Abstract
Aim
The Bruton's tyrosine kinase (BTK) inhibitor Ibrutinib (PCI-32765) is effective in patients with multiple myeloma, non-Hodgkin lymphoma and chronic lymphoblastic leukemia. We previously showed that primary cells of children with TCF3-PBX1 positive B-cell precursor acute lymphoblastic leukemia (BCP-ALL) express BTK and are sensitive to ibrutinib in vitro. However, preclinical studies in mice are lacking that justify clinical implementation.
Methods
Immunocompromised NSG mice were engrafted with a luciferase-positive TCF3-PBX1 leukemic cell line or primary leukemic cells and treated with ibrutinib or placebo. Additionally, primary cells were exposed in vitro to 4 main induction drugs as monotherapy and in combination with ibrutinib.
Results
Treatment with ibrutinib of mice engrafted with a TCF3-PBX1 cell line, TCF3-PBX1 positive or TCF3-PBX1 negative primary leukemic cells did not result in prolonged life span compared to placebo treated mice. In vitro sensitivity to ibrutinib was unaltered in leukemic cells obtained from engrafted mice compared to the original material. However, ibrutinib treatment did not affect leukemic cell viability and tumor outgrowth, nor could lymphocytosis be detected. Ibrutinib was biologically active, since hCD19+ cells harvested from ibrutinib treated mice had no detectable levels of phospho-BTK at tyrosine 223 (pBTK Y223), whereas pBTK Y223 was still detectable in placebo treated cases. In combination tests, we noticed an antagonistic effect of ibrutinib on vincristine sensitivity, which was not observed for prednisolone, L-asparaginase and daunorubicin.
Conclusions
We conclude that ibrutinib is not the precision medicine of choice for TCF3-PBX1 positive BCP-ALL.
Introduction
Around 5% of all pediatric cases of B-cell precursor acute lymphoblastic leukemia (BCP-ALL) are caused by a rearrangement of TCF3 (E2A) [1], with the vast majority of cases having a t(1;19) [2], resulting in a TCF3-PBX1 fusion protein. This subtype is generally considered as prognostically favorable as patients have a 5 year overall survival of >95% [3,4].
The good clinical outcome of patients with BCP-ALL is achieved by a regime of intense chemotherapeutic drug combinations for two years or longer. Although highly efficacious, the current treatment has short-term and long term side effects, which can be very severe, like osteonecrosis and cardiac malfunctioning [5,6].
In the Dutch ALL-10 protocol, most children with TCF3-PBX1 BCP-ALL had detectable minimal residual disease (MRD) levels after induction therapy, and as a result were stratified into the medium-risk arm with more intense therapy [4].
A simple reduction of therapy does not seem feasible since previous studies have shown that if TCF3-PBX1 BCP-ALL relapses, it does so at an early time point within 2.5 years after diagnosis [[7], [8], [9], [10], [11]], hinting at an aggressive regrowth potential of residual TCF3-PBX1 BCP-ALL cells.
Reduction of the side-effects of the current treatment without endangering the favorable outcome, therefore calls for a more targeted therapy for TCF3-PBX1 BCP-ALL.
TCF3-PBX1 BCP-ALL cells have an active preB-cell receptor (preBCR) pathway. Cells are arrested at an immature stage of B-cell differentiation with an in-frame IGH VDJ recombination but lack of light chain rearranged IGK/IGL genes, have high cytoplasmic Igμ and express several other components of the preBCR pathway, including Bruton's tyrosine kinase (BTK) [12]. BTK is specifically required for preBCR and BCR signaling [13,14] and can be successfully inhibited by ibrutinib (PCI-32765), a small molecule inhibitor that binds covalently to the cysteine 481 residue of BTK and blocks downstream signaling [15].
Ibrutinib has been shown to be of clinical relevance in patients with multiple myeloma [16], B-cell non-Hodgkin lymphoma [17] and chronic lymphoblastic leukemia (CLL) [[17], [18], [19]] with either relapsed or newly diagnosed disease. However, in children with BCP-ALL, no conclusive evidence has been presented so far that justifies the implementation in treatment. Here, we aimed to provide pre-clinical data for the effect of ibrutinib on TCF3-PBX1 positive BCP-ALL cells xenografted in immunocompromised mice. We show that ibrutinib, although still efficacious, did not prolong the survival of treated mice nor reduced tumor burden in mice engrafted with leukemic cell lines and with primary patients' cells. Moreover, we provide evidence that ibrutinib has an antagonistic effect on vincristine in vitro, suggesting a combination of both therapeutics in the clinic is not desirable.
Material and methods
Primary leukemic cells
Primary human BCP-ALL cells were isolated from bone marrow and/or peripheral blood from children (1–18 years) with BCP-ALL at diagnosis. Written informed consent was obtained from parents or guardians to use excess of diagnostic material for research purposes, as approved by the institutional review board and in accordance with the Declaration of Helsinki. Mononuclear cells were isolated and processed as described previously [20]. When necessary, samples were enriched towards >90% leukemic cells by depletion of non-leukemic cells using immunomagnetic beads [21]. Cytogenetic subtype was characterized using established diagnostic workflows, including tests for the recurrent translocations ETV6-RUNX1, BCR-ABL1, KMT2A-AF4, TCF3-PBX1, and high hyperdiploidy [22]. Patient characteristics were provided by the central study center of DCOG, The Hague, the Netherlands. Leukemic cells were stored frozen in liquid nitrogen at 50–100 million cells per vial. Upon thawing a vial for an experiment, the percentage of dead cells was determined using a Trypan blue staining and percentage of leukemic cells was determined by May-Grünwald Giemsa staining.
