Abstract
Background
Dietary fibers (DFs) are known as potential formulations in human health due to their beneficial effects in control of life-threatening chronic diseases including cardiovascular disease (CVD), diabetes mellitus, obesity and cancer. In recent decades scientists around the globe have shown tremendous interest to evaluate the interplay between DFs and gastrointestinal (GIT) microbiota. Evidences from various epidemiological and clinical trials have revealed that DFs modulate formation and metabolic activities of the microbial communities residing in the human GIT which in turn play significant roles in maintaining health and well-being. Furthermore, interestingly, a rapidly growing literature indicates success of DFs being prebiotics in immunomodulation, namely the stimulation of innate, cellular and humoral immune response, which could also be linked with their significant roles in modulation of the probiotics (live beneficial microorganisms).
Scope and approach
The main focus of the current review is to expressively highlight the importance of DFs being prebiotics in human health in association with their influence on gut microbiota. Now in order to significantly achieve the promising health benefits from these prebiotics, it is aimed to develop novel formulations to enhance and scale up their efficacy. Therefore, finally, herein unlike previously published articles, we highlighted different kinds of prebiotic and probiotic formulations which are being regarded as hot research topics among the scientific community now a days.
Conclusion
The information in this article will specifically provide a platform for the development of novel functional foods the demands for which has risen drastically in recent years.
Keywords: Dietary fiber, Human health, Prebiotics, Gut micro-biota, Encapsulation, Formulations
1. Introduction
The word dietary fiber (DF), denoting undigestible extremely intricate carbohydrates and lignins, was first described by Eben Hipsley in his article after seeing people consuming diets high in fiber-rich foods (Hipsley, 1953). In a more elaborated way, DFs comprises a large group of carbohydrates having varied physiochemical characteristics and therefore, also include both fructo and galacto oligosaccharides (Korcz, Kerényi, & Varga, 2018). Generally DFs are categorized into two classes i.e as soluble DFs (SDFs) including inulin or beta-glucan, and insoluble DFs (ISDFs) such as cellulose which is a structural carbohydrate not used for energy but mostly constitutes cell wall materials in plant cells (Prasad & Bondy, 2019). Whether SDFs or ISDFs, both these kinds of DFs have a range of anticipated functions. For example, due to their partial fermentation which provides bulk to the luminal materials and boost off colonic transit time, ISDFs play a critical role in safeguarding colonic epithelium from the harmful effects of different carcinogenic compounds ingested to the body (Kunzmann et al., 2015).
It is presumed that human gastrointestinal tract (GIT) is home to about 1014 bacterial cells, with the most populated and diversified community residing in the large intestine or colon. The presence of these bacterial colonies in the gut has been known to put forward a potential impact on human health. And it could be due to their capability to readily ferment the SDFs into short chain fatty acids (SCFAs), mainly including acetate, propionate and butyrate, as well as other metabolites which in turn confer potential health benefits (Koh, De Vadder, Kovatcheva-Datchary, & Bäckhed, 2016). Also, these colonic bacteria are known to have strong influences on metabolic processes and modulation of the immune system and provide significant protection against different pathogenic organisms while modulating GIT growth and development (Sugihara, Morhardt, & Kamada, 2019). The SDFs are also known to enhance bioavailability of beneficial natural flavonoids such quercetins (Trakooncharoenvit, Tanaka, Mizuta, Hira, & Hara, 2019).
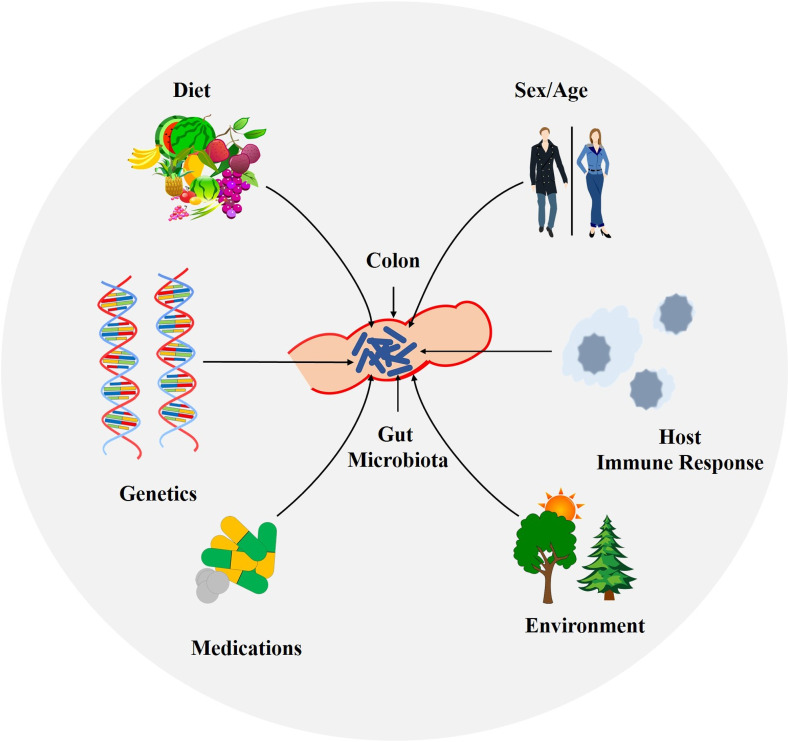
Previous literature shows that DFs have significant beneficial roles in the control of chronic diseases e.g cancer, type 2 diabetes, obesity, cardiovascular diseases (CVDs) etc, which is particularly linked to their impact on the gut microbiota (Slavin, 2013; Soliman, 2019). If left untreated, the aforementioned conditions could significantly subsidize in the progression of many life threatening chronic diseases including cancer, insulin resistance, type 2 diabetes and CVD, pro-inflammatory activity, inflammatory bowel disease (IBD) etc (Yoo & Kim, 2016). It is generally believed that the bacterial flora residing in the GIT vary from to person to person, however, mainly it remains stable over time periods (Rodríguez et al., 2015). In this perspective, a range of factors have been known to influence this useful bacterial flora in our GIT. These predominantly include genetical background of the individuals, their physiology, and surrounding environmental factors like living conditions and use of different medications (Fig. 1 ). Nevertheless, among these factors, diet is regarded as the most important environmental factor known to arbitrate their composition as well as other metabolic activities (Greenblum, Turnbaugh, & Borenstein, 2012). Furthermore, in recent decades, DFs have attracted considerable attentions owing to their significant roles being “prebiotics”. Prebiotics a group of DFs and are defined as “selectively fermented complex ingredients/carbohydrates that cause and promote specific changes, in the structure and/or function of the GIT bacterial flora, thereby exerting potential health benefit(s) upon host health.” (Gibson et al., 2010). Being food for probiotics, these prebiotics in combination with probiotics have shown significant antiviral efficacy. For example, in children with acute RotaVirus (RV) gastroenteritis, the oral administration of a mixture of Bifidobacterium lactis B94 and inulin as prebiotic showed a shorter duration of RV acute watery diarrhea (Islek et al., 2014). Also, milk fermented with B. breve C50, Streptococcus thermophilus 065, and combined with prebiotics prevents RV-induced diarrhea when fed to suckling rats (Rigo-Adrover et al., 2019).
Fig. 1.
Key factors influencing the composition as well as metabolic activities of gut microbiota.
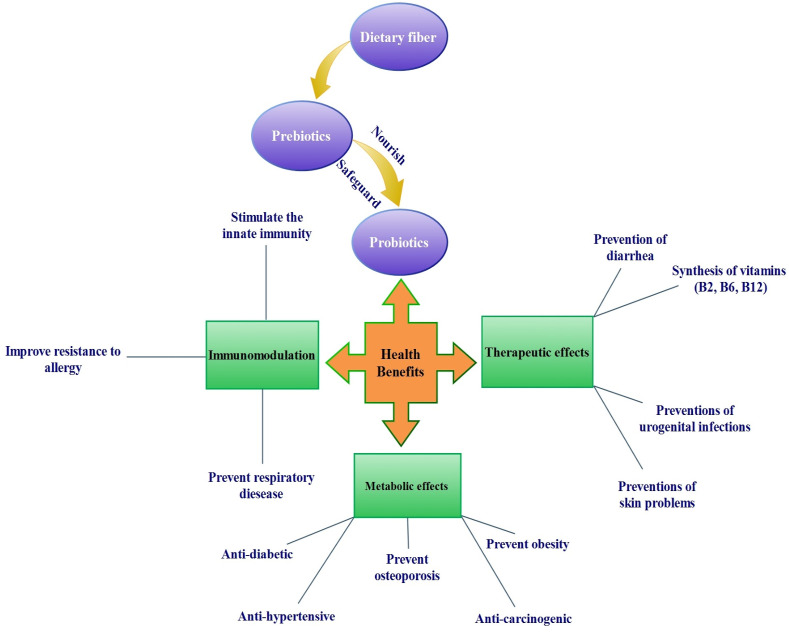
In light of the potential health benefits of prebiotics & probiotic given in Fig. 2 , the main aim of the current review is to highlight the importance of DFs in human health with presenting a critical background on its role as prebiotics.
Fig. 2.
Roles and potential health benefits of prebiotics & probiotics.
1.1. Effects of DFs in control of chronic diseases
As stated earlier, diets with high fiber contents are asserted to present significant protective effects against chronic diseases such as metabolic syndrome including type 2 diabetes (M. Müller, Canfora, & Blaak, 2018), CVD and obesity (M. Müller et al., 2018). Substantial research has been carried out so far stating DFs as important formulation in the control and treatment of these pathologies (Prasad & Bondy, 2019). In this context, in our previous review we also presented the health benefits of DFs particularly konjac glucomannan (KGM) with critical focus on its role in control of diabetes (Shah et al., 2015). It is thought that the benefit of DFs in control of diabetes is connected with their fermentation by gut microbiota to short chain fatty acids (SCFAs), the deficiency of which may lead to the development and progression of the disease (L. Zhao et al., 2018). Although most of the research community assumes that the detailed mood of actions of DFs in safeguarding against these pathologies is up to some extent complicated and not fully known, still there are evidences describing the presence of β-glucans in some DFs (e.g.oats) linked to curb the levels of total cholesterol and low density lipoprotein (LDL) cholesterol (Korcz et al., 2018). In particular instance, SDFs being more viscous are known to control and lower postprandial blood cholesterol and sugar rises by influencing their absorption from the small intestine because of the formation of gels by these fibers (Mackie, Bajka, & Rigby, 2016). Similarly, another mechanism in lowering blood cholesterol levels by SDFs involve their capability to attenuate the quantity of bile reabsorbed in the intestines thereby elevating the excretion of bile in feces (Threapleton et al., 2013). And in order to cope with this loss, the liver stimulates the release of extra bile salts via enhancing the production of LDL cholesterol receptors, which skillfully perform separation of LDL molecules in the blood stream (Jesch & Carr, 2017). Consequently, the elevated bile salts production by the liver causes isolation of more LDL molecules from the blood. Furthermore, in another way SDFs and indigestible starch molecules go through fermentation by intestinal bacterial flora resulting in the productions of SCFAs, which in turn aid in attenuating circulating cholesterol levels in blood (Threapleton et al., 2013).
