Abstract
Introduction
With almost 50% of cases preventable and the Australian National Bowel Cancer Screening Program in place, colorectal cancer (CRC) is a prime candidate for investment to reduce the cancer burden. The challenge is determining effective ways to reduce morbidity and mortality and their implementation through policy and practice. Pathways-Bowel is a multistage programme that aims to identify best-value investment in CRC control by integrating expert and end-user engagement; relevant evidence; modelled interventions to guide future investment; and policy-driven implementation of interventions using evidence-based methods.
Methods and analysis
Pathways-Bowel is an iterative work programme incorporating a calibrated and validated CRC natural history model for Australia (Policy1-Bowel) and assessing the health and cost outcomes and resource use of targeted interventions. Experts help identify and prioritise modelled evaluations of changing trends and interventions and critically assess results to advise on their real-world applicability. Where appropriate the results are used to support public policy change and make the case for optimal investment in specific CRC control interventions. Fourteen high-priority evaluations have been modelled or planned, including evaluations of CRC outcomes from the changing prevalence of modifiable exposures, including smoking and body fatness; potential benefits of daily aspirin intake as chemoprevention; increasing CRC incidence in people aged <50 years; increasing screening participation in the general and Aboriginal and Torres Strait Islander populations; alternative screening technologies and modalities; and changes to follow-up surveillance protocols. Pathways-Bowel is a unique, comprehensive approach to evaluating CRC control; no prior body of work has assessed the relative benefits of a variety of interventions across CRC development and progression to produce a list of best-value investments.
Ethics and dissemination
Ethics approval was not required as human participants were not involved. Findings are reported in a series of papers in peer-reviewed journals and presented at fora to engage the community and policymakers.
Keywords: colorectal cancer, prevention, early detection, screening
Strengths and limitations of this study.
Pathways-Bowel leverages a fully calibrated natural history microsimulation model for colorectal cancer (CRC) (Policy1-Bowel) to model evaluations of existing and hypothetical trends and interventions to improve CRC outcomes for Australians.
It aims to bridge the gap between end-user priorities, epidemiological and statistical research outputs, and practical applicability from health, resource and health system cost perspectives.
Findings from the Pathways-Bowel programme are applicable to Australia; however, the flexibility of Policy1-Bowel enables its future adaptation to other settings where location-specific data are available.
The predictive modelling used is limited by and dependent on the available data sources and assumptions made when empirical data are absent.
The overarching Pathways programme generates evidence on the best-value investments or ‘best buys’ in cancer control across multiple cancers to inform future decision making.
Introduction
Background
Colorectal cancer (CRC) was the third most commonly diagnosed cancer in Australia in 2017, with estimated incidence of 63.4 per 100 000 and 45.8 per 100 000 in men and women, respectively.1 2 A small proportion of CRC cases are found in higher-risk patients and associated with strong family history of CRC or hereditary syndromes. Lynch syndrome and familial adenomatous polyposis account for ~3% and less than 1% of new CRC cases, respectively.3–5 The Australian Burden of Disease Study found there were over 95 000 years of healthy life lost due to CRC in 2015, which accounted for 2% of the total disease burden in Australia.6 From 1982 to 2015, CRC incidence and mortality rates decreased (from 58.3 to 57.4 and from 32.3 to 19.2 per 100 000, respectively),7 with noted gender, socioeconomic and geographical disparities in these reductions.8–11 The 5-year overall survival from CRC in Australia increased from 51% in 1985–1989 to 70% in 2010–2014.12 A recent analysis highlighted increasing CRC incidence in people under 50 years of age, which could be partially attributable to the rising prevalence of harmful risk factors, but there are, as yet, no confirmed causes.13 Nearly half (49.8%) of new CRC cases in Australia are attributable to known modifiable risk factors14 and therefore could be influenced by primary prevention interventions. Evidence on policies and interventions for preventing CRC through lifestyle change varies widely between risk factors. On current evidence, the best buy in CRC control is increasing participation in Australia’s National Bowel Cancer Screening Program (NBCSP)15; however, fewer than half the eligible population are participating. From 2020, all Australians aged 50–74 will be invited to participate in biennial screening using an immunochemical faecal occult blood test (iFOBT).12 Further decreases in incidence and mortality of 23% and 36%, respectively, are anticipated by 2040 at current participation rates with full implementation of the NBCSP.15
Pathways
‘Pathways to a cancer-free future’ (‘Pathways’) is a programme of research developed to focus investment where the biggest impact can be made at a population level. It aims to identify the best-value investments, or ‘best buys’, in cancer control to inform future decision making. First described in relation to cervical cancer,16 the Pathways model is now being applied to five major cancers—cervical, lung, colorectal, prostate and breast cancer—and to cancers relating to Lynch syndrome, and early work has commenced in melanoma and cancers of the ovary and liver. Pathways-Bowel refers to the programme focused on CRC, with a detailed assessment of high-risk individuals with Lynch syndrome and other Lynch-related cancers incorporating evidence-based intervention implementation currently under way,17 18 as part of Pathways-Lynch. Pathways-Bowel will span the CRC control continuum from primary prevention to survivorship. It aims to model comparative evaluations of CRC interventions guided by the best available evidence to underpin future research investment and policy implementation. The aim of the current article is to outline the design and objectives of Pathways-Bowel. Pathways-Bowel will inform ongoing and planned modelled evaluations of CRC interventions by integrating expert and end-user engagement; relevant evidence; modelled interventions to guide future investment; and policy-driven implementation of interventions using evidence-based methods.
