Abstract
Induced ketosis (or ketone body ingestion) can ameliorate several changes associated with neuroprogressive disorders, including schizophrenia, bipolar disorder, and major depressive disorder. Thus, the effects of glucose hypometabolism can be bypassed through the entry of beta-hydroxybutyrate, providing an alternative source of energy to glucose. The weight of evidence suggests that induced ketosis reduces levels of oxidative stress, mitochondrial dysfunction, and inflammation—core features of the above disorders. There are also data to suggest that induced ketosis may be able to target other molecules and signaling pathways whose levels and/or activity are also known to be abnormal in at least some patients suffering from these illnesses such as peroxisome proliferator-activated receptors, increased activity of the Kelch-like ECH-associated protein/nuclear factor erythroid 2-related factor 2, Sirtuin-1 nuclear factor-κB p65, and nicotinamide adenine dinucleotide (NAD). This review explains the mechanisms by which induced ketosis might reduce mitochondrial dysfunction, inflammation, and oxidative stress in neuropsychiatric disorders and ameliorate abnormal levels of molecules and signaling pathways that also appear to contribute to the pathophysiology of these illnesses. This review also examines safety data relating to induced ketosis over the long term and discusses the design of future studies.
Introduction
Diet-induced ketosis and/or ingestion of ketone bodies (KBs) is an established treatment for children (Neal et al., 2008, 2009) and adults with pharmacologically resistant epilepsy (Klein et al., 2014; Liu et al., 2018). Research teams have reported some success in ameliorating the severity of symptoms in neurodegenerative diseases, most notably in patients with mild cognitive impairment or early Alzheimer’s disease (for review, see Lange et al., 2017), more recently Parkinson’s disease (Phillips et al., 2018), and autistic spectrum disorders (for review, see Elamin et al., 2017). There is also some, albeit limited, evidence that nutritional ketosis may reduce symptoms in some patients with schizophrenia (SZ) (Włodarczyk et al., 2018), bipolar disorder (BPD) (Phelps et al., 2013), and major depressive disorder (MDD) (Brietzke et al., 2018) (Bostock et al., 2017a). It is worthy of note that MDD, BPD, and SZ are being increasingly described as neuroprogressive disorders to reflect progressive neuroanatomical and cognitive decline driven by many common factors present in each illness such as inflammation in the periphery and the brain, nitroxidative stress mitochondrial dysfunction coupled with disrupted tryptophan metabolism, and deficiencies in glutamatergic neurotransmission and neurotropin activity and increased production of cortisone coupled with impaired performance of glucocorticoid receptors (Berk et al., 2011; Maes et al., 2011; Davis et al., 2014; Haroon and Miller, 2017). The presence of these abnormalities in each neuroprogressive illness is unsurprising as evidence suggests that they may be the result of mitochondrial dysfunction oxidative stress and mitochondrial dysfunction in the periphery and in the brain (Morris et al., 2015a, 2017, 2019b). This may be of considerable clinical relevance as evidence suggests that many of the effects of induced ketosis would appear to be desirable as far as the treatment of these illnesses is concerned. Readers interested in the biochemistry underpinning the development of induced ketosis are invited to consult Figure 1.
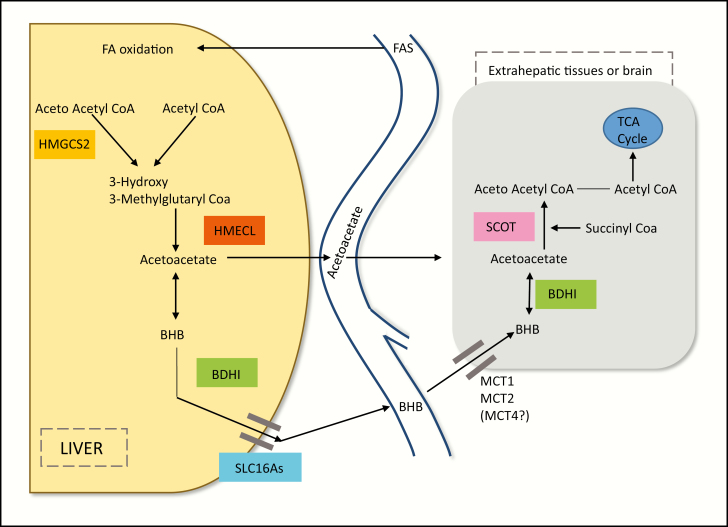
Figure 1.
The biochemistry of ketogenesis. Prolonged glucose restriction leads to an increased glucagon insulin ratio, relieving the inhibition of adipose triglyceride lipase and hormone-sensitive lipase, which are key enzymes in the production of free fatty acids (FFAs) in adipocytes and their subsequent release into the peripheral circulation. Decreased levels of insulin and glucose also combine to relieve the inhibition of carnitine acyltransferase 1 in the liver, which governs the uptake of FFAs into mitochondria, and to increase levels of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) while reducing levels of oxaloacetate, as it is used as a precursor for the manufacture of glucose. The net effect of these changes is increased glucagon-mediated transport of FFAs into the liver and increased uptake into mitochondria where they are used for the manufacture of acetyl coenzyme A (acetyle-CoA). In normal conditions, this would enter the tricarboxylic acid (TCA) cycle, but in an environment of reduced oxaloacetate the only metabolic pathway open to the molecule is ketogenesis involving the formation of ketone bodies via a series of steps. In the first of these reactions, Acetyl-CoA (AcCoA) is converted to acetoacetyl CoA in a reaction enabled by 3-Ketothiolase. This molecular intermediate is then converted to HMG CoA by HMG-CoA synthase, which is constitutively expressed in mitochondria. The final reaction in this pathway is the cleavage of HMG-CoA by HMG-CoA Lyase to produce acetoacetate. beta-hydroxybutyrate (BHB) may then be formed by the reversible reduction of acetoacetate mediated by 3-hydroxybutyrate dehydrogenase, and acetone may be produced by the thermodynamically favorable decarboxylation of aceoaetic acid (AA). Egress of these ketone bodies from the liver is facilitated by the transporter Solute Carrier Family 16, Member 6 (SLC16A6). Their subsequent entry into peripheral tissues and brain facilitated by monocarboxylic acid transporters ultimately serves as a source of AcCoA for the TCA cycle. Once in situ, BHB may be reconverted to acetoacetate in a reaction enabled by the same enzyme. However, from that point, ketolysis and utilization of ketone bodies display major biochemical differences compared with ketogenesis. In particular, Succinyl-CoA transfers its CoA group to acetoacetate to produce acetoacetyl-CoA in a reaction enabled by the enzyme succinyl-CoA:3-ketoacid coenzyme A transferase (also known as OXCT1 or SCOT), bypassing the irreversible step in ketogenesis catalyzed by HMG-CoA synthase and thereby preventing the development of a futile cycle of hepatic BHB synthesis and utilization.
For example, there is accumulating preclinical and clinical evidence that dietary ketosis results in the amelioration of oxidative stress, mitochondrial dysfunction, and inflammation in the periphery and in the brain of animals and humans (Jarrett et al., 2008; Nylen et al., 2009; Milder et al., 2010; Milder and Patel, 2012; Kim et al., 2015; Greco et al., 2016; Hasan-Olive et al., 2019). This could provide a possible approach that might reduce the magnitude of inflammation oxidative stress and mitochondrial dysfunction, which may be the core drivers of many of the symptoms associated with neuroprogressive disorders (Morris and Berk, 2015; Morris et al., 2015b).
