Abstract
Background
During the current COVID-19 pandemic, a link between acute cardiac injury and COVID-19 infection has been observed. There is currently no consensus on the incidence of cardiac injury, its relationship to prognosis, or its possible cause. In this article we provide a comprehensive review and meta-analysis of the incidence, comorbidities, outcomes, and possible mechanisms of acute cardiac injury in COVID-19 patients.
Methods
We searched PubMed and Embase for studies that evaluated cardiac injury in hospitalized COVID-19 patients. Data on demographic information, comorbidities, and relevant laboratory values were extracted and a meta-analysis was performed.
Results
Sixteen studies from China, Italy, and the United States with 2224 patients were included in this meta-analysis. The incidence of cardiac injury was 24.4% (542/2224 patients) in hospitalized COVID-19 patients. The all-cause mortality in patients with cardiac injury was 72.6% (odds ratio, 17.32; 95% confidence interval, 9.21-32.57) compared with those without cardiac injury (14.5%). In subgroup analyses, factors associated with increased risk of developing cardiac injury were older age and history of hypertension, and chronic obstructive respiratory disease.
Conclusions
Cardiac injury is common in hospitalized COVID-19 patients and is significantly associated with mortality. Patients who were older with hypertension and chronic obstructive respiratory disease were prone to develop cardiac injury. Early screening, triage, and cardiac monitoring are recommended for these patients.
Résumé
Contexte
Durant la pandémie de COVID-19 qui sévit actuellement, un lien a été observé entre l’infection par le virus de la COVID-19 et l’apparition de lésions cardiaques aiguës. À l’heure actuelle, il n’y a pas de consensus quant à l’incidence de telles lésions cardiaques, à leur lien avec le pronostic ou à leur cause possible. Nous présentons ici une revue exhaustive et une méta-analyse de l’incidence des lésions cardiaques aiguës chez les patients atteints de COVID-19 ainsi que des affections concomitantes, des issues et des mécanismes qui pourraient y être associés.
Méthodologie
Nous avons effectué une recherche dans les bases de données PubMed et Embase afin de recenser les études évaluant les lésions cardiaques chez les patients atteints de COVID-19 admis à l’hôpital. Les caractéristiques démographiques des patients ainsi que les données sur les affections concomitantes et d’autres paramètres de laboratoire pertinents ont été extraites des études recensées, puis soumises à une méta-analyse.
Résultats
La méta-analyse portait sur les données de 16 études réalisées en Chine, en Italie et aux États-Unis qui regroupaient 2 224 patients au total. L’incidence des lésions cardiaques chez les patients atteints de COVID-19 hospitalisés s’établissait à 24,4 % (542 patients sur 2 224). La mortalité toutes causes confondues chez les patients présentant des lésions cardiaques était de 72,6 % (rapport de cotes : 17,32; intervalle de confiance à 95 % : 9,21 à 32,57) comparativement à 14,5 % chez les patients sans lésions cardiaques. Dans les analyses par sous-groupe, les facteurs associés à un risque accru d’apparition de lésions cardiaques étaient l’âge plus avancé et des antécédents d’hypertension, ainsi que la présence d’une maladie respiratoire obstructive chronique.
Conclusions
Les lésions cardiaques sont fréquentes chez les patients atteints de COVID-19 hospitalisés et sont associées de manière significative à la mortalité. Les patients plus âgés ayant des antécédents d’hypertension et atteints d’une maladie respiratoire obstructive chronique étaient plus susceptibles de présenter des lésions cardiaques. Un dépistage précoce, un triage approprié et une surveillance cardiaque sont recommandés chez ces patients.
COVID-19 is caused by the SARS-CoV-2 and is the most recently discovered member of the coronavirus family. Since late December 2019, COVID-19 has caused initially a local epidemic in Wuhan, China and subsequently a global pandemic involving 212 countries, areas, or territories. Approximately 3.5 million cases and 250,000 deaths were recorded worldwide as of May 2020.1 Increasing experience with this disease has led to the understanding that it is not just an illness of the respiratory system, but that there are significant cardiovascular effects. Recent works by Huang et al.2 and Guo et al.3 not only showed that a considerable number of hospitalized patients diagnosed with COVID-19 exhibited cardiac injury (represented by elevation of cardiac biomarkers such as troponin), but that those who developed cardiac injury had a significantly higher mortality rate than those without. Moreover, a multivariable analysis of 416 patients showed that cardiac injury was independently associated with an increased risk of mortality in patients with COVID-19.4 Therefore, the purpose of this study was to present a systematic review and meta-analysis of the literature to investigate the incidence of cardiac injury, its correlations with comorbidities and outcomes, and its possible mechanisms in the global COVID-19 population.
