Abstract
BACKGROUND
Intracerebral hemorrhage (ICH) is characterized by a 30-d mortality rate of 40% and significant disability for those who survive.
OBJECTIVE
To investigate the initial safety concerns of histotripsy mediated clot liquefaction and aspiration in a porcine ICH model. Histotripsy is a noninvasive, focused ultrasound technique that generates cavitation to mechanically fractionate tissue. Histotripsy has the potential to liquefy clot in the brain and facilitate minimally invasive aspiration.
METHODS
About 1.75-mL clots were formed in the frontal lobe of the brain (n = 18; n = 6/group). The centers of the clots were liquefied with histotripsy 48 h after formation, and the content was either evacuated or left within the brain. A control group was left untreated. Pigs underwent magnetic resonance imaging (MRI) 7 to 8 d after clot formation and were subsequently euthanized. Neurological behavior was assessed throughout. Histological analysis was performed on harvested brains. A subset of pigs underwent acute analysis (≤6 h).
RESULTS
Histotripsy was able to liquefy the center of clots without direct damage to the perihematomal brain tissue. An average volume of 0.9 ± 0.5 mL was drained after histotripsy treatment. All groups showed mild ischemia and gliosis in the perihematomal region; however, there were no deaths or signs of neurological dysfunction in any groups.
CONCLUSION
This study presents the first analysis of histotripsy-based liquefaction of ICH in vivo. Histotripsy safely liquefies clots without significant additional damage to the perihematomal region. The liquefied content of the clot can be easily evacuated, and the undrained clot has no effect on pig survival or neurological behavior.
Keywords: Focused ultrasound, Histotripsy, Thrombolysis, Intracerebral hemorrhage, Neurosurgery
ABBREVIATIONS
- CPP
cerebral perfusion pressure
- FLAIR
fluid-attenuated inversion recovery
- FSE
fast-spin echo
- FUS
focused ultrasound
- ICH
intracerebral hemorrhage
- ICP
intracranial pressure
- MERGE
multi-echo recombined gradient echo
- MRgFUS
magnetic resonance-guided FUS
- MRI
magnetic resonance imaging
- PRF
pulse-repetition frequencies
- RBCs
red blood cells
- ROIs
ring-shaped regions of interest
Intracerebral hemorrhage (ICH) is a devastating form of hemorrhagic stroke, leading to a 30-d mortality of 40% and significant disability for those who survive.1,2 The primary neurologic injury induced by ICH occurs immediately and is characterized by the mass effect and increased intracranial pressure (ICP), which, in turn, leads to a reduction in cerebral perfusion pressure (CPP), causing further ischemic injuries.3,4 The secondary injury to the brain develops in the days following the hemorrhage and is characterized by the toxic effects associated with clot metabolism and processes associated with the inflammatory and complement systems’ response to erythrolysis.3 In particular, the infusion of hemolysates into the brain has shown damaging effects in rats and pigs with the primary injury mechanism attributed to iron overload and the generation of reactive oxygen species.3 Thus, the immediate removal of intracranial blood products reduces brain pressure and removes products responsible for secondary cerebral injuries.
Many techniques have been developed to remove ICH with the goal of removing the maximal amount of clot in the most minimally invasive manner. Currently, surgeons utilize craniotomy, endoscopic, and lytic approaches in evacuating the hematoma. The craniotomy-based approach is maximally invasive, whereas the endoscopic approach is less invasive, but still requires traversing normal tissue with a large port. The intraclot lytic method is being tested in the MISTIE III trial; however, this approach relies on an evacuation process that takes days and cannot be initiated immediately. There is an unmet need for a minimally invasive method that can be applied immediately following ICH and allow evacuation of clot within minutes.
Histotripsy is a novel noninvasive, focused ultrasound (FUS) technique that differs from magnetic resonance-guided FUS (MRgFUS) in its therapeutic mechanism and possesses advantages for rapid liquefaction and removal of ICH. Instead of using long ultrasound pulses at moderate amplitude to heat and thermally necrose tissue, histotripsy uses very short (<0.1%), high-amplitude ultrasound pulses (>27 MPa) applied from outside the body to generate a cluster of targeted, focused cavitation microbubbles (bubble cloud) without the use of extrinsically administered microbubbles.5-8 The rapid bubble expansion and collapse create significant stress and strain in tissue that mechanically fractionate it into an acellular liquid.9 Recent studies have shown the ability for transcranial clot liquefaction through humans skulls in vitro, with liquefaction rates in the range of 2 to 5 mL/min.10,11 The liquefied volume can be drained through a burr hole in the skull via catheter. It should be noted that, in this study, craniotomy is performed because the geometry of the pig skull is not amenable for ultrasound propagation. In humans, craniotomy will not be needed, as in vitro studies already shows that histotripsy can be applied from outside the intact human skull to liquefy the clot.
