Abstract
Although survival has been the focus of aging research for many years, the field is rapidly evolving towards incorporating healthspan and health indices in studies that explore aging-related outcomes. Frailty is one such measure that is tightly correlated with human aging. Several frailty measures have been developed that focus on phenotypes of aging, including physical, cognitive and metabolic health that define healthspan. The extent at which cumulative deficits associated with frailty predict functional characteristics of healthy aging and longevity is currently unknown. A growing consensus for the use of animal models has emerged to evaluate a composite measure of frailty that provides a translational basis to understanding human frailty. In this review, we will focus on the impact of several anti-aging interventions, some of which have been characterized as caloric restriction (CR) mimetics such as metformin, rapamycin, resveratrol as well as more novel approaches that are emerging in the field - nicotinamide adenine dinucleotide precursors, small molecule activators of sirtuins, and senolytics - on a number of frailty measurements associated with aging-related outcomes in mice and discuss the translatability of such measures to human frailty.
Keywords: Aging, frailty, mouse models, interventions
1. INTRODUCTION
Frailty is classified as one of the most important risk factors for mortality in older adults and is defined as a ‘state of high vulnerability’, correlated with advancing age and detrimental health outcomes when compared to individuals who are not frail [1]. A significant proportion (~ 25–50%) of individuals who are above the age of 85 and other vulnerable populations, such as ethnic minorities and women, are disproportionally living with frailty [2] [3], a condition that is associated with comorbidity and disability [4]. Alas, the etiology of frailty remains poorly understood [5]. Studying the burden of frailty in humans has been challenging – particularly due to the ethical, logistical, and biological complications associated with working with older adults [6]. As human life expectancy is increasing by 2 to 3 months every year [7], age-related disorders and disabilities invariably bring with them significant social, medical, and economic challenges that need to be addressed urgently.
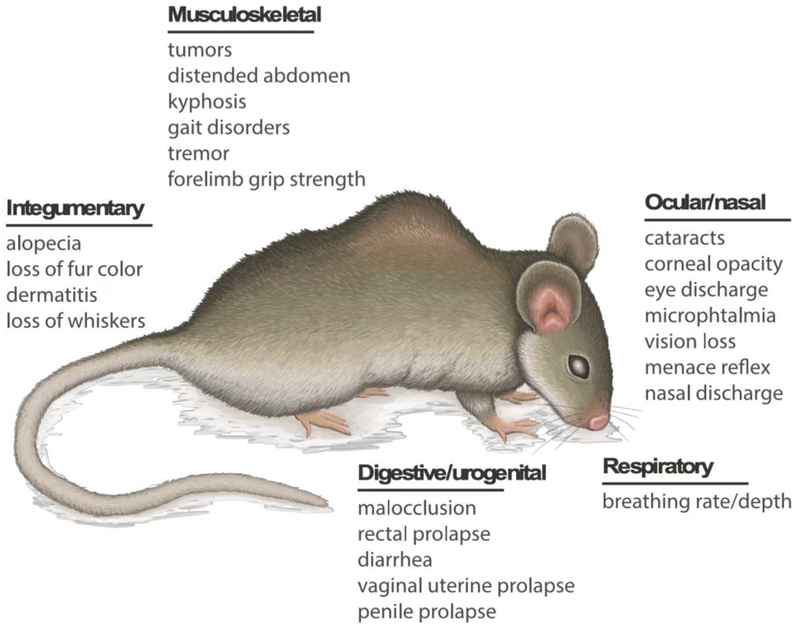
Animal models and interventions conducted on them provide an opportunity to study the biological basis of aging and potential ways to delay the onset of aging-related phenotypes [8]. Such studies are also inherently cross-translational in nature as knowledge gathered from animal models can be directly applied to human aging and vice versa. A frailty index score that encompasses various parameters of frailty allows to assess whether an individual is frail or not [1]. Parks and colleagues first implemented a frailty index in mice [9], which has garnered great interest in the aging field for the last several years. Factors related to integument, physical/musculoskeletal, vestibulocochlear/auditory, ocular/nasal, digestive/urogenital, respiratory, and aspects of physical discomfort are used to assess the frailty index in mice (Figure 1). Similar to the human frailty index, a higher index score is associated with lower survivability in mice [10]. These main categories of frailty assessment are further subdivided into characteristics in order to calculate a comprehensive score for each category. These characteristics are as follows: 1) Integument evaluates alopecia, loss of fur color, amount of dermatitis, loss of whiskers, and the general condition of the coat; 2) physical/musculoskeletal parameters examine tumors, distended abdomen, kyphosis (abnormal spinal curvature), gait disorders, tremor, forelimb grip strength, and overall body condition score; 3) the vestibulocochlear/auditory section of the frailty index reflects on vestibular disturbance and hearing loss; 4) ocular/nasal segment explores cataracts, corneal opacity, eye discharge, microphthalmia, vision loss, menace reflex, and nasal discharge; 5) digestive/urogenital section evaluates any malocclusions, rectal prolapse, diarrhea, and vaginal/uterine or penile prolapse; 6) breathing rate/depth is evaluated; and also 7) any physical discomfort as evidenced by the mouse grimace scale and piloerection. Along with these indices, mouse weight and body temperature are also scored.
Figure 1.
