Abstract
Significance: Toxic epidermal necrolysis (TEN) and Steven–Johnson syndrome (SJS) are potentially fatal acute mucocutaneous vesiculobullous disorders. Evidence to date suggests that outcomes for patients with both TEN and SJS are largely dependent on stopping the causative agent, followed by supportive care and appropriate wound management in a specialized burns unit. These are life-threatening conditions characterized by widespread full-thickness cutaneous and mucosal necrosis. This article outlines the approach to holistic management of such patients, in a specialized unit, highlighting various practical aspects of wound care to prevent complications such as infection, mucosal and adhesions, and ocular scaring.
Recent Advances: There is improved understanding of pain and morbidity with regard to the type and frequency of dressing changes. More modern dressings, such as nanocrystalline, are currently favored as they may be kept in situ for longer periods. The most recent evidence on systemic agents, such as corticosteroids and cyclosporine, and novel treatments, are also discussed.
Critical Issues: Following cessation of the culprit trigger, management in a specialized burns unit is the most important management step. It is now understood that a multidisciplinary team is essential in the care of these patients. Following admission of such patients, dermatology, ear, nose, and throat surgery, ophthalmology, urology, colorectal surgery, and gynecology should all be consulted to prevent disease sequelae.
Future Directions: Looking forward, research is aimed at achieving prospective data on the efficacy of systemic immunomodulating agents and dressing types. Tertiary centers with burns units should develop policies for such patients to ensure that the relevant teams are consulted promptly to avoid mucocutaneous complications.
Keywords: toxic epidermal necrolysis, Steve–Johnson syndrome, drug reaction

Olivia A. Charlton, BAS, MBBS, MPH
Scope and Significance
Toxic epidermal necrolysis (TEN) and Steven–Johnson syndrome (SJS) are rare and life-threatening mucocutaneous conditions caused by immune system activation most commonly initiated by certain drugs and their metabolites. They are characterized by widespread keratinocyte death resulting in full-thickness denudation of the skin and mucosa, which leaves the patient highly susceptible to sepsis with a mortality rate of 30%.1 Here we summarize the evidence on management of the SJS/TEN wound and various oral treatments.
Translational Relevance
This review highlights various approaches derived from interdisciplinary collaboration between clinicians and researchers. Understanding the TEN/SJS wound and potential complications has allowed the proposition of novel dressings and systemic treatments, which are discussed.
Clinical Relevance
There remains controversy around the nursing and medical treatment of these patients, including the role of corticosteroids, intravenous immunoglobulin (IVIG), and other experimental treatments. Based on the current speculation about the pathogenesis of TEN/SJS, numerous treatments have been proposed for their anti-inflammatory and immunomodulatory effects. Here we discuss the details of practical approaches to nursing care in a specialized unit and the role of various systemic agents.
Background
TEN and SJS are considered a disease continuum, distinguished largely by their severity, as determined by the percentage of body surface area (BSA) affected by erosive blistering. Here we use the term TEN/SJS to refer to TEN, SJS, and TEN/SJS overlap. The more severe presentation TEN is always initiated by drugs, but the milder presentation SJS can often by caused by infectious agents. The mainstay of treatment of TEN/SJS is early identification and cessation of the culprit trigger and subsequent supportive care in a specialized burns intensive care unit (ICU) or a similar high-dependency unit. The exact mechanism of the disease is still not fully understood and there is little convincing evidence for the efficacy of systemic treatment. In addition, the rarity of the disease has meant randomized control trials are difficult to conduct. Recently, immunotherapy for melanoma and lymphoma patients has been implicated in cases of TEN.2,3
Epidemiology
TEN/SJS are rare, with reports ranging from two to seven cases per million people per year.4–10 SJS, the less severe form, is three times more common than TEN. Both are more common in women than men, and people of all ages are affected.11 Patients with a malignancy, particularly hematologic cancers,12 and human immunodeficiency virus (HIV) are also at increased risk of SJS/TEN.8 The mortality rate for TEN is 50% and 10% for SJS, with a combined rate of 30%. Increased age (more than 70) and comorbidities are associated with greater mortality.8
Genome-wide studies have identified populations at increased risk of TEN/SJS, for example, the HLA-B 15:02 allele confers increased risk in the Han Chinese population in the context of carbamazepine use.13 Furthermore the HLA-B 58:01 allele has been linked with allopurinol-induced TEN/SJS in European and Asian populations.14
Pathogenesis
Medications are the most common trigger for TEN/SJS and will usually trigger disease within 8 weeks in both adults and children; however, the typical exposure period is 4 days to 4 weeks. A history of tolerated use of a medication makes it less likely as a trigger. Common medication triggers include nonsteroidal anti-inflammatories, allopurinol, anticonvulsants such as lamotrigine, phenytoin, and carbamazepine, antibacterial sulfonamides, and the antiretroviral nevirapine.15 Other less strongly associated medications include antibiotics such as doxycycline, ciprofloxacin, and amoxicillin, and there is a suspected association with other agents such as pantoprazole, glucocorticoids, and terbinafine to name a few.15 Targeted immunotherapy and more conventional cancer medications have also been implicated in TEN/SJS cases, such as vemurafenib, ipilimumab, pembrolizumab, nivoliumab,16 thalidomide, and tamoxifen.17
Mycoplasma pneumoniae infection is the second-most common trigger of SJS, more so in the pediatric population. However, in more than a third of cases, a trigger is not found.18 Other triggers have been reported such as herbal medicine,19,20 vaccinations,21,22 systemic diseases, and contrast agents, however, whether such cases were in fact caused by these agents is not clear.
