Abstract
Various agents are currently under evaluation as potential treatments in the fight against coronavirus disease 2019 (COVID-19). Plasma from patients that have overcome COVID-19 infection, referred to as convalescent plasma, is a treatment option with considerable background in viral diseases such as Spanish influenza, H1N1, Ebola, Severe Acute Respiratory Syndrome (SARS), and Middle East Respiratory Syndrome (MERS). Although convalescent plasma has historically proven beneficial in the treatment of some viral diseases, its use is still explorative in the context of COVID-19. To date, preliminary evidence from case series is favorable as significant clinical, biochemical improvement and hospital discharge have been reported. A detailed overview of randomized as well non-randomized trials of treatment with convalescent plasma, which have been registered worldwide, is provided in this review. Based on these studies, data from thousands of patients is anticipated in the near future. Convalescent plasma seems to be a safe option, but potential risks such as transfusion-related acute lung injury and antibody-dependent enhancement are discussed. Authorities including the Food and Drug Administration (FDA), and scientific associations such as the International Society of Blood Transfusion (ISBT) and the European Blood Alliance (EBA), have provided guidance into the selection criteria for donors and recipients. A debatable, pivotal issue pertains to the optimal timing of convalescent plasma transfusion. This treatment should be administered as early as possible to maximize efficacy, but at the same time be reserved for severe cases. Emerging risk stratification algorithms integrating clinical and biochemical markers to trace the cases at risk of significant deterioration can prove valuable in this direction.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia was first noted in Wuhan (China) in December 20191 and the disease induced by the virus has been termed coronavirus infectious disease 2019 (COVID-19). To date, various treatment regimens are being evaluated as potential tools in COVID-19 in addition to the standard supportive care including oxygen supply, intensive care admission, or even extracorporeal membrane oxygenation for critically ill patients.2 Among agents, antiviral drugs such as remdesivir,3 lopinavir/ritonavir,4 the antimalarial agent hydroxychloroquine in combination with azithromycin,5 and monoclonal antibodies, such the anti-interleukin-6 receptor tocilizumab,6–8 are currently under evaluation for treatment of COVID-19.
Plasma from patients that have overcome COVID-19 infection, namely convalescent plasma, is a treatment with considerable historical background in other diseases, but still explorative in the context of SARS-CoV-2. In a pandemic, convalescent plasma could provide an easily accessible source of antiviral antibodies. Indeed, fresh frozen plasma (FFP) is an established treatment in many clinical indications with a well-known safety profile. The present article summarizes available evidence about convalescent plasma in COVID-19, registered trials, and guidance from authorities, providing a critical overview of published studies and perspectives.
Historical evidence for convalescent plasma in other epidemics
In recent history, convalescent plasma has been successfully used in viral outbreaks and epidemics. In as early as the 1918–1925 Spanish influenza pandemic, studies evaluated convalescent blood products to treat pneumonia due to Spanish influenza in hospitals, presenting assessment versus a control or comparison group. A meta-analysis conducted almost a century later (2006) showed a sizable reduction in overall crude fatality rate, from 37% among controls to 16% among patients treated with convalescent plasma. Benefit was maximized among patients receiving the treatment early, namely within the first four days of pneumonia complications.9 Although these early epidemiological studies had been rather rudimentary in their design and were not blinded, randomized, nor placebo-controlled, they underlined the beneficial role of convalescent plasma that prompted modern researchers to support a role of this regimen in a possible future H5N1 influenza pandemic. Convalescent serum had also been used during the first half of the 20th century for measles,10 poliomyelitis,11 and mumps.12
Several decades later, in the context of pandemic influenza A (H1N1) 2009 virus infection, convalescent plasma treatment was able to significantly reduce respiratory tract viral load, serum cytokine response (interleukin-6, interleukin-19, tumor necrosis factor-alpha), and mortality in a comparative study recruiting 99 patients. In that study, the decrease in mortality was rather impressive, as the odds of death decreased by 80%.13 A subsequent systematic review and meta-analysis synthesized 32 studies of severe acute respiratory syndrome (SARS) coronavirus infection and severe influenza and highlighted the consistent evidence for a reduction in mortality, especially in case of early administration of convalescent plasma and hyperimmune immunoglobulin after symptom onset. The meta-analysis confirmed the sizable reduction in the odds of mortality, pointing to a decrease by 75% in the odds of death.14
In the case of Middle East Respiratory Syndrome (MERS), a protocol of convalescent plasma therapy for patients with the disease was established in 2015. According to this protocol, subjects with an anti-MERS-coronavirus indirect fluorescent antibody titer of 1:160 or more would be screened for eligibility for plasma donation in line with standard donation criteria, provided that they were free of clinical or laboratory evidence of active MERS infection.15 Nevertheless, challenges of this approach were highlighted in the Korean MERS outbreak where Ko et al supported that donor plasma with a neutralization activity of a titer 1:80 or more in the plaque reduction neutralization test should be adopted, whereas ELISA IgG could provide an alternative for the neutralization test in conditions where the former is not available.16
In the context of Ebola virus, a meta-analysis of clinical studies was conducted during the West Africa Ebola virus disease outbreak, gathering a pool of 1147 individuals. This analysis acknowledged that studies were considerably limited by the lack of randomization.17 A large, non-randomized study of 84 patients pointed to a non-significant 7% decrease in death risk following transfusion of up to 500 mL of convalescent plasma, albeit with unknown levels of neutralizing antibodies.18 Nevertheless, transfusion of convalescent plasma or whole blood collected from patients that recovered from Ebola virus has been recommended by the World Health Organization as an empirical treatment for the Ebola outbreaks, with provision of guidance also about the selection of donors, screening, and handling of blood and plasma units.19
Convalescent plasma in COVID-19 disease: emerging evidence from published studies
Table 1 presents the results of published studies that have evaluated transfusion of convalescent plasma in COVID-19 patients.20–26 Shen et al20 first presented the experience on critically ill patients in Shenzhen (China). All patients received a single dose of 400 mL convalescent plasma from donors with neutralizing antibody titer ranging between 1:80 and 1:480. After the transfusion, the titers of anti-SARS-CoV-2 IgG and IgM in recipients increased in a time-dependent manner. Three patients were discharged, whereas the remaining 2 remained hospitalized until the end of the study, with improvement in body temperature and clinical and biochemical indices. Notably, convalescent plasma was administered relatively late during the disease in all patients, namely between days 19 and 22 of hospitalization, except for 1 patient who received the treatment on day 10.20 All patients at the time of convalescent plasma administration had detectable IgM and IgG antibodies and neutralizing antibodies titers ranging from 1:40 to 1:160. All titers of antibodies increased in a time-dependent manner after transfusion.