Systemic effects of frailty in aging mouse. The main categories of frailty assessment are subdivided into characteristics in order to calculate a comprehensive score for each category.
The overarching goal of developing a mouse frailty index is to justify its incorporation into intervention studies aimed at evaluating age-related phenotypic outcomes with direct translatability into human frailty in the elderly. This article reviews and evaluates the effectiveness of few proposed anti-aging interventions aiming to extend healthy lifespan to specific frailty-related measures. Many of these small molecules were first developed as CR and fasting mimetics [11]. While CR has also been shown to be associated with improvement in frailty in mice [6, 12] in several studies, these will not be discussed in detail in this review. We focused on mouse intervention studies with small molecules that have utilized frailty indices as a measure of efficacy in combatting aging-related phenotypes. A summary of these studies can be found in Table 1.
TABLE 1:
Major frailty-related findings from aging interventions using Metformin, Rapamycin, Resveratrol, NAD precursors, SIRT1 activators, and Senolytics
| Ref. | Strain | Age of Onset | Sex | Intervention | Dose, Route of Exposure, Duration | Frailty Index Measures |
|---|---|---|---|---|---|---|
| (18) | C57BL/6 | 104-weeks-old | M | Metformin | AIN-93G diet supplemented with 1% metformin every other week (EOW) or for 2 consecutive weeks each month (2WM) for the remainder of their lives | Performance on the rotarod and forelimb grip strength measures were undistinguishable compared to their SD controls. Tests were performed at 17 weeks of intermittent metformin treatment. Longitudinal measures are needed to determine effects on healthspan. |
| (16) | C57BL/6 | 12-month-old | M | Metformin | HFD based on AIN-93 G supplemented with 1.0% metformin for 6 months | Attenuated the decline in motor performance |
| (17) | C57BL/6 | 54-week-old | M | Metformin | Daily treatment with 0.1% metformin in diet until natural death | Improved physical performance Decreased incidence of cataract |
| (41) | C57BL/6J | 1-year-old | M | Nicotinamide (NAM) | 62-week NAM supplementation (0.5 and 1.0 g/kg of diet) in SD or HFD for the remainder of their lives | HFD-fed mice with supplemen tation of NAM, improved healthspan parameters, specifically: Motor coordination via rotarod testing and locomotor activity (both total distance and average speed) during open field testing |
| (45) | Nampt-rod/-rod mice backcrossed onto C57BL/6J | 6-week-old | N/A | NMN | NMN was administered via daily i.p. injection (150 mg/kg) for 4 weeks | Vision loss was rescued via significant recovery of scotopic and photopic retinal function |
| (44) | Nampt knockout (mNKO) | 3-month-old | M & F | NR & NAM | NR chloride and NAM were dissolved weekly in drinking water (12mM) and provided ad libitum | Intervention restored exercise performance and endurance in KO mice |
| (43) | WldS, BalbC, and CBA mice on C57BL/6J background | 8–10-week-old | N/A | NR | NR was administered by i.p. twice daily for a period of 5 days prior to noise exposure and then 14 days thereafter | NR treatment provided protection against hearing loss in all tested mouse strains |
| (29) | C57BL/6J | 4-month-old | M & F | Rapamycin | Microencapsulated rapamycin (14ppm) continued throughout life | No differences in stride length or age-related hearing loss Worsening of motor function in males Increased grip strength in females Spontaneous activity greater in females |
| (24) | C57BL/6Nia | 19-month-old | M & F | Rapamycin | Rapamycin supplemented in diet (14 ppm) | Stride length improved at 32 mo. of age Better motor function for both males and females No effect on grip strength regardless of sex |
| (27) | C57BL/6J | 24-month-old | F | Rapamycin | Oral delivery of microencapsulated rapamycin (14 ppm in diet) for 3 months | Significant reduction in kyphosis Index at 27-months of age Rapamycin increased resistance to muscle fatigue |
| (23) | UM-HET3 | 20–22-month-old | M & F | Rapamycin | Mice exposed to rapamycin at doses of 4.7, 14, or 42 ppm for 9 months | Higher incidence of testicular degeneration (13% in the controls and 83% in all rapamycin-treated groups) and cataracts in treated males Lower incidence of lesions within uterine lining with highest dose of rapamycin |
| (26) | C57BL/6JNia | 20–21-month-old | M & F | Rapamycin | Daily intraperitoneal (i.p.) injections of 8 mg/kg rapamycin for 90 days. | Significant increase in muscle function |
| (34) | C57BL/6nia | 1-year-old | M | RSV | 2 doses of RSV (5.2 ± 0.1 and 22.4 ± 0.4 mg.kg-1/day) added to standard and high-calorie diets for 6 months | Improved endurance/motor skills Increased survival in high-calorie fed mice supplemented with RSV compared to their high calorie only counterparts. |
