Abstract
Introduction
Osteoporosis and bone fractures are common in chronic obstructive pulmonary disease (COPD) and contribute significantly to morbidity and mortality. Current national guidance on COPD management recommends addressing bone health in patients, however, does not detail how. This consensus outlines key elements of a structured approach to managing bone health and fracture risk in patients with COPD.
Methods
A systematic approach incorporating multifaceted methodologies included detailed patient and healthcare professional (HCP) surveys followed by a roundtable meeting to reach a consensus on what a pathway would look like.
Results
The surveys revealed that fracture risk was not always assessed despite being recognised as an important aspect of COPD management by HCPs. The majority of the patients also stated they would be receptive to discussing treatment options if found to be at risk of osteoporotic fractures. Limited time and resource allocation were identified as barriers to addressing bone health during consultations. The consensus from the roundtable meeting was that a proactive systematic approach to assessing bone health should be adopted. This should involve using fracture risk assessment tools to identify individuals at risk, investigating secondary causes of osteoporosis if a diagnosis is made and reinforcing non-pharmacological and preventative measures such as smoking cessation, keeping active and pharmacological management of osteoporosis and medicines management of corticosteroid use. Practically, prioritising patients with important additional risk factors, such as previous fragility fractures, older age and long-term oral corticosteroid use for an assessment, was felt required.
Conclusion
There is a need for integrating fracture risk assessment into the COPD pathway. Developing a systematic and holistic approach to addressing bone health is key to achieving this. In tandem, opportunities to disseminate the information and educational resources are also required.
Keywords: COPD, fracture risk, osteoporosis, bone health
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Key Points
Fracture risk in patients with COPD is not always comprehensively addressed, despite its importance being highlighted by healthcare professionals.
A proactive systematic approach to addressing bone health in people with COPD should be adopted where possible. Time and resource are major barriers.
Whilst assessment for osteoporosis should be carried out for all patients with COPD, this may not be feasible. Prioritizing a risk assessment in patients with important additional risk factors such as previous fragility fractures, older age and on long-term oral corticosteroids may be the pragmatic approach.
Fracture risk assessment should be carried out in all newly diagnosed patients with COPD within 6–12 months of diagnosis.
Annual COPD reviews provide a suitable setting in which bone health can be addressed for patients with established COPD, provided healthcare professionals receive adequate training and resources.
Fracture risk prediction tools, such as FRAX® and QFracture®, should be used to identify high-risk patients and aid targeted use of DEXA scans.
Other secondary causes of osteoporosis are common and should be investigated accordingly where osteoporosis is detected in patients with COPD.
Management of osteoporosis in COPD should focus on conservative measures such as smoking cessation and exercise in addition to pharmacological therapy.
Patient information on bone health is required.
Background
Osteoporotic fractures are common worldwide,1–3 have a debilitating impact on patients and in the UK confer a significant economic burden on the National Health Service (NHS).3,4 More than half of adult women and one in five men are believed to sustain one or more fragility fractures in their lifetime.5 It was estimated that in 2010 these fractures cost the NHS £4.4 billion and contributed to increased pain, disability and mortality for patients.3 Chronic obstructive pulmonary disease (COPD), primarily a respiratory condition but with known co-morbidities and extra-pulmonary manifestations, is linked to an increased risk of osteoporosis and fractures.6–10 When bone densitometry has been systematically performed, up to a third of patients with COPD have evidence of osteoporosis.7 Furthermore, the risk of falls, which is an important determinate of fragility fractures,11 is also increased in patients with COPD likely owing to a range of factors including impaired balance,12 poor muscle strength13 and cognitive deficits.14 As a result, people with COPD experience a greater risk of hip fracture than their matched counterparts without COPD.15 In one cohort, nearly half of patients with a surgical hip fracture had a diagnosis of COPD amongst their co-morbidities.16 The impact of fragility fractures specifically in people with COPD is of particular importance, owing to its contribution to morbidity and mortality.17,18 Osteoporosis-related vertebral fractures can limit lung function through associated kyphosis. In addition, rib fractures that are known to cause chest pain may lead to hypoventilation and reduced sputum clearance, with the possibility of worsening exacerbations.18 Given at least 1.2 million people in the UK are currently living with COPD,19 fracture risk in the context of COPD represents a significant and common clinical problem, yet the clinical pathway for this is far from clear.
National institute of clinical excellence (NICE) guidelines for osteoporosis recommend that COPD is a secondary cause of osteoporosis and therefore that a systematic assessment of fracture risk is required.20 This systematic assessment is not incorporated into either the NICE COPD guidelines 2010 or 201821,22 but they do refer to consideration of fracture risk in people with repeated oral corticosteroids. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019 strategy on management of COPD recommends active assessment and appropriate treatment of osteoporosis but it does not describe how and when this assessment should be carried out.23
Whilst the endeavour for clinicians should be to recognise bone health within COPD care, the lack of information detailing a systematic approach may make this challenging. There is a need for integrating osteoporosis assessment into the COPD pathway and managing both falls and fracture risk for people with COPD. Meanwhile, any change in the COPD pathway to accommodate this needs to be feasible as well as be acceptable to healthcare professionals (HCPs), carers responsible for people with COPD, and the patients themselves.
Aims and Objectives
To reach consensus on practically incorporating the assessment and management of bone health and fracture risk in patients with COPD, through a series of coordinated mixed method approaches, including:
Seeking opinion of people living with COPD of bone health with respect to knowledge, experience and views.
Seeking opinion from HCPs to determine knowledge of, experiences and clinical practices associated with managing bone health and fracture risks in COPD patients.
Collating surveys with available published evidence to form the basis of a roundtable meeting with key stakeholders including many multidisciplinary HCPs across respiratory, bone disease specialities in primary and secondary care to reach the consensus.
In addition, the aim was to discuss how the awareness of bone health in people with COPD could be raised with both patients and healthcare professionals and how the training could be delivered to healthcare professionals who manage patients with COPD.
Method
Ethical Approval
The survey protocol and plan were reviewed by the University of Nottingham research ethics department and the outcome was that this did not need a full ethics committee review or approval as the participants were providing their expert knowledge and experience to inform about the topic rather than being the subject of the topic in question (Ethics reference number: 81–1807).
