Significance
Cilia are composed of hundreds of proteins whose identities and functions are far from being completely understood. In this study, we determined that calmodulin-regulated spectrin-associated protein 3 (CAMSAP3) plays an important role for the function of motile cilia in multiciliated cells (MCCs). Global knockdown of CAMSAP3 protein expression in mice resulted in defects in ciliary structures, polarity, and synchronized beating in MCCs. These animals also displayed signs and symptoms reminiscent of primary ciliary dyskinesia (PCD), including a mild form of hydrocephalus, subfertility, and impaired mucociliary clearance that leads to hyposmia, anosmia, rhinosinusitis, and otitis media. Functional characterization of CAMSAP3 enriches our understanding of the molecular mechanisms underlying the generation and function of motile cilia in MCCs.
Keywords: CAMSAP3, motile cilia, primary ciliary dyskinesia, central MT pair, basal body orientation
Abstract
Synchronized beating of cilia on multiciliated cells (MCCs) generates a directional flow of mucus across epithelia. This motility requires a “9 + 2” microtubule (MT) configuration in axonemes and the unidirectional array of basal bodies of cilia on the MCCs. However, it is not fully understood what components are needed for central MT-pair assembly as they are not continuous with basal bodies in contrast to the nine outer MT doublets. In this study, we discovered that a homozygous knockdown mouse model for MT minus-end regulator calmodulin-regulated spectrin-associated protein 3 (CAMSAP3), Camsap3tm1a/tm1a, exhibited multiple phenotypes, some of which are typical of primary ciliary dyskinesia (PCD), a condition caused by motile cilia defects. Anatomical examination of Camsap3tm1a/tm1a mice revealed severe nasal airway blockage and abnormal ciliary morphologies in nasal MCCs. MCCs from different tissues exhibited defective synchronized beating and ineffective generation of directional flow likely underlying the PCD-like phenotypes. In normal mice, CAMSAP3 localized to the base of axonemes and at the basal bodies in MCCs. However, in Camsap3tm1a/tm1a, MCCs lacked CAMSAP3 at the ciliary base. Importantly, the central MT pairs were missing in the majority of cilia, and the polarity of the basal bodies was disorganized. These phenotypes were further confirmed in MCCs of Xenopus embryos when CAMSAP3 expression was knocked down by morpholino injection. Taken together, we identified CAMSAP3 as being important for the formation of central MT pairs, proper orientation of basal bodies, and synchronized beating of motile cilia.
Microtubules (MTs) are dynamic, polymeric polar tubes: The fast-growing plus end leads to MT elongation, while the slow-growing minus end attaches to various cellular structures such as basal bodies. CAMSAP3 (calmodulin-regulated spectrin-associated protein 3), also called Marshalin (1), is an MT minus-end regulator that stabilizes MTs (2, 3). CAMSAP3 has multiple protein–protein interaction domains (SI Appendix, Fig. S1A) that permit interactions with other partners to regulate MT networks, organelle positioning, and MT polarity (1–7). In addition, CAMSAP3 is involved in the formation of MTs from a noncentrosomal MT-organizing center (ncMTOC) as CAMSAP3-coated MTs are stable and capable of acting as seeds for MT elongation (8, 9). For example, CAMSAP3 is involved in forming ncMTOCs in preimplantation embryos when the centrosome, a prevalent MTOC, is not present (10). Because CAMSAP3 is widely expressed, knockout or reducing Camsap3 expression was reported to have multiple defects in placenta (11), neurons (8, 12), and enterocytes of the gastrointestinal tract (13, 14). Unexpectedly, we discovered that CAMSAP3 is also expressed in multiciliated cells (MCCs).
MCCs are restricted to luminal surfaces as in the nasal cavity, trachea, middle ear, Eustachian tube, reproductive tract, and ventricles of the brain (15). Each MCC displays hundreds of motile cilia protruding from basal bodies under the apical membrane. A complicated transcriptome and multitier pathways are devoted to MT-based multiciliogenesis (16–19). Mature motile cilia are thought to consist of over 600 proteins, whose identities and functions are far from being completely understood. Because cilia are highly conserved structures, dysfunction of motile cilia impacts a diverse set of tissues and causes primary ciliary dyskinesia (PCD). This complex disorder can include embryonic lethality, infertility, hydrocephalus, and dysfunctional mucus clearance, which leads to anosmia or hyposmia, sinusitis, respiratory distress, otitis media, and hearing loss (15). A key step in understanding the mechanism(s) underlying PCD is to identify the components that participate in the generation of motile cilia, so that their functions can be characterized in vivo. Due to the importance of cilia in developmental signaling, mutations that affect all cilia are often embryonically lethal, making detailed analysis challenging.
To produce a directional fluid flow that transports materials across the epithelium, hundreds of cilia on MCCs need to move in a synchronized manner. A unidirectional array of basal bodies in MCCs is required to achieve synchronized beating (16, 20, 21). This polarized array is achieved by a combination of planar cell polarity (PCP) signaling, cytoskeletal dynamics, and hydrodynamic forces (22). Unlike most primary cilia, which lack a central pair, motile cilia maintain a “9 + 2” MT configuration in their axonemes with two MT singlets surrounded by nine MT doublets (18). The central MTs and their associated proteins form the central apparatus (23), which is essential for planar motion rather than rotational beating (24). Unlike the outer nine MT doublets, the central MT pair is not continuous with MT triplets in the basal body (25). How the central MT pair is assembled, and what components are required, is still not fully understood.
In this study, we investigated a knockdown (KD) mouse model, Camsap3tm1a, that we rederived on CBA/CaJ and FVB murine strain backgrounds. Homozygous Camsap3tm1a/tm1a mice exhibited multiple pathological phenotypes. Many of these phenotypes are similar to those of PCD, including subfertility in both sexes and severe nasal airway blockage leading to coughing, sneezing, hyposmia, and rhinosinusitis. Since PCD is a condition caused by motile cilia defects and CAMSAP3-coated MTs are known to be involved in many cellular functions (4–10), we suspected that CAMSAP3 plays an important role(s) for motile cilia function. To test this possibility, we investigated the function of MCCs in different tissues. Our data show that CAMSAP3 localizes to the base of axonemes and at the basal bodies in normal MCCs. In contrast, the majority of cilia in Camsap3tm1a/tm1a lack the central MT pair in their axoneme and display disorientated basal bodies and defects in ciliary motion, which is no longer synchronized. The latter phenotype likely underlies their PCD-like behaviors. Since multiciliogenesis is remarkably well conserved throughout eukaryotes (26), we further investigated CAMSAP3’s function in MCCs in early Xenopus embryos. Xenopus embryos injected with Camsap3 morpholinos to knock down expression also exhibited defective ciliary function and abnormal morphology. Taken together, our data suggest that CAMSAP3 is required for normal motile cilia function.
Results
Strain-Dependent Subviability of the CAMSAP3-KD Mouse Model Camsap3tm1a.
Originally, a Camsap3tm1a mouse model on the C57BL6N background was created using the “knockout first, conditional-ready” strategy (27). A targeted trap allele was inserted into the intron between exons 6 and 7 of the Camsap3 gene to knock out Camsap3 expression through RNA processing (28, 29) without deleting any exons (Fig. 1A). However, Camsap3tm1a/tm1a showed preweaning mortality and very low reproductive rates. Among 104 mice, Camsap3tm1a breeders on the C57B6N background had only two Camsap3tm1a/tm1a pups (SI Appendix, Table S1). This observation was consistent with the available data on Deciphering the Mechanisms of Developmental Disorders (DMDD; https://dmdd.org.uk/), which indicated that Camsap3tm1a/tm1a were subviable. In addition, we did not observe obvious behavioral or gross structural differences between wild-type (WT) and Camsap3tm1a/+ mice. In order to obtain more Camsap3tm1a/tm1a, we rederived this line on CBA/CaJ and FVB murine backgrounds. Camsap3tm1a mice on the FVB background overcame the severe subviability issue in the original background and were born at Mendelian ratios: 1:2:1 for WT, heterozygote (Camsap3tm1a/+), and homozygote (Camsap3tm1a/tm1a) (SI Appendix, Table S1).