With the current study, we present the first analysis of histotripsy-based liquefaction of ICH in vivo. There is significant concern that the lysed blood products generated by histotripsy treatment will cause severe neurological injury.3,12-16 A previous study reported that lysed blood directly injected into the pig brain with the similar volume (∼2 mL), as used in this study, lead to significant cerebral injury and even death.16 Thus, we have set to investigate the safety of histotripsy clot liquefaction in a well-established porcine ICH model.17 The primary goals of this study were the following: (1) to evaluate initial safety concerns of histotripsy in liquefying ICH utilizing MR imaging, histopathology, and porcine neurological functioning and (2) to determine ability to evacuate the histotripsy-treated ICH utilizing a simple drainage technique.
METHODS
Animals
The protocols involved in this study were approved by the pertinent group at our university. Male and female pigs (30-35 kg) were obtained from an authorized dealer.
Experimental Groups and Study Overview
Pigs were divided into 3 groups: 2 treatment groups and 1 control group (n = 6/group). In one treatment group, the clot was liquefied with histotripsy, and the liquefied contents were evacuated with a needle and syringe immediately following treatment (treatment-drained). In the second treatment group, the clot was liquefied, and the contents were left within the brain (treatment-undrained). The timeline of the study can be seen in Figure 1. In a randomized order, craniotomies were performed, and hematomas were formed within the brain on day 1. For treatment groups, clots were liquefied with histotripsy 48 h after they were formed (day 3). Ideally, a 24-h treatment window would have been used, but because of the significant surgical burden of the craniotomy and clot injection, pigs were given 48 h to recover. To assess the degree to which pigs tolerated the clots that underwent histotripsy liquefaction (drained and undrained) relative to those that did not receive histotripsy treatment, pigs were survived 5 to 6 d following treatment. Magnetic resonance imaging (MRI) was performed on a subset (n = 4-6/group) of each group on day 8 or 9. Pigs were euthanized directly following MRI, and brains were fixed and sectioned for histology.
Figure 1.
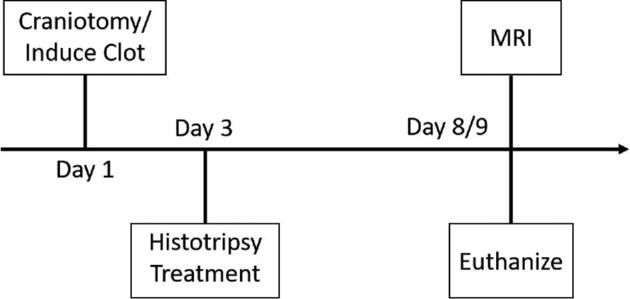
The timeline of the study. For the treatment-drained pigs, the volume was drained directly following histotripsy treatment on day 3.
Porcine ICH Model
All surgical procedures during the clot formation were performed using aseptic techniques. Pigs were intubated and maintained on isoflurane gas. A transcutaneous, intra-arterial catheter was placed into the common femoral artery of the pig to allow for removal of arterial blood for ICH formation. Following the placement of a longitudinal incision in the scalp, the skin and periosteum were separated, and a 5-cm craniotomy was performed. The craniotomy was necessary to provide an acoustic window for histotripsy therapy, as the geometry of the pig skull is not conducive to ultrasound transmission.
Immediately following the craniotomy, clots were formed in the cerebral white matter using the porcine lobar ICH model described by Wagner et al.17 A 20-guage needle was connected to the arterial line via extension tubing and fed through an infusion pump (Baxter, Deerfield, Illinois). The needle was inserted to a depth of 2 to 5 mm into the white matter of the left frontal lobe, approximately 2 mm laterally from the longitudinal fissure and 1 cm anterior of the ventricles. About 1.75 mL of blood was infused over 13 min. This relatively small volume was chosen with consideration to the size of the pig brain and has been established as a standard volume for this ICH model.17 Although slightly larger volumes have been reported using this model,16 we observed an increased likelihood of pig death when the volume was increased much beyond 1.75 mL. Following clot formation, the incision was sutured closed.
Neurological Evaluation
The neurological status of each pig was evaluated according to a grading scale similar to that used by Tanaka et al and Yamaguchi et al.18,19 To assess neurological status of the pigs, scores were generated from a 25-point scale: 0 (no deficit) and 25 (severe deficit). Neurological signs for the grading scale included appetite (4 points), standing position (5), head position (2), utterance (2), gait (3), motor function (fore and hind limbs, 4 each), and facial paralysis (1). Neurological behavior was evaluated daily starting with a preoperative evaluation on the clot formation date.
Histotripsy Treatment
Histotripsy treatment was applied to anesthetized pigs through the scalp using a 1-MHz, 8-element, handheld transducer (Figure 2). A phased-array, B-mode ultrasound probe was inserted coaxially into the transducer to allow treatment targeting and monitoring. The histotripsy transducer was coupled to the pig brain via degassed saline placed on top of the pig head. The transducer was guided by hand using B-mode guidance during all treatments. The cavitation activity generated by histotripsy appears intensely hyperechoic with time-varying brightness on B-mode, allowing it to be easily identified and monitored during treatment.