Survey for People with COPD
The survey was developed by the research team and refined and piloted with the help of two members of different regional support groups for people with respiratory disease: “Breathe Easy”. Both members were trained in patient and public engagement; one a patient advocate and the other a person with a chronic lung condition. The aims of the survey and confirmation of anonymity were introduced at the start of the survey. This allowed implied consent. The survey (see online supplement 1) comprised of two sections: 1) participants characteristics including diagnosis and experiences of fractures and 2) knowledge and experience, including the link between COPD and bone thinning and of any care/treatment in relation to bone thinning. There were several questions with a choice of responses as well as opportunity for free text throughout the survey.
A link to the “live” patient survey (via onlinesurveys.ac.uk) was emailed to “Breathe Easy” groups across the East Midlands alongside hard copies of the surveys posted for distribution at their monthly meetings. Pre-paid envelopes were provided for completed questionnaires to be returned. The NIHR Nottingham Biomedical Research Centre (BRC) Respiratory theme posted the link on their website, Facebook and Twitter accounts, and the research team promoted via their personal work-related Twitter accounts. This reached a wider geographical area than East-Midlands. Online responses were downloaded directly from onlinesurveys.ac.uk into STATA and paper copies were manually entered into STATA by one of the researchers. Quality checks of data inputted were performed.
Survey for Healthcare Professionals
The survey for HCPs was also developed by the research team and piloted by four independent HCPs for content, ease of use, timing and accuracy and then refined prior to launch on onlinesurveys.ac.uk. The survey introduction outlined the aims of the project along with the online survey and confirmed that the survey was anonymous. Questions were themed across four sections:
Participants’ characteristics;
Knowledge of the link between COPD and bone thinning;
Beliefs and attitudes towards their role in its treatment; and
Practice in treating/discussing the link (see online supplement 2)
Over 160 HCPs were identified as potential recipients through the teams’ professional networks; all had experience in managing patients with COPD or lung disease, across a number of healthcare roles in the UK. In many instances, respiratory disease was not the core part of their job role. The identified HCPs were sent an introductory email containing a link to the survey and politely asked to forward the questionnaire link onto others who might be eligible to complete it. The survey was also promoted on social media (Facebook and Twitter) through Primary Care Respiratory Society UK (PCRS UK), East Midlands Thoracic Society (EMTS), National Institute for Health Research (NIHR), Nottingham BRC Respiratory theme, and the research team’s work-related accounts. Information regarding the survey was also posted on the PCRS UK website.
Both surveys were only available in English and data collection took place over a four-week period during July and August 2018 in parallel. Participants were asked to contact the research team if they had any concerns, wanted to be involved in further work or wished to receive a summary of the findings.
Data Analysis
Quantitative and qualitative content analysis was used to collate written comments by patients and HCPs in their respective survey as it enabled researchers to establish a set of categories (in this instance, themes derived from the responses to questions) and then count up the number of instances that fall into this category.24 One researcher read all comments for each question and preliminary themes were derived from grouping similar responses. The number of comments under each theme were then calculated.
Roundtable Session with Key Stakeholders to Gather Consensus on Managing Bone Health and Fracture Risk in Patients with COPD
Multidisciplinary HCPs involved in either COPD care or osteoporosis were invited to participate in a structured roundtable discussion on this topic in September 2018. Invites were made to give balanced representation across primary and secondary care and across multidisciplinary roles (nursing, physician, physiotherapy and pharmacy). The invitees were from different regions of the country including London, East Midlands, West Midlands and Somerset. Some members in the consensus meeting also had subspecialist interests in areas relevant to the subject matter; one physician was a geriatric consultant representing the Nottingham Osteoporosis Support Group and another was a GP present on behalf of PCRS. The meeting room hire and light refreshments were supported through funding by the British Lung Foundation (BLF). A summary of the results from both surveys was disseminated out to all delegates before the meeting. More in-depth results and further questions were sent out to three HCPs who could not attend in person but were able to provide comprehensive feedback, which was then incorporated into the roundtable discussion by two members of the research team. At the meeting, a detailed background of published literature was presented followed6–8,15,20,22,23,25,26–34 by an in-depth presentation of the surveys (full raw datasets were available for review too). The group was then divided into two with a facilitator each to carry out a structured discussion process with regards to a pathway addressing bone health. Key questions discussed within the groups included:
“Should we be offering assessment of fracture risk to all patients with COPD?”
“What should the patient pathway look like?”
“Are there any other considerations of bone health?”
Discussions were audio recorded for researcher reference, but no direct quotes are used in the write-up and anonymity was maintained. The content of the structured discussions was analysed for common themes relating to the key questions. Given that this was a consensus report on key elements of a structured approach to managing bone health and fracture risk in patients with COPD rather than development of national guidelines, the decision outcomes were not formally graded according to literature evidence but were a representation of the invitees’ recommendations, focusing on pragmatic and practical opportunities to addressing bone health.
Results
Patient Survey
Demographics
Of the 51 respondents in the patient survey, 41 had a diagnosis of COPD (or classed themselves as having chronic bronchitis or emphysema) and were included in the analysis. The other 10 respondents who had bronchiectasis, asthma, pulmonary fibrosis, sleep apnea or raised hemidiaphragm were excluded from the formal analysis but their responses were reviewed for any salient points that might need to be discussed later. More than half [n=24 (59%)] of the patients with COPD were female.
The vast majority (n=35) of the patients were ex-smokers with a further 3 patients never smoked and 3 current smokers. Most were over the age of 60 years; 51% 60 to 74 years and 46% 75+ years. Five (12%) of the patients had a diagnosis of osteoporosis and 11 (27%) were on bone protection treatment (Table 1).
Table 1.
Survey Responses from Patients with COPD
| N= 41 | % | |
|---|---|---|
| Who Do You See for Your Lung Condition? | ||
| (Patients Can Select More Than One Option) | ||
| GP | 29 | 71 |
| Hospital doctor | 12 | 29 |
| COPD nurse | 25 | 61 |
| Respiratory nurse | 12 | 29 |
| Have You Ever Completed Pulmonary Rehabilitation?a | ||
| Yes | 34 | 83 |
| No | 7 | 17 |
| Since Being Diagnosed, Have You Ever Broken a Bone? | ||
| Yes | 5 | 12 |
| No | 36 | 88 |
| Have You Got a Diagnosis of Osteoporosis? | ||
| Yes | 5 | 12 |
| No | 26 | 63 |
| Do not know | 10 | 25 |
| Are You on Tablets for Osteoporosis? | ||
| Yes | 11 | 27 |
| No | 28 | 68 |
| Do not know | 2 | 5 |
| Do You Take Tablets Containing Vitamin D? | ||
| Yes | 14 | 34 |
| No | 23 | 56 |
| Do not know | 4 | 10 |
Notes: aSix- to eight-week-long exercise-based programme consisting of strength and endurance training, nutritional counselling, self-management strategies and breathing technique advice, prescribed to patients with cardio-respiratory disease.