Table 1.
Studies and Main Findings of Published Studies Examining Convalescent Plasma in COVID-19 Patients.
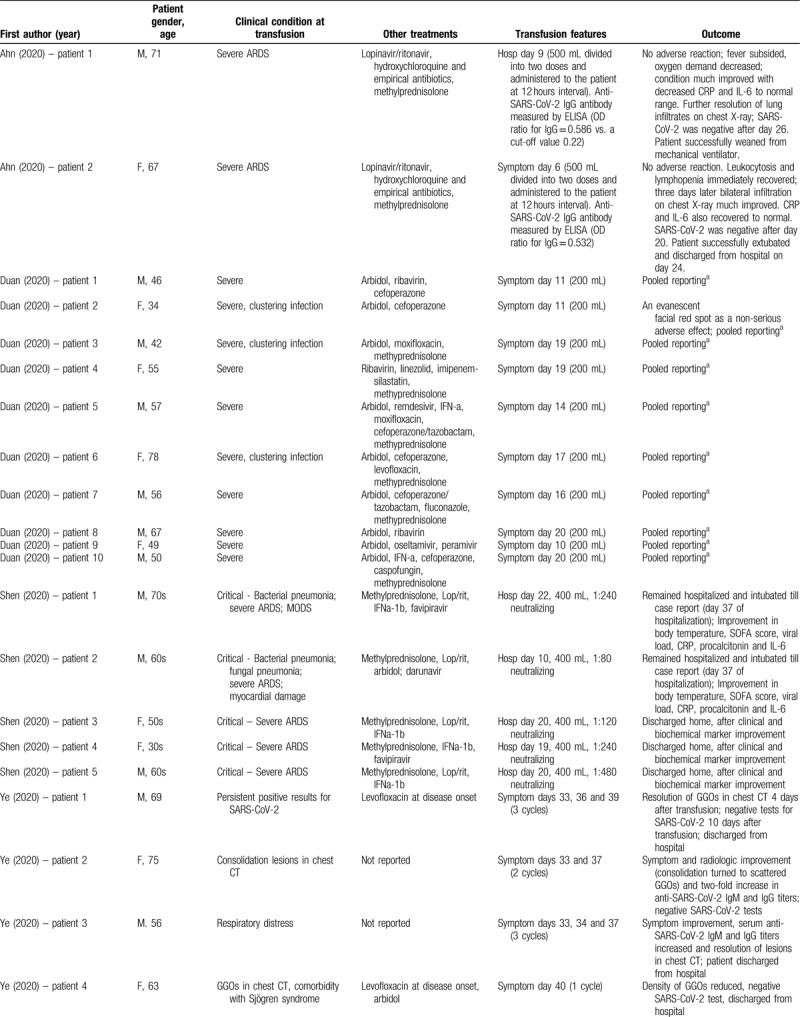
Zhang et al21 adopted a richer regimen of convalescent plasma transfusions in their series of four COVID-19 patients in Guangdong (China), with total volumes administered ranging between 200 and 2400 mL. All patients in severe condition received convalescent plasma after day 12 of hospitalization. Very positive outcomes were reported, as three of 4 patients were discharged from the hospital, whereas the remaining one was finally PCR negative for the virus and was transferred to an unfenced intensive care unit. Two cases produced anti-SARS-CoV-2 IgG approximately 14 days after transfusion.21
The largest series published up to date is the one by Duan et al,22 encompassing 10 COVID-19 patients in China, administering convalescent plasma with neutralizing antibody titers above 1:640. Among them, those who received the transfusion relatively early during the disease (days 10 and 11 from initiation of symptoms) showed a rapid increase in lymphocyte counts, decrease in serum CRP levels, and a notable remission of lung lesions in CT. The neutralizing antibody titers of 5 patients increased rapidly up to 1:640, whereas 4 patients remained at the same high level (1:640) after transfusion. Overall, the results were excellent, as no deaths were noted, 3 patients were discharged, and the remaining 7 were ready for discharge; this is in contrast to a historical control group presented by the study authors, where a death rate of 30% was noted. No serious adverse effects were documented following transfusion of convalescent plasma; only a minor effect was reported by a patient, namely an evanescent facial red spot.22
The study by Ye et al23 in Wuhan (China) encompassed 6 patients and examined the transfusion of convalescent plasma in a spectrum of clinical scenarios, including persistent SARS-CoV-2 detection, consolidation or extensive lesions in chest CT, comorbidity with Sjögren syndrome and post-discharge positivity to SARS-CoV-2; improvement and promising results were noted in all cases. Similarly, Zhang et al24 reported improvement in a patient from Nanjing (China) receiving convalescent plasma. Ahn et al25 provided the first report on 2 cases from Korea, where the administration of convalescent plasma was linked to beneficial effects, namely successful weaning from mechanical ventilator in one patient and hospital discharge in the other patient. On the other hand, the study by Zeng et al,26 from Zhengzhou (China), published in late April, 2020, highlighted the limitations of the new treatment; administration of convalescent plasma in critical, end-stage respiratory failure, at a late stage during the course of the disease (median: 21.5 days after first detection) was associated with suppression of viral shedding but ultimately death in 5 out of 6 examined cases.26
In line with the published case series concerning the optimal timing of convalescent plasma administration, a recent review by Tiberghien et al27 has presented a strategy of administration for high-risk patients (older than age 70 or dependent on oxygen with a baseline oxygen saturation <94%). According to preliminary remarks by the aforementioned research team, early treatment with convalescent plasma (not later than day 5) is preferred before seroconversion for SARS-CoV-2, which may occur on days 6 to 12. A regimen proposed by Tiberghien et al is the slow transfusion of two plasma units, under careful monitoring, for patients whose weight is within the 50 to 80 kg range. The volume is adjusted according to weight, whereas a second infusion of 2 additional units 1 or 2 days later can also be considered.27 The need for early administration is in line with observations in other diseases, such as pneumococcal pneumonia, where no benefit is noted if the antibody is administered after day 3 of the disease.28,29
In light of the promising evidence from case series presented above, there is a clear need for randomized controlled trials on large patient numbers to evaluate the efficacy of the process. Apart from sample size and the non-comparative, non-randomized study design, numerous limitations hamper the interpretation of the aforementioned studies, such as the superimposition of effects mediated by other antiviral treatments, antibiotics, and glucocorticoids administered concomitantly with convalescent plasma. As a whole, these studies indicate that patients receiving transfusions earlier than 14 days post infection may benefit from convalescent plasma treatment.
