Abstract
Obesity is associated with increased risk and aggressiveness of many types of cancer. Women with obesity and breast cancer are more likely to be diagnosed with larger and higher-grade tumors and have higher incidence of metastases than lean individuals. Increasing evidence indicates that obesity includes systemic, chronic low-grade inflammation, and that adipose tissue can act as an important endocrine site, secreting a variety of substances that may regulate inflammation, immune response, and cancer predisposition. Obesity-associated inflammation appears to be initially mediated by macrophage infiltration into adipose tissue. Macrophages can surround damaged or necrotic adipocytes, forming “crown-like” structures (CLS). CLS are increased in breast adipose tissue from breast cancer patients and are more abundant in patients with obesity conditions. Moreover, the CLS index-ratio from individuals with obesity seems to influence breast cancer recurrence rates and survival. In this review, we discuss the most recent cellular and molecular mechanisms involved in CLS establishment in the white adipose tissue of women with obesity and their implications for breast cancer biology. We also explain how CLS influence the tumor microenvironment and affect breast cancer behavior. Targeting breast adipose tissue CLS can be a crucial therapeutic tool in cancer treatment, especially in patients with obesity.
Keywords: Adipocytes, Adipose tissue, Breast neoplasms, Macrophages, Obesity
INTRODUCTION
Overweight and obesity are major risk factors for cancer and chronic diseases such as diabetes, insulin resistance, and cardiovascular disease [1]. These conditions are currently on the rise in low- and middle-income countries. Obesity is characterized by an excessive accumulation of adipose tissue accompanied by systemic chronic inflammation, and it is associated with several types of cancer: breast, ovarian, liver, and pancreatic cancers, among others [2,3]. Overweight and obesity are defined by body mass index (BMI), the ratio between an individual's weight (in kilograms) and the square of their height (in meters). A person with a BMI of 25 or more is considered overweight, and a BMI of 30 or more is considered obese, according to the World Health Organization (WHO) classification [4]. However, BMI is an imprecise tool to determine obesity status, since it does not provide a quantitative analysis of the adiposity directly. Instead, it correlates with a few direct measures of body fat content [5]. In contrast, computed tomography is capable of providing an estimate of the amount of fat stored in different adipose tissue compartments. However, this technique is not routinely used in the diagnosis of obesity [6].
Adipose tissue is an endocrine and immune organ [7,8] composed of an intricate network of heterogeneous cell types, including infiltrating immune cells, such as lymphocytes (T and B cells), mast cells, and antigen-presenting leukocytes (macrophages and dendritic cells). Granulocytes, fibroblasts, endothelial cells, extracellular matrix (ECM), and other stromal components are also present [9,10]. This complex network may have a dramatic impact on carcinogenesis and tumor promotion [8,9,11,12]. Obesity has been associated with a higher risk of developing breast cancer, particularly in postmenopausal women, as well as with a worse disease outcome for women of all ages [13].
Breast cancer is the most common cancer in women worldwide [14,15,16]. Several risk factors for breast cancer are well-established by epidemiologic studies and include exogenous hormones, family history of cancer, genetic traits, race, ethnicity, and physical inactivity [16]. There are three surface receptors commonly used to characterize breast cancer: the estrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2). According to their presence or absence, breast cancer can be classified into ER+/−, PR+/−, and HER2+/−. Triple negative breast cancer (TNBC) is characterized by the absence of ER-/PR-/HER2- receptors [17]. TNBC is considered more aggressive with a poorer prognosis than other types of breast cancer, mainly because there are fewer targeted medicines that treat triple-negative breast cancer [18]. Systemic effects of adiposity are believed to be implicated in breast cancer development and aggressiveness, and may involve breast fat [19].
Adipocytes secrete many paracrine and endocrine hormones, as well as adipokines, and are classified into four types: white, brown, beige, and pink [8,20]. These regulate local and systemic metabolism and inflammation [8,9,11,21]. In mammary tumors, white adipocytes are the major component of the stromal microenvironment, and they can accelerate cancer progression by releasing free fatty acids and producing several inflammatory cytokines [22], inducing tumor proliferation and angiogenesis [8,9,11,23].
Expansion of white adipose tissue, characterized by white adipocyte hyperplasia (an increase in adipocyte number) and/or hypertrophy (an increase in adipocyte size), promotes inflammation mediated by macrophage infiltration, activation, and polarization [24]. Adipose tissue macrophages (ATMs) are highly inflammatory and can secrete proinflammatory cytokines such as tumor necrosis factor α (TNF-α). ATMs likely contribute to propagation of the recruitment of additional macrophages by releasing chemokines such as monocyte chemoattractant protein-1 and chemokine (C-C motif) ligand 2 (MCP1/CCL2). An increase of ATMs can result in the formation of crown-like structures (CLS) that surround dead adipocytes [25,26,27]. During obesity, ATM infiltration positively correlates with adipocyte size and CLS density [27,28].