Knowledge
Patients were asked to give their response to their understanding of osteoporosis in COPD and their experiences. More than half of the cohort replied “Don’t know” when asked about their understanding of osteoporosis in COPD. The majority of patients (58%) stated they had not previously been assessed for osteoporosis or its risk factors by an HCP. When asked how receptive they would be to discuss treatment options if at risk of osteoporosis, 73% of patients replied they would be either very or moderately receptive. Furthermore, if provided with a leaflet on osteoporosis, respondents would be particularly interested in reading about the causes, diagnosis and the treatment involved, especially what lifestyle changes they could make to improve their bone health.
Healthcare Professional Survey
Demographics
There were 147 responses to the HCP survey however two participants were excluded from the analysis as they reported not seeing/assessing any patients with COPD. Of the included 145, 79% were female and the majority were from the Midlands (70%) with 2% from overseas (all Europe). The breakdown of job roles was as follows: 41% nurse with experience in managing COPD, 28% hospital doctor, 12% GP, and 19% other HCPs. The majority of the HCPs (85%) had worked in the NHS for more than 10 years. The proportion of patients who had COPD from their full case load were <25%: 25%; 25–49%: 23%; 50–74%: 22% and ≥75%: 30%.
Knowledge
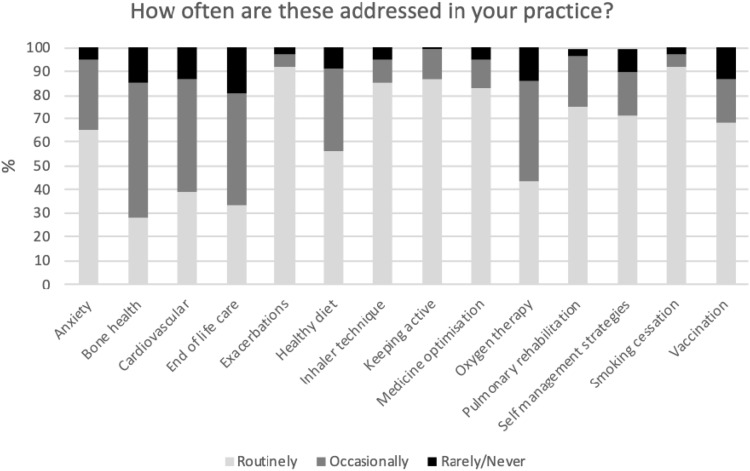
When asked what proportion of patients with COPD were at risk of osteoporosis, the responses were varied: <20% 10%; 21–40% 23%; 41–60% 34% and 61–75% 12%. More than half of HCPs (61%) had heard of the fracture risk assessment scores, Frax® and QFracture®. On a scale of 1–10 of the importance of bone health (1 being not important at all and 10 being extremely important), 74% of the respondents scored it 7 or greater. Meanwhile, an overwhelming majority (95%) thought bone health was under-addressed. As for where discussions around bone health should occur, the annual COPD assessment (88%), hospital out-patient appointment (77%), follow-up after an exacerbation (61%) were the most popular answers (participants could select more than one answer). How often the various aspects of COPD care were commonly advised upon is summarised in Figure 1.
Figure 1.
A bar chart showing how often topics in the management of COPD are addressed during consultations as reported by healthcare professionals.
When bone health was discussed in a COPD consultation, nearly three quarters of HCPs discussed it broadly and a few referred for a dual-energy X-ray absorptiometry (DEXA) bone densitometry or assessed fracture risk. Only 6% did a formal fracture risk score (such as Frax®) routinely. Less than 50% either “routinely” or “occasionally” referred patients for a bone DEXA scan, the majority (81%) thought patients were at least somewhat receptive to discussing bone health.
HCPs felt the top managements or therapies offered to COPD patients with an increased fracture risk were “brief advice” (51%), oral calcium and vitamin D (58%), diet advice (57%), increase physical activity (54%), and a DEXA bone scan (52%) (participants could select more than one answer). Half (48%) of respondents offered bisphosphonates to COPD patients viewed to be at an increased fracture risk.
From the free text responses, the two biggest barriers for HCPs in providing advice on fracture risk/risk of osteoporosis were time (44% of free text response raised this as an issue) and knowledge/guidance of how to deal with the issues (42% of responses). Other issues to a lesser extent included access to DEXA and information overload for patient.
Half of HCPs (49%) highlighted the importance of more training to facilitate discussions with patients to assess bone health, fracture risk and offer more general advice in this area. On top of this, nearly half (42%) of HCPs said in this instance a patient information leaflet would be valuable. The other request was providing clear local or national guidance.
Roundtable Consensus Meeting
Discussion of Patient and HCP Surveys
There were 13 professionals (including two methodologists) at the meeting and a further three (one GP with a specialist interest in respiratory medicine, one consultant integrated respiratory physician and one respiratory pharmacy consultant) were unable to attend but offered comments prior to the meeting on the survey results and questions to be posed.
Overwhelmingly, the view of the attendees was that the profile of bone health in patients with COPD needs to be raised. The striking mismatch in the survey by HCPs was highlighted; that most respondents felt assessment of bone health during consultations is “important” or “very important” but also that the subject is under-addressed. Reasons for such a discrepancy were further explored; it was felt that the current challenges of insufficient allocated time with patients, lack of resources particularly availability of DEXA scanning and educational needs for clinicians and awareness for patients prevented consideration of a robust systematic approach.
Ongoing discussion followed that the lack of a single patient record traversing primary and secondary care in the NHS may lead to a risk of omission or duplication. Furthermore, many felt there was insufficient time currently in a COPD review held in the primary care setting for core elements to be addressed; already having to prioritise between inhaler technique checks, health promotion advice, addressing patients’ ideas and concerns, reviewing lung function and assessing for other important co-morbidities, such as cardiovascular disease. Patients self-completing some questions prior to the consultation and software extracting and populating the fracture risk questions where possible were suggested solutions. These questions could include the clinical risk factors incorporated into the fracture risk assessment tools, such as smoking and alcohol status, history of previous fractures, family history (parent) of hip fractures, comorbidities (rheumatoid arthritis and other secondary causes of osteoporosis) and glucocorticoid use.
“Should We Be Offering Assessment of Fracture Risk to All Patients with COPD?”