Mechanism of actions
Convalescent plasma may offer various beneficial actions in COVID-19 disease (Fig. 1). First and foremost, the apparent mechanism pertains to the fact that antibodies from convalescent plasma can suppress viremia.1 Similar to the strategies implemented in the SARS epidemic, theoretically the administration of convalescent plasma at the early stage of the disease would be more effective.30 Viremia peak is noted in the first week of infection in the majority of viral illnesses and a primary immune response of the host is usually developed by days 10 to 14 of infection31 (beginning somewhat earlier according to other researchers),27 signaling the clearance of the viruses. Other potential mechanisms include antibody-dependent cellular cytotoxicity, complement activation and phagocytosis (ADCP).29 Moreover, the presence of non-neutralizing antibodies binding to the pathogens may also be helpful.32 In any case, the administered antibody modifies inflammatory response and this can be optimally achieved during the early response, even at the asymptomatic stage.33 It has also been suggested that apart from the direct anti-viral properties, plasma components can provide other beneficial actions, such as restoring coagulation factors.34
Figure 1.
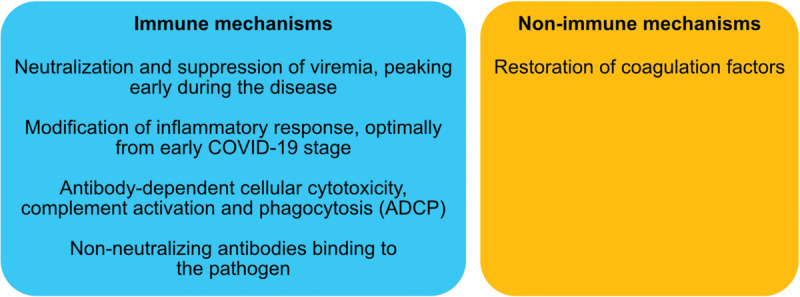
Potential mechanisms of action of convalescent plasma in COVID-19.
So far, information on immune response to SARS-CoV-2 is rather limited. According to studies in process, a detailed analysis of 9 cases with mild upper respiratory tract symptoms revealed that seroconversion occurred 6 to 12 days after onset of symptoms, while antibodies were not detectable between day 3 and 6, and all patients showed neutralizing antibodies after 2 weeks. Seroconversion coincided with a slow but steady decline of sputum viral load.35 In another study, the majority of PCR-confirmed SARS-CoV-2-infected persons seroconverted by 2 weeks after disease onset.36 A study on 173 COVID-19 patients showed that the presence of antibodies was less than 40% within the first week since onset, increasing to 94.3% for IgM and 79.8% for IgG since day 15 after onset, and higher titer of total antibodies correlated with worsened clinical classification.37 To further assess the time for seroconversion and its correlation with disease severity and antibody titers, additional longitudinal studies evaluating large numbers of serum samples from COVID-19 patients with a broad spectrum of clinical symptoms are needed.
Registered trials on convalescent plasma in COVID-19 disease
Tracing the progression of research on the potential utilization of convalescent plasma in COVID-19, 11 studies were identified in the clinicaltrials.gov register and their main features are summarized in Table 2 . Of these 11 studies, 6 were single-arm, 4 were randomized comparative studies, and 1 pertained to the expanded access status for convalescent plasma (NCT04338360). Various countries have been implicated in these studies, including the USA, China, Colombia, Iran, Italy, Mexico, and the Netherlands. If completed, these studies would examine a minimum total of 1106 patients. At present, 5 studies are either recruiting, enrolling by invitation, or active and providing expanded access; six studies are not yet recruiting.
Table 1 (Continued).
Studies and Main Findings of Published Studies Examining Convalescent Plasma in COVID-19 Patients.
