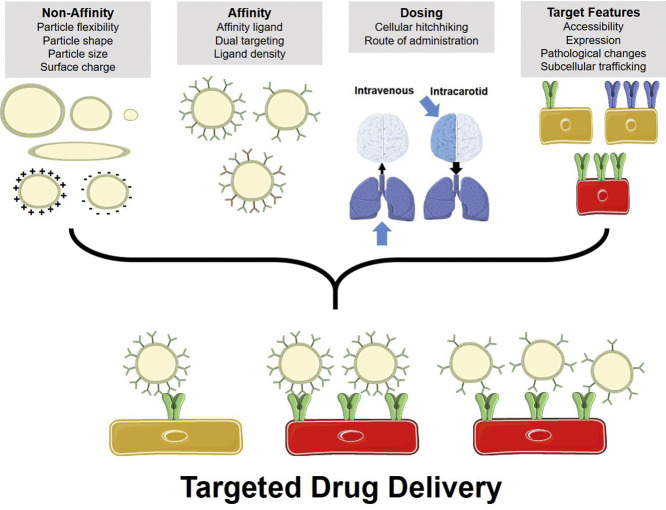
Abstract
The bloodstream is the main transporting pathway for drug delivery systems (DDS) from the site of administration to the intended site of action. In many cases, components of the vascular system represent therapeutic targets. Endothelial cells, which line the luminal surface of the vasculature, play a tripartite role of the key target, barrier, or victim of nanomedicines in the bloodstream. Circulating DDS may accumulate in the vascular areas of interest and in off-target areas via mechanisms bypassing specific molecular recognition, but using ligands of specific vascular determinant molecules enables a degree of precision, efficacy, and specificity of delivery unattainable by non-affinity DDS. Three decades of research efforts have focused on specific vascular targeting, which have yielded a multitude of DDS, many of which are currently undergoing a translational phase of development for biomedical applications, including interventions in the cardiovascular, pulmonary, and central nervous systems, regulation of endothelial functions, host defense, and permeation of vascular barriers. We discuss the design of endothelial-targeted nanocarriers, factors underlying their interactions with cells and tissues, and describe examples of their investigational use in models of acute vascular inflammation with an eye on translational challenges.
Keywords: Vascular system, Endothelium, Nanocarriers, Inflammation
Graphical abstract
1. Introduction: Vascular endothelium and drug delivery
Circulation of blood through the vascular system represents the main mechanism for distribution of pharmacological formulations administered via intravascular, intramuscular, and other types of injections, and delivered via oral, pulmonary, nasal, sublingual, and other routes. Blood flow transports drugs and drug delivery systems (DDS). The rapidly growing roster of DDS devised to improve pharmacokinetics (PK), tissue distribution, and effects of drugs includes molecular carriers (e.g., antibodies, peptides, sugars, polyethylene glycol (PEG)), multimolecular carriers (e.g., liposomes and other nanocarriers), cells, and fragments derived from cells.
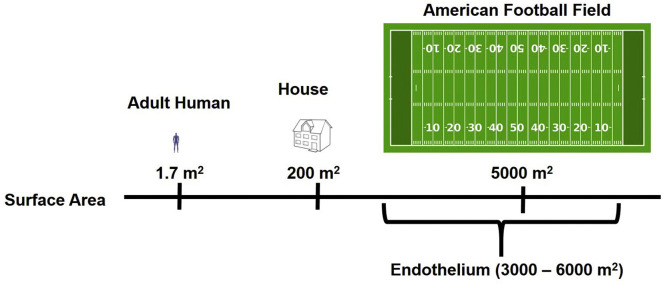
The endothelium exists as a lining surrounding the lumen of all blood vessels – arteries, arterioles, capillaries, venules, and veins separating blood from tissues. The degree of restriction of access to extravascular sites depends on the permeability characteristics of endothelial cells typical of a given vascular area under conditions of a specific pathology, as well as PK and other features of circulating agents, such as size. Generally, endothelium less effectively restricts diffusion to tissues of small drugs vs. large DDS. Yet, even large, micron-sized, long-circulating DDS having favorable PK enter some extravascular sites normally (e.g. hepatic sinuses) or in pathology (e.g. sites of edema and hemorrhage). Furthermore, endothelium, which represents an enormously extended surface area accessible to blood (3000–6000 m2, Fig. 1 ) [1], is the key target for therapeutic interventions in many conditions.
Fig. 1.
The endothelium provides an extremely large surface area for drug delivery.
Endothelial cells are the primary component of the endothelial lining of the circulatory systems (blood and lymphatic), heart (chambers and valves), and cavities in the central nervous system (brain ventricles). These highly specialized cells function in concert with other components of the barrier to form the endothelium. The tunica intima consists of endothelial cells and a basal membrane, which is the only component of the endothelium in capillaries. However, in larger vessels (e.g. veins and arteries), the tunica intima is surrounded by a layer known as the tunica media, which consists of smooth muscle cells, which regulate features such as vascular compliance. In different portions of the vasculature, the tunica media has different compositions. Finally, the tunica adventitia is a layer that consists of microvasculature within the vessel wall, such as the vasa vasorum [2].
The phenotype of endothelial cells is different within organs and larger vessels. For example, in tissues such as liver and spleen, the endothelium has large openings (hundreds of nanometers – microns in size) permitting movement of larger objects out of the bloodstream. Another specialized endothelium is that found in the glomerulus, where fenestrae are found that permit small solutes to be filtered into the renal tubules, while excluding proteins and cells larger than 5–10 nm in diameter [3]. A feature of endothelial cells within the lung and heart is the presence of a large number of caveolae (“little caves”), which seem to play a role in transendothelial transport.
In certain tissues, such as the brain, the endothelium forms an integral part of a very tight barrier, evolved to restrict passage of all but necessary substances, and, as such, lacks features such as fenestrae [4]. Results of recent studies indicate that, contrary to the previous conventional notion that the CNS vasculature lacks caveolae, the arterioles in the brain do contain abundant caveolae [5]. It is important to keep in mind, however, that caveolae have multiple functions, which likely vary in specific vascular areas, organs and endothelial phenotypes. These functions, in addition to the transport into and across the endothelial monolayer, include sensing of hydrodynamic and other physical forces, regulation of numerous signaling pathways. Thus, caveolae in the CNS arterioles seem to exert sensor rather than transport functions, consistent with the notion of restrictive transport across the BBB.
The endothelium is central in supporting the transport functions of the circulatory system, such as: tissue delivery of nutrients/oxygen, waste removal, and immune surveillance. It also controls vascular permeability, adhesiveness, contractility and angiogenesis, blood clotting and fluidity, and blood/tissue exchanges [6]. As the endothelium is the critical interface between the bloodstream and extravascular sites in tissues, it serves as a key site for pharmacological interventions in inflammation, sepsis, acute respiratory distress syndrome (ARDS), blood clotting disorders, ischemia-reperfusion (I/R), hypertension, atherosclerosis, restenosis, diabetes, arthritis, tumor growth, and many others. Targeted drug delivery to endothelial cells has great potential in improving clinical outcomes in these severe pathologies [[7], [8], [9]].
2. Non-affinity-based targeting
Two distinct strategies to accumulate DDS in certain parts of the circulatory system employ either non-affinity- or affinity-based targeting (Table 1 ).
Table 1.
Comparison of targeting characteristics between passive vs. active approaches. Passive targeting may be reached by mechanical entrapment or non-specific features of DDS surface, while active delivery may be systemic (ubiquitous target expression) or local (tissue-specific target enrichment).
| Non-Affinity |
Affinity |
|||
|---|---|---|---|---|
| Carrier size or aggregation | Carrier surface features | Systemic Delivery |
Site-Specific | |
| Mechanism | Mechanical uptake in microvasculature down-stream injection (first pass) | Charge, Glycocalyx, ECM, PS, integrins |
Binding to EC surface determinant epitopes |
Using ligands of locally enriched epitopes or/and first pass |
| Addressing to vascular areas and EC types | Pre-capillary arterioles | Limited if any | Pan-endothelial or first pass in an organ | Diverse |
| Sub-cellular addressing |
None | None or non-specific internalization | Cell surface and interior | As in systemic |
| Specificity | Low | Low | Variable to very high | High to very high |
Abbreviations used in table: ECM: extracellular matrix, PS: phosphatidylserine, EC: endothelial cell, DDS: drug delivery systems.
DDS may accumulate both in sites of interest and in off-target counterparts via binding or retention mediated by mechanisms with variable degrees of specificity. DDS endowed with specific affinity bind to components selectively or preferentially expressed in the target cell, tissue, or organ. This is essentially “active” (affinity-based) targeting, the subject of this paper. Conjugation to such affinity ligands using chemical or recombinant techniques yields DDS with targeting characteristics determined by the features of the binding epitope (surface density, accessibility, etc.), ligand (affinity, specificity, etc.), DDS (size, rigidity, etc.) and configuration of the conjugate (ligand density, spatial freedom, etc.). Alternative approaches bypass the need for cognizant molecular recognition of specific target determinants. For example, large particles (diameter > 10–30 μm) will lodge in the first microvascular bed encountered following injection (capillaries have a diameter of just a few μm). Mechanical retention also underlies uptake of nanoparticles aggregating in blood or forming large complexes with blood components after injection.
Some DDS have a relatively promiscuous affinity to a wide spectra of tissue counterparts. Cationic DDS bind to the glycocalyx and other anionic components of the plasma membrane of both endothelial and blood cells. DDS that are surface-decorated with Annexin V bind to membranes of apoptotic cells via exposed phosphatidylserine. While these approaches do have some specific affinity to components of the tissue, they have relatively little selectivity, hence we define these as non-affinity targeting strategies [10,11].
DDS may accumulate in some vascular areas via interactions with components of blood having natural affinity to these sites. For example, uptake of lipid nanoparticles (LNP) by hepatocytes occurs via a fortuitous interaction with apolipoprotein E (apoE), delivering LNP to the corresponding apoE receptors [12]. Opsonization, i.e., interaction of DDS with complement and IgG, favors clearance by cells comprising the reticuloendothelial system (RES) [13]. Further, reversible binding to blood cells (RBC, WBC) favors delivery of nanoparticles and other DDS to some vascular areas, organs, and pathological sites [[14], [15], [16]]. Understanding of regulation of these fortuitous hitchhiking mechanisms may enable one to employ this type of drug delivery in a more cognizant and controlled fashion.
Due to unpredictable scenarios of retention, delivery, and uptake of DDS in the vasculature, several groups have introduced and are currently in pursuit of methodologies that utilize in vivo screening of libraries of carrier formulations [[17], [18], [19]]. The outcomes, dependent on the screening approach (e.g. detection of biological activity of cargo or directly tracing a labeled component of the DDS), may yield exciting formulation variants displaying unusual tropisms to either normal or pathologically-altered organs. These discovery-driven approaches yield intriguing and often enigmatic results.
However, the DDS discussed above afford relatively limited, if any, control over addressing cargoes to specific vascular areas, cells, or cellular compartments of interest. DDS relying on size- and charge-mediated retention in the lumen of the first pass vascular areas are unlikely to enable control over cellular distribution and uptake vs. release of cargoes [20]. Engaging unknown components of the target might lead to unintended side effects. The utility of DDS evolving from discovery-driven, fortuitous, and non-specific methods for vascular drug delivery is hanging on the results of the ensuing efforts to identify target molecules and cells, as well as pharmacokinetics, biodistribution, toxicity, mechanism of delivery, and other parameters that may or may not permit emerging carriers to achieve their envisioned clinical applications.
3. Use of specific affinity ligands for targeting
Alternatively, to achieve efficient, selective targeting, one can employ ligands of surface determinants specific to or enriched in the area of interest [6,[21], [22], [23], [24], [25]]. Conjugation of DDS to affinity ligands using chemical or recombinant techniques is a common approach used to achieve specific targeting. Immunostaining, flow cytometry, Western blotting, PCR, phage display, functional genomics, and proteomics are useful approaches used to detect proteins enriched in the tissues of interest. Many moieties (e.g., antibody-derived, natural ligands, peptides, etc.) provide mechanisms for target recognition and have been used as ligands for vascular DDS [[26], [27], [28], [29], [30]].
The selection criteria of target determinants for drug delivery include: A) sufficient selectivity, specificity, and surface density of target epitopes in the desired site of action; B) accessibility of target epitopes to the intended carrier; C) engagement of target by the carrier must not induce any adverse effects; D) the target must provide the appropriate sub-cellular addressing of the carrier; and, E) the target should be useful in providing the appropriate kinetics of onset and duration and magnitude of effect to achieve the desired therapeutic outcomes.
3.1. Approaches for target identification
The hypothesis-driven approach focuses on target molecules meeting the criteria of utility in a given pathology, based on their location in the body, cell surface density, specificity for the pathologically-altered region, function, membrane dynamics (clustering, internalization and shedding), and so on. This knowledge provides investigators with a rationale for choosing the target, targeting ligand, and configuration of the DDS.
The first two published reports of vascular targeting provide examples of the two primary approaches for target selection – hypothesis- and discovery-driven. Kennel, Huang, and co-workers obtained a monoclonal antibody from mice following immunization with rat lung homogenate that provided pulmonary targeting of immunoliposomes after IV injection [31,32]. Further research identified the target as thrombomodulin (TM), an endothelial transmembrane glycoprotein that converts thrombin [33] to exert anti-inflammatory and anti-thrombotic functions [34]. In clinical scenarios, meddling with TM is likely to be dangerous and produce severe adverse effects [35,36].
Meanwhile, Sergei Danilov obtained monoclonal antibodies to ACE (angiotensin-converting enzyme, an endothelial transmembrane glycoprotein that cleaves Ang I into a vasoconstricting and pro-inflammatory peptide Ang II) to test the hypothesis that ACE can be used for endothelial targeting. Indeed, anti-ACE antibodies accumulated in lungs after IV injection. Targeting to ACE, reported by Danilov and co-workers concomitantly with the Kennel and Huang paper, enabled delivery of drugs, carriers, imaging probes, and gene therapies to the pulmonary endothelium of multiple animal species, ranging from mice to humans, consistent with the high expression of ACE in the pulmonary vasculature [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]].
It was observed that interaction of some anti-ACE antibodies with ACE lead to its shedding from the cell surface [47,48]. Extensive clinical use of ACE inhibitors implies that this effect may be tolerable or possibly beneficial in both hypertension and inflammation, and related conditions [21,49,50]. Anti-ACE mAbs were used for gene delivery in treatment of spontaneous [51] and hypoxic pulmonary hypertension [52]. In translational studies, no obvious adverse effects were observed following injection of anti-ACE in animals and humans [44,47].
3.2. Target determinants for vascular targeting
Three decades of research efforts pursuing both hypothesis- and discovery-driven approaches have yielded the roster of endothelial surface molecules that might serve as targets for drug delivery. These surface determinants include enzymes, cell adhesion molecules (CAM), integrins, receptors, transporting molecules, and other molecules, may localize to different regions of the endothelial cell membrane and to different areas of the vasculature (Table 2 ).
Table 2.
Target determinants for drug delivery to the endothelium.
| Protein | Status, organ & vessel type | Cellular localization | Function(s) | Surface density | Internalization pathway | Changes in inflammation |
|---|---|---|---|---|---|---|
| ACE/CD143 | Constitutive Pulmonary microvasculature |
Apical surface | Peptidase, converts Ang I into Ang II, cleaves bradykinin | Intermediate | Yes. Clathrin endocytosis? | Suppressed |
| PECAM/CD31 | Constitutive Systemic Pan-endothelial |
Intercellular borders | Signaling Adhesion & migration of WBC | Very high (up to 106/cell) | Not free ligands. DDS induce CAM-endocytosis |
Stable |
| ICAM-1/CD54 | Constitutive Lungs |
Lipid rafts & tetraspanin microdomains on cell surface | As above Immunologic synapse formation. Ligand of LFA-1, MAC-1 |
High | Similar to above | Elevated in inflammation |
| VCAM-1/CD106 | Inducible CNS, skin, kidney |
Apical surface | As above | Low | Clathrin endocytosis | Highly elevated in inflammation |
| E-selectin/ CD62E | Strictly inducible Microvasculature |
Apical surface | Signaling. WBC rolling, blood clotting | Intermediate | Clathrin endocytosis | |
| P-selectin/ CD62P | Strictly inducible Microvasculature |
Weibel-Palade bodies of EC, alpha-granules of platelets | Blood clotting, binding platelets, signaling, WBC rolling | As above | Unclear | De-encrypted & Inducible in inflammation & clotting |
| APP2/ XPNPEP2 | Constitutive Lung, heart, kidneys Alveolar & cardiac microvasculature |
Caveolae | Peptidase Decays substance P Transcytosis |
High | Constitutive caveolar endocytosis | Unclear |
| PLVAP/PV1 | As above | Caveolae and fenestrae | Structure aperture; signaling | As above and spleen & kidney | Constitutive caveolar endocytosis | |
| TfR/CD71 | Ubiquitously distributed | Cell surface | Cellular uptake of iron into specialized endosomes | Regulated by cellular iron | Receptor-mediated endocytosis; transcytosis regulator | Stable |
| APN/CD13 [358] | Vasculature upon angiogenesis in tissue remodeling and tumors | Apical surface | Peptidase of Ang III and IV, peptides, chemokines |
Unclear | Clathrin- and/or caveola-dependent [358] | Increased, shedded |
| Integrins [359,360] αvβ3, αvβ5, α5β1 | Enriched in tumor vessels and other types of angiogenesis | Cell surface | Adhesion receptors for extracellular ligands; signaling; angiogenesis. | Variable | Type-specific (i.e. caveolin-dependent for α5β1, etc.) [361] | Activated |
| VE-cadherin/ CD144 [362] | Constitutive, exclusively endothelial |
Intercellular junctions | Cohesion and organization of the intercellular junctions; signaling; vascular permeability regulation | High | Clathrin-mediated endocytosis (p120-catenin- or phosphorylation-induced) | Decreased, causing high vessel permeability |
| TEM1/CD248 [363] | Constitutive in stromal fibroblasts. Expressed in tumor endothelial cells, but not in normal endothelium | Cell surface | Tumor angiogenesis | Variable | Unclear | Unclear |
| GP90/CD44 [364] | Most epithelial and lymphoid tissues. Avesicular zone of pulmonary endothelium [99] | Cell surface | Cell adhesion and migration; immune response; tumor angiogenesis. Hyalorunan receptor | Variable | Non-classical CD44-mediated endocytosis of hyalorunan [365] | Increased |
Abbreviations used in table: ACE: angiotensin-converting enzyme, Ang I: angiotensin I, Ang II, angiotensin II, PECAM: platelet-endothelial cell adhesion molecule, WBC: white blood cell, DDS: drug delivery system, CAM: cell adhesion molecule, ICAM-1: intercellular adhesion molecule 1, LFA-1: lymphocyte function-associated antigen 1, MAC-1: macrophage-1 antigen, VCAM-1: vascular cell adhesion molecule 1, CNS: central nervous system, EC: endothelial cell, APP2/XPNPEP2: aminopeptidase P, PLVAP: plasmalemma vesicle-associated protein, TfR: transferrin receptor, APN: aminopeptidase N, VE-cadherin: vascular endothelial cadherin, TEM1: tumor endothelial marker 1.
Ideal target determinants would be selectively expressed and accessible in the target site. However, there is no specific conventionally accepted gradation of the surface density of cellular epitopes, for many reasons including rather relative character of the results provided by many methods (FACS, immunostaining, ELISA) and mostly enigmatic clustering of the epitopes. It seems reasonable to categorize epitope density based on the Bmax (maximal number of binding sites per cell determined using directly radiolabeled ligands), as high (105 and above), intermediate (103–105 sites per cell) and low (less than few thousands per cell).
3.2.1. Constitutive vs. inducible determinants
Under pathological conditions, endothelial cells undergo many changes that could impact targeting. Some constitutive surface molecules, such as TM, are shed from the endothelium in pathologies. For example, following acute lung injury, delivery of anti-TM mAbs to the lung was reduced by 50% [53]. This observation is in agreement with the notion that TM is shed by pulmonary endothelial cells following an inflammatory insult [54]. Another classical endothelial target, ACE, is also shed by the endothelium in pathologies [55]. This process has been shown to be mediated by several agents, including pro-inflammatory cytokines and oxidants, resulting in reduced uptake of ACE-targeted agents [56,57]. In thoracic imaging studies, it was revealed that sarcoidosis led to reduced pulmonary uptake of anti-ACE in patients [44]. Animal studies have revealed a similar reduction in lung targeting of anti-ACE in several models, including pulmonary edema, ischemia-reperfusion, and endotoxemia [[57], [58], [59]].
Disappearance of endothelial determinants in pathologies occurs via multiple mechanisms. They include activation on the cell surface specific proteases and peptidases that cleave off transmembrane glycoproteins such as ACE and TM [48,60,61]. In addition, enzymes cleaving chondroitin sulfate, heparan sulfate, and other sugar moieties from ACE, TM, and many other endothelial surface glycoproteins shed these components of glycocalyx, which unmasks new binding sites on normally hidden epitopes. Reactive oxygen species, proteases, and other highly aggressive entities, including hypochlorous acid (bleach), released by activated leukocytes modify endothelial membrane components directly and via damage to endothelial cells themselves [[62], [63], [64]].
Stable constitutive molecules, such as Platelet-Endothelial Cell Adhesion Molecule (PECAM), can be used for prophylactic and/or therapeutic delivery [39,49]. Inflammation enhances Intercellular Adhesion Molecule 1 (ICAM-1) level in many cell types, including endothelia [65,66]. Pathological endothelia commonly express inducible markers including Aminopeptidase N (APN), Tumor Endothelial Marker 1 (TEM-1), Vascular Cell Adhesion Molecule 1 (VCAM-1), and E- and P-selectins [25,67]. These determinants are preferable for diagnostic imaging and therapeutic interventions due to their specificity for pathologically-altered endothelium [6,23,[68], [69], [70]]. Selectins exposed by pathological endothelium facilitate adhesion of WBC and platelets [71]. The kinetics of upregulation of surface epitopes are dependent on the mechanism of increased surface exposure. For example, P-selectin is mobilized from intracellular stores within 10–30 min [72]. However, E-selectin [73] and VCAM-1 [74] surface expression requires hours to increase due to the requirement for de novo protein synthesis (Fig. 2 ).
