiratory Failure | 14 | 25,167 | 64 (81) | REF | REF | REF | REF |
| Gastrointestinal | 3 | 5,915 | 56 (66) | −9 (−27, 11) | 0.40 | −9 (−46, 28) | 0.64 |
| Sepsis | 7 | 12,980 | 49 (67) | −15 (−44, 15) | 0.33 | −18 (−39, 4) | 0.11 |
| Cardiovascular | 4 | 10,738 | 58 (63) | −6 (−32, 21) | 0.68 | −11 (−27, 5) | 0.18 |
| Other | 6 | 11,921 | 43 (54) | −21 (−39, −2) | 0.03 | −21 (−44, 3) | 0.08 |
| ICU Variables | |||||||
| Time of day | |||||||
| 06:00–09:59 | - | 12,836 | 59 (76) | REF | REF | REF | REF |
| 10:00–13:59 | - | 11,769 | 63 (83) | 5 (−1.6, 11) | 0.14 | 2 (−3, 7) | 0.45 |
| 14:00–17:59 | - | 14,261 | 61 (75) | 4 (−3, 11) | 0.27 | 2 (−3, 6) | 0.42 |
| 18:00–21:59 | - | 13,443 | 63 (83) | 4 (−5.3, 13) | 0.40 | 0 (−6, 7) | 0.96 |
| 22:00–01:59 | - | 9,329 | 50 (65) | −4 (−13, 5) | 0.38 | −5 (−12, 3) | 0.19 |
| 02:00–05:59 | - | 8,017 | 50 (67) | −6 (−13, 1) | 0.07 | −5 (−11, 2) | 0.16 |
| Average Daily SOFA Tertile | |||||||
| 0.0 – 3.5 | 13 | 30,605 | 58 (71) | REF | REF | REF | REF |
| 3.6 – 7.9 | 11 | 22,809 | 62 (75) | 3 (−19, 26) | 0.77 | −3 (−26, 20) | 0.78 |
| 8.0 – 18.3 | 10 | 12,876 | 39 (57) | −19 (−34, −4) | 0.01 | −22 (−43, −2) | 0.03 |
| Mechanically Ventilated | |||||||
| Never | 23 | 54,114 | 54 (66) | REF | REF | REF | REF |
| Ever | 11 | 12,607 | 63 (87) | 9 (−22, 40) | 0.56 | 0 (−27, 28) | 0.98 |
| Received Continuous Sedation | |||||||
| Never | 27 | 59,101 | 56 (71) | REF | REF | REF | REF |
| Ever | 7 | 7,620 | 49 (64) | −7 (−21, 7) | 0.32 | −9 (−43, 24) | 0.60 |
| Restrained | |||||||
| Never | 31 | 64,220 | 56 (70) | REF | REF | REF | REF |
| Ever | 3 | 2,501 | 38 (62) | −19 (−32, −4) | 0.01 | 2 (−34, 37) | 0.93 |
| All Subjectsc | 34 | 66,721 | 55 (70) | ||||
Abbreviations: ICU = Intensive Care Unit; BMI = Body Mass Index; SOFA = Sequential Organ Failure Assessment
Within-patient standard deviation
Derived using predictive margins of a Poisson regression of non-zero activity counts per epoch, using clustering to account for within-patient correlation of activity levels. All covariates included in multivariable Poisson model.
For reference, a healthy adult undergoing 24-hour actigraphy exhibits 51% non-zero epochs with a mean±SD non-zero activity of 132±141movements, with 138±143 movements from 06:00–09:59, 140±137 from 10:00–13:59, 108±108 from 14:00–17:59, 158±166 from 18:00–21:59, 38±75 from 22:00–01:59, and 143±149 from 02:00–05:59.