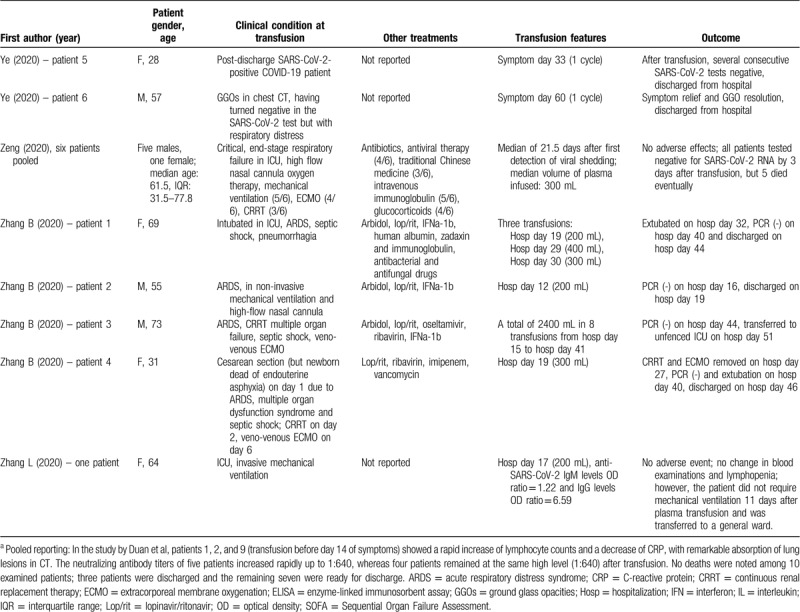
Table 2 (Continued).
Studies Registered in Clinicaltrials.gov, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 09, 2020). Withdrawn Studies were not Included.
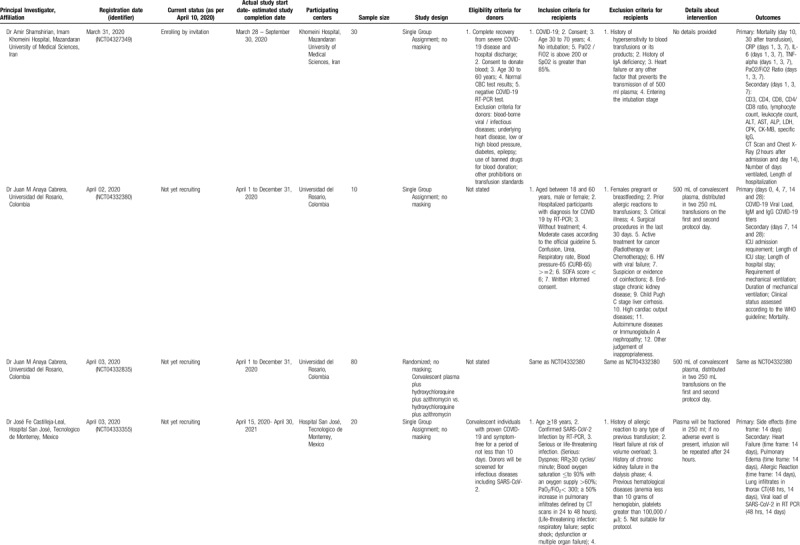
Table 2 (Continued).
Studies Registered in Clinicaltrials.gov, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 09, 2020). Withdrawn Studies were not Included.
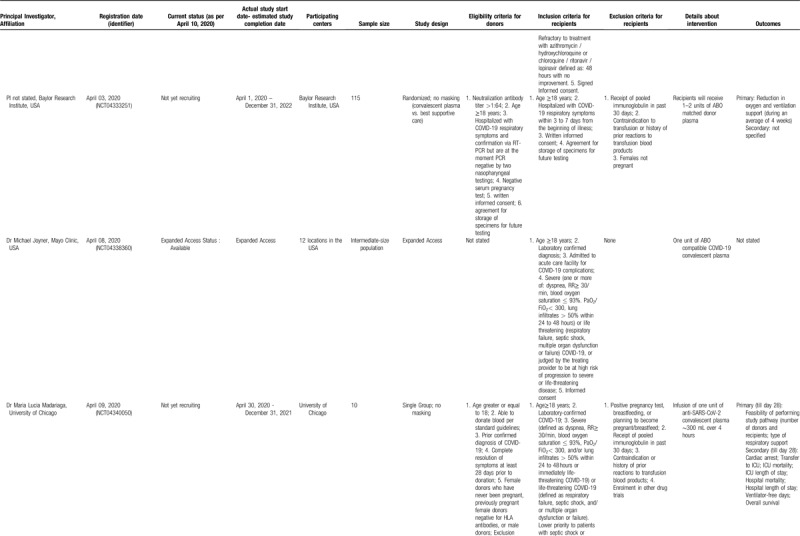
Table 2 (Continued).
Studies Registered in Clinicaltrials.gov, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 09, 2020). Withdrawn Studies were not Included.
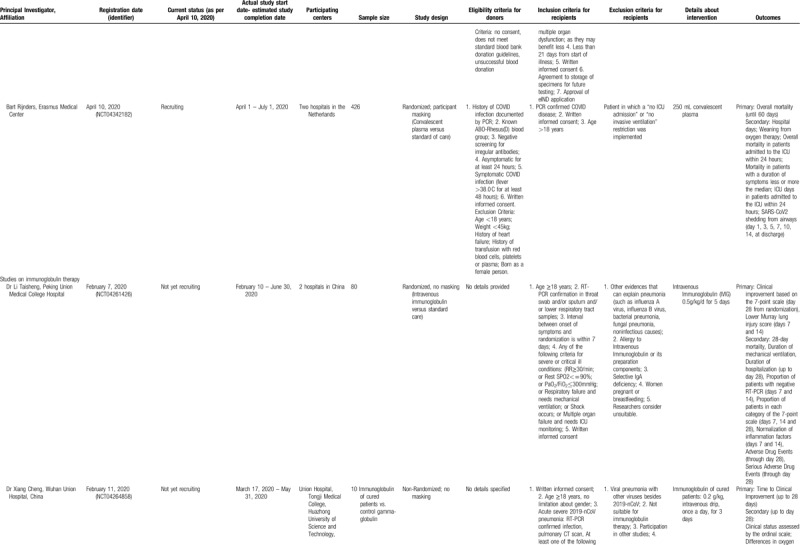
Table 2 (Continued).
Studies Registered in Clinicaltrials.gov, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 09, 2020). Withdrawn Studies were not Included.

