Abstract
Despite efforts to develop new antibiotics, antibacterial resistance still develops too fast for drug discovery to keep pace. Often, resistance against a new drug develops even before it reaches the market. This continued resistance crisis has demonstrated that resistance to antibiotics with single protein targets develops too rapidly to be sustainable. Most successful long-established antibiotics target more than one molecule or possess targets, which are encoded by multiple genes. This realization has motivated a change in antibiotic development toward drug candidates with multiple targets. Some mechanisms of action presuppose multiple targets or at least multiple effects, such as targeting the cytoplasmic membrane or the carrier molecule bactoprenol phosphate and are therefore particularly promising. Moreover, combination therapy approaches are being developed to break antibiotic resistance or to sensitize bacteria to antibiotic action. In this Review, we provide an overview of antibacterial multitarget approaches and the mechanisms behind them.
Keywords: polypharmacology, multifunctional antibiotic, multiresistant bacteria, antibiotic combination therapy, synergy
Antimicrobial resistance has developed into a global healthcare crisis that has culminated in the emergence of multidrug-resistant bacteria that are no longer treatable with any common antibiotic.1 For example, recently emerging Neisseria gonorrhoeae strains resistant to third-generation carbapenems led to a number of untreatable gonorrhea infections in the UK.2 Despite considerable investments, the development of innovative, resistance-breaking antibiotics still progresses too slowly.3,4 For a long time, antibiotics with one specific protein target were highly sought after in drug design and screening efforts. Single target drugs were propagated as the ideal antibiotics with the argument that a high target specificity equals less side effects. This resulted in a predominantly genomic-driven approach to antibiotic discovery, where single protein targets were evaluated, while compounds with multiple or complex targets were regarded to be unspecific and unsuitable for further development. However, the last decades have shown that resistance to antibiotics with specific single protein targets develops too fast to be sustainable, and the realization emerged that candidates with multiple targets are worth pursuing for their slower resistance development rates.5−7 In fact, long-established antibiotic drugs with great clinical success rarely target only one specific molecule. For example, β-lactam antibiotics typically target more than one transpeptidase, and quinolones inhibit both topoisomerases II and IV.8,9 Antibiotics that truly have one single protein target, such as sulfonamides targeting an enzyme involved in folate synthesis or rifampicin targeting RNA polymerase, are famous for high resistance development and are therefore usually administered in combination regimes.10,11
Naturally occurring antibiotics often do not have strict single targets either. The most common class of natural antimicrobial substances are antimicrobial peptides, which occur in virtually all organisms. These molecules predominantly target complex bacterial structures, e.g., the cell membrane, the cell wall, or both.12,13 Moreover, antibiotic producers often produce a mix of structurally related compounds. This is, for example, the case for gramicidin (contain gramicidin A–C), tyrothricin (contains gramicidin A–C and tyrocidine A–D), and polymyxins (contains multiple peptides with small structural variations depending on the polymyxin type).14,15 Such small structural variations can result in slightly different target interactions16−20 and may complicate resistance development by target alterations and antibiotic-cleaving or scavenging molecules. Naturally occurring mixes have been used in clinical settings with great success and in some cases are combined with each other or other antibiotics like neomycin.21,22 It is quite common in clinical practice to use antibiotic combinations for different reasons. For example, trimethoprim is combined with sulfonamides to prevent quick resistance development by a single target mutation, and erythromycin is often given together with penicillin for their synergistic effect.23 Since more and more pathogens become resistant to individual antibiotics, it has become pivotal to thoroughly explore multitarget approaches to combat resistant bacteria. In this Review, we provide an overview of multitarget antibiotics and combination approaches that are in current clinical use or have a chance to be applied in clinical settings in the future.
Part 1: Multitarget Compounds
Generally, the ability of a single drug to interact with more than one specific target is called polypharmacology.24 When talking about multitarget antibiotics, we distinguish between different levels of multitargeting. In this Review, we use the terminology intrinsically multi-effective, multitarget, and multifunctional. Intrinsically multi-effective compounds may have a single target, but this molecule or structure is involved in multiple processes, which are all affected by its inhibition. Multitarget antibiotics can be divided into multitarget compounds that target more than one isoenzyme or closely related proteins of the same pathway and multitarget compounds that bind to different molecules involved in separate cellular processes. Multifunctional compounds have a direct antibiotic target but an additional indirect antibacterial activity, e.g., immunomodulatory properties. Table 1 gives an overview of prominent antibiotics of these categories that are used in the clinic.
Table 1. Examples for Clinically Used Antibiotics with Multiple Mechanisms of Action.
| antibiotic | targets | mechanism of action | ref |
|---|---|---|---|
| Intrinsically Multi-effective | |||
| daptomycin | phosphatidylglycerol, fluid lipid domains | binds to phosphatidylglycerol, inserts into fluid lipid domains that harbor the cell wall synthesis machinery, immediately inhibits cell wall and membrane synthesis; prolonged treatment results in partial membrane depolarization and impairs several other membrane-bound processes | (25, 26) |
| gramicidin S | membrane | induces membrane phase separation causing inhibition of cell envelope synthesis and cell division | (20) |
| vancomycin | lipid II | binds to lipid II, thereby inhibits peptidoglycan synthesis and depletes the pool of bactoprenol phosphate, additionally resulting in the inhibition of wall teichoic acid synthesis | (27, 28) |
| bacitracin | bactoprenol pyrophosphate | depletes the pool of bactoprenol phosphate resulting in inhibition of peptidoglycan and wall teichoic acid synthesis | (29) |
| nitrofurantoin | cellular macromolecules | generates reactive oxygen species, which damage cellular macromolecules including DNA and membrane lipids | (30, 31) |
| acyldepsipeptides | Clp protease | deregulates the Clp protease resulting in unspecific degradation of a variety of proteins | (32, 33) |
| bedaquiline | ATP synthase | inhibits ATP synthase, depleting the ATP pool and resulting in the inhibition of all energy-consuming cellular processes | (34) |
| Multitarget | |||
| penicillin | penicillin-binding proteins | inhibits multiple penicillin-binding proteins | (8) |
| ciprofloxacin | topoisomerase II and IV | inhibits topoisomerase II and IV | (9) |
| tetracycline | ribosome and membrane | blocks attachment of loaded aminoacyl tRNA to the A-site of the ribosome; also impairs membrane function | (31, 35) |
| polymyxin B | outer and inner membrane | permeabilizes both the outer and inner membrane of Gram-negative bacteria | (36) |
| tyrocidine | membrane and probably DNA | forms defined ion-conducting membrane pores; probably additionally binds to DNA | (20, 37, 38) |
| Multifunctional | |||
| clindamycin | 50S rRNA | anti-inflammatory | (39, 40) |
| clofazimine | guanine | anti-inflammatory | (41−43) |
| dapsone | dihydropteroate synthase | anti-inflammatory and immunomodulatory | (44, 45) |
| macrolides | 50S rRNA | anti-inflammatory and immunomodulatory | (46−48) |
| metronidazole | DNA | anti-inflammatory | (49, 50) |
| rifampicin | DNA-dependent RNA polymerase | anti-inflammatory and immunomodulatory | (51, 52) |
| tetracycline | ribosome and membrane | anti-inflammatory | (53−55) |
Intrinsically Multi-effective
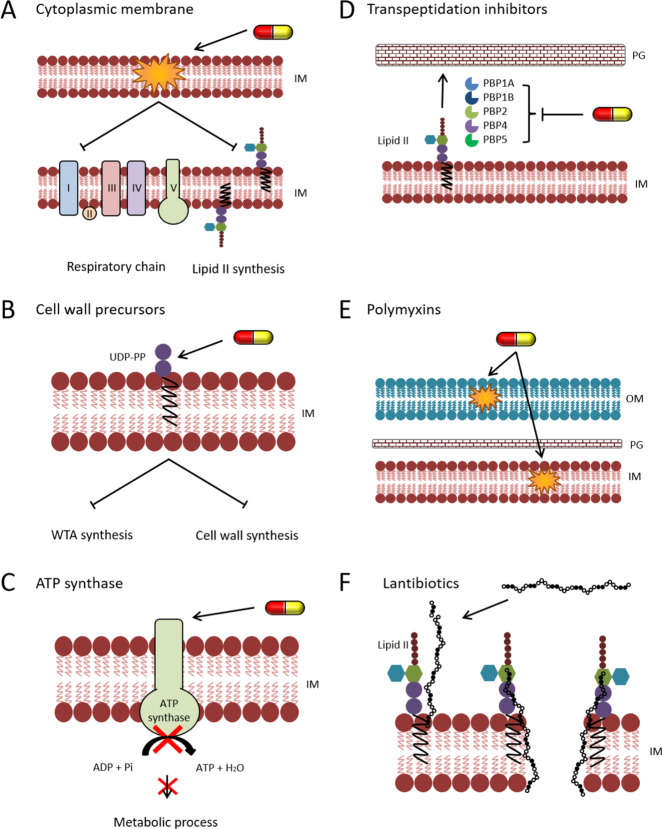
The most common antimicrobial target, whose inhibition results in a multitude of cellular effects, is the cytoplasmic membrane. It is the target of many natural antibiotics, most prominently host defense peptides.12 The cytoplasmic membrane harbors essential cellular processes, such as the respiratory chain and cell wall synthesis (Figure 1A), and many more important components, including nutrient uptake, secretion, and stress response systems. Thus, membrane-targeting antibiotics have the potential to (i) inhibit processes essential for survival, (ii) reduce bacterial fitness, (iii) impair virulence, and (iv) interfere with stress adaptation. Depending on how an antibiotic interferes with the membrane, few or a whole array of membrane-bound processes can be affected.20 Several membrane-active antibiotics are used in the clinic, although only a handful are applied systemically. For some of them, their multiple effects on bacterial cells have been studied.
Figure 1.
Examples for multiple mechanisms of action of antibiotics. (A–C) Antibiotics with intrinsically multi-effective properties. (A) Disrupting cytoplasmic membrane integrity, e.g., by gramicidin S, affects membrane-bound processes, most prominently respiration and lipid II synthesis. (B) Binding of antibiotics to lipid II or its carrier molecule undecaprenyl(pyro)phosphate (here: bacitracin binding UDP-PP) depletes the carrier pool, affecting both the synthesis of wall teichoic acids (WTA) and lipid II. (C) Inhibition of ATP synthase, e.g., by bedaquiline, leads to the depletion of the cellular ATP pool and thus inhibition of multiple metabolic processes. (D–F) Antibiotics with multiple targets. (D) β-Lactams (here imipenem) typically inhibit more than one penicillin-binding protein (PBP). (E) Polymyxins like polymyxin B or colistin disrupt both the outer and inner membrane of Gram-negative bacteria. (F) Type A lantibiotics like nisin bind to lipid II and use it as a docking molecule to form a transmembrane pore.
Many membrane-active antibiotics dissipate the membrane potential. This depolarization has a number of downstream effects due to the depletion of ATP and the subsequent impairment of ATP-dependent processes. Moreover, it leads to the displacement of peripheral membrane proteins that bind to the membrane surface by electrostatic interactions, such as the cell division proteins FtsA and MinD.56,57 However, not all membrane effects can be explained by membrane depolarization. Thus, the membrane pore-forming peptides tyrocidine A and C not only dissipate the membrane potential and lead to leakage of ions and small molecules but also reduce membrane fluidity, diminish membrane domains, and affect the localization of several membrane-bound processes, including cell division, peptidoglycan synthesis, phospholipid synthesis, respiration, and ATP synthesis.20 Such effects are not observed when the membrane potential is dissipated by specific ionophores.56,57 The structurally similar peptide gramicidin S, which does not form distinct membrane pores and has milder effects on membrane potential and fluidity, only affects cell division and cell envelope synthesis proteins.20,58 Daptomycin, which is one of the few systemically applied membrane-targeting antibiotics, has recently been described to interfere with both peptidoglycan and phospholipid synthesis by interfering with fluid membrane microdomains that harbor the lateral cell wall synthesis machinery.25,31,59
Similar results have been obtained for host defense peptides and experimental compounds that are not yet in clinical application. For example, human β-defensin 3 forms a dimeric raft-like structure with two α-helices anchoring it to the hydrocarbon layer and binds to negatively charged lipid head goups.60 It has also been shown to bind the cell wall precursor lipid II with low affinity, probably due to electrostatic interactions with the pyrophosphate group.61 Thus, the defensin is attracted to sites of active cell division and cell wall synthesis, where its presence disturbs the interactions of the complex peptidoglycan synthetic machinery, which was coined the “sand in the gearbox” effect.61 The structurally similar human β-defensin 2 binds to distinct membrane foci at nascent cell division septa, where it disturbs the function of SecA and sortase A, which specifically localize to these sites and are involved in the secretion of virulence factors.62 Other membrane-active compounds have been observed to have broader effects. For example, the membrane-disruptive peptides TC19 and TC84, which are derived from the microbicidal blood platelet protein thrombocidin,63 lead to large-scale disruption of membrane organization and affect a multitude of cellular processes, including cell division, peptidoglycan synthesis, phospholipid synthesis, respiration, ATP synthesis, and spore outgrowth.64−66 Such a broad panel of effects raises the following questions: which ones are due to the multifaceted mechanisms of the compounds and which are merely a consequence of cell death? It is therefore pivotal to examine the mechanism of a compound after short treatment times with sublethal concentrations.4 The comparison of the effects observed under these conditions with those occurring at lethal concentrations or after prolonged treatment can give insight into which effects lead to cell death and which are a consequence thereof. In the case of the TC19 and TC84 peptides, membrane depolarization, rigidification, and phase separation were already observed at sublethal concentrations and increased at lethal concentrations, while other observations like protein delocalization and intracellular content leakage were mainly observed at lethal concentrations or after prolonged treatment.64,65 This suggests that the primary mechanism of these peptides is the disruption of membrane organization. Similarly, depolarization and limited ion leakage are observed with daptomycin at high concentrations and long treatment times. However, at inhibitory, and even lethal, concentrations these effects do not occur, suggesting that increased membrane permeability is a consequence of cell death and not the primary mechanism by which it occurs.67
However, broad effects do not necessarily need to be a mere consequence of membrane disruption. Similar effects have been achieved by other compounds with quite creative molecular mechanisms. For example, the cyclic hexapeptide cWFW induces large-scale phase separation sorting peripheral and integral membrane proteins into two distinct domains,68 and the plant-derived antibiotic candidate rhodomyrtone forms large membrane vesicles that irreversibly trap both peripheral and transmembrane proteins.69
Another molecular target that intrinsically presupposes multiple effects is the peptidoglycan precursor lipid II, which is the antibiotic target of the glycopeptide antibiotics vancomycin, telavancin, oritavancin, dalbavancin, and teicoplanin.70−73 Lipid II is also the target of a variety of lantibiotics, most prominently the food preservative nisin. While nisin and other type A lantibiotics, such as gallidermin and epidermin, additionally target the cell membrane,13,74,75 type B lantibiotics like mersacidin do not seem to have additional membrane effects.74,76 Though the application of lantibiotics for antimicrobial coatings, e.g., for implants, is being explored,77 none of them is currently used in a clinical setting. Lipid II is synthesized in the cytosol and attached to a lipid carrier molecule, undecaprenol phosphate, yielding the peptidoglycan building block lipid II. Lipid II flips over the membrane to the cell surface, and when the building block is incorporated into the cell wall, undecaprenyl phosphate flips back over the membrane to the cytosolic side, where it can undergo another cycle of peptidoglycan synthesis.78 However, undecaprenyl phosphate is also used in the biosynthesis of wall teichoic acids, an important component of the Gram-positive bacterial cell wall.78,79 The binding of antibiotics to lipid II not only directly prevents the incorporation of peptidoglycan building blocks into the cell wall but also depletes the undecaprenyl phosphate pool and thus concomitantly impairs the wall teichoic acid synthesis. Likewise, compounds that bind undecaprenyl phosphate (e.g., friulimicin B) or undecaprenol pyrophosphate (bacitracin) simultaneously inhibit both processes (Figure 1B). Moreover, cell wall synthesis is coupled to other cellular processes, most prominently cell division, and the binding of antibiotics to the sites of cell wall synthesis is likely to have additional “sand in the gearbox” effects.61 Due to their typically large size and/or hydrophobic or amphipathic properties, membrane and cell wall-targeting antibiotics rarely cross the outer membrane of Gram-negative bacteria and are mostly used against and studied in Gram-positives. However, the general principle of the intrinsically multiple effects of such compounds is the same in all organisms.
These effects have also been predicted for other multiprotein machineries like the respiratory chain. Indeed, the inhibition of respiration and/or ATP synthase and subsequent depletion of ATP pools have been observed for several compounds, whose primary target is the cell membrane.58,69,80−82 Membrane potential and respiration are closely intertwined, and the impairment of one can lead to the inhibition of the other.83,84 Either way, both will have downstream effects on the performance of ATP synthase and the availability of ATP for important cellular processes (Figure 1C). Currently, no respiratory chain inhibitor is available as an antibiotic on the market, but with bedaquiline, the first ATP synthase inhibitor was marketed for the treatment of tuberculosis in 2012.34 The impairment of the electron transport chain can also generate reactive oxygen species and thus cause oxidative stress.85 This leads to widespread oxidative damage to cellular macromolecules, including DNA, proteins, and the cell membrane.86 Oxidative damage is also a key mechanism of killing pathogenic bacteria by the immune system that is mediated by neutrophils.87 While the generation of reactive oxygen species may be a side effect of membrane-targeting antibiotics, there is one antibiotic in the clinic that makes targeted use of oxidative stress. Nitrofurantoin, which is used against urinary tract infections, is a pro-drug that is activated by cellular nitrofuran reductases resulting in reactive intermediates that damage cellular macromolecules, especially DNA.30,31
Another mechanism that should be mentioned in this category is the deregulation of the ClpP protease by acyldepsipeptide antibiotics. ClpP takes part in both the general degradation of misfolded proteins and the regulated proteolysis of a range of substrates, including transcription factors and regulatory proteins. Acyldepsipeptides deactivate the tight control mechanisms that normally ensure that only such proteins that are either defective or supposed to be degraded as part of a regulatory cascade are degraded by ClpP. This leads to the uncontrolled proteolysis of intact cytosolic proteins and among them the key cell division protein FtsZ.32,33,88
Translation inhibitors that target the bacterial ribosome always bind to rRNAs, which are encoded in multiple copies in the bacterial genome.5,89 While they only have one binding site, their resistance development rates are reduced, since simple point mutations are unlikely to affect all gene copies.5 Translation inhibitors are also intrinsically multi-effective antibiotics, since an impaired ability to produce proteins hampers multiple cellular processes including the ability to elicit an appropriate stress response and thus prevents stress adaptation. Next to β-lactams, ribosome inhibitors constitute the most successful antibiotic class in the clinic, underlining the potential of compounds with multiple targets.