Methods and analysis
Study design
Pathways as an overarching programme was previously described.16 Since that time, Pathways has changed from a staged approach to a more iterative process. As modelled evaluation results become available, they are immediately reviewed and disseminated as appropriate to support potential policy change.
Patient and public involvement statement
Multisectoral stakeholder involvement in Pathways-Bowel is achieved via a multidisciplinary Scientific Advisory Committee (SAC) including academics, clinicians, consumers and advisers on policy. The SAC is designed to ensure involvement from relevant stakeholders outside the core research team and confirm the relevance of modelled evaluations and their translation. The SAC was first convened to discuss CRC in March 2018. Since then, members continue to be consulted based on their area of expertise to guide, critique and support the programme and its recommendations, thus ensuring there is involvement from interested parties throughout the process.
Processes and analysis for modelled evaluations of interventions
Modelling platform: Policy1-Bowel
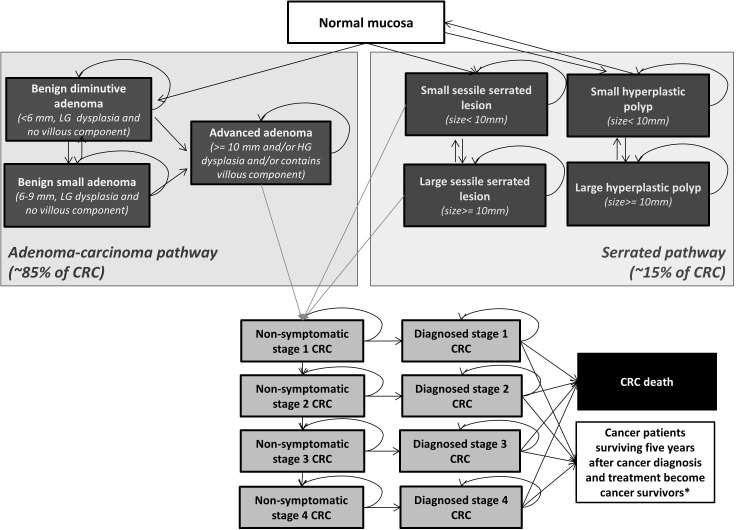
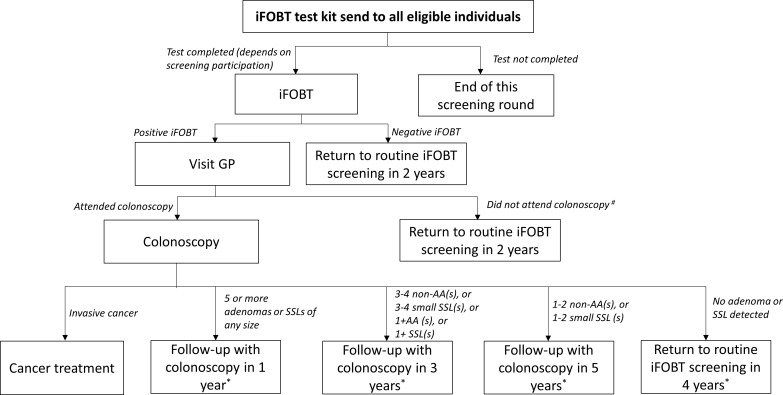
We use a previously developed microsimulation model platform, Policy-1 Bowel, to perform predictive modelled evaluations of CRC interventions in Australia.19 Policy1-Bowel is a comprehensive platform that synthesises clinical, epidemiological, demographic, behavioural and economic data and has been used to simulate the impact of CRC screening in Australia.15 Existing Policy1-Bowel evaluations have assessed a range of screening scenarios and provided estimates of CRC outcomes, resource utilisation and costs. They have, for example, analysed the use of various CRC screening test technologies and target age ranges for the NBCSP to inform Australian guidelines.20 21 The model platform is implemented in C++ and includes several interconnected elements to evaluate the NBCSP. It incorporates the development of CRC from adenoma (via the adenoma-carcinoma pathway) and sessile serrated lesions (via the serrated pathways) and survival from CRC (see figure 1). Policy1-Bowel then incorporates screening for average-risk people, including postscreening diagnosis, treatment and surveillance (figure 2 summarises the current NBCSP screening delivery pathways included). As evaluations are conducted, single-cohort or multiple-cohort approaches are used to simulate the development of polyps and CRC, screening, diagnosis and other downstream NBCSP processes in the target population over a time period of interest. The resulting evaluation is informed by Australian-specific demographic data and economic and health utilities data obtained from national and international literature (including cost and quality-adjusted life years) to produce cross-sectional results for the population. For modelled evaluations of CRC interventions, data are sourced from national surveys and data collection agencies (eg, Australian Institute of Health and Welfare: AIHW) and the published literature including meta-analyses, systematic reviews, randomised controlled trials, cohort studies and other relevant publications. Where empirical data are not available, the SAC and other experts are consulted to guide the assumptions used.
Figure 1.
Schematic diagram of the Policy1-Bowel microsimulation model platform. *Cancer patients surviving five years after diagnosis and treatment become cancer survivors. Cancer survivors in the model were assumed to have no additional risk of death due to colorectal cancer compared with the average population with no colorectal cancer. CRC, colorectal cancer; HG, high grade; LG, low grade.
Figure 2.
Screening delivery pathway (based on NBCSP) modelled in the Policy1-Bowel microsimulation model platform. #Including people who were not recommended to attend colonoscopy due to coexistent disease or other health issues and people who did not comply with GP’s colonoscopy referral. *Barclay, K. Cancer Council Australia Surveillance Colonoscopy Guidelines Working Party. Algorithm for colonoscopic surveillance intervals–adenomas, 2013. Available at: http://www.gastroservices.com.au/pdf/algorithm-for-colonoscopic-surveillance-intervals-adenomas.pdf (accessed 28 December 2016). AA, advanced adenoma; GP, general practitioner; iFOBT, immunochemical faecal occult blood test; NBCSP, National Bowel Cancer Screening Program; SSL, sessile serrated lesion.
