Abstract
Objective: Mcl-1 overexpression confers acquired resistance to Bcl-2 inhibitors in non-small cell lung cancer (NSCLC), but no direct Mcl-1 inhibitor is currently available for clinical use. Thus, novel therapeutic strategies are urgently needed to target Mcl-1 and sensitize the anti-NSCLC activity of Bcl-2 inhibitors.
Methods: Cell proliferation was measured using sulforhodamine B and colony formation assays, and apoptosis was detected with Annexin V-FITC staining. Gene expression was manipulated using siRNAs and plasmids. Real-time PCR and Western blot were used to measure mRNA and protein levels. Immunoprecipitation and immunofluorescence were used to analyze co-localization of dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A) and Mcl-1.
Results: Suppression of DYRK1A resulted in reduced Mcl-1 expression in NSCLC cells, whereas overexpression of DYRK1A significantly increased Mcl-1 expression. Suppression of DYRK1A did not alter Mcl-1 mRNA levels, but did result in an accelerated degradation of Mcl-1 protein in NSCLC cells. Furthermore, DYRK1A mediated proteasome-dependent degradation of Mcl-1 in NSCLC cells, and DYRK1A co-localized with Mcl-1 in NSCLC cells and was co-expressed with Mcl-1 in tumor samples from lung cancer patients, suggesting that Mcl-1 may be a novel DYRK1A substrate. We showed that combined therapy with harmine and Bcl-2 antagonists significantly inhibited cell proliferation and induced apoptosis in NSCLC cell lines as well as primary NSCLC cells.
Conclusions: Mcl-1 is a novel DYRK1A substrate, and the role of DYRK1A in promoting Mcl-1 stability makes it an attractive target for decreasing Bcl-2 inhibitor resistance.
Keywords: DYRK1A, Mcl-1, NSCLC, combination, Bcl-2 inhibitor
Introduction
The B-cell lymphoma-2 (Bcl-2) family of proteins are characterized by four homology domains, BH1-BH4, and are subdivided into two major groups. The first of these is the anti-apoptotic subgroup, which includes Bcl-2 and Mcl-1. The second is the proapoptotic subgroup, which includes Bax-like molecules (such as Bax and Bak) that contain the BH1-3 domains, as well as BH3-only proteins (such as Bid and Bad)1. When BH3-only proteins are activated, their amphipathic α-helical BH3 domain inserts into a hydrophobic groove on Bcl-2 anti-apoptotic proteins, subsequently initiating apoptosis2. Small molecule Bcl-2 inhibitors are promising for the treatment of hematological malignancies in addition to other cancers3. ABT-737 (Navitoclax), the first BH3 mimetic, has shown promising effects in clinical trials for patients with relapsed chronic lymphocytic leukemia (CLL)4. Consequently, ABT-199 (Venetoclax) became the first BH3 mimetic approved for cancer treatment by the Food and Drug Administration (FDA) on April 11, 20165. ABT-199 is an oral Bcl-2 specific inhibitor and is approved to treat CLL patients with the 17p deletion who have received at least one prior therapy6,7. Because Bcl-2 inhibitors bind to Mcl-1 with low affinity, Mcl-1 overexpression confers acquired resistance to Bcl-2 inhibitors in multiple cancer types, including non-small cell lung cancer (NSCLC) and acute myeloid leukemia8,9. NSCLC is the most common malignancy worldwide and accounts for ~85% of all lung cancers, with small cell lung cancer comprising ∼15%10. In addition, Mcl-1 is a novel biomarker of poor prognoses for NSCLC patients11. However, there is no direct Mcl-1 inhibitor currently available in clinical use. Thus, novel therapeutic strategies are urgently needed to target Mcl-1 and sensitize the anti-cancer activities of Bcl-2 inhibitors for effective treatment of NSCLC.
DYRK1A is located on the critical region of Down syndrome (DS) chromosome 21, and its overexpression in DS patients contributes to cognitive impairments12. In addition, DYRK1A is thought to be involved in neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease13. DYRK1A is also overexpressed in multiple human malignancies, including hematological and brain cancers, where it contributes to tumor growth by manipulating cell cycle progression14–16. Thus, its role in multiple human diseases makes DYRK1A an attractive target for therapeutic drugs17. Harmine, a β-carboline alkaloid, is a selective ATP-competitive inhibitor of DYRK1A, which acts by binding to the ATP-binding pocket of DYRK1A18. Inhibition of DYRK1A with harmine reverses resistance to multiple anti-cancer drugs including mitoxantrone, camptothecin, and AZD929119,20.
DYRK1A is a serine/threonine kinase that regulates diverse pathways involved in cell differentiation and apoptosis, gene transcription and splicing, and the cell cycle21. DYRK1A phosphorylates NFATc1, p27, EGFR, and c-myc at serine or threonine residues22. Here, we showed that Mcl-1 was a novel DYRK1A substrate, and DYRK1A suppression sensitized NSCLC cells to Bcl-2 inhibitors.
Materials and methods
Materials
DMEM/F-12, RPMI-1640, and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, NY, USA). Harmine was provided by MedChemExpress (HY-N0737A; Monmouth Junction, NJ, USA). ABT-737 (S1002) and ABT-199 (S8048) were provided by Selleck Chemicals (Houston, TX, USA). The Annexin V-FITC apoptosis kit was provided by Becton Dickinson (556547; Franklin Lakes, NJ, USA). The primary antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was provided by Santa Cruz Biotechnology (sc-32233; Santa Cruz, CA, USA). The primary antibodies against Bcl-2 (ab32124) and Bcl-xL (ab32370) were provided by Abcam (Cambridge, MA, USA). The primary antibodies against Mcl-1 (94296), α/β-Tubulin (2148S), caspase-3 (9662S), phospho-Mcl-1 (Ser159/Thr163; 4579S), DYRK1A (2771S), and cleaved-PARP (9541S) were provided by Cell Signaling Technology (Danvers, MA, USA). The horseradish peroxidase-labeled secondary anti-mouse (AS003) and anti-rabbit (AS014) antibodies were provided by ABclonal Technology (Woburn, MA, USA). The primary antibodies were diluted with Primary Antibody Dilution Buffer (P0023A; Beyotime, Shanghai, China) at a ratio of 1:1,000.
Isolation of primary tumor cells from NSCLC patients
Tumor samples were collected from NSCLC patients and informed consent was obtained from all participants. All patient-related research was approved by the Clinical Ethics Committee of Hangzhou First People’s Hospital before study initiation (Approval No. 2016/21-01)20. The research was conducted according to the World Medical Association Declaration of Helsinki.
Cell culture
Human lung carcinoma cell lines (A549, NCI-H1299, and NCI-H460) were provided by Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). A549, NCI-H460, and NCI-H1299 cells were cultured in RPMI-1640 media with 10% FBS, and primary NSCLC cells were cultured in DMEM/F-12 with 10% FBS. The cells were maintained in humidified conditions (37 °C, 5% CO2).