Multiple Targets in the Same Pathway
Most of the antibiotics described above target one specific cellular structure, which itself is involved in different processes. However, there are also several antibiotics that bind to two or more distinct molecules. We will first discuss those compounds that target related proteins of the same pathway. The most extreme examples for this are the β-lactam antibiotics, which typically target more than one penicillin-binding protein (PBP) (Figure 1D).8 For example, Escherichia coli has 8 PBPs, 6 of which are inhibited by penicillin G.90−92 Other examples include quinolone antibiotics, which target both topoisomerase II and IV involved in DNA supercoiling and resolving DNA concatemers, respectively,9 and the cell wall synthesis inhibitors fosfomycin and d-cycloserine. Gram-positive bacteria possess two copies of MurA, the first enzyme in the lipid II synthesis pathway, and fosfomycin inhibits both isoenzymes.93d-Cycloserine competitively inhibits both alanine racemase and d-ala-d-ala ligase, which converts l-alanine to the d-alanine dipeptide that is part of the peptidoglycan interpeptide bridge.94 Similarly, platencin, a fatty acid synthesis inhibitor that attracted significant attention upon its discovery but then encountered several hurdles in preclinical development,95 is a dual inhibitor of the FabF and FabH enzymes.96,97
Multiple Targets in Different Pathways
Several antibiotics have been found that inhibit two or more distinct targets that are not closely related components of the same pathway or process. Not only is it more difficult to acquire target-based resistance in two different molecules, but also bacteria need to react with two different stress responses to mitigate antibiotic action, hampering their capabilities for efficient stress adaptation; thus, these antibiotics have an additional advantage.
There are several examples for such compounds in the clinic, and new molecules with multiple targets are regularly discovered. One example are the polymyxins, in particular polymyxin B and colistin, which are used as last resort antibiotics against multiresistant Gram-negative infections.98 These polypeptides target both the outer and the inner membrane of Gram-negative bacteria99,100 (Figure 1E). Another example is clofazimine, which is used to treat leprosy.101 This antibiotic binds to guanine bases in bacterial DNA but also increases phospholipase A1 activity, leading to toxic overproduction of lysophospholipids.102 The atypical tetracycline chelocardin, which has recently attracted renewed interest for clinical development,103,104 inhibits the bacterial ribosome and additionally depolarizes the cytoplasmic membrane.105 Tetracycline itself, while not leading to depolarization, has recently been shown to disturb membrane organization in addition to translation inhibition.31 The same was observed for anhydrotetracycline, suggesting that this dual activity could be a general activity of the tetracycline class.31 It would be reasonable to assume that membrane activity may be a general consequence of translation inhibition, since ribosomes associate with the cell membrane to couple translation to protein secretion.106 However, other ribosome inhibitors did not visibly disturb the cell membrane, and a ribosomal protein mutant that diminishes tetracycline binding to the ribosome showed the same tetracycline-induced membrane effects as the wild-type.31 These findings suggest that the membrane activity of tetracycline is independent from translation inhibition and thus a separate secondary target. Similarly, tyrocidines, which primarily disrupt cytoplasmic membrane integrity, have long ago been proposed to bind DNA as secondary target. This notion, which was derived from test tube interactions of the peptides with isolated DNA,38 was only recently supported by in vivo experiments.20
Another very prominent example are antibiotics with a dual mechanism on cell wall synthesis and the cell membrane. This is relatively common, since cell wall synthesis inhibitors often target membrane-bound steps of this pathway. Thus, both targets are in close proximity. The most prominent example for this is the lantibiotic nisin, which is not used clinically but is a common food preservative (Figure 1F). Nisin has a two-step mechanism of action. It first establishes contact with lipid II, inhibiting peptidoglycan synthesis. It then inserts into the lipid bilayer to form a transmembrane pore.107−109 More recently, it was found that nisin also binds to undecaprenyl phosphate, adding another structure to its list of molecular targets.110 While nisin is able to bind to negatively charged lipids in the absence of lipid II or undecaprenyl phosphate,111 it needs the lipid-coupled cell wall precursor as a docking molecule to form a transmembrane pore. Other lantibiotics like gallidermin can impair both targets independently.112 This property of lantibiotics also inspired the development of dual-function vancomycin derivatives. Telavancin and oritavancin carry a hydrocarbon tail, which allows them to insert into the cell membrane after binding lipid II. Thereby, they disrupt the permeability barrier, a feature that vancomycin is lacking and that restores activity against strains with high-level vancomycin resistance.7,71 Daptomycin could be included in this group as well, since it likewise inserts into the cell membrane and impairs cell wall synthesis.25 However, at least to our current knowledge, it only binds a single molecular target, namely, phosphatidylglycerol-containing lipids.113−116
Designer Dual-Target Compounds
Inspired by the clinical success of these multitarget antibiotics, approaches have been undertaken to develop dual-target molecules. These can either be stable hybrids combining two functions in one molecule or cleavable compounds, whereby the prodrug has a different target than the cleavage product.117,118 In the past, cleavable compounds have essentially behaved like simple prodrugs for the cleavage product rather than eliciting their intended dual effects. Hence, newer efforts rather focus on stable hybrids.118−120 These approaches have been recently reviewed in detail,117,121 but we want to give a brief overview of the molecules that are currently underway in clinical development (Table 2).
Table 2. Antibiotic Hybrid Molecules Currently under Clinical Development.
| compound | antibiotic 1 inhibited process 1 | antibiotic 2 inhibited process 2 | stage | ref |
|---|---|---|---|---|
| cadazolid | quinolone topoisomerases II and IV | oxazolidinone translation | phase III | (122) |
| cefilavancin (TD-1792) | vancomycin lipid II | cephalosporin PBPs | phase III | (123) |
| DNV3837 (MCB-3681)a | fluoroquinolone topoisomerases II and IV | oxazolidinone translation | phase II | (124) |
| TNP-2092 (CBR-2092) | rifamycin RNA polymerase | quinolone topoisomerases II and IV | phase II | (120) |
| TD-1607 | glycopeptide lipid II | cephalosporin PBPs | phase I | (125) |
MCB-3681 was developed into the prodrug MBB-3837, which was renamed DNV3837 after Morphochem was acquired by Deinove.
Cadazolid is a stable hybrid of a quinolone and oxazolidinone and currently in phase III clinical trials for the treatment of Clostridium difficile infections.122,126 It primarily inhibits translation but also topoisomerase activity and shows low resistance development rates.127 Cefilavancin is a stable hybrid molecule of vancomycin and a third-generation cephalosporin, which is currently undergoing phase III clinical trials for complicated skin and soft tissue infections.121 This hybrid compound is active against both methicillin and vancomycin-resistant Staphylococcus aureus, yet it has not been verified to which extent it inhibits lipid II and PBPs.128,129 MCB-3681 is a stable fluoroquinolone–oxazolidinone hybrid similar to cadazolid.124 It is active against strains that are resistant to both ciprofloxacin and linezolid.130 MCB-3681 was scheduled to undergo phase II clinical trials since 2015 and was granted fast track status by the FDA in 2016.124 Instead, it was further developed into a prodrug molecule, MCB-3837. In 2018, its developing company Morphochem was acquired by Deinove, and the compound, now under the name DNV3837, finally went into phase II clinical trials for treatment of C. difficile infections in 2019.131 The study is planned to be completed in the summer of 2020.132 TNP-2092 is a stable rifamycin–quinolone hybrid currently in phase II trials for prosthetic joint infections.120 It is active against a range of quinolone and rifamycin-resistant strains.120 TNP-2092 is also being evaluated against Helicobacter pylori infections yet at a preclinical stage.133 TD-1607 is a stable glycopeptide–cephalosporin hybrid similar to cefilavancin. Two phase I clinical trials have been completed, yet results remain to be published.121,125
A different hybrid approach was followed by the company Visterra, who developed an ultranarrow spectrum hybrid molecule against P. aeruginosa infections by coupling a specific antibody that targets cell surface glycan molecules with an antimicrobial peptide.134 Here, the hybrid molecule does not possess two antibacterial targets, but the antibody strategy allows more targeted treatment by increasing the local peptide concentration at the cell surface and bringing the antibiotic group close to its target.
Another approach to new multifunctional antibiotic candidates against Gram-negative bacteria based on the polymyxin lead structure has recently been published.135 These compounds are chimeric peptidomimetics combining features of polymyxin A with the outer membrane protein-targeting cyclic peptide murepavadin. They permeabilize the outer membrane by targeting lipopolysaccharides, inhibit the outer membrane protein complex Bam, which is involved in the folding and insertion of β-barrel proteins in the outer membrane, and permeabilize the inner membrane.135 One of these compounds is currently undergoing preclinical toxicology studies.135
It will be exciting to see whether these hybrid molecules will receive FDA approval and how they will ultimately perform in the clinic.
Multifunctional Compounds
The last category of multitarget molecules that we want to discuss are multifunctional compounds, which in addition to their antibacterial properties possess a second, unrelated activity that is beneficial for treatment. Most prominently, such compounds may have anti-inflammatory or immunomodulatory properties (Table 1). This is well-described for a range of antibiotics, and some are even used to treat conditions unrelated to infections due to these properties. This is, for example, the case for macrolides, which are used to treat diffuse panbronchiolitis,47 dapsone, which has anti-inflammatory and immunomodulatory effects and is, for example, used against dermatitis herpetiformis,136 or rifampicin, used against pruritus caused by primary biliary cholangitis.137 For other indications, the dual effects of these compounds are crucial for their therapeutic efficacy. For example, clindamycin is used to treat acne for both its direct antibacterial action and its ability to decrease swelling and inflammation by modulating cytokine production.40,138 Acne is a very common indication for combining antibacterial and anti-inflammatory activities. Other antibiotics with this dual function are metronidazole, tetracycline, macrolides, and dapsone, which are all used to treat acne.48 Clofazimine, which is used against leprosy, already has two distinct antibacterial targets, guanine bases and phospholipase A2.102 In addition to these activities, it also reduces neutrophil mobility, which is beneficial for treating patients with a type 2 lepra reaction characterized by strong inflammation.139,140 The range of antibiotics with immunomodulatory and anti-inflammatory effects and the detailed use of these compounds have been thoroughly reviewed before48,141−143 and should not be described here in more detail. However, it should be noted that daptomycin has been implicated to have immunomodulatory effects as well.144 Daptomycin already has multiple effects on bacterial cells, and both tetracycline and clofazimine have two distinct antibacterial targets. This illustrates that multiple activities are quite common in clinically successful antibiotics.
Part 2: Antibiotic Combinations
Combination regimes are nowadays quite common in the clinic, normally to potentiate activity against multiresistant strains and prevent resistance development. A common regime is, for example, the combination of sulfonamides with trimethoprim in urinary tract infections (Figure 2).
Figure 2.
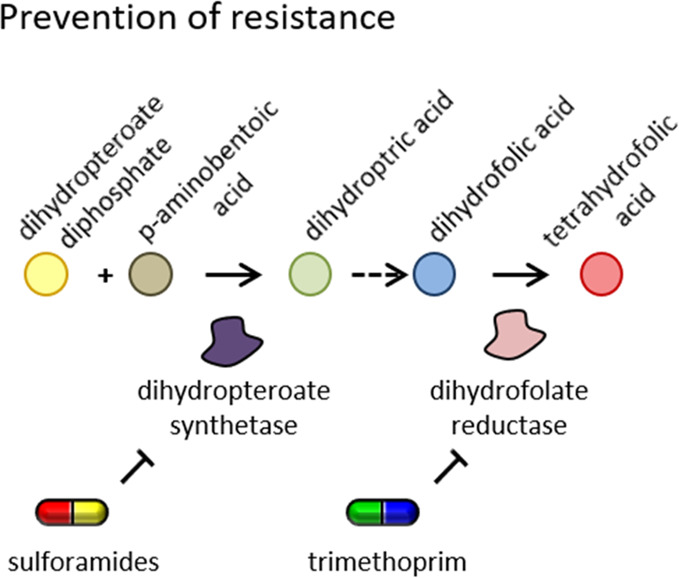
Prevention of resistance development by combination therapy. Sulfonamide antibiotics are typically given together with trimethoprim to prevent fast target mutation of a single enzyme.
Table 3 gives an overview of well-characterized antibiotic combinations and their effects. A significant amount of research is being conducted to find combinations that are more effective than monotherapy or that are resistance-breaking. When two antibiotics are combined, they are usually either indifferent in their activity or more effective in killing bacteria due to additive effects. However, if the effects of the combination exceed the expected additive effects, the combination is called synergistic. This is, for example, the case for daptomycin and β-lactams.145 The opposite effect is called antagonistic and is observed, e.g., with the antifungal drugs amphotericin B and ravuconazole.146 Synergistic drug combinations are highly sought after, since they allow one to lower drug doses and thus may reduce side effects. A different approach to combination strategies is using potentiators, sometimes also called antibiotic adjuvants. These are compounds that by themselves have little or no antibacterial activity but enhance the effects of antibiotics when administered in combination. This is, for example, the case for resistance-breaking compounds like β-lactamase inhibitors.147 Such compounds typically target either acquired or intrinsic antibiotic resistance mechanisms. There have also been approaches to develop inhibitors of horizontal gene transfer to prevent the spread of resistance genes.148 In the following, we will discuss these different approaches to antibiotic combination therapy.
Table 3. Examples for Well-Characterized Antibiotic Combinationsa.
| antibiotic 1 | antibiotic 2 | mechanism of combination | ref |
|---|---|---|---|
| synergistic | |||
| d-cycloserine inhibits peptidoglycan synthesis | epigallocatechin gallate binds to and disrupts the peptidoglycan layer | d-cycloserine inhibits lipid II synthesis, and epigallocatechin gallate disrupts cell wall peptidoglycan | (149) |
| ampicillinb inhibition of PBPs | daptomycin inhibition of membrane and cell wall synthesis | daptomycin affects membrane organization, which might interfere with the function of PBPs; it also inhibits lipid II synthesis by abolishing membrane binding of MurG | (25, 150, 151) |
| rifampicinb RNA synthase inhibition | fusidic acid ribosome inhibition | mechanism unknown, but it has been observed in vitro that DNA-dependent RNA-polymerase is inhibited by the elongation factor T | (152−155) |
| erythromycinb ribosome inhibition | penicillin inhibition of PBPs | inhibition of translation might deplete β-lactamases | (153, 156, 157) |
| additive | |||
| ampicillinb inhibition of PBPs | imipenem inhibition of PBPs | both antibiotics bind to the same site of PBP2A but with low affinity | (150, 158, 159) |
| azithromycinb ribosome inhibition | imipenem inhibition of PBPs | inhibition of translation might deplete PBPs, requiring lower doses of imipenem | (160, 161) |
| indifferent | |||
| sulphamethoxazoleb inhibits dihydropteroate synthetase | trimethoprim inhibits dihydrofolate reductase | both compounds target folate synthesis but at different steps; a combination is given to prevent rapid resistance development to a single drug rather than increase activity | (162−164) |
| potentiative | |||
| amoxicillineb inhibition of PBPs | clavulanate β-lactamase inhibitor | clavulanate inhibits the β-lactamase that degrades amoxicilline | (165, 166) |
PBP: penicillin-binding protein.
Combination in clinical use.
Antibiotic Synergy
Synergistic compound combinations are known against Gram-positive and Gram-negative bacteria and mycobacteria. However, the exact mechanisms of antibiotic synergy are mostly unknown. While there have been attempts to explain synergy, these have rarely been experimentally proven. However, there are four widely accepted categories of possible synergistic mechanisms for which examples are known (Figure 3).
Figure 3.
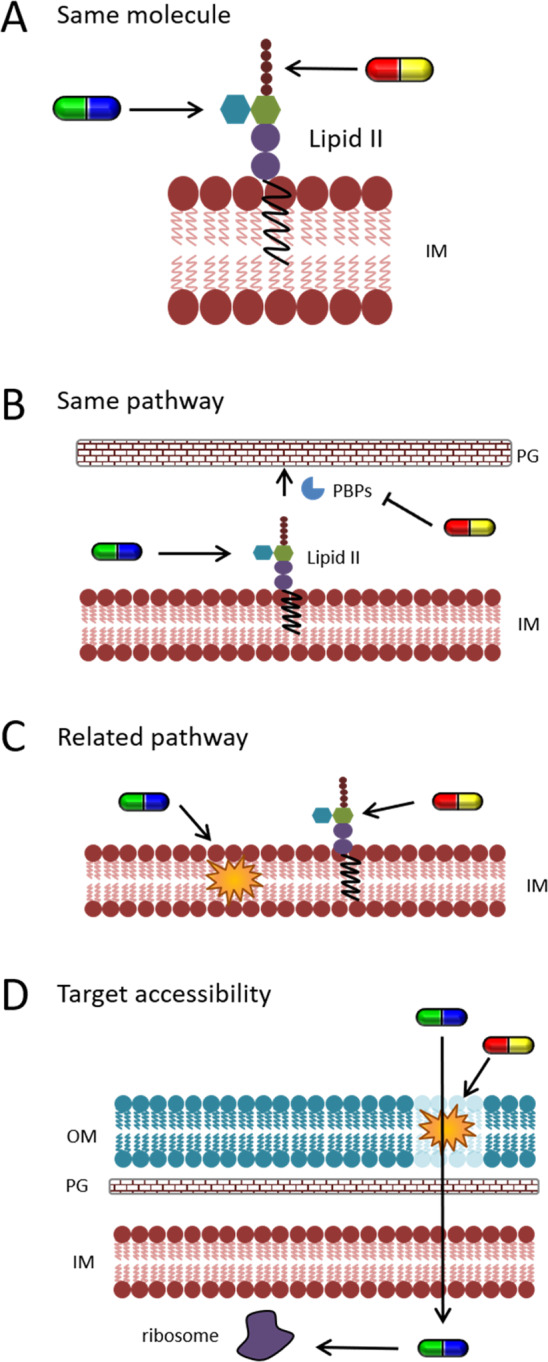
Mechanisms of synergy. (A) Targeting the same molecule (here, plectasin in red–yellow and dalbavancin in green–blue), (B) targeting the same pathway (here, plectasin in red–yellow and moenomycin in green–blue), (C) targeting a related process (here, LL-37 in green–blue and teicoplanin in red–yellow), and (D) improving target accessibility (here, colistin in red–yellow and minocycline in green–blue).