A cardinal point pertains to the timing of convalescent plasma administration in the study protocols, but the majority of studies did not provide this information. However, some studies necessitated an interval of 3 to 7 days from the beginning of illness (NCT04333251) or, less strictly, acute respiratory distress syndrome (ARDS) lasting less than 10 days (NCT04321421). Variable degrees of severity have been adopted as inclusion criteria; some studies focus on severe cases, whereas there were also studies focusing on less severe cases, as intubation (NCT04327349) or critical illness (NCT04332380) was an exclusion criterion. The total amount of convalescent plasma ranges from 1 to 3 units.
A particularly interesting study protocol (NCT04323800) involves the use of convalescent plasma as a prophylaxis for COVID-19. According to this protocol, convalescent plasma administration will be tested within 120 hours after high-risk contact exposure with a person with confirmed COVID-19. Individuals at high risk for a severe COVID-19 illness will be recruited, including elderly subjects and patients suffering from chronic conditions. This strategy of prophylaxis has been successfully implemented in the prevention of other viral diseases via passive immunity, such as the case of administration of hepatitis B immune globulin, human rabies immune globulin, and polyclonal hyperimmune globulin for respiratory syncytial virus (RSV), or more recently palivizumab, a humanized murine monoclonal antibody for high-risk infants.29,38 In addition to these studies, reports in the media have been appearing about other countries, such as Canada, starting to test convalescent plasma in COVID-19.39
Concerning immunoglobulin therapy in COVID-19, 2 studies were identified (Table 2 , lower panels). The first one (NCT04261426) focuses on early administration of the immunoglobulin in severe cases, adopting an eligibility interval between the onset of symptoms and randomization that should not surpass 7 days. The second (NCT04261426) is a small exploratory study on 10 patients with the aim to evaluate this experimental treatment in severe COVID-19 pneumonia cases in China.
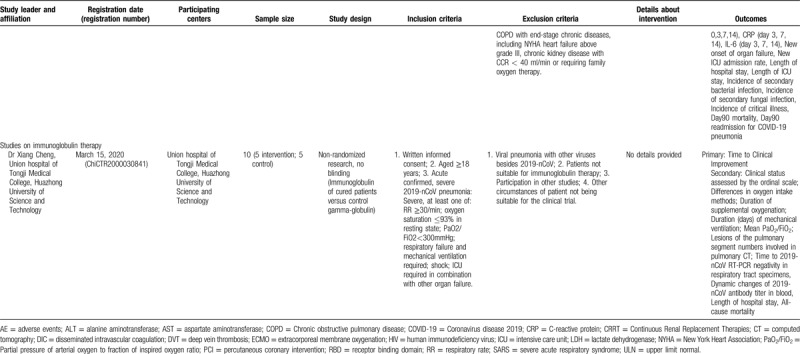
The examination of studies registered in the Chinese Clinical Trial Registry (Table 3 ) revealed a different pattern. In the registry, 10 studies on convalescent plasma were identified; among them, the majority (6 trials) were randomized comparative (vs conventional treatment with/without ordinary plasma), three were non-randomized comparative, and only was a single-arm trial. If all studies are complete, a total of 680 patients will have been evaluated. All studies are focusing primarily on severe or critical cases; among the exclusion criteria, pregnancy, lactation, immunoglobulin allergy/allergy to plasma, immunoglobulin A deficiency, and other contraindications for plasma transfusion (such as heart failure) have been often stated. Interestingly, some studies (ChiCTR2000029757, ChiCTR2000030010, ChiCTR2000030702, ChiCTR2000030929) provide shock and disseminated intravascular coagulation as an exclusion criterion. No details have been provided regarding the selection of donors. Regarding immunoglobulin therapy for COVID-19, the identified study in the Chinese Clinical Trial Registry (ChiCTR20000308410) seemed to correspond to the previously presented study in clinicaltrials.gov by the same principal investigator (NCT04264858).
Table 2.
Studies Registered in Clinicaltrials.gov, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 09, 2020). Withdrawn Studies were not Included.
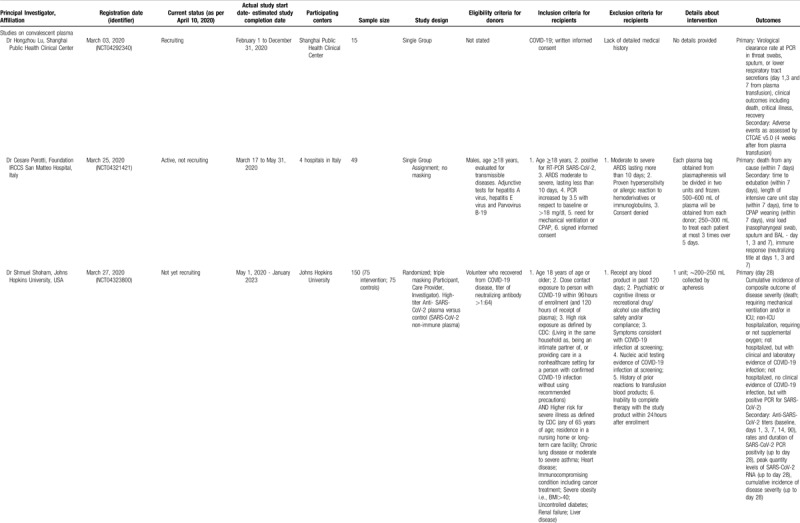
Table 3.
Studies Registered in the Chinese Clinical Trial Registry, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 10, 2020). Cancelled Studies have not been Included.