| (37) | C57BL/6J | 16-month-old | M | RSV | Diet supplementation with 25 mg/kg RSV for 4 weeks | Mice on either RSV or exercise training or the combination of exercise + RSV had greater forelimb grip strength than control group. Mice on exercise alone and exercise + RSV performed significantly better during an endurance swimming test than controls |
| (36) | ICR | 6-week-old | M | RSV | Oral administration of RSV at 25, 50, and 125 mg/kg/day in standard laboratory diet for 21 days | Significantly longer exhaustive swimming time for mice with the lowest RSV dose than vehicle control group |
| (6) | C57BL/6J | 18-month-old | M & F | RSV | RSV (100 mg/kg) was supplemented in diet AIN-93G die fed AL, until they reached 24 month-of-age | 6 months of RSV treatment significantly reduced the FI from ~0.2 to ~0.18 in C57BL/6J mice compared to their control counterparts Reported was a significant increase in the proportion of mice with a frailty score greater than 0 for corneal opacity in the SD-fed group (34%) compared to the RSV diet group (0%) |
| (57) | C57BL/6 | 24-month-old | M | Ruxolitinib | Treatment of aged mice with JAK inhibitor in diet (60 mg/kg BW) for a duration of 10 weeks | Treatment with JAK 1/2 inhibitor significantly enhanced physical function in aged mice, specifically: Motor coordination, grip strength measures, hanging time, and frequency of rearing |
| (53) | BubR1H/H and INK-ATTAC. All mice were on a mixed 129 × C57BL/6 × FVB genetic background | 3-week-old | F | Senolytic AP20187 | Mice were treated with AP20187 (0.2 μg.g−1 BW) by i.p. injection every 3rd day for beginning at 3 weeks of age until 8 months old | Treatment significantly delayed onset of cataracts & lordokyphosis Better treadmill exercise performance (both duration and distance travelled) in treated mice, indicative of an attenuation of sarcopenia and preservation of muscle fibers |
| (53) | BubR1H/H and INK-ATTAC. All mice were on a mixed 129 × C57BL/6 × FVB genetic background | 5-month-old | F | Senolytic AP20187 | Mice were treated with AP20187 (0.2 μg/g BW) by i.p. injection | Cataract formation was not reduced in treated mice and remained unchanged Improvement in treadmill exercise testing was still prominent in 10-month-old timepoint |
| (55) | syngeneic C57BL/6 mice | 6-month-old | M | Senolytics | Transplantation of control and doxorubicin-treated preadipocytes isolated from luciferase-expressing transgenic (LUC+) mice into syngeneic mice via i.p. injection. Physical performance tests were evaluated 1 month later | Physical dysfunction was induced as evidenced by significantly lower maximal walking speed on rotarod, grip strength, and hanging endurance compared to controls |
| (58) | Ercc1 −/Δ mice in an f1 background (C57Bl/6:FVB/n) | 6 weeks-of age | M & F | Senolytics | 17-DMAG (10 mg/kg) was administered 3x/week every 3 weeks (intermittent treatment) beginning at 6-weeks-of-age via oral gavage | Treatment significantly reduced incidence of kyphosis, loss of forelimb grip strength, coat condition, gait disorder, and overall body condition |
| (56) | Ink-Attac mice on a C57BL/6 × BALB/c background | 2.5–8 months of age | M | Senolytics | Mice were treated with PBS or 2 U/kg bleomycin via aerosolized i.p. injection and then randomized. Vehicle, AP20187 (10 mg/kg via i.p. injection), or the combination of Dasatinib (5 mg/kg) + Quercetin (50 mg/kg) delivered via oral gavage, termed DQ) was administered. | Exercise capacity was evaluated on a motorized treadmill. Mice treated with bleomycin alone ran shorter mean and maximal distances as compared to all other groups. Mice on bleomycin + AP20187 and bleomycin + DQ ran further than mice on bleomycin alone even though all three groups’ performance was subpar to their vehicle controls |
| (48) | C57BL/6J | 6-month-old | M | SRT2104 | Mice were fed AIN-930 diet supplemented with SRT2104 (100 mg/kg/bw) for the remainder of their lives | Treatment correlates with higher endurance on treadmill Increased performance on rotarod |
| (49) | C57BL/6J | 6-month-old | M | SRT1720 | Mice were fed either HFD or SD supplemented with SRT1720 (100 mg/kg/bw) for the reminder of their lives | SRT1720 significantly increased motor coordination and balance Significant reduction in cataract formation at 105 weeks-of-age |
List of abbreviations: AL, ad libidum; CR, calorie restriction; DQ, combination of dasatinib and quercetin; F, females; FI, frailty index; HFD, high fat diet; M, males; NAM, nicotinamide; NR, nicotinamide riboside; NMN, nicotinamide mononucleotide; RSV, resveratrol; SD, standard diet. NA, not available.