CLS formation in the breast adipose tissue is related to the production of several immunomodulatory molecules which may favor breast cancer cell proliferation and progression. Here, we provide an overview of the mechanisms involved in CLS formation and their correlation with breast cancer. Moreover, we discuss the role of CLS in breast cancer prognosis, clinical-pathological parameters, and therapeutic approaches.
ADIPOSE TISSUE-ASSOCIATED MACROPHAGES AND CROWN-LIKE STRUCTURES
Adipose tissue is heterogeneous and contains a diversified pattern of immune cells and adipocytes depending on the anatomical localization. During weight gain, adipose deposits expand beyond the tissue capacity, resulting in white adipose tissue dysfunction and secretion of a greater number of inflammatory cytokines [29]. This moves the tissue toward a phenotype with more pro-inflammatory immune cells inducing a higher inflammatory response [30].
Adipose tissue can increase in size (hypertrophy) and in number of adipocytes (hyperplasia), accompanied by structural and cellular changes in the tissue in response to excess energy. These changes include fibrosis, local inflammation, infiltration of immune cells, polarization of macrophages from an anti-inflammatory to a pro-inflammatory phenotype, adipocyte death, hypoxia, and mechanical stress in the ECM [31,32].
Excessively hypertrophied adipocytes can induce secretion of chemotactic factors and promote recruitment of immune cells to the adipose tissue. Elevated expression of monocyte chemoattractant protein-1 (MCP-1) and chemokine (C-C motif) ligand 2 (CCL2) in the white adipose tissue (WAT) of subjects with obesity can prompt an increased influx of monocytes that will differentiate into macrophages to migrate and infiltrate into WAT, setting up a feed-forward inflammatory process [33].
The newly recruited monocytes then become polarized, turning into either proinflammatory M1 macrophages (or classically activated macrophages) or into anti-inflammatory M2 macrophages (alternatively activated macrophages) [34]. Macrophage populations can be distinguished based on the expression of surface markers and their location; for example, CD206, CD163, arginase-1, Mg1l, and IL-10 characterize the M2 macrophage population, which is involved in adipocyte tissue remodeling. M1 macrophages can be distinguished based on iNOS, CD86, and CD80 markers, while also expressing genes such as interleukin-6 (IL-6) and TNF-α [35]. Adipose tissue from lean individuals presents more M2 than M1 macrophages, while adipose tissue from obese individuals present more abundant M1 compared to M2 macrophages [36]. Macrophages in lean adipose tissue play an important role in maintaining the function and homeostasis of the tissue through phagocytosis of dead adipocytes, secretion of anti-inflammatory cytokines, and regulation of iron flux, which plays an important role in adipogenesis [37,38].
In conditions of obesity and overweight, M1 macrophages can accumulate and upregulate CD11c and F4/80 [39], producing proinflammatory factors that potentiate inflammation and insulin resistance [40]. M1 macrophages can deregulate adipocyte signaling processes, increase the production of reactive oxygen species, and secrete proinflammatory cytokines associated with oxidative stress and tissue destruction [40]. M1 macrophages in adipose tissue can form a characteristic “crown-like structure (CLS)” around the necrotic, hypertrophied, and dying adipocytes that need to be resorbed [27,28,41,42]. Electron microscopic analysis of human and mouse adipose tissue in conditions of obesity showed ruptured adipocyte membranes and the presence of cellular debris, dilated endoplasmic reticulum, and cytoplasmic lipid droplets, suggesting necrosis [43]. CLS is consider a hallmark of the proinflammatory process in adipose tissue, characterized by adipocyte cell death and intense release of free fatty acids, and infiltration of other immune cells such as lymphocytes, neutrophils, and mast cells, promoting and maintaining the exacerbated inflammatory state [44]. Moreover, adipose tissue macrophages are an important source of the proinflammatory cytokines TNF-α and IL-6, which can block the insulin receptors, leading to insulin resistance [45].
However, a deeper characterization is needed of the adipocyte cell death type that occurs during obesity; how this influences the polarization of macrophages is not yet clear. Even though CLS is a proinflammatory microenvironment, debate about the M1 or M2 profiles of macrophages in obesity is ongoing. Recent work indicates that a complex mixture of M1 and M2 macrophage phenotypes can be observed in white adipose tissue during obesity [46], indicating that macrophages cannot be classified using the simple dual M1/M2 model. Importantly, excess accumulation of adipose tissue produces a proinflammatory “metabolically activated” macrophage (MMe) phenotype, mechanistically distinct from M1 or M2 activation [47,48]. It was demonstrated that MMe accumulation in mammary adipose tissue promotes the establishment of TNBC during obesity, indicating that the metabolic status of these macrophages under conditions of weight gain may be crucial to cancer progression [49].