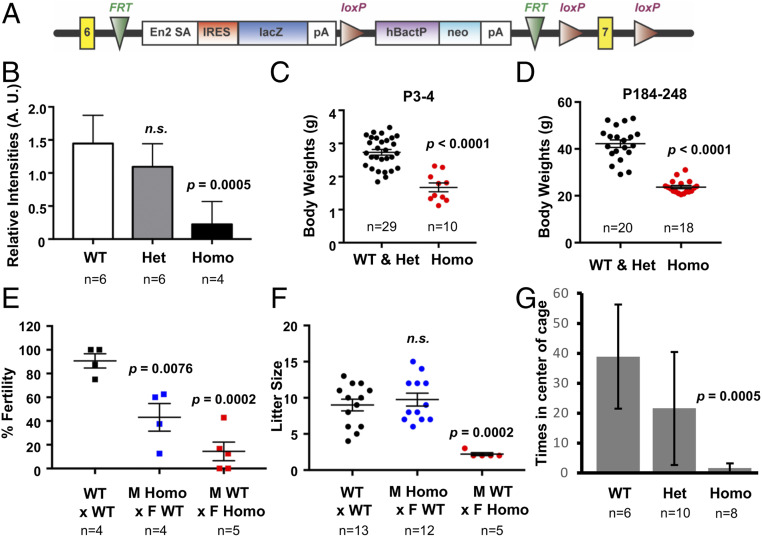
Fig. 1.
Phenotypes of the Camsap3tm1a mouse model. (A) Structure of the Camsap3tm1a allele with an RNA-processing signal/lacZ-trapping element inserted between exons 6 and 7 (indicated as yellow boxes) of the Camsap3 gene. (B) CAMSAP3 expression is significantly decreased in Camsap3tm1a/tm1a. Relative intensities in arbitrary units of CAMSAP3 bands from three independent Western blots using brain lysates from P10 to 11 littermates. Intensities are normalized to tubulin-loading control bands (detected by anti–α-tubulin). (C and D) Body weight is significantly decreased in Camsap3tm1a/tm1a. Weight of neonatal mice ages P3 to 4 (C) and older adult mice ages P184 to 248 (D) versus genotype. (E and F) Fertility and litter size are significantly decreased in Camsap3tm1a/tm1a. Fertility rate (E) and mean litter sizes (F) for breeding pairs of the indicated genotypes. M, male; F, female. (G) Food finding is significantly decreased in older adult Camsap3tm1a/tm1a. Mean number of times mice were found in the center of a cage with hidden food versus genotype. Het, Camsap3tm1a/+; Homo, Camsap3tm1a/tm1a. n, number of animals; n.s., not statistically significant. Error bars are standard deviations from the means.
We confirmed that Camsap3 transcripts were indeed decreased in Camsap3tm1a/tm1a in both FVB and CBA/CaJ backgrounds (SI Appendix, Fig. S2 A–C) but not completely absent as reported in other tm1a alleles using the same strategy (29). To test whether CAMSAP3 proteins were present in Camsap3tm1a/tm1a, brain lysates from male and female littermates were analyzed using anti–CAMSAP3-M, whose antigen specificity was verified by both immunofluorescence (IF) and Western blot analyses (SI Appendix, Fig. S1). Although the degree of CAMSAP3 reduction varied among individuals from the same litter (Fig. 1B and SI Appendix, Fig. S2 D–F), the reduction in CAMSAP3 protein expression was statistically significant in Camsap3tm1a/tm1a. Hence, the Camsap3tm1a mouse model is hypomorphic for CAMSAP3 but not a null.
We observed domed heads, a mild form of hydrocephalus, in some postnatal Camsap3tm1a/tm1a mice on the CBA/CaJ background (SI Appendix, Fig. S3A) but seldom observed this change in animals on the FVB background. However, snout asymmetry in Camsap3tm1a/tm1a was observed for both CBA/CaJ and FVB strain backgrounds (SI Appendix, Fig. S3B), although the penetrance was incomplete. Heterozygotes of all strains were indistinguishable from WT. These data reinforce the notion that genetic background impacts the severity of phenotypes observed in Camsap3tm1a/tm1a mice.
Characterization of Camsap3tm1a Mice.
We examined Camsap3tm1a at three stages: neonatal (postnatal day [P] ∼3), adolescent/young adult (3 wk to 3 mo), and older mice (>6 mo). Camsap3tm1a/tm1a consistently exhibited some of the phenotypes that are often associated with PCD regardless of sex and strain backgrounds. Heterozygotes were indistinguishable from wild type.
-
1)
Low body weight. Camsap3tm1a/tm1a were born smaller than their WT and Camsap3tm1a/+ littermates, and remained smaller into adulthood. The mean weights at P3 to 4 in g ± SD were as follows: WT and Camsap3tm1a/+, 2.7 ± 0.5; Camsap3tm1a/tm1a, 1.7 ± 0.4 (Fig. 1C); at P184 to 248, WT and Camsap3tm1a/+, 42.23 ± 7.077; Camsap3tm1a/tm1a, 23.7 ± 2.862 (Fig. 1D). Unpaired t test with Welch’s correction revealed statistically significant reduction in Camsap3tm1a/tm1a for both age groups.
-
2)
Subfertility in both sexes. Compared with WT, average fertility was significantly reduced for male Camsap3tm1a/tm1a and even further reduced for female Camsap3tm1a/tm1a (Fig. 1E). Mean ± SD are as follows: WT x WT, 90.63 ± 11.97; male Camsap3tm1a/tm1a x female WT, 43.13 ± 23.31; female Camsap3tm1a/tm1a x male WT, 14.40 ± 17.56. One-way ANOVA with Dunnett’s multiple-comparison test showed that both male and female Camsap3tm1a/tm1a had significantly lower fertility (P values are as indicated). Furthermore, average litter sizes (Fig. 1F) were similar between WT and Camsap3tm1a/tm1a males but significantly decreased for female Camsap3tm1a/tm1a compared with WT x WT. Mean litter sizes ± SD are as follows: WT x WT, 9.0 ± 2.9; male Camsap3tm1a/tm1a x female WT, 9.8 ± 3.1; male WT x female Camsap3tm1a/tm1a, 2.2 ± 0.4. One-way ANOVA with Dunnett’s multiple-comparison test revealed that male Camsap3tm1a/tm1a do not have significant differences, while female Camsap3tm1a/tm1a had significantly fewer pups per litter.
-
3)
Coughing and sneezing. Camsap3tm1a/tm1a produced audible “wheezing” in both sexes (Movie S1). The amplitudes of the soundtracks in Camsap3tm1a/tm1a were larger than for their age-matched WT (SI Appendix, Fig. S4). Sneezing was also audible in Camsap3tm1a/tm1a mice (Audio S1).
-
4)
Olfactory dysfunction. Mice were placed in a cage with hidden food to test olfactory function (buried food assay). Young adult mice were able to find the hidden food regardless of their genotype, suggesting that they had functional olfaction (WT, n = 7; Camsap3tm1a/+, n = 9; Camsap3tm1a/tm1a, n = 16). In contrast, when older mice were tested, none of the Camsap3tm1a/tm1a could find food, while all control mice (WT and Camsap3tm1a/+) did (Fig. 1G), suggesting Camsap3tm1a/tm1a progressively lost their sense of smell. One-way ANOVA was performed to compare the mean number of times mice were found in the center of a cage with hidden food. The Tukey–Kramer test indicated that WT mice were not significantly different from Camsap3tm1a/+, but both WT and Camsap3tm1a/+ were significantly different from Camsap3tm1a/tm1a (P = 0.0005).
