Abstract
Purpose
Adipose-derived mesenchymal stem cells (MSCs) are attractive biological agents in regenerative medicine. To optimize cell therapies, it is necessary to determine the most effective delivery method for MSCs. Therefore, we evaluated the biological properties of MSCs after exposure to various temperatures to define optimal storage conditions prior to therapeutic delivery of MSCs.
Design
Prospective observational study
Methods and materials
Adherent and non-adherent MSCs were incubated at multiple temperatures (i.e., 4, 23 and 37°C) in Lactated Ringers (LR) solution lacking essential cell growth ingredients, or in culture media which is optimized for cell growth. Cells were assessed either after the temperature changes (4 hours) or after recovery (24 hours). Metabolic activity of MSCs, cell number and expression of representative mRNA biomarkers were evaluated to assess the biological effects of temperature. We monitored changes in mRNAs expression related to cytoprotective- or stress-related responses (e.g., FOS, JUN, ATF1, ATF4, EGR1, EGR2, MYC), proliferation (e.g., HIST2H4, CCNB2), and extracellular matrix production (ECM; e.g., COL3A1, COL1A1) by quantitative real time reverse-transcriptase polymerase chain reaction (RT-qPCR) analysis.
Results
Our study demonstrates that storing MSCs in Lactated Ringers (LR) solution for 4 hours decreases cell number and metabolic activity. The number of viable MSCs decreased significantly when cultured at physiological temperature (37 °C) and severe hypothermia (4°C), while cells grown at ambient temperature (23°C) exhibited the least detrimental effects. There were no appreciable biological differences in mRNA markers for proliferation or ECM deposition at any of the temperatures. However, biomarkers related to cytoprotective- or stress-responses were selectively elevated depending on temperature or media type (i.e., LR versus standard media).
Conclusion
The biological impact of nutrient-free media and temperature changes after 4 hours exposure persists after a 24 hour recovery period. Hence, storage temperature and media conditions should be optimized to improve effective dosing of MSCs.
Keywords: Mesenchymal stem cell, stem cell therapy, hypothermia, hypoxia, cell stress, connective tissue diseases, musculoskeletal conditions, quality improvement and patient safety, basic science
Introduction
Degenerative diseases of the musculoskeletal system are a major source of chronic pain and disability in the general population and cause a significant burden to health care systems worldwide, particularly in developed countries. The most common problems include primary and secondary osteoarthrosis of knee, hip and other joints, degenerative disc disease and spondylarthrosis.[1] All of these disorders significantly limit mobility and cause a decline in quality of life, especially in elderly patients.
Possible treatment approaches of musculoskeletal problems are considered individually, ranging from least invasive (e.g., physical therapy and pharmacotherapy) to more invasive (e.g., injections or surgeries). Our group examines a number of skeletal degenerative diseases that affect cartilaginous tissues in the articular joints and spine [2–4] that may benefit from stem cell therapies [5–7]. In musculoskeletal regenerative medicine, cell therapy is rapidly gaining traction and has become a prevalent treatment modality that may alleviate pain and combat disease progression. Recent studies have demonstrated that therapeutic effects of mesenchymal stem cells may be due to the release of bioactive molecules rather than functioning as a source of new cells incorporated into healing tissues[8]. Additional work supports these findings and suggests that stem cells mitigate degeneration by providing anti-inflammatory or trophic signals [9–11].
Various clinical trials have explored effects of culture-expanded adipose-derived mesenchymal stem cells MSCs [12–15]. In clinical settings, it is important to provide consistent cell doses for proposed therapeutic effect. Similarly the retention of overall MSC quality during the storage, transport and clinical application is crucial for reproducibility of clinical trials. There is a paucity of data on the biological properties of adipose-derived MSCs and how their phenotype may change from the moment when the cells leave a ‘good manufacturing practice’ (GMP) facility until they are injected into patients. In order to eliminate potential detrimental effects caused by environmental factors that may occur during preparation for cell therapy, our group has extensively explored a number of these effects, such as exposure to preservatives [16], contrast agents [17], hypoxia [18], needle passage [19], various growth surfaces [20, 21], as well as the cytotoxicity of local anesthetics [22]. Here, we examined whether the viability and metabolic activity of MSCs may be compromised by other environmental factors. Because MSCs undergo temperature and media changes during the various stages prior to delivery, we considered that these experimental variables could affect the potency and/or dosing of MSCs during the delivery process.
To address the hypothesis that ambient temperatures and media changes during clinical delivery may affect the viability, metabolic activity, and gene expression signatures of MSCs, we examined survival and metabolic activity of MSCs incubated in both nutrient-rich and non-nutritious solutions within ambient temperatures that are commonly encountered in the clinical setting. Temperatures we considered were body core temperature (37°C), moderate hypothermia (23°C), or severe hypothermia (4°C). We demonstrated that metabolic activity and MSC number are altered with changing temperature with a concurrent temperature-dependent change in the expression of stress-response related markers. MSCs are particularly sensitive to temperature changes when suspended in nutrient–free solutions (e.g., Lactate Ringers solution) that are used during clinical delivery. The latter finding may necessitate a reappreciation of standard operating procedures for MSC-based cell therapies.
Methods and materials
Cell isolation
Human adipose-derived MSCs from fat biopsies were harvested for research use from consenting patients during elective surgeries with approval from the Mayo Clinic Institutional Review Board (IRB). MSCs from three donors (#258, #283, #211) were cultured in platelet lysate containing zoonotic free culture media as previously reported [23, 24]. These cells were extensively characterized for multilineage potential [25] and their molecular characteristics by RNA-sequencing [26, 27]. MSCs were expanded in standard tissue culture media optimal for this cell type which is Advanced Minimum Essential Medium (Advanced MEM, Life Technologies, Grand Island, NY), supplemented with 5% human platelet lysate (PLTMax; Mill Creek Life Sciences, Rochester, MN), 2mM Glutamax (Life Technologies), 2U/mL heparin, and 1% Pen-Strep (100 U/mL penicillin, 100g/mL streptomycin; Cellgro, Thermo Fisher Scientific, Dublin, Ireland). Following several passaging steps, the MSCs were frozen for storage in Cryostore cell cryopreservation media (Sigma-Aldrich, St.Louis, MO) in liquid nitrogen. For the purpose of our experiments, individual frozen batches of MSCs (passage 6 or 7) were thawed and expanded in a cell culture at 95% humidity, 5% CO2 and standard incubator temperature (37°C). Upon retrieval from liquid nitrogen storage, MSCs were allowed to recover from cell stresses due to the freeze/thaw procedure and expanded for at least 48 hours in 175 cm2 flasks in standard tissue culture media until they reached passage 8. The MSCs were detached using TrypLE Express (Thermo Fisher Scientific). MSCs were seeded at 104 cells/cm2 in 12 well-plates (Costar, Thermo Fisher Scientific) and allowed to attach and recover for 1 day after which culture media was aspirated prior to experimentation.