CROWN-LIKE STRUCTURES AND BREAST CANCER: CLINICAL-PATHOLOGICAL PARAMETERS AND PROGNOSIS
CLS are related to free fatty acid release in adipose tissue, NF-κB activation, and generation of a pro-inflammatory microenvironment [50]. For these reasons, CLS are often used as a biomarker of adipose tissue inflammation [51]. Adipose tissue inflammation associated with metabolic syndrome may favor breast cancer development and progression [52]. Given the important biological function of CLS, methods have been developed to measure these structures by light microscopy, and an index has been created to quantify CLS severity on a scale ranging from 0 to 1.0 [30]. CLS were found to be related to several stages of cancer formation and progression in different types of cancer, including breast cancer in women [53] and men [54], squamous cell carcinoma [55], endometrial cancer [56], prostate cancer [57], and hepatocellular cancer [58].
Consistent with clinical and experimental observations, the prognostic value of CLS in breast cancer may vary according to race/ethnicity [59], menopausal status [60,61], tumor subtype [62], presence of fibrosis [63], increased mammary tumor vascular density [64], resistance to therapy [65], and treatment responsiveness [66]. The breast cancer microenvironment is highly heterogeneous, composed of several immune cell types and molecules that are reprogrammed to sustain tumor growth and spread. The most abundant immune cells are macrophages, and more than 50% of macrophages are tumor-associated macrophages (TAMs) [67].
During carcinogenesis, circulating monocytes are recruited by tumor-derived chemoattractants including CCL2 (MCP-1) and CSF-1, and also differentiate into TAM. An enhanced understanding of how obesity modulates M1 and M2 macrophage density and function in human breast tissue is important, since most of TAM are composed of M2 macrophages [68] and could play a role in tumorigenesis by suppressing anti-tumor immune responses [69,70]. TAM are found along the tumor-stroma border, an area characterized by improved fibrotic ECM remodeling [71], allowing macrophages with an M1 profile to polarize to M2 through contact with molecules that mimic biochemical and biophysical alterations of ECM [72], thus contributing to worse cancer clinical outcomes [73].
The TAM with an M1 profile exhibit antitumor properties that identify and destroy cancer cells via phagocytosis and cytotoxicity. However, experimental data suggest that during tumor initiation, proinflammatory macrophages might promote malignant transformation through mutagenic reactive species of oxygen and nitrogen [74]. Additionally, the presence of inflammatory configurations is related to NF-κB activation and enhanced levels of proinflammatory mediators, including TNF-α, IL-6, and cyclooxygenase-2 (COX-2)-derived prostaglandin E2 (PGE2) [30]. These mediators can act to upregulate the transcription of the CYP19 gene encoding aromatase, leading to estrogen production [75] and worse breast cancer prognosis.
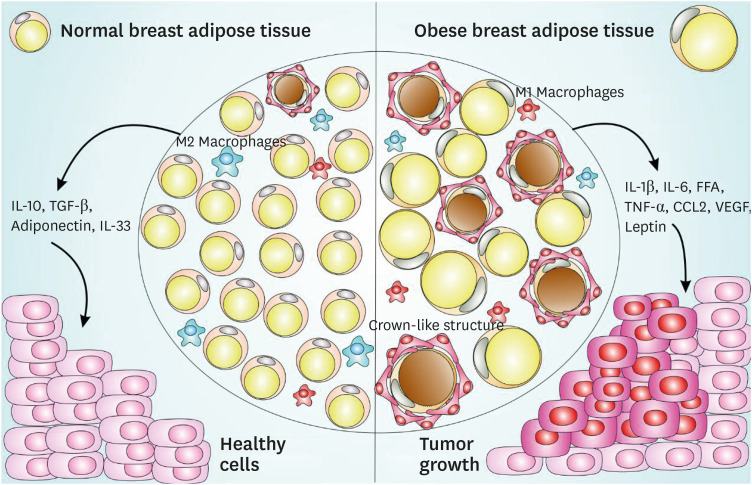
The presence of more CD68 and CD163 macrophage markers in breast adipose tissue is correlated with increased numbers of cancer-associated adipocytes in the breast cancer microenvironment [76,77], and these events are related to decreased survival in breast cancer patients [62,76,78]. These findings indicate that CLS can affect cancer development and impact breast cancer patients' overall survival (Figure 1).
