This cohort study examines brain autopsy data for the presence of quadruple misfolded proteins (tau, amyloid-β, α-synuclein, and transactive response DNA-binding protein 43) and their association with cognitive decline in older adults.
Key Points
Question
How frequently are both comorbid α-synuclein and transactive response DNA-binding protein 43 observed simultaneously in brains of individuals with Alzheimer disease (defined by amyloid-β and tau proteins), and do the characteristics associated with the presence of quadruple misfolded proteins differ from those of other proteinopathies?
Findings
In this cohort study of brain autopsy data from 375 older adults, quadruple misfolded proteins (tau, amyloid-β, α-synuclein, and transactive response DNA-binding protein 43) were commonly detected. Mild cognitive impairment transitioned to dementia most rapidly for those with these 4 proteinopathies, which were present in 19% of individuals with dementia.
Meaning
This study suggests that quadruple misfolded proteins are a common but underappreciated phenotype that is associated with impaired cognition.
Abstract
Importance
Quadruple misfolded proteins (tau neurofibrillary tangles, amyloid-β [Aβ], α-synuclein, and transactive response DNA-binding protein 43 [TDP-43]) in the same brain are relatively common in aging. However, the clinical presentation, associated factors, frequency in community-based cohorts, genetic characteristics, and cognitive trajectories associated with the quadruple misfolded proteins phenotype are not well understood.
Objective
To describe the quadruple misfolded proteins phenotype, including the trajectories of global cognition, in an autopsy cohort.
Design, Setting, and Participants
This retrospective cohort study used brain autopsy data from the University of Kentucky Alzheimer Disease Center (UK-ADC) Brain Bank. Participants were deceased individuals who were enrolled in a longitudinal community-based cohort study of aging and dementia in central Kentucky conducted by the UK-ADC. Included participants were enrolled in the UK-ADC cohort between January 1, 1989, and December 31, 2017; aged 55 years or older at baseline; and followed up for a mean duration of 10.4 years. The participants had Alzheimer disease pathology (tau and Aβ), α-synuclein, and TDP-43 data, along with Braak neurofibrillary tangle stage I to VI. Data analysis was conducted between February 1, 2019, and September 30, 2019.
Main Outcomes and Measures
Frequency of quadruple misfolded proteins was estimated, and proteinopathy group characteristics and associations with global cognition were evaluated. Multinomial logistic regression was used to estimate the association of proteinopathy group with participant characteristics, including age at death, sex, and apolipoprotein ε4 (APOE ε4) allele. Generalized estimating equations were used to estimate the probability of obtaining Mini-Mental State Examination (MMSE) scores within the normal cognition (27-30 points) and severe impairment (≤13 points) ranges during the 12 years before death.
Results
The final sample included 375 individuals (mean [SD] age at death, 86.9 [8.0] years); 232 women [61.9%]). Quadruple misfolded proteins were detected in 41 of 214 individuals with dementia (19.2%). Overall, 46 individuals (12.3%) had quadruple misfolded proteins, whereas 143 individuals (38.1%) had 3 proteinopathies. Dementia frequency was highest among those with quadruple misfolded proteins (41 [89.1%]), and participants with quadruple misfolded proteins had the lowest final mean (SD) MMSE scores of 13.4 (9.8) points. Adjusting for age at death and sex, the APOE ε4 allele was associated with higher odds of quadruple misfolded proteins (adjusted odds ratio, 2.55; 95% CI, 1.16- 5.62; P = .02). The quadruple misfolded proteins group had both the lowest probability of obtaining MMSE scores in the normal cognition range, even 12 years before death, and the highest probability of having MMSE scores in the severe impairment range.
Conclusions and Relevance
Quadruple misfolded proteins appear to be a common substrate for cognitive impairment and to be associated with an aggressive course of disease that typically ends with severe dementia. The prevalence of comorbid α-synuclein and TDP-43 with Alzheimer disease pathology (tau and Aβ) may complicate efforts to identify therapies to treat and prevent Alzheimer disease.
Introduction
Amyloid-β (Aβ) plaques and tau neurofibrillary tangles (NFTs) define Alzheimer disease neuropathological change (ADNC).1 Other misfolded proteins, including α-synuclein and transactive response DNA-binding protein 43 (TDP-43), also commonly occur in old age.2,3,4 Aberrant α-synuclein has been associated with Parkinson disease, dementia with Lewy bodies, and multiple system atrophy,5 whereas TDP-43 has been associated with multiple neurological diseases,6 the most common of which was designated as limbic-predominant, age-related TDP-43 encephalopathy.7,8
Neuropathological studies report that all 4 proteinopathies (Aβ, tau, α-synuclein, and TDP-43) coexist in aged human brains.2,3,4,9,10,11,12,13,14,15,16,17,18 We use the term quadruple misfolded proteins to describe this phenomenon. Other proteinopathies are associated with increased dementia risk.10,17 For example, ADNC-associated cognitive impairment has been associated with neocortical NFT density.19 The association of comorbid ADNC and α-synuclein with ADNC is well documented9,20,21: compared with ADNC and dementia with Lewy bodies pathology, pure dementia with Lewy bodies and ADNC were associated with improved memory and global cognition.22,23 The TDP-43 proteinopathy with ADNC also occurs3,7,24,25,26 and appears to be a factor in cognitive impairment.3,6,24,26,27,28,29 In addition, TDP-43 is associated with memory loss and medial-temporal atrophy in persons with ADNC7,30,31,32 and may preferentially change episodic and working memory.29
Few studies have investigated the quadruple misfolded proteins phenotype.3,4,16 Cognitive impairment is exacerbated by the presence of quadruple misfolded proteins compared with the presence of 1 to 3 proteinopathies.4,13,16 In this cohort study, we identified the prevalence and characteristics of the quadruple misfolded proteins phenotype in deceased research volunteers with brain autopsy data. We evaluated demographic and neuropathological characteristics, cognitive diagnoses, and global cognition trajectories in late life.