Policy1-Bowel validation
Extensive calibration of the model has been carried out against a wide range of current NBCSP outputs and other Australian data sources.15 The model has also been validated against the findings of other well-established microsimulation models and multiple large randomised controlled trials with long-term follow-up. Further details of the model used in this work, and descriptions of its development, parameterisation, data sources, calibration and validation outcomes, have been published previously and technical appendices are available.15 19–21
Economic analysis
The modelled evaluations result in economic analyses to develop a business case for investment. Pathways-Bowel (and all Pathways) uses a common framework so the best-value investment, or ‘best buy’, can be compared within and between analyses. This framework is in development and will be based on similar initiatives internationally.22 The populations of interest are average-risk Australians and subgroups relevant to the modelled evaluations. For each evaluation, several primary outcomes are considered, including the following:
Health benefits, for example, reduction in lifetime risk of CRC incidence and mortality.
Harms, for example, hospitalisations and adverse events of colonoscopy.
Resource use, for example, health costs of CRC (hospitals, workforce, screening and diagnostic tests, programme communications and so on).
Health economic outcomes, for example, discounted and undiscounted lifetime cost, life years, quality-adjusted life years, disability-adjusted life years and cost-effectiveness.
For each intervention, the primary outcomes listed may be expanded or differ. The comparator for analyses is the general population or specific subgroup of interest without the influence of the intervention being assessed. The potential harms associated with interventions are often minimal, but Pathways will enable their characterisation and quantification. For example, the health benefits and harms for screening would also include colonoscopy-related adverse events and number needed to colonoscope per CRC death prevented.
A health services perspective is applied, and efforts are being made to expand to the societal perspective, including characterisation of out-of-pocket expenses. From a health services perspective, costs incurred by governments and the health system over a person’s lifetime are incorporated. For each evaluation, multiple time horizons may be chosen as appropriate to the specific intervention, but the common time horizon is to 2050 (as this timeline indicates a change within a generation). In terms of an indicative willingness-to-pay (WTP) threshold, $30 000–$50 000 per life year saved has previously been used for evaluations of interventions for CRC and cervical cancer.20 23 24 In Pathways, a 5% annual discount rate and the indicative WTP threshold of $30 000–$50 000 per life year saved are used, with alternative WTP thresholds included for comparability. Our focus is to quantify and compare cost-effectiveness in all our analyses. One-way and probabilistic sensitivity analyses and uncertainty analyses will be conducted as required to assess the impact of model parameter uncertainties on the key model findings.
Rationale for modelled evaluations of CRC interventions
Under the guidance of the SAC, a list of priority modelled evaluations for CRC interventions was compiled. Evaluations are preceded by exploratory scoping reviews of the literature to identify potential interventions, and are escalated to a full systematic review to source evidence for predictive modelling as required and determined by the SAC. The ongoing and planned interventions are listed in table 1 and represent the first series of evaluations. Broadly, these evaluations cover interventions to reduce CRC risk, interventions in light of changing incidence trends, modifications to the NBCSP via target age groups, increased participation and alternative screening methods, and improved surveillance management. Findings have and will continue to be reviewed by the SAC as required. At a later date, these evaluations will grow and could include topics of growing public interest, such as the promotion of healthy diet, and extend to later stages of CRC control as evidence becomes available.
Table 1.
Priority modelled evaluations for CRC interventions
| Evaluation | Focus area | Status |
| Impact* of changing smoking prevalence on CRC. | Reducing risk of CRC. | Ongoing. |
| Impact* of changing body fatness prevalence and distribution on CRC. | Reducing risk of CRC. | Ongoing. |
| Impact* of daily aspirin prophylaxis on CRC. | Reducing risk of CRC. | Ongoing. |
| Impact* of NBCSP in the long term due to the increasing CRC incidence in younger cohorts. | NBCSP outcomes: changing temporal trends. | Ongoing. |
| Impact* of extending the NBCSP to younger ages for birth cohorts with increasing CRC rate. | NBCSP outcomes: changing temporal trends. | Ongoing. |
| Impact* of extending the NBCSP to people aged 40–49 years for the Aboriginal and Torres Strait Islander peoples. | NBCSP outcomes: targeting population subgroups with different CRC risk profiles. | Complete. |
| Impact* of extending the NBCSP to younger (40–49 years) and/or older (75–84 years) ages of average-risk Australians. | NBCSP outcomes: targeting NBCSP participation to a broader age range. | Published. |
| Impact* of the NBCSP at currently observed rates in the long term. | NBCSP outcomes: long-term NBCSP participation. | Published. |
| Impact* of increasing NBCSP participation to 60% and 70%. | NBCSP outcomes: increasing NBCSP participation rates. | Published. |
| Impact* of optimising NBCSP adherence (iFOBT screening and diagnostic assessment) to 90% and quantifying a threshold for cost-effective investment towards improving NBCSP adherence. | NBCSP outcomes: increasing NBCSP participation rates. | Published. |
| Impact* of mass media campaigns aimed at increasing participation in NBCSP. | NBCSP outcomes: increasing NBCSP participation rates. | Published. |
| Impact* of including twice-off screening colonoscopies at ages 40 and 60 in addition to the current NBCSP. | NBCSP outcomes: alternative screening methods. | Published. |
| Impact* of 13 alternative screening approaches involving use of iFOBT, colonoscopy, sigmoidoscopy, CT colonography, faecal DNA and plasma DNA for the NBCSP. | NBCSP outcomes: alternative screening methods. | Published. |
| Impact* of modifications to colonoscopic surveillance protocols, especially the newly ratified Australian colonoscopy surveillance guidelines to the previous guidelines. | NBCSP outcomes: modifying colonoscopic surveillance management. | Ongoing. |
*The impact of listed evaluations assessed in terms of health outcomes, resource use and costs.
