Abstract
Objective
The purpose of this study was to examine the use of multiple mobile health technologies to generate and transmit data from diverse patients with type 2 diabetes mellitus (T2DM) in between clinic visits. We examined the data to identify patterns that describe characteristics of patients for clinical insights.
Methods
We enrolled 60 adults with T2DM from a US healthcare system to participate in a 6-month longitudinal feasibility trial. Patient weight, physical activity, and blood glucose were self-monitored via devices provided at baseline. Patients also responded to biweekly medication adherence text message surveys. Data were aggregated in near real-time. Measures of feasibility assessing total engagement in device submissions and survey completion over the 6 months of observation were calculated.
Results
It was feasible for participants from different socioeconomic, educational, and racial backgrounds to use and track relevant diabetes-related data from multiple mobile health devices for at least 6 months. Both the transmission and engagement of the data revealed notable patterns and varied by patient characteristics.
Discussion
Using multiple mobile health tools allowed us to derive clinical insights from diverse patients with diabetes. The ubiquitous adoption of smartphones across racial, educational, and socioeconomic populations and the integration of data from mobile health devices into electronic health records present an opportunity to develop new models of care delivery for patients with T2DM that may promote equity as well.
Keywords: diabetes mellitus, type 2 mobile health, self-management
INTRODUCTION
In our current healthcare system, healthcare is organized primarily around episodic interactions with patients. The challenge with episodic care for a chronic illness, such as type 2 diabetes mellitus (T2DM), is that patients do not receive interventions when they need them most—when a problem is about to occur or is occurring. To illustrate, guidelines from the American Diabetes Association suggest patients visit their doctors every 3 to 6 months to assess their blood sugar values, medication regimen, and daily behaviors, such as diet and exercise.1 However, this is already too late if a problem manifested several months before and has led to continuously high blood sugar. Patients need to be empowered to manage their T2DM in their daily environments, adapt to challenges when they occur, and to have clinicians intervene in near real-time.
Because the majority of T2DM care occurs in outpatient settings, mobile health (mHealth) technologies may have a great impact in improving care delivery and health outcomes. mHealth involves the use of mobile devices to support healthy behaviors through continuous health monitoring.2–5 Mobile devices include mobile phones and sensors that are worn, carried, or accessed by individuals during normal daily activities.6,7 In the United States alone, more than 96% of adults own a cellphone, and 81% of those are smartphones.8 These statistics are similar across racial/ethnic and urban/rural demographics. Further, 95% of low-income US residents own cellphones, and 70% of those are smartphones. Low-income racial/ethnic minorities are more likely than low-income whites to own mobile devices and to use features such as text messaging or smartphone applications.8
Mobile devices allow medical professionals to gather more precise information related to patients’ health in their everyday environments. The ability to generate and gather data from patients in their environments also allows us to apply analytical techniques to gain data-driven clinical insights (ie, risk prediction, monitoring for remission and relapse, diagnoses).9,10 Further, these insights can be delivered to clinicians and to individual patients as needed and across geographic locations. As such, mHealth has the potential to facilitate delivery of more precise T2DM treatment and self-management assistance to a broad population. Thus, as the integration of data from mHealth tools into electronic health records (EHRs) advances, an understanding of how to use these data for diabetes management is needed.
The purpose of this study was to examine the use of multiple mHealth technologies to generate and transmit data from diverse and underserved patients with T2DM to healthcare professionals in between clinic visits. We examined the data to begin identifying patterns that describe characteristics of patients for clinical insights. Specifically, the study aims were to: 1) examine the feasibility of having patients self-monitor multiple types of diabetes-related data (blood glucose, weight, physical activity, medication adherence) using mHealth technologies (wireless glucometer, cellular scale, wrist-worn accelerometer, and medication adherence text message surveys) for 6 months; and 2) examine trajectories and patterns of diabetes-related variables (blood glucose, weight, physical activity, medication adherence).
MATERIALS AND METHODS
Study design
With institutional review board approval, we enrolled 60 adults with T2DM to participate in this 6-month longitudinal observational feasibility trial. The full study protocol including software development are previously reported.11,12 Patients were recruited from a primary care clinic that cares for underserved populations and is associated with an academic medical center in the southeastern United States, and through local advertisements. We defined underserved as patients who are racial/ethnic minorities, low income, or Medicaid-eligible.13 Eligible participants met the following inclusion criteria: 1) ages ≥ 18 years, 2) able to speak and read English, 3) diagnosed with T2DM, 4) prescribed to monitor their blood sugar at least weekly, 5) were on diabetes-related medication, and 6) owned a smartphone. We targeted patients with varying levels of diabetes control and included at least 30 patients with a hemoglobin A1c (HbA1c) > 7.5%. Patients were not required to have Wi-Fi or in-home Internet; a smartphone with an Internet connection was adequate. A research nurse identified patients by reviewing the EHR, assessing for documented illiteracy, and then sending them an invitation to participate. Those interested contacted the nurse, were screened over the phone, and a baseline in person appointment was scheduled.