Experimental Design for Cell Treatment
Fresh culture media or Lactated Ringers solution were added so that each plate contained triplicates with 1 ml of solution. Plates with control cells were maintained in the incubator for the whole experimental period without any culture media change or other handling. Culture media and LR were prechilled or pre-warmed to the desired temperatures before the experiment, therefore by design causing rapid rather than gradual temperature changes at the time of addition to the cells. MSCs were incubated for 4 hours (hrs) at distinct temperatures and either immediately analyzed or transferred to the incubator to temperature equilibration and recovery for another 24 hrs. These cells were given fresh culture media so that those that contained LR could restore their metabolism with access to standard nutrition optimized for cell growth.
We selected three storage temperatures (i.e., 4, 23, 37°C) that are relevant to clinical settings and are easily reproducible in the laboratory. Temperature-specific environments were simulated with adherent MSC cultured on standard tissue culture plates or with non-adherent MSCs maintained in plastic tubes. Adherent cells were used to reflect standard growth conditions as clinical grade cells in GMP facilities and non-adherent to simulate cells loaded in a syringe for stem cell therapy injection. For adherent cells, one plate was placed on ice in a cooler box (4°C group), one plate at ambient temperature on the bench surface (23°C group), and two plates remained in the tissue culture incubator (37°C groups). The latter two plates were either subjected to mock treatment (to account for effects of handling cells in a tissue culture hood), or always maintained in the incubator. For non-adherent cells, conical plastic tubes (Falcon, Thermo Fisher Scientific) were immersed in temperature-controlled water baths to simulate the effects of temperature on non-adherent MSCs. All plates except for a plate with control cells were sealed with Parafilm (Bemis NA, Neenah, WI) and all tubes were covered with a lid to model limited gas exchange expected in the closed environment of cells stored in syringes. Also, the sealed vessels eliminate potential differences due to reduced oxygen levels in different experimental environments, while control cells were maintained with access to oxygen and 5% CO2.
In the second experimental setting, culture expanded cells were enzymatically detached and divided in 3×15 ml conical plastic Falcon tubes or in 1.5ml Eppendorf tubes. This measure was taken in order to simulate the storage of cells in the syringe before application in the clinical environment, where the cells are non-adherent. Tubes with MSCs were centrifuged and culture media aspirated. The pellet of cells was re-suspended with 10 ml Lactated Ringers at: 4, 23, 37°C and incubated in these temperatures for 4 hrs in concentrations of 15 −20 × 104 cells per ml. Following the incubation, part of the cell solution was centrifuged and used for RNA extraction and part was used for re-plating for a metabolic activity MTS assay at 104 cells/cm2. After the initial experimental modulation of temperature, no additional calibration of cell counts was performed. Thus, our end-point results reflect the cumulative effects of both initial temperature changes and the recovery period on the final number of cells.
Metabolic activity assays were performed immediately after the experiment in the adherent cells as well as in the recovery period at 24 hrs. Following the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-car-boxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) colorimetric assay (CellTiter 96® Aqueous One Solution, Promega), MSCs were fixed overnight using 10% neutral buffered saline (NBF) and stained with Hoechst stain 33258 (Santa Cruz Biotechnology, Dallas, TX) to observe nuclear DNA.
Gene expression analysis
Real time reverse transcriptase quantitative PCR (RT-qPCR) analysis of human adipose-derived MSCs was performed to assess cell stress-related responses, proliferation and extracellular matrix production using mRNA biomarkers. We extracted RNA using Tri Reagent (Zymo Research, Irvine, CA) immediately after experimental incubation. RNA was isolated using Direct-zol RNA MiniPrep (Zymo Research Irvine, CA) and RNA yield was determined by Nanodrop 2000 (Thermo Scientific, Waltham, MA). Reverse-transcription was performed to obtain cDNA using SuperScript III (Invitrogen Carlsbad, CA). Gene expression was quantified with RT-qPCR in CFX384 Real-Time system (BioRad Hercules, CA) using 3.3ng cDNA per 10μl reaction with QuantiTect SYBR green PCR kit (Qiagen Valencia, CA). Primers’ sequences are given in Table 1. Results are normalized to EEF1A1 rather than GAPDH within each sample and data analyzed with 2^ (-ΔΔ Ct) method. We note that mRNA expression of GAPDH (which encodes the glycolytic enzyme glyceraldehyde dehydrogenase) may change with metabolic status, while EEF1A1 (which encodes the translational elongation factor 1α1) is expected to be less variable (see Fig. 5). Our RNA-seq data for adipose-derived MSCs from >50 patients show that mRNAs for GAPDH and EEF1A1 are highly abundant, but EEF1A1 is more abundantly expressed, has a lower coefficient of variation and is less variable than GAPDH when cells exhibit reduced cell proliferation ([27] and unpublished observations).
Table 1.