Figure 2.
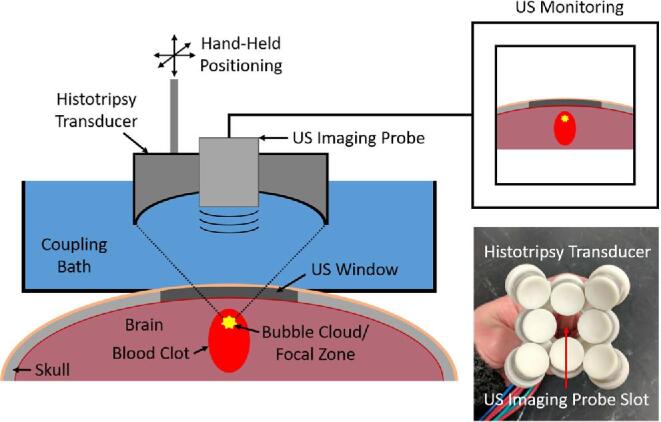
An experimental schematic of the histotripsy treatment.
Histotripsy treatments were delivered using ultrasound pulses with peak-negative pressures of 30 to 40 MPa at pulse-repetition frequencies (PRF) of 40 to 60 Hz. Prior to treatment, the bulk clot volume appeared hyperechoic on B-mode ultrasound. The central portion of the clot was treated until the targeted region went from hyperechoic (intact clot) to hypoechoic (liquefied clot), as observed via B-mode. A 1-mm to 2-mm rim of untreated clot was intentionally left at the clot boundary as a safety margin. In the treatment-drained group, the hypoechoic liquefied content was drained via an 18-gauge needle and 3-mL syringe following treatment. In the treatment-undrained group, the liquefied content was left within the brain for the duration of the porcine survival.
In Vivo Magnetic Resonance Imaging (MRI) Protocol
Samples from each group (n = 4-6/group) were evaluated on a 3T MRI scanner (MR750, General Electric, Waukesha, Wisconsin) 7 to 8 d after the hematoma was formed. The imaging protocol included axial T2 fast-spin echo (FSE), T2 fluid-attenuated inversion recovery (FLAIR), T2*-weighted multi-echo recombined gradient echo (MERGE), and T1 sequences. To examine perihematomal edema, ring-shaped regions of interest (ROIs) on the FLAIR images in the periphery of the hematoma were compared to similar shaped ROIs made on the contralateral side of the brain in an identical anatomical site. MRI evaluations were performed by a board-certified neuroradiologist who was blinded to the treatment and control groups.
Histological Analysis
Following MR scans, pigs were euthanized. Following euthanasia, the heads were removed and fixed whole in 10% phosphate-buffered formalin (Fisher Chemical, Pittsburgh, Pennsylvania). Following fixation, the brains were removed and sectioned around the location of the ICH and stained with hematoxylin and eosin (H&E), Perls’ Prussian blue, CD45, and neurofilament 200.
Acute Treatment
A subset of acute treatments was performed to more effectively highlight the immediate effects of histotripsy on the clot and surrounding brain tissue. For the acute subset, treatment was applied directly after the infused blood had coagulated. The liquefied contents were left undrained, and the pigs were euthanized within 1 h following the completion of treatment. Fixation followed the same procedures described above. The fixed brains were imaged using a T2-weighted FSE sequence on a 7T MRI (Varian, Palo Alto, California) and sectioned around the location of the ICH.
Statistical Methods
Within each group, pair-wise, independent 2-tailed t-tests (α = 0.05) were used to compare the signal intensity in ROIs made in the perihematomal region on FLAIR images to that in ROIs made on the contralateral side of the brain. Pair-wise comparisons of the signal intensity in the perihematomal ROI normalized by the mean signal intensity in the contralateral side of the brain were made between each group using independent 2-tailed t-tests (α = 0.05).
RESULTS
Acute Treatment
In acute pigs, the untreated clot was fully hypointense on MRI (Figure 3A), which suggested a coagulated acute clot.20 This contrasted with the hyperintense core and hypointense rim of the clot treated with histotripsy (Figure 3B), which indicated the region of lysed red blood cells (RBCs) surrounded by a rim of unlysed, intact clot.20 The H&E-stained sections of the brain from the untreated and treated pig showed a fully coagulated acute clot (Figure 3C) and a homogenized, acellular core surrounded by an intact rim (Figure 3D), respectively. There was no evident damage to the surrounding perihematomal region based on the H&E-stained sections.
Figure 3.