No doubt while speaking about CVD, the term “obesity” can't be ignored as it is one of the main reasons for CVD related morbidities and mortalities. Till date, a substantial amount of research work has been reported dealing with interventional human trials to determine the potential effects of DFs rich diets or DFs supplements in weight loss management. The reason could be the indigestible characteristic of DFs which prolong their transit time in the intestinal lumen and thereby induce greater satiety compared with simple and digestible polysaccharides which are readily digested. Furthermore, DFs could also be involved in prolongation of meal intervals and induce an enhanced mastication with presumable cephalic and peripheral effects on satiety. Interestingly and more healthfully diets rich in DFs have a lesser energy bulk and could influence palatability of foods, which could ultimately lead to a lower calories intake (Benton & Young, 2017). Another mechanism related to appetite reducing effects of DFs, is based on stimulating the signaling of GIT satiation peptide hormones such as glucagon-like peptide (GLP-1) and peptide YY (PYY) or glucose dependent insulinotropic peptide (GIP) by DFs which result from their fermentation in the large intestine by gut microbiota (Lim & Poppitt, 2019). Han et al. (2017) found that both oat and wheat fiber significantly reduced body and adipose tissues weight in the rats fed with high fat diet (HFD). Also compared to HFD group, cereal DFs increased protein expressions involved in the lipolysis and browning process. From their results, they concluded that cereal DFs promoted adipocyte lipolysis by the cAMP–PKA–HSL pathway and enhanced WAT browning by activation of UCP1, and consequently reduced visceral fat mass in response to HFD feeding (Han et al., 2017). It is crucial to mention here that although research studies conducted on animal experimental models usually have more pronounced effects, however, the amount of fibers generally used in these studies are also higher compared to the levels used in human clinical trials, particularly in cases for prebiotics, where the dose is generally 40-fold higher on the basis of body weight (Makki, Deehan, Walter, & Bäckhed, 2018).
The preceding results imply that DFs have shown significant effects in control and treatment of different pathological conditions, still there are reports where contradictory results have been observed in the treatment groups in comparison to the control ones. For example, in a study conducted to evaluate the effects of fibers intake on inflammatory markers (IM) in post-menopausal women, it was found that women in the higher fibers intake group (24.7 g/day) had lower plasma IL-6 and TNF-α-R2 (receptor 2 of the TNF-α) levels as compared to those with lower ingestion (7.7 g/day). However, there was no association between fibers and c-reactive protein (CRP) in both the groups (Grundy, Brewer, Cleeman, Smith, & Lenfant, 2004). Likewise, in another study individuals from different ethnical groups, who consumed more fibers (1.39 g/serving of food) compared to those with the less consumption (0.02 g/serving) were favored with lower CRP values (King et al., 2007). Even higher intake of DFs (43.8 g/day) as compared to lower intake (8.2 g/day) didn't show significant effects on IM in the two experimental groups, except for the lower values of homocysteine among several individuals analyzed in the study. And hence, the authors of the study concluded that there could be many influential factors such as group homogeneity, better conditions of the subjects assessed to get fiber-rich diets, statistical errors in the measures of randomization, that affected outcomes of the study (Jensen et al., 2006). As human trials with consumption of DFs dosage greater than 50 g/day exhibited significant improvements in the assessed health markers (S. J. O'Keefe et al., 2015; L. Zhao et al., 2018), it could be postulated that the health benefits of DFs are strongly co-related with their daily intake and it is suggested to be greater than 50 g, which could be achieved not only by regular food but also with the addition of supplements (S. J. D. O'Keefe, 2018). However, in modern world there could be problems in tolerating such a higher dose and could lead to a number of gut related undesirable conditions like flatulence, bloating, stomach pain, diarrhea, and constipation (Grabitske & Slavin, 2009).
1.2. Colonic microflora and fermentation of DFs
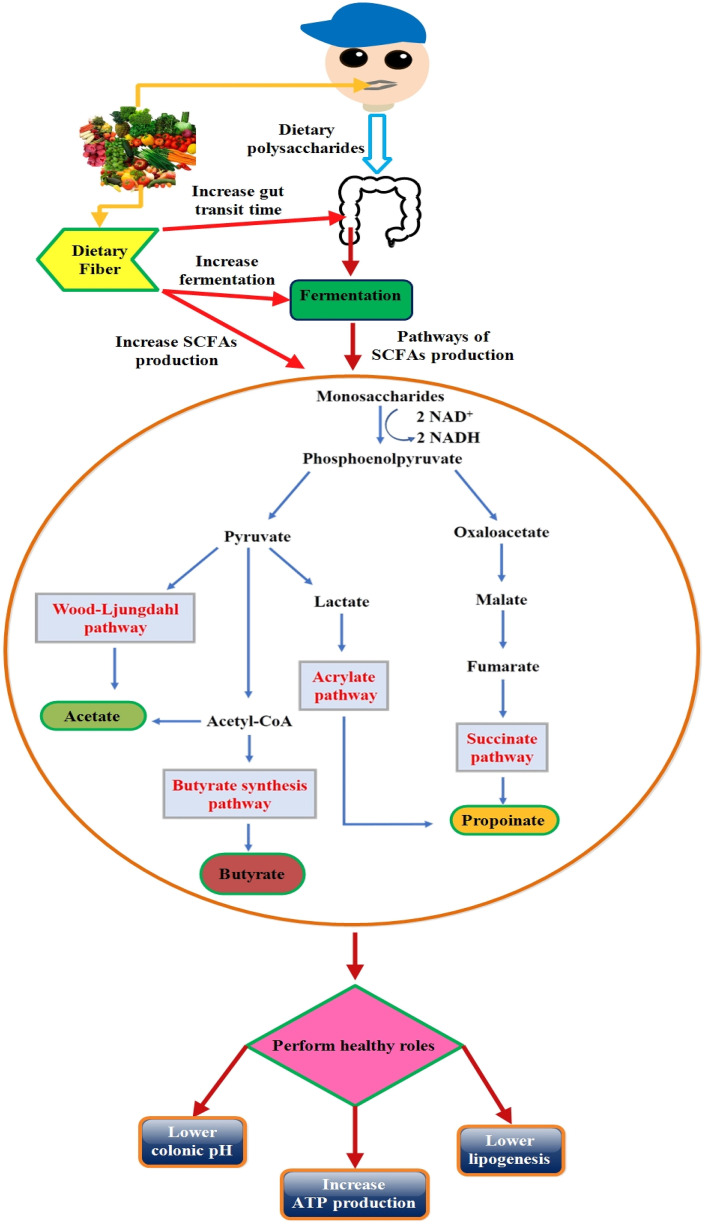
As stated earlier, the human gut is a natural habitat for trillion of microorganisms which not only contribute a significant role in promoting GIT health itself but also play their potential part in general health well beings. The diversity and constitution of bacterial flora residing in the GIT significantly differ from person to person and is greatly affected by many factors including age (Mariat et al., 2009), genetics (Khachatryan et al., 2008) and Neolithic settlements (Larsen et al., 2010). However, among them, dietary habits are regarded as one of the most crucial factors which can be modified accordingly by selecting the diets rich in complex undigestible components (resistant starches), unable to be digested by human enzymes but rather by these bacteria for their growth and energy (Milani et al., 2017). As schematically shown in Fig. 3 , these undigested complex and resistant starches arriving the colon of large intestine encounter anaerobic fermentation producing vital SCFAs (mainly acetate, propionate and butyrate) (Canfora, Meex, Venema, & Blaak, 2019). Broadly speaking, each of the produced SCFAs has a different and individual role in exerting healthy effects but in general they perform a fundamental collective role. For example being weak acids, all of the produced SCFAs cause in decreasing the colonic pH which is of potential advantage to prohibit the growth of harmful pathogenic bacteria such as Enterobacteraciae due to their sensitivity to low pH (den Besten et al., 2013). More specifically, on individual scale, it has been researched that after being transported to liver, propionate efficiently contribute in gluconeogenesis to produce glucose from non-carbohydrate sources (i.e. proteins or lipids). Similarly, acetate is carried in the blood to the peripheral tissues, where it is involved in enhancing ATPs production via citric acid cycle thereby scaling up fermentation proficiency (Gonzalez-Garcia et al., 2017). On the other hand, butyrate is known as an important calorie source for the colonic epithelial cells instead of other energy sources like glucose and glutamine. Butyrate is thought to be a fundamental nutrient executing the metabolic activity and growth of colonocytes and may work as a prime safeguarding factor against colonic disturbances (Murugesan et al., 2018). These claims were further backed by the results stating a significant reduction in butyrate-producing bacteria in feces of individuals suffering from ulcerative colitis and colon cancers in comparison to the healthy subjects (Sivaprakasam et al., 2016; L.; Zhao et al., 2018). Also, butyrate has shown the capability of promoting metabolic disorders (CVD and related disorders) via seral mechanisms including AMPK activation and GLUT4 expression in the adipose tissue, combating high fat diet (HFD) incited gut microbiome dysbiosis, and stimulating resolvin E1 and lipoxin biosynthesis (Gao et al., 2019). Its noteworthy to mention that the roles and actions of SCFAs are also highly controversial. And it has been known that in mice, SCFAs usually evoke positive effects on disease phenotype but in humans, this effect is not as straightforward (Chambers, Preston, Frost, & Morrison, 2018). Also, SCFA turnover is very high and local, hence measures of SCFA in plasma/feces should be interpreted with great caution since it is unlikely to be an accurate representation of SCFAs metabolism (Venegas et al., 2019).
Fig. 3.
Interplay between dietary fiber and colonic microbiome.
1.3. Effects of DFs on composition of gut microbiome
Previous literature has acknowledged that the kind of dietary substrate as well as the overall DFs in the diet are the two factors that potentially affect the composition of gut microbiota. Consequently, this alteration in bacterial composition in the gut by DFS in turn influences several important processes such as pH and amounts of SCFAs, which can ultimately even have a strong impact on the bacterial composition itself. This provided scientists around the world with an interesting background to identify and comprehensively understand the relationship between different kinds of DFs and the gut microbiota. One research group analyzed and compared the fecal bacterial contents of children from two different ethnic groups i.e European children (EU) and children from a rural African village of Burkina Faso (BF), which is the area where the diet is generally believed to contain higher amounts of fibers, as occurred during the early human settlements from the time when agriculture came into being. They found significant differences in the gut microbiota of the two selected groups as analyzed by high-throughput 16S rDNA sequencing and biochemical analyses. Furthermore, they also determined a significant difference in the production of short-chain fatty acids (P < 0.001) in BF than in EU children (De Filippo et al., 2010). Another research group described the same phenomenon by determining the effects of a highly branched randomly linked polysaccharide i.e Polydextrose, and a SDF derived from corn containing oligosaccharides with random glycosyl bonds. Performing 454 pyrosequencing, these fibers exhibited a favorable move in the gut microbiota of the adult population thereby hallmarking them with potential application as prebiotics (Hooda et al., 2012). Inulin a potential DF based prebiotic is a carbohydrate formed by β-D (2 → 1) linked fructose oligomers with terminal β-D fructose or α-D glucose units joined by α-D (1 → 2) linkage which has gained tremendous attention in functional foods development (Alvarez-Sabatel, de Marañón, & Arboleya, 2018; J.; Xu et al., 2016). On large industrial scale, inulin obtained from dahlia, chicory and Jerusalem artichoke and is well known prebiotic having astonishing beneficial characteristics which made it interesting candidate to be extensively used in formulating functional foods (W. Xu et al., 2018). It has shown to aid in the prevention of diarrhea associated with enteral nutrition and to boost up GIT health (Esmaeilnejad Moghadam, Keivaninahr, Fouladi, Rezaei Mokarram, & Nazemi, 2019; Silva, Guimarães, Costa, Cruz, & Meireles, 2019), to enhance stability and protection of probiotics (Krithika & Preetha, 2019; Peredo, Beristain, Pascual, Azuara, & Jimenez, 2016), to reduce fat contents of the food products (Glisic et al., 2019) etc. The principal product obtained by inulin fermentation is butyrate, which is thought to be the result of two different mechanisms. The first pathway involves the condensation of two molecules of acetyl coenzyme A and the reduction of them to butyryl-CoA. In the other connected metabolic pathway, the generated butyryl-CoA:acetate CoA transferase can move the CoA to extrinsic acetate, resulting in the production of butyrate and acetyl-CoA (Karimi, Azizi, Ghasemlou, & Vaziri, 2015). In earlier times Hannah et al. determined the influence of agave inulin on gut microbiome. Agave inulin, which is consisted of linear and branched fructose chains, connected with β-2,1 and β −2,6 linkages, is considered different from other inulin kinds of fibers both in chemical composition as well as botanical origin. After feeding to healthy individuals with different doses in a randomized, double-blind, placebo-controlled, 3-period, crossover trial, it was concluded that agave inulin supplementation significantly altered the GIT microbiota composition and activity in the selected healthy individuals (Holscher et al., 2015). In the next year, Birgitte and colleagues., evaluated the effects of dietary inulin, cellulose or brewers spent grain (BSG) on intestinal tumorigenesis and cecal microbiota. Their results demonstrated that the type of DF contributes a potential role in the progression of colorectal cancer; however the protective effects of dietary inulin against colonic tumorigenesis is strongly linked with the profound alterations in the ceacal microbiota profile (Moen et al., 2016). Nevertheless, the impact of different dietary trials in clinical practice have shown controversial results due to a significant variability in individual response against the tested diet, which could be linked to variations in their gut microbiome as stated earlier that gut microbiome vary from person to person. Last year Biesiekierski and colleagues published a comprehensive review on these issues by citing studies dealing with the predictive dimensions of baseline microbiome for clinical response to dietary trials in two particular pathological conditions, i.e. obesity and IBS (Biesiekierski, Jalanka, & Staudacher, 2019). Therefore, it is still a challenging task and needs further large-scale interventions to address and provide solid grounds for these claims that can be significantly undertaken in clinical practices.