The pathogenesis of TEN/SJS is incompletely understood. It is considered a T cell-mediated, type IV hypersensitivity response.23 It is postulated that the reaction is initiated by an immune response, in which an antigenic drug/host tissue complex is made. Following this, there are various theories about events leading to stimulation of T cells, such as the hapten/prohapten theory, altered peptide theory, drug immune receptor theory, and an altered T cell receptor repertoire theory.20
Studies assessing the immunophenotype of blister fluid reinforce it as a cell-mediated cytotoxic reaction against keratinocytes, resulting in widespread apoptosis.24 Epidermal necrosis is understood as the result of a cumulative effect of risks, T cell clonotypes, HLA alleles, drug structure, and drug metabolism.20 The proposed mechanisms can be classified as intrinsic and extrinsic. Intrinsic refers to the production of toxic metabolites by keratinocytes, which then produce reactive oxygen species and ultimately lead to the production of TNF (tumor necrosis factor) alpha, causing further damage. The extrinsic pathway includes cytotoxic lymphocytes, monocytes, granulysin, perforin, granzyme, and Fas/Fas ligand interactions.20
In the hapten/prohapten theory, it is the metabolite not the drug that is antigenic. The drug is metabolized to form a hapten, which then stimulates the immune system. Carrier proteins then bind neoantigens, which are presented via antigen-presenting cells, to T cells.20 In the altered peptide model, the causative drug binds to a specific HLA, which then forms a complex, before the binding of other peptides. Thus, the display to T cell receptors is altered and a different T cell may be triggered.25 In the pharmacological interaction theory, the drug itself binds T cell receptors, resulting in the activation of specific T cells. Ko et al. highlighted that drugs triggering TEN/SJS have the capacity to directly kill keratinocytes by binding the T cell receptor and MHC class I, ultimately resulting in massive clonal expansion of cytotoxic T cells, and indirectly through dispersement of toxic soluble mediators such as granulysin, a cytolytic protein.26 The final theory postulated relates to an altered T cell receptor repertoire, where the drug binds a TCR and thus alters its structure.27
More recently, IL-15 is thought to play a central role, expressed by both immune and nonimmune cells, it activates NK cells, CD8+ cells, dendritic cells, and macrophages. IL-15 signals through the JAK-STAT pathway and then has downstream effects on the PI3K/AKT/mTOR pathway responsible for the IL-15-mediated effect on NK and CD8+ cells.28 It has been demonstrated that cytotoxic T cells target the drug rather than its metabolites, are drug specific, and HLA class I restricted.29,30
Mediators of nonapoptotic cell death pathways involving Fas-ligand, TNF-alpha, granzyme B, and perforin have been documented in high concentrations in blister fluid and mononuclear cells.31,32
Clinical Features
The clinical manifestations of TEN/SJS include a prodrome of fever and malaise for several days, followed by a rapidly progressing symmetric macular exanthem with mucosal involvement. Lesions are initially ill-defined erythematous macules, with purpuric centers that coalesce. Atypical lesions may appear targetoid. The skin is painful and out of proportion with the clinical appearance. The characteristic features then evolve within days; blistering and detachment of the epidermis with light pressure (known as the Nikolsky sign).33 The widespread shedding results in exposed and inflamed dermis that is very painful for the patient. The lesional skin is similar to a large wound as there has been loss of the epidermis with subsequent exposed dermis, with considerable fluid loss and risk of infection (Fig. 1).
Figure 1.
Widespread epidermal detachment with exposed dermis, affecting >30% of the total body surface area, consistent with a diagnosis of TEN. (a) Upper limb. (b) Torso. TEN, toxic epidermal necrolysis.
Defining disease as SJS or TEN can be difficult in areas with spotty lesions. In SJS, <10% of BSA is affected by skin detachment, 10–30% is an overlap area between SJS and TEN, and >30% is defined as TEN (Figs. 2 and 3).34
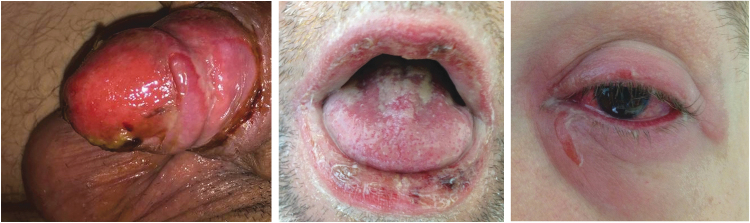
Figure 2.
TEN affecting mucosal sites. (a) Penis, (b) mouth, and (c) eyes.
Figure 3.
(a) Wound care for the torso in TEN/SJS. (b) Area-specific wound care in TEN/SJS. SJS, Steven–Johnson syndrome.
The severity of illness score for toxic epidermal necrolysis (SCORTEN), first proposed in 2000,30 is used in the determination of severity, as determined partially by the degree of skin detachment. Others factors such as age (>40), malignancy, tachycardia (>120 bpm), serum glucose (> 14 mM), serum bicarbonate (<20 mM), and serum urea (>10 mM) also contribute to the final score representative of the severity of illness. The SCORTEN is highly predictive of mortality.1 Other severity of illness scores exist, such as the modified APACHE (Acute Physiology and Chronic Health Evaluation) II and ICNARC (Intensive Care National Audit & Research Center) score. However, an ICU-based study in the United Kingdom compared its application in 145 patients and found no statistically significant difference in the outcome when comparing scoring systems, and that the SCORTEN was much easier to apply.35
Two modified SCORTEN systems were developed by Sorrell et al. for application in a pediatric population; however, these were not shown to be superior to the original adult score.36 A recent Brazilian study also found the SCORTEN to be valid in patients with acquired immunodeficiency syndrome (AIDS).37
The final diagnosis is made by corresponding these clinical features with histopathological findings, namely widespread epidermal necrosis, subepidermal blisters, apoptotic keratinocytes, and usually sparse dermal inflammatory infiltrate.38 Unlike autoimmune blistering disorders, direct immunofluorescence will not show immunoglobulin deposition.
There are considerable long-term sequelae from the initial insult of TEN/SJS. For patients who survive the acute stages of the disease, there is long recovery with multiple possible complications from the resultant mucosal scarring and adhesions.
Oral and genital (Fig. 2a, b) mucosa is involved in 90% of cases with complications from the shedding of all epidermal surfaces, including oral mucosa, larynx, cornea, urethra, vagina, labia, and scrotal skin, resulting in sequelae such as acute respiratory distress, gastrointestinal disturbance, and genitourinary denudation and dysfunction.39 Ocular involvement and subsequent scarring are common, leading to chronic ocular discomfort and potential loss of vision (Fig. 2c).