Figure 1. CLS-mediated signaling pathway in normal and obese adipose tissue and impact on breast cancer. Normal breast adipose tissue is characterized by the presence of smaller and less numerous white adipocytes, fewer crown-like structures, a prevalent M2 macrophages subset, with the secretion of anti-inflammatory cytokines such as IL-10, TGF-β, IL-33, and adiponectin. Obese breast adipose tissue is characterized by larger size and more abundant white adipocytes, with the secretion of pro-inflammatory cytokines such as IL-1β, IL-6, FFA, TNF-α, macrophages-chemoattractant CCL-2 chemokine, angiogenesis inducers such as VEGF, a prevalent M1 macrophages subset and an increased production of leptin, a negative feedback signal in the regulation of energy balance. This obese breast adipose tissue provides a favorable microenvironment to cancer establishment and progression.
CLS = crown-like structures; IL = interleukin; TGF = transforming growth factor; FFA = free fatty acid; TNF = tumor necrosis factor; CCL = chemokine (C-C motif) ligand; VEGF = vascular endothelial growth factor.
CLS are also correlated with race with regard to breast cancer prognosis. Iyengar et al. [59] reported that menopausal Taiwanese women had pathologically enlarged adipocytes and increased presence of CLS in breast tissue, despite having a lower BMI than Caucasian women in the United States. In contrast, in Hispanic/Latin breast cancer survivors who underwent mastectomy, CLS were absent in 35% of patients with grade II and III obesity [79], indicating that a subset of patients with normal BMI and breast cancer can also present signs of inflammation and metabolic abnormalities (Table 1).
Table 1. Clinical and experimental studies in breast cancer and crown-like structures.
| Reference | Model | Target | Results |
|---|---|---|---|
| Morris et al., 2011 [53] | Human | Aromatase activity; adipocyte size; serum inflammation marker; CLS-B index | CLS-B correlated with BMI and adipocyte size |
| Subbaramaiah et al., 2011 [91] | Animals | NF-kB Activation; TNF-α, IL-1β, and COX-2 levels; aromatase activity; CLS | Obesity-inflammation-aromatase axis in the mammary gland and WAT, and its association with CLS, increased risk of ER breast cancer |
| Subbaramaiah et al., 2012 [75] | Human | Aromatase activity; CLS-B index | CLS-B index as an improved correlation marker of breast cancer and obesity |
| Iyengar et al., 2015 [60] | Human | CLS-B presence; Circulating inflammatory mediators | CLS-B is associated with metabolic syndrome and worse breast cancer prognosis |
| Cowen et al., 2015 [64] | Animal | Effect of a high-fat/high-calorie diet on mammary carcinogenesis | Breast adipose tissue inflammation induced by diet increases tumor vascular density |
| Seo et al., 2015 [63] | Animal; in vitro and human | Role of obesity in interstitial fibrosis in mammary fat | α-SMA levels correlated with CLS-B contribute to increased levels of matrix extracellular remodeling in obesity |
| Koru-Sengul et al., 2016 [62] | Human | Association between the number of TAM and/or CLS and ethnicities | Race is associated with the numbers of TAM and CLS in breast cancer |
| Mullooly et al., 2017 [89] | Human | CLS-B and sex steroid hormones in breast adipose tissue | CLS were not related to hormone levels or tumor characteristics, but were associated with hormone ratios |
| Vaysse et al., 2017 [90] | Human | CLS-B associated with mammary adipocyte size, body composition, and serum biomarkers | CLS was associated with systemic markers |
| Brown et al., 2017 [61] | Human | Effect of menopause on breast aromatase expression in relation to BMI, CLS and systemic markers of metabolic dysfunction | Postmenopausal women had higher BMI and presence of CLS than did premenopausal women |
| Cha et al., 2018 [76] | Human | Macrophage infiltration and identification of CLS status | CD68+ and/or CD163+ macrophages and CLS are present in adipose tissue near the breast cancer lesion |
| Iyengar et al., 2018 [59] | Human | CLS compared in Taiwanese vs Caucasian women | Compared with Caucasians, Taiwanese women had larger breast adipocytes despite lower BMI after adjusting for BMI and menopausal status |
| Greenlee et al., 2019 [79] | Human | Inflammation and BMI in Hispanic/Latina breast cancer patients | Prevalence of CLS-B was associated with Hispanic/Latina patients |
| Springer et al., 2019 [73] | Human and mice | Extracellular matrix affects macrophage phenotype | Fibrotic extracellular matrix remodeling promotes a TAM phenotype of macrophages in CLS |
CLS-B = crown-like structures in breast; BMI = body mass index; TNF-α = tumor necrosis factor α; IL = interleukin; COX-2 = cyclooxygenase-2; WAT = white adipose tissue; ER = estrogen receptor; α-SMA = α-smooth muscle actin; TAM = tumor-associated macrophage.
