Abstract
Background
The aim of this study was to determine the frequency of anti‐cardiolipin antibodies (aCL) and anti‐β2 glycoprotein I antibodies (aβ2GPI) among Tunisian patients with rheumatoid arthritis (RA).
Methods
Ninety RA patients with positive anti‐cyclic citrullinated antibodies (anti‐CCP) and 90 healthy blood donors (HBD) were studied. aCL and aβ2GPI of isotype IgG, IgA and IgM were detected by ELISA.
Result
The frequency of antiphopholipid antibodies (aPL) (aCL and/or aβ2GPI) was significantly higher in patients with RA than in HBD (35.5% vs 11.1%, P = .0001). The frequencies of aCL and aβ2GPI were significantly higher in patients than in healthy subjects (15.5% vs 5.5%, P = .04 and 32.2% vs 11.1%, P = .0005 respectively). aβ2GPI‐IgA were significantly more frequent in patients than in the control group (26.7% vs 7.8%, P = .0007). In patients, aβ2GPI‐IgA were significantly more frequent than aβ2GPI‐IgG (26.7% vs. 6.7%, P = .0003) and aβ2GPI‐IgM (26.7% vs 5.6%, P = .0001). In RA patients, the frequency of aβ2GPI was significantly higher than that of aCL (32.2% vs 15.5%, P = .008). aβ2GPI‐IgA was significantly more frequent than aCL‐IgA (26.7% vs 4.4%, P = .00005). The average titer of anti‐CCP in aPL positive patients was significantly higher than in aPL negative patients (170.6 ± 50 RU/mL vs 147.7 ± 51 RU/mL, P = .04). Significant correlation was found between aβ2GPI‐IgA and anti‐CCP (r = .235, P = .026).
Conclusions
aPL and particularly aβ2GPI‐IgA are frequent in RA and are correlated with anti‐CCP.
Keywords: anti‐cardiolipin antibodies, anti‐β2 glycoprotein I antibodies, rheumatoid arthritis, Tunisia
1. INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, inflammatory joint disease of autoimmune nature. The ethiopathogenic mechanisms involved are complex and include gut dysbiosis.1 RA is characterized by autoantibodies production (rheumatoid factor (RF) and anti–citrullinated protein antibody (ACPA)). RA can lead to accumulating joint damage and irreversible disability.2
Antiphospholipid antibodies (aPL) are a heterogeneous group of antibodies that have been associated with thrombotic or obstetrical events in patients with antiphospholipid syndrome (APS).3 These antibodies can occur not only in APS but also in a variety of autoimmune, malignant, and infectious diseases.4 In fact, the definition of clinically significant aPL positivity is not well established.3
The most commonly detected aPL antibodies are lupus anticoagulant, anti‐cardiolipin antibodies (aCL) and anti‐β2 glycoprotein I (aβ2GPI). In RA, several studies determined the frequency of aCL and aβ2GPI.5, 6, 7, 8, 9, 10, 11 However, to our knowledge, aβ2GPI‐IgA has been determined in only three studies.7, 8, 9 Furthermore, the frequency of aPL antibodies is not known in RA in Tunisia. So, the aim of our study is to evaluate the frequency of aCL (IgG, IgA, IgM) and aβ2GPI (IgG, IgA, IgM) in a cohort of RA patients without looking for APS.
2. MATERIALS AND METHODS
2.1. Patients
In our retrospective study, sera of 90 RA patients, with positive anti‐cyclic citrullinated antibodies (anti‐CCP), were included from the database of our Immunology laboratory. Sera were collected between 2017 and 2018 from four hospitals in the center of Tunisia. Patients were diagnosed with RA according to American College of Rheumatology/European League Against Rheumatism (ACR/EULAR).12
Sera of sex‐matched 90 healthy blood donors (HBD) served as normal controls. All sera of control group were tested for anti‐CCP and RF.
All sera were stored at −80°C until the use. Ethical committee of our hospital gave approval for this study.