Methods
Participants
We obtained brain autopsy data of long-term participants in a community-based cohort study of aging and dementia in central Kentucky conducted by the University of Kentucky Alzheimer Disease Center (UK-ADC).33,34 These research volunteers were recruited through community outreach, local press, or broadcast media; enrolled between January 1, 1989, and December 31, 2017; aged 55 years or older at baseline; followed up for a mean duration of 10.4 years; and autopsied. The UK-ADC Brain Bank that we used also contains autopsy data from a non–UK-ADC cohort. We included individuals with Braak NFT stage I or higher given the near-universal presence of tau pathology in older adults and its association with cognition.19,35,36 We excluded individuals with Down syndrome or frontotemporal lobar degeneration (FTLD); FTLD was excluded because of its rarity in the underlying population despite its relevance to protein misfolding.7 Individuals with brain cancer were also excluded. In addition, available data were needed on all proteinopathies under study. Neuropathological assessments were performed with blinding of clinical information. The University of Kentucky Institutional Review Board approved the study procedures. Participants provided written informed consent.
Cognitive Diagnoses and Evaluations
Cognitive diagnoses were based on annual examinations and were described previously.37,38 Participant cognition was classified as normal, mild cognitive impairment (MCI), impaired (but not MCI), or dementia. Normal cognition was defined as intact functional ability and performance on neurocognitive tests within expected ranges for age and years of education, and MCI was defined as objective cognitive impairment (score of >1.5 SD below the expected mean) or cognitive complaint, intact global cognition, no or minimal functional impairment, and no evidence of dementia.39 Impaired cognition was defined according to the Uniform Data Set, a standard data protocol used by Alzheimer disease centers.40 Participants with impaired cognition exhibited MCI features on clinical examination, but neurodegenerative or cerebrovascular disease was not suspected in these individuals. Standard criteria were used to determine dementia.41
Annual cognitive evaluations included the Mini-Mental State Examination (MMSE).42 The MMSE scores, which have been consistently collected at UK-ADC since 1989, range from 0 to 30 points.42 For analysis, we classified MMSE scores as follows: 27-30 points as normal cognition and 13 points or less as severe impairment. These cutoff points were based on the guidelines published by Folstein et al42 and on the cutoff points for severe dementia used in clinical trials.43 Indicators for MMSE score of 13 points or less were imputed for 24 participants with missing scores for more than 3 years before death and with a last observed MMSE score of 16 points or less, assuming that the MMSE score decreased approximately 3 points per year.44
Neuropathological Assessments
Brain autopsies were performed as described previously.34,45 Briefly, for autopsies performed before 2012 (n = 203), Bielschowsky silver stains were used to detect neuritic plaques (NPs) and diffuse plaques (DPs), and Gallyas silver stains46 were used to detect NFTs in accordance with the 1997 National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease.47 For autopsies performed beginning in 2012 (n = 172), immunohistochemical stains were used to detect Aβ deposits, and phospho–tau antibody ([PHF-1] a gift from Peter Davies, PhD, The Feinstein Institutes for Medical Research, Manhasset, New York) was used to visualize NPs and NFTs (per the 2012 National Institute on Aging–Alzheimer’s Association guidelines1), with digital pathological methods used for lesion detection and counting.48
Immunohistochemistry detection of α-synuclein aggregates, visualized with mouse monoclonal antibody (clone KM51, Novocastra; Leica Biosystems), were assessed using established diagnostic criteria.5 Evaluation of TDP-43 immunoreactive inclusions was performed on 5-μm-thick sections cut on slides (ProbeOn; Thermo Fisher Scientific). Rat anti-phospho TDP-43 (clone 1D3; Millipore) was used after antigen retrieval in a decloaking chamber and formic acid pretreatment. Secondary antibody reaction used the avidin-biotin complex kit (Vectastain ABC Kit; Vector Laboratories). Details were reported previously.27
Proteinopathy Groups
Participants were grouped on the basis of the presence of proteinopathies: Aβ, tau, α-synuclein, and TDP-43. Tau was considered present when Braak NFT stage was I or higher, whereas Aβ was present when the CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) ratings for DPs or NPs were at least sparse.49 Meanwhile, α-synuclein was considered present if Lewy bodies were detected in the neocortex, medial temporal lobe, or amygdala. In defining proteinopathy groups, we did not consider Lewy bodies in the brainstem only (n = 7) as having α-synuclein. Previous work showed that brainstem-only Lewy bodies were not associated with cognitive impairment.27 TDP-43 inclusion bodies were considered present for TDP-43 proteinopathy when detected in the left or right hippocampus. Previous work suggested that amygdala-only TDP-43 was not associated with odds of dementia or cognitive impairment.31
In addition, we divided participants with pure ADNC into 2 groups according to Braak NFT stage (stage I to IV and stage V to VI). Participants were classified into 7 proteinopathy groups: (1) tau alone; (2) tau and TDP-43; (3) tau Braak stage I to IV and Aβ; (4) tau Braak stage V to VI and Aβ; (5) tau, Aβ, and α-synuclein; (6) tau, Aβ, and TDP-43; and (7) quadruple misfolded proteins (tau, Aβ, TDP-43, and α-synuclein).
Cerebrovascular pathology was not considered in proteinopathy group definitions. We assessed gross diagnosis of atherosclerosis severity (all vessels ≥50% vs <50% occluded), microscopic diagnosis of brain arteriolosclerosis (moderate or severe vs none or mild), and brain infarcts (stage 0, none; stage 1, microinfarcts or lacunar or large infarcts; and stage 2, both microinfarcts and lacunar or large infarcts) within proteinopathy groups.16 In addition, the right and left hippocampi were evaluated for hippocampal sclerosis.50 Presence of argyrophilic grain disease was assessed in cornu ammonis, subiculum, and entorhinal regions.
Covariates
Age at death, sex (reference group: male), years of education, and apolipoprotein (APOE) ε4 allele were covariates of interest. The APOE (OMIM 107741) genotype was converted to a dummy indicator for 1 or more ɛ4 alleles.