CRC, colorectal cancer; iFOBT, immunochemical faecal occult blood test; NBCSP, National Bowel Cancer Screening Program.
While Policy1-Bowel has been used to evaluate the NBCSP, it is a flexible and dynamic model which can be adapted to incorporate both alternative screening interventions as well as interventions addressing other stages of the CRC continuum. Policy1-Bowel proves a critical tool for assessing the ‘best buys’ for CRC. The following section outlines how Pathways-Bowel is being used in the contexts of primary prevention, screening and early detection and treatment for CRC.
Primary prevention: reducing risk
Promoting healthy behaviours and reducing risk through primary prevention can play an important role in CRC control.14 Targeted primary prevention interventions to reduce CRC risk could address any or all of the following: tobacco use, alcohol use, body fatness, insufficient physical activity, insufficient dietary fibre intake, and excess red and processed meat intake.25–27 Except for tobacco use, the prevalence of these risk factors has increased in Australians in recent decades, and for some key risk factors, such as body fatness, prevalence in children is rising and calls for action are increasing.28 The 2017 Australian clinical practice guidelines for CRC recommend low-dose daily aspirin use for all people aged 50–70, as evidence suggests its potential effectiveness in CRC primary prevention.29 30 More recent studies have begun exploring the role of the gut microbiota in the development of CRC, which can be indirectly affected by diet.31 In practice, evidence-based interventions addressing these risk factors are challenging to comprehensively evaluate without information on medium-term to long-term CRC outcomes. Pathways-Bowel will synthesise the available evidence from national and international data sources and published evidence to estimate the likely impact on CRC outcomes in the future for modelled evaluations. Initially, the priorities in this area (table 1) are (1) changing smoking prevalence; (2) changing body fatness prevalence and distribution; and (3) impact of daily aspirin prophylaxis. Other behaviours, such as alcohol consumption and diet, may be added at a later stage.
Screening and early detection: NBCSP outcomes
Identification and removal of precancerous adenomas can prevent CRC development, and early detection of malignancies improves survival. The technology for these interventions is effective, available, affordable and acceptable, making CRC an ideal candidate for an organised population screening programme.32 The NBCSP participation rate over the 2016–2017 period was about 40% nationally.12 Current reported rates of colonoscopy for assessment of individuals with a positive NBCSP-iFOBT test are approximately 66%, with known under-reporting.12 Recommended screening for people at intermediate or high CRC risk due to family history of CRC or hereditary syndromes is more intense, beginning at a younger age, and may include iFOBT and colonoscopy screening depending on level of risk, informed by evidence.33 In addition, ongoing surveillance of individuals with either a positive iFOBT or polyps removed at colonoscopy follows varying management recommendations based on individual risk and colonoscopy results.34
National reports issued by the AIHW, along with other studies, have drawn attention to NBCSP participation disparities by gender, geographical location, Indigenous status, place of birth and language spoken at home.12 35–37 Interventions to promote CRC screening that are used revolve largely around general population awareness and health organisation or practitioner endorsement of participation and follow-up.38–40 Efforts are now being made by government and not-for-profit organisations to improve NBCSP participation.41–43 Such interventions are likely to be cost-effective investments.41 42 Evaluations of interventions to support compliance with recommendations for screening, follow-up and surveillance and to assess the best use of existing health resources could also be conducted, when data on the performance of these interventions are available.
Australia has a national organised, federally funded screening programme that began in 2006. It has undergone phased roll-out, nearing full implementation, and should be taken into account in any modelled evaluation. The Pathways-Bowel priority areas cover predictive modelling of the NBCSP outcomes under a range of conditions or changes in the external environment or programme (table 1). These scenarios are (1) changing temporal incidence trends; (2) targeting NBCSP participation in population subgroups; (3) targeting NBCSP participation to a broader age range; (4) long-term NBCSP participation at varying rates; (5) NBCSP participation increased by simulated mass media campaigns; (6) using alternative technologies; and (7) modifying surveillance management.
Treatment
Once diagnosed, surgery is generally considered as initial treatment, with or without adjuvant chemotherapy or radiation therapy.44 45 The goal of surgery is to remove any tumour as well as surrounding tissue either laparoscopically or via traditional open surgery.46 Variations in treatment pathways more often relate to adjuvant chemotherapy where there are differences in guidelines and outcomes based on stage, location and genetic mutations. Metastatic disease is treated with systemic chemotherapy and biological therapies. Bevacizumab, added to the Pharmaceutical Benefit Schedule (PBS) for Australian Government subsidies in 2009, can be used in addition to chemotherapy in metastatic CRC cases and has been found to prolong both progression-free survival (from 7.1 to 9.7 months) and overall survival (from 17.7 to 20.5 months) in first-line and second-line therapy.47 Cetuximab and panitumumab are also PBS-subsidised for use in patients with rat sarcoma viral oncogene homologue (RAS) wild-type CRC.48 Besides these, there have been few modifications to the PBS related directly to CRC therapies. Immunotherapy has proven effective in early and advanced microsatellite unstable CRC tumours, which can comprise 15% of all CRC or more for those under 50 years.49 Research continues in this area with an active interest in the concept of personalised medicine with therapies for specific CRC subtypes, including the possible use of organoids to predict therapy response.49 50 There have been calls for further research into several new immune agents and other therapies that could change patient outcomes. In future, evaluations of treatment options and their associated outcomes can be conducted as part of Pathways-Bowel to determine both the therapeutic effectiveness and cost-effectiveness of existing and novel therapies as evidence becomes available in Australia.