DISCUSSION
This evaluation was performed as a secondary exploratory analysis of a feasibility study of actigraphy in medical ICU (MICU) patients, with the goal of characterizing inactivity and activity among critically ill subpopulations. Our analysis of nearly 190,000 30-second epochs of actigraph data demonstrated that MICU patients are profoundly inactive, registering zero movements for two-thirds of the time recorded, including 61% during waking hours. Moreover, when patients were moving, their non-zero activity levels were lower than previously-used activity cutoffs for sedentary behavior.23,24 Notably, activity levels were low for all patients, including those who were younger and who never received mechanical ventilation, continuous sedative infusions, or restraints during the actigraphy recording period. This finding was surprising, as most patients (91%) were able to walk before ICU admission. These findings suggest that ICU hospitalization itself contributes to inactivity for critically ill patients, irrespective of patient-specific factors.
While our study involved a MICU population, prior studies involving actigraphy-based activity measurement in the critically ill have occurred in general ICU populations13 and in those recovering from cardiac surgery25,26 and neuromuscular blockade.27 Similar to our findings, these prior studies demonstrated low daytime,13,25,28,29 nighttime,25,28,29 and mean 24-hour21,26,27 activity levels, ranging from 25 to 418, 2 to 19, and 9 to 1455 movements per 15 to 60 second epoch, respectively, along with 44 to 71% daytime and 74 to 91% nighttime zeroes.25,29
Building on prior research, we introduced non-zero activity levels as a simple, but potentially important metric of movement intensity. We demonstrated that even with epochs with zero activity removed from the equation, patients generally exhibited low activity levels. Our method of measuring both inactivity and non-zero activity, rather than mean activity alone, could help more clearly define patient activity patterns, movement intensity, and associated outcomes. Not surprisingly, patients with the highest organ failure scores, and those receiving mechanical ventilation tended to be more inactive and when they moved, they moved less. While our study did not differentiate voluntary from involuntary (i.e., tremors) patient movement and movement related to patient-care activities, given the low levels of activity in the study, removing these movements would result in even lower activity levels.
Notably, we were surprised to observe that younger, less sick, non-mechanically ventilated, non-sedated, and non-restrained patients were also vulnerable to inactivity and low activity; these patients were profoundly motionless when compared to non-ICU inpatients and older community-dwelling adults.19,30–36 While our study was not powered to identify all subgroup differences, the raw values suggest relatively small differences across many subgroups. Prior studies involving actigraphy defined “sedentary” behavior as ≤200 movements per 60 second epoch, far exceeding the activity counts of nearly all of our ICU patients, even with zeros removed.23,24 Given all the data describing associations between low activity and post-intensive care syndrome, this finding reinforces the need for early mobility efforts in the ICU, including in younger and non-mechanically ventilated patients with lower acuity illness. Barriers in this population are less likely to be related to patient illness such as hemodynamic instability or excessive devices, and highlight institutional and process issues such as limited staff, difficulty in coordination can contribute to low activity states. Such priorities have support from recent ICU clinical practice guidelines, and have been associated with reduced mechanical ventilation duration, ICU length of stay and improved post-ICU functional outcomes.3,8,37–39
As expected, we observed more inactivity during the nighttime hours (22:00 to 01:59 and 02:00 to 05:59) as compared to daytime hours. In our initial feasibility study we utilized manufacterer-provided software to demonstrate that 72% of epochs were defined as sleep, including 80% of nighttime (22:00 to 06:00) and 67% of daytime (07:00 to 19:00) epochs.22 However, this 24-hour “sleep” amount was unlikely to reflect true sleep, and in this analysis we therefore did not attempt to differentiate between sleep and wake.19,22 Notably, zero-activity and low non-zero activity occupied the majority of the 24-hour day, suggesting that robust circadian patterns of inactivity and activity were absent and small daytime and nighttime differences were not clinically meaningful. Recent attention has highlighted circadian rhythm misalignment as a common, deleterious and potentially modifiable problem in critically ill patients.40–42 However, measuring circadian rhythms, whether via polysomnography or laboratory biomarkers, is extremely complicated in the ICU setting.14,19,43–45 In non-ICU patients, advanced analyses of actigraphy-based patterns of rest and activity have been used to measure circadian rhythm alignment and misalignment.11,17,19,25,46–51 To better understand and improve circadian rhythm misalignment in the ICU, future investigations could build on existing actigraphy-based investigations, including more advanced analyses of inactivity and non-zero activity levels.