Statistical Analysis
Characteristics of participants by proteinopathy group were assessed with analysis of variance or χ2 tests. Time in MCI and dementia states was calculated on the basis of the dates of clinical diagnosis. When a participant transitioned to a new diagnosis between annual visits, the diagnosis date was taken as the midpoint between the 2 visits. Time in cognitive state that was consistent throughout follow-up (eg, dementia at baseline) was taken as the difference between the date of death and UK-ADC study enrollment date. We repeated the analyses on a restricted cohort of participants who began follow-up with normal cognition.
Multivariable multinomial logistic regression was used to estimate the association between demographic characteristics and proteinopathy groups. Tau Braak stage I to IV and Aβ was the largest proteinopathy group and served as the reference. Adjusted odds ratios (AORs) with 95% CIs were obtained from the logistic regression model, which included age at death, sex, and APOE ε4 allele indicator.
To evaluate the association of the proteinopathies with global cognition over time, we used multivariable logistic regression with generalized estimating equations with a first-order autoregressive (AR[1]) working correlation structure. With this approach, we estimated the probability that individuals in the proteinopathy groups obtained MMSE scores within the normal range at each assessment in the 12 years before death (based on data availability), adjusting for age at death, sex, APOE ɛ4 allele, and years of education. We used the same approach to estimate the probability that individuals would obtain MMSE scores in the severe impairment range. The reference group was, again, tau Braak stage I to IV and Aβ.
To assess potential misclassification owing to group definitions in the current study, we examined amygdalar TDP-43 proteinopathy in a convenience sample of 47 individuals (of 234 [20.1%]) without hippocampal TDP-43. In addition, because the definition of Aβ positivity could include individuals with low Aβ, we examined the joint distribution of NP and DP ratings to ascertain the frequency of individuals with sparse numbers of both NP and DP.
All data were analyzed using SAS, version 9.4 (SAS Institute Inc). Statistical significance was set at α = .05. Data analysis was conducted between February 1, 2019, to September 30, 2019.
Results
The final sample included 375 individuals (eFigure 1 in the Supplement). The mean (SD) age at death was 86.9 (8.0) years and ranged from 82.7 (10.3) years in the tau, Aβ, and α-synuclein proteinopathy group to 89.7 (6.7) years in the tau and TDP-43 group. Participants were predominantly women (232 [61.9%]); were predominantly white individuals (363 [96.8%]), which was consistent with the underlying population; and had a mean (SD) 15.6 (3.1) years of education (Table 1).
Table 1. Participant Characteristics by Proteinopathy Groupa.
| Variable | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 375) | Tau (n = 24) | Tau Braak stage I to IV and Aβ (n = 81) | Tau Braak stage V to VI and Aβ (n = 57) | Tau and TDP-43 (n = 24) | Tau, Aβ, and αSyn (n = 72) | Tau, Aβ, and TDP-43 (n = 71) | QMP (n = 46) | |
| Age at death, mean (SD), y | 86.9 (8.0) | 87.0 (6.6) | 88.7 (7.0) | 86.1 (7.9) | 89.7 (6.7) | 82.7 (10.3) | 89.2 (5.6) | 86.3 (7.6) |
| Female sex | 232 (61.9) | 16 (66.7) | 48 (59.3) | 37 (64.9) | 18 (75.0) | 41 (56.9) | 44 (61.9) | 28 (60.9) |
| White race/ethnicity | 363 (96.8) | 24 (100) | 78 (96.3) | 56 (96.3) | 23 (95.8) | 69 (95.8) | 70 (98.6) | 43 (93.5) |
| Education, mean (SD), y | 15.6 (3.1) | 15.1 (2.6) | 16.0 (2.9) | 14.9 (3.5) | 16.2 (2.3) | 16.0 (2.9) | 15.4 (3.4) | 15.3 (3.4) |
| APOE ε4 allele | 137 (36.5) | 1 (4.2) | 21 (25.9) | 30 (52.6) | 3 (12.5) | 30 (41.7) | 29 (40.8) | 23 (50.0) |
| MMSE | 20.0 (9.7) | 26.6 (4.2) | 26.2 (5.1) | 17.4 (9.3) | 20.5 (8.9) | 19.4 (10.1) | 15.7 (10.2) | 13.4 (9.8) |
| Last clinical cognitive diagnosis | ||||||||
| Normal | 104 (27.7) | 16 (66.7) | 48 (59.3) | 7 (12.3) | 8 (33.3) | 14 (19.4) | 6 (8.5) | 5 (10.9) |
| Impaired | 10 (2.7) | 3 (12.5) | 3 (3.7) | 1 (1.8) | 0 | 3 (4.2) | 0 | 0 |