Sample
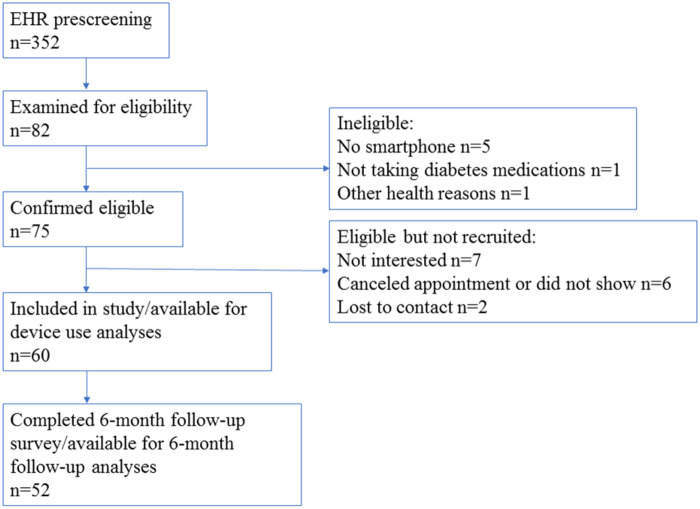
Our goal was to obtain information critical to planning a larger trial and to understand how to use data from this study in clinical practice. Accordingly, our sample size (N = 60) was based on our aims to determine feasibility and acceptability of using multiple devices, not on the power to detect significant effects of self-monitoring on outcomes (Figure 1).
Figure 1.
Participant flow in accordance with STROBE guidelines (13).
Baseline assessment
Following in person informed consent, we administered surveys to record demographic factors (Table 1) and evaluated patients’ perceived usefulness and ease of use of mobile technologies using 2 validated 6-item scales14 that were modified to specify mobile technologies. We also collected data from patients’ EHRs (ie, HbA1c, height, weight, blood pressure, heart rate, and medications).
Table 1.
Mobile devices and data collection
| Data | Instrument | Description | Data points |
|---|---|---|---|
| Activity | Triaxial accelerometer and associated fitness app by Fitbit | Tracks data on the frequency and timing of steps | Daily: steps, minutes sedentary, minutes active, distance traveled |
| Weight | Cellular-enabled Scale by BodyTrace | Tracks weight | Daily weight |
| Glucose | Food and Drug Administration-approved wireless glucometer by iHealth | Tracks blood glucose readings | As prescribed by primary care physician, at least weekly |
| Medication adherence | Self-report via short message service text message15 | Medication adherence over the last week | Baseline, biweekly up to 6 months |
| Hemoglobin A1c | EHR laboratory results | Average level of blood sugar over the previous 3 months | As available at baseline and 3- and 6-months post baseline from EHR |
Orientation occurred in a private room following an investigator-developed protocol and took up to 1.5 hours. This included reviewing and having patients demonstrate device usage, downloading and registering the associated apps on their smartphones, troubleshooting, and answering questions. The nurse was available via e-mail, telephone, or to schedule in person assistance throughout the study. Technical support included mechanical and data tracking issues such as meter malfunctions and device pairing failures. The nurse checked weekly if data was being transmitted to the research team. If there was no data for the past week, an e-mail was sent, and, if no response, a phone call was made to participants.
Device monitoring
For 6 months, weight, physical activity, and blood glucose were self-monitored via devices provided at baseline (Table 1). Patients were asked to monitor weight using a cellular-enabled scale by BodyTrace. They were advised to weigh in daily because more frequent weighing promotes better weight outcomes.16,17 Patients were instructed to wear a Fitbit Alta, a reliable and validated triaxial accelerometer, for activity monitoring.18,19 The Fitbit tracked daily number of steps, distance traveled, and activity intensity and provided feedback on these data points to patients. The Fitbit was tethered to the Fitbit app via Bluetooth on the participants’ smartphones.
Glucose readings were tracked using a US Food and Drug Administration-approved glucometer by iHealth (model BG5). Participants were instructed to monitor glucose based on their healthcare providers’ recommendations which was once per week at a minimum. The glucometer was tethered via Bluetooth to a companion smartphone app, which acted as an automatic logbook to store readings, notes, and medication dosages. Patients were given sufficient test strips to allow testing at their recommended frequencies for 6 months.