Cell lines
The human acute lymphoblastic leukemia cell line RCH-ACV was purchased from the Leibniz Institute DSMZ-German Collection of Micro-organisms and Cell lines (DSMZ, Braunschweig, Germany) and cultured in RPMI-1640 medium, supplemented with 10% fetal calf serum (Bodinco BV, Alkmaar, Netherlands), 100 units/mL penicillin, 100 μg/mL streptomycin and 0.125 μg/mL fungizone (Life Technologies, Bleiswijk, Netherlands). The identity of the cell line was verified by DNA fingerprinting by short tandem repeat method (STR) at purchase and throughout culture for consistency.
Lentiviral transduction
RCH-ACV cells were made to express luciferase by transduction of the FUW-Luc-mCherry-puro vector (obtained from Dr. A. Kung, DFCI, Boston). The vector was transfected in HEK293T cells, together with psPAX2 (Addgene plasma 12260; Addgene, Cambridge, MA, USA) and pMD2.G (Addgene plasmid 12259) using X-tremeGene 9 DNA transfection reagent (Roche, Woerden, The Netherlands). Virus was collected on the second and third day after transfection and concentrated by ultracentrifugation at 32.000 rpm, 4 °C for 2 h (Optima L-90K Ultracentrifuge; Beckman Coulter, Woerden, The Netherlands). Concentrated virus was aliquoted and stored at −80 °C. RCH-ACV cells were transduced by spin-infection. Positive cells were selected by puromycin resistance and verified by DNA fingerprint. Luciferase and mCherry expression were confirmed by a luminescence assay (E1500; Promega, Leiden, The Netherlands) and flow cytometry respectively.
Ex vivo drug resistance assay
The ex vivo cytotoxicity of all therapeutics was determined using the MTT assay. The assay conditions were essentially the same as described before [23]. Concentration of the therapeutics ranged from: Ibrutinib 0.08–40 μM (Janssen, Leiden, The Netherlands), daunorubicin 0.002–2 μg/mL (Cerubidine; Rhone Poulenc Rorer, Amstelveen, The Netherlands), prednisolone disodium phosphate 0.06–250 μg/mL (Bufa pharmaceutical Products, Uitgeest, The Netherlands), vincristine 0.05–50 μg/mL (Oncovin; Eli Lilly, Nieuwegein, The Netherlands), L-asparaginase 0.003–10 IU/mL (Medac; Medac, Hamburg, Germany). All tests were performed in 96-well round-bottomed microculture plates, cells were exposed to ten different concentrations of ibrutinib, six different concentrations of the other four drugs, or to culture medium only. For the synergy tests, cells were exposed to six concentrations of daunorubicin, prednisolone, vincristine or L-asparaginase with or without 4 μM Ibrutinib. After 4 days of culture at 37 °C and 5% CO2, 50 mg 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT, 5 mg/mL; Sigma-Aldrich, Zwijndrecht, The Netherlands) was added to each well and incubated for 6 h at 37 °C. The formed formazan molecules (by viable cells only) were dissolved in acidified isopropanol (0·04 M HCl) and the quantity of formazan was measured spectrophotometrically at 562 nm. The optical density (OD) is linearly related to the number of viable cells in each well [23]. The LC50 value represents the drug concentration which killed 50% of the cells compared with the number of cells that were present in the control wells without drug. Primary patient samples of all eleven patients were tested, but six were not informative.
Xenografts and in vivo ibrutinib treatment
Primary leukemic cells were injected intra-femoral in 7–12 weeks old female NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice (Charles River, Wilmington, Massachusetts, USA; eight NSG mice per patient, later divided over placebo and ibrutinib treatment groups), as approved by the animal ethics committee (EMC 3117). Xenografted mice were monitored for successful engraftment using weight and human CD19-positivity in the peripheral blood (Brilliant Violet 421™ anti-human CD19 Antibody, Biolegend, San Diego, California, United States) was determined by flow cytometry using a MACSQuant analyzer (Miltenyi Biotec, Leiden, The Netherlands).
Treatment of mice engrafted with primary leukemic cells started at a percentage of human CD19 in the blood >1%, with daily intraperitoneal injections of 12 mg/kg ibrutinib in 50% polyethylene glycol (PEG 400, PanReac AppliChem, Barcelona, Spain) for the ibrutinib group or 50% PEG for the placebo group. Mice were treated for a maximum of 12 weeks. Treatment with a higher dose of ibrutinib (25 mg/kg) caused high mortality within 24 h in engrafted NSG mice, and was therefore not further investigated.
Luciferase-positive RCH-ACV cells were engrafted similar to the procedure used for primary leukemic cells. Xenografted mice were monitored for human CD19-positivity in the peripheral blood and bioluminescence determined by intravenous injection of luciferin (RediJect D-Luciferin Bioluminescent Substrate, Perkin-Elmer, Waltham, Massachusetts, United States) and subsequent measurement of released photons using the IVIS in vivo imaging system 200 (Xenogen, now: Perkin-Elmer). Treatment with 12 mg/kg ibrutinib or placebo started 7 days after engraftment until overt leukemia was reached.
After treatment xenografted mice were sacrificed and blood plasma was collected by centrifugation of peripheral blood obtained via heart puncture. Additionally, cells were harvested from bone marrow, spleen and peripheral blood. Cells were assessed for human CD19 percentage by flow cytometry, viably frozen or processed for protein isolation.