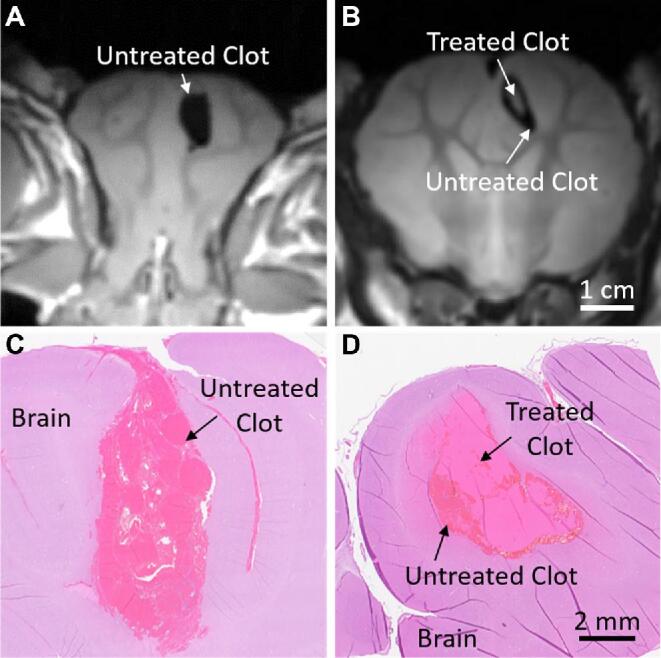
The T2-weighted FSE MRI of Ex Vivo, formalin-fixed acute clot and brain for the A, untreated pig and B, treated pig. H&E-stained sections of the acute clot and brain for the C, untreated pig, and D, treated pig. Sections from the untreated pigs showed fully intact coagulated clots, whereas the clots treated with histotripsy were characterized by an acellular, homogenized core surrounded by an intact rim of untreated clot.
Neurological Evaluation
There were no significant neurological differences between any of the groups. For each category other than appetite, all pigs received a net score of 0. Appetite scores fluctuated between 0 and 3, but the fluctuations appeared random and showed no relation to a specific group.
Evacuation of the Liquefied Clot after Histotripsy
Directly following histotripsy treatment, the liquefied core of clot was evacuated using a simple drainage technique (Figure 4). Prior to histotripsy treatment, the clots were fully hyperechoic on B-mode (Figure 4A). As histotripsy was applied, the core of the clot transformed from a hyperechoic to hypoechoic appearance, indicating liquefaction (Figure 4B). Inserting the needle to the center and pulling back on the plunger of the syringe easily evacuated the liquid core of the clot. It was not possible to remove the intact hyperechoic regions of the clot. After evacuating the core, a hyperechoic strip corresponding to the intact rim of the clot was left behind (Figure 4C). On average, the volume drained after treatment was 0.9 ± 0.5 mL (Table 1). For pig 6, the reported volume drained was slightly greater than that of the initial clot formed, which was caused by the accidental suction of some of the fluid filling the space between the brain and scalp. Excluding this data point, the average volume drained was 0.7 ± 0. 2 mL.
Figure 4.
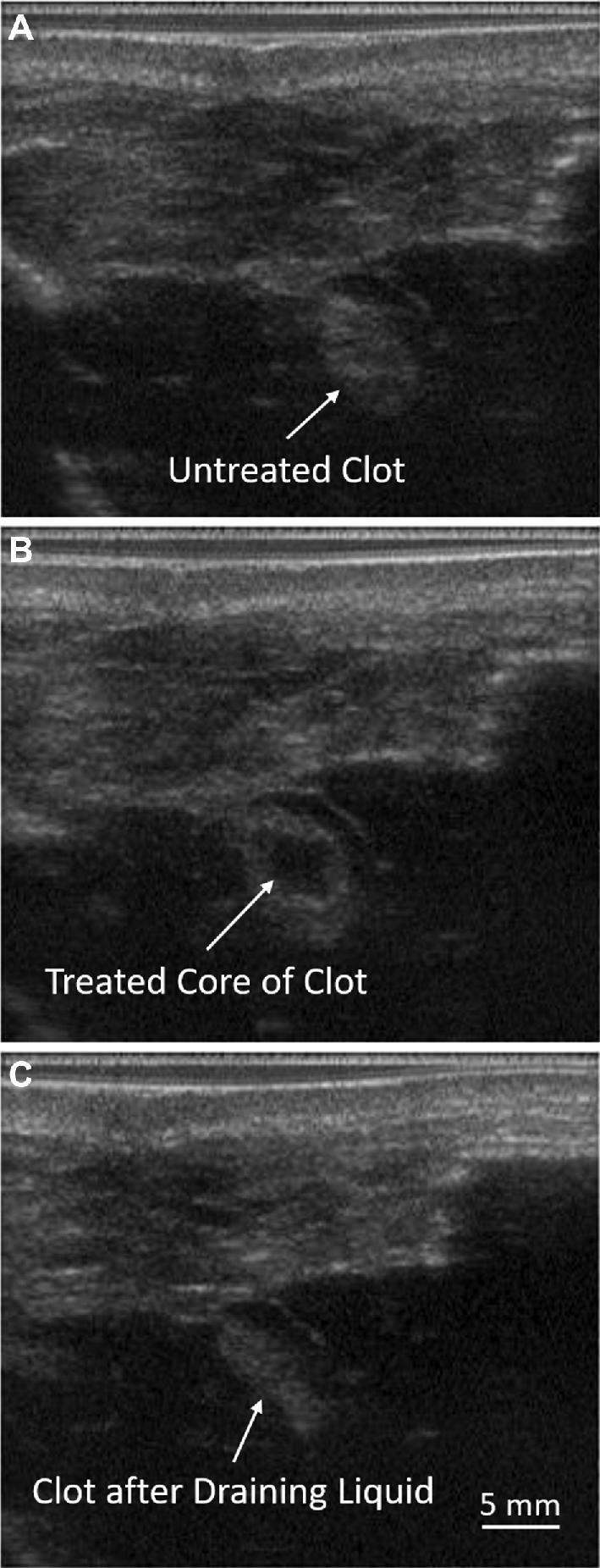
B-mode ultrasound images of the clot from a treatment-drained pig A, before treatment, B, after treatment, and C, after drainage. Histotripsy treatment produced a hypoechoic core of liquefied clot that was able to be evacuated through a simple drainage technique using a needle and syringe.