Consideration of the genitourinary tract is important as adhesions can cause significant functional complications. Genitourinary involvement occurs in approximately one-third of TEN/SJS patients. Basal keratinocytes are present in the keratinized stratified squamous epithelium of the external genitalia and both the stratified squamous and pseudostratified columnar epithelium of the urethra, and hence are at risk of being involved. Women must be monitored for vulvar ulceration, synechiae of the vulva and vagina, and vaginal stenosis. There should be prompt involvement of a gynecology team at the point of admission. In rare cases, significant obstruction develops, resulting in hydrocolpos.29 Vaginal dilators and potent topical corticosteroids have a role in preventing stenosis in some patients, to minimize the chances of requiring surgical intervention. Vaginal and urethral adhesions may require regular manual lysis, and close gynecological monitoring in female patients is important from the early stages of the disease.40 Genital disease is painful, results in scarring, and may affect normal sexual activity. Men must be monitored for erosive balanitis and phimosis.41 The role of catheterization is unclear.42 It is unknown whether this might prevent or potentiate strictures, and facilitate infection.43 However, in many patients with extensive disease who are intubated and receiving potent analgesia, catheterization is necessary to monitor fluid balance and treat urinary retention.31
Early oral and upper airway involvement is typically characterized by dysphagia, excessive salivation, and painful oral ulceration and crusting at the vermillion border.44 The epithelial involvement can extend down to the larynx, with inflammation and edema compromising airway patency. Bronchial epithelial sloughing may also occur, affecting the lower respiratory tract with edema, infection, and atelectasis.45,46 Williams et al. recently developed criteria for intubation, in a retrospective study of 40 patients with SJS and TEN. On the basis of their findings, they have suggested that there must be oral involvement plus one of the following: (1) initial total body surface area (TBSA) >70%, (2) progression of TBSA sloughed while in hospital, >15% from days 1 to 3, (3) underlying neurologic diagnosis inhibiting protection of airway, or (4) visualization of airway involvement with direct laryngoscopy.47
Ocular complications occur in approximately half of patients with TEN/SJS (Fig. 2c).48 In the more acute stages, severe ocular complications include epithelial defects of the ocular surface (conjunctiva and cornea), and conjunctivitis with pseudomembrane.49 In rare cases, patients may first present for medical attention with ocular erythema as the initial sign of SJS/TEN.50 Under blue illumination with fluorescein staining, large areas of confluent corneal and conjunctival epithelial loss will be seen.38 Opportunistic infection is frequently observed with methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE).35 Long-term complications include vision loss,51 permanent dry eye, trichiasis, and conjunctival invasion into the cornea.52
Management
The multisystem involvement and high morbidity and mortality of TEN/SJS necessitate high-dependency, multidisciplinary care of these patients in a specialized burns unit or ICU. The most important aspect of the management of TEN/SJS remains; early diagnosis, identification and withdrawal of culprit medication, supportive care, and specialist wound and multidisciplinary care.
Supportive care
The components of supportive care of TEN/SJS patients are similar to that of a severe burns patient. They include nutritional support due to the high catabolic state of these often septic patients, fluid and electrolyte monitoring and replacement due to multiorgan involvement and high fluid loss, thermoregulation, and adequate analgesia. The involvement of respiratory mucosa may require intubation and sedation for analgesia (Fig. 4). Multisystem involvement also necessitates early multidisciplinary involvement from gynecology, urology, colorectal, ear, nose, and throat (ENT) surgeons, and ophthalmology to help prevent sequelae of the disease. These patients are often managed in an induced coma for up to 4 weeks to manage the severe pain of this condition.
Figure 4.
TEN affecting the face, eyes, lips, oral mucosa, and respiratory tract, necessitating intubation.
Prompt identification and management of secondary infection in this cohort are imperative. Sepsis and pneumonia are the most common complications in TEN/SJS.53 A recent Japanese study compared the sensitivity of procalcitonin (PCT) and C-reactive protein (CRP) in detecting systemic infection.54 They demonstrated that PCT levels were significantly higher in patients with systemic bacterial infections, than in patients with only superficial cutaneous infection or no infection. The PCT and SCORETEN were also positively correlated. This was the first study to highlight PCT as a superior diagnostic tool and further prospective multicenter studies are required.
TEN/SJS: the wound
Specialized wound care in a burns unit setting is preferable for these patients. These patients require early isolation and barrier precautions to prevent infection. Dressings also prevent further mechanical trauma. Although TEN/SJS patients are managed in a similar way to burns patients, the wound is fundamentally different, with only involvement of the epidermis. Thus, TEN/SJS do not cause scarring. The type of dressings used varies greatly among different centers. Some centers use the detached epidermal sheets as a type of biological dressing.55 However, once there is extensive necrosis, the risk of infection may necessitate the debridement of necrotic tissue. The overall goals are avoidance of infection, avoidance of further trauma, management of exudate, and prevention of maceration (Fig. 3).
Care of the wound involves expressing fluid from blisters, cleaning with an antibacterial solution, and application of a dressing. Areas of intact skin require a thick layer of emollient containing corticosteroid. Absorbent pads are then applied to areas that are weeping, and the area is wrapped. Eyes must be regularly cleansed and reviewed by an ophthalmology service. Lubricating eye drops are to be applied every 1–2 h and white soft paraffin applied to the eyelids. Corticosteroid eye drops and possibly antibiotic eye drops may also be used. Careful attention to the urogenital region is required. In men, the foreskin must be regularly retracted and cleansed. Manual severing of adhesions may be necessary. Similarly in women, the vulva and vagina must be regularly examined and manual lysis of synechia may be required. A dilator may be considered to prevent vaginal stenosis (Fig. 3).
In the context of early surgical debridement, it is important to consider that while the wound in TEN/SJS is treated similarly to a burn, they have different pathomechanisms, that is, the epidermal process is more dynamic in TEN/SJS. This is important as the time to maximum detachment in TEN/SJS is approximately 7–10 days, and thus, at the outset it is difficult to determine the TBSA to be involved. Thus, early debridement may involve unnecessary removal of epidermis. This is especially relevant in the use of more modern techniques discussed below such as xenografts, as operative debridement is often required to promote adherence of the biologic membranes.56
Currently, nanocrystalline gauze materials containing silver are increasingly replacing petrolatum-impregnated gauze for the coverage of denuded skin, although controlled trials have not been performed.57 A single multisite study has also recommend the use of silicone silver foam dressings with silver sulfate in burns patients,58 which may be applicable in TEN/SJS, demonstrated successfully by McCarthy and Donovan.59 The benefit of the foam dressings being decreased frequency of dressing changes, and thus associated pain and skin trauma. There is some caution with using silver dressings over extensive exposed due to the theoretical risk of absorption. However, there is a lack of evidence that proposed absorption causes any clinical problems.60 The advantage of nanocrystalline dressings is they can be left in situ for up to 7 days, and this decreases the pain from handling the patient. Biosynthetic skin substitutes have also been investigated as alternative dressings, including “Biobrane” that has been found to reduce pain, eliminate need for further dressings allowing early physiotherapy.61
Castillo et al.62 conducted a literature review in 2018 to compare the efficacy of different dressing types in TEN/SJS. They included 22 studies that examined the time to re-epithelialization. They noted that these studies included simple older style dressings such as ointment with bandages and more modern biosynthetic and silver impregnated dressings. While no significant difference was found in healing time, more modern dressings could be left in place for longer and thus facilitated improved patient comfort. This reinforces the benefits of the abovementioned use of nanocrystalline dressings. While further studies are required to establish what chemical properties of dressings, if any, will facilitate reduced healing time, it is evident that dressings that can be left in situ for longer are preferable.
Systemic Therapy
Currently, there is insufficient evidence to support active medical therapy for TEN/SJS beyond that of supportive care. The comparison of clinical studies is difficult due to lack of uniform reporting and measurement standards at different centers. Also, the rarity of the disease has meant that large-scale studies are difficult to perform.