Protein isolation and western blot analysis
Frozen cell pellets of primary leukemic cells, RCH-ACV and harvested xenografted cells were lysated using lysis buffer with protease and phosphatase inhibitors. Protein concentration was determined by the BCA method (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and 25 μg lysate was loaded on 10% mini-PROTEAN TGX precast protein gels (Biorad, Veenendaal, The Netherlands) and transferred onto nitrocellulose membranes (Bio-rad). Primary antibody incubation was performed according to manufacturer's protocol. Anti-BTK (#8547S) and Anti-phospho-BTK-Tyr223 (#5082S) were obtained from Cell Signaling Technology (Danvers, Massachusetts, USA). Anti-β-actin (ab6276) was obtained from Abcam (Cambridge, UK). Blots were incubated with secondary antibodies (IRDye 680 CW or 800 CW-labeled anti-rabbit and IRDye 680 CW or 800 CW-labeled anti-mouse; LI-COR Biosciences, Leusden, The Netherlands) and scanned with the Odyssey infrared imaging system (LI-COR Biosciences).
Results
Outgrowth of RCH-ACV in vivo remains unaltered by ibrutinib treatment
To explore the potency of ibrutinib in vivo, we generated murine xenografts with the human TCF3-PBX1 positive cell line RCH-ACV. RCH-ACV cells were sensitive to ibrutinib in vitro with an IC50 of 2.8 μM (95% CI, 2.2–3.7 μM, Fig. 1A). The cell line was made transgenic for mCherry and luciferase and retained sensitivity to ibrutinib (IC50 of 1.16 μM, 95% CI: 1.2–2.7 μM, Fig. 1A). This cell line was injected directly into the femur of NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice. Intrafemoral injection resulted in a swift engraftment in the bone marrow. RCH-ACV rapidly populated the mouse, typically spread to the liver, but not the spleen, and mice died around 25 days after engraftment due to hepatic complications. Due to this swift spread and short life span, we started daily treatment with 12 mg/kg ibrutinib or placebo through intraperitoneal injection 7 days after intrafemoral injection, even before human CD19 positive (hCD19+) cells could be detected in the peripheral blood (Fig. 1B), however isolated cells from the liver died immediately after isolation for both ibrutinib and placebo treated mice, and thus could not be used for further analysis.
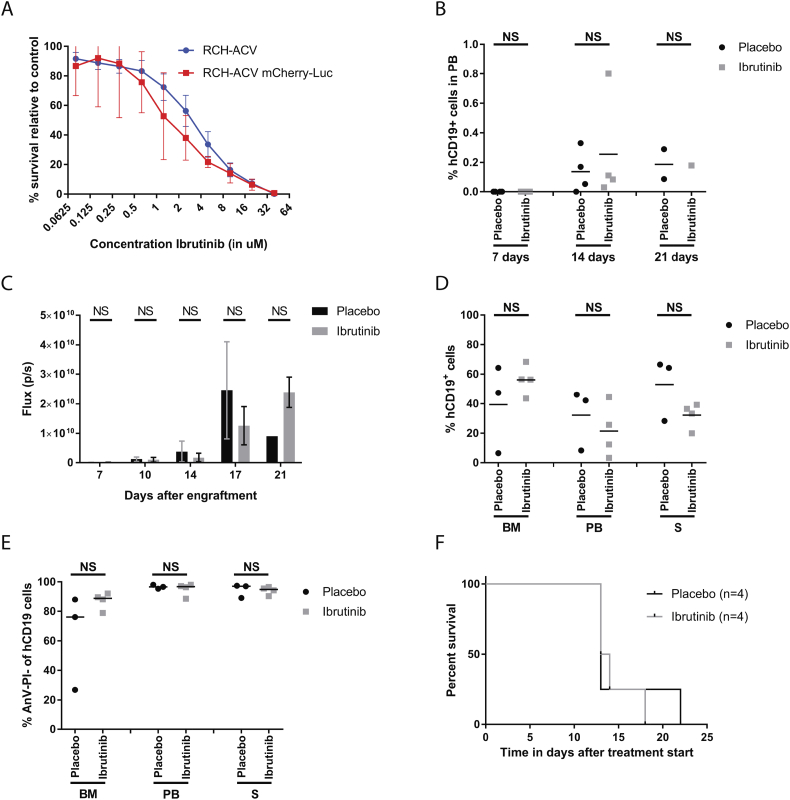
Fig. 1.
In vivo ibrutinib efficacy in xenografts of RCH-ACV mCherry-Luc cell lines. (A) Sensitivity to different concentrations of ibrutinib for RCH-ACV cell line transgenic for mCherry-Luc (red line, n = 3) in comparison to the parental cell line (blue line, n = 10) measured in an MTT assay. Mean and standard error are depicted. (B) Percentage of human CD19 positive cells (hCD19+) detected with flow cytometry in peripheral blood (PB) of engrafted mice at 7, 14 and 21 days after RCH-ACV injection. Treatment with ibrutinib (grey squares) or placebo (black circles) was started at day 7 immediately after PB extraction for flow cytometry (n = 4 for 7 and 14 days for both treatment groups, n = 2 for placebo and n = 1 for ibrutinib at 21 days). (C) Average total flux in photons per seconds (p/s) per mouse for placebo (black circles, n = 4 for days 7 till 17, n = 1 for day 21) and for ibrutinib (grey squares, n = 4 for days 7 till 17, n = 2 for day 21) treated groups. (D) Percentage of hCD19+ cells in bone marrow (BM), peripheral blood (PB) and spleen (S) after mice were sacrificed and organs harvested for placebo treated mice (black circles, n = 3) and ibrutinib treated mice (grey squares, n = 4). (E) Percentage of living hCD19+ cells in the harvested organs, cells were negative for AnnexinV-AF647 (AnV-) and propidium iodine (PI-) for placebo treated mice (black circles, n = 3) and ibrutinib treated mice (grey squares, n = 4). (F) Kaplan Meier curve for placebo (black line, n = 4) and ibrutinib (grey line, n = 4) treated mice engrafted with RCH-ACV mCherry-Luc. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
During treatment, all mice were monitored weekly for the presence of hCD19+ cells in peripheral blood by flow cytometry. Although ibrutinib causes lymphocytosis in CLL patients [24], we could detect only very low percentage of human cells in the blood during treatment, with no significant difference between ibrutinib and placebo treated mice at any of the measured time points (Fig. 1B).