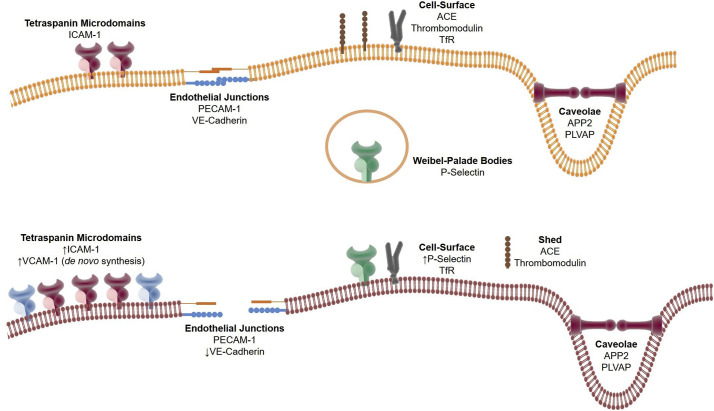
Fig. 2.
Inflammation affects surface expression and cellular localization of endothelial targets. Upper panel: Receptor expression on quiescent endothelium. Certain proteins are localized to specific cell surface microdomains, such as tetraspanin-rich (ICAM-1), cell-cell junctions (PECAM-1, VE-Cadherin), and caveolae (APP2, PLVAP), while others may be found throughout the cell surface (ACE, thrombomodulin, TfR). Lower panel: Receptor expression, localization, and accessibility change upon endothelial activation. ICAM-1 and VCAM-1 expression in the tetraspanin domains is increased, largely via de novo synthesis. Accessibility to endothelial junction proteins PECAM-1 and VE-Cadherin may change due to reduced cell-cell interactions. P-selectin is upregulated on the membrane through mobilization of intracellular stores found in Weibel-Palade Bodies. Other proteins such as ACE and thrombomodulin are lost from the cell surface through a shedding mechanism.
In massive hemorrhage, sepsis, severe sterile tissue injury, such as reperfusion or cytokine release syndrome, and other pathological conditions, endothelial cells cease their quiescent phenotype. Instead of maintaining blood fluidity and confinement to the vascular space, pathological endothelium aggravates thrombosis and inflammation. It promotes blood clotting via exposure of phosphatidylserine and exteriorization of von Willebrand Factor and P-selectin [75]. Activated endothelial cells release chemoattractants and cytokines luring migrating host defense cells and the luminal surface becomes pro-adhesive to leukocytes and blood components and the monolayer loses the barrier function as endothelial cells contract and the VE-cadherin “zipper” opens up [76,77]. These changes, concomitant with loss of anti-thrombotic and anti-inflammatory mechanisms, including TM/APC, CD39, and nitric oxide (converted into dangerous peroxinitrite ONOO− by elevated influx of reactive oxygen species) ignite and propagate the vicious cycle of acute vascular damage, multi-organ failure, and demise [[78], [79], [80]]. Rapidly emerging pathological and clinical data implicate this transformation in the pathogenesis of COVID-19, in particular contributing to severe morbidity and mortality ensuing from the pulmonary microvascular injury [[81], [82], [83]].
Beyond the endothelium, activated platelets also serve as a target for P-selectin binding agents [84]. In general, both selectins (e.g., P-selectin, E-selectin) and molecules such as VCAM-1 have a low and transient surface expression on endothelium. Because of this, targeting to these epitopes could provide excellent specificity in diagnostic imaging using radioisotopes [68] or ultrasound contrasts [84,85] to visualize pathologically activated endothelium. In general, these molecules are more closely associated with activated endothelia, in tissues such as the skin [86]. VCAM-1 is expressed relatively selectively in the inflamed cerebrovascular endothelium and VCAM-targeted DDS selectively and effectively target drugs, including mRNA, to the blood-brain barrier and normalize its pathological alterations in mouse models [87] (Fig. 3 ).
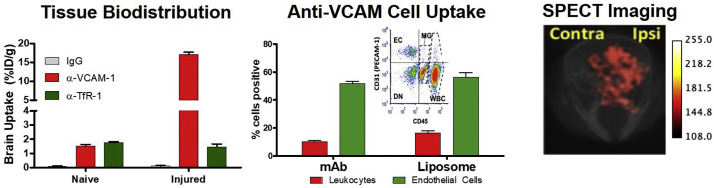
Fig. 3.
Targeting to VCAM-1 enables selective delivery to the inflamed cerebral vasculature. Left panel: Absolute uptake of anti-VCAM-1 mAb in the brain of mice injured via an intrastriatal injection of TNF-α exceeds that of the ‘gold standard’ for brain delivery, Transferrin Receptor (TfR), by an order of magnitude. Middle panel: Flow cytometry on brain homogenates reveals that over 50% of endothelial cells are positive for VCAM-targeted agents (either mAb or liposome) following IV injection. Following IV injection of fluorescently labeled mAbs and liposomes under the same conditions as in the left panel, brains were disaggregated and stained to determine mAb/liposome association with leukocytes and endothelial cells. Inset: Typical dot plot showing cell types identified via this approach. Right panel: SPECT imaging of VCAM-targeted liposomes labeled with 111In demonstrates selective uptake in the injured hemisphere of the brain. Units in the scale bar are presented as arbitrary intensity units. Figures adapted from [87]. Colors are consistent across panels. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Hydrodynamic conditions may also modulate endothelial processing of targeted carriers [88,89]. It was observed that nanoparticles targeted to either ICAM or PECAM had reduced uptake by endothelial cells following flow adaptation [90,91], and their uptake in endothelium in vivo appears to be inferior in arterioles vs. capillaries [91]. In contrast, acute increses in shear stress stimulates endocytosis of PECAM-targeted nanocarriers [90]. Activation of endothelial cells using cytokines (e.g. TNF-α) leads to enhanced internalization of ICAM-1-targeted nanoparticles, compared to naïve, quiescent cells [91].
3.2.2. Cellular localization of ligands and ligand-coated DDS
Endothelial surface determinants localize to diverse domains of the plasmalemma. Proteins such as PECAM and VE-cadherin function to maintain the endothelial barrier, and, as such, are often found at inter-endothelial junctions [92,93]. On the other hand, VCAM-1 and ICAM-1, which function in leukocyte adhesion and migration, are largely found on the apical surface of the membrane within specific micro-domains [[94], [95], [96]], or ‘rafts’ [97]. Within the pulmonary endothelium, GP85 is found on the luminal surface within an organelle-free portion of the cell [98,99].
Various forms of endocytosis (e.g. clathrin, etc.) are used by endothelial cells to internalize ligands bound to selectins [[100], [101], [102]]. This process allows entry of a variety of E-selectin targeted agents, such as liposomes [103], drugs [103,104], and nucleic acids [105]. However, not all endothelial surface epitopes are internalized as efficiently. Studies revealed that anti-PECAM mAbs are not internalized and that anti-ICAM-1 mAbs are internalized, but are subsequently recycled back to the cell surface [106]. This feature of CAMs is desirable for therapeutics that require prolonged exposure in the vessel lumen, such as anti-thrombotics [107]. It should be noted that this process can be modulated by the avidity of the drug carrier, as multivalent protein conjugates and nanoparticles targeted to PECAM and ICAM-1 are able to enter endothelial cells via an inducible CAM-mediated endocytosis mechanism [108]. By tuning the valency of a DDS, sub-cellular delivery of CAM-targeted agents can be controlled, to a certain extent.
Accessibility of particles to specific endothelial epitopes is dependent on the localization of the epitope within the membrane and vascular conditions [109]. Epitopes that may not be suitable for affinity targeting include those that are masked by the glycocalyx, located deep within cell-cell junctions, or are inside membrane invaginations [110]. Pathological changes in tissues can alter the accessibility of target epitopes. A feature of certain pathologies is the shedding of the endothelial glycocalyx, which may increase accessibility of certain target epitopes, such as ICAM-1 [111]. However, the endothelial surface may become coated with leukocytes and thrombi, which would reduce accessibility to target determinants [58]. Recent advances in techniques such as proteomics [112] in vivo phage display [[113], [114], [115]] have provided additional insights into accessibility of membrane targets. As carrier size increases, accessibility to epitopes becomes increasingly more important (e.g., accessibility is less of a concern for peptides than for immunoliposomes).
Proteins located within caveolae, such as Aminopeptidase P (APP) and Plasmalemma Vesicle Associated Protein-1 (PLVAP) [116], are accessible only to relatively small drug carriers. This is due to the nature of the flask-shaped membrane invaginations that are characteristic of caveolae. It is generally thought that the caveolar mouth has a diameter of about 50 nm [117,118]. Functions of caveolae include endothelial trafficking and cellular signaling [[119], [120], [121], [122], [123], [124]]. A key feature of caveolae that distinguishes them from other cholesterol-rich domains (e.g., lipid rafts) is the presence of the protein caveolin-1. Caveolar pathway(s) transport ligands including albumin [125] and chemically-modified albumin [126] across the endothelium [127]. In rats, mAbs directed against several caveolar antigens are able to efficiently target and transmigrate across the pulmonary vasculature, consistent with the large number of caveolae present in the pulmonary endothelium [6].
However, conjugation of APP and PLVAP antibodies with nanoparticles larger than 50-70 nm obliterates targeting, due to the presence of the caveolar neck [128]. It has been reported that caveolae are able to merge, forming “caveolosomes”, which are capable of internalizing large particles. However, these experiments were perfomed in vitro using static cell culture systems, which may not be reflective of true in vivo endothelial physiology [116,129,130]. The spatial accessibility of caveolar targets to circulating nanocarriers is a hot topic. For example, flexible anti-PLVAP nanogels with a diameter of 150–300 nm enter caveolae and accumulate in the lungs, whereas their rigid nanoparticle counterparts do not [131].
Ferritin-based nanocarriers have been investigated as a DDS that can be used in a modular and stimuli-responsive manner [132]. Ferritin particles conjugated with anti-PLVAP accumulate in the lungs after intravenous injection and enter caveolae, since their size does not exceed 20 nm. Since the ferritin carriers are so small, conjugation of the ligand and enzymatic cargo to external and internal facets of ferritin nanocages, respectively, improves targeting [133]. Interestingly, ferritin nanocarriers targeted to ICAM also appear to have greater access to the endothelial surface in the pulmonary vasculature compared to larger carriers [134,135].
3.3. Intracellular endothelial delivery
A conventional approach to achieve intracellular delivery utilize affinity ligands capable of anchoring DDS to epitopes that are permissive of endocytosis. Diverse endothelial determinants have been implicated in internalization [50,[136], [137], [138]]. In general, identification and selection of affinity ligands is empirical in nature [139,140]. Approaches such as phage display facilitate selection of internalizable ligands [[141], [142], [143]]. In one example, phage display was used to select several VCAM-1-binding peptides [144]. Peptides that were rapidly endocytosed were used for molecular imaging of vascular inflammation [86,144]. Antibodies to APP, PLVAP or GP90 enter caveolae [6] and those to E-selectin or VCAM-1 [103,104] enter clathrin-mediated endocytosis [101,102].
In some cases, coating carriers with affinity ligands permits internalization to occur at least as efficiently as monovalent ligands, if not more efficiently. This may occur due to multivalent carriers eliciting strong cell signaling and actin rearrangement [145]. Both VCAM-1 antibodies [104,146] and VCAM-1 targeted DDS have been reported to enter endothelial clathrin-mediated endocytosis [86,144]. Anti-transferrin receptor (TfR) mAbs bound to TfR and free transferrin also enter endothelial cells via clathrin-mediated endocytosis [147,148]. However, coupling ligands to carriers may actually reduce cellular uptake due to their exceeding size limits of endocytosis. Caveolar and clathrin-mediated endocytosis have size limits of 50–70 [149] and 200–300 nm [70], respectively. Findings made using in vitro, static systems should be taken with a grain of salt, as they may use non-physiologically-relevant endocytic pathways that have not been confirmed in vivo thus far [127].
The uptake and trafficking of ICAM-targeted DDS vary. Endothelial cells are not effective in internalizing monovalent fusion proteins that do not initiate clustering of epitopes, whereas bivalent anti-ICAM and anti-ICAM conjugates recycle rapidly to the luminal surface, unlike multivalent nanoparticles targeted to ICAM, which traffic to the lysosomes [91,106,108,[150], [151], [152], [153], [154]]. Binding to different epitopes may also change the efficiency of entrance into cells [110]. Nanocarrier geometry may also affect uptake and subcellular trafficking [108,155]. For example, disk shaped particles targeted to ICAM-1 have a lower rate of endocytosis compared to spheres of a similar size. Once inside the cell, the rate of lysosomal trafficking is inversely related to size (e.g., small particles traffick more quickly) [155].
Silvia Muro, whose studies greatly advanced the field of endothelial intracellular delivery, devised a novel DDS platform for ICAM-1 targeted delivery of nanocarriers loaded with enzyme replacement therapies for lysosomal storage diseases (LSD) [153,156]. LSDs are morbid conditions caused by mutation of lysosomal enzymes [157]. In LSD, enzyme replacement therapy (ERT) is the standard of care. Clinically, this approach is applied by chronic injections of recombinant forms of the deficient lysosomal enzyme [158]. The systemically injected proteins are then internalized by cells in a manner dependent on mannosylation of proteins, via either mannose receptor and/or mannose-6-phosphate receptor [159]. Currently approved therapies for treatment of LSD include ERT, substrate reduction inhibitors, and small molecule chaperones [[160], [161], [162]]. Type B Niemann-Pick disease (NPD) is caused by deficiency/malfunction of the protein acid sphingomyelinase (ASM). Deficiency of ASM results in increased deposition of sphingomyelin and cholesterol enzymes [157].
In LSD, endothelial cells are often a key pharmacologic target [156], but endothelial delivery of ERT is not very effective [161,163]. It has been reported that endothelial cells have increased ICAM-1 expression in many LSD, which may provide a route for improved delivery of recombinant enzymes [164,165]. Delivery of ERT using ICAM-1-targeted nanoparticles has been shown to improve endothelial targeting and pharmacologic effects in several animal models of LSD [153,166,167]. In a mouse model of Pömpe disease, an ~600-fold improvement in lung delivery was observed for ICAM-1-targeted acid α-glucosidase (the enzyme deficient in this disease), relative to untargeted enzyme [168]. By adjusting geometric features of the carrier, delivery via ICAM-1 targeting can be optimized. Use of relatively small (100–200 nm), spherical carriers provided maximal delivery to the lysosomes as compared to particles with other features (e.g., discoidal, micron-sized/spherical) [169].
The Muro group has also identified that interactions between nanocarriers and endothelial ICAM-1 results in activation of enzymes responsible for sphingomyelin metabolism in the membrane and endocytosis of a wide range of sizes of targeted particles [170]. However, this pathway is dependent on sphingomyelinase expression and coating of particles with both anti-ICAM-1 and ASM permits internalization in NPD [166]. Additionally, it was shown that larger carriers (>200 nm) coated with ASM and anti-TfR were also internalized [171].
CAM-mediated endocytosis achieved via targeting to ICAM-1 has been shown to be more efficient than clathrin-mediated endocytosis via TfR targeting in delivery of enzymes to the lysosome [154]. However, dual targeting to ICAM-1 and TfR provided a different tissue distribution than either mono- or non-targeted carriers [171]. The utility and significance of these findings represent an area of active investigations [156].
4. Interaction of DDS with endothelial targets: vascular targeting modulation
Nanocarriers can be decorated by ligands in many ways. Interplay between features of the carrier (e.g., size, shape, flexibility, etc.), the ligand (e.g., affinity, density, etc.), and the target epitope (e.g., accessibility, density, etc.) creates a complex matrix of parameters controlling interactions between a DDS and its target.
4.1. Dual targeting
Carriers co-coated by antibodies to both inducible and stable high-density molecules can, in theory, selectively bind to pathological cells [172,173]. Dual P-selectin/ICAM-1 targeted particles carrying equimolar ratios of both ligands were reported to bind to the inflamed endothelium better than mono-targeted particles [172,174,175]. This dual targeting approach improved imaging in mouse models of inflammation using contrast enhancing particles targeted to either P-selectin and VCAM [176] or P-selectin and ICAM-1 [177]. Dual targeting of anti-ICAM/anti-TfR nanoparticles has shown that individual ligands could promote targeting to a specific tissue vasculature (ICAM-1: inflamed lungs, TfR: brain) [171].
4.2. Collaborative endothelial targeting
Dual therapeutic targeting to vascular lumen can be achieved by utilizing a recently discovered phenomenon where distinct monoclonal antibodies directed to adjacent epitopes in the extracellular region of PECAM stimulate binding of each other [178]. The effect was dubbed Collaborative Enhancement of Paired Affinity Ligands, or CEPAL, and is dissimilar to generally observed binding patterns of ligands to same target molecule inhibiting binding of each other [48,179,180]. This unusual finding can be explained by increased accessibility of an epitope to its ligand due to conformational changes in the PECAM molecule induced by binding of a paired “stimulatory” ligand [181]. In-depth investigation of mechanisms underlying this effect revealed several findings: CEPAL is independent of PECAM-PECAM homophilic interactions in the plasma membrane and can be observed with recombinant PECAM protein [181]. Given the known roles of PECAM-1 in promoting endothelial quiescence and in maintaining cell junction integrity [182], investigations of possible unintended effects of CEPAL on vascular function have shown only modest or insignificant effects. Interestingly, these effects were observed in response to antibodies to PECAM-1, whether given solo or in pairs [183].
CEPAL was applied to achieve enhanced effects of protein therapeutics using anti-PECAM scFv fusion proteins [178]. A key advantage of this dual targeting strategy was demonstrated by co-delivery of thrombomodulin (TM) and its natural endothelial binding partner, endothelial protein C receptor (EPCR) using paired anti-PECAM scFv fusions. Co-administered scFv/TM and scFv/EPCR partnered in activation of protein C (PC) and reduced markers of lung inflammation in a model of acute lung injury [184]. Interestingly, the CEPAL effect also increased delivery of PECAM-targeted nanocarriers, a finding that opens up a new avenue for the field of nanomedicine in optimizing and enhancing delivery of diverse therapeutic cargoes encapsulated in endothelial-targeted nanocarriers [185]. The CEPAL mechanism, occurring by exposure of partially occult epitopes on PECAM protein, may be a generalizable phenomenon and is likely to occur for other molecules and antibody pairs.
4.3. Ligand surface density
A key parameter controlling targeted nanocarrier delivery is the surface density of the affinity ligand. However, selection of the optimal density is not a straightforward process, as this value is dependent on features of the carrier, the affinity ligand, and the target epitope. In other words, selection of the maximal surface density may not be the optimal strategy for achieving the maximal selectivity and magnitude of drug delivery. For example, an intermediate ligand density has been shown to provide enhanced selectivity of targeting [186,187]. An excessive ligand density (“ligand overcrowding”) impedes congruency of ligand and target molecules [186,188]. Selectivity of both delivery and imaging of vascular inflammation has been shown to be improved via reduction of surface density to an optimal value [128,189]. Recent studies have shown that ligand density can play a role not only in selectivity of delivery, but also in sub-cellular trafficking. Delivery of ICAM-1-targeted nanocarriers across the blood-brain barrier was demonstrated to be optimal at an intermediate ligand density, which balanced efficient uptake and detachment from target following transport [150].
4.4. Carrier geometry and flexibility
In addition to a carrier's affinity, its geometry and flexibility regulate pharmacokinetics, circulation, tissue permeation, and interactions with cells [[190], [191], [192], [193], [194]]. Carrier size is a factor in route of administration, target access, and pharmacokinetics. Carriers smaller than 20–30 nm can be administered via diverse routes, while larger carriers generally require vascular injection for systemic administration [193,[195], [196], [197], [198]]. Once in circulation, carriers of small (<10 nm) diameter are prone to rapid renal clearance [199]. A size range of ~100–200 nm generally avoids filtration to the space of Disse in the liver and entrapment in the splenic sinusoids, giving longer circulation and more opportunities for target engagement [200]. For instance, it has been noted that larger ICAM- and PECAM-targeted particles have enhanced uptake in the RES, and a reduction of specificity of vascular delivery relative to untargeted carriers [155,201]. Indeed, at larger extremes of injectable particle size (>1–3 μm), both greater splenic retention and non-specific entrapment in lung capillaries is observed [[202], [203], [204]].
Larger carriers generally have lesser access to targets and adhered carriers may experience greater dragging force from blood, leading to target detachment [198,205,206]. For instance, larger carriers were shown to be incapable of targeting PLVAP, expressed in caveolae in lung endothelium [128]. However, in studies of targeting to PECAM, increasing carrier size from 40 nm to 300 nm increased specific adhesion in the lungs, implying that greater engagement with accessible endothelial targets may be achieved for larger carriers [201].
Nanocarrier shape, and interplay between shape and size, is also an important factor in targeting behavior [192,207]. Carriers that are non-spherical in nature may circulate longer than similarly sized spheres due to reduced recognition by host defense cells [109,208]. Discoid, ellipsoid, and filamentous carriers have prolonged circulation relative to spheres [155,209,210], in part due to reduced uptake by macrophages [211]. Rods and discoid particles avoid side effects associated with engagement with host defense cells in the lungs, unlike spherical carriers [210].
Eluding clearance from circulation and non-specific uptake may lead to non-spherical nanocarriers with more specific targeting to vascular endothelium. ICAM-targeted disks adhered to the pulmonary vascular endothelium more specifically than spherical counterparts [155]. Non-spherical and spherical particles have distinct propensity for margination in flow [212,213], modulating their affinity interactions under flow [[214], [215], [216]] and facilitating efficient non-spherical particle targeting to endothelial cells in the lungs and brain [217]. However, at an extreme of carrier elongation, worm-like filomicelles align with flow and avoid binding to vascular cells [218]. Filomicelles targeted to ICAM-1 had similar specificity of targeting as spheres, but a lower magnitude of uptake in the lung [209].
The above discussion of shape and size largely presumes rigid particle geometry, but many nanocarriers are capable of dynamic changes in shape and size. Methods for adjusting the flexibility of nanocarriers have been described for a variety of materials, with specific focus on lipid and polymeric vesicles [195,[219], [220], [221], [222]]. Mechanical flexibility has a clear impact on systemic and target-specific behaviors of nanoparticles.
