Abstract
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi is one of the most important neglected parasitic diseases in the Americas. Vaccines represent an attractive complementary or even an alternative for the control of T. cruzi infection and pre-clinical studies in mice demonstrated that trypomastigote surface antigen (TSA-1) and the flagellar calcium-binding (Tc24) parasite antigens are promising candidates for vaccine development. We performed here the first evaluation of the safety and immunogenicity of two recombinant vaccine antigens (named TSA1-C4 and Tc24-C4) in naïve non-human primates. Three rhesus macaques received 3 doses of each recombinant protein, formulated with E6020 (Eisai Co., Ltd.), a novel Toll-like receptor-4 agonist, in a stable emulsion. All parameters from blood chemistry and blood cell counts were stable over the course of the study and unaffected by the vaccine. A specific IgG response against both antigens was detectable after the first vaccine dose, and increased with the second dose. After three vaccine doses, stimulation of PBMCs with a peptide pool derived from TSA1-C4 resulted in the induction of TSA1-C4-specific TNFα-, IL-2-and IFNγ-producing CD4+ in one or two animals while stimulation with a peptide pool derived from Tc24-C4 only activated IFNγ-producing CD4+T cells in one animal. In two animals there was also activation of TSA1-C4-specific IL2-producing CD8+ T cells. This is the first report of the immunogenicity of T. cruzi-derived recombinant antigens formulated as an emulsion with a TLR4 agonist in a non-human primate model. Our results strongly support the need for further evaluation of the preventive efficacy of this type of vaccine in non-human primates and explore the effect of the vaccine in a therapeutic model of naturally-infected Chagasic non-human primates, which would strengthen the rationale for the clinical development as a human vaccine against Chagas disease.
1. Introduction
Chagas disease, also known as American trypanosomiasis, is a vector-borne zoonotic disease caused by the protozoan parasite Trypanosoma cruzi. It is one of the most important parasitic diseases in the Americas, causing 0.55 million disability-adjusted life years lost, and 10,600 annual deaths [1–3], and an estimated $7.2 billion in annual economic losses [3].
The disease begins by an acute phase of a few weeks duration with non-specific signs and symptoms, followed by a chronic phase initially asymptomatic but 20–30% of patients will develop cardiac alterations leading to chagasic cardiomyopathy, associated with arrhythmias, which are sometimes fatal, aneurysms, or a dilated cardiomyopathy and eventually cardiac failure [4]. Some patients may also present a digestive form of the disease, with megaesophagous or megacolon. Disease progression is associated with increased mortality [5–7]. Current drug treatments with benznidazole or nifurtimox are effective during the acute phase, but treatment outcomes can be very variable during the chronic phase [8, 9], and do not halt or slow the progression of fibrotic heart disease in patients showing signs and symptoms of Chagasic cardiomyopathy [10]. In addition, these drugs are associated with adverse effects that can be severe and often lead to treatment interruption. Therefore, there have been some efforts at exploring complementary or alternative treatment regimens and combination therapies to reduce treatment duration and drug doses, with the objective of maintaining therapeutic efficacy while reducing adverse effects [9].
Therapeutic vaccines may represent an attractive complementary or alternative for the treatment or control of T. cruzi infection [11]. Indeed, extensive proof-of-principle studies have shown that different vaccine formulations can control a T. cruzi infection in mice (reviewed in [12]), and reduce fibrosis [13]. They are also effective in regimens of vaccine-linked chemotherapy [14]. These studies have shown that while antibodies may help clear circulating blood parasites, the induction of a cellular immune response, and particularly the activation of cytotoxic CD8+ T cells, is also critical for the control of T. cruzi infection, as the parasite is mostly intracellular in its mammalian hosts [15, 16]. Additional economic modeling demonstrates that a Chagas disease vaccine would contribute to lowering health care costs associated with the disease [17–19].
Trypomastigote surface antigen (TSA-1) and the flagellar calcium-binding Tc24 parasite antigens have emerged as promising candidates for further vaccine development of a therapeutic vaccine against T. cruzi [20]. Process development for the large-scale production of both recombinant antigens has been initiated and both antigens have been found able to at least partially control T. cruzi infection in mice when formulated with a variety of Th1 adjuvants, including the Toll-like receptor-4 agonist, E6020 (Eisai Co., Ltd.) [21–24]. Specific mutations have been made in both proteins, to facilitate production and increase stability, while maintaining immunogenicity in mice [25–27]. The native antigens are also able to recall an antigen-specific immune response in Chagasic patients [28], indicating that they are adequately processed by the human immune system during natural infection. As a key step prior to clinical trials, we performed here the first evaluation of the safety and immunogenicity of recombinant TSA1-C4 and Tc24-C4 antigens formulated in a stable emulsion that also contained E6020 adjuvant in naïve non-human primates. Macaques are indeed a very valuable model that has repeatedly found to be a much better predictor of immune responses in humans than mouse models [29–32]. In particular, macaques naturally infected with T. cruzi also present an immune response that mirrors that observed in human Chagasic patients [33], as well as comparable cardiac alterations [34].
