Abstract
This single-center prospective clinical trial evaluated the combination of nivolumab plus bendamustine (NB) as a salvage regimen in classical Hodgkin lymphoma patients after failure of nivolumab monotherapy. A total of 30 patients received nivolumab (3 mg/kg) on D1,14 and bendamustine (90 mg/m2) on D1, 2 of a 28-day cycle for up to 3 cycles. The ORR was 87% with 57% CR, 30% PR. With median follow-up of 25 months, the estimated 2-year OS was 96,7% (95% CI, 90.2%–100%), PFS was 23,3% (95% CI, 8.2%–38.4%) median PFS was 10.2 months (95% CI, 7.7–14.2 months) with median DOR 6.6 months (95% CI 3.9–11.6 months). Ten patients (33.3%) experienced grade 3 to 4 AE during therapy. Infections were most common AEs of the combined therapy. NB was a highly efficient salvage regimen in relapsed/refractory cHL with a manageable toxicity profile and modest potential for achievement of long-term remission. Registered at www.clinicaltrials.gov (#NCT0334365).
Introduction
In spite of developments in the treatment of classical Hodgkin lymphoma, patients with relapsed refractory disease (r/r cHL) constitute up to 30% cases and have unfavorable prognosis in case of high-dose chemotherapy and autologous transplantation failure.1–5 Recent advances in the treatment of r/r cHL were achieved with the accumulation of data regarding classical Hodgkin lymphoma inflammatory microenvironment, demonstrating the key importance of PD-L1/2-PD-1 axis in the immune evasion of Hodgkin Reed Sternberg cells and creating the rationale for the use of immune checkpoint inhibitors (ICI).6 Currently, the US Food and Drug Administration approved 2 anti-PD-1 antibodies for the treatment of cHL, including nivolumab and pembrolizumab. While ICI demonstrated unprecedented activity in this patient population, only about one-third of patients achieve a complete remission after PD-1 inhibitors therapy.7,8 Prolonged follow up demonstrated that a major proportion of patients ultimately relapse after nivolumab and pembrolizumab treatment, with a median PFS of 15 to 17 months.8,9 These results were also confirmed in real-life clinical practice.10 Despite ongoing discussion about the response assessment and observed cases of tumor flare, nivolumab pivotal trial showed that patients with disease progression as best response to nivolumab therapy have dismal prognosis with 12-month OS of 59%.9 The optimal treatment for this severely pretreated patients’ population is yet to be defined and represent an unmet medical need.
One potential approach to increase the efficiency of immunotherapy is the introduction of combination with another targeted therapy or chemotherapy.11,12 In preclinical models, chemotherapy had shown enhancement of the antigenicity and immunogenicity of the tumor, elimination of immunosuppressive cellular components of the microenvironment followed by immune shift.13,14 There are also experimental and practical observations demonstrating the emerging role of lymphodepletion in cancer immunotherapy.15,16 Based on these observations the combination of immune checkpoint inhibitors with chemotherapy was tested and granted accelerated FDA approval for patients with non-squamous non-small cell lung cancer.12
In the limited population of 7 patients, the nivolumab combination with ICE regimen showed high efficiency (100% ORR) as a first salvage therapy.17 Chemosensitization was also demonstrated in the retrospective analysis of chemotherapy efficiency in Hodgkin lymphoma patients who lost response to nivolumab, performed by The Lymphoma Study Association.18 In this retrospective trial subsequent chemotherapy or chemotherapy-anti-PD-1 combination helped to achieve an overall response rate (ORR) of 67%. Other retrospective analysis of 17 centers in Canada and the USA demonstrated ORR to the subsequent treatment of 52%, with the response of post ICI treatment correlating with the response to ICI therapy.19 To date there is no representative prospective data regarding the efficiency of nivolumab-chemotherapy combination in patients with resistant and refractory classical Hodgkin lymphoma. Bendamustine is a bifunctional alkylating agent which is effective as monotherapy or in combination with BV in patients with r/r cHL and which induces an early sustained lymphodepletion20,21; therefore, having the potential to enhance the effect of the nivolumab. To assess the safety and efficiency of the nivolumab-bendamustine (NB) combination, we conducted the prospective clinical trial (NCT03343652) of combined chemo-immunotherapy in patients with r/r cHL after the failure of nivolumab monotherapy.