Generally, greater flexibility correlates to prolonged circulation of nanocarriers [195,218,219]. In part, prolonged circulation of soft nanoparticles may be attributable to reduced non-specific uptake in phagocytic cells [223,224]. In macrophages and other immune cells, harder particles are internalized more readily than soft particles [195,219,224,225]. A relationship between particle flexibility and ease of particle uptake may also be emerging in results with endothelial cells, where in vitro and in silico studies show passive and affinity-mediated nanoparticle uptake being impeded in softer particles [195,219,226]. Further, recent results show that harder (GPa bulk modulus) non-targeted 200 nm spheres associated with and transported across brain endothelium in a microfluidic model to a greater degree than softer (MPa bulk modulus) spheres [227].
Changes in the mechanical properties of nanoparticles have yielded changes in affinity-directed targeting behavior. For PEG hydrogel nanoparticles, softer nanoparticles outperformed similar rigid particles in targeting to ICAM in the lungs [195]. However, in vivo observations with soft nanoparticles must consider the impact of prolonged circulation times on the extent of engagement with target molecules. The softer PEG nanoparticles also maintained higher circulating concentrations, meaning the lung to blood localization ratio was largely unaffected by flexibility of the ICAM-targeted particles in this case. Controlling for this factor, a microfluidic study showed that soft disc nanoparticles outperform rigid counterpart discs in adhesion to a target surface under flow [216]. It is hypothesized that this result may reflect the capacity of soft particles for increased target engagement and for resistance to target disengagement via fluid forces on the nanoparticles.
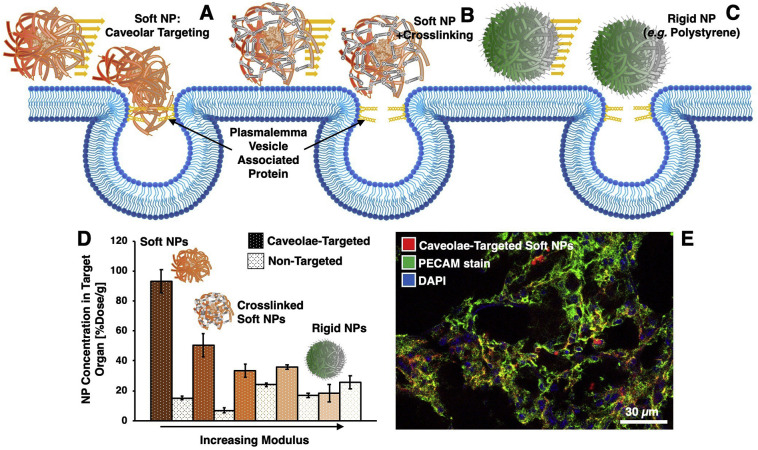
A specific application for soft particles in endothelial targeting was noted in studies of nanoparticles targeted to PLVAP. As noted above, PLVAP localizes to caveolae in the lungs and targeting to caveolae is limited for particles larger than the caveolar neck (>50-100 nm) [128]. However, soft (~50 kPa) dextran nanogels of 150–300 nm diameter successfully targeted to PLVAP in the lungs (Fig. 4 ), where analogous rigid polystyrene particles, functionalized with the same antibody, did not [131]. Further, tuning the modulus of the dextran nanogels via crosslinking resulted in abrogation of PLVAP targeting in inverse proportion to Young's modulus of the crosslinked particles [228] (Fig. 4D). Therefore, engineering of nanoparticle softness may affect particle access to caveolae, representing an important route for internalization in endothelial cells and possibly for transcytosis.
Fig. 4.
Carrier flexibility modulates engagement of targeted carriers with caveolar plasmalemma vesicle associated protein (PLVAP) in lung endothelium. Carrier mechanical properties can affect how well carriers access targets (e.g. by soft particles “squeezing” into small spaces, as depicted in (a)). PLVAP is located in a sterically concealed position, the caveolar neck, in lung endothelial cells. Soft dextran particles (a) were able to target PLVAP more effectively than crosslinked dextran particles (b) or rigid polystyrene particles (c) of similar size. As evaluated in radioisotope tracing (d) and fluorescence (e) studies, PLVAP targeting efficacy in the lungs decreased as nanoparticle Young's modulus (as determined by AFM) increased (d). Adapted from [131,228].
5. Modulating vascular targeting via control of route and PK
5.1. Local infusion
Compared to other routes of administration, the intravenous route has a distinct advantage in pulmonary delivery, as the lungs receive the entire first pass of venous blood [229]. In addition to first pass benefits, delivery to the pulmonary endothelium is particularly attractive, as it comprises ¼ of the entire surface area of endothelium. Because of the high perfusion of lungs and large endothelial surface area, delivery to endothelial antigens (e.g. ICAM-1, PECAM) is pharmacokinetically favored in the pulmonary vasculature.
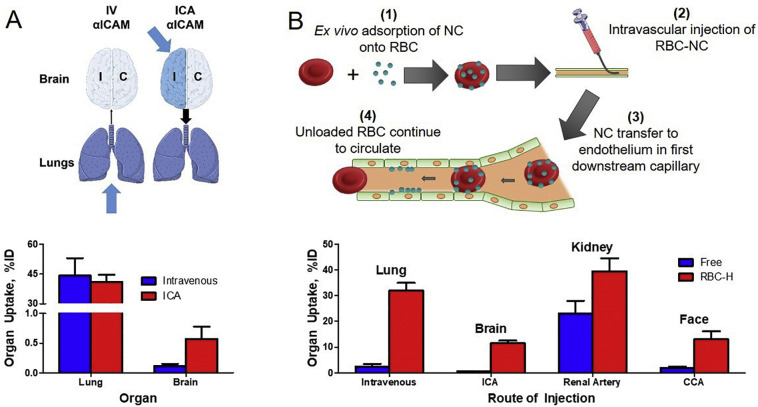
An infusion via vascular catheters in conduit vessels favors uptake in the microvasculature immediately downstream of the injection site. This principle was successfully employed for PECAM and ICAM-targeted DDS: local infusion enhanced uptake in the vasculature in the brain, mesentery, and heart in several species, including mice, rats, dogs, and pigs [[229], [230], [231]] (Fig. 5a). Further, infusion in the isolated organs bypasses numerous factors impeding targeting and boosts local delivery, providing mechanistic insights and potential utility of vascular targeting for improving grafts for organ transplantation.
Fig. 5.
Utility of the first-pass effect in tissue targeting. A: Intravenous (IV) infusion of ICAM-targeted nanocarriers (NC) results in a large portion of the dose being deposited in the lungs. On the other hand, infusion into the internal carotid artery bypasses the lung first pass and permits delivery to the brain. B: Ex vivo adsorption of untargeted NC onto RBC, termed RBC hitchhiking (RBC–H), allows transfer of NC to endothelium in the first capillary downstream of the injection site. This technology has been shown to increase delivery to several tissues by varying the injection site. Additional abbreviations used in figure: ICA (internal carotid artery), CCA (common carotid artery). Figures adapted from [231,232].
5.2. RBC Hitchhiking
The intravascular route can be further manipulated by using red blood cells (RBC) as a transport vehicle for DDS that are both actively and passively targeted [232,233]. Nanocarrier DDS can massively increase the mass of drug delivered to the target organ, but are not without their inherent problems, such as short half-life due to rapid clearance by the reticuloendothelial system [234]. The liver, spleen, and intravascular leukocytes, aided by the complement cascade, all contribute to this rapid clearance.
Red blood cell hitchhiking (RBC—H) was devised to evade this rapid clearance by the RES. Early studies used passive adsorption to non-covalently attach polystyrene nanoparticles onto RBCs and found that the half-life of RBC-adsorbed nanocarriers was longer than that of free nanoparticles. Additionally, the lung-to-clearing organ (liver & spleen) ratios were significantly improved using RBC-H [232]. This landmark work; however, was limited to rigid nanoparticles having no drug delivery utility, nonspecific attachment to RBC, and a circulation time of less than 24 h. Notably, increased delivery to the target tissue was achieved by injecting RBC-coupled nanoparticles immediately upstream of target organ, thus increasing first-pass delivery (Fig. 5b).
Upon careful loading that does not compromise RBC biocompatibility, RBC carrying surface-bound cargoes do not accumulate in any organ, including the reticuloendothelial system. However, the surface-bound cargoes that are loaded on RBC in a reversible fashion detach from RBC and transfer to the microvascular bed encountered by RBC. In the case of intravascular injections of RBC carrying such cargoes, the first major microvascular bed downstream injection site, i.e., first pass. In IV injection this is the pulmonary vasculature.
Additional studies incorporated biocompatible nanocarriers into RBC-H using liposomes, polymeric nanocarriers, and adeno-associated virus (AAV). The first pass effect was again amplified using RBC-H with 40-fold increase in lung targeting compared to free nanocarrier after intravenous injection. Importantly, this effect was also observed after intra-arterial injection via the carotid artery and measuring uptake in the immediate downstream organ, the brain, achieving brain uptake 143-fold than that of free nanocarriers [232]. Studies have shown therapeutic benefit using this technique in vivo in mouse models of acute pulmonary embolism and lung metastasis [232,233].
RBC-H has shown many advantages over intravenous delivery of free nanocarriers, but is not without room for improvement. The initial work focused largely on first-pass delivery to the immediate downstream organ, relying on the interaction of RBCs with the endothelium to encourage mechanical dissociation of nanoparticle from RBC vehicle. The promise of RBC-H as a drug delivery system that successfully evades the RES will likely need to incorporate better control over the RBC-nanoparticle association (and thus dissociation) and effect on the carrier RBC. One method to increase control of RBC-nanocarrier interaction is the use of targeted binding rather than passive adsorption. The interaction of RBC with RBC membrane targeting moieties has been characterized using drug-coupled fusion proteins. Specifically, anti-RBC-membrane scFv fused to thrombomodulin were evaluated in vitro in a microfluidic system. While some targeting to certain RBC surface epitopes caused pathologic increases in membrane rigidity (Band 3/ Glycophorin A), targeting to others (RhCE) did not. Importantly, therapeutic efficacy of fusion-TM was retained when compared to soluble TM [235]. Future generations of RBC-H may utilize nanocarriers targeted to the RBC membrane to improve control of RBC-nanocarrier association, dissociation, and ultimately prolong time in circulation beyond the first pass effect.
5.3. Molecular engineering of targeting ligands
Many groups utilize antibody fragments (e.g. Fab, scFv, nanobody, etc.) as affinity ligands to achieve targeted drug delivery. Despite many advantages, such as ease of production in microbial systems and lack of Fc fragment-induced toxicities, these fragments are often inferior to full mAbs as isolated targeting ligands due to rapid blood clearance and poor stability of binding due to their monovalent nature. Additionally, commonly used amine-based conjugation strategies often inhibit the function of these small affinity ligands [236]. As such, there have been a myriad of protein engineering strategies proposed to improve the drug delivery properties of antibody fragments.
Improving circulation time of affinity ligands can be critical in conferring them with favorable biodistribution properties for therapeutic applications. This has been achieved in many ways, including fusion to long-circulating proteins (IgG and albumin) [[237], [238], [239], [240]] or attachment of hydrophilic polymers (PEG) [241,242] to reduce renal elimination. Fusion to proteins such as albumin or albumin-binding molecules confers a long half-life, in part, due to interactions with the neonatal Fc receptor (FcRn), which serves as a salvage receptor preventing the intracellular catabolism of albumin [243] and IgG [[244], [245], [246]]. We have recently reported that fusion of a VCAM-1-targeting nanobody to an albumin-binding nanobody leads to 25–40-fold improvements in uptake in target tissues over a 24 h period, relative to the anti-VCAM-1 nanobody alone [247].
To overcome challenges in coupling of therapeutic cargoes to small affinity ligands, site-specific conjugation strategies are becoming more prevalent. These typically involve building in motifs for conjugation near the C-terminus of the antibody fragment. Some of the more common strategies for site-specific conjugation of antibodies and fragments include Sortase A transpeptidation (recognizing the sequence LPXTG) [248] and conjugation to unpaired cysteines introduced into the molecule. These strategies have been successfully used to attach therapeutic cargoes and radiolabels to targeting ligands without adverse effects on their function [236,[249], [250], [251], [252], [253]]. In addition to engineering of antibody fragments, our group has recently reported a strategy for engineering full-length mAbs for site-specific modification by Sortase A via CRISPR/Cas9-mediated engineering of hybridoma cells [254,255]. The use of site-specific bioconjugation strategies permits oriented coupling of targeting ligands on the surface of nanoparticles, which has been demonstrated to improve their specificity for endothelial targets [252,256].
Nucleic acid aptamers are a recently developed class of affinity ligands that selectively bind to targets with high affinity via unique three-dimensional structures [257]. Aptamers are short, single-stranded DNA oligonucleotides that can be developed for a variety of target molecules using techniques analogous to phage display for antibody selection [258]. Aptamers directed against TfR have been used to deliver therapeutics both to [259] and across [260] the blood-brain barrier in animal models. This presents another potential approach for selective delivery to the endothelium.
6. Envisioned biomedical utility in acute vascular pathologies
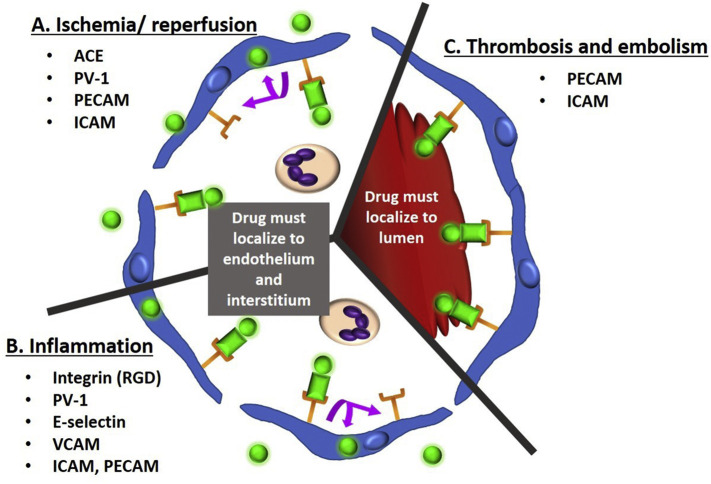
Investigations in both animal models and in patients [6,19,41,44,45,69,138,[261], [262], [263]] have demonstrated affinity targeting to both normal and pathologically altered endothelium [40,[264], [265], [266]]. The delivery of enzyme replacement therapies to improve lysosome storage disease treatment was discussed above in the section on intracellular endothelial delivery. Here we provide a brief overview of a few areas of potential medical utility of this approach, with a focus on thrombosis, acute vascular inflammation, and ischemia (Fig. 6 ). Applications of endothelial targeting in select pathologies are summarized in Table 3 .
Fig. 6.
Endothelial receptors for conjugate targeting in acute vascular pathology. Shown is a small vessel or capillary in cross-section, lined by endothelial cells (blue). Receptors (orange) are bound by drug conjugate (green circle) plus targeting moiety/ nanocarrier (green rectangle). Many receptors including PECAM and ICAM enable both intravascular and extravascular drug targeting. Factors that promote intracellular uptake (with retention of drug conjugate and recycling of receptor) include multivalent targeting moieties and low-flow state. Factors that modify uptake include ligand density, carrier geometry, and size. Ischemia/reperfusion injury (A) and acute vascular inflammation (B) both result in increased leukocyte recruitment and leaky tight junctions between endothelial cells. Treatment requires drug to be delivered intracellularly to the endothelium and to the interstitial space. Drug targeting to the specific receptors shown has resulted in treatment effect in animal models of these pathologies. (C) Shown is a blood clot within the lumen. Thrombosis and embolism both require the drug to remain in the vessel lumen to achieve direct therapeutic effect. Drug targeting to the receptors shown has resulted in treatment effect in animal models of thrombosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Examples of endothelial-targeted drug delivery in non-oncology settings.
| Pathology | Target Epitope(s) | Drug(s) | DDS | Ref |
|---|---|---|---|---|
| Pulmonary Embolism | PECAM-1 ICAM-1 N/A |
scuPA tPA Reteplase |
Fusion Protein Protein Conjugate RBC-H |
[267,268] [269] [232] |
| Ischemic Stroke | PECAM-1 | scuPA | Fusion Protein | [230] |
| Lung Transplant | ACE PECAM-1 |
Catalase Catalase |
Protein Conjugate Protein Conjugate |
[324] [278,366] |
| Acute Lung Injury | PECAM-1 ICAM-1 PLVAP |
Catalase Thrombomodulin MJ33 EUK-134 SOD/Catalase Thrombomodulin Dexamethasone SOD |
Protein Conjugate Fusion Protein Liposomes Liposomes Nanoparticles Fusion Protein Nanogel Protein Conjugate |
[321,332] [270] [333] [205] [367] [271] [273] [318] |
| Type B NPD | ICAM-1 | Acid Sphingomyelinase | Nanocarrier | [166] |
| Neurovascular Inflammation | VCAM-1 | Thrombomodulin mRNA | LNP | [87] |
| Fabry Disease | ICAM-1 | α-Galactosidase A | Nanocarriers | [167] |
| Glomerulonephritis | E-Selectin | Dexamethasone DnlκB Transgene |
Liposome Adenovirus |
[284,285] [289] |
| Arthritis | Integrin | Dexamethasone | Liposome | [291] |
| Atherosclerosis | VCAM-1 | Prostaglandins | Liposome | [293] |
| Pulmonary Hypertension | ACE | Tph1 shRNA | Adenovirus | [323] |
| Pulmonary Ischemia-Reperfusion | ACE | Catalase | Protein Conjugate | [325] |
| Endotoxemia (IP LPS) | VCAM-1 | Dexamethasone | SAINT-O-Somes | [368] |
| Idiopathic Pulmonary Fibrosis | PLVAP | PGE2 | Protein Conjugate | [369] |
Abbreviations used in table: ACE: angiotensin-converting enzyme, ICAM-1: intercellular adhesion molecule-1, LNP: lipid nanocarrier, NPD: Neimann-Pick disease, PECAM-1: platelet-endothelial cell adhesion molecule-1, PGE2: prostaglandin E2, PLVAP: plasmalemma vesicle-associated protein, RBC–H: red blood cell hitchhiking, scuPA: single chain urokinase-like plasminogen activator, SOD: superoxide dismutase, tPA: tissue-type plasminogen activator.
Endothelial targeted DDS can deliver a great variety of anti-thrombotic agents, with different mechanisms for release and or activation at the intended site. They include plasminogen activators [230,[267], [268], [269]] and thrombomodulin [64,[270], [271], [272]] fused with scFv binding to surface endothelial epitopes and exert their activities in the lumen directly. Alternatively, DDS targeted to the same epitopes carrying encapsulated small drug molecules such as anti-inflammatory drugs (which have both direct and indirect anti-thrombotic effects) employ release of drugs from the cell-bound DDS either in blood or in endothelial cells [205,273]. Further, delivery of mRNA for TM (and likely other anti-thrombotic proteins) deliver their cargo into the cytosol of endothelial cells enabling synthesis and luminal exposure of the therapeutic transgene [87].
6.1. Acute thrombosis
In many pathologies, affected blood vessels have an increased propensity for thrombosis, in no small part due to suppression of the endothelium's natural anti-thrombotic mechanisms [274]. Delivery of ATA, such as TM or plasminogen activators (tissue type, tPA, or urokinase, uPA) to the luminal surface of the endothelium may help to subside some pro-thrombotic states. These results have been supplemented by nucleic acid-based delivery of these proteins [275].
In cell-based models, targeting anticoagulants to activated endothelium using anti-E-selectin mAbs was shown to inhibit thrombin expression, providing proof-of-principle of this approach [67]. As investigations moved into rodent studies, both tPA and uPA were shown to be concentrated within the lungs by targeting to ACE; however, there were limited pharmacologic benefits due to rapid endocytosis [99,276]. However, delivery of ATA to non-internalizing endothelial epitopes (e.g., ICAM-1, PECAM) permitted not only efficient targeting of the lung, but also clot dissolution [269]. Fusion proteins between anti-PECAM scFv and ATA were able to provide thromboprophylaxis due to prolonged residence in the pulmonary lumen [267,277,278]. Local infusion of scFv/uPA via the carotid artery prevented cerebrovascular thrombosis [230]. Fusions containing uPA mutant activated by thrombin [[279], [280], [281]] had similar high degree of lung uptake and thromboprophylaxis, with additional improvements in reductions of fibrin deposition and improvement of arterial oxygen tension relative to a non-thrombin-dependent scFv/uPA [268].
In rat models, pharmacologic benefits have been observed using both recombinant soluble TM [34] and tissue factor-targeted TM [282], but this fusion rapidly disappeared from the vascular lumen. PECAM-targeted scFv/TM fusion accumulated in the pulmonary vasculature, and reduced both thrombosis and other tissue damage in mice, without causing bleeding [270]. By fusing TM with scFvs directed against PECAM and ICAM, the antithrombotic and anti-inflammatory effects of untargeted soluble TM are dwarfed, due to prolonged endothelial anchoring of TM [268]. Targeting scFv/TM fusions to ICAM afforded more potent protection than to PECAM, consistent with the notion of ICAM localization close to the TM cofactor EPCR in the plasmalemma [271]. As discussed above, by taking advantage of the CEPAL effect observed for PECAM-targeted ligands, TM and EPCR were able to be co-delivered, providing a substantially increased pharmacologic effect. This benefit was likely due to enhanced targeting (CEPAL effect) and optimal special arrangement of TM and EPCR, allowing enhanced interactions between the partner molecules [184]. Thrombin-activated, targeted ATA have the potential to provide a safe thrombophrophlyactic option in patients at high risk of acute thrombosis.
6.2. Inflammation
Thrombosis and inflammation are closely intertwined. Endothelium controls vascular permeability of leukocytes in response to tissue damage and infection, but also become a victim of injurious inflammatory mediators. Targeting anti-inflammatory drugs to protect the endothelium from friendly fire without impeding host defense is an attractive strategy. Inducible and constitutive endothelial determinants involved in inflammation appear to be good targets for this approach.