2. Materials and methods
2.1. Study design
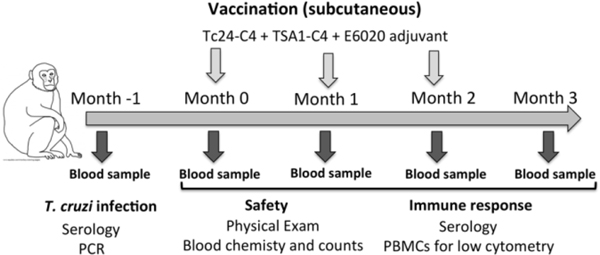
Animals were housed at the Tulane National Primate Research Center (TNPRC) under the care of TNPRC veterinarians, in accordance with the standards incorporated in the Guide for the Care and Use of Laboratory Animals and with the approval of Tulane Institutional Animal Care and Use Committee (IACUC). All procedures were performed on sedated animals. The study followed the ARRIVE guidelines for animal research [35]. Following confirmatory negative diagnostic for T. cruzi infection based on serology and PCR, three naïve male rhesus macaques (Macaca mulatta) 4–5 years old were enrolled in the study. A baseline blood sample was taken, and animals were then vaccinated with three doses of the vaccine, one month apart (Figure 1). A blood sample was collected in EDTA at each vaccination, and one month after the last vaccine dose to evaluate safety and immunogenicity of the vaccine. General physical examination was also performed at each time point.
Figure 1. Summary of study design.
2.2. Expression and purification of recombinant antigens
Recombinant Tc24-C4 (lot #Tc24-C4160117CIFP-02) and TSA1-C4 (lot #TSA1C4– 280518-AAP-2) proteins were produced in Escherichia coli following established processes described in detail before [24, 27]. These correspond to mutated antigens in which cysteine residues have been replaced [25–27]. TSA1-C4 was produced in 10L batch culture, purified by ion-exchange chromatography, followed by a refolding step, and a final resuspension in PBS. Tc24-C4 was also produced in a 10L fermenter, and purified on a GE Healthcare Q Sepharose XL column, followed by a 4 LGE Healthcare Sephacryl S-200 High Resolution column, and a final elution in PBS. Final purity of both antigens was >95% and endotoxin content was <10 EU/mg of protein. Purified proteins were stored in PBS at −20°C until used.
2.3. Vaccine formulation and immunizations
Each vaccine dose consisted of 100 μg of recombinant protein with 25 μg of E6020 (Eisai Co., Ltd.), a novel Toll-like receptor-4 agonist, in a stable emulsion [36]. Each antigen was mixed with the adjuvant just prior to immunization, which was performed subcutaneously in a volume of 500 μl. Tc24-C4 was administered over the right quadriceps, and TSA1-C4 over the left quadriceps in sedated animals, to avoid any potential interactions between the two proteins if mixed together.
2.4. Vaccine safety
General physical examination of the animals, including body weight and temperature, was performed at each time point to assess the general health following vaccination. Blood chemistry and cell counts were also performed at each time points to assess vaccine safety. Blood chemistry included Na, K, Cl, proline, albumin, globulin, blood urea nitrogen, glucose and creatinine. Cell counts included neutrophils, lymphocytes, monocytes, eosinophils and basophils.
2.5. Evaluation of vaccine-specific humoral response
Antigen-specific antibody responses were measured in plasma samples through western blot and ELISA. For western blot, 2 μg of whole T. cruzi parasite lysate from strain WB1 as well as 12 ng of Tc24-C4 and 18 ng of TSA1-C4 vaccine antigens were separated on 12% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes using a Bio-Rad mini protein-II wet transfer unit. The transferred membranes were incubated with 5% nonfat dried milk dissolved in PBS-T buffer for 1 hour blocking at room temperature, followed by incubation with rhesus plasma samples (1:500 dilution, in blocking buffer) overnight at 4°C with gentle agitation. Membranes were washed three times with PBS-T buffer and incubated with a 1:10,000 dilution of peroxidase-labeled secondary antibody anti-rhesus IgG (Sigma-Aldrich, St. Louis, MO) for 1 hour and washed four times. Signal detection was performed with an enhanced chemiluminescence kit (Clarity Western ECL Substrate kit, Bio-Rad, Hercules, CA). Images were captured using Image Quant LAS 4000, with exposure times of 2 minutes.
For ELISA, ninety-six well microplates were coated overnight at 4°C with 0.5 μg/well of Tc24-C4 or 0.1 μg/well of TSA1-C4 vaccine antigens in carbonate buffer, washed three times with PBS, and blocked with 1% BSA and 0.05% Tween 20 in PBS for 1h at 37°C. After three additional washes, serial dilutions of rhesus plasma ranging from 1:500 to 1:200,000 were added in duplicate wells and incubated for 1h at 37°C. Wells were then washed 3 times, and incubated with a peroxidase-labeled antibody against rhesus IgG at a 1:10,000 dilution, for 30 min at 37°C. After three final washes, 3,3’,5,5’–tetramethylbenzidine substrate in DMSO and phosphate-citrate buffer (pH 5.0) with 30% hydrogen peroxide were added and incubated for 30 min at room temperature in the dark. Reactions were stopped with 2M H2SO4, and plates were read at 450 nm in an ELISA plate reader. Positive responses to calculate end-point antibody titers were defined using a threshold corresponding to the mean of negative controls plus two standard deviations.