This healthier phenotype presented by some individuals with obesity is called metabolically healthy obesity, characterized by a lower degree of systemic inflammation, favorable inflammatory and hormonal profiles, normal adipokine secretion patterns, and reduced levels of ectopic and visceral fat storage [80,81]. A subgroup of normal-weight individuals with abnormal metabolic parameters (those exhibiting metabolically unhealthy non-obesity or metabolically obese normal weight) has also been described [82]. However, metabolically healthy obese people may be more prone to the risk of obesity-associated cancer mortality [83,84].
CROWN-LIKE STRUCTURES AND BREAST CANCER TREATMENT
Since the presence of CLS in breast adipose tissue involves the modulation of several components such as proinflammatory immunological cells, proinflammatory cytokines, adipocyte cell death, release of free fatty acids, and different hormones, it is important to note that any anti-cancer therapy targeting CLS can be potentially mediated by all these parameters. Endocrine therapy is one treatment tool for breast cancer, as it blocks estrogenic activity and suppress adipose tissue aromatization of androgens to estrogens [85]. Although aromatase inhibitors reduce circulating estrogens in most cases, conditions of overweight and obesity in patients with breast cancer (premenopausal and postmenopausal) make patients more prone to a higher risk of recurrence and resistance to therapy [86]. Estrogen replacement therapy in ovariectomized mice with obesity revealed that treatment with 17β-estradiol is enough to attenuate weight gain, reduce production of inflammatory markers and number of CLS [87], and reduce the expression of genes related to inflammation (Cd68, Mcp1, and Tnf) [88].
The presence of CLS and inflammatory mediators in breast adipose tissue in women with both breast cancer and obesity is associated with changes in intracellular signaling and significant changes in cell dysfunction [30]. The CLS are implicated not only in inflammation, rather than in obesity alone, but also as drivers of aromatase activity in the breast via a complex and dynamic system of paracrine interactions between macrophages and other cells [53,75], a process also associated with increased estrogen-to-androgen ratios [89]. These findings underscore the role of CLS as a potential booster of estrogenic signaling and may be crucial for endocrine therapy selection during breast cancer treatment [90]. In vitro experiments further defined the pathways mediating this effect, showing that macrophage COX-2 expression and PGE2 production promote estrogen receptor (ERα) target gene expression [91]. Based on these results, post-menopausal patients with obesity and breast cancer may benefit from an aromatase inhibitor/COX-2 inhibitor combination treatment during breast cancer [92].
Supplementation of breast cancer patients with omega-3 fatty acids can also modulate CLS in breast cancer tissue and serve as an important adjuvant in the treatment of this cancer. There are multiple preclinical and epidemiologic studies suggesting that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) attenuate inflammation and reduce risks for breast cancer [93,94]. In vitro studies also corroborate the anti-tumor effects of omega-3 against human breast cancer cells [95,96]. In a study using rodents, omega-3 supplementation significantly decreased the number and size of CLS and F4/80+ macrophages and decreased expression of proinflammatory mediators including Ptgs2, IL-6, CCL2, TNF-α, NF-κB, and interferon-γ proteins in the mammary fat pad [97].
CLS can be also modulated by other compounds such as polyphenols (resveratrol) decreasing inflammation in breast tissue and potentially affecting breast cancer. Supplementation with resveratrol in the diet of mice with obesity and breast cancer resulted in lower numbers of CLS and decreased proinflammatory cytokine gene expression [66].
Since CLS can be also be identified in a significant proportion of normal-BMI women undergoing mastectomy for breast cancer risk reduction or therapy, CLS may be also important in breast cancer treatment in normal weight women [98], not only in obese breast cancer patients.
To date, at least three clinical trials have advanced in the investigation of adiposity and inflammation to evaluate the risk factors associated with metabolic profile and tumor growth, which influence disease-free survival, overall mortality, and breast-cancer-specific mortality (NCT02240836; NCT03091842; NCT02598557). The forthcoming results of these studies should elucidate the relationship of CLS function to prognosis in different subtypes of breast cancer, providing new therapeutic approaches.
CONCLUSION
CLS are increased in breast adipose tissue from breast cancer patients and are particularly abundant in patients with conditions of obesity. Breast cancer associated with chronic obesity is most common in postmenopausal women. In this review, we discussed how CLS are related to the inflammation status of breast adipose tissue, and how these events can be decisive for breast cancer development and treatment, especially in obese women.
To improve interventions to prevent and treat breast cancer, a better understanding of the physiological and molecular mechanisms involved in breast tissue inflammation is crucial. It is important to understand that breast adipose tissue CLS are important keys to breast tissue inflammation and, consequently, may directly influence the development and treatment of breast cancer.
Targeting breast adipose tissue CLS can be a prominent therapeutic tool during cancer treatment, especially in patients with obesity. Therefore, tests based on body adipose tissue composition and inflammation in daily medical practice could be very effective to better stratify patients and direct combinatorial approaches in clinically relevant ways toward breast cancer treatment.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Magalhães KG.