1.4. Whole grains and gut microbiome
According to AACCI, the whole grains (WGs) can be defined as the “integral, ground, cracked or flaked fruit of the grain whose original three parts, the starchy endosperm, germ and bran, are present in the same relative proportions as they exist in the intact grain” (AACCI & (American Association of Cereal Chemists International), 1999). Generally speaking, the WGs constitute a huge group of staple foods that includes wheat, corn, rice, oats, barley, rye and so on (Hui et al., 2019). It is evident from the available literature that WGs confer a broad spectrum of potential health benefits due to their richness in bioactive compounds which may significantly affect processes like energy metabolism, weight regulation and food intake (Koecher, McKeown, Sawicki, Menon, & Slavin, 2019). One possible mechanism by which WGs confer benefits maybe be associated with their capability of modulation gut microbiota where DFs are regarded as the fundamental factor to stimulate this modulation. It is postulated that the fiber contents of the WGs imparts enhanced viscosity to the digesta and lower glucose as well as cholesterol levels by binding to the bile acids in the small intestine (Wu, Inui, & Chen, 2020), thereby implying DFs and whole grains based diets very important in diabetes management to aid in glycaemic, cholestrol as well as body weight, and inflammation controls (Reynolds, Akerman, & Mann, 2020; Wang et al., 2020). Similarly, another pivotal role of WGs involve their ability to curb protein fermentation by the gut microbiota, which can ultimately lead to protein fermentation accountable for unwanted fermentation metabolites and harmful impacts on the host (Russell et al., 2011). Also, another noteworthy characteristic of the WGs is their richness in resistant starch (RS) which is particularly butyrogenic and thus scale up the increased production of butyrate by the bacterial fermentation (Gong et al., 2018). And the bacterial fermentation of the undigestible components of WGs in the GIT has been agreed upon to be partly associated with the healthful effects of WGs (Koecher et al., 2019). In addition, the WGs have also demonstrated an anti-inflammatory activity and hence can be of significant benefit in lessening of metabolic inflammation responsible for metabolic disorders (McCarty & Assanga, 2018) as has been mentioned in a meta-analysis of 9 randomized trials (Y. Xu et al., 2018). This specific significance of WGs become particularly applicable in light of recent research that designated a strong connection of gut bacteria involvement in metabolic disorders (Gregor & Hotamisligil, 2011). Therefore, in 2013, a research group evaluated the influence of the diet supplemented with WGs on gut microbiota in healthy human subjects. They also explored a possible connection between metabolic and immunological advancements observed in response to the experimental diet. Their results stated that short-term utilization of the WGs significantly modified the composition of the gut microbiota which as a consequence interestingly supported in excelling host physiological actions connected with metabolic dysfunctions in humans (Martínez et al., 2013). In the year 2017, Sally and his research group evaluated the effects of two different types of diets 1) rich in WGs and 2) rich in refined grains (RGs) on immune and inflammatory responses, gut microbiota, and microbial products in healthy adults while maintaining subject body weights. Presenting their results, they stated that the short-term consumption of WGs had significant effect on the studied parameters in comparison to the RGs diets. The WGs maintenance diet increased stool weight and frequency and had modest positive impacts on gut microbiota, SCFAs, effector memory T cells, and the acute innate immune response and no effect on other markers of cell-mediated immunity or systemic and gut inflammation (Vanegas et al., 2017). In the following year, researchers from Japan in a randomized double-blind study investigated the effects of WGs wheat bread on visceral fat obesity in Japanese individuals. After 12 weeks of experimental trials, the subjects who consumed WG wheat bread showed significant and safe reductions in visceral fat areas (Kikuchi et al., 2018). Similar phenomenon was also described by another group in a randomized control showing the role of WGs to promote gastrointestinal health in overweight and obese individuals (Kopf et al., 2018). In the following year, the effects of dietary WG, fruit, and vegetables on weight and inflammatory biomarkers in 75 overweight and obese women were assessed in a randomized control trial. Significant modifications in serum biomarkers (CRP, TNF-α, IL-6, D-dimer, and serum fibrinogen) after consuming the trial diet for 10 weeks suggested WG based diet to support in attenuating inflammatory degrees and control following unpleasant health outcomes (Arabzadegan et al., 2019, pp. 1–9). WG millet has also shown significant impact on lipid metabolism in both the in vitro cells and the in vivo rats (fed with high fat diet) experimental models. Associated with its lowering lipid storage, total cholesterol and triglyceride contents in the cells, it was found that WG millet exhibit hypolipidemic characteristics by modulating lipid metabolism concomitant genes expression or composition of gut microflora (Li et al., 2019). However, in the same year another research group found that whole barley had the potential in preventing obesity and dyslipidemia in germ free C57BL/6J obese mice with or without the involvement of gut microbiota related mechanisms (Gong, Wang, Sun, Wang, & Sun, 2019). These observations were further supported by another randomized cross-over trial stating body weight and systemic low-grade inflammation lowering effects of WG without major modifications in the composition of gut microbiota (Roager et al., 2019; Wu et al., 2020). Nevertheless, most recently, Zhu and colleagues found that a fiber rich Med diet changed human gut microbiota composition and its metabolites after consumption of just 4 days in comparison to an animal fat–rich, low-fiber fast food diet (Zhu et al., 2020). The preceding discussion hallmarks a wide range of health benefits conferred by different WGs. In order to elucidate the extent to which WGs offer these healthy impacts, Hui et al. categorized the lipid monitoring effects of different WGs by performing a network meta-analysis of fifty-five studies including a total of 3900 participants. They found oat bran to be the most effective than barley, brown rice, wheat and wheat bran which were not reported with significant effects on blood lipid profiles (Hui et al., 2019). Moreover, the efficacy of WGs in prevention of cancer has also been extensively research previously. In this perspective, in the cancer prevention study-II nutrition cohort, it has been found that higher WGs intake was linked with lower incidence of colorectal cancer risks in older US men (Um et al., 2019) and gastric cancer risks as summarized in a meta-analysis of observational data from a registered study (Y. Xu, Yang, Du, Li, & Zhou, 2019).
1.5. Prebiotic effects of DFs
Prebiotics are known as the “selectively fermented food ingredients that allow particular modifications both in the composition and/or performance of the gut microflora, thereby imparting health benefits to the host” (Gibson et al., 2010). This definition elaborates that not all DFs can be put together in the group of prebiotics; however, most of the prebiotics can be regarded as DFs (Slavin, 2013). Scientifically, food constituents can be classified as prebiotics if they proficiently perform these actions; (i) must resist gastric acidity, hydrolysis by human enzymes, and gastrointestinal absorption; (ii) must be fermented by the gut microflora; (iii) must stimulate the growth and activity of gut microflora associated with health (Davani-Davari et al., 2019). Till date a substantial research has stamped prebiotics as important formulations contributing to the well-being of human health. For example, they have been claimed to promote gut epithelial barrier concerns, prevent apoptosis of intestinal epithelial cells, and attenuate intestinal inflammation and carcinogenesis (Baliou et al., 2019). Similarly, prebiotics were also found to lower down obesity in experimental obese mice by affecting the gene expression style occurring in their white adipose tissues, and therefore resulting in increasing lipolysis, a decreased adipogenesis and elevated metabolic response to leptin (E. M. Dewulf et al., 2011). Furthermore, prebiotics potentially contribute in ameliorating metabolic disorders e.g. type 2 diabetes and CVD via promoting and stimulating gut microbiota, which in turn stimulates insulin-signaling and cholesterol-lowering characteristics (Yoo & Kim, 2016). It is believed that the suggested pathways could comprise the secretion of gut peptides with incretin role, such as glucagon-like peptide 1, which takes part in the development of hepatic insulin resistance (T. D. Müller et al., 2019). Prebiotics have also exhibited shielding effects on liver, because of their capability to prevent the hepatic storage of TAG and/or cholesterol in the liver tissue known as steatosis (Loman, Hernández-Saavedra, An, & Rector, 2018).
In light of the preceding discussion, it can be stated that consumption of prebiotics in addition to DFs is considered as an avenue in the modulation of gut microbiota. The natural sources of prebiotics with their ample quantities include foods such as asparagus, leeks, garlic, onion, Jerusalem artichokes, oats and soybeans so on (Davani-Davari et al., 2019). Popular prebiotics, such as lactulose, galacto oligosaccharides and inulin-type fructans (eg, inulin, oligofructose and fructo-oligosaccharides), have been shown to perform a critical role in stimulating beneficial alternation in the gut microbiota composition as well as function including SCFAs production and modulating appetite and satiety) (Bindels, Delzenne, Cani, & Walter, 2015). Earlier studies have recognized the reality that oligosaccharides, particularly fructo oligosaccharides (FOS) and inulin are key candidates acknowledged for their prebiotic roles. Outcomes of randomized control trials have revealed that these oligosaccharides enhance production of Bifidobacterium (a genus of oligosaccharides fermenting gut microflora) as well as Lactobacillus species (Tojo et al., 2014). Nevertheless, lately some other bacterial species such as Roseburia intestinalis, Ruminococcus bromii and Faecalibacterium prausnitzii are evolving as bacteria linked with good health (Conlon & Bird, 2015). This assures that prebiotic treatment is not involved just in boosting Bifidobacterium, but also endorses other species displaying anti-inflammatory characteristics (Faecalibacterium prausnitzii) and weight control (Akkermansia muciniphila) (Zhang, Li, Cheng, Buch, & Zhang, 2019). This claim was further supported by the findings of Dewulf et al. presenting human subjects. They issued their results with a statement that even though the rise in Bifidobacteria remains the foremost and common signature of the prebiotic approach, a complex modulation of the gut microbial ecology also happens upon prebiotic treatment in obese individuals (E. Dewulf et al., 2012). A summary of clinical trials based on pre & probiotics applications conducted in aim to treat different diseases during the past decade mostly during the last five years has been outlined in Table 1 .
Table 1.
