ABSTRACT
Background
Very-long-chain SFAs (VLCSFAs), such as arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0), have demonstrated inverse associations with cardiometabolic conditions, although more evidence is needed to characterize their relation with risk of type 2 diabetes (T2D). In addition, little is known regarding their potential dietary and lifestyle predictors.
Objective
We aimed to examine the association of plasma and erythrocyte concentrations of VLCSFAs with incident T2D risk.
Methods
We used existing measurements of fatty acid concentrations in plasma and erythrocytes among 2854 and 2831 participants in the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS), respectively. VLCSFAs were measured using GLC, and individual fatty acid concentrations were expressed as a percentage of total fatty acids. Incident T2D cases were identified by self-reports and confirmed by a validated supplementary questionnaire. Cox proportional hazards regression was used to evaluate the association between VLCSFAs and T2D, adjusting for demographic, lifestyle, and dietary variables.
Results
During 39,941 person-years of follow-up, we documented 243 cases of T2D. Intakes of peanuts, peanut butter, vegetable fat, dairy fat, and palmitic/stearic (16:0–18:0) fatty acids were significantly, albeit weakly, correlated with plasma and erythrocyte VLCSFA concentrations (|rs| ≤ 0.19). Comparing the highest with the lowest quartiles of plasma concentrations, pooled HRs (95% CIs) were 0.51 (0.35, 0.75) for arachidic acid, 0.43 (0.28, 0.64) for behenic acid, 0.40 (0.27, 0.61) for lignoceric acid, and 0.41 (0.27, 0.61) for the sum of VLCSFAs, after multivariate adjustments for demographic, lifestyle, and dietary factors. For erythrocyte VLCSFAs, only arachidic acid and behenic acid concentrations were inversely associated with T2D risk.
Conclusions
Our findings suggest that, in US men and women, higher plasma concentrations of VLCSFAs are associated with lower risk of T2D. More research is needed to understand the mechanistic pathways underlying these associations.
Keywords: very-long-chain saturated fatty acids, type 2 diabetes, prospective cohort, biomarker, arachidic acid, behenic acid, lignoceric acid
Introduction
Type 2 diabetes (T2D) is a leading public health issue with numerous complications ranging from cardiovascular disease, renal disease, and retinopathy to amputations (1). Numerous dietary, genetic, lifestyle, and metabolic risk factors of T2D have been identified in large epidemiologic studies (2). However, circulating fatty acids, exogenously ingested or endogenously synthesized, have been explored only recently as potential risk factors for T2D (3).
Very-long-chain SFAs (VLCSFAs) are a unique group of SFAs with chain length ≥20, including arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0). VLCSFAs are present in low concentrations in food products such as peanuts and peanut butter, canola oil, macadamia nuts, and dairy fat (4). However, the main source of these fatty acids in mammalian tissues is endogenous synthesis by elongation in the endoplasmic reticulum from SFAs with a chain length of 12:0–18:0 (lauric acid to stearic acid) (5). This synthesis is mediated via a set of 7 elongases of very long chain fatty acids (ELOVL 1–7) embedded in the endoplasmic reticulum (5). In multiple epidemiologic studies, VLCSFAs were inversely associated with multiple cardiovascular health outcomes (6–8). However, the association between circulating concentrations of VLCSFAs and T2D (9–11) did not yield entirely consistent results. For example, the European Prospective Investigation into Cancer and Nutrition (EPIC)–InterAct study (11) and Cardiovascular Health Study (CHS) (9) have demonstrated inverse associations for all VLCSFAs in plasma with diabetes, although the association for erythrocyte VLCSFA concentrations was unclear in the EPIC-Potsdam study (10).
In the current investigation, we aimed to examine prospective associations of concentrations of VLCSFAs in both plasma and erythrocytes with T2D risk in US men and women. We hypothesized that arachidic acid, behenic acid, and lignoceric acid concentrations in plasma and erythrocyte membranes were inversely associated with T2D risk. We also explored dietary and lifestyle predictors of these fatty acids.
Methods
Study population
The Nurses’ Health Study (NHS) was established in 1976 with a recruitment of 121,700 female nurses ages 30–55 y. The Health Professionals Follow-Up Study (HPFS) started in 1986, recruiting 51,529 male health professionals who were 40–75 y of age. In both cohorts, questionnaires were administered biennially to gather and update information on lifestyle practices and occurrence of chronic diseases. A subset of participants from the NHS and another from the HPFS, whose lifestyle and dietary variables were similar to those of the overall cohorts, provided blood samples in 1990 and 1994, respectively (12).
Study design
For this analysis, we used existing measurements of plasma and erythrocyte fatty acid concentrations in 2 nested case-control studies of cardiovascular disease (CVD) in the NHS and HPFS. For the original CVD case-control studies, participants were all free of CVD and cancer at the time of blood draw (6). In the current analysis, we further excluded men and women who had diagnoses of diabetes at blood draw, participants who had missing fatty acid data, and participants with missing diabetes diagnostic date. We further excluded plasma and erythrocyte VLCSFA outlier measurements (9 for NHS and 8 for HPFS VLCSFA plasma values and 1 for NHS and 2 for HPFS erythrocyte values) identified using the Generalized Extreme Studentized deviate method (13). After exclusions, data from 2854 participants with plasma fatty acids and 2831 participants with erythrocyte fatty acids were available for analysis and constituted the current study population. The study protocol was approved by the institutional review boards of Brigham and Women's Hospital and the Harvard TH Chan School of Public Health. Written informed consent was obtained for blood collection and assessments of biomarkers.
Measurement of circulating biomarkers of VLCSFAs
Participants’ blood samples were returned to the laboratory with a cold pack via overnight courier and most of the samples arrived within 24 h. Upon arrival, samples were centrifuged, divided into aliquots for plasma, buffy coat, and erythrocytes, and stored in liquid nitrogen freezers at ≤−130°C until analysis in 2010–2011 (14, 15). Previous studies have demonstrated that fatty acids in serum phospholipids stored at −80°C for ≤12 y showed minimal degradation and are suitable for use in epidemiologic studies (16, 17). Fatty acid concentrations in total plasma (including all lipid subfractions, such as cholesterol ester, phospholipids, triglycerides, and free fatty acids) and erythrocytes were analyzed by GLC (18). Procedures for fatty acid analyses in the NHS and HPFS have been published previously (15, 19). The mean intra-assay CVs derived from measurements of quality control samples were 5.3% for arachidic acid, 6.0% for behenic acid, and 7.7% for lignoceric acid for NHS assessments of plasma fatty acids. Corresponding CVs in NHS erythrocyte measurements were 7.2%, 10.6%, and 11.2%. For the HPFS samples, the mean intra-assay CVs for quality controls in plasma samples were 9.3% for arachidic acid, 11.2% for behenic acid, and 13.4% for lignoceric acid. Corresponding CVs in HPFS erythrocyte measurements were 12.2%, 14.6%, and 18.2%. CVs for other fatty acids ranged from 3% to 16% for SFAs and MUFAs, 3% to 6% for PUFAs, and 10% to 15% for trans fatty acids (TFAs). Concentrations of individual circulating fatty acids were expressed as a percentage of total fatty acids in plasma or erythrocyte membranes and we used the circulating biomarkers of arachidic acid, behenic acid, lignoceric acid, and the sum of these 3 fatty acids as the exposures of interest (19).