The use of liposomes targeted to E-selectin has shown utility in delivering dexamethasone (DEX) to a variety of tissues in animal models of chronic inflammation. For example, these particles showed accumulation in skin and kidneys in models of dermal [283] and renal [284] inflammation. In the latter model, this treatment conferred anti-inflammatory effects in mice [284] and in rats [285]. Within minutes of injection, similar formulations accumulated in the inflamed eye and reduced the level of inflammatory markers [286]. Similar DDS were used for nucleic acid delivery to the kidney and tumors [287,288]. Both E-selectin and ICAM-1 have been used as target molecules for delivery of siRNA in vitro [289,290]. Coupling of an E-selectin targeting ligand to adenovirus allowed targeting to the kidneys and displayed functional activity in a glomerulonephritis model [289].
Delivery of DEX liposomes to integrins was achieved in a rat arthritis model, using RGD peptides. These particles provided improved protection when compared to untargeted particles [291]. A similar strategy was also used to delivery anti-inflammatory siRNA in animals [292]. Delivery of the anti-inflammatory prostaglandin, PGE2, was achieved by targeting to VCAM-1. Chronic administration of VCAM-1-targeted particles provided improved PGE2 delivery and therapeutic benefits in a mouse model of ‘atherosclerosis’ [293]. Targeting dexamethasone-loaded liposomes and nanogels to ICAM, PECAM and PLVAP supersedes protective effects of untargeted counterparts in a mouse model of ARDS [273,294].
6.3. Inflammatory signaling and reactive oxygen species
A plethora of recent findings have revealed close links between endocytosis and signaling pathways that have changed our understanding of the role that endocytosis plays in a cell's make-up [295,296]. Traditionally, endocytosis and signaling were considered as distinct processes. However, it has now become clear that receptor-regulated endocytosis is critical for modulation of numerous signaling pathways, including attenuation of signal, its prolongation, activation, signal distinction from several possible paths, etc. [297]. This is also the case for many cytokine receptors, including TNFR1 and IL-1R [298]. TNFR1 activated by interaction with TNF co-localizes with caveolin in lipid raft and receptor complex internalization may modulate TNF signaling outcome [[299], [300], [301]]. Similarly, IL-1R translocates to caveolae upon activation and receptor internalization is required for complete NF-κB signaling processes [302,303].
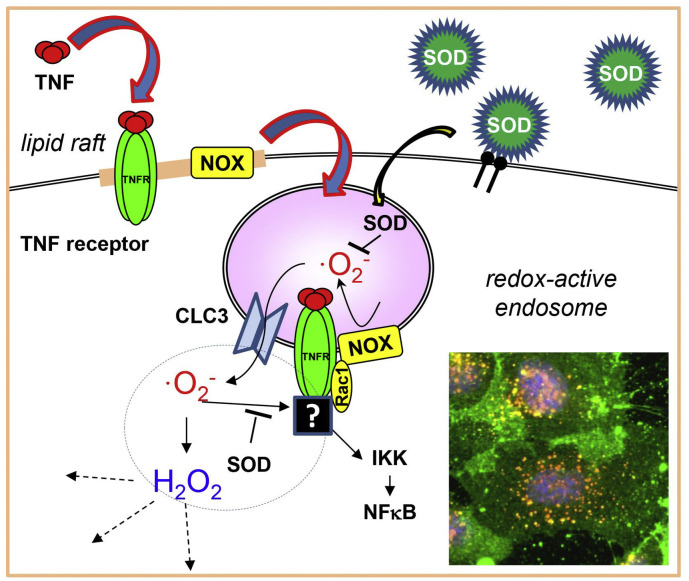
Redox-active endosomes (i.e. containing reactive oxygen species (ROS)) may play an important role in pro-inflammatory signaling [304,305] indicating potential therapeutic applications of antioxidant drug delivery [306]. PECAM- and ICAM-targeted delivery of SOD is able to considerably block the inflammatory response caused by cytokines and by TLR activation [[307], [308], [309]]. In addition, caveolar targeting of nanocarriers may have significant potential, particularly for endothelial and trans-endothelial delivery, due to the abundance of caveolae in endothelial cells [310]. Effective caveolar targeting has been demonstrated using albumin-coated nanocarriers [130]. The profound involvement of caveolae in cellular signaling [311] should be of particular interest for the drug delivery field (Fig. 7 ).
Fig. 7.
Role of ROS in cytokine- and TLR-induced signaling and potential use of intracellular antioxidant delivery for anti-inflammatory protection. Cytokine or PAMP binds to counterpart receptor, causing NOX activation and the complex internalization forming redox-active endosome. Generated ROS transfers into cytosol via CLC3 channel and stimulates proinflammatory NFκB cascade. Delivery of antioxidant to the endosome or overexpression of cytosolic SOD can reduce the inflammatory signaling and attenuate cellular injury [307,308]. Inset shows SOD-loaded nanoparticles (green) enter endosomes (red), SOD-containing endosomes seen as yellow. Cell nuclei are shown blue. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The role of localization and receptor trafficking of TLRs (and particularly TLR4 as the best characterized TLR) was clarified from recent works [312,313]. However, the mechanisms of TLRs endocytosis and its exact role in cellular response to pro-inflammatory signals still require more studies for our understanding. Moreover, it may be cell-specific. Indeed, TLR4 endocytosis in macrophages is not required for their response. In contrast, astrocytes internalized activated TLR4 by both caveolae and clathrin endocytosis, and caveolar endocytosis is required for both MyD88- and TRIF-dependent signaling pathways [314], while epithelial cells are characterized by intracellular localization of TLR4 at Golgi apparatus and CD14-dependent trafficking of LPS via clathrin-dependent endocytosis [315,316]. Interestingly, the proinflammatory effects of LPS are significantly attenuated in caveolin-1 knockout mice. NF-κB activation by LPS is decreased; ICAM expression is lower as well as PMN accumulation in lungs, while NO level is higher in Cav-1−/− mice compared to wild type [317] suggesting an important role of caveolae in LPS-induced response in vivo. Indeed, caveolar targeting and delivery of SOD using PLVAP has a significant advantage compared to less specific delivery via PECAM and ICAM [133,318]. Caveolar targeting potentially can deliver SOD right to the signaling endosomes responsible for activation of NFκB signal transduction pathway.
6.4. Ischemia-reperfusion and acute oxidative stress
Endothelial involvement in oxidative mechanisms of inflammation and other conditions is not limited to mediation of pro-inflammatory signaling via endosomal pathways discussed above. Endothelial cells are common victims of ROS in ischemia-reperfusion injury, acute inflammation, and infections. ROS-quenching enzymes, catalase and SOD, conjugated with mAbs directed against several endothelial targets (PECAM, ICAM-1, ACE) improved protection in several models of acute oxidative stress compared to untargeted formulations [263,277,278,[319], [320], [321], [322], [323], [324], [325], [326], [327], [328], [329]].
PECAM- and ICAM-targeted catalase protected against H2O2-induced vascular leak [330], alleviated vascular [331] and oxidative stresses [332], and pulmonary ischemia-reperfusion injuries [278,321]. These results include lung transplantation in multiple animal models [324,325]. Targeting to PECAM and ICAM of SOD, liposomal SOD, and liposomal SOD/catalase mimetic, as well as inhibitors of ROS-producing enzymes, reduced ROS toxicities in endothelial cells [205,306,326], normalized vasoconstriction in mice [321], attenuated VEGF-induced endothelial leakage [330], and endotoxin-induced acute pulmonary inflammation in animals [333].
Pharmacological agents decelerating vesicular trafficking prolong endothelial antioxidant protection conferred by ICAM- and PECAM-targeted catalase and SOD via delaying their lysosomal degradation [106,152]. Targeting to PECAM of catalase loaded into nanocarriers selectively permeable for ROS provided prolonged protection [[334], [335], [336], [337]].
Overall, delivery of both antioxidants and anti-inflammatories to the endothelium provides a variety of mechanistically precise, spatiotemporally controlled protective effects in the variety of animal models emulating pathological pathways typical of acute human diseases and conditions associated with or driven by vascular oxidative stress, ischemia-reperfusion and inflammation.
7. Current challenges and perspectives
7.1. Modeling of vascular targeting
Many reasons drive attempts to minimize in vivo investigations by pursuing in vitro models, despite the fact that no in vitro system fully recapitulates the situation in vivo and most, if not all, lack effects of an organism on behavior of a drug or DDS systemically (PK, clearance, effect of pathology, as well as humoral, nervous and defense factors) and locally (cellular microenvironment, histological, and pathological factors). In case of vascular targeting, these challenges are aggravated by rapid phenotypic bastardization of cultivated endothelial cells, which quickly lose specific markers like ACE [338].
The introduction of HUVEC (human umbilical vein endothelial cells) as an in vitro endothelial model by Eric Jaffe, Michael Gimbrone, Jordan Pober and colleagues nearly half a century ago was a truly major breakthrough [339]. Further advances, such as adaptation of the Boyden's chamber to study endothelial permeability, upgrades of static cell cultures to flow-adapted and flow-exposed cell cultures, microfluidic systems and flow chambers with cells grown on semi-permeable supports, and recent advent of the “organ on the chip”, including exquisite 3D multichannel models of vascular segments have yielded significant results [91,272,[340], [341], [342], [343]].
In vitro models are useful to address specific questions, such as analysis of hydrodynamic parameters and effects of flow adaptation on binding, uptake, vesicular transport, and effects of DDS targeted to endothelium. Static 2D cultures are useful to determine specificity and other parameters of binding DDS to target vs. non-target cells (Kd, Bmax, kinetics of binding and dissociation).
Sometimes, there is no choice but to retreat to in vitro models, since our methods do not work in vivo. Thus, parameters of resolution, dynamics, objectivity, and attribution to histological and cellular structures of current methods afford rather limited information on DDS sub-cellular addressing and intracellular transport. Here, endothelial cell culture seems to be a reasonable resolution. More technically challenging models of perfusion of isolated vessels and organs provide a valuable resource, permitting real-time direct measurements of the uptake, tissue localization, and activity of a DDS in conditions maximally close to in vivo [39,319].
Computational modeling and systems biology represent further interesting in silico approaches. Major challenges in this area include variable compatibility and quality of the feed data, oversimplification of conditions, parameters, and margins, and relatively arbitrary selection of the focus and readouts. Yet, mutually enriching collaboration of computational researchers with experimentalists and physicians may enable modeling with high predictive value that allows for a reduction of in vivo experimentation [[344], [345], [346], [347]].
7.2. Characterization and analysis of PK/BD
A key consideration in development of a DDS is selection of appropriate methodologies for characterization of blood and tissue pharmacokinetics. An ideal bioassay would have the capacity to reliably quantify DDS in all relevant matrices (e.g. whole blood, plasma, tissues, etc.) in an unbiased and highly precise manner.
There are several methods that are often utilized to describe PK/biodistribution of DDS, each with their own unique strengths and weaknesses. Measurement of radioisotope-labeled DDS can be considered as the ‘gold standard’ for assessment of blood and tissue PK [348,349]. Radioisotope-based measurements do not require tissue processing, have no background signal, and can be directly related to the injected dose, allowing calculation of key PK parameters with no intrinsic bias. However, a key drawback of radioisotope labeling is the potential introduction of artifacts due to the labeling process, including, but not limited to, changes in PK due to introduction of the label and loss of the label from the DDS. By labeling and tracing multiple components of the DDS, these artifacts can be minimized.
A second approach that is often reported in the literature is optical tracing of fluorescent components of a DDS. While fluorescent labels are often useful for visualizing distribution on a sub-tissue level, the presence of high (and variable) background signals in biological matrices and non-linear signal vs. concentration relationships reduces their utility in measuring PK/BD.
Finally, mass spectrometry (MS) is being used frequently for PK measurements in both preclinical and clinical investigations [[350], [351], [352]]. Due to the complex nature of many DDS, it would be challenging to validate an assay for direct measurement of unlabeled DDS. However, MS should be considered as the gold standard for assessment of PK/BD of small molecule cargo drugs, as it is able to make highly precise measurements in both blood and tissues.
Collection of high quality PK/BD data permits development of mathematical models that can be used to guide further development and optimization of DDS. The types of models that can be developed range in terms of complexity and degree of mechanistic information, from empirical models that simply describe the concentration vs. time curve, to physiologically-based models that are able to predict drug behavior. At the preclinical academic level, more mechanistic models are often useful as tools to predict the impact of DDS properties and pathology on behavior, permitting optimization of a delivery strategy early in development.
Additionally, physiologically-based models have the potential for relatively straightforward scaling to higher species, permitting prediction of clinical PK/PD profiles [[353], [354], [355]]. Once a DDS moves into the clinical domain, modeling often shifts to simpler models, with the goal of identifying patient-specific factors, termed covariates that may impact drug behavior and patient outcomes. This application of population PK is then used to identify special patient populations (e.g. renal failure, pediatrics, obesity, etc.) that may require dose adjustments from the ‘standard’ dose [356,357].
7.3. Benefit/risk ratio appraisal: Omnes viae Romam ducunt
Ultimately, preclinical studies culminate in analysis of the beneficial vs. unintended and adverse effects of the devised DDS in animal models. Admittedly, no animal model fully recapitulates human diseases, but, as physicians know, no individual patient's condition fully recapitulates another patient's condition. Of course, the closer to human the species is and the closer the pathogenesis is to the relevant human condition, the greater the confidence in the translatability of results.
Parameters of the amplitude, timing of the onset, and duration of the desirable vs. unintended effects are the key readouts deciding the fate of a DDS. Statistics are necessary, but not always sufficient to prove the therapeutic significance. The rigor and scrutiny of characterization of DDS effects are painstaking. These studies, performed in healthy and sick animals in an obligatory double-blinded manner, must include direct comparison of experimental groups with controls including healthy and sick animals treated with: A) Untargeted agents - free and loaded in untargeted DDS; B) A mixture of targeted DDS and free drug; C) Drugless targeted and untargeted DDS. Not all, but many studies appraising benefit/risk ratio of vascular targeting in fact meet these draconian criteria, giving a certain level of comfort and confidence that this drug delivery approach will inevitably reach the clinical utility.
8. Conclusions
The wealth of knowledge garnered in three decades of studies of vascular targeting is enormous. These studies yielded diverse endothelial target molecules for drug delivery, multitudes of ligands, nanocarriers, and methodologies for their conjugation and assembly. These means provide specific and effective targeting of drugs to, into and across of the endothelia in several important organs and vascular areas of significant biomedical interest. It seems safe to state that the general progress in this area of the drug delivery field is impressive, encouraging and brings us to the brink of industrial development and clinical translation.
These studies have also advanced our understanding of physiological and pathological processes localized in or involving endothelial interface, especially in the lungs and brain. Targeted pharmacological interventions designed to yield functional responses in the specific components and compartments of vascular system and blood represent valuable investigational tools. Indeed, both positive and negative outcomes of such interventions may give mechanistic insight about the role of implicated molecules and cells, on the sine qua none condition that delivery, localization and activity of the targeted pharmacological agent are confirmed “beyond any reasonable doubt”, and therefore, the lack of the effect is attributable to the intricacies of the pathophysiological mechanism, not failure of delivery.
Notwithstanding, the main biomedical purpose of drug delivery is, of course, advancement of the treatment of patients - diagnostic, prophylaxis and therapy. The pressure to come up with the winning formulations is palpable, but it would be a mistake to put on the back burner, suspend or abandon devising and research of new DDS, especially in academia, for the sake of concentrating efforts on the industrial development and clinical testing of DDS showing favorable profile in experimental settings. It is unpredictable which specific DDS iterations will emerge as new therapeutic options. Yet, focus on promising candidates seems timely and necessary. Selection of these lead candidates is a complex and multifaceted interdisciplinary affair that must involve basic scientists, clinical investigators, industrial, and regulatory counterparts.
Acknowledgements
Funding: This work was supported by the National Institutes of Health [grant numbers 1R01HL126874-01A1 (VRM), 1R01HL125462-02 (VRM), 1R01HL128398-02 (VRM), 1R01HL143806-01 (VRM), 5T32HL007586-34 (LTF), and 5T32HL007971-19 (RYK)].