Summary of clinical trials based on applications of prebiotics and probiotics aimed for the treatments of different diseases.
| S. No | Study design | Name of disease | No. of patients | Age group (in years) | Duration of the study | Name of pre & prebiotic used | Control group | Main outcome of the study | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Randomized Control Trial (RCT) | Irritable bowel syndrome (IBS) | 39 | 19–75 | 4 weeks | Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium breve, B. actis, B. longum, and Streptococcus thermophilus | Placebo | Significantly enhanced the fecal contents of most probiotic strains. Promoted diarrhea-symptom scores in the IBS patients. |
Yoon et al. (2015) |
| 2 | Double Blind Clinical Trial | Inflammatory bowel disease (IBD) | 105 | 36–38 | 8 weeks | Lactobacillus acidophilus La-5 and Bifidobacterium BB-12 | Placebo | Significantly increased the numbers of Lactobacillus, Bifidobacterium, and Bacteroides in the treated group | Shadnoush, Hosseini et al. (2015) |
| 3 | Randomized, Double-Blind, Placebo-Controlled Trial | Infantile colic | 24 | <1 (3 weeks-6 months) | 21 days | Lactobacillus reuteri DSM 17938 | Placebo | Significantly improved colic symptoms | Chau, Lau et al. (2015) |
| 4 | RCT | Necrotizing enterocolitis (NEC) | 400 | <1 (<32 weeks) | 8 weeks | Bifidobacteriumlactis as probiotic and inulin as prebiotic | Placebo | Probiotic (Bifidobacterium lactis) and synbiotic (Bifidobacterium lactis plus inulin) but not prebiotic (inulin) alone decrease NEC | Dilli, Aydin et al. (2015) |
| 5 | RCT | Nonalcoholic fatty liver disease (NAFLD) | 42 | 18–65 | 8 weeks | Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophilus | Placebo | Significantly reduced insulin, insulin resistance, TNF-a, and IL-6 decreased | Sepideh, Karim et al. (2016) |
| 6 | RCT | Rheumatoid arthritis (RA) | 46 | 20–80 | 8 weeks | Lactobacillus casei 01 | Placebo | No significant effect on oxidative status was observed between the treated and placebo groups | Vaghef-Mehrabany, Homayouni-Rad et al. (2016) |
| 7 | Randomized, Double-Blind, Placebo-Controlled Trial |
RA | 30 | 25–70 | 8 weeks |
Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum |
Placebo | Significantly improved Disease Activity Score of 28 Joints (DAS-28). Significantly lowered serum insulin levels, homeostatic model assessment-B cell function (HOMA-B), and serum high-sensitivity C-reactive protein (hs-CRP) concentrations |
Zamani, Golkar et al. (2016) |
| 8 | Randomized, Double-Blind, Placebo-Controlled Trial |
Alzheimer's Disease (AD) | 60 | 60–95 | 12 weeks | Lactobacillusacidophilus, Lactobacilluscasei,Bifidobacteriumbifidum, andLactobacillusfermentum | Placebo | Significantly improved Mini-mental state examination (MMSE)score in the treated group. No considerable effect on oxidative stress and inflammation, fasting plasma glucose, and other lipid profiles was observed |
Akbari, Asemi et al. (2016) |
| 9 | Randomized, Double-Blind, Placebo-Controlled Trial |
Parkinson disease (PD) | 120 | 71.8 ± 7.7 | 4 weeks |
Streptococcus salivarius subsp thermophilus, Enterococcus faecium, Lactobacillus rhamnosus GG, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp bulgaricus, and Bifidobacterium as probiotics and Psyllium fiber as prebiotic |
Placebo | Significantly improved constipation in PD patients. | Barichella, Pacchetti et al. (2016) |
| 10 | Randomized, Double-Blind, Placebo-Controlled Trial | Overweight diabetic patients with coronary heart disease (CHD). | 30 | 40–85 | 12 weeks | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, as probiotics and Inulin as prebiotic | Placebo | Significantly decreased plasma glucose, serum insulin concentrations. Improved insulin sensitivity. |
Tajabadi-Ebrahimi, Sharifi et al. (2017) |
| 11 | Double-Blind, Placebo-Controlled Trial | IBS | 54 | 18–70 | 8 weeks |
Lactobacillus rhamnosus L. casei, L. paracasei, L. plantarum L. acidophilus, Bifidobacterium bifidum, B. longum, B. breve, B. infantis, Streptococcus thermophilus, L. bulgaricus and Lactococcus lactis |
Placebo | No significant difference was observed between the treated and placebo groups | Hod, Sperber et al. (2017) |
| 12 | Randomized Triple-Blind Placebo-Controlled Trial |
NAFLD | 64 | 10–18 | 12 weeks | Lactobacillus acidophilus, Bifidobacterium lactis, Bifidobacterium bifidum, Lactobacillus rhamnosus | Placebo | Significantly decreased levels of alanine aminotransferase, mean aspartate aminotransferase, mean cholesterol, low-density lipoprotein-C, and triglycerides as well as waist circumference in the intervention group. No significant change in weight, body mass index, and body mass index z score was observed between the two groups. |
Famouri, Shariat et al. (2017) |
| 13 | Double-Blind, Placebo-Controlled Trial | Overweight or obesity | 22 | 7–12 | 12 weeks | Oligofructose-enriched inulin | Placebo | Significantly decreased body weight z-score, percent body fat, and percent trunk fat and levels of interleukin 6 and serum triglycerides in the treated group. | Nicolucci, Hume et al. (2017) |
| 14 | Randomized, Double-Blind, Placebo-Controlled Trial | IBS | 22 | 30–50 | 10 weeks | Bifidobacterium longum NCC3001 (BL) | Placebo | BL reduced depression but not anxiety scores and increases quality of life in patients with IBS | Pinto-Sanchez, Hall et al. (2017) |
| 15 | Randomized Double-Blind Placebo-Controlled Clinical Trial | Diabetes | 60 | 54.0 ± 16.0 | 12 weeks | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum | Placebo | Significantly decreased fasting plasma glucose, homeostasis model of assessment-estimated insulin resistance, homeostasis model of assessment-estimated beta-cell function and HbA1c, and improved quantitative insulin sensitivity check index. Significantly reduced serum high-sensitivity C-reactive protein, plasma malondialdehyde, subjective global assessment scores and total iron binding capacity, and a significant increase in plasma total antioxidant capacity |
Soleimani, Mojarrad et al. (2017) |
| 16 | Randomized, Double-Blind Trial | Overweight or obesity | 21 | 20–59 | 8 weeks | Lactobacillus acidophilus and casei; Lactococcus lactis; Bifidobacterium bifidum and lactis | Placebo | Significantly reduced the waist circumference, waist-height ratio, conicity index, and plasma polyunsaturated fatty acids and increased the activity of glutathione peroxidase. | Gomes, de Sousa et al. (2017) |
| 17 | Double Blind Randomized Clinical Trial | NAFLD | 75 | 20–60 | 12 weeks | Bifidobacterium longum (BL) and Lactobacillus acidophilus as probiotic and inulin high performance (HP) as prebiotic | Placebo | Supplementation with probiotics and/or prebiotics improved amino transferase enzymes, and supplementation with probiotics or pro- and prebiotics recovered the grade of fatty liver in NAFLD patients. | Javadi, Ghavami et al. (2017) |
| 18 | Randomized, Double-Blind, Placebo-Controlled Trial | Overweight and obese type-2 diabetes | 60 | 30–55 | 45 days | Sodium butyrate and inulin | Placebo | Significantly improved Akkermansia muciniphila percent change. Significant decreased TNF-α mRNA, hs-CRP, MDA and diastolic blood pressure levels in the treated groups |
Roshanravan, Mahdavi et al. (2017) |
| 19 | Randomized Controlled Clinical Trial | Diabetic kidney disease (DKD) | 48 | 32–68 | 8 weeks | L plantarum A7 | Placebo | Improved oxidative stress factors among DKD patients | Miraghajani, Zaghian et al. (2017) |
| 20 | Double Blind Randomized Clinical Trial | NAFLD | 84 | 20–60 | 12 weeks | BL as probiotic and inulin as prebiotic | Placebo | Probiotic and prebiotic supplementation improved serum lipid profile and insulin resistance markers in NAFLD patients. | Javadi, Ghavami et al. (2018) |
| 21 | Placebo-Controlled, Double-Blind Randomized Controlled Trial |
Major depressive disorder | 81 | 18–50 | 8 weeks | Lactobacillus helveticus and B.L. as probiotic and galactooligosaccharide as prebiotic | Placebo | Improved Beck Depression Inventory (BDI) score in the treatment group | Kazemi, Noorbala et al. (2019) |
| 22 | Single-Blind, Parallel, Randomized, Placebo-Controlled Trial | Overweight or obesity | 41 | 30–65 | 12 weeks | Bacillus coagulans BC30, 6086 as probiotic and β-glucans as prebiotic | Placebo | The formulated synbiotic pasta showed synbiotic exhibited beneficial effects on plasma resistin, plasma LDL/HDL cholesterol ratio, and plasma hs-CRP | Angelino, Martina et al. (2019) |
| 23 | Double-Blind, Placebo-Controlled, Randomized Clinical Trial | Overweight or obesity | 50 | 20–60 | 12 weeks |
B. breve CBT BR3, L. plantarum CBT LP3 as probiotic and fructo-oligosaccharide as prebiotic |
Placebo | Significantly improved obesity-related markers in obese people | Song, Han et al. (2020) |
1.6. Anti-viral efficacy of prebiotics & probiotics
Nowadays a strong and efficient inner defense mechanism (immunity) is more and more important than ever before. In this perspective, balanced nutrition and wise selection of nutrients can be a crucial strategy to significantly aid in excelling our immune system. These facts are backed by different research works which have shown that prebiotics and probiotics modulate the gut microbiota and interact with innate and adaptive immunity. This could be due to the existence of the major part of immune systems in the large intestine which is home to trillions of beneficial micro-organisms (microbiome) (Wells, 2011). A substantial research work has been done in this area to provide scientific proofs for these claims. For example, in a randomized placebo-control trial, it was hypothesized that early prebiotic or probiotic supplementation would reduce the risk of virus-associated respiratory tract infections (RTIs) during the first year of life in a cohort of preterm infants. The results concluded that supplementation with prebiotics galacto oligosaccharide and polydextrose mixture at ratio1:1 and a probiotic (Lactobacillus rhamnosus GG, ATCC 53103) significantly reduced the incidence of (RTIs) in these children as compared to the control placebo group receiving (microcrystalline cellulose). And hence it was suggested that gut microbiota modification with specific prebiotics and probiotics might offer a novel and cost-effective means to reduce the risk of rhinovirus infections (Luoto et al., 2014). The same probiotic bacterium (Lactobacillus rhamnosus) also showed to enhance macrophage viability for HSV-1 (herpes simplex virus type 1) elimination and activation against HSV-1 more effectively than non-probiotic Escherichia coli (Khani, Motamedifar, Golmoghaddam, Hosseini, & Hashemizadeh, 2012). Another study determined the anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity. The results concluded potential protective effects of the orally administrated (10 mg/mouse per d) probiotics bacteria against influenza virus infection in mouse model infected intranasally with influenza virus (H1N1) (Goto et al., 2013). Rotavirus (RV) infection, the underlying cause of diarrhea and vomiting leads to sever dehydration in patients specially children and therefore is consider the second leading cause of mortality in children < 5 years of age (Yen et al., 2014). One of the possible biological pathways inducing diarrhea due to the infection is nonstructural protein (NSP4) production that causes Ca2+ -dependent transepithelial secretion. Therefore, the antiviral activity of four probiotic metabolites (Lactobacillus casei and Bifidobacterium species) was evaluated using in vitro RV infection model. The findings demonstrated that probiotic metabolites were capable to interfere with the final amount of intracellular NSP4 protein and a successful Ca2+ regulation, thereby suggesting probiotics based novel approach against the RV infection (Olaya Galan et al., 2016). In 2017, researchers from China evaluated the effects of probiotic bacteria Lactobacillus plantarum strain N4(Lp) against transmissible gastroenteritis coronavirus. The results found a significant viral yields reduction in the treatment groups due to the optimal inhibition of viral RNA replication (Yang et al., 2017). Last year in another randomized, placebo-controlled trial with 258 healthy children aged 3–6 years consuming 6 g/day prebiotic inulin-type fructans or maltodextrina, it was found that supplementing these children during a cold season with the prebiotic significantly reduced febrile episodes requiring medical attention and lowered the incidence of sinusitis, which could be attributed to the modulation of specific gut microbiota by this prebiotic (Soldi et al., 2019). In the same year, another research group evaluated the in vitro immunomodulatory and anti- Campylobacter jejuni activities of probiotic lactobacilli spp. (L. salivarius, L. johnsonii, L. reuteri, L. crispatus, and L. gasseri). The results demonstrated that lactobacilli carry differential antagonistic effects against C. jejuni and vary in their ability to trigger off innate responses (Taha-Abdelaziz et al., 2019).