2.6. Evaluation of vaccine-specific cellular immune responses
Vaccine-induced cellular immune responses were assessed by flow cytometric analysis of intracellular cytokine secretion in stimulated peripheric blood mononuclear cells (PBMCs). Briefly, PBMCs were purified from blood samples through a Ficoll-hypaque centrifugation, and then cryopreserved. Cryopreserved cells were thawed on the same day as stimulation and re-suspended in complete medium (RPMI-1640, 2 mM/L L-glutamine, 10% heat-inactivated fetal bovine serum, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 10mM HEPES). Prior to stimulation, BD Falcon tubes were coated with GAM (Goat Anti-Mouse IgG (H+L) Kierkegaard and Perry Lab, Ics., Gaithersburg, MD) using 2.5μg GAM/tube in 1.0mL of 50mM TRIS (pH 8.6) and incubation at 37°C for 1 hour. After twice-washing with PBS, anti-CD28 and anti-CD49d antibodies (10ng/mL) were added in 1mL of PBS and incubated at 37°C for 1 hour to facilitate cross-linking. Following this, excess co-stimulatory antibodies were removed, and a minimum of 1.5 million cells per test were added to each BD Falcon tube and were stimulated with pools of peptides predicted to be T cell epitopes from Tc24 and TSA1, respectively (Table 1), and incubated overnight at 37°C with 5% CO2. Epitope predictions were made for MAMU A*01, A*02, A*11, B*01, and B*17 alleles using NetCTLpan, NetMHCpan, MHC-I Binding Prediction and IEBD analysis algorithms, and the 20 top ranked peptides from each program were compared. Based on this, consensus peptides predicted by all programs were selected. Unstimulated control cells were treated with media only, and positive control cells were stimulated with Staphylococcal enterotoxin B (SEB) (100ng/mL).
Table 1.
Peptides derived from TSA1 and Tc24 antigens
| Source antigen | Peptide ID | Sequence |
|---|---|---|
| TSA1 | TSAP97 | LSSSDIVAGY |
| TSA1 | TSAP122 | KEYWQAHTV |
| TSA1 | TSAP167 | REIDDYIWKA |
| TSA1 | TSAP176 | AEAWNIKVI |
| TSA1 | TSAP265 | WEIPGGVSSV |
| TSA1 | TSAP377 | RTFHLGPFSV |
| TSA1 | TSAP334 | RVDALITATI |
| TSA1 | TSAP392 | KTFANTLLY |
| TSA1 | TSAP400 | YSDDALHLL |
| TSA1 | TSAP497 | RATWPVNSRW |
| TSA1 | TSAP514 | VDYNFTIVAM |
| TSA1 | TSAP551 | GLSYGAGGKW |
| TSA1 | TSAP547 | RTKLIGLSY |
| TSA1 | TSAP534 | STPLLGASL |
| TSA1 | TSAP580 | REYQVALMLQ |
| TSA1 | TSAP616 | WTEFSHFYF |
| Tc24 | Tc24P81 | DEFTPRVRDIT |
| Tc24 | Tc24P92 | KRAFDKARAL |
| Tc24 | Tc24P110 | SEDFVEFLEFR |
| Tc24 | Tc24P114 | VEFLEFRLML |
| Tc24 | Tc24P117 | LEFRLMLCYI |
| Tc24 | Tc24P127 | YDFFELTVMFDE |
| Tc24 | Tc24P153 | KRAVPKLEAWG |
| Tc24 | Tc24P164 | AKVEDPAALFK |
| Tc24 | Tc24P183 | SVTFDEFAAW |
| Tc24 | Tc24P189 | FAAWASAVKL |
After overnight stimulation, cells were washed with PBS and stained with a live/dead amine-reactive dye in PBS, washed, and surface stained with a cocktail of surface antibodies (Table 2) for 20 minutes in the dark at room temperature in 2% FCS-PBS. Samples were resuspended in Fixation/Permeabilization solution (BD Biosciences, San Jose, CA) and incubated for 20 minutes at room temperature, followed by washing with Perm/Wash Buffer (BD Biosciences, San Jose, CA). Intracellular antibodies in cocktail (Table 2) were added to the pellet for 20 minutes at room temperature, and then washed with Perm/Wash and resuspended in Stabilizing Fixative (BD Biosciences, San Jose, CA) until flow cytometry acquisition on a BD LSR Fortessa at the TNPRC Flow Cytometry Core Facility. Flow cytometry analysis was performed using the FlowJo 10.6.1 software (BD Biosciences).
Table 2.
Antibodies used for flow cytometric analysis of T cells.
| Marker | Fluorochrome | Clone | Manufacturer | Catalog # | Staining |
|---|---|---|---|---|---|
| CD3 | Alexa Fluor 488 | SP34–2 | Becton Dickinson |
557705 | Surface |
| IL-2 | PerCP-Cy5.5 | MQ1-17H12 | Becton Dickinson |
560708 | Intracellular |
| TNFα | Alexa Fluor 700 | MAB11 | Becton Dickinson |
557996 | Intracellular |
| Live-Dead | Aqua Dead Cell Stain Kit |
ThermoFisher | L34965 | Live-dead | |
| CD8 | Brilliant Violet 605 | SK1 | Becton Dickinson |
564115 | Surface |
| CD4 | Brilliant Violet 650 | L200 | Becton Dickinson |
563737 | Surface |
| CD69 | PE | FN50 | Becton Dickinson |
560968 | Intracellular |
| IFN𝛾 | PE-Cy7 | B27 | Becton Dickinson |
557643 | Intracellular |
Single cell lymphocytes were selected based on time, single cells, SSC and FSC lymphocyte parameters, and the live/dead discriminator prior to gating CD3+ T cells. Cells were further gated on CD4+ and CD8+ activated T cells producing IFNγ, IL-2 and TNFα. The frequency of antigen-specific cytokine producing CD4+ and CD8+ T cells was calculated by subtracting the background response observed with unstimulated cells (medium alone), and a positive response was defined as a frequency being greater than two-fold of the mean frequency of unstimulated cells.