Despite this, there are numerous immunosuppressive and immunomodulating treatments that have been proposed, including corticosteroids, IVIG, cyclosporine, and TNF antagonists. The use of these agents is based on the evidence that various cytotoxic proteins and cytokines such as soluble Fas ligand, perforin/granzyme, tumor necrosis factor-alpha, TNF-related apoptosis inducing ligand, and most importantly granulysin are mediators for the widespread keratinocyte apoptosis in TEN/SJS.63
Immunosuppression (corticosteroids, cyclosporine)
The role of systemic corticosteroids in the treatment of TEN/SJS has long been debated as to whether it is beneficial or detrimental. Advocates of corticosteroids state high doses early in the disease are useful to inhibit inflammation and have been shown to decrease some of the biomarkers (IFN-IL-6) of inflammation in TEN/SJS.64 The counter argument is that patients on high-dose steroids are at greater risk of complications such as prolonged wound healing leading to increased risk of infection, occult early signs of sepsis, severe gastrointestinal bleeding, and increased mortality.65 There are also theoretical concerns that corticosteroids may have some proapoptotic effects, based on the findings that corticosteroids may downregulate NF-κB activity in the presence of an abundance of TNF and consequently promote apoptosis.66
A large retrospective European study of 281 German and French patients named EuroSCAR found a trend for beneficial effect of corticosteroid over the group of patients treated with supportive care alone.67 However, the subsequent RegiSCAR study that involved 442 patients with TEN and SJS did find a significant improved survival rate in the patients treated with corticosteroids versus supportive care alone.12 There were limitations to this study in that patients were treated in different ways and some already immunomodulated at the start of the reaction. Also, there was a noted lack of detailed information about the course of the reaction, and therefore, the investigators had several limitations on the validity of their findings.38 This highlights the need for international multicenter standardized diagnostic criteria and reporting standards in TEN to allow for rigorous international studies.
The questionable attributes of oral corticosteroids in the treatment of TEN have been affirmed in a number of studies. A large retrospective review of 366 patients for ocular sequelae found that there was no significant change in ocular outcomes in response to corticosteroids.68 In addition, a case/control study that compared 92 TEN patients who were already on high doses of corticosteroid for pre-existing conditions at the onset of TEN versus 321 randomly selected TEN patients and found that the prophylactic exposure did not affect the severity or mortality of the disease.10 Therefore, given that there is not a clear benefit to the use of corticosteroids and there is a theoretical risk, the use of corticosteroids in TEN cannot be recommended.69
Cyclosporine
Cyclosporine is relatively less studied compared with corticosteroids in TEN/SJS. However, there are several case series and reports of its efficacy in slowing disease progression. Cyclosporine has an established suppressive action on activated T lymphocytes.70 Cytotoxic T cell-derived granulysin has been recognized as the key mediator in the disseminated keratinocyte apoptosis seen in TEN/SJS. Therefore, there is a plausible theoretical benefit to its early administration in the disease process. There have been a number of small case series and individual case reports that have had favorable outcomes for patients treated with cyclosporine (Table 1). Of particular note was the recent study by Lee, through which 24 patients received cyclosporine (3 mg/kg per day); there were only 3 deaths, despite SCORTEN-predicted rate of 7.2, versus the comparison group of 20 patients treated supportively, where 6 deaths occurred in line with SCORTEN-predicted 5.9.46 Lee et al.46 chose the dose of 3 mg/kg based on a regimen published by a French referral center for toxic bullous disorders. Valeyrie-Allanore et al. had shown this dose to reduce both mortality and rate of progression of detachment.71 The favorable outcome in the cyclosporine-treated group warrants further randomized studies to validate these findings. The 2017 JAMA systematic review found that there was some evidence for both corticosteroids and cyclosporine; however, these findings need further substantiation.72
Table 1.
Summary of Studies Investigating Cyclosporine in Toxic Epidermal Necrolysis
| Study | Year | No. of patients | Dose (mg/kg) | No. of deaths | SCORTEN-expected mortality | Reported benefit |
|---|---|---|---|---|---|---|
| Arevelo et al.99 | 2000 | 11 | 3 | 0 | ND | Yes |
| Reese et al.100 | 2011 | 4 | 0 | ND | Yes | |
| Valeyrie-Allanore et al.71 | 2010 | 29 | 3 | 0 | 2.75 | Yes |
| Singh et al.101 | 2013 | 11 | 3 | 0 | ND | Yes |
| Kirchhoff et al.102 | 2014 | 16 | 5–7 | 1 | 2.4 | Yes |
| Lee et al.103 | 2017 | 44 | 3 | 3 | 7.2 | Yes |
ND, not done; SCORTEN, severity of illness score for toxic epidermal necrolysis.
Immunomodulating: IVIG
There was a promising in vitro study that showed IVIG could effectively block the interaction of the Fas receptor with Fas ligand, a reported pathway of keratinocyte death.73 There was subsequent interest in clinical studies for IVIG in the treatment of TEN to prevent disease progression. However, the subsequent clinical trials examining the efficacy of IVIG have not convincingly proved its efficacy.
A large study that included 48 patients with SJS/TEN and TEN suggested some benefit from IVIG at a dose of 3 g/kg over 3 days.74 Another single-center study showed outcomes for patients with SJS/TEN and TEN treated at a lower dose of IVIG (0.4–1 g/kg dose). This Miami-based study that involved 16 consecutively treated patients had significantly decreased mortality using the SCORTEN-predicted mortality tool.75
Despite these positive studies and a sound theoretical basis for the efficacy of IVIG, a more recent systematic review and meta-analysis concluded that the current evidence overall does not support a clinical benefit.76 The lack of evidence for the efficacy of IVIG has been affirmed in two subsequent studies. A study including 23 patients with TEN treated with IVIG versus supportive care alone showed no improved survival.77 More recently, a larger retrospective analysis of 64 patients (including SJS/TEN and TEN) treated at a single center showed no improved survival from treatment with IVIG regardless of whether it was high (>3 g/kg) or low dose (<3 g/kg). The uncertainty around the use of IVIG was highlighted in recent U.K. guidelines for management of TEN that stated there were strong advocates for and against the use of IVIG.78 This highlights the need for more rigorous trials in this area.