Tumor load over time could be detected by bioluminescence, since engrafted RCH-ACV cells were transgenic for luciferase. Mice were monitored twice per week, but no significant difference between ibrutinib and placebo treated groups could be identified at any of the timepoints (Fig. 1C, Suppl Fig. 1A). Furthermore, no significant difference in tumor load between ibrutinib and placebo treated mice could be detected in harvested organs (Fig. 1D). Viability of the hCD19+ cells in the different organs remained unaltered (Fig. 1E), as did the viability of murine hematopoietic mCD45+ cells (Suppl Fig. 1B), suggesting ibrutinib did not cause cell death in human cells in vivo nor affected healthy cells of the host.
Treatment with ibrutinib did not result in a prolonged life span for xenografted mice (median survival time after treatment start (MST): 13.5 days for 12 mg/kg ibrutinib) compared to placebo (MST: 13 days, p = 0.74, Fig. 1F).
Ibrutinib does not prevent outgrowth of primary pediatric leukemia in vivo
To elucidate if ibrutinib may successfully contribute to the cure of patients with TCF3-PBX1 positive BCP-ALL and to exclude possible bias of cell line models, we engrafted leukemic cells from four patients with newly diagnosed TCF3-PBX1 positive BCP-ALL (BCP-ALL #1-4, Suppl Table S1) and two TCF3-PBX1 negative BCP-ALL (BCP-ALL #5&6) into NSG mice.
To mimic the clinical situation as well as possible, treatment with 12 mg/kg ibrutinib or placebo started when percentage of hCD19+ cells in the peripheral blood exceeded 1%. Treatment with 12 mg/kg ibrutinib did not result in a prolonged life span for mice engrafted with primary TCF3-PBX1 positive BCP-ALL cells compared to placebo control (Fig. 2A). In contrast, in mice engrafted with one out of two TCF3-PBX1 negative we observed a prolonged lifespan of 15 days (BCP-ALL #6: MST: 57.5 days, Fig. 2B) compared to placebo control (MST: 42.5 days, p = 0.03), while this was not observed in BCP-ALL #5. Strikingly, responsiveness of patient #6 to ibrutinib is not BTK dependent, since cells of neither patient #5 nor patient #6 have high total BTK or detectable levels of phosphorylated BTK (Fig. 3B).
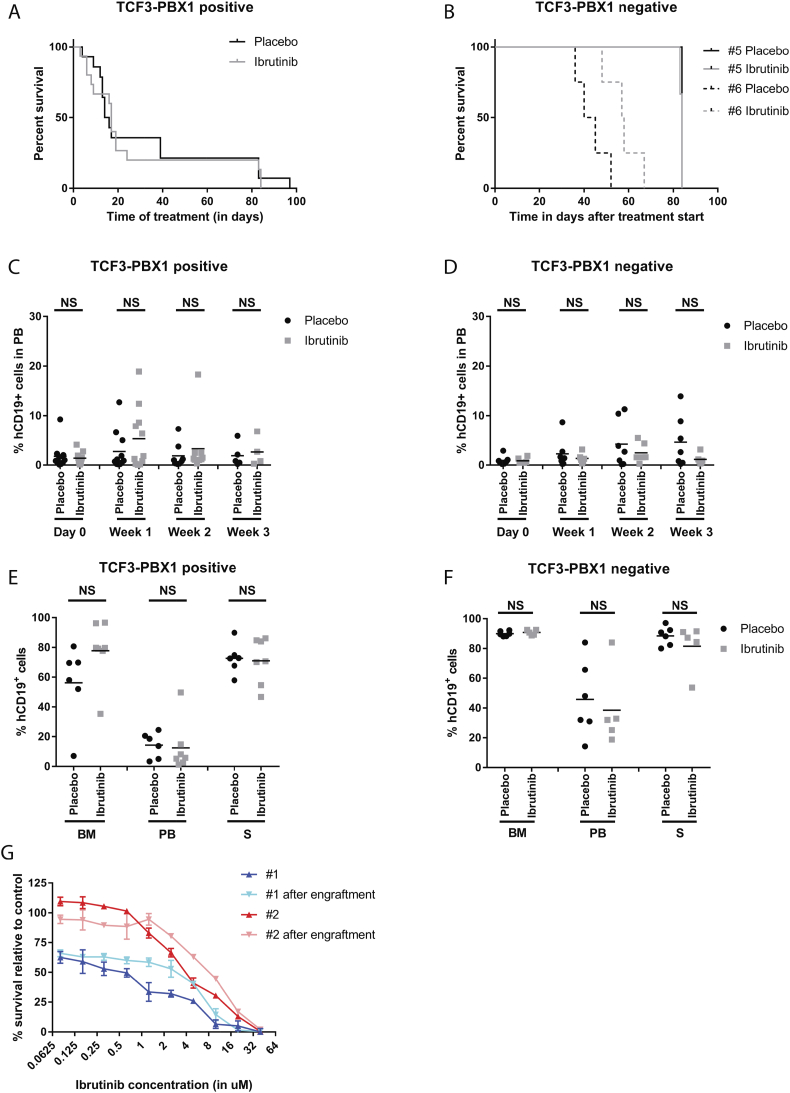
Fig. 2.