Our study was motivated, in part, by rising interest in ICU-based mobility efforts aimed at preventing adverse outcomes associated with prolonged bedrest.1,5,38,39,52–54 While such efforts are of interest to clinicians, quantification of their impact is difficult, especially when utilizing patient- or staff-reported activity levels. Past reviews of activity measurements in patients have demonstrated agreement of actigraphic or accelerometer based measurements of activity with respect to observation,14,47,55 EEG,47 and PSG.12,31,43,56 Recent research has demonstrated that wrist accelerometer-based movements not only correlate with observer-recorded patient behavior maps,57 but also energy expenditure and functional outcomes.58,59 Hence, wrist accelerometers are now available that can decipher positions and movements (i.e., lying, sitting, standing, and walking) based on activity patterns.24 However, the device used in our study did not have such capabilities, thus preventing us from better understanding the content and characteristics of specific characteristics of recorded activity periods. Nevertheless, given its affordably and ability to non-invasively and continuously track activity, actigraphy poses an attractive option large-scale use in critically-ill patients and, with the development of more advanced interpretation algorithms, a valuable tool to inform ICU mobility efforts. Nevertheless, given its affordably and ability to non-invasively and continuously track activity, actigraphy poses an attractive option large-scale use in critically-ill patients and, with the development of more advanced interpretation algorithms, a valuable tool to inform ICU mobility efforts.
Using actigraphy, our analysis made apparent that inactivity is a serious issue in critically ill patients, given the high frequency of zero-activity epochs and low non-zero activity intensity overall. Several studies have explored the culture of immobility in the critical care environment.1,3,6,7 Though barriers to mobility are generally perceived to be low by providers, patient, environment, cultural and process-related barriers are common and often hinder these efforts.1,6,7 Actigraphy, particularly in real-time, may enlighten providers regarding the extent of patient immobility, which could in turn motivate rehabilitation interventions. Additionally, large-scale activity data could stress to health system leadership the importance of patient mobility, helping to jumpstart efforts to address barriers, including formation of interdisciplinary teams, identification of champions, and staff-wide education on safety and benefits of early mobility.60
Key strengths of our study including enrollment of consecutive ICU patients, epoch-by-epoch analysis, and a separate analysis of zero and non-zero activity epochs. In addition, we introduce a novel tool in analyzing activity patterns by actigraphy – the percentage of zero vs. non-zero activity. However, our study also limitations, including a small sample size (thus impacting the power to detect all important effects) and a relatively short 48-hour recording duration. Additionally, with use of one actigraph model, our findings were confined to the specific sensitivities of that device. To minimize device-related bias, we analyzed gross activity levels instead of using processed software-based activity metrics, and placed the devices according to standard practice. Future efforts could evaluate different actigraphs for measurement differences. Next, because patients were not observed during the recording period, our study could not distinguish between voluntary and involuntary (i.e., in setting of tremor) patient movements and movements associated with patient care. While our research staff diligently monitored actigraphs for position and placement, future efforts could include direct observation to differentiate actual from artifactual movement. Nevertheless, despite these limitations, our study provides a foundation for future studies involving actigraphy to evaluate patient inactivity and activity in the ICU setting. Further research is necessary in this area to evaluate what activity levels may be optimal in the critical care setting. We emphasize that more studies such as this may inform future research in ICU activity and mobility.