3. Results
3.1. Vaccine safety
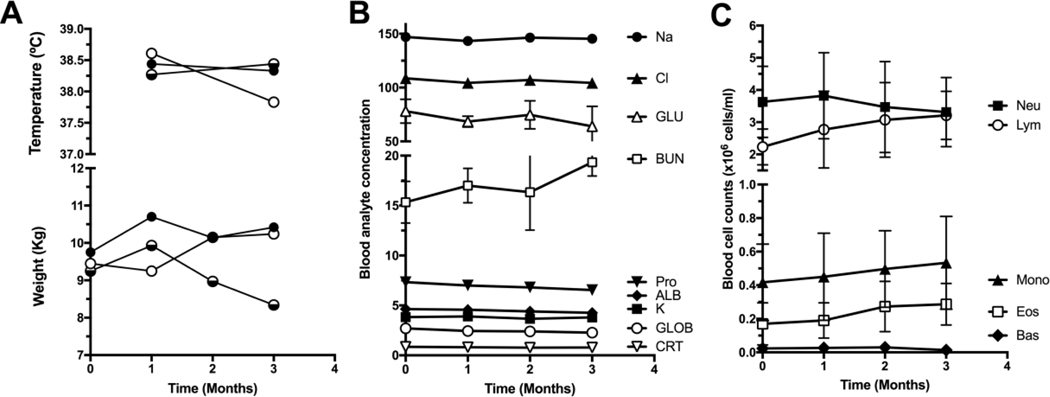
General health of the vaccinated rhesus was unaffected by the vaccine. Their body weights were stable over the 3 months duration of the experiment and body temperature remained normal (Figure 2A). All parameters from blood chemistry (Figure 2B) and blood cell counts (Figure 2C) were also stable over the course of the study and unaffected by each of the vaccine doses, indicating an overall excellent safety profile.
Figure 2. General health and blood parameters.
(A) Body weight and temperature, (B) blood chemistry and (C) blood cell counts were evaluated during the course of the study. All values are within the normal range. Data are presented as mean ± standard deviation (SD), except in (A) where data from individual animals are shown. GLU: glucose; BUN: blood urea nitrogen; Pro: proline; ALB: albumin; GLOB: Globin; CRT: Creatinine; Neu: neutrophils; Lym: lymphocytes; Mono: monocytes; Eos: eosinophils; Bas: basophils.
3.2. Vaccine humoral response
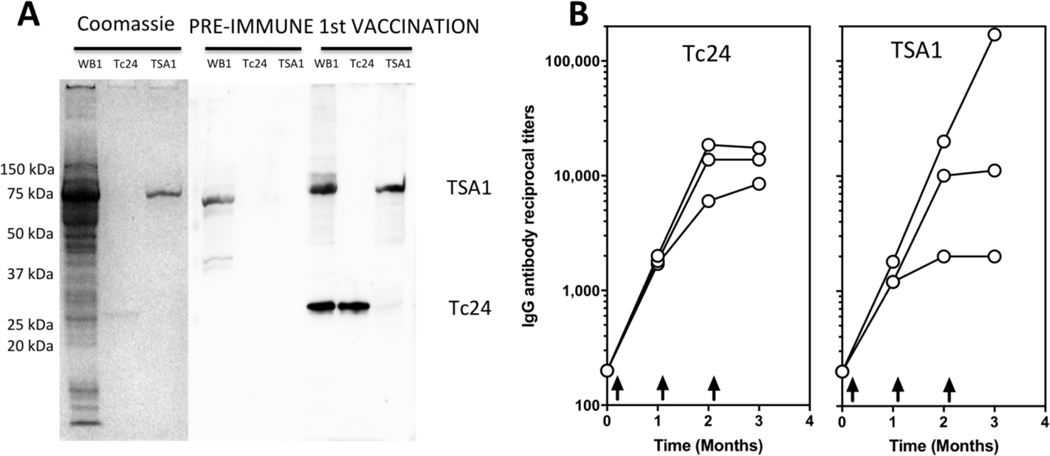
Analysis of the humoral response by western blot indicated that naïve animals, that were confirmed to be seronegative and PCR negative for T. cruzi infection, had no detectable antibodies against Tc24-C4 or TSA1-C4 antigens at baseline, but antigen-specific IgG were detectable one month after the first vaccine dose (Figure 3A). Importantly, these antibodies also recognized the respective wild type antigens present in a whole T. cruzi parasite lysate. Analysis of the time course of antigen-specific antibody titers by ELISA indicated that these strongly increased for both vaccine antigens following the first and second vaccine dose, but then remained high and stable after the third vaccine dose (Figure 3B). Titers above 1:10,000 were measured in most animals for both antigens. Only one animal presented a further increase in IgG titer against TSA1 after the third dose. Also, antibody titers against Tc24-C4 appeared to be somewhat more homogenous than that against TSA1-C4 which were more variable, particularly after the third vaccine dose. Together, these data confirmed the induction of a strong humeral response against both vaccine antigens in naïve rhesus macaques.
Figure 3. Induction of antigen-specific antibodies in vaccinated rhesus macaques.
(A) Western blot indicating the presence of specific antibodies against Tc24-C4 and TSA1-C4 antigens following the first vaccine dose in Rhesus LB83. These antibodies also recognize the corresponding native antigens in a parasite lysate (WB1 strain). (B) Time course of antigen-specific IgG titers in vaccinated rhesus, measured by ELISA. Arrows in the graph indicate the timing of the three vaccine doses. Data points represent individual Rhesus monkeys.