The sulforhodamine B (SRB) assay
Cell viability was measured using the SRB assay. Cells (5 × 103) were seeded on 96-well plates and treated with compounds for 72 h. The cells were fixed with 10% (w/v) trichloroacetic acid overnight, washed 5 times with deionized water, and dried at 60 °C. After the plates were entirely dry, cells were stained using 100 μL 0.4% SRB solution for 30 min, washed using 1% (v/v) acetic acid, and dried at 60 °C. Protein-bound dye was dissolved with a Tris-based solution and cell viability was measured at 540 nm using a multi-scan spectrum.
Colony formation assay
NSCLC cells were cultured on 6-well plates (1,000 cells per well) overnight, incubated with the compounds for 14 days, fixed with 4% paraformaldehyde for 15 min, and stained using Giemsa solution for another 15 min. Colonies were photographed using ChemiDoc XPS (Bio-Rad, Hercules, CA, USA).
Apoptosis assay
Cells were collected, washed with phosphate-buffered saline (PBS), stained using the Annexin V-FITC Apoptosis Kit (BD Biosciences, Franklin Lakes, NJ, USA), and detected using flow cytometry. Briefly, 1 × 105 cells were harvested and resuspend in 200 μL of Annexin V binding buffer containing 5 μL of Annexin V-FITC for 15 min at room temp in the dark. And 5 μL of 0.5 mg/mL propidium iodide solution was added before flow cytometer detection.
Silencing of gene expression with small interfering RNA (siRNA)
The cells were seeded on 6-well plates, cultured overnight, and transfected the next day with DYRK1A siRNA using Oligofectamine (Invitrogen, Waltham, MA, USA). Twenty nM siRNA/well was used in a 6-well plate with 100 μL jetPRIME buffer and 2 μL jetPRIME. After 48 h of incubation, the cells were harvested for further analysis. The efficiency of transfection was assessed by Western blot. DYRK1A siRNA was obtained from GenePharma (Shanghai, China), and the sense sequences were as follows: siDYRK1A-1: 5′-AUGGAGCUAUGGACGUUAATT-3′, siDYRK1A-2: 5′-AAACUCGAAUUCAACCUUATT-3′, negative control: 5′-UUCUCCGAACGUGUCACGUTT-3′.
Real-time reverse transcription PCR
Total cellular RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA), precipitated with isopropyl alcohol, and rinsed with 70% ethanol. Single stranded cDNA was generated using oligo (dT) priming, followed by SYBR Green-based real-time reverse transcription PCR (Bio-Rad Laboratories, Hercules, CA, USA). After the primers were received, they were centrifuged and then diluted to 10 μM as stock solutions. SYBR Green qPCR Master Mix Kit was used to establish a 10 μL reaction system [cDNA: 1.0 μL, primer-forward: 1.0 μL, primer-reverse: 1.0 μL, ddH20: 1.8 μL, ROX Reference Dye (50×): 0.2 μL, SYBR Green (2×): 5 μL]. Amplification conditions were 95 °C for 15 min, followed by 40 thermal cycles of 15 s at 95 °C, 30 s at 58 °C, and 30 s at 72 °C. A standard curve-based method was used to measure the expression of the indicated mRNA. The primers used in the present study were as follows: DYRK1A, 5′-TCTGGGTATTCCACCTGCTC-3′ (forward), 5′-GTCCTCCTGTTTCCACTCCA-3′ (reverse); Mcl-1, 5′-GGGCAGGATTGTGACTCTCATT-3′ (forward), 5′-GATGCAGCTTTCTTGGTTTATGG-3′ (reverse); GAPDH, 5′-GAGTCAACGGATTTGGTCGT-3′ (forward), 5′-TTGATTTTGGAGGGATCTCG-3′ (reverse).
Plasmid transfection
NSCLC cells were cultured on 6-well plates overnight, and transfection was performed the next day using jetPRIME (Polyplus, Illkirck, France) according to the manufacturer’s instructions. Briefly, 1 μg plasmid was diluted into 100 μL jetPRIME® buffer. Then, 2 μL jetPRIME® was added, vortexed, and incubated for 10 min at room temperature. Transfection mix (100 μL/well) was added to the cells.
Western blot analysis
Cells were lysed using RIPA Buffer (Thermo Fisher Scientific, Waltham, MA, USA). Total protein (20 μg) was subjected to 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membranes were blocked and then incubated with primary and secondary antibodies. Protein expression was analyzed using an enhanced chemiluminescence (ECL, Abbkine, Wuhan, China) system.
Immunoprecipitation
Cell lysates were centrifuged at 10,000 × g for 30 min at 4 °C, incubated with primary antibody using a slow rotation for 4 h, and then incubated with protein G magnetic beads for 1 h at 4 °C. The beads were washed a minimum of five times, mixed with loading buffer, heated to 95 °C for 5 min, followed by Western blot analysis.
Immunofluorescence
Cells were seeded into 96-well plates and cultured overnight. The next day, the cells were fixed with 4% paraformaldehyde for 30 min at room temperature, washed with PBS, and permeabilized with 200 μL of 0.3% Triton X-100 in PBS for 30 min. Cells were washed again with PBS, blocked with 1% bovine serum albumin in PBS for 30 min, incubated with primary antibodies at 4 °C overnight, washed three times with PBS, and incubated with fluorescent dye-conjugated secondary antibodies for 1 h in the dark. Nuclei were stained with 4′,6-diamidino-2-phenylindole for 5 min in the dark. Cells were visualized and photographed using a fluorescence microscope.