CONCLUSION
We performed a detailed analysis of ~190,000 30-second epochs of actigraphy data from 34 MICU patients, and demonstrated that nearly all patients were profoundly inactive, with ~60% of 30-second epochs equaling zero movements. When moving, patients exhibited markedly low levels of non-zero activity. Importantly, we observed profound inactivity and low activity at all times of the day, and in patients with lower acuity illness, including those who were younger and never received continuous sedative infusions or restraints. These findings suggest that irrespective of patient-specific factors, ICU hospitalization alone contributes to inactivity and low activity, highlighting an intriguing area of investigation and improvement.
Highlights:
Medical ICU patients demonstrate profound inactivity, and are making no movements about 2/3 of the time
Activity levels were lowest in patients who were non-ambulatory prior to ICU admission and in patients with the highest severity of illness
Inactivity was more prevalent in patients receiving mechanical ventilation and in sicker patients
Sedation status, age, or presence of restraints did not contribute to differences in activity level
Funding source:
During this project, B.B.K. was supported by a grant through the UCLA Clinical Translational Research Institute (CTSI) and the National Institutes of Health/National Center for Advancing Translational Sciences [grant number UL1TR000124]; he is currently supported by a Paul B. Beeson Career Development Award through the National Institutes of Health/National Institute on Aging [grant number K76AG059936]. J.L.M. is supported by the National Heart Lung and Blood Institute [grant number K24143055]. D.M.N. is the principal investigator on a NIH-funded, multi-centered randomized trial (R01HL132887) evaluating nutrition and exercise in acute respiratory failure. For purposes of this multi-site trial, Baxter Healthcare Corporation has provided an unrestricted research grant and donated amino acid product. In addition, two study sites (not this university or site) have received an equipment loan from Reck Medical Devices. E.C. is supported by a grant through the National Heart Lung and Blood Institute [grant number AG061384].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to declare
REFERENCES
- 1.Goodson CM, Friedman LA, Mantheiy E, et al. Perceived Barriers to Mobility in a Medical ICU: The Patient Mobilization Attitudes & Beliefs Survey for the ICU. J Intensive Care Med. October 2018:088506661880712. doi: 10.1177/0885066618807120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolley SE, Bunnell AE, Hough CL. ICU-Acquired Weakness. 2016. doi: 10.1016/j.chest.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashem MD, Parker AM, Needham DM. Early Mobilization and Rehabilitation of Patients Who Are Critically Ill. Chest. 2016;150(3):722–731. doi: 10.1016/j.chest.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–183. doi: 10.1007/s00134-016-4612-0 [DOI] [PubMed] [Google Scholar]
- 5.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure*. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e [DOI] [PubMed] [Google Scholar]
- 6.Eakin MN, Ugbah L, Arnautovic T, Parker AM, Needham DM. Implementing and sustaining an early rehabilitation program in a medical intensive care unit: A qualitative analysis. J Crit Care. 2015;30(4):698–704. doi: 10.1016/j.jcrc.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 7.Dubb R, Nydahl P, Hermes C, et al. Barriers and Strategies for Early Mobilization of Patients in Intensive Care Units. Ann Am Thorac Soc. 2016;13(5):724–730. doi: 10.1513/AnnalsATS.201509-586CME [DOI] [PubMed] [Google Scholar]