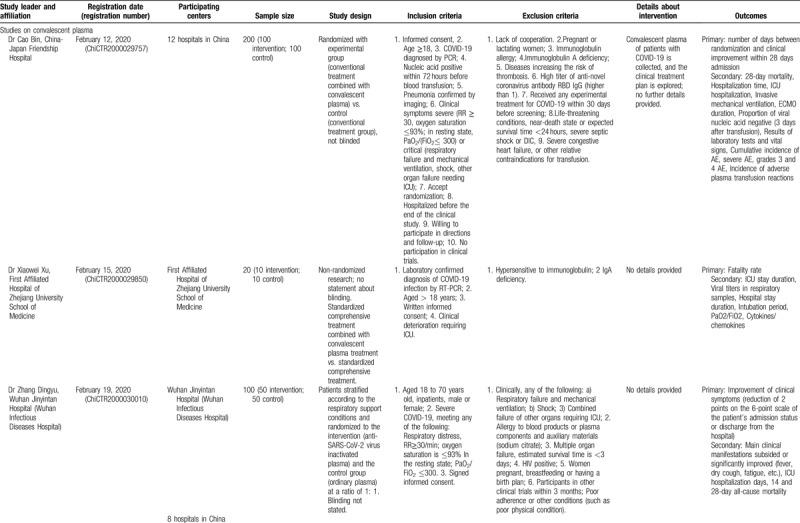
Table 3 (Continued).
Studies Registered in the Chinese Clinical Trial Registry, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 10, 2020). Cancelled Studies have not been Included.
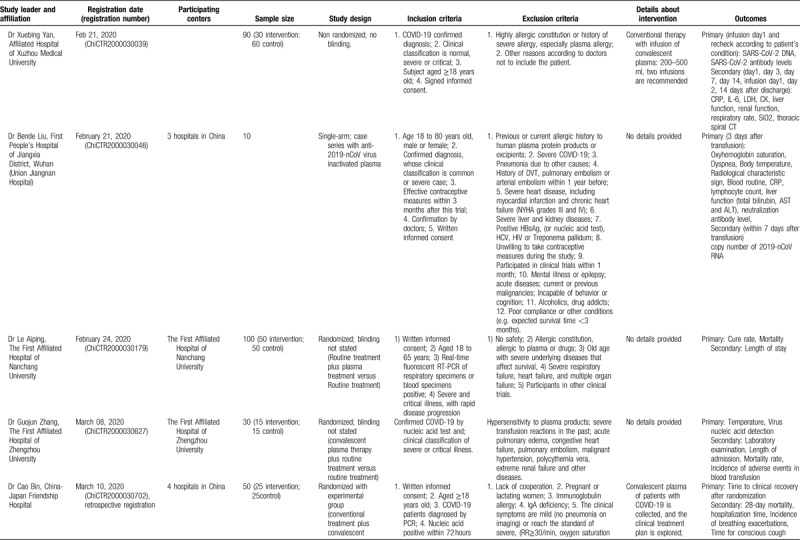
Table 3 (Continued).
Studies Registered in the Chinese Clinical Trial Registry, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 10, 2020). Cancelled Studies have not been Included.
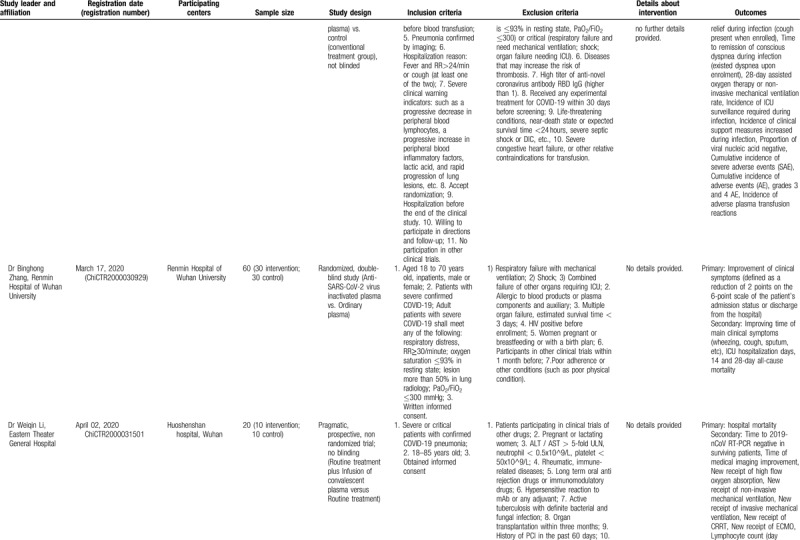
Table 3 (Continued).
Studies Registered in the Chinese Clinical Trial Registry, to Evaluate Convalescent Plasma or Immunoglobulin Therapy in COVID-19 Patients (updated on April 10, 2020). Cancelled Studies have not been Included.
Current situation – American and European framework for donors
On March 24, 2020, the Food and Drug Administration (FDA) took an important step facilitating access to COVID-19 convalescent plasma to be used in COVID-19 patients at a serious or immediately life-threatening stage of the disease, allowing the process of single patient emergency Investigational New Drug Applications (referred to as eINDs) under Title 21 of the Code of Federal Regulations (CFR) 312.310. Under this process, convalescent plasma can be used for the treatment of an individual patient by a licensed physician upon the authorization of the FDA.