Ascertainment of T2D cases
Incident cases of T2D were identified by self-reports on the mail questionnaires and confirmed by a supplementary questionnaire inquiring after diagnosis date, symptoms, blood glucose concentrations, and medication use. Self-reported diagnosis of T2D was confirmed using the following criteria from the National Diabetes Data Group up until 1998 (20): 1) manifestation of classic symptoms such as excessive thirst, polyuria, weight loss, and hunger, in conjunction with elevated fasting glucose concentrations ≥140 mg/dL (7.77 mmol/L) or nonfasting glucose concentrations ≥200 mg/dL (11.1 mmol/L); 2) asymptomatic but elevated plasma glucose on 2 separate occasions or abnormal glucose-tolerance test results; or 3) receiving any hypoglycemic treatment for diabetes. After 1998, a fasting glucose concentration ≥126 mg/dL (6.99 mmol/L) was adopted per the new diagnostic criteria of the American Diabetes Association. This supplementary questionnaire has been validated in previous studies with ≥97% questionnaire-confirmed cases reconfirmed through medical record review by a blinded study physician (21, 22). In another validation study, medical record review documented that 129 of 130 participants who reported a negative screening of diabetes had fasting plasma glucose concentrations <126 mg/dL (23). This low level of false negative diabetes status was probably due to the fact that all participants were health professionals with ready access to health care.
Covariate assessment
In the biennial follow-up questionnaires, we inquired about information on risk factors for chronic diseases, such as body weight, cigarette smoking, physical activity, and medication use. Among the NHS participants, we ascertained menopausal status, postmenopausal hormone use, and oral contraceptive use in the questionnaires. Diet was assessed using FFQs including 131 items administered in 1984, 1986, and every 4 y thereafter in both cohorts. To measure diet quality, we used the 2010 Alternative Healthy Eating Index (AHEI) (24).
Statistical analyses
We used a Cox proportional hazards regression to model the associations of interest, and person-time was counted from the time of blood draw until T2D diagnosis, death, or censoring at return of the last questionnaire through 2012, whichever came first. For this analysis, we categorized participants into quartiles of arachidic acid, behenic acid, and lignoceric acid, and their sum, respectively. To examine linear trend, we assigned the median intake within each quartile and modeled this variable continuously. Fatty acids were also evaluated continuously to estimate associations per 1-SD change of fatty acids. To increase statistical power, we used data from both CVD cases and controls. To evaluate potential heterogeneity of associations between CVD cases and controls, we examined the significance of interaction terms of VLCSFAs (per SD change) and case-control status of the original CVD studies, and all the interaction terms were not statistically significant (P > 0.05), suggesting that the main associations were unlikely dependent on CVD case-control status.
The basic model was adjusted for age and BMI. The multivariate model was further adjusted for race/ethnicity (white, nonwhite), physical activity (metabolic equivalents per week), smoking (never, former, current), alcohol use (in g/d), family history of diabetes, parental history of myocardial infarction, menopausal status, postmenopausal hormone use (in the NHS), baseline diagnosis of hypertension, hypercholesterolemia, glycemic load, AHEI, and total energy intake. The full multivariate model also adjusted for biomarkers of linoleic acid (18:2), biomarkers of dairy intake [pentadecylic acid (15:0), margaric acid (17:0), and trans-palmitoleic acid (16:1n–7)], and elaidic acid (trans-18:1) and linoleaidic acid (trans-18:2). The associations for each VLCSFA and T2D risk were modeled separately for each cohort, and the composite HRs were estimated by pooling the data from both cohorts and analyzing the data using a Cox model stratified by sex.
Spearman partial correlation coefficients adjusted for age and BMI among CVD controls were used to evaluate correlations between plasma and erythrocyte VLCSFAs and dietary and lifestyle variables averaged for the 2 FFQ cycles closest to the date of blood draw: 1986 and 1990 for the NHS and 1990 and 1994 for the HPFS. We used linear regression to estimate the variation of VLCSFAs explained by the dietary and lifestyle predictors. We also conducted a sensitivity analysis to address the potential issue of reverse causation by excluding cases that occurred in the first 2 or 4 y after blood sampling. In addition, we performed an analysis restricted to CVD control participants. We also conducted a sensitivity analysis where we further adjusted the full multivariable model for intakes of the main dietary sources of VLCSFAs, including peanuts, peanut butter, dairy fat, and vegetable fat.
All P values were 2-sided, and 95% CIs were calculated for HRs. Data were analyzed with the Statistical Analysis Systems software package, version 9.4 (SAS Institute, Inc.).
Results
During 39,941 person-years of follow-up, we documented 243 confirmed cases of T2D in the analysis of plasma VLCSFAs (136 in the NHS and 107 in the HPFS) and 245 cases in the analysis of erythrocyte VLCSFAs (133 in the NHS and 112 in the HPFS). Baseline characteristics of NHS and HPFS participants are shown in Table 1. In the NHS, the mean plasma concentrations of arachidic acid, behenic acid, and lignoceric acid were 0.20%, 0.52%, and 0.40%, respectively. In the HPFS the mean plasma concentrations of arachidic acid, behenic acid, and lignoceric acid were 0.18%, 0.46%, and 0.38%, respectively. Overall, the concentrations of VLCSFAs in erythrocytes were higher than their plasma counterparts.
TABLE 1.
Baseline characteristics of 1392 women and 1462 men with plasma fatty acid measurements in the Nurses’ Health Study (1990) and Health Professionals Follow-Up Study (1994)1
| Women (n = 1392) | Men (n = 1462) | |
|---|---|---|
| Age, y | 60.4 ± 6.4 | 65.0 ± 8.6 |
| Age range, y | 43–70 | 47–81 |
| Race/ethnicity, % | ||
| Caucasian | 99.3 | 93.6 |
| African American | 0.3 | 0.1 |
| Asian/other | 0.4 | 6.3 |
| Weight status, % | ||
| Normal (BMI <25) | 55.4 | 42.1 |
| Overweight (BMI 25 to <30) | 31.5 | 47.1 |
| Obese (BMI ≥30) | 13.1 | 10.8 |
| BMI, kg/m2 | 25.3 ± 4.5 | 25.8 ± 3.3 |
| Smoking status, % | ||
| Current smoker | 18.3 | 8.2 |
| Past smoker | 40.2 | 49.4 |
| Never smoker | 41.5 | 42.5 |
| Physical activity, MET-h/wk | 16.4 ± 19.4 | 36.4 ± 39.0 |
| Medical history | ||
| Hypertension, % | 22.5 | 25.1 |
| Hypercholesterolemia, % | 35.9 | 26.8 |
| Family history of diabetes, % | 26.9 | 22.9 |
| Dietary factors | ||
| Total energy, kcal/d | 1765 ± 507 | 2046 ± 622 |
| Peanuts, g/d | 2.2 ± 5.9 | 4.9 ± 10.9 |
| Peanut butter, g/d | 4.0 ± 8.4 | 2.8 ± 6.1 |
| Fruits, servings/d | 1.7 ± 1.2 | 1.8 ± 1.5 |
| Vegetables, servings/d | 3.9 ± 2.2 | 4.1 ± 2.3 |
| Unprocessed meats, servings/d | 0.9 ± 0.5 | 0.9 ± 0.6 |
| Processed meats, servings/d | 0.2 ± 0.3 | 0.3 ± 0.4 |
| Coffee, mL/d | 369 ± 377 | 469 ± 410 |
| Alcohol, g/d | 5.7 ± 10.3 | 12.2 ± 16.2 |
| AHEI | 48.9 ± 10.3 | 48.6 ± 10.4 |
| Plasma fatty acids, % of total fatty acids | ||
| Arachidic acid | 0.20 ± 0.1 | 0.18 ± 0.1 |
| Behenic acid | 0.52 ± 0.2 | 0.46 ± 0.2 |
| Lignoceric acid | 0.40 ± 0.2 | 0.38 ± 0.2 |
| Erythrocyte fatty acids, % of total fatty acids | ||
| Arachidic acid | 0.44 ± 0.1 | 0.39 ± 0.1 |
| Behenic acid | 1.51 ± 0.4 | 1.58 ± 0.3 |
| Lignoceric acid | 3.12 ± 0.9 | 3.88 ± 0.8 |
Values are means ± SDs for continuous variables and percentages for categorical variables. BMI in kg/m2. AHEI, Alternative Healthy Eating Index; MET, metabolic equivalent.