2.2. Methods
2.2.1. aCL assays
Serum samples were evaluated for aCL‐IgG, IgA, and IgM by using a commercial enzyme‐linked immunosorbent assay (ELISA) (Orgentec Diagnostika®) as we have described it previously.13 Results were expressed as arbitrary units with a cutoff of positivity of 10 U/mL for IgA and IgG and 7 U/mL for IgM following the manufacturer's instructions.
2.2.2. aβ2GPI assays
The determination of aβ2GPI IgG, IgA, and IgM were carried out with a commercial ELISA (Orgentec Diagnostika®) using a purified human β2GPI as we have described it previously.13 Results were expressed as arbitrary units with a cutoff for positivity of 8 U/mL following the manufacturer's instructions.
2.2.3. RF assays
Serum samples were evaluated for IgG, IgA, and IgM‐FR by using a commercial ELISA (Orgentec Diagnostika®) as we have described it previously.14 Results were expressed as arbitrary units following the manufacturer's instructions.
2.2.4. Anti‐CCP assays
Anti‐CCP was detected by using a commercially available second‐generation ELISA (Euroimmun®) as we have described it previously.15 Results were expressed as arbitrary units with a cutoff for positivity of 5 RU/mL according to the manufacturer's instructions.
2.2.5. Statistical analysis
The comparison of frequencies of aPL was performed using Chi‐square or Fisher's test. The variables were tested for normality using the Kolmogorov‐Smirnov test. To compare the mean titer of anti‐CCP between positive and negative aPL patients, we used a parametric Student's t test. Correlation study between aβ2GPI‐IgA and anti‐CCP was done by calculating Spearmans's correlation coefficient. A P‐value <.05 was considered significant.
3. RESULTS
The characteristics of patients and normal controls are presented in Table 1.
Table 1.
Characteristics of RA patients and the control group
| RA patients (n = 90) | Control group (n = 90) | |
|---|---|---|
| Sex‐ratio | 1.6 | 1.6 |
| (F/M) | (56/34) | (56/34) |
| Mean age | 53 ± 15 y | 37 ± 11 y |
| Age range | 22‐83 y | 20‐64 y |
| Positive anti‐CCP |
100% (90/90) |
3.3% (3/90) |
| Positive IgG‐RF |
78.8% (71/90) |
2.2% (2/90) |
| Positive IgA‐RF |
78.8% (71/90) |
0% (0/90) |
| Positive IgM‐RF |
90% (81/90) |
5.5% (5/90) |
aCL and aβ2GPI frequencies are summarized in Table 2. The frequency of having any type of aPL (aCL and/or aβ2GPI) was significantly higher in patients with RA than in HBD (35.5% vs 11.1%, P = .0001).
Table 2.
Frequency of aCL and aβ2GPI in patients with RA and in the control group
| Autoantibodies | RA patients (n = 90) | Control group (n = 90) | P |
|---|---|---|---|
| aPL (aCL or aβ2GPI) |
35.5% (32/90) |
11.1% (10/90) |
.0001 |
| aCL (IgG, IgA or IgM) |
15.5%**** (14/90) |
5.5% (5/90) |
.04 |
| aCL‐IgG |
8.9% (8/90) |
2.2% (2/90) |
NS |
| aCL‐IgA |
4.4% * (4/90) |
2.2% (2/90) |
NS |
| aCL‐IgM |
6.7% (6/90) |
4.4% (4/90) |
NS |
| aβ2GPI (IgG, IgA, or IgM) |
32.2%**** (29/90) |
11.1% (10/90) |
.0005 |
| aβ2GPI‐IgG |
6.7%** (6/90) |
3.3% (3/90) |
NS |
| aβ2GPI‐IgA |
(24/90) |
7.8% (7/90) |
.0007 |
| aβ2GPI‐IgM |
5.6%*** (5/90) |
4.4% (4/90) |
NS |
Comparison between aCL‐IgA and aβ2GPI‐IgA (P = .00005).