1.7. Prebiotics and probiotics based formulations (emulsions & nano, microparticles)
In recent years a greater attention has been attracted by different kinds of prebiotic formulations. Among them, nowadays the most favorable and predominantly practiced one is the encapsulation of these prebiotics into emulsions or nano & microparticles based systems (summarized in Table 2 ). Encapsulation is a versatile technique used to enclose the encapsulants within polymeric solid, liquid or semi-solid shells in order to protect them from the harsh environments (e.g light, oxygen, pH, heat etc) as well as to control their release. For example, Vito et al. produced emulsion filled gels (EFG) based on inulin and extra virgin olive oil, by means of both mechanical shearing and ultrasound homogenization (Paradiso et al., 2015). Similar study was also conducted by another research group which evaluated the stability, consistency, and oil oxidation of emulsion filled gel prepared by inulin and rice bran oil using ultrasonic radiation (Nourbehesht, Shekarchizadeh, & Soltanizadeh, 2018). In order to enhance microbiological, nutritional, and sensory quality of strawberry juices after 2 weeks of refrigerated storage, another study group reported the enrichment of the juices with prebiotic fiber (inulin and oligofructose) and their preservation treatment (with ultrasound and geraniol). As a result, combining preservation treatment (geraniol) with prebiotics (inulin and oligofructose) showed to be an efficient strategy to control the native microflora, as well as, to inhibit inoculated pathogens in strawberry juice during 2 weeks of refrigerated storage (Cassani, Tomadoni, Moreira, & Agüero, 2018). Gum odina (GOd) a plant-derived gum, which is non-toxic in nature and is capable of enhancing immune system of the body due to its prebiotic action (Mitra et al., 2016). Aditya et al. developed a multiple emulsion (W/O/W) of lamivudine using a GOd to increase bioavailability and patient compliances of the encapsulated drug. GOd was employed to stabilize both the interfaces of liquid membrane in both the external and internal aqueous phases. Their results provided a promising scope for GOd stabilized W/O/W multiple emulsions in antiviral therapies (Jena, Nayak, De, Mitra, & Samanta, 2018). Addition of prebiotic ingredients also reduce the health risk of certain foods associated with their high contents of sodium, fats, fatty acid fatty acid profile rich in saturated fatty acids and cholesterol, which can increase the incidence of chronic disease such as coronary heart disease (CVD), obesity, high blood cholesterol and cancer. Within this context Maria et al. evaluated the effect of the addition of various prebiotic fibers on the rheological and technological properties and the microstructure of an emulsified meat product (bologna). They concluded that the simultaneous addition of a partial level of cassava starch and the prebiotic fibers would improve the stability of the meat emulsions, allowing the reliable production of a healthier bologna sausage (Felisberto, Galvão, Picone, Cunha, & Pollonio, 2015).
Table 2.
Summary of recent advancements in prebiotics and probiotics formulations
| S. No | Name of prebiotic or probiotic used | Formulations | Potentials of the formulations | References |
|---|---|---|---|---|
| 1 | Lactobacillus gasseri (L) and Bifidobacterium bifidum (B) as probiotics and quercetin as prebiotic | alginate-chitosan capsules | To enhance survival of the probiotic bacteria and keeping intact the prebiotic during exposure to the adverse conditions of the GIT | Chávarri, Marañón et al. (2010) |
| 2 | Inulin, FOS, polydextrose, and resistant starch | Meat emulsion | To reduce sodium chloride and pork-back-fat contents of the emulsified meat product (bologna) | Felisberto, and et al (2015) |
| 3 | Probiotic Bifidobacterium BB-12 and prebiotics inulin and polydextrose | Microcapsules | To improve stability of the microcapsules and protect the encapsulated probiotic | Pinto, Fritzen-Freire et al. (2015) |
| 4 | Inulin | Emulsion filled gels | To reduce fat content in the foods | Paradiso, and et al (2015) |
| 5 | Lactobacillus salivarius NRRL B-30514 as probiotic and pectin as prebiotics | Emulsion | To enhance viability of the encapsulated probiotic bacteria | Zhang, Lin et al. (2015) |
| 6 | Inulin | Emulsion | To enhance the retention and antioxidant efficacy of geranylgeraniol (bioactive compound) | Silva, Zabot et al. (2016) |
| 7 | Inulin | Nanocomplexes | Protection and delivery of encapsulated resveratrol and to enhance the prebiotic efficacy of the complexes | Ha, Jeon et al. (2016) |
| 8 | Inulin | Microcapsules | To enhance protection and retention of the encapsulated thymol | Zabot, Silva et al. (2016) |
| 9 | Lactobacillus salivarius NRRL B-30514 as probiotic and sugar beet pectin as prebiotics | Emulsion | To enhance viability of the encapsulated probiotic bacteria | Zhang, Lin et al. (2016) |
| 10 | Potato starch (PS), Plantago psyllium (PSY) and Inulin (IN)) as prebiotics and Lactobacillus casei Shirota (Lc) and Lactobacillus plantarum (Lp33 and Lp17) as probiotics | Microcapsules | To protect the encapsulated bacteria and enhance their viability | Peredo, Beristain, et al. (2016) |
| 11 | Lactic acid bacteria as probitioc and cactus pear peel or apple marc flours as prebiotics | Microcapsules | To protect the encapsulated bacteria and enhance their viability | Serrano-Casas, Pérez-Chabela et al. (2017) |
| 12 | Inulin (IN), and oligofructose (OL) | Emulsion | To enhance storage, release and antioxidant properties of the encapsulated lime essential oil | Campelo-Felix, Souza et al. (2017) |
| 13 | Bacillus coagulans as probitioc and Pectin as prebiotic | bionanocomposite | To enhance protection of the encapsulated bacteria | Khorasani and Shojaosadati (2017) |
| 14 | Lactobacillus rhamnosus | Emulsion | To enhance viability of the encapsulated probiotic bacteria | Marino, Innocente et al. (2017) |
| 15 | Gum odina (GO) | Emulsion | To increase bioavailability and patient compliances of the encapsulated anti-retroviral drug lamivudine | Jena, and et al (2018) |
| 16 | Inulin | Emulsion | To produce reduced fat mayonnaises | Calligaris, Marino et al. (2018) |
| 17 | Escherichia coli O157:H7 and Listeria innocua as probitoics and Inulin and oligofructose as prebiotics | Strawberry juices | To enhance quality parameters (microbiological, antioxidant capacity indicators, and sensory) of the juices and enhance safety and protection of the encapsulants | Cassani, and et al (2018) |
| 18 | Inulin | Soursop flavored whey beverage | To scale up the physical stability of the system | Guimarães, Silva et al. (2018) |
| 19 | Inulin | Whey beverage | To scale up kinetic stability of the system | Guimarães, Silva et al. (2018) |
| 20 | Inulin | Emulsion filled gel | To enhance stability and consistency of the synthesized system | Nourbehesht, and et al (2018) |
| 21 | Lactobacillus rhamnosus | Emulsion | To obtaining a low-saturated fat ice cream functionalized with probiotic bacteria | Calligaris, Marino et al. (2018) |
| 22 | Inulin | Emulsion-filled gel | To produce fermented sausages with reduced fat and improved stability as well as fatty acid composition, | Glisic, Baltic et al. (2019) |
| 23 | Inulin | Emulsion | To enhance the stability and thickening of the synthesized system | López-Castejón, Bengoechea et al. (2019) |
| 24 | Inulin | Emulsion | To enhance the stability and protein digestibility of the formulated enteral formulas | Souza et al. (2019) |
| 25 | Lactobacillus acidophilus as probitoc and rice bran, inulin and hi-maize as prebitotics | Alginate microparticles | To enhance viability of the encapsulated probiotic bacteria | Poletto, Fonseca et al. (2019) |
| 26 | Enterococcus faceium as probiotic and Inulin as prebiotic | Nanoemulsion | To enhance viability and protection of the encapsulated probiotic bacteria | Krithika and Preetha (2019) |
| 27 | Saccharomyces boulardii and Enterococcus faecium | Emulsion | To enhance growth and survival of the encapsulated proboiotic bacteria | Qi, Liang et al. (2019) |
| 28 | Inulin | Liposome and emulsion gels | To enhance oxidative stability of the synthesized system | Li, Gunenc et al. (2020) |
| 29 | octenyl-succinic anhydride (OSA) starch, Inulin (IN), maltodextrin (MD), chitosan (CS) | Nanocapsules | To improve the physicochemical stability and solubility of the encapsulated algal oil | Wang et al. (2020) |
| 30 | Lactobacillus reuteri | Emulsion | To enhance protection and viability of the encapsulated probiotic bacteria | Zhao, Huang et al. (2020) |
| 31 | Lactic acid bacteria | Alginate–pectin gels microcapsules | To enhance protection and viability of the encapsulated bacteria and thereby enhance nutritional value of the cooked sausages | (Barragán-Martínez, Totosaus et al., 2019) |
| 32 | Inulin and konjac glucomannan | Emulsion | To scale up the stability and rheological properties of the emulsions | Xu, Xiong et al. (2020) |
| 33 | Lactobacillus acidophilus LA-5 as probiotic and hi-maize, inulin, and rice bran as prebiotics | pectin microparticles | To enhance protection and viability of the encapsulated bacteria | Raddatz, Poletto et al. (2020) |
Interestingly, this technology has shown promising effects not only for the prebiotics but also for probiotics simultaneously. Probiotics derived from a Greek word meaning “for life” and are living organisms which confer potential health benefits including the alleviation of digestive discomfort, reduction of the duration of childhood diarrhea, regulation of intestinal immunity, and improvement of symptoms of the irritable bowel syndrome (Hill et al., 2014). However, to significantly achieve these health benefits associated with probiotics, their protection against processing and storage conditions as well as those encountered during passage through human gut is inevitable. At this point, encapsulation technology has been proven to be effective in protecting probiotics from these adverse conditions, while maintaining their viability and functionality (Raddatz et al., 2020; M.; Zhao et al., 2020). So far a number of different encapsulation based formulations have been implied in food industry to enhance protection and viability of the encapsulated probiotic bacteria and thereby scale up the nutritional value and functional properties of the produced products. The presence of prebiotics in these systems perform several pivotal roles. For example, these prebiotics increase stability of the synthesized systems, aid in developing of novel food products with reduced fats and salt contents and enhance protection and viability of the encapsulated probiotics bacteria. These approaches are of particular importance in the production of novel functional foods the demands for which have increased significantly in recent years. A summary of these formulations developed in recent years with their respective potentials has been given in Table 1.