- 8.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 9.Tipping CJ, Bailey MJ, Bellomo R, et al. The ICU Mobility Scale Has Construct and Predictive Validity and Is Responsive. A Multicenter Observational Study. Ann Am Thorac Soc. 2016;13(6):887–893. doi: 10.1513/AnnalsATS.201510-717OC [DOI] [PubMed] [Google Scholar]
- 10.Raj R, Ussavarungsi K, Nugent K. Accelerometer-based devices canbe used to monitor sedation/agitation in the intensive care unit. J Crit Care. 2014;29(5):748–752. doi: 10.1016/j.jcrc.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 11.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruse D, Thibaut A, Demertzi A, et al. Actigraphy assessments of circadian sleep-wake cycles in the Vegetative and Minimally Conscious States. BMC Med. 2013;11(1):18. doi: 10.1186/1741-7015-11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mary B, Grap J, Borchers CT, Munro CL, Elswick RK. Actigraphy in the Critically Ill: Correlation with Activity, Agitation, and Sedation. 2005;14(1). [PubMed] [Google Scholar]
- 14.Verceles AC, Hager ER. Use of Accelerometry to Monitor Physical Activity in Critically Ill Subjects: A Systematic Review. Respir Care. 2015;60(9):1330–1336. doi: 10.4187/respcare.03677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino M, Li Y, Rueschman MN, et al. Measuring Sleep: Accuracy, Sensitivity, and Specificity of Wrist Actigraphy Compared to Polysomnography. Sleep. November 2013. doi: 10.5665/sleep.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakazaki K, Kitamura S, Motomura Y, et al. Validity of an algorithm for determining sleep/wake states using a new actigraph. J Physiol Anthropol. 2014;33(1):31. doi: 10.1186/1880-6805-33-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller KL, Juliff L, Gore CJ, Peiffer JJ, Halson SL. Software thresholds alter the bias of actigraphy for monitoring sleep in team-sport athletes. J Sci Med Sport. January 2017. doi: 10.1016/j.jsams.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 18.Mistraletti G, Taverna M, Sabbatini G, et al. Actigraphic monitoring in critically ill patients: Preliminary results toward an “observation-guided sedation.” J Crit Care. 2009;24(4):563–567. doi: 10.1016/j.jcrc.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Schwab KE, Ronish B, Needham DM, To AQ, Martin JL, Kamdar BB. Actigraphy to Evaluate Sleep in the Intensive Care Unit: A Systematic Review. Ann Am Thorac Soc. June 2018:AnnalsATS.201801–004OC. doi: 10.1513/AnnalsATS.201801-004OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: Comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008;34(11):2076–2083. doi: 10.1007/s00134-008-1180-y [DOI] [PubMed] [Google Scholar]
- 21.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. doi: 10.5665/sleep.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamdar BB, Kadden DJ, Vangala S, et al. Feasibility of Continuous Actigraphy in Patients in a Medical Intensive Care Unit. Am J Crit Care. 2017;26(4):329–335. doi: 10.4037/ajcc2017660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heesch KC, Hill RL, Aguilar-Farias N, Van Uffelen JGZ, Pavey T. Validity of objective methods for measuring sedentary behaviour in older adults: a systematic review. Int J Behav Nutr Phys Act. 2018;15(1). doi: 10.1186/s12966-018-0749-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee I-M, Shiroma EJ, Evenson KR, Kamada M, LaCroix AZ, Buring JE. Accelerometer-Measured Physical Activity and Sedentary Behavior in Relation to All-Cause Mortality: The Women’s Health Study. Circulation. 2018;137(2):203–205. doi: 10.1161/CIRCULATIONAHA.117.031300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osse RJ, Tulen JHM, Hengeveld MW, Bogers AJJC. Screening methods for delirium: early diagnosis by means of objective quantification of motor activity patterns using wrist-actigraphy. Interact Cardiovasc Thorac Surg. 2008;8(3):344–348. doi: 10.1510/icvts.2008.192278 [DOI] [PubMed] [Google Scholar]