2. FRAILTY IN MOUSE AGING INTERVENTION STUDIES
A). Metformin
Studies in humans have shown that diabetic patients are more likely to have poor muscle health and to develop neurodegeneration as well as loss of executive function [13]. These impediments beg the question whether metformin, a widely prescribed oral anti-diabetic drug, might be useful in improving such outcomes in people with type 2 diabetes and improve aging in general. The recent plans for establishing the clinical trial known as ‘Targeting Aging with Metformin’ (TAME; American Federation for Aging Research initiative) reflects this interest [14]. The ability of metformin to improve lifespan and health in humans has led to the conduct of animal studies to determine dose effectiveness and safety, physiological/metabolic effects, and mechanisms of action of this biguanide [15] [16]. Martin-Montalvo and colleagues evaluated mouse healthspan and endpoints related to frailty by assessing the impact of long-term diet supplementation with metformin in C57BL/6 mice [15]. Middle-aged mice treated with metformin performed significantly better on rotarod and treadmill tests and exhibited significantly greater open field test locomotor activity than the control group [17]. Overall health was improved in response to metformin treatment as evidenced by an increase in insulin sensitivity and mitochondrial function, and reduction in chronic inflammation and cataract progression rate [15]. In a second study, 12-month-old C57BL/6 mice on HFD supplemented with 1% metformin performed significantly better on the rotarod test compared to mice on HFD alone [16]. Moreover, chronic, intermittent treatment with 1% metformin significantly improved the average hanging test performance in mice while removal of the drug from the diet led to a weakened functional ability vis-à-vis elevated cage top or accelerating rotarod tests [18]. However, other studies did not substantiate these findings and, instead, suggested that metformin may exert harmful effects by reducing visual acuity of aged male mice upon addition of metformin in drinking water [19]. Other studies in mice fed a HFD also reveal adverse effects of co-treatment with metformin – exacerbation of damage caused to sciatic nerve fibers [20] and incidence of porcelain gallbladder [21]. The exact conditions under which beneficial effects of metformin far exceed the risks is currently an area of scientific investigation. In particular, the doses of metformin used in these animal studies generally far exceed the recommended daily metformin therapy in patients with type 2 diabetes, putting into question the translatability of these findings. Hence, future studies should include more frailty-related outcomes in response to clinically-relevant doses of metformin and dosing schedules.
B). Rapamycin
Rapamycin is currently being used as an immunosuppressant drug during organ transplant. It inhibits the cellular mTOR complex, which acts as a master regulator of protein synthesis and redox sensing. The beneficial effects of rapamycin in improving healthspan and lifespan in mice [22] [23] [24] come with some undesirable effects [25]. Yet, rapamycin has shown promise in several mouse longevity studies that have utilized frailty indices as endpoints. Intraperitoneal daily injection of rapamycin for 90 days improved parameters of muscle function in middle-aged (20–21 mo-old) C57BL/6Nia mice, accompanied by significantly better scores on both grip strength and rotarod tests than control mice [26]. Additionally, improvements in stride length and rotarod performance were found in C57BL/6Nia mice of both sexes with rapamycin supplementation in the same study. The increased frequency of exercise in the treated group suggests that rapamycin may provide greater endurance and resistance to muscle fatigue. A significant reduction of the kyphosis index was observed when 24-month-old C57BL/6J mice were treated with an oral formulation of microencapsulated rapamycin for a 3-month period [27]. However, these findings are not universal as rapamycin treatment has been found to elicit deleterious effects on some frailty-related outcomes. Rapamycin dose-dependently promoted higher occurrence of cataracts and sex-cell/testicular degeneration in male mice after a 9-month treatment [23]. In the same study, however, the authors observed that the age-associated decline in spontaneous in-cage activity was mitigated by rapamycin in a sex-dependent fashion, with male mice being affected at a lower dose of the drug than females.
Frailty in mice has also been utilized to better gauge biological age. A physiological frailty index was developed to estimate the biological age of a cohort of mice following treatment with rapamycin as an anti-aging drug. The micellar nanoformulation of rapamycin, rapatar, has been found to significantly improve this frailty index only in HFD-fed male mice [28]. In the HFD male mice, the frailty index score reduced from ~0.3 to ~0.25 with rapatar treatment. In HFD females, the baseline frailty score was higher and rapatar treatment did not result in a change in score. Similar “sex-specific” effects of rapamycin have been previously reported when chronic rapamycin treatment was initiated in 4-mo-old male and female C57BL/6J mice for the duration of their life [29]. In this study, no significant differences in rotarod performance among females were reported while males on rapamycin performed poorly. Moreover, the rapamycin-treated females displayed higher grip strength at all ages with greater spontaneous activity in the older females compared to age-matched controls. No such benefits were noted in males on rapamycin. Regardless of sex, rapamycin did not alter cochlear neurons, outer hair cells, or inner hair cells, and no difference in stride length was observed.
To gain further insights into the cellular mechanisms underlying the physiological manifestations of age-associated frailty, one should consider assessing the effects of molecules such as rapamycin in longevity studies aimed at preventing or delaying the development of frailty. Rapamycin significantly improves neuromuscular coordination without reduction in inflammaging in a mouse model with genetically enhanced NF-κB activity [30]. NF-κB is a redox-sensitive transcription factor that increases expression of pro-inflammatory factors implicated in age-dependent muscle loss, a strong risk factor for frailty [31]. Whether such observations stem from crosstalk between mTOR and signaling pathways implicated in inflammation is currently not known. Yet, we surmise that the molecular mechanisms regulating frailty can be elucidated through the use of small molecules such as rapamycin. In addition to mTOR and NF-κB, there are other age-associated signaling pathway targets, such as Nrf2 and AMPK, that can be studied using pharmacologic and nonpharmacologic strategies.
Overall, results from these animal studies show the ability of rapamycin to promote lifespan and healthspan extension by mitigating frailty-related end points. Despite these advances, more studies are required to better understand the mechanisms of action of molecules like rapamycin and the sex-specific effects that this compound has on frailty.