This question was divided up into sections according to the nature of patients, as outlined below:
Current COPD diagnosis: There was a view that more could be done within the current system but that it was currently aspirational to deliver a systematic approach to bone health to all patients with already diagnosed COPD. However, a pro-active (eg assess early and prevent) rather than a re-active (eg treat once fracture occurs) was agreed. It was proposed that for people currently diagnosed with COPD the annual review would be an ideal setting to assess bone health. However, in such a setting if a systematic review for osteoporosis and fracture risk has not occurred before, the practical approach would be to search for those at most risk (previous low impact fractures, current smokers, low body mass index (BMI), severe COPD, frequent steroid courses) and prioritise their review. The aim would be to eventually provide a comprehensive review for all people with COPD.
New COPD diagnosis: Following discussion, a view held across the majority of the attendees was that bone health should be assessed at around the time of COPD diagnosis (during a 6–12 month period) and thereafter as part of the considerations at annual COPD reviews every 3–5 years.
All COPD patients: It was suggested that settings such as a pulmonary rehabilitation programme, review by the community respiratory teams and secondary care outpatient clinics, could offer an alternative opportunity for ascertaining fracture risk for patients but that these do not systematically address the COPD population as a whole.
Cautions: Consideration was given to patients where it may not be appropriate to undergo comprehensive investigations or treatment, such as those receiving palliative end of life care. Furthermore, if a patient had already sustained a fragility fracture, it was felt that they did not need to undergo a further fracture risk score but should be considered high risk and managed accordingly.
It was debated whether all people with COPD starting bone protection medication should have a DEXA bone densitometry alongside the fracture risk score with clear information on when to repeat. One argument was that baseline bone mineral density (BMD) measurements would be needed if treatment response was to be monitored. Furthermore, DEXA scans are relatively cheap and easy to arrange. However, current availability of scanning, especially if large numbers were referred may delay obtaining results and consequently treatment.
“What Would the Patient Pathway Look Like?”
The following recommendations were made for patients aged 50 years and over:
- Assess bone health within 6−12 months of a new COPD diagnosis.
- Then, regular review (at least every 3–5 years, likely during a COPD annual review to enable treatment to be considered and started or potentially stopped)
- For patients who have an established diagnosis of COPD, the annual review is ideal for assessing bone health but other opportunities such as hospital COPD out-patient appointments and pulmonary rehabilitation also provide opportune times and it is every HCP working with patients with COPD responsibility.
- Regular review should be carried out every 3–5 years during the annual review.
If already sustained a fragility fracture – consider the person high risk and establish on therapy.
A history of fragility fractures can be usually elicited during the clinical consultation, but given the asymptomatic nature of vertebral fractures, some patients may remain undiagnosed. With this in mind, review previous radiological imaging or organise appropriate vertebral X-rays if there is a clinical suspicion (loss in height) to identify vertebral fractures.
For all others, to complete a fracture risk score.
Consider a DEXA bone densitometry for those in intermediate range or where considering pharmacological treatment.
Consider how to tell patients of the risk.
Consider risk in the context of other co-morbidities.
Special Considerations
Consider all patients high risk regardless of age if there is a previous history of fragility fractures
Patients younger than 50 years with multiple other risk factors (but no previous fragility fracture), a decision on treatment decisions is based on clinical judgement
- Patients on maintenance (7.5mg or more for more than 3 months) or frequent (3 or more) use of systemic glucocorticoids consider
- If over 70 years old – consider high risk
- If 50–70 years old, assess fracture risk using fracture risk calculator
- For those <50 years old, treatment decisions on clinical judgement
The International Osteoporosis Foundation (IOF)–European Calcified Tissue Society recommends initiating bisphosphonates for patients on long-term steroids above the age of 70 years or who are on high dose steroids (more than 7.5mg/day of prednisolone or equivalent).35,36
The group agreed that for those with diagnosed osteoporosis, other secondary causes for osteoporosis should be addressed. Basic blood tests pertaining to secondary causes should be considered in those with evidence of osteoporosis (Table 2).
Table 2.
List of Suggested Investigations When Considering Secondary Causes of Osteoporosis Besides COPD
| Assess Other Potential Causes of Secondary Osteoporosis |
|---|
The following investigations should be considered in order to identify other causes of secondary osteoporosis where osteoporosis is diagnosed:
|
Table 3 highlights management options available for patients at risk of fragility fractures. There was agreement that this included pharmacological therapy but also non-pharmacological options were imperative. Such a consultation discussing treatment needs a receptive patient and an informed clinician. Monitoring the side effect profile of bisphosphonates was also suggested, especially gastro-oesophageal reflux disease (GORD) and osteonecrosis of the jaw; the former being a relatively common side effect also usually prevalent in COPD and the latter a serious complication of long-term bisphosphonate use.23,37 Reasons for a referral to a metabolic bone disease specialist are also stated in Table 4.
Table 3.
Management Options That Need to Be Considered for Patients at Risk of Fragility Fractures
Management for “at risk” of fracture group is holistic and includes recommendation of
|
Table 4.
Reasons for Referral to Metabolic Bone Disease Specialist
|
In order to raise the profile of bone health, the group felt there needs to be better health promotion for patients and more educational opportunities for HCPs. Suggestions discussed included whether it could be in the BLF passport alongside other common and clinically relevant co-morbidities, developing a comorbidities/bone health leaflet for patients and an educational campaign for HCPs in order to contextualise the benefits of treatment. Another recommendation was to calculate the cost–benefits of reducing fracture risk in patients with COPD and numbers needed to treat to emphasise the importance of fracture risk assessment and treatment to GPs.
Discussion
Osteoporotic fractures in the general population are extremely debilitating.1–3 Within the context of COPD they play a significant role in contributing to disease burden.17,18 Yet, there is limited guidance on how to address bone health within the COPD pathway. Whilst the NICE guidelines on osteoporosis emphasize the need for assessment of fracture risk in all patients with COPD,20 there is a lack of information on how this should be implemented into the COPD assessments and when. Furthermore, much of the literature assessing fracture risk in COPD is carried out in a select population, with little literature on a validated systematic process of assessing fracture risk. This statement outlines the key elements needed for a structured approach to addressing bone health, developed through a series of multifaceted methodologies consisting of patient and HCP surveys and a roundtable meeting to reach a consensus on the issue.