- 26.Redeker NS, Mason DJ, Wykpisz E, Glica B, Miner C. First postoperative week activity patterns and recovery in women after coronary artery bypass surgery. Nurs Res. 43(3):168–173. http://www.ncbi.nlm.nih.gov/pubmed/8183659. Accessed March 21, 2019. [PubMed] [Google Scholar]
- 27.Whetstone Foster JG, Clark AP. Functional recovery after neuromuscular blockade in mechanically ventilated critically ill patients. Hear Lung. 2006;35(3):178–189. doi: 10.1016/j.hrtlng.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 28.Mistraletti G, Taverna M, Sabbatini G, et al. Actigraphic monitoring in critically ill patients: Preliminary results toward an “observation-guided sedation.” J Crit Care. 2009;24(4):563–567. doi: 10.1016/j.jcrc.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 29.Osse RJ, Tulen JHM, Bogers AJJC, Hengeveld MW. Disturbed circadian motor activity patterns in postcardiotomy delirium. Psychiatry Clin Neurosci. 2009;63(1):56–64. doi: 10.1111/j.1440-1819.2008.01888.x [DOI] [PubMed] [Google Scholar]
- 30.Reyes S, Algarin C, Bunout D, Peirano P. Sleep/wake patterns and physical performance in older adults. Aging Clin Exp Res. 2013;25(2):175–181. doi: 10.1007/s40520-013-0028-7 [DOI] [PubMed] [Google Scholar]
- 31.Martin JL, Fiorentino L, Jouldjian S, Josephson KR, Alessi CA. Sleep Quality in Residents of Assisted Living Facilities: Effect on Quality of Life, Functional Status, and Depression. J Am Geriatr Soc. 2010;58(5):829–836. doi: 10.1111/j.1532-5415.2010.02815.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chien M-Y, Chen H-C. Poor Sleep Quality is Independently Associated with Physical Disability in Older Adults. J Clin Sleep Med. 2015;11(3):225–232. doi: 10.5664/jcsm.4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denehy L, Lanphere J, Needham DM. Ten reasons why ICU patients should be mobilized early. Intensive Care Med. 2017;43(1):86–90. doi: 10.1007/s00134-016-4513-2 [DOI] [PubMed] [Google Scholar]
- 34.Beveridge C, Knutson K, Spampinato L, et al. Daytime physical activity and sleep in hospitalized older adults: Association with demographic characteristics and disease severity. J Am Geriatr Soc. 2015;63(7):1391–1400. doi: 10.1111/jgs.13520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang YL, Liu RY, Wang QS, Van Someren EJW, Xu H, Zhou JN. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;76(4–5):597–603. doi: 10.1016/S0031-9384(02)00733-3 [DOI] [PubMed] [Google Scholar]
- 36.Althoff T, Sosič R, Hicks JL, King AC, Delp SL, Leskovec J. Large-scale physical activity data reveal worldwide activity inequality. Nature. 2017;547(7663):336–339. doi: 10.1038/nature23018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–183. doi: 10.1007/s00134-016-4612-0 [DOI] [PubMed] [Google Scholar]
- 38.Denehy L, Lanphere J, Needham DM. Ten reasons why ICU patients should be mobilized early. Intensive Care Med. 2017;43(1):86–90. doi: 10.1007/s00134-016-4513-2 [DOI] [PubMed] [Google Scholar]
- 39.Brower RG. Consequences of bed rest. Crit Care Med. 2009;37(10 Suppl):S422–8. doi: 10.1097/CCM.0b013e3181b6e30a [DOI] [PubMed] [Google Scholar]
- 40.Korompeli A, Muurlink O, Kavrochorianou N, Katsoulas T, Fildissis G, Baltopoulos G. Circadian disruption of ICU patients: A review of pathways, expression, and interventions. J Crit Care. 2017;38:269–277. doi: 10.1016/j.jcrc.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 41.Oldham MA, Lee HB, Desan PH. Circadian Rhythm Disruption in the Critically Ill. Crit Care Med. 2016;44(1):207–217. doi: 10.1097/CCM.0000000000001282 [DOI] [PubMed] [Google Scholar]