Survivorship
With 70% 5-year overall survival (2010–2014) and declines in mortality predicted to continue,12 survivorship issues are growing in relevance and importance. Most evidence is focused on patient surveillance for recurrence, with differences across available guidelines on the frequency and timing of follow-up tests.51 Survivorship issues include physical, psychological and social challenges, as well as ongoing healthcare needs.51–54 Australian evidence has suggested care is highly variable in CRC survivors and disparities by socioeconomic group are apparent.53 American guidelines for CRC survivorship have highlighted the role of risk-based healthcare, and there has been a shift in focus to improving patient outcomes through survivorship care plans and coordinated care.51 52 Evaluations of survivorship issues and related interventions to improve outcomes will be integrated into future versions of Pathways-Bowel.
Preliminary results
This programme formalises an existing ongoing body of research which has already produced outputs. Work initially focused on evaluating the NBCSP using both predictive modelling and epidemiological research.15 20 21 37 41 An evaluation of NBCSP effectiveness and cost-effectiveness at various participation levels showed that increasing participation from 40% to 60% would prevent 83 800 deaths from 2015 to 2040 and reduce annual expenditure on CRC control within a decade of full NBCSP roll-out.15 We also explored the impact of optimistic NBCSP adherence rates, possibly beyond those achievable in practice, to determine whether the impact of such an intervention is substantial and worth pursuing further.55
Alternative screening methods using different NBCSP screening modalities or different screening age groups have also been evaluated.20 21 The alternative technologies evaluated were plasma DNA testing, faecal DNA testing, CT colonography, flexible sigmoidoscopy and colonoscopy.21 Extensions to the target age range for the general population included extending to people in their 40s and/or people in their 80s.20 Considering the health outcomes and cost-effectiveness, the studies concluded that the planned NBCSP using biennial iFOBT and targeting people aged 50–74 years is currently the best option for CRC screening in Australia, and achieving higher screening participation within that age range can save more lives and improve the long-term cost-effectiveness.20 21 These results had a direct impact on clinical practice and policy as they were used to inform the 2017 ‘Clinical practice guidelines for the prevention, early detection and management of colorectal cancer’, approved by the National Health and Medical Research Council, and guided recommendations for the NBCSP.29
In addition to the planned modelled evaluations, epidemiological data will also be assessed to quantify and characterise screening occurring outside the NBCSP, which is thought to be considerable and may impact estimates of the benefits of increasing participation in NBCSP.56 Further work has been undertaken and continues to inform guidelines and policy in areas of CRC management, such as the updated national surveillance colonoscopy guidelines.34 As additional evidence accumulates for potential interventions, these will be explored in future modelled evaluations and used to inform guidelines and policy change discussions. Notably, the Policy1-Bowel platform was used to evaluate a recent pilot mass media campaign aimed at increasing NBCSP participation; its results prompted a $10 million government investment in a national mass media campaign.43 This provides a clear demonstration on the usefulness of Pathways-Bowel in guiding investment and policy implementation.41–43
Discussion
The proven ability and future capacity of the Pathways programme to identify the best-value investments in cancer control is critical in public health decision making. Pathways is a way to assess the impact of many more interventions than could be subject to clinical trials; the interventions can even be complementary, provided they are anchored in the real world. Internationally it has been recognised that demonstrating the cost-effectiveness of public health interventions helps to underpin commitment from policymakers and funders.57 However, the varying methods by which interventions are evaluated make them difficult to compare and subject to methodological confounding.57
Pathways-Bowel is a unique, evidence-based, comprehensive approach to CRC control initially focused on screening interventions and their effectiveness in relation to the evolving knowledge of the natural history of CRC. There is relevant evidence in CRC, but no prior body of work has assessed the relative benefits of interventions across the CRC spectrum in a systematic way using a health economics framework and producing ‘best buys’ for the nation. By providing uniformly obtained, high-quality evidence guided by a standardised framework, which is in development, Pathways-Bowel has the capacity to drive CRC control change and improve outcomes for Australians across the entire spectrum of risk.
Pathways-Bowel engages and involves researchers, clinicians, consumers, policymakers and other key stakeholders from its outset and throughout the process. Findings are presented so stakeholders can use the information to guide policy change priorities, funding recommendations and decisions, and evidence-based advocacy for improved outcomes. Early results are integrated with policy and advocacy efforts through local independent cancer control agencies with a track record in changing policy. The findings may also identify areas where further research could facilitate evaluations and guide research priority setting by funders.
The predictive modelling used in the Pathways programme is not without its limitations. It is dependent on the available data sources and assumptions made in the absence of robust data. In Australia we are fortunate to have high-quality data available on CRC incidence and mortality and regular monitoring reports made publicly available on the performance of the NBCSP. These data have been used to develop a robust and sound Policy1-Bowel platform. Nevertheless, the modelled results remain predictions. It is through extensive validation with trial outcomes, continual improvement of the model and input of updated real-world observational information as it becomes available that the outputs are strengthened.