Antitumor necrosis factor agents—thalidomide, infliximab
The only known randomized control trial for TEN that studied thalidomide versus placebo found 10 deaths out of a cohort of 12 treated patients, versus 3 out of 10 deaths in the placebo group, resulting in early termination of the study.79 The proposed mechanism for the deleterious effect of thalidomide was the paradoxical increase of TNF-alpha production observed in plasma concentrations of the treated patient in this study. Despite this cautionary trial, there has been a subsequent report of infliximab (another TNF antagonist) as a single dose (5 mg/kg) administered on day 4 of rash to a 50-year-old woman with rapidly progressing TEN (affecting >35% BSA), who had rapid cessation of spread of rash following treatment.80 There is also a case series of 10 patients with SJS/TEN treated with a single dose of etanercept (50 mg subcutaneous injection) with all patients surviving despite an SCORTEN-predicted mortality of 50%.81 Etanercept was also recently shown to be effective in a child, an 11-year-old female, with SJS that had progressed despite methylprednisolone and cyclosporine.82 While TNF-alpha antagonists have been shown to be effective at halting the progression of TEN/SJS, they are potent immunosuppressants and the risk of infection must be considered in each patient. Further studies are required to characterize the place of TNF-alpha inhibition in TEN/SJS.83
Granulocyte colony-stimulating factor
The use of granulocyte colony-stimulating factor (G-CSF) has come about in an effort to boost bioregeneration and promote the re-epithelialization healing stage of TEN. It has been proposed that by accelerating healing, the complications of infection and sepsis-related death are avoided. The acceleration of wound healing with G-CSF has been seen in other conditions such as chronic leg ulcers.84 However, the mechanism of action remains unknown. It was proposed as a useful adjunct in two cases of severe TEN with concurrent neutropenia that showed rapid re-epithelialization following administration of G-CSF.85 There has also been a successful pediatric case report of a 7-year-old girl with TEN (affecting up to 70% BSA epidermal loss), complicated by neutropenia, who had normalization of white cell count after 48 h of rG-CSF (Neupogen), 500,000 units/kg per day by intravenous infusion on 4 successive days and complete recovery with no reported sequelae.86 There have been very few subsequent reports of the efficacy of G-CSF to validate these case reports.
Emerging and Experimental Treatments
There are a number of experimental treatments that have been reported for the topical treatment of TEN/SJS, including different wound dressing mediums and topical therapies. One example of this is the use of porcine xenografts, shown in a retrospective study to have significantly improved intravenous fluid requirements, pain and analgesia requirements in 8 patients treated versus those in the control group; however, the mortality rate was identical in both groups (12.5%).87 A 2018 systematic review assessed the role of biological skin substitutes in TEN and SJS, looking at 29 cases of xenograft use, in which the average TBSA involvement was 73.87%.88 Their review highlighted that only 2 of the 29 subjects died (10%), compared with the reported mortality figures of 50% in patients with such extensive disease. Further studies are, however, required to validate these findings.
Another new topical therapy that has been tested for the prevention of the long-term ocular complications of TEN/SJS is the use of cultivated oral mucosal epithelial transplantation (COMET). One case series reported the successful treatment of three patients with SJS who underwent COMET and, despite all patients experiencing the perioperative complication of methicillin-resistant staphylococcal aureus infection, they all had complete re-epithelialization and avoidance of vision loss.89
A second novel topical treatment for the ocular complications of TEN/SJS is the use of amniotic membrane in conjunction with topical corticosteroids. An ophthalmic case series found that covering the entire ocular surface (as opposed to partial coverage) with amniotic membrane coupled with application of topical corticosteroids was associated with preservation of visual acuity and the entire ocular surface in six patients. These ocular case series have promising results for the use of novel dressings in TEN and highlight the need for further studies in this area. Porcine amnion has also been use successfully on the torso and face in TEN.90
Activated protein C contributes to maintaining and promoting skin barrier integrity. It increases proliferation and migration of keratinocytes, junction proteins, and anti-inflammatory mediators, while decreasing apoptosis and inflammatory mediators, thereby enhancing the physical and immunological barrier.91 In animal models, it has been shown to induce re-epithelialization in full-thickness biopsies. It has also been used topically in humans in pressure sores,92 diabetic ulcers,93 and pyoderma gangrenosum-associated ulcers.94 Given its success in other areas, it has been postulated that activated protein C may have a future role in the management of patients with TEN/SJS.
There has been a single case report of extracorporeal therapy, in the form of hemodialysis, which has been used effectively to halt progression of TEN.95 In this case, bullae formation was seen to cease shortly after the initiation of therapy. It is unclear whether this was the product of expedited drug clearance, or possibly removal of uremic toxins contributing to cellular dysfunction. This same group reported success in a patient with linear immunoglobulin A bullous dermatosis and acute generalized exanthematous pustulosis. These findings are preliminary and isolated, however, it has been suggested that hemodialysis may have a role in patients in which the triggering agent is not clear.
Other targets being investigated in preclinical wound and burn studies may also be later considered for application in TEN/SJS. Morasso and colleagues have examined the rapid healing of oral mucosa and the central role of SOX 2 in increased cell migration.96 Studies analyzing angiogenesis and the role of pigment epithelium-derived growth factor by Michalczyk et al. address the concept of wound resolution and healing.97 Yang et al. have demonstrated the effect of IL-27 on epidermal proliferation, specifically skin wound closure, and antiviral host defense.98 Such preliminary findings may eventuate in clinical applications with implications for future therapeutic use in TEN/SJS.
Conclusion
TEN and SJS are severe and life-threatening conditions. Early diagnosis, elimination of the culprit medication, and supportive care in a specialized facility are the cornerstones of management for these patients. There is minimal supporting evidence for the use of systemic treatment for TEN/SJS. This is due, in part, to the rarity of the disease and the lack of randomized control trials.
Take-Home Messages
TEN/SJS are potentially fatal acute mucocutaneous vesiculobullous disorders.
Early elimination of the culprit medication is essential.
In SJS <10% of TBSA is affected by skin detachment, 10–30% is an overlap area between SJS and TEN, and >30% is defined as TEN.
These patients require specialized nursing care in a burns unit, with early isolation and barrier precautions to prevent infection.
More modern dressings such as nanocrystalline may be kept in situ for longer periods, thus reducing pain and morbidity.
Systemic agents such as cyclosporine may have a role, however, large, randomized controlled trials are required to validate findings from smaller studies.
Multisystem involvement necessitates early multidisciplinary involvement from gynecology, urology, colorectal, ENT surgeons, and ophthalmology to help prevent sequelae of the disease.
Acknowledgments and Funding Sources
There are no sources of funding to declare.
ABBREVIATIONS AND ACRONYMS
- BSA
body surface area
- COMET
cultivated oral mucosal epithelial transplantation
- ENT
ear, nose, and throat
- G-CSF
granulocyte colony-stimulating factor
- ICU
intensive care unit
- IVIG
intravenous immunoglobulin
- PCT
procalcitonin
- SCORTEN
severity of illness score for toxic epidermal necrolysis
- SJS
Steven–Johnson syndrome
- TBSA
total body surface area
- TEN
toxic epidermal necrolysis
- TNF
tumor necrosis factor
Author Disclosure and Ghostwriting
C.J. and A.C. have shares in a company with an interest in a variant of activated protein C to treat diabetic wounds. No other competing financial interests exist.