- Data curation: Faria SS, Heyn GS, Almeida RN.
- Formal analysis: Faria SS, Heyn GS, Almeida RN.
- Supervision: Magalhães KG.
- Writing - original draft: Faria SS, Corrêa LH, Heyn GS, Sant'Ana LP, Almeida RN.
- Writing - review & editing: Magalhães KG.
References
- 1.Langin D. In and out: adipose tissue lipid turnover in obesity and dyslipidemia. Cell Metab. 2011;14:569–570. doi: 10.1016/j.cmet.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankovic N, Geelen A, Winkels RM, Mwungura B, Fedirko V, Jenab M, et al. Adherence to the WCRF/AICR dietary recommendations for cancer prevention and risk of cancer in elderly from Europe and the United States: a meta-analysis within the CHANCES project. Cancer Epidemiol Biomarkers Prev. 2017;26:136–144. doi: 10.1158/1055-9965.EPI-16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi B, Steiss D, Garcia-Rivas J, Kojaku S, Schnall P, Dobson M, et al. Comparison of body mass index with waist circumference and skinfold-based percent body fat in firefighters: adiposity classification and associations with cardiovascular disease risk factors. Int Arch Occup Environ Health. 2016;89:435–448. doi: 10.1007/s00420-015-1082-6. [DOI] [PubMed] [Google Scholar]
- 5.González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJ, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 6.Parikh AM, Coletta AM, Yu ZH, Rauch GM, Cheung JP, Court LE, et al. Development and validation of a rapid and robust method to determine visceral adipose tissue volume using computed tomography images. PLoS One. 2017;12:e0183515. doi: 10.1371/journal.pone.0183515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Corrêa LH, Heyn GS, Magalhaes KG, Corrêa The impact of the adipose organ plasticity on inflammation and cancer progression. Cells. 2019;8:E662. doi: 10.3390/cells8070662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrêa LH, Corrêa R, Farinasso CM, de Sant'Ana Dourado LP, Magalhães KG. Adipocytes and macrophages interplay in the orchestration of tumor microenvironment: new implications in cancer progression. Front Immunol. 2017;8:1129. doi: 10.3389/fimmu.2017.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc. 2001;60:319–328. doi: 10.1079/pns200192. [DOI] [PubMed] [Google Scholar]
- 11.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139–154. doi: 10.1038/s41574-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin PJ, Stambolic V. Impact of the obesity epidemic on cancer. Annu Rev Med. 2015;66:281–296. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- 13.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 15.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 16.Coughlin SS. Epidemiology of breast cancer in women. 2019. http://link.springer.com/10.1007/978-3-030-20301-6_2. [DOI] [PubMed] [Google Scholar]
- 17.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geyer FC, Pareja F, Weigelt B, Rakha E, Ellis IO, Schnitt SJ, et al. The spectrum of triple-negative breast disease. Am J Pathol. 2017;187:2139–2151. doi: 10.1016/j.ajpath.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soguel L, Durocher F, Tchernof A, Diorio C. Adiposity, breast density, and breast cancer risk: epidemiological and biological considerations. Eur J Cancer Prev. 2017;26:511–520. doi: 10.1097/CEJ.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cinti S. Adipose organ development and remodeling. Compr Physiol. 2018;8:1357–1431. doi: 10.1002/cphy.c170042. [DOI] [PubMed] [Google Scholar]
- 21.Scherer PE. The multifaceted roles of adipose tissue-therapeutic targets for diabetes and beyond: the 2015 banting lecture. Diabetes. 2016;65:1452–1461. doi: 10.2337/db16-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blücher C, Stadler SC. Obesity and breast cancer: current insights on the role of fatty acids and lipid metabolism in promoting breast cancer growth and progression. Front Endocrinol (Lausanne) 2017;8:293. doi: 10.3389/fendo.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3:545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127:74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book. 2013;33:46–51. doi: 10.1200/EdBook_AM.2013.33.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 32.Saltiel AR, Olefsky JM, Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henninger AM, Eliasson B, Jenndahl LE, Hammarstedt A. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLoS One. 2014;9:e105262. doi: 10.1371/journal.pone.0105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh M, Suganami T, Hachiya R, Ogawa Y. Adipose tissue remodeling as homeostatic inflammation. Int J Inflamm. 2011;2011:720926. doi: 10.4061/2011/720926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 36.Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. The macrophage switch in obesity development. Front Immunol. 2016;6:637. doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Hubler MJ, Peterson KR, Hasty AH. Iron homeostasis: a new job for macrophages in adipose tissue? Trends Endocrinol Metab. 