2. Conclusions
From the above detailed descriptions, a conclusion can be withdrawn with the facts that DFs and complex resistant undigestible carbohydrates, that withstand digestion by host enzymes in the upper GIT play a pivotal role in driving bacterial fermentation in the large intestine. It could be due to the production of SCFAs from these DFs by the gut microbiota, which in turn paly significant roles in promoting health and well-being. The production of SCFAs mainly depends on dietary substrate, which affects the composition of the bacterial flora, transit time through the colon and luminal pH (which are all interlinked variables). In short, the type of DFs arriving the colon has a remarkable impact on the performance and composition of bacterial communities, thereby influencing the production of SCFAs, the balance of which is vital to maintain gut health. Among all the produced SCFAs, butyrate may be regarded as the most beneficial one for colonic health, which could be due to is principal energy source for the colonycytes as well as its role in modulating cell turnover in the colon. This implies that ensuring an adequate supply of DFs, such that to maintain raised butyrate levels is throughout the entire length of the large intestine, can be a beneficial strategy in the prevention of colon cancer, usually originated in the distal colon. However, interestingly maintaining an optimal levels of all the three SCFAs may have a broader range of health benefits including impacting the progressions of metabolic syndrome such as type 2 diabetes, obesity and CVD. Profound knowledge of particular DFs that develop and stimulate SCFAs particularly butyrate and propionate production, with reducing secondary bile acid conversion and elevating bile acid binding and excretion, would ultimately lead to enhanced defense against both colon cancer and metabolic syndrome. Furthermore, weight loss formulas that are based on lowered carbohydrate and calories intake should maintain ample amounts of DFs to sustain gut health. In addition, being prebiotics and as a food for probiotics, DFs play inevitable role in the development of novel functional foods and hence various formulations have been focused tremendously in recent years. These pre and probiotics-based formulations are aimed to enhance stability of the synthesized systems, protect the encapsulants and promote viability and functionality of the encapsulated probiotics. Therefore, it is strongly recommended that future work is needed to; i) develop novel pre & probiotic based formulations with promising characteristics, ii) evaluate the in vivo efficacy as well as dosage optimization of the designed systems in animal models and iii) carry out clinical trials to find out the significance of these formulations in human subjects suffering from different pathologies.
Declaration of competing interest
There is no conflict of interest in this article.
Acknowledgment
The author is grateful to the Ministry of Education, Youth and Sports of the Czech Republic-the CENAKVA project (LM2018099), CENAKVA Center Development (CZ.1.05/2.1.00/19.0380) and Biodiversity (CZ.02.1.01/0.0/0.0/16_025/0007370) and National Agency for Agricultural Research (QK1810296). The author is very much grateful to Dr. Sophie Dominique Alix Bedel for her encouragement and support in writing this article.
References
- AACCI, American Association of Cereal Chemists International Whole grain definition. Cereal Foods World. 1999;45:79. [Google Scholar]
- Akbari E., et al. Effect of probiotic supplementation on cognitive function and metabolic status in alzheimer's disease: A randomized, double-blind and controlled trial. Frontiers in Aging Neuroscience. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Sabatel S., de Marañón I.M., Arboleya J.-C. Impact of oil and inulin content on the stability and rheological properties of mayonnaise-like emulsions processed by rotor-stator homogenisation or high pressure homogenisation (HPH) Innovative Food Science & Emerging Technologies. 2018;48:195–203. [Google Scholar]
- Angelino D., et al. Glucose-and lipid-related biomarkers are affected in healthy obese or hyperglycemic adults consuming a whole-grain pasta enriched in prebiotics and probiotics: A 12-week randomized controlled trial. Journal of Nutrition. 2019;149(10):1714–1723. doi: 10.1093/jn/nxz071. [DOI] [PubMed] [Google Scholar]
- Arabzadegan N., Daneshzad E., Fatahi S., Moosavian S.P., Surkan P.J., Azadbakht L. Bulimia and Obesity; 2019. Effects of dietary whole grain, fruit, and vegetables on weight and inflammatory biomarkers in overweight and obese women. Eating and Weight Disorders-Studies on Anorexia. [DOI] [PubMed] [Google Scholar]
- Baliou S., Adamaki M., Spandidos D.A., Kyriakopoulos A.M., Christodoulou I., Zoumpourlis V. The microbiome, its molecular mechanisms and its potential as a therapeutic strategy against colorectal carcinogenesis. World Academy of Sciences Journal. 2019;1(1):3–19. [Google Scholar]
- Barichella M., et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology. 2016;87(12):1274–1280. doi: 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- Barragán Martínez L.P., et al. Probiotication of cooked sausages employing agroindustrial coproducts as prebiotic co‐encapsulant in ionotropic alginate–pectin gels. International Journal of Food Science and Technology. 2019;55(3):1088–1096. [Google Scholar]
- Benton D., Young H.A. Reducing calorie intake may not help you lose body weight. Perspectives on Psychological Science : A Journal of the Association for Psychological Science. 2017;12(5):703–714. doi: 10.1177/1745691617690878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.-J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesiekierski J.R., Jalanka J., Staudacher H.M. Can gut microbiota composition predict response to dietary treatments? Nutrients. 2019;11(5):1134. doi: 10.3390/nu11051134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels L.B., Delzenne N.M., Cani P.D., Walter J. Towards a more comprehensive concept for prebiotics. Nature Reviews Gastroenterology & Hepatology. 2015;12(5):303. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- Calligaris S., et al. Potential application of monoglyceride structured emulsions as delivery systems of probiotic bacteria in reduced saturated fat ice cream. Lebensmittel-Wissenschaft & Technologie. 2018;96:329–334. [Google Scholar]
- Campelo-Felix P.H., et al. Prebiotic carbohydrates: Effect on reconstitution, storage, release, and antioxidant properties of lime essential oil microparticles. Journal of Agricultural and Food Chemistry. 2017;65(2):445–453. doi: 10.1021/acs.jafc.6b04643. [DOI] [PubMed] [Google Scholar]
- Canfora E.E., Meex R.C., Venema K., Blaak E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nature Reviews Endocrinology. 2019;15(5):261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- Cassani L., et al. Improving quality parameters of functional strawberry juices: Optimization of prebiotic fiber enrichment and geraniol treatment. Food and Bioprocess Technology. 2018;11(11):2110–2124. [Google Scholar]
- Chambers E.S., Preston T., Frost G., Morrison D.J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Current Nutrition Reports. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau K., et al. Probiotics for infantile colic: A randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. The Journal of Pediatrics. 2015;166(1):74–78. doi: 10.1016/j.jpeds.2014.09.020. e71. [DOI] [PubMed] [Google Scholar]
- Chávarri M., et al. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. International Journal of Food Microbiology. 2010;142(1-2):185–189. doi: 10.1016/j.ijfoodmicro.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Conlon M.A., Bird A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7(1):17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davani-Davari D., Negahdaripour M., Karimzadeh I., Seifan M., Mohkam M., Masoumi S.J., et al. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods (Basel, Switzerland) 2019;8(3):92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S.…Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf E., Cani P., Claus S., Neyrinck A., Puylaert P., Glenn G.…Delzenne N. INULIN-TYPE fructans with prebiotic properties lessen endotoxemia and modulate host metabolism by changing gut microbiota composition in obese women: 749 accepted poster. Obesity Facts. 2012;5:200–201. [Google Scholar]
- Dewulf E.M., Cani P.D., Neyrinck A.M., Possemiers S., Van Holle A., Muccioli G.G.…Sohet F.M. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. The Journal of Nutritional Biochemistry. 2011;22(8):712–722. doi: 10.1016/j.jnutbio.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Dilli D., et al. The propre-save study: Effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. The Journal of Pediatrics. 2015;166(3):545–551. doi: 10.1016/j.jpeds.2014.12.004. e541. [DOI] [PubMed] [Google Scholar]
- Esmaeilnejad Moghadam B., Keivaninahr F., Fouladi M., Rezaei Mokarram R., Nazemi A. Inulin addition to yoghurt: Prebiotic activity, health effects and sensory properties. International Journal of Dairy Technology. 2019;72(2):183–198. [Google Scholar]
- Famouri F., et al. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. Journal of Pediatric Gastroenterology and Nutrition. 2017;64(3):413–417. doi: 10.1097/MPG.0000000000001422. [DOI] [PubMed] [Google Scholar]
- Felisberto M.H.F., et al. Effect of prebiotic ingredients on the rheological properties and microstructure of reduced-sodium and low-fat meat emulsions. LWT-Food Science and Technology. 2015;60(1):148–155. [Google Scholar]
- Gao F., Lv Y., Long J., Chen J., He J., Ruan X., et al. Natural fermentation product butyrate improves the metabolic disorder and gut microbiome dysbiosis in mice induced by a high-fat diet. Frontiers in Pharmacology. 2019;10:1040. doi: 10.3389/fphar.2019.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G.R., Scott K.P., Rastall R.A., Tuohy K.M., Hotchkiss A., Dubert-Ferrandon A.…Loh G. Dietary prebiotics: Current status and new definition. Food Science & Technology Bulletin: Functional Foods. 2010;7(1):1–19. [Google Scholar]
- Glisic M., Baltic M., Glisic M., Trbovic D., Jokanovic M., Parunovic N., et al. Inulin‐based emulsion‐filled gel as a fat replacer in prebiotic‐and PUFA‐enriched dry fermented sausages. International Journal of Food Science and Technology. 2019;54(3):787–797. [Google Scholar]
- Gomes A.C., et al. The additional effects of a probiotic mix on abdominal adiposity and antioxidant status: A double‐blind, randomized trial. Obesity. 2017;25(1):30–38. doi: 10.1002/oby.21671. [DOI] [PubMed] [Google Scholar]
- Gong L., Cao W., Chi H., Wang J., Zhang H., Liu J., et al. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Research International. 2018;103:84–102. doi: 10.1016/j.foodres.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Gong L., Wang T., Sun C., Wang J., Sun B. Whole barley prevents obesity and dyslipidemia without the involvement of the gut microbiota in germ free C57BL/6J obese mice. Food and Function. 2019;10(11):7498–7508. doi: 10.1039/c9fo01268k. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia R.A., McCubbin T., Navone L., Stowers C., Nielsen L.K., Marcellin E. Microbial propionic acid production. Fermentation. 2017;3(2):21. [Google Scholar]
- Goto H., Sagitani A., Ashida N., Kato S., Hirota T., Shinoda T., et al. Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity. British Journal of Nutrition. 2013;110(10):1810–1818. doi: 10.1017/S0007114513001104. [DOI] [PubMed] [Google Scholar]
- Grabitske H.A., Slavin J.L. Gastrointestinal effects of low-digestible carbohydrates. Critical Reviews in Food Science and Nutrition. 2009;49(4):327–360. doi: 10.1080/10408390802067126. [DOI] [PubMed] [Google Scholar]