-
5)
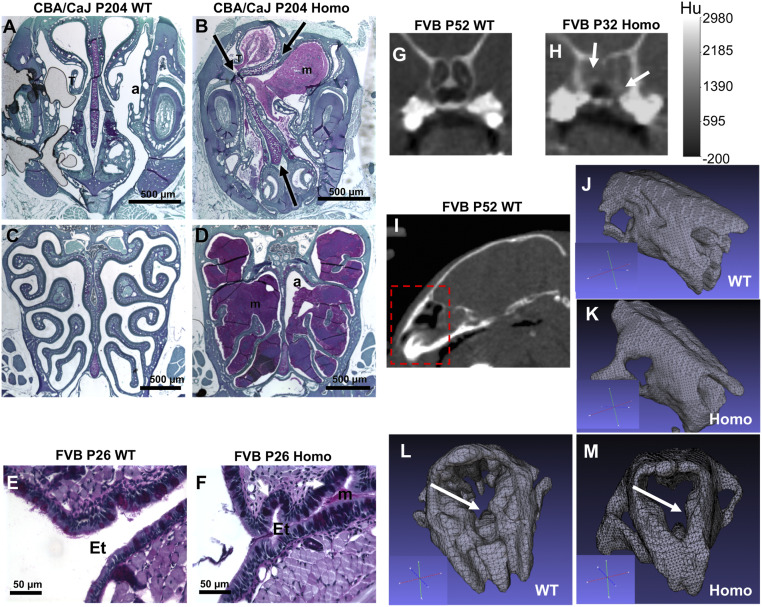
Mucin accumulation and rhinosinusitis. Animals at different ages showed mucin accumulation to various degrees (n = 10). For older mice, all Camsap3tm1a/tm1a had accumulated mucin that almost completely obstructed the nasal passage, as detected by periodic acid Schiff (PAS) staining (Fig. 2B). The nasal septum was noticeably deviated (arrows in Fig. 2B) and the turbinates were displaced in Camsap3tm1a/tm1a as compared with WT (Fig. 2A). Mucin accumulation was also found around the ethmoid turbinates that are enriched in the olfactory epithelium (Fig. 2D) and maxillary sinus (SI Appendix, Fig. S5). Accumulated mucin also extended to the Eustachian tube, even in young adult Camsap3tm1a/tm1a mice (Fig. 2F), but not in WT (Fig. 2 C and E). For young adult mice, microcomputed tomography (micro-CT) and MRI were performed to reveal nasal blockage and structural abnormalities (WT, n = 1; Camsap3tm1a/+, n = 2; Camsap3tm1a/tm1a, n = 3). MRI images show the airway blockage in Camsap3tm1a/tm1a but not in WT (Fig. 2 G and H and SI Appendix, Fig. S6). Three-dimensional (3D) rendered images including bone and cartilaginous tissues (Fig. 2 I–M) from micro-CT scans showed that the olfactory cavity is clearly more structured in WT (Fig. 2L) as compared with Camsap3tm1a/tm1a (Fig. 2M). In neonatal mice, however, we did not observe accumulated mucus in the nasal cavity in either WT or Camsap3tm1a/tm1a (SI Appendix, Figs. S8 A and B and S10 A and B), suggesting that Camsap3tm1a/tm1a mice were not born with mucin hyperproduction. Accumulated mucin likely results in the structural abnormality as well as coughing and sneezing symptoms (SI Appendix, Fig. S4 and Audio S1) observed in Camsap3tm1a/tm1a mice. In addition, the nasal cavity of Camsap3tm1a/tm1a exhibited signs of inflammation, as accumulated mucin was populated by leukocytes (SI Appendix, Fig. S7 B and C), suggesting chronic rhinosinusitis.
-
6)
Olfactory sensory neuron (OSN) degeneration. Since progressive loss of olfaction in Camsap3tm1a/tm1a mice could also relate to neural defects, we examined OSNs in neonatal and young and older adult animals (n = 3 each for WT and Camsap3tm1a/tm1a). OSNs were stained using the anti-olfactory marker protein antibody. WT mice showed a pseudostratified olfactory epithelium of normal thickness, with mature OSNs and axon bundles at all ages (SI Appendix, Fig. S8 A, C, E, and G). However, extensive thinning of the olfactory epithelium and fewer mature OSNs were present in Camsap3tm1a/tm1a at P1 and 67 with complete loss of OSNs at P204 (SI Appendix, Fig. S8 B, D, F, and H). The progressive loss of OSNs is consistent with impaired olfaction observed in the behavioral testing of Camsap3tm1a/tm1a (Fig. 1G). Mucus blockage (labeled “b”) is evident in both young and older adult Camsap3tm1a/tm1a mice (SI Appendix, Fig. S8 F and H), but not in neonates.
Fig. 2.
Camsap3tm1a/tm1a mice have obstructed nasal cavities and abnormal nasal cavity structure. (A–D) Mucin blocks the nasal airway and obstructs the olfactory epithelium in older Camsap3tm1a/tm1a mice. PAS staining for glycoproteins in coronal nasal sections in WT (A) showing a patent anterior nasal airway (A) with a central bony septum at the level of the vomeronasal organ compared with mucin buildup (B) (purple) in Camsap3tm1a/tm1a with a deviated septum (arrows). WT (C) patent nasal airway with a central septum at a level where the ethmoid turbinates (T) are covered primarily with olfactory sensory epithelium compared with Camsap3tm1a/tm1a (D) with almost total airway (a) blockage with mucin (purple). (Scale bars, 500 µm.) (E–H) Mucin blocks the nasal airway of young adult Camsap3tm1a/tm1a mice. PAS staining shows a normal opening to the Eustachian tube (Et) in WT mouse (E), while its Camsap3tm1a/tm1a littermate exhibited mucin accumulation (m) and folding of epithelial cell layers (white arrows) in the Eustachian tube (F). (Scale bars, 50 µm.) Micro-CT images showing dark and radiopaque regions at the back of the nasal cavity in WT (G) and Camsap3tm1a/tm1a (H), respectively. White arrows indicate blockage. A calibration bar is as indicated. Hu, Hounsfield units. (I–M) Representative rendered 3D images obtained by thresholding of CT data in WT and Camsap3tm1a/tm1a are shown. Snout details from the region outlined in red in I are shown for different orientations for WT (J and L) and Camsap3tm1a/tm1a (K and M) mice. An orientation axis for each figure is shown in the corresponding inset. The white arrows in (L) and (M) point at the snout region with most structural differences between the WT and Camsap3tm1a/tm1amouse.
Knockdown of CAMSAP3 Results in Abnormal Cilia on MCCs and Impaired Mucociliary Transport.