mRNA primer sequences
| mRNA primers sequences | Primers, 5 ’–3’ | Primers, 3 ’–5’ |
|---|---|---|
| Gene | Forward | Reverse |
| GAPDH | ATGTTCGTCATGGGTGTGAA | TGTGGTCATGAGTCCTTCCA |
| EEF1A1 | TGTCGTCATTGGACACGTAGA | ACGCTCAGCTTTCAGTTTATCC |
| FOSB | GCTGCAAGATCCCCTACGAAG | ACGAAGAAGTGTACGAAGGGTT |
| JUNB | TGGCCCAGCTCAAACAGAAG | CAGAAGGCGTGTCCCTTGAC |
| JUND | GTGAAGACCCTCAAGAGTCAGA | GACGTGGCTGAGGACTTTCT |
| EGR1 | ACCCCTCTGTCTACTATTAAGGC | TGGGACTGGTAGCTGGTATTG |
| EGR2 | ATTCTGAGGCCTCGCAAGTA | GCTTATGCCCAGTGTGGATT |
| COL1A1 | GTAACAGCGGTGAACCTGG | CCTCGCTTTCCTTCCTCTCC |
| COL3A1 | TTGAAGGAGGATGTTCCCATCT | ACAGACACATATTTGGCATGGTT |
| ATF1 | CTGGAGTTTCTGCTGCTGTC | GGCAATGGCAATGTACTGTC |
| ATF4 | ATGACCGAAATGAGCTTCCTG | CTGGAGAACCCATGAGGTTTG |
| CMYC | CGGATTCTCTGCTCTCCTCGAC | CCTCCAGCAGAAGGTGATCCA |
| HIST2H4 | AGCTGTCTATCGGGCTCCAG | CCTTTGCCTAAGCCTTTTCC |
| CCNB2 | CCGACGGTGTCCAGTGATTT | TGTTGTTTTGGTGGGTTGAACT |
Figure 5:
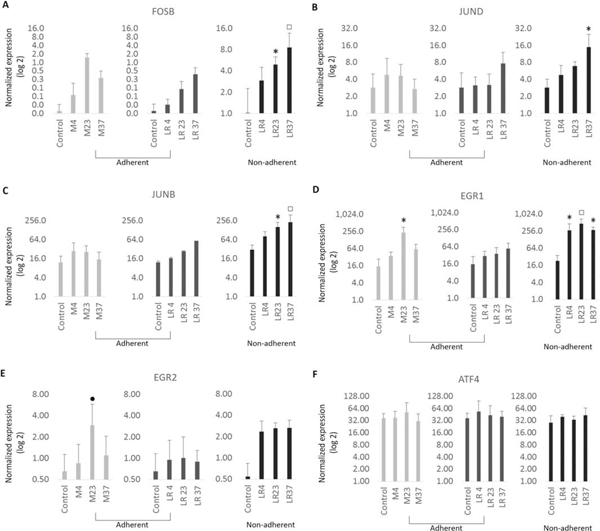
Effects of temperature changes on mRNA expression of MSCs
The graphs show mRNA expression levels immediately after exposure to temperatures for cyto-protective response genes: immediate early response genes (A, B, C), transcription factors (D, E), and constitutive response (F). The data represent mean values from biological triplicates of three cell lines.
*p<.05, □ p<. 01, ●p<.0001, One-way ANOVA with Tukey’s post-hoc test, n=9 for each cell line
Metabolic activity and numbers of cells
Metabolic activity testing was performed using colorimetric assays (MTS assay Kit, Cell Titer 96 Non-radioactive cell proliferation assay, Promega). Cells were rinsed with PBS (Phosphate Buffered Saline) and incubated with MTS reagents for 1.5–2 hrs. After incubation, 100μl of media was transferred to a 96-well plate and evaluated by absorbance measurements at 490nm using the Spectra Max Plus Plate Reader (Molecular Devices, Sunnyvale, CA). Signals obtained for each type of media were used as blank background readings and subtracted during numerical analysis. Metabolic activity of experimental groups was normalized relative to that of control cells and presented as percentage.
To assess the number of cells remaining in the wells after incubation, nuclear staining was performed using Hoechst 33258 (Santa Cruz Biotechnology, Dallas, TX) after MTS assays and/or Live Dead Cytotoxicity assay (Thermo Fisher Scientific) with a separate set of MSCs plated for this staining only. Cells were analyzed immediately with Live solution from Live/Dead cytotoxicity assay or rinsed with PBS and fixed using 10% Neutral Buffered Formalin for 24 hrs. In cells that were analyzed using Live stain, the solution was diluted in PBS and added to the wells so that final concentration of the stain is 0.5 ul/ml. For Hoechst stain, following another PBS wash, Hoechst 33258 nuclear stain diluted in PBS was used at 0.1μl/ml concentration. Fluorescence intensity was quantified with a fluorescence reader (Infinite Pro 2000; Tecan, Austria) at wavelengths of 340/460 for Hoechst and of 465/633 for Live stain. After subtracting background values of each sample, the overall level of Hoechst staining and Live staining was normalized by expressing values as a percentage of control cells.
Statistical analysis
All biological analyses were performed using biological triplicates from multiple cell lines of adipose-derived MSCs. Statistical analysis was performed using Graph Pad Prism Version 7.05 (GraphPad, La Jolla, CA). One-way ANOVA and Tukey post-hoc test were used to compare proportions of surviving cells and metabolic activity and RNA expression levels. The p-value 0.01 was considered as significant. The results were presented as mean and standard deviation.
Results
The logistic path for delivery of MSCs in clinical gene therapies is that MSCs are dislodged from monolayers and stored in syringes on ice until injection into patients. This clinical scenario was simulated in our experimentation by generating MSCs that were left in suspension in nutrient-poor Lactated Ringers and exposed to temperatures of 4, 23, 37°C. MTS metabolic assays were performed to assess changes in metabolic activity of these non-adherent nutrient-deprived MSCs. To dissect the effects related to temperature, nutrient deprivation and cell adhesion, we also carried out MTS assays with adherent cells in standard nutrient-rich culture media that is used for GMP production of clinical grade MSCs (i.e., advanced MEM with 5% platelet lysate). Measurements for biological activities and molecular parameters were made immediately after 4 hr and after a subsequent 24 hr period of recovery.
Acute effects of temperature and media conditions on metabolic activity and cell number
We first examined the metabolic activity of adherent MSCs in Lactated Ringers solution immediately following a 4 hr exposure to different temperatures. When cells are switched from standard culture media (M Control 37) to Lactated Ringers solution (LR) but maintained at 37°C, there is a clear decrease in the metabolic activity of adherent MSCs (Fig. 1A). This effect is due to the removal of essential metabolic ingredients (e.g., glucose and amino acids) that isotonic biochemical solution Lactated Ringers lacks. When temperatures are lowered to 4 or 23°C (LR4 and LR23), we observe that the decline in metabolic activity is less pronounced. Thus, the deleterious effect of Lactated Ringers solution is more prominent at normal physiological temperatures. To assess effects of Lactated Ringers solution on cell number at different temperatures, we monitored the number of viable cells using Live/Dead cytotoxicity staining (Fig. 1B). Regardless of temperature, incubation in Lactated Ringers solution reduced cell numbers, indicating that cells are perishing due to the lack of nutrition. Remarkably, while cell death due to nutrient deprivation is prominent at 4 and 37°C (LR4 and LR37), cells appear to be modestly protected at ambient temperature (LR23), while maximal cell loss (to 40–50%) is observed at 4°C. These data could suggest that hypothermic storage of MSCs in Lactated Ringers solution at 4°C may dramatically reduce the effective dose of MSCs.