Methods
Data source and searches
We performed a systematic literature search in PubMed and Embase. We used the following search terms: (“coronavirus”) and (“cardiac injury” or “myocardial injury” or “myocarditis” or “troponin”). The search was limited to Chinese and English and all articles from January 1, 2020 to May 30, 2020 were reviewed.
Study selection, data extraction, and definitions
Original studies that reported cardiac injury as elevated troponin levels were included. Review articles, meta-analysis and case reports were excluded. An aggregate data meta-analysis was performed. The extracted data included the number of patients enrolled in each study, age, sex, comorbidities (coronary artery disease [CAD], diabetes [DM], hypertension [HTN], and chronic obstructive pulmonary disease [COPD]), laboratory values (C-reactive protein [CRP], procalcitonin, and N-terminal pro brain natriuretic peptide [NT-proBNP]), mortality, and other outcome measures including intensive care unit (ICU) admission, respiratory failure in need of mechanical ventilation, and shock.
The primary outcome was defined as all-cause mortality. If mortality in patients with or without cardiac injury was not directly reported, the mortality rate of patients with cardiac injury was calculated as the ratio of deceased patients with cardiac injury to the overall number of patients with reported cardiac injury. The secondary outcome was defined as any one of death, ICU admission, respiratory failure in need of mechanical ventilation, or shock.5
Statistical analysis
Review Manager 5.3 (Cochrane, London, United Kingdom) was used to perform data analysis. Continuous variables were extracted and analyzed using standardized mean difference (SMD). Median with interquartile range was converted to mean with SD using the equations delineated by Hozo et al.6 Dichotomous variables were analyzed using the Mantel-Haenszel method and the random effects model. A 95% confidence interval (CI) was selected and the results were expressed as odds ratio (OR). P values of < 0.05 were considered statistically significant. Statistical heterogeneity was evaluated using the I2 statistic. Publication bias was evaluated using funnel plots. Sensitivity analyses were performed to identify potential heterogeneity by leaving out each study.
Results
Study characteristics
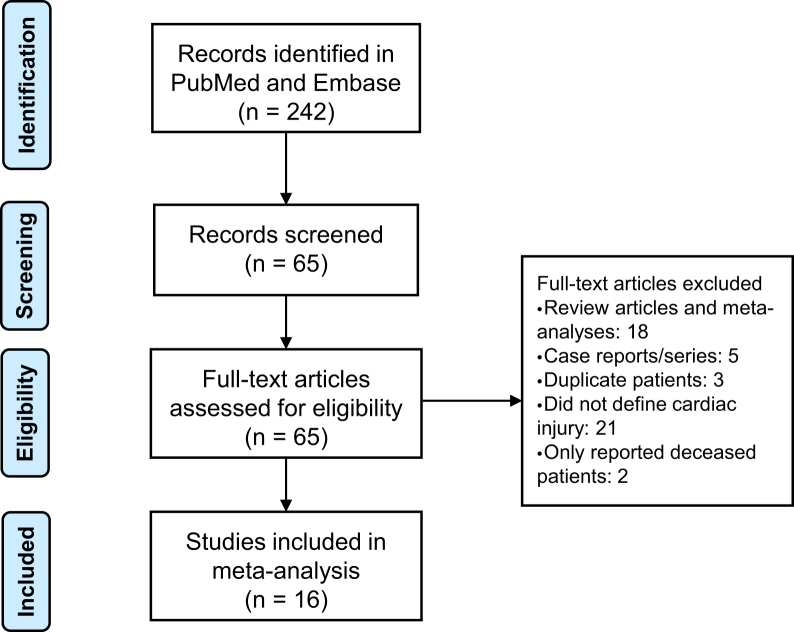
A total of 242 abstracts resulted with the search term as of May 30, 2020. Each abstract was reviewed by the authors and 65 studies that reported cardiac injury or troponin were reviewed in full. Figure 1 shows the literature search and selection process. Serial original articles with overlapping patient series (many from early reports because most early data originated from Wuhan, China) were compared, and only those with the largest series of patients were included. Sixteen studies were included in our analysis and their study characteristics are listed in Table 1. Cardiac injury was defined as elevated serum troponin level. Because of differences in the types of troponin tested at each centre and sensitivity of testing, specific troponin cutoff criteria for each included study is also included in Table 1.