C). Resveratrol
Resveratrol (RSV) is a naturally-occurring compound with antioxidative properties, found in plants, particularly in grapes. In humans, several studies have suggested its beneficial effects on lifespan as well as on frailty [32]. The role of this small biomolecule in extending lifespan has also been reported in lower organisms such as Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster [33]. A number of studies have also been conducted with RSV in rodents, with promising results. One-year-old male C57BL/6Nia mice fed daily a high-calorie diet supplemented with RSV exhibited longer lifespan and performed equally well as their standard diet (SD)-fed littermates on rotarod test [34]. In this study, RSV treatment of 21–24-month-old mice on SD displayed better balance and motor coordination than their age-matched controls [35]. A delayed onset of age-related cataracts was also reported in RSV-treated mice at 30 months of age compared to controls [35]. Several frailty parameters were assessed in 18-month old C57BL/6J mice under caloric restriction and after a 6-month diet supplementation with RSV [6]. Significant improvement in overall frailty index scores (~0.2 in control compared to ~0.18 in RSV) was observed in C57BL/6J mice treated with RSV compared to their SD controls. Whether and how small molecules such as RSV can be successfully combined with other interventions that have shown promising results in aging studies, such as dietary restriction, metformin, rapamycin, and others (see below) is an area that warrants further research.
The impact of RSV treatment on physical performance was also investigated in aged male ICR mice. A 21-day RSV treatment improved time to exhaustion in a swimming exercise performance test, and it also dose-dependently increased grip strength compared to sedentary control mice [36]. In this study, RSV improved exercise-induced fatigue together with a reduction in blood lactate, ammonia, and glucose through molecular mechanisms that are not well understood. In another study, 16-month old C57BL/6J mice that were on a diet supplemented with 25 mg/kg RSV for 4 weeks showed enhanced performance in forelimb grip strength and endurance swimming test when combined with exercise, compared to the no RSV control groups [37]. Future studies should aim at determining a selective dose-response effectiveness of RSV in order to provide beneficial physiological effects during physical activity. Although the translational impact of longevity studies in lower organisms is tantalizing, it is important to implement new studies in animal models that recapitulate human aging and associated frailty outcomes.
D). Nicotinamide adenine dinucleotide (NAD) precursors
NAD primarily acts as a co-enzyme in redox reactions and functions as a mediator in glucose and lipid metabolism and mitochondrial function, interacting especially with sirtuins, poly(ADP-ribose) polymerases (PARPs), and the cyclic ADP-ribose synthases (CD38, CD157) [38] [39]. The decline in NAD levels during aging is triggered by decreased synthesis and/or increased NAD+ consumption [40], and the supplementation with nicotinamide (NAM), a NAD+ precursor, has been shown to improve healthspan in adult mice, although lifespan was not affected [41]. Given the involvement of NAD in aging and health, pharmacological approaches that rely on small molecules that alter NAD and NADH levels have been recently tested in aging intervention studies with promising but variable results. Within these studies, there is some evidence to suggest that NAD might be involved in altering frailty measures in mice. For example, hearing loss is associated with decreased levels of NAD [42] and administration of nicotinamide riboside, a NAD+ precursor, prevented noise-induced hearing loss by reducing neurite degeneration through SIRT3 pathway [43]. Age-related sarcopenia, characterized by the loss of muscle mass and function, is associated with decreased physical performance. Muscle-specific deletion of the gene encoding nicotinamide phosphoribosyltransferase (Nampt), a major enzyme involved in NAD+ synthesis and metabolism, causes age-associated muscle decline, which can be improved by NR supplementation [44]. Furthermore, an age-dependent drop in NAD+ levels has been associated with vision loss. Supplementation with nicotinamide mononucleotide (NMN), a second NAD precursor, restored vision in wild type and Nampt knockout mice [45]. Collectively, intervention by small molecules that alter NAD levels represents an active area of research and future studies should specifically explore how frailty indices are affected by them.
E). Synthetic sirtuin activators
Sirtuins are NAD+-dependent protein deacetylases that have recently garnered great interest in the aging field. Interestingly, serum sirtuin levels have been recently proposed as protein markers of age-related frailty in humans [46]. Mice overexpressing SIRT1 have an increased healthspan, as evidenced by improved glucose metabolism and lower senescence but without lifespan extension [47]. Long-term diet supplementation with the small molecule SIRT1 activator SRT2104 in male C57BL/6J mice leads to higher endurance on the treadmill and motor performance on the rotarod without impacting spontaneous/voluntary activity [48]. These phenotypic changes coupled with molecular changes suggest the suppression of inflammatory pathways and preservation of muscle and bone mass. Interestingly, a number of SIRT1 activators have been tested in clinical trials (e.g., NCT00937326, NCT00933062, NCT01018017) against a range of diseases, including type 2 diabetes, and are now completed, highlighting the potential clinical significance of this class of compounds. A second small allosteric activator for SIRT1, known as SRT1720, significantly increased motor coordination and balance in male C57BL/6J mice on standard diet at both 13 and 18 months of age, with a positive trend at 24 months [49]. In the same study, a significant reduction in cataract formation was also observed in SRT1720-treated mice at 105 weeks of age [49]. The effects of sirtuins within the context of other indices of frailty have also been reported. For instance, SIRT1-deficient mice exhibit impaired ocular morphogenesis and retinal development [50], and upregulation of SIRT1 confers protection against various ocular diseases such as age-related cataracts [51]. Yet, the beneficial effects of these sirtuin activators might be dependent on factors such as age and exposure to external stressors.