Figure 1:
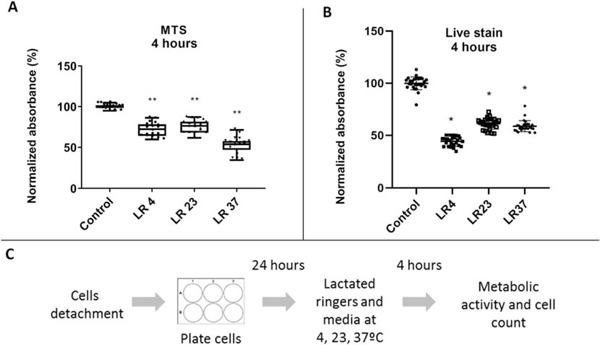
Effects of temperature changes on adherent MSCs viability and metabolic activity immediately after experimental treatment
This figure illustrates metabolic activity (A) and cell counts (B) immediately after 4 hour exposure to temperatures (4°C, 23°C, 37°C) in Lactated Ringers. The design of the experiment is shown (C). MTS assay was used to assess metabolic activity and Live/Dead stain for cell quantification. The data represent mean values from biological triplicates from two and three cell lines.
LR= Lactated Ringers, MTS = MTS assay; *p<.01, ** p<.0001, One-way ANOVA with Tukey’s post-hoc test, n=18(MTS, 4hrs), n=27(Live stain as part of the Live/Dead Cytotoxicity assay, 4hrs)
Assessment of metabolic recovery after hypothermia followed by extended incubation under standard culture conditions.
To assess whether MSCs are temporarily or permanently compromised in their metabolic ability, we incubated cells for 24 hours in standard culture media after initial exposure to Lactated Ringers solution at varying temperatures. Cells exposed to temperatures of, respectively, 4°C and 23°C (LR4 and LR23), did not return to baseline metabolic activity after 24 hours of post-incubation under standard cell culture conditions (Fig. 2A). However, MSCs that were initially incubated at 37°C in Lactated Ringers solution (LR37) displayed a heterogeneous response with some cell cultures having only partial to no restoration of metabolic activity while others demonstrated full recovery (Fig. 2A). Analysis of Hoechst staining revealed that the low temperatures groups (LR4 and LR23) appeared to regain cell numbers to some extent, while the physiological temperature group (LR37) did not (Fig. 2B). Regardless of temperature, it appears that cells have a limited capacity to restore metabolic activity upon pre-incubation in Lactate Ringers solution and that nutrient deprivation at physiological temperature (LR37) is least conductive for maintenance of metabolic activity and cell viability. Notably, cell storage at ambient temperatures in nutrient poor media (LR23) may not only protect cells from metabolic activity loss (see Fig. 1), but perhaps also be beneficial for more optimal recovery.
Figure 2:
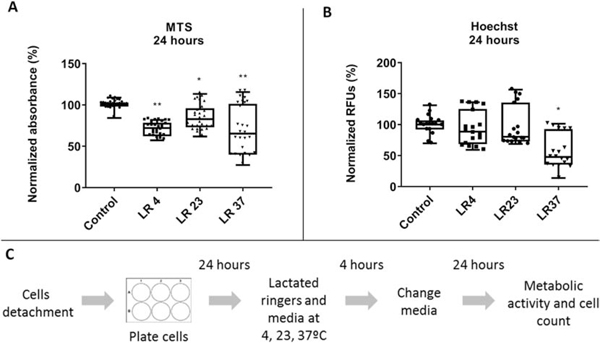
Effects of temperature changes on adherent MSCs viability and metabolic activity at 24 hours after experimental treatment
This figure shows metabolic activity (A) and cell counts (B) at 24 hours after exposure to temperatures in Lactated Ringers. The design of the experiment is shown (C). MTS assay was used to assess metabolic activity and Hoechst stain for cell quantification. The data represent mean values from biological triplicates from two and three cell lines.
LR= Lactated Ringers, MTS = MTS assay, Hoechst = Hoechst 33258 nuclear stain; *p<.01, ** p<.0001, One-way ANOVA with Tukey’s post-hoc test, n=27(MTS, 24hrs), n=18(Hoechst, 24hrs)
To further investigate whether the significant changes in metabolic activity and cell number were due to LR exposure rather than temperature changes, we repeated the previous set of experiments in standard culture media containing a proper nutritional profile. Metabolic activity and viability of adherent cells cultured in LR or standard culture media was assessed 24 hours after exposure to temperature changes (Fig. 3). As expected, cells cultured in growth media and exposed to 37°C (M37) had similar metabolic activity and cell number as control cells. However, MSCs exposed to 4°C and 23°C had significantly lower viability (Fig. 3A) and metabolic activity (Fig. 3B) than the control group in standard culture media. The pattern and extent of these changes resembles that of cells exposed to 4 and 23°C (Fig. 3A, B) in LR solution. Together, these data demonstrate that nutritional differences between LR and standard culture media contribute minimally to the biological changes seen upon temperature variation.
Figure 3:
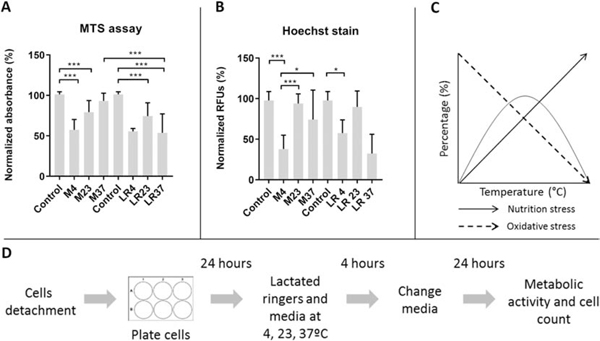
Effects of temperature changes on adherent MSCs in standard culture media and in Lactated Ringers at 24 hours after experimental treatment
This figure shows metabolic activity (A) and cell counts (B) of the adherent MSCs exposed to 4 hour incubation in temperatures (4, 23, 37°C ) in standard culture media and in Lactated Ringers following a 24 hour recovery period. The diagram of nutrition and oxidative stress (C) shows the suggested mechanism behind the observed results where cells in the 4°C and 37°C show lower viability and metabolic activity than 23°C. The design of the experiment is shown (D). MTS assay was used to assess metabolic activity and Hoechst stain for cell quantification. The data represent mean values from biological triplicates of three cell lines.