Biweekly medication adherence survey
Patients also received a text message every 2 weeks with a link to a medication adherence survey. Participants clicked on the link, which took them to a web-based survey linked to a secure REDCap (Research Electronic Data Capture) database, an electronic data capture tool hosted at [blinded] University.20,21 We used a 3-item measure of medication nonadherence by Voils et al.15 Participants were asked if, in the past 7 days, they 1) took all doses of My Diabetes Medication, 2) missed or skipped at least 1 dose of My Diabetes Medication, and 3) were not able to take all of My Diabetes Medication. Response options ranged from never (0) to always (5) (Cronbach’s alpha = 0.84).15 Response items were scored to assess the extent of nonadherence.
Six-month assessment
After 6 months, participants were sent a link via e-mail to complete a follow-up survey using REDCap on their perceived usefulness and ease of use of the multiple devices. Participants who completed the study and the final survey were able to keep all of the mobile devices.
Feasibility measurements
Feasibility in this study measured the following: 1) the ability to recruit a diverse sample; 2) the means to retain patients in the study for 6 months; 3) the capacity to assess engagement in daily measures of weight, physical activity, and blood glucose and in biweekly measures of medication adherence over 6 months; and 4) the perceived usefulness and ease of use of the devices.
Data analysis
Measures of feasibility that assessed overall engagement in device submissions were calculated by the percentage of daily measures of weight, activity, and blood glucose and biweekly medication adherence surveys that were completed for the 6 months of observation. We also tested whether this differed by age (< 55 years vs ≥ 55 years), HbA1c group (< 7 vs ≥ 7, respectively), and race (White vs Black or other non-White race), using independent samples t-tests. The age of 55 is a common cutoff for older adults and HbA1c ≤ 7% is the usual target for patients with T2DM.
To examine trends of engagement over 6 months, we calculated the percentage of daily measures of weight, activity, and blood glucose completed in each biweekly period and produced empirical summary plots. To illustrate change in biweekly engagement by predictors, we conducted empirical plot summaries of biweekly engagement for weight, activity, and blood glucose. These consisted of plots of mean percentages completed at each of 13 biweekly periods. At each time point, bars representing 1 standard error below and above the mean are presented. To examine trends of biweekly engagement over 6 months, by age, HbA1c, and race, we used linear mixed models. For each measure (weight, activity, and blood glucose), we nested repeated observations of biweekly engagement within individuals. Predictors included age, HbA1c group, and race and were tested in separate models. Time was also included to assess change in biweekly engagement. This included interactions between time and each predictor, to assess differences in change in biweekly engagement over time by age, HbA1c group, and race. Models were tested for covariates and then adjusted. Covariates were centered on income, education, and sex with males as the reference group. Given that the effect of young age on device engagement may be confounded by high HbA1c, models of age as a predictor also included HbA1c as a covariate.
At baseline and the 6-month follow-up, we used a 6-item scale assessing the perceived usefulness of the devices in improving diabetes self-management. Items were on a scale of 1 (strongly agree) to 7 (strongly disagree) scale, and scale scores were computed by averaging items. Internal reliability was good at both time points (baseline: α = 0.98; 6-month follow-up: α = 0.96). Ease of use was also assessed with a 6-item 1- to 7-point scale to measure perceived ability to learn and become skillful at using the devices with good internal reliability (baseline: α = 0.97; 6-month follow-up: α = 0.96). The change in these measures between baseline and 6-month follow-up was tested using paired-samples t-tests. Descriptive statistics were used to summarize demographic characteristics. Analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
We enrolled participants from February 2017 to November 2017 (Figure 1 and Table 2). Most participants were female (71%), identified as Black or African American (60%), and the mean age was 54.68. Around one-quarter (27%) of participants completed high school or less, 40% had some college education, and 28% had a bachelor’s degree or higher. Approximately 54% of participants reported a household income of less than $50 000. The average years of being diagnosed with T2DM was 11 (SD 6.7). No participants had documented illiteracy in the EHR. Most participants (87%) had used a smartphone for over 2 years. However, only up to 35% of participants had used apps or wearable devices to track their health.
Table 2.
Patient characteristics at baseline (N = 60)
| Mean | SD | |
|---|---|---|
| Age | 54.68 | (11.70) |
| Weight (pounds) | 220.2 | (52.09) |
| BMI | 36.27 | (7.82) |
| Years of T2DM diagnosis | 10.59 | (6.65) |
| HbA1c | 8.18 | 1.90 |
| n | % | |
| Female | 43 | (71.67) |
| Race (%) | ||
| Black or African American | 36 | (60.00) |
| White | 21 | (35.00) |
| Other or more than 1 race | 3 | (500) |
| Hispanic or Latino | 2 | (3.33) |
| Education | ||
| High school or less | 14 | (23.33) |
| Some college but no degree | 20 | (33.33) |
| Associates or Bachelor’s degree | 13 | (21.66) |
| Graduate degree | 9 | (15.00) |
| Household income < $49 000 per year | 31 | (52.53) |
| Cell phone type | ||
| iPhone | 31 | (51.67) |
| Android | 28 | (46.67) |
| Used a smartphone for more than 2 years | 52 | (86.67) |
| Has ever used an app to track | ||
| Blood sugar | 7 | (11.67) |
| Weight | 8 | (13.33) |
| Physical activity | 21 | (35.00) |
| Medication | 5 | (8.33) |
| Has ever used a wearable device to track physical activity | 18 | (30.00) |
Technical and other support
During the study, 35 patients (58%) reached out to the nurse about a technical issue, such as devices not syncing or logins not working. This occurred approximately 100 times 2–3 times per week. Patients were also contacted by the nurse a similar number of times due to data not being received. A quarter of the issues were technical and others were due to patient behaviors, such as forgetting to use the device or not charging it.