One possible synergistic mechanism is the inhibition of the same target molecule at multiple binding sites (Figure 3A). An example for this is the synergy of plectasin with teicoplanin and dalbavancin, which all bind the peptidoglycan precursor lipid II.167 However, this is not a universal phenomenon, and other antibiotics that target the same molecule might only have additive effects. This is, for example, the case for the PBP inhibitors ampicillin and imipenem.150 Another possibility for synergy is that two compounds inhibit independent targets in the same pathway (Figure 3B). This is for example the case for d-cycloserine, an antituberculosis drug that inhibits an intracellular step of peptidoglycan synthesis,168 and epigallocatechin gallate, a compound found in tea that is thought to disrupt cell wall peptidoglycan.149,169 Epigallocatechin gallate also acts synergistically with β-lactam antibiotics149 and inhibits β-lactamase activity.170 Another example for this synergistic mechanism is again plectasin, which also acts synergistically with the glycosyltransferase inhibitor moenomycin.167 However, plectasin does not show synergy with other cell wall synthesis inhibitors like vancomycin or penicillin G, demonstrating that the exact mechanisms underlying synergy are little understood.167 Synergy can also be observed between compounds that inhibit related processes (Figure 3C). This can often be observed for antibiotics that target cell wall synthesis and membrane-active compounds, e.g., β-lactams and daptomycin150,171,172 or the cationic antimicrobial peptide LL-37 and the lipid II-binding antibiotic teicoplanin.173 In some cases, synergy is also observed between antibiotics with different targets in unrelated pathways as observed with penicillin and the ribosome inhibitor erythromycin. Here, it has been suggested that the depletion of penicillin-binding proteins by translation inhibition might be the underlying mechanism.157 However, a similar combination of azithromycin (ribosome inhibitor) and imipenem (β-lactam) only results in additive effects.174 The last documented mechanism underlying antibiotic synergy is the improvement of target accessibility (Figure 3D). This is, for example, observed with the outer membrane-permeabilizing peptide colistin in combination with drugs that have inner membrane-bound or cytosolic targets, such as the β-lactam meropenem, the ribosome inhibitor minocycline, and fosfomycin, which inhibits the first enzyme in the lipid II pathway.175 This is an important anti-Gram-negative strategy that will be discussed in more detail in the following chapter. Antibiotic synergy has attracted significant attention over the last years, and efforts are being undertaken to discover new synergistic combinations that can be useful for clinical applications.176−180
Permeabilizers
In addition to synergistic antibiotic combinations, potentiative combination approaches have recently attracted increased attention. Potentiators typically target intrinsic or acquired antibiotic resistance mechanisms to sensitize or resensitize bacteria to existing antibiotics (Figure 4).
Figure 4.
Mechanisms of resistance-breaking and antibiotic-potentiating compounds. (A) Cell envelope permeabilizers, (B) antibiotic sensitizers, (C) β-lactamase inhibitors, (D) inhibitors of aminoglycoside-modifying enzymes, (E) efflux pump inhibitors, and (F) biofilm inhibitors. red–yellow: antibiotic; magenta–turquoise: potentiator.
The advantage of these molecules is that they can potentially increase the activity of a whole array of existing antibiotics. One class of potentiators are cell envelope permeabilizers, especially outer membrane-permeabilizing compounds (Figure 4A). These are particularly interesting, since the outer membrane is the main reason why most antibiotics are ineffective against Gram-negative bacteria. The inactivation of this permeability barrier could make almost the whole array of current antibiotic drugs available for treating these infections, instead of the handful that we have at our disposal now. Two antibiotics in current clinical use are known to target the outer membrane of Gram-negative bacteria, colistin and polymyxin B. Both of them display synergy with antibiotics with intracellular targets, which can at least partially be attributed to increased outer membrane permeability.175,181,182 Many other outer membrane-permeabilizing agents are known, for example, chelators like EDTA, metal ions, and certain antimicrobial peptides, but many of these have little clinical promise.183−188 However, several compounds have recently been described that could make the step into clinical practice in the future. For example, the antiprotozoal drug pentamidine has been identified as an outer membrane-permeabilizing agent. It was shown to potentiate the activity of typical Gram-positive-only drugs against Gram-negative bacteria in vitro and in a systemic Acinetobacter baumannii mouse infection model. Importantly, this activity was retained in colistin-resistant strains.189,190 Since pentamidine has already been in clinical use for the treatment of trypanosomiasis, leishmaniasis, and babesiosis since 1937, its safety and side effects have been extensively assessed. Recent efforts have yielded lipophilic vancomycin analogues that were able to permeabilize both the inner and outer membrane of Gram-negative bacteria while retaining their ability to inhibit peptidoglycan activity.191
Attempts to fuse outer membrane-penetrating peptide sequences to the lantibiotic nisin, which by itself is not active against Gram-negative bacteria due to its inability to reach its inner membrane target, have resulted in hybrid molecules with significantly increased anti-Gram-negative activity.192,193 A similar approach is the hybridization of antibiotics with the aminoglycoside tobramycin.194 Tobramycin is known as a ribosome inhibitor but at higher concentrations primarily attacks and permeabilizes the outer membrane.195,196 It has been conjugated with efflux pump inhibitors,197 quinolones,198,199 and the chelator cyclam.200 The resulting conjugates turned out to be potent antibiotic adjuvants and increased the activity of tetracyclines, fluoroquinolones, and β-lactams against Gram-negative bacteria by increasing uptake and limiting efflux. This is achieved by a multifaceted mechanism involving outer membrane permeabilization, impairment of efflux pumps, and depolarization of the inner membrane.117,194,197−200 Similar results have been obtained for tobramycin dimers.201
Another very interesting example are short peptide sequences derived from the cell cycle proteins FtsA and MreB from E. coli. These proteins bind to the cytoplasmic membrane with an amphipathic α helix motif. Peptides derived from these membrane-anchoring sequences not only display potent activity against E. coli and other Gram-negative bacteria but also significantly increase their outer membrane permeability.186,202,203
Few compounds are known that selectively permeabilize the outer membrane, and most known compounds also permeabilize the inner membrane. Such dual membrane activity can often be a sign for poor selectivity for bacteria, and side effects are likely. This is, for example, the case for colistin and polymyxin B and the reason why they are only employed as the last resort antibiotics for otherwise resistant infections.98,204−206 However, colistin nonapeptide and polymyxin B hepta-, octa-, and nonapeptide are derivatives of these compounds that retain their membrane-permeabilizing but not antibacterial activity, indicating high selectivity.184 Such derivatives, particularly polymyxin B nonapeptide, have been shown to act as strong potentiators of antibiotics with otherwise poor anti-Gram-negative activity.184,207,208 At the same time, it is much less toxic than full-length polymyxin B.184,209,210 While these properties have been demonstrated by many different studies, it is not used for combination therapy in the clinic.211 However, a polymyxin derivative with similar properties as polymyxin B nonapeptide, NAB741, has been developed and successfully passed its first phase I clinical trial.211 Compounds like polymyxin B nonapeptide or NAB741 hold great promise as potentiators of a wide range of antibiotics that would otherwise be ineffective against Gram-negative infections. It remains exciting to see how this type of compound proceeds in future clinical trials.
Sensitizers
A different strategy to enhance the performance of existing antibiotic drugs is to sensitize bacteria to their action. This can be achieved by inhibiting bacterial stress response systems that allow bacteria to adapt and survive antibiotic exposure (Figure 4B). Many studies on bacterial stress responses have proposed this as an option for future drug development, yet this has almost never been pursued. One exception is the bacterial SOS response to DNA damage. DNA damage is sensed by the RecA protein, which induces autoproteolysis of the LexA repressor, leading to derepression of DNA repair genes. It has been shown that genetically impairing this stress response mechanism leads to reversion of quinolone resistance in E. coli.212 Few inhibitors of DNA repair have been verified so far. For example, p-coumaric acid was found to interfere with the DNA-binding ability of RecA in Listeria monocytogenes and increased the activity of ciprofloxacin against this pathogen.213 Another study found that zinc inhibits the ability of RecA to bind to single-stranded DNA, suggesting a potential application for zinc ionophores.214 However, the effectivity and cytotoxicity of zinc ionophores would depend on the external zinc concentration,215 which may limit this approach to topical applications or targeted drug delivery and release approaches. A large-scale screen for inhibitors of LexA autoproteolysis has yielded promising lead structures for the inhibition of this step of SOS response activation.216 Similarly, IMP-1700, a rationally designed inhibitor of DNA repair, was later verified to target the AddAB DNA repair complex and sensitized multidrug-resistant S. aureus to ciprofloxacin.217
Another example for this strategy was identified by a transposon screen for increased tobramycin sensitivity in Pseudomonas aeruginosa. This screen identified the two-component stress response system AmgRS as a potential target for combination therapy with aminoglycoside antibiotics.218 It was later found that the RNA polymerase inhibitor rifampicin was also a potent inhibitor of this stress response system and potentiated the activity of aminoglycosides like tobramycin, amikacin, gentamycin, and neomycin against P. aeruginosa.219,220
A multitude of stress response systems have been identified that play a role for antibiotic adaptation and resistance, yet little effort has been put into identifying inhibitors of these systems to be used as antibiotic potentiators. In view of the urgent need for novel antibacterial strategies, this possibility deserves more attention.
Resistance Breakers
A similar strategy, yet much more exploited, is the direct targeting of bacterial resistance mechanisms (Table 4).
Table 4. Examples for Resistance-Breaking Compounds.
| resistance breaker | antibiotic | mechanism | ref |
|---|---|---|---|
| clavulanatea | amoxicilline | β-lactamase inhibitor | (165, 166) |
| avibactama | ceftazidime, ceftaroline, aztreonam | β-lactamase inhibitor | (221) |
| vaborbactama | Meropenem | β-lactamase inhibitor | (221) |
| tazobactama | ceftolozane | β-lactamase inhibitor | (221) |
| compound 1 | amikacin | inhibitor of aminoglycoside-modifying enzymes | (222) |
| PAβN | erythromycin, chloramphenicol | efflux pump inhibitor | (223) |
| verapamil | bedaquiline, ofloxacin | efflux pump inhibitor | (224, 225) |
| IMP-1700 | quinolones | sensitizer (SOS response) | (212, 217) |
| colistina | rifampin | permeabilizer | (226−228) |
| dispersin B | several possible | biofilm inhibitor | (229−231) |
| dehydrocrepenyc acid | several possible | inhibitor of horizontal gene transfer | (232) |
| streptazolin | several possible | immunomodulator | (233) |
Combination in clinical use.
The most prominent example for this is the inhibition of β-lactamase activity234 (Figure 4C). Having pioneered the field, it was the first resistance-breaking strategy to be applied in the clinic. It is also the only combination of antibiotic and potentiator in current clinical use apart from synergistic combinations with the outer membrane-permeabilizing peptides colistin and polymyxin B. The oldest combination of β-lactam antibiotic and β-lactamase inhibitor is amoxicillin–clavulanate, which was approved for clinical use in 1981. It is still in use today and remains the only one that is orally available.235 Since then, several other β-lactamase inhibitors have been identified, and eight combinations are currently on the market: amoxicillin–clavulanate, ticarcillin–clavulanate, ampicillin–sulbactam, cefoperazone–sulbactam, piperacillin–tazobactam, ceftolozane–tazobactam, ceftazidime–avibactam, and Meropenem–vaborbactam.221,235 Several other combinations are in different stages of clinical development.221
A similar strategy is the inhibition of aminoglycoside-modifying enzymes, which are responsible for the majority of cases of aminoglycoside resistance (Figure 4D). Different enzymes have been described that acetylate different sites of aminoglycoside antibiotics,236 some of which are inhibited by Cu2+, Zn2+, and Cd2+ ions.237 Inhibitors of these enzymes have been identified by molecular docking studies with virtual compound libraries and by screening libraries for in vitro enzyme inhibition.222,238,239 One of these has been confirmed to restore the activity of amikacin against a resistant Acinetobacter baumannii strain.222 However, none of these compounds is close to clinical development at the present time.
The most important intrinsic resistance mechanism, next to the outer membrane permeability barrier, is constituted by antibiotic efflux pumps. Particularly, Gram-negative bacteria possess different multidrug efflux pumps that can export a wide variety of antibiotics and antimicrobial molecules. The most prominent efflux pump type is the Gram-negative-specific resistance-nodulation-division (RND) superfamily, in particular the well-characterized AcrAB-TolC pump.240 A wide variety of antibiotics can be the substrate of these export systems, and their overexpression leads to high level drug resistance.240 This makes efflux pumps one of the biggest challenges in overcoming antibiotic resistance and at the same time an attractive target for resistance-breaking antibiotic potentiators (Figure 4E). Since the discovery of the very first efflux pump inhibitors reserpine and verapamil against the S. aureus efflux pump NorA in 1991,240 a multitude of inhibitors have been identified for both Gram-positive and Gram-negative efflux pumps. Many of them have been found to increase the activity of drugs, which are normally ineffective due to active export. These molecules have been extensively reviewed elsewhere.240−244 Despite these efforts, no efflux pump inhibitor has advanced to clinical development yet. Common limitations of these molecules appear to be a high toxicity for mammalian cells, low in vivo efficacy, and insufficient spectrum coverage.240,245 More research into this topic is needed to fully understand how multidrug efflux pumps work and how they synergize with other resistance mechanisms, such as the outer membrane, to go forward with effective inhibitor design.
Prevention of Horizontal Gene Transfer
A very different strategy to target antibiotic resistance is inhibiting horizontal gene transfer. Target mutations are only one way for bacteria to become antibiotic resistant. The much more threatening possibility is the spread of resistance genes that are encoded on plasmids or other mobile genetic elements within a bacterial population. This is especially critical for otherwise slowly occurring resistance mechanisms like enzymatic resistance.246 Importantly, the human (or animal) microbiota can act as a reservoir for resistance plasmids that may spread to pathogenic bacteria during infection. Thus, the administration of an inhibitor of horizontal gene transfer in combination with antibiotic treatment could decrease the incidence of resistance transfer from a reservoir to an infectious strain.247 This is an interesting concept but remains to be tested in infection experiments. Several compounds have been identified that inhibit horizontal gene transfer, yet in the majority of cases, this activity turned out to be due to secondary effects.248 However, a small number of specific inhibitors have been found. For example, unsaturated fatty acid species from tropical fruits and their synthetic derivatives were able to inhibit the transfer of the most important resistance-related plasmid types in several Gram-negative pathogens.232,249 Likewise, a mutation of a competence-stimulating peptide from Streptococcus pneumoniae has resulted in peptide versions that reduced competence and, thus, horizontal gene transfer.250 As mentioned earlier, zinc was able to inhibit the bacterial SOS response, which interestingly, also impaired horizontal gene transfer between enterobacteria.214 Secretion systems could be a promising target for such molecules as well. Thus, the inhibition of type IV secretion, which is involved in conjugative DNA transfer, has been shown to be inhibited by small peptidomimetic compounds.251 While more research is needed to verify the feasibility of these approaches in clinical settings, it is certainly an interesting new combination therapy strategy that deserves further exploration.
Biofilm Inhibitors
One big problem in the clinic is the production of biofilms by bacteria like S. aureus and P. aeruginosa, and biofilm inhibitors are potent potentiators for antibiotics used against such infections (Figure 4F). Strategies to prevent biofilm formation or disperse mature biofilms can commonly be divided into three categories: the inhibition of adhesion or extracellular matrix production, the inhibition of quorum sensing, and the dispersion of the extracellular matrix.252 Several antimicrobial peptides, for example, LL-37, prevent adhesion of bacterial cells by inhibiting the initiation of biofilm production.253 Furanones are structurally similar to the quorum sensing molecule N-acyl-homoserine-lactone and are thought to competitively inhibit binding to their cognate transcriptional regulator.253 Several other analogues of quorum sensing molecules have been patented, yet studies on their clinical potential are yet to come.254 The most prominent biofilm-dispersing agent is the glycoside hydrolase dispersin B, which directly targets the extracellular matrix.230 Further small molecule inhibitors have been identified for all these mechanisms.254−257
Biofilm inhibitors are particularly relevant in the context of medical devices like catheters and of chronic lung infections, with P. aeruginosa being the most problematic pathogen due to its high intrinsic antibiotic resistance and the emergence of totally drug-resistant isolates.258 Several compounds have been described that could be used against P. aeruginosa biofilms. Interestingly, biofilm-degrading enzymes have been shown to remain active when immobilized on a surface, suggesting future applications in medical device technologies.259 Short glycans have been shown to disrupt P. aeruginosa biofilms and subsequently increase the efficacy of antibiotics against it.260 The small peptide 1018 targets the stringent response (p)ppGpp signaling, which is involved in biofilm formation, and acts as a potent biofilm-dispersing agent not only for P. aeruginosa but also for other pathogens including Klebsiella pneumoniae and S. aureus.261 Peptide 1018 also resulted in decreased virulence of P. aeruginosa in a murine skin infection model.262 Similarly, small molecule quorum sensing inhibitors have been shown to increase the efficacy of tobramycin against P. aeruginosa in a foreign-body infection model in mice.263 Micafungin, which inhibits the synthesis of the pseudomonal cell wall component 1,3-β-d-glucan, was demonstrated to prevent P. aeruginosa biofilm formation and improved the outcome of antibiotic therapy in P. aeruginosa-infected mice, particularly when combined with the fluoroquinolone levofloxacin.264 Interestingly, when the fatty acid synthesis inhibitor triclosan, which has been commonly used in hygiene products like toothpaste, was applied together with aminoglycosides like tobramycin, a strong antibiofilm activity was observed. Neither of these compounds have an effect on biofilms by themselves, and the mechanism behind this adjuvant activity is unknown.265 So far, no biofilm inhibitor has made the transition to the clinic, yet a number of preclinical studies show the promise of using such agents as antibiotic potentiators in the future.