Methods
Study design
This was a phase 2, single-arm, open-label study. The patients included were at least 18 years of age with a histological diagnosis of classical HL, relapsed or refractory to at least 2 lines of previous therapy, including treatment with nivolumab. All eligible patients had active disease following previous therapy. Additional criteria included Karnofsky index of more than 30%, no uncontrolled bacterial or fungal infection at the time of enrollment, no requirement for vasopressor support, pregnancy, active or prior documented autoimmune disease requiring systemic treatment. Patients with prior exposure to bendamustine, and those who underwent previous allogeneic hematopoietic stem cell transplantation (alloHSCT) could be included in the study. This study was performed in accordance with the Declaration of Helsinki and approved by the institutional review board. All enrolled patients gave written informed consent. The patients received a combination therapy of IV nivolumab infusions in the dose of 3 mg/kg on day 1,14 and IV bendamustine infusions in the dose of 90 mg/kg on day 1, 2 of a 28-day cycle for up to 3 cycles in the absence of tumor progression or treatment intolerance. After the combined treatment the response was assessed by total-body PET/CT scan with the LYmphoma Response to Immunomodulatory therapy Criteria (LYRIC) by investigators. The disease status was assessed every 3 months during year 1 and every 6 months during follow up longer than 1 year or earlier in case of special indications (before alloHSCT, or the initiation of another treatment regimens). After the end of study treatment patients could undergo allogeneic stem cell transplantation or other consolidation therapy off the study at the discretion of the treating physician. Adverse events (AEs) were monitored from baseline through the end-of-treatment visit within one year after the end of the study treatment or until the initiation of additional therapy and graded according to Common Terminology Criteria for Adverse Events (CTCAE) v 4.03.
Statistical analysis
The primary efficacy endpoint was the overall response rate during combination therapy, defined as proportion of patients with complete response (CR) or partial response (PR) in measurable lesions by LYRIC criteria. The efficacy and safety evaluable population included those patients who received at least 1 cycle of combined therapy. To evaluate the best response, all assessments during combination therapy were analyzed up to the initiation of other therapy. Secondary endpoints included frequency of grade 3 or higher treatment-related adverse events by NCI CTCAE 4.03 grades, duration of response (DOR) defined as time from initial objective response to documented disease progression or death, progression-free survival (PFS) defined as the time from the first dose of combination therapy to disease progression, relapse or death, event-free survival (EFS) defined as the time from the first dose of combination therapy to disease progression, relapse, death or initiation of other therapy and overall survival (OS) defined as the time from the first dose of combination therapy to death from any reason. In each survival outcome, data were censored at the date of last contact for patients who have not experienced the events of interest during their follow-up.
Data analysis was performed using SAS and SPSS software. The survival were analyzed using Kaplan-Meier method with 95% CIs estimates. The descriptive statistics methods were applied when appropriate. The impact of clinical factors on response was tested with Chi-square and Kruskal-Wallis tests. Both OS and PFS were censored at the date of the last contact. The difference in OS and PFS was tested with a log-rank test.