Comparison between aβ2GPI‐IgG and aβ2GPI‐IgA (P = .0003).
Comparison between aβ2GPI‐IgM and aβ2GPI‐IgA (P = .0001).
Comparison between aCL and aβ2GPI (P = .008).
In RA patients, the frequency of aβ2GPI was significantly higher than that of aCL (32.2% vs 15.5%, P = .008). aβ2GPI‐IgA was significantly more frequent than aCL‐IgA (26.7% vs 4.4%, P = .00005).
Distribution of titers of aCL and aβ2GPI in positive aPL patients is presented in Figure 1.
Figure 1.
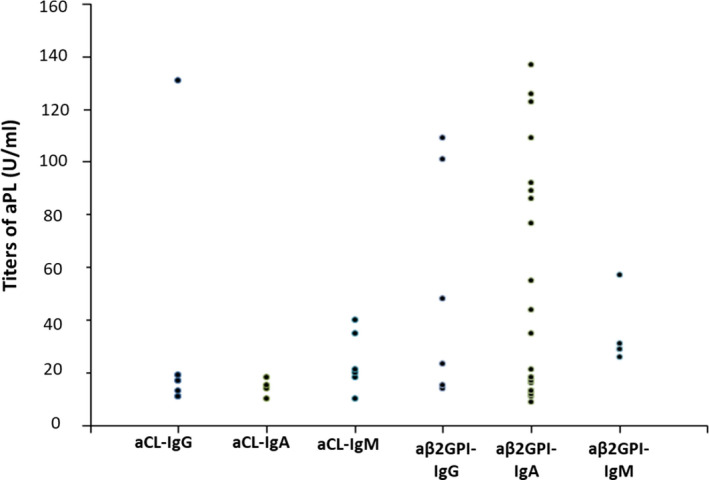
Distribution of titers of aCL and aβ2GPI in positive aPL patients
3.1. Frequencies of aCL‐IgG, IgA, and IgM
The frequency of aCL (IgG, IgA, or IgM) was significantly higher in RA patients than in controls (15.5% vs 5.5%, P = .04).
3.2. Frequencies of aβ2GPI‐IgG, IgA, and IgM
The frequency of aβ2GPI (IgG, IgA, or IgM) was significantly higher in RA patients than in the control group (32.2% vs 11.1%, P = .0005). aβ2GPI‐IgA was significantly more frequent in RA patients than in HBD (26.7% vs 7.8%, P = .0007). In RA patients, aβ2GPI‐IgA was significantly more frequent than aβ2GPI‐IgG (26.7% vs 6.7%, P = .0003) and aβ2GPI‐IgM (26.7% vs 5.6%, P = .0001).
3.3. Frequency of aPL according to sex
In RA patients, the frequency of aPL was not statistically different between females and males (32.1% and 41.2%, respectively) (Table 3). The frequency of aPL was significantly higher in female patients than in healthy females (32.1% vs 8.9%, P = .004). Male patients had a significantly higher frequency of aPL than healthy males (41.2% vs 14.7%, P = .02). In females, aβ2GPI and aβ2GPI‐IgA were significantly more frequent in patients than in healthy subjects (30.3% vs 8.9%, P = .007 and 23.2% vs 7.1%, P = .03, respectively). The same results were obtained for males (35.3% vs 11.8%, P = .04 for aβ2GPI and 32.3% vs 8.8%, P = .03 for aβ2GPI‐IgA).
Table 3.