Prolonged ingestion of a ketogenic diet (KD) or the ketone body, beta-hydroxybutyrate (BHB) leads to a global upregulation of peroxisome proliferator-activated receptors (PPARs) and increased activity of the Kelch-like ECH-associated protein-1/Nrf-2 system throughout the brain, at least as far as rodent data are concerned (Jeong et al., 2011; Simeone et al., 2017a; Knowles et al., 2018). Ingestion of a KD or BHB also decreases levels of nuclear factor (NF)-κB p65, most notably in microglia (Fu et al., 2014, 2015; Harun-Or-Rashid and Inman, 2018). There is a wealth of in vivo data associating the entry of KBs into glial cells and neurons, facilitated by monocarboxylate transporters, with the upregulation of several transcription factors, cofactors, and enzymes such as Sirtuin-1 (SIRT-1), SIRT-3, and Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1-alpha (Scheibye-Knudsen et al., 2014; McCarty et al., 2015; Elamin et al., 2018b; Hasan-Olive et al., 2019), which play a major role in regulating energy production, multiple aspects of cellular metabolism, and cellular redox status (Gano et al., 2014; Newman and Verdin, 2014; Veech et al., 2017; Miller et al., 2018).
These results offer the potential for ameliorating the effects of other likely sources of pathology in neuroprogressive disorders such as the inhibition of the Nrf-2 system and the compensatory antioxidant response (Genc and Genc, 2009; Martin-Hernandez et al., 2018; Zhang et al., 2018; Morris et al., 2019d). Elevated NF-KB activity is also thought to be a factor involved in driving the high levels of neuroinflammation, which in turn, is thought to play a major role in the pathophysiology, and possibly the pathogenesis, of neuroprogressive disorders (Thibaut, 2017; for review, see Kopitar-Jerala, 2015). Similarly, several research teams have reported decreased PPARγ activity and/or levels in the brains of individuals diagnosed with BPD (Nierenberg et al., 2018) and first-episode SZ (García-Bueno et al., 2014). This may also be of pathophysiological importance as it suggests impaired fatty acid oxidation and a failure to adjust energy supply in the face of changing metabolic environments (Poulsen et al., 2012; Chowdhury et al., 2018). This may be of paramount significance as fatty acid oxidation is a vital process enabling the maintenance of brain function and neural survival in an environment of glucose hypometabolism observed in SZ (Seethalakshmi et al., 2006), BPD (Fabrazzo, 2018), and MDD (Su et al., 2014) and is increasingly considered to be a factor in the pathogenesis and pathophysiology of these illnesses. Dysregulated or reduced SIRT-1 signaling also appears to be a characteristic feature of neuroprogressive diseases (Kishi et al., 2011; Nivoli et al., 2016; Lu et al., 2018; for review, see Alageel et al., 2018). The same appears to be true for PGC 1 alpha (for review, see Morris et al., 2013). These findings also suggest the presence of impaired energy metabolism and a failure of antioxidant systems in patients suffering from neuroprogressive disorders (Morris et al., 2019a).
The question arises as to the mechanisms underpinning these multidimensional, and potentially highly beneficial, effects that stem from induced ketosis or BHB administration, and the first objective of this paper aims to answer this question. In doing so, we will first focus on the properties of BHB as a free radical scavenger and activator of histone deacetylase (Shimazu et al., 2013; Kong et al., 2017; Wang et al., 2017) and then move on to discuss the plethora of favorable biochemical changes in antioxidant and bioenergetic profiles resulting from the upregulation of nicotinamide adenine dinucleotide+ (NAD+) that has been repeatedly observed in the brains of animals and in humans following protracted ketosis or ketonemia (Grabacka et al., 2016b; Elamin et al., 2017, 2018a; Xin et al., 2018). The physiological role of all the molecules discussed above is depicted in Table 1.
Table 1.
Physiological Roles of Signalling Molecules Commonly Cited in the Body of the Paper
| Molecules | Physiological role |
|---|---|
| NF-κB | Represents family of 5 structurally similar inducible transcription factors (p50, p52, RelA, RelB, and c-Rel) whose activity governs that of plethora of genes involved in effecting or regulating immune and inflammatory pathways and modulating several aspects of mitochondrial performance and energy production. |
| Nrf-2 | A transcription factor that, once translocated to nucleus, associates with small Maf proteins and subsequently binds to ARE in promoter regions of target genes involved in cellular antioxidant response network, stimulating their transcription. |
| KEAP-1 | A cysteine-rich molecule that binds to Nrf-2 in cytoplasm, promoting its degradation by ubiquitin proteasome pathway. |
| PPARα and PPARγ | Ligand-governed members of nuclear hormone receptor superfamily. Their activation generally increases expression of genes by binding to PPREs within their promoter regions in tandem with a retinoid X receptor. In certain circumstances, activation of PPARα or PPARγ may inhibit expression of gene clusters via interaction with other molecules such as NF-KB, SIRT-1, and PGC 1 alpha. PPARα and PPARγ activation provokes range of antioxidant, antiapoptotic, and antiinflammatory effects and plays major role in regulation of metabolism and mitochondrial dynamics. |
| FOXO | A FOXO class member of FOX protein family of transcription factors widely distributed in periphery and brain. Plays major role in regulating antioxidant responses, metabolism, energy production, and autophagy, including mitophagy and mitogenesis, by targeting promoter sequences on plethora of genes, generally leading to upregulation. This may be alone or in combination with range of other enzymes or coactivators such as SIRT-3, AMPK, and PGC 1alpha. |
| Sirtuins | Mammalian SIRTs function as NAD+-dependent deacylases and play many roles in regulating expression of genes involved in energy metabolism, cellular survival, inflammation, circadian rhythm regulation, and DNA repair. SIRT1 is found in cytosol and nucleus, modulates activity of transcription factors such as NF-KB p53, FOXOs, PPARs PGC1α, and PARP1. SIRT-3 is located in mitochondria and plays indispensable role in energy production and protecting organelles against oxidative and nitrosative stress. |
| Peroxisome proliferator-activated receptor-gamma coactivator | PGC-1alpha is a member of large family of transcription coactivators that acts as key player in regulation of energy metabolism by increasing mitochondrial biogenesis and stimulating mitochondrial respiration. Increased activity of this molecule also upregulates mitochondrial and cellular antioxidant responses. |
Abbreviations: AMPK, AMP-activated protein kinase; ARE, antioxidant response element; FOX, forkhead box; KEAP1, Kelch ECH associating protein 1; NAD, Nicotinamide adenine dinucleotide; NF-κB, Nuclear factor-κB; Nrf-2, nuclear factor erythroid 2-related factor 2; PARP1, Poly (ADP-ribose) polymerase 1; PGC 1 alpha, Pparg coactivator 1 alpha; PPARalpha, Peroxisome proliferator-activated receptor alpha; PPRAgamma, Peroxisome proliferator-activated receptor gamma; PPREs, Peroxisome proliferator hormone response elements; SIRT-1, Sirtuin 1; SIRT-3, Sirtuin 3.
In addition, there is an increasing use of induced ketosis or BHB ingestion in the treatment of other illnesses other than epilepsy encouraged by several factors, including data from large prospective cohort studies suggesting that moderate or high carbohydrate intake is associated with significantly higher rates of mortality compared with diets low in carbohydrate content over an 8-year period (Dehghan et al., 2017). Such an increase in use has produced a large volume of efficacy and safety data following long-term administration in illnesses and conditions such as type 2 diabetes, metabolic syndrome, and obesity (Gupta et al., 2017). This is of particular importance when considering the long-term use of a KD or BHB supplementation in neuroprogressive disorders as these abnormalities are present in such patients at significantly higher levels than age- and sex-matched population norms (for review, see Morris et al., 2019c). Hence, reviewing the efficacy and safety evidence available from the long-term induction of ketosis and/or ketonemia in metabolic disorders and, indeed, epilepsy before arriving at a projection of the relative risks and benefits of each approach will be the second objective of this paper.