Both surveys highlighted that bone health and fracture risk in patients with COPD is not comprehensively addressed during chronic disease management and consultations, despite being recognised as an important issue. There are a number of important factors that are likely to contribute to this including system-wide, educational, awareness and also lack of clear guidance. Even for patients who have sustained a fragility fracture, studies have shown that treatment initiation is inadequate.38–40 Further, for many patients, osteoporosis is asymptomatic which means a pro-active approach is required to identify who might be at risk.41 Certainly, for people with COPD, there is currently no guidance on how to assess bone health during a routine consultation but where it is systematically done, it can identify those at risk.6,15 However, there are many other priorities at the annual assessment such as review of exacerbation history, symptoms, assessment of lung function, advising on smoking cessation and inhaler technique. Developing a systematic approach to assessing and addressing bone health across primary and secondary care could improve identification of those who are at risk of fractures and who requires treatment in a timely manner and thereby reduce fractures. The big question remains as to how this can be conducted and not at the detriment of other elements of patient care. Whilst elements of the fracture risk score can be completed through a semi-automated or a patient self-completed questionnaire, clearly, the impact on discussing results and the subsequent pathway needs time and specialist education.
Another important barrier to addressing bone health is limited opportunity for learning and minimal guidance for HCPs on practically assessing and treating osteoporosis in patients with COPD. This is not unique to COPD.42,43 Whilst consensus statements such as this highlight this knowledge gap in order to address and aim to improve learning opportunities, clearly, this is some step from implementing bone health assessment into routine clinical care and that implementation step is imperative.44 National Osteoporosis Guideline Group (NOGG) recommends management of osteoporosis to be incorporated into the training programmes of all relevant disciplines.41 Educational campaigns for HCPs, highlighting the burden of disease, importance of fracture risk assessment tools and cost–benefit of treatment could help raise awareness. Signposting HCPs to websites and e-learning packages with information on osteoporosis may also facilitate their understanding and an outcome of this consensus is for the committee to work with stakeholders.
Whilst the opinion of the consensus group was that a systematic approach to bone health and fracture risk should be provided to all patients with a current diagnosis of COPD, the feasibility was questioned. In light of this, a staged approach to implementation was discussed, starting off with higher risk groups that can be determined from GP computer records such as patients with previously recorded fragility fracture,45 older age20,46 and those on maintenance or frequent oral glucocorticoids.15,47 COPD-specific characteristics relating to severity of disease, such as airflow obstruction, are also associated with osteoporosis and fracture risk and there is an argument that these patients would also need priority for assessment.7,48 However, the decision for assessment and/or treatment needs to be made on an individual basis, as there may be patients in whom fracture risk assessment or therapy might not be appropriate (eg those receiving end of life care).
The roundtable group decision to assess fracture risk within 6–12 months of a new diagnosis of COPD was a pragmatic decision so as not to overburden the patients following a COPD diagnosis. For patients with established COPD, the annual review is considered the optimal opportunity to assess co-morbidities in general and reinforce lifestyle modifications such as smoking cessation and exercise rehabilitation, which are relevant for both COPD and osteoporosis.22 Therefore, it is intuitive to address bone health within this setting. Other points of care, where periodical reviews of COPD related risk factors and self-management strategies are carried out, can also be useful in addressing bone health. Pulmonary rehabilitation is an important setting, where the risk factors common to fragility fractures are already assessed. It also offers an opportunity to emphasise the benefits of strength training and nutrition on not only COPD but also its associated co-morbidities like osteoporosis.49
The pathway highlights the need for a fracture risk assessment tool, which can quantify risk and aid in the targeted use of DEXA scans. Fracture risk assessment tools such as FRAX® and QFracture® are two validated models that are recommended by national osteoporosis guidelines.20,41 Both are straightforward to complete although the QFracture® is more comprehensive, factoring in history of falls and COPD within the fracture risk calculations.50,51 Both tools have been shown to reasonably predict fracture risk in COPD in line with non-COPD populations.15 The recommendation is to assess fracture risk rather than relying solely on BMD to govern treatment irrespective of which assessment tool is used. Where a patient has an indeterminate risk or pharmacotherapy is considered to be started, a DEXA bone scan to permit monitoring is recommended.41
For those where osteoporosis is detected through DEXA scanning or identification of a fragility fracture, it is important to consider alternative secondary causes other than COPD. Certainly, vitamin D deficiency, secondary hyperparathyroidism and hypogonadism are common in COPD.32,48,52,53 Previous studies have advocated screening of secondary causes through medical history, physical examination and biochemical tests.54,55 Hence, the consensus includes recommendations of other investigations.
Former fragility fractures warrant consideration of “high risk” in their own right: Vertebral and hip fractures are more common in COPD than in the general population.15,56 As well as increasing the risk of further fragility fractures, vertebral fractures have also been shown to reduce lung function even when asymptomatic.53 Diagnosis of a new vertebral fracture would allow prompt initiation of secondary prevention treatment, bypassing the pathway for risk assessment. Whilst routine assessment for vertebral fractures is not appropriate, it could be considered if there is a clinical suspicion and certainly opportunistically looked for when imaging for other reasons is available.
The consensus group did not diverge from standard pharmacotherapy pathways for those requiring treatment. There is an overwhelming amount of evidence demonstrating the protective effect of bisphosphonates for those at risk or with osteoporosis,57–59 although the evidence in COPD is minimal with no studies studying fracture prevention and only one small study demonstrating a modest improvement in BMD for those on treatment.60 Recommendation for monitoring GORD as a side effect of bisphosphonates was based on the condition being common within COPD and associated with an increased risk of exacerbations.61 Not only can this negatively impact lung function and quality of life,62 but the use of rescue courses of oral steroids during exacerbations may further impair bone health. Repletion of vitamin D levels prior to initiating bisphosphonates is also an important aspect of the management. Vitamin D insufficiency in patients on bisphosphonates appears to be associated with a poorer response to the antiresorptive medication.63 This may be in part due to vitamin D deficiency causing secondary hyperparathyroidism, which increases bone resorption, hence counteracting the protective effects of antiresorptives.64
The consensus group also flagged important non-pharmacological options, some COPD specific. The recommendation for people at high or intermediate risk of fracture to stop smoking was based on indirect evidence from 10 cohort studies (n = 59,232), suggesting that smoking cessation may reduce the risk of osteoporotic fragility fracture in women.65 Furthermore, a meta-analysis on smoking and BMD showed that bone loss was greater in current smokers than non-smokers by an additional 2% every 10 years increase in age.66 Similarly, exercise is shown to reduce the risk of major osteoporotic fractures by reducing the risk of falls and independently preserving bone density. A Cochrane systematic review that assessed the evidence from 159 randomised control trials (n = 79,193) on interventions for preventing falls in older people living in the community reported that exercise reduces the rate of falls and the risk of fracture.67 In a randomised control trial, lung transplantation patients who underwent six months of exercise significantly gained BMD, whilst the patients who were not prescribed exercise (controls) lost BMD.68 Whilst such non-pharmacological strategies should be advocated in all patients with COPD regardless of fracture risk, the knowledge that there is an increased risk of fractures may aid in adherence to such lifestyle modifications.