Frequency of aPL according to sex
| Autoantibodies | Females | Males | ||||
|---|---|---|---|---|---|---|
| RA patients (n = 56) | Control group (n = 56) | P | RA patients (n = 34) | Control group (n = 34) | P | |
| aPL |
32.1% (18/56) |
8.9% (5/56) |
.004 |
41.2% (14/34) |
14.7% (5/34) |
.02 |
| aCL |
16.1% (9/56) |
5.3% (3/56) |
NS |
14.7% (5/34) |
5.9% (2/34) |
NS |
| aβ2GPI |
30.3% (17/56) |
8.9% (5/56) |
.007 |
35.3% (12/34) |
11.8% (4/34) |
.04 |
| aβ2GPI‐IgA |
23.2% (13/56) |
7.1% (4/56) |
.03 |
32.3% (11/34) |
8.8% (3/34) |
.03 |
3.4. Association between aPL and RA antibodies
The average titer of anti‐CCP in aPL positive patients was significantly higher than in aPL negative patients (170.6 RU/mL ± 50 vs 147.7 ± 51 RU/mL, P = .04) (Figure 2).
Figure 2.
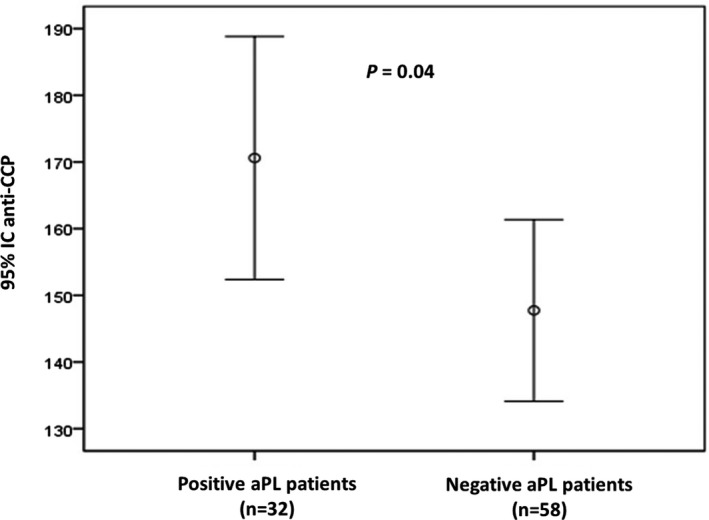
Association between anti‐CCP mean titer and aPL in RA patients
No significant difference was found in the average titer of RF (IgG, IgA, or IgM) between positive and negative aPL patients.
Significant correlation was found between titers of aβ2GPI‐IgA and titers of anti‐CCP (r = .235, P = .026).
4. DISCUSSION
This study provides evidence for an increased frequency of aPL (aCL and/or aβ2GPI) in patients with RA compared to the control group (35.5% vs 11.1%; P = .0001). The frequency of aPL in our RA patients is similar to that found by Pahor et al7 (35.5% and 37%, respectively) and higher than those found by Ambrozic et al8 and Palomo et al9 (23% and 19.1%, respectively). The frequency of aCL in our study is similar to that of Merkel et al10 (15.5% and 15.7%, respectively) but lower than that of Wolf et al11 (32%). This discrepancy could be explained by the difference between the epidemiological characteristics of RA patients included and the methods used for aPL measurement (Table 4).
Table 4.