According to the FDA, eligible donors could be recovered COVID-19 patients who had been proven positive either by a diagnostic test such as nasopharyngeal swab at the time of illness, or antibody-positive patients on whom a diagnostic test had not been performed during their illness. The level of neutralizing antibody titers should be greater than 1:160, whereas a titer of 1:80 could be deemed acceptable if alternative matching units are not available. Symptoms should have resolved completely at least 28 days prior to donation; alternatively, a symptom-free interval of at least 14 days prior to donation and negative results in one or more nasopharyngeal swabs or in blood-based molecular diagnostic tests are necessitated. Male donors are eligible; special attention is paid to female donors who should be negative for human leukocyte antigen (HLA) antibodies in case of previous pregnancy. General donor eligibility requirements along with the additional criteria for plasmapheresis should be also met, including infection status control. The FDA has also provided guidance on blood establishment standards, labeling, and recordkeeping.40
Regarding donors, the International Society of Blood Transfusion (ISBT) Working Party set an interval of 14 days or more after full recovery and necessitated a non-reactivity of the sample for human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), syphilis, and locally transmitted infections. As a means to avoid the incidence of transfusion-related acute lung injury (TRALI)—a serious condition emerging within 6 hours from transfusion—donors should preferably be either males or females who have never been pregnant, including abortions.41 On April 4, 2020, the European Commission issued the guidance document on the collection and transfusion of convalescent COVID-19 plasma, adopting similar criteria regarding donor eligibility. Notably, the titers of neutralizing antibodies for donors according to the document were set at a level greater than 1:320, although it was recognized that lower thresholds could also be effective.42
Convalescent plasma recipients and blood establishments
According to the FDA, eligible recipients of convalescent plasma should be COVID-19 positive patients with severe disease (dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio less than 300, and/or lung infiltrates > 50% within 24 to 48 hours) or a life-threatening disease (respiratory failure, septic shock, multiple organ dysfunction) who have given informed consent for the procedure.40 Nevertheless, as evidenced by the registered trials (Tables 2 and 3), a wide variety of selection criteria have been envisaged. Following the FDA's National Expanded Access Treatment Protocol, 2115 sites have registered to participate to convalescent plasma administration by April 27, 2020, enrolled a total of 5,968 patients, with 2576 receiving convalescent plasma.43
Convalescent plasma administration seems to be a safe procedure free from serious adverse effects. Meticulous selection of donors can minimize the risk of TRALI. Another potentially concerning phenomenon pertains to antibody-dependent enhancement (ADE) of coronavirus entry. This has been reported in viral diseases and refers to an enhancement of disease in the presence of certain antibodies.44 Keeping in mind the high titers of neutralizing antibodies that convalescent plasma includes against the same virus (SARS-CoV-2), as well as the previously documented safe experience in SARS and MERS, the occurrence of ADE does not seem to represent a major problem; however, surveillance is warranted.29
In accordance to the Infectious Diseases Society of America Guidelines on the treatment and management of patients with COVID-19 infection, convalescent plasma is recommended in the framework of a clinical trial.45 Similarly, the International Society of Blood Transfusion (ISBT) Working Party on Global Blood Safety has underlined that the clinical use of convalescent plasma should be performed as an experimental therapy, ideally in the context of an organized research trial.
Plasma should be collected in certified blood establishments through legally approved blood collection or plasmapheresis equipment by trained professionals; 200 to 600 mL of plasma can be collected and in most cases, the interval between potential subsequent new donations should be longer than 7 days. Regarding collection workflow, the existing blood transfusion infrastructure can be useful. However, along with increased numbers of survivors, the increasing pool of potential donors may entail logistical challenges, spanning the assessment of donor eligibility, coordination of donor recruitment and collections, as well as transfusion.32
Facing the COVID-19 pandemic, the European Blood Alliance (EBA), together with the European Commission's Directorate-General for Informatics (DIGIT) and Directorate-General for Health and Food Safety (DG SANTE), has been developing an open database hosted on a platform by the European Commission with the aim to collect, monitor, and share all information on convalescent plasma.46 Blood establishments will organize collection, enter donor data in the database to supply plasma to hospitals, and research projects and the industry; afterwards, the patient outcomes of transfusion can serve as the basis of aggregated data, reports, and pragmatic assessment of convalescent plasma effectiveness. According to the guidance document by the European Commission, the data should include gender, age, comorbidities, time point of transfusion, number, volume and antibody titer of the unit, other therapies administered, clinical symptoms (prior to transfusion, 5 days later and at discharge for survivors), serious adverse events, and length of hospitalization.42
Critical appraisal, perspectives, and conclusions
As of April 2020, more than 350,000 people have recovered from COVID-19 worldwide.47 These individuals may offer a valuable pool of a life-saving treatment for future patients during the pandemic. Asymptomatic, antibody positive carriers may also prove helpful as donors of the disease. For instance, there is anecdotal evidence that in Northern Italy among 60 volunteer blood donors in the town of Castiglione d’ Adda, Lombardy, 67% were antibody positive although asymptomatic; nevertheless, their specific antibody titers were not declared in detail.48,49 If proven in larger cohorts, these results may be promising in terms of identifying a large number of eligible convalescent plasma donors. Thorough research into the evaluation of humoral response and neutralizing antibodies50 in the context of COVID-19 seems an important step in designing strategies pertaining to convalescent plasma.
Until now the number of COVID-19 patients with known outcomes of convalescent plasma administration is particularly limited, stemming from 6 case series.20–26 The follow-up of cases reaching hospitalizations of 51 days21 and 60 days after onset of symptoms23 highlights the need for adequate observation, but also underlines the time needed from the early, sizable Chinese cohorts (reportedly reaching 245 COVID-19 patients, with improvement in 91 of them)51 to provide robust results in relevant scientific publications.
A pivotal and controversial point is the time of convalescent plasma administration in COVID-19; that should be as early as possible to maximize efficacy, but at the same time oriented to severe cases. To this direction, the examination of risk markers and integrating clinical (gender, age, comorbidities) and biochemical aspects in a comprehensive risk stratification can provide a valuable tool for decision making, promptly tracing those patients with forthcoming poor prognosis who would most need early intervention with convalescent plasma. Emerging markers with such potential are lymphocytopenia, elevated procalcitonin, ferritin, D-dimer, and C-reactive protein.52
Along with the evaluation of convalescent plasma from blood donors, the plasma industry could take future steps, manufacturing concentrated hyperimmune globulin preparations that contain standardized antibody doses and could provide a further reach in terms of health setting administering therapy.34 As a whole, the promising results of convalescent plasma transfusion could change the course of COVID-19. The formulation, namely convalescent plasma or hyperimmune globulin, as well as the optimal time frame, remain to be identified in the future.
Footnotes
Citation: Psaltopoulou T, Sergentanis TN, Pappa V, Politou M, Terpos E, Tsiodras S, Pavlakis GN, Dimopoulos MA. The Emerging Role of Convalescent Plasma in the Treatment of COVID-19. HemaSphere, 2020;4:3:(e409). http://dx.doi.org/10.1097/HS9.0000000000000409
Theodora Psaltopoulou and Theodoros N. Sergentanis contributed equally to this work.