Results
Patients characteristics
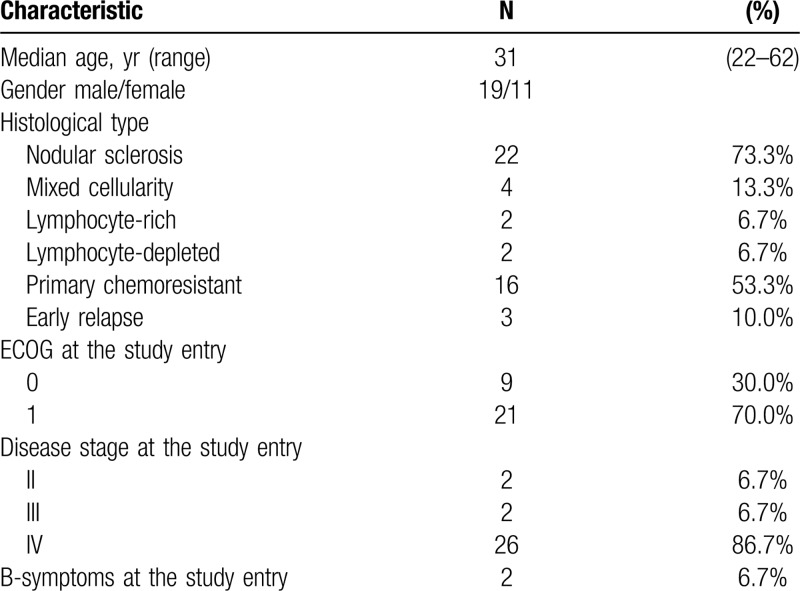
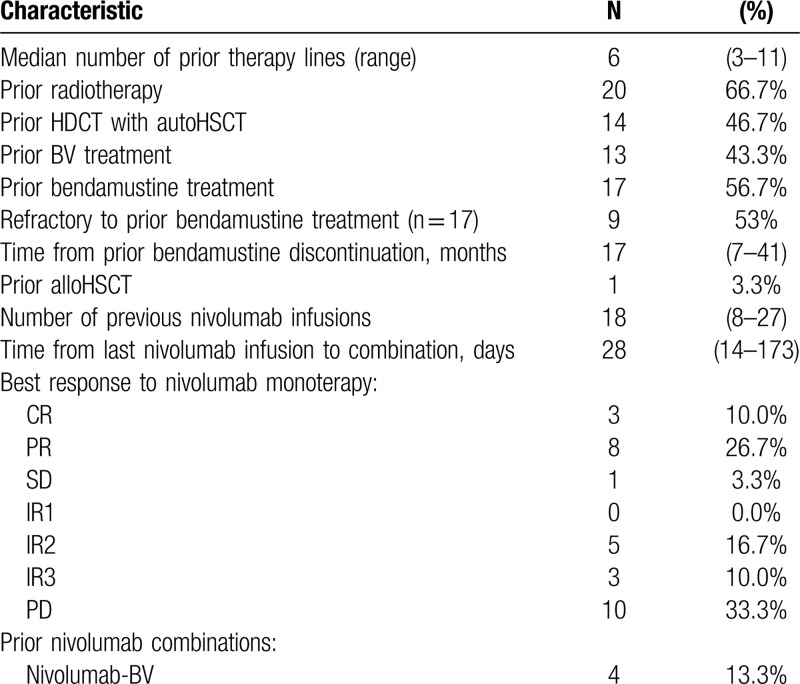
A total of 30 patients with resistant and refractory classical HL were enrolled in the study between May 2017 and November 2017. Demographic characteristics and clinical data are summarized in Table 1. All patients were previously treated with nivolumab in the dose of 3 mg/kg body weight every 2 weeks and assessed by PET/CT scan using LYRIC criteria during Russian named patient program. All patients received nivolumab as an immediate prior therapy. The median number of nivolumab infusions was 18 (8–27). The best overall response (BOR) to nivolumab monotherapy was a complete response (CR) in 3 (10%) patients, partial response (PR) in 8 (27%) patients, stable disease in 1 (3%) patient, indeterminate response (IR) in 8 (27%) patients (IR type 2 in 5 (17%), IR type 3 in 3 (10%)). Ten (33%) patients had disease progression as BOR to nivolumab therapy. Median time from the last nivolumab infusion to NB combination initiation was 28 days (14–173). At the study start, all patients had measurable tumor lesions with disease status of PD in 17 (56%), IR in 9 (30%), SD in 1 (3), PR in 3 (10%) patients according to LYRIC criteria, which corresponds to PD in 26 (86%) of patients according to Lugano criteria. The median number of previous therapy lines was 6 (6–11). Although all (100%) patients had measurable disease at the study entry, their condition was satisfactory with only 2 (7%) patients having B-symptoms and clinical signs of disease at the start of combined treatment. Seventeen (57%) patients received bendamustine-containing regimens during prior treatment and were either refractory (9/17, 53%) or relapsed after that therapy. Median time from prior bendamustine treatment to NB initiation was 17 months (7–41). Additional characteristics regarding the prior treatment of the analyzed population are summarized in Table 2.