Role of BHB in Free Radical Scavenging
Several research teams have produced in vivo data demonstrating that BHB and acetoacetate (ACA) administration can reduce oxidative stress by scavenging hydroxyl radicals and superoxide ions in various regions of the central nervous system (CNS), including the hippocampus and the neocortex. This results in reduced lipid peroxidation, improved ATP generation, and abrogation of glutamate excitotoxicity and synaptic dysfunction (Massieu et al., 2003; Maalouf et al., 2007; Haces et al., 2008; Julio-Amilpas et al., 2015). These findings have also been reported by research teams examining the effects of BHB and/or ACA on whole extracted neurons or CNS mitochondria in vitro (Maalouf et al., 2007; Haces et al., 2008; Maalouf and Rho, 2008; Julio-Amilpas et al., 2015). Reactive oxygen species (ROS) scavenging capacity also most likely underpins reports that KBs have the capacity to correct defective autophagy and ameliorate the effects of endoplasmic reticulum stress and the associated unfolded protein response (Camberos-Luna et al., 2016; Soejima et al., 2018).
BHB and Upregulation of Uncoupling Proteins
Induced ketosis or BHB administration is associated with increased expression of a number of uncoupling proteins (UCPs), most notably UCP2, although UCP4 and UCP5 also appear to be upregulated, in the periphery and in the brain (Grav et al., 2003; Sullivan et al., 2004; Fisler and Warden, 2006; Malingriaux et al., 2013; Hasan-Olive et al., 2019). Increased activity of UCPs can diminish the mitochondrial membrane potential (ΔΨ), resulting in a decrease in ROS production. This has been associated with increased resistance to kainic acid-induced seizures (Kovac et al., 2012). Increased activity of UCPs leads to the uncoupling of oxidative phosphorylation and ATP production by allowing a partial dissipation of ΔΨ or ΔpH by allowing the entry of protons from the inner membrane space at sites other than at ATP synthase (Kovac et al., 2012). This is of importance as ΔΨ and Δp are the drivers of electron transfer from complexes I, III, and IV to oxygen in the mitochondrial matrix (MM) (Brand et al., 2004) with the resulting formation of superoxide ions (Brand et al., 2004), and unsurprisingly the upregulation of UCPs is associated with a reduction in mitochondrial ROS (mtROS) production (Echtay, 2007; Mailloux and Harper, 2011). Given the decrease in ΔΨ and Δp, there is clearly a possibility that elevated UCP production could seriously compromise ATP production as discussed above. However, UCP elevation appears to be a very efficient defense against the advent of oxidative stress as a very modest dissipation of Δp results in a large decrease in mtROS production and hence does not necessarily result in a significant decrease in the production of ATP (Votyakova and Reynolds, 2001; Lambert and Brand, 2004).
Ketone Bodies (KBs) as Hydroxycarboxylic acid receptor 2 (HCA2) Ligands
In vivo data supplied by several research teams have established the role of BHB as a ligand for the G-protein-coupled HCA2 (Newman and Verdin, 2014; Guo et al., 2018; Trotta et al., 2019). BHB engagement with HCA2 has a major ameliorative effect on inflammation by inhibiting endoplasmic reticulum stress, resulting in decreased assembly of nucleotide-binding domain-like receptor protein 3 (Guo et al., 2018; Trotta et al., 2019). Details of the inhibitory effect of HCA2 activation on the suppression of inflammatory signaling in the periphery and brain may be obtained by reference to the work of (Graff et al., 2016).
The weight of evidence suggests that in vivo BHB engagement of HCA2 in activated microglia reduces activity of the pro-inflammatory enzymes Prostaglandin-endoperoxide synthase 2 (COX-2) and Inducible nitric oxide synthase (iNOS) by reducing degradation of NF of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha and inhibiting the nuclear translocation of NF-κB, thereby preventing the action of the transcription factor in initiating and maintaining the transcription of proinflammatory cytokines and a range of other inflammatory molecules (Fu et al., 2014, 2015). Data also suggest that the downregulation of nucleotide-binding domain-like receptor protein 3 activity and decreased levels of Interleukin 1 beta (IL-1β) concomitant with reduced neuroinflammation seen in the CNS of study animals following BHB ingestion is mediated via HCA2 (Youm et al., 2015; Yamanashi et al., 2017; Trotta et al., 2019).
BHB as A Class I and II Deacetylase Inhibitor
Background
BHB acts as a class I and II deacetylase inhibitor that increases global levels of acetylation in vivo in a dose-dependent manner, resulting in the increased expression of specific genes involved in stimulating cellular antioxidant defenses and in an amelioration of oxidative stress (Shimazu et al., 2013; Kong et al., 2017; Wang et al., 2017). The weight of evidence suggests that such inhibition is associated with increased transcription and/or activity of metallothionein II, mitochondrial Superoxide Dismutase 2 (SOD2), catalase, Forkhead box O (FOXO) 3a, and Nrf2, (Shimazu et al., 2013; Wei et al., 2014; Nagao et al., 2016). Increased activity of FOXO3a and Nrf2 has significance as far as a global cellular antioxidant response is concerned, which we discuss below. These signaling pathways and the molecules involved are represented diagrammatically in Figure 2.
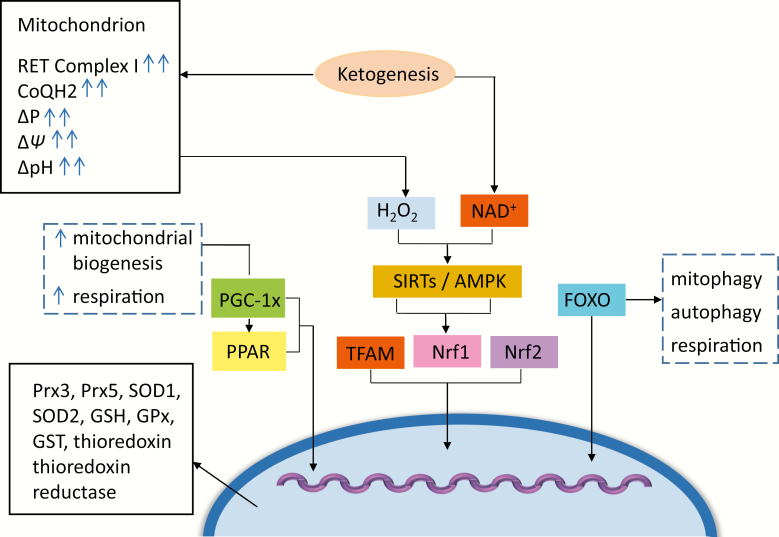
Figure 2.
Mitochondrion. Glucose restriction and BHB oxidation leads to an increase in NAD+ and upregulated AMP-activated protein kinase (AMPK), with “downstream” activation of silent mating type information regulation 2 homologue 1 and 3 (SIRT1 and 3), peroxisome proliferator-activated receptor γ (PPARy), peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), forkhead box O 3a (FOXO3a), and nuclear factor erythroid-derived 2-like 2 (NFE2L2). The cooperative activity of these enzymes and signalling systems ultimately result in increased transcription of genes related to oxidative capacity, mitochondrial uncoupling, and antioxidant defenses as detailed in the text. Fatty acid oxidation subsequent to ketolysis in an environment of glucose restriction decreases the ratio of NADH/FADH2, leading to the overreduction of the CoQ/CoQH2 couple due to an excess of electrons entering the ETC at complex II. This scenario may provoke increases in reverse electron transport and increased ROS production in the form of superoxide radicals at complex I of the ETC. The subsequent dismutation of superoxide to hydrogen peroxide in the mitochondrial matrix and “spill over” into the cytoplasm offer another mechanism whereby mitochondria to nuclear signaling activates a transcriptional response almost identical to the one initiated by glucose restriction and BHB formation described above. This response is likely to be a major player in increasing the cellular antioxidant response in stressed mitochondria characteristic of neuroprogressive disorders.