Baseline characteristics according to quartiles of each VLCSFA are shown in Supplemental Tables 1 and 2 (for the NHS and HPFS, respectively). Each VLCSFA and their sum were positively associated with physical activity, and inversely associated with BMI, hypertension, hypercholesterolemia, and family history of diabetes. Within each cohort, Spearman partial correlation coefficients (rs) ranged between 0.68 and 0.92 among plasma VLCSFAs. The correlations among erythrocyte VLCSFAs ranged from 0.31 to 0.83. Each VLCSFA was moderately, negatively correlated with biomarkers of myristic acid (14:0) and palmitic acid (16:0), and positively correlated with stearic acid. Weak correlations were found for VLCSFAs with trans-elaidic acid, trans-palmitoleic acid, EPA, and DHA (Supplemental Tables 3 and 4).
Table 2 shows the HRs of T2D by quartiles of plasma VLCSFA concentrations. In the fully adjusted multivariate model, comparing the highest with the lowest quartiles of plasma VLCSFAs, the pooled HRs (95% CIs) were 0.51 (0.35, 0.75; P-trend < 0.001) for arachidic acid; 0.43 (0.28, 0.64; P-trend < 0.001) for behenic acid; 0.40 (0.27, 0.61; P-trend < 0.001) for lignoceric acid; and 0.41 (0.27, 0.61; P-trend < 0.001) for the sum of the 3 VLCSFAs. We observed similar associations for each VLCSFA when examined as per-SD change. The pooled HRs (95% CIs) of T2D for each SD increase were 0.74 (0.64, 0.85) for arachidic acid; 0.72 (0.62, 0.82) for behenic acid; 0.68 (0.58, 0.79) for lignoceric acid; and 0.69 (0.60, 0.80) for the sum of the 3 VLCSFAs.
TABLE 2.
Risk of incident type 2 diabetes according to circulating plasma FA biomarkers of arachidic acid, behenic acid, and lignoceric acid among 1392 women in the NHS (n = 136 cases) and 1462 men in the HPFS (n = 107 cases)
| Cohort-specific FA quartiles | |||||
|---|---|---|---|---|---|
| FA | 1 | 2 | 3 | 4 | P for trend5 |
| Arachidic acid, NHS | |||||
| % of total FA, mean ± SD1 | 0.14 ± 0.02 | 0.18 ± 0.01 | 0.22 ± 0.01 | 0.27 ± 0.03 | |
| Cases/person-years | 53/6178 | 38/6021 | 22/5976 | 23/5583 | |
| Age- and BMI-adjusted HR | Reference | 0.91 (0.59, 1.39) | 0.53 (0.32, 0.88) | 0.61 (0.37, 1.00) | 0.01 |
| Multivariable HR 12 | Reference | 0.88 (0.57, 1.36) | 0.57 (0.34, 0.95) | 0.59 (0.35, 0.98) | 0.02 |
| Multivariable HR 23 | Reference | 0.81 (0.52, 1.25) | 0.53 (0.32, 0.89) | 0.53 (0.31, 0.89) | 0.01 |
| Arachidic acid, HPFS | |||||
| % of total FA, mean ± SD1 | 0.13 ± 0.02 | 0.17 ± 0.01 | 0.20 ± 0.01 | 0.25 ± 0.03 | |
| Cases/person-years | 48/4179 | 31/4071 | 19/3919 | 18/4014 | |
| Age- and BMI-adjusted HR | Reference | 0.68 (0.43, 1.08) | 0.49 (0.29, 0.85) | 0.46 (0.26, 0.79) | 0.002 |
| Multivariable HR 12 | Reference | 0.67 (0.42, 1.07) | 0.46 (0.26, 0.79) | 0.42 (0.24, 0.74) | 0.001 |
| Multivariable HR 23 | Reference | 0.67 (0.42, 1.08) | 0.47 (0.27, 0.81) | 0.46 (0.26, 0.81) | 0.002 |
| Arachidic acid, pooled4 | Reference | 0.80 (0.58, 1.10) | 0.59 (0.41, 0.84) | 0.51 (0.35, 0.75) | <0.001 |
| Behenic acid, NHS | |||||
| % of total FA, mean ± SD1 | 0.32 ± 0.07 | 0.46 ± 0.03 | 0.57 ± 0.04 | 0.76 ± 0.10 | |
| Cases/person-years | 50/5859 | 44/6301 | 26/6018 | 16/5579 | |
| Age- and BMI-adjusted HR | Reference | 1.10 (0.72, 1.67) | 0.64 (0.39, 1.04) | 0.42 (0.24, 0.75) | 0.001 |
| Multivariable HR 12 | Reference | 1.10 (0.72, 1.67) | 0.68 (0.41, 1.11) | 0.42 (0.24, 0.76) | 0.001 |
| Multivariable HR 23 | Reference | 1.08 (0.71, 1.65) | 0.61 (0.37, 1.01) | 0.37 (0.21, 0.67) | <0.001 |
| Behenic acid, HPFS | |||||
| % of total FA, mean ± SD1 | 0.27 ± 0.07 | 0.42 ± 0.04 | 0.55 ± 0.03 | 0.73 ± 0.10 | |
| Cases/person-years | 44/4018 | 33/4200 | 22/4011 | 17/3954 | |
| Age- and BMI-adjusted HR | Reference | 0.74 (0.46, 1.17) | 0.57 (0.34, 0.96) | 0.47 (0.27, 0.84) | 0.01 |
| Multivariable HR 12 | Reference | 0.72 (0.45, 1.16) | 0.51 (0.30, 0.86) | 0.45 (0.25, 0.81) | 0.002 |
| Multivariable HR 23 | Reference | 0.80 (0.50, 1.28) | 0.57 (0.33, 0.98) | 0.51 (0.28, 0.93) | 0.01 |
| Behenic acid, pooled4 | Reference | 0.92 (0.68, 1.26) | 0.57 (0.40, 0.81) | 0.43 (0.28, 0.64) | <0.001 |
| Lignoceric acid, NHS | |||||
| % of total FA, mean ± SD1 | 0.22 ± 0.05 | 0.34 ± 0.03 | 0.44 ± 0.03 | 0.63 ± 0.10 | |
| Cases/person-years | 57/6217 | 43/5902 | 21/6009 | 15/5630 | |
| Age- and BMI-adjusted HR | Reference | 0.99 (0.66, 1.48) | 0.47 (0.29, 0.79) | 0.38 (0.22, 0.68) | <0.001 |
| Multivariable HR 12 | Reference | 0.99 (0.66, 1.49) | 0.51 (0.30, 0.84) | 0.39 (0.21, 0.69) | <0.001 |
| Multivariable HR 23 | Reference | 0.94 (0.62, 1.42) | 0.45 (0.27, 0.76) | 0.34 (0.19, 0.61) | <0.001 |
| Lignoceric acid, HPFS | |||||
| % of total FA, mean ± SD1 | 0.22 ± 0.05 | 0.35 ± 0.03 | 0.46 ± 0.03 | 0.62 ± 0.09 | |
| Cases/person-years | 51/4069 | 26/4154 | 23/4090 | 16/3871 | |
| Age- and BMI-adjusted HR | Reference | 0.52 (0.32, 0.84) | 0.51 (0.31, 0.85) | 0.41 (0.23, 0.73) | 0.001 |
| Multivariable HR 12 | Reference | 0.56 (0.34, 0.93) | 0.51 (0.30, 0.84) | 0.44 (0.24, 0.78) | 0.002 |
| Multivariable HR 23 | Reference | 0.60 (0.36, 1.00) | 0.58 (0.34, 0.97) | 0.47 (0.26, 0.86) | 0.01 |
| Lignoceric acid, pooled4 | Reference | 0.74 (0.54, 1.00) | 0.47 (0.33, 0.68) | 0.40 (0.27, 0.61) | <0.001 |
| Sum (arachidic acid + behenic acid + lignoceric acid), NHS | |||||