Statistical analysis
The results are expressed as the mean ± SD. The data presented were obtained at least three times. The synergistic effects of harmine plus ABT-199/ABT-737 were quantitatively determined by calculating the combination index (CI) values using Calcusyn software. A CI value < 0.9 indicated synergism; 0.9–1.1, additive; > 1.1, antagonism. A two-tailed Student’s t-test was used to compare the difference between two groups. A value of P < 0.05 was accepted as statistically significant, and the levels of significance were indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
DYRK1A upregulates Mcl-1 expression in NSCLC cells
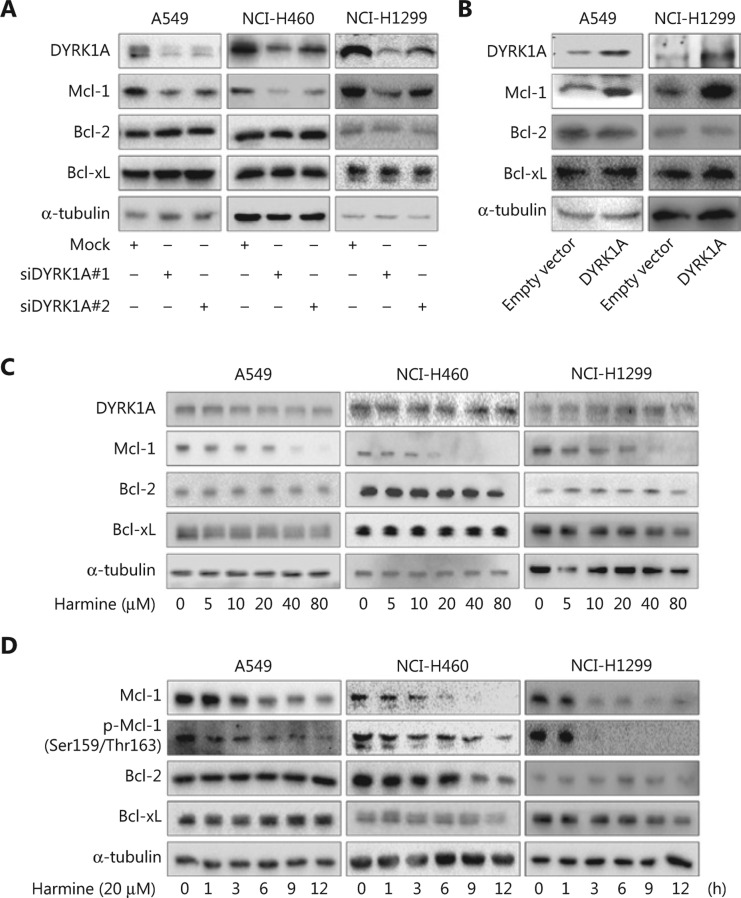
DYRK1A expression was knocked down in NSCLC cell lines using siRNA, and DYRK1A knockdown resulted in decreased Mcl-1 expression in NSCLC cells (Figure 1A). In contrast, overexpression of DYRK1A in NSCLC cells significantly increased Mcl-1expression (Figure 1B). Expression of other Bcl-2 family members, such as Bcl-2 and Bcl-xL, was not altered with DYRK1A knockdown or overexpression in NSCLC cells (Figure 1A and 1B). Treatment of NSCLC cells with harmine, a DYRK1A inhibitor, resulted in a dose- and time-dependent inhibition of Mcl-1 expression (Figure 1C and 1D). However, Bcl-2 and Bcl-xl expression was not changed when DYRK1A was inhibited with harmine in NSCLC cells (Figure 1C and 1D). These data suggested that DYRK1A upregulated Mcl-1 expression in NSCLC cells.
Figure 1.
DYRK1A regulates the expression of Mcl-1 in NSCLC cells. (A) NSCLC cells were transfected with control siRNA and DYRK1A siRNA for 48 h, and the expression of DYRK1A and Bcl-2 family members were detected by Western blot. (B) NSCLC cells were transfected with empty vector or DYRK1A plasmid for 48 h, and the expression of DYRK1A and Bcl-2 family members were detected by Western blot. (C) NSCLC cells were treated with harmine at the indicated concentrations for 24 h, and the expression of the indicated proteins were detected by Western blot. (D) NSCLC cells were treated with 20 μM harmine for 1, 3, 6, 9, and 12 h, and the expression of the indicated proteins was detected by Western blot.
DYRK1A promotes the stability of Mcl-1 in NSCLC cells
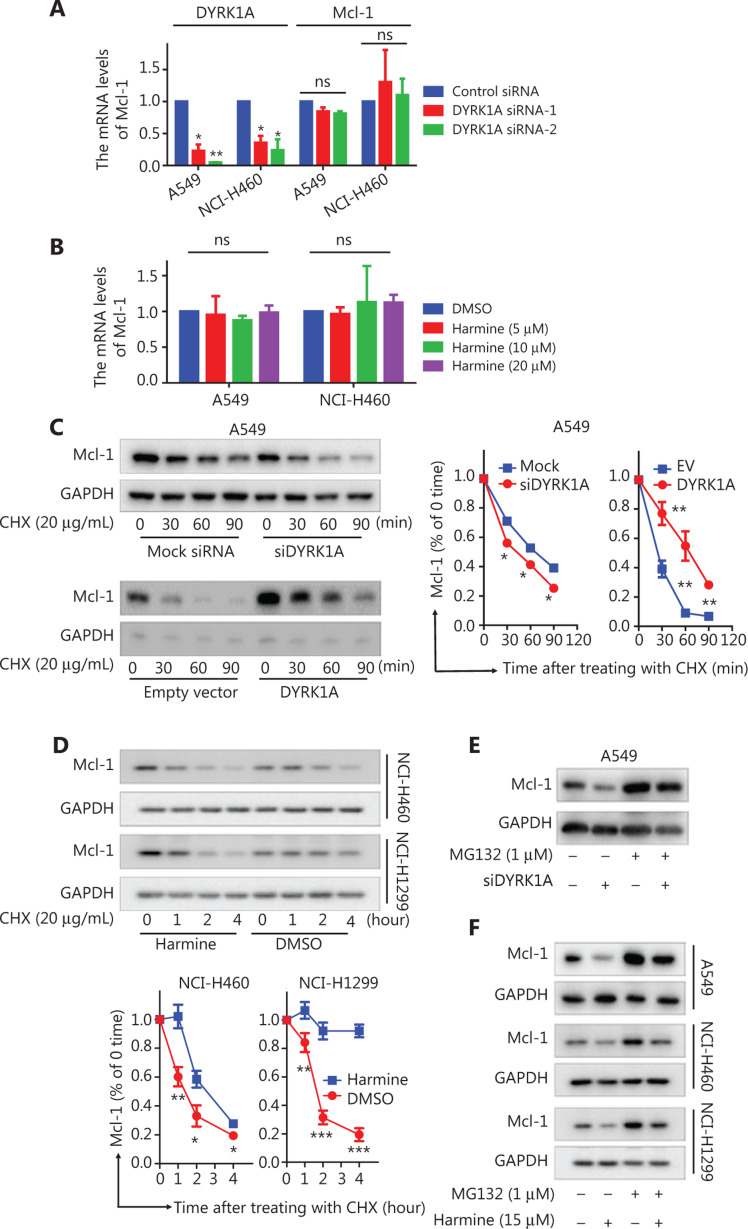
To investigate whether DYRK1A regulates Mcl-1 protein expression at the transcriptional level, Mcl-1 mRNA expression was measured in NSCLC cells after treatment with siDYRK1A or harmine. After treatment with siRNA, depletion of DYRK1A did not inhibit Mcl-1 mRNA expression (Figure 2A, the Mcl-1 mRNA levels in the DYRK1A siRNA group vs. control siRNA group, P > 0.05). Likewise, inhibition of DYRK1A with harmine did not change Mcl-1 mRNA expression (Figure 2B). Next, we hypothesized that DYRK1A might promote the degradation of Mcl-1 in NSCLC cells. Indeed, depletion of DYRK1A resulted in an accelerated degradation of Mcl-1 protein in A549 cells when new protein synthesis was blocked by cycloheximide (CHX). In contrast, overexpression of DYRK1A in NSCLC cells significantly reduced Mcl-1 protein degradation in NSCLC cells (Figure 2C, the Mcl-1 protein levels in DYRK1A overexpression group vs. control group, P < 0.01). Inhibition of DYRK1A with harmine resulted in accelerated degradation of Mcl-1 in NSCLC cells in the presence of CHX (Figure 2D). Furthermore, treatment of cells with the proteasome inhibitor, MG132, prevented the degradation of Mcl-1 that was induced by DYRK1A depletion or inhibition (Figure 2E and 2F). These data demonstrated that DYRK1A promoted stability of Mcl-1 in NSCLC cells.