Figure 1.
Flow chart of study selection process.
Table 1.
Summary of included studies
| Reference | Hospital | Type | COVID-19 patient selection | Number of patients | Number of cardiac injury | Death | Definition of cardiac injury |
|---|---|---|---|---|---|---|---|
| Aggarwal et al.9 | Unity Point Clinic, New York, United States | Retrospective | Until 4/4/2020; all hospitalized patients | 16 | 3 | 3 | Hs TnT > the 99th percentile upper reference limit |
| Cecconi et al.11 | Humanitas Research Hospital, Milan, Italy | Retrospective | 2/22/2020 to 3/22/2020; all patients | 206 | 57 | 36 | TnI > normal range (19.8 ng/L) |
| Chen et al.21 | Tongji Hospital Wuhan, Wuhan, China | Retrospective | 1/13/2020 to 2/12/2020; all deceased or discharged patients | 274 | 89 | 113 | Cardiac biomarkers (eg, TnI) > the 99th percentile upper reference limit |
| Deng et al.14 | Hankou and Caidian branch of Tongji Hospital (and) Hankou branch of Central Hospital of Wuhan, Wuhan, China | Retrospective | 1/1/2020 to 2/21/2020; all deceased or discharged patients | 225 | 66 | 109 | Hs TnI > the 99th percentile upper reference limit (> 28 pg/mL) |
| Yang et al.17 | Jin Yin-Tan Hospital, Wuhan, China | Retrospective | 12/24/2019 to 1/26/2020; all critically ill patients | 52 | 12 | 32 | Hs TnI > the upper limit of the reference range (> 28 pg/mL) |
| Zhou et al.15 | Jin Yin-Tan Hospital (and) Wuhan Pulmonary Hospital, Wuhan, China | Retrospective | 12/29/2019 to 1/31/2020; all discharged or deceased patients | 191 | 33 | 54 | Cardiac biomarkers (eg, Hs TnI) > 99th percentile upper reference limit |
| Guo et al.3 | Seventh Hospital of Wuhan, Wuhan, China | Retrospective | 1/23/2020 to 2/23/2020 all patients | 187 | 52 | 43 | TnT > 99th percentile upper reference limit |
| Shi et al.4 | Renmin Hospital of Wuhan University, Wuhan, China | Retrospective | 1/20/2020 to 2/10/2020; all patients | 416 | 82 | 57 | Hs TnI > 99th percentile upper reference limit |
| Huang et al.2 | Jin Yin-Tan Hospital, Wuhan, China | Prospective | 12/16/2019 to 1/2/2020; all patients | 41 | 5 | 6 | Hs TnI > 99th percentile upper reference limit (> 28 pg/mL) |
| Wang et al.18 | Zhongnan Hospital of Wuhan University, Wuhan, China | Retrospective | 1/1/2020 to 1/28/2020; all patients | 138 | 10 | 6 | Hs TnI > 99th percentile upper reference limit (> 28 pg/mL) |
| Chen et al.20 | Hankou, Zhongfa Xin Cheng and Guang Gu branches of Tongji Hospital, Wuhan, China | Retrospective | 1/2020 to 2/2020; all patients | 150 | 22 | 11 | TnI > 99th percentile upper reference limit |
| Zhou et al.16 | West District of Union Hospital of Tongji Medical College, Wuhan, China | Retrospective | 2/5/2020 to 2/13/2020; all patients | 34 | 9 | Not reported | TnI > 26.2 ng/L |
| He et al.19 | Tongji Hospital Zhong Fa Xin Cheng Branch, Wuhan, China | Retrospective | 2/3/2020 to 2/24/2020; severely or critically ill patients | 54 | 24 | 26 | Troponin > 3 times upper reference limit |
| Wei et al.13 | Public Health Clinical Centre of Chengdu and West China Hospital of Sichuan University, Sichuan, China | Prospective | 1/16/2020 to 3/10/2020; all patients | 101 | 16 | 3 | Hs TnT > the institutional upper limit of normal (14 pg/mL) |
| Zheng et al.10 | First Affiliated Hospital of Zhejiang University, Hangzhou, China | Retrospective | 1/22/2020 to 3/5/2020; ICU patients | 34 | 13 | 0 | Hs TnI > 99th percentile upper reference limit (> 28 pg/mL) |
| Ni et al.12 | Central Hospital of Wuhan, Wuhan, China | Retrospective | 1/28/2020 to 3/16/2020; all patients | 176 | 49 | 60 | TnI > the 99th percentile upper reference limit |
Hs TnT, Troponin T; ICU, intensive care unit; TnI, Troponin I.