In terms of health economics, the health services perspective used limits the interpretation of results. Economic modelling, by itself, does not explicitly aid policymakers to maximise equity. However, more broadly, the Pathways-Bowel programme of research embeds equity as a pillar. Through Pathways, standard economic analyses are complemented by systematic predictive modelling for specific groups and issues. Although applicable to the Australian general population, the outcomes can be evaluated for other contexts where data are available. Aboriginal and Torres Strait Islander peoples, for example, have seen varying trends in CRC incidence and mortality over time when compared with the Australian population, with significant increase in incidence, no statistically significant trend in mortality and a lower 5-year relative survival (58%).58 Evaluations have been done for this population group to assess the impact of NBCSP screening from ages 40 to 74, and modelling of subgroups can be extended to culturally and linguistically diverse populations living in Australia as required. Overall, the flexibility of the modelling platform used in Pathways-Bowel allows for its application to other settings in the future, both for developed and developing countries, and this has already begun for China.
While the current focus is on prevention and screening, Pathways-Bowel and the Policy1-Bowel platform have the flexibility to evaluate diagnosis, treatment and survivorship interventions as evidence is gathered. The capacity of the model is continually being extended and strengthened with each new modelled evaluation performed. There is much promise in current research to identify optimal approaches to population-based screening for CRC in Australia. The Pathways programme has already been established based on a comprehensive analysis of the associated benefits, harms and costs. The next step, implementation of interventions and policies into practice, is crucial for ensuring the benefits of optimal approaches are realised by the Australian population, and has begun for patients with Lynch syndrome.18 Evidence-based approaches to inform ‘best buys’ in policy reform, accounting for context, system complexity and stakeholder perspectives, are a fundamental prerequisite for successful and sustained translation of discoveries into real-world settings. The Pathways programme presents the opportunity to continually optimise evidence-based support for cancer control interventions.
Ethics and dissemination
The Pathways-Bowel protocol for modelled evaluations has been reviewed and approved by the SAC. No human participants are involved to perform modelled evaluations and therefore human research ethics committee was not required. Where epidemiological analyses are planned and require ethics approval, it will be sought. No deviations from the protocol will be made without prior review and approval of the relevant working party leads from the SAC.
Study status
Pathways-Bowel officially commenced in early 2017 and is an ongoing collaboration with the SAC and other CRC specialist researchers. As results become available, they are reviewed and prepared for peer-reviewed publication. The status is outlined in table 1. The expected completion date for the currently outlined evaluations is 2023.
Supplementary Material
Footnotes
Twitter: @ettofel, @jbin_lew, @njt14, @adessaix, @ProfJonEmery, @Doctor_IMF, @BonnyEconomist, @Aung_Ko_Win, @danworthley
Contributors: KC conceived Pathways and developed the scope of evaluations with EF, JBL, JW, EH and MC and input on data interpretation from all SAC members: KBu, HH, NT, EB, KBa, KBr, AB, RC, JC, AD, HE, JE, IMF, PG, CaH, ChH, MAJ, JGK, MAL, BL, GM, SM, BP, DJSJ, LT, KT, MAW, RLW, AKW, DLW, BKA and FAM. EF authored the manuscript with input from all coauthors: JBL, JW, EH, MC, KBu, HH, NT, EB, KBa, KBr, AB, RC, JC, AD, HE, JE, IMF, PG, CaH, ChH, MAJ, JGK, MAL, BL, GM, SM, BP, DJSJ, LT, KT, MAW, RLW, AKW, DLW, BKA, FAM and KC. All authors critically reviewed and contributed to the final manuscript.
Funding: This work was supported by Cancer Council New South Wales (CCNSW).
Competing interests: KC, EF, JW, JBL, EH, MC, NT, KBu and HH receive salary support from CCNSW. KC is co-PI of unrelated investigator-initiated trial of cervical screening in Australia (‘Compass’) conducted by the Victorian Cytology Service, which has received funding contribution from Roche Molecular Systems and Ventana, USA.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Australian Institute of Health and Welfare Cancer in Australia. Canberra: AIHW, 2019. [Google Scholar]
- 2.Australian Institute of Health and Welfare Cancer in Australia: actual incidence data from 1982 to 2013 and mortality data from 1982 to 2014 with projections to 2017. Asia Pac J Clin Oncol 2018;14:5–15. 10.1111/ajco.12761 [DOI] [PubMed] [Google Scholar]
- 3.Lynch HT, Lynch PM, Lanspa SJ, et al. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 2009;76:1–18. 10.1111/j.1399-0004.2009.01230.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karstensen JG, Burisch J, Pommergaard H-C, et al. Colorectal cancer in individuals with familial adenomatous polyposis, based on analysis of the Danish polyposis registry. Clin Gastroenterol Hepatol 2019;17:2294–300. 10.1016/j.cgh.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 5.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 2008;26:5783–8. 10.1200/JCO.2008.17.5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Australian Institute of Health and Welfare Australian burden of disease study: impact and causes of illness and death in Australia 2015. Canberra: AIHW, 2019. [Google Scholar]
- 7.Australian Institute of Health and Welfare Australian cancer incidence and mortality (ACIM) books, 2019. Available: http://www.aihw.gov.au/acim-books/ [Accessed 31 Jan 2019].