3.3. Vaccine cellular response
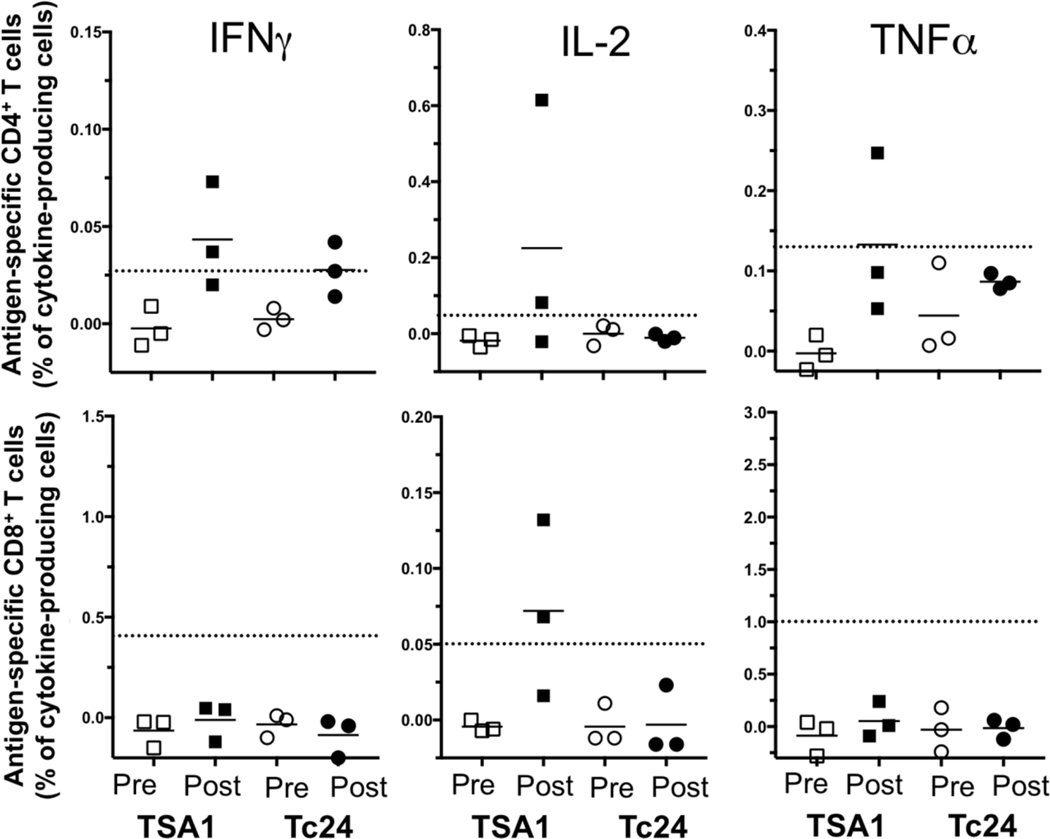
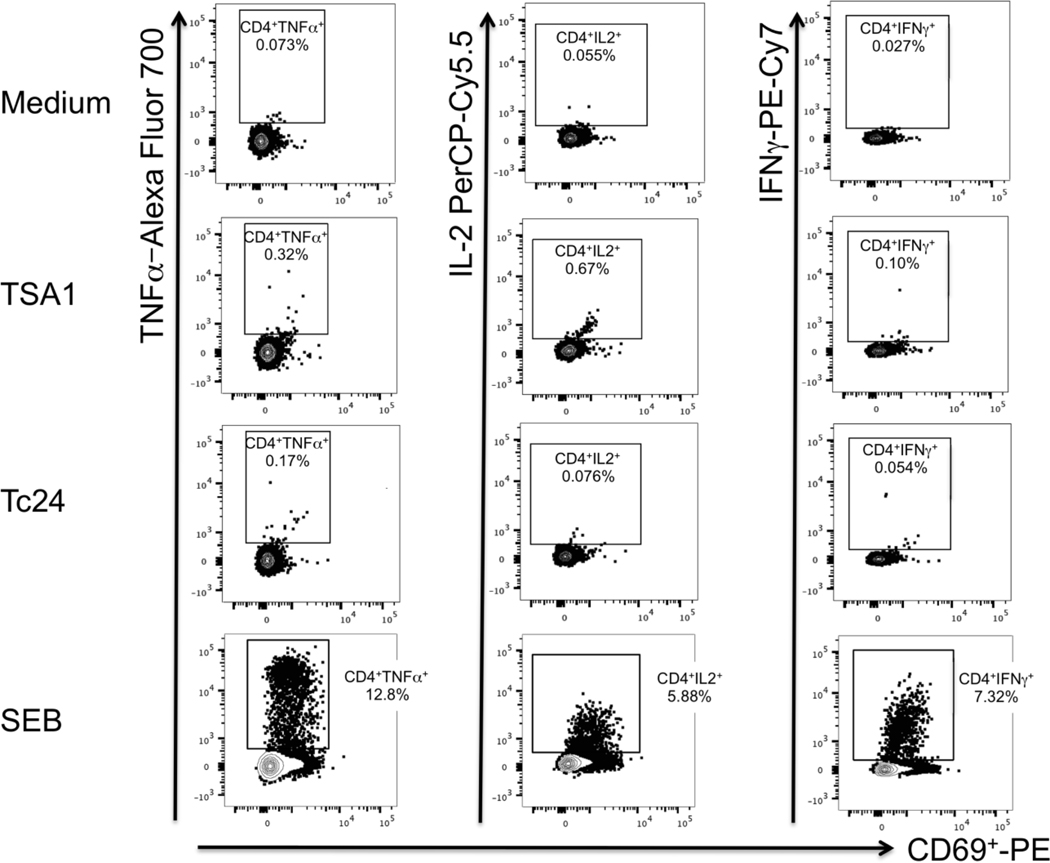
Evaluation of the cellular immune responses was performed by flow cytometry analysis of in vitro stimulated PBMCs, which were collected before vaccination and one month after the third vaccine dose. Stimulation with a peptide pool derived from TSA1-C4 resulted in a noticeable induction of CD4+ T cells producing TNFα, IL-2, and IFNγ with positive responses detected in one or two vaccinated animals, depending on the cytokine (Figure 4). Stimulation with a peptide pool derived from Tc24-C4 resulted in a noticeable induction of CD4+ T cells producing IFNγ in two vaccinated animals, but no IL-2 and TNFα producing CD4+ T cells were detected. In addition, an antigen-specific positive CD8+ response with IL-2 production was detected in two vaccinated animals following stimulation with the peptide pool derived from TSA1-C4 but not that from Tc24-C4 (Figure 4). Remarkably, one animal showed induction of all three cytokines from CD4+ T cells in addition to IL-2 expression from CD8+ T cells after stimulation with Tc24-C4 (Figure 5). Together, these data indicate the induction of at least some cellular immune response against the peptide pools derived from TC24-C4 and TSA1-C4 following vaccination.