References
- 1.Wolinsky H. A proposal linking clearance of circulating lipoproteins to tissue metabolic activity as a basis for understanding atherogenesis. Circ. Res. 1980;47:301–311. doi: 10.1161/01.res.47.3.301. [DOI] [PubMed] [Google Scholar]
- 2.Aird W.C. Endothelium and haemostasis. Hamostaseologie. 2015;35:11–16. doi: 10.5482/HAMO-14-11-0075. [DOI] [PubMed] [Google Scholar]
- 3.Molema G., Aird W.C. Vascular heterogeneity in the kidney. Semin. Nephrol. 2012;32:145–155. doi: 10.1016/j.semnephrol.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Aird W.C. Phenotypic heterogeneity of the endothelium: I, Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 5.Chow B.W., Nunez V., Kaplan L., Granger A.J., Bistrong K., Zucker H.L., Kumar P., Sabatini B.L., Gu C. Caveolae in CNS arterioles mediate neurovascular coupling. Nature. 2020;579:106–110. doi: 10.1038/s41586-020-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntosh D.P., Tan X.Y., Oh P., Schnitzer J.E. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cybulsky M.I., Gimbrone M.A., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 8.Kiseleva R.Y., Glassman P.M., Greineder C.F., Hood E.D., Shuvaev V.V., Muzykantov V.R. Targeting therapeutics to endothelium: are we there yet? Drug Deliv Transl Res. 2018;8:883–902. doi: 10.1007/s13346-017-0464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai Y., Akita H., Harashima H. Targeting tumor endothelial cells with nanoparticles. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20235819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farshbaf M., Davaran S., Zarebkohan A., Annabi N., Akbarzadeh A., Salehi R. Significant role of cationic polymers in drug delivery systems. Artif Cells Nanomed Biotechnol. 2018;46:1872–1891. doi: 10.1080/21691401.2017.1395344. [DOI] [PubMed] [Google Scholar]
- 11.Kolodgie F.D., Petrov A., Virmani R., Narula N., Verjans J.W., Weber D.K., Hartung D., Steinmetz N., Vanderheyden J.L., Vannan M.A., Gold H.K., Reutelingsperger C.P., Hofstra L., Narula J. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation. 2003;108:3134–3139. doi: 10.1161/01.CIR.0000105761.00573.50. [DOI] [PubMed] [Google Scholar]
- 12.Akinc A., Querbes W., De S., Qin J., Frank-Kamenetsky M., Jayaprakash K.N., Jayaraman M., Rajeev K.G., Cantley W.L., Dorkin J.R., Butler J.S., Qin L., Racie T., Sprague A., Fava E., Zeigerer A., Hope M.J., Zerial M., Sah D.W., Fitzgerald K., Tracy M.A., Manoharan M., Koteliansky V., Fougerolles A., Maier M.A. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D., Hu Q., Song Y.K. Liposome clearance from blood: different animal species have different mechanisms. Biochim. Biophys. Acta. 1995;1240:277–284. doi: 10.1016/0005-2736(95)00184-0. [DOI] [PubMed] [Google Scholar]
- 14.Anselmo A.C., Gupta V., Zern B.J., Pan D., Zakrewsky M., Muzykantov V., Mitragotri S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7:11129–11137. doi: 10.1021/nn404853z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naumenko V., Nikitin A., Garanina A., Melnikov P., Vodopyanov S., Kapitanova K., Potashnikova D., Vishnevskiy D., Alieva I., Ilyasov A., Eletskaya B.Z., Abakumov M., Chekhonin V., Majouga A. Neutrophil-mediated transport is crucial for delivery of short-circulating magnetic nanoparticles to tumors. Acta Biomater. 2020;104:176–187. doi: 10.1016/j.actbio.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Hao J., Chen J., Wang M., Zhao J., Wang J., Wang X., Li Y., Tang H. Neutrophils, as "Trojan horses", participate in the delivery of therapeutical PLGA nanoparticles into a tumor based on the chemotactic effect. Drug Deliv. 2020;27:1–14. doi: 10.1080/10717544.2019.1701141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y., Love K.T., Dorkin J.R., Sirirungruang S., Zhang Y., Chen D., Bogorad R.L., Yin H., Chen Y., Vegas A.J., Alabi C.A., Sahay G., Olejnik K.T., Wang W., Schroeder A., Lytton-Jean A.K., Siegwart D.J., Akinc A., Barnes C., Barros S.A., Carioto M., Fitzgerald K., Hettinger J., Kumar V., Novobrantseva T.I., Qin J., Querbes W., Koteliansky V., Langer R., Anderson D.G. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci U S A. 2014;111:3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guimaraes P.P.G., Zhang R., Spektor R., Tan M., Chung A., Billingsley M.M., El-Mayta R., Riley R.S., Wang L., Wilson J.M., Mitchell M.J. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J. Control. Release. 2019;316:404–417. doi: 10.1016/j.jconrel.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajotte D., Arap W., Hagedorn M., Koivunen E., Pasqualini R., Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J. Clin. Invest. 1998;102:430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danhier F., Feron O., Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Muzykantov V.R. Immunotargeting of drugs to the pulmonary vascular endothelium as a therapeutic strategy. Pathophysiology. 1998;5:15–33. [Google Scholar]
- 22.Oh P., Li Y., Yu J., Durr E., Krasinska K.M., Carver L.A., Testa J.E., Schnitzer J.E. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 23.Schnitzer J.E. Vascular targeting as a strategy for cancer therapy. N. Engl. J. Med. 1998;339:472–474. doi: 10.1056/NEJM199808133390711. [DOI] [PubMed] [Google Scholar]
- 24.Kennel S.J., Lee R., Bultman S., Kabalka G. Rat monoclonal antibody distribution in mice: an epitope inside the lung vascular space mediates very efficient localization. Int J Rad Appl Instrum B. 1990;17:193–200. doi: 10.1016/0883-2897(90)90147-s. [DOI] [PubMed] [Google Scholar]
- 25.Spragg D.D., Alford D.R., Greferath R., Larsen C.E., Lee K.D., Gurtner G.C., Cybulsky M.I., Tosi P.F., Nicolau C., Gimbrone M.A., Jr. Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8795–8800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang R.B., Mocherla S., Heslinga M.J., Charoenphol P., Eniola-Adefeso O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery. Mol. Membr. Biol. 2010;27:312–327. doi: 10.3109/09687688.2010.522117. [DOI] [PubMed] [Google Scholar]
- 27.Corti A., Pastorino F., Curnis F., Arap W., Ponzoni M., Pasqualini R. Targeted drug delivery and penetration into solid tumors. Med. Res. Rev. 2012;32:1078–1091. doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- 28.Wickline S.A., Neubauer A.M., Winter P.M., Caruthers S.D., Lanza G.M. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J. Magn. Reson. Imaging. 2007;25:667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 29.Noble C.O., Kirpotin D.B., Hayes M.E., Mamot C., Hong K., Park J.W., Benz C.C., Marks J.D., Drummond D.C. Development of ligand-targeted liposomes for cancer therapy. Expert Opin. Ther. Targets. 2004;8:335–353. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- 30.Xia W., Low P.S. Folate-targeted therapies for cancer. J. Med. Chem. 2010;53:6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 31.Hughes B.J., Kennel S., Lee R., Huang L. Monoclonal antibody targeting of liposomes to mouse lung in vivo. Cancer Res. 1989;49:6214–6220. [PubMed] [Google Scholar]
- 32.Maruyama K., Kennel S.J., Huang L. Lipid composition is important for highly efficient target binding and retention of immunoliposomes. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5744–5748. doi: 10.1073/pnas.87.15.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin F.A., Murphy R.P., Cummins P.M. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1585–H1597. doi: 10.1152/ajpheart.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esmon C.T. Inflammation and the activated protein C anticoagulant pathway. Semin. Thromb. Hemost. 2006;32(Suppl. 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 35.Brisson C., Archipoff G., Hartmann M.L., Hanau D., Beretz A., Freyssinet J.M., Cazenave J.P. Antibodies to thrombomodulin induce receptor-mediated endocytosis in human saphenous vein endothelial cells. Thromb. Haemost. 1992;68:737–743. [PubMed] [Google Scholar]
- 36.Guermazi S., Mellouli F., Trabelsi S., Bejaoui M., Dellagi K. Anti-thrombomodulin antibodies and venous thrombosis. Blood Coagul. Fibrinolysis. 2004;15:553–558. doi: 10.1097/00001721-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Balyasnikova I.V., Metzger R., Visintine D.J., Dimasius V., Sun Z.L., Berestetskaya Y.V., McDonald T.D., Curiel D.T., Minshall R.D., Danilov S.M. Selective rat lung endothelial targeting with a new set of monoclonal antibodies to angiotensin I-converting enzyme. Pulm. Pharmacol. Ther. 2005;18:251–267. doi: 10.1016/j.pupt.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Balyasnikova I.V., Sun Z.L., Metzger R., Taylor P.R., Vicini E., Muciaccia B., Visintine D.J., Berestetskaya Y.V., McDonald T.D., Danilov S.M. Monoclonal antibodies to native mouse angiotensin-converting enzyme (CD143): ACE expression quantification, lung endothelial cell targeting and gene delivery. Tissue Antigens. 2006;67:10–29. doi: 10.1111/j.1399-0039.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 39.Danilov S.M., Gavrilyuk V.D., Franke F.E., Pauls K., Harshaw D.W., McDonald T.D., Miletich D.J., Muzykantov V.R. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1335–L1347. doi: 10.1152/ajplung.2001.280.6.L1335. [DOI] [PubMed] [Google Scholar]
- 40.Danilov S.M., Martynov A.V., Klibanov A.L., Slinkin M.A., Sakharov I., Malov A.G., Sergienko V.B., Vedernikov A., Muzykantov V.R., Torchilin V.P. Radioimmunoimaging of lung vessels: an approach using indium-111-labeled monoclonal antibody to angiotensin-converting enzyme. J. Nucl. Med. 1989;30:1686–1692. [PubMed] [Google Scholar]
- 41.Danilov S.M., Muzykantov V.R., Martynov A.V., Atochina E.N., Sakharov I., Trakht I.N., Smirnov V.N. Lung is the target organ for a monoclonal antibody to angiotensin-converting enzyme. Lab. Investig. 1991;64:118–124. [PubMed] [Google Scholar]
- 42.Erdos E.G. Angiotensin I converting enzyme and the changes in our concepts through the years. Lewis K. Dahl memorial lecture. Hypertension. 1990;16:363–370. doi: 10.1161/01.hyp.16.4.363. [DOI] [PubMed] [Google Scholar]
- 43.Heitsch H., Brovkovych S., Malinski T., Wiemer G. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 44.Muzykantov V.R., Danilov S.M. Targeting of radiolabeled monoclonal antibody against ACE to the pulmonary endothelium. In: Torchilin V., editor. Targeted Delivery of Imaging Agents. CRC Press; Roca Baton, Florida: 1995. pp. 465–485. [Google Scholar]
- 45.Reynolds P.N., Nicklin S.A., Kaliberova L., Boatman B.G., Grizzle W.E., Balyasnikova I.V., Baker A.H., Danilov S.M., Curiel D.T. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat. Biotechnol. 2001;19:838–842. doi: 10.1038/nbt0901-838. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds P.N., Zinn K.R., Gavrilyuk V.D., Balyasnikova I.V., Rogers B.E., Buchsbaum D.J., Wang M.H., Miletich D.J., Grizzle W.E., Douglas J.T., Danilov S.M., Curiel D.T. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol. Ther. 2000;2:562–578. doi: 10.1006/mthe.2000.0205. [DOI] [PubMed] [Google Scholar]
- 47.Danilov S., Atochina E., Hiemisch H., Churak-ova T., Moldobayeva A., Sakharov I., Deichman G., Ryan U., Muzykantov V.R. Interaction of mAb to angiotensin-converting enzyme (ACE) with antigen in vitro and in vivo: antibody targeting to the lung induces ACE antigenic modulation. Int. Immunol. 1994;6:1153–1160. doi: 10.1093/intimm/6.8.1153. [DOI] [PubMed] [Google Scholar]
- 48.Balyasnikova I.V., Karran E.H., Albrecht R.F., 2nd, Danilov S.M. Epitope-specific antibody-induced cleavage of angiotensin-converting enzyme from the cell surface. Biochem. J. 2002;362:585–595. doi: 10.1042/0264-6021:3620585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christofidou-Solomidou M., Kennel S., Scherpereel A., Wiewrodt R., Solomides C.C., Pietra G.G., Murciano J.C., Shah S.A., Ischiropoulos H., Albelda S.M., Muzykantov V.R. Vascular immunotargeting of glucose oxidase to the endothelial antigens induces distinct forms of oxidant acute lung injury: targeting to thrombomodulin, but not to PECAM-1, causes pulmonary thrombosis and neutrophil transmigration. Am. J. Pathol. 2002;160:1155–1169. doi: 10.1016/S0002-9440(10)64935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muzykantov V.R. Targeting of superoxide dismutase and catalase to vascular endothelium. J. Control. Release. 2001;71:1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 51.Miller W.H., Brosnan M.J., Graham D., Nicol C.G., Morecroft I., Channon K.M., Danilov S.M., Reynolds P.N., Baker A.H., Dominiczak A.F. Targeting endothelial cells with adenovirus expressing nitric oxide synthase prevents elevation of blood pressure in stroke-prone spontaneously hypertensive rats. Mol. Ther. 2005;12:321–327. doi: 10.1016/j.ymthe.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds A.M., Xia W., Holmes M.D., Hodge S.J., Danilov S., Curiel D.T., Morrell N.W., Reynolds P.N. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1182–L1192. doi: 10.1152/ajplung.00020.2006. [DOI] [PubMed] [Google Scholar]
- 53.Shuvaev V.V., Christofidou-Solomidou M., Scherpereel A., Simone E., Arguiri E., Tliba S., Pick J., Kennel S., Albelda S.M., Muzykantov V.R. Factors modulating the delivery and effect of enzymatic cargo conjugated with antibodies targeted to the pulmonary endothelium. J. Control. Release. 2007;118:235–244. doi: 10.1016/j.jconrel.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perkowski S., Sun J., Singhal S., Santiago J., Leikauf G.D., Albelda S.M. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am. J. Respir. Cell Mol. Biol. 2003;28:682–696. doi: 10.1165/rcmb.4692. [DOI] [PubMed] [Google Scholar]
- 55.Beneteau-Burnat B., Baudin B. Angiotensin-converting enzyme: clinical applications and laboratory investigations on serum and other biological fluids. Crit. Rev. Clin. Lab. Sci. 1991;28:337–356. doi: 10.3109/10408369109106868. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe K., Lam G., Keresztes R.S., Jaffe E.A. Lipopolysaccharides decrease angiotensin converting enzyme activity expressed by cultured human endothelial cells. J. Cell. Physiol. 1992;150:433–439. doi: 10.1002/jcp.1041500228. [DOI] [PubMed] [Google Scholar]
- 57.Atochina E.N., Hiemisch H.H., Muzykantov V.R., Danilov S.M. Systemic administration of platelet-activating factor in rat reduces specific pulmonary uptake of circulating monoclonal antibody to angiotensin-converting enzyme. Lung. 1992;170:349–358. doi: 10.1007/BF00177581. [DOI] [PubMed] [Google Scholar]
- 58.Muzykantov V.R., Puchnina E.A., Atochina E.N., Hiemish H., Slinkin M.A., Meertsuk F.E., Danilov S.M. Endotoxin reduces specific pulmonary uptake of radiolabeled monoclonal antibody to angiotensin-converting enzyme. J. Nucl. Med. 1991;32:453–460. [PubMed] [Google Scholar]
- 59.Atochina E.N., Muzykantov V.R., Al-Mehdi A.B., Danilov S.M., Fisher A.B. Normoxic lung ischemia/reperfusion accelerates shedding of angiotensin converting enzyme from the pulmonary endothelium. Am. J. Respir. Crit. Care Med. 1997;156:1114–1119. doi: 10.1164/ajrccm.156.4.96-12116. [DOI] [PubMed] [Google Scholar]
- 60.Danilov S.M., Wade M.S., Schwager S.L., Douglas R.G., Nesterovitch A.B., Popova I.A., Hogarth K.D., Bhardwaj N., Schwartz D.E., Sturrock E.D., Garcia J.G. A novel angiotensin I-converting enzyme mutation (S333W) impairs N-domain enzymatic cleavage of the anti-fibrotic peptide, AcSDKP. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christiaans S.C., Wagener B.M., Esmon C.T., Pittet J.F. Protein C and acute inflammation: a clinical and biological perspective. Am J Physiol Lung Cell Mol Physiol. 2013;305:L455–L466. doi: 10.1152/ajplung.00093.2013. [DOI] [PubMed] [Google Scholar]
- 62.Lipowsky H.H., Lescanic A. Inhibition of inflammation induced shedding of the endothelial glycocalyx with low molecular weight heparin. Microvasc. Res. 2017;112:72–78. doi: 10.1016/j.mvr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lipowsky H.H. Relative shedding of glycosaminoglycans from the endothelial glycocalyx during inflammation and their contribution to stiffness of the glycocalyx. Biorheology. 2019;56:191–205. doi: 10.3233/BIR-190225. [DOI] [PubMed] [Google Scholar]
- 64.Carnemolla R., Greineder C.F., Chacko A.M., Patel K.R., Ding B.S., Zaitsev S., Esmon C.T., Muzykantov V.R. Platelet endothelial cell adhesion molecule targeted oxidant-resistant mutant thrombomodulin fusion protein with enhanced potency in vitro and in vivo. J. Pharmacol. Exp. Ther. 2013;347:339–345. doi: 10.1124/jpet.113.205104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muro S., Muzykantov V.R. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr. Pharm. Des. 2005;11:2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 66.Koning G.A., Schiffelers R.M., Storm G. Endothelial cells at inflammatory sites as target for therapeutic intervention. Endothelium. 2002;9:161–171. doi: 10.1080/10623320213631. [DOI] [PubMed] [Google Scholar]
- 67.Kiely J.M., Cybulsky M.I., Luscinskas F.W., Gimbrone M.A., Jr. Immunoselective targeting of an anti-thrombin agent to the surface of cytokine-activated vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1995;15:1211–1218. doi: 10.1161/01.atv.15.8.1211. [DOI] [PubMed] [Google Scholar]
- 68.Keelan E.T., Harrison A.A., Chapman P.T., Binns R.M., Peters A.M., Haskard D.O. Imaging vascular endothelial activation: an approach using radiolabeled monoclonal antibodies against the endothelial cell adhesion molecule E-selectin. J. Nucl. Med. 1994;35:276–281. [PubMed] [Google Scholar]
- 69.Huang X., Molema G., King S., Watkins L., Edgington T.S., Thorpe P.E. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science. 1997;275:547–550. doi: 10.1126/science.275.5299.547. [DOI] [PubMed] [Google Scholar]
- 70.Muro S., Koval M., Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Curr. Vasc. Pharmacol. 2004;2:281–299. doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- 71.Springer T.A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 72.Doerschuk C.M., Quinlan W.M., Doyle N.A., Bullard D.C., Vestweber D., Jones M.L., Takei F., Ward P.A., Beaudet A.L. The role of P-selectin and ICAM-1 in acute lung injury as determined using blocking antibodies and mutant mice. J. Immunol. 1996;157:4609–4614. [PubMed] [Google Scholar]
- 73.Kishimoto T.K., Rothlein R. Integrins, ICAMs, and selectins: role and regulation of adhesion molecules in neutrophil recruitment to inflammatory sites. Adv Pharmacol. 1994;25:117–169. doi: 10.1016/s1054-3589(08)60431-7. [DOI] [PubMed] [Google Scholar]
- 74.Albelda S.M. Endothelial and epithelial cell adhesion molecules. Am. J. Respir. Cell Mol. Biol. 1991;4:195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 75.Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front. Biosci. 2006;11:59–80. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- 76.Langer H.F., Chavakis T. Leukocyte-endothelial interactions in inflammation. J. Cell. Mol. Med. 2009;13:1211–1220. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reglero-Real N., Colom B., Bodkin J.V., Nourshargh S. Endothelial cell junctional adhesion molecules: role and regulation of expression in inflammation. Arterioscler. Thromb. Vasc. Biol. 2016;36:2048–2057. doi: 10.1161/ATVBAHA.116.307610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daiber A., Steven S., Weber A., Shuvaev V.V., Muzykantov V.R., Laher I., Li H., Lamas S., Munzel T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017;174:1591–1619. doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ince C., Mayeux P.R., Nguyen T., Gomez H., Kellum J.A., Ospina-Tascon G.A., Hernandez G., Murray P., De Backer D., Workgroup A.X. The endothelium in Sepsis. Shock. 2016;45:259–270. doi: 10.1097/SHK.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., Nishigaki I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gustafson D., Raju S., Wu R., Ching C., Veitch S., Rathnakumar K., Boudreau E., Howe K.L., Fish J.E. Overcoming Barriers: The Endothelium As a Linchpin of Coronavirus Disease 2019 Pathogenesis? Arterioscler Thromb Vasc Biol. 2020 doi: 10.1161/ATVBAHA.120.314558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindner J.R., Song J., Christiansen J., Klibanov A.L., Xu F., Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104:2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 85.Lindner J.R., Klibanov A.L., Ley K. Targeting inflammation. In: Muzykantov V.R., Torchilin V.P., editors. Biomedical Aspects of Drug Targeting. Kluwer Academic Pub; Boston: 2003. pp. 149–172. [Google Scholar]
- 86.Tsourkas A., Shinde-Patil V.R., Kelly K.A., Patel P., Wolley A., Allport J.R., Weissleder R. In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug. Chem. 2005;16:576–581. doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 87.Marcos-Contreras O.A., Greineder C.F., Kiseleva R.Y., Parhiz H., Walsh L.R., Zuluaga-Ramirez V., Myerson J.W., Hood E.D., Villa C.H., Tombacz I., Pardi N., Seliga A., Mui B.L., Tam Y.K., Glassman P.M., Shuvaev V.V., Nong J., Brenner J.S., Khoshnejad M., Madden T., Weissmann D., Persidsky Y., Muzykantov V.R. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 2020;117:3405–3414. doi: 10.1073/pnas.1912012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samuel S.P., Jain N., O’Dowd F., Paul T., Kashanin D., Gerard V.A., Gun’ko Y.K., Prina-Mello A., Volkov Y. Multifactorial determinants that govern nanoparticle uptake by human endothelial cells under flow. Int. J. Nanomedicine. 2012;7:2943–2956. doi: 10.2147/IJN.S30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terrisse A.D., Puech N., Allart S., Gourdy P., Xuereb J.M., Payrastre B., Sie P. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J. Thromb. Haemost. 2010;8:2810–2819. doi: 10.1111/j.1538-7836.2010.04088.x. [DOI] [PubMed] [Google Scholar]
- 90.Han J., Zern B.J., Shuvaev V.V., Davies P.F., Muro S., Muzykantov V. Acute and chronic shear stress differently regulate endothelial internalization of Nanocarriers targeted to platelet-endothelial cell adhesion Molecule-1. ACS Nano. 2012;6:8824–8836. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhowmick T., Berk E., Cui X., Muzykantov V.R., Muro S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J. Control. Release. 2012;157:485–492. doi: 10.1016/j.jconrel.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newman P.J., Albelda S.M. Cellular and molecular aspects of PECAM-1. Nouv. Rev. Fr. Hematol. 1992;34(Suppl):S9–13. [PubMed] [Google Scholar]
- 93.Giannotta M., Trani M., Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 94.Barreiro O., Yanez-Mo M., Sala-Valdes M., Gutierrez-Lopez M.D., Ovalle S., Higginbottom A., Monk P.N., Cabanas C., Sanchez-Madrid F. Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood. 2005;105:2852–2861. doi: 10.1182/blood-2004-09-3606. [DOI] [PubMed] [Google Scholar]
- 95.Cook-Mills J.M., Marchese M.E., Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011;15:1607–1638. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Almenar-Queralt A., Duperray A., Miles L.A., Felez J., Altieri D.C. Apical topography and modulation of ICAM-1 expression on activated endothelium. Am. J. Pathol. 1995;147:1278–1288. [PMC free article] [PubMed] [Google Scholar]