Figure 2.
DYRK1A promotes the stability of Mcl-1 in NSCLC cells. (A) NSCLC cells were transfected with control siRNA and DYRK1A siRNA for 48 h, and real-time reverse transcriptase PCR analyses were used to detect the mRNA levels of DYRK1A and Mcl-1. (B) NSCLC cells were treated with harmine for 24 h, and real-time reverse transcriptase PCR analyses were used to detect the mRNA levels of Mcl-1. (C) A549 cells were transfected with siDYRK1A or DYRK1A plasmid for 48 h. Then, cells were treated with cycloheximide (CHX; 20 μg/mL) to block new protein synthesis, and the degradation of Mcl-1 was detected by Western blot. (D) NSCLC cells were treated with CHX (20 μg/mL) in the absence or presence of 15 μM harmine for 1, 2, 3, and 4 h, after which Western blot analysis was performed to measure Mcl-1 levels. (E) A549 cells were transfected with siDYRK1A or mock siRNA for 48 h, and cells were exposed to MG132 (1 μM) or dimethylsulfoxide (DMSO) for 24 h, then Western blot analysis was performed to measure Mcl-1 levels. (F) NSCLC cells were treated with MG132 (1 μM) and/or harmine (15 μM) for 24 h, then Western blot analysis was performed to measure Mcl-1 levels. Significant results are presented as ns non-significant, * P < 0.05, ** P < 0.01, or *** P < 0.001.
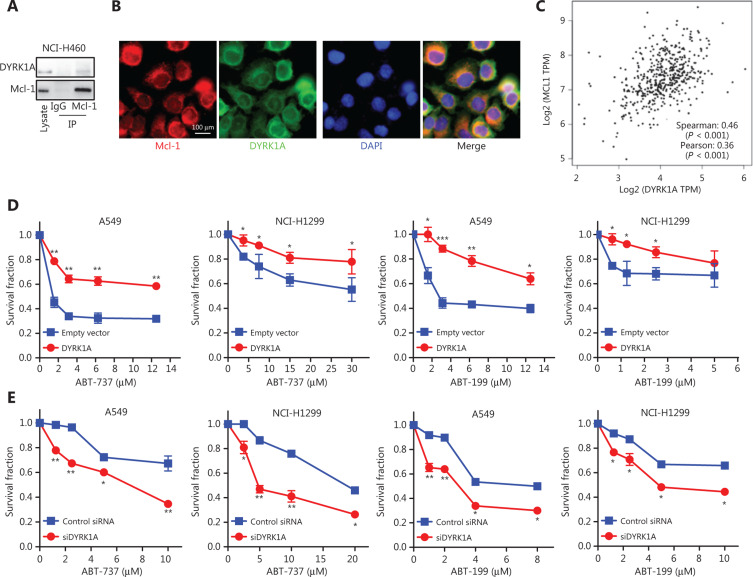
DYRK1A directly interacts with Mcl-1 in NSCLC cells and is co-expressed with Mcl-1 in tumor samples from lung cancer patients
DYRK1A regulates phosphorylation of many substrates and is involved in controlling a variety of molecular processes17. Phosphorylation of Mcl-1 at Thr-163 increases protein stability and is responsible for chemoresistance in NSCLC cells23. We found that treatment of cells with harmine significantly reduced Mcl-1 phosphorylation (Thr-163; Figure 1D). Thus, we hypothesized that DYRK1A interacted with Mcl-1 to regulate its phosphorylation. Indeed, our data suggested that DYRK1A directly interacted with Mcl-1 in A549 cells, and co-localization of DYRK1A with Mcl-1 was observed in A549 cells using a dual immunofluorescent assay (Figure 3A and 3B). Next, co-expression of DYRK1A and Mcl-1 was analyzed in NSCLC patient samples. We found that DYRK1A was co-expressed with Mcl-1 in tumor samples from lung cancer patients (Figure 3C)24. Mcl-1 overexpression confers acquired resistance to Bcl-2 inhibitors, and therefore we hypothesized that DYRK1A overexpression would also hinder the anti-cancer effects of Bcl-2 inhibitors. Overexpression of DYRK1A by transfecting DYRK1A plasmid decreased the anti-proliferative effects of Bcl-2 inhibitors in both A549 and NCI-H1299 cells (Figure 3E). In contrast, suppression of DYRK1A by transfecting with DYRK1A siRNA enhanced the anti-proliferative effects of Bcl-2 inhibitors in NSCLC cells (Figure 3F). These data suggested that overexpression of DYRK1A and Mcl-1 promoted acquired resistance to Bcl-2 inhibitors in NSCLC cells.
Figure 3.
DYRK1A directly interacts with Mcl-1 in NSCLC cells and co-expresses with Mcl-1 in tumor samples from lung cancer patients. (A) The interaction between DYRK1A and Mcl-1 was determined using immunoprecipitation in NCI-H460 cells. (B) The interaction between DYRK1A and Mcl-1 was tested using immunofluorescence in A549 cells. Scale bar: 100 μm. (C) The co-expression data were collected from GEPIA (http://gepia.cancer-pku.cn). Gene: DYRK1A and Mcl-1; Database: LUAD Tumor. (D) NSCLC cells were transfected with empty vector or DYRK1A plasmid for 24 h, seeded on 96-well plates overnight, and treated with Bcl-2 inhibitors (ABT-737 or ABT-263) at indicated concentrations for 72 h. Then, the Sulforhodamine B (SRB) assay was used to evaluate the cell viability. (E) NSCLC cells were transfected with DYRK1A siRNA or mock siRNA for 24 h, seeded on 96-well plates overnight, and treated with Bcl-2 inhibitors (ABT-737 or ABT-263) at indicated concentrations for 72 h. Then, the SRB assay was used to evaluate the cell viability. Significant results are presented as * P < 0.05, ** P < 0.01, or *** P < 0.001.