In otherwise healthy individuals treated for osteoporosis, repeat DEXA scan is usually recommended after a minimum of two years of anti-resorptive treatment, as it takes a minimum of two years to have a significant change in BMD.20 Duration of anti-resorptive therapy is usually between 3 and 5 years and is based on evidence showing positive effects of therapy at reducing fracture risk without increasing the prevalence of long-term side effects.41 NOGG recommends continuation of treatment in patients who obtain a fracture despite being on treatment or in whom the bone mineral density T score remains less than or equal to −2.5.41
The majority of the patients completing the survey had a limited understanding of the impact of fracture risk and osteoporosis in COPD. This is an important issue as patients may not know they have the disease until a fracture occurs. Engagement with primary prevention through weight-bearing exercises, smoking cessation and adequate nutritional intake would only be possible if patients understand the implications of the disease in COPD. Park et al demonstrated an improvement in osteoporosis self-efficacy, fall-self efficacy, and calcium and vitamin D intake for community-dwelling elderly participants after an individualised educational intervention consisting of brochures and formal exercise programmes.69 The consensus suggested developing a bone health leaflet, which would particularly emphasise the importance of lifestyle modifications in improving BMD and fracture risk. Working with organisations such as BLF and Royal Osteoporosis Society to co-ordinate educational events at focus group meetings could help raise awareness in the community.
We acknowledge the limitations of our methodology. Whilst the roundtable meeting was open to a variety of multidisciplinary staff and several stakeholder invites were prompted from the open free-text box on the survey, the process did not involve open applications and selection. Further, there was an East Midlands predominance. Similarly, the majority of the respondents for the HCP survey were also from the Midlands. Connotations here may be that the views and suggestions made regarding management of osteoporosis in COPD may not reflect the clinical practice across the UK. Furthermore, the recommendations made at the consensus meeting were not graded according to the level of evidence that form the basis of the suggestions. However, the lack of evidence in many areas prompted the consensus as opposed to formal guidelines at this stage.
Conclusion
Osteoporosis is a common and debilitating condition in patients with COPD. Whilst the importance of fracture prevention within these patients is accepted, this does not currently translate universally into clinical practice. Limited knowledge of fracture assessment and lack of time to prioritise bone health during consultations are the main HCP barriers to this. Development of a consensus document incorporating the current literature on managing osteoporosis in COPD and outlining important elements of a systematic and holistic approach to addressing bone health for individuals with COPD is key to overcoming these barriers but requires opportunities to disseminate the information, educational resources and time for it to be implemented. In tandem, patient information on bone health is key.
Acknowledgments
We thank the British Lung Foundation for financial support for the project. Thank you too to the Primary Care Respiratory Society team for dissemination of the HCP survey through social media and their newsletter to members.
The people present at the roundtable were Leah Jayes, Tricia Mckeever, Charlotte Bolton, Steve Holmes, Ayushman Gupta, Opinder Sahota, Melissa Canavan, Sally Singh, Elizabeth Towlson, Helen Ward, Rebekah Hooker, Eve Elliott and Jane Scullion. In addition, Kelvin Lim, Anna Murphy and Sarah Elkin contributed to the discussions, gave detailed input and helped develop the consensus statement but could not attend in person.
Many thanks to Barbara Preston and Teresa Burgoyne for working with us on the design of the patient survey and facilitating distribution to patients at Breathe Easy groups. Many thanks to all the patients and HCP who completed the relevant survey.
Charlotte Bolton, Tricia McKeever and Ayushman Gupta are supported by the NIHR Nottingham BRC respiratory theme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author Contributions
AG and LRJ are co-first authors. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. In particular, CEB, TMM and JS wrote the original proposal and sought funding from the British Lung Foundation. LRJ, TMM and CEB were responsible for design of the survey, plan and they organised the roundtable discussion. LRJ oversaw the conduct of the 2 surveys and collection of results. TMM carried out the data analysis for the surveys. AG and LJ were involved in the initial drafting of the report (different sections). CEB was the lead and guarantor for the project.
Disclosure
SH is funded for 1 session per month to work as a clinical commissioner for respiratory in Somerset CCG and works as a general practitioner partner in Somerset (five sessions per week). He is also a member of the NHS England Cardiovascular and Respiratory Programme Strategic Board and has worked as an unpaid advisor for the British Lung Foundation and Royal College of General Practitioners at a national level.
KL owns a share in Primary Integrated Community Service (PICS) that employs respiratory nurses in the community and is also the medical director of PICS.
JS has no direct conflict of interest with this publication but has received support or honorarium from Astra Zeneca, Nutricia, ARNS, Chiesi, Mundipharma, ROCHE, Boehringer Ingelheim, Teva, PCRS-UK, MIMS, Mark Allen Group, ADMIT, NIP and Mylan.
Prof. CEB reports grants from British Lung Foundation, during the conduct of the study.
All authors declare they have no other conflicts of interest in relation to this article.