Frequency of aPL in patients with RA in Literature
| Authors | Number of patients | aPL (%) | aCL‐IgG (%) | aCL‐IgA (%) | aCL‐IgM (%) | aβ2GPI‐IgG (%) | aβ2GPI‐IgA (%) | aβ2GPI‐IgM (%) |
|---|---|---|---|---|---|---|---|---|
| Merkel et al10 | 70 | 15.7 | 11.4 | 0 | 4.3 | ‐ | ‐ | ‐ |
| Wolf et a11 | 173 | 32 | 20 | ‐ | 16 | ‐ | ‐ | ‐ |
| Ambrozic et al8 | 53 | 23 | 8 | 17 | 8 | 6 | 8 | 11 |
| Pahor et al7 | 70 | 37 | 12.8 | ‐ | 4.3 | 10 | 25.7 | 2.8 |
| Palomo et al9 | 84 | 19.1 | 8.3 | 0 | 2.4 | 7.2 | 0 | 4.8 |
| Our study | 90 | 35.5 | 8.9 | 4.4 | 6.7 | 6.7 | 26.7 | 5.6 |
In the present study, the frequency of aβ2GPI is similar to that found by Pahor et al7 (32.2% and 30%, respectively). In our RA group, IgA was the predominant isotype of aβ2GPI and its frequency is similar to that of Pahor et al7 (26.7% and 25.7%, respectively). The frequency of aβ2GPI‐IgA is higher than that found by Ambrozic et al and Palomo et al8, 9 (8% and 0%, respectively) (Table 4). The predominance of IgA class of aβ2GPI in our RA patients is in agreement with our previous studies on the frequency of aβ2GPI in other autoimmune diseases (Table 5).13, 16, 17, 18 Indeed, it has been reported that IgA is the predominant isotype of aPL antibodies in Afro‐Caribbeans19 and also in Afro‐Americans.20
Table 5.
Predominance of aβ2GPI‐IgA in our previous studies
| Authors | Autoimmune diseases | aβ2GPl‐IgG (%) | aβ2GPl‐IgA (%) | aβ2GPl‐IgM (%) |
|---|---|---|---|---|
| Mankaï et al16 | Celiac disease | 1.6 | 14.3 | 1.6 |
| Mankaï et al17 | Systemic lupus erythematosus | 19.8 | 50.9 | ‐ |
| Mankaï et al13 | Primary biliary cholangitis | 12.5 | 62.5 | 21.2 |
| Mankaï et al18 | Antiphospholipid syndrome | 22 | 83.1 | ‐ |
| Present study | Rheumatoid arthritis | 6.7 | 26.7 | 5.6 |
Alessandri et al21 reported anti‐mutated citrullinated vimentin antibodies (anti‐MCV), autoantibodies of RA, in APS and we found aβ2GPI in RA. Moreover, Alessandri et al21 found a correlation between anti‐MCV and arthritis in APS patients and we found a correlation between aβ2GPI and anti‐CCP in RA patients. So, we tried to know if there is a similarity between these two diseases. Interestingly, dysbiosis of gut microbiota was described not only in RA1, 22 but also in APS.23 This dysbiosis induces not only protein citrullination22 but also a conformational change of β2GPI, that exposes a cryptic epitopes in domain I of β2GPI23 and therefore aβ2GPI synthesis.24
In our RA patients, we found a high frequency of aβ2GPI and a correlation between aβ2GPI‐IgA and anti‐CCP. So, the question arises: are aβ2GPI‐IgA implicated in the pathogenesis of arthritis in RA? During RA, gut microbiota dysbiosis may cause the activation of innate‐like T cells, which can be skewed toward a pro‐inflammatory state and contribute to inflamed joint tissue.25 Aberrant epigenetic changes (histone modifications, DNA methylation, and miRNAs) are implicated in inflammatory joints of RA.26 Moreover, phospholipid transfer protein is highly expressed in joints and its activity in synovial fluid is elevated and correlated with pro‐inflammatory cytokines (Il‐1 β, Il‐6) and, therefore, it may directly trigger inflammation.27 Surprisingly, during joint inflammation, enzymatically activated β2GPI is transformed from closed conformation to an open hockey stick‐like conformation. The resulting aβ2GPI is responsible for cartilage degradation of phospholipid bilayers and, therefore, boundary‐lubricating ability is deactivated.28 Moreover, through multiple mechanisms, aPL activity results not only in vasculopathy, thrombosis, and pregnancy complications but also in inflammation.3 So, could aβ2GPI be both the cause and the consequence of the inflammation in the synovial joints?