Figure 4. Cytokine-producing T cells in vaccinated rhesus macaques.
The cellular immune response was evaluated before vaccination (Pre, open symbols) and one month after the third vaccine doses (Post, closed symbols). The frequency of antigen-specific (Squares: Tc24-C4; Circles: TSA1-C4) cytokine producing CD4+ (Top row of panels) and CD8+ T cells (Bottom row of panels) was calculated by subtracting the background frequency of unstimulated cells to that of peptide-stimulated cells. The dotted horizontal lines indicate the cut-off to consider a positive response, defined as two-fold the frequency of cytokine-producing unstimulated cells. Data points represent individual rhesus macaques.
Figure 5. Dot plots of cytokine-producing CD4+ T cells.
Cells were analyzed by flow cytometry, and CD4+ T cells were gated as described in the methods section. Responses from rhesus KL72 are shown for unstimulated control cells (Medium, top row of panels), TSA1 peptide pool (upper middle row of panels), Tc24 peptide pool (lower middle row of panels), and SEB (bottom row of panels) stimulation. Percentages of cytokine-producing CD4+ T cells are indicated in each panel for TNFα (Left column panels), IL-2 (middle column panels) and IFNγ (Right column panels).
4. Discussion
There is a critical need to develop novel tools for the control Chagas disease, to overcome the shortcomings of current drug treatments and complement vector control interventions [11]. While extensive studies in mouse models have provided a strong rationale for the development of a human vaccine against T. cruzi based on Tc24 and TSA1 [20], the extrapolation of these encouraging results to humans remains uncertain. Because Rhesus macaques living in outdoor primate colonies are both known natural hosts of T. cruzi [37], and have frequently been found to be better predictors of human responses to vaccines than mouse models, we evaluated here for the first time the safety and immunogenicity of two recombinant vaccine candidates in these non-human primates.
Our preliminary evaluation of the safety of the vaccine revealed no evidence of toxicity, as all vaccinated animals remained healthy, and no alterations in liver enzymes or kidney function were detected following three vaccine doses. Blood cell counts were also unaffected, further confirming that the vaccine was very well tolerated, as expected with recombinant proteins [38] and the E6020 adjuvant formulation [36]. To our knowledge this is the first report of safety and immunogenicity for a vaccine formulated with E6020 in a non-human primate model. While further studies are needed for an in-depth evaluation of the safety and toxicity of these two recombinant vaccines, these results are thus encouraging. Future studies should also assess potential local skin reactions at the site of vaccine injection.
In terms of immunogenicity, a strong antigen-specific antibody response was detected against each Tc24-C4 and TSA1-C4 antigens as early as one month after the first dose of the vaccine. Importantly, the response to each antigen was of a comparable magnitude, reaching end-point titers above 1:10,000 in most animals for both antigens after 2–3 vaccine doses. Furthermore, while these antibodies were induced by exposure to the mutated vaccine antigens, in which cysteine residues had been replaced [25–27], the antibodies also recognized the corresponding native antigens from a whole parasite lysate. This is in agreement with previous results in mice indicating that these mutations did not affect the immunogenicity of the recombinant proteins, but improved protein stability and the production process [25–27].
While antibodies may help clear circulating blood parasites, it is well established that a cellular immune response is also needed for the control of T. cruzi infection, as the parasite is mostly intracellular in its mammalian hosts [15, 16]. Analysis of T cell responses following vaccination of rhesus macaques confirmed the induction of a limited cellular immune response to the peptide epitope pools derived from both antigens, confirming that they are adequately processed by the immune system of these monkeys. Importantly, there seem to be differences in the profile of T cell activation by each peptide pool, with Tc24-C4 peptides only activating IFNγ-producing CD4+ T cells, while TSA1-C4 peptides also activating IL-2-and TNFα-producing CD4+ T cells, which suggests that the combination of both antigens in a vaccine may broaden the immune response, which may be important for the control of T. cruzi parasites. In addition, we detected positive TSA1-specific CD8+ T cell response in two of the vaccinated animals. While we do not know which peptides in our pools contributed to T cell stimulation, and can expect that additional epitopes are present in these proteins, the further identification of individual T cell epitopes from each antigen may allow a finer evaluation of the complete cellular immune response induced by these antigens, and of their possible differences in immunogenicity in non-human primates. Together with the detection of specific antibodies and a recall cellular immune response to Tc24 and TSA1 in Chagasic patients from southern Mexico [28], these results provide a strong rationale for further evaluation of these antigens in non-human primates including those that are naturally infected with T. cruzi and ultimately transition into phase I clinical trials.