| % of total FA, mean ± SD1 | 0.70 ± 0.14 | 1.00 ± 0.06 | 1.22 ± 0.08 | 1.63 ± 0.20 | |
| Cases/person-years | 56/6046 | 40/6235 | 26/5879 | 14/5597 | |
| Age- and BMI-adjusted HR | Reference | 0.89 (0.59, 1.34) | 0.59 (0.37, 0.95) | 0.35 (0.19, 0.62) | <0.001 |
| Multivariable HR 12 | Reference | 0.94 (0.62, 1.42) | 0.63 (0.39, 1.02) | 0.35 (0.19, 0.63) | <0.001 |
| Multivariable HR 23 | Reference | 0.93 (0.61, 1.42) | 0.57 (0.35, 0.92) | 0.31 (0.17, 0.57) | <0.001 |
| Sum (arachidic acid + behenic acid + lignoceric acid), HPFS | |||||
| % of total FA, mean ± SD1 | 0.63 ± 0.13 | 0.94 ± 0.08 | 1.20 ± 0.07 | 1.57 ± 0.20 | |
| Cases/person-years | 49/4053 | 28/4156 | 21/4078 | 18/3896 | |
| Age- and BMI-adjusted HR | Reference | 0.56 (0.35, 0.90) | 0.51 (0.30, 0.86) | 0.46 (0.26, 0.80) | 0.003 |
| Multivariable HR 12 | Reference | 0.60 (0.37, 0.81) | 0.50 (0.29, 0.85) | 0.46 (0.26, 0.80) | 0.002 |
| Multivariable HR 23 | Reference | 0.63 (0.39, 1.03) | 0.55 (0.32, 0.95) | 0.50 (0.28, 0.88) | 0.01 |
| Sum (arachidic acid + behenic acid + lignoceric acid), pooled4 | Reference | 0.73 (0.54, 1.00) | 0.54 (0.37, 0.77) | 0.41 (0.27, 0.61) | <0.001 |
Values are means ± SDs or HRs (95% CIs). FA, fatty acid; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Model adjusted for age, BMI, race (white, nonwhite), smoking status (never, former, current, missing), physical activity (metabolic equivalents/wk), alcohol (g/d), family history of diabetes (yes, no, missing), parental history of myocardial infarction (yes, no, missing), hypercholesterolemia (yes, no), hypertension (yes, no), menopausal status in NHS (pre, post), postmenopausal hormone use in NHS (no, yes, missing), Alternative Healthy Eating Index, glycemic load (continuous), and total energy (kcal/d).
Included multivariate model 1 covariates and further adjusted for plasma concentrations of trans-elaidic acid, trans-linoleaidic acid, and the sum of pentadecylic acid, margaric acid, and trans-palmitoleic acid.
Pooled estimates and P values were calculated by combining the participant data from both cohorts and further adjusting multivariate model 1 by sex.
P for trend was calculated by assigning the median values to each quartile and modeling the median values as a continuous variable.
Table 3 shows the associations for erythrocyte VLCSFAs. In the fully adjusted multivariate model, comparing the highest with the lowest quartiles of erythrocyte biomarker concentrations, the pooled HRs (95% CIs) of T2D were 0.58 (0.39, 0.87; P-trend = 0.01) for arachidic acid; 0.51 (0.34, 0.76; P-trend = 0.001) for behenic acid; 0.95 (0.61, 1.46; P-trend = 0.95) for lignoceric acid; and 0.79 (0.52, 1.21; P-trend = 0.94) for the sum of the 3 VLCSFAs. The associations were similar for each VLCSFA examined continuously as per-SD change. The pooled HRs (95% CIs) of T2D for each SD increase in erythrocyte VLCSFAs were 0.80 (0.69, 0.92) for arachidic acid; 0.74 (0.64, 0.86) for behenic acid; 0.99 (0.84, 1.17) for lignoceric acid; and 0.90 (0.77, 1.05) for the sum of the 3 VLCSFAs.
TABLE 3.
Risk of incident type 2 diabetes according to circulating erythrocyte FA biomarkers of arachidic acid, behenic acid, and lignoceric acid among 1314 women in the NHS (n = 133 cases) and 1517 men in the HPFS (n = 112 cases)1
| Cohort, specific FA quartiles | |||||
|---|---|---|---|---|---|
| FA | 1 | 2 | 3 | 4 | P for trend5 |
| Arachidic acid, NHS | |||||
| % of total FA, mean ± SD | 0.35 ± 0.03 | 0.42 ± 0.01 | 0.47 ± 0.01 | 0.55 ± 0.05 | |
| Cases/person-years | 53/5848 | 39/5681 | 28/5304 | 13/5543 | |
| Age- and BMI-adjusted HR | Reference | 0.72 (0.47, 1.09) | 0.56 (0.36, 0.89) | 0.30 (0.16, 0.56) | <0.001 |
| Multivariable HR 12 | Reference | 0.81 (0.53, 1.23) | 0.60 (0.38, 0.97) | 0.32 (0.17, 0.60) | <0.001 |
| Multivariable HR 23 | Reference | 0.85 (0.55, 1.31) | 0.61 (0.38, 0.98) | 0.31 (0.17, 0.58) | <0.001 |
| Arachidic acid, HPFS | |||||
| % of total FA, mean ± SD | 0.32 ± 0.03 | 0.36 ± 0.01 | 0.39 ± 0.01 | 0.45 ± 0.04 | |
| Cases/person-years | 33/4393 | 27/4231 | 30/4109 | 30/4116 | |
| Age- and BMI-adjusted HR | Reference | 0.96 (0.57, 1.60) | 1.00 (0.60, 1.65) | 1.11 (0.67, 1.82) | 0.71 |
| Multivariable HR 12 | Reference | 0.87 (0.52, 1.47) | 0.89 (0.54, 1.50) | 1.09 (0.65, 1.83) | 0.78 |
| Multivariable HR 23 | Reference | 0.90 (0.53, 1.52) | 0.92 (0.54, 1.55) | 1.08 (0.63, 1.85) | 0.82 |
| Arachidic acid, pooled4 | Reference | 0.87 (0.62, 1.23) | 0.93 (0.66, 1.33) | 0.58 (0.39, 0.87) | 0.01 |
| Behenic acid, NHS | |||||
| % of total FA, mean ± SD | 1.06 ± 0.18 | 1.42 ± 0.07 | 1.65 ± 0.07 | 1.98 ± 0.18 | |
| Cases/person-years | 46/5824 | 33/5488 | 31/5610 | 23/5453 | |
| Age- and BMI-adjusted HR | Reference | 0.93 (0.59, 1.46) | 0.88 (0.55, 1.40) | 0.62 (0.38, 1.03) | 0.08 |
| Multivariable HR 12 | Reference | 1.05 (0.67, 1.67) | 0.97 (0.61, 1.56) | 0.65 (0.38, 1.08) | 0.13 |
| Multivariable HR 23 | Reference | 0.68 (0.41, 1.13) | 0.60 (0.35, 1.01) | 0.35 (0.19, 0.65) | 0.001 |
| Behenic acid, HPFS | |||||
| % of total FA, mean ± SD | 1.22 ± 0.14 | 1.50 ± 0.06 | 1.67 ± 0.05 | 1.94 ± 0.16 | |
| Cases/person-years | 41/4099 | 28/4352 | 26/4084 | 25/4304 | |
| Age- and BMI-adjusted HR | Reference | 0.66 (0.41, 1.06) | 0.68 (0.41, 1.11) | 0.56 (0.33, 0.93) | 0.03 |