- 8.Yu XQ, O'connell DL, Gibberd RW, et al. A population-based study from New South Wales, Australia 1996-2001: area variation in survival from colorectal cancer. Eur J Cancer 2005;41:2715–21. 10.1016/j.ejca.2005.05.018 [DOI] [PubMed] [Google Scholar]
- 9.Jong KE, Smith DP, Yu XQ, et al. Remoteness of residence and survival from cancer in New South Wales. Med J Aust 2004;180:618–22. 10.5694/j.1326-5377.2004.tb06123.x [DOI] [PubMed] [Google Scholar]
- 10.Yu XQ, O'Connell DL, Gibberd RW, et al. Assessing the impact of socio-economic status on cancer survival in New South Wales, Australia 1996-2001. Cancer Causes Control 2008;19:1383–90. 10.1007/s10552-008-9210-1 [DOI] [PubMed] [Google Scholar]
- 11.Stanbury JF, Baade PD, Yu Y, et al. Cancer survival in New South Wales, Australia: socioeconomic disparities remain despite overall improvements. BMC Cancer 2016;16:48. 10.1186/s12885-016-2065-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Australian Institute of Health and Welfare National bowel cancer screening program: monitoring report. Canberra: AIHW, 2019. [Google Scholar]
- 13.Feletto E, Yu XQ, Lew J-B, et al. Trends in colon and rectal cancer incidence in Australia from 1982 to 2014: analysis of data on over 375,000 cases. Cancer Epidemiol Biomarkers Prev 2019;28:83–90. 10.1158/1055-9965.EPI-18-0523 [DOI] [PubMed] [Google Scholar]
- 14.Whiteman DC, Webb PM, Green AC, et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health 2015;39:477–84. 10.1111/1753-6405.12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lew J-B, St John DJB, Xu X-M, et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National bowel cancer screening program in Australia: a modelling study. Lancet Public Health 2017;2:e331–40. 10.1016/S2468-2667(17)30105-6 [DOI] [PubMed] [Google Scholar]
- 16.Velentzis LS, Smith MA, Simms KT, et al. Pathways to a cancer-free future: a protocol for modelled evaluations to maximize the future impact of interventions on cervical cancer in Australia. Gynecol Oncol 2019;152:465–71. 10.1016/j.ygyno.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 17.Kang Y-J, Killen J, Caruana M, et al. The predicted impact and cost-effectiveness of systematic testing of people with incident colorectal cancer for Lynch syndrome. Med J Aust 2020;212:72–81. 10.5694/mja2.50356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow A, Hogden E, Kang Y-J, et al. Comparing theory and non-theory based implementation approaches to improving referral practices in cancer genetics: a cluster randomised trial protocol. Trials 2019;20:373. 10.1186/s13063-019-3457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Council NSW Policy1, 2019. Available: http://www.policy1.org/models/bowel [Accessed 21 Aug 2019].
- 20.Lew J-B, St John DJB, Macrae FA, et al. Benefits, harms, and cost-effectiveness of potential age extensions to the National bowel cancer screening program in Australia. Cancer Epidemiol Biomarkers Prev 2018;27:1450–61. 10.1158/1055-9965.EPI-18-0128 [DOI] [PubMed] [Google Scholar]
- 21.Lew J-B, St John DJB, Macrae FA, et al. Evaluation of the benefits, harms and cost-effectiveness of potential alternatives to iFOBT testing for colorectal cancer screening in Australia. Int J Cancer 2018;143:269–82. 10.1002/ijc.31314 [DOI] [PubMed] [Google Scholar]
- 22.Emerson J, Panzer A, Cohen JT, et al. Adherence to the iDSI reference case among published cost-per-DALY averted studies. PLoS One 2019;14:e0205633. 10.1371/journal.pone.0205633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simms KT, Laprise J-F, Smith MA, et al. Cost-effectiveness of the next generation nonavalent human papillomavirus vaccine in the context of primary human papillomavirus screening in Australia: a comparative modelling analysis. Lancet Public Health 2016;1:e66–75. 10.1016/S2468-2667(16)30019-6 [DOI] [PubMed] [Google Scholar]
- 24.Lew J-B, Simms KT, Smith MA, et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: effectiveness and economic assessment for the National cervical screening program. Lancet Public Health 2017;2:e96–107. 10.1016/S2468-2667(17)30007-5 [DOI] [PubMed] [Google Scholar]
- 25.World cancer research fund / American Institute for cancer research Continuous update project report. diet, nutrition, physical activity, and colorectal cancer. World Cancer Research Fund, 2017. [Google Scholar]
- 26.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans IARC Monographs: Personal habits and indoor combustions : A review of human carcinogens. Lyon: International Agency for Research on Cancer, 2012. [PMC free article] [PubMed] [Google Scholar]
- 27.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer — viewpoint of the IARC Working group. N Engl J Med 2016;375:794–8. 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Australian Institute of Health and Welfare Risk factors contributing to chronic disease. Canberra: AIHW, 2012. [Google Scholar]
- 29.Cancer Council Australia Colorectal Cancer Guidelines Working Party Clinical practice guidelines for the prevention, early detection and management of colorectal cancer, 2017. Available: https://wiki.cancer.org.au/australia/Guidelines:Colorectal_cancer [Accessed 3 Jan 2018].
- 30.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 2012;13:518–27. 10.1016/S1470-2045(12)70112-2 [DOI] [PubMed] [Google Scholar]
- 31.Hullar MAJ, Burnett-Hartman AN, Lampe JW, et al. Gut microbes, diet, and cancer. Cancer Treat Res 2014;159:377–99. 10.1007/978-3-642-38007-5_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keum N, Giovannucci EL. Epidemiology of Colorectal Cancer : Loda M, Mucci L, Mittelstadt M, et al., Pathology and epidemiology of cancer. Cham: Springer, 2017: 391–407. [Google Scholar]
- 33.Jenkins MA, Ait Ouakrim D, Boussioutas A, et al. Revised Australian national guidelines for colorectal cancer screening: family history. Med J Aust 2018;209:455–60. 10.5694/mja18.00142 [DOI] [PubMed] [Google Scholar]
- 34.Cancer Council Australia Surveillance Colonoscopy Guidelines Working Party Clinical practice guidelines for surveillance colonoscopy, 2019. Available: https://wiki.cancer.org.au/australiawiki/index.php?oldid=200728 [Accessed 29 Mar 2019].