F). Senolytics
In normal aging and age-related diseases, cellular senescence imposes irreversible growth arrest on cells while triggering a biologically-specific phenotype marked by enhanced p16INK4a expression, higher senescence-associated beta galactosidase expression as well as a senescence-associated secretory phenotype (SASP). The ensuing secretion of cytokines, chemokines, and proteases contributes to create an inflammatory milieu associated with tissue aging and age-related diseases such as frailty [52]. Genetic manipulation that targets senescent cells improves age-related phenotypes and frailty [53] while the combination of two senolytic drugs, dasatinib and quercetin, was found to improve the physical capacity and total work performance of mice previously irradiated to induce senescence [54]. The same senolytic combination was recently reported to slow physical dysfunction with regard to walking speed, hanging endurance, grip strength, treadmill endurance, and daily activity caused by the transplantation of senescent cells in young and old mice [55]. In another study, AP20187 and a combination of dasatinib and quercetin were shown to improve running time in bleomycin-treated mice that exhibited lung fibrosis through alleviation of senescence [56].
The JAK/STAT pathway is involved in cytokine production and signaling and contributes to the transcription of genes implicated in SASP and age-related tissue dysfunction. Administration of a JAK1/2 inhibitor effectively improved physical activity, rearing, ambulation counts, hanging endurance, and grip strength in 24-months-old mice [57]. However, the molecular pathways that contribute to senescence are not well understood and represents an area of active research. A recent screening assay for identification of potential senotherapeutic drugs found two inhibitors of the molecular chaperone HSP90 as having senolytic activity in vitro and in vivo. Oral administration of the HSP90 inhibitor, 17-DMAG, which is also a water-soluble analog of Geldanamycin, extended healthspan in a mouse model of a human progeroid syndrome when started at 6 weeks of age, with improvement in kyphosis, dystonia, tremor, forelimb grip strength, coat condition, ataxia, and gait compared to age-matched controls [58]. Given that many of the senolytic molecules that have been identified thus far may have pleiotropic mechanisms of action, they could potentially be utilized to address a plethora of complexities associated with aging-related phenotypes, including frailty. However, well-designed and well-executed pre-clinical studies, in mice and in other model organisms, are imperative for promising senolytics to be advanced to the clinic.
G). Other interventions
Although the aforementioned lifespan/healthspan extension interventions have specifically looked at frailty indices, there are also other pharmacological agents that may offer promising effects against frailty. To this end, some drugs that have been in clinical use for several decades have been specifically evaluated for their effects on frailty. Lifelong treatment with the angiotensin-converting enzyme (ACE) inhibitor ramipril extends longevity in hypertensive rats, matching that of the normotensive control group [59]. In another study, chronic treatment with enalapril, another ACE inhibitor, for 4 months delayed the onset of age-related frailty in middle-aged and old female C57BL/6J mice while conferring benefits only in the oldest group (25 mo.) of males [10]. For example, enalapril lowered the fraily index from ~0.2 to ~0.15 in middle aged female mice, but not in middle aged males. Lower overall frailty scores were reported in middle-aged females after 3–4 months of treatment with enalapril compared to age-matched controls, an effect not observed in male mice [10]. A reduction in vision loss was also apparent in 21-month-old females on enalapril. Administration of the antihypertensive drug, losartan, for 4 months was associated with an increase in locomotor activity in 18-month-old C57BL/6 mice as measured by treadmill time, standing and travelling activity, but without improvement in grip strength [60]. The antidiabetic drug, acarbose, has well validated anti-aging effects in mice [61], and it has been determined that acarbose treatment significantly attenuates psoriasis-like inflammation in BALB/c mice [62]. It appears, however, that rapamycin, but not acarbose treatment, attenuates outer hair cell loss in 9 −10-month-old UM-HET3 mice of both sexes [63].
The National Institute on Aging Intervention Testing Program (NIA ITP) reported that 17-α estradiol increases median lifespan in male but not female HET3 mice by 12% [64]. However, specific frailty parameters were not evaluated in the study. Another estrogen, 17-β estradiol, promotes the regrowth of normally pigmented hair shafts after chemotherapy-induced alopecia [65] while conferring better visual acuity in rats with high intraocular pressure [66]. However, the use of estrogens for the improvement of age-related diseases has been widely debated, especially with regard to 17-β estradiol due to its carcinogenicity [67]. Small molecules of natural origin have also been studied within the context of frailty in animal models. For example, Icariin, a natural flavonoid, increased neuromuscular coordination and is accompanied by improvement in rotarod performance [68]. The potential of natural compounds to promote overall health benefits highlights the need to conduct further studies on how plant extracts can be formulated for medical and aging research [69].
3. CONCLUSIONS
Utilizing small molecules to delay the aging process and improve aging-related outcomes is currently a flourishing area of research. Evaluation of the recent literature suggests that there is immense value in incorporating the mouse frailty index in these research efforts. Frailty measurements can potentially be utilized as reliable markers of aging. When frailty-related endpoints are coupled with data on survival, metabolism, and molecular signatures, they can be collectively used as predictors of lifespan and overall health. Several different mouse frailty indices that focus on different endpoints have been proposed [70] [71]. Establishing detailed and consistent protocols on animal frailty assessment that are uniform across institutions and personnel, is a key factor that needs to be addressed in frailty research. Furthermore, implementing a more specific frailty index tailored to different mouse sexes and strains will allow for better accuracy. We recommend greater use of the frailty index in mouse studies that test novel small molecules and novel combinations in aging interventions. It is imperative that the mechanisms of actions of these compounds and druggable molecular targets be identified, where applicable. Enhancement of wellbeing and quality of life can have a profound impact on health objectives of societies. In order to successfully tackle the challenges that prevent us from achieving this, well-designed and thoughtfully executed animal research studies that encompass indices of human aging, such as frailty, are required.