| MCI | 45 (12.0) | 2 (8.3) | 14 (17.3) | 7 (12.3) | 5 (20.8) | 11 (15.3) | 6 (8.5) | 0 |
| Dementia | 214 (57.1) | 3 (12.5) | 16 (19.8) | 41 (71.9) | 11 (45.8) | 44 (61.1) | 58 (81.7) | 41 (89.1) |
| Braak NFT stage | ||||||||
| I | 35 (9.3) | 10 (41.7) | 0 | 0 | 8 (33.3) | 13 (18.1) | 3 (4.2) | 1 (2.2) |
| II | 77 (20.5) | 10 (41.7) | 40 (49.4) | 0 | 11 (45.8) | 9 (12.5) | 3 (4.2) | 4 (8.7) |
| III | 43 (11.5) | 2 (8.3) | 23 (28.4) | 0 | 4 (16.7) | 5 (6.9) | 4 (5.6) | 5 (10.9) |
| IV | 36 (9.6) | 2 (8.3) | 18 (22.2) | 0 | 1 (4.2) | 4 (5.6) | 7 (9.8) | 4 (8.7) |
| V | 87 (23.2) | 0 | 0 | 27 (47.4) | 0 | 20 (27.8) | 31 (43.7) | 9 (19.6) |
| VI | 97 (25.9) | 0 | 0 | 30 (52.6) | 0 | 21 (29.2) | 23 (32.4) | 23 (50.0) |
| Atherosclerosis (vessels occluded) | ||||||||
| <50% | 149 (39.7) | 12 (50.0) | 29 (35.8) | 27 (47.4) | 11 (45.8) | 31 (43.1) | 25 (35.2) | 14 (30.4) |
| ≥50% | 222 (59.2) | 12 (50.0) | 52 (64.2) | 30 (52.6) | 13 (54.2) | 40 (55.6) | 45 (63.4) | 30 (65.2) |
| Arteriolosclerosis | ||||||||
| None/mild | 238 (64.5) | 17 (70.8) | 49 (60.5) | 36 (63.2) | 15 (62.5) | 52 (72.2) | 38 (53.5) | 31 (67.4) |
| Moderate/severe | 92 (24.5) | 5 (20.8) | 24 (29.6) | 12 (21.1) | 6 (25.0) | 18 (25.0) | 16 (22.5) | 11 (23.9) |
| Brain infarctsb | ||||||||
| Stage 0 | 205 (54.7) | 14 (58.3) | 41 (50.6) | 29 (50.9) | 10 (41.7) | 51 (70.8) | 34 (47.9) | 26 (56.5) |
| Stage 1 | 118 (31.5) | 5 (20.8) | 24 (29.6) | 16 (28.1) | 12 (50.0) | 19 (26.4) | 27 (38.0) | 15 (32.6) |
| Stage 2 | 52 (13.9) | 5 (20.8) | 16 (19.8) | 12 (21.1) | 2 (8.3) | 2 (2.8) | 10 (14.1) | 5 (10.9) |
| HS | 92 (24.5) | 0 | 1 (1.2) | 1 (1.8) | 13 (56.5) | 3 (4.2) | 41 (56.9) | 33 (71.7) |
| AGD | 48 (12.8) | 5 (21.7) | 16 (19.5) | 5 (8.8) | 2 (8.7) | 4 (5.6) | 11 (15.3) | 5 (10.4) |
| Time in state, mean (SD), y | ||||||||
| MCI | 2.9 (2.4) | 4.1 (3.6) | 3.0 (2.4) | 2.9 (2.0) | 3.0 (1.9) | 2.9 (3.2) | 3.0 (2.4) | 1.7 (0.6) |
| Dementia | 5.4 (3.4) | 8.9 (2.9) | 4.7 (2.8) | 3.9 (2.8) | 4.9 (2.5) | 5.3 (4.1) | 5.8 (3.3) | 6.2 (3.5) |
Abbreviations: AGD, argyrophilic grain disease; APOE ε4, apolipoprotein ε4 allele; αSyn, α-synuclein; Aβ, amyloid-β; HS, hippocampal sclerosis; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination score; NFT, neurofibrillary tangle; QMP, quadruple misfolded protein; TDP-43, transactive response DNA-binding protein 43.
Missing data are reported in eTables 2 and 3 in the Supplement.
Stage 0: none; stage 1: microinfarcts or lacunar/large infarcts; and stage 2: both microinfarcts and lacunar/large infarcts.
Presence of multiple proteinopathies was common, with just 24 individuals (6.4%) having tau alone (eFigure 2 in the Supplement). Two proteinopathies were detected in 162 of 375 individuals (43.2%), 3 proteinopathies in 143 individuals (38.1%), and quadruple misfolded proteins in 46 individuals (12.3%). Overall, α-synuclein was present in 117 individuals (31.2%), TDP-43 in 141 (37.6%), and tau and Aβ in 327 (87.2%). Individuals with quadruple misfolded proteins had a higher frequency of Braak stage VI (23 [50.0%), as did individuals with 3 proteinopathies (44 [approximately 30%]).
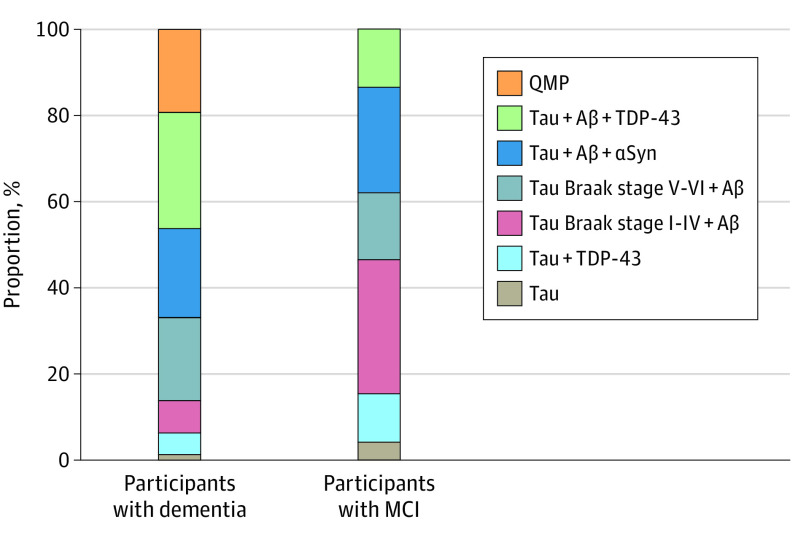
Dementia was diagnosed in 214 of 375 participants (57.1%), and 104 (27.7%) participants retained normal cognition (Table 1). Dementia prevalence was highest in the quadruple misfolded proteins group (41 [89.1%]), followed by tau, Aβ, and TDP-43 group (58 [81.7%]); tau Braak stage V to VI and Aβ group (41 [71.9%]); and tau, Aβ, and α-synuclein group (44 [61.1%]). Among all participants with dementia (Figure 1), quadruple misfolded proteins were present in 41 of 214 individuals (19.2%); tau, Aβ, and TDP-43 in 58 individuals (27.1%); tau, Aβ, and α-synuclein in 44 individuals (20.6%); and tau Braak stage V to VI and Aβ in 41 individuals (19.2%).
Figure 1. Distribution of Proteinopathy Groups Among Participants With Dementia and Participants With Mild Cognitive Impairment (MCI) Proximate to Death.
Aβ indicates amyloid-β; αSyn, α-synuclein; QMP, quadruple misfolded protein; and TDP-43, transactive response DNA-binding protein 43.