Incidence and outcomes of hospitalized COVID-19 patients with cardiac injury
The pooled overall incidence of cardiac injury in hospitalized COVID-19 patients was 24.4% (542/2224 patients). The incidence of cardiac injury in COVID-19 patients from China was 24.1% (482/2002 patients) compared with the rest of the world (27.0%, 60/222 patients). Supplemental Table S1 includes a compilation of the incidence of cardiac injury in hospitalized COVID-19 patients.
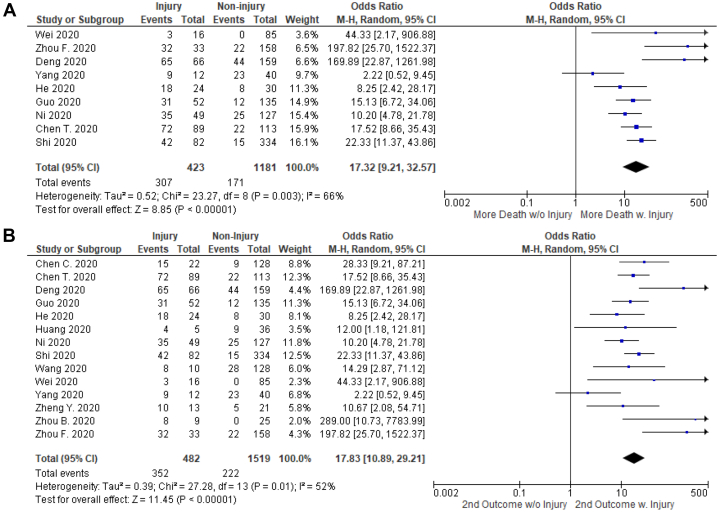
The primary outcome of all-cause mortality in hospitalized COVID-19 patients with cardiac injury was 72.6% (307/423 patients) compared with a mortality rate of 14.5% (171/1181 patients) in patients without cardiac injury (OR, 17.32; 95% CI, 9.21-32.57; I2 = 66%; Z= 8.85; P < 0.00001) as shown in Figure 2A.
Figure 2.
Comparison of primary and secondary outcomes in hospitalized COVID-19 patients with or without cardiac injury. (A) Cardiac injury and primary outcome; (B) Cardiac injury and secondary outcome (combined death, intensive care unit admission, respiratory failure in need of mechanical ventilation, or shock). Hospitalized COVID-19 patient with cardiac injury had significantly higher rates of mortality and poor outcomes compared with those without.
The rate of secondary outcome of death, ICU admission, respiratory failure in need of mechanical ventilation, or shock in hospitalized COVID-19 patients with cardiac injury was 73.0% (352/482 patients) compared with 14.6% (222/1519) in those without cardiac injury (OR, 17.83; 95% CI, 10.89-29.21; I2 = 52%; Z = 11.45; P < 0.00001) as shown in Figure 2B. Funnel plots in Supplemental Figure S1 show symmetrical distribution of effect sizes in the studies included in the primary and secondary outcomes analyses.