Multidrug Approaches
Combination therapy approaches may involve more than two compounds. This is, for example, very common in tuberculosis treatment regimes, which often consist of a combination of rifampicin, isoniazid, ethambutol, and pyrazinamide266 but has also been employed for other bacterial infections.267 A newly emerging approach is the combination of an antibiotic with more than one potentiator. This has, for example, been explored for the combination of β-lactams with β-lactamase inhibitors and permeabilizers such as dimeric tobramycin and tobramycin–cyclam.200,201 However, such combinations are not currently used in the clinic.
Challenges of Combination Therapy
To date, antibiotic combination therapy is rather a last resort for severe infections than a standard therapy option. It is normally only employed for infections with multiresistant bacteria that cannot be treated with monotherapy, for latent infections like tuberculosis, or for antibiotics that have a high risk of encountering resistance when applied alone, such as trimethoprim–sulfonamide or β-lactam−β-lactamase combinations. Just as multitarget antibiotics are more effective than single-target antibiotics,267 combination therapy is superior over monotherapy in terms of patient outcomes, at least when it comes to infections with multiresistant pathogens.268 The current developments on antibiotic adjuvants have shown great promise for new combination strategies being added to our clinically available repertoire soon. However, combination therapies also face challenges.
One important limitation is the development of clinically useful formulations and treatment regimes. Finding the right dosing, treatment intervals, and total treatment duration can already be challenging for single drugs and gets much more complicated with each additional drug added to the regime, since each individual drug has its own pharmacodynamic and -kinetic properties and its own side effects. Matching these to elicit the desired outcome in patients can be a serious challenge.267,269 A solution for this limitation could be antibiotic hybrids as discussed in Designer Dual-Target Compounds. However, hybrid molecules may encounter other challenges like solubility and uptake.125
Another risk of combination therapy is antimicrobial resistance. While many empirical studies have found that drug combinations suppress resistance,198,269−276 other studies have warned that under certain conditions antibiotic combinations might even promote faster resistance development. This is a particular concern for synergistic drug combinations. While they allow one to lower the dose and thus reduce side effects,277,278 they come with an inherent risk of treatment failure through resistance: If resistance to one drug occurs, synergistic effects are lost, resulting in the low-dose exposure to a single antibiotic.279−283 Even with nonsynergistic antibiotic combinations, the risk for increased resistance development is evident. A recent study found that the development of tolerance, i.e., the ability of bacteria to survive longer under antibiotic exposure without changing the antibiotic’s minimal inhibitory concentration, to one drug may promote the transmission of resistance to the second drug.284 These unwanted effects of combination treatments seem to be due to the prolonged survival of bacteria and their exposure to sublethal antibiotic concentrations. Therefore, the concept of “hitting them fast and hard”, which has been proposed to be a key to successful antibiotic treatment, should be paid particular attention in combination regimes, even if lowering drug doses can be tempting to reduce adverse effects.6,276
Conclusion
Many fascinating approaches have been and are currently being developed to target multiple molecules in bacterial cells. From antibiotics that have multiple downstream effects, true multitargeting compounds, and dual-activity hybrid molecules to combination approaches with antibiotic adjuvants, all of these strategies have shown a certain promise and give hope for one to see light at the end of the tunnel of antibiotic development. Recent research gives the concept of polypharmacology another dimension by designing antibiotic adjuvants that possess multiple mechanisms themselves, such as outer membrane permeabilization and efflux pump inhibition, which synergistically increase the intracellular antibiotic concentration.197 While more research is needed to truly understand the complex mechanisms underlying some of these multiple activities, they have already taught us one important thing, namely, that we should not shy away from complex mechanisms and multiple targets but try to exploit their full potential for future drug development.
Acknowledgments
M.W. received funding from Chalmers University of Technology and the Swedish Research Council (VR Starting Grant 2019-04521). The funders had no role in the design of the paper, in the writing of the manuscript, or in the decision to publish the results.
Author Contributions
Conceptualization, writing, and visualization: D.A.G. and M.W.
The authors declare no competing financial interest.
References
- Sprenger M.; Fukuda K. (2016) New Mechanisms, New Worries. Science (Washington, DC, U. S.) 351, 1263–1264. 10.1126/science.aad9450. [DOI] [PubMed] [Google Scholar]
- WHO . (accessed 2020-03-10) Antimicrobial resistance fact sheet, http://www.who.int/mediacentre/factsheets/fs194/en/.
- Ventola C. L. (2015) The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 40, 277–283. [PMC free article] [PubMed] [Google Scholar]
- Wenzel M.; Bandow J. E. (2011) Proteomic Signatures in Antibiotic Research. Proteomics 11, 3256–3268. 10.1002/pmic.201100046. [DOI] [PubMed] [Google Scholar]
- Brötz-Oesterhelt H.; Brunner N. (2008) How Many Modes of Action Should an Antibiotic Have?. Curr. Opin. Pharmacol. 8, 564–573. 10.1016/j.coph.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Gajdács M. (2019) The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules 24, 892. 10.3390/molecules24050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L. L. (2007) Multi-Targeting by Monotherapeutic Antibacterials. Nat. Rev. Drug Discovery 6, 41–55. 10.1038/nrd2202. [DOI] [PubMed] [Google Scholar]
- Scheffers D.-J.; Pinho M. G. (2005) Bacterial Cell Wall Synthesis: New Insights from Localization Studies. Microbiol. Mol. Biol. Rev. 69, 585–607. 10.1128/MMBR.69.4.585-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K.; Malik M.; Kerns R. J.; Zhao X. (2008) Quinolone-Mediated Bacterial Death. Antimicrob. Agents Chemother. 52, 385–392. 10.1128/AAC.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. (2007) Bacterial RNA Polymerase: A Promising Target for the Discovery of New Antimicrobial Agents. Curr. Opin. Investig. Drugs 8, 600–607. [PubMed] [Google Scholar]
- Sköld O. (2000) Sulfonamide Resistance: Mechanisms and Trends. Drug Resist. Updates 3, 155–160. 10.1054/drup.2000.0146. [DOI] [PubMed] [Google Scholar]
- Yeaman M. R.; Yount N. Y. (2003) Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 55, 27–55. 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- Brotz H.; Josten M.; Wiedemann I.; Schneider U.; Gotz F.; Bierbaum G.; Sahl H. G. (1998) Role of Lipid-Bound Peptidoglycan Precursors in the Formation of Pores by Nisin, Epidermin and Other Lantibiotics. Mol. Microbiol. 30, 317–327. 10.1046/j.1365-2958.1998.01065.x. [DOI] [PubMed] [Google Scholar]
- Velkov Y., Thompson P., Azad M., Roberts K., and Bergen P. (2019) History, Chemistry and Antibacterial Spectrum. In Polymyxin Antibiotics: From Laboratory Bench to Bedside (Li J., Nation R., and Kaye K., Eds.) 1st ed., Vol. 1145, p 17, Spinger Nature, Switzerland AG, 10.1007/978-3-030-16373-0_3. [DOI] [PubMed] [Google Scholar]
- Tang X.-J.; Thibault P.; Boyd R. K. (1992) Characterisation of the Tyrocidine and Gramicidin Fractions of the Tyrothricin Complex from Bacillus Brevis Using Liquid Chromatography and Mass Spectrometry. Int. J. Mass Spectrom. Ion Processes 122, 153–179. 10.1016/0168-1176(92)87015-7. [DOI] [Google Scholar]
- Loll P. J.; Upton E. C.; Nahoum V.; Economou N. J.; Cocklin S. (2014) The High Resolution Structure of Tyrocidine A Reveals an Amphipathic Dimer. Biochim. Biophys. Acta, Biomembr. 1838, 1199–1207. 10.1016/j.bbamem.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyuki G.; Jackson G. E.; Venter G. A.; Kover K. E.; Szilagyi L.; Rautenbach M.; Spathelf B. M.; Bhattacharya B.; van der Spoel D. (2013) Beta-Sheet Structures and Dimer Models of the Two Major Tyrocidines, Antimicrobial Peptides from Bacillus Aneurinolyticus. Biochemistry 52, 7798–7806. 10.1021/bi401363m. [DOI] [PubMed] [Google Scholar]
- Leussa A. N.-N.; Rautenbach M. (2014) Detailed SAR and PCA of the Tyrocidines and Analogues towards Leucocin A-Sensitive and Leucocin A-Resistant Listeria Monocytogenes. Chem. Biol. Drug Des. 84, 543–557. 10.1111/cbdd.12344. [DOI] [PubMed] [Google Scholar]
- Spathelf B. M.; Rautenbach M. (2009) Anti-Listerial Activity and Structure-Activity Relationships of the Six Major Tyrocidines, Cyclic Decapeptides from Bacillus Aneurinolyticus. Bioorg. Med. Chem. 17, 5541–5548. 10.1016/j.bmc.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Wenzel M.; Rautenbach M.; Vosloo J. A.; Siersma T.; Aisenbrey C. H. M.; Zaitseva E.; Laubscher W. E.; van Rensburg W.; Behrends J.; Bechinger B.; Hamoen L. W. (2018) The Multifaceted Antibacterial Mechanisms of the Pioneering Peptide Antibiotics Tyrocidine and Gramicidin S. mBio 9, e00802-18 10.1128/mBio.00802-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm J.; Fuchs K.; Stammer H.; Schumacher-Stimpfl A.; Milde J. (2018) Efficacy and Safety of a Triple Active Sore Throat Lozenge in the Treatment of Patients with Acute Pharyngitis: Results of a Multi-Centre, Randomised, Placebo-Controlled, Double-Blind, Parallel-Group Trial (DoriPha). Int. J. Clin. Pract. 72, e13272 10.1111/ijcp.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosscha M. I.; van Dissel J. T.; Kuijper E. J.; Swart W.; Jager M. J. (2004) The Efficacy and Safety of Topical Polymyxin B, Neomycin and Gramicidin for Treatment of Presumed Bacterial Corneal Ulceration. Br. J. Ophthalmol. 88, 25–28. 10.1136/bjo.88.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J.; Zhu F.; Ma X.; Cao Z. W.; Li Y. X.; Chen Y. Z. (2009) Mechanisms of Drug Combinations: Interaction and Network Perspectives. Nat. Rev. Drug Discovery 8, 111. 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- Reddy A. S.; Zhang S. (2013) Polypharmacology: Drug Discovery for the Future. Expert Rev. Clin. Pharmacol. 6, 41–47. 10.1586/ecp.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A.; Wenzel M.; Strahl H.; Grein F.; Saaki T. N. V; Kohl B.; Siersma T.; Bandow J. E.; Sahl H.-G.; Schneider T.; Hamoen L. W. (2016) Daptomycin Inhibits Cell Envelope Synthesis by Interfering with Fluid Membrane Microdomains. Proc. Natl. Acad. Sci. U. S. A. 113, E7077–E7086. 10.1073/pnas.1611173113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachmann A. B.; Sevim E.; Gaballa A.; Popham D. L.; Antelmann H.; Helmann J. D. (2011) Reduction in Membrane Phosphatidylglycerol Content Leads to Daptomycin Resistance in Bacillus Subtilis. Antimicrob. Agents Chemother. 55, 4326–4337. 10.1128/AAC.01819-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E. (1989) Structure, Biochemistry and Mechanism of Action of Glycopeptide Antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 8, 943–950. 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- Singh M.; Chang J.; Coffman L.; Kim S. J. (2017) Hidden Mode of Action of Glycopeptide Antibiotics: Inhibition of Wall Teichoic Acid Biosynthesis. J. Phys. Chem. B 121, 3925–3932. 10.1021/acs.jpcb.7b00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J.; Strominger J. L. (1971) Mechanism of Action of Bacitracin: Complexation with Metal Ion and C55-Isoprenyl Pyrophosphate. Proc. Natl. Acad. Sci. U. S. A. 68, 3223–3227. 10.1073/pnas.68.12.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y.; McCalla D. R. (1975) Effect of Activated Nitrofurans on DNA. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 402, 142–149. 10.1016/0005-2787(75)90032-5. [DOI] [PubMed] [Google Scholar]
- Wenzel M.; Dekker M. P.; Wang B.; Burggraaf M. J.; Bitter W.; van Weering J. R. T.; Hamoen L. W. (2019) New Flat Embedding Method for Transmission Electron Microscopy Reveals an Unknown Mechanism of Tetracycline. bioRxiv 820191. 10.1101/820191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brötz-Oesterhelt H.; Beyer D.; Kroll H.-P.; Endermann R.; Ladel C.; Schroeder W.; Hinzen B.; Raddatz S.; Paulsen H.; Henninger K.; Bandow J. E.; Sahl H.-G.; Labischinski H. (2005) Dysregulation of Bacterial Proteolytic Machinery by a New Class of Antibiotics. Nat. Med. 11, 1082–1087. 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]
- Sass P.; Josten M.; Famulla K.; Schiffer G.; Sahl H.-G.; Hamoen L.; Brotz-Oesterhelt H. (2011) Antibiotic Acyldepsipeptides Activate ClpP Peptidase to Degrade the Cell Division Protein FtsZ. Proc. Natl. Acad. Sci. U. S. A. 108, 17474–17479. 10.1073/pnas.1110385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoghare S. (2013) Bedaquiline: A New Drug Approved for Treatment of Multidrug-Resistant Tuberculosis. Indian J. Pharmacol. 45, 536–537. 10.4103/0253-7613.117765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I.; Roberts M. (2001) Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 65, 232–60. 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M.; Bader J. (1976) Action of Polymyxin B on Bacterial Membranes. Binding Capacities for Polymyxin B of Inner and Outer Membranes Isolated from Salmonella Typhimurium G30. Arch. Microbiol. 109, 51–58. 10.1007/BF00425112. [DOI] [PubMed] [Google Scholar]
- Bohg A.; Ristow H. (1987) Tyrocidine-Induced Modulation of the DNA Conformation in Bacillus Brevis. Eur. J. Biochem. 170, 253–258. 10.1111/j.1432-1033.1987.tb13693.x. [DOI] [PubMed] [Google Scholar]
- Ristow H.; Schazschneider B.; Vater J.; Kleinkauf H. (1975) Some Characteristics of the DNA-Tyrocidine Complex and a Possible Mechanism of the Gramicidin Action. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 414, 1–8. 10.1016/0005-2787(75)90120-3. [DOI] [PubMed] [Google Scholar]
- Weinkle A. P.; Doktor V.; Emer J. (2015) Update on the Management of Rosacea. Clin., Cosmet. Invest. Dermatol. 8, 159–177. 10.2147/CCID.S58940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosso J. Q.; Schmidt N. F. (2010) A Review of the Anti-Inflammatory Properties of Clindamycin in the Treatment of Acne Vulgaris. Cutis 85, 15–24. [PubMed] [Google Scholar]
- Goihman-Yahr M.; Pari T.; George S.; Jacob M.; Chandi S. M.; Pulimood S.; Rajagopalan B. (1996) Malignant Pyoderma Responding to Clofazimine. Int. J. Dermatol. 35, 757. 10.1111/j.1365-4362.1996.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Seukeran D. C.; Stables G. I.; Cunliffe W. J.; Sheehan-Dare R. A. (1999) The Treatment of Acne Agminata with Clofazimine. Br. J. Dermatol. 141, 596–597. 10.1046/j.1365-2133.1999.03084.x. [DOI] [PubMed] [Google Scholar]
- Gomez-De la Fuente E.; del Rio R.; Rodriguez M.; Guerra A.; Rodriguez-Peralto J. L.; Iglesias L. (2000) Granuloma Faciale Mimicking Rhinophyma: Response to Clofazimine. Acta Derm.-Venereol. 80, 144. [PubMed] [Google Scholar]
- Prendiville J. S.; Logan R. A.; Russell-Jones R. (1988) A Comparison of Dapsone with 13-Cis Retinoic Acid in the Treatment of Nodular Cystic Acne. Clin. Exp. Dermatol. 13, 67–71. 10.1111/j.1365-2230.1988.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Tan B. B.; Lear J. T.; Smith A. G. (1997) Acne Fulminans and Erythema Nodosum during Isotretinoin Therapy Responding to Dapsone. Clin. Exp. Dermatol. 22, 26–27. 10.1046/j.1365-2230.1997.1830600.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Boado Y. S.; Rubin B. K. (2008) Macrolides as Immunomodulatory Medications for the Therapy of Chronic Lung Diseases. Curr. Opin. Pharmacol. 8, 286–291. 10.1016/j.coph.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Keicho N.; Kudoh S. (2002) Diffuse Panbronchiolitis: Role of Macrolides in Therapy. Am. J. Respir. Med. 1, 119–131. 10.1007/BF03256601. [DOI] [PubMed] [Google Scholar]