Among those with a final diagnosis of MCI (n = 45), none had quadruple misfolded proteins (although 5 participants died with normal cognition), whereas 14 (31.1%) had tau Braak stage I to IV and Aβ; 11 (24.4%) had tau, Aβ, and α-synuclein; 7 (15.6%) had tau Braak stage V to VI and Aβ; and 6 (13.3%) had tau, Aβ, and TDP-43 (Figure 1). Among participants with an initial diagnosis of normal cognition (n = 228), 14 of 83 individuals (16.9%) with a final diagnosis of dementia had quadruple misfolded proteins (eFigure 3 in the Supplement).
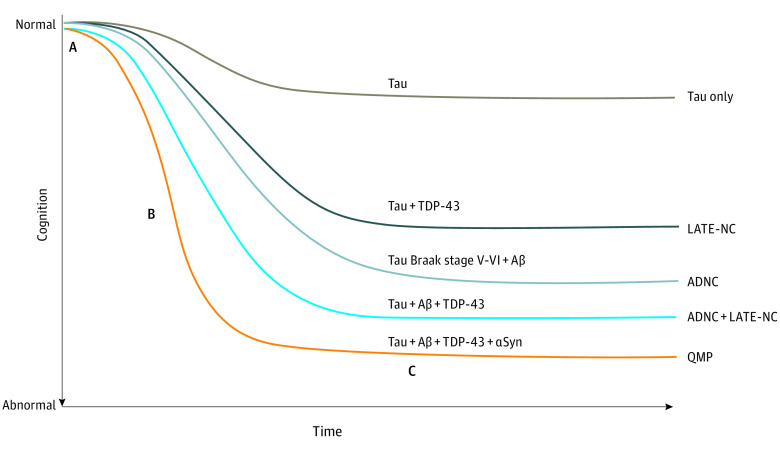
APOE ε4 allele was common in persons with quadruple misfolded proteins (23 of 46 [50.0%]), similar to those in the tau Braak stage V to VI and Aβ group (30 of 57 [52.6%]). In contrast, APOE ε4 allele was observed in a single participant (4.2%) in the tau alone group and in 3 of 24 participants (12.5%) in the tau and TDP-43 group. Mean (SD) time spent in the MCI state was shortest among those in the quadruple misfolded proteins group (1.7 [0.6] years) (Table 1) and among those with initial normal cognition (1.8 [0.6] years) (eTable 1 in the Supplement). This finding suggests a more aggressive disease course for individuals with quadruple misfolded proteins (Figure 2).
Figure 2. Hypothesized Patterns of Late-Life Cognitive Decline Associated With Specific Proteinopathies.
Quadruple misfolded protein (QMP) is distinguished by early decline in cognition (A), fast transition through mild cognitive impairment of approximately 1.7 years (B), and long duration of severely abnormal cognition (C). Aβ indicates amyloid-β; ADNC, Alzheimer disease neuropathological change; αSyn, α-synuclein; LATE-NC, limbic-predominant, age-related TDP-43 encephalopathy neuropathological change; and TDP-43, transactive response DNA-binding protein 43.
Cerebrovascular burden (atherosclerosis, arteriolosclerosis, and infarcts) was similar across groups (Table 1), although atherosclerosis was most prevalent in the quadruple misfolded proteins group (30 of 46 [65.2%]) and in those who started follow-up with normal cognition (15 of 19 [79.0%]) (Table 1; eTable 1 in the Supplement). As expected, hippocampal sclerosis was most common in participants with TDP-43. The highest proportion of hippocampal sclerosis was in the quadruple misfolded proteins group (33 [71.7%) (Table 1). This finding may be relevant to a previous finding that more clinically severe cases of limbic-predominant age-related TDP-43 encephalopathy–neuropathological change are more likely to have hippocampal sclerosis pathology.7
The lowest final mean (SD) MMSE score was observed in the quadruple misfolded proteins group (13.4 [9.8] points), which was significantly lower than in any other group (P < .001). Although the final mean (SD) MMSE scores in the tau alone group (26.6 [4.2] points) and tau Braak stage I to IV and Aβ group (26.2 [5.1] points) indicated generally intact cognition at death, participants in all other groups had mean MMSE scores lower than 21, indicating moderate to severe dementia (Table 1). Among participants with initially normal cognition, the final mean (SD) MMSE score was also lowest among those in the quadruple misfolded proteins group (18.9 [9.0] points) (eTable 1 in the Supplement).
With a 5-year increase in age at death, participants were less likely to have quadruple misfolded proteins (AOR, 0.82; 95% CI, 0.63-1.08; P = .15) or tau, Aβ, and α-synuclein (AOR, 0.61; 95% CI, 0.48-0.78; P < .001) compared with tau Braak stage I to IV and Aβ (reference). APOE ε4 allele was associated with higher odds of having quadruple misfolded proteins (AOR, 2.55; 95% CI, 1.16-5.62; P = .02); tau Braak stage V to VI and Aβ (AOR, 3.45; 95% CI, 1.61-7.39; P < .001); and tau, Aβ, and TDP-43 (AOR, 2.34; 95% CI, 1.15-4.77; P = .02). Sex was not significantly associated with the proteinopathy groups (Table 2).
Table 2. Association of Participant Characteristics With Proteinopathy Groupa.
| Variable | Proteinopathy group | Adjusted OR (95% CI) |
|---|---|---|
| Age at death (5-y increase) | Tau alone | 0.71 (0.51-1.00) |
| Tau Braak stage V to VI and Aβ | 0.85 (0.66-1.11) | |
| Tau and TDP-43 | 1.02 (0.69-1.49) | |
| Tau, Aβ, and αSyn | 0.61 (0.48-0.78) | |
| Tau, Aβ, and TDP-43 | 1.15 (0.89-1.50) | |
| QMP | 0.82 (0.63-1.08) | |
| Female sex | Tau alone | 1.65 (0.60-4.51) |
| Tau Braak stage V to VI and Aβ | 1.48 (0.69-3.15) | |
| Tau and TDP-43 | 1.87 (0.65-5.38) | |
| Tau, Aβ, and αSyn | 1.41 (0.70-2.87) | |
| Tau, Aβ, and TDP-43 | 1.00 (0.50-1.99) | |
| QMP | 1.18 (0.55-2.57) | |
| APOE ε4 allele | Tau alone | 0.10 (0.01-0.77) |
| Tau Braak stage V to VI and Aβ | 3.45 (1.61-7.39) | |
| Tau and TDP-43 | 0.43 (0.11-1.62) | |
| Tau, Aβ, and αSyn | 1.53 (0.73-3.19) | |
| Tau, Aβ, and TDP-43 | 2.34 (1.15-4.77) | |
| QMP | 2.55 (1.16-5.62) |
Abbreviations: Aβ, amyloid-β; APOE ε4, apolipoprotein ε4; αSyn, α-synuclein; OR, odds ratio; QMP, quadruple misfolded protein; TDP-43, transactive response DNA-binding protein 43.