- 42.Knauert MP, Haspel JA, Pisani MA. Sleep Loss and Circadian Rhythm Disruption in the Intensive Care Unit. Clin Chest Med. 2015;36(3):419–429. doi: 10.1016/j.ccm.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 43.Watson PL, Pandharipande P, Gehlbach BK, et al. Atypical Sleep in Ventilated Patients: Empirical Electroencephalography Findings and the Path Toward Revised ICU Sleep Scoring Criteria. Crit Care Med. 2013;41(8):1958–1967. doi: 10.1097/CCM.0b013e31828a3f75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamdar BB, Needham DM, Collop NA. Sleep Deprivation in Critical Illness. J Intensive Care Med. 2012;27(2):97–111. doi: 10.1177/0885066610394322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163(2):451–457. doi: 10.1164/ajrccm.163.2.9912128 [DOI] [PubMed] [Google Scholar]
- 46.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 47.Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997;20(1):24–27. doi: 10.1124/dmd.107.016501.CYP3A4-Mediated [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuster-Garcia E, Bresó A, Miranda JM, García-Gómez JM. Actigraphy Pattern Analysis for Outpatient Monitoring In: Methods in Molecular Biology (Clifton, N.J.). Vol 1246 ; 2015:3–17. doi: 10.1007/978-1-4939-1985-7_1 [DOI] [PubMed] [Google Scholar]
- 49.Boyne K, Sherry DD, Gallagher PR, Olsen M, Brooks LJ. Accuracy of computer algorithms and the human eye in scoring actigraphy. Sleep Breath. 2013;17(1):411–417. doi: 10.1007/s11325-012-0709-z [DOI] [PubMed] [Google Scholar]
- 50.James BD, Boyle PA, Bennett DA, Buchman AS. Total daily activity measured with actigraphy and motor function in community-dwelling older persons with and without dementia. Alzheimer Dis Assoc Disord. 2012. doi: 10.1097/WAD.0b013e31822fc3cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18(4):288–302. http://www.ncbi.nlm.nih.gov/pubmed/7618029. [DOI] [PubMed] [Google Scholar]
- 52.Hashem MD, Parker AM, Needham DM. Early Mobilization and Rehabilitation of Patients Who Are Critically Ill. Chest. 2016;150(3):722–731. doi: 10.1016/j.chest.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraser D, Spiva L, Forman W, Hallen C. Original research: Implementation of an early mobility program in an ICU. Am J Nurs. 2015;115(12):49–58. doi: 10.1097/01.NAJ.0000475292.27985.fc [DOI] [PubMed] [Google Scholar]
- 54.Barr J, Fraser GL, Puntillo K, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72 [DOI] [PubMed] [Google Scholar]
- 55.Schwab KE, To AQ, Chang J, et al. The use of actigraphy to evaluate physical activity in the intensive care unit: A systematic review. Am J Respir Crit Care Med. 2018;197(MeetingAbstracts) http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L622967708. [Google Scholar]
- 56.Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: Algorithm development. J Sleep Res. 2010;19(4):612–619. doi: 10.1111/j.1365-2869.2010.00835.x [DOI] [PubMed] [Google Scholar]
- 57.Valkenet K, Bor P, van Delft L, Veenhof C. Measuring physical activity levels in hospitalized patients: a comparison between behavioural mapping and data from an accelerometer. Clin Rehabil. March 2019:269215519836454. doi: 10.1177/0269215519836454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beach LJ, Fetterplace K, Edbrooke L, et al. Measurement of physical activity levels in the Intensive Care Unit and functional outcomes: An observational study. J Crit Care. 2017;40:189–196. doi: 10.1016/j.jcrc.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 59.Phane Mandigout S, Lacroix J, Atrice Ferry B, Vuillerme N, Compagnat M, Daviet J-C. Can energy expenditure be accurately assessed using accelerometry-based wearable motion detectors for physical activity monitoring in post-stroke patients in the subacute phase? doi: 10.1177/2047487317738593 [DOI] [PubMed] [Google Scholar]
- 60.Azuh O, Gammon H, Burmeister C, et al. Benefits of Early Active Mobility in the Medical Intensive Care Unit: A Pilot Study. Am J Med. 2016;129(8):866–871.e1. doi: 10.1016/j.amjmed.2016.03.032 [DOI] [PubMed] [Google Scholar]