- 35.Weber MF, Chiew M, Feletto E, et al. Cancer screening among immigrants living in urban and regional Australia: results from the 45 and up study. Int J Environ Res Public Health 2014;11:8251–66. 10.3390/ijerph110808251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber MF, Banks E, Smith DP, et al. Cancer screening among migrants in an Australian cohort; cross-sectional analyses from the 45 and up study. BMC Public Health 2009;9:144. 10.1186/1471-2458-9-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He E, Lew J-B, Egger S, et al. Factors associated with participation in colorectal cancer screening in Australia: results from the 45 and up study cohort. Prev Med 2018;106:185–93. 10.1016/j.ypmed.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 38.White B, Power E, Ciurej M, et al. Piloting the impact of three interventions on guaiac faecal occult blood test uptake within the NHS bowel cancer screening programme. Biomed Res Int 2015;2015:1–11. 10.1155/2015/928251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonah L, Pefoyo AK, Lee A, et al. Evaluation of the effect of an audit and feedback reporting tool on screening participation: the primary care screening activity report (PCSAR). Prev Med 2017;96:135–43. 10.1016/j.ypmed.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 40.ACE Bowel Screening Cluster Interventions to increase bowel screening uptake: final report, 2017. Available: http://www.cancerresearchuk.org/sites/default/files/interventions_to_increase_bowel_screening_uptake_v1.0.pdf
- 41.Worthington J, Feletto E, Lew J-B, et al. Evaluating health benefits and cost-effectiveness of a mass-media campaign for improving participation in the National bowel cancer screening program in Australia. Public Health 2020;179:90–9. 10.1016/j.puhe.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 42.Durkin SJ, Broun K, Wakefield MA. Mass media campaign impact on overall and subgroup participation rates in the Australian national bowel cancer screening program: a field experiment 2019. [DOI] [PMC free article] [PubMed]
- 43.Australian Government Department of Health $10 million to boost bowel cancer screening participation, 2019. Available: https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/10-million-to-boost-bowel-cancer-screening-participation [Accessed 29 Mar 2019].
- 44.Aran V, Victorino AP, Thuler LC, et al. Colorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer 2016;15:195–203. 10.1016/j.clcc.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 45.Young JM, Leong DC, Armstrong K, et al. Concordance with national guidelines for colorectal cancer care in New South Wales: a population-based patterns of care study. Med J Aust 2007;186:292–5. 10.5694/j.1326-5377.2007.tb00903.x [DOI] [PubMed] [Google Scholar]
- 46.Kuhry E, Schwenk WF, Gaupset R, et al. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev 2008;2:CD003432. 10.1002/14651858.CD003432.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner ADADW, Arnold D, Grothey AAG, et al. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev 2009:CD005392. 10.1002/14651858.CD005392.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Australian Government Department of Health Pharmaceutical benefits scheme (PBS) | cetuximab, panitumumab and bevacizumab for metastatic colorectal cancer, 2018. Available: http://www.pbs.gov.au/info/industry/listing/participants/public-release-docs/2018-02/metastatic-colorectal-cancer-february-2018 [Accessed 15 Aug 2019].
- 49.Kalyan A, Kircher S, Shah H, et al. Updates on immunotherapy for colorectal cancer. J Gastrointest Oncol 2018;9:160–9. 10.21037/jgo.2018.01.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooft SN, Weeber F, Dijkstra KK, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med 2019;11:eaay2574. 10.1126/scitranslmed.aay2574 [DOI] [PubMed] [Google Scholar]
- 51.Jorgensen ML, Young JM, Solomon MJ. Optimal delivery of colorectal cancer follow-up care: improving patient outcomes. Patient Relat Outcome Meas 2015;6:127–38. 10.2147/PROM.S49589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Shami K, Oeffinger KC, Erb NL, et al. American cancer Society colorectal cancer survivorship care guidelines. CA Cancer J Clin 2015;65:427–55. 10.3322/caac.21286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young JM, Durcinoska I, DeLoyde K, et al. Patterns of follow up and survivorship care for people with colorectal cancer in New South Wales, Australia: a population-based survey. BMC Cancer 2018;18:339. 10.1186/s12885-018-4297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drury A, Payne S, Brady A-M. Cancer survivorship: advancing the concept in the context of colorectal cancer. Eur J Oncol Nurs 2017;29:135–47. 10.1016/j.ejon.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 55.Worthington J, Lew J-B, Feletto E, et al. Improving Australian national bowel cancer screening program outcomes through increased participation and cost-effective investment. PLoS One 2020;15:e0227899. 10.1371/journal.pone.0227899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grogan PB, Olver IN. A bowel cancer screening plan at last. Med J Aust 2014;201:435–6. 10.5694/mja14.01089 [DOI] [PubMed] [Google Scholar]
- 57.Owen L, Morgan A, Fischer A, et al. The cost-effectiveness of public health interventions. J Public Health 10.1093/pubmed/fdr075 [DOI] [PubMed] [Google Scholar]
- 58.Australian Institute of Health and Welfare Cancer in aboriginal & torres strait islander people of Australia, 2018. Available: https://www.aihw.gov.au/reports/cancer/cancer-in-indigenous-australians/contents/table-of-contents [Accessed 15 May 2018].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.