- 97.Wright M.D., Moseley G.W., van Spriel A.B. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens. 2004;64:533–542. doi: 10.1111/j.1399-0039.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 98.Ghitescu L., Jacobson B.S., Crine P. A novel, 85 kDa endothelial antigen differentiates plasma membrane macrodomains in lung alveolar capillaries. Endothelium. 1999;6:241–250. doi: 10.3109/10623329909053414. [DOI] [PubMed] [Google Scholar]
- 99.Murciano J.C., Harshaw D.W., Ghitescu L., Danilov S.M., Muzykantov V.R. Vascular immunotargeting to endothelial surface in a specific macrodomain in alveolar capillaries. Am. J. Respir. Crit. Care Med. 2001;164:1295–1302. doi: 10.1164/ajrccm.164.7.2010076. [DOI] [PubMed] [Google Scholar]
- 100.Kuijpers T.W., Raleigh M., Kavanagh T., Janssen H., Calafat J., Roos D., Harlan J.M. Cytokine-activated endothelial cells internalize E-selectin into a lysosomal compartment of vesiculotubular shape. A tubulin-driven process. J. Immunol. 1994;152:5060–5069. [PubMed] [Google Scholar]
- 101.Straley K.S., Green S.A. Rapid transport of internalized P-selectin to late endosomes and the TGN: roles in regulating cell surface expression and recycling to secretory granules. J. Cell Biol. 2000;151:107–116. doi: 10.1083/jcb.151.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.von Asmuth E.J., Smeets E.F., Ginsel L.A., Onderwater J.J., Leeuwenberg J.F., Buurman W.A. Evidence for endocytosis of E-selectin in human endothelial cells. Eur. J. Immunol. 1992;22:2519–2526. doi: 10.1002/eji.1830221009. [DOI] [PubMed] [Google Scholar]
- 103.Kessner S., Krause A., Rothe U., Bendas G. Investigation of the cellular uptake of E-selectin-targeted immunoliposomes by activated human endothelial cells. Biochim. Biophys. Acta. 2001;1514:177–190. doi: 10.1016/s0005-2736(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 104.Everts M., Kok R.J., Asgeirsdottir S.A., Melgert B.N., Moolenaar T.J., Koning G.A., van Luyn M.J., Meijer D.K., Molema G. Selective intracellular delivery of dexamethasone into activated endothelial cells using an E-selectin-directed immunoconjugate. J. Immunol. 2002;168:883–889. doi: 10.4049/jimmunol.168.2.883. [DOI] [PubMed] [Google Scholar]
- 105.Harari O.A., Wickham T.J., Stocker C.J., Kovesdi I., Segal D.M., Huehns T.Y., Sarraf C., Haskard D.O. Targeting an adenoviral gene vector to cytokine-activated vascular endothelium via E-selectin. Gene Ther. 1999;6:801–807. doi: 10.1038/sj.gt.3300898. [DOI] [PubMed] [Google Scholar]
- 106.Muro S., Gajewski C., Koval M., Muzykantov V.R. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105:650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 107.Ding B.S., Dziubla T., Shuvaev V.V., Muro S., Muzykantov V.R. Advanced drug delivery systems that target the vascular endothelium. Mol. Interv. 2006;6:98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 108.Muro S., Wiewrodt R., Thomas A., Koniaris L., Albelda S.M., Muzykantov V.R., Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J. Cell Sci. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 109.Howard M., Zern B.J., Anselmo A.C., Shuvaev V.V., Mitragotri S., Muzykantov V. Vascular targeting of nanocarriers: perplexing aspects of the seemingly straightforward paradigm. ACS Nano. 2014;8:4100–4132. doi: 10.1021/nn500136z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garnacho C., Albelda S.M., Muzykantov V.R., Muro S. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J. Control. Release. 2008;130:226–233. doi: 10.1016/j.jconrel.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mulivor A.W., Lipowsky H.H. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 112.Durr E., Yu J., Krasinska K.M., Carver L.A., Yates J.R., Testa J.E., Oh P., Schnitzer J.E. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat. Biotechnol. 2004;22:985–992. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- 113.Tradonsky A., Rubin T., Beck R., Ring B., Seitz R., Mair S. A search for reliable molecular markers of prognosis in prostate cancer: a study of 240 cases. Am. J. Clin. Pathol. 2012;137:918–930. doi: 10.1309/AJCPF3QWIG8FWXIH. [DOI] [PubMed] [Google Scholar]
- 114.Wood K.C., Azarin S.M., Arap W., Pasqualini R., Langer R., Hammond P.T. Tumor-targeted gene delivery using molecularly engineered hybrid polymers functionalized with a tumor-homing peptide. Bioconjug. Chem. 2008;19:403–405. doi: 10.1021/bc700408r. [DOI] [PubMed] [Google Scholar]
- 115.Valadon P., Garnett J.D., Testa J.E., Bauerle M., Oh P., Schnitzer J.E. Screening phage display libraries for organ-specific vascular immunotargeting in vivo. Proc. Natl. Acad. Sci. U. S. A. 2006;103:407–412. doi: 10.1073/pnas.0506938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oh P., Borgstrom P., Witkiewicz H., Li Y., Borgstrom B.J., Chrastina A., Iwata K., Zinn K.R., Baldwin R., Testa J.E., Schnitzer J.E. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotech. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Minshall R.D., Tiruppathi C., Vogel S.M., Niles W.D., Gilchrist A., Hamm H.E., Malik A.B. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J. Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vogel S.M., Easington C.R., Minshall R.D., Niles W.D., Tiruppathi C., Hollenberg S.M., Parrillo J.E., Malik A.B. Evidence of transcellular permeability pathway in microvessels. Microvasc. Res. 2001;61:87–101. doi: 10.1006/mvre.2000.2274. [DOI] [PubMed] [Google Scholar]
- 119.Riezman H., Woodman P.G., van Meer G., Marsh M. Molecular mechanisms of endocytosis. Cell. 1997;91:731–738. doi: 10.1016/s0092-8674(00)80461-4. [DOI] [PubMed] [Google Scholar]
- 120.Predescu D., Palade G.E. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am. J. Phys. 1993;265:H725–H733. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- 121.Stan R.V. Structure and function of endothelial caveolae. Microsc. Res. Tech. 2002;57:350–364. doi: 10.1002/jemt.10089. [DOI] [PubMed] [Google Scholar]
- 122.Minshall R.D., Tiruppathi C., Vogel S.M., Malik A.B. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem. Cell Biol. 2002;117:105–112. doi: 10.1007/s00418-001-0367-x. [DOI] [PubMed] [Google Scholar]
- 123.Schnitzer J.E. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv. Drug Deliv. Rev. 2001;49:265–280. doi: 10.1016/s0169-409x(01)00141-7. [DOI] [PubMed] [Google Scholar]
- 124.Schnitzer J.E., McIntosh D.P., Dvorak A.M., Liu J., Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- 125.John T.A., Vogel S.M., Tiruppathi C., Malik A.B., Minshall R.D. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284:L187–L196. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 126.Predescu D., Predescu S., Malik A.B. Transport of nitrated albumin across continuous vascular endothelium. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13932–13937. doi: 10.1073/pnas.212253499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parton R.G., Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 128.Simone E.A., Zern B.J., Chacko A.M., Mikitsh J.L., Blankemeyer E.R., Muro S., Stan R.V., Muzykantov V.R. Endothelial targeting of polymeric nanoparticles stably labeled with the PET imaging radioisotope iodine-124. Biomaterials. 2012;33:5406–5413. doi: 10.1016/j.biomaterials.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iversen T.G., Frerker N., Sandvig K. Uptake of ricinB-quantum dot nanoparticles by a macropinocytosis-like mechanism. J. Nanobiotechnol. 2012;10:33. doi: 10.1186/1477-3155-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Z., Tiruppathi C., Minshall R.D., Malik A.B. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano. 2009;3:4110–4116. doi: 10.1021/nn9012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Myerson J.W., Braender B., McPherson O., Glassman P.M., Kiseleva R.Y., Shuvaev V.V., Marcos-Contreras O., Grady M.E., Lee H.S., Greineder C.F., Stan R.V., Composto R.J., Eckmann D.M., Muzykantov V.R. Flexible nanoparticles reach sterically obscured endothelial targets inaccessible to rigid nanoparticles. Adv. Mater. 2018;30 doi: 10.1002/adma.201802373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khoshnejad M., Parhiz H., Shuvaev V.V., Dmochowski I.J., Muzykantov V.R. Ferritin-based drug delivery systems: hybrid nanocarriers for vascular immunotargeting. J. Control. Release. 2018;282:13–24. doi: 10.1016/j.jconrel.2018.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shuvaev V.V., Khoshnejad M., Pulsipher K.W., Kiseleva R.Y., Arguiri E., Cheung-Lau J.C., LeFort K.M., Christofidou-Solomidou M., Stan R.V., Dmochowski I.J., Muzykantov V.R. Spatially controlled assembly of affinity ligand and enzyme cargo enables targeting ferritin nanocarriers to caveolae. Biomaterials. 2018;185:348–359. doi: 10.1016/j.biomaterials.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khoshnejad M., Greineder C.F., Pulsipher K.W., Villa C.H., Altun B., Pan D.C., Tsourkas A., Dmochowski I.J., Muzykantov V.R. Ferritin Nanocages with biologically orthogonal conjugation for vascular targeting and imaging. Bioconjug. Chem. 2018;29:1209–1218. doi: 10.1021/acs.bioconjchem.8b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khoshnejad M., Shuvaev V.V., Pulsipher K.W., Dai C., Hood E.D., Arguiri E., Christofidou-Solomidou M., Dmochowski I.J., Greineder C.F., Muzykantov V.R. Vascular accessibility of endothelial targeted ferritin nanoparticles. Bioconjug. Chem. 2016;27:628–637. doi: 10.1021/acs.bioconjchem.5b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Christofidou-Solomidou M., Muzykantov V.R. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5:47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 137.Pasqualini R., Arap W., McDonald D.M. Probing the structural and molecular diversity of tumor vasculature. Trends Mol. Med. 2002;8:563–571. doi: 10.1016/s1471-4914(02)02429-2. [DOI] [PubMed] [Google Scholar]
- 138.Pasqualini R., McDonald D.M., Arap W. Vascular targeting and antigen presentation. Nat. Immunol. 2001;2:567–568. doi: 10.1038/89704. [DOI] [PubMed] [Google Scholar]
- 139.Tagliabue E., Centis F., Campiglio M., Mastroianni A., Martignone S., Pellegrini R., Casalini P., Lanzi C., Menard S., Colnaghi M.I. Selection of monoclonal antibodies which induce internalization and phosphorylation of p185HER2 and growth inhibition of cells with HER2/NEU gene amplification. Int. J. Cancer. 1991;47:933–937. doi: 10.1002/ijc.2910470625. [DOI] [PubMed] [Google Scholar]
- 140.Feero W.G., Li S., Rosenblatt J.D., Sirianni N., Morgan J.E., Partridge T.A., Huang L., Hoffman E.P. Selection and use of ligands for receptor-mediated gene delivery to myogenic cells. Gene Ther. 1997;4:664–674. doi: 10.1038/sj.gt.3300453. [DOI] [PubMed] [Google Scholar]
- 141.Zhou Y., Zhao L., Marks J.D. Selection and characterization of cell binding and internalizing phage antibodies. Arch. Biochem. Biophys. 2012;526:107–113. doi: 10.1016/j.abb.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou Y., Zou H., Zhang S., Marks J.D. Internalizing cancer antibodies from phage libraries selected on tumor cells and yeast-displayed tumor antigens. J. Mol. Biol. 2010;404:88–99. doi: 10.1016/j.jmb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heitner T., Moor A., Garrison J.L., Marks C., Hasan T., Marks J.D. Selection of cell binding and internalizing epidermal growth factor receptor antibodies from a phage display library. J. Immunol. Methods. 2001;248:17–30. doi: 10.1016/s0022-1759(00)00340-9. [DOI] [PubMed] [Google Scholar]
- 144.Kelly K.A., Allport J.R., Tsourkas A., Shinde-Patil V.R., Josephson L., Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ. Res. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 145.Muro S. Challenges in design and characterization of ligand-targeted drug delivery systems. J. Control. Release. 2012;164:125–137. doi: 10.1016/j.jconrel.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ricard I., Payet M.D., Dupuis G. VCAM-1 is internalized by a clathrin-related pathway in human endothelial cells but its alpha 4 beta 1 integrin counter-receptor remains associated with the plasma membrane in human T lymphocytes. Eur. J. Immunol. 1998;28:1708–1718. doi: 10.1002/(SICI)1521-4141(199805)28:05<1708::AID-IMMU1708>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 147.Hanover J.A., Willingham M.C., Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- 148.Cabezon I., Manich G., Martin-Venegas R., Camins A., Pelegri C., Vilaplana J. Trafficking of gold nanoparticles coated with the 8D3 anti-transferrin receptor antibody at the mouse blood-brain barrier. Mol. Pharm. 2015;12:4137–4145. doi: 10.1021/acs.molpharmaceut.5b00597. [DOI] [PubMed] [Google Scholar]
- 149.Carver L.A., Schnitzer J.E. Caveolae: mining little caves for new cancer targets. Nat. Rev. Cancer. 2003;3:571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 150.Manthe R.L., Loeck M., Bhowmick T., Solomon M., Muro S. Intertwined mechanisms define transport of anti-ICAM nanocarriers across the endothelium and brain delivery of a therapeutic enzyme. J. Control. Release. 2020;324:181–193. doi: 10.1016/j.jconrel.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Muro S., Cui X., Gajewski C., Murciano J.C., Muzykantov V.R., Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol. 2003;285:C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 152.Muro S., Mateescu M., Gajewski C., Robinson M., Muzykantov V.R., Koval M. Control of intracellular trafficking of ICAM-1-targeted nanocarriers by endothelial Na+/H+ exchanger proteins. Am J Physiol Lung Cell Mol Physiol. 2006;290:L809–L817. doi: 10.1152/ajplung.00311.2005. [DOI] [PubMed] [Google Scholar]
- 153.Muro S., Schuchman E.H., Muzykantov V.R. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis. Mol. Ther. 2006;13:135–141. doi: 10.1016/j.ymthe.2005.07.687. [DOI] [PubMed] [Google Scholar]
- 154.Papademetriou J., Garnacho C., Serrano D., Bhowmick T., Schuchman E.H., Muro S. Comparative binding, endocytosis, and biodistribution of antibodies and antibody-coated carriers for targeted delivery of lysosomal enzymes to ICAM-1 versus transferrin receptor. J. Inherit. Metab. Dis. 2012;36(3):467–477. doi: 10.1007/s10545-012-9534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Muro S., Garnacho C., Champion J.A., Leferovich J., Gajewski C., Schuchman E.H., Mitragotri S., Muzykantov V.R. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Muro S. New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders. Wiley Interdis. Rev. 2010;2:189–204. doi: 10.1002/wnan.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beutler E. Lysosomal storage diseases: natural history and ethical and economic aspects. Mol. Genet. Metab. 2006;88:208–215. doi: 10.1016/j.ymgme.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 158.Grabowski G.A. Delivery of lysosomal enzymes for therapeutic use: glucocerebrosidase as an example. Expert Opin Drug Deliv. 2006;3:771–782. doi: 10.1517/17425247.3.6.771. [DOI] [PubMed] [Google Scholar]
- 159.Du H., Levine M., Ganesa C., Witte D.P., Cole E.S., Grabowski G.A. The role of mannosylated enzyme and the mannose receptor in enzyme replacement therapy. Am. J. Hum. Genet. 2005;77:1061–1074. doi: 10.1086/498652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Desnick R.J., Schuchman E.H. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet. 2002;3:954–966. doi: 10.1038/nrg963. [DOI] [PubMed] [Google Scholar]
- 161.Miranda S.R., He X., Simonaro C.M., Gatt S., Dagan A., Desnick R.J., Schuchman E.H. Infusion of recombinant human acid sphingomyelinase into niemann-pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 2000;14:1988–1995. doi: 10.1096/fj.00-0014com. [DOI] [PubMed] [Google Scholar]
- 162.Garbade S.F., Zielonka M., Mechler K., Kolker S., Hoffmann G.F., Staufner C., Mengel E., Ries M. FDA orphan drug designations for lysosomal storage disorders - a cross-sectional analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bae J.S., Jang K.H., Schuchman T.R., Jin H.K. Comparative effects of recombinant acid sphingomyelinase administration by different routes in niemann-pick disease mice. Exp. Anim. 2004;53:417–421. doi: 10.1538/expanim.53.417. [DOI] [PubMed] [Google Scholar]
- 164.Muro S. VCAM-1 and ICAM-1. In: Aird W.C., editor. Endothelial Biomedicine. Cambridge University Press; 2007. pp. 1058–1070. [Google Scholar]
- 165.DeGraba T., Azhar S., Dignat-George F., Brown E., Boutiere B., Altarescu G., McCarron R., Schiffmann R. Profile of endothelial and leukocyte activation in Fabry patients. Ann. Neurol. 2000;47:229–233. [PubMed] [Google Scholar]
- 166.Garnacho C., Dhami R., Simone E., Dziubla T., Leferovich J., Schuchman E.H., Muzykantov V., Muro S. Delivery of acid sphingomyelinase in normal and niemann-pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J. Pharmacol. Exp. Ther. 2008;325:400–408. doi: 10.1124/jpet.107.133298. [DOI] [PubMed] [Google Scholar]
- 167.Hsu J., Serrano D., Bhowmick T., Kumar K., Shen Y., Kuo Y.C., Garnacho C., Muro S. Enhanced endothelial delivery and biochemical effects of α-galactosidase by ICAM-1-targeted nanocarriers for Fabry disease. J. Control. Release. 2011;149:323–331. doi: 10.1016/j.jconrel.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hsu J., Northrup L., Bhowmick T., Muro S. Enhanced delivery of α-glucosidase for Pompe disease by ICAM-1-targeted nanocarriers: comparative performance of a strategy for three distinct lysosomal storage disorders. Nanomedicine. 2012;8:731–739. doi: 10.1016/j.nano.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Solomon M., Muro S. Lysosomal enzyme replacement therapies: historical development, clinical outcomes, and future perspectives. Adv. Drug Deliv. Rev. 2017;118:109–134. doi: 10.1016/j.addr.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Serrano D., Bhowmick T., Chadha R., Garnacho C., Muro S. Intercellular adhesion molecule 1 engagement modulates sphingomyelinase and ceramide, supporting uptake of drug carriers by the vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2012;32:1178–1185. doi: 10.1161/ATVBAHA.111.244186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ansar M., Serrano D., Papademetriou I., Bhowmick T.K., Muro S. Biological functionalization of drug delivery carriers to bypass size restrictions of receptor-mediated endocytosis independently from receptor targeting. ACS Nano. 2013;7:10597–10611. doi: 10.1021/nn404719c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Omolola Eniola A., Hammer D.A. In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. Biomaterials. 2005;26:7136–7144. doi: 10.1016/j.biomaterials.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 173.Papademetriou I.T., Garnacho C., Schuchman E.H., Muro S. In vivo performance of polymer nanocarriers dually-targeted to epitopes of the same or different receptors. Biomaterials. 2013;34:3459–3466. doi: 10.1016/j.biomaterials.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun D., Nakao S., Xie F., Zandi S., Schering A., Hafezi-Moghadam A. Superior sensitivity of novel molecular imaging probe: simultaneously targeting two types of endothelial injury markers. FASEB J. 2010;24:1532–1540. doi: 10.1096/fj.09-148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eniola A.O., Willcox P.J., Hammer D.A. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys. J. 2003;85:2720–2731. doi: 10.1016/s0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McAteer M.A., Schneider J.E., Ali Z.A., Warrick N., Bursill C.A., von zur Muhlen C., Greaves D.R., Neubauer S., Channon K.M., Choudhury R.P. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28:77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weller G.E., Villanueva F.S., Tom E.M., Wagner W.R. Targeted ultrasound contrast agents: in vitro assessment of endothelial dysfunction and multi-targeting to ICAM-1 and sialyl Lewisx. Biotechnol. Bioeng. 2005;92:780–788. doi: 10.1002/bit.20625. [DOI] [PubMed] [Google Scholar]
- 178.Chacko A.M., Nayak M., Greineder C.F., Delisser H.M., Muzykantov V.R. Collaborative enhancement of antibody binding to distinct PECAM-1 epitopes modulates endothelial targeting. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Danilov S., Jaspard E., Churakova T., Towbin H., Savoie F., Wei L., Alhenc-Gelas F. Structure-function analysis of angiotensin I-converting enzyme using monoclonal antibodies. Selective inhibition of the amino-terminal active site. J. Biol. Chem. 1994;269:26806–26814. [PubMed] [Google Scholar]
- 180.Gordon K., Balyasnikova I.V., Nesterovitch A.B., Schwartz D.E., Sturrock E.D., Danilov S.M. Fine epitope mapping of monoclonal antibodies 9B9 and 3G8 to the N domain of angiotensin-converting enzyme (CD143) defines a region involved in regulating angiotensin-converting enzyme dimerization and shedding. Tissue Antigens. 2010;75:136–150. doi: 10.1111/j.1399-0039.2009.01416.x. [DOI] [PubMed] [Google Scholar]
- 181.Kiseleva R., Greineder C.F., Villa C.H., Hood E.D., Shuvaev V.V., Sun J., Chacko A.-M., Abraham V., DeLisser H.M., Muzykantov V.R. Mechanism of collaborative enhancement of binding of paired antibodies to distinct epitopes of platelet endothelial cell adhesion Molecule-1. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Newman P.J. The role of PECAM-1 in vascular cell biology. Ann. N. Y. Acad. Sci. 1994;714:165–174. doi: 10.1111/j.1749-6632.1994.tb12041.x. [DOI] [PubMed] [Google Scholar]
- 183.Kiseleva R.Y., Greineder C.F., Villa C.H., Marcos-Contreras O.A., Hood E.D., Shuvaev V.V., DeLisser H.M., Muzykantov V.R. Vascular endothelial effects of collaborative binding to platelet/endothelial cell adhesion molecule-1 (PECAM-1) Sci. Rep. 2018;8:1510. doi: 10.1038/s41598-018-20027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Greineder C.F., Brenza J.B., Carnemolla R., Zaitsev S., Hood E.D., Pan D.C., Ding B.S., Esmon C.T., Chacko A.M., Muzykantov V.R. Dual targeting of therapeutics to endothelial cells: collaborative enhancement of delivery and effect. FASEB J. 2015;29:3483–3492. doi: 10.1096/fj.15-271213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chacko A.M., Han J., Greineder C.F., Zern B.J., Mikitsh J.L., Nayak M., Menon D., Johnston I.H., Poncz M., Eckmann D.M., Davies P.F., Muzykantov V.R. Collaborative enhancement of endothelial targeting of Nanocarriers by modulating platelet-endothelial cell adhesion Molecule-1/CD31 epitope engagement. ACS Nano. 2015;9:6785–6793. doi: 10.1021/nn505672x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Elias D.R., Poloukhtine A., Popik V., Tsourkas A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine. 2013;9:194–201. doi: 10.1016/j.nano.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gu F., Zhang L., Teply B.A., Mann N., Wang A., Radovic-Moreno A.F., Langer R., Farokhzad O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fakhari A., Baoum A., Siahaan T.J., Le K.B., Berkland C. Controlling ligand surface density optimizes nanoparticle binding to ICAM-1. J. Pharm. Sci. 2011;100:1045–1056. doi: 10.1002/jps.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zern B.J., Chacko A.-M., Liu J., Greineder C.F., Blankemeyer E.R., Radhakrishnan R., Muzykantov V. Reduction of nanoparticle avidity enhances the selectivity of vascular targeting and PET detection of pulmonary inflammation. ACS Nano. 2013;7:2461–2469. doi: 10.1021/nn305773f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Decuzzi P., Pasqualini R., Arap W., Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm. Res. 2009;26:235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 191.Ma D., Tian S., Baryza J., Luft J.C., DeSimone J.M. Reductively responsive hydrogel nanoparticles with uniform size, shape, and Tunable composition for systemic siRNA delivery in vivo. Mol. Pharm. 2015;12(10):3518–3526. doi: 10.1021/acs.molpharmaceut.5b00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Myerson J.W., Anselmo A.C., Liu Y., Mitragotri S., Eckmann D.M., Muzykantov V.R. Non-affinity factors modulating vascular targeting of nano- and microcarriers. Adv. Drug Deliv. Rev. 2016;99:97–112. doi: 10.1016/j.addr.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Perry J.L., Kai M.P., Reuter K.G., Bowerman C., Christopher Luft J., DeSimone J.M. Calibration-quality cancer nanotherapeutics. Cancer Treat. Res. 2015;166:275–291. doi: 10.1007/978-3-319-16555-4_12. [DOI] [PubMed] [Google Scholar]