While we immunized each antigen separately and in different sites for this co-administration study, due to the lack of data on potential interactions between the two proteins, it would be key that in the future we could also evaluate a simpler administration of a combined formulation of the antigens, as well as optimize further the vaccine formulation evaluating alternative immunostimulants, doses and route of immunization. In mouse models, immunization with mixtures of TSA1-C4 and Tc24-C4 suggest that these two antigens can indeed be combined (Dumonteil et al., unpublished data), and several Th1 adjuvants such as monophosphoryl lipid A (MPLA) or glucopyranosyl lipid A (GLA, IDRI) can potentiate vaccine efficacy in a similar manner as E6020 [24]. Studies in non-human primates would also allow evaluating vaccine efficacy for the control of T. cruzi infection and the development of the chronic Chagasic cardiomyopathy, either as a preventative vaccines or as a therapeutic vaccines.
5. Conclusion
In conclusion, we tested here for the first time the safety and immunogenicity of two co-administered recombinant vaccines against T. cruzi in rhesus macaques. The vaccines were safe as did not induce any alterations in blood chemistry and cell counts, indicating normal liver and kidney function. The vaccines also induced high IgG levels against Tc24-C4 and TSA1-C4 proteins, as well some limited antigen-specific cytokine-producing T cells. These preliminary results support the further assessment of the breadth of the cellular immune response induced by the vaccines and the evaluation of their efficacy in non-human primates, to strengthen the rationale for its clinical development as a human vaccine against Chagas disease.
Acknowledgements
We thank the Division of Veterinary Medicine of the Tulane National Primate Research Center for expert animal care and Theresa Glissman for administrative support.
6. Funding sources
This work was funded by grant #187714 from the Carlos Slim Foundation via Baylor College of Medicine, grant #632083 from Tulane University School of Public Health and Tropical Medicine, and the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the NIH through grant P51 OD011104 to the Tulane National Primate Research Center.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Declaration of interests
☐ X The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Global burden of disease collaboration. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hotez PJ, Alvarado M, Basanez MG, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS neglected tropical diseases. 2014;8:e2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].WHO. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Weekly epidemiological record. 2015;90:33–43. [PubMed] [Google Scholar]
- [4].Rassi A Jr., Marin JAN, Rassi A. Chronic Chagas cardiomyopathy: a review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Memorias do Instituto Oswaldo Cruz. 2017;112:224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nadruz W Jr., Gioli-Pereira L, Bernardez-Pereira S, Marcondes-Braga FG, Fernandes-Silva MM, Silvestre OM, et al. Temporal trends in the contribution of Chagas cardiomyopathy to mortality among patients with heart failure. Heart. 2018;104:1522–8. [DOI] [PubMed] [Google Scholar]
- [6].Simoes TC, Borges LF, Parreira de Assis AC, Silva MV, Dos Santos J, Meira KC. Chagas disease mortality in Brazil: A Bayesian analysis of age-period-cohort effects and forecasts for two decades. PLoS neglected tropical diseases. 2018;12:e0006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Capuani L, Bierrenbach AL, Pereira Alencar A, Mendrone A Jr., Ferreira JE, Custer B, et al. Mortality among blood donors seropositive and seronegative for Chagas disease (1996–2000) in Sao Paulo, Brazil: A death certificate linkage study. PLoS neglected tropical diseases. 2017;11:e0005542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cardoso CS, Ribeiro ALP, Oliveira CDL, Oliveira LC, Ferreira AM, Bierrenbach AL, et al. Beneficial effects of benznidazole in Chagas disease: NIH SaMi-Trop cohort study. PLoS neglected tropical diseases. 2018;12:e0006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kratz JM, Garcia Bournissen F, Forsyth CJ, Sosa-Estani S. Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert Rev Clin Pharmacol. 2018;11:943–57. [DOI] [PubMed] [Google Scholar]
- [10].Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr., Rosas F, et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. The New England journal of medicine. 2015;373:1295–306. [DOI] [PubMed] [Google Scholar]
- [11].Dumonteil E, Herrera C. Ten years of Chagas disease research: Looking back to achievements, looking ahead to challenges. PLoS neglected tropical diseases. 2017;11:e0005422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Quijano-Hernandez I, Dumonteil E. Advances and challenges towards a vaccine against Chagas disease. Human Vaccines. 2011;7:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barry MA, Versteeg L, Wang Q, Pollet J, Zhan B, Gusovsky F, et al. A therapeutic vaccine prototype induces protective immunity and reduces cardiac fibrosis in a mouse model of chronic Trypanosoma cruzi infection. PLoS neglected tropical diseases. 2019;13:e0007413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jones K, Versteeg L, Damania A, Keegan B, Kendricks A, Pollet J, et al. Vaccine-Linked Chemotherapy Improves Benznidazole Efficacy for Acute Chagas Disease. Infection and immunity. 2018;86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kumar S, Tarleton RL. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite immunology. 1998;20:207–16. [DOI] [PubMed] [Google Scholar]