Demographic characteristics, comorbidities, and cardiac injury
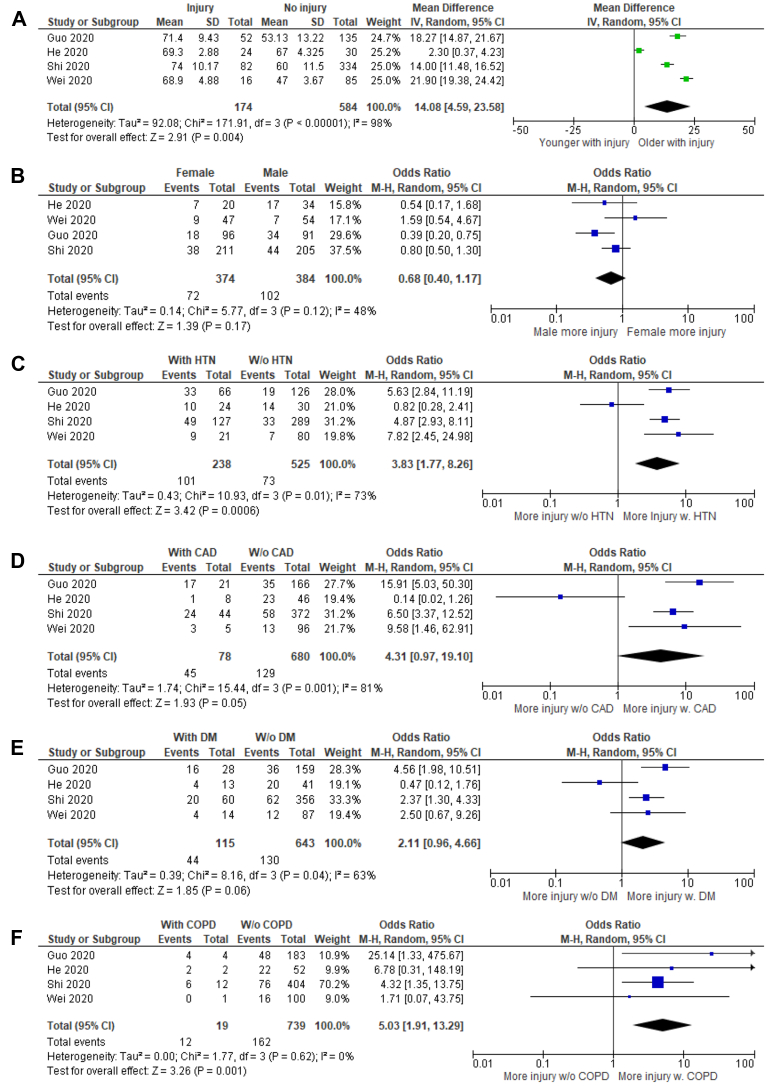
Patients with cardiac injury were older (SMD, 2.13; 95% CI, 0.98-3.28; I2 = 96%; Z = 3.63; P = 0.0003) and consisted of fewer women (OR, 0.68; 95% CI, 0.40-1.17; I2 = 48%; Z = 1.39; P = 0.17). However, the gender discrepancy was not statistically significant (Fig. 3A and B).
Figure 3.
Comparison of demographic characteristics and comorbidities in hospitalized COVID-19 patients with or without cardiac injury: (A) age; (B) sex; (C) hypertension (HTN); (D) coronary artery disease (CAD); (E) diabetes (DM); and (F) chronic obstructive pulmonary disease (COPD). COVID-19 patients who were older and had history of HTN and/or COPD exhibited increased odds of developing cardiac injury during hospitalization. The effects of sex, CAD, and DM on cardiac injury did not reach statistical significance.
Predictors of cardiac injury in hospitalized COVID-19 patients included a history of HTN (OR, 3.83; 95% CI, 1.77-8.26; I2= 73%; Z = 3.42; P = 0.0006) and COPD (OR, 5.03; 95% CI, 1.91-13.29; I2= 0%; Z = 3.26; P = 0.001). Comorbidities such as CAD (OR, 4.31; 95% CI, 0.97-19.10; I2= 81%; Z = 1.93; P = 0.05) and DM (OR, 2.11; 95% CI, 0.96-4.66; I2= 63%; Z = 1.85; P = 0.06) were associated with greater likelihood of developing cardiac injury but did not reach statistical significance (Fig. 3C-F).
Cardiac injury and laboratory values
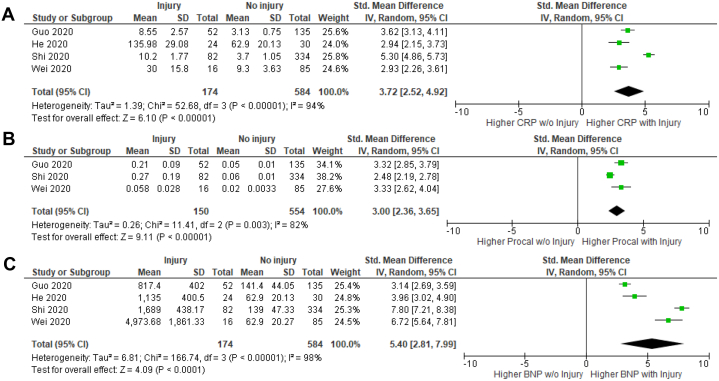
The correlation of select laboratory parameters and cardiac injury was examined (Fig. 4). Hospitalized COVID-19 patients with cardiac injury had significantly higher CRP (SMD, 3.72; 95% CI, 2.52-4.92; I2 = 94%; Z = 6.10; P < 0.00001), procalcitonin (SMD, 3.00; 95% CI, 2.36-3.65; I2 = 82%; Z = 9.11; P < 0.00001), and NT-proBNP (SMD, 5.40; 95% CI, 2.81-7.99; I2 = 98%; Z = 4.09; P < 0.0001) levels.