TABLE 1.
The Volume of Clot Drained from Each Treatment-Drained Pig following Histotripsy
| Pig 1 | Pig 2 | Pig 3 | Pig 4 | Pig 5 | Pig 6* | Mean | Std Dev | |
|---|---|---|---|---|---|---|---|---|
| Volume Drained (mL) | 0.5 | 0.7 | 0.7 | 1 | 0.7 | 1.8 | 0.9 | 0.5 |
*The slightly greater volume drained was caused by suction of some of the fluid filling the space between the brain and scalp on withdrawal of the needle from the brain.
Abbreviation: Std Dev, standard deviation.
MRI
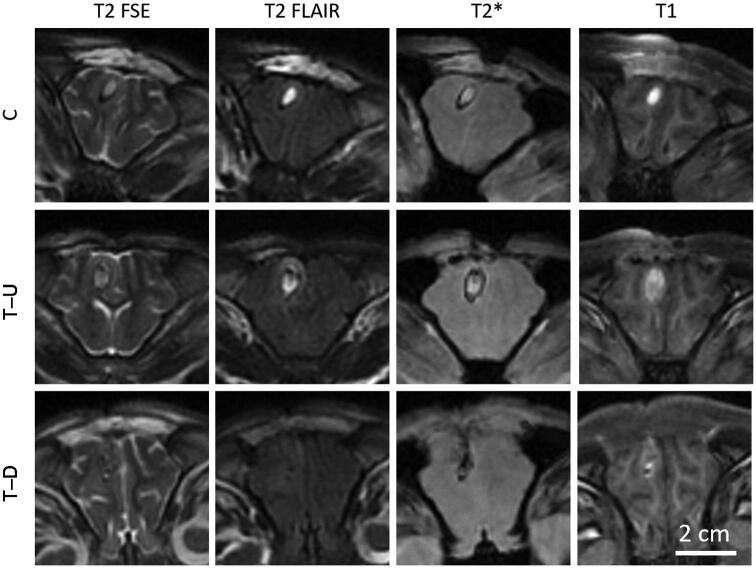
All pigs from each group survived 7 to 8 d after clot formation, at which point MRI was performed (Figure 5). Clots in the control and treatment-undrained groups had a T2 signature typical of a chronic stage ICH with a central hyperintense region surrounded by a hypointense boundary.20 Similar image characteristics were observed in T2*-weighted images, whereas the T1-weighted images were characterized primarily with a hyperintense region across the entire clot. The T2 FLAIR images showed a hyperintense ring surrounding the clot, indicating the presence of edema. In contrast to the control and treatment-undrained group, the treatment-drained group showed sections where the central region of the clot was successfully evacuated after histotripsy treatment. In these sections, the signal from the hematoma region was more isointense on T2- and T1-weighted images with hypointense spots.
Figure 5.
MRI of the brain sections with clot from a control (C), treatment-undrained (T-U), and treatment-drained (T-D) pig.
Edema in the perihematomal region was quantified via the T2 FLAIR images. For each group, the perihematomal region showed higher T2 FLAIR signal intensity as compared to the contralateral side (Table 2) (P value < .05), which was expected because of the presence of the hematoma.21 Normalized pair-wise comparisons of T2 FLAIR signal in the perihematomal region showed no significant changes between groups (P value > .05).
TABLE 2.