Upregulation of Nrf2
Oxidative modification of selected cysteine thiol groups on Kelch-like ECH-associated protein (KEAP-1) is probably the most prevalent mechanism enabling dissociation of the Nrf2/KEAP-1 dimer in the cytosol enabling Nrf2 translocation to the nucleus (for review, see Morris et al., 2018). However, KEAP-1 dissociation and Nrf2 activation can also result from several covalent modifications of the latter, including phosphorylation and, in this case, acetylation (for review, see Ma, 2013).
Activation of Nrf2 leads to increased activity of gamma-glutamylcysteine ligase, which is the rate-limiting enzyme as far as glutathione synthesis is concerned, and the cysteine/glutamate antiporter (Xc−), which ensures adequate levels of intracellular cysteine, the rate-limiting substrate for glutathione synthesis and reduces the efflux of oxidized glutathione into the extracellular environment, thereby preserving levels of glutathione within the cell (Steele et al., 2013; Ishii and Mann, 2014). Increased translocation of Nrf2 into the nucleus also leads to increased transcription of glutathione peroxidase, which plays an indispensable role in the reduction of highly toxic membrane lipid peroxides and maintenance of levels of hydrogen peroxide within physiological limits (Banning et al., 2005; Jablonska et al., 2015). Cytosolic mitochondrial and plasma membrane-bound and mitochondrial glutathione transferases are also upregulated by Nrf2 (Bartolini et al., 2015). These enzymes are important not least because they play an indispensable role in detoxifying electrophiles and phase 1-modified xenobiotics via conjugation prior to export into the extracellular environment (McWalter et al., 2004). These enzymes also exert other cytoprotective effects (Bartolini et al., 2015). Nrf2 also upregulates glutathione reductase activity, which provides another avenue for maintaining levels of glutathione (Harvey et al., 2009). Increased Nrf2 activity also upregulates the thioredoxin system increasing levels and activity of thioredoxin (TRX) and thioredoxin reductase 1 (Tanito et al., 2007; Im et al., 2012; Cebula et al., 2015). Nrf2, TRX, and thioredoxin reductase 1 also engage in complex self-sustaining, mutually reinforcing signaling with reductions in TRX1 activity leading to increasing Nrf2 activity and upregulated TRX activity increasing the transcriptional efficiency of Nrf2 (Li et al., 2016; Sueblinvong et al., 2016).
While there are many publications discussing the apex role of Nrf2 as the “master regulator” of cellular antioxidant defenses, the behavior of Nrf2 as an indispensable regulator of energy production is probably underdiscussed. There are accumulating data that this molecule also acts as an important regulator of mitochondrial structure and respiration via increasing fatty acid oxidation and ATP production via several different mechanisms following physical attachment to the outer mitochondrial membrane (Dinkova-Kostova and Abramov, 2015). Subsequent to such attachment, Nrf2 negatively regulates acetyl-CoA carboxylase, ATP-citrate lyase, stearoyl-CoA desaturase, and fatty acid synthase, which are all crucial enzymes enabling fatty acid synthesis. Importantly, a reduction in malonyl-CoA increases mitochondrial fatty acid oxidation, as this enzyme negatively regulates carnitine palmitoyltransferase 1 (Holmström et al., 2016). Nrf2 also plays a major role in maintaining the efficiency of the electron transfer chain (ETC) in an environment of chronic oxidative and nitrosative stress by stabilizing cytochrome b, cytochrome c, and cytochrome c oxidase (Venditti et al., 2009; Strom et al., 2016). The activity of this transcription factor also exerts a positive effect on mitochondrial dynamics by promoting association into networks and inducing mitophagy via mechanisms that are independent of mitochondrial membrane dissipation or the interplay between PTEN-induced putative kinase 1 and parkin (Holmström et al., 2016).
Upregulation of FOXO3a
This transcription factor has a well-documented positive effect on the expression of a wide range of cellular antioxidant enzymes and other functional proteins, such as glutathione S-transferase, GPx1, GPx4, thioredoxin, thioredoxin reductases, peroxiredoxins (Prx) Prx3 and Prx5, selenoprotein P, metallothioneins I and II, and caeruloplasmin (Greer et al., 2007; Tan et al., 2008), as well as increasing the expression and activity of SOD2 and catalase (Greer et al., 2007; Li et al., 2009; Olmos et al., 2009; for review, see Klotz et al., 2015).
FOXO3a increases mitochondrial biogenesis and expression of transcription factor A, mitochondrial (Tseng et al., 2013). Furthermore, FOXO3a induces widespread mitochondrial gene expression (Peserico et al., 2013). This property is of importance as many such genes are involved in regulating mitochondrial mass, mitochondrial morphology, mitophagy, mitochondrial fusion and fission, and the production of ATP (Tseng et al., 2013; Zhou et al., 2017). While the effect on mitochondrial genes is broadly positive as far as improved organelle performance is concerned, somewhat counterintuitively, increased extra-mitochondrial FOXO3a activity may inhibit the activity of some nuclear genes involved in mitochondrial function, which could adversely affect ATP generation, at least in some circumstances (Ferber et al., 2012). However, the survival value of this action is emphasized by data highlighting a slight decrease in energy production but a reduction in mtROS production to a level below baseline (Ferber et al., 2012). The capacity of FOXO3a to regulate the balance between mtROS production and ATP production appears to be particularly important in stressed neurons and plays a major role in promoting their survival (Hagenbuchner and Ausserlechner, 2013).
Consequences of BHB Oxidation, Decreased Glycolysis, and Increased NAD+ Levels
BHB and NAD+ “sparing”
Several research teams have reported an upregulation of NAD+ levels in the brains of their study animals and in humans following protracted ketosis (Grabacka et al., 2016b; Elamin et al., 2017, 2018a; Xin et al., 2018). Other authors have reported the inhibition or termination of glycolysis in the brains of human volunteers consuming a KD (Roy et al., 2012; Zhang et al., 2013b; for review, see Courchesne-Loyer et al., 2017a). These observations are interconnected and stem from relative differences in the number of NAD+ molecules reduced to NADH in the process of forming acetyl-CoA via glycolysis or BHB oxidation, as we explain below.
Briefly, the irreversible oxidation of glyceraldehyde-3 phosphatase to produce 3-phosphoglycerate, which is an indispensable step in the formation of pyruvate, is enabled by the action of glyceraldehyde-3 phosphate dehydrogenase. This enzyme requires NAD+ as a co-substrate leading to the reduction of 2 molecules of this cofactor to NADH per molecule of glyceraldehyde-3 phosphatase oxidized (Newman and Verdin, 2014; Elamin et al., 2018a). Once formed, pyruvate and NADH are translocated into the MM via the action of pyruvate translocase and several NAD+/NADH redox shuttles, for example, the malate-aspartate system, resulting in a relative depletion of the cytosolic NAD+ pool (Stein and Imai, 2012). Once in situ in the MM, NADH molecules are utilized as reducing equivalents for the tricarboxylic acid (TCA) cycle while each molecule of pyruvate is oxidized to 2 molecules of acetyl-CoA via the action of pyruvate dehydrogenase, whose action requires the reduction of 2 molecules of NAD+ per molecule of pyruvate oxidized. The oxidation of BHB to ACA and acetyl-CoA, on the other hand, is enabled by BHB dehydrogenase and succinyl-CoA:3 oxoacid (or ketoacid) CoA transferase and only requires the reduction of 2 molecules of NAD+ per 2 molecules of ACA formed as BHB dehydrogenase is the only NAD+-consuming enzyme in the process (Grabacka et al., 2016b; Puchalska and Crawford, 2017). This is clearly a very brief description of the biochemistry involved in glucose and BHB oxidation, but the key point to reemphasize is that the termination of glycolysis and increased oxidation of BHB subsequent to the advent of induced ketosis (Roy et al., 2012; Zhang et al., 2013b; Courchesne-Loyer et al., 2017a) effectively leads to an increased level of NAD+, which has a number of profound bioenergetic and metabolic consequences described in detail below (Elamin et al., 2018b).