None of the other authors has any disclosures. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Olivia A. Charlton, BAS, MBBS, MPH, is a Dermatology Registrar at Lotus Dermatology in Newcastle. Victoria Harris, MBBS, M Phil, LLB (Hons), is a Dermatology Registrar at the Prince of Wales Hospital. Kevin Phan, MD, BSc (Adv), MSc, MPhil, is a Dermatology Resident at Liverpool Hospital, Sydney. Erin Mewton, BN, Postgraduate Certification in Tissue Viability, is the Dermatology CNC at Royal North Shore Hospital, and has extensive experience managing TEN/SJS patients. Chris Jackson, BAppSc, MAppSc, PhD, runs a research team involved with the clinical departments of Rheumatology, the Severe Burns Unit, Dermatology, and Endocrinology at the University of Sydney. He has a particular research focus in the areas of inflammation and wound healing. Alan Cooper, BSc, MBBS, FACD, is the head of Dermatology Department at Royal North Shore Hospital and the Clinical Professor of Dermatology at the Sydney Medical School of the University of Sydney. He is actively involved in clinical and laboratory research through Royal North Shore Hospital, and is the current chairman of Epiderm, the Australian Dermatology Research and Education Foundation, which supports dermatology research centers throughout the country.
References
- 1. Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000;115:149–153 [DOI] [PubMed] [Google Scholar]
- 2. Griffin LL, Cove-Smith L, Alachkar H, Radford JA, Brooke R, Linton KM. Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD-1 inhibitor) for lymphoma. JAAD Case Rep 2018;4:229–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CB, Wu MY, Ng CY, et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag Res 2018;10:1259–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rzany B, Mockenhaupt M, Baur S, et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol 1996;49:769. [DOI] [PubMed] [Google Scholar]
- 5. Strom BL, Carson JL, Halpern AC, et al. Using a claims database to investigate drug-induced Stevens-Johnson syndrome. Stat Med 1991;10:565. [DOI] [PubMed] [Google Scholar]
- 6. Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol 1990;126:43. [PubMed] [Google Scholar]
- 7. Schöpf E, Stühmer A, Rzany B, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol 1991;127:839. [DOI] [PubMed] [Google Scholar]
- 8. Frey N, Jossi J, Bodmer M, et al. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the UK. J Invest Dermatol 2017;137:1240. [DOI] [PubMed] [Google Scholar]
- 9. Roujeau JC, Guillaume JC, Fabre JP, et al. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981–1985. Arch Dermatol 1990;126:37–42 [DOI] [PubMed] [Google Scholar]
- 10. Yang MS, Lee JY, Kim J, et al. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: a nationwide population-based study using national health insurance database in Korea. PLoS One 2016;11:e0165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013;133:1197. [DOI] [PubMed] [Google Scholar]
- 12. Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol 2016;136:1387. [DOI] [PubMed] [Google Scholar]
- 13. Harris V, Jackson C, Cooper A. Review of toxic epidermal necrolysis. Int J Mol Sci 2016;17:2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med 2011;364:1134–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128:35. [DOI] [PubMed] [Google Scholar]
- 16. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer 2017;41:125. [DOI] [PubMed] [Google Scholar]
- 17. Madabhavi I, Revannasiddaiah S, Patel A, Anand A. Toxic epidermal necrolysis with the use of tamoxifen. BMJ Case Rep 2015;2015 [Epub ahead of print]; DOI: 10.1136/bcr-2014-209102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 2010;88:60. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury AD, Oda M, Markus AF, et al. Herbal medicine induced Stevens-Johnson syndrome: a case report. Int J Paediatr Dent 2004;14:204. [DOI] [PubMed] [Google Scholar]
- 20. Kumar Das K, Khondokar S, Rahman A, Chakraborty A. Unidentified drugs in traditional medications causing toxic epidermal necrolysis: a developing country experience. Int J Dermatol 2014;53:510–515 [DOI] [PubMed] [Google Scholar]
- 21. Ball R, Ball LK, Wise RP, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis after vaccination: reports to the vaccine adverse event reporting system. Pediatr Infect Dis J 2001;20:219. [DOI] [PubMed] [Google Scholar]
- 22. Oda T, Sawada Y, Okada E, et al. Stevens-Johnson syndrome after influenza vaccine injection. J Investig Allergol Clin Immunol 2017;27:274. [DOI] [PubMed] [Google Scholar]
- 23. Kinoshita Y, Saeki H. A review of the pathogenesis of toxic epidermal necrolysis. J Nippon Med Sch 2016;83:216–222 [DOI] [PubMed] [Google Scholar]
- 24. Correia O, Delgado L, Ramos JP, et al. Cutaneous T-cell recruitment in toxic epidermal necrolysis. Further evidence of CD8+ lymphocyte involvement. Arch Dermatol 1993;129:466. [PubMed] [Google Scholar]
- 25. Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-Johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol 2015;16:475–493 [DOI] [PubMed] [Google Scholar]
- 26. Ko TM, Chung WH, Wei CY, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol 2011;128:1266. [DOI] [PubMed] [Google Scholar]
- 27. Chung WH, Wang CW, Dao RL. Severe cutaneous adverse drug reactions. J Dermatol 2016;43:758–766 [DOI] [PubMed] [Google Scholar]
- 28. Su SC, Mockenhaupt M, Wolkenstein P, et al. Interleukin-15 is associated with severity and mortality in Stevens-Johnson syndrome/toxic epidermal necrolysis. J Invest Dermatol 2017;137:1065–1073 [DOI] [PubMed] [Google Scholar]
- 29. Nassif A, Bensussan A, Boumsell L, et al. Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J Allergy Clin Immunol 2004;114:1209. [DOI] [PubMed] [Google Scholar]
- 30. Roujeau JC, Bricard G, Nicolas JF. Drug-induced epidermal necrolysis: important new piece to endthe puzzle. J Allergy Clin Immunol 2011;128:1277. [DOI] [PubMed] [Google Scholar]
- 31. Posadas SJ, Padial A, Torres MJ, et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol 2002;109:155. [DOI] [PubMed] [Google Scholar]
- 32. de Araujo E, Dessirier V, Laprée G, et al. Death ligand TRAIL, secreted by CD1a+ and CD14+ cells in blister fluids, is involved in killing keratinocytes in toxic epidermal necrolysis. Exp Dermatol 2011;20:107. [DOI] [PubMed] [Google Scholar]
- 33. Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol 2007;56:181–200 [DOI] [PubMed] [Google Scholar]