- Greenblum S., Turnbaugh P.J., Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proceedings of the National Academy of Sciences. 2012;109(2):594–599. doi: 10.1073/pnas.1116053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Brewer H.B., Cleeman J.I., Smith S.C., Lenfant C. Definition of metabolic syndrome. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Guimarães J.T., et al. Physicochemical changes and microbial inactivation after high-intensity ultrasound processing of prebiotic whey beverage applying different ultrasonic power levels. Ultrasonics Sonochemistry. 2018;44:251–260. doi: 10.1016/j.ultsonch.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Ha H.-K., et al. Physicochemical characterization and potential prebiotic effect of whey protein isolate/inulin nano complex. Korean Journal for Food Science of Animal Resources. 2016;36(2):267. doi: 10.5851/kosfa.2016.36.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.-F., Jiao J., Zhang W., Xu J.-Y., Zhang W., Fu C.-L., et al. Lipolysis and thermogenesis in adipose tissues as new potential mechanisms for metabolic benefits of dietary fiber. Nutrition. 2017;33:118–124. doi: 10.1016/j.nut.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Hipsley E.H. Dietary “fibre” and pregnancy toxaemia. British Medical Journal. 1953;2(4833):420. doi: 10.1136/bmj.2.4833.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hod K., et al. A double‐blind, placebo‐controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea‐predominant IBS. Neuro-Gastroenterology and Motility. 2017;29(7) doi: 10.1111/nmo.13037. [DOI] [PubMed] [Google Scholar]
- Holscher H.D., Bauer L.L., Gourineni V., Pelkman C.L., Fahey G.C., Jr., Swanson K.S. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. Journal of Nutrition. 2015;145(9):2025–2032. doi: 10.3945/jn.115.217331. [DOI] [PubMed] [Google Scholar]
- Hooda S., Boler B.M.V., Serao M.C.R., Brulc J.M., Staeger M.A., Boileau T.W.…Swanson K.S. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. Journal of Nutrition. 2012;142(7):1259–1265. doi: 10.3945/jn.112.158766. [DOI] [PubMed] [Google Scholar]
- Hui S., Liu K., Lang H., Liu Y., Wang X., Zhu X., et al. Comparative effects of different whole grains and brans on blood lipid: A network meta-analysis. European Journal of Nutrition. 2019;58(7):2779–2787. doi: 10.1007/s00394-018-1827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islek A., Sayar E., Yilmaz A., Baysan B.O., Mutlu D., Artan R. The role of Bifidobacterium lactis B94 plus inulin in the treatment of acute infectious diarrhea in children. Turkish Journal of Gastroenterology. 2014;25(6):628–633. doi: 10.5152/tjg.2014.14022. [DOI] [PubMed] [Google Scholar]
- Javadi L., et al. The effect of probiotic and/or prebiotic on liver function tests in patients with nonalcoholic fatty liver disease: A double blind randomized clinical trial. Iranian Red Crescent Medical Journal. 2017;19(4) [Google Scholar]
- Javadi L., et al. 2018. Correction: The potential role of probiotics or/and prebiotic on serum lipid profile and insulin resistance in non-alcoholic fatty liver disease: A double blind randomized clinical trial. [Google Scholar]
- Jena A.K., et al. Development of lamivudine containing multiple emulsions stabilized by gum odina. Future Journal of Pharmaceutical Sciences. 2018;4(1):71–79. [Google Scholar]
- Jensen M.K., Koh-Banerjee P., Franz M., Sampson L., Grønbaek M., Rimm E.B. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. American Journal of Clinical Nutrition. 2006;83(2):275–283. doi: 10.1093/ajcn/83.2.275. [DOI] [PubMed] [Google Scholar]
- Jesch E.D., Carr T.P. Food ingredients that inhibit cholesterol absorption. Preventive Nutrition and Food Science. 2017;22(2):67–80. doi: 10.3746/pnf.2017.22.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi R., Azizi M.H., Ghasemlou M., Vaziri M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: A review. Carbohydrate Polymers. 2015;119:85–100. doi: 10.1016/j.carbpol.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Kazemi A., et al. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clinical Nutrition. 2019;38(2):522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Khachatryan Z.A., Ktsoyan Z.A., Manukyan G.P., Kelly D., Ghazaryan K.A., Aminov R.I. Predominant role of host genetics in controlling the composition of gut microbiota. PloS One. 2008;3(8) doi: 10.1371/journal.pone.0003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khani S., Motamedifar M., Golmoghaddam H., Hosseini H.M., Hashemizadeh Z. In vitro study of the effect of a probiotic bacterium Lactobacillus rhamnosus against herpes simplex virus type 1. Brazilian Journal of Infectious Diseases. 2012;16(2):129–135. doi: 10.1016/S1413-8670(12)70293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorasani A.C., Shojaosadati S.A. Starch-and carboxymethylcellulose-coated bacterial nanocellulose-pectin bionanocomposite as novel protective prebiotic matrices. Food Hydrocolloids. 2017;63:273–285. [Google Scholar]
- Kikuchi Y., Nozaki S., Makita M., Yokozuka S., Fukudome S.-i., Yanagisawa T., et al. Effects of whole grain wheat bread on visceral fat obesity in Japanese subjects: A randomized double-blind study. Plant Foods for Human Nutrition. 2018;73(3):161–165. doi: 10.1007/s11130-018-0666-1. [DOI] [PubMed] [Google Scholar]
- King D.E., Egan B.M., Woolson R.F., Mainous A.G., 3rd, Al-Solaiman Y., Jesri A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Archives of Internal Medicine. 2007;167(5):502–506. doi: 10.1001/archinte.167.5.502. [DOI] [PubMed] [Google Scholar]
- Koecher K.J., McKeown N.M., Sawicki C.M., Menon R.S., Slavin J.L. Effect of whole-grain consumption on changes in fecal microbiota: A review of human intervention trials. Nutrition Reviews. 2019;77(7):487–497. doi: 10.1093/nutrit/nuz008. [DOI] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Kopf J.C., Suhr M.J., Clarke J., Eyun S.-i., Riethoven J.-J.M., Ramer-Tait A.E., et al. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: A randomized controlled trial. Nutrition Journal. 2018;17(1):72. doi: 10.1186/s12937-018-0381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korcz E., Kerényi Z., Varga L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food and Function. 2018;9(6):3057–3068. doi: 10.1039/c8fo00118a. [DOI] [PubMed] [Google Scholar]
- Krithika B., Preetha R. Formulation of protein based inulin incorporated synbiotic nanoemulsion for enhanced stability of probiotic. Materials Research Express. 2019;6(11):114003. [Google Scholar]
- Kunzmann A.T., Coleman H.G., Huang W.-Y., Kitahara C.M., Cantwell M.M., Berndt S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. American Journal of Clinical Nutrition. 2015;102(4):881–890. doi: 10.3945/ajcn.115.113282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N., Vogensen F.K., Van Den Berg F.W., Nielsen D.S., Andreasen A.S., Pedersen B.K.…Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2) doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., et al. Developing emulsion gels by incorporating Jerusalem artichoke inulin and investigating their lipid oxidative stability. Food Production, Processing and Nutrition. 2020;2(1):1–11. [Google Scholar]
- Lim J.J., Poppitt S.D. How satiating are the ‘Satiety'Peptides: A problem of pharmacology versus physiology in the development of novel foods for regulation of food intake. Nutrients. 2019;11(7):1517. doi: 10.3390/nu11071517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Yu W., Guan X., Huang K., Liu J., Liu D., et al. Effects of millet whole grain supplementation on the lipid profile and gut bacteria in rats fed with high-fat diet. Journal of Functional Foods. 2019;59:49–59. [Google Scholar]
- Loman B.R., Hernández-Saavedra D., An R., Rector R.S. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutrition Reviews. 2018;76(11):822–839. doi: 10.1093/nutrit/nuy031. [DOI] [PubMed] [Google Scholar]
- López-Castejón M.L., et al. Characterization of prebiotic emulsions stabilized by inulin and β-lactoglobulin. Food Hydrocolloids. 2019;87:382–393. [Google Scholar]
- Luoto R., Ruuskanen O., Waris M., Kalliomäki M., Salminen S., Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial. The Journal of Allergy and Clinical Immunology. 2014;133(2):405–413. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie A., Bajka B., Rigby N. Roles for dietary fibre in the upper GI tract: The importance of viscosity. Food Research International. 2016;88:234–238. [Google Scholar]
- Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host & Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Mariat D., Firmesse O., Levenez F., Guimarăes V., Sokol H., Doré J., et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiology. 2009;9(1):123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M., et al. Viability of probiotic Lactobacillus rhamnosus in structured emulsions containing saturated monoglycerides. Journal of Functional Foods. 2017;35:51–59. [Google Scholar]
- Martínez I., Lattimer J.M., Hubach K.L., Case J.A., Yang J., Weber C.G., et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. The ISME Journal. 2013;7(2):269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty M.F., Assanga S.B.I. Ferulic acid may target MyD88-mediated pro-inflammatory signaling–Implications for the health protection afforded by whole grains, anthocyanins, and coffee. Medical Hypotheses. 2018;118:114–120. doi: 10.1016/j.mehy.2018.06.032. [DOI] [PubMed] [Google Scholar]
- Milani C., Duranti S., Bottacini F., Casey E., Turroni F., Mahony J., et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiology and Molecular Biology Reviews : Microbiology and Molecular Biology Reviews. 2017;81(4) doi: 10.1128/MMBR.00036-17. e00036-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraghajani M., et al. The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: A randomized controlled clinical trial. Journal of Renal Nutrition. 2017;27(5):317–324. doi: 10.1053/j.jrn.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Mitra D., Jena A.K., De A., Das M., Das B., Samanta A. Prebiotic potential of gum odina and its impact on gut ecology: In vitro and in vivo assessments. Food and Function. 2016;7(7):3064–3072. doi: 10.1039/c5fo01581b. [DOI] [PubMed] [Google Scholar]
- Moen B., Henjum K., Måge I., Knutsen S.H., Rud I., Hetland R.B., et al. Effect of dietary fibers on cecal microbiota and intestinal tumorigenesis in azoxymethane treated A/J Min/+ mice. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Canfora E.E., Blaak E.E. Gastrointestinal transit time, glucose homeostasis and metabolic health: Modulation by dietary fibers. Nutrients. 2018;10(3):275. doi: 10.3390/nu10030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T.D., Finan B., Bloom S.R., D'Alessio D., Drucker D.J., Flatt P.R.…Tschöp M.H. Glucagon-like peptide 1 (GLP-1) Molecular Metabolism. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan S., Nirmalkar K., Hoyo-Vadillo C., García-Espitia M., Ramírez-Sánchez D., García-Mena J. Gut microbiome production of short-chain fatty acids and obesity in children. European Journal of Clinical Microbiology & Infectious Diseases. 2018;37(4):621–625. doi: 10.1007/s10096-017-3143-0. [DOI] [PubMed] [Google Scholar]
- Nicolucci A.C., et al. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153(3):711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- Nourbehesht N., et al. Investigation of stability, consistency, and oil oxidation of emulsion filled gel prepared by inulin and rice bran oil using ultrasonic radiation. Ultrasonics Sonochemistry. 2018;42:585–593. doi: 10.1016/j.ultsonch.2017.12.029. [DOI] [PubMed] [Google Scholar]
- O'Keefe S.J.D. The need to reassess dietary fiber requirements in healthy and critically ill patients. Gastroenterology Clinics of North America. 2018;47(1):219–229. doi: 10.1016/j.gtc.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe S.J., Li J.V., Lahti L., Ou J., Carbonero F., Mohammed K.…Zoetendal E.G. Fat, fibre and cancer risk in African Americans and rural Africans. Nature Communications. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya Galan N., Ulloa Rubiano J., Velez Reyes F., Fernandez Duarte K., Salas Cardenas S., Gutierrez Fernandez M. In vitro antiviral activity of Lactobacillus casei and Bifidobacterium adolescentis against rotavirus infection monitored by NSP 4 protein production. Journal of Applied Microbiology. 2016;120(4):1041–1051. doi: 10.1111/jam.13069. [DOI] [PubMed] [Google Scholar]
- Paradiso V.M., et al. Production and characterization of emulsion filled gels based on inulin and extra virgin olive oil. Food Hydrocolloids. 2015;45:30–40. [Google Scholar]
- Peredo A., Beristain C., Pascual L., Azuara E., Jimenez M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. Lebensmittel-Wissenschaft & Technologie. 2016;73:191–196. [Google Scholar]
- Pinto-Sanchez M.I., et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459. doi: 10.1053/j.gastro.2017.05.003. e448. [DOI] [PubMed] [Google Scholar]