BHB and Increased NADH Oxidation in Mitochondria
There is also evidence to suggest that BHB increases NADH oxidation in mitochondria, leading to an increased NAD+/NADH ratio within organelles in the brain (Maalouf et al., 2007; Zhang et al., 2013a; Pawlosky et al., 2017). This would appear to be due, at least in part, to increases in complex I integrity and activity (Hughes et al., 2014; Frey et al., 2017; Paleologou et al., 2017). BHB also appears to stimulate the activity of succinate dehydrogenase (Tieu et al., 2003). An increased supply of succinate following its preferential oxidation over glucose may bypass complex I abnormalities by supplying electrons directly to complex II (Tieu et al., 2003; Sullivan et al., 2004). There is also some evidence to suggest that BHB may exert a direct and stimulatory effect on succinate dehydrogenase activity (Balietti et al., 2010). The potential benefit of BHB as the predominant source of electrons and reducing equivalents in an environment of ETC dysfunction or inhibition is further emphasized by data supplied by Kim and fellow workers, who demonstrated that complex II inhibition could be mitigated following BHB administration in the hippocampal neurons of their study animals (Kim et al., 2010). It would also appear that BHB oxidation increases the energetic status of electrons entering complex I and the redox difference between the NAD+/NADH couple and the CoQ/CoQH2 couple (Veech, 2004) with the effect of increasing the Gibbs free energy of hydrolysis of ATP molecules ultimately produced (Sato et al., 1995).
Consequences of Increased NAD+/NADH Ratio
The increase in the NAD+/NADH ratio facilitated in the cytosolic and mitochondrial compartments following preferential BHB oxidation and/or increased BHB activity is of paramount importance. Increased NAD+ levels stimulate oxidative phosphorylation, ETC activity, and ATP production and affect multiple dimensions of cellular metabolism that govern adaptation in performance in response to different environmental conditions and changes in nutrient availability (Blacker and Duchen, 2016; Xiao et al., 2018; for review, see Stein and Imai, 2012). NAD+ may be phosphorylated in the cytosol and the NADP+-NADPH couple plays a major role in redox homeostasis in the periphery and the brain (Ying, 2008; Yang and Sauve, 2016; for review, see Xiao et al., 2018).
NAD+ levels and activity also modulate calcium homeostasis, regulate mitochondrial transition pore opening, and influence the transcription and posttranscriptional modification of hundreds of cellular proteins by activating PARP-1 and the sirtuin family of class III histone deacetylases, most notably the cytosolic SIRT-1 and the mitochondrial SIRT-3 (Kincaid and Bossy-Wetzel, 2013; Elamin et al., 2018a). Readers interested in more information regarding the location and mode of operation of individual sirtuins are referred to a comprehensive review on the subject by (Parodi-Rullán et al., 2018). It is important to note at the outset of this section that authors have reported the upregulation of SIRT-3 in the brains of their study animals following administration of a KD and/or BHB, which supports the data supplied by the authors above (Yin et al., 2015; Hasan-Olive et al., 2019). However, there is some suggestion that levels of SIRT-3 may be reduced in the periphery following BHB and/or KD intake in an environment where SIRT-1 (Srivastava et al., 2013) and SIRT-5 (Hutfles et al., 2017) are increased, for reasons that are not entirely clear (Srivastava et al., 2013). There is also some suggestion that SIRT-1 may be more sensitive than SIRT-3 to changes in NAD+ levels. However, we will discuss the actions of SIRT-3 before moving on to discuss the consequences of SIRT-1 activation as the activity of the former explains, at least in part, many of the apparent effects of BHB on the ETC and overall ATP production as detailed below.
Increased Activity of SIRT-3
The activation of SIRT-3 has profound effects on the mitochondrial acetylome, affecting thousands of acyl groups on proteins performing essential functions in energy production and metabolic adaptation to a changing cellular environment. This leads to a global reprogramming of the mitochondrial proteome aimed at adapting to changes in energy demand, fuel supply, and redox status (Dittenhafer-Reed et al., 2015).
Many of the effects of BHB on the ETC and ATP production discussed above can be explained by the activation of SIRT-3 by elevated levels of NAD+ (Lombard et al., 2011). For example, SIRT-3 can bind to complexes I and II leading to an increase in their activity (Ahn et al., 2008; Cimen et al., 2010). The precise targets of SIRT-3 as far as complex I is concerned have not been fully elucidated, but direct binding to its NDUFA9 subunit appears to be one of the mechanisms involved (Ahn et al., 2008). Deacetylation/acetylation patterns of complex I subunits regulate basal ATP levels (Ahn et al., 2008). In terms of complex II, on the other hand, the stimulatory effect of SIRT-3 activation appear to be mediated by increased acetylation and activation of succinate dehydrogenase and succinate dehydrogenase flavoprotein (Cimen et al., 2010; Finley et al., 2011).
SIRT-3 also acts as the main regulator of fatty acid oxidation by reversible acetylation of long-chain acyl-CoA dehydrogenase (Hirschey et al., 2010a). This sirtuin also binds and deacetylates acetyl-CoA synthetase 2 (Hallows et al., 2006; Hirschey et al., 2010b) and thus plays an indispensable role in regulating the production of acetyl-CoA in the periphery and the CNS (Dittenhafer-Reed et al., 2015). Evidence suggests that this sirtuin exerts stimulatory effects on the TCA cycle by increasing the activity of glutamate dehydrogenase, resulting in enhanced metabolism of glutamate into α-ketoglutarate (Schlicker et al., 2008). SIRT-3 may also deacetylate isocitrate dehydrogenase 2, leading to an increase in the enzyme’s activity that also results in increased production of α-ketoglutarate (Yu et al., 2012; Sheng et al., 2015). Considered as a whole, the weight of evidence suggests that elevated SIRT-3 activity stimulates the TCA cycle by increasing the flux of carbon and the levels of metabolites that play an important role in regulating TCA cycle activity (Verdin et al., 2010; Finley and Haigis, 2012).
SIRT-3 also plays an important role in the regulation of mitochondrial redox homeostasis via increased acetylation and activity of mitochondrial SOD-2 (Kong et al., 2010; Zhang et al., 2016) and FOXO3a (Tseng et al., 2013; Rangarajan et al., 2015); the latter is capable of initiating a cascade of antioxidant enzymes and systems as detailed above. The cited study conducted by Rangarajan and fellow workers is of interest from the perspective of neuroprogressive diseases as the results demonstrate an increase in FOXO3a activity mediated by SIRT-3 in microglia in vivo (Rangarajan et al., 2015). Similarly, the study conducted by Zhang and others is of interest because the authors report an in vivo SIRT-3–mediated increase in FOXO3a activity in neurons (Zhang et al., 2016). There is also evidence to suggest that SIRT-3 activation may lead to the upregulation of glutathione peroxide, but it is not clear whether this is a direct effect or secondary to the activation of FOX03a (Kong et al., 2010). Readers interested in an in-depth treatment of the role of SIRT-3 in the maintenance of mitochondrial homeostasis are invited to consult an excellent review of the subject by (Kincaid and Bossy-Wetzel, 2013).