References
- 1.Coughlan T, Dockery F. Osteoporosis and fracture risk in older people. Clin Med (Lond). 2014;14(2):187–191. doi: 10.7861/clinmedicine.14-2-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roux C, Wyman A, Hooven FH, et al. Burden of non-hip, non-vertebral fractures on quality of life in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). Osteoporos Int. 2012;23(12):2863–2871. doi: 10.1007/s00198-012-1935-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svedbom A, Hernlund E, Ivergard M, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013;8:137. doi: 10.1007/s11657-013-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burge RT, Worley D, Johansen A, Bhattacharyya S, Bose U. The cost of osteoporotic fractures in the UK: projections for 2000–2020. J Med Econ. 2001;4(1–4):51–62. doi: 10.3111/200104051062 [DOI] [Google Scholar]
- 5.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517–522. doi: 10.1016/S8756-3282(01)00614-7 [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Greening NJ, Evans RA, Samuels A, Toms N, Steiner MC. Prospective risk of osteoporotic fractures in patients with advanced chronic obstructive pulmonary disease. Chron Respir Dis. 2019;16:1479972318769763. doi: 10.1177/1479972318769763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton CE, Ionescu AA, Shiels KM, et al. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(12):1286–1293. doi: 10.1164/rccm.200406-754OC [DOI] [PubMed] [Google Scholar]
- 8.Sabit R, Bolton CE, Edwards PH, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(12):1259–1265. doi: 10.1164/rccm.200701-067OC [DOI] [PubMed] [Google Scholar]
- 9.Duckers JM, Evans BA, Fraser WD, Stone MD, Bolton CE, Shale DJ. Low bone mineral density in men with chronic obstructive pulmonary disease. Respir Res. 2011;12:101. doi: 10.1186/1465-9921-12-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J. 2009;34(1):209–218. doi: 10.1183/09031936.50130408 [DOI] [PubMed] [Google Scholar]
- 11.Hakamy A, Bolton CE, Gibson JE, McKeever TM. Risk of fall in patients with COPD. Thorax. 2018;73(11):1079–1080. doi: 10.1136/thoraxjnl-2017-211008 [DOI] [PubMed] [Google Scholar]
- 12.Beauchamp MK, Hill K, Goldstein RS, Janaudis-Ferreira T, Brooks D. Impairments in balance discriminate fallers from non-fallers in COPD. Respir Med. 2009;103(12):1885–1891. doi: 10.1016/j.rmed.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 13.Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36(1):81–88. doi: 10.1183/09031936.00104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922. doi: 10.1183/09031936.00125109 [DOI] [PubMed] [Google Scholar]
- 15.Akyea RK, McKeever TM, Gibson J, Scullion JE, Bolton CE. Predicting fracture risk in patients with chronic obstructive pulmonary disease: a UK-based population-based cohort study. BMJ Open. 2019;9(4):e024951. doi: 10.1136/bmjopen-2018-024951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan EA, Radcliff TA, Henderson WG, et al. Improving hip fractures outcomes for COPD patients. COPD. 2013;10(1):11–19. doi: 10.3109/15412555.2012.723072 [DOI] [PubMed] [Google Scholar]
- 17.Buss L, McKeever T, Nightingale J, et al. Hip fracture outcomes in patients with chronic obstructive pulmonary disease. Br J Anaesth. 2018;121(6):1377–1379. doi: 10.1016/j.bja.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 18.Sarkar M, Bhardwaj R, Madabhavi I, Khatana J. Osteoporosis in chronic obstructive pulmonary disease. Clin Med Insights Circ Respir Pulm Med. 2015;9:CCRPM. S22803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.British Lung Foundation. Chronic obstructive pulmonary disease (COPD) statistics; 2019. Available from: https://statistics.blf.org.uk/copd. Accessed April29, 2020.
- 20.National Institute of Clinical Excellence. NICE (CG146) Osteoporosis: Assessing the Risk of Fragility Fracture; 2012. Amended in 2017. [PubMed] [Google Scholar]
- 21.National Institute of Clinical Excellence. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care; 2010. [PubMed] [Google Scholar]
- 22.National Institute of Clinical Excellence. NICE (NG115) Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management; 2018. [PubMed] [Google Scholar]
- 23.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of COPD (2019 Report); 2019:130–134 [Google Scholar]
- 24.Silverman D. Interpreting Qualitative Data. Sage; 2015. [Google Scholar]
- 25.Silva DR, Coelho AC, Dumke A, et al. Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care. 2011;56(7):961–968. doi: 10.4187/respcare.01056 [DOI] [PubMed] [Google Scholar]
- 26.McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(3 Pt 1):704–709. doi: 10.1164/ajrccm.157.3.9703080 [DOI] [PubMed] [Google Scholar]
- 27.Shane E, Silverberg SJ, Donovan D, et al. Osteoporosis in lung transplantation candidates with end-stage pulmonary disease. Am J Med. 1996;101(3):262–269. doi: 10.1016/S0002-9343(96)00155-6 [DOI] [PubMed] [Google Scholar]
- 28.Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med. 2003;114(1):10–14. doi: 10.1016/S0002-9343(02)01297-4 [DOI] [PubMed] [Google Scholar]
- 29.Jorgensen NR, Schwarz P, Holme I, Henriksen BM, Petersen LJ, Backer V. The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med. 2007;101(1):177–185. doi: 10.1016/j.rmed.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 30.Miller J, Edwards LD, Agusti A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. doi: 10.1016/j.rmed.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 31.Ferguson GT, Calverley PMA, Anderson JA, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the TOwards a Revolution in COPD health study. Chest. 2009;136(6):1456–1465. doi: 10.1378/chest.08-3016 [DOI] [PubMed] [Google Scholar]
- 32.Graat-Verboom L, Smeenk FW, van den Borne BE, et al. Risk factors for osteoporosis in Caucasian patients with moderate chronic obstructive pulmonary disease: a case control study. Bone. 2012;50(6):1234–1239. doi: 10.1016/j.bone.2012.02.638 [DOI] [PubMed] [Google Scholar]
- 33.Jaramillo JD, Wilson C, Stinson DS, et al. Reduced bone density and vertebral fractures in smokers. Men and COPD Patients at increased risk. Ann Am Thorac Soc. 2015;12(5):648–656. doi: 10.1513/AnnalsATS.201412-591OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greulich T, Weist BJD, Koczulla AR, et al. Prevalence of comorbidities in COPD patients by disease severity in a German population. Respir Med. 2017;132:132–138. doi: 10.1016/j.rmed.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 35.Lekamwasam S, Adachi JD, Agnusdei D, et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23(9):2257–2276. doi: 10.1007/s00198-012-1958-1 [DOI] [PubMed] [Google Scholar]