DYRK1A inhibition sensitizes NSCLC cells to Bcl-2 inhibitors
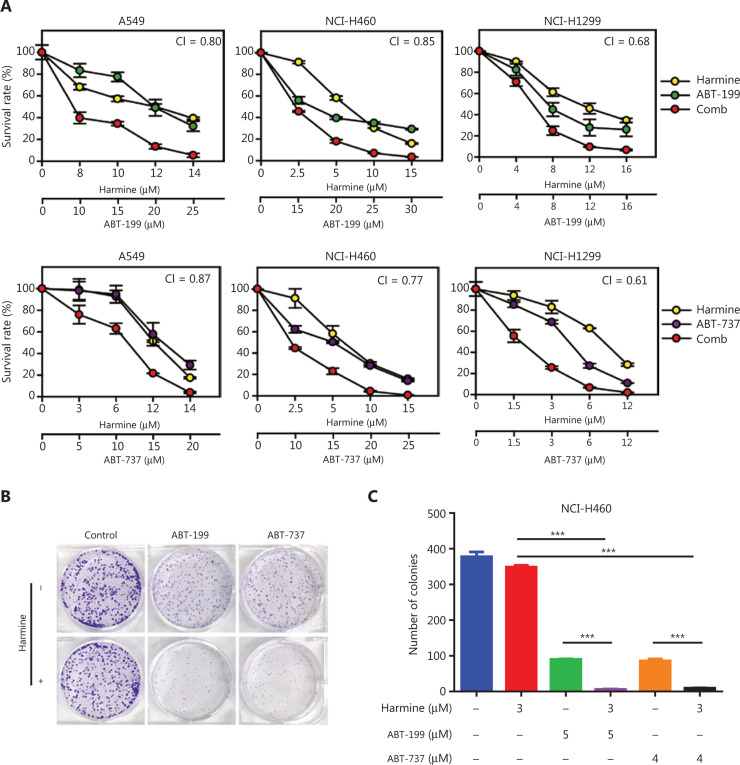
Next, we hypothesized that DYRK1A inhibition would sensitize NSCLC cells to Bcl-2 inhibitors via suppression of Mcl-1. As expected, we observed synergistic cytotoxic effects when the DYRK1A inhibitor, harmine, was combined with either ABT-737 or ABT-199 in NSCLC cells, with mean CI values below 0.9 (Figure 4A). In contrast, treatment of cells with a combination of harmine and Bcl-2 antagonists significantly suppressed NCI-H460 cell colony formation compared to treatment with harmine alone or Bcl-2 antagonists alone (Figure 4B and 4C, combination therapy vs. monotherapy, P < 0.001). Thus, combined treatment with harmine and Bcl-2 antagonists dramatically reduced NSCLC cell proliferation.
Figure 4.
DYRK1A inhibitor, harmine, enhances the anti-proliferation effects of Bcl-2 inhibitors in NSCLC cells. (A) NSCLC cells were treated with DYRK1A inhibitor harmine and/or Bcl-2 inhibitor (ABT-737 or ABT-263) at the indicated concentrations for 72 h, and then the proliferation of NSCLC cells was measured with a SRB assay. The mean combination index (CI) values are shown. (B and C) Colony formation assays were used to detect the proliferation of NCI-H460 cells. NCI-H460 cells were incubated with DYRK1A inhibitor, harmine, and/or Bcl-2 inhibitor (ABT-737 or ABT-263) at the indicated concentrations for 14 days, the cells were then stained with Giemsa solution, and the colony numbers were counted. Significant results are presented as *** P < 0.001.
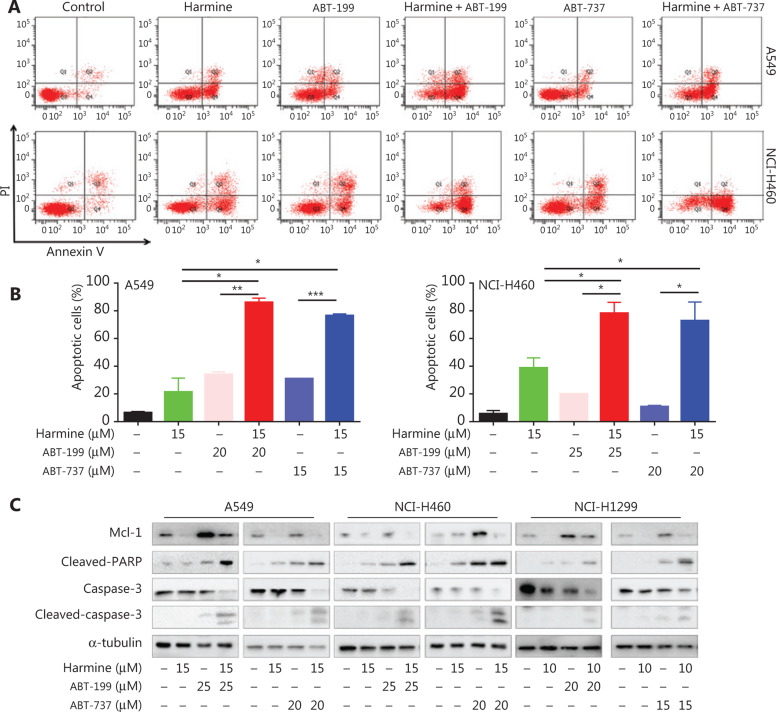
To determine whether harmine could enhance Bcl-2 antagonist-mediated apoptosis, we compared the effect of the Bcl-2 antagonist treatment with or without harmine on NSCLC cells. Harmine and Bcl-2 antagonists each elicited apoptosis as single agents, but a more profound effect was observed using combined treatment in NSCLC cells (Figure 5A and 5B, combination therapy vs. monotherapy, P < 0.05). Furthermore, PARP and caspase-3 showed greater activation with combined treatment compared to single treatment (Figure 5C). Treatment of NSCLC cells with Bcl-2 antagonist alone resulted in increased expression of Mcl-1, whereas Mcl-1 expression was markedly reduced following combined treatment with harmine and either ABT-737 or ABT-199. These data demonstrated that harmine enhanced Bcl-2 antagonist-mediated apoptosis in NSCLC cells by reducing Mcl-1 expression.
Figure 5.
DYRK1A inhibitor, harmine, enhances the apoptosis induced by Bcl-2 inhibitors in NSCLC cells. (A and B) NSCLC cells were incubated with harmine and/or Bcl-2 inhibitor at the indicated concentrations for 48 h, and then the apoptosis was measured by Annexin V-FITC staining. (C) NSCLC cells were incubated with harmine and/or Bcl-2 inhibitor at the indicated concentrations for 48 h, and then Western blot was used to detect the expression of the indicated proteins. Significant results are presented as * P < 0.05, ** P < 0.01, or *** P < 0.001.