Overall engagement over 6 months by subgroups
Table 3 includes the percent of device engagement by age, HbA1c, and race. Overall, engagement was highest for Fitbit, followed by glucose and then weight (Figure 2). In the overall sample, 53.83% of daily weight submissions were completed. The percent of daily weight submissions that were completed differed by age group (t[57] = −2.23, P = .03), with those younger than 55 having lower engagement (M = 43.30%, SD = 32.90%) than those 55 and older (M = 61.87%, SD = 30.94%). The percent of daily weight submissions that were completed did not differ by HbA1c group (t[57] = 1.58, P = .12), but did differ by race (t[58] = 3.13, P = .003), in that Black participants had lower engagement (M = 44.80%, SD = 34.25%) than White participants (M = 70.60%, SD = 21.35%). In the sample overall, 87.45% of daily Fitbit submissions were completed; engagement did not differ by age group (t[57] = 0.60, P = .55), HbA1c group (t[57] = 0.83, P = .41), or race (t[57] = 0.09, P = .93)). For blood glucose engagement, 60.99% were completed. Blood glucose engagement did not differ by age (t[57] = −1.75, P = .09). Engagement was lower among patients with high HbA1c (HbA1c < 7: M = 76.22%, SD = 29.08%; HbA1c ≥ 7.0: M = 55.90%, SD = 30.60%; t[57] = 2.30, P = .03). When race was considered, engagement was lower among Black and other minority participants (Black, or other non-White race: M = 55.04%, SD = 32.44%) than for Whites (M = 72.03%, SD = 26.0%; t[58] = 2.07, P = .04). Overall, 71.19% of medication adherence surveys were completed, but engagement did not differ by age group (t[57] = 0.12, P = .91), HbA1c group (t[57] = 1.30, P = .20), or race (t[57] = 1.38, P = .17).
Table 3.
Total percentage engagement over 6 months, overall and by age, HbA1c group, and race
| Outcome | Overall | Age group |
HbA1c at baseline |
Race |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | < 55% | > = 55% | t value | P value | < 7% | > = 7% | t value | P value | White | Black | t value | P value | |
| Weight | 53.83% | 43.30% | 61.87% | −2.23 | .03 | 64.64% | 49.61% | 1.58 | .12 | 70.60% | 44.80% | 3.13 | .003 |
| Activity | 87.45% | 87.89% | 87.28% | 0.60 | .55 | 88.24% | 85.78% | 0.83 | .41 | 87.52% | 87.42% | 0.09 | .93 |
| Glucose | 60.99% | 53.52% | 67.63% | −1.75 | .09 | 76.22% | 55.90% | 2.30 | .03 | 72.03% | 55.04% | 2.07 | .04 |
| Adherence | 71.19% | 71.69% | 70.81% | 0.12 | .91 | 78.73% | 68.13% | 1.30 | .20 | 78.02% | 67.41% | 1.38 | .17 |
Figure 2.
Overall engagement across devices.
Trend for biweekly proportion of engagement by subgroups
Table 4 reports results from linear mixed models regressing biweekly weight, activity, and blood glucose engagement over 6 months for age group. Weight engagement was 25% lower at baseline in patients younger than 55 (b = 0.25, P = .006, Figure 3.1). This finding was robust to inclusion of covariates (b = 0.21, P = .03). The interaction between age and time was not significant in unadjusted or adjusted models (b = −0.01, P = .20; b = −0.01, P = .27), which indicates that the slope of biweekly engagement over 6 months did not differ by age group. Biweekly Fitbit activity engagement indicated nonsignificant effects for age group (unadjusted: b = −0.03, P = .58; adjusted: b = −0.04, P = .42) and for age group by time (unadjusted: b = 0.003, P = .69; adjusted: b = 0.005, P = .49; Figure 3.2). Biweekly blood glucose engagement indicated a nonsignificant effect for age group in unadjusted and adjusted models (b = 0.13, P = .19; b = 0.09, P = .36). The interaction between age group and time was nonsignificant (unadjusted: b = −0.0002, P = .98; adjusted: b = 0.0009, P = .94).
Table 4.