- 36.Lekamwasam S, Adachi JD, Agnusdei D, et al. An appendix to the 2012 IOF-ECTS guidelines for the management of glucocorticoid-induced osteoporosis. Arch Osteoporos. 2012;7:25–30. doi: 10.1007/s11657-012-0070-7 [DOI] [PubMed] [Google Scholar]
- 37.Janovska Z. Bisphosphonate-related osteonecrosis of the jaws. A severe side effect of bisphosphonate therapy. Acta Medica (Hradec Kralove). 2012;55(3):111–115. doi: 10.14712/18059694.2015.47 [DOI] [PubMed] [Google Scholar]
- 38.Andrade SE, Majumdar SR, Chan KA, et al. Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med. 2003;163(17):2052–2057. doi: 10.1001/archinte.163.17.2052 [DOI] [PubMed] [Google Scholar]
- 39.Freedman KB, Kaplan FS, Bilker WB, Strom BL, Lowe RA. Treatment of osteoporosis: are physicians missing an opportunity? J Bone Joint Surg Am. 2000;82-a(8):1063–1070. doi: 10.2106/00004623-200008000-00001 [DOI] [PubMed] [Google Scholar]
- 40.Gong HS, Oh WS, Chung MS, Oh JH, Lee YH, Baek GH. Patients with wrist fractures are less likely to be evaluated and managed for osteoporosis. J Bone Joint Surg Am. 2009;91(10):2376–2380. doi: 10.2106/JBJS.H.01871 [DOI] [PubMed] [Google Scholar]
- 41.Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. doi: 10.1007/s11657-017-0324-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fogelman Y, Goldshtein I, Segal E, Ish-Shalom S. Managing osteoporosis: a survey of knowledge, attitudes and practices among primary care physicians in Israel. PLoS One. 2016;11(8):e0160661. doi: 10.1371/journal.pone.0160661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neuner JM, Schapira MM. The importance of physicians’ risk perception in osteoporosis treatment decision making. J Clin Densitom. 2012;15(1):49–54. doi: 10.1016/j.jocd.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 44.Chenot R, Scheidt-Nave C, Gabler S, Kochen M, Himmel W. German primary care doctors’ awareness of osteoporosis and knowledge of national guidelines. Exp Clin Endocrinol Diabetes. 2007;115(09):584–589. doi: 10.1055/s-2007-981454 [DOI] [PubMed] [Google Scholar]
- 45.Kanis J, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35(2):375–382. doi: 10.1016/j.bone.2004.03.024 [DOI] [PubMed] [Google Scholar]
- 46.Curtis EM, van der Velde R, Moon RJ, et al. Epidemiology of fractures in the United Kingdom 1988–2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. doi: 10.1016/j.bone.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Staa T, Leufkens H, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology. 2000;39(12):1383–1389. doi: 10.1093/rheumatology/39.12.1383 [DOI] [PubMed] [Google Scholar]
- 48.Okazaki R. COPD and bone. Clin Calcium. 2016;26(8):1195–1200. [PubMed] [Google Scholar]
- 49.Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042–1048. doi: 10.1183/09031936.00203809 [DOI] [PubMed] [Google Scholar]
- 50.Kanis JA. FRAX® Fracture Risk Assessment Tool - University of Sheffield, 2008. [Google Scholar]
- 51.ClinRisk. Qfracture®-2016 Risk Calculator; 2016. [Google Scholar]
- 52.Førli L, Halse J, Haug E, et al. Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J Intern Med. 2004;256(1):56–62. doi: 10.1111/j.1365-2796.2004.01337.x [DOI] [PubMed] [Google Scholar]
- 53.Lehouck A, Boonen S, Decramer M, Janssens W. COPD, bone metabolism, and osteoporosis. Chest. 2011;139(3):648–657. doi: 10.1378/chest.10-1427 [DOI] [PubMed] [Google Scholar]
- 54.Romme EA, Geusens P, Lems WF, et al. Fracture prevention in COPD patients; a clinical 5-step approach. Respir Res. 2015;16:32. doi: 10.1186/s12931-015-0192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazokopakis EE, Starakis IK. Recommendations for diagnosis and management of osteoporosis in COPD men. ISRN Rheumatol. 2011;2011:901416. doi: 10.5402/2011/901416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papaioannou A, Parkinson W, Ferko N, et al. Prevalence of vertebral fractures among patients with chronic obstructive pulmonary disease in Canada. Osteoporos Int. 2003;14(11):913–917. doi: 10.1007/s00198-003-1449-5 [DOI] [PubMed] [Google Scholar]
- 57.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/S0140-6736(96)07088-2 [DOI] [PubMed] [Google Scholar]
- 58.Delmas P, Recker RR, Chesnut C, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15(10):792–798. doi: 10.1007/s00198-004-1602-9 [DOI] [PubMed] [Google Scholar]
- 59.Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9):604–610. doi: 10.1056/NEJM200008313430902 [DOI] [PubMed] [Google Scholar]
- 60.Smith B, Laslett L, Pile K, et al. Randomized controlled trial of alendronate in airways disease and low bone mineral density. Chron Respir Dis. 2004;1(3):131–137. doi: 10.1191/1479972304cd025oa [DOI] [PubMed] [Google Scholar]
- 61.Ingebrigtsen TS, Marott JL, Vestbo J, Nordestgaard BG, Hallas J, Lange P. Gastro-esophageal reflux disease and exacerbations in chronic obstructive pulmonary disease. Respirology (Carlton, Vic). 2015;20(1):101–107. doi: 10.1111/resp.12420 [DOI] [PubMed] [Google Scholar]
- 62.Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113–118. doi: 10.1183/09059180.00002610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carmel AS, Shieh A, Bang H, Bockman RS. The 25(OH)D level needed to maintain a favorable bisphosphonate response is >/=33 ng/mL. Osteoporos Int. 2012;23(10):2479–2487. doi: 10.1007/s00198-011-1868-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barone A, Giusti A, Pioli G, et al. Secondary hyperparathyroidism due to hypovitaminosis D affects bone mineral density response to alendronate in elderly women with osteoporosis: a randomized controlled trial. J Am Geriatr Soc. 2007;55(5):752–757. doi: 10.1111/j.1532-5415.2007.01161.x [DOI] [PubMed] [Google Scholar]
- 65.Kanis JA, Johnell O, Odén A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155–162. doi: 10.1007/s00198-004-1640-3 [DOI] [PubMed] [Google Scholar]
- 66.Law M, Hackshaw A. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841–846. doi: 10.1136/bmj.315.7112.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitchell MJ, Baz MA, Fulton MN, Lisor CF, Braith RW. Resistance training prevents vertebral osteoporosis in lung transplant recipients. Transplantation. 2003;76(3):557–562. doi: 10.1097/01.TP.0000076471.25132.52 [DOI] [PubMed] [Google Scholar]
- 69.Park K-S, Yoo J-I, Kim H-Y, Jang S, Park Y, Ha Y-C. Education and exercise program improves osteoporosis knowledge and changes calcium and vitamin D dietary intake in community dwelling elderly. BMC Public Health. 2017;17(1):966. doi: 10.1186/s12889-017-4966-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- British Lung Foundation. Chronic obstructive pulmonary disease (COPD) statistics; 2019. Available from: https://statistics.blf.org.uk/copd. Accessed April29, 2020.