DYRK1A inhibition sensitizes primary NSCLC cells to Bcl-2 inhibitors by reducing Mcl-1 expression
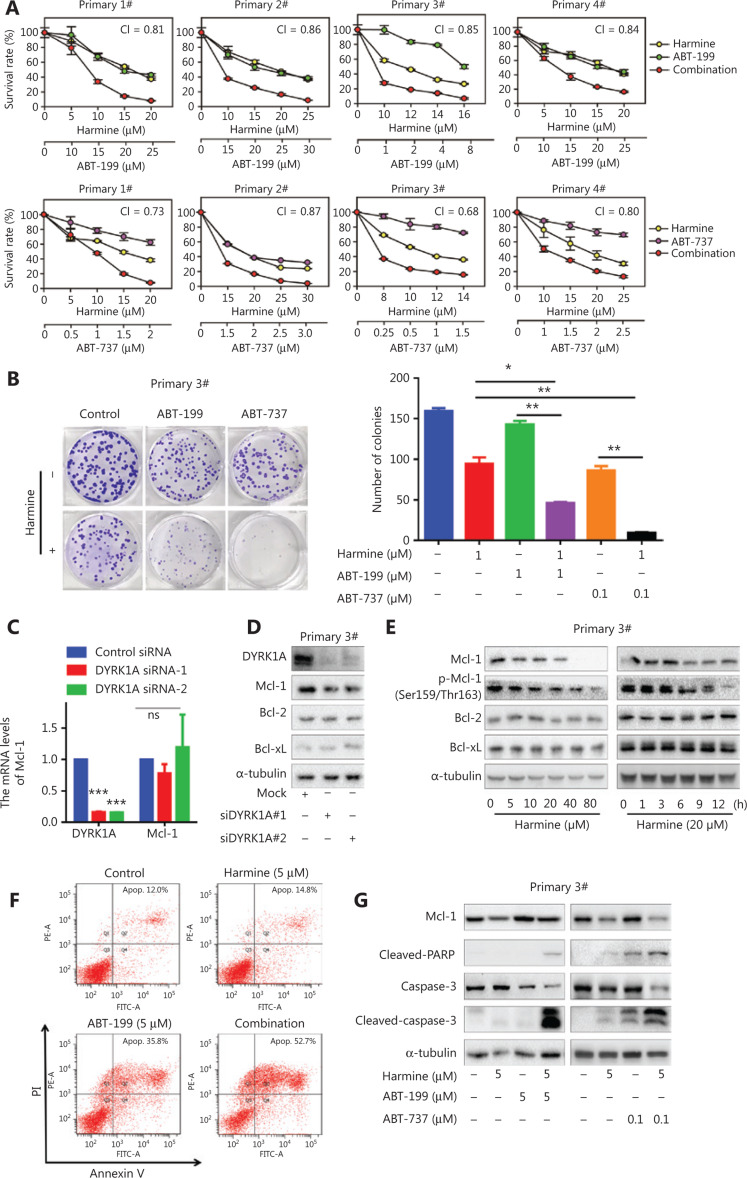
Finally, to evaluate the clinical potential of combined harmine and Bcl-2 antagonist therapy, we established four primary NSCLC cancer cell lines from lung cancer patients. Combined treatment of primary NSCLC cell lines with harmine and Bcl-2 antagonist showed synergistic, anti-proliferative effects with the CI values below 0.9 (Figure 6A). Furthermore, combined treatment with harmine and Bcl-2 antagonist significantly suppressed primary NSCLC cell colony formation when compared to treatment with single agents (Figure 6B, combination therapy vs. monotherapy, P < 0.05). Although suppression of DYRK1A with siRNA had no effect on the level of Mcl-1 mRNA (Figure 6C), suppression of DYRK1A expression with siRNA or harmine reduced Mcl-1 protein expression in primary NSCLC cells (Figure 6D and 6E). In addition, harmine treatment enhanced Bcl-2 inhibitor-induced apoptosis in primary NSCLC cells by suppressing Mcl-1 expression (Figure 6F and 6G). Together, these data demonstrated that DYRK1A suppression enhanced the anti-NSCLC activity of Bcl-2 antagonists by reducing Mcl-1 expression, both in NSCLC cell lines and primary NSCLC cells.
Figure 6.
DYRK1A inhibition sensitizes primary NSCLC cells to Bcl-2 inhibitors via regulating Mcl-1. (A) Primary NSCLC cells were treated with harmine and/or Bcl-2 inhibitor at the indicated concentrations for 72 h, and then the proliferation of NSCLC cells was measured with a SRB assay. The mean combination index (CI) values are shown. (B) Primary NSCLC cells were incubated with harmine and/or Bcl-2 inhibitor at the indicated concentrations for 14 days, the cells were then stained with Giemsa solution, and the colony numbers were counted. (C) Primary NSCLC cells (#3) were transfected with control siRNA and DYRK1A siRNA for 48 h, and real-time reverse transcriptase PCR analyses were used to detect the mRNA levels of DYRK1A and Mcl-1. (D) Primary NSCLC cells (#3) were transfected with control siRNA and DYRK1A siRNA for 48 h, and the expression of DYRK1A and Bcl-2 family members were detected by Western blot. (E) Left panel: primary NSCLC cells (#3) were treated with harmine at the indicated concentration for 24 h, and the expression of the indicated proteins were detected by Western blot. Right panel: primary NSCLC cells (#3) were treated with 20 μM harmine for 1, 3, 6, 9, and 12 h, and the expression of the indicated proteins was detected by Western blot. (F) Primary NSCLC cells (#3) were incubated with harmine and/or ABT-199 at the indicated concentrations for 48 h, and then apoptosis was measured by Annexin V-FITC staining. (G) Primary NSCLC cells (#3) were incubated with harmine and/or Bcl-2 inhibitor at the indicated concentrations for 48 h, and then Western blot was used to detect the expression of the indicated proteins. Significant results are presented as * P < 0.05, ** P < 0.01.
Discussion
Mcl-1 is an anti-apoptotic protein that plays a critical role in the development of resistance to anti-cancer drugs, including epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and Bcl-2 inhibitors25. Although direct Mcl-1 inhibitors are currently in preclinical and clinical development, none has been approved by the Food and Drug Administration. This highlights the need for alternative therapeutics that target the Mcl-1 pathway26. Mcl-1 is degraded in cancer cells after effective therapeutic treatment, and defective Mcl-1 degradation is correlated with intrinsic and acquired drug resistance27. In this study, we demonstrated that overexpression of DYRK1A in NSCLC cells significantly increased Mcl-1 expression, whereas suppression of DYRK1A inhibited Mcl-1 expression, indicating that DYRK1A is an upstream activator of Mcl-1 in NSCLC cells, including adenocarcinoma and squamous cell carcinoma (Figure 1 and Supplementary Figure S1). Furthermore, DYRK1A and Mcl-1 proteins showed co-localization in NSCLC cells and co-expressed in tumor samples from lung cancer patients, revealing that Mcl-1 may be a novel DYRK1A substrate. Therefore, we hypothesized that DYRK1A suppression would sensitize cancer cells to Bcl-2 inhibitor treatment by suppressing Mcl-1 expression. Indeed, treatment of NSCLC cells with harmine, a DYRK1A inhibitor, resulted in induction of apoptosis and suppression of cell proliferation following Bcl-2 inhibitor treatment. Furthermore, harmine enhanced the anti-NSCLC activity of Bcl-2 inhibitors in four primary NSCLC cell lines. In addition, our data showed that treatment of NSCLC cells with Bcl-2 inhibitor alone resulted in increased Mcl-1 expression, but combined treatment with harmine suppressed this increase in Mcl-1 expression. These data demonstrated that Mcl-1 was involved in the synergistic effect that was observed with combined harmine and Bcl-2 inhibitor treatment in NSCLC cells.
Phosphorylation of Mcl-1 at Thr-163 by extracellular signal-regulated kinase leads to an increase in the stability of Mcl-1 protein and contributes to the development of acquired resistance to Bcl-2 inhibitors28,29. Phosphorylation of Mcl-1 at Thr-163 also serves as a primer for subsequent glycogen synthase kinase-3 (GSK3)-mediated phosphorylation at Ser-159, which ultimately leads to Mcl-1 ubiquitination and subsequent proteasomal degradation30–32. We demonstrated that DYRK1A suppression by siRNA or harmine decreased the phosphorylation of Mcl-1 at Ser159/Thr163. DYRK1A suppression also accelerated Mcl-1 degradation, indicating that DYRK1A mediated proteasome-dependent degradation of Mcl-1. Furthermore, DYRK1A likely directly bound and phosphorylated Mcl-1, thereby enhancing Mcl-1 protein stability and contributing to the development of acquired resistance to Bcl-2 inhibitors. In this regard, our findings suggested that targeting of DYRK1A may serve as a novel approach to reduce Mcl-1 protein stability in NSCLC cells and thereby prevent Bcl-2 inhibitor resistance. However, further studies are required to identify the binding site between DYRK1A and Mcl-1.