Table 1.
Demographic and Clinical Characteristics.
Table 2.
Prior Treatments Characteristics.
Efficacy
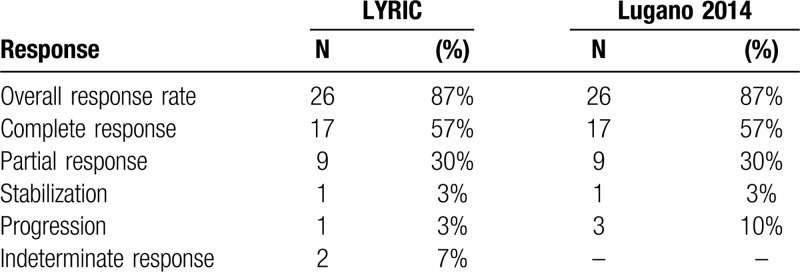
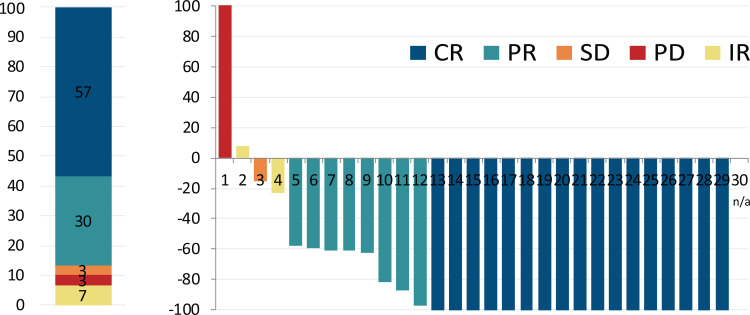
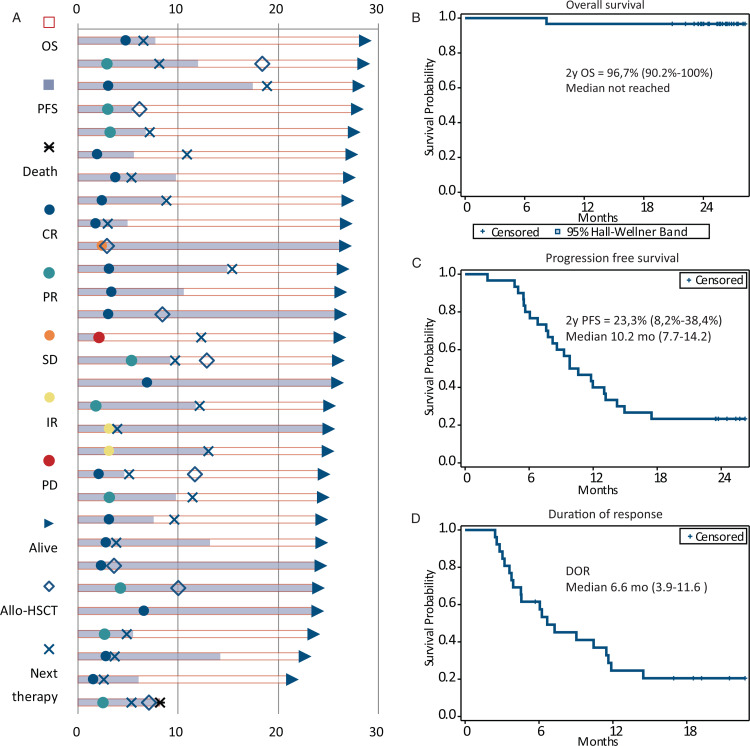
All patients were included in the efficacy analysis. At the time of analysis, the median follow up was 25 months (range 8–28). The overall response rate during nivolumab-bendamustine treatment was 87%. The response structure according to LYRIC, as well as Lugano criteria are summarized in Table 3, and presented in Figure 1A and Supplementary Figure 1 respectively. There was no difference in the BOR regarding previous treatment with bendamustine, bendamustine response, BOR to nivolumab, or disease status and the study start. Mean reduction of tumor volume (Fig. 1B) after the combined treatment was 70% (95% CI, 52%–88%).