- Pradhan S.; Madke B.; Kabra P.; Singh A. L. (2016) Anti-Inflammatory and Immunomodulatory Effects of Antibiotics and Their Use in Dermatology. Indian J. Dermatol. 61, 469–481. 10.4103/0019-5154.190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeverbeke T.; Lequen L.; de Barbeyrac B.; Labbe L.; Bebear C. M.; Morrier Y.; Bannwarth B.; Bebear C. M.; Dehais J. (1998) Propionibacterium Acnes Isolated from Synovial Tissue and Fluid in a Patient with Oligoarthritis Associated with Acne and Pustulosis. Arthritis Rheum. 41, 1889–1893. . [DOI] [PubMed] [Google Scholar]
- Nishimuta K.; Ito Y. (2003) Effects of Metronidazole and Tinidazole Ointments on Models for Inflammatory Dermatitis in Mice. Arch. Dermatol. Res. 294, 544–551. 10.1007/s00403-002-0381-4. [DOI] [PubMed] [Google Scholar]
- Mendonca C. O.; Griffiths C. E. M. (2006) Clindamycin and Rifampicin Combination Therapy for Hidradenitis Suppurativa. Br. J. Dermatol. 154, 977–978. 10.1111/j.1365-2133.2006.07155.x. [DOI] [PubMed] [Google Scholar]
- Mela M.; Mancuso A.; Burroughs A. K. (2003) Review Article: Pruritus in Cholestatic and Other Liver Diseases. Aliment. Pharmacol. Ther. 17, 857–870. 10.1046/j.1365-2036.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- Sarici G.; Cinar S.; Armutcu F.; Altinyazar C.; Koca R.; Tekin N. S. (2010) Oxidative Stress in Acne Vulgaris. J. Eur. Acad. Dermatol. Venereol. 24, 763–767. 10.1111/j.1468-3083.2009.03505.x. [DOI] [PubMed] [Google Scholar]
- Webster G. F.; Leyden J. J.; McGinley K. J.; McArthur W. P. (1982) Suppression of Polymorphonuclear Leukocyte Chemotactic Factor Production in Propionibacterium Acnes by Subminimal Inhibitory Concentrations of Tetracycline, Ampicillin, Minocycline, and Erythromycin. Antimicrob. Agents Chemother. 21, 770–772. 10.1128/AAC.21.5.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore R.; Kovach R.; Walker C.; Thomas J.; Bradshaw M.; Leyden J.; Powala C.; Ashley R. (2003) Effects of Subantimicrobial-Dose Doxycycline in the Treatment of Moderate Acne. Arch. Dermatol. 139, 459–464. 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- Strahl H.; Hamoen L. W. (2010) Membrane Potential Is Important for Bacterial Cell Division. Proc. Natl. Acad. Sci. U. S. A. 107, 12281–12286. 10.1073/pnas.1005485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl H.; Burmann F.; Hamoen L. W. (2014) The Actin Homologue MreB Organizes the Bacterial Cell Membrane. Nat. Commun. 5, 3442. 10.1038/ncomms4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel M.; Chiriac A. I.; Otto A.; Zweytick D.; May C.; Schumacher C.; Gust R.; Albada H. B.; Penkova M.; Krämer U.; Erdmann R.; Metzler-Nolte N.; Straus S. K.; Bremer E.; Becher D.; Brötz-Oesterhelt H.; Sahl H.-G.; Bandow J. E. (2014) Small Cationic Antimicrobial Peptides Delocalize Peripheral Membrane Proteins. Proc. Natl. Acad. Sci. U. S. A. 111, E1409–18. 10.1073/pnas.1319900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J.; Pogliano N.; Silverman J. A. (2012) Daptomycin-Mediated Reorganization of Membrane Architecture Causes Mislocalization of Essential Cell Division Proteins. J. Bacteriol. 194, 4494–4504. 10.1128/JB.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgera F.; Antcheva N.; Pacor S.; Quaroni L.; Berti F.; Vaccari L.; Tossi A. (2008) Structuring and Interactions of Human Beta-Defensins 2 and 3 with Model Membranes. J. Pept. Sci. 14, 518–523. 10.1002/psc.981. [DOI] [PubMed] [Google Scholar]
- Sass V.; Schneider T.; Wilmes M.; Körner C.; Tossi A.; Novikova N.; Shamova O.; Sahl H. G. (2010) Human β-Defensin 3 Inhibits Cell Wall Biosynthesis in Staphylococci. Infect. Immun. 78, 2793–2800. 10.1128/IAI.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandaswamy K.; Liew T. H.; Wang C. Y.; Huston-Warren E.; Meyer-Hoffert U.; Hultenby K.; Schröder J. M.; Caparon M. G.; Normark S.; Henriques-Normark B.; Hultgren S. J.; Kline K. A. (2013) Focal Targeting by Human β-Defensin 2 Disrupts Localized Virulence Factor Assembly Sites in Enterococcus Faecalis. Proc. Natl. Acad. Sci. U. S. A. 110, 20230–20235. 10.1073/pnas.1319066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakman P. H. S.; Krijgsveld J.; de Boer L.; Nguyen L. T.; Boszhard L.; Vreede J.; Dekker H. L.; Speijer D.; Drijfhout J. W.; te Velde A. A.; Crielaard W.; Vogel H. J.; Vandenbroucke-Grauls C. M. J. E.; Zaat S. A. J. (2011) Native Thrombocidin-1 and Unfolded Thrombocidin-1 Exert Antimicrobial Activity via Distinct Structural Elements. J. Biol. Chem. 286, 43506–43514. 10.1074/jbc.M111.248641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omardien S.; Drijfhout J. W.; Vaz F. M.; Wenzel M.; Hamoen L. W.; Zaat S. A. J.; Brul S. (2018) Bactericidal Activity of Amphipathic Cationic Antimicrobial Peptides Involves Altering the Membrane Fluidity When Interacting with the Phospholipid Bilayer. Biochim. Biophys. Acta, Biomembr. 1860, 2404. 10.1016/j.bbamem.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Omardien S.; Drijfhout J. W.; van Veen H.; Schachtschabel S.; Riool M.; Hamoen L. W.; Brul S.; Zaat S. A. J. (2018) Synthetic Antimicrobial Peptides Delocalize Membrane Bound Proteins Thereby Inducing a Cell Envelope Stress Response. Biochim. Biophys. Acta, Biomembr. 1860, 2416–2427. 10.1016/j.bbamem.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Omardien S.; Drijfhout J. W.; Zaat S. A.; Brul S. (2018) Cationic Amphipathic Antimicrobial Peptides Perturb the Inner Membrane of Germinated Spores Thus Inhibiting Their Outgrowth. Front. Microbiol. 9, 2277. 10.3389/fmicb.2018.02277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. A.; Wenzel M. (2020) More Than a Pore: A Current Perspective on the In Vivo Mode of Action of the Lipopeptide Antibiotic Daptomycin. Antibiotics (Basel, Switz.) 9, 17. 10.3390/antibiotics9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinpflug K.; Wenzel M.; Krylova O.; Bandow E. J.; Dathe M.; Strahl H. (2017) Antimicrobial Peptide CWFW Kills by Combining Lipid Phase Separation with Autolysis. Sci. Rep. 7, 44332. 10.1038/srep44332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeloh D.; Tipmanee V.; Jim K. K.; Dekker M. P.; Bitter W.; Voravuthikunchai S. P.; Wenzel M.; Hamoen L. W. (2018) The Novel Antibiotic Rhodomyrtone Traps Membrane Proteins in Vesicles with Increased Fluidity. PLoS Pathog. 14, e1006876 10.1371/journal.ppat.1006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanel G. G.; Schweizer F.; Karlowsky J. A. (2012) Oritavancin: Mechanism of Action. Clin. Infect. Dis. 54, S214–S219. 10.1093/cid/cir920. [DOI] [PubMed] [Google Scholar]
- Higgins D. L.; Chang R.; Debabov D. V.; Leung J.; Wu T.; Krause K. M.; Sandvik E.; Hubbard J. M.; Kaniga K.; Schmidt D. E.; Gao Q.; Cass R. T.; Karr D. E.; Benton B. M.; Humphrey P. P. (2005) Telavancin, a Multifunctional Lipoglycopeptide, Disrupts Both Cell Wall Synthesis and Cell Membrane Integrity in Methicillin-Resistan Staphylococcus Aureus. Antimicrob. Agents Chemother. 49, 1127–1134. 10.1128/AAC.49.3.1127-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti A.; Cassani G. (1985) Synthesis and Characterization of D-Alanyl-D-Alanine-Agarose. Appl. Biochem. Biotechnol. 11, 101–109. 10.1007/BF02798542. [DOI] [Google Scholar]
- Cheng M.; Ziora Z. M.; Hansford K. A.; Blaskovich M. A.; Butler M. S.; Cooper M. A. (2014) Anti-Cooperative Ligand Binding and Dimerisation in the Glycopeptide Antibiotic Dalbavancin. Org. Biomol. Chem. 12, 2568–2575. 10.1039/C3OB42428F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel M.; Kohl B.; Münch D.; Raatschen N.; Albada H. B.; Hamoen L.; Metzler-Nolte N.; Sahl H. G.; Bandow J. E. (2012) Proteomic Response of Bacillus Subtilis to Lantibiotics Reflects Differences in Interaction with the Cytoplasmic Membrane. Antimicrob. Agents Chemother. 56, 5749–5757. 10.1128/AAC.01380-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli R. R.; Schneider T.; Sahl H.-G.; Wiedemann I. (2006) Insights into in Vivo Activities of Lantibiotics from Gallidermin and Epidermin Mode-of-Action Studies. Antimicrob. Agents Chemother. 50, 1449–1457. 10.1128/AAC.50.4.1449-1457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brötz H.; Bierbaum G.; Leopold K.; Reynolds P. E.; Sahl H. G. (1998) The Lantibiotic Mersacidin Inhibits Peptidoglycan Synthesis by Targeting Lipid II. Antimicrob. Agents Chemother. 42, 154–160. 10.1128/AAC.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J.-B.; Seyer D.; Jouenne T.; Thébault P. (2017) Elaboration of Antibacterial Plastic Surfaces by a Combination of Antiadhesive and Biocidal Coatings of Natural Products. Colloids Surf., B 156, 186–193. 10.1016/j.colsurfb.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Ulm H.; Schneider T. (2016) Targeting Bactoprenol-Coupled Cell Envelope Precursors. Appl. Microbiol. Biotechnol. 100, 7815–7825. 10.1007/s00253-016-7732-0. [DOI] [PubMed] [Google Scholar]
- Weidenmaier C.; Peschel A. (2008) Teichoic Acids and Related Cell-Wall Glycopolymers in Gram-Positive Physiology and Host Interactions. Nat. Rev. Microbiol. 6, 276–287. 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- Kepplinger B.; Morton-Laing S.; Seistrup K. H.; Marrs E. C. L.; Hopkins A. P.; Perry J. D.; Strahl H.; Hall M. J.; Errington J.; Allenby N. E. E. (2018) Mode of Action and Heterologous Expression of the Natural Product Antibiotic Vancoresmycin. ACS Chem. Biol. 13, 207–214. 10.1021/acschembio.7b00733. [DOI] [PubMed] [Google Scholar]
- Wenzel M.; Patra M.; Senges C. H. R.; Ott I.; Stepanek J. J.; Pinto A.; Prochnow P.; Vuong C.; Langklotz S.; Metzler-Nolte N.; Bandow J. E. (2013) Analysis of the Mechanism of Action of Potent Antibacterial Hetero-Tri-Organometallic Compounds: A Structurally New Class of Antibiotics. ACS Chem. Biol. 8, 1442–1450. 10.1021/cb4000844. [DOI] [PubMed] [Google Scholar]
- Andrés M. T.; Fierro J. F. (2010) Antimicrobial Mechanism of Action of Transferrins: Selective Inhibition of H+-ATPase. Antimicrob. Agents Chemother. 54, 4335–4342. 10.1128/AAC.01620-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarkina N.; Konstantinov A. A. (2002) Stimulation of Menaquinone-Dependent Electron Transfer in the Respiratory Chain of Bacillus Subtilis by Membrane Energization. J. Bacteriol. 184, 5339–5347. 10.1128/JB.184.19.5339-5347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirawski J.; Unden G. (1998) Menaquinone-Dependent Succinate Dehydrogenase of Bacteria Catalyzes Reversed Electron Transport Driven by the Proton Potential. Eur. J. Biochem. 257, 210–215. 10.1046/j.1432-1327.1998.2570210.x. [DOI] [PubMed] [Google Scholar]
- Mesa-Arango A. C.; Trevijano-Contador N.; Roman E.; Sanchez-Fresneda R.; Casas C.; Herrero E.; Arguelles J. C.; Pla J.; Cuenca-Estrella M.; Zaragoza O. (2014) The Production of Reactive Oxygen Species Is a Universal Action Mechanism of Amphotericin B against Pathogenic Yeasts and Contributes to the Fungicidal Effect of This Drug. Antimicrob. Agents Chemother. 58, 6627–6638. 10.1128/AAC.03570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T.; Nakayama-Imaohji H.; Elahi M.; Tada A.; Toyonaga E.; Yamasaki H.; Okazaki K.; Miyoshi H.; Tsuchiya K.; Kuwahara T. (2018) Lhistidine Augments the Oxidative Damage against Gram-negative Bacteria by Hydrogen Peroxide. Int. J. Mol. Med. 41, 2847–2854. 10.3892/ijmm.2018.3473. [DOI] [PubMed] [Google Scholar]
- Beavers W. N.; Skaar E. P. (2016) Neutrophil-Generated Oxidative Stress and Protein Damage in Staphylococcus Aureus. Pathog. Dis. 74, ftw060. 10.1093/femspd/ftw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein J.; Hoffmann A.; Lilie H.; Schmidt R.; Rubsamen-Waigmann H.; Brotz-Oesterhelt H.; Mogk A.; Turgay K. (2009) The Antibiotic ADEP Reprogrammes ClpP, Switching It from a Regulated to an Uncontrolled Protease. EMBO Mol. Med. 1, 37–49. 10.1002/emmm.200900002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappenbach J. A.; Saxman P. R.; Cole J. R.; Schmidt T. M. (2001) Rrndb: The Ribosomal RNA Operon Copy Number Database. Nucleic Acids Res. 29, 181–184. 10.1093/nar/29.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. (1977) Properties of the Penicillin-Binding Proteins of Escherichia Coli K12. Eur. J. Biochem. 72, 341–352. 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Denome S. A.; Elf P. K.; Henderson T. A.; Nelson D. E.; Young K. D. (1999) Escherichia Coli Mutants Lacking All Possible Combinations of Eight Penicillin Binding Proteins: Viability, Characteristics, and Implications for Peptidoglycan Synthesis. J. Bacteriol. 181, 3981–3993. 10.1128/JB.181.13.3981-3993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaoglu O.; Carlson E. E. (2015) Profiling of β-Lactam Selectivity for Penicillin-Binding Proteins in Escherichia Coli Strain DC2. Antimicrob. Agents Chemother. 59, 2785–2790. 10.1128/AAC.04552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W.; Brown J. R.; Sylvester D. R.; Huang J.; Chalker A. F.; So C. Y.; Holmes D. J.; Payne D. J.; Wallis N. G. (2000) Two Active Forms of UDP-N-Acetylglucosamine Enolpyruvyl Transferase in Gram-Positive Bacteria. J. Bacteriol. 182, 4146–4152. 10.1128/JB.182.15.4146-4152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M.; Kawahara Y.; Ichikawa A.; Matoba Y.; Matsuo H.; Lee D.-G.; Kumagai T.; Sugiyama M. (2004) Self-Protection Mechanism in D-Cycloserine-Producing Streptomyces Lavendulae. Gene Cloning, Characterization, and Kinetics of Its Alanine Racemase and D-Alanyl-D-Alanine Ligase, Which Are Target Enzymes of D-Cycloserine. J. Biol. Chem. 279, 46143–46152. 10.1074/jbc.M404603200. [DOI] [PubMed] [Google Scholar]
- Rudolf J. D.; Dong L.-B.; Shen B. (2017) Platensimycin and Platencin: Inspirations for Chemistry, Biology, Enzymology, and Medicine. Biochem. Pharmacol. 133, 139–151. 10.1016/j.bcp.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasuriya H.; Herath K. B.; Zhang C.; Zink D. L.; Basilio A.; Genilloud O.; Diez M. T.; Vicente F.; Gonzalez I.; Salazar O.; Pelaez F.; Cummings R.; Ha S.; Wang J.; Singh S. B. (2007) Isolation and Structure of Platencin: A FabH and FabF Dual Inhibitor with Potent Broad-Spectrum Antibiotic Activity. Angew. Chem., Int. Ed. 46, 4684–4688. 10.1002/anie.200701058. [DOI] [PubMed] [Google Scholar]
- Wang J.; Kodali S.; Lee S. H.; Galgoci A.; Painter R.; Dorso K.; Racine F.; Motyl M.; Hernandez L.; Tinney E.; et al. (2007) Discovery of Platencin, a Dual FabF and FabH Inhibitor with in Vivo Antibiotic Properties. Proc. Natl. Acad. Sci. U. S. A. 104, 7612–7616. 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkov T.; Roberts K. D.; Nation R. L.; Thompson P. E.; Li J. (2013) Pharmacology of Polymyxins: New Insights into an “old” Class of Antibiotics. Future Microbiol. 8, 711–724. 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L.; Wan M.; Zhang S.; Gao L.; Fang W. (2020) Polymyxin B Loosens Lipopolysaccharide Bilayer but Stiffens Phospholipid Bilayer. Biophys. J. 118, 138–150. 10.1016/j.bpj.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deris Z. Z.; Akter J.; Sivanesan S.; Roberts K. D.; Thompson P. E.; Nation R. L.; Li J.; Velkov T. (2014) A Secondary Mode of Action of Polymyxins against Gram-Negative Bacteria Involves the Inhibition of NADH-Quinone Oxidoreductase Activity. J. Antibiot. 67, 147–151. 10.1038/ja.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart M. C., Kouimtzi M., and Hill S. (2009) WHO Model Formulary 2008, WHO, Geneva. [Google Scholar]
- Arbiser J. L.; Moschella S. L. (1995) Clofazimine: A Review of Its Medical Uses and Mechanisms of Action. J. Am. Acad. Dermatol. 32, 241–247. 10.1016/0190-9622(95)90134-5. [DOI] [PubMed] [Google Scholar]
- Lesnik U.; Lukezic T.; Podgorsek A.; Horvat J.; Polak T.; Sala M.; Jenko B.; Harmrolfs K.; Ocampo-Sosa A.; Martinez-Martinez L.; Herron P. R.; Fujs S.; Kosec G.; Hunter I. S.; Muller R.; Petkovic H. (2015) Construction of a New Class of Tetracycline Lead Structures with Potent Antibacterial Activity through Biosynthetic Engineering. Angew. Chem., Int. Ed. 54, 3937–3940. 10.1002/anie.201411028. [DOI] [PubMed] [Google Scholar]