Measurements Made from the ROIs Drawn on T2-Weighted FLAIR Images
| FLAIR | |||
|---|---|---|---|
| Ipsilateral | Contralateral | (Ipsilateral/Contralateralavg) | |
| C (n = 5) | 718 ± 168 | 478 ± 54 | 1.50 ± 0.35 |
| T-U (n = 6) | 722 ± 130 | 513 ± 60 | 1.40 ± 0.25 |
| T-D (n = 5) | 620 ± 103 | 402 ± 45 | 1.54 ± 0.26 |
Abbreviations: avg, average; C, control; FLAIR, fluid-attenuated inversion recovery; T-D, treatment-drained; T-U, treatment-undrained.
Histology
Brains were sectioned around the location of the ICH and stained. In the control group (Figure 6A), the histology showed well-formed organizing clots with overall normal brain parenchyma surrounding the clot. There was some evidence of cortical ischemia and gliosis, as normally seen in ICH. There was mild lymphocyte and macrophage infiltration at the periphery of the clot with minimal extension into the cortex. Axonal spheroids, indicative of axonal disruption, were concentrated around the periphery with a high concentration at the distal end of the clot. The Perls’ stain highlighted a circumferential ring of hemosiderin at the clot periphery. The treatment-undrained group (Figure 6B) showed ischemia and gliosis surrounding the clot with hemosiderin deposits at the periphery. The portion of clot remaining in the treatment-drained group (Figure 6C) showed similar characteristics to that of the other groups. In 1 pig from both treatment groups, there was evidence of mild subarachnoid inflammation and hemorrhage, likely from prefocal cavitation, which can occur when the transducer focus is moved close to the boundary of the brain.
Figure 6.
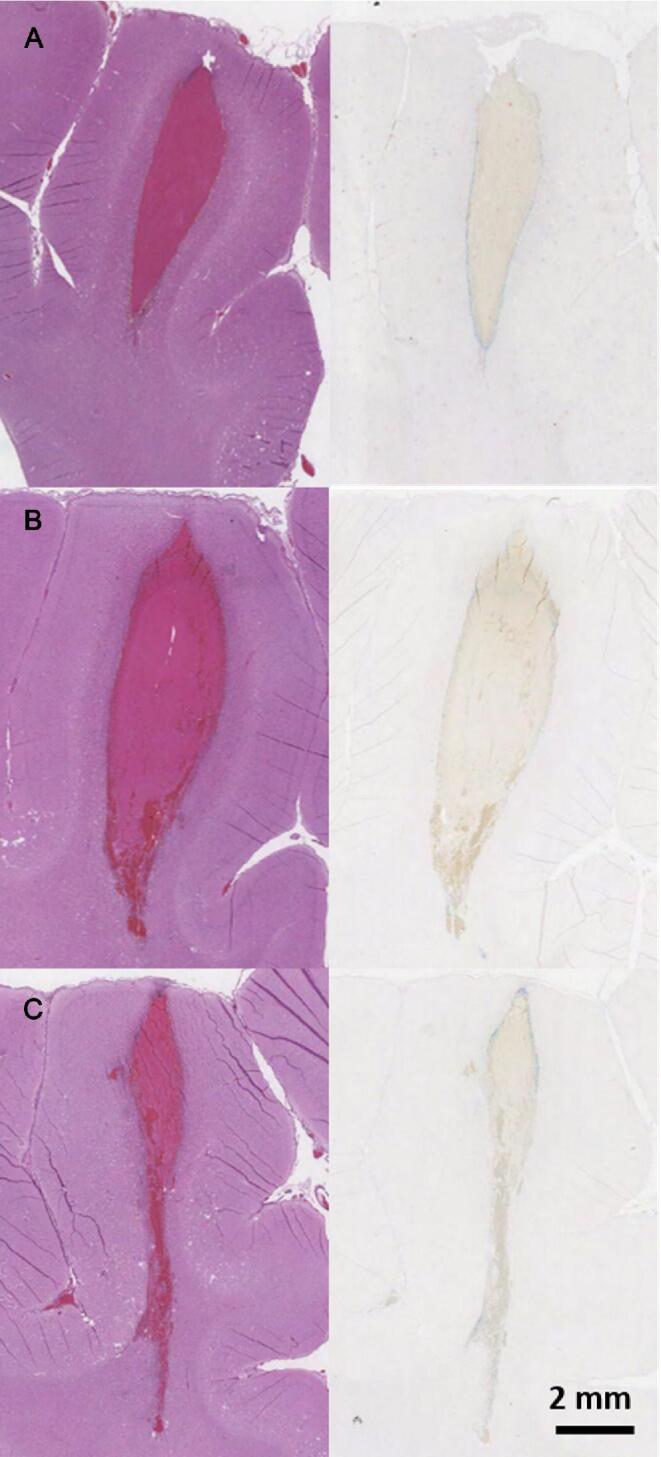
H&E (left) and Perls’ Prussian blue (right) stains of brain sections with clot from a control A, treatment-undrained B, and treatment-drained C pig. The blue color in the Perls’ Prussian blue indicated hemosiderin deposition.