Table 3.
Best Response Structure.
Figure 1.
Best overall response and change of tumor load during therapy. (Left) Structure of best overall response (BOR) during combined treatment. The colors represent the BOR during nivolumab treatment. Numbers represent the percent of patients with particular response type. (Right) Best change from baseline in tumor load for all evaluable cases. Negative values indicate maximum tumor reduction, and positive values indicate tumor increase. N/a: unmeasurable lesions at restaging.
The patients’ response, additional treatments and outcomes are presented in Figure 2A. With a median follow-up time of 25 (range 8–28) months from initiation of combination therapy, the estimated 2-year overall survival (OS) was 96.7% (95% CI, 90.2%–100%) (Fig. 2B). At the time of analysis 23.3% (95% CI, 8.2%–38.4%) of patients were alive and free of progression with median progression free survival of 10.2 months (95% CI, 7.7–14.2 months) from initiation of combination therapy (Fig. 2C). In 26 patients with objective response to treatment, median duration of response was 6.6 months (95% CI 3.9–11.6 months) (Fig. 2D). Only 2 (6.7%) of patients were alive and free of disease progression with no additional treatment after NB treatment with median EFS of 6.6 months (95% CI, 4.8–9.2 months) (Supplementary Figure 2).
Figure 2.
Response characteristics among all patients and treatment outcomes. (A) Response characteristics and outcomes in all patients. (B) Overall survival for the whole patient group. Values are medians and 95% CIs at 25 months. (C) Progression-free survival (PFS) in whole patient group. Values are medians and 95% CIs at 25 months. (D) Duration of response (PFS) in all patients. Values are medians and 95% CIs at 25 months.
Factors influencing the prognosis
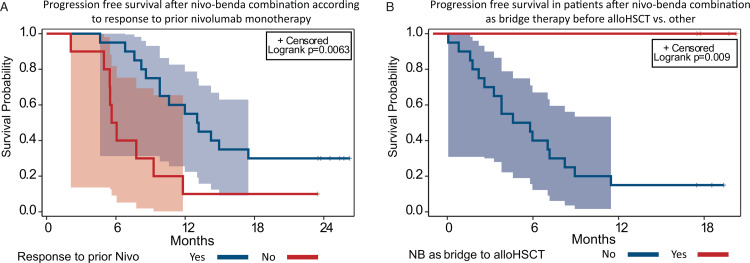
Since there was only one patient dead, the statistical analysis of factors influencing OS was not possible. The influence of set of clinical factors on PFS was analyzed (Supplementary Table 1). Previous bendamustine treatment, as well as response to bendamustine did not affect the PFS after NB therapy (Supplementary Figure 3). The best response to previous nivolumab monotherapy had influence on PFS (p = 0.02). Patients that had disease progression as BOR to nivolumab monotherapy had significantly worse PFS of 10.0% (95% CI, 0%–28.6%) with a median of 5.9 months (95% CI 2.1–9.3 months) vs 30% (95% CI, 10%–50%) with a median 13.1 months (95% CI, 8.6–N/A months) in patients with other types of response (p = 0.006) (Fig. 3A). Although BOR to NB was a significant factor in analysis across all responses (p = 0.0001), remarkably, CR had not improved the PFS after combination therapy compared to other types of responses (CR vs PR+SD+IR) (p = 0.91) (Supplementary Figure 4).