2015;26:101–109. doi: 10.1016/j.tem.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, et al. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One. 2016;11:e0154003. doi: 10.1371/journal.pone.0154003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murano I, Rutkowski JM, Wang QA, Cho YR, Scherer PE, Cinti S. Time course of histomorphological changes in adipose tissue upon acute lipoatrophy. Nutr Metab Cardiovasc Dis. 2013;23:723–731. doi: 10.1016/j.numecd.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I, et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54:2423–2436. doi: 10.1194/jlr.M038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cildir G, Akıncılar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor α inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiwari P, Blank A, Cui C, Schoenfelt KQ, Zhou G, Xu Y, et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J Exp Med. 2019;216:1345–1358. doi: 10.1084/jem.20181616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lees T, Cullinane A, Condon A, Shabaan AM, Humphries MP, Speirs V. Characterising the adipose-inflammatory microenvironment in male breast cancer. Endocr Relat Cancer. 2018;25:773–781. doi: 10.1530/ERC-17-0407. [DOI] [PubMed] [Google Scholar]
- 55.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berstein LM, Iyevleva AG, Mukhina MS, Vasilyev DA, Poroshina TE. Features of omental adipose tissue in endometrial cancer patients with ‘standard’ or ‘metabolically healthy’ obesity: associations with tumor process characteristics. Springerplus. 2016;5:1900. doi: 10.1186/s40064-016-3582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyazawa M, Subbaramaiah K, Bhardwaj P, Zhou XK, Wang H, Falcone DJ, et al. Pioglitazone inhibits periprostatic white adipose tissue inflammation in obese mice. Cancer Prev Res (Phila) 2018;11:215–226. doi: 10.1158/1940-6207.CAPR-17-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lade A, Noon LA, Friedman SL. Contributions of metabolic dysregulation and inflammation to nonalcoholic steatohepatitis, hepatic fibrosis, and cancer. Curr Opin Oncol. 2014;26:100–107. doi: 10.1097/CCO.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iyengar NM, Chen IC, Zhou XK, Giri DD, Falcone DJ, Winston LA, et al. Adiposity, inflammation, and breast cancer pathogenesis in Asian women. Cancer Prev Res (Phila) 2018;11:227–236. doi: 10.1158/1940-6207.CAPR-17-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iyengar NM, Morris PG, Zhou XK, Gucalp A, Giri D, Harbus MD, et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res (Phila) 2015;8:349–358. doi: 10.1158/1940-6207.CAPR-14-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown KA, Iyengar NM, Zhou XK, Gucalp A, Subbaramaiah K, Wang H, et al. Menopause is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J Clin Endocrinol Metab. 2017;102:1692–1701. doi: 10.1210/jc.2016-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Glück S, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat. 2016;158:113–126. doi: 10.1007/s10549-016-3847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cowen S, McLaughlin SL, Hobbs G, Coad J, Martin KH, Olfert IM, et al. High-fat, high-calorie diet enhances mammary carcinogenesis and local inflammation in MMTV-PyMT mouse model of breast cancer. Cancers (Basel) 2015;7:1125–1142. doi: 10.3390/cancers7030828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giles ED, Wellberg EA, Astling DP, Anderson SM, Thor AD, Jindal S, et al. Obesity and overfeeding affecting both tumor and systemic metabolism activates the progesterone receptor to contribute to postmenopausal breast cancer. Cancer Res. 2012;72:6490–6501. doi: 10.1158/0008-5472.CAN-12-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossi EL, Khatib SA, Doerstling SS, Bowers LW, Pruski M, Ford NA, et al. Resveratrol inhibits obesity-associated adipose tissue dysfunction and tumor growth in a mouse model of postmenopausal claudin-low breast cancer. Mol Carcinog. 2018;57:393–407. doi: 10.1002/mc.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 68.Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sousa S, Brion R, Lintunen M, Kronqvist P, Sandholm J, Mönkkönen J, et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015;17:101. doi: 10.1186/s13058-015-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018;23:1239–1248. doi: 10.1016/j.celrep.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Springer NL, Iyengar NM, Bareja R, Verma A, Jochelson MS, Giri DD, et al. Obesity-associated extracellular matrix remodeling promotes a macrophage phenotype similar to tumor-associated macrophages. Am J Pathol. 2019;189:2019–2035. doi: 10.1016/j.ajpath.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 75.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Cha YJ, Kim ES, Koo JS. Tumor-associated macrophages and crown-like structures in adipose tissue in breast cancer. Breast Cancer Res Treat. 2018;170:15–25. doi: 10.1007/s10549-018-4722-1. [DOI] [PubMed] [Google Scholar]