Finally, while SIRT-3 is highly sensitive to changes in NAD+ levels and thus a pivotal metabolic sensor, the enzyme may also be activated by several other mechanisms. For example, the transcription of SIRT-3 may be activated by the physical engagement of the alpha subunit of Nrf-2 or PGC-1α, so increased levels and activity of both transcription factors can also induce increased activity of mitochondrial SIRT-3 (Satterstrom et al., 2015; Zhang et al., 2016). In addition, several authors have reported that the antioxidant effects of SIRT-3 in the brain are mediated, at least in part, via the activation of PGC-1α (Zhang et al., 2016).
Activation of SIRT-1
It should be noted that PGC-1α activity may also be increased in vivo by upregulation of SIRT-1, which is also activated by increased levels of NAD+ secondary to BHB oxidation so that the activation of SIRT-3 by PGC-1α is also dependent on ketolysis (Wang et al., 2015). Similarly, SIRT-1 may also acetylate and activate Nrf-2 and initiate a positive interaction between the 2 molecular entities, which plays a major reinforcing role in maintaining cellular antioxidant systems and fostering energy production in the face of increasing oxidative and nitrosative stress (Huang et al., 2017). Increased activity of SIRT-1 has been consistently reported in the brains of study animals following protracted ingestion of a KD or infusion of BHB (Scheibye-Knudsen et al., 2014; McCarty et al., 2015; Elamin et al., 2018b). There have also been reports of elevated SIRT-1 in the periphery under the same conditions (Srivastava et al., 2013).
Several authors have reported an association between SIRT-1 activation and an increase in mitochondrial biogenesis and beta-oxidation of fatty acids, with the latter facilitated via different mechanisms. These include increased fatty acid uptake into cells via the CD36 membrane transporter coupled with elevated influx of these molecules into mitochondria via upregulation of various carnitine palmitoyl transferase enzymes, including carnitine palmitoyltransferase 1 (Wanders et al., 2010; Liu et al., 2012; Vachharajani et al., 2014; for review, see Qu et al., 2016). Upregulated SIRT-1 activity also exerts several positive effects on mitochondria aimed at maximizing the efficiency of energy production. For example, SIRT-1 upregulation induces a signaling cascade of protein-protein interactions, ultimately aimed at terminating ATP generation via glycolysis and increasing ATP generation via fatty acid oxidation. Mechanisms involved in engineering this metabolic switch include suppression of lactate production (Elhanati et al., 2016; Long et al., 2017), downregulation of the glucose transporter 1, and inhibition of hypoxia-inducible factor 1-alpha signaling (Sebastian et al., 2012). SIRT-1 upregulation also offers a degree of mitochondrial protection in an environment of increasing oxidative and nitrosative stress. The primary mechanisms involved are the stimulation of cellular antioxidant defenses, via Nrf-2 activation, and promoting favorable mitochondrial dynamics such as increasing mitophagy, thereby removing damaged and dysfunctional mitochondria and maintaining mitochondrial membrane potential and so inhibiting the opening of the mitochondrial transition pore (Price et al., 2012b; Song et al., 2017).
AMPK Activation
The weight of evidence suggests that levels and activity of adenosine monophosphate-activated protein kinase (AMPK) are increased in the brains of rodents following ingestion of a KD or BHB (Laeger et al., 2010; Genzer et al., 2015; Paoli et al., 2015). This also seems to be true of peripheral tissues (Kennedy et al., 2007; Badman et al., 2009). As previously discussed, evidence suggests that 1 route driving AMPK activation is increased activity of SIRT-1 (Price et al., 2012a). However, the relationship between SIRT-1 and AMPK is complex, and recent reports note an increase in SIRT-1 mRNA levels following AMPK activation (Dong et al., 2018). This may be due in part to the ability of AMPK to upregulate NAD+ by increasing levels of nicotinamide phosphoribosyl transferase (Fulco et al., 2008; Cantó et al., 2009). Furthermore, these molecular players seem to engage in a complex pattern of crosstalk with each molecule reinforcing and perpetuating the activity of the other and ultimately acting in partnership to regulate cellular bioenergetics and metabolism (for review, see Ruderman et al., 2010). The ability of AMPK to activate SIRT-1 is important because AMPK activation is part of the starvation response that is activated by the advent of dietary induced ketosis and increased levels of BHB (for review, see Newman and Verdin, 2014). Hence this mechanism may provide a route for the activation of SIRT-1 that is independent of BHB oxidation per se. SIRT-3 and AMPK can also engage in mutual activation and mutually reinforcing crosstalk aimed at regulating multiple dimensions of mitochondrial redox homeostasis and energy production (Chen et al., 2018; Zhao et al., 2018). Increased AMPK also increases the nuclear translocation of Nrf-2 (Joo et al., 2016) and would appear to increase its activity as a transcription factor, providing yet another route for increasing Nrf-2 activity stemming from induced ketosis (Mo et al., 2014).
Upregulation of PGC-1α
While SIRT1, SIRT-3, and AMPK clearly have some direct effects on cellular energy production and mitochondrial survival, they also exert combined effects by deacylating and phosphorylating PGC-1α, leading to the activation of the latter molecule (Cantó et al., 2009; Kong et al., 2010). There is also some evidence to suggest that AMPK increases the expression of PGC-1α via a mechanism that remains to be delineated (Cantó et al., 2009). The activation of PGC-1α in turn positively modulates mitochondrial dynamics and increases mitochondrial biogenesis, oxidative phosphorylation, and oxygen consumption via a number of different mechanisms (Scarpulla, 2011; LeBleu et al., 2014).
One mechanism underpinning the positive effects of PGC-1α on energy production is the increased transcription of proteins involved in mitochondrial biogenesis and respiration (Valle et al., 2005; Lagouge et al., 2006). However, this protein also exerts a number of other beneficial effects on mitochondrial dynamics by inducing positive changes in the expression and activity of mitofusin-2 (MFN2), mitochondrial dynamin like GTPase (OPA1), dynamin related protein 1(Drp-1), and mitochondrial fission 1 protein (FIS-1) (Peng et al., 2017). The presence of data demonstrating the positive effects of PGC-1α in restoring the balance between mitochondrial fusion and fission in neurons also speaks to the potential merits of the KD as an adjunctive therapy in patients with neuroprogressive illnesses characterized by bioenergetic dysregulation (Dabrowska et al., 2015).
PGC-1α also increases the transcription of antioxidant proteins, including SOD1 (St-Pierre et al., 2006), SOD2 (St-Pierre et al., 2006), catalase (Valle et al., 2005), GPx (St-Pierre et al., 2003), thioredoxins (Valle et al., 2005), TRXR (Valle et al., 2005), Prx3 (Valle et al., 2005), and Prx5 (Valle et al., 2005) as well as the mitochondrial uncoupling proteins UCP2 (St-Pierre et al., 2003, 2006) and UCP3 (St-Pierre et al., 2003, 2006).