In vivo ibrutinib efficacy in xenografts of primary BCP-ALL samples. (A) Kaplan Meier curve for all TCF3-PBX1 positive engraftments treated with placebo (black line, n = 11) and 12 mg/kg ibrutinib (grey line, n = 11). (B) Kaplan Meier curve for TCF3-PBX1 negative cases BCP-ALL #5 (solid lines, n = 4 per treatment group) and BCP-ALL #6 (dotted lines, n = 4 per treatment group) engraftments treated with placebo (black lines) and 12 mg/kg ibrutinib (grey line). (C) Percentage of human CD19 positive cells (hCD19+) detected with flow cytometry in peripheral blood (PB) of in mice engrafted with TCF3-PBX1 positive cells at day 0 and 1, 2 and 3 weeks of treatment with ibrutinib (grey squares, n = 11 for day 0 and week 1, n = 9 for week 2, n = 4 for week 3) or placebo (black circles, n = 11 for day 0 and week 1, n = 8 for week 2, n = 5 for week 3). Treatment was started at day 1. Mean per treatment group is displayed. (D) Idem for TCF3-PBX1 negative cells, for ibrutinib (grey squares, n = 6 for all timepoints) or placebo (black circles, n = 7 for all timepoints). (E) Percentage of hCD19+ cells in bone marrow (BM), peripheral blood (PB) and spleen (S) of TCF3-PBX1 positive cells for placebo (black circles, n = 6) and ibrutinib (grey squares, n = 7). (F) Idem for TCF3-PBX1 negative cells, for placebo (black circles, n = 5) and ibrutinib (grey squares, n = 5). (G) Sensitivity of BCP-ALL # 1 (blue lines) and BCP-ALL #2 (red lines) to different concentrations of ibrutinib measured in MTT assays, for samples taken before injection into mice (#1: dark blue, #2 dark red) and after isolation from engrafted mice (#1: light blue, #2 light red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
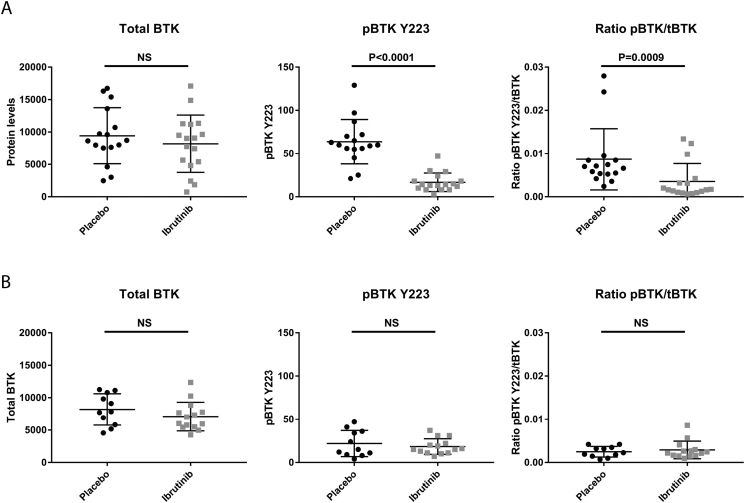
Fig. 3.
BTK protein levels after in vivo placebo and ibrutinib treatment. (A–F) Protein levels detected on western blot of total BTK (tBTK), BTK phosphorylated at tyrosine 223 (pBTK Y223) and the ratio between total BTK and BTK phosphorylated at tyrosine 223 (ratio pBTK Y223/tBTK) for lysates of cells harvested from organs of placebo (black circles) and ibrutinib (grey squares) treated mice for TCF3-PBX1 positive (A) and TCF3-PBX1 negative (B) engraftments. Mean and SD per treatment group are displayed.
Similar to the results with RCH-ACV, no signs of lymphocytosis were detected in 12 mg/kg ibrutinib treated mice compared to placebo (Fig. 2C+D), nor any differences in tumor load or viability of hCD19+ or mCD45+ cells in harvested organs for the TCF3-PBX1 positive ALL or TCF3-PBX1 negative ALL (Fig. 2E+F, Suppl Fig. 2).
However, engrafted primary TCF3-PBX1 positive cells were confirmed to retain sensitivity to ibrutinib in vitro when isolated from the mouse after successful engraftment and cytotoxicity was similar between engrafted cells and the original sample from patients (Fig. 2G), suggesting that the intrinsic response to ibrutinib was not affected in these cells.