NSCLC patients harboring EGFR-activating mutations derive greater benefit from EGFR-TKI therapy than from chemotherapy, either in first-line or subsequent lines of treatment33. However, the overall EGFR mutation rate is only 16.7% in NSCLC patients and most NSCLC patients harboring EGFR wild-type have poor responses to EGFR-TKI therapy. Thus, platinum-based chemotherapy is still the standard therapeutic regimen for these patients34. For these reasons, there is an urgent need to develop novel targeted therapeutic strategies for NSCLC patients with wild-type EGFR. Our previous work has demonstrated that DYRK1A positively regulates EGFR/Met via regulating the expression and activation of STAT3, and that suppression of DYRK1A enhances the anti-cancer activity of EGFR-TKIs in wild-type EGFR NSCLC cells via inhibiting the STAT3 signaling pathway20. Furthermore, inhibition of DYRK1A suppresses angiogenesis and tumor growth via the p53 signaling pathway in EGFR wild-type NSCLC cells35. These findings suggested that targeting of DYRK1A could be a novel, effective strategy for treatment of NSCLC patients with EGFR wild-type. In the present study, we demonstrated that combined treatment with a DYRK1A inhibitor and a Bcl-2 inhibitor produced a synergistic, anti-proliferative effect in EGFR wild-type NSCLC cell lines (NCI-H1299, A549, and NCI-H460) as well as in primary NSCLC cells (derived from three EGFR wild-type NSCLC patients and one patient with an EGFR exon 20 insertion mutation resistant to EGFR-TKI treatment; Table 1)36. Thus, this combined treatment regimen provides an attractive therapeutic strategy for the treatment of wild-type EGFR NSCLC patients.
Table 1.
Primary NSCLC patient information
| Primary cells | Gender | Age (years) | Pathology | Immunohistochemistry | EGFR mutation |
|---|---|---|---|---|---|
| LS-1# | Male | 58 | Metastatic lung adenocarcinoma | TTF1[+], Napsin A[+], CK7[+], CK20[+], GFAP[−], Ki-67[+,15%-20%] | EGFR (wt) |
| LS-2# | Female | 52 | Peripulmonary moderately to poorly differentiated lung adenocarcinoma | CK7[+], CK20[−], CK5/6[−], P63[−], Napsin A[+], TTF1[+], ALK[0], Ki-67[+,2%-5%] | EGFR (20 lns) |
| LS-3# | Male | 71 | Invasive lung adenocarcinoma | ROS1(−), c-Met(++,80%), CK5/6(−), CK7(+), P40(−), P63(−), TTF1(+), Napsin A(+) | EGFR (wt) |
| LS-4# | Male | 71 | Invasive lung adenocarcinoma | CK20(−), CDX-2(−), c-Met(+,10%), CK5/6(−), ROS1(+,5%), CK7(−), P40(−), P63(−), TTF1(+), Napsin A(+), ALK(D5F3)(−) | EGFR (wt) |
EGFR, epidermal growth factor receptor; wt, wild-type.
Conclusions
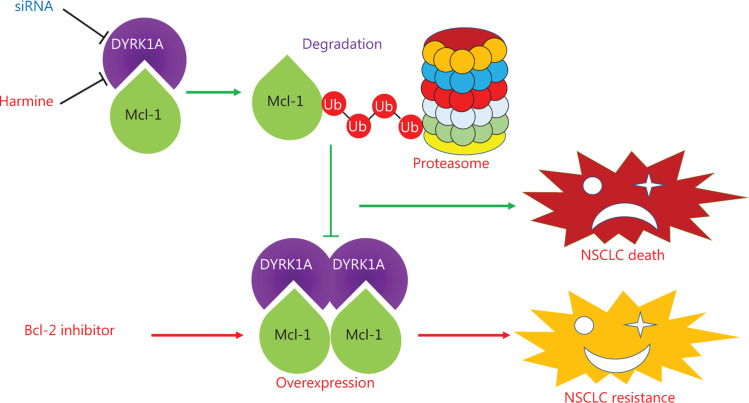
In summary, we showed that either genetic or pharmacological inhibition of DYRK1A selectively perturbed the stability of Mcl-1. Furthermore, inhibition of DYRK1A enhanced the anti-NSCLC activity of Bcl-2 inhibitors by inhibiting Mcl-1 expression. Our data identified Mcl-1 as a novel substrate of DYRK1A and highlighted DYRK1A as a potential therapeutic target for conquering Bcl-2 inhibitor resistance in NSCLC (Figure 7).
Figure 7.
The summary of DYRK1A in regulating Mcl-1 and anti-NSCLC effects of Bcl-2 inhibitors. DYRK1A promotes the stability of Mcl-1, and genetic or pharmacological inhibition of DYRK1A promotes the proteasome-dependent degradation of Mcl-1 in NSCLC cells. Mcl-1 overexpression confers acquired resistance to Bcl-2 inhibitors in NSCLC. Thus, DYRK1A suppression sensitizes NSCLC cells to Bcl-2 inhibitors treatment via regulating Mcl-1.