- Pinto S.S., et al. Potential use of whey concentrate and prebiotics as carrier agents to protect Bifidobacterium-BB-12 microencapsulated by spray drying. Food Research International. 2015;67:400–408. [Google Scholar]
- Poletto G., et al. Encapsulation of Lactobacillus acidophilus and different prebiotic agents by external ionic gelation followed by freeze-drying. Ciência Rural. 2019;49(2) [Google Scholar]
- Prasad K.N., Bondy S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioactive Carbohydrates and Dietary Fibre. 2019;17 doi: 10.1016/j.mad.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Qi W., et al. Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. Journal of Food Science and Technology. 2019;56(3):1398–1404. doi: 10.1007/s13197-019-03616-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raddatz G.C., et al. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Research International. 2020;130 doi: 10.1016/j.foodres.2019.108902. [DOI] [PubMed] [Google Scholar]
- Reynolds A.N., Akerman A.P., Mann J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Medicine. 2020;17(3) doi: 10.1371/journal.pmed.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo-Adrover M.d.M., Knipping K., Garssen J., van Limpt K., Knol J., Franch À., et al. Prevention of rotavirus diarrhea in suckling rats by a specific fermented milk concentrate with prebiotic mixture. Nutrients. 2019;11(1):189. doi: 10.3390/nu11010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roager H.M., Vogt J.K., Kristensen M., Hansen L.B.S., Ibrügger S., Mærkedahl R.B., et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut. 2019;68(1):83–93. doi: 10.1136/gutjnl-2017-314786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J.M., Murphy K., Stanton C., Ross R.P., Kober O.I., Juge N., et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microbial Ecology in Health and Disease. 2015;26 doi: 10.3402/mehd.v26.26050. 26050-26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshanravan N., et al. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. Journal of Cardiovascular and Thoracic Research. 2017;9(4):183. doi: 10.15171/jcvtr.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W.R., Gratz S.W., Duncan S.H., Holtrop G., Ince J., Scobbie L., et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. American Journal of Clinical Nutrition. 2011;93(5):1062–1072. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- Sepideh A., et al. Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: A double-blind randomized clinical trial. Journal of the American College of Nutrition. 2016;35(6):500–505. doi: 10.1080/07315724.2015.1031355. [DOI] [PubMed] [Google Scholar]
- Serrano-Casas V., et al. Improvement of lactic acid bacteria viability in acid conditions employing agroindustrial co-products as prebiotic on alginate ionotropic gel matrix co-encapsulation. Journal of Functional Foods. 2017;38:293–297. [Google Scholar]
- Shadnoush M., et al. Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: A double-blind, placebo-controlled clinical trial. Korean Journal of Gastroenterology. 2015;65(4):215–221. doi: 10.4166/kjg.2015.65.4.215. [DOI] [PubMed] [Google Scholar]
- Shah B.R., Li B., Wang L., Liu S., Li Y., Wei X., et al. Health benefits of konjac glucomannan with special focus on diabetes. Bioactive Carbohydrates and Dietary Fibre. 2015;5(2):179–187. [Google Scholar]
- Silva E.K., et al. Biopolymer-prebiotic carbohydrate blends and their effects on the retention of bioactive compounds and maintenance of antioxidant activity. Carbohydrate Polymers. 2016;144:149–158. doi: 10.1016/j.carbpol.2016.02.045. [DOI] [PubMed] [Google Scholar]
- Silva E.K., Guimarães J.T., Costa A.L.R., Cruz A.G., Meireles M.A.A. Non-thermal processing of inulin-enriched soursop whey beverage using supercritical carbon dioxide technology. The Journal of Supercritical Fluids. 2019;154 [Google Scholar]
- Sivaprakasam S., Gurav A., Paschall A., Coe G., Chaudhary K., Cai Y.…Huang L. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis. 2016;5(6) doi: 10.1038/oncsis.2016.38. e238-e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldi S., Vasileiadis S., Lohner S., Uggeri F., Puglisi E., Molinari P., et al. Prebiotic supplementation over a cold season and during antibiotic treatment specifically modulates the gut microbiota composition of 3-6 year-old children. Beneficial Microbes. 2019;10(3):253–263. doi: 10.3920/BM2018.0116. [DOI] [PubMed] [Google Scholar]
- Soleimani A., et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney International. 2017;91(2):435–442. doi: 10.1016/j.kint.2016.09.040. [DOI] [PubMed] [Google Scholar]
- Soliman G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients. 2019;11(5):1155. doi: 10.3390/nu11051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E.-J., et al. Effect of probiotics on obesity-related markers per enterotype: A double-blind, placebo-controlled, randomized clinical trial. The EPMA Journal. 2020;11(1):31–51. doi: 10.1007/s13167-020-00198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M.W.S.d., et al. Effect of inulin, medium-chain triglycerides and whey protein isolate on stability and in vitro digestibility of enteral nutrition formulas. Food Science and Technology. 2019 doi: 10.1590/fst.23619. ((AHEAD)) [DOI] [Google Scholar]
- Taha-Abdelaziz K., Astill J., Kulkarni R.R., Read L.R., Najarian A., Farber J.M., et al. In vitro assessment of immunomodulatory and anti-Campylobacter activities of probiotic lactobacilli. Scientific Reports. 2019;9(1):1–15. doi: 10.1038/s41598-019-54494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajabadi-Ebrahimi M., et al. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Experimental and Clinical Endocrinology & Diabetes. 2017;125:21–27. doi: 10.1055/s-0042-105441. 01. [DOI] [PubMed] [Google Scholar]
- Threapleton D.E., Greenwood D.C., Evans C.E., Cleghorn C.L., Nykjaer C., Woodhead C.…Burley V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ. 2013;347:f6879. doi: 10.1136/bmj.f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo R., Suárez A., Clemente M.G., de los Reyes-Gavilán C.G., Margolles A., Gueimonde M., et al. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World Journal of Gastroenterology: WJG. 2014;20(41) doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakooncharoenvit A., Tanaka S., Mizuta E., Hira T., Hara H. Water-soluble dietary fibers enhance bioavailability of quercetin and a fiber derived from soybean is most effective after long-term feeding in rats. European Journal of Nutrition. 2019:1–10. doi: 10.1007/s00394-019-01992-9. [DOI] [PubMed] [Google Scholar]
- Um C.Y., Campbell P.T., Carter B., Wang Y., Gapstur S.M., McCullough M.L. Association between grains, gluten and the risk of colorectal cancer in the cancer prevention study-II nutrition cohort. European Journal of Nutrition. 2019:1–11. doi: 10.1007/s00394-019-02032-2. [DOI] [PubMed] [Google Scholar]
- Vaghef-Mehrabany E., et al. Effects of probiotic supplementation on oxidative stress indices in women with rheumatoid arthritis: A randomized double-blind clinical trial. Journal of the American College of Nutrition. 2016;35(4):291–299. doi: 10.1080/07315724.2014.959208. [DOI] [PubMed] [Google Scholar]
- Vanegas S.M., Meydani M., Barnett J.B., Goldin B., Kane A., Rasmussen H.…Jonnalagadda S. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. American Journal of Clinical Nutrition. 2017;105(3):635–650. doi: 10.3945/ajcn.116.146928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas D.P., Marjorie K., Landskron G., González M.J., Quera R., Dijkstra G.…Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology. 2019;10 doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., et al. Prebiotic carbohydrates: Effect on physicochemical stability and solubility of algal oil nanoparticles. Carbohydrate Polymers. 2020;228 doi: 10.1016/j.carbpol.2019.115372. [DOI] [PubMed] [Google Scholar]
- Wang W., Li J., Chen X., Yu M., Pan Q., Guo L. Whole grain food diet slightly reduces cardiovascular risks in obese/overweight adults: A systematic review and meta-analysis. BMC Cardiovascular Disorders. 2020;20(1):1–11. doi: 10.1186/s12872-020-01337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J.M. Paper presented at the Microbial cell factories. 2011. Immunomodulatory mechanisms of lactobacilli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.-C., Inui A., Chen C.-Y. Weight loss induced by whole grain-rich diet is through a gut microbiota-independent mechanism. World Journal of Diabetes. 2020;11(2):26. doi: 10.4239/wjd.v11.i2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Chen D., Liu C., Wu X.-Z., Dong C.-X., Zhou J. Structural characterization and anti-tumor effects of an inulin-type fructan from Atractylodes chinensis. International Journal of Biological Macromolecules. 2016;82:765–771. doi: 10.1016/j.ijbiomac.2015.10.082. [DOI] [PubMed] [Google Scholar]
- Xu W., et al. Stability, microstructural and rheological properties of complex prebiotic emulsion stabilized by sodium caseinate with inulin and konjac glucomannan. Food Hydrocolloids. 2020;105 [Google Scholar]
- Xu W., Huang K., Jin W., Luo D., Liu H., Li Y., et al. Catalytic and anti-bacterial properties of biosynthesized silver nanoparticles using native inulin. RSC Advances. 2018;8(50):28746–28752. doi: 10.1039/c8ra03386b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Wan Q., Feng J., Du L., Li K., Zhou Y. Whole grain diet reduces systemic inflammation: A meta-analysis of 9 randomized trials. Medicine. 2018;97(43) doi: 10.1097/MD.0000000000012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Yang J., Du L., Li K., Zhou Y. Association of whole grain, refined grain, and cereal consumption with gastric cancer risk: A meta‐analysis of observational studies. Food Science and Nutrition. 2019;7(1):256–265. doi: 10.1002/fsn3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Song H., Wang L., Dong W., Yang Z., Yuan P., et al. Antiviral effects of a probiotic metabolic products against transmissible gastroenteritis coronavirus. Journal of Problem Health. 2017;5(3):1–6. [Google Scholar]
- Yen C., Tate J.E., Hyde T.B., Cortese M.M., Lopman B.A., Jiang B.…Parashar U.D. Rotavirus vaccines: Current status and future considerations. Human Vaccines & Immunotherapeutics. 2014;10(6):1436–1448. doi: 10.4161/hv.28857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J.Y., Kim S.S. Probiotics and prebiotics: Present status and future perspectives on metabolic disorders. Nutrients. 2016;8(3):173. doi: 10.3390/nu8030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H., et al. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Journal of Clinical Biochemistry and Nutrition. 2015;57(2):129–134. doi: 10.3164/jcbn.15-14. 15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabot G.L., et al. Replacing modified starch by inulin as prebiotic encapsulant matrix of lipophilic bioactive compounds. Food Research International. 2016;85:26–35. doi: 10.1016/j.foodres.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Zamani B., et al. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double‐blind, placebo‐controlled trial. International Journal of Rheumatic Diseases. 2016;19(9):869–879. doi: 10.1111/1756-185X.12888. [DOI] [PubMed] [Google Scholar]
- Zhang Y., et al. The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Research International. 2015;71:9–15. [Google Scholar]
- Zhang Y., et al. S/O/W emulsions prepared with sugar beet pectin to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocolloids. 2016;52:804–810. [Google Scholar]
- Zhang T., Li Q., Cheng L., Buch H., Zhang F. Akkermansia muciniphila is a promising probiotic. Microbial Biotechnology. 2019;12(6):1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., et al. Probiotic encapsulation in water-in-water emulsion via heteroprotein complex coacervation of type-A gelatin/sodium caseinate. Food Hydrocolloids. 2020;105 [Google Scholar]
- Zhao L., Zhang F., Ding X., Wu G., Lam Y.Y., Wang X.…Ma J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- Zhu C., Sawrey-Kubicek L., Beals E., Rhodes C.H., Houts H.E., Sacchi R., et al. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and mediterranean diet in four days: A pilot study. Nutrition Research. May 2020;77:62–72. doi: 10.1016/j.nutres.2020.03.005. [DOI] [PubMed] [Google Scholar]