- [16].Bustamante J, Tarleton R. Reaching for the Holy Grail: insights from infection/cure models on the prospects for vaccines for Trypanosoma cruzi infection. Memorias do Instituto Oswaldo Cruz. 2015;110:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee BY, Bacon KM, Wateska AR, Bottazzi ME, Dumonteil E, Hotez PJ. Modeling the Economic Value of a Chagas’ Disease Therapeutic Vaccine. Hum Vaccines Immunother. 2012;8:1293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee BY, Bacon KM, Connor DL, Willig AM, Bailey RR. The potential economic value of a Trypanosoma cruzi (Chagas disease) vaccine in Latin America. PLoS neglected tropical diseases. 2010;4:e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bartsch SM, Bottazzi ME, Asti L, Strych U, Meymandi S, Falcon-Lezama JA, et al. Economic value of a therapeutic Chagas vaccine for indeterminate and Chagasic cardiomyopathy patients. Vaccine. 2019;37:3704–14. [DOI] [PubMed] [Google Scholar]
- [20].Dumonteil E, Bottazzi ME, Zhan B, Heffernan MJ, Jones K, Valenzuela JG, et al. Accelerating the development of a therapeutic vaccine for human Chagas disease: rationale and prospects. Expert review of vaccines. 2012;11:1043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martinez-Campos V, Martinez-Vega P, Ramirez-Sierra MJ, Rosado-Vallado M, Seid CA, Hudspeth EM, et al. Expression, purification, immunogenicity, and protective efficacy of a recombinant Tc24 antigen as a vaccine against Trypanosoma cruzi infection in mice. Vaccine. 2015;33:4505–12. [DOI] [PubMed] [Google Scholar]
- [22].Barry MA, Wang Q, Jones KM, Heffernan MJ, Buhaya MH, Beaumier CM, et al. A therapeutic nanoparticle vaccine against Trypanosoma cruzi in a BALB/c mouse model of Chagas disease. Human vaccines & immunotherapeutics. 2016;12:976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Konduri V, Halpert MM, Liang D, Levitt JM, Cruz-Chan JV, Zhan B, et al. Genetic Adjuvantation of a Cell-Based Therapeutic Vaccine for Amelioration of Chagasic Cardiomyopathy. Infection and immunity. 2017;85:e00127–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de la Cruz JJ, Villanueva-Lizama L, Dzul-Huchim V, Ramírez-Sierra MJ, Martinez-Vega P, Rosado-Vallado M, et al. Production of recombinant TSA-1 and evaluation of its potential for the immuno-therapeutic control of Trypanosoma cruzi infection in mice. Human vaccines & immunotherapeutics. 2019;15:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Seid CA, Jones KM, Pollet J, Keegan B, Hudspeth E, Hammond M, et al. Cysteine mutagenesis improves the production without abrogating antigenicity of a recombinant protein vaccine candidate for human chagas disease. Human vaccines & immunotherapeutics. 2017;13:621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gunter SM, Jones KM, Seid CA, Essigmann HT, Zhan B, Strych U, et al. Mutations to Cysteine Residues in the Trypanosoma cruzi B-Cell Superantigen Tc24 Diminish Susceptibility to IgM-Mediated Hydrolysis. The Journal of parasitology. 2017;103:579–83. [DOI] [PubMed] [Google Scholar]
- [27].Biter AB, Weltje S, Hudspeth EM, Seid CA, McAtee CP, Chen WH, et al. Characterization and Stability of Trypanosoma cruzi 24-C4 (Tc24-C4), a Candidate Antigen for a Therapeutic Vaccine Against Chagas Disease. J Pharm Sci. 2018;107:1468–73. [DOI] [PubMed] [Google Scholar]
- [28].Villanueva-Lizama L, Cruz-Chan JV, Cetina-Aguilar AC, Herrera-Sanchez LF, Rodriguez-Perez JM, Rosado-Vallado ME, et al. Trypanosoma cruzi vaccine candidate antigens Tc24 and TSA-1 recall memory immune response associated with HLA-A and –B supertypes in Chagasic chronic patients from Mexico. PLoS neglected tropical diseases. 2018;12:e0006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Phares TW, May AD, Genito CJ, Hoyt NA, Khan FA, Porter MD, et al. Rhesus macaque and mouse models for down-selecting circumsporozoite protein based malaria vaccines differ significantly in immunogenicity and functional outcomes. Malaria journal. 2017;16:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cawlfield A, Genito CJ, Beck Z, Bergmann-Leitner ES, Bitzer AA, Soto K, et al. Safety, toxicity and immunogenicity of a malaria vaccine based on the circumsporozoite protein (FMP013) with the adjuvant army liposome formulation containing QS21 (ALFQ). Vaccine. 2019;37:3793–803. [DOI] [PubMed] [Google Scholar]
- [31].Cox LA, Olivier M, Spradling-Reeves K, Karere GM, Comuzzie AG, VandeBerg JL. Nonhuman Primates and Translational Research-Cardiovascular Disease. ILAR J. 2017;58:235–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kennedy RC, Shearer MH, Hildebrand W. Nonhuman primate models to evaluate vaccine safety and immunogenicity. Vaccine. 1997;15:903–8. [DOI] [PubMed] [Google Scholar]
- [33].Vitelli-Avelar DM, Sathler-Avelar R, Mattoso-Barbosa AM, Gouin N, Perdigao-de-Oliveira M, Valerio-Dos-Reis L, et al. Cynomolgus macaques naturally infected with Trypanosoma cruzi-I exhibit an overall mixed pro-inflammatory/modulated cytokine signature characteristic of human Chagas disease. PLoS neglected tropical diseases. 2017;11:e0005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Espinola Carvalho CM, Ribeiro Andrade MC, Salles Xavier S, Riccioppo Mangia RH, Carvalho Britto C, Jansen AM, et al. Chronic Chagas’ disease in rhesus monkeys (Macaca mulatta): evaluation of parasitemia, serology, electrocardiography, echocardiography, and radiology. The American journal of tropical medicine and hygiene. 2003;68:683–91. [PubMed] [Google Scholar]
- [35].Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS biology. 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ishizaka ST, Hawkins LD. E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert review of vaccines. 2007;6:773–84. [DOI] [PubMed] [Google Scholar]
- [37].Dorn PL, Daigle ME, Combe CL, Tate AH, Stevens L, Phillippi-Falkenstein KM. Low prevalence of Chagas parasite infection in a nonhuman primate colony in Louisiana. Journal of the American Association for Laboratory Animal Science : JAALAS. 2012;51:443–7. [PMC free article] [PubMed] [Google Scholar]
- [38].Nascimento IP, Leite LC. Recombinant vaccines and the development of new vaccine strategies. Braz J Med Biol Res. 2012;45:1102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]