Supporting Information
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant Nos. 81702887, 81272473), the Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province (Grant No. 2020E10021), the Key Medical Disciplines of Zhejiang Province (Grant No. 2018-2-3), the Hangzhou Major Science and Technology Project (Grant No. 20172016A01), the Zhejiang Provincial Foundation of Natural Science (Grant No. LY19H310004), the Scientific and Technological Developing Scheme of Hangzhou City (Grant No. 20191203B49), the Science Research Foundation of Zhejiang Health Bureau (Grant No. 2020RC026), the High-level Talents Coming Back from Abroad Innovation, the Entrepreneurship Program in Hangzhou, and the Teachers Research Fund of Zhejiang University City College (Grant No. J-19006).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Chiu WT, Chang HA, Lin YH, Lin YS, Chang HT, Lin HH, et al. Bcl(−)2 regulates store-operated Ca(2+) entry to modulate ER stress-induced apoptosis. Cell Death Discov. 2018;4:37. doi: 10.1038/s41420-018-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lever JR, Fergason-Cantrell EA. Allosteric modulation of sigma receptors by BH3 mimetics ABT-737, ABT-263 (Navitoclax) and ABT-199 (Venetoclax). Pharmacol Res. 2019;142:87–100. doi: 10.1016/j.phrs.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Cheng L, Shen K, Jin H, Li H, Cheng Y, et al. Efficacy and safety of Bcl-2 inhibitor venetoclax in hematological malignancy: a systematic review and meta-analysis of clinical trials. Front Pharmacol. 2019;10:697. doi: 10.3389/fphar.2019.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero J, Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018;25:56–64. doi: 10.1038/cdd.2017.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25:27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks ED. Venetoclax: first global approval. Drugs. 2016;76:979–87. doi: 10.1007/s40265-016-0596-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW, et al. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther. 2011;10:1264–75. doi: 10.1158/1535-7163.MCT-10-1091. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Wan J, Zhang W, Hao S. MCL-1 or BCL-xL-dependent resistance to the BCL-2 antagonist (ABT-199) can be overcome by specific inhibitor as single agents and in combination with ABT-199 in acute myeloid leukemia cells. Leuk Lymphoma. 2019;60:2170–80. doi: 10.1080/10428194.2018.1563694. [DOI] [PubMed] [Google Scholar]
- 10.Arcaro A. Targeted therapies for small cell lung cancer: where do we stand? Crit Rev Oncol Hematol. 2015;95:154–64. doi: 10.1016/j.critrevonc.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Wen Q, Zhan Y, Zheng H, Zang H, Luo J, Zhang Y, et al. Elevated expression of mcl-1 inhibits apoptosis and predicts poor prognosis in patients with surgically resected non-small cell lung cancer. Diagn Pathol. 2019;14:108. doi: 10.1186/s13000-019-0884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TL, Duchon A, Manousopoulou A, Loaec N, Villiers B, Pani G, et al. Correction of cognitive deficits in mouse models of down syndrome by a pharmacological inhibitor of dyrk1a. Dis Model Mech. 2018;11 doi: 10.1242/dmm.035634. DOI: 10.1242/dmm.035634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latour A, Gu Y, Kassis N, Daubigney F, Colin C, Gausseres B, et al. LPS-induced inflammation abolishes the effect of DYRK1A on IkB stability in the brain of mice. Mol Neurobiol. 2019;56:963–75. doi: 10.1007/s12035-018-1113-x. [DOI] [PubMed] [Google Scholar]
- 14.Jarhad DB, Mashelkar KK, Kim HR, Noh M, Jeong LS. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors as potential therapeutics. J Med Chem. 2018;61:9791–810. doi: 10.1021/acs.jmedchem.8b00185. [DOI] [PubMed] [Google Scholar]
- 15.Abbassi R, Johns TG, Kassiou M, Munoz L. Dyrk1a in neurodegeneration and cancer: molecular basis and clinical implications. Pharmacol Ther. 2015;151:87–98. doi: 10.1016/j.pharmthera.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Luna J, Boni J, Cuatrecasas M, Bofill-De Ros X, Nunez-Manchon E, Gironella M, et al. DYRK1A modulates c-MET in pancreatic ductal adenocarcinoma to drive tumour growth. Gut. 2019;68:1465–76. doi: 10.1136/gutjnl-2018-316128. [DOI] [PubMed] [Google Scholar]
- 17.Arbones ML, Thomazeau A, Nakano-Kobayashi A, Hagiwara M, Delabar JM. DYRK1A and cognition: a lifelong relationship. Pharmacol Ther. 2019;194:199–221. doi: 10.1016/j.pharmthera.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Adayev T, Wegiel J, Hwang YW. Harmine is an ATP-competitive inhibitor for dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A). Arch Biochem Biophys. 2011;507:212–8. doi: 10.1016/j.abb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Wink M. The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother Res. 2010;24:146–9. doi: 10.1002/ptr.2860. [DOI] [PubMed] [Google Scholar]
- 20.Li YL, Ding K, Hu X, Wu LW, Zhou DM, Rao MJ, et al. DYRK1A inhibition suppresses STAT3/EGFR/Met signalling and sensitizes EGFR wild-type NSCLC cells to AZD9291. J Cell Mol Med. 2019;23:7427–37. doi: 10.1111/jcmm.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TL, Fruit C, Herault Y, Meijer L, Besson T. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors: a survey of recent patent literature. Expert Opin Ther Pat. 2017;27:1183–99. doi: 10.1080/13543776.2017.1360285. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Wang K, Chen S, Sun Q, Zhang Y, Chen L, et al. NFATc1 phosphorylation by DYRK1A increases its protein stability. PLoS One. 2017;12:e0172985. doi: 10.1371/journal.pone.0172985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu DW, Chen CY, Chu CL, Lee H. Paxillin confers resistance to tyrosine kinase inhibitors in EGFR-mutant lung cancers via modulating BIM and Mcl-1 protein stability. Oncogene. 2016;35:621–30. doi: 10.1038/onc.2015.120. [DOI] [PubMed] [Google Scholar]
- 24.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao F, Gong YB, Kang XH, Lu ZH, Wang Y, Zhao KL, et al. Degradation of MCL-1 by bufalin reverses acquired resistance to osimertinib in EGFR-mutant lung cancer. Toxicol Appl Pharmacol. 2019;379:114662. doi: 10.1016/j.taap.2019.114662. [DOI] [PubMed] [Google Scholar]
- 26.Kour S, Rana S, Contreras JI, King HM, Robb CM, Sonawane YA, et al. CDK5 inhibitor downregulates Mcl-1 and sensitizes pancreatic cancer cell lines to Navitoclax. Mol Pharmacol. 2019;96:419–29. doi: 10.1124/mol.119.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong J, Wang P, Tan S, Chen D, Nikolovska-Coleska Z, Zou F, et al. Mcl-1 degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res. 2017;77:2512–21. doi: 10.1158/0008-5472.CAN-16-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazumder S, Choudhary GS, Al-Harbi S, Almasan A. Mcl-1 phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Res. 2012;72:3069–79. doi: 10.1158/0008-5472.CAN-11-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nifoussi SK, Ratcliffe NR, Ornstein DL, Kasof G, Strack S, Craig RW. Inhibition of protein phosphatase 2A (PP2A) prevents Mcl-1 protein dephosphorylation at the Thr-163/Ser-159 phosphodegron, dramatically reducing expression in Mcl-1-amplified lymphoma cells. J Biol Chem. 2014;289:21950–9. doi: 10.1074/jbc.M114.587873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–4. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 32.Gores GJ, Kaufmann SH. Selectively targeting Mcl-1 for the treatment of acute myelogenous leukemia and solid tumors. Genes Dev. 2012;26:305–11. doi: 10.1101/gad.186189.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrelli F, Borgonovo K, Cabiddu M, Barni S. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small-cell lung cancer: a meta-analysis of 13 randomized trials. Clin Lung Cancer. 2012;13:107–14. doi: 10.1016/j.cllc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhao N, Zhang XC, Yan HH, Yang JJ, Wu YL. Efficacy of epidermal growth factor receptor inhibitors versus chemotherapy as second-line treatment in advanced non-small-cell lung cancer with wild-type EGFR: a meta-analysis of randomized controlled clinical trials. Lung Cancer. 2014;85:66–73. doi: 10.1016/j.lungcan.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Dai F, Chen Y, Song Y, Huang L, Zhai D, Dong Y, et al. A natural small molecule harmine inhibits angiogenesis and suppresses tumour growth through activation of p53 in endothelial cells. PLoS One. 2012;7:e52162. doi: 10.1371/journal.pone.0052162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Reva BA, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12:220–9. doi: 10.1158/1535-7163.MCT-12-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.