- Herrmann J.; Lukezic T.; Kling A.; Baumann S.; Huttel S.; Petkovic H.; Muller R. (2016) Strategies for the Discovery and Development of New Antibiotics from Natural Products: Three Case Studies. Curr. Top. Microbiol. Immunol. 398, 339–363. 10.1007/82_2016_498. [DOI] [PubMed] [Google Scholar]
- Stepanek J. J.; Lukezic T.; Teichert I.; Petkovic H.; Bandow J. E. (2016) Dual Mechanism of Action of the Atypical Tetracycline Chelocardin. Biochim. Biophys. Acta, Proteins Proteomics 1864, 645–654. 10.1016/j.bbapap.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Herskovits A. A.; Bibi E. (2000) Association of Escherichia Coli Ribosomes with the Inner Membrane Requires the Signal Recognition Particle Receptor but Is Independent of the Signal Recognition Particle. Proc. Natl. Acad. Sci. U. S. A. 97, 4621–4626. 10.1073/pnas.080077197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhr E.; Sahl H. G. (1985) Mode of Action of the Peptide Antibiotic Nisin and Influence on the Membrane Potential of Whole Cells and on Cytoplasmic and Artificial Membrane Vesicles. Antimicrob. Agents Chemother. 27, 841–845. 10.1128/AAC.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukink E.; Wiedemann I.; van Kraaij C.; Kuipers O. P.; Sahl H. G.; de Kruijff B. (1999) Use of the Cell Wall Precursor Lipid II by a Pore-Forming Peptide Antibiotic. Science 286, 2361–2364. 10.1126/science.286.5448.2361. [DOI] [PubMed] [Google Scholar]
- Hasper H. E.; de Kruijff B.; Breukink E. (2004) Assembly and Stability of Nisin-Lipid II Pores. Biochemistry 43, 11567–11575. 10.1021/bi049476b. [DOI] [PubMed] [Google Scholar]
- ’t Hart P.; Oppedijk S. F.; Breukink E.; Martin N. I. (2016) New Insights into Nisin’s Antibacterial Mechanism Revealed by Binding Studies with Synthetic Lipid II Analogues. Biochemistry 55, 232–237. 10.1021/acs.biochem.5b01173. [DOI] [PubMed] [Google Scholar]
- Bonev B. B.; Chan W. C.; Bycroft B. W.; Roberts G. C.; Watts A. (2000) Interaction of the Lantibiotic Nisin with Mixed Lipid Bilayers: A 31P and 2H NMR Study. Biochemistry 39, 11425–11433. 10.1021/bi0001170. [DOI] [PubMed] [Google Scholar]
- Christ K.; Al-Kaddah S.; Wiedemann I.; Rattay B.; Sahl H.-G.; Bendas G. (2008) Membrane Lipids Determine the Antibiotic Activity of the Lantibiotic Gallidermin. J. Membr. Biol. 226, 9–16. 10.1007/s00232-008-9134-4. [DOI] [PubMed] [Google Scholar]
- Muraih J. K.; Palmer M. (2012) Estimation of the Subunit Stoichiometry of the Membrane-Associated Daptomycin Oligomer by FRET. Biochim. Biophys. Acta, Biomembr. 1818, 1642–1647. 10.1016/j.bbamem.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Muraih J. K.; Pearson A.; Silverman J.; Palmer M. (2011) Oligomerization of Daptomycin on Membranes. Biochim. Biophys. Acta, Biomembr. 1808, 1154–1160. 10.1016/j.bbamem.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Muraih J. K.; Harris J.; Taylor S. D.; Palmer M. (2012) Characterization of Daptomycin Oligomerization with Perylene Excimer Fluorescence: Stoichiometric Binding of Phosphatidylglycerol Triggers Oligomer Formation. Biochim. Biophys. Acta, Biomembr. 1818, 673–678. 10.1016/j.bbamem.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Kreutzberger M. A.; Pokorny A.; Almeida P. F. (2017) Daptomycin-Phosphatidylglycerol Domains in Lipid Membranes. Langmuir 33, 13669–13679. 10.1021/acs.langmuir.7b01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domalaon R.; Idowu T.; Zhanel G. G.; Schweizer F. (2018) Antibiotic Hybrids: The Next Generation of Agents and Adjuvants against Gram-Negative Pathogens?. Clin. Microbiol. Rev. 31, e00077-17 10.1128/CMR.00077-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H.; Bertasso A.; Chan K. K.; Chapman J. S.; Cleeland R.; Cummings L. M.; Dix B. A.; Keith D. D. (1989) Mode of Action of the Dual-Action Cephalosporin Ro 23–9424. Antimicrob. Agents Chemother. 33, 1067–1071. 10.1128/AAC.33.7.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone G. W.; Zhang Q.; Castillo R.; Doppalapudi V. R.; Bueno A. R.; Lee J. Y.; Li Q.; Sergeeva M.; Khambatta G.; Georgopapadakou N. H. (2004) Mechanism of Action of NB2001 and NB2030, Novel Antibacterial Agents Activated by Beta-Lactamases. Antimicrob. Agents Chemother. 48, 477–483. 10.1128/AAC.48.2.477-483.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.; Lynch A. S. (2016) Development of a Dual-Acting Antibacterial Agent (TNP-2092) for the Treatment of Persistent Bacterial Infections. J. Med. Chem. 59, 6645–6657. 10.1021/acs.jmedchem.6b00485. [DOI] [PubMed] [Google Scholar]
- Gupta V.; Datta P. (2019) Next-Generation Strategy for Treating Drug Resistant Bacteria: Antibiotic Hybrids. Indian J. Med. Res. 149, 97–106. 10.4103/ijmr.IJMR_755_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres B. T.; Basseres E.; Alam M. J.; Garey K. W. (2017) Cadazolid for the Treatment of Clostridium Difficile. Expert Opin. Invest. Drugs 26, 509–514. 10.1080/13543784.2017.1304538. [DOI] [PubMed] [Google Scholar]
- Stryjewski M. E.; Potgieter P. D.; Li Y.-P.; Barriere S. L.; Churukian A.; Kingsley J.; Corey G. R. (2012) TD-1792 versus Vancomycin for Treatment of Complicated Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 56, 5476–5483. 10.1128/AAC.00712-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb E. (accessed 2020-03-10) FDA grants QIDP and Fast Track Designations to MCB3837, Morphochem’s novel intravenous antibacterial to treat C. difficile infections, https://www.tvm-lifescience.com/fda-grants-qidp-fast-track-designations-mcb3837-morphochems-novel-intravenous-antibacterial-treat-c-difficile-infections/.
- Parkes A. L.; Yule I. A. (2016) Hybrid Antibiotics - Clinical Progress and Novel Designs. Expert Opin. Drug Discovery 11, 665–680. 10.1080/17460441.2016.1187597. [DOI] [PubMed] [Google Scholar]
- Locher H. H.; Seiler P.; Chen X.; Schroeder S.; Pfaff P.; Enderlin M.; Klenk A.; Fournier E.; Hubschwerlen C.; Ritz D.; Kelly C. P.; Keck W. (2014) In Vitro and in Vivo Antibacterial Evaluation of Cadazolid, a New Antibiotic for Treatment of Clostridium Difficile Infections. Antimicrob. Agents Chemother. 58, 892–900. 10.1128/AAC.01830-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher H. H.; Caspers P.; Bruyère T.; Schroeder S.; Pfaff P.; Knezevic A.; Keck W.; Ritz D. (2014) Investigations of the Mode of Action and Resistance Development of Cadazolid, a New Antibiotic for Treatment of Clostridium Difficile Infections. Antimicrob. Agents Chemother. 58, 901–908. 10.1128/AAC.01831-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthner K. D.; Vidaillac C.; Cheung C. M.; Rybak M. J. (2010) In Vitro Activity of the New Multivalent Glycopeptide-Cephalosporin Antibiotic TD-1792 against Vancomycin-Nonsusceptible Staphylococcus Isolates. Antimicrob. Agents Chemother. 54, 3799–3803. 10.1128/AAC.00452-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais J.; Lewis S. R.; Krause K. M.; Benton B. M. (2012) Antistaphylococcal Activity of TD-1792, a Multivalent Glycopeptide-Cephalosporin Antibiotic. Antimicrob. Agents Chemother. 56, 1584–1587. 10.1128/AAC.05532-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrovskaya V.; Baasov T. (2010) Dual-Acting Hybrid Antibiotics: A Promising Strategy to Combat Bacterial Resistance. Expert Opin. Drug Discovery 5, 883–902. 10.1517/17460441.2010.508069. [DOI] [PubMed] [Google Scholar]
- Deinove . (accessed 2020-03-10) DNV3837/DNV3681: First-in-class antibiotic candidate, https://www.deinove.com/en/antibiotics/portfolio/dnv3837/dnv3681.
- Deinove . (accessed 2020-03-10) An Exploratory, Open-Label, Oligo-Center Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of Intravenous DNV3837 in Subjects With Clostridium Difficile Infection, https://clinicaltrials.gov/ct2/show/NCT03988855?term=DNV3681&draw=2&rank=1.
- Wang B.; Zhao Q.; Yin W.; Yuan Y.; Wang X.; Wang Y.-H.; Wang H.; Ye W.; Chen S.; Guo H.-L.; Xie Y. (2018) In-Vitro Characterisation of a Novel Antimicrobial Agent, TNP-2092, against Helicobacter Pylori Clinical Isolates. Swiss Med. Wkly. 148, w14630 10.4414/smw.2018.14630. [DOI] [PubMed] [Google Scholar]
- Burrows L. L. (2018) The Therapeutic Pipeline for Pseudomonas Aeruginosa Infections. ACS Infect. Dis. 4, 1041–1047. 10.1021/acsinfecdis.8b00112. [DOI] [PubMed] [Google Scholar]
- Luther A.; Urfer M.; Zahn M.; Muller M.; Wang S.-Y.; Mondal M.; Vitale A.; Hartmann J.-B.; Sharpe T.; Monte F. Lo; et al. (2019) Chimeric Peptidomimetic Antibiotics against Gram-Negative Bacteria. Nature 576, 452–458. 10.1038/s41586-019-1665-6. [DOI] [PubMed] [Google Scholar]
- Zhu Y. I.; Stiller M. J. (2001) Dapsone and Sulfones in Dermatology: Overview and Update. J. Am. Acad. Dermatol. 45, 420–434. 10.1067/mjd.2001.114733. [DOI] [PubMed] [Google Scholar]
- Trivedi H. D.; Lizaola B.; Tapper E. B.; Bonder A. (2017) Management of Pruritus in Primary Biliary Cholangitis: A Narrative Review. Am. J. Med. 130, 744.e1–744.e7. 10.1016/j.amjmed.2017.01.037. [DOI] [PubMed] [Google Scholar]
- Nakano T.; Hiramatsu K.; Kishi K.; Hirata N.; Kadota J.-I.; Nasu M. (2003) Clindamycin Modulates Inflammatory-Cytokine Induction in Lipopolysaccharide-Stimulated Mouse Peritoneal Macrophages. Antimicrob. Agents Chemother. 47, 363–367. 10.1128/AAC.47.1.363-367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg C. E.; Gatner E. M.; Imkamp F. M.; Anderson R. (1982) Effects of Clofazimine Alone or Combined with Dapsone on Neutrophil and Lymphocyte Functions in Normal Individuals and Patients with Lepromatous Leprosy. Antimicrob. Agents Chemother. 21, 693–697. 10.1128/AAC.21.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatner E. M.; Anderson R.; van Remsburg C. E.; Imkamp F. M. (1982) The in Vitro and in Vivo Effects of Clofazimine on the Motility of Neutrophils and Transformation of Lymphocytes from Normal Individuals. Lepr. Rev. 53, 85–90. 10.5935/0305-7518.19820010. [DOI] [PubMed] [Google Scholar]
- Zimmermann P.; Ziesenitz V. C.; Curtis N.; Ritz N. (2018) The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. Front. Immunol. 9, 302. 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale T. R.; Tan J. S. (2005) Nonantimicrobial Effects of Antibacterial Agents. Clin. Infect. Dis. 40, 127–135. 10.1086/426545. [DOI] [PubMed] [Google Scholar]
- Tauber S. C.; Nau R. (2008) Immunomodulatory Properties of Antibiotics. Curr. Mol. Pharmacol. 1, 68–79. 10.2174/1874467210801010068. [DOI] [PubMed] [Google Scholar]
- Ye Y.; Xia Z.; Zhang D.; Sheng Z.; Zhang P.; Zhu H.; Xu N.; Liang S. (2019) Multifunctional Pharmaceutical Effects of the Antibiotic Daptomycin. BioMed Res. Int. 2019, 8609218. 10.1155/2019/8609218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S. C. J.; Zasowski E. J.; Trinh T. D.; Lagnf A. M.; Bhatia S.; Sabagha N.; Abdul-Mutakabbir J. C.; Alosaimy S.; Mynatt R. P.; Davis S. L.; Rybak M. J. (2019) Daptomycin plus Beta-Lactam Combination Therapy for Methicillin-Resistant Staphylococcus Aureus Bloodstream Infections: A Retrospective, Comparative Cohort Study. Clin. Infect. Dis. ciz746 10.1093/cid/ciz746. [DOI] [PubMed] [Google Scholar]
- Meletiadis J.; Petraitis V.; Petraitiene R.; Lin P.; Stergiopoulou T.; Kelaher A. M.; Sein T.; Schaufele R. L.; Bacher J.; Walsh T. J. (2006) Triazole-Polyene Antagonism in Experimental Invasive Pulmonary Aspergillosis: In Vitro and in Vivo Correlation. J. Infect. Dis. 194, 1008–1018. 10.1086/506617. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bello C. (2017) Antibiotic Adjuvants - A Strategy to Unlock Bacterial Resistance to Antibiotics. Bioorg. Med. Chem. Lett. 27, 4221–4228. 10.1016/j.bmcl.2017.08.027. [DOI] [PubMed] [Google Scholar]
- Kwapong A. A.; Stapleton P.; Gibbons S. (2019) Inhibiting Plasmid Mobility: The Effect of Isothiocyanates on Bacterial Conjugation. Int. J. Antimicrob. Agents 53, 629–636. 10.1016/j.ijantimicag.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Zhao W. H.; Hu Z. Q.; Okubo S.; Hara Y.; Shimamura T. (2001) Mechanism of Synergy between Epigallocatechin Gallate and Beta-Lactams against Methicillin-Resistant Staphylococcus Aureus. Antimicrob. Agents Chemother. 45, 1737–1742. 10.1128/AAC.45.6.1737-1742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand K. H.; Houck H. (2004) Daptomycin Synergy with Rifampicin and Ampicillin against Vancomycin-Resistant Enterococci. J. Antimicrob. Chemother. 53, 530–532. 10.1093/jac/dkh104. [DOI] [PubMed] [Google Scholar]
- Paul T. R.; Venter A.; Blaszczak L. C.; Parr T. R. J.; Labischinski H.; Beveridge T. J. (1995) Localization of Penicillin-Binding Proteins to the Splitting System of Staphylococcus Aureus Septa by Using a Mercury-Penicillin V Derivative. J. Bacteriol. 177, 3631–3640. 10.1128/JB.177.13.3631-3640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebricher C. K.; Druminski M. (1980) Inhibition of RNA Polymerase Activity by the Escherichia Coli Protein Biosynthesis Elongation Factor Ts. Proc. Natl. Acad. Sci. U. S. A. 77, 866–869. 10.1073/pnas.77.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasher M. A.; Hay R. J. (1998) Synergy of Antibiotics against Streptomyces Somaliensis Isolates in Vitro. J. Antimicrob. Chemother. 41, 281–284. 10.1093/jac/41.2.281. [DOI] [PubMed] [Google Scholar]
- Lowe P. A.; Malcolm A. D. (1976) Rifampicin Binding as a Probe for Subunit Interactions in Escherchia Coli RNA Polymerase. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 454, 129–137. 10.1016/0005-2787(76)90360-9. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S.; Lee H.; Ochoa S. (1974) Inhibition of Polypeptide Chain Initiation in Escherichia Coli by Elongation Factor G. Proc. Natl. Acad. Sci. U. S. A. 71, 2928–2931. 10.1073/pnas.71.8.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinos G. P.; Connell S. R.; Nierhaus K. H.; Kalpaxis D. L. (2003) Erythromycin, Roxithromycin, and Clarithromycin: Use of Slow-Binding Kinetics to Compare Their in Vitro Interaction with a Bacterial Ribosomal Complex Active in Peptide Bond Formation. Mol. Pharmacol. 63, 617–623. 10.1124/mol.63.3.617. [DOI] [PubMed] [Google Scholar]
- Allen N. E.; Epp J. K. (1978) Mechanism of Penicillin-Erythromycin Synergy on Antibiotic-Resistant Staphylococcus Aureus. Antimicrob. Agents Chemother. 13, 849–853. 10.1128/AAC.13.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignard B.; Entenza J. M.; Moreillon P. (2005) Beta-Lactams against Methicillin-Resistant Staphylococcus Aureus. Curr. Opin. Pharmacol. 5, 479–489. 10.1016/j.coph.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brandt C. M.; Rouse M. S.; Laue N. W.; Stratton C. W.; Wilson W. R.; Steckelberg J. M. (1996) Effective Treatment of Multidrug-Resistant Enterococcal Experimental Endocarditis with Combinations of Cell Wall-Active Agents. J. Infect. Dis. 173, 909–913. 10.1093/infdis/173.4.909. [DOI] [PubMed] [Google Scholar]
- Drew R. H.; Gallis H. A. (1992) Azithromycin--Spectrum of Activity, Pharmacokinetics, and Clinical Applications. Pharmacotherapy 12, 161–173. 10.1002/j.1875-9114.1992.tb04504.x. [DOI] [PubMed] [Google Scholar]
- Ono S.; Muratani T.; Matsumoto T. (2005) Mechanisms of Resistance to Imipenem and Ampicillin in Enterococcus Faecalis. Antimicrob. Agents Chemother. 49, 2954–2958. 10.1128/AAC.49.7.2954-2958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W.; Hamilton-Miller J. M. (1993) Reassessment of the Rationale for the Combinations of Sulphonamides with Diaminopyrimidines. J. Chemother. 5, 465–469. 10.1080/1120009X.1993.11741097. [DOI] [PubMed] [Google Scholar]
- Voeller D.; Kovacs J.; Andrawis V.; Chu E.; Masur H.; Allegra C. (1994) Interaction of Pneumocystis Carinii Dihydropteroate Synthase with Sulfonamides and Diaminodiphenyl Sulfone (Dapsone). J. Infect. Dis. 169, 456–459. 10.1093/infdis/169.2.456. [DOI] [PubMed] [Google Scholar]