Figure 4.
Comparison of laboratory values in hospitalized COVID-19 patients with or without cardiac injury: (A) C-reactive protein (CRP); (B) Procalcitonin (Procal); and (C) N-Terminal pro brain natriuretic peptide (BNP). Hospitalized COVID-19 patients with cardiac injury had significantly increased CRP, procalcitonin, and BNP compared with those without.
Sensitivity analysis
Sensitivity analyses were performed by leaving out each study. Excluding any one of the studies did not significantly influence the direction or magnitude of the results.
Discussion
Our meta-analysis of the currently published data has shown that: (1) a considerable amount of hospitalized COVID-19 patients developed cardiac injury (24.4%) and the incidence was similar between patients from China and other parts of the world; (2) hospitalized COVID-19 patients who developed cardiac injury exhibited significantly higher rates of mortality (72.6%) compared with those without; and (3) hospitalized COVID-19 patients who were older and had comorbidities including HTN, and COPD showed an increased risk of developing cardiac injury.
Incidence of cardiac injury in COVID-19 patients
Since the outbreak, cardiac injury in COVID-19 patients who needed inpatient care has been described. Cardiac injury in the form of myopericarditis were reported from China and Europe.7,8 These case reports should ring a bell on the potential involvement of the cardiovascular system in a wider range of COVID-19 patients worldwide. More than a dozen studies have reported incidence of cardiac injury in hospitalized COVID-19 patients, ranging from 7% to 44%.2, 3, 4,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 However, no consensus was reached on the overall incidence of cardiac injury in these patients. Li et al.22 performed a meta-analysis and attempted to determine the incidence of cardiac injury. However, only 2 studies (179 patients) were analyzed and the overall incidence of cardiac injury was 8% (15/179). Although 2 other studies with more patients were included, only elevated creatine kinase levels were reported, which was not specific for cardiac injury and could not represent true incidence.
With more evidence available, all of the current studies that reported cardiac injury were included and incidence of cardiac injury in hospitalized COVID-19 patients was determined as 24.4% from a total of 2224 patients. Moreover, the incidence was similar between patients from China (24.1%) and other parts of the world (27.0%).
Demographic characteristics and comorbidities associated with cardiac injury
Age has been illustrated as an indicator of outcomes in this current COVID-19 outbreak because the general public often has the notion that this is a disease of the elderly. Whether there is a connection between age and the development of cardiac injury was also studied. Our pooled analysis was consistent with other studies in that COVID-19 patients who developed cardiac injury were significantly older (SMD, 2.13; 95% CI, 0.98-3.28; P = 0.0003). Besides age, male sex has also been associated with poor outcomes in COVID-19 patients. A preprint meta-analysis of 77,932 patients by Wei et al. showed that male patients had significantly higher risks of developing severe cases (OR, 1.63; 95% CI, 1.28-2.06) and death (OR, 1.71; 95% CI, 1.51-1.93).23 However, Shi et al. and Guo et al. reported more male patients in the cardiac injury group.3,4 The pooled analysis of 4 studies did not achieve statistical significance. Thus, whether male patients were more likely to develop cardiac injury still needs more evidence.
Several studies have investigated cardiovascular comorbidities in COVID-19 patients. In the meta-analysis of 6 studies by Li et al.,22 the prevalence of HTN, DM, and cardio-cerebral vascular diseases were 17.1%, 9.7%, and 16.4%, respectively. Chen et al.21 reported that hospitalized COVID-19 patients with cardiovascular comorbidities were more likely to develop cardiac complications. In the multivariable analysis carried out by Wang et al.,24 history of cardiovascular disease was an independent predictor for death. This was consistent with another multivariate analysis from Chen et al.20 On the other end of the spectrum, Guo et al.3 pointed out that a significant number of patients with these comorbidities did not progress to cardiac injury and had relatively favourable outcomes. According to China CDC Weekly in February,25 80.9% of all COVID-19 patients (hospitalized and nonhospitalized) with cardiovascular comorbidities had only mild symptoms with no mortality, 13.8% severe symptoms, and only 4.7% critically ill among 44,672 confirmed cases. Consistent with previous reports, hospitalized COVID-19 patients with history of HTN and COPD were more likely to develop cardiac injury in our analyses. Whether CAD or DM was independently associated with higher incidence of cardiac injury in COVID-19 patients still needs further investigation. Only a trend for difference without statistical significance was revealed in this pooled analysis for cardiac injury in patients with CAD or DM compared with those without.