All authors contributed to data collection and writing of this review article.
The authors have no conflicts of interest to disclose.
References
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis. 2020;101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Wang Y, Wen D, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michot JM, Albiges L, Chaput N, et al. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020 April 2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Song K, Tong F, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020 March 29. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luke TC, Kilbane EM, Jackson JL, et al. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. [DOI] [PubMed] [Google Scholar]
- 10.Park WH, Freeman RG. The prophylactic use of measles convalescent serum. JAMA. 1926;87:556–558. [Google Scholar]
- 11.Park WH. Therapeutic use of antipoliomyelitis serum in preparalytic cases of poliomyelitis. JAMA. 1932;99:1050–1053. [Google Scholar]
- 12.Rambar AC. Mumps: use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Child. 1946;71:1–13. [PubMed] [Google Scholar]
- 13.Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arabi Y, Balkhy H, Hajeer AH, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko JH, Seok H, Cho SY, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617–622. [DOI] [PubMed] [Google Scholar]
- 17.Dodd LE, Follmann D, Proschan M, et al. A meta-analysis of clinical studies conducted during the West Africa Ebola virus disease outbreak confirms the need for randomized control groups. Sci Transl Med. 2019;11:eaaw1049. [DOI] [PubMed] [Google Scholar]
- 18.van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease. 2014; https://www.who.int/csr/resources/publications/ebola/convalescenttreatment/en/. Accessed April 08, 2020. [Google Scholar]
- 20. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020 Mar 27. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020 March 31. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;177:9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020 April 15. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Pang R, Xue X, et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging (Albany NY). 2020;12:6536–6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn JY, Sohn Y, Lee SH, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020 April 29. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tiberghien P, de Lambalerie X, Morel P, et al. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how. Vox Sang. 2020 April 2. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Xiong J, Bao L, et al. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020 April 7. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casadevall A, Pirofski LA. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24:474–478. [DOI] [PubMed] [Google Scholar]
- 34. Roback JD, Guarner J. Convalescent Plasma to Treat COVID-19: Possibilities and Challenges. JAMA. 2020 March 27. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Woelfel R, Corman M, Guggemos W, et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. 2020; . Accessed April 12, 2020. [DOI] [Google Scholar]
- 36. Okba NMA, Muller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020 April 8. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 March 28. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luke TC, Casadevall A, Watowich SJ, et al. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010;38 (4 Suppl):e66–e73. [DOI] [PubMed] [Google Scholar]
- 39. The Globe and Mail. Canada begins clinical trial of experimental COVID-19 treatment using plasma from recovered individuals. 2020; https://www.theglobeandmail.com/canada/article-canada-begins-clinical-trial-of-experimental-covid-19-treatment-using/. Accessed April 10, 2020. [Google Scholar]
- 40. Food and Drug Administration. Revised Information for Investigational COVID-19 Convalescent Plasma. 2020; https://www.fda.gov/vaccinesblood-biologics/investigational-new-drug-ind-or-device-exemptionide-process-cber/revised-information-investigational-covid-19-convalescent-plasma. Accessed April 08, 2020. [Google Scholar]
- 41. Epstein J, Burnouf T. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. 2020; https://ipfa.nl/wpcontent/uploads/2020/03/Points_to_consider_in_the_preparation_of_COVID_convalescent_plasma_-_200331_ISBT_WP_GBS_Final-1.pdf. Accessed April 10, 2020. [Google Scholar]
- 42. European Commission. An EU programme of COVID-19 convalescent plasma collection and transfusion. Guidance on collection, testing, processing, storage, distribution and monitored use. 2020; https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf. Accessed April 10, 2020. [Google Scholar]
- 43. Rubin R. Testing an old therapy against a new disease: convalescent plasma for COVID-19. JAMA. 2020 April 30. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 44.Wan Y, Shang J, Sun S, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020;94:e02015–e02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19 Infection. 2020; https://www.idsociety.org/practiceguideline/covid-19-guideline-treatment-and-management/. Accessed April 12, 2020. [Google Scholar]
- 46. European Blood Alliance. COVID-19 and blood establishments. Update April 08, 2020. 2020; https://europeanbloodalliance.eu/covid19-and-blood-establishment/. Accessed April 10, 2020. [Google Scholar]
- 47. Johns Hopkins University of Medicine. Coronavirus Resource Center. 2020; https://coronavirus.jhu.edu/map.html. Accessed April 10, 2020. [Google Scholar]
- 48. Medicine OU-TCfE-B. COVID-19: What proportion are asymptomatic? 2020; https://www.cebm.net/2020/04/covid-19-what-proportion-are-asymptomatic/. Accessed April 08, 2020. [Google Scholar]
- 49. Serra M. Coronavirus, Castiglione d’Adda è un caso di studio: “Il 70% dei donatori di sangue è positivo” [article in Italian]. 2020; https://www.lastampa.it/topnews/primo-piano/2020/04/02/news/coronavirus-castiglione-d-adda-e-un-caso-di-studio-il-70-dei-donatori-di-sangue-epositivo-1.38666481. Accessed April 08, 2020. [Google Scholar]
- 50.Felber BK, Pavlakis GN. HIV vaccine: better to start together? Lancet HIV. 2019;6:e724–e725. [DOI] [PubMed] [Google Scholar]
- 51. Xinhuanet. China puts 245 COVID-19 patients on convalescent plasma therapy. 2020; http://www.xinhuanet.com/english/2020-02/28/c_138828177.htm. Accessed April 08, 2020. [Google Scholar]
- 52. Terpos E, Ntanasis-Stathopoulos I, Kastritis E, et al. Haematological findings and complications of COVID-19. Am J Hematol. 2020 April 13. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