Ibrutinib is biologically active in NSG mice
The biological activity of ibrutinib in our in vivo model, was confirmed in hCD19+ cells harvested from treated mice for inhibition of BTK. TCF3-PBX1 positive hCD19+ cells harvested from 12 mg/kg ibrutinib treated mice have no detectable levels above background of phospho-BTK at tyrosine 223 (pBTK Y223), whereas pBTK was still detectable in placebo treated cases (Fig. 3A, p < 0.0001). Total BTK remained unaltered in both ibrutinib and placebo treated samples (Fig. 3A, p = 0.56). As a consequence, the ratio between pBTK Y223 and total BTK was significantly lower in TCF3-PBX1 positive hCD19+ cells isolated from ibrutinib treated mice compared to those of placebo treated mice (Fig. 3A, p = 0.0009). In the TCF3-PBX1 negative cells, no differences in total BTK levels or ratio between pBTK and total BTK could be detected between ibrutinib and placebo treated mice, while levels of pBTK Y223 did not reach above background in both treatment groups (Fig. 3B, p = 0.24, p = 0.88 and p = 0.999 respectively)
Ibrutinib antagonizes vincristine in vitro
Finally, we examined if ibrutinib could enhance in vitro functionality of four main chemotherapeutics currently used to treat children with BCP-ALL. In four out of five primary patient cells we found an increase in survival of cells when treated with a combination of ibrutinib and vincristine, compared to monotherapy for either drug (Fig. 4A). Antagonism between ibrutinib and vincristine was seen in both TCF3-PBX1 positive and –negative samples and was independent of BTK levels.
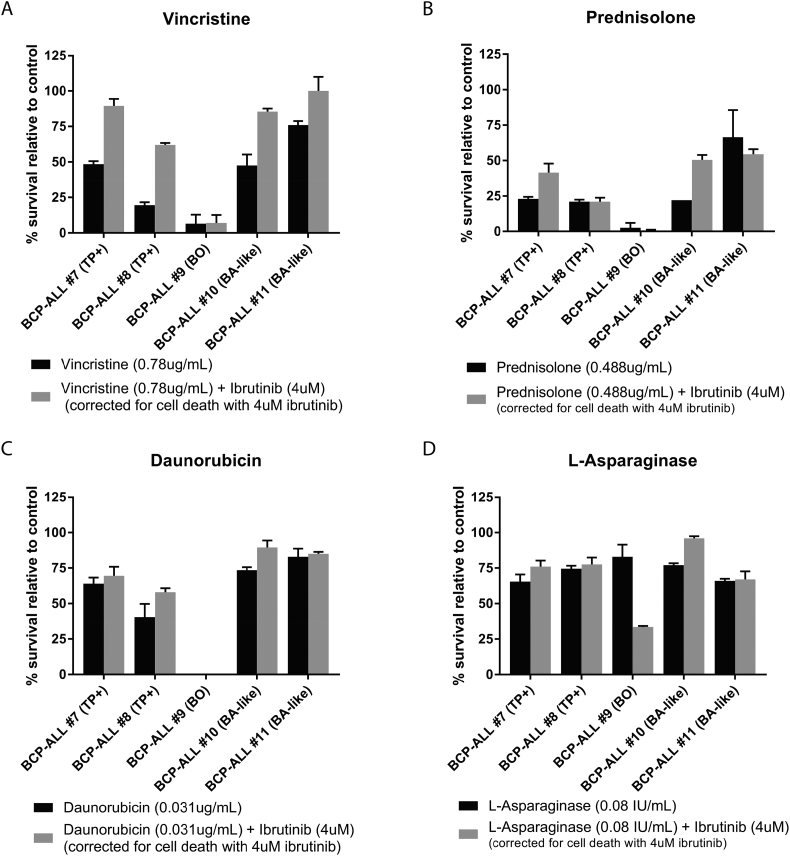
Fig. 4.
In vitro sensitivity to main therapeutics and ibrutinib in primary BCP-ALL samples. (A–D) In vitro sensitivity of five primary BCP-ALL samples measured in MTT assays to 0.78 μg/mL vincristine (A), 0.488 μg/mL prednisolone (B), 0.031 μg/mL daunorubicin (C) and 0.08 IU/mL asparaginase (D). Either as a single agent (black bars) or in combination with 4 μM ibrutinib (grey bars). Primary cells were TCF3-PBX1 positive (TP+, BCP-ALL #7 and #8), B-Other (BO, BCP-ALL #9) or BCR-ABL1-like (BA-like, BCP-ALL #10 and #11). Error and average survival relative to survival with no drug (for single agent) or survival with 4 μM ibrutinib (drug combination testing). Survival with 4 μM ibrutinib as a single agent ranged between 66 and 103% compared to survival of placebo treated cells.
No marked changes in cell survival were found in combining ibrutinib with prednisolone, daunorubicin or L-asparaginase (Fig. 4B–D), except for a marked sensitizing effect of ibrutinib on L-asparaginase in one B-other case (BCP-ALL #9).
Discussion
Personalized therapies, specifically designed to target oncogenic pathways unaffected in healthy cells, may be of great added value to standard chemotherapy for children with BCP-ALL. These new agents could be key to prevent outgrowth of residual cells and/or salvage therapy for relapsed cases. Additionally, they might be able to replace or warrant a reduction of highly toxic therapeutics currently used, decreasing side effects. Ibrutinib has been hailed to be such a new agent; well tolerated, minimal side effects and targeting a molecule almost exclusively activated in the cancerous B-cell.
Although a very good response to ibrutinib is observed in adult patients with CLL [[17], [18], [19]] and results have been promising in vitro with pediatric BCP-ALL [12], here we show that ibrutinib does not prevent the outgrowth of primary BCP-ALL cells in vivo. Furthermore, ibrutinib was not efficacious in mice engrafted with TCF3-PBX1 positive nor TCF3-PBX1 negative leukemia. This lack of anti-tumor efficacy could not be attributed to a lack of ibrutinib activity in mice, since the pBTK levels in leukemic cells of treated mice were clearly reduced compared to those in the placebo treated group.