- 34. Roujeau JC. Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol 1997;24:726. [DOI] [PubMed] [Google Scholar]
- 35. George SM, Harrison DA, Welch CA, Nolan KM, Friedmann PS. Dermatological conditions in intensive care: a secondary analysis of the Intensive Care National Audit and Research Centre (ICNARC) Case Mix Programme database. Crit Care 2008;12(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sorrell J, Anthony L, Rademaker A, et al. Score of toxic epidermal necrosis predicts the outcomes of pediatric epidermal necrolysis. Pediatr Dermatol 2017;34:433–437 [DOI] [PubMed] [Google Scholar]
- 37. Wambier CG, Hoekstra TA, Wambier SPF, et al. Epidermal necrolysis: SCORTEN performance in AIDS and non-AIDS patients. An Bras Dermatol 2019;94:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cote B, Wechsler J, Bastuji-Garin S, Assier H, Revuz J, Roujeau JC. Clinicopathologic correlation in erythema multiforme and Stevens-Johnson syndrome. Arch Dermatol 1995;131:1268–1272 [DOI] [PubMed] [Google Scholar]
- 39. Downey A, Jackson C, Harun N, et al. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol 2012;66:995–1003 [DOI] [PubMed] [Google Scholar]
- 40. Meneux E, Paniel BJ, Pouget F, et al. Vulvovaginal sequelae in toxic epidermal necrolysis. J Reprod Med 1997;42:153–156 [PubMed] [Google Scholar]
- 41. Revuz J, Penso D, Roujeau JC, et al. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol 1987;123:1160–1165 [DOI] [PubMed] [Google Scholar]
- 42. Van Batavia JP, Chu DI, Long CJ, Jen M, Canning DA, Weiss DA. Genitourinary involvement and management in children with Stevens–Johnson syndrome and toxic epidermal necrolysis. J Pediatr Urol 2017;13:490..e1–490.e7. [DOI] [PubMed] [Google Scholar]
- 43. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol 2013;69:187..e1–187.e16. [DOI] [PubMed] [Google Scholar]
- 44. Calcaterra T, Strahan R. Stevens-Johnson syndrome; oropharyngeal manifestations. Arch Otolaryngol 1971;93:37–41 [DOI] [PubMed] [Google Scholar]
- 45. Brogan TV. Epidermal necrolysis: a skin disease to take your breath away. Crit Care Med 2014;42:210–211 [DOI] [PubMed] [Google Scholar]
- 46. Lebargy F, Wolkenstein P, Gisselbrecht M, et al. Pulmonary complications in toxic epidermal necrolysis: a prospective clinical study. Intensive Care Med 1997;23:1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Williams R, Hodge J, Ingram W. Indications for intubation and early tracheostomy in patients with Stevens–Johnson syndrome and toxic epidermal necrolysis. Am J Surg 2016;211:684–688 [DOI] [PubMed] [Google Scholar]
- 48. Ueta M. Results of detailed investigations into Stevens-Johnson syndrome with severe ocular complications. Invest Ophthalmol Vis Sci 2018;59:DES183–DES191 [DOI] [PubMed] [Google Scholar]
- 49. Sotozono C, Ueta M, Koizumi N, et al. Diagnosis and treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis with ocular complications. Ophthalmology 2009;116:685–690 [DOI] [PubMed] [Google Scholar]
- 50. Barry R, Zanetto U, Kolli S, Morjaria R. Toxic epidermal necrolysis: the red eye and red herrings in casualty. BMJ Case Rep 2018. [Epub ahead of print]; DOI: 10.1136/bcr-2018-225861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Magina S, Lisboa C, Leal V, et al. Dermatological and ophthalmological sequels in toxic epidermal necrolysis. Dermatology 2003;207:33–36 [DOI] [PubMed] [Google Scholar]
- 52. Sotozono C, Ang LP, Koizumi N, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology 2007;114:1294–1302 [DOI] [PubMed] [Google Scholar]
- 53. Yamane Y, Matsukura S, Watanabe Y, et al. Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients—treatment and outcome. Allergol Int 2016;65:74–81 [DOI] [PubMed] [Google Scholar]
- 54. Wang Q, Tian X, Liu W, Zhang L. Procalcitonin as a diagnostic indicator for systemic bacterial infections in patients with Stevens–Johnson syndrome/toxic epidermal necrolysis. J Dermatol 2018;45:989–993 [DOI] [PubMed] [Google Scholar]
- 55. Dorasfshar AH, Dickie SR, Cohn AB, et al. Antishear therapy for toxic epidermal necrolysis: an alternative treatment approach. Plast Recontr Surg 2008;122:154. [DOI] [PubMed] [Google Scholar]
- 56. Lee H. Wound management strategies in Stevens-Johnson sundrome/toxic epidermal necrolysis: an unmet need. J Am Acad Dermatol 2018;79:e87–e88 [DOI] [PubMed] [Google Scholar]
- 57. Dalli RL, Kumar R, Kennedy P, et al. Toxic epidermal necrolysis/Stevens-Johnson syndrome: current trends in management. ANZ J Surg 2007;77:671. [DOI] [PubMed] [Google Scholar]
- 58. Silverstein P, Heimbach D, Meites H, et al. An open, parallel, randomized, comparative, multicenter study to evaluate the cost-effectiveness, performance, tolerance, and safety of a silver-containing soft silicone foam dressing (intervention) vs silver sulfadiazine cream. J Burn Care Res 2011;32:617–626 [DOI] [PubMed] [Google Scholar]
- 59. McCarthy K, Donovan R. Management of a patient with toxic epidermal necrolysis using silicone transfer foam dressings and a secondary absorbent dressing. J Wound Ostomy Continence Nurs 2016;43:650–651 [DOI] [PubMed] [Google Scholar]
- 60. Vlachou E, Chipp E, Shale E, et al. The safety of nanocyrstalline silver dressings on burns: a study of systemic silver absorption. Burns 2007;33:979–985 [DOI] [PubMed] [Google Scholar]
- 61. Boorboor P, Vogt PM, Becara FG, et al. Toxic epidermal necrolysis: use of Biobrane or skin coverage reduces pain, improves mobilization and decreases infection in elderly patients. Burns 2008;34:487. [DOI] [PubMed] [Google Scholar]
- 62. Castillo B, Vera N, Ortega-Loayza AG, Seminario-Vidal L. Wound care for Stevens-Johnson syndrome and toxic epidermal necrolysis. J Am Acad Dermatol 2018;79:764..e1–767.e1. [DOI] [PubMed] [Google Scholar]
- 63. Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008;14:1343–1350 [DOI] [PubMed] [Google Scholar]
- 64. Hirahara K, Kano Y, Sato Y, et al. Methylprednisolone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis: clinical evaluation and analysis of biomarkers. J Am Acad Dermatol 2013;69:496–498 [DOI] [PubMed] [Google Scholar]
- 65. Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg 1986;204:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Antwerp DJ, Martin SJ, Kafri T, et al. Suppression of TNF-alpha-induced cell death. Science 1996;274:782–784 [DOI] [PubMed] [Google Scholar]
- 67. Schneck J, Stat D, Fagot JP, et al. Effects of treatment on the mortality of Stevens- Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol 2008;34:33–40 [DOI] [PubMed] [Google Scholar]