Gut microbiota dysbiosis1, 22 is associated with an intestinal barrier dysfunction.29 Alterations in gut permeability may allow intraluminal compounds entry the mucosal site, and this may be a trigger cause of an autoimmune reaction. In the gut, there is not only microbiota but also mycobiota (fungal community).30 Two major genera in the mycobiota were Candida and Saccharomyces.31 Because of a leaky gut, Saccharomyces cerevisiae arrives to the mucosa and induces the synthesis of antibodies to Saccharomyces cerevisiae named ASCA.30 ASCA has been described in RA.32
Interestingly, cross‐reactive epitopes on β2GPI and the phosphopeptidomannan part of the cell wall of Saccharomyces cerevisiae have been described.33 In the same way, we have previously demonstrated a high frequency of ASCA in patients with aβ2GPI.18 So could we imagine that aβ2GPI, that we have detected in RA in the present study, are ASCA and are implicated in the pathogenesis of RA? Fascinatingly, a strong similarity between the sequence of autoantigens of RA and mannan expressed by the cell wall of Saccharomyces cerevisiae has been described.34 So, ASCA could bind to citrullinated peptides or to β2GPI in joints, inducing complement activation. Another possibility is that these antibodies bind to mannan of the yeast which arrived from the mycobiota until the joint via the vascular compartment because of a leaky intestinal wall observed in RA. Surprisingly, a new model of chronic arthritis induced by mannan from Saccharomyces cerevisiae has been discovered. This model involves both macrophages which express mannose receptor and complement cascade.35
Our study presents some limitations: 1‐ It is a retrospective one, so we do not have data on clinical manifestations and correlation between aβ2GPI‐IgA and any clinical feature of RA could not be studied. 2‐ Our study lacks an experimental demonstration on a possible pathogenic mechanism of aβ2GPI in RA.
5. CONCLUSION
In conclusion, we found a significantly higher frequency of aβ2GPI in RA patients in comparison to the healthy subjects and we tried to explain why these antibodies are produced in RA. We could hypothesize, as said Hippocrates "all disease starts in the gut", that RA begins in the gut by: (a) Microbiota which induces joint inflammation, protein citrullination, aβ2GPI synthesis, and intestinal barrier dysfunction. (b) Mycobiota which induces synthesis of antibodies (ASCA) who recognize self antigens such as β2GPI and citrillunated proteins. In Tunisia, stress,36 smoking,37 and high prescription of antibiotics38 trigger gut microbiota dysbiosis and high bread consumption trigger a mycobiota rich in Saccharomyces cerevisiae. All these factors combined with a high frequency of consanguineous marriage39 could explain the high frequency of RA in our country.
CONFLICT OF INTEREST
None of the authors have conflicts of interest to declare.
ACKNOWLEDGMENTS
This study is supported by Unité de recherche, Auto‐immunité et Allergie (03/UR/07‐02), Faculté de Pharmacie de Monastir, Université de Monastir, Tunisia.
Melayah S, Changuel M, Mankaï A, Ghedira I. IgA is the predominant isotype of anti‐β2 glycoprotein I antibodies in rheumatoid arthritis. J Clin Lab Anal. 2020;34:e23217 10.1002/jcla.23217
REFERENCES
- 1. Horta‐Baas G, Romero‐Figueroa MDS, Montiel‐Jarquín AJ, Pizano‐Zárate ML, García‐Mena J, Ramírez‐Durán N. Intestinal dysbiosis and rheumatoid arthritis: a link between gut microbiota and the pathogenesis of rheumatoid arthritis. J Immunol Res. 2017;2017:4835189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 3. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378(21):2010‐2021. [DOI] [PubMed] [Google Scholar]