Figure 3.
Progression free survival regarding best reponse to nivolumab monotherapy and alloHSCT consolidation. A. Progression-free survival (PFS) in patients with progression as best response achieved during nivolumab monotherapy vs other patients. B. Progression-free survival (PFS) in patients with nivolumab-bendamustine as bridge to allogeneic stem cell transplantations vs other patients.
Follow up and additional treatment
Overall, 27 patients received additional therapy after the study treatment, including 9 patients that underwent allogeneic stem cell transplantation. The design of the trial allowed the additional therapy after the end of study treatment at the discretion of the treating physician. The type of next treatment in the analyzed population summarized in the Supplementary Table 2. Five patients had undergone the alloHSCT as the consolidation of response achieved with NB combination regimen and therefore having combination as the bridge therapy before alloHSCT. The median time from NB treatment to alloHSCT was 6 months (2–10 months). To reduce the bias during assessment of the role of alloHSCT, the landmark analysis was performed. The landmark analysis of alloHSCT consolidation role included patients who sustained response at 6 months after NB combination and included 4 patients in alloHSCT group vs 20 with other treatment strategies. None of the patients in alloHSCT group had disease relapse or progression (with median PFS not reached) versus 15% PFS (95% CI, 0–30.4%) with a median 5.2 months (95% CI, 2.2–8.2 months) in other strategies (p = 0.009) (Fig. 3B). In 9 patients (6 CR, 2 PR, 1 IR) additional treatment was performed for maintenance/consolidation of achieved response: in 4 nivolumab monotherapy was continued, 3 received other nivolumab combination, 1 BV and 1 received chemotherapy. The median time from NB treatment to initiation of additional therapy was 4 months (2–6 months). To reduce the bias during assessment of the additional treatment influence, the landmark analysis was performed. The landmark analysis excluded patients with consolidation of response with alloHSCT as well as patients progressed before landmark time (4 months). There were no benefit regarding PFS in patients receiving consolidation/maintenance treatment: 13.3% (95% CI, 0%–30.5%) with a median 5.8 months (95% CI, 2.8–9,0) vs 11,1% (95% CI, 0%–31.6%) with a median 5.8 months (95% CI, 1.0–10.2 months) (p = 0.77) (Supplementary Figure 5).
At the moment of last follow up, 29 patients remain alive. One death in a patient with PR after the treatment was associated with early complications after haploidentical stem cell transplantation and was not attributed to early toxicity of the combined treatment nor lymphoma progression.
Toxicity
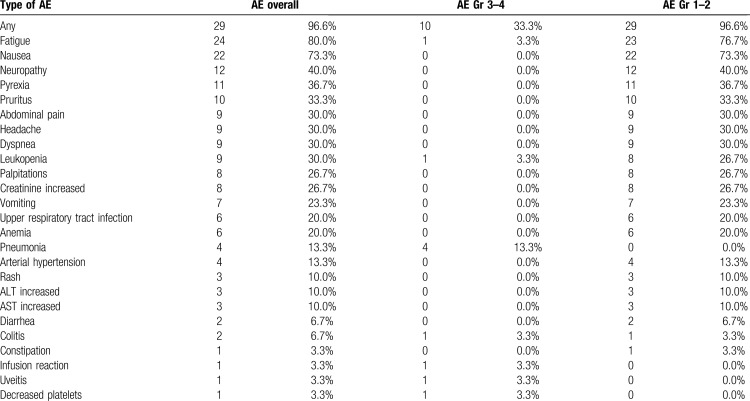
Adverse events during nivolumab-bendamustine combination treatment were summarized in Table 4. A total of 29 (96.6%) patients had adverse events of any grade, 10 patients (33.3%) experienced a severe grade 3 or 4 AE. There were no cases of fatal toxicities. The most common adverse event was fatigue (24, 80.0%), followed by nausea (22, 73.3%) neuropathy (12, 40.0%) and pyrexia during first 3 to 4 days after the infusion (11, 36.7%). The most frequent severe AE was pneumonia, observed in 4 patients (13.3%). Severe toxicities were associated with the prior therapy intensity: patients with severe AE had significantly higher number of previous therapy lines (p = 0.04). The structure of AE's considered as immune-related presented in Supplementary Table 3. In case of severe immune related adverse events, combination treatment was discontinued, treatment with methylprednisolone 1 mg/kg was initiated with complete resolution of observed AE's. Among patients enrolled in the study 6 patients (20%) had hypothyroidism associated with previous nivolumab monotherapy with none of the cases worsen during combination treatment.