- 77.Cha YJ, Koo JS. Expression of autotaxin-lysophosphatidate signaling-related proteins in breast cancer with adipose stroma. Int J Mol Sci. 2019;20:20. doi: 10.3390/ijms20092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quail DF, Olson OC, Bhardwaj P, Walsh LA, Akkari L, Quick ML, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol. 2017;19:974–987. doi: 10.1038/ncb3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greenlee H, Shi Z, Hibshoosh H, Giri DD, Ahmed A, Williams S, et al. Obesity-associated breast inflammation among Hispanic/Latina breast cancer patients. Cancer Prev Res (Phila) 2019;12:21–30. doi: 10.1158/1940-6207.CAPR-18-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14:219–227. doi: 10.1007/s11154-013-9252-x. [DOI] [PubMed] [Google Scholar]
- 81.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 82.Samocha-Bonet D, Dixit VD, Kahn CR, Leibel RL, Lin X, Nieuwdorp M, et al. Metabolically healthy and unhealthy obese--the 2013 Stock Conference report. Obes Rev. 2014;15:697–708. doi: 10.1111/obr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oh CM, Jun JK, Suh M. Risk of cancer mortality according to the metabolic health status and degree of obesity. Asian Pac J Cancer Prev. 2014;15:10027–10031. doi: 10.7314/apjcp.2014.15.22.10027. [DOI] [PubMed] [Google Scholar]
- 84.Blüher M. Are metabolically healthy obese individuals really healthy? Eur J Endocrinol. 2014;171:R209–19. doi: 10.1530/EJE-14-0540. [DOI] [PubMed] [Google Scholar]
- 85.Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sini V, Lunardi G, Cirillo M, Turazza M, Bighin C, Giraudi S, et al. Body mass index and circulating oestrone sulphate in women treated with adjuvant letrozole. Br J Cancer. 2014;110:1133–1138. doi: 10.1038/bjc.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhardwaj P, Du B, Zhou XK, Sue E, Giri D, Harbus MD, et al. Estrogen protects against obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila) 2015;8:751–759. doi: 10.1158/1940-6207.CAPR-15-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhardwaj P, Ikeda T, Zhou XK, Wang H, Zheng XE, Giri DD, et al. Supplemental estrogen and caloric restriction reduce obesity-induced periprostatic white adipose inflammation in mice. Carcinogenesis. 2019;40:914–923. doi: 10.1093/carcin/bgz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mullooly M, Yang HP, Falk RT, Nyante SJ, Cora R, Pfeiffer RM, et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 2017;19:8. doi: 10.1186/s13058-016-0791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaysse C, Lømo J, Garred Ø, Fjeldheim F, Lofteroed T, Schlichting E, et al. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer. 2017;3:19. doi: 10.1038/s41523-017-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Bowers LW, Brenner AJ, Hursting SD, Tekmal RR, deGraffenried LA. Obesity-associated systemic interleukin-6 promotes pre-adipocyte aromatase expression via increased breast cancer cell prostaglandin E2 production. Breast Cancer Res Treat. 2015;149:49–57. doi: 10.1007/s10549-014-3223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paixão EM, Oliveira AC, Pizato N, Muniz-Junqueira MI, Magalhães KG, Nakano EY, et al. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naïve breast cancer patients: a randomized double-blind controlled trial. Nutr J. 2017;16:71. doi: 10.1186/s12937-017-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fabian CJ, Kimler BF, Phillips TA, Box JA, Kreutzjans AL, Carlson SE, et al. Modulation of breast cancer risk biomarkers by high-dose omega-3 fatty acids: phase II pilot study in premenopausal women. Cancer Prev Res (Phila) 2015;8:912–921. doi: 10.1158/1940-6207.CAPR-14-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pizato N, Luzete BC, Kiffer LF, Corrêa LH, de Oliveira Santos I, Assumpção JA, et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8:1952. doi: 10.1038/s41598-018-20422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pizato N, Kiffer LF, Luzete BC, Assumpção JA, Correa LH, Melo HA, et al. Omega 3-DHA and Delta-Tocotrienol modulate lipid droplet biogenesis and lipophagy in breast cancer cells: the impact in cancer aggressiveness. Nutrients. 2019;11:1199. doi: 10.3390/nu11061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khadge S, Thiele GM, Sharp JG, McGuire TR, Klassen LW, Black PN, et al. Long-chain omega-3 polyunsaturated fatty acids modulate mammary gland composition and inflammation. J Mammary Gland Biol Neoplasia. 2018;23:43–58. doi: 10.1007/s10911-018-9391-5. [DOI] [PubMed] [Google Scholar]
- 98.Iyengar NM, Brown KA, Zhou XK, Gucalp A, Subbaramaiah K, Giri DD, et al. Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev Res (Phila) 2017;10:235–243. doi: 10.1158/1940-6207.CAPR-16-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]