- 4. Gómez‐Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun. 2014;48–49:20‐25. [DOI] [PubMed] [Google Scholar]
- 5. Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8(2):100‐108. [DOI] [PubMed] [Google Scholar]
- 6. Kim KJ, Baek IW, Park KS, Kim WU, Cho CS. Association between antiphospholipid antibodies and arterial thrombosis in patients with rheumatoid arthritis. Lupus. 2017;26(1):88‐94. [DOI] [PubMed] [Google Scholar]
- 7. Pahor A, Hojs R, Holc I, et al. Antiphospholipid antibodies as a possible risk factor for atherosclerosis in patients with rheumatoid arthritis. Immunobiology. 2006;211(9):689‐694. [DOI] [PubMed] [Google Scholar]
- 8. Ambrozic A, Bozic B, Hojnik M, Kveder T, Rozman B. Antiphospholipid antibodies and rheumatoid arthritis. Ann Rheum Dis. 2002;61(1):85‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20(5):190‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merkel PA, Chang Y, Pierangeli SS, Convery K, Harris EN, Polisson RP. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101(6):576‐583. [DOI] [PubMed] [Google Scholar]
- 11. Wolf P, Gretler J, Aglas F, Auer‐Grumbach P, Rainer F. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131(1):48‐51. [DOI] [PubMed] [Google Scholar]
- 12. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569‐2581. [DOI] [PubMed] [Google Scholar]
- 13. Mankaï A, Manoubi W, Ghozzi M, Melayah S, Sakly W, Ghedira I. High frequency of antiphospholipid antibodies in primary biliary cirrhosis. J Clin Lab Anal. 2015;29(1):32‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sghiri R, Bouajina E, Bargaoui D, et al. Value of anti‐mutated citrullinated vimentin antibodies in diagnosing rheumatoid arthritis. Rheumatol Int. 2008;29(1):59‐62. [DOI] [PubMed] [Google Scholar]
- 15. Sghiri R, Bouagina E, Zaglaoui H, et al. Diagnostic performances of anti‐cyclic citrullinated peptide antibodies in rheumatoid arthritis. Rheumatol Int. 2007;27(12):1125‐1130. [DOI] [PubMed] [Google Scholar]
- 16. Mankaï A, Achour A, Thabet Y, Manoubia W, Sakly W, Ghedira I. Anti‐cardiolipin and anti‐beta 2‐glycoprotein I antibodies in celiac disease. Pathol Biol. 2012;60(5):291‐295. [DOI] [PubMed] [Google Scholar]
- 17. Mankaï A, Sakly W, Thabet Y, Achour A, Manoubi W, Ghedira I. Anti‐Saccharomyces cerevisiae antibodies in patients with systemic lupus erythematosus. Rheumatol Int. 2013;33(3):665‐669. [DOI] [PubMed] [Google Scholar]
- 18. Mankaï A, Layouni S, Ghedira I. Anti Saccharomyces cerevisiae antibodies in patients with anti‐β2 Glycoprotein I antibodies. J Clin Lab Anal. 2016;30(6):818‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molina JF, Gutierrez‐Ureña S, Molina J, et al. Variability of anticardiolipin antibody isotype distribution in 3 geographic populations of patients with systemic lupus erythematosus. J Rheumatol. 1997;24(2):291‐296. [PubMed] [Google Scholar]
- 20. Diri E, Cucurull E, Gharavi AE, et al. Antiphospholipid (Hughes') syndrome in African‐Americans: IgA aCL and abeta 2 glycoprotein‐I is the most frequent isotype. Lupus. 1999;8(4):263‐268. [DOI] [PubMed] [Google Scholar]
- 21. Alessandri C, Agmon‐Levin N, Conti F, et al. Anti‐mutated citrullinated vimentin antibodies in antiphospholipid syndrome: diagnostic value and relationship with clinical features. Immunol Res. 2017;65(2):524‐531. [DOI] [PubMed] [Google Scholar]