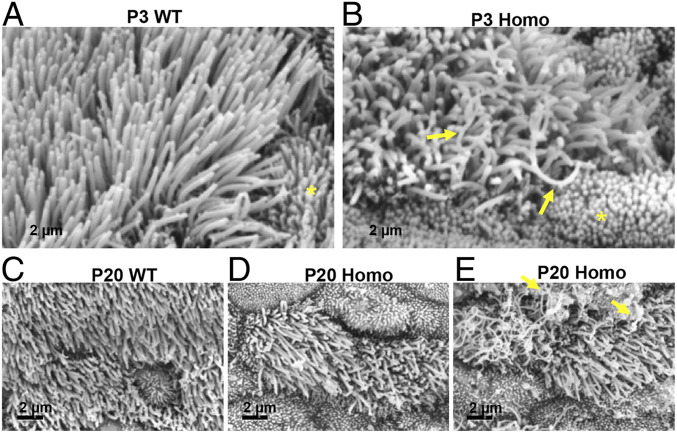
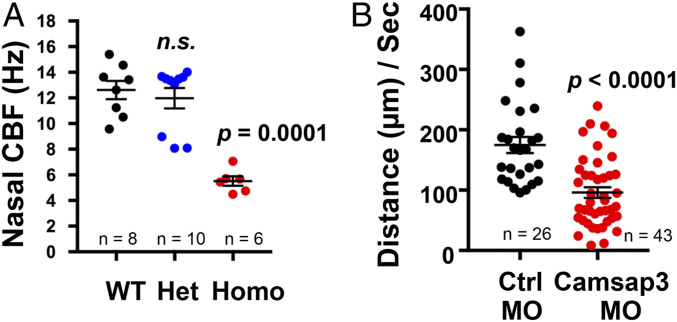
The several PCD-like phenotypes observed in Camsap3tm1a/tm1a prompted us to hypothesize that CAMSAP3 plays important roles in motile cilia. Therefore, we examined the nasal respiratory tract of Camsap3tm1a mice in more detail. Samples from the nasal cavity of Camsap3tm1a/tm1a and WT (n = 4 each) mice at P3 and 20 were examined using scanning electron microscopy (SEM). Cilia of MCCs from Camsap3tm1a/tm1a (Fig. 3 B and D and SI Appendix, Fig. S9 C and D) were in general shorter compared with WT littermates (Fig. 3 A and C and SI Appendix, Fig. S9 A and B) and were heterogeneous in length and shape. Some cilia were curved and much longer than other cilia on the same cell (Fig. 3B and SI Appendix, Fig. S9D, arrows). In contrast, cilia from WT were straight and of comparable lengths (Fig. 3 A and C and SI Appendix, Fig. S9 A and B). At P20, the apical surfaces of MCCs in Camsap3tm1a/tm1a mice were often covered with a layer of fibrous threads (Fig. 3E, arrows and SI Appendix, Fig. S9C), likely to be mucin-based structures (30, 31). These fibrous threads were seldom found in cilia of P3 samples, suggesting that mucus accumulation was minimal in the nasal cavity at this age. To test whether MCCs have normal mucociliary transport function, we examined ciliary motility of freshly dissected tissues from similar areas of the nasal cavity, tympanic cavity, and trachea of WT, Camsap3tm1a/+, and Camsap3tm1a/tm1a at ages ranging from neonate to young adult. The isolated tissues were washed several times to remove mucus before recording. Similar to a previous report (32), the average cilia beat frequency (CBF) determined at P3 for both WT and Camsap3tm1a/+ was ∼12 Hz. In Fig. 4A, CBFs for motile cilia in nasal tissues at P3 in WT, Camsap3tm1a/+, and Camsap3tm1a/tm1a mice were determined from time-lapse images. Mean ± SD for WT was 12.62 ± 2.03; for Camsap3tm1a/+, 11.98 ± 2.51; and for Camsap3tm1a/tm1a, 5.52 ± 0.90. The CBFs in Camsap3tm1a/+ were not statistically different from WT, but both were significantly greater than in Camsap3tm1a/tm1a (one-way ANOVA with Dunnett’s multiple-comparison test, P = 0.0001). A rhythmic beating with a coordinated motion was observed in MCCs of WTs and Camsap3tm1a/+, but Camsap3tm1a/tm1a showed only patches of cilia with slow movements in the nasal cavity (Movies S2 and S3), tympanic cavity of the middle ear (Movies S4 and S5), and trachea (Movies S6 and S7). As shown in Movies S2–S7, directional flow of particles across the field of view was observed in WT and Camsap3tm1a/+ (Movies S2, S4, and S6). Cilia of MCCs from Camsap3tm1a/tm1a exhibited noncoordinated beating with various speeds (Movie S3) and sometimes they were motionless (Movies S5 and S7), that is, directional flow was reduced or not observed. The lack of directional flow is an indicator that mucociliary clearance was compromised in Camsap3tm1a/tm1a mice.
Fig. 3.
MCCs from Camsap3tm1a/tm1a have abnormal ciliary morphology. SEM images of nasal MCCs from WT (A and C) and its Camsap3tm1a/tm1a littermate (B, D, and E) at P3 and 20. Arrows in B point to long and curved cilia in Camsap3tm1a/tm1a mice. Nearby microvilli are indicated by asterisks. (E) The surface of MCCs from a Camsap3tm1a/tm1a at P20. Yellow arrows indicate the layer of fibrous substances. (Scale bars, 2 µm.)
Fig. 4.
Reduction of CAMSAP3 causes impaired mucociliary transport. (A) Nasal ciliary beat frequency in hertz (beats per second) for motile cilia of nasal tissues at age P3 versus genotype. Replicates (n) are as indicated. (B) Velocity of fluorescent beads in micrometers per second of control (Ctrl) Xenopus embryos injected with morpholinos versus Camsap3 morpholino-injected embryos. n, number of fluorescent beads.
Because CAMSAP3 is expressed in different tissues and cells, it is possible that reduced clearance observed in MCCs of Camsap3tm1a/tm1a is secondary to other defects. For example, increased mucin secretion by potentially dysfunctional submucosal glands and/or goblet cell hyperplasia and mucin accumulation can affect cilia structure and mucociliary clearance. Therefore, we examined goblet cells from neonatal and older adult mice. Goblet cell hyperplasia and excess mucin were not observed in neonatal Camsap3tm1a/tm1a when compared with WT mice at P1 using PAS staining for mucus (SI Appendix, Figs. S8 A and B and S10 A and B). In contrast, defects in ciliary motion and cilia morphology were consistently observed in neonates (Figs. 3B and 4A and SI Appendix, Fig. S9D). These data suggest that impaired mucociliary transport was not due to excess mucin at birth. However, accumulated mucin (Fig. 2 B and D, purple) and goblet cell hyperplasia were observed but only in adult Camsap3tm1a/tm1a mice (SI Appendix, Fig. S10 C and D).
To further verify CAMSAP3’s role in motile cilia function, morpholino-mediated anti-Camsap3 oligomers (Camsap3-MO) were used to knock down CAMSAP3 expression in MCCs located on the skin of Xenopus embryos. Morpholino-mediated random oligomers (control-MOs) were used as negative controls. The mucociliary epithelium of Xenopus is similar to mouse, with the important distinction that it occurs on the outer surface of the embryo. Therefore, unlike the constrained internal environment of the mouse, mucosal blockage is not likely to represent a serious issue. Although cilia morphology varied (n = 7), that of MCCs with Camsap3-MO injection appeared to have fewer and shorter cilia as compared with MCCs with control-MO injection (SI Appendix, Fig. S11). To measure cilia-generated flow, fluorescent beads were added onto the surface of Xenopus embryos injected with control- or Camsap3-MOs and imaged. As shown in Fig. 4B and Movies S8 and S9, fluorescent beads on Camsap3-MO–injected embryos exhibited statistically slower movement as compared with beads on control-MO–injected embryos. Each dot represents the distance of directional movement for each individual fluorescent bead. Data are collected from 9 control-MO– and 14 Camsap3-MO–injected Xenopus embryos in two independent experiments. Mean ± SD for control-MO, 174.80 ± 68.39; Camsap3-MO, 96.01 ± 57.86; *P < 0.0001 (unpaired t test). These data further indicate that CAMSAP3 is required for the coordinated motile ciliary movements that underlie mucociliary transportation.