The different potency of ibrutinib in CLL and BCP-ALL might be caused by an intrinsic difference between these two forms of leukemia. CLL cells are more differentiated compared to BCP-ALL, already having an activated mature BCR-signaling pathway. TCF3-PBX1+ BCP-ALL is characterized by a VDJ rearranged heavy chain only and lack of further processing of the kappa and lambda light chain resulting in a differentiation arrest at the preBCR level [12]. In an in vivo model preBCR+ ALL cells may therefore need a different micro-environmental factor to expand than mature BCR+ CLL cells. This might cause a difference in cellular dependence on BTK signaling, explaining the difference in efficacy of ibrutinib in both cell types.
Exposure of different cell lines to various TKI inhibitors, showed all tested TCF3-PBX1 positive cell lines to be sensitive to ibrutinib in vitro [25]. Although treatment with 75 mg/kg resulted in a delay in outgrowth of leukemia, some marked differences are that a different mouse strain was used as well as a different TCF3-PBX1 positive cell line (ICN12). More importantly, ibrutinib has also previously been tested in vivo in a murine ALL model, using the same cell line as described in this study, RCH-ACV [26]. A crucial difference in experimental setup of both studies may account for the 3-days prolonged lifespan observed in the study of Kim et al. and the delayed outgrowth of ICN12 in the study of Geng et al. Ibrutinib has been reported to affect migration of ALL cells to the bone marrow by influencing CXCR4 signaling [27]. In the studies by Kim et al. and Geng et al., ibrutinib was given only 24 h after the injection of ALL cells, which may have inhibited CXCR4 and thus migration to the bone marrow (the preferred niche of BCP-ALL cells) thereby delaying the outgrowth of leukemic cells. However, patients with BCP-ALL present in clinic with a fully established leukemia in the bone marrow and inhibiting CXCR4 will not have a dramatic effect. We therefore started Ibrutinib treatment when the leukemia was fully settled. Our data show that ibrutinib as a single agent does not inhibit the expansion of leukemic cells once the BCP-ALL leukemia has homed to and settled in the bone marrow and extramedullary organs.
We also investigated whether ibrutinib might work synergistic to current chemotherapy. In this study we noticed a marked sensitizing effect only for L-asparaginase in one out of five tested patient samples, but not for the other drugs. Strikingly, we found an antagonistic effect between ibrutinib and vincristine in the majority of patient samples tested, for which the causative relationship remains to be determined.
In conclusion, our data show that monotherapy with ibrutinib does not delay outgrowth and is not effective in in vivo mouse models with a fully established human BCP-ALL. This opposes our observation that ibrutinib is cytotoxic to leukemic cells in monoculture, suggesting that the (mouse) leukemic niche protects against the cytotoxic effect of ibrutinib. The lack of strong synergy with 3 out of 4 tested induction drugs (prednisolone, daunorubicin and L-asparaginase) and impaired sensitivity to vincristine indicates that ibrutinib is not a preferred precision medicine in TCF3-PBX1 positive BCP-ALL despite target presence.
Author contributions statement
Cesca van de Ven: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Project administration, Funding acquisition. Aurélie Boeree: Software, Investigation, Data Curation, Writing - Review & Editing, Visualization, Project administration. Femke Stalpers: Validation, Investigation, Writing - Review & Editing. Michel Zwaan: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Funding acquisition. Monique den Boer: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank all members of the BCP-ALL research group of Princess Máxima Center for Pediatric Oncology for their help in processing leukemic samples, Alex Q. Hoogkamer for help with analysis and all members of the EDC of the Erasmus MC for their help with animal experiments. No writing assistance was used for this article. This work was financially supported by the Dutch Cancer Society (project EMCR 2014-6998). PCI-32765 (ibrutinib) has been provided by Janssen, Leiden, The Netherlands.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2020.100817.