| Multivariable HR 12 | Reference | 0.61 (0.38, 1.00) | 0.59 (0.36, 0.98) | 0.50 (0.30, 0.85) | 0.01 |
| Multivariable HR 23 | Reference | 0.68 (0.42, 1.13) | 0.67 (0.40, 1.14) | 0.60 (0.34, 1.06) | 0.07 |
| Behenic acid, pooled4 | Reference | 0.84 (0.59, 1.18) | 0.69 (0.47, 1.00) | 0.51 (0.34, 0.76) | 0.001 |
| Lignoceric acid, NHS | |||||
| % of total FA, mean ± SD | 2.03 ± 0.36 | 2.81 ± 0.16 | 3.38 ± 0.18 | 4.31 ± 0.52 | |
| Cases/person-years | 38/5877 | 22/5640 | 37/5396 | 36/5463 | |
| Age- and BMI-adjusted HR | Reference | 0.58 (0.34, 0.98) | 1.25 (0.79, 1.98) | 0.99 (0.63, 1.57) | 0.50 |
| Multivariable HR 12 | Reference | 0.61 (0.36, 1.05) | 1.39 (0.87, 2.21) | 1.02 (0.64, 1.63) | 0.45 |
| Multivariable HR 23 | Reference | 0.46 (0.26, 0.82) | 0.97 (0.57, 1.65) | 0.65 (0.37, 1.16) | 0.50 |
| Lignoceric acid, HPFS | |||||
| % of total FA, mean ± SD | 2.84 ± 0.42 | 3.72 ± 0.16 | 4.21 ± 0.14 | 4.87 ± 0.33 | |
| Cases/person-years | 33/4210 | 23/3985 | 37/4371 | 27/4274 | |
| Age- and BMI-adjusted HR | Reference | 0.80 (0.47, 1.36) | 1.03 (0.64, 1.66) | 0.78 (0.47, 1.31) | 0.51 |
| Multivariable HR 12 | Reference | 0.80 (0.47, 1.37) | 0.95 (0.59, 1.54) | 0.69 (0.41, 1.16) | 0.24 |
| Multivariable HR 23 | Reference | 0.93 (0.53, 1.62) | 1.29 (0.76, 2.19) | 1.04 (0.57, 1.90) | 0.68 |
| Lignoceric acid, pooled4 | Reference | 1.07 (0.75, 1.55) | 1.18 (0.80, 1.75) | 0.95 (0.61, 1.46) | 0.95 |
| Sum (arachidic acid + behenic acid + lignoceric acid), NHS | |||||
| % of total FA, mean ± SD | 3.52 ± 0.54 | 4.68 ± 0.23 | 5.48 ± 0.25 | 6.71 ± 0.65 | |
| Cases/person-years | 40/5682 | 27/5705 | 32/5534 | 34/5455 | |
| Age- and BMI-adjusted HR | Reference | 0.68 (0.42, 1.12) | 0.99 (0.62, 1.58) | 0.90 (0.57, 1.42) | 0.92 |
| Multivariable HR 12 | Reference | 0.77 (0.47, 1.27) | 1.10 (0.68, 1.77) | 0.94 (0.58, 1.50) | 0.95 |
| Multivariable HR 23 | Reference | 0.54 (0.32, 0.93) | 0.72 (0.42, 1.25) | 0.56 (0.32, 0.98) | 0.13 |
| Sum (arachidic acid + behenic acid + lignoceric acid), HPFS | |||||
| % of total FA, mean ± SD | 4.47 ± 0.56 | 5.59 ± 0.21 | 6.25 ± 0.19 | 7.15 ± 0.46 | |
| Cases/person-years | 33/4175 | 28/4031 | 30/4310 | 29/4325 | |
| Age- and BMI-adjusted HR | Reference | 0.96 (0.58, 1.59) | 0.92 (0.56, 1.52) | 0.79 (0.47, 1.32) | 0.38 |
| Multivariable HR 12 | Reference | 0.96 (0.58, 1.61) | 0.83 (0.50, 1.37) | 0.72 (0.43, 1.22) | 0.19 |
| Multivariable HR 23 | Reference | 1.14 (0.67, 1.94) | 1.10 (0.63, 1.90) | 1.07 (0.59, 1.94) | 0.82 |
| Sum (arachidic acid + behenic acid + lignoceric acid), pooled4 | Reference | 1.09 (0.76, 1.56) | 1.09 (0.74, 1.59) | 0.79 (0.52, 1.21) | 0.94 |
Values are means ± SDs or HRs (95% CIs). FA, fatty acid; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Model adjusted for age, BMI, race (white, nonwhite), smoking status (never, former, current, missing), physical activity (metabolic equivalents/wk), alcohol (g/d), family history of diabetes (yes, no, missing), parental history of myocardial infarction (yes, no, missing), hypercholesterolemia (yes, no), hypertension (yes, no), menopausal status in NHS (pre, post), postmenopausal hormone use in NHS (no, yes, missing), Alternative Healthy Eating Index, glycemic load (continuous), and total energy (kcal/d).
Included multivariate model 1 covariates and further adjusted for erythrocyte concentrations of trans-elaidic acid, trans-linoleaidic acid, and the sum of pentadecylic acid, margaric acid, and trans-palmitoleic acid.
Pooled estimates and P values were calculated by combining the participant data from both cohorts and further adjusting multivariate model 1 by sex.
P for trend was calculated by assigning the median values to each quartile and modeling the median values as a continuous variable.
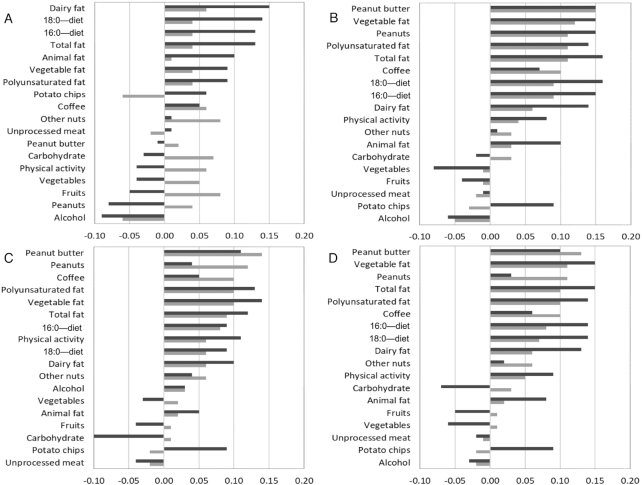
Cross-sectional Spearman partial correlations between each plasma VLCSFA biomarker and dietary and lifestyle variables were calculated in both the NHS and HPFS (Figure 1). In the NHS, biomarkers of behenic acid and lignoceric acid and the sum of VLCSFAs were positively correlated with intakes of peanuts, peanut butter, coffee, total fat, PUFAs, and vegetables. Behenic acid was positively correlated with dietary intakes of palmitic/stearic acid. Lignoceric acid was positively correlated with dietary palmitic acid. In the HPFS, behenic acid, lignoceric acid, and the sum of VLCSFAs were positively correlated with intakes of peanut butter and potato chips and with physical activity. Each fatty acid and their sum were positively correlated with intakes of total fat, dairy fat, vegetable fat, PUFAs, and dietary palmitic acid and stearic acid. Fish and vegetable intake was inversely correlated with behenic acid concentrations, whereas the correlations were positive for intakes of French fries and processed meat. Similar results were observed for erythrocyte VLCSFAs and intakes of peanut butter, total dairy, and coffee, as well as correlations between behenic acid and dietary palmitic/stearic acid, total fat, dairy fat, and animal fat in the NHS and HPFS (Supplemental Figure 1). Despite the statistical significance, all correlations were weak (|rs| ≤ 0.19), and the dietary and lifestyle predictors explained only a small proportion of overall variation of VLCSFAs, with the percentage ranging from 1.53% to 1.61%.