CAMSAP3 Is Located at the Basal Bodies and at the Base of Axonemes of Motile Cilia in Nasal MCCs.
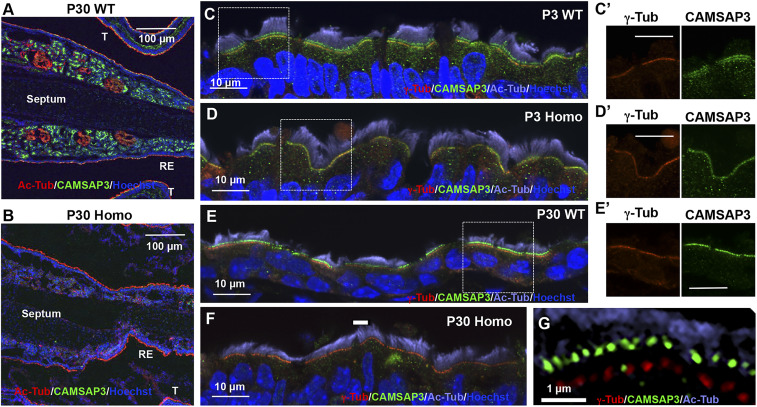
To understand the role of CAMSAP3 in motile cilia function, the nasal cavity was examined using anti–CAMSAP3-M. Anti–γ-tubulin (γ-Tub) was used as a basal body marker, while anti–acetylated-α-tubulin (Ac-Tub) was used to label cilia. CAMSAP3 distributions were compared among nasal tissue samples from WT, Camsap3tm1a/+, and Camsap3tm1a/tm1a mice collected at different ages ranging from P3 to 30 (WT, n = 9; Camsap3tm1a/+, n = 3; Camsap3tm1a/tm1a, n = 9). CAMSAP3 staining was found at the apical surface of respiratory epithelia, as well as in the acini of submucosal glands that lie within WT mucociliary epithelia (Fig. 5A and SI Appendix, Fig. S12 A and C). Lack of staining in Camsap3tm1a/tm1a (Fig. 5B and SI Appendix, Fig. S12B) suggests that CAMSAP3 proteins are absent or significantly reduced in the nasal cavity of Camsap3tm1a/tm1a.
Fig. 5.
CAMSAP3 is located at the base of axonemes and at the basal bodies. (A–F) Immunofluorescent images of nasal MCCs from WT (A, C, and E) and Camsap3tm1a/tm1a (B, D, and F) at P3 (C and D) and 30 (A, B, and E–G) are shown. Antibodies include anti–CAMSAP3-M (green), anti–γ-tubulin (red), anti–acetylated-α-tubulin (red in A and B and violet in C–G), and Hoechst 33342 (blue, nuclei). Respiratory epithelium (RE). (G) SIM image showing the upper CAMSAP3 line (green dots) and lower line of basal bodies (γ-Tub, red dots). (Scale bars, 100 µm [A and B], 10 µm [C–F], 5 µm [C′, D′, and E′], and 1 µm [G].) C′, D′, and E′ are enlarged images of the boxed regions in C–E showing anti–γ-tubulin (red) and anti–CAMSAP3-M (green) channels.
For MCCs in the respiratory epithelia, CAMSAP3 staining (green) showed two distinct lines with similar staining intensity in all WT samples in neonatal mice at P3 (Fig. 5C). 1) The upper line of staining is between basal bodies (γ-Tub, red) and axonemes (Ac-Tub, violet). 2) The lower line of staining overlaps with γ-tubulin staining (Fig. 5 C and C′). For Camsap3tm1a/tm1a littermates, some samples did not have CAMSAP3 staining at all, while others had only the lower line (CAMSAP3) that overlapped with γ-tubulin staining, the basal body marker (Fig. 5 D and D′). As animals became older, most of the MCCs from Camsap3tm1a/tm1a did not show CAMSAP3 staining (Fig. 5F). However, cells with CAMSAP3 signals were occasionally found aligning with the γ-tubulin staining (lower line) (SI Appendix, Fig. S13B). This is consistent with the notion that Camsap3tm1a/tm1a is a hypomorphic model. In other words, we did not observe the upper line of CAMSAP3 in MCCs from Camsap3tm1a/tm1a regardless of age. In contrast, the upper line of CAMSAP3 was present in all WT and Camsap3tm1a/+ samples. At P30, most WT MCCs had only the upper line, although a few MCCs had double lines with some CAMSAP3 staining located along basal bodies as judged by merged images with γ-tubulin staining (Fig. 5 E, E′, and G). Using structured illumination microscopy (SIM), a separation between green CAMSAP3 dots and red γ-tubulin dots was clearly observed (Fig. 5G). The distance between the green CAMSAP3 dots in the upper line and the basal bodies stained with γ-tubulin was measured to be 545 ± 133 nm (mean ± SD; n = 306) (Fig. 5G). Because CAMSAP3 and CAMSAP2 are similar in structure and function (8, 33), anti–CAMSAP3-M recognizes CAMSAP2 protein, albeit with a lower affinity (SI Appendix, Fig. S1C). To exclude the possibility that CAMSAP3 signals in MCCs are CAMSAP2, we examined CAMSAP2 expression patterns in MCCs of WT, Camsap3tm1a/+, and Camsap3tm1a/tm1a at P3 and 30 using anti-CAMSAP2. As shown in SI Appendix, Fig. S14, MCCs from WT and Camsap3tm1a/tm1a have similar CAMSAP2 staining patterns, which are completely different from those of CAMSAP3. In fact, no signals were detected at the axoneme base by anti-CAMSAP2. In summary, CAMSAP3 is expressed between the basal body and the axoneme, where the central MT pair is initiated, as well as at the basal bodies in young animals.
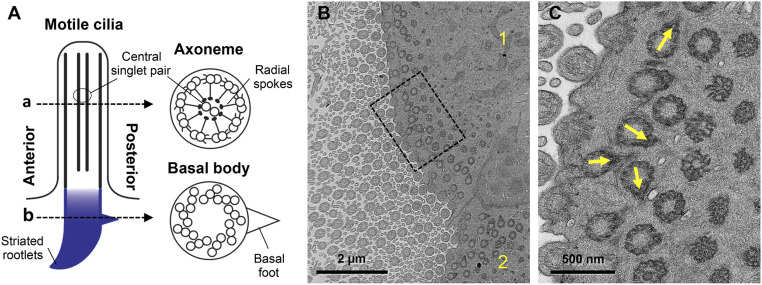
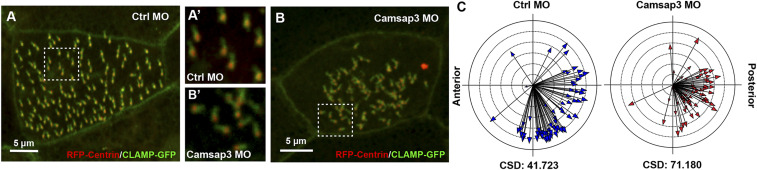
Knockdown of CAMSAP3 Results in Loss of Basal Body Polarity in MCCs.