In addition to demographic characteristics and comorbidities, substantially elevated levels of NT-proBNP were also observed in patients who developed cardiac injury during their hospitalization. This pointed to one possible explanation of the high mortality rate in patients with cardiac injury: acute decompensated heart failure. In cases reported by many groups, patients sometimes developed heart failure and required inotropic support.18,19 Zhou et al.15 reported that 23% of hospitalized COVID-19 patients (44/191 patients) developed heart failure symptoms. This ratio increased to 52% in those who died (28/54 patients). Guo et al.3 not only reported a positive correlation of Troponin T (TnT) levels in COVID-19 patients with NT-proBNP (β = 0.613; P < 0.001), but also showed a dynamic increase of NT-proBNP during the hospital course exclusively in patients who eventually died. Together with our analysis, these data proposed a new area of investigation of whether COVID-19 patients with cardiac injury died from heart failure.
Cardiac injury in hospitalized COVID-19 patients is correlated with higher mortality rates
Cardiac injury and its association with patient outcomes was also examined. Shi et al.4 and Chen et al.20 performed multivariable regression analysis and both reported that cardiac injury was an independent predictor of mortality. However, in the analysis performed by Wang et al.,24 cardiac injury was only predictive of fatal outcomes in univariable analysis but failed to achieve significance in multivariable analysis. Lippi et al. conducted a preliminary meta-analysis of troponin levels in COVID-19 patients and reported that it was increased in COVID-19 patients with severe disease compared with those without.26
In this analysis, 14 studies were integrated to investigate how cardiac injury affected outcomes in hospitalized COVID-19 patients. It was discovered that hospitalized COVID-19 patients with cardiac injury had a significantly increased mortality rate compared with those without, with an OR of 17.32. The mortality rate of hospitalized COVID-19 patients with cardiac injury was 72.6%. This mortality rate was significantly higher than any mortality rates reported so far. Guo et al.3 described a mortality rate of 69.44% (25/36 patients) for patients who developed cardiac injury and also had underlying cardiovascular diseases and 37.50% (6/16 patients) for those with cardiac injury but without cardiovascular comorbidities. Shi et al.4 reported a mortality rate of 51.2% (42/82 patients) in patients with cardiac injury. This extremely high rate of mortality among patients with cardiac injury is alarming. Revisiting the studies included in our analysis, we discovered that in 2 studies—Zhou et al.15 and Deng et al.14—the death rate among patients with cardiac injury were particularly high: 97.0% (32/33 patients) and 98.5% (65/66 patients), respectively. These extreme rates might have skewed the overall mortality rate higher and needs to be considered with caution. When the 2 studies with high mortality rates (Zhou et al.15 and Deng et al.14) were excluded in the analysis, the overall rate of primary outcome was 64.8% (210/324 patients), with an OR of 12.83 (95% CI, 7.93-20.75; P < 0.00001) compared with patients without injury. The rate of secondary outcomes was 66.6% (255/383 patients), with an OR of 14.46 (95% CI, 9.76-21.42; P < 0.00001) compared with patients without injury. Primary and secondary outcomes were similar in direction and slightly smaller in magnitude. It is important to note that these high rates of mortality were only associated with COVID-19 patients who were hospitalized and could not be applied to those who did not require hospitalization.
Possible mechanisms of cardiac injury in COVID-19 patients
Currently several editorials and review articles have elaborated on the possible etiologies of cardiac injury. The mainstream hypotheses include: (1) damage caused by cytokine storm triggered by the virus22; (2) type II demand ischemia due to hypoxemia caused by the dominating respiratory failure27; and (3) direct myocardial injury by viral infiltration.28
A few autopsy reports were available that described pathological involvement of the heart. Xu et al.29 observed the presence of interstitial mononuclear inflammatory infiltrates in the heart but no substantial tissue damage. Another Chinese pathology report by Yao et al.30 described 3 patients who died of COVID-19 in Chongqing, China. They observed enlarged and necrotic cardiomyocytes with infiltrative phagocytes and rare CD4+ T cells in all 3 patients. The hypothesis of cytokine storm as a cause of cardiac injury was solidified by Huang et al.,2 whose study revealed higher plasma levels of interleukin (IL)-2, IL-7, IL-10, granulocyte colony-stimulating factor, interferon gamma-induced protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein 1-A, and tumour necrosis factor-α in COVID-19 patients admitted to the ICU. This analysis was also consistent with this hypothesis by showing significantly elevated inflammatory marker levels including CRP and procalcitonin in patients with cardiac injury compared with those without. To further elucidate the cytokine storm hypothesis, more studies are needed to compare inflammatory marker levels in COVID-19 patients with or without cardiac injury.