Biweekly engagement regressed on (1) time and age group, (2) time and HbA1c group, and (3) time and race
| Weight |
Activity |
Glucose |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||||
| Est. | P value | Est. | P value | Est. | P value | Est. | P value | Est. | P value | Est. | P value | |
| Age | ||||||||||||
| Intercept | 0.45 | < .001 | 0.56 | < .001 | 0.88 | < .001 | 0.88 | < .001 | 0.61 | < .001 | 0.77 | < .001 |
| Time | −0.0005 | .94 | −0.0007 | .92 | −0.0004 | .94 | −0.0004 | .94 | −0.01 | .20 | −0.01 | .19 |
| Age group | 0.25 | .006 | 0.21 | .03 | −0.03 | .58 | −0.04 | .42 | 0.13 | .19 | 0.09 | .36 |
| Time × Age group | −0.01 | .20 | −0.01 | .27 | 0.003 | .69 | 0.005 | .49 | −0.0002 | .98 | 0.0009 | .94 |
| HbA1c | ||||||||||||
| Intercept | 0.69 | < .001 | 0.66 | < .001 | 0.81 | < .001 | 0.81 | < .001 | 0.84 | < .001 | 0.84 | < .001 |
| Time | −0.006 | .46 | −0.004 | .66 | 0.009 | .16 | 0.01 | .12 | −0.02 | .13 | −0.02 | .13 |
| HbA1c group | −0.14 | .19 | −0.12 | .27 | 0.07 | .19 | 0.07 | .23 | −0.22 | .03 | −0.24 | .03 |
| Time × HbA1c group | −0.001 | .88 | −0.004 | .72 | −0.01 | .15 | −0.01 | .16 | 0.006 | .63 | 0.007 | .58 |
| Race | ||||||||||||
| Intercept | 0.68 | < .001 | 0.60 | < .001 | 0.87 | < .001 | 0.88 | < .001 | 0.80 | < .001 | 0.74 | < .001 |
| Time | 0.004 | .60 | 0.006 | .41 | 0.0 | .99 | 0.0005 | .93 | −0.01 | .17 | −0.01 | .16 |
| Race | −0.14 | .15 | −0.10 | .36 | −0.02 | .80 | −0.01 | .80 | −0.18 | .07 | −0.18 | .08 |
| Time × Race | −0.02 | .06 | −0.02 | .04 | 0.002 | .81 | 0.003 | .71 | 0.003 | .82 | 0.003 | .77 |
Note: Age: younger than 55 (reference group), 55 or older; Covariates: HbA1c at baseline, income (centered), education (centered), and sex (reference group: males). HbA1c group: less than 7 (reference group), 7 or greater. Covariates: income (centered), education (centered), and sex (reference group: males). Race: White (reference group), Black and other non-White race. Covariates: income (centered), education (centered), and sex (reference group: males).
Figure 3.
Biweekly missingness of age group by (3.1) scale weights, (3.2) Fitbit activity, (3.3) glucose readings missingness.
Table 4 presents results for mixed models of biweekly weight, activity, and blood glucose engagement over 6 months for the HbA1c group. Results indicated a nonsignificant main effect of HbA1c in unadjusted and adjusted models of weight engagement (b = −0.14, P = .19; b = −0.12, P = .27). The interaction between HbA1c and time was nonsignificant in predicting weight engagement (b = −0.001, P = .88; b = −0.004, P = .72; Figure 4.1). For Fitbit activity engagement, the main effect of HbA1c was nonsignificant (b = 0.07, P = .19; b = 0.07, P = .23) as was the interaction between HbA1c and time (b = −0.01, P = .15; b = −0.01, P = .16; Figure 4.2). For blood glucose engagement, the main effect of HbA1c was significant in unadjusted and adjusted models (b = −0.22, P = .03; b = −0.24, P = .03; Figure 4.3), indicating that blood glucose engagement was 22% lower at baseline in those with an HbA1c of 7 or higher. In contrast, the interaction between HbA1c and time was nonsignificant for this outcome (b = 0.006, P = .63; b = 0.007, P = .58).
Figure 4.
Biweekly missingness by HbA1c group and (4.1) scale weights, (4.2) Fitbit activity, (4.3) glucose readings.
Table 4 presents results for mixed models of biweekly weight, activity, and blood glucose engagement by race. For weight engagement, the main effect of race was nonsignificant in unadjusted and adjusted models (b = −0.14, P = .15; b = −0.10, P = .36), and the interaction between race and time was non-significant in the unadjusted model (b = −0.02, P = .06). However, this interaction was significant in the adjusted model (b = −0.02, P = .04). This indicates the slope of engagement was lower among Black participants and others of non-White race (Figure 5.1). For activity engagement, the main effect of race was non-significant (b = −0.02, P = .80; b = −0.01, P = .80), as was the interaction between race and time (b = 0.002, P = .81; b = 0.003, P = .71; Figure 5.2). For blood glucose engagement, the main effect of race was nonsignificant (b = −0.18, P = .07; b = −0.18, P = .08) as was the interaction between race and time (b = 0.003, P = .82; b = 0.003, P = .77; Figure 5.3).