The motile cilium is a complex organelle formed by the axoneme and emanates from the basal body (Fig. 6A). Basal bodies have an electron-dense side projection called the basal foot that points in the direction of the effective stroke, as well as a striated rootlet that projects in the opposite direction and is thought to anchor the cilium inside the cell. Both the basal foot and the striated rootlet are reliable determinants of cilia polarity (34). In WT tissues including the nasal cavity, basal feet are unidirectional (20, 21). Although PCP signaling instructs MCC polarity, hydrodynamic forces driven by cilia motility are critical for coordinating cilia polarity along the axis of cell polarity (35–37). Additionally, the basal feet and their orientation are critical for the synchronized beating needed for mucociliary transportation (30, 38). Based on the location of CAMSAP3 (Fig. 5), we forecast that lack of CAMSAP3 in MCCs may change the orientation of basal bodies. To test this possibility, the basal feet (position “b” in Fig. 6A) in MCCs of WT and Camsap3tm1a/tm1a were examined by transmission electron microscopy (TEM). Although the basal feet were not unidirectional in the nasal MCCs of Camsap3tm1a/tm1a (Fig. 6 B and C, yellow arrows), they were nicely aligned in WT (SI Appendix, Fig. S15). Since ciliogenesis is well-conserved throughout eukaryotes (26, 39), we further investigated the orientation of cilia in MCCs of Xenopus embryos, where polarity has been extensively studied (22). Specifically, control- or Camsap3-MOs were injected, together with messenger ribonucleic acids (mRNAs) for RFP-centrin (a basal body marker) and CLAMP-GFP (a striated rootlet marker). We observed a uniform array of cilia in control-MO–injected embryos (Fig. 7 A and A′), while in Camsap3-MO–injected embryos the cilia orientation was disorganized (Fig. 7 B and B′). The phenotype was quantified by measuring cilia orientation (Fig. 7C). In these graphs, the direction of the arrow represents the mean orientation of the cilia within a given cell and is a readout of cell polarity. Additionally, the length of the arrow represents variation around the mean such that a long arrow represents a coordinated cell. Circular SD (CSD) is a metric for cumulative variation and increases from 41.72 in the control-MO to 71.18 in the Camsap3-MO in four independent experiments (control-MO embryos, n = 8; Camsap3-MO embryos, n = 11; P < 0.0001). Importantly, while the cilia are disorganized in Camsap3-MO cells, the mean cilia orientation is still in the posterior direction, indicating that PCP-driven cell polarity is maintained. Taken together, our data suggest that reduced CAMSAP3 expression results in the loss of basal body polarity. Motility defects likely underlie the inability of cilia to refine their orientation, consistent with what has been observed in PCD-related mutations (35).
Fig. 6.
Reduction of CAMSAP3 expression in mice disrupts the polarity of basal bodies in MCCs. (A) Motile cilium structure. (B) A representative image showing disorientated basal feet found in two MCCs (indicated with numbers 1 and 2) in the nasal cavity of Camsap3tm1a/tm1a (P30). (Scale bar, 2 µm.) (C) High-magnification image of the boxed region in B. Yellow arrows indicate basal feet with various orientations. (Scale bar, 500 nm.)
Fig. 7.
Reduction of CAMSAP3 expression disrupts the polarity of basal bodies in MCCs of Xenopus embryos. Confocal images of Xenopus embryos injected with RNAs for RFP-centrin and CLAMP-GFP together with either control- (A) or Camsap3-MO (B). Boxed regions from A and B are shown in A′ and B′, respectively. (Scale bars, 5 µm.) (C) Quantification of MCC polarity. Each arrow represents the mean cilia orientation for a given cell, while arrow length represents 1/r, namely the variation in orientation within the cell (long arrows indicate low variation and short arrows indicate high variation).
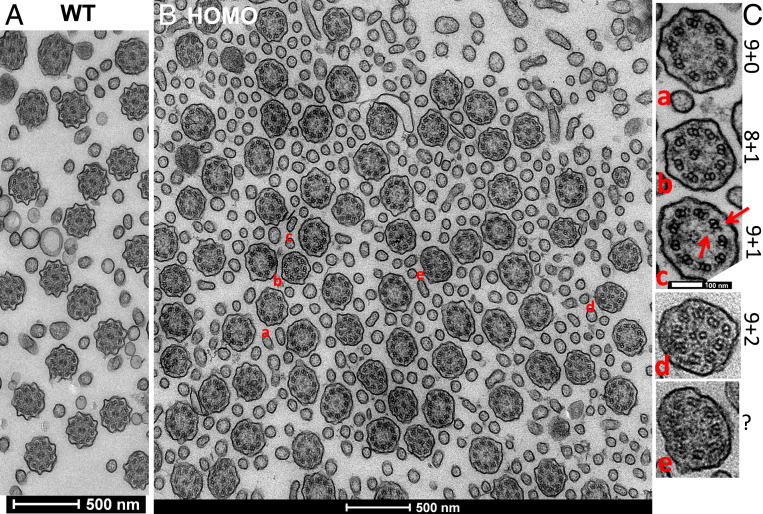
Central MT Pairs Are Rare in Cilia of MCCs from Camsap3tm1a/tm1a Mice.
The axoneme of MCCs is composed of the 9 + 2 MT skeleton, while basal bodies have nine triplet MTs and lack a central MT (Fig. 6A). Since CAMSAP3 was also located at the base of axonemes (Fig. 5), where the central MT pair is initiated, and CAMSAP3 proteins can act as seeds for MT elongation (8, 10), we examined axoneme structure (“a” position in Fig. 6A) in MCCs of WT and Camsap3tm1a/tm1a using TEM (Fig. 8). Although both inner and outer dynein arms (red arrows in Fig. 8) were present in the nine MT doublets, we discovered several structural abnormalities in the axonemes of MCCs from Camsap3tm1a/tm1a (Fig. 8B). The phenotypes were divided into five groups, as shown in Fig. 8C: 1) 9 + 0, in which the axoneme has only outer doublets (Fig. 8A); 2) 8 + 1, in which the axoneme has eight outer doublets and one doublet MT mislocalized at the center (Fig. 8B); 3) 9 + 1, in which the axoneme has nine outer doublets and one inner singlet (Fig. 8C); 4) 9 + 2, in which the axoneme has nine outer doublets and two normal MT singlets (Fig. 8D); and 5) unclassified (Fig. 8E). Among 128 axonemes examined, 64.1% were type 1 (9 + 0); 13.3% were type 2 (8 + 1); 11.7% were type 3 (9 + 1); 5.5% were type 4 (9 + 2); and 5.4% were unclassified. In other words, most of the cilia (89.1%) in Camsap3tm1a/tm1a had abnormal central MT pairs or were missing the central pair entirely, suggesting that CAMSAP3 is needed for central MT-pair formation.
Fig. 8.
CAMSAP3 is needed for the formation of central MT pairs in axonemes. TEM images showing MT arrays in axonemes from an adult P30 WT (A) compared with a P30 Camsap3tm1a/tm1a (B). Five different axoneme MT configurations (a–e) are listed in C. Red arrows point to inner and outer dynein arms in outer MT doublets. (Scale bars, 500 nm [A and B] and 100 nm [C].)
Discussion
The results of this study are summarized as follows. 1) CAMSAP3-KD mice exhibit some PCD-like phenotypes. 2) Knocking down CAMSAP3 protein levels produces structural and functional defects in motile cilia of MCCs in both mice and frogs. 3) CAMSAP3 is expressed along the basal bodies and at the base of axonemes in MCCs. 4) MCCs in CAMSAP3-KD mice show disorientated basal bodies and lack the central MT pair in the axoneme. Collectively, our data suggest that CAMSAP3 plays an important role in forming central MT pairs, orienting the basal bodies in MCCs, and facilitating cilia motility.
Our study of Camsap3-KD mice on three different backgrounds revealed that CAMSAP3-related phenotypes were strain-dependent. The C57BL/6 (B6) strain appears more vulnerable to ciliary defects as previously reported in transgenic models for PCD (40). In fact, mRNA expression profiles for PCD models on B6 and other strains are significantly different in several gene clusters, including genes involved in cilia formation and function (41). Hence, further investigation is needed to understand how genetic modifications relate to specific PCD-like phenotypes.