Once activated, PGC-1α interacts with the PPAR family of nuclear receptors and the FOXO family of transcription factors, thereby modulating their activity and location (Olmos et al., 2009; Wang, 2010) to influence expression of a variety of bioenergetic and antioxidant proteins (Puigserver and Spiegelman, 2003; Corona and Duchen, 2015). The combined effects of PGC-1α and PPARγ are mediated by complex formation (Olmos et al., 2013), and this physical interaction is pivotal in inducing the expression of a plethora of enzymes governing fatty acid metabolism and ketogenesis, which are characteristic of a KD (for review, see Grabacka et al., 2016a). Clearly, this route of FOXO activation differs from the increased acetylation induced by BHB directly acting as a deacetylase inhibitor, but the consequences are very much the same. Activated PGC-1α also increases expression of Nrf-2 via a mechanism dependent on p53, p38, and GSK3β (Aquilano et al., 2013; Choi et al., 2017). The activation and nuclear translocation of this “master regulator” of cellular antioxidant defenses is also induced by upregulated activity of SIRT-1 (Chai et al., 2018) and PPARγ co-activator 1-α (Cherry et al., 2014).
Activation of PPARγ
The upregulation of PPARγ has been reported in the brains of study animals within a few days of the advent of a ketotic state (Grabacka et al., 2016b; Simeone et al., 2017b; Knowles et al., 2018). There are several mechanisms that could drive this phenomenon such as the activation of PGC-1α (Yun et al., 2018) and SIRT-1 (Fujita and Yamashita, 2018). However, the transcription factor is the prime regulator of ketogenesis and ketolysis and may be upregulated by the presence of high levels of fatty acids or KBs (Grabacka et al., 2016b; Simeone et al., 2017b; Knowles et al., 2018).
PPARγ may bind to genes and recruit transcriptional corepressor complexes that have the effect of repressing gene expression. Alternatively, it may bind to and/or sequestrate cofactors necessary for the activation of genes via a series of protein-protein interactions in a phenomenon described as transrepression (Tyagi et al., 2011; Polvani et al., 2012).
PPARγ activation has a well-documented role in reducing levels of inflammation in the brain and in the periphery by inhibiting the transcription of cytokines by sequestrating transcription factors such as activator protein 1 (AP-1), Signal transducer and activator of transcription 1 (STAT-1), and Nuclear factor of activated T-cells (NFAT), which positively regulate their expression (Yang et al., 2008; Tyagi et al., 2011). Perhaps unsurprisingly, increased activity of this nuclear hormone receptor also inhibits NF-κB–mediated inflammatory signaling via several mechanisms, which include the upregulation of NF kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, a negative regulator of NF-κB (Scirpo et al., 2015) and acting as an E3 ligase, thereby increasing the proteosomal degradation of NF-κB p65 (Hou et al., 2012). PPARγ also appears to bind to the NF-κB p65 promoter region and directly suppresses the transcription of this subunit (Park et al., 2009; Remels et al., 2009). The various inhibitory effects of PPARγ on NF-κB are of particular interest as far as reducing neuroinflammation is concerned as they appear to be 1 element enabling PPAR to act as a major player in inducing antiinflammatory phenotypes in microglia (Cullingford, 2008; Jeong et al., 2011; Sikder et al., 2018).
PPARγ is also another major regulator of cellular redox status and acts at the junction of several signaling pathways and is involved in the upregulation of FOXO3a and Nrf-2 (Polvani et al., 2012). The upregulation of PPAR is associated with increased levels and activity of SOD, catalase, GPx, glutathione, UCP2, and Heme oxygenase-1 (HO-1) (Kim and Yang, 2013; Sekulic-Jablanovic et al., 2017). It could be argued that this antioxidant effect is exercised via stimulation of Nrf-2 and FOXO3a signaling, but PPAR/RXR dimers seem to have independent effects on upregulating several players in the cellular antioxidant defense network via binding to PPAR responsive elements of genes such as HO-1, Catalase (CAT), and SOD (Kim and Yang, 2013; Ndisang, 2014; Sekulic-Jablanovic et al., 2017).
PPARγ upregulation can also positively regulate oxidative metabolism by stimulating the transcription of genes governing rates of mitochondrial glucose metabolism and mitochondrial beta-oxidation of free fatty acids (Monsalve et al., 2013; Corona and Duchen, 2016). These activities are largely carried out via the activation of PGC-1α, and readers interested in the pathways involved are invited to consult the work of (Fan and Evans, 2015) and (Govindarajulu et al., 2018).
Future Directions and Conclusions
Given the data discussed above, it is reasonable to conclude that a therapeutic intervention based on induced ketosis could potentially alleviate many of the elements known to be involved in the pathophysiology, and possibly the pathogenesis, of neuroprogressive illnesses. However, studies relating to the use of the approach in such illnesses are currently limited to very small prospective or retrospective open label studies or internet-based analysis of patient feedback (Bostock et al., 2017b; Brietzke et al., 2018), clearly indicating a need for well-designed and adequately powered randomized blinded controlled trails assessing efficacy and safety. We now move on to considering major issues that need to be taken into account while designing such studies, with the main focus being safety and compliance.
Generally, dietary-induced ketosis in rodents involves the use of commercial preparations at approximately 5.2 Kcal/g with 70% fat, 20% protein, and 10% carbohydrate. The sources of fat are usually Medium-chain triglycerides (MCTs) coupled with range of oils such as canola oil or flax seed oil. Casein is normally the sole source of protein source and maltodextrin usually serves as the sole source of carbohydrates (Hyatt et al., 2016). A classical or modified KD is usually started in hospital when children are concerned, but adults usually commence such diets in the community (Kossoff and Hartman, 2012). The use of KB supplements can largely avoid the side effects associated with the transition to ketosis often described in the grey literature as “keto flu.” The classical KD diet, with a very high intake of fatty foods, may produce a range of gastrointestinal side effects such as nausea, vomiting, dehydration, constipation, low appetite, and, most commonly, diarrhea (Włodarek, 2019). Other minor side effects include headache, muscle cramps, rashes, general weakness, and halitosis (Harvey et al., 2018). Unsurprisingly, the therapeutic utility of the unmodified KD may be limited by issues of poor tolerability and compliance from the perspective of patients and caregivers. In fact, a recent study based on the meta-analysis of 45 studies concluded that the compliance rate of children on a classical KD for the treatment of refractory epilepsy over a 2-year period was 29% (Cai et al., 2017). An earlier meta-analysis reported a somewhat higher but still troublesome compliance rate of 42% for adults over the same time period (Ye et al., 2015). This is comparable with the compliance rate of adults consuming a classical KD for the treatment of type 2 diabetes (Stern et al., 2004; Westman et al., 2008), which is obviously relevant from the perspective of treating patients with neuroprogressive disorders who will probably require long-term if not continuous consumption of this diet. The use of medium chain triglycerides to induce ketosis has allowed the ingestion of significantly lower levels of dietary fat compared with the 3 or 4:1 fat to carbohydrate and protein ratios used in the orthodox or classical KD, which has improved tolerability somewhat, although the high “dropout rates” observed in studies still remain primarily caused by unpleasant gastric side effects (Harvey et al., 2018; Taylor et al., 2018).
There are also concerns among many clinicians regarding the safety of a high-lipid diet due to the potential of increasing total Low-density lipoprotein (LDL), and Very low-density lipoprotein (VLDL) levels, potentially increasing the patient’s risk of developing obesity, insulin resistance, metabolic syndrome, Type 2 diabetes (T2D), and cardiovascular disease (Kosinski and Jornayvaz, 2017; Bolla et al., 2019). Indeed, there is evidence that the use of the classical KD may induce dyslipidemia, at least in the short term (Kossoff et al., 2018), and that this phenomenon may also apply to individuals on a MCT-based KD as there is a report of increased LDL and total cholesterol in healthy volunteers consuming such a diet (Tholstrup et al., 2004). This is particularly relevant as far as patients suffering from neuroprogressive disorders are concerned as there is a wealth of evidence showing an increased prevalence of metabolic abnormalities and cardiovascular disease in such individuals compared with age and sex norms even before the instigation of any therapeutic interventions (for review, see Morris et al., 2019c). Clearly, patient safety is paramount, and hence we will examine evidence regarding the effects of prolonged induced ketosis before examining ways of improving patient tolerance.