- 194.Roberts R.A., Eitas T.K., Byrne J.D., Johnson B.M., Short P.J., McKinnon K.P., Reisdorf S., Luft J.C., DeSimone J.M., Ting J.P. Towards programming immune tolerance through geometric manipulation of phosphatidylserine. Biomaterials. 2015;72:1–10. doi: 10.1016/j.biomaterials.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Anselmo A.C., Zhang M., Kumar S., Vogus D.R., Menegatti S., Helgeson M.E., Mitragotri S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano. 2015;9:3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 196.Mahmud A., Discher D.E. Lung vascular targeting through inhalation delivery: insight from filamentous viruses and other shapes. IUBMB Life. 2011;63:607–612. doi: 10.1002/iub.481. [DOI] [PubMed] [Google Scholar]
- 197.Oltra N.S., Nair P., Discher D.E. From stealthy polymersomes and filomicelles to “self” peptide-nanoparticles for cancer therapy. Annu Rev Chem Biomol Eng. 2014;5:281–299. doi: 10.1146/annurev-chembioeng-060713-040447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pillai J.D., Dunn S.S., Napier M.E., DeSimone J.M. Novel platforms for vascular carriers with controlled geometry. IUBMB Life. 2011;63:596–606. doi: 10.1002/iub.497. [DOI] [PubMed] [Google Scholar]
- 199.Choi H.S., Liu W., Misra P., Tanaka E., Zimmer J.P., Itty Ipe B., Bawendi M.G., Frangioni J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 201.Shuvaev V.V., Ilies M.A., Simone E., Zaitsev S., Kim Y., Cai S., Mahmud A., Dziubla T., Muro S., Discher D.E., Muzykantov V.R. Endothelial targeting of antibody-decorated polymeric Filomicelles. ACS Nano. 2011;5:6991–6999. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chen L.T. Microcirculation of the spleen: and open or closed circulation? Science. 1978;201:157–159. doi: 10.1126/science.663644. [DOI] [PubMed] [Google Scholar]
- 203.Slack J.D., Kanke M., Simmons G.H., DeLuca P.P. Acute hemodynamic effects and blood pool kinetics of polystyrene microspheres following intravenous administration. J. Pharm. Sci. 1981;70:660–664. doi: 10.1002/jps.2600700621. [DOI] [PubMed] [Google Scholar]
- 204.Illum L., Davis S.S. The targeting of drugs parenterally by use of microspheres. J. Parenter. Sci. Technol. 1982;36:242–248. [PubMed] [Google Scholar]
- 205.Howard M.D., Greineder C.F., Hood E.D., Muzykantov V.R. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J. Control. Release. 2014;177:34–41. doi: 10.1016/j.jconrel.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Anselmo A.C., Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. J. Control. Release. 2014;190:531–541. doi: 10.1016/j.jconrel.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu Y., Tan J., Thomas A., Ou-Yang D., Muzykantov V.R. The shape of things to come: importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012;3:181–194. doi: 10.4155/tde.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mitragotri S., Lahann J. Materials for drug delivery: innovative solutions to address complex biological hurdles. Adv. Mater. 2012;24:3717–3723. doi: 10.1002/adma.201202080. [DOI] [PubMed] [Google Scholar]
- 209.Shuvaev V.V., Ilies M.A., Simone E., Zaitsev S., Kim Y., Cai S., Mahmud A., Dziubla T., Muro S., Discher D.E., Muzykantov V.R. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano. 2011;5:6991–6999. doi: 10.1021/nn2015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wibroe P.P., Anselmo A.C., Nilsson P.H., Sarode A., Gupta V., Urbanics R., Szebeni J., Hunter A.C., Mitragotri S., Mollnes T.E., Moghimi S.M. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat. Nanotechnol. 2017;12:589–594. doi: 10.1038/nnano.2017.47. [DOI] [PubMed] [Google Scholar]
- 211.Sharma G., Valenta D.T., Altman Y., Harvey S., Xie H., Mitragotri S., Smith J.W. Polymer particle shape independently influences binding and internalization by macrophages. J. Control. Release. 2010;147:408–412. doi: 10.1016/j.jconrel.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Swaminathan T.N., Mukundakrishnan K., Hu H.H. Sedimentation of an ellipsoid inside an infinitely long tube at low and intermediate Reynolds numbers. J. Fluid Mech. 2006;551:357–385. [Google Scholar]
- 213.Shah S., Liu Y., Hu W., Gao J. Modeling particle shape-dependent dynamics in nanomedicine. J. Nanosci. Nanotechnol. 2011;11:919–928. doi: 10.1166/jnn.2011.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Doshi N., Prabhakarpandian B., Rea-Ramsey A., Pant K., Sundaram S., Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J. Control. Release. 2010;146:196–200. doi: 10.1016/j.jconrel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Doshi N., Orje J.N., Molins B., Smith J.W., Mitragotri S., Ruggeri Z.M. Platelet mimetic particles for targeting thrombi in flowing blood. Adv. Mater. 2012;24:3864–3869. doi: 10.1002/adma.201200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Anselmo A.C., Modery-Pawlowski C.L., Menegatti S., Kumar S., Vogus D.R., Tian L.L., Chen M., Squires T.M., Sen Gupta A., Mitragotri S. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8:11243–11253. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kolhar P., Anselmo A.C., Gupta V., Pant K., Prabhakarpandian B., Ruoslahti E., Mitragotri S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10753–10758. doi: 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Geng Y., Dalhaimer P., Cai S., Tsai R., Tewari M., Minko T., Discher D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Anselmo A.C., Mitragotri S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Deliv. Rev. 2017;108:51–67. doi: 10.1016/j.addr.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 220.Gunawan R.C., Auguste D.T. The role of antibody synergy and membrane fluidity in the vascular targeting of immunoliposomes. Biomaterials. 2010;31:900–907. doi: 10.1016/j.biomaterials.2009.09.107. [DOI] [PubMed] [Google Scholar]
- 221.Guo P., Liu D., Subramanyam K., Wang B., Yang J., Huang J., Auguste D.T., Moses M.A. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 2018;9:130. doi: 10.1038/s41467-017-02588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Eckmann D.M., Composto R.J., Tsourkas A., Muzykantov V.R. Nanogel Carrier Design for Targeted Drug Delivery. J. Mater. Chem. B. 2014;2:8085–8097. doi: 10.1039/C4TB01141D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Key J., Palange A.L., Gentile F., Aryal S., Stigliano C., Di Mascolo D., De Rosa E., Cho M., Lee Y., Singh J., Decuzzi P. Soft discoidal polymeric Nanoconstructs resist macrophage uptake and enhance vascular targeting in Tumors. ACS Nano. 2015;9:11628–11641. doi: 10.1021/acsnano.5b04866. [DOI] [PubMed] [Google Scholar]
- 224.Banquy X., Suarez F., Argaw A., Rabanel J.-M., Grutter P., Bouchard J.-F., Hildgen P., Giasson S. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. Soft Matter. 2009;5:3984–3991. [Google Scholar]
- 225.Beningo K.A., Wang Y.L. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 2002;115:849–856. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
- 226.Sun J., Zhang L., Wang J., Feng Q., Liu D., Yin Q., Xu D., Wei Y., Ding B., Shi X., Jiang X. Tunable rigidity of (polymeric core)-(lipid shell) nanoparticles for regulated cellular uptake. Adv. Mater. 2015;27:1402–1407. doi: 10.1002/adma.201404788. [DOI] [PubMed] [Google Scholar]
- 227.M. Nowak, T.D. Brown, A. Graham, M.E. Helgeson, S. Mitragotri, Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow, Bioeng. Transl. Med., n/a e10153. [DOI] [PMC free article] [PubMed]
- 228.Myerson J.W., McPherson O., DeFrates K.G., Towslee J.H., Marcos-Contreras O.A., Shuvaev V.V., Braender B., Composto R.J., Muzykantov V.R., Eckmann D.M. Cross-linker-modulated Nanogel flexibility correlates with Tunable targeting to a sterically impeded endothelial marker. ACS Nano. 2019;13:11409–11421. doi: 10.1021/acsnano.9b04789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scherpereel A., Rome J.J., Wiewrodt R., Watkins S.C., Harshaw D.W., Alder S., Christofidou-Solomidou M., Haut E., Murciano J.C., Nakada M., Albelda S.M., Muzykantov V.R. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J. Pharmacol. Exp. Ther. 2002;300:777–786. doi: 10.1124/jpet.300.3.777. [DOI] [PubMed] [Google Scholar]
- 230.Danielyan K., Ding B.S., Gottstein C., Cines D.B., Muzykantov V.R. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J. Pharmacol. Exp. Ther. 2007;321:947–952. doi: 10.1124/jpet.107.120535. [DOI] [PubMed] [Google Scholar]
- 231.Marcos-Contreras O.A., Brenner J.S., Kiseleva R.Y., Zuluaga-Ramirez V., Greineder C.F., Villa C.H., Hood E.D., Myerson J.W., Muro S., Persidsky Y., Muzykantov V.R. Combining vascular targeting and the local first pass provides 100-fold higher uptake of ICAM-1-targeted vs untargeted nanocarriers in the inflamed brain. J. Control. Release. 2019;301:54–61. doi: 10.1016/j.jconrel.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Brenner J.S., Pan D.C., Myerson J.W., Marcos-Contreras O.A., Villa C.H., Patel P., Hekierski H., Chatterjee S., Tao J.Q., Parhiz H., Bhamidipati K., Uhler T.G., Hood E.D., Kiseleva R.Y., Shuvaev V.S., Shuvaeva T., Khoshnejad M., Johnston I., Gregory J.V., Lahann J., Wang T., Cantu E., Armstead W.M., Mitragotri S., Muzykantov V. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018;9:2684. doi: 10.1038/s41467-018-05079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhao Z., Ukidve A., Gao Y., Kim J., Mitragotri S. Erythrocyte leveraged chemotherapy (ELeCt): Nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci Adv. 2019;5 doi: 10.1126/sciadv.aax9250. eaax9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Glassman P.M., Muzykantov V.R. Pharmacokinetic and Pharmacodynamic properties of drug delivery systems. J. Pharmacol. Exp. Ther. 2019;370:570–580. doi: 10.1124/jpet.119.257113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Villa C.H., Pan D.C., Johnston I.H., Greineder C.F., Walsh L.R., Hood E.D., Cines D.B., Poncz M., Siegel D.L., Muzykantov V.R. Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv. 2018;2:165–176. doi: 10.1182/bloodadvances.2017011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Greineder C.F., Villa C.H., Walsh L.R., Kiseleva R.Y., Hood E.D., Khoshnejad M., Warden-Rothman R., Tsourkas A., Muzykantov V.R. Site-specific modification of single-chain antibody fragments for bioconjugation and vascular Immunotargeting. Bioconjug. Chem. 2018;29:56–66. doi: 10.1021/acs.bioconjchem.7b00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schulte S. Half-life extension through albumin fusion technologies. Thromb. Res. 2009;124(Suppl. 2):S6–S8. doi: 10.1016/S0049-3848(09)70157-4. [DOI] [PubMed] [Google Scholar]
- 238.Sleep D., Cameron J., Evans L.R. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta. 2013;1830:5526–5534. doi: 10.1016/j.bbagen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 239.Sung C., Nardelli B., LaFleur D.W., Blatter E., Corcoran M., Olsen H.S., Birse C.E., Pickeral O.K., Zhang J., Shah D., Moody G., Gentz S., Beebe L., Moore P.A. An IFN-beta-albumin fusion protein that displays improved pharmacokinetic and pharmacodynamic properties in nonhuman primates. J. Interf. Cytokine Res. 2003;23:25–36. doi: 10.1089/10799900360520423. [DOI] [PubMed] [Google Scholar]
- 240.Cox G.N., Smith D.J., Carlson S.J., Bendele A.M., Chlipala E.A., Doherty D.H. Enhanced circulating half-life and hematopoietic properties of a human granulocyte colony-stimulating factor/immunoglobulin fusion protein. Exp. Hematol. 2004;32:441–449. doi: 10.1016/j.exphem.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 241.Chinol M., Casalini P., Maggiolo M., Canevari S., Omodeo E.S., Caliceti P., Veronese F.M., Cremonesi M., Chiolerio F., Nardone E., Siccardi A.G., Paganelli G. Biochemical modifications of avidin improve pharmacokinetics and biodistribution, and reduce immunogenicity. Br. J. Cancer. 1998;78:189–197. doi: 10.1038/bjc.1998.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Harris J.M., Martin N.E., Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 2001;40:539–551. doi: 10.2165/00003088-200140070-00005. [DOI] [PubMed] [Google Scholar]
- 243.Chaudhury C., Mehnaz S., Robinson J.M., Hayton W.L., Pearl D.K., Roopenian D.C., Anderson C.L. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ghetie V., Hubbard J.G., Kim J.K., Tsen M.F., Lee Y., Ward E.S. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 245.Junghans R.P., Anderson C.L. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Israel E.J., Wilsker D.F., Hayes K.C., Schoenfeld D., Simister N.E. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology. 1996;89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Glassman P.M., Walsh L.R., Villa C.H., Marcos-Contreras O.A., Hood E.D., Muzykantov V.R., Greineder C.F. Molecularly engineered nanobodies for tunable pharmacokinetics and drug delivery. Bioconjug. Chem. 2020;31:1144–1155. doi: 10.1021/acs.bioconjchem.0c00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mao H., Hart S.A., Schink A., Pollok B.A. Sortase-mediated protein ligation: a new method for protein engineering. J. Am. Chem. Soc. 2004;126:2670–2671. doi: 10.1021/ja039915e. [DOI] [PubMed] [Google Scholar]
- 249.Beerli R.R., Hell T., Merkel A.S., Grawunder U. Sortase enzyme-mediated generation of site-specifically conjugated antibody drug conjugates with high in vitro and in vivo potency. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Massa S., Vikani N., Betti C., Ballet S., Vanderhaegen S., Steyaert J., Descamps B., Vanhove C., Bunschoten A., van Leeuwen F.W., Hernot S., Caveliers V., Lahoutte T., Muyldermans S., Xavier C., Devoogdt N. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: a versatile strategy for multiple molecular imaging modalities. Contrast Media Mol Imaging. 2016;11:328–339. doi: 10.1002/cmmi.1696. [DOI] [PubMed] [Google Scholar]
- 251.van Lith S.A., van Duijnhoven S.M., Navis A.C., Leenders W.P., Dolk E., Wennink J.W., van Nostrum C.F., van Hest J.C. Legomedicine-a versatile chemo-enzymatic approach for the preparation of targeted dual-Labeled llama antibody-nanoparticle conjugates. Bioconjug. Chem. 2017;28:539–548. doi: 10.1021/acs.bioconjchem.6b00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hood E.D., Greineder C.F., Shuvaeva T., Walsh L., Villa C.H., Muzykantov V.R. Vascular targeting of Radiolabeled liposomes with bio-orthogonally conjugated ligands: single chain fragments provide higher specificity than antibodies. Bioconjug. Chem. 2018;29:3626–3637. doi: 10.1021/acs.bioconjchem.8b00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Massa S., Xavier C., De Vos J., Caveliers V., Lahoutte T., Muyldermans S., Devoogdt N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug. Chem. 2014;25:979–988. doi: 10.1021/bc500111t. [DOI] [PubMed] [Google Scholar]
- 254.Khoshnejad M., Brenner J.S., Motley W., Parhiz H., Greineder C.F., Villa C.H., Marcos-Contreras O.A., Tsourkas A., Muzykantov V.R. Molecular engineering of antibodies for site-specific covalent conjugation using CRISPR/Cas9. Sci. Rep. 2018;8:1760. doi: 10.1038/s41598-018-19784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Khoshnejad M., Brenner J.S., Parhiz H., Muzykantov V.R. CRISPR/Cas9-mediated genetic engineering of hybridomas for creation of antibodies that allow for site-specific conjugation. Methods Mol. Biol. 2019;2033:81–93. doi: 10.1007/978-1-4939-9654-4_7. [DOI] [PubMed] [Google Scholar]
- 256.Roki N., Tsinas Z., Solomon M., Bowers J., Getts R.C., Muro S. Unprecedently high targeting specificity toward lung ICAM-1 using 3DNA nanocarriers. J. Control. Release. 2019;305:41–49. doi: 10.1016/j.jconrel.2019.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Stoltenburg R., Reinemann C., Strehlitz B. SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007;24:381–403. doi: 10.1016/j.bioeng.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 258.Ku T.H., Zhang T., Luo H., Yen T.M., Chen P.W., Han Y., Lo Y.H. Nucleic acid aptamers: an emerging tool for biotechnology and biomedical sensing. Sensors (Basel) 2015;15:16281–16313. doi: 10.3390/s150716281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hu J., Al-Waili D., Hassan A., Fan G.C., Xin M., Hao J. Inhibition of cerebral vascular inflammation by brain endothelium-targeted oligodeoxynucleotide complex. Neuroscience. 2016;329:30–42. doi: 10.1016/j.neuroscience.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Macdonald J., Denoyer D., Henri J., Jamieson A., Burvenich I.J.G., Pouliot N., Shigdar S. Bifunctional aptamer-doxorubicin conjugate crosses the blood-brain barrier and selectively delivers its payload to EpCAM-positive tumor cells. Nucleic Acid Ther. 2020;30:117–128. doi: 10.1089/nat.2019.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Goetz D.J., el-Sabban M.E., Hammer D.A., Pauli B.U. Lu-ECAM-1-mediated adhesion of melanoma cells to endothelium under conditions of flow. Int. J. Cancer. 1996;65:192–199. doi: 10.1002/(SICI)1097-0215(19960117)65:2<192::AID-IJC11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 262.Stan R.V., Ghitescu L., Jacobson B.S., Palade G.E. Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein. J. Cell Biol. 1999;145:1189–1198. doi: 10.1083/jcb.145.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Muzykantov V.R., Atochina E.N., Ischiropoulos H., Danilov S.M., Fisher A.B. Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5213–5218. doi: 10.1073/pnas.93.11.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 265.Trubetskoy V.S., Torchilin V.P., Kennel S., Huang L. Cationic liposomes enhance targeted delivery and expression of exogenous DNA mediated by N-terminal modified poly(L-lysine)-antibody conjugate in mouse lung endothelial cells. Biochim. Biophys. Acta. 1992;1131:311–313. doi: 10.1016/0167-4781(92)90030-4. [DOI] [PubMed] [Google Scholar]
- 266.Maruyama K., Takizawa T., Yuda T., Kennel S.J., Huang L., Iwatsuru M. Targetability of novel immunoliposomes modified with amphipathic poly(ethylene glycol)s conjugated at their distal terminals to monoclonal antibodies. Biochim. Biophys. Acta. 1995;1234:74–80. doi: 10.1016/0005-2736(94)00263-o. [DOI] [PubMed] [Google Scholar]
- 267.Ding B.S., Gottstein C., Grunow A., Kuo A., Ganguly K., Albelda S.M., Cines D.B., Muzykantov V.R. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106:4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ding B.S., Hong N., Murciano J.C., Ganguly K., Gottstein C., Christofidou-Solomidou M., Albelda S.M., Fisher A.B., Cines D.B., Muzykantov V.R. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood. 2008;111:1999–2006. doi: 10.1182/blood-2007-07-103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Murciano J.C., Muro S., Koniaris L., Christofidou-Solomidou M., Harshaw D.W., Albelda S.M., Granger D.N., Cines D.B., Muzykantov V.R. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101:3977–3984. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 270.Ding B.S., Hong N., Christofidou-Solomidou M., Gottstein C., Albelda S.M., Cines D.B., Fisher A.B., Muzykantov V.R. Anchoring fusion thrombomodulin to the endothelial lumen protects against injury-induced lung thrombosis and inflammation. Am. J. Respir. Crit. Care Med. 2009;180:247–256. doi: 10.1164/rccm.200809-1433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Greineder C.F., Chacko A.M., Zaytsev S., Zern B.J., Carnemolla R., Hood E.D., Han J., Ding B.S., Esmon C.T., Muzykantov V.R. Vascular immunotargeting to endothelial determinant ICAM-1 enables optimal partnering of recombinant scFv-thrombomodulin fusion with endogenous cofactor. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Greineder C.F., Johnston I.H., Villa C.H., Gollomp K., Esmon C.T., Cines D.B., Poncz M., Muzykantov V.R. ICAM-1-targeted thrombomodulin mitigates tissue factor-driven inflammatory thrombosis in a human endothelialized microfluidic model. Blood Adv. 2017;1:1452–1465. doi: 10.1182/bloodadvances.2017007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ferrer M.C., Shuvaev V.V., Zern B.J., Composto R.J., Muzykantov V.R., Eckmann D.M. Icam-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Esmon C.T. Inflammation and thrombosis. J. Thromb. Haemost. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 275.Dichek D.A., Anderson J., Kelly A.B., Hanson S.R., Harker L.A. Enhanced in vivo antithrombotic effects of endothelial cells expressing recombinant plasminogen activators transduced with retroviral vectors. Circulation. 1996;93:301–309. doi: 10.1161/01.cir.93.2.301. [DOI] [PubMed] [Google Scholar]
- 276.Muzykantov V.R., Barnathan E.S., Atochina E.N., Kuo A., Danilov S.M., Fisher A.B. Targeting of antibody-conjugated plasminogen activators to the pulmonary vasculature. J. Pharmacol. Exp. Ther. 1996;279:1026–1034. [PubMed] [Google Scholar]
- 277.Muzykantov V.R., Christofidou-Solomidou M., Balyasnikova I., Harshaw D.W., Schultz L., Fisher A.B., Albelda S.M. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kozower B.D., Christofidou-Solomidou M., Sweitzer T.D., Muro S., Buerk D.G., Solomides C.C., Albelda S.M., Patterson G.A., Muzykantov V.R. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003;21:392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 279.Husain S.S. Single-chain urokinase-type plasminogen activator does not possess measurable intrinsic amidolytic or plasminogen activator activities. Biochemistry. 1991;30:5797–5805. doi: 10.1021/bi00237a024. [DOI] [PubMed] [Google Scholar]
- 280.Ichinose A., Fujikawa K., Suyama T. The activation of pro-urokinase by plasma kallikrein and its inactivation by thrombin. J. Biol. Chem. 1986;261:3486–3489. [PubMed] [Google Scholar]
- 281.Yang W.P., Goldstein J., Procyk R., Matsueda G.R., Shaw S.Y. Design and evaluation of a thrombin-activable plasminogen activator. Biochemistry. 1994;33:p606–p612. doi: 10.1021/bi00174a043. [DOI] [PubMed] [Google Scholar]