Table 4.
Adverse Events Observed in the Analyzed Population.
Discussion
This report presents the first results of a prospective trial assessing the efficacy and toxicity of NB combination in patients relapsed or refractory to nivolumab monotherapy. Overall, the combination of nivolumab and bendamustine was highly efficient with ORR and CR rates of 87% and 57% respectively and an excellent 2-years overall survival of 96.7%. The structure of response differs favorably from the reported responses to the bendamustine monotherapy (ORR 53%, CR 33%) in comparable patients’ population.20 In spite of this, the study clearly demonstrates that this combination has only modest potential for long-term control of the disease. While most patients had received additional therapy to consolidate the achieved response, the 2 year PFS was only 23.3% (95% CI, 8.2%–38.4%) with a median duration of response of 6.6 months (95% CI 3.9–11.6 months). At the time of analysis, only 2 patients (6.7%) were free of disease relapse/progression with no additional therapy.
In general, the combination has an acceptable toxicity profile. For most patients the adverse effects were limited to flu-like syndrome that included fatigue, nausea and pyrexia, with no signs of active infection, possibly representing the immune activation caused by ICI and chemotherapy combination. In the structure of severe toxicities, the infectious complications were the most important with pneumonia observed in 4 (13.3%) patients. In this heavily pretreated patient population, the risk of severe adverse events increased with number of previous therapy lines. The number of immune-related adverse events was in line with expected rate demonstrated for the nivolumab monotherapy.9,10 Severe immune adverse events were limited to grade 3 and completely resolved after glucocorticosteroid therapy.
Prognostic factors analysis showed that the key prognostic factor for PFS was the best response during prior nivolumab therapy. Patients that had disease progression as BOR to nivolumab monotherapy have significantly worse prognosis regarding the progression free survival (p = 0.006). Importantly, the previous bendamustine treatment had no influence on BOR and PFS after combination therapy.
Because this combination has only limited potential for cure, one of the main conclusions of the study is the need for consolidation after NB treatment. The study failed to show any benefit (regarding PFS and DOR) from further maintenance treatment with nivolumab monotherapy or nivolumab based combinations. In contrast, consolidation of response achieved with NB by allogeneic stem cell transplantation significantly improved the outcomes of patients. To address the possible selection bias, the landscape analysis was used to assess the influence of alloHSCT. Hence, nivolumab-bendamustine combination can be considered as efficient bridge therapy with potential for disease control before alloHSCT.
Important point for discussion is an actual need for combined treatment in analyzed patients population, as opposed to chemotherapy alone or continuation of nivolumab. The examination of T-cell subsets in lymphoma patients demonstrated that the effect of PD-1 blockade on immune cells populations can be detected at least in 6 to 9 months after discontinuation of immunotherapy.22 These observations can explain the chemosensitization effect significantly deferred from the cessation of ICI and increased chemotherapy potential with no additional anti-PD-1 treatment.18,19 Treatment beyond progression is an alternative viable option for this patient population as the data from phase 2 CheckMate 205 trial demonstrated the benefit of nivolumab continuation despite progression defined by conventional criteria.9 Results of the current study, along with LYSA retrospective trial that showed higher activity of concomitant use of chemotherapy with PD-1 blockade (86% ORR) versus chemotherapy alone (59% ORR),18 create rationale for combined therapy approach. Therefore to date, the optimal strategy for patients with r/r cHL after nivolumab failure is not defined and should be tested in the future prospective trials.