Appendix A. Supplementary data
Supplementary material
References
- 1.Pui C.H., Carroll W.L., Meshinchi S., Arceci R.J. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J. Clin. Oncol. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber K.E., Harrison C.J., Broadfield Z.J., Stewart A.R., Wright S.L., Martineau M. Molecular cytogenetic characterization of TCF3 (E2A)/19p13.3 rearrangements in B-cell precursor acute lymphoblastic leukemia. Genes Chromosom. Cancer. 2007;46:478–486. doi: 10.1002/gcc.20431. [DOI] [PubMed] [Google Scholar]
- 3.Sutton R., Venn N.C., Law T., Boer J.M., Trahair T.N., Ng A. A risk score including microdeletions improves relapse prediction for standard and medium risk precursor B-cell acute lymphoblastic leukaemia in children. Br. J. Haematol. 2018;180:550–562. doi: 10.1111/bjh.15056. [DOI] [PubMed] [Google Scholar]
- 4.Pieters R., de Groot-Kruseman H., Van der Velden V., Fiocco M., van den Berg H., de Bont E. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study ALL10 from the Dutch childhood oncology group. J. Clin. Oncol. 2016;34:2591–2601. doi: 10.1200/JCO.2015.64.6364. [DOI] [PubMed] [Google Scholar]
- 5.Cooper S.L., Brown P.A. Treatment of pediatric acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2015;62:61–73. doi: 10.1016/j.pcl.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness K.K., Armenian S.H., Kadan-Lottick N., Gurney J.G. Adverse effects of treatment in childhood acute lymphoblastic leukemia: general overview and implications for long-term cardiac health. Expert. Rev. Hematol. 2011;4:185–197. doi: 10.1586/ehm.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen M.K., Autio K., Barbany G., Borgstrom G., Cavelier L., Golovleva I. Paediatric B-cell precursor acute lymphoblastic leukaemia with t(1;19)(q23;p13): clinical and cytogenetic characteristics of 47 cases from the Nordic countries treated according to NOPHO protocols. Br. J. Haematol. 2011;155:235–243. doi: 10.1111/j.1365-2141.2011.08824.x. [DOI] [PubMed] [Google Scholar]
- 8.Kager L., Lion T., Attarbaschi A., Koenig M., Strehl S., Haas O.A. Incidence and outcome of TCF3-PBX1-positive acute lymphoblastic leukemia in Austrian children. Haematologica. 2007;92:1561–1564. doi: 10.3324/haematol.11239. [DOI] [PubMed] [Google Scholar]
- 9.Asai D., Imamura T., Yamashita Y., Suenobu S., Moriya-Saito A., Hasegawa D. Outcome of TCF3-PBX1 positive pediatric acute lymphoblastic leukemia patients in Japan: a collaborative study of Japan Association of Childhood Leukemia Study (JACLS) and Children's Cancer and Leukemia Study Group (CCLSG) Cancer Med. 2014;3:623–631. doi: 10.1002/cam4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeha S., Pei D., Raimondi S.C., Onciu M., Campana D., Cheng C. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23:1406–1409. doi: 10.1038/leu.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irving J.A., Enshaei A., Parker C.A., Sutton R., Kuiper R.P., Erhorn A. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2016;128:911–922. doi: 10.1182/blood-2016-03-704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Veer A., van der Velden V.H., Willemse M.E., Hoogeveen P.G., Petricoin E.F., Beverloo H.B. Interference with pre-B-cell receptor signaling offers a therapeutic option for TCF3-rearranged childhood acute lymphoblastic leukemia. Blood Cancer J. 2014;4 doi: 10.1038/bcj.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bubien J.K., Watson B., Khan M.A., Langloh A.L., Fuller C.M., Berdiev B. Expression and regulation of normal and polymorphic epithelial sodium channel by human lymphocytes. J. Biol. Chem. 2001;276:8557–8566. doi: 10.1074/jbc.M008886200. [DOI] [PubMed] [Google Scholar]
- 14.Satterthwaite A.B., Witte O.N. The role of Bruton's tyrosine kinase in B-cell development and function: a genetic perspective. Immunol. Rev. 2000;175:120–127. [PubMed] [Google Scholar]
- 15.Honigberg L.A., Smith A.M., Sirisawad M., Verner E., Loury D., Chang B. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rushworth S.A., Bowles K.M., Barrera L.N., Murray M.Y., Zaitseva L., MacEwan D.J. BTK inhibitor ibrutinib is cytotoxic to myeloma and potently enhances bortezomib and lenalidomide activities through NF-kappaB. Cell. Signal. 2013;25:106–112. doi: 10.1016/j.cellsig.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Advani R.H., Buggy J.J., Sharman J.P., Smith S.M., Boyd T.E., Grant B. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponader S., Chen S.S., Buggy J.J., Balakrishnan K., Gandhi V., Wierda W.G. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd J.C., Furman R.R., Coutre S.E., Burger J.A., Blum K.A., Coleman M. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boer J.M., Steeghs E.M., Marchante J.R., Boeree A., Beaudoin J.J., Beverloo H.B. Tyrosine kinase fusion genes in pediatric BCR-ABL1-like acute lymphoblastic leukemia. Oncotarget. 2017;8:4618–4628. doi: 10.18632/oncotarget.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soler G., Radford-Weiss I., Ben-Abdelali R., Mahlaoui N., Ponceau J.F., Macintyre E.A. Fusion of ZMIZ1 to ABL1 in a B-cell acute lymphoblastic leukaemia with a t(9;10)(q34;q22.3) translocation. Leukemia. 2008;22:1278–1280. doi: 10.1038/sj.leu.2405033. [DOI] [PubMed] [Google Scholar]
- 22.Den Boer M.L., van Slegtenhorst M., De Menezes R.X., Cheok M.H., Buijs-Gladdines J.G., Peters S.T. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10:125–134. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaspers G.J., Pieters R., Van Zantwijk C.H., De Laat P.A., De Waal F.C., Van Wering E.R. In vitro drug sensitivity of normal peripheral blood lymphocytes and childhood leukaemic cells from bone marrow and peripheral blood. Br. J. Cancer. 1991;64:469–474. doi: 10.1038/bjc.1991.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd J.C., Furman R.R., Coutre S.E., Flinn I.W., Burger J.A., Blum K.A. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng H., Hurtz C., Lenz K.B., Chen Z., Baumjohann D., Thompson S. Self-enforcing feedback activation between BCL6 and pre-B cell receptor signaling defines a distinct subtype of acute lymphoblastic leukemia. Cancer Cell. 2015;27:409–425. doi: 10.1016/j.ccell.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim E., Hurtz C., Koehrer S., Wang Z., Balasubramanian S., Chang B.Y. Ibrutinib inhibits pre-BCR(+) B-cell acute lymphoblastic leukemia progression by targeting BTK and BLK. Blood. 2017;129:1155–1165. doi: 10.1182/blood-2016-06-722900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Rooij M.F., Kuil A., Geest C.R., Eldering E., Chang B.Y., Buggy J.J. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material