- 282.Wang Y.X., Wu C., Vincelette J., Martin-McNulty B., Alexander S., Larsen B., Light D.R., McLean K. Amplified anticoagulant activity of tissue factor-targeted thrombomodulin: in-vivo validation of a tissue factor-neutralizing antibody fused to soluble thrombomodulin. Thromb. Haemost. 2006;96:317–324. doi: 10.1160/TH06-04-0219. [DOI] [PubMed] [Google Scholar]
- 283.Everts M., Koning G., Kok R., Ásgeirsdóttir S., Vestweber D., Meijer D.F., Storm G., Molema G. In vitro cellular handling and in vivo targeting of E-selectin-directed Immunoconjugates and Immunoliposomes used for drug delivery to inflamed endothelium. Pharm. Res. 2003;20:64–72. doi: 10.1023/a:1022298725165. [DOI] [PubMed] [Google Scholar]
- 284.Asgeirsdottir S.A., Kamps J.A., Bakker H.I., Zwiers P.J., Heeringa P., van der Weide K., van Goor H., Petersen A.H., Morselt H., Moorlag H.E., Steenbergen E., Kallenberg C.G., Molema G. Site-specific inhibition of glomerulonephritis progression by targeted delivery of dexamethasone to glomerular endothelium. Mol. Pharmacol. 2007;72:121–131. doi: 10.1124/mol.107.034140. [DOI] [PubMed] [Google Scholar]
- 285.Asgeirsdottir S.A., Zwiers P.J., Morselt H.W., Moorlag H.E., Bakker H.I., Heeringa P., Kok J.W., Kallenberg C.G., Molema G., Kamps J.A. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Renal Physiol. 2008;294:F554–F561. doi: 10.1152/ajprenal.00391.2007. [DOI] [PubMed] [Google Scholar]
- 286.Hashida N., Ohguro N., Yamazaki N., Arakawa Y., Oiki E., Mashimo H., Kurokawa N., Tano Y. High-efficacy site-directed drug delivery system using sialyl-Lewis X conjugated liposome. Exp. Eye Res. 2008;86:138–149. doi: 10.1016/j.exer.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 287.Zuckerman J.E., Choi C.H.J., Han H., Davis M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lee J.B., Zhang K., Tam Y.Y.C., Tam Y.K., Belliveau N.M., Sung V.Y.C., Lin P.J.C., LeBlanc E., Ciufolini M.A., Rennie P.S., Cullis P.R. Lipid nanoparticle siRNA systems for silencing the androgen receptor in human prostate cancer in vivo. Int. J. Cancer. 2012;131:E781–E790. doi: 10.1002/ijc.27361. [DOI] [PubMed] [Google Scholar]
- 289.Kuldo J.M., Asgeirsdottir S.A., Zwiers P.J., Bellu A.R., Rots M.G., Schalk J.A., Ogawara K.I., Trautwein C., Banas B., Haisma H.J., Molema G., Kamps J.A. Targeted adenovirus mediated inhibition of NF-kappaB-dependent inflammatory gene expression in endothelial cells in vitro and in vivo. J. Control. Release. 2013;166:57–65. doi: 10.1016/j.jconrel.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 290.Asgeirsdottir S.A., Talman E.G., de Graaf I.A., Kamps J.A., Satchell S.C., Mathieson P.W., Ruiters M.H., Molema G. Targeted transfection increases siRNA uptake and gene silencing of primary endothelial cells in vitro--a quantitative study. J. Control. Release. 2010;141:241–251. doi: 10.1016/j.jconrel.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 291.Koning G.A., Schiffelers R.M., Wauben M.H.M., Kok R.J., Mastrobattista E., Molema G., ten Hagen T.L.M., Storm G. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate–containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheumat. 2006;54:1198–1208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- 292.Vader P., Crielaard B.J., van Dommelen S.M., van der Meel R., Storm G., Schiffelers R.M. Targeted delivery of small interfering RNA to angiogenic endothelial cells with liposome-polycation-DNA particles. J. Control. Release. 2012;160:211–216. doi: 10.1016/j.jconrel.2011.09.080. [DOI] [PubMed] [Google Scholar]
- 293.Homem de Bittencourt P.I., Lagranha D.J., Maslinkiewicz A., Senna S.M., Tavares A.M., Baldissera L.P., Janner D.R., Peralta J.S., Bock P.M., Gutierrez L.L., Scola G., Heck T.G., Krause M.S., Cruz L.A., Abdalla D.S., Lagranha C.J., Lima T., Curi R. LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis. 2007;193:245–258. doi: 10.1016/j.atherosclerosis.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 294.Hegeman M.A., Cobelens P.M., Kamps J., Hennus M.P., Jansen N.J.G., Schultz M.J., van Vught A.J., Molema G., Heijnen C.J. Liposome-encapsulated dexamethasone attenuates ventilator-induced lung inflammation. Br. J. Pharmacol. 2011;163:1048–1058. doi: 10.1111/j.1476-5381.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Scita G., Di Fiore P.P. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 296.Sigismund S., Confalonieri S., Ciliberto A., Polo S., Scita G., Di Fiore P.P. Endocytosis and signaling: cell logistics shape the eukaryotic cell plan. Physiol. Rev. 2012;92:273–366. doi: 10.1152/physrev.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sorkin A., von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Cendrowski J., Maminska A., Miaczynska M. Endocytic regulation of cytokine receptor signaling. Cytokine Growth Factor Rev. 2016;32:63–73. doi: 10.1016/j.cytogfr.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 299.D’Alessio A., Al-Lamki R.S., Bradley J.R., Pober J.S. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am. J. Pathol. 2005;166:1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.D’Alessio A., Kluger M.S., Li J.H., Al-Lamki R., Bradley J.R., Pober J.S. Targeting of tumor necrosis factor receptor 1 to low density plasma membrane domains in human endothelial cells. J. Biol. Chem. 2010;285:23868–23879. doi: 10.1074/jbc.M110.122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Legler D.F., Micheau O., Doucey M.A., Tschopp J., Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 302.Blanco A.M., Perez-Arago A., Fernandez-Lizarbe S., Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J. Neurochem. 2008;106:625–639. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- 303.Hansen B., Dittrich-Breiholz O., Kracht M., Windheim M. Regulation of NF-kappaB-dependent gene expression by ligand-induced endocytosis of the interleukin-1 receptor. Cell. Signal. 2013;25:214–228. doi: 10.1016/j.cellsig.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 304.Spencer N.Y., Engelhardt J.F. The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies. Biochemistry. 2014;53:1551–1564. doi: 10.1021/bi401719r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ushio-Fukai M. Compartmentalization of redox Signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shuvaev V.V., Muzykantov V.R. Targeted modulation of reactive oxygen species in the vascular endothelium. J. Control. Release. 2011;153:56–63. doi: 10.1016/j.jconrel.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Shuvaev V.V., Han J., Tliba S., Arguiri E., Christofidou-Solomidou M., Ramirez S.H., Dykstra H., Persidsky Y., Atochin D.N., Huang P.L., Muzykantov V.R. Anti-inflammatory effect of targeted delivery of SOD to endothelium: mechanism, synergism with NO donors and protective effects in vitro and in vivo. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shuvaev V.V., Han J., Yu K.J., Huang S., Hawkins B.J., Madesh M., Nakada M., Muzykantov V.R. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25:348–357. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Shuvaev V.V., Muro S., Arguiri E., Khoshnejad M., Tliba S., Christofidou-Solomidou M., Muzykantov V.R. Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates. J. Control. Release. 2016;234:115–123. doi: 10.1016/j.jconrel.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wang Z., Tiruppathi C., Cho J., Minshall R.D., Malik A.B. Delivery of nanoparticle: complexed drugs across the vascular endothelial barrier via caveolae. IUBMB Life. 2011;63:659–667. doi: 10.1002/iub.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Parton R.G., del Pozo M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 312.McGettrick A.F., O’Neill L.A. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr. Opin. Immunol. 2010;22:20–27. doi: 10.1016/j.coi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 313.Hornef M.W., Henriques-Normark B., Normark S. The function and biological role of toll-like receptors in infectious diseases: an update. Curr. Opin. Infect. Dis. 2008;21:304–312. doi: 10.1097/QCO.0b013e3282f88ba3. [DOI] [PubMed] [Google Scholar]
- 314.Pascual-Lucas M., Fernandez-Lizarbe S., Montesinos J., Guerri C. LPS or ethanol triggers clathrin- and rafts/caveolae-dependent endocytosis of TLR4 in cortical astrocytes. J. Neurochem. 2014;129:448–462. doi: 10.1111/jnc.12639. [DOI] [PubMed] [Google Scholar]
- 315.Guillot L., Medjane S., Le-Barillec K., Balloy V., Danel C., Chignard M., Si-Tahar M. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J. Biol. Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 316.Hornef M.W., Normark B.H., Vandewalle A., Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J. Exp. Med. 2003;198:1225–1235. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Garrean S., Gao X.P., Brovkovych V., Shimizu J., Zhao Y.Y., Vogel S.M., Malik A.B. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J. Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 318.Shuvaev V.V., Kiseleva R.Y., Arguiri E., Villa C.H., Muro S., Christofidou-Solomidou M., Stan R.V., Muzykantov V.R. Targeting superoxide dismutase to endothelial caveolae profoundly alleviates inflammation caused by endotoxin. J. Control. Release. 2018;272:1–8. doi: 10.1016/j.jconrel.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Atochina E.N., Balyasnikova I.V., Danilov S.M., Granger D.N., Fisher A.B., Muzykantov V.R. Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am. J. Phys. 1998;275:L806–L817. doi: 10.1152/ajplung.1998.275.4.L806. [DOI] [PubMed] [Google Scholar]
- 320.Scherpereel A., Wiewrodt R., Christofidou-Solomidou M., Gervais R., Murciano J.C., Albelda S.M., Muzykantov V.R. Cell-selective intracellular delivery of a foreign enzyme to endothelium in vivo using vascular immunotargeting. FASEB J. 2001;15:416–426. doi: 10.1096/fj.00-0022com. [DOI] [PubMed] [Google Scholar]
- 321.Shuvaev V.V., Christofidou-Solomidou M., Bhora F., Laude K., Cai H., Dikalov S., Arguiri E., Solomides C.C., Albelda S.M., Harrison D.G., Muzykantov V.R. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharmacol. Exp. Ther. 2009;331:404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Nowak K., Kolbel H.C., Metzger R.P., Hanusch C., Frohnmeyer M., Hohenberger P., Danilov S.M. Immunotargeting of the pulmonary endothelium via angiotensin-converting-enzyme in isolated ventilated and perfused human lung. Adv. Exp. Med. Biol. 2013;756:203–212. doi: 10.1007/978-94-007-4549-0_26. [DOI] [PubMed] [Google Scholar]
- 323.Morecroft I., White K., Caruso P., Nilsen M., Loughlin L., Alba R., Reynolds P.N., Danilov S.M., Baker A.H., Maclean M.R. Gene therapy by targeted adenovirus-mediated knockdown of pulmonary endothelial Tph1 attenuates hypoxia-induced pulmonary hypertension. Mol. Ther. 2012;20:1516–1528. doi: 10.1038/mt.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nowak K., Hanusch C., Nicksch K., Metzger R.P., Beck G., Gebhard M.M., Hohenberger P., Danilov S.M. Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies. Eur. J. Cardiothorac. Surg. 2010;37:859–863. doi: 10.1016/j.ejcts.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 325.Nowak K., Weih S., Metzger R., Albrecht R.F., 2nd, Post S., Hohenberger P., Gebhard M.M., Danilov S.M. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2007;293:L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 326.Shuvaev V.V., Tliba S., Nakada M., Albelda S.M., Muzykantov V.R. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J. Pharmacol. Exp. Ther. 2007;323:450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 327.Muzykantov V.R., Sakharov D.V., Domogatsky S.P., Goncharov N.V., Danilov S.M. Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am. J. Pathol. 1987;128:276–285. [PMC free article] [PubMed] [Google Scholar]
- 328.Sakharov D.V., Muzykantov V.R., Domogatsky S.P., Danilov S.M. Protection of cultured endothelial cells from hydrogen peroxide-induced injury by antibody-conjugated catalase. Biochim. Biophys. Acta. 1987;930:140–144. doi: 10.1016/0167-4889(87)90025-5. [DOI] [PubMed] [Google Scholar]
- 329.Sweitzer T.D., Thomas A.P., Wiewrodt R., Nakada M.T., Branco F., Muzykantov V.R. PECAM-directed immunotargeting of catalase: specific, rapid and transient protection against hydrogen peroxide. Free Radic. Biol. Med. 2003;34:1035–1046. doi: 10.1016/s0891-5849(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 330.Han J., Shuvaev V.V., Muzykantov V.R. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J. Pharmacol. Exp. Ther. 2011;338:82–91. doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Muzykantov V.R., Atochina E.N., Kuo A., Barnathan E.S., Notarfrancesco K., Shuman H., Dodia C., Fisher A.B. Endothelial cells internalize monoclonal antibody to angiotensin-converting enzyme. Am. J. Phys. 1996;270:L704–L713. doi: 10.1152/ajplung.1996.270.5.L704. [DOI] [PubMed] [Google Scholar]
- 332.Christofidou-Solomidou M., Scherpereel A., Wiewrodt R., Ng K., Sweitzer T., Arguiri E., Shuvaev V., Solomides C.C., Albelda S.M., Muzykantov V.R. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2003;285:L283–L292. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 333.Hood E.D., Greineder C.F., Dodia C., Han J., Mesaros C., Shuvaev V.V., Blair I.A., Fisher A.B., Muzykantov V.R. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J. Control. Release. 2012;163(2):161–169. doi: 10.1016/j.jconrel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Dziubla T.D., Karim A., Muzykantov V.R. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J. Control. Release. 2005;102:427–439. doi: 10.1016/j.jconrel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 335.Simone E.A., Dziubla T.D., Arguiri E., Vardon V., Shuvaev V.V., Christofidou-Solomidou M., Muzykantov V.R. Loading PEG-catalase into filamentous and spherical polymer nanocarriers. Pharm. Res. 2009;26:250–260. doi: 10.1007/s11095-008-9744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Simone E.A., Dziubla T.D., Discher D.E., Muzykantov V.R. Filamentous polymer Nanocarriers of Tunable stiffness that encapsulate the therapeutic enzyme catalase. Biomacromolecules. 2009;10(6):1324–1330. doi: 10.1021/bm900189x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Dziubla T.D., Shuvaev V.V., Hong N.K., Hawkins B.J., Madesh M., Takano H., Simone E., Nakada M.T., Fisher A., Albelda S.M., Muzykantov V.R. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials. 2008;29:215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Balyasnikova I.V., Danilov S.M., Muzykantov V.R., Fisher A.B. Modulation of angiotensin-converting enzyme in cultured human vascular endothelial cells. In Vitro Cell Dev Biol Anim. 1998;34:545–554. doi: 10.1007/s11626-998-0114-x. [DOI] [PubMed] [Google Scholar]
- 339.Jaffe E.A., Nachman R.L., Becker C.G., Minick C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Han J., Zern B.J., Shuvaev V.V., Davies P.F., Muro S., Muzykantov V. Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1. ACS Nano. 2012;6:8824–8836. doi: 10.1021/nn302687n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Thomas A., Daniel Ou-Yang H., Lowe-Krentz L., Muzykantov V.R., Liu Y. Biomimetic channel modeling local vascular dynamics of pro-inflammatory endothelial changes. Biomicrofluidics. 2016;10 doi: 10.1063/1.4936672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Uhl C.G., Muzykantov V.R., Liu Y. Biomimetic microfluidic platform for the quantification of transient endothelial monolayer permeability and therapeutic transport under mimicked cancerous conditions. Biomicrofluidics. 2018;12 doi: 10.1063/1.5000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Paek J., Park S.E., Lu Q., Park K.T., Cho M., Oh J.M., Kwon K.W., Yi Y.S., Song J.W., Edelstein H.I., Ishibashi J., Yang W., Myerson J.W., Kiseleva R.Y., Aprelev P., Hood E.D., Stambolian D., Seale P., Muzykantov V.R., Huh D. Microphysiological engineering of self-assembled and Perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano. 2019;13:7627–7643. doi: 10.1021/acsnano.9b00686. [DOI] [PubMed] [Google Scholar]
- 344.Liu J., Weller G.E., Zern B., Ayyaswamy P.S., Eckmann D.M., Muzykantov V.R., Radhakrishnan R. Computational model for nanocarrier binding to endothelium validated using in vivo, in vitro, and atomic force microscopy experiments. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16530–16535. doi: 10.1073/pnas.1006611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ayyaswamy P.S., Muzykantov V., Eckmann D.M., Radhakrishnan R. Nanocarrier hydrodynamics and binding in targeted drug delivery: challenges in numerical Modeling and experimental validation. J Nanotechnol Eng Med. 2013;4 doi: 10.1115/1.4024004. 101011-1010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ramakrishnan N., Tourdot R.W., Eckmann D.M., Ayyaswamy P.S., Muzykantov V.R., Radhakrishnan R. Biophysically inspired model for functionalized nanocarrier adhesion to cell surface: roles of protein expression and mechanical factors. R. Soc. Open Sci. 2016;3:160260. doi: 10.1098/rsos.160260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ranganathan A., Campo J., Myerson J., Shuvaev V., Zern B., Muzykantov V., Eckmann D.M. Fluorescence microscopy imaging calibration for quantifying Nanocarrier binding to cells during shear flow exposure. J. Biomed. Nanotechnol. 2017;13:737–745. doi: 10.1166/jbn.2017.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pan H., Myerson J.W., Hu L., Marsh J.N., Hou K., Scott M.J., Allen J.S., Hu G., San Roman S., Lanza G.M., Schreiber R.D., Schlesinger P.H., Wickline S.A. Programmable nanoparticle functionalization for in vivo targeting. FASEB J. 2013;27:255–264. doi: 10.1096/fj.12-218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Deng H., Konopka C.J., Cross T.L., Swanson K.S., Dobrucki L.W., Smith A.M. Multimodal Nanocarrier probes reveal superior biodistribution quantification by isotopic analysis over fluorescence. ACS Nano. 2020;14:509–523. doi: 10.1021/acsnano.9b06504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Cuyckens F. Mass spectrometry in drug metabolism and pharmacokinetics: current trends and future perspectives. Rapid Commun. Mass Spectrom. 2019;33(Suppl. 3):90–95. doi: 10.1002/rcm.8235. [DOI] [PubMed] [Google Scholar]
- 351.Mou S., Huang Y., Rosenbaum A.I. ADME considerations and bioanalytical strategies for pharmacokinetic assessments of antibody-drug conjugates. Antibodies (Basel) 2018;7 doi: 10.3390/antib7040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.de Maar J.S., Sofias A.M., Porta Siegel T., Vreeken R.J., Moonen C., Bos C., Deckers R. Spatial heterogeneity of nanomedicine investigated by multiscale imaging of the drug, the nanoparticle and the tumour environment. Theranostics. 2020;10:1884–1909. doi: 10.7150/thno.38625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Glassman P.M., Chen Y., Balthasar J.P. Scale-up of a physiologically-based pharmacokinetic model to predict the disposition of monoclonal antibodies in monkeys. J. Pharmacokinet. Pharmacodyn. 2015;42:527–540. doi: 10.1007/s10928-015-9444-y. [DOI] [PubMed] [Google Scholar]
- 354.Glassman P.M., Balthasar J.P. Physiologically-based pharmacokinetic modeling to predict the clinical pharmacokinetics of monoclonal antibodies. J. Pharmacokinet. Pharmacodyn. 2016;43:427–446. doi: 10.1007/s10928-016-9482-0. [DOI] [PubMed] [Google Scholar]
- 355.Kagan L., Gershkovich P., Wasan K.M., Mager D.E. Dual physiologically based pharmacokinetic model of liposomal and nonliposomal amphotericin B disposition. Pharm. Res. 2014;31:35–45. doi: 10.1007/s11095-013-1127-z. [DOI] [PubMed] [Google Scholar]
- 356.Sun H., Fadiran E.O., Jones C.D., Lesko L., Huang S.M., Higgins K., Hu C., Machado S., Maldonado S., Williams R., Hossain M., Ette E.I. Population pharmacokinetics. A regulatory perspective. Clin. Pharmacokinet. 1999;37:41–58. doi: 10.2165/00003088-199937010-00003. [DOI] [PubMed] [Google Scholar]
- 357.Joerger M. Covariate pharmacokinetic model building in oncology and its potential clinical relevance. AAPS J. 2012;14:119–132. doi: 10.1208/s12248-012-9320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Miki T., Takegami Y., Okawa K., Muraguchi T., Noda M., Takahashi C. The reversion-inducing cysteine-rich protein with Kazal motifs (RECK) interacts with membrane type 1 matrix metalloproteinase and CD13/aminopeptidase N and modulates their endocytic pathways. J. Biol. Chem. 2007;282:12341–12352. doi: 10.1074/jbc.M610948200. [DOI] [PubMed] [Google Scholar]
- 359.Harburger D.S., Calderwood D.A. Integrin signalling at a glance. J. Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 361.Moreno-Layseca P., Icha J., Hamidi H., Ivaska J. Integrin trafficking in cells and tissues. Nat. Cell Biol. 2019;21:122–132. doi: 10.1038/s41556-018-0223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Gavard J. Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adhes. Migr. 2014;8:158–164. doi: 10.4161/cam.29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Fujii S., Fujihara A., Natori K., Abe A., Kuboki Y., Higuchi Y., Aizawa M., Kuwata T., Kinoshita T., Yasui W., Ochiai A. TEM1 expression in cancer-associated fibroblasts is correlated with a poor prognosis in patients with gastric cancer. Cancer Med. 2015;4:1667–1678. doi: 10.1002/cam4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Senbanjo L.T., Chellaiah M.A. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Jiang H., Peterson R.S., Wang W., Bartnik E., Knudson C.B., Knudson W. A requirement for the CD44 cytoplasmic domain for hyaluronan binding, pericellular matrix assembly, and receptor-mediated endocytosis in COS-7 cells. J. Biol. Chem. 2002;277:10531–10538. doi: 10.1074/jbc.M108654200. [DOI] [PubMed] [Google Scholar]
- 366.Preissler G., Loehe F., Huff I.V., Ebersberger U., Shuvaev V.V., Bittmann I., Hermanns I., Kirkpatrick J.C., Fischer K., Eichhorn M.E., Winter H., Jauch K.W., Albelda S.M., Muzykantov V.R., Wiewrodt R. Targeted endothelial delivery of nanosized catalase immunoconjugates protects lung grafts donated after cardiac death. Transplantation. 2011;92:380–387. doi: 10.1097/TP.0b013e318226bc6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Hood E.D., Chorny M., Greineder C.F., S.A. I, R.J. Levy, V.R. Muzykantov Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials. 2014;35:3708–3715. doi: 10.1016/j.biomaterials.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Li R., Kowalski P.S., Morselt H.W.M., Schepel I., Jongman R.M., Aslan A., Ruiters M.H.J., Zijlstra J.G., Molema G., van Meurs M., Kamps J. Endothelium-targeted delivery of dexamethasone by anti-VCAM-1 SAINT-O-Somes in mouse endotoxemia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Marchetti G.M., Burwell T.J., Peterson N.C., Cann J.A., Hanna R.N., Li Q., Ongstad E.L., Boyd J.T., Kennedy M.A., Zhao W., Rickert K.W., Grimsby J.S., Dall'Acqua W.F., Wu H., Tsui P., Borrok M.J., Gupta R. Targeted drug delivery via caveolae-associated protein PV1 improves lung fibrosis. Commun Biol. 2019;2:92. doi: 10.1038/s42003-019-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]