CAMSAP3 is widely expressed in different tissues and cell types. Therefore, the phenotypes reported in this study may relate to several deficiencies that occur in multiple cells/tissues in addition to motile cilia defects. For example, preweaning mortality was suggested to be caused by a defect in the placenta (11). An abnormal placenta may also contribute to the lower litter size that was only observed in Camsap3tm1a/tm1a females in addition to dysfunctional motile cilia located in the reproductive tract (Fig. 1F). Although accumulated mucus or change in airflow in the nasal cavity often results in unusual nasal structures (42) that are also found in other PCD mice (43), CAMSAP3 may have other functions that contribute to craniofacial abnormalities, requiring systematic investigation in Camsap3tm1a mice.
The central MT pair is critical for MCCs to generate the coordinated planar motion required for mucus clearance (23, 24). One of the long-standing questions in ciliogenesis is how the central MT pair is assembled and what components are involved. We found that the MT-minus end regulator CAMSAP3 localizes to where the central pair initiates, suggesting a role in central-pair formation. Analysis of patients with PCD has identified a subset of structural defects associated with the disease. In particular, patients have been identified with mutations in hydin, Rsph4A, and Rsph9, all of which lead to central-pair defects (44–47). In this report, we identify CAMSAP3 as an additional potential candidate to be included in this class of mutations. Intriguingly, the central MT pairs extend from a region near the transition zone, which is ∼500 nm in length (23, 48, 49). It is of interest, therefore, that CAMSAP3 is located more than 500 nm away from the center of the basal bodies, that is, at the distal end of the transition zone. CAMSAP3 is also known to interact with the MT-severing protein katanin, mutations of which have been shown to cause central-pair defects in Tetrahymena (6, 50, 51). It is, therefore, tempting to speculate that the balance between the nucleation regulated by CAMSAP3 and the destruction regulated by katanin represents a critical nexus for the control of central-pair formation.
MT networks in individual cells are organized to perform different cellular functions. For neurons, CAMSAP3 sustains nonacetylated MTs in order to maintain neuronal polarity (12). For epithelial cells, CAMSAP3 is reported to regulate the polarity of MTs and influence the distribution of organelles, such as Golgi and nuclei, as reported in Camsap3-knockout (KO) and Camsap3-knockin (Camsap3dc/dc) mouse models, as well as in several cell lines (1, 13, 14, 52). However, mispositioning of organelles was not obvious in MCCs of Camsap3tm1a/tm1a (Fig. 5) because the basal bodies were all located under the axoneme at the apical cortex, and nuclei appeared on the basal sides of MCCs. One possibility for the lack of abnormal organelle distribution in MCCs of Camsap3tm1a/tm1a is that CAMSAP2 could compensate for some functions of CAMSAP3 to maintain the MT networks of MCCs as previously reported in other cells (33). Indeed, we found similar staining patterns in Camsap3tm1a/tm1a and their WT littermates (SI Appendix, Fig. S14), showing that CAMSAP2 is expressed throughout the cytoplasm in neonates and colocalizes with the basal bodies at the apical cortex in adults. Further investigation is needed to understand the roles of CAMSAP2 and CAMSAP3 for establishing and maintaining differentiated MCCs.
Dense MT networks at the apical cortex of MCCs control basal body positioning and are essential for synchronizing motile ciliary movements (30, 38, 53). However, it is not fully known how the MT networks are formed around basal bodies. In addition to localizing to the base of the axoneme, we found that CAMSAP3 is present in neonates along the basal bodies (Fig. 5 C and C′), suggesting that CAMSAP3 could play an important role in establishing MT networks at the apical cortex. It is known that apical and subapical actin networks are also important for establishing ciliary spacing and polarity, as well as for coordinating metachronal synchrony (22, 38, 54). Since CAMSAP3 can influence apical actin networks through its interaction with ACF7 (5, 55), we suspect that CAMSAP3 may facilitate the establishment and coordination of cytoskeletal networks in the apical cortex through its interaction with both MTs and actin filaments. Understanding how CAMSAP3 facilitates the establishment and coordination of cytoskeletal networks requires further study.
Motile cilia are only present in specific tissues and cell types. In contrast, most cells in our body have primary cilia that are sensory organelles. A majority of primary cilia have MTs in the 9 + 0 configuration, that is, they do not have a central MT pair. As shown in SI Appendix, Fig. S16, CAMSAP3 signals do not colocalize with the basal bodies of primary cilia. In addition, the reduction of CAMSAP3 in Camsap3tm1a/tm1a shows that the location and morphology of basal bodies in primary cilia on cochlear epithelial cells are similar to those of WT. This observation is consistent with previous reports showing that CAMSAP3 is not centrosome-bound. In fact, CAMSAP3 is often found along noncentrosomal MTs or in pericentrosomal areas, where it is involved in releasing MTs from the centrosome (4, 8, 51, 56). We also acknowledge that a minority of primary cilia, like OSNs, can display the 9 + 2 configuration. At present, it is not known whether OSNs in Camsap3tm1a/tm1a mice retain normal 9 + 2 cilia, but these animals were born with fewer OSNs (SI Appendix, Fig. S8 A–D). Although OSN loss in older adult Camsap3tm1a/tm1a mice (SI Appendix, Fig. S8H) could be a consequence of mucus obstruction (Fig. 2) and/or inflammatory changes (SI Appendix, Fig. S7) due to dysfunctional MCCs of Camsap3tm1a/tm1a, the abnormality of OSNs observed in neonates is clearly unrelated to motile cilia dysfunction of MCCs. In addition, CAMSAP3 might serve to maintain OSN polarity by regulating MT stability, as observed in hippocampal neurons (12). In other words, OSN loss observed in Camsap3tm1a/tm1a mice is likely a combination of defects in several different kinds of cells. To determine how lack of CAMSAP3 in OSNs contributes to their loss in Camsap3tm1a/tm1a will require a specific conditional KO model.
In conclusion, CAMSAP3 is involved in many physiological functions through its associated protein partners, suggesting that this minus-end MT regulator may influence ciliogenesis and cilia function via several different mechanisms, including microtubule organization, material trafficking, organelle positioning, and so forth. Hence, divergent phenotypes are to be expected when conditional knockout mouse models become available to investigate CAMSAP3’s role in specific cells and tissues. Nevertheless, the data obtained from this study identify CAMSAP3 as a critical component of motile cilia, providing scientific knowledge that is fundamental to the basic biology regarding cilia function.
Materials and Methods
Camsap3tm1a mice are described in SI Appendix. The full procedures for evaluation of olfaction, measurements of ciliary motion and ciliary orientation, sound recording and analysis, histochemistry, TEM, SEM, micro-CT and MRI, IF, RT-PCR, Western blot, cell culture, and transfection are provided in SI Appendix, where the statistical analyses are also described.
Data and Materials Availability.
Data generated or analyzed during this study are included in this article and its supplementary files. All data, including the original images from micro-CT and MRI, will be made available upon request to the corresponding author.
Supplementary Material
Acknowledgments
We thank Drs. Jonathan Siegel, Donna Whitlon (Northwestern University), and Masatoshi Takeichi (RIKEN Center for Developmental Biology) for providing instruments and reagents, and Mr. Taewoo Kim (Northwestern University) for participating in the data analysis. Imaging work was performed at the Northwestern University Center for Advanced Microscopy, which is supported by National Cancer Institute (NCI) grant CCSG P30 CA060553 and National Institutes of Health NIH grant 1S10OD016342-01. MRI and CT scans were conducted at the Center for Translational Imaging Small Animal Imaging Laboratory. Rederivation of Camsap3tm1a lines was performed by the Transgenic and Targeted Mutagenesis Laboratory of Northwestern University. This research was funded by the Knowles Hearing Center (to J.Z.) and by National Institute on Deafness and Other Communication Disorders (NIDCD) grant R56 DC011813 (to J.Z.), grant DC000089 (to M.A.C.), and grant GM089970 (to B.J.M.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. H.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907335117/-/DCSupplemental.