FIGURE 1.
Spearman partial correlations between circulating plasma fatty acid biomarkers of arachidic acid (A), behenic acid (B), lignoceric acid (C), and the sum of very-long-chain SFAs (D) and dietary factors at baseline among 692 participants in the NHS and 672 participants in the HPFS. Correlations were adjusted for age, total energy intake, and BMI. Fatty acid concentrations were assessed from the plasma fractions of HPFS and NHS participants. Dietary and nutrient factors were assessed using the mean of self-reported responses in 1990 and 1994 in the HPFS (black bars) and 1986 and 1990 in the NHS (gray bars). HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent; NHS, Nurses’ Health Study.
In a sensitivity analysis, we excluded T2D cases diagnosed within the first 2 or 4 y after blood draw and the associations between each VLCSFA and T2D risk largely persisted, with wider CIs because of there being fewer cases included (data not shown). In another sensitivity analysis conducted among CVD controls only, the inverse associations for each plasma VLCSFA biomarker and T2D risk persisted but they lost statistical significance owing to the diminished statistical power. Comparing the highest with the lowest quartiles, the pooled HRs (95% CIs) of T2D were 0.75 (0.41, 1.41; P-trend = 0.30) for arachidic acid; 0.58 (0.30, 1.13; P-trend = 0.08) for behenic acid; 0.63 (0.34, 1.19; P-trend = 0.10) for lignoceric acid; and 0.66 (0.35, 1.27; P-trend = 0.13) for the sum of the 3 VLCSFAs. In another sensitivity analysis, we also adjusted for intakes of the main dietary sources of VLCSFAs, including peanuts, peanut butter, dairy fat, and vegetable fat, and the results were largely unchanged (data not shown).
Discussion
Plasma concentrations of arachidic acid, behenic acid, lignoceric acid, and the sum of these VLCSFAs were significantly associated with a lower risk of T2D after multivariate adjustment of covariates in 2 cohorts of US men and women. For erythrocyte biomarkers, only arachidic acid and behenic acid were significantly associated with a lower risk of T2D. Cross-sectionally, we observed weak yet significant positive correlations between plasma and erythrocyte concentrations of VLCSFAs and various dietary factors, including intakes of peanuts and peanut butter, coffee, and dietary fats. In addition, plasma VLCSFAs were also positively correlated with physical activity levels.
Our findings of inverse associations between plasma concentrations of VLCSFAs and diabetes risk are in line with previous studies. In the EPIC-InterAct study, inverse associations with T2D risk were observed for all 3 plasma VLCSFA biomarkers: HRs (95% CIs) of T2D per SD change were 0.78 (0.68, 0.88) for arachidic acid, 0.81 (0.71, 0.93) for behenic acid, and 0.75 (0.64, 0.88) for lignoceric acid (11). In the CHS, inverse associations were also found between plasma concentrations of VLCSFAs and T2D risk; the HRs (95% CIs) comparing the highest with the lowest quartiles were 0.53 (0.37, 0.77) for arachidic acid; 0.67 (0.47, 0.94) for behenic acid, and 0.63 (0.45, 0.89) for lignoceric acid (9). In contrast, findings regarding erythrocyte VLCSFAs are mixed. Similar to our findings, in the EPIC-Potsdam study, erythrocyte concentrations of arachidic acid were inversely associated with T2D risk (RR for highest vs. lowest quintiles: 0.66; 95% CI: 0.47, 0.94), although a significant positive association was observed for lignoceric acid (RR for highest vs. lowest quintiles: 1.56; 95% CI: 1.11, 2.21) (10). In a case-control study in the Hunter Community Study, concentrations of lignoceric acid in whole blood were associated with a reduced T2D risk but other VLCSFAs were not measured (25). Lastly, a pooled analysis from 12 cohorts (VLCSFA contents measured in total plasma or plasma phospholipids in 7 studies) reported inverse associations of each VLCSFA measured in various blood compartments, including total plasma, plasma phospholipids, and erythrocyte fractions, with T2D risk. The pooled HRs (95% CIs) for IQR difference were 0.78 (0.70, 0.87) for arachidic acid, 0.84 (0.77, 0.91) for behenic acid, and 0.75 (0.69, 0.83) for lignoceric acid. In this analysis, no significant heterogeneity by blood compartments was observed (18). Taken together, these data suggest that VLCSFAs, especially those in plasma, were consistently associated with a lower risk of T2D. Of note, the VLCSFAs in plasma were also inversely associated with other cardiometabolic outcomes, including ischemic heart disease in the case-control studies in the NHS and HPFS (6), sudden cardiac arrest (8), and atrial fibrillation (7).
In the current study, the associations for plasma VLCSFAs are in general stronger than for those in erythrocyte membranes. The reason underlying the weaker associations for erythrocyte VLCSFAs is unknown, although this may be ascribed to factors such as the uptake and release of these fatty acids from erythrocyte membranes to plasma, which can influence their bioavailability and biological functions in metabolic processes at target tissues. Evidence from feeding trials where patients were randomly assigned to supplementation with long-chain PUFAs showed that fatty acid uptake into erythrocyte cell membranes depends on chain length and duration of supplementation (26, 27). Fatty acid chain length also affects their rate of release from cell membrane phospholipids into plasma through phospholipase A2 (28). Fatty acids with shorter chain lengths are released more readily (28, 29), which may partially explain the resemblance of associations for arachidic acid and behenic acid, but not lignoceric acid, between erythrocyte membranes and plasma. In addition, it should be also noted that CVs for erythrocyte measurements were higher than for plasma (especially for lignoceric acid), and thus measurement error may contribute to the nonsignificant associations observed for lignoceric acid and the sum of VLCSFAs, which is largely driven by lignoceric acid.
The current study is among the first to explore dietary and lifestyle predictors of VLCSFAs. We observed significant correlations between plasma behenic acid and lignoceric acid and intakes of peanuts and peanut butter, probably because these foods contain arachidic acid and behenic acid (4). These data are consistent with those from feeding trials, where peanut intake led to higher plasma concentrations of behenic acid, lignoceric acid, and cerotic acid (26:0) after 2–8 h of intake (30). Positive associations between nut and seed intake and VLCSFA concentrations in plasma were also found in the EPIC-InterAct study (11), although whether such an observation was driven by peanut intake is unclear. In the current study, we also observed significant positive correlations with intakes of vegetable fat and potato chips, which may be explained by the fact that canola oil contains arachidic acid and behenic acid and this oil is commonly used in the manufacture of potato chips (4). The positive correlations between dairy fat intake and arachidic acid and behenic acid in the HPFS could be possibly explained by the fact that dairy fat is among the food sources of all 3 VLCSFAs (4). In the EPIC-InterAct study, intake of dairy products, milk, and butter was also positively correlated with VLCSFA concentrations in plasma (11). The positive correlation with coffee in the NHS is unexpected because coffee does not contain these fatty acids, although we cannot exclude the possibility that milk or cream added to coffee may partially explain the associations. Of other factors, alcohol intake was inversely associated with VLCSFAs and physical activity was positively associated with these fatty acids, although the biological explanations underlying these correlations are unknown. Nonetheless, the correlations between VLCSFAs and these dietary factors were quite weak, and adjustment for these factors did not attenuate the strong inverse associations of VLCSFAs.