LR= Lactated Ringers, M = standard culture media, MTS = MTS assay, Hoechst = Hoechst nuclear stain 33258; *p<.05, ** p<.01, ***p<.001, One-way ANOVA with Tukey’s post-hoc test, n=9 (adherent)
Injections during stem cell therapy are performed with non-adherent cells in suspension. Compared to adherent cells, non-adherent cells may be exposed to additional stress due to substrate detachment and loss of cell-to-cell contact. The effects of this additional handling and non-adherent storage within the syringe on the biological state of MSCs need to be addressed. Therefore, we examined whether non-adherent cells have the same cellular properties upon temperature changes as adherent MSCs (Fig. 4). We note that non-adherent cells cannot be maintained in nutrient-rich cell culture media, because these conditions produced non-specific cell aggregates that compromise metabolic read-outs (data not shown). Hence, treatment combinations were restricted to culture conditions using Lactated Ringers which most closely mimic current standard operating procedures for MSC delivery. Results from these experiments demonstrated that temperature changes in non-adherent MSCs lead to alterations in metabolic activity (Fig. 4A) and cell viability (Fig. 4B) of non-adherent MSCs that are comparable to those observed for adherent cells and that these biological effects persist for at least one day after the initial exposure (triplicates from two cell lines) (p<.0001)(Fig. 4). Similar to results for adherent cells, these results are expected from deprivation of nutrients present in cell culture media and apparently occur independent of cell contact with a substratum. However, recovery appears to be more pronounced if the incubation temperatures are 4 and 23°C. Ambient temperature (23°C) appears to be the least harmful temperature for short term storage of MSCs. Cells at physiological temperature (37°C) displayed a major decline in metabolic activity and viability suggesting that optimal temperatures for cell growth are causing a rapid decline in cell survival of non-adherent cells if subjected to starvation. These data with non-adherent cells are consistent with the previous results obtained by testing adherent cells. Taken together, it appears that LR decreases the metabolic activity of cells in a temperature dependent manner, but this decrease is not further exacerbated by lack of cell adherence.
Figure 4:
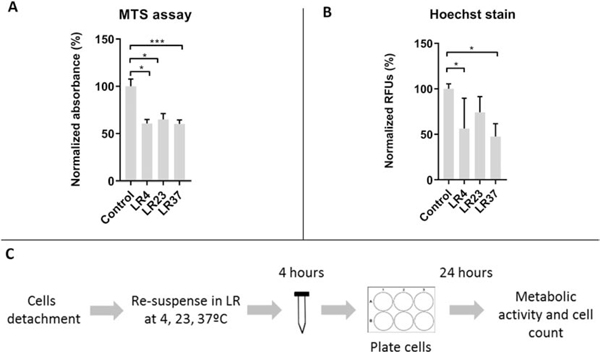
Effects of temperature changes on non-adherent MSCs in Lactated Ringers at 24 hours after experimental treatment
This figure shows metabolic activity (A) and cell counts (B) of the non-adherent MSCs exposed to 4 hour incubation in temperatures (4, 23, 37°C ) in Lactated Ringers following a 24 hour recovery period in standard culture media. The data represent mean values from biological triplicates of two cell lines.
LR= Lactated Ringers, MTS = MTS assay, Hoechst = Hoechst 33258 nuclear stain; *p<.05, ** p<.01, ***p<.001, One-way ANOVA with Tukey’s post-hoc test, n=6 (non-adherent)
Analysis of mRNA expression
The temperature changes during cell handling and storage may induce major cell stress and provoke cytoprotective responses and changes in the biological behavior of MSCs that may decrease the effective dosing and therapeutic effects of MSCs. To assess for stress responses due to temperature shifts in standard nutrient-rich media and nutrient-free Lactated Ringers, we performed mRNA expression analysis using RT-qPCR. We focused on expression of stress-related genes and cytoprotective markers that mediate the ‘immediate early response’ (transcription factors FOS, FOSB, JUN, JUNB, JUND, EGR1, EGR2, MYC) and constitutive proteins (transcription factors ATF1 and ATF4)(Fig. 5; 6A; 7A, B, C); we note that molecular changes and signaling cascades stimulated by expression of stress response genes in response to temperature or nutrient changes may result in cell cycle inhibition, proliferation arrest, and/or apoptosis [28]. We observed distinctly higher expression of multiple genes related to the AP-1 transcription factor complex (i.e., FOSB, JUNB, JUND) in all cells cultured on Lactate Ringers compared to control MSCs in standard culture media. Expression is proportional with increasing temperature and similar under all experimental conditions (Fig. 5 A, B, C). These data show that nutrition deprived cells exposed to physiological temperatures undergo significant cell stress, potentially leading to cell death.
Figure 6:
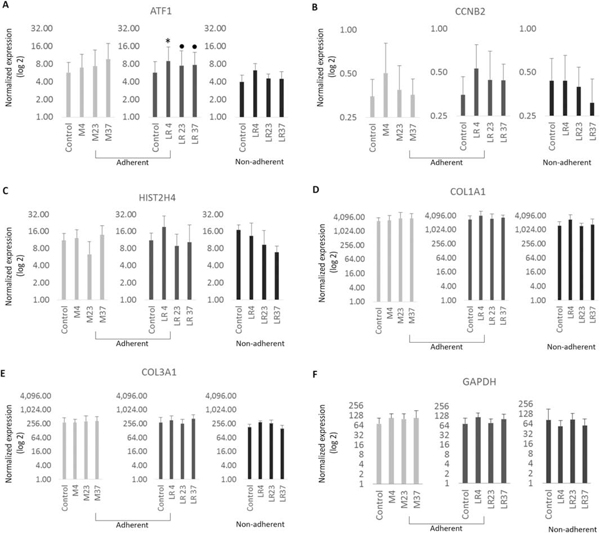
Effects of temperature changes on mRNA expression of MSCs
The graphs show mRNA expression levels immediately after exposure to temperatures for cyto-protective response genes: constitutive response (A), as well proliferation genes (B, C) and ECM (D, E). The data represent mean values from biological triplicates of three cell lines.