Figure 5.
Biweekly missingness by race and (5.1) scale weights, (5.2) Fitbit activity, (5.3) glucose readings.
We also assessed baseline-to-6-month differences in the perceived usefulness and ease of using the multiple devices. Perceived usefulness did not change significantly (t = 0.52, P = .60) from baseline (1.86) to 6 months (1.78). However, perceived ease of use did improve (t = 2.41, P = .02) from baseline (2.22) to 6 months (2.41).
DISCUSSION
Determining whether it is feasible for patients to use multiple mobile devices to self-manage their diabetes is an important first step in developing effective personalized care delivery strategies that use multiple types of near real-time data from patients in their everyday environment. It also is an important step in understanding if multiple devices are appropriate for integration into formal care delivery systems including EHRs. Our findings indicate it is feasible for participants from different socioeconomic, educational, and racial backgrounds, including underserved populations in which the incidence of diabetes may be higher, to use and track relevant diabetes-related data from multiple devices for at least 6 months. Participants used devices that sent physiological and behavioral data over time, thus providing a more complete picture of their health in between clinic visits. Patient-generated health data have been shown to provide value to both patients and healthcare professionals by providing deeper insight into a patient’s condition and insights into health in between clinic visits.22 Those who completed surveys at both baseline and at 6 months reported high usefulness of the devices. Further, ease of use improved from baseline to 6 months, suggesting that by using the devices patients became increasingly familiar with them.
Nevertheless, the data revealed notable patterns particularly among age, race, and HbA1c level. Generally, the Fitbit was used consistently, and data was received from most participants throughout the study, indicating that participants may generally enjoy using this tool. Engagement data from the glucometer and cellular scale revealed different patterns and decreased over time. Younger individuals, those with high HbA1c, and patients who identified as Black, had less engagement with these tools. While we do not know precisely why this was the case, receipt of time-sensitive data from these tools warrants further clinical investigation. It has been reported that stigma disproportionately affects patients with diabetes who have higher BMI, HbA1c, and poorer self-reported blood glucose control.23 It has also been reported that there is additional stigma associated with diabetes in Black communities due to a variety of reasons, including racial discrimination, by healthcare providers, among others.24 Further, while the results that younger people were less likely to be engaged with the technology over time may be surprising, they also had higher HbA1c levels, and research shows that young people can be less engaged with their health.25 Interestingly, because the Fitbit activity often revealed different patterns from the other devices, use of multiple devices may be needed to elucidate hidden patterns in complex chronic illnesses, such as T2DM. Further, these tools could play an important role in identifying patients who need healthcare resources the most.
T2DM is an increasingly common chronic illness, and mobile and digital health tools are being used more and more to help improve diabetes-related outcomes and management. Studies on the use of tools—such as wireless glucometers, activity trackers, weight scales, and apps to log behaviors (such as diet and medication taking)—show promise in improving health outcomes.26–32 Our study is among the first to seek answers to the many questions related to integrating patient data from multiple mobile devices into diabetes self-management care-delivery models. Integration of these data into EHRs is advancing due to the wide access to and reach of mHealth tools among diverse populations. Questions, such as how long patients will track multiple types of diabetes-related data, how healthcare providers are able to receive the data, which strategies will best help patients self-manage their diabetes, and how clinicians might effectively guide patients to better manage their disease in near real time, will need to be addressed in the emerging era of digital health. Further, the ability to identify trends and predict outcomes from data, such as from the multiple devices used in this study, is a complex process and remains a challenge for both patients and clinicians.33 Patients may not always transmit enough quality data for them to be useful for use in prediction algorithms and clinical decision support tools. There are also limits to the amount of information that humans can process at a given time,34 and this may vary by health literacy and numeracy.35
Finally, the ability of medical professionals to gather more precise information related to patients’ health in their everyday environments will allow us to create digital phenotypes. This is defined as the quantification of the individual-level human phenotype in situ, using data from personal digital devices36 and sensors. This growing area of research will be useful in the future for prediction of disease and wellness progression for individuals and for predicting trends at the population level. Challenges moving forward for usefulness in clinical practice will include making sense of the deluge of data from many devices, data wrangling, and maintaining patient privacy, among others.
Limitations
This was an observational study with no intervention intended to influence health outcomes. This study was limited by its sample size, which limits generalizability and the ability to make statistical conclusions. Self-monitoring occurred for 6 months and, thus, does not allow us to examine long-term patterns in a complex chronic illness, such as diabetes. Those who enrolled in the study may have been biased towards perceptions of device usefulness from the beginning. Additionally, we considered the use of a smart pill bottle for medication tracking. However, this would have added burden and complexity of another technology, challenges with monitoring multiple and diverse types of medications, and added costs. Thus, we chose a low-cost method of a short text message survey that, if feasible, is easily scaled. Finally, we did not integrate these data into an EHR to assess clinical use of the data within the health system.