The mechanistic pathways underlying the observed associations for arachidic acid, behenic and lignoceric acid and T2D risk are not well established. VLCSFAs may modulate insulin sensitivity through multiple mechanisms. VLCSFAs are constituents of ceramides when they are attached to sphingosine (5, 31). The chain length of the fatty acid side groups may determine the ceramides’ effects on insulin sensitivity in peripheral tissues. For instance, ceramides that incorporate SFAs such as palmitic acid and stearic acid are associated with increased insulin resistance (32), and inhibition of glucose uptake by decreasing the translocation of glucose transporters 1 and 4 (GLUT1 and GLUT4) to plasma membranes (33). In contrast, ceramides with very-long-chain sphingolipid species are inversely associated with hepatic insulin resistance in rodent models (34, 35). These findings are supported by epidemiologic evidence suggesting that the ratio of ceramides with side groups 18:0 to 16:0 is a strong predictor of T2D, whereas the ratio of ceramides with acyl chains 18:0 to 24:0 or 24:1 was not associated with T2D risk (36).
Another potential mechanism might be related with the metabolism of these fatty acids. VLCSFAs undergo β-oxidation in peroxisomes because mitochondria lack the very-long-chain acyl-CoA synthetase (37). The resulting medium-chain acyl-CoA β-oxidation products can act as precursors in the synthesis of plasmalogens in peroxisomes and undergo further modifications in the endoplasmic reticulum (37, 38). Plasmalogens are glycerophospholipid constituents of cell membranes and may be preferentially oxidized to spare the oxidation of PUFAs and other membrane components, and stop the propagation of lipid peroxidation (38).
The third potential mechanism could be through the unique regulation of β-oxidation of VLCSFAs which is modulated by peroxisome proliferator–activated receptor (PPAR)δ as opposed to other fatty acids, which are modulated by PPARα (39). Potential interactions between VLCSFAs and PPARδ gene variants have been reported in the NHS and HPFS in relation with ischemic heart disease (6). PPARδ is widely expressed in many tissues including muscle, liver, and adipose tissue where it can regulate energy balance (39) and stimulate β-oxidation, triglyceride, and glucose utilization in adipose tissue (39, 40). PPARδ agonists have shown improvements in insulin sensitivity in oral-glucose-tolerance tests in mouse models (41) and reverse metabolic abnormalities in obese men, including fasting triglycerides, insulin, and LDL concentrations, but the mechanisms are still unclear (42). PPARδ activation reduces the production of proinflammatory cytokines involved in insulin resistance and improves the functions of pancreatic β-cells (43).
Conversely, insulin sensitivity may result in the reduction of VLCSFA production through the activation of mammalian target of rapamycin complex 1 (mTORC1). mTORC1 plays an important role in nutrient sensing and in the regulation of multiple metabolic processes (44, 45). Elevated intakes of glucose and amino acids, in addition to high concentrations of proinflammatory cytokines, insulin, and insulin growth factors, promote mTORC1 activity (44–46). Over-activation of mTORC1 further promotes insulin resistance in peripheral tissues through inhibition of insulin signaling (47–51). Interestingly, over-activation of mTORC1 also downregulates ELOVL1 expression and VLCSFA synthesis (52, 53). Despite these pathways suggesting that VLCSFAs may just serve as markers of existing insulin sensitivity, the robust associations observed in the sensitivity analysis after we excluded the diabetes cases that occurred during early follow-up argue against this possibility. Nonetheless, more evidence from molecular epidemiologic studies and basic science research is needed to further elucidate the mechanisms underlying our observations.
This study has several strengths, including long duration of follow-up, examination of both plasma and erythrocyte VLCSFA concentrations, and the examination of diet/lifestyle associations with VLCSFAs. Our study also has some limitations. First, relatively large laboratory measurement errors for erythrocyte VLCSFA assessments may lead to attenuation of the true associations for erythrocyte VLCSFAs in this prospective study. Second, the study participants were exclusively middle-aged and older health professionals, and >95% of them are of European ancestry. These facts may limit the generalizability of the results to other populations. Third, owing to the observational nature of these studies, the reported associations will not necessarily entail causal interpretation. Although the adjustment of multiple covariates may help better control for confounding, we cannot exclude the role of residual and/or unmeasured confounding in our findings.
In summary, in US men and women, plasma concentrations of VLCSFAs are associated with a lower risk of T2D. These associations are independent of established and potential dietary and lifestyle risk factors of T2D, as well as dietary sources of these fatty acids, and robust in various sensitivity analyses. The inverse associations with other cardiometabolic diseases observed in previous studies call for more research to understand the mechanistic pathways behind these associations.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—AVAK, FBH, and QS: participated in study conception and design; FBH and DM: obtained funding and provided oversight; AVAK: performed the data analysis; JDF: supervised the laboratory procedures and quality control; AVAK and QS: drafted the manuscript, are the guarantors of this work, and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; VSM, BR, FS, QS, WCW, and FBH: provided critical feedback on revisions and other intellectual input; all authors participated in critical revisions and read and approved the final manuscript.
Notes
Parts of this study were presented in abstract form at the Society of Epidemiologic Research meeting, Seattle, WA, 19–22 June, 2017.
Supported by NIH grants CA186107, CA176726, CA167552, DK082486, DK112940, and DK120870 and by National Institute of Diabetes and Digestive and Kidney Diseases T32 DK007703 and by National Cancer Institute T32 CA009001 (to AVAK).
Author disclosures: AVAK, VSM, JDF, FS, BR, KMR, WCW, and DM, no conflicts of interest. QS reported receiving ad hoc consulting fees from Emavant Solutions GmbH. FBH has received research support from the California Walnut Commission and lecture fees from Standard Process and Metagenics.
Supplemental Tables 1–4 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AHEI, Alternative Healthy Eating Index; CHS, Cardiovascular Health Study; CVD, cardiovascular disease; ELOVL, elongase of very long chain fatty acids; EPIC, European Prospective Investigation into Cancer and Nutrition; GLUT, glucose transporter; HPFS, Health Professionals Follow-Up Study; mTORC1, mammalian target of rapamycin complex 1; NHS, Nurses’ Health Study; PPAR, peroxisome proliferator–activated receptor; TFA, trans fatty acid; T2D, type 2 diabetes; VLCSFA, very-long-chain SFA.