*p<.05, □ p<. 01, ●p<.0001, One-way ANOVA with Tukey’s post-hoc test, n=9 for each cell line
Figure 7:
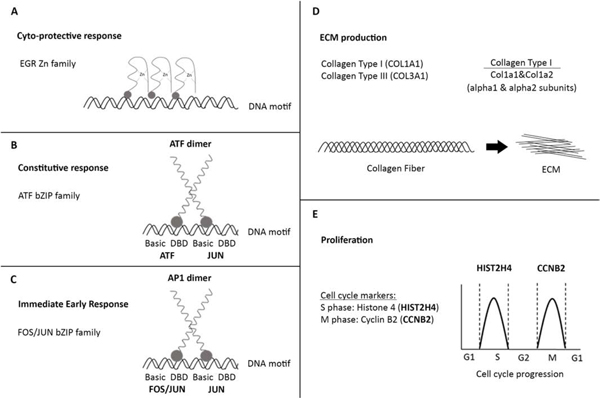
Diagram of PCR biomarkers
The diagram shows graphical representation of biomarkers for cyto-protective genes (A, B, C), for ECM markers (D) and proliferation markers (E). EGR gene family encodes for TF that bind as zinc finger proteins to DNA. ATF dimer is formed by 2 ATF or ATF and JUN proteins that belong to bZIP family of TF. Genes FOSB and/or JUNB and/or JUND together form an AP1 dimer (activator protein 1) which serves as a TF that regulates gene expression by binding to DNA by bZIP domain. COL1A1 gene encodes for alfa 1 subunit of collagen 1 and together with alfa 2 subunit (COL1A2 gene) they form procollagen 1 molecule. A collagen fiber is made from triple-stranded pro-collagen molecules, multiple fibers form the ECM. HIST2H4 is S phase marker (DNA replication) of the cell cycle and CCNB2 is M phase (mitosis) marker.
ECM=Extracellular matrix, DBD=DNA-binding domain, bZIP (leucine zipper), TF=transcription factor
Markers linked to proliferation and cell cycle progression were also evaluated by mRNA expression analysis. For these studies, we monitored the mRNA levels of S-phase related gene HIST2H4, which encodes the nucleosomal protein histone H4 that packages newly replicated DNA, and the mitosis-specific cyclin Cyclin B (CCNB2), which together with the cyclin dependent kinase CDK1 controls cell division (Fig.6 B,C; 7 E). Both HIST2H4 and CCNB2 exhibited similar patterns of expression in all experimental conditions, although we noted modest changes in the expression of the two genes at 4°C, but these changes did not reach statistical significance. Thus, a temporary temperature change during 4hrs of incubation without nutrition has no appreciable effects on cell proliferation.
Recruitment of MSCs into damaged tissues may modulate extracellular matrix production (ECM) during deposition of fibrotic tissues that provide stress shielding during tissue repair. To evaluate the ability of MSCs to produce extracellular matrix proteins (ECM) following temperature changes, we assessed mRNA expression of COL1A1 and COL3A1 (Fig.6 D, E, 7E). Expression of both COL1A1 and COL3A1 appeared to be moderately increased at 4°C compared to control cells, although again these results did not reach statistically significance. Therefore, we conclude that the ability of MSCs to produce a collagenous ECM is not altered by changes in temperature.
Discussion
Cell therapies in musculoskeletal regenerative medicine employ a wide variety of stem cells. Pluripotent embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSC) may have significant regenerative potential through their ability to generate any tissue cell type (including somatic and germ line cells) however these stem cell types are either encumbered by ethical controversies (e.g., ESCs) or incomplete re-programming (e.g., iPSCs). Somatic multipotent stem/stromal cells such as MSCs have more realistic clinical potential in therapeutic applications, because they are easily harvested from the vascular stroma of bone marrow, adipose tissue or umbilical cord tissue. Adipose-derived MSCs cells are preferentially used since the isolation procedure is the least invasive and relatively painless [29]. Another advantage is their rapid in vitro expansion which reduces risk of their clonal chromosomal and phenotypic changes. These cells represent normal diploid human cells that cease proliferation due to contact inhibition [27]. More importantly, adipose-derived MSCs are cultured in human platelet lysate and their zoonotic free production as high quality GMP-compliant MSCs permits direct clinical applications [23].
The success of cell-based therapies relies in part on certification of clinical grade MSCs that conform to standardized MSC release criteria. However, MSCs are characterized under optimal cell culture conditions and this biological status does not necessarily resemble the status of cells delivered at the point of care. Standard production of MSCs occurs under nutrient-rich, adherent and static conditions that mimic a normal physiological environment at body temperature (37°C). However, when MSCs are delivered during therapy, a number of non-physiological events may compromise the dosing and/or biological activity of MSCs. Potentially adverse steps in the logistic chain of stem cell delivery include (i) hypothermic temperature (4°C) due to storage at on ice, (ii) nutrient deprivation and loss of cell adherence due to suspension of MSCs in Lactate Ringers solution, (iii) potential cytotoxicity of local anesthetics, preservatives and contrast agents during injection, (iv) hypoxia due to storage in syringes, (v) pressure changes due to needle passage, and (iv) environmental interactions with synovial fluid and joint tissues. This study examined the effects of modulating storage temperature on MSC metabolic activity, viability and gene expression of cytoprotective, proliferation and ECM related genes.
MSCs frequently encounter major changes in temperatures. Beyond rapid freezing in storage medium for long-term storage which may alter MSC viability [16], MSCs are stored on ice for short term storage on the day of the injection. However, storage on ice may induce hypothermia and alter the biological state of the cells in a manner that reduces their effective dosing. There is a robust evidence that hypothermia (<35°C) in general may preserve cells and tissues during hypoxia by decreasing metabolic requirements of cells [30]. Preservation of cells may be facilitated by cell cycle arrest, because hypothermia blocks cell cycle progression ([28, 31, 32]). Hypothermia is widely used for neuroprotection [33] such as for prevention of hypoxic ischemic encephalopathy in newborns [34] or brain damage caused by injury or stroke [35], preservation of transplanted organs [36] and during cardiopulmonary resuscitation [30]. While cellular changes caused by hyperthermia are well understood, our knowledge of the effects of sub-physiological temperatures on cells is limited and detailed mechanisms are not well understood [32]. Cells exposed to certain temperatures or temperatures exhibit a range of molecular changes, including oxidative stress [37, 38], differences in dissolved oxygen [32], as well as the emergence of heat-sensitive factors like heat shock proteins [39] or cold shock proteins [32, 40], that reflect significant cell stress and may induce apoptosis. MSCs exposed to hypothermia experience a major exposure to oxidative stress due to the production of reactive oxygen species (ROS) [37]. This overproduction of ROS may eventually lead to cell apoptosis if it surpasses certain threshold levels [41].