- Greenwood D.; O’Grady F. (1976) Activity and Interaction of Trimethoprim and Sulphamethoxazole against Escherichia Coli. J. Clin. Pathol. 29, 162–166. 10.1136/jcp.29.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura M.; Nakazawa H.; Hashimoto T.; Mitsuhashi S. (1980) Combined Antibacterial Activity of Amoxicillin with Clavulanic Acid against Ampicillin-Resistant Strains. Antimicrob. Agents Chemother. 17, 908–911. 10.1128/AAC.17.6.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R. N.; Carmine A.; Heel R. C.; Morley P. A.; Speight T. M.; Avery G. S. (1981) Amoxycillin/Clavulanic Acid: A Review of Its Antibacterial Activity, Pharmacokinetics and Therapeutic Use. Drugs 22, 337–362. 10.2165/00003495-198122050-00001. [DOI] [PubMed] [Google Scholar]
- Breidenstein E. B. M.; Courvalin P.; Meziane-Cherif D. (2015) Antimicrobial Activity of Plectasin NZ2114 in Combination with Cell Wall Targeting Antibiotics Against VanA-Type Enterococcus Faecalis. Microb. Drug Resist. 21, 373–379. 10.1089/mdr.2014.0221. [DOI] [PubMed] [Google Scholar]
- Schneider T.; Sahl H. G. (2010) An Oldie but a Goodie - Cell Wall Biosynthesis as Antibiotic Target Pathway. Int. J. Med. Microbiol. 300, 161–169. 10.1016/j.ijmm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Steinmann J.; Buer J.; Pietschmann T.; Steinmann E. (2013) Anti-Infective Properties of Epigallocatechin-3-Gallate (EGCG), a Component of Green Tea. Br. J. Pharmacol. 168, 1059–1073. 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.-H.; Hu Z.-Q.; Hara Y.; Shimamura T. (2002) Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus Aureus. Antimicrob. Agents Chemother. 46, 2266–2268. 10.1128/AAC.46.7.2266-2268.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti A. D.; Theisen E.; Sauer J.-D.; Nonejuie P.; Olson J.; Pogliano J.; Sakoulas G.; Nizet V.; Proctor R. A.; Rose W. E. (2016) Penicillin Binding Protein 1 Is Important in the Compensatory Response of Staphylococcus Aureus to Daptomycin-Induced Membrane Damage and Is a Potential Target for Beta-Lactam-Daptomycin Synergy. Antimicrob. Agents Chemother. 60, 451–458. 10.1128/AAC.02071-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. R.; Barber K. E.; Raut A.; Aboutaleb M.; Sakoulas G.; Rybak M. J. (2015) Beta-Lactam Combinations with Daptomycin Provide Synergy against Vancomycin-Resistant Enterococcus Faecalis and Enterococcus Faecium. J. Antimicrob. Chemother. 70, 1738–1743. 10.1093/jac/dkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppen B. C.; Mulder P. P. G.; de Boer L.; Riool M.; Drijfhout J. W.; Zaat S. A. J. (2019) Synergistic Microbicidal Effect of Cationic Antimicrobial Peptides and Teicoplanin against Planktonic and Biofilm-Encased Staphylococcus Aureus. Int. J. Antimicrob. Agents 53, 143–151. 10.1016/j.ijantimicag.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Fernandez-Cuenca F.; Martinez-Martinez L.; Pascual A.; Perea E. J. (2003) In Vitro Activity of Azithromycin in Combination with Amikacin, Ceftazidime, Ciprofloxacin or Imipenem against Clinical Isolates of Acinobacter Baumannii. Chemotherapy 49, 24–26. 10.1159/000069774. [DOI] [PubMed] [Google Scholar]
- Sertcelik A., Baran I., Akinci E., Mumcuoglu I., and Bodur H. (2019) Synergistic Activities of Colistin Combinations with Meropenem, Sulbactam, Minocycline, Disodium Fosfomycin, or Vancomycin Against Different Clones of Carbapenem-Resistant Acinetobacter Baumannii Strains. Microb. Drug Resist. 10.1089/mdr.2019.0088. [DOI] [PubMed] [Google Scholar]
- Langeveld W. T.; Veldhuizen E. J. A.; Burt S. A. (2014) Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 40, 76–94. 10.3109/1040841X.2013.763219. [DOI] [PubMed] [Google Scholar]
- Owen L.; Laird K. (2018) Synchronous Application of Antibiotics and Essential Oils: Dual Mechanisms of Action as a Potential Solution to Antibiotic Resistance. Crit. Rev. Microbiol. 44, 414–435. 10.1080/1040841X.2018.1423616. [DOI] [PubMed] [Google Scholar]
- Mathur H.; Field D.; Rea M. C.; Cotter P. D.; Hill C.; Ross R. P. (2017) Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective. Front. Microbiol. 8, 1205. 10.3389/fmicb.2017.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolska K. I.; Grzes K.; Kurek A. (2012) Synergy between Novel Antimicrobials and Conventional Antibiotics or Bacteriocins. Pol. J. Microbiol. 61, 95–104. 10.33073/pjm-2012-012. [DOI] [PubMed] [Google Scholar]
- Wittekind M.; Schuch R. (2016) Cell Wall Hydrolases and Antibiotics: Exploiting Synergy to Create Efficacious New Antimicrobial Treatments. Curr. Opin. Microbiol. 33, 18–24. 10.1016/j.mib.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Zusman O.; Avni T.; Leibovici L.; Adler A.; Friberg L.; Stergiopoulou T.; Carmeli Y.; Paul M. (2013) Systematic Review and Meta-Analysis of in Vitro Synergy of Polymyxins and Carbapenems. Antimicrob. Agents Chemother. 57, 5104–5111. 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard J. R.; Nation R. L.; Tsuji B. T. (2016) Synergistic Combinations of Polymyxins. Int. J. Antimicrob. Agents 48, 607–613. 10.1016/j.ijantimicag.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakomi H.-L.; Saarela M.; Helander I. M. (2003) Effect of EDTA on Salmonella Enterica Serovar Typhimurium Involves a Component Not Assignable to Lipopolysaccharide Release. Microbiology 149, 2015–2021. 10.1099/mic.0.26312-0. [DOI] [PubMed] [Google Scholar]
- Vaara M. (1992) Agents That Increase the Permeability of the Outer Membrane. Microbiol. Rev. 56, 395–411. 10.1128/MMBR.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellio P.; Luzi C.; Mancini A.; Cracchiolo S.; Passacantando M.; Di Pietro L.; Perilli M.; Amicosante G.; Santucci S.; Celenza G. (2018) Cerium Oxide Nanoparticles as Potential Antibiotic Adjuvant. Effects of CeO2 Nanoparticles on Bacterial Outer Membrane Permeability. Biochim. Biophys. Acta, Biomembr. 1860, 2428–2435. 10.1016/j.bbamem.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Saikia K.; Chaudhary N. (2018) Antimicrobial Peptides from C-Terminal Amphipathic Region of E. Coli FtsA. Biochim. Biophys. Acta, Biomembr. 1860, 2506–2514. 10.1016/j.bbamem.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Wiese A.; Gutsmann T.; Seydel U. (2003) Towards Antibacterial Strategies: Studies on the Mechanisms of Interaction between Antibacterial Peptides and Model Membranes. J. Endotoxin Res. 9, 67–84. 10.1179/096805103125001441. [DOI] [PubMed] [Google Scholar]
- Vaara M.; Porro M. (1996) Group of Peptides That Act Synergistically with Hydrophobic Antibiotics against Gram-Negative Enteric Bacteria. Antimicrob. Agents Chemother. 40, 1801–1805. 10.1128/AAC.40.8.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M.; Kamei R.; Komagoe K.; Inoue T.; Yamada K.; Katsu T. (2012) In Situ Potentiometric Method to Evaluate Bacterial Outer Membrane-Permeabilizing Ability of Drugs: Example Using Antiprotozoal Diamidines. J. Microbiol. Methods 91, 497–500. 10.1016/j.mimet.2012.09.033. [DOI] [PubMed] [Google Scholar]
- Stokes J. M.; MacNair C. R.; Ilyas B.; French S.; Cote J.-P.; Bouwman C.; Farha M. A.; Sieron A. O.; Whitfield C.; Coombes B. K.; Brown E. D. (2017) Pentamidine Sensitizes Gram-Negative Pathogens to Antibiotics and Overcomes Acquired Colistin Resistance. Nat. Microbiol. 2, 17028. 10.1038/nmicrobiol.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlagadda V.; Manjunath G. B.; Sarkar P.; Akkapeddi P.; Paramanandham K.; Shome B. R.; Ravikumar R.; Haldar J. (2016) Glycopeptide Antibiotic To Overcome the Intrinsic Resistance of Gram-Negative Bacteria. ACS Infect. Dis. 2, 132–139. 10.1021/acsinfecdis.5b00114. [DOI] [PubMed] [Google Scholar]
- Zhou L.; van Heel A. J.; Montalban-Lopez M.; Kuipers O. P. (2016) Potentiating the Activity of Nisin against Escherichia Coli. Front. Cell Dev. Biol. 4, 7. 10.3389/fcell.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Montalban-Lopez M.; Kuipers O. P. (2018) Increasing the Antimicrobial Activity of Nisin-Based Lantibiotics against Gram-Negative Pathogens. Appl. Environ. Microbiol. 84, e00052-18 10.1128/AEM.00052-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F. (2019) Enhancing Uptake of Antibiotics into Gram-Negative Bacteria Using Nonribosome-Targeting Aminoglycoside-Based Adjuvants. Future Med. Chem. 11, 1519–1522. 10.4155/fmc-2019-0131. [DOI] [PubMed] [Google Scholar]
- Raulston J. E.; Montie T. C. (1989) Early Cell Envelope Alterations by Tobramycin Associated with Its Lethal Action on Pseudomonas Aeruginosa. Microbiology 135, 3023–3034. 10.1099/00221287-135-11-3023. [DOI] [PubMed] [Google Scholar]
- Bulitta J. B.; Ly N. S.; Landersdorfer C. B.; Wanigaratne N. A.; Velkov T.; Yadav R.; Oliver A.; Martin L.; Shin B. S.; Forrest A.; Tsuji B. T. (2015) Two Mechanisms of Killing of Pseudomonas Aeruginosa by Tobramycin Assessed at Multiple Inocula via Mechanism-Based Modeling. Antimicrob. Agents Chemother. 59, 2315–2327. 10.1128/AAC.04099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Goswami S.; Gorityala B. K.; Domalaon R.; Lyu Y.; Kumar A.; Zhanel G. G.; Schweizer F. (2017) A Tobramycin Vector Enhances Synergy and Efficacy of Efflux Pump Inhibitors against Multidrug-Resistant Gram-Negative Bacteria. J. Med. Chem. 60, 3913–3932. 10.1021/acs.jmedchem.7b00156. [DOI] [PubMed] [Google Scholar]
- Gorityala B. K.; Guchhait G.; Goswami S.; Fernando D. M.; Kumar A.; Zhanel G. G.; Schweizer F. (2016) Hybrid Antibiotic Overcomes Resistance in P. Aeruginosa by Enhancing Outer Membrane Penetration and Reducing Efflux. J. Med. Chem. 59, 8441–8455. 10.1021/acs.jmedchem.6b00867. [DOI] [PubMed] [Google Scholar]
- Gorityala B. K.; Guchhait G.; Fernando D. M.; Deo S.; McKenna S. A.; Zhanel G. G.; Kumar A.; Schweizer F. (2016) Adjuvants Based on Hybrid Antibiotics Overcome Resistance in Pseudomonas Aeruginosa and Enhance Fluoroquinolone Efficacy. Angew. Chem., Int. Ed. 55, 555–559. 10.1002/anie.201508330. [DOI] [PubMed] [Google Scholar]
- Idowu T.; Ammeter D.; Arthur G.; Zhanel G. G.; Schweizer F. (2019) Potentiation of Beta-Lactam Antibiotics and Beta-Lactam/Beta-Lactamase Inhibitor Combinations against MDR and XDR Pseudomonas Aeruginosa Using Non-Ribosomal Tobramycin-Cyclam Conjugates. J. Antimicrob. Chemother. 74, 2640–2648. 10.1093/jac/dkz228. [DOI] [PubMed] [Google Scholar]
- Idowu T.; Zhanel G. G.; Schweizer F. (2020) A Dimer, but Not Monomer, of Tobramycin Potentiates Ceftolozane against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas Aeruginosa and Delays Resistance Development. Antimicrob. Agents Chemother. 64, e02055-19 10.1128/AAC.02055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia K.; Chaudhary N. (2018) Interaction of MreB-Derived Antimicrobial Peptides with Membranes. Biochem. Biophys. Res. Commun. 498, 58–63. 10.1016/j.bbrc.2018.02.176. [DOI] [PubMed] [Google Scholar]
- Saikia K.; Sravani Y. D.; Ramakrishnan V.; Chaudhary N. (2017) Highly Potent Antimicrobial Peptides from N-Terminal Membrane-Binding Region of E. Coli MreB. Sci. Rep. 7, 42994. 10.1038/srep42994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M. E.; Kasiakou S. K. (2006) Toxicity of Polymyxins: A Systematic Review of the Evidence from Old and Recent Studies. Crit. Care 10, R27–R27. 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattimo M. de F. F.; Watanabe M.; da Fonseca C. D.; Neiva L. B. de M.; Pessoa E. A.; Borges F. T. (2016) Polymyxin B Nephrotoxicity: From Organ to Cell Damage. PLoS One 11, e0161057 10.1371/journal.pone.0161057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirri G.; Giuliani A.; Nicoletto S. F.; Pizzuto L.; Rinaldi A. C. (2009) Lipopeptides as Anti-Infectives: A Practical Perspective. Cent. Eur. J. Biol. 4, 258–273. 10.2478/s11535-009-0031-3. [DOI] [Google Scholar]
- Vaara M.; Vaara T. (1983) Polycations Sensitize Enteric Bacteria to Antibiotics. Antimicrob. Agents Chemother. 24, 107–113. 10.1128/AAC.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M.; Vaara T. (1983) Sensitization of Gram-Negative Bacteria to Antibiotics and Complement by a Nontoxic Oligopeptide. Nature 303, 526–528. 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Keirstead N. D.; Wagoner M. P.; Bentley P.; Blais M.; Brown C.; Cheatham L.; Ciaccio P.; Dragan Y.; Ferguson D.; Fikes J.; et al. (2014) Early Prediction of Polymyxin-Induced Nephrotoxicity with next-Generation Urinary Kidney Injury Biomarkers. Toxicol. Sci. 137, 278–291. 10.1093/toxsci/kft247. [DOI] [PubMed] [Google Scholar]
- Danner R. L.; Joiner K. A.; Rubin M.; Patterson W. H.; Johnson N.; Ayers K. M.; Parrillo J. E. (1989) Purification, Toxicity, and Antiendotoxin Activity of Polymyxin B Nonapeptide. Antimicrob. Agents Chemother. 33, 1428–1434. 10.1128/AAC.33.9.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. (2019) Polymyxin Derivatives That Sensitize Gram-Negative Bacteria to Other Antibiotics. Molecules 24, 249. 10.3390/molecules24020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recacha E.; Machuca J.; Diaz de Alba P.; Ramos-Guelfo M.; Docobo-Perez F.; Rodriguez-Beltran J.; Blazquez J.; Pascual A.; Rodriguez-Martinez J. M. (2017) Quinolone Resistance Reversion by Targeting the SOS Response. mBio 8, e00971-17. 10.1128/mBio.00971-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha D.; Patil K. N. (2019) P-Coumaric Acid Inhibits the Listeria Monocytogenes RecA Protein Functions and SOS Response: An Antimicrobial Target. Biochem. Biophys. Res. Commun. 517, 655–661. 10.1016/j.bbrc.2019.07.093. [DOI] [PubMed] [Google Scholar]
- Crane J. K.; Cheema M. B.; Olyer M. A.; Sutton M. D. (2018) Zinc Blockade of SOS Response Inhibits Horizontal Transfer of Antibiotic Resistance Genes in Enteric Bacteria. Front. Cell. Infect. Microbiol. 8, 410. 10.3389/fcimb.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J.; Moyer A.; Peng B.; Wu J.; Hannafon B. N.; Ding W.-Q. (2014) Chloroquine Is a Zinc Ionophore. PLoS One 9, e109180 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C. Y.; Culyba M. J.; Selwood T.; Kubiak J. M.; Hostetler Z. M.; Jurewicz A. J.; Keller P. M.; Pope A. J.; Quinn A.; Schneck J.; Widdowson K. L.; Kohli R. M. (2018) Inhibitors of LexA Autoproteolysis and the Bacterial SOS Response Discovered by an Academic-Industry Partnership. ACS Infect. Dis. 4, 349–359. 10.1021/acsinfecdis.7b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. S. Q.; Ha K. P.; Clarke R. S.; Gavin L.-A.; Cook D. T.; Hutton J. A.; Sutherell C. L.; Edwards A. M.; Evans L. E.; Tate E. W.; Lanyon-Hogg T. (2019) Identification of a Potent Small-Molecule Inhibitor of Bacterial DNA Repair That Potentiates Quinolone Antibiotic Activity in Methicillin-Resistant Staphylococcus Aureus. Bioorg. Med. Chem. 27, 114962. 10.1016/j.bmc.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Hinz A.; Bauerle E.; Angermeyer A.; Juhaszova K.; Kaneko Y.; Singh P. K.; Manoil C. (2009) Targeting a Bacterial Stress Response to Enhance Antibiotic Action. Proc. Natl. Acad. Sci. U. S. A. 106, 14570–14575. 10.1073/pnas.0903619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K.; Gilmour C.; Farha M. A.; Mullen E.; Lau C. H.-F.; Brown E. D. (2016) Potentiation of Aminoglycoside Activity in Pseudomonas Aeruginosa by Targeting the AmgRS Envelope Stress-Responsive Two-Component System. Antimicrob. Agents Chemother. 60, 3509–3518. 10.1128/AAC.03069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikalauskas A.; Parkins M. D.; Poole K. (2017) Rifampicin Potentiation of Aminoglycoside Activity against Cystic Fibrosis Isolates of Pseudomonas Aeruginosa. J. Antimicrob. Chemother. 72, 3349–3352. 10.1093/jac/dkx296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docquier J.-D.; Mangani S. (2018) An Update on Beta-Lactamase Inhibitor Discovery and Development. Drug Resist. Updates 36, 13–29. 10.1016/j.drup.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Chiem K.; Jani S.; Fuentes B.; Lin D. L.; Rasche M. E.; Tolmasky M. E. (2016) Identification of an Inhibitor of the Aminoglycoside 6’-N-Acetyltransferase Type Ib [AAC(6’)-Ib] by Glide Molecular Docking. MedChemComm 7, 184–189. 10.1039/C5MD00316D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya O.; Warren M. S.; Lee A.; Galazzo J.; Fronko R.; Lee M.; Blais J.; Cho D.; Chamberland S.; Renau T.; Leger R.; Hecker S.; Watkins W.; Hoshino K.; Ishida H.; Lee V. J. (2001) Identification and Characterization of Inhibitors of Multidrug Resistance Efflux Pumps in Pseudomonas Aeruginosa: Novel Agents for Combination Therapy. Antimicrob. Agents Chemother. 45, 105–116. 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.; Cohen K. A.; Winglee K.; Maiga M.; Diarra B.; Bishai W. R. (2014) Efflux Inhibition with Verapamil Potentiates Bedaquiline in Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 58, 574–576. 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.; Jadaun G. P. S.; Ramdas Srivastava K.; Chauhan V.; Mishra R.; Gupta K.; Nair S.; Chauhan D. S.; Sharma V. D.; Venkatesan K.; Katoch V. M. (2011) Effect of Efflux Pump Inhibitors on Drug Susceptibility of Ofloxacin Resistant Mycobacterium Tuberculosis Isolates. Indian J. Med. Res. 133, 535–540. [PMC free article] [PubMed] [Google Scholar]