There is now a large and accumulating body of evidence associating short-term prolonged ingestion of a KD with significant weight loss in obese adults, which is accompanied by a decrease in systemic inflammation, reduced insulin resistance, and, on balance, an increased capacity for exercise (Paoli, 2014; Paoli et al., 2015; Hall and Chung, 2018; Bolla et al., 2019; for review, see Murphy and Jenkins, 2019). There are also several studies reporting favorable effects on metabolic syndrome, including evidence of reversal (Staverosky, 2016; Gibas and Gibas, 2017; Gershuni et al., 2018). Remarkably, there is evidence to suggest that the improvement in all parameters of metabolic syndrome in patients ingesting an MCT-based KD may be greater than in patients consuming a low-fat diet combined with engaging in rigorous exercise regimes (Gibas and Gibas, 2017). Perhaps unsurprisingly, given the data discussed thus far, there is also an accumulating body of evidence associating the short-term or prolonged consumption of a MCT-based or classical KD with a clinically significant decline in HbA1c and improvements in glycemic control in patients with T2D (Westman et al., 2008; Hussain et al., 2012; Krebs et al., 2013; Goday et al., 2016). In addition, there are a number of studies reporting a significantly reduced need for medication in T2D patients on a KD compared with patients on high-carbohydrate or low-fat diets (for review, see Westman et al., 2008).
However, consideration of the data provided by some of these studies suggests that adults with metabolic abnormalities should be careful to avoid long chain triglycerides and/or saturated fats as a vehicle to induce ketosis as this may result in elevated levels of LDL (Westman et al., 2008; Brinkworth et al., 2009). This is consistent with data obtained from studies involving children with pharmaceutically resistant epilepsy where researchers have reported significant increases in LDL, VLDL, and total cholesterol in their study participants (Kwiterovich, 2003; Groesbeck et al., 2006; Nizamuddin et al., 2008; Güzel et al., 2016; Zamani et al., 2016). However, in most cases, these increases appear to be short term and transitory and normalize within 12 months (Kwiterovich, 2003; Groesbeck et al., 2006; Liu et al., 2013; Kapetanakis et al., 2014). This would also appear to be the case for individuals with preexisting hyperlipidemia prior to the commencement of the classical KD (Liu et al., 2013). In addition, there is evidence that children who have been on the classical KD for 6 years display no evidence of hyperlipidemia (Groesbeck et al., 2006).
From the perspective of chronic administration in adult patients with neuroprogressive illnesses, it also seems reassuring to note that even in a scenario where the use of a classical KD leads to increases in LDL cholesterol in patients with T2D, there would appear to be an even greater relative increase in HDL cholesterol and reduction in levels of triglycerides, greater increase in HDL cholesterol, and a decrease in serum triglycerides (Westman et al., 2008; Brinkworth et al., 2009). Other authors have also reported increases in HDL levels (Sharman et al., 2002; Foster et al., 2003; Samaha et al., 2003; Volek et al., 2005; Dashti et al., 2006; Brinkworth et al., 2009; Tay et al., 2014) following prolonged diet-induced ketosis in human studies and reductions in triglyceride in conjunction with increased levels of HDL (Foster et al., 2003; Samaha et al., 2003; Dashti et al., 2006; Brinkworth et al., 2009; Tay et al., 2014). For the sake of completeness, it should also be noted that a solitary study has reported a reduction in LDL cholesterol in patients consuming a MCT-based KD (Dashti et al., 2006).
Further reassurance may be obtained by the results of a recent meta-analysis of 13 randomized controlled studies that concluded that MCT ingestion has at minimum no adverse effects on the lipid profiles of humans in the short or longer term (Mumme and Stonehouse, 2015). It is also noteworthy that the addition of MCTs to a classical KD diet is often used in clinical practice to ameliorate dyslipidemia induced by a classical KD (for review, see Kossoff et al., 2018). There is also a growing body of evidence to suggest that the beneficial effects of MCTs are at least equal to those provided by extra virgin olive oil (St-Onge et al., 2008; Namayandeh et al., 2013; Chinwong et al., 2017; Khaw et al., 2018). Readers interested in the evidence relating to the beneficial effects of olive oil on human lipid profiles are referred to an excellent treatment of the subject by (Høstmark and Haug, 2013). There is also evidence to suggest that that caprylic to capric ratio in MCT preparations is an important element underpinning their favorable or neutral effect on lipid profiles, with high levels of caprylic acid being particularly important (for review, see Bach et al., 1996). It is also reassuring to note that use of modified tricaprylin and other MCT-based KDs has also produced encouraging results in the treatment of Alzheimer’s disease, with significant (albeit modest) improvements in memory scores and overall cognitive functions in patients with mild or moderate disease without any significant adverse effects on any metabolic parameters (Reger et al., 2004; Henderson et al., 2009; Taylor et al., 2018; Ota et al., 2019). Similar results have also been achieved via the use of MCT-based KDs in patients with mild cognitive impairment (Krikorian et al., 2012).
There may be an opportunity to further reduce the amounts of MCT oil consumed each day, thereby minimizing gastrointestinal side effects and aiding compliance, by using caprylic MCTs as these molecules are about 3 times more ketogenic than their capric equivalents and upwards of 6 times more ketogenic than C12 MCTs, thereby offering the prospect of therapeutic levels of BHB from reduced amounts of MCT oil ingested (St-Pierre et al., 2019). Side effects may also be minimized via the emulsification of MCT oils before ingestion, which also offers the prospect of ketosis achieved by lower daily amounts of these oils as emulsified MCTs may increase plasma ketones twofold compared with an equivalent amount of nonemulsified MCT oils (Courchesne-Loyer et al., 2017b). In addition, there are also data to suggest that the use of emulsified MCT oil as the means of inducing ketosis can reduce overall side effects by some 50% compared with an equivalent amount of nonesterified oil (Courchesne-Loyer et al., 2017b). However, despite such approaches, poor compliance remains a major issue with classical and MCT-based KDs, and it is also fair to say that long-term prospective studies to assess the effects of induced ketosis over several years of continuous consumption have not yet been carried out (Kossoff et al., 2018; Murphy and Jenkins, 2019).
These enduring problems with tolerability and lingering concerns over the development of dyslipidemia in long-term use have led to an increasing research focus on the use of exogenous ketone supplements in the form of KB salts and esters, with common examples being 1, 3-butanediol monoester of BHB and glyceryl-tris-3-hydroxybutyrate, which provide high levels of BHB directly to the body without the need to induce ketogenesis and thus without elevations in free fatty acids and, perhaps more importantly thus far, no evidence of induced dyslipidemia (Hashim and VanItallie, 2014; Veech, 2014). In fact, there is accumulating evidence to suggest that orally administered KB esters or salts result in the inhibition of adipocyte lipolysis (Evans et al., 2017; for review, see Pinckaers et al., 2017) and the inhibition of cholesterol synthesis (Kemper et al., 2015).
The research focus on KB supplementation is not just concerned with improving safety; however, there is accumulating evidence that the administration of R-1, 3-butanediol from ketone monoesters can produce plasma levels of KBs in humans that are at least as high as those produced by the most rigorous consumption of the classical KD (Clarke et al., 2012; Hashim and VanItallie, 2014; Stubbs et al., 2017). For example, at a single dose of 395 mg/kg, KB supplementation in human adults can increase levels of BHB in the plasma to 3.3 mmol/L whether administered as a capsule or in a drink (Clarke et al., 2012; Stubbs et al., 2017). In addition, there are data to suggest that the administration of