CONCLUSION
Diabetes is difficult to manage because it requires daily, if not more frequent, monitoring along with behavioral change. Patients must adapt their diet, physical activity, and medication dosage based on blood glucose values; continually assess for both acute and chronic complications; and develop strategies to address psychosocial issues and concerns. mHealth technologies may facilitate self-management among diverse patients with diabetes by capturing diabetes-related data in their everyday environments and providing direct feedback to patients. Monitoring of these data in near real-time by patients and transmitting them to healthcare providers, can foster collaborative work and change care delivery from infrequent episodic delivery to care at the time when patients need it most. Using multiple tools also allows us to capture a more complete picture of patients’ health, the ability to more accurately decipher patterns within the data that elucidate health of populations, and to make more informed data-driven decisions for clinical care and patient self-management.
As health systems around the world incorporate EHRs that integrate with mHealth devices, similar data as presented in this study will increasingly be incorporated into healthcare. Because of the wide access to mobile devices across racial, educational, and socioeconomic populations, this presents an opportunity to develop new models of care delivery for patients with T2DM that may promote equity as well.
FUNDING
This work was supported by a grant from the US National Institutes of Health, the National Institute of Nursing Research (NINR 1R15NR015890), and a [blinded] University Data+ grant to RJS. DS was supported by the Building Interdisciplinary Careers in Women’s Health career development award (K12HD043446). MJC was supported by a Career Development Award from Veterans Affairs Health Services Research and Development (CDA 13-261). Support for AL was provided by the US Department of Veterans Affairs Office of Academic Affiliations (TPH 21-000) and the [blinded] Health Services Research Center of Innovation funding (CIN 13-410). Support for JV was provided by the US National Institutes of Health (NINR F31NR018100). The content is solely the responsibility of the authors and does not necessarily reflect the position or policy of [blinded] University, the US Department of Veterans Affairs, or the US government.
AUTHOR CONTRIBUTIONS
RJS was the principal investigator, led this study team, and was responsible for all aspects of research design, methods, analysis, and dissemination. RJS, DS, JMC, AV, and QY substantially contributed to the research methods, acquisition of data, analysis, and interpretation and also participated in all aspects of writing the manuscript. DH, JV, AAL, MJ, and JS contributed to the analysis, and interpretation of data, and participated in all aspects of writing the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Martin Streicher from the [blinded] Global Digital Health Science Center for programming and iHealth for their generosity in donating devices. The authors would also like to thank Karen Judge and Jane Shealy for their editorial assistance.
CONFLICT OF INTEREST STATEMENT
DS is a consultant with Omada Health. There are no other competing interests to declare.
REFERENCES
- 1.American Diabetes Association. Standards of medical care in diabetes—2019 abridged for primary care providers. Clin. Diabetes 2019; 37 (1): 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rice R, Katz J.. Comparing Internet and mobile phone usage: digital divides of usage, adoption and dropouts. Telecommun Policy 2003; 27 (8–9): 597–623. [Google Scholar]
- 3. Ling R. The Mobile Connection: The Cell Phone’s Impact on Society. San Francisco: Morgan Kaufmann Publishers; 2004. [Google Scholar]
- 4.Shaw RJ, Bonnet JP, Modarai F, George A, Shahsahebi M. Mobile health technology for personalized primary care medicine. Am J Med 2015; 128 (6): 555–7. [DOI] [PubMed] [Google Scholar]
- 5. Goggin G. Cell Phone Culture: Mobile Technology in Everyday Life. New York: Routledge; 2006. [Google Scholar]
- 6. Kumar S, Nilsen WJ, Abernethy A, et al. Mobile health technology evaluation: the mHealth evidence workshop. Am J Prev Med 2013; 45 (2): 228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. mHealth: New Horizons for Health through Mobile Technologies: Based on the Findings of the Second Global Survey on eHealth. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 8.Pew Research Center. Mobile Fact Sheet. Washington, DC: Author; 2019. [Google Scholar]
- 9. Insel TR. Digital phenotyping: technology for a new science of behavior. JAMA 2017; 318 (13): 1215–6. [DOI] [PubMed] [Google Scholar]
- 10. Jain SH, Powers BW, Hawkins JB, Brownstein JS.. The digital phenotype. Nat Biotechnol 2015; 33 (5): 462–3. [DOI] [PubMed] [Google Scholar]
- 11. Wood E, Yang Q, Steinberg D, et al. Diabetes mobile care: aggregating and visualizing data from multiple mobile health technologies. AMIA Jt Summits Transl Sci Proc 2019; 2019: 202–11. [PMC free article] [PubMed] [Google Scholar]
- 12. Shaw RJ, Barnes A, Steinberg D, et al. Enhancing diabetes self-management through collection and visualization of data from multiple mobile health technologies: protocol for a development and feasibility trial. JMIR Res Protoc 2019; 8 (6): e13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healh Resources & Services Administration. Medically Underserved Areas and Populations (MUA/Ps); 2019. https://bhw.hrsa.gov/shortage-designation/muap Accessed January 2020.