HIGHLIGHTS.
Frailty is increased during human aging and, therefore, has been evaluated as a target for anti-aging interventions
Aspects of the mouse frailty index have been utilized to quantify the efficacy of anti-aging interventions in vivo
While studies investigating metformin, rapamycin, resveratrol, NAD+ precursors, sirtuin activators, and senolytics have incorporated some indices of the mouse frailty index, opportunities to expand its use exist
Funding sources:
Intramural Research Program, NIA/NIH
ABBREVIATIONS
- ACE
angiotensin converting enzyme
- AL
ad libidum
- AMPK
5’-AMP activated protein kinase
- CR
calorie restriction
- DQ
combination of dasatinib and quercetin
- FI
frailty index
- HFD
high fat diet
- NAD
nicotinamide adenine dinucleotide
- NAM
nicotinamide
- NR
nicotinamide riboside
- NMN
nicotinamide mononucleotide
- RSV
resveratrol
- SD
standard diet
- NA
not available
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kane AE, et al. , Implementation of the mouse frailty index. Can J Physiol Pharmacol, 2017. 95(10): p. 1149–1155. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch C, et al. , The association of race with frailty: the cardiovascular health study. Ann Epidemiol, 2006. 16(7): p. 545–53. [DOI] [PubMed] [Google Scholar]
- 3.Gordon EH, et al. , Sex differences in frailty: A systematic review and meta-analysis. Exp Gerontol, 2017. 89: p. 30–40. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, et al. , Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci, 2001. 56(3): p. M146–56. [DOI] [PubMed] [Google Scholar]
- 5.Strawbridge WJ, et al. , Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci, 1998. 53(1): p. S9–16. [DOI] [PubMed] [Google Scholar]
- 6.Kane AE, et al. , Impact of Longevity Interventions on a Validated Mouse Clinical Frailty Index. J Gerontol A Biol Sci Med Sci, 2016. 71(3): p. 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Zglinicki T, et al. , Frailty in mouse ageing: A conceptual approach. Mech Ageing Dev, 2016. 160: p. 34–40. [DOI] [PubMed] [Google Scholar]
- 8.de Cabo R, et al. , The search for antiaging interventions: from elixirs to fasting regimens. Cell, 2014. 157(7): p. 1515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parks RJ, et al. , A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci, 2012. 67(3): p. 217–27. [DOI] [PubMed] [Google Scholar]
- 10.Keller K, et al. , Chronic treatment with the ACE inhibitor enalapril attenuates the development of frailty and differentially modifies pro-and anti-inflammatory cytokines in aging male and female C57BL/6 mice. J Gerontol A Biol Sci Med Sci, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingram DK, et al. , Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci, 2004. 1019: p. 412–23. [DOI] [PubMed] [Google Scholar]
- 12.Arum O, et al. , Prevention of neuromusculoskeletal frailty in slow-aging ames dwarf mice: longitudinal investigation of interaction of longevity genes and caloric restriction. PLoS One, 2013. 8(10): p. e72255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanase T, et al. , Frailty in elderly diabetes patients. Endocr J, 2018. 65(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 14.Barzilai N, et al. , Metformin as a Tool to Target Aging. Cell Metab, 2016. 23(6): p. 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anisimov VN, et al. , If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY), 2011. 3(2): p. 148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allard JS, et al. , Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res, 2016. 301: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Montalvo A, et al. , Metformin improves healthspan and lifespan in mice. Nat Commun, 2013. 4: p. 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfaras I, et al. , Health benefits of late-onset metformin treatment every other week in mice. NPJ Aging Mech Dis, 2017. 3: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thangthaeng N, et al. , Metformin Impairs Spatial Memory and Visual Acuity in Old Male Mice. Aging Dis, 2017. 8(1): p. 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciric D, et al. , Metformin exacerbates and simvastatin attenuates myelin damage in high fat diet-fed C57BL/6 J mice. Neuropathology, 2018. 38(5): p. 468–474. [DOI] [PubMed] [Google Scholar]
- 21.Dorvash MR, et al. , Metformin treatment prevents gallstone formation but mimics porcelain gallbladder in C57Bl/6 mice. Eur J Pharmacol, 2018. 833: p. 165–172. [DOI] [PubMed] [Google Scholar]
- 22.Harrison DE, et al. , Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 2009. 460(7253): p. 392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson JE, et al. , Rapamycin slows aging in mice. Aging Cell, 2012. 11(4): p. 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. , Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci, 2014. 69(2): p. 119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmon AB, About-face on the metabolic side effects of rapamycin. Oncotarget, 2015. 6(5): p. 2585–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitto A, et al. , Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flynn JM, et al. , Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell, 2013. 12(5): p. 851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoch MP, et al. , Physiological frailty index (PFI): quantitative in-life estimate of individual biological age in mice. Aging (Albany NY), 2017. 9(3): p. 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer KE, et al. , Health Effects of Long-Term Rapamycin Treatment: The Impact on Mouse Health of Enteric Rapamycin Treatment from Four Months of Age throughout Life. PLoS One, 2015. 10(5): p. e0126644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correia-Melo C, et al. , Rapamycin improves healthspan but not inflammaging in nfkappab1(−/−) mice. Aging Cell, 2018: p. e12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakuma K, Aoi W, and Yamaguchi A, Current understanding of sarcopenia: possible candidates modulating muscle mass. Pflugers Arch, 2015. 467(2): p. 213–29. [DOI] [PubMed] [Google Scholar]