- Hogg G. M.; Barr J. G.; Webb C. H. (1998) In-Vitro Activity of the Combination of Colistin and Rifampicin against Multidrug-Resistant Strains of Acinetobacter Baumannii. J. Antimicrob. Chemother. 41, 494–450. 10.1093/jac/41.4.494. [DOI] [PubMed] [Google Scholar]
- MacGowan A. P.; Rynn C.; Wootton M.; Bowker K. E.; Holt H. A.; Reeves D. S.; et al. (1999) In Vitro Assessment of Colistin’s Antipseudomonal Antimicrobial Interactions with Other Antibiotics. Clin. Microbiol. Infect. 5, 32–36. 10.1111/j.1469-0691.1999.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Petrosillo N.; Chinello P.; Proietti M. F.; Cecchini L.; Masala M.; Franchi C.; Venditti M.; Esposito S.; Nicastri E. (2005) Combined Colistin and Rifampicin Therapy for Carbapenem-Resistant Acinetobacter Baumannii Infections: Clinical Outcome and Adverse Events. Clin. Microbiol. Infect. 11, 682–683. 10.1111/j.1469-0691.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- Kerrigan J. E.; Ragunath C.; Kandra L.; Gyemant G.; Liptak A.; Janossy L.; Kaplan J. B.; Ramasubbu N. (2008) Modeling and Biochemical Analysis of the Activity of Antibiofilm Agent Dispersin B. Acta Biol. Hung. 59, 439–451. 10.1556/ABiol.59.2008.4.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu N.; Thomas L. M.; Ragunath C.; Kaplan J. B. (2005) Structural Analysis of Dispersin B, a Biofilm-Releasing Glycoside Hydrolase from the Periodontopathogen Actinobacillus Actinomycetemcomitans. J. Mol. Biol. 349, 475–486. 10.1016/j.jmb.2005.03.082. [DOI] [PubMed] [Google Scholar]
- Brindle E. R.; Miller D. A.; Stewart P. S. (2011) Hydrodynamic Deformation and Removal of Staphylococcus Epidermidis Biofilms Treated with Urea, Chlorhexidine, Iron Chloride, or DispersinB. Biotechnol. Bioeng. 108, 2968–2977. 10.1002/bit.23245. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lopez R.; Machon C.; Longshaw C. M.; Martin S.; Molin S.; Zechner E. L.; Espinosa M.; Lanka E.; de la Cruz F. (2005) Unsaturated Fatty Acids Are Inhibitors of Bacterial Conjugation. Microbiology 151, 3517–3526. 10.1099/mic.0.28216-0. [DOI] [PubMed] [Google Scholar]
- Perry J. A.; Koteva K.; Verschoor C. P.; Wang W.; Bowdish D. M. E.; Wright G. D. (2015) A Macrophage-Stimulating Compound from a Screen of Microbial Natural Products. J. Antibiot. 68, 40–46. 10.1038/ja.2014.83. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bello C.; Rodriguez D.; Pernas M.; Rodriguez A.; Colchon E. (2020) Beta-Lactamase Inhibitors To Restore the Efficacy of Antibiotics against Superbugs. J. Med. Chem. 63, 1859. 10.1021/acs.jmedchem.9b01279. [DOI] [PubMed] [Google Scholar]
- Drawz S. M.; Bonomo R. A. (2010) Three Decades of β-Lactamase Inhibitors. Clin. Microbiol. Rev. 23, 160–201. 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau-Tsodikova S.; Labby K. J. (2016) Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. MedChemComm 7, 11–27. 10.1039/C5MD00344J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Green K. D.; Johnson B. R.; Garneau-Tsodikova S. (2015) Inhibition of Aminoglycoside Acetyltransferase Resistance Enzymes by Metal Salts. Antimicrob. Agents Chemother. 59, 4148–4156. 10.1128/AAC.00885-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. L.; Tran T.; Adams C.; Alam J. Y.; Herron S. R.; Tolmasky M. E. (2013) Inhibitors of the Aminoglycoside 6’-N-Acetyltransferase Type Ib [AAC(6’)-Ib] Identified by in Silico Molecular Docking. Bioorg. Med. Chem. Lett. 23, 5694–5698. 10.1016/j.bmcl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. D.; Chen W.; Garneau-Tsodikova S. (2012) Identification and Characterization of Inhibitors of the Aminoglycoside Resistance Acetyltransferase Eis from Mycobacterium Tuberculosis. ChemMedChem 7, 73–77. 10.1002/cmdc.201100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-Z.; Plesiat P.; Nikaido H. (2015) The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev. 28, 337–418. 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.; Gupta V. K.; Pathania R. (2019) Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside. Indian J. Med. Res. 149, 129–145. 10.4103/ijmr.IJMR_2079_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handzlik J.; Matys A.; Kieć-Kononowicz K. (2013) Recent Advances in Multi-Drug Resistance (MDR) Efflux Pump Inhibitors of Gram-Positive Bacteria S. Aureus. Antibiotics 2, 28–45. 10.3390/antibiotics2010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler G.; Kincses A.; Gajdacs M.; Amaral L. (2017) New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria. Molecules 22, 468. 10.3390/molecules22030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L.; Wu X. (2016) Development of Efflux Pump Inhibitors in Antituberculosis Therapy. Int. J. Antimicrob. Agents 47, 421–429. 10.1016/j.ijantimicag.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Schillaci D.; Spano V.; Parrino B.; Carbone A.; Montalbano A.; Barraja P.; Diana P.; Cirrincione G.; Cascioferro S. (2017) Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 60, 8268–8297. 10.1021/acs.jmedchem.7b00215. [DOI] [PubMed] [Google Scholar]
- Soucy S. M.; Huang J.; Gogarten J. P. (2015) Horizontal Gene Transfer: Building the Web of Life. Nat. Rev. Genet. 16, 472–482. 10.1038/nrg3962. [DOI] [PubMed] [Google Scholar]
- Graf F. E.; Palm M.; Warringer J.; Farewell A. (2019) Inhibiting Conjugation as a Tool in the Fight against Antibiotic Resistance. Drug Dev. Res. 80, 19–23. 10.1002/ddr.21457. [DOI] [PubMed] [Google Scholar]
- Cabezón E.; de la Cruz F.; Arechaga I. (2017) Conjugation Inhibitors and Their Potential Use to Prevent Dissemination of Antibiotic Resistance Genes in Bacteria. Front. Microbiol. 8, 2329. 10.3389/fmicb.2017.02329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getino M.; Sanabria-Rios D. J.; Fernandez-Lopez R.; Campos-Gomez J.; Sanchez-Lopez J. M.; Fernandez A.; Carballeira N. M.; de la Cruz F. (2015) Synthetic Fatty Acids Prevent Plasmid-Mediated Horizontal Gene Transfer. mBio 6, e01032-15 10.1128/mBio.01032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.; Lau G. W. (2011) Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in Streptococcus Pneumoniae by a Modified Competence Stimulating Peptide. PLoS Pathog. 7, e1002241 10.1371/journal.ppat.1002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer C. L.; Good J. A. D.; Kumar S.; Krishnan K. S.; Gaddy J. A.; Loh J. T.; Chappell J.; Almqvist F.; Cover T. L.; Hadjifrangiskou M. (2016) Peptidomimetic Small Molecules Disrupt Type IV Secretion System Activity in Diverse Bacterial Pathogens. mBio 7, e00221-16 10.1128/mBio.00221-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi L.; Maisetta G.; Esin S.; Batoni G. (2017) Combination Strategies to Enhance the Efficacy of Antimicrobial Peptides against Bacterial Biofilms. Front. Microbiol. 8, 2409. 10.3389/fmicb.2017.02409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R.; Tiwari M.; Donelli G.; Tiwari V. (2018) Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 9, 522–554. 10.1080/21505594.2017.1313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Zhang L.; Zhang M.; Liu H.; Lu P.; Lin K. (2018) Quorum Sensing Inhibitors: A Patent Review (2014–2018). Expert Opin. Ther. Pat. 28, 849–865. 10.1080/13543776.2018.1541174. [DOI] [PubMed] [Google Scholar]
- Xiang H.; Cao F.; Ming D.; Zheng Y.; Dong X.; Zhong X.; Mu D.; Li B.; Zhong L.; Cao J.; Wang L.; Ma H.; Wang T.; Wang D. (2017) Aloe-Emodin Inhibits Staphylococcus Aureus Biofilms and Extracellular Protein Production at the Initial Adhesion Stage of Biofilm Development. Appl. Microbiol. Biotechnol. 101, 6671–6681. 10.1007/s00253-017-8403-5. [DOI] [PubMed] [Google Scholar]
- Wang J.; Nong X.-H.; Amin M.; Qi S.-H. (2018) Hygrocin C from Marine-Derived Streptomyces Sp. SCSGAA 0027 Inhibits Biofilm Formation in Bacillus Amyloliquefaciens SCSGAB0082 Isolated from South China Sea Gorgonian. Appl. Microbiol. Biotechnol. 102, 1417–1427. 10.1007/s00253-017-8672-z. [DOI] [PubMed] [Google Scholar]
- Wunnoo S.; Saising J.; Voravuthikunchai S. P. (2017) Rhodomyrtone Inhibits Lipase Production, Biofilm Formation, and Disorganizes Established Biofilm in Propionibacterium Acnes. Anaerobe 43, 61–68. 10.1016/j.anaerobe.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Falagas M. E.; Bliziotis I. A. (2007) Pandrug-Resistant Gram-Negative Bacteria: The Dawn of the Post-Antibiotic Era?. Int. J. Antimicrob. Agents 29, 630–636. 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Asker D.; Awad T. S.; Baker P.; Howell P. L.; Hatton B. D. (2018) Non-Eluting, Surface-Bound Enzymes Disrupt Surface Attachment of Bacteria by Continuous Biofilm Polysaccharide Degradation. Biomaterials 167, 168–176. 10.1016/j.biomaterials.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Pritchard M. F.; Powell L. C.; Jack A. A.; Powell K.; Beck K.; Florance H.; Forton J.; Rye P. D.; Dessen A.; Hill K. E.; Thomas D. W. (2017) A Low-Molecular-Weight Alginate Oligosaccharide Disrupts Pseudomonal Microcolony Formation and Enhances Antibiotic Effectiveness. Antimicrob. Agents Chemother. 61, 61. 10.1128/AAC.00762-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Nunez C.; Reffuveille F.; Haney E. F.; Straus S. K.; Hancock R. E. W. (2014) Broad-Spectrum Anti-Biofilm Peptide That Targets a Cellular Stress Response. PLoS Pathog. 10, e1004152 10.1371/journal.ppat.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer D.; Wolfmeier H.; Bains M.; Hancock R. E. W. (2017) Synthetic Peptides to Target Stringent Response-Controlled Virulence in a Pseudomonas Aeruginosa Murine Cutaneous Infection Model. Front. Microbiol. 8, 1867. 10.3389/fmicb.2017.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L. D.; van Gennip M.; Jakobsen T. H.; Alhede M.; Hougen H. P.; Hoiby N.; Bjarnsholt T.; Givskov M. (2012) Synergistic Antibacterial Efficacy of Early Combination Treatment with Tobramycin and Quorum-Sensing Inhibitors against Pseudomonas Aeruginosa in an Intraperitoneal Foreign-Body Infection Mouse Model. J. Antimicrob. Chemother. 67, 1198–1206. 10.1093/jac/dks002. [DOI] [PubMed] [Google Scholar]
- Kissoyan K. A. B.; Bazzi W.; Hadi U.; Matar G. M. (2016) The Inhibition of Pseudomonas Aeruginosa Biofilm Formation by Micafungin and the Enhancement of Antimicrobial Agent Effectiveness in BALB/c Mice. Biofouling 32, 779–786. 10.1080/08927014.2016.1199021. [DOI] [PubMed] [Google Scholar]
- Maiden M. M.; Hunt A. M. A.; Zachos M. P.; Gibson J. A.; Hurwitz M. E.; Mulks M. H.; Waters C. M. (2018) Triclosan Is an Aminoglycoside Adjuvant for Eradication of Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 62, e00146-18 10.1128/AAC.00146-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington R. J.; Melander C. (2013) Combination Approaches to Combat Multidrug-Resistant Bacteria. Trends Biotechnol. 31, 177–184. 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M.; Wright G. D. (2019) Drug Combinations: A Strategy to Extend the Life of Antibiotics in the 21st Century. Nat. Rev. Microbiol. 17, 141–155. 10.1038/s41579-018-0141-x. [DOI] [PubMed] [Google Scholar]
- Schmid A.; Wolfensberger A.; Nemeth J.; Schreiber P. W.; Sax H.; Kuster S. P. (2019) Monotherapy versus Combination Therapy for Multidrug-Resistant Gram-Negative Infections: Systematic Review and Meta-Analysis. Sci. Rep. 9, 15290. 10.1038/s41598-019-51711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L.; Hope W.; MacGowan A.; Louie A. (2016) Suppression of Emergence of Resistance in Pathogenic Bacteria: Keeping Our Powder Dry, Part 1. Antimicrob. Agents Chemother. 60, 1194–1201. 10.1128/AAC.02231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Patel G.; Huprikar S.; Calfee D. P.; Jenkins S. G. (2009) Decreased Susceptibility to Polymyxin B during Treatment for Carbapenem-Resistant Klebsiella Pneumoniae Infection. J. Clin. Microbiol. 47, 1611–2. 10.1128/JCM.02466-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss C. H.; Muhlebach M. S. (2011) Review: Staphylococcus Aureus and MRSA in Cystic Fibrosis. J. Cystic Fibrosis 10, 298–306. 10.1016/j.jcf.2011.06.002. [DOI] [PubMed] [Google Scholar]
- McCaughey G.; Diamond P.; Elborn J. S.; McKevitt M.; Tunney M. M. (2013) Resistance Development of Cystic Fibrosis Respiratory Pathogens When Exposed to Fosfomycin and Tobramycin Alone and in Combination under Aerobic and Anaerobic Conditions. PLoS One 8, e69763 10.1371/journal.pone.0069763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monedero I.; Caminero J. A. (2010) Management of Multidrug-Resistant Tuberculosis: An Update. Ther. Adv. Respir. Dis. 4, 117–127. 10.1177/1753465810365884. [DOI] [PubMed] [Google Scholar]
- (2013) Heterogeneity of Selection and the Evolution of Resistance. Trends Ecol. Evol. 28, 110–118. 10.1016/j.tree.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Takesue Y.; Nakajima K.; Ichiki K.; Ishihara M.; Wada Y.; Takahashi Y.; Tsuchida T.; Ikeuchi H. (2010) Impact of a Hospital-Wide Programme of Heterogeneous Antibiotic Use on the Development of Antibiotic-Resistant Gram-Negative Bacteria. J. Hosp. Infect. 75, 28–32. 10.1016/j.jhin.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Raymond B. (2019) Five Rules for Resistance Management in the Antibiotic Apocalypse, a Road Map for Integrated Microbial Management. Evol. Appl. 12, 1079–1091. 10.1111/eva.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M.; Lador A.; Grozinsky-Glasberg S.; Leibovici L. (2014) Beta Lactam Antibiotic Monotherapy versus Beta Lactam-Aminoglycoside Antibiotic Combination Therapy for Sepsis. Cochrane database Syst. Rev. (1), CD003344. 10.1002/14651858.CD003344.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma P. D.; Cosgrove S. E.; Maragakis L. L. (2012) Combination Therapy for Treatment of Infections with Gram-Negative Bacteria. Clin. Microbiol. Rev. 25, 450–470. 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M.; Levin B. R. (1997) The Population Dynamics of Antimicrobial Chemotherapy. Antimicrob. Agents Chemother. 41, 363–373. 10.1128/AAC.41.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond B.; Wright D. J.; Crickmore N.; Bonsall M. B. (2013) The Impact of Strain Diversity and Mixed Infections on the Evolution of Resistance to Bacillus Thuringiensis. Proc. R. Soc. London, Ser. B 280, 20131497. 10.1098/rspb.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Miller R.; Laehnemann D.; Jansen G.; Fuentes-Hernandez A.; Rosenstiel P.; Schulenburg H.; Beardmore R. (2013) When the Most Potent Combination of Antibiotics Selects for the Greatest Bacterial Load: The Smile-Frown Transition. PLoS Biol. 11, e1001540 10.1371/journal.pbio.1001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean R. C.; Hall A. R.; Perron G. G.; Buckling A. (2010) The Population Genetics of Antibiotic Resistance: Integrating Molecular Mechanisms and Treatment Contexts. Nat. Rev. Genet. 11, 405–414. 10.1038/nrg2778. [DOI] [PubMed] [Google Scholar]
- Hegreness M.; Shoresh N.; Damian D.; Hartl D.; Kishony R. (2008) Accelerated Evolution of Resistance in Multidrug Environments. Proc. Natl. Acad. Sci. U. S. A. 105, 13977–13981. 10.1073/pnas.0805965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Gefen O.; Ronin I.; Bar-Meir M.; Balaban N. Q. (2020) Effect of Tolerance on the Evolution of Antibiotic Resistance under Drug Combinations. Science 367, 200–204. 10.1126/science.aay3041. [DOI] [PubMed] [Google Scholar]