- 68. Power WJ, Ghoraishi M, Merayo-Lloves J, et al. Analysis of the acute ophthalmic manifestations of erythema multiforme/toxic epidermal necrolysis disease spectrum. Ophthalmology 1995;102:1669–1676 [DOI] [PubMed] [Google Scholar]
- 69. Chave TA, Mortimer NJ, Sladden MJ, et al. Toxic epidermal necrolysis: current evidence, practical management and future directions. Br J Dermatol 2005;153:241–253 [DOI] [PubMed] [Google Scholar]
- 70. Emmel EA, Verweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science 1989;246:1617. [DOI] [PubMed] [Google Scholar]
- 71. Valeyrie-Allanore L, Wolkenstein P, Brochard L, et al. Open trial of cyclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 2010;163:847–853 [DOI] [PubMed] [Google Scholar]
- 72. Zimmermann S, Sekula P, Venhoff M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol 2017;153:514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Viard I, Wehrli P, Bullani R, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998;282:490–493 [DOI] [PubMed] [Google Scholar]
- 74. Prins C, Kerdel F, Padilla S, et al. Treatment of toxic epidermal necrolyis with high-dose intravenous imunoglobulins. Multicenter retrospective analysis of 48 consecutive cases. Arch Dermatol 2003;139:26–32 [DOI] [PubMed] [Google Scholar]
- 75. Trent J, Kirsner R, Romanelli P, et al. Analysis of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis using SCORTEN. Arch Dermatol 2003;139:39–43 [DOI] [PubMed] [Google Scholar]
- 76. Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol 2012;167:424–434 [DOI] [PubMed] [Google Scholar]
- 77. Firoz BF, Henning JS JS, Zarazabal LA, et al. Toxic epidermal necrolysis: five years of treatment experience from a burn unit. J Am Acad Dermatol 2012;67:630–635 [DOI] [PubMed] [Google Scholar]
- 78. Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol 2016;174:1194–1227 [DOI] [PubMed] [Google Scholar]
- 79. Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998;352:1–4 [DOI] [PubMed] [Google Scholar]
- 80. Fischer M, Fiedler E, Marsch WC, et al. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol 2002;146:707–709 [DOI] [PubMed] [Google Scholar]
- 81. Paradisi A, Beni D, Bergamo F, et al. Entanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol 2014;71:278–283 [DOI] [PubMed] [Google Scholar]
- 82. Gavigan G, Kanigsberg N, Ramien M. Pediatric Steven-Johnson syndrome/toxic epidermal necrolysis halted by etanercept. J Cutan Med Surg 2018;22:514–515 [DOI] [PubMed] [Google Scholar]
- 83. Woolridge KF, Boler PL, Lee BD. Tumour necrosis factor alpha inhibitors in the treatment of toxic epidermal necrolysis. Cutis 2018;101:e15–e21 [PubMed] [Google Scholar]
- 84. Cianfarani F, Tommasi R, Failla CM, et al. Granulocyte/macrophage colony stimulating factor treatment of human chronic ulcers promotes angiogenesis associated de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol 2006;154:34–41 [DOI] [PubMed] [Google Scholar]
- 85. De Sica-Chapman A W illiams G, Soni N. Granulocyte colonoy-stimulating factor in toxic epidermal necrolysis (TEN) and Chelsea Westminster TEN protocol. Br J Dermatol 2010;162:860–865 [DOI] [PubMed] [Google Scholar]
- 86. Goulden V, Goodfield M. Recombinant granulocyte colony-stimulating factor in the management of toxic epidermal necrolysis. Br J Dermatol 1996;135:305–306 [PubMed] [Google Scholar]
- 87. Young JB, Gondek SP, Troche M. The use of porcine xenografts in patients with toxic epidermal necrolysis. Burns 2016;42:1728–1733 [DOI] [PubMed] [Google Scholar]
- 88. Paggiaro AO, E Silva Filho ML, de Carvalho VF, Isaac C, Gemperli R. The role of biological skin substitutes in Steven-Johnson syndrome: systematic review. Plast Surg Nurs 2018;38:121–127 [DOI] [PubMed] [Google Scholar]
- 89. Sotozono C, Inatomi T, Nakamur T. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol 2014;92;e447–e453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Klama-Baryla A, Labus W, Kitala D, Kraut M, Nowak M, Kawecki M. Experience in using fetal membranes: present and new perspectives. Transplant Proc 2018;50:2188–2194 [DOI] [PubMed] [Google Scholar]
- 91. Xue M, Jackson CJ. Novel functions of the anticoagulant activated protein C in maintaining skin barrier integrity to impact on skin disease. Pathobiology 2015;82:100–106 [DOI] [PubMed] [Google Scholar]
- 92. Wijewardena A, Lajevardi SS, Vandervord E, et al. Activated protein C to heal pressure ulcers. Int Wound J 2016;13:986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Whitmont K, Reid I, Tritton S, et al. Treatment of chronic leg ulcers with topical activated protein C. Arch Dermatol 2008;144:1479–1483 [DOI] [PubMed] [Google Scholar]
- 94. Kapila S, Reid I, Dixit S, et al. The use of dermal injection of activated protein C for treatment of large chronic wounds secondary to pyoderma gangrenosum. Clin Exp Dermatol 2014;39:785–790 [DOI] [PubMed] [Google Scholar]
- 95. El-Azhary R, Wang M, Wentworth A, Hickson L. Treatment of severe drug reactions by hemodialysis. Int J Dermatol 2018;57:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Iglesias-Bartolome R, Uchiyama A, Molinolo A, et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci Transl Med 2018;10:eaap8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Michalczyk E, Chen L, Fine D, et al. Pigment epithelium-derived factor (PEDF) as a regulator of wound angiogenesis. Nat Sci Rep 2018;8:11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yang B, Suwanpradid J, Sanchez-Lagunes R, et al. IL-27 facilitates skin wound healing through induction of epidermal proliferation and host defense. J Invest Dermatol 2017;137:1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Arevelo JM, Lorente JA, Gonzalez-Herrada C, Jimenez-Reyes J. Treatment of toxic epidermal necrolysis with cyclosporine A. J Trauma 2000;48:473–478 [DOI] [PubMed] [Google Scholar]
- 100. Reese D, Henning JS, Rockers K, et al. Cyclosporine for SJS/TEN: a case series and review of the literature. Cutis 2011;87:24. [PubMed] [Google Scholar]
- 101. Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venerol Leprol 2013;79:686–692 [DOI] [PubMed] [Google Scholar]
- 102. Kirchhof M, Miliszewski M, Sikora S, et al. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol 2014;71:941–947 [DOI] [PubMed] [Google Scholar]
- 103. Lee HY, Fook-Chong S, Koh HY, et al. Cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis: restrospective analysis of a cohort treated in a specialized referral centre. J Am Acad Dermatol 2017;76:106. [DOI] [PubMed] [Google Scholar]