- 22. Picchianti‐Diamanti A, Rosado MM, D'Amelio R. Infectious agents and inflammation: the role of microbiota in autoimmune arthritis. Front Microbiol. 2018;8:2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruff WE, Vieira SM, Kriegel MA. The role of the gut microbiota in the pathogenesis of antiphospholipid syndrome. Curr Rheumatol Rep. 2015;17(1):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yin D, de Laat B, Devreese KMJ, Kelchtermans H. The clinical value of assays detecting antibodies against domain I of β2‐glycoprotein I in the antiphospholipid syndrome. Autoimmun Rev. 2018;17(12):1210‐1218. [DOI] [PubMed] [Google Scholar]
- 25. Mortier C, Govindarajan S, Venken K, Elewaut D. It takes "guts" to cause joint inflammation: role of innate‐like T cells. Front Immunol. 2018;9:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Araki Y, Mimura T. The mechanisms underlying chronic inflammation in rheumatoid arthritis from the perspective of the epigenetic landscape. J Immunol Res. 2016;2016:6290682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Audo R, Deckert V, Daien CI, et al. Phospholipid transfer protein (PLTP) exerts a direct pro‐inflammatory effect on rheumatoid arthritis (RA) fibroblasts‐like‐synoviocytes (FLS) independently of its lipid transfer activity. PLoS ONE. 2018;13(3):e0193815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pawlak Z, Mrela A, Kaczmarek M, Cieszko M, Urbaniak W. Natural joints: Boundary lubrication and antiphospholipid syndrome (APS). Biosystems. 2019;177:44‐47. [DOI] [PubMed] [Google Scholar]
- 29. Julio‐Pieper M, Bravo JA. Intestinal barrier and behavior. Int Rev Neurobiol. 2016;131:127‐141. [DOI] [PubMed] [Google Scholar]
- 30. Mukherjee PK, Sendid B, Hoarau G, Colombel JF, Poulain D, Ghannoum MA. Mycobiota in gastrointestinal diseases. Nat Rev Gastro Enterol Hepatol. 2015;12(2):77‐87. [DOI] [PubMed] [Google Scholar]
- 31. Motooka D, Fujimoto K, Tanaka R, et al. Fungal ITS1 deep‐sequencing strategies to reconstruct the composition of a 26‐species community and evaluation of the gut mycobiota of healthy Japanese individuals. Front Microbiol. 2017;8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dai H, Li Z, Zhang Y, Lv P, Gao XM. Elevated levels of serum IgA against Saccharomyces cerevisiae mannan in patients with rheumatoid arthritis. Cell Mol Immunol. 2009;6(5):361‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krause I, Blank M, Cervera R, et al. Cross‐reactive epitopes on beta2‐glycoprotein‐I and Saccharomyces cerevisiae in patients with the antiphospholipid syndrome. Ann N Y Acad Sci. 2007;1108:481‐488. [DOI] [PubMed] [Google Scholar]
- 34. Rinaldi M, Perricone R, Blank M, Perricone C, Shoenfeld Y. Anti‐Saccharomyces cerevisiae autoantibodies in autoimmune diseases: from bread baking to autoimmunity. Clin Rev Allergy Immunol. 2013;45(2):152‐161. [DOI] [PubMed] [Google Scholar]
- 35. Hagert C, Sareila O, Kelkka T, et al. Chronic active arthritis driven by macrophages without involvement of T cells: a novel experimental model of rheumatoid arthritis. Arthritis Rheumatol. 2018;70(8):1343‐1353. [DOI] [PubMed] [Google Scholar]
- 36. Ouanes S, Bouasker A, Ghachem R. Psychiatric disorders following the Tunisian revolution. J Ment Health. 2014;23(6):303‐306. [DOI] [PubMed] [Google Scholar]
- 37. Fakhfakh R, Hsairi M, Maalej M, Achour N, Nacef T. Tobacco use in Tunisia: behaviour and awareness. Bull World Health Organ. 2002;80(5):350‐356. [PMC free article] [PubMed] [Google Scholar]
- 38. Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463‐E3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romdhane L, Mezzi N, Hamdi Y, El‐Kamah G, Barakat A, Abdelhak S. Consanguinity and inbreeding in health and disease in north African populations. Annu Rev Genomics Hum Genet. 2019;20:155‐179. [DOI] [PubMed] [Google Scholar]