Tau Braak stage I to IV and Aβ group was the reference. Results are from multinomial logistic regression, with age at death, sex, and APOE ε4 allele as covariates. Nineteen participants were excluded from this analysis because of missing data (eTables 2 and 3 in the Supplement).
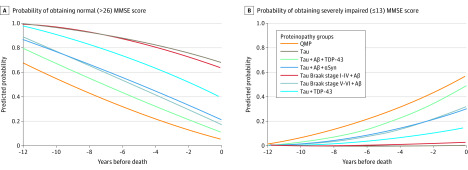
The estimated probabilities of obtaining MMSE scores categorized as normal cognition and severely impaired cognition are shown in Figure 3. For example, 6 years before death, the mean (SD) estimated probability of an MMSE score in the normal cognition range (27-30 points) was lowest in the quadruple misfolded proteins group (0.33 [0.24]), followed by tau, Aβ, and TDP-43 group (0.49 [0.12]); tau, Aβ, and α-synuclein group (0.57 [0.15]); and tau Braak stage V to VI and Aβ group (0.59 [0.16]; P < .001 for all proteinopathy group β coefficients). Moreover, 6 years before death, the mean [SD] probability of an MMSE score in the severe impairment range (≤13 points) was highest in the quadruple misfolded proteins group (0.16 [0.10]; P < .001), followed by tau, Aβ, and TDP-43 group (0.10 [0.05]; P < .001); tau, Aβ, and α-synuclein group (0.07 [0.09]; P = .04); and tau Braak stage V to VI and Aβ group (0.05 [0.05]; P = .004).
Figure 3. Mean Estimated Probabilities of Obtaining Normal or Severely Impaired Mini-Mental State Examination (MMSE) Score Ranges in the 12 Years Before Death.
Aβ indicates amyloid-β; αSyn, α-synuclein; QMP, quadruple misfolded protein; and TDP-43, transactive response DNA-binding protein 43.
To assess how proteinopathy definitions may have altered the results, we performed 2 additional analyses. First, we assessed a convenience sample of 47 individuals without hippocampal TDP-43 proteinopathy, and TDP-43 was detected in the amygdala of 19 participants (40.4%). By proteinopathy group of the sampled cases, TDP-43 in the amygdala was detected in 12 of 23 participants (52.2%) in the tau, Aβ, and α-synuclein group; 3 of 8 participants (37.5%) in the tau Braak stage I to IV and Aβ group; 4 of 13 participants (30.8%) in the tau Braak stageV to VI and Aβ group; and none in 3 participants assessed in the tau group. Twelve of 327 participants (3.7%) with Aβ proteinopathy had only sparse numbers of both NPs and DPs: quadruple misfolded proteins (n = 3); tau, Aβ, and α-synuclein (n = 1); and tau Braak stage I to IV and Aβ (n = 8).
Discussion
In this cohort study, we investigated quadruple misfolded proteins and other proteinopathy combinations in a cohort of 375 deceased individuals with autopsy data. At least 3 proteinopathies were observed in 50% of brains. Quadruple misfolded proteins were observed in 19.2% of individuals with dementia, which was the same proportion of participants with dementia who had pure ADNC with Braak stage V to VI. In addition, quadruple misfolded proteins were associated with severe cognitive impairment at least 12 years before death.
Participants with 3 or more proteinopathies tended to have high Braak NFT stages (V-VI). Higher Braak stage in these groups complicates the interpretation of the association among risk factors, cognition, and comorbid brain pathologies because it raises the question of which primary factor (the Braak stage or the number and combination of proteinopathies) is associated with cognitive decline. Participants with 3 proteinopathies tended to have poorer global cognition earlier than with the presence of only tau and Aβ and were likely to have higher Braak stages.
Previous studies have reported cognitive decline associated with the presence of mixed pathologies,3,4,16,17,26 with study-to-study differences in methods and proteinopathies,3,4,17,24 the assessment and inclusion of cerebrovascular pathologies,12,16,17 and hippocampal sclerosis.12,17 In the present sample, as in other community-based cohorts,31 FTLD in old age was rare (with an estimated incidence of 8.9 of 100 000 in individuals aged 60 to 69 years51; no incidence data are available for older age groups) and was not found in brains of individuals who began follow-up with normal cognition.7 Individuals with FTLD-TDP with data in the UK-ADC Brain Bank were recruited from a dementia clinic. We excluded 6 individuals with FTLD-TDP in the study; none had the quadruple misfolded proteins phenotype. No discernible overlap in any FTLD feature was observed in these individuals other than presence of TDP-43 proteinopathy, which is now detected in many different neurological diseases outside of the amyotrophic lateral sclerosis–FTLD spectrum.52
Cognitive impairment was associated with quadruple misfolded proteins at autopsy, with 89.1% of participants developing dementia and some experiencing profound impairment (as measured by MMSE scores) up to 12 years before death. This finding suggests that quadruple misfolded proteins occur before end-stage ADNC (ie, before high Braak stage). Consistent with this hypothesis, the MCI-to-dementia transition was, on average, fastest in the quadruple misfolded proteins group (Figure 3).