DISCUSSION
This study presents the first analysis of histotripsy-based liquefaction of ICH in vivo. Clot cores were accurately targeted and successfully liquefied using histotripsy without significant damage to overlying or surrounding perihematomal cerebral tissue, as evident on histology and MRI. The liquefied content of the treated cores was easily evacuated with a simple drainage technique and, when left undrained, had no effect on pig survival and no measured effect on neurological behavior. This study addresses the initial safety concerns of using histotripsy for ICH treatment.
The precision of histotripsy treatment was exemplified by the fact that we were able to intentionally leave a 1- to 2-mm rim of clot intact, which minimized the possibility of causing immediate, direct damage to the perihematomal region via the cavitation activity of the bubble cloud. In a clinical case, it is hypothesized that this would prevent rebleed, as the vessel responsible for a rebleed should be at the periphery. Although there was evidence of mild subarachnoid damage in 2 of the treatment pigs, likely from prefocal cavitation, it was small in extent and confined to small regions at the periphery of the brain, with no evidence of neurological compromise. However, the presence of this raises some safety concerns, and further studies may be needed to understand its long-term effects and its effect on rebleed. This could be done using a bacterial collagenase porcine ICH model.22 The liquefied core was easily drained using a simple drainage technique. Given the small initial volume of the clots (1.75 mL) and the fact that we intentionally left a rim of clot intact, only about half of the initial clot volume was liquefied and subsequently drained. Consequently, a significant portion of the initial clot remained within the brain after evacuation of the treated portion. The small clot volume is a limitation of the porcine model because of the small size of the pig brain. With larger clots typical of human ICH, with which early intervention is likely a necessity (≥30 mL),1 a larger proportion of the clot can be targeted while leaving the same size rim intact, and thus, a larger percentage of the clot can be liquefied and evacuated.
Previous studies injecting 2.5 mL of lysed blood into the porcine brain have revealed significant cerebral injury with erythrolysis, including poor neurological functioning and death.3,16 The adverse outcome is thought to be caused by the toxic effect of the hemolysates. In this study, the lysed blood contents generated with histotripsy showed some cerebral swelling and gliosis, but no effect on survival or neurological function. The hemosiderin was confined mostly to the rim of the clot, and there was no observed difference in edema. As other studies report much more striking effects because of the introduction of lysed blood into the brain,12-16 the minimal effect observed here could be related to the rim of intact clot left untreated, which may have served as a barrier between the liquefied clot and surrounding brain tissue.
Given prior studies,10,11 as well as the current in vivo study, histotripsy may prove to be a unique approach to rapid liquefaction and evacuation of ICH in a minimally invasive manner. In comparison to MRgFUS, which uses longer pulses and requires MRI, histotripsy can treat large10 and superficial regions23 in shorter durations of time. As MRgFUS relies on thermal absorption to necrose tissue in the targeted region, the therapy must be delivered slowly, typically on the order of ≤0.2 mL/min, to avoid perilesional and skull heating. Overall, to achieve safe liquefaction at clinically relevant timescales, MRgFUS therapy is restricted to small, central volumes within the brain. In a previous in vitro histotripsy ICH study, 40 mL clots were liquefied in 20 min (2 mL/min) by applying histotripsy transcranially through human skulls while the skull heating remained within a clinically safe range.10 The goal is to develop this minimally invasive technology without reliance on MRI. Patients with primary ICH who do not have a vascular lesion as evident on CT will be candidates for histotripsy treatment of ICH. Localization can be done by utilizing a preoperative CT and neuronavigation to place the transducer focus at the center of the ICH. Real-time treatment monitoring will be done via cavitation mapping through the skull using the histotripsy transducer itself.24 Post-treatment, the ICH will be immediately evacuated utilizing a previously placed catheter hydrophone,11 and the drain will be left in place for further drainage.
The current study has limitations, and additional work is necessary to further demonstrate the clinical efficacy and safety of histotripsy for use in ICH treatment. Although this model is well established, the small ICH volume limits the clinical relevance for therapeutic effects. Large ICH models (human cadavers or skull phantoms) with ICH at different anatomical locations are needed to capture human ICH experience. Furthermore, longer-term survival studies (up to 3 mo) are needed to understand the long term safety of histotripsy treatment. Additionally, as the pig skull is not conducive to the transmission of focus ultrasound, a craniotomy was necessary, and effects of skull heating and associated safety implications at the skull/brain interface could not be addressed. In humans, the craniotomy will not be needed. The effect to the skull and the brain close to the skull will be investigated using the human cadaver model.