The limitation of this report was the significant proportion of patients that received additional treatment after assessment of response to NB. Additional therapy was performed at discretion of treating physician based on the response to NB, number of prior therapies, availability of HLA matched donors and the preference of the patient. While it hampers the PFS and DOR analysis, in this severely pre-treated cHL patients population, in which all of the patients already received an anti-PD-1 therapy and most were treated with bendamustine, the restriction of additional therapy after the end of study treatment (and assessment of response) was considered unethical, especially in patients with residual tumor. In the current study, this limitation was specifically addressed by analysis of event-free survival, in which additional treatment was considered as an event. Also, due to the limitation of the analyzed population (30 patients) the results of the subgroup comparisons and landmark analyses should be interpreted with caution.
In summary, the combination of nivolumab and bendamustine as salvage therapy in r/r cHL after failure of nivolumab monotherapy is highly active, has acceptable toxicity profile, the modest potential to induce the durable remissions and can be considered as an efficient bridge therapy before alloHSCT in selected patients. Future studies with longer follow up and expanded patient population could determine the place of this combination in the treatment of patients with r/r cHL.
Supplementary Material
Footnotes
Citation: Lepik KV, Mikhailova NB, Kondakova EV, Zalyalov YR, Fedorova LV, Tsvetkova LA, Kotselyabina PV, Borzenkova ES, Babenko EV, Popova MO, Darskaya EI, Baykov VV, Moiseev IS, Afanasyev BV. A Study of Safety and Efficacy of Nivolumab and Bendamustine (NB) in Patients With Relapsed/Refractory Hodgkin Lymphoma After Nivolumab Monotherapy Failure. HemaSphere, 2020;00:00. http://dx.doi.org/10.1097/HS9.0000000000000401
This study was supported by “AdVita” charitable foundation.
The authors have no conflicts of interest to disclose.
References
- 1.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–2071. [DOI] [PubMed] [Google Scholar]
- 3.Arai S, Fanale M, Devos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533. [DOI] [PubMed] [Google Scholar]
- 4.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134:1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36:1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepik KV, Mikhailova NB, Moiseev IS, et al. Nivolumab for the treatment of relapsed and refractory classical Hodgkin lymphoma after ASCT and in ASCT-naïve patients. Leuk Lymphoma. 2019;4:1–4. [DOI] [PubMed] [Google Scholar]
- 11.Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazzari C, Karachaliou N, Bulotta A, et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther Adv Med Oncol. 2018;10:1758835918762094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the Nuclear Factor- κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. [DOI] [PubMed] [Google Scholar]
- 14.Liu WM, Fowler DW, Smith P, et al. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearl TJ, Jing W, Gershan JA, et al. Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol. 2013;190:5620–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svoboda J, Ballard H, Chong E, et al. Use of bendamustine for lymphodepletion before tisagenlecleucel (anti-CD19 CAR T cells) for aggressive B-cell lymphomas. Blood. 2019;134(Supplement_1):1606. [Google Scholar]
- 17.Herrera A, Chen R, Palmer J, et al. PET-adapted nivolumab or nivolumab plus ICE as first salvage therapy in relapsed or refractory hodgkin lymphoma. Blood. 2019;134(Supplement_1):239.31076442 [Google Scholar]
- 18.Rossi C, Gilhodes J, Maerevoet M, et al. Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory Hodgkin lymphoma: a series from Lysa centers. Am J Hematol. 2018;8:1042–1049. [DOI] [PubMed] [Google Scholar]
- 19.Carreau NA, Pail O, Armand P, et al. Checkpoint blockade therapy may sensitize hodgkin lymphoma to subsequent therapy. Blood. 2018;132:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31:456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–317. [DOI] [PubMed] [Google Scholar]
- 22.Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood. 2017;129:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.