References
- 1. International Diabetes Federation. Diabetes atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 2. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang WS, de Oliveira Otto MC, Kröger J et al.. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Department of Agriculture (USDA). USDA food composition databases. Published by USDA Agricultural Research Service; Washington, DC: [cited 2018 Apr 1]. Available from: http://ndb.nal.usda.gov/ndb/foods. [Google Scholar]
- 5. Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem. 2012;152:387–95. [DOI] [PubMed] [Google Scholar]
- 6. Malik VS, Chiuve SE, Campos H, Rimm EB, Mozaffarian D, Hu FB, Sun Q. Circulating very-long chain saturated fatty acids and incident coronary heart disease in U.S. men and women. Circulation. 2015;132(4):260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fretts AM, Mozaffarian D, Siscovick DS, Djousse L, Heckbert SR, King IB, McKnight B, Sitlani C, Sacks FM, Song X et al.. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3:e000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lemaitre RN, King IB, Rice K, McKnight B, Sotoodehnia N, Rea TD, Johnson CO, Raghunathan TE, Cobb LA, Mozaffarian D et al.. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot Essent Fat Acids. 2014;91:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB, Song X, Djousse L, Siscovick DS, McKnight B et al.. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kröger J, Zieteman V, Enzenback C, Weikert C, Jansen EHJM, Döring F, Joost H-G, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and nutrition (EPIC)-Potsdam study. Am J Clin Nutr. 2011;93:127–42. [DOI] [PubMed] [Google Scholar]
- 11. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, Crowe FL, Huerta JM, Amiano P, Boeing H et al.. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–9. [DOI] [PubMed] [Google Scholar]
- 13. Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25:165–72. [Google Scholar]
- 14. Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, Campos H. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–81. [DOI] [PubMed] [Google Scholar]
- 15. Zock PL, Mensink RP, Harryvan J, De Vries JHM, Katan MB. Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. Am J Epidemiol. 1997;145:1114–22. [DOI] [PubMed] [Google Scholar]
- 16. Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, Sacks FM, Stampfer MJ. Effect of transport conditions on the stability of biochemical markers in blood. Clin Chem. 1989;35:2313–6. [PubMed] [Google Scholar]
- 17. Zeleniuch-Jacquotte A, Chajès V, Van Kappel A, Riboli E, Toniolo P. Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr. 2000;54:367–72. [DOI] [PubMed] [Google Scholar]
- 18. Fretts AM, Imamura F, Marklund M, Micha R, Wu JHY, Murphy RA, Chien KL, McKnight B, Tintle N, Forouhi NG et al.. Associations of circulating very-long-chain saturated fatty acids and incident type 2 diabetes: a pooled analysis of prospective cohort studies. Am J Clin Nutr. 2019;109:1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr. 2007;86:929–37. [DOI] [PubMed] [Google Scholar]
- 20. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–57. [DOI] [PubMed] [Google Scholar]
- 21. Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–8. [DOI] [PubMed] [Google Scholar]
- 22. Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–8. [DOI] [PubMed] [Google Scholar]
- 23. Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–6. [DOI] [PubMed] [Google Scholar]
- 24. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alhazmi A, Stojanovski E, Garg ML, McEvoy M. Fasting whole blood fatty acid profile and risk of type 2 diabetes in adults: a nested case control study. PLoS Med. 2014;9:e97001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vidgren HM, Agren JJ, Schwab U, Rissanen T, Hänninen O, Uusitupa MI. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids. 1997;32:697–705. [DOI] [PubMed] [Google Scholar]
- 27. Eltweri AM, Thomas AL, Fisk HL, Arshad A, Calder PC, Dennison AR, Bowrey DJ. Plasma and erythrocyte uptake of omega-3 fatty acids from an intravenous fish oil based lipid emulsion in patients with advanced oesophagogastric cancer. Clin Nutr. 2017;36:768–74. [DOI] [PubMed] [Google Scholar]
- 28. Jorgensen K, Davidsen J, Mouritsen OG. Biophysical mechanisms of phospholipase A2 activation and their use in liposome-based drug delivery. FEBS Lett. 2002;531:23–7. [DOI] [PubMed] [Google Scholar]
- 29. Gabriel NE, Roberts MF. Short-chain lecithin/long-chain phospholipid unilamellar vesicles: asymmetry dynamics, and enzymatic hydrolysis of the short-chain component. Biochemistry. 1987;26:2432–40. [DOI] [PubMed] [Google Scholar]
- 30. Lam C, Wong D, Cederbaum S, Lim B, Qu Y. Peanut consumption increases levels of plasma very long chain fatty acids in humans. Mol Genet Metab. 2012;107:620–2. [DOI] [PubMed] [Google Scholar]
- 31. Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL et al.. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–94. [DOI] [PubMed] [Google Scholar]
- 33. Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–95. [DOI] [PubMed] [Google Scholar]
- 34. Montgomery MK, Brown SHJ, Lim XY, Fiveash CE, Osborne B, Bentley NL, Braude JP, Mitchell TW, Coster ACF, Don AS et al.. Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: a beneficial role for very long-chain sphingolipid species. Biochim Biophys Acta Mol Cell Biol Lipids. 2016;1861:1828–39. [DOI] [PubMed] [Google Scholar]
- 35. Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51:50–62..< /bib> [DOI] [PubMed] [Google Scholar]
- 36. Hilvo M, Salonurmi T, Havulinna AS, Kauhanen D, Pedersen ER, Tell GS, Meyer K, Teeriniemi A-M, Laatikainen T, Jousilahti P et al.. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia. 2018;61(6):1424–34. [DOI] [PubMed] [Google Scholar]
- 37. Wanders RJA. Metabolic functions of peroxisomes in health and disease. Biochimie. 2014;98:36–44. [DOI] [PubMed] [Google Scholar]
- 38. Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822:1442–52. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y-X, Lee C-H, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113:159–70. [DOI] [PubMed] [Google Scholar]
- 40. Roberts LD, Murray AJ, Menassa D, Ashmore T, Nicholls AW, Griffin JL. The contrasting roles of PPARδ and PPARγ in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue. Genome Biol. 2011;12:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee C-H, Olson P, Hevener A, Mehl I, Chong L-W, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM et al.. PPARδ regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2006;103:3444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riserus U, Sprecher D, Johnson T, Olson E, Hirschberg S, Liu A, Fang Z, Hegde P, Richards D, Sarov-Blat L et al.. Activation of peroxisome proliferator–activated receptor (PPAR)δ promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–9. [DOI] [PubMed] [Google Scholar]
- 43. Salvadó L, Serrano-Marco L, Barroso E, Palomer X, Vázquez-Carrera M. Targeting PPARβ/δ for the treatment of type 2 diabetes mellitus. Expert Opin Ther Targets. 2012;16:209–23. [DOI] [PubMed] [Google Scholar]
- 44. Ong PS, Wang LZ, Dai X, Tseng SH, Loo SJ, Sethi G. Judicious toggling of mTOR activity to combat insulin resistance and cancer: current evidence and perspectives. Front Pharmacol. 2016;7:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang P, Drackley JK, Stamey-Lanier JA, Keisler D, Loor JJ. Effects of level of nutrient intake and age on mammalian target of rapamycin, insulin, and insulin-like growth factor-1 gene network expression in skeletal muscle of young Holstein calves. J Dairy Sci. 2013;97:383–91. [DOI] [PubMed] [Google Scholar]
- 46. Guillén C, Benito M. MTORC1 overactivation as a key aging factor in the progression to type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2018;9:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–81. [DOI] [PubMed] [Google Scholar]
- 48. Kozma SC, Auwerx J, Allegrini PR, Fumagalli S, Frigerio F, Watanabe M, Sticker M, Picard F, Joaquin M, Hee Um S et al.. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:485. [DOI] [PubMed] [Google Scholar]
- 49. Bae JY, Shin KO, Woo J, Woo SH, Jang KS, Lee YH, Kang S. Exercise and dietary change ameliorate high fat diet induced obesity and insulin resistance via mTOR signaling pathway. J Exerc Nutr Biochem. 2016;20:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tandelilin AAK, Hirase T, Hudoyo AW, Cheng J, Toyama K, Morisaki H, Morisaki T. AMPD1 regulates mTORC1-p70 S6 kinase axis in the control of insulin sensitivity in skeletal muscle. BMC Endocr Disord. 2015;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. [DOI] [PubMed] [Google Scholar]
- 52. Wang W, He Q, Guo Z, Yang L, Bao L, Bao W, Zheng X, Wang Y, Wang Z. Inhibition of mammalian target of rapamycin complex 1 (mTORC1) downregulates ELOVL1 gene expression and fatty acid synthesis in goat fetal fibroblasts. Int J Mol Sci. 2015;16:16440–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guo Z, Wang Y, Feng X, Bao C, He Q, Bao L, Hao H, Wang Z. Rapamycin inhibits expression of elongation of very-long-chain fatty acids 1 and synthesis of docosahexaenoic acid in bovine mammary epithelial cells. Asian-Australas J Anim Sci. 2016;29:1646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.