Estimation of the group cognitive trajectories was aided by the relatively long follow-up (mean duration of 10.4 years). These data provide the basis for a novel hypothesis that quadruple misfolded proteins have a more aggressive phenotype from the early stages of the disease rather than accruing additional pathologies only after ADNC has progressed to high levels. About 10% of these participants died with normal cognition, and previous research has shown quadruple misfolded proteins were present in persons with apparently normal cognition.16 In the present study, all individuals with quadruple misfolded proteins who had normal cognition at the last visit before death had lower Braak NFT stages (I-III), had no APOE ε4 allele, and were predominantly male (4 of 5 participants). These individuals may represent an early stage of quadruple misfolded proteins, but there are complexities: clinical presentation of proteinopathy combinations may be cohort specific and depend on other currently unknown factors. Older cohorts that survive into advanced old age, like those in the UK-ADC study, may be more likely to experience multiple proteinopathies than younger cohorts.
As previously described,4,16 APOE appeared to be associated with multiple proteinopathies in this study, particularly those proteinopathy combinations including Aβ plaques. Carriers of APOE ε4 allele not only had increased odds of tau and Aβ, an expected result, but also had higher odds of tau, Aβ, and α-synuclein; tau, Aβ, and TDP-43; and quadruple misfolded proteins. Unlike previous studies, this study did not find evidence that the ε4 allele was associated with tau or TDP-43 in the absence of Aβ,28,53 but the sample size was relatively small.
The temporality of protein misfolding may play a clinically important and differentiating role in disease progression. Autopsy data, although cross-sectional by nature, are compatible with the hypothesis that Aβ aggregates precede, and perhaps stimulate or exacerbate, the widespread misfolding of tau, TDP-43, and α-synuclein.13 These results suggest that TDP-43 pathology may be associated with poor global cognition.
Limitations
This study has limitations. The cohort comprised primarily older adult, white, and well-educated participants, which limit generalizability of the findings. Some studies have reported that black54,55 and Hispanic55 persons are more likely to have mixed ADNC. Furthermore, although the sample size was relatively large for an autopsy-based study, it underpowered some intergroup comparisons. The sample size also limited our ability to assess associations with other participant characteristics, such as medical history and environmental risk factors. In particular, the association of traumatic brain injury with quadruple misfolded proteins and multiple proteinopathies needs to be addressed in future research.
We did not use data on TDP-43 pathology in brain regions other than the hippocampus. In a future study, we will examine the role of limbic-predominant, age-related TDP-43 encephalopathy neuropathological change stages 1 and 3 in the disease course of individuals with mixed pathology. The convenience sample analyses suggest that 30% to 40% of individuals without hippocampal TDP-43 pathology may have TDP-43 in the amygdala, although the amygdalar TDP-43 pathology was often sparse. However, the convenience sample was about 5 years younger than the overall cohort but had a higher proportion of Braak stage V (similar in all other characteristics), which suggests that the true proportion of participants with amygdalar TDP-43 but without hippocampal TDP-43 is higher than the estimate. Furthermore, the definition of Aβ positivity could include individuals with little amyloid, and we did not include quantitative measures of amyloid in the analyses. The analyses showed few individuals in any group with low Aβ; thus, we believe that adjusting for quantitative Aβ in the models would not change the results meaningfully.
Conclusions
The presence of multiple proteinopathies, particularly the quadruple misfolded proteins phenotype, appeared to have been associated with the cognitive decline in deceased individuals who participated in a longitudinal community-based study at the UK-ADC. Most individuals who had quadruple misfolded proteins had dementia, and none died with MCI. These observations have potentially significant implications for clinical practice and public health, given that strategies to prevent or manage AD dementia may be complicated by the unrecognized presence of multiple additional neuropathologies.
eFigure 1. Flow Diagram Included Cases
eTable 1. Proteinopathy Case Group Characteristics Who Began Follow-Up While Normal (n = 228)
eFigure 2. Bar Graph Showing Distribution of Proteinopathy Case Groups Among Participants (n = 375)
eFigure 3. Bar Graph Showing Distribution of Proteinopathy Case Groups for Those Participants Who Began Follow-Up While Normal (n = 228), and With Last Clinical Diagnosis of Dementia and MCI
eTable 2. Frequency of Missing Data in All Participants (n = 375)
eTable 3. Frequency of Missing Data in All Participants Who Began Follow-Up While Normal (n = 228)
References
- 1.Montine TJ, Phelps CH, Beach TG, et al. ; National Institute on Aging; Alzheimer’s Association . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284-294. doi: 10.1016/j.brainres.2007.09.048 [DOI] [PubMed] [Google Scholar]
- 3.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J. TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. 2017;27(4):472-479. doi: 10.1111/bpa.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181-2193. doi: 10.1093/brain/awy146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKeith IG, Dickson DW, Lowe J, et al. ; Consortium on DLB . Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. doi: 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 6.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-133. doi: 10.1126/science.1134108 [DOI] [PubMed] [Google Scholar]
- 7.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527. doi: 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134(pt 5):1506-1518. doi: 10.1093/brain/awr053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther. 2014;6(9):82. doi: 10.1186/s13195-014-0082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197-2204. doi: 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 11.Abner EL, Kryscio RJ, Schmitt FA, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol. 2017;81(4):549-559. doi: 10.1002/ana.24903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171-186. doi: 10.1007/s00401-017-1717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spires-Jones TL, Attems J, Thal DR. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017;134(2):187-205. doi: 10.1007/s00401-017-1709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suemoto CK, Ferretti-Rebustini RE, Rodriguez RD, et al. Neuropathological diagnoses and clinical correlates in older adults in Brazil: a cross-sectional study. PLoS Med. 2017;14(3):e1002267. doi: 10.1371/journal.pmed.1002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanskanen M, Mäkelä M, Notkola I-L, et al. Population-based analysis of pathological correlates of dementia in the oldest old. Ann Clin Transl Neurol. 