CONCLUSION
This study presents the first analysis of histotripsy-based liquefaction of ICH in vivo. We aimed to investigate the initial safety concerns of histotripsy clot liquefaction in a well-established porcine ICH model. The liquefied content of the treated cores was easily evacuated with a simple drainage technique and, when left undrained, had no effect on pig survival and no measured effect on neurological behavior. Further safety studies are needed to understand the long-term safety (up to 3 mo) associated with using this technology as well as to understand prefocal cavitation and address the risk of rebleeds.
Disclosures
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) under Award Number R21-NS093121 and from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the NIH under Award Number R01-EB008998. Drs Timothy L Hall and Zhen Xu have financial and/or other relationships with HistoSonics. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Notes
Portions of this work were presented at the 6th World Intracranial Hemorrhage Conference in Baltimore, Maryland, May 1-3, 2017 and the International Stroke Conference in Honolulu, Hawaii, February 6-8, 2019.
REFERENCES
- 1. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987-993. [DOI] [PubMed] [Google Scholar]
- 2. Flaherty ML, Haverbusch M, Sekar P et al.. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66(8):1182-1186. [DOI] [PubMed] [Google Scholar]
- 3. Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53-63. [DOI] [PubMed] [Google Scholar]
- 4. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450-1460. [DOI] [PubMed] [Google Scholar]
- 5. Xu Z, Ludomirsky A, Eun LY et al.. Controlled ultrasound tissue erosion. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2004;51(6):726-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: the role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am. 2005;117(1):424-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts WW, Hall TL, Ives K, Wolf JS, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175(2):734-738. [DOI] [PubMed] [Google Scholar]
- 8. Xu Z, Owens G, Gordon D, Cain C, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121(6):742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin KW, Kim Y, Maxwell A et al.. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2014;61(2):251-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerhardson T, Sukovich JR, Pandey AS, Hall TL, Cain CA, Xu Z. Effect of frequency and focal spacing on transcranial histotripsy clot liquefaction, using electronic focal steering. Ultrasound Med Biol. 2017;43(10):2302-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerhardson T, Sukovich JR, Pandey AS, Hall TL, Cain CA, Xu Z. Catheter hydrophone aberration correction for transcranial histotripsy treatment of intracerebral hemorrhage: proof-of-concept. IEEE Trans Ultrason Ferroelectr Freq Control 2017;64(11):1684-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage. Stroke. 2001;32(12):2932-2938. [DOI] [PubMed] [Google Scholar]
- 13. Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89(6):991-996. [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 2002;953(1-2):45-52. [DOI] [PubMed] [Google Scholar]
- 15. Matz PG, Weinstein PR, Sharp FR. Heme oxygenase-1 and heat shock protein 70 induction in glia and neurons throughout rat brain after experimental intracerebral hemorrhage. Neurosurgery. 1997;40(1):152-162. [DOI] [PubMed] [Google Scholar]
- 16. Wagner KR, Xi G, Hua Y, de Courten-Myers GM, Broderick JP. Blood components and acute white matter edema development following intracerebral hemorrhage: are hemolysates edemogenic? Stroke. 2000;31:345. [Google Scholar]
- 17. Wagner KR, Xi G, Hua Y et al.. Lobar intracerebral hemorrhage model in pigs. Stroke. 1996;27(3):490-497. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka Y, Imai H, Konno K et al.. Experimental model of lacunar infarction in the gyrencephalic brain of the miniature pig. Stroke. 2008;39(1):205-212. [DOI] [PubMed] [Google Scholar]
- 19. Yamaguchi M, Zhou C, Heistad DD, Watanabe Y, Zhang JH. Gene transfer of extracellular superoxide dismutase failed to prevent cerebral vasospasm after experimental subarachnoid hemorrhage. Stroke. 2004;35(11):2512-2517. [DOI] [PubMed] [Google Scholar]
- 20. Huisman TAGM. Intracranial hemorrhage: ultrasound, CT and MRI findings. Eur Radiol. 2005;15(3):434-440. [DOI] [PubMed] [Google Scholar]
- 21. Dul K, Drayer BP. CT and MR imagining of intracerebral hemorrhage. In: Kase CS, Caplan LR, eds. Intracerebral Hemorrhage. Boston: Butterworth-Heinemann;1994:73-98. [Google Scholar]
- 22. Mun-Bryce S, Wilkerson AC, Papuashvili N, Okada YC. Recurring episodes of spreading depression are spontaneously elicited by an intracerebral hemorrhage in the swine. Brain Res. 2001;888(2):248-255. [DOI] [PubMed] [Google Scholar]
- 23. Sukovich JR, Xu Z, Hall TL, Allen SP, Cain CA. Treatment envelope of transcranial histoptripsy applied without aberration correction. J Acoust Soc Am. 2016;140(4):3031. [Google Scholar]
- 24. Sukovich JR, Xu Z, Hall TL, Macoskey J, Cain CA. Transcranial histotripsy acoustic-backscatter localization and aberration correction for volume treatments. J Acoust Soc Am. 2017;141(5):3490. [Google Scholar]