In this study, we evaluated both mild (23°C) and severe (4°C) hypothermia in either nutrient-rich standard culture media or nutrient-deficient Lactated Ringers solution. Our data show that metabolic activity and cell number of MSCs is reduced, and a cellular stress response as measured by mRNA biomarkers is increased, when cells are maintained in nutrient-deficient Lactated Ringers at physiological temperature (37°C). Likewise, metabolic activity and cell number of MSCs decrease, and molecular stress response increase, when cells are maintained in mild or severe hypothermic temperatures (4°C and 23°C). In contrast to these findings, we observed that the detrimental effects of low nutrition were less pronounced in mild hypothermia (23°C).
Our results are consistent with a conceptual model that considers the opposing effects of temperature and nutrient deprivation on the metabolic activity of mitochondria that is linked to oxidative stress responses. First, molecular and oxidative stress is expected to be minimal under normal physiological temperature and nutrient conditions when mitochondria function optimally. However, stress responses are gradually activated as MSCs become severely hypothermic and the normal metabolic functions of mitochondria may be compromised (at 4°C). Molecular and oxidative stress responses due to nutrient deprivation are expected to be most severe at 37°C when mitochondria are primed for optimal metabolic activity but lack essential ingredients (e.g., glucose). Yet, nutritional stress is less apparent when mitochondrial activity declines due to hypothermia. Consequently, maintenance of nutrient deprived MSCs at ambient temperature (23°C) may represent a biological optimum for cell survival perhaps because mild hypothermia reduces mitochondrial stress linked to nutrient deprivation (Figure 3C).
In hypothermia, as the metabolic activity decreases, the progression of cell cycle and proliferation becomes inhibited. Underhill and colleagues showed that cooling of HeLa cervical carcinoma cells for 24 hrs reduces proliferation by 25% at 32°C and 50% at 10°C [42]. Both mild and severe hypothermia can synchronize cells in a resting phase, because hypothermia prolongs the G1 phase and arrests the cell cycle in the G2 phase [31]. Release of such growth arrested synchronized cells by re-incubating cells at 37°C results in abundant mitotic activity [31]. Our experimentation examined differences in metabolic activity and cell count both immediately after cold exposure and after a 24 hr recovery period at 37°C. We observed increase in cell number upon recovering cells at normal physiological temperature which may be possibly explained by increased mitosis. However, it is plausible that the differences in the ability to provoke a hypothermia-induced cell cycle arrest may be related to differences in cell types [31], especially because HeLa cells are highly robust cancer cells, while MSCs are diploid cells with more normal cell growth characteristics.
Conclusion
Our study shows that the metabolic activity and cell counts of MSCs are both severely decreased after incubation in nutrient-deficient Lactated Ringers solution, or upon reduction of ambient temperatures to generate mild (23°C) or severe (4°C) hypothermic conditions. The most optimal condition for MSCs in Lactated Ringers appears to be under mild hypothermia at ambient temperature, because this condition minimizes loss of cells and overall metabolic activity. Clinical grade MSCs are currently delivered for therapeutic use in syringes on ice, a condition that based on our results is not optimal for their delivery. Therefore, our laboratory results suggest that this standard method for therapeutic delivery of MSCs perhaps should be reconsidered to reduce temperature changes and transfer time from GMP production to delivery in the patient.
Acknowledgments
We thank the members of our research group, including David Deyle, Roman Thaler, Joselin S. Jerez Ortega, Catalina Galeano-Garces and Daniela Galeano-Garces for general support of this project and stimulating discussions. This work was supported in part by National Institutes of Health grants R01 AR049069 (AJvW). We also appreciate the generous philanthropic support of William H. and Karen J. Eby, and the charitable foundation in their names.
Abbreviations:
- C
Celsius
- CDK1
Cyclin-Dependent Kinase
- CO2
Carbon Dioxide
- DNA
Deoxyribonucleic Acid
- ECM
Extracellular Matrix Proteins
- EEF1A1
Elongation Factor 1-Alpha 1
- ESCs
Embryonic Stem Cells
- g/mL
Gram Per Milliiter
- GAPDH
Glyceraldehyde 3-Phosphate Dehydrogenase
- GMP
Good Manufacturing Practice
- iPSCs
Induced Pluripotent Stem Cells
- IRB
Institutional Review Board
- LR
Lactated Ringers
- mRNA
Messenger RNA
- MSC
Mesenchymal Stem Cells
- nm
Nanomolar
- PBS
Phosphate-Buffered Saline
- RNA
Ribonucleic Acid
- U/mL
Units Per Millilitre
- μl
Microliter
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
☐ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Eva Kubrova, Wenchun Qu, M. Lizeth Galvan, Christopher R. Paradise, Juan Yang, Allan B. Dietz, Amel Dudakovic, Jay Smith, and Andre J. van Wijnen2
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Litwic A, et al. , Epidemiology and burden of osteoarthritis. Br Med Bull, 2013. 105: p. 185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riester SM, et al. , RNA sequencing identifies gene regulatory networks controlling extracellular matrix synthesis in intervertebral disk tissues. J Orthop Res, 2018. 36(5): p. 1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y, et al. , RNA-seq analysis of clinical-grade osteochondral allografts reveals activation of early response genes. J Orthop Res, 2016. 34(11): p. 1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewallen EA, et al. , The synovial microenvironment of osteoarthritic joints alters RNA-seq expression profiles of human primary articular chondrocytes. Gene, 2016. 591(2): p. 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riester SM, et al. , Safety Studies for Use of Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells in a Rabbit Model for Osteoarthritis to Support a Phase I Clinical Trial. Stem Cells Transl Med, 2017. 6(3): p. 910–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, et al. , Efficacy of intervertebral disc regeneration with stem cells - a systematic review and meta-analysis of animal controlled trials. Gene, 2015. 564(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 7.Gou S, et al. , Stem cell therapy for intervertebral disk regeneration. Am J Phys Med Rehabil, 2014. 93(11 Suppl 3): p. S122–31. [DOI] [PubMed] [Google Scholar]