- 14. Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q 1989; 13 (3): 319–40. [Google Scholar]
- 15. Voils CI, Maciejewski ML, Hoyle RH, et al. Initial validation of a self-report measure of the extent of and reasons for medication nonadherence. Med Care 2012; 50 (12): 1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wing RR, Papandonatos G, Fava JL, et al. Maintaining large weight losses: the role of behavioral and psychological factors. J Consult Clin Psychol 2008; 76 (6): 1015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steinberg DM, Bennett GG, Askew S, Tate DF.. Weighing every day matters: daily weighing improves weight loss and adoption of weight control behaviors. J Acad Nutr Diet 2015; 115 (4): 511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sasaki JE, Hickey A, Mavilia M, et al. Validation of the Fitbit wireless activity tracker for prediction of energy expenditure. J Phys Act Health 2015; 12 (2): 149–54. [DOI] [PubMed] [Google Scholar]
- 19. de Zambotti M, Goldstone A, Claudatos S, Colrain IM, Baker FC.. A validation study of Fitbit Charge 2 compared with polysomnography in adults. Chronobiol Int 2018; 35 (4): 465–76. [DOI] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42 (2): 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen DJ, Keller SR, Hayes GR, Dorr DA, Ash JS, Sittig DF.. Integrating patient-generated health data into clinical care settings or clinical decision-making: lessons learned from project healthdesign. JMIR Hum Factors 2016; 3 (2): e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu NF, Brown AS, Folias AE, et al. Stigma in people with type 1 or type 2 diabetes. Clin Diabetes 2017; 35 (1): 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shiyanbola OO, Ward E, Brown C.. Sociocultural influences on African Americans’ representations of type 2 diabetes: a qualitative study. Ethn Dis 2018; 28 (1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stroud C, Walker LR, Davis M, Irwin C Jr. Investing in the health and well-being of young adults. J Adolesc Health 2015; 56 (2): 127–9. [DOI] [PubMed] [Google Scholar]
- 26. Offringa R, Sheng T, Parks L, Clements M, Kerr D, Greenfield MS.. Digital diabetes management application improves glycemic outcomes in people with type 1 and type 2 diabetes. J Diabetes Sci Technol 2018; 12 (3): 701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitsiou S, Pare G, Jaana M, Gerber B.. Effectiveness of mHealth interventions for patients with diabetes: an overview of systematic reviews. PLoS One 2017; 12 (3): e0173160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berman MA, Guthrie NL, Edwards KL, et al. Change in glycemic control with use of a digital therapeutic in adults with type 2 diabetes: cohort study. JMIR Diabetes 2018; 3 (1): e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veazie S, Winchell K, Gilbert J, et al. 2018; 33 (7): 1167–76. [DOI] [PMC free article] [PubMed]
- 30. Bonn SE, Alexandrou C, Steiner KH, et al. App-technology to increase physical activity among patients with diabetes type 2-the DiaCert-study, a randomized controlled trial. BMC Public Health 2018; 18 (1): 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marcolino MS, Oliveira JAQ, D'Agostino M, Ribeiro AL, Alkmim MBM, Novillo-Ortiz D.. The impact of mHealth interventions: systematic review of systematic reviews. JMIR mHealth Uhealth 2018; 6 (1): e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veazie S, Winchell K, Gilbert J, et al. Rapid evidence review of mobile applications for self-management of diabetes. J Gen Intern Med 2018; 33 (7): 1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mamykina L, Levine ME, Davidson PG, Smaldone AM, Elhadad N, Albers DJ.. Data-driven health management: reasoning about personally generated data in diabetes with information technologies. J Am Med Inform Assoc 2016; 23 (3): 526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Halford GS, Baker R, McCredden JE, Bain JD.. How many variables can humans process? Psychol Sci 2005; 16 (1): 70–6. [DOI] [PubMed] [Google Scholar]
- 35. Rothman RL, Montori VM, Cherrington A, Pignone MP.. Perspective: the role of numeracy in health care. J Health Commun 2008; 13 (6): 583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Torous J, Kiang MV, Lorme J, Onnela J-P.. New tools for new research in psychiatry: a scalable and customizable platform to empower data driven smartphone research. JMIR Mental Health 2016; 3 (2): e16. [DOI] [PMC free article] [PubMed] [Google Scholar]