- 32.Rabassa M, et al. , Association of habitual dietary resveratrol exposure with the development of frailty in older age: the Invecchiare in Chianti study. Am J Clin Nutr, 2015. 102(6): p. 1534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bass TM, et al. , Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev, 2007. 128(10): p. 546–52. [DOI] [PubMed] [Google Scholar]
- 34.Baur JA, et al. , Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 2006. 444(7117): p. 337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson KJ, et al. , Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab, 2008. 8(2): p. 157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu RE, et al. , Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules, 2013. 18(4): p. 4689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kan NW, et al. , Effects of Resveratrol Supplementation and Exercise Training on Exercise Performance in Middle-Aged Mice. Molecules, 2016. 21(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canto C, Menzies KJ, and Auwerx J, NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab, 2015. 22(1): p. 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdin E, NAD(+) in aging, metabolism, and neurodegeneration. Science, 2015. 350(6265): p. 1208–13. [DOI] [PubMed] [Google Scholar]
- 40.Yaku K, Okabe K, and Nakagawa T, NAD metabolism: Implications in aging and longevity. Ageing Res Rev, 2018. 47: p. 1–17. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell SJ, et al. , Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab, 2018. 27(3): p. 667–676 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HJ, et al. , NAD(+) Metabolism in Age-Related Hearing Loss. Aging Dis, 2014. 5(2): p. 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown KD, et al. , Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab, 2014. 20(6): p. 1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frederick DW, et al. , Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab, 2016. 24(2): p. 269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin JB, et al. , NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell Rep, 2016. 17(1): p. 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar R, et al. , Identification of serum sirtuins as novel noninvasive protein markers for frailty. Aging Cell, 2014. 13(6): p. 975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herranz D, et al. , Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun, 2010. 1: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercken EM, et al. , SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell, 2014. 13(5): p. 787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell SJ, et al. , The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep, 2014. 6(5): p. 836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng HL, et al. , Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A, 2003. 100(19): p. 10794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mimura T, et al. , The role of SIRT1 in ocular aging. Exp Eye Res, 2013. 116: p. 17–26. [DOI] [PubMed] [Google Scholar]
- 52.Tchkonia T, et al. , Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest, 2013. 123(3): p. 966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker DJ, et al. , Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature, 2011. 479(7372): p. 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Y, et al. , The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell, 2015. 14(4): p. 644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu M, et al. , Senolytics improve physical function and increase lifespan in old age. Nat Med, 2018. 24(8): p. 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schafer MJ, et al. , Cellular senescence mediates fibrotic pulmonary disease. Nat Commun, 2017. 8: p. 14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu M, et al. , JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A, 2015. 112(46): p. E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuhrmann-Stroissnigg H, et al. , Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun, 2017. 8(1): p. 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linz W, et al. , Long-term ACE inhibition doubles lifespan of hypertensive rats. Circulation, 1997. 96(9): p. 3164–72. [DOI] [PubMed] [Google Scholar]
- 60.Lin CH, et al. , Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp Gerontol, 2014. 58: p. 174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strong R, et al. , Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell, 2016. 15(5): p. 872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen HH, et al. , Oral administration of acarbose ameliorates imiquimod-induced psoriasis-like dermatitis in a mouse model. Int Immunopharmacol, 2016. 33: p. 70–82. [DOI] [PubMed] [Google Scholar]
- 63.Altschuler RA, et al. , Rapamycin but not acarbose decreases age-related loss of outer hair cells in the mouse Cochlea. Hear Res, 2018. 370: p. 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrison DE, et al. , Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell, 2014. 13(2): p. 273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohnemus U, et al. , Topical estrogen accelerates hair regrowth in mice after chemotherapy-induced alopecia by favoring the dystrophic catagen response pathway to damage. J Invest Dermatol, 2004. 122(1): p. 7–13. [DOI] [PubMed] [Google Scholar]
- 66.Prokai-Tatrai K, et al. , 17beta-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol Pharm, 2013. 10(8): p. 3253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almeida M, et al. , Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol Rev, 2017. 97(1): p. 135–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang SQ, et al. , Icariin, a natural flavonol glycoside, extends healthspan in mice. Exp Gerontol, 2015. 69: p. 226–35. [DOI] [PubMed] [Google Scholar]
- 69.Palliyaguru DL, Singh SV, and Kensler TW, Withania somnifera: From prevention to treatment of cancer. Mol Nutr Food Res, 2016. 60(6): p. 1342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H, et al. , Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci, 2014. 69(12): p. 1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Graber TG, et al. , Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci, 2015. 70(9): p. 1045–58. [DOI] [PMC free article] [PubMed] [Google Scholar]