References
- 1.Zheng J. et al., Marshalin, a microtubule minus-end binding protein, regulates cytoskeletal structure in the organ of Corti. Biol. Open 2, 1192–1202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin S. S., Vale R. D., Patronin regulates the microtubule network by protecting microtubule minus ends. Cell 143, 263–274 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendershott M. C., Vale R. D., Regulation of microtubule minus-end dynamics by CAMSAPs and Patronin. Proc. Natl. Acad. Sci. U.S.A. 111, 5860–5865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng W., Mushika Y., Ichii T., Takeichi M., Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 135, 948–959 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Ning W. et al., The CAMSAP3-ACF7 complex couples noncentrosomal microtubules with actin filaments to coordinate their dynamics. Dev. Cell 39, 61–74 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Jiang K. et al., Structural basis of formation of the microtubule minus-end-regulating CAMSAP-katanin complex. Structure 26, 375–382.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Takahashi S. et al., Cadherin 23-C regulates microtubule networks by modifying CAMSAP3’s function. Sci. Rep. 6, 28706 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang K. et al., Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev. Cell 28, 295–309 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Nashchekin D., Fernandes A. R., St Johnston D., Patronin/shot cortical foci assemble the noncentrosomal microtubule array that specifies the Drosophila anterior-posterior axis. Dev. Cell 38, 61–72 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zenker J. et al., A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science 357, 925–928 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Perez-Garcia V. et al., Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature 555, 463–468 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pongrakhananon V. et al., CAMSAP3 maintains neuronal polarity through regulation of microtubule stability. Proc. Natl. Acad. Sci. U.S.A. 115, 9750–9755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toya M. et al., CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 113, 332–337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muroyama A., Terwilliger M., Dong B., Suh H., Lechler T., Genetically induced microtubule disruption in the mouse intestine impairs intracellular organization and transport. Mol. Biol. Cell 29, 1533–1541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spassky N., Meunier A., The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18, 423–436 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Brooks E. R., Wallingford J. B., Multiciliated cells. Curr. Biol. 24, R973–R982 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stubbs J. L., Vladar E. K., Axelrod J. D., Kintner C., Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat. Cell Biol. 14, 140–147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choksi S. P., Lauter G., Swoboda P., Roy S., Switching on cilia: Transcriptional networks regulating ciliogenesis. Development 141, 1427–1441 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Boutin C. et al., A dual role for planar cell polarity genes in ciliated cells. Proc. Natl. Acad. Sci. U.S.A. 111, E3129–E3138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Iongh R., Rutland J., Orientation of respiratory tract cilia in patients with primary ciliary dyskinesia, bronchiectasis, and in normal subjects. J. Clin. Pathol. 42, 613–619 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herawati E. et al., Multiciliated cell basal bodies align in stereotypical patterns coordinated by the apical cytoskeleton. J. Cell Biol. 214, 571–586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner M. E., Mitchell B. J., Understanding ciliated epithelia: The power of Xenopus. Genesis 50, 176–185 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loreng T. D., Smith E. F., The central apparatus of cilia and eukaryotic flagella. Cold Spring Harb. Perspect. Biol. 9, a028118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng J. et al., Microtubule-bundling protein Spef1 enables mammalian ciliary central apparatus formation. J. Mol. Cell Biol. 11, 67–77 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Lechtreck K.-F., Gould T. J., Witman G. B., Flagellar central pair assembly in Chlamydomonas reinhardtii. Cilia 2, 15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell D. R., Speculations on the evolution of 9+2 organelles and the role of central pair microtubules. Biol. Cell 96, 691–696 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skarnes W. C. et al., A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474, 337–342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Testa G. et al., A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis 38, 151–158 (2004). [DOI] [PubMed] [Google Scholar]
- 29.White J. K. et al.; Sanger Institute Mouse Genetics Project , Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell 154, 452–464 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clare D. K. et al., Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat. Commun. 5, 4888 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostedgaard L. S. et al., Gel-forming mucins form distinct morphologic structures in airways. Proc. Natl. Acad. Sci. U.S.A. 114, 6842–6847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chilvers M. A., O’Callaghan C., Analysis of ciliary beat pattern and beat frequency using digital high speed imaging: Comparison with the photomultiplier and photodiode methods. Thorax 55, 314–317 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka N., Meng W., Nagae S., Takeichi M., Nezha/CAMSAP3 and CAMSAP2 cooperate in epithelial-specific organization of noncentrosomal microtubules. Proc. Natl. Acad. Sci. U.S.A. 109, 20029–20034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park T. J., Mitchell B. J., Abitua P. B., Kintner C., Wallingford J. B., Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 40, 871–879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell D. R., The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv. Exp. Med. Biol. 607, 130–140 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell B. et al., The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr. Biol. 19, 924–929 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guirao B. et al., Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341–350 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Kunimoto K. et al., Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell 148, 189–200 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Zhang S., Mitchell B. J., Basal bodies in Xenopus. Cilia 5, 2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finn R., Evans C. C., Lee L., Strain-dependent brain defects in mouse models of primary ciliary dyskinesia with mutations in Pcdp1 and Spef2. Neuroscience 277, 552–567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKenzie C. W. et al., Strain-specific differences in brain gene expression in a hydrocephalic mouse model with motile cilia dysfunction. Sci. Rep. 8, 13370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coppola D. M., Craven B. A., Seeger J., Weiler E., The effects of naris occlusion on mouse nasal turbinate development. J. Exp. Biol. 217, 2044–2052 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Ibañez-Tallon I., Gorokhova S., Heintz N., Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum. Mol. Genet. 11, 715–721 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Jeanson L. et al., RSPH3 mutations cause primary ciliary dyskinesia with central-complex defects and a near absence of radial spokes. Am. J. Hum. Genet. 97, 153–162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castleman V. H. et al., Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am. J. Hum. Genet. 84, 197–209 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lechtreck K.-F., Gould T. J., Witman G. B., Flagellar central pair assembly in Chlamydomonas reinhardtii. Cilia 2, 15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawe H. R., Shaw M. K., Farr H., Gull K., The hydrocephalus inducing gene product, Hydin, positions axonemal central pair microtubules. BMC Biol. 5, 33 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dean S., Moreira-Leite F., Varga V., Gull K., Cilium transition zone proteome reveals compartmentalization and differential dynamics of ciliopathy complexes. Proc. Natl. Acad. Sci. U.S.A. 113, E5135–E5143 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang T. T. et al., Superresolution pattern recognition reveals the architectural map of the ciliary transition zone. Sci. Rep. 5, 14096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma N. et al., Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 178, 1065–1079 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong C. et al., CAMSAP3 accumulates in the pericentrosomal area and accompanies microtubule release from the centrosome via katanin. J. Cell Sci. 130, 1709–1715 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Wang J. et al., CAMSAP3-dependent microtubule dynamics regulates Golgi assembly in epithelial cells. J. Genet. Genomics 44, 39–49 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Werner M. E. et al., Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol. 195, 19–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tateishi K., Nishida T., Inoue K., Tsukita S., Three-dimensional organization of layered apical cytoskeletal networks associated with mouse airway tissue development. Sci. Rep. 7, 43783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noordstra I. et al., Control of apico-basal epithelial polarity by the microtubule minus-end-binding protein CAMSAP3 and spectraplakin ACF7. J. Cell Sci. 129, 4278–4288 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Rao A. N. et al., Sliding of centrosome-unattached microtubules defines key features of neuronal phenotype. J. Cell Biol. 213, 329–341 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.