- 8.Dietz AB, Padley DJ, and Gastineau DA, Infrastructure development for human cell therapy translation. Clin Pharmacol Ther, 2007. 82(3): p. 320–4. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI and Correa D, The MSC: an injury drugstore. Cell Stem Cell, 2011. 9(1): p. 11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y, et al. , Trophic Effects of Mesenchymal Stem Cells in Tissue Regeneration. Tissue Eng Part B Rev, 2017. 23(6): p. 515–528. [DOI] [PubMed] [Google Scholar]
- 11.Caplan AI and Dennis JE, Mesenchymal stem cells as trophic mediators. J Cell Biochem, 2006. 98(5): p. 1076–84. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Olmo D, et al. , Expanded Adipose-Derived Stem Cells for the Treatment of Complex Perianal Fistula: a Phase II Clinical Trial. Diseases of the Colon & Rectum, 2009. 52(1): p. 79–86. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez O, et al. , Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One, 2018. 13(5): p. e0195891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar H, et al. , Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res Ther, 2017. 8(1): p. 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arjmand B, et al. , Concomitant Transurethral and Transvaginal-Periurethral Injection of Autologous Adipose Derived Stem Cells for Treatment of Female Stress Urinary Incontinence: A Phase One Clinical Trial. Acta Med Iran, 2017. 55(6): p. 368–374. [PubMed] [Google Scholar]
- 16.Pollock K, et al. , Improved Post-Thaw Function and Epigenetic Changes in Mesenchymal Stromal Cells Cryopreserved Using Multicomponent Osmolyte Solutions. Stem Cells Dev, 2017. 26(11): p. 828–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu T, et al. , Cytotoxic Effects of Nonionic Iodinated Contrast Agent on Human Adipose-Derived Mesenchymal Stem Cells. Pm r, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galeano-Garces C, et al. , Molecular Validation of Chondrogenic Differentiation and Hypoxia Responsiveness of Platelet-Lysate Expanded Adipose Tissue-Derived Human Mesenchymal Stromal Cells. Cartilage, 2017. 8(3): p. 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onishi K, et al. , Human Adipose-Derived Mesenchymal Stromal/Stem Cells Remain Viable and Metabolically Active Following Needle Passage. Pm r, 2016. 8(9): p. 844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y, et al. , Extracellular matrix protein production in human adipose-derived mesenchymal stem cells on three-dimensional polycaprolactone (PCL) scaffolds responds to GDF5 or FGF2. Gene Rep, 2018. 10: p. 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewallen EA, et al. , Osteogenic potential of human adipose-tissue-derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium. Gene, 2016. 581(2): p. 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu T, et al. , Cytotoxicity of Local Anesthetics in Mesenchymal Stem Cells. Am J Phys Med Rehabil, 2018. 97(1): p. 50–55. [DOI] [PubMed] [Google Scholar]
- 23.Crespo-Diaz R, et al. , Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant, 2011. 20(6): p. 797–811. [DOI] [PubMed] [Google Scholar]
- 24.Mader EK, et al. , Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med, 2013. 11: p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudakovic A, et al. , Histone deacetylase inhibition destabilizes the multi-potent state of uncommitted adipose-derived mesenchymal stromal cells. J Cell Physiol, 2015. 230(1): p. 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camilleri ET, et al. , Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res Ther, 2016. 7(1): p. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudakovic A, et al. , High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem, 2014. 115(10): p. 1816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neutelings T, et al. , Effects of mild cold shock (25 degrees C) followed by warming up at 37 degrees C on the cellular stress response. PLoS One, 2013. 8(7): p. e69687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabol RA, et al. , Therapeutic Potential of Adipose Stem Cells. Adv Exp Med Biol, 2018. [DOI] [PubMed] [Google Scholar]
- 30.Polderman KH, Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: Indications and evidence. Intensive Care Med, 2004. 30(4): p. 556–75. [DOI] [PubMed] [Google Scholar]
- 31.Rieder CL and Cole RW, Cold-shock and the Mammalian cell cycle. Cell Cycle, 2002. 1(3): p. 169–75. [PubMed] [Google Scholar]
- 32.Al-Fageeh MB and Smales CM, Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J, 2006. 397(2): p. 247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drury PP, et al. , Mechanisms of hypothermic neuroprotection. Clin Perinatol, 2014. 41(1): p. 161–75. [DOI] [PubMed] [Google Scholar]
- 34.McAdams RM and Juul SE, Neonatal Encephalopathy: Update on Therapeutic Hypothermia and Other Novel Therapeutics. Clin Perinatol, 2016. 43(3): p. 485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Worp HB, et al. , Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain, 2007. 130(Pt 12): p. 3063–74. [DOI] [PubMed] [Google Scholar]
- 36.Belzer FO and Southard JH, Principles of solid-organ preservation by cold storage. Transplantation, 1988. 45(4): p. 673–6. [DOI] [PubMed] [Google Scholar]
- 37.Blagojevic DP, Grubor-Lajsic GN, and Spasic MB, Cold defence responses: the role of oxidative stress. Front Biosci (Schol Ed), 2011. 3: p. 416–27. [DOI] [PubMed] [Google Scholar]
- 38.Rauen U, et al. , Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species. Faseb j, 1999. 13(1): p. 155–68. [DOI] [PubMed] [Google Scholar]
- 39.Garrido C, et al. , Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun, 2001. 286(3): p. 433–42. [DOI] [PubMed] [Google Scholar]
- 40.Fujita J, Cold shock response in mammalian cells. J Mol Microbiol Biotechnol, 1999. 1(2): p. 243–55. [PubMed] [Google Scholar]
- 41.Redza-Dutordoir M. and Averill-Bates DA, Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta, 2016. 1863(12): p. 2977–2992. [DOI] [PubMed] [Google Scholar]
- 42.Underhill MF and Smales CM, The cold-shock response in mammalian cells: investigating the HeLa cell cold-shock proteome. Cytotechnology, 2007. 53(1–3): p. 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