2017;4(3):154-165. doi: 10.1002/acn3.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wennberg AM, Whitwell JL, Tosakulwong N, et al. The influence of tau, amyloid, alpha-synuclein, TDP-43, and vascular pathology in clinically normal elderly individuals. Neurobiol Aging. 2019;77:26-36. doi: 10.1016/j.neurobiolaging.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology. 2015;85(6):535-542. doi: 10.1212/WNL.0000000000001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle PA, Yang J, Yu L, et al. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain. 2017;140(3):804-812. doi: 10.1093/brain/aww341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362-381. doi: 10.1097/NEN.0b013e31825018f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dugger BN, Adler CH, Shill HA, et al. ; Arizona Parkinson’s Disease Consortium . Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord. 2014;20(5):525-529. doi: 10.1016/j.parkreldis.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson PT, Kryscio RJ, Jicha GA, et al. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73(14):1127-1133. doi: 10.1212/WNL.0b013e3181bacf9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson PT, Kryscio RJ, Abner EL, et al. Acetylcholinesterase inhibitor treatment is associated with relatively slow cognitive decline in patients with Alzheimer’s disease and AD + DLB. J Alzheimers Dis. 2009;16(1):29-34. doi: 10.3233/JAD-2009-0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014;127(6):811-824. doi: 10.1007/s00401-014-1269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson AC, Dugger BN, Dickson DW, Wang D-S. TDP-43 in aging and Alzheimer’s disease - a review. Int J Clin Exp Pathol. 2011;4(2):147-155. [PMC free article] [PubMed] [Google Scholar]
- 26.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139(11):2983-2993. doi: 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20(1):66-79. doi: 10.1111/j.1750-3639.2008.00244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wennberg AM, Tosakulwong N, Lesnick TG, et al. Association of apolipoprotein E ε4 with transactive response DNA-binding protein 43. JAMA Neurol. 2018;75(11):1347-1354. doi: 10.1001/jamaneurol.2018.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70(11):1418-1424. doi: 10.1001/jamaneurol.2013.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology. 2017;88(7):653-660. doi: 10.1212/WNL.0000000000003610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun. 2018;6(1):33. doi: 10.1186/s40478-018-0531-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70(19 pt 2):1850-1857. doi: 10.1212/01.wnl.0000304041.09418.b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt FA, Nelson PT, Abner E, et al. University of Kentucky Sanders-Brown healthy brain aging volunteers: donor characteristics, procedures and neuropathology. Curr Alzheimer Res. 2012;9(6):724-733. doi: 10.2174/156720512801322591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66(12):1136-1146. doi: 10.1097/nen.0b013e31815c5efb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maass A, Landau S, Baker SL, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448-463. doi: 10.1016/j.neuroimage.2017.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abner EL, Kryscio RJ, Cooper GE, et al. Mild cognitive impairment: statistical models of transition using longitudinal clinical data. Int J Alzheimers Dis. 2012;2012:291920. doi: 10.1155/2012/291920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abner EL, Nelson PT, Schmitt FA, et al. Self-reported head injury and risk of late-life impairment and AD pathology in an AD center cohort. Dement Geriatr Cogn Disord. 2014;37(5-6):294-306. doi: 10.1159/000355478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240-246. doi: 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 40.National Alzheimer’s Coordinating Center . NACC Uniform Data Set: researchers data dictionary. Version 3.0. Published March 2015. Accessed January 11, 2019. https://www.alz.washington.edu/WEB/rdd_uds.pdf
- 41.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994. [Google Scholar]
- 42.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 43.Ravina B, Cummings J, McDermott M, Poole RM, eds. Clinical Trials in Neurology: Design, Conduct, Analysis. Cambridge University Press; 2012. doi: 10.1017/CBO9781139032445 [DOI] [Google Scholar]
- 44.Clark CM, Sheppard L, Fillenbaum GG, et al. Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol. 1999;56(7):857-862. doi: 10.1001/archneur.56.7.857 [DOI] [PubMed] [Google Scholar]
- 45.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63(1):38-46. doi: 10.1001/archneur.63.1.38 [DOI] [PubMed] [Google Scholar]
- 46.Gallyas F. Silver staining of Alzheimer’s neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung. 1971;19(1):1-8. [PubMed] [Google Scholar]
- 47.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18(4)(suppl):S1-S2. [PubMed] [Google Scholar]
- 48.Neltner JH, Abner EL, Schmitt FA, et al. Digital pathology and image analysis for robust high-throughput quantitative assessment of Alzheimer disease neuropathologic changes. J Neuropathol Exp Neurol. 2012;71(12):1075-1085. doi: 10.1097/NEN.0b013e3182768de4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479-486. doi: 10.1212/WNL.41.4.479 [DOI] [PubMed] [Google Scholar]
- 50.Nelson PT, Gal Z, Wang WX, et al. TDP-43 proteinopathy in aging: associations with risk-associated gene variants and with brain parenchymal thyroid hormone levels. Neurobiol Dis. 2019;125:67-76. doi: 10.1016/j.nbd.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. 2013;25(2):130-137. doi: 10.3109/09540261.2013.776523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chornenkyy Y, Fardo DW, Nelson PT. Tau and TDP-43 proteinopathies: kindred pathologic cascades and genetic pleiotropy. Lab Invest. 2019;99(7):993-1007. doi: 10.1038/s41374-019-0196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang HS, Yu L, White CC, et al. Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE ε4 haplotype status: a community-based cohort study. Lancet Neurol. 2018;17(9):773-781. doi: 10.1016/S1474-4422(18)30251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528-534. doi: 10.1212/WNL.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Filshtein TJ, Dugger BN, Jin LW, et al. Neuropathological diagnoses of demented Hispanic, black, and non-Hispanic white decedents seen at an Alzheimer’s disease center. J Alzheimers Dis. 2019;68(1):145-158. doi: 10.3233/JAD-180992 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram Included Cases
eTable 1. Proteinopathy Case Group Characteristics Who Began Follow-Up While Normal (n = 228)
eFigure 2. Bar Graph Showing Distribution of Proteinopathy Case Groups Among Participants (n = 375)
eFigure 3. Bar Graph Showing Distribution of Proteinopathy Case Groups for Those Participants Who Began Follow-Up While Normal (n = 228), and With Last Clinical Diagnosis of Dementia and MCI
eTable 2. Frequency of Missing Data in All Participants (n = 375)
eTable 3. Frequency of Missing Data in All Participants Who Began Follow-Up While Normal (n = 228)