In April 2020, direct involvement of coronavirus particles in the myocardium was reported in an Italian patient.31 The patient had significantly increased hs Troponin I and collapsed into cardiogenic shock. As indicated by Dr Libby in his recent perspective,32 mechanisms of cardiac injury in COVID-19 patients lie on a spectrum. On one end, direct viral invasion could cause fulminant myocarditis as shown in the Italian case. On the other end, preexisting lesions in susceptible patients could be exacerbated by cytokine storm from inflammatory responses. Together with increased oxygen requirements in patients with respiratory distress, type II demand ischemia should also be kept in mind.
Recommendations for managing hospitalized COVID-19 patients with cardiac injury
Because of the high risk of mortality in hospitalized COVID-19 patients with cardiac injury, early screening with cardiac biomarkers, electrocardiograms, and echocardiograms is warranted in patients who are at higher risk of developing cardiac injury. These patients are older and often have history of HTN, and COPD. We hope early detection with appropriate triage and support would help reduce the incidence of cardiac injury in hospitalized COVID-19 patients and the dangerously high rate of mortality.
Limitations
This study is limited in several ways. First, a limited number of studies reported cardiac injury in COVID-19 patients. Second, only 4 studies directly compared hospitalized COVID-19 patients with or without cardiac injury. Moreover, a small number of studies did not define or had different diagnostic criteria of cardiac injury and therefore could not be included in the analysis. Third, most studies reported their continuous variables as median with interquartile range. To analyze some of these continuous variables, conversion to median with SD was necessary, which might introduce bias in our results.
Conclusion
Our meta-analysis revealed that cardiac injury is common in hospitalized COVID-19 patients and its incidence is similar in the Chinese and the Western population. The overall mortality of patients with cardiac injury was alarmingly high. Cardiac injury was significantly associated with older age and comorbidities including HTN and COPD. Early screening, triage, and support is warranted to help reduce the incidence of cardiac injury in hospitalized COVID-19 patients and its associated mortality.
Funding Sources
The authors report no funding sources.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported above has adhered to the ethical guidelines from the authors' institutions.
See page 393 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.06.010.
Supplementary Material
References
- 1.Boukhris M., Hillani A., Moroni F. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol. 2020;36:1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of the People’s Republic of China Diagnosis and treatment protocol for novel coronavirus pneumonia (6th edition) [in Chinese] www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml Available at:
- 6.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin [epub ahead of print]. Eu Heart J, doi:10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed]
- 8.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [e-pub ahead of print]. JAMA Cardiol https://doi.org/10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed]
- 9.Aggarwal S., Garcia-Telles N., Aggarwal G. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl) 2020;7:91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y., Sun L., Xu M. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ Sci B. 2020;21:378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecconi M., Piovani D., Brunetta E. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for COVID-19 infection in Lombardy, Italy. J Clin Med. 2020;9:1548. doi: 10.3390/jcm9051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni W., Yang X., Liu J. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol. 2020;76:124–125. doi: 10.1016/j.jacc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei J., Huang F., Xiong T. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106:1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng Y., Liu W., Liu K. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133:1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou B., She J., Wang Y., Ma X. The clinical characteristics of myocardial injury in severe and very severe patients with 2019 novel coronavirus disease. J Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Resp Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X.W., Lai J.S., Cheng J. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E011. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 20.Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 21.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei X., Xiao Y.-T., Wang J. 2020. Sex differences in severity and mortality among patients with COVID-19: evidence from pooled literature analysis and insights from integrated bioinformatic analysis. arXiv:2003.13547. [Google Scholar]
- 24.Wang L., He W., Yu X. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.China CDC Weekly The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) China, 2020. http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 Available at: [PMC free article] [PubMed]
- 26.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Z.Y., Qian H.Y. Myocardial injury in patients with COVID-19 pneumonia [in Chinese] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E006. doi: 10.3760/cma.j.issn.cn112148-20200220-00106. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Resp Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao X.H., Li T.Y., He Z.C. A pathological report of three COVID-19 cases by minimally invasive autopsies [in Chinese] Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 31.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby P. The heart in COVID-19. JACC Basic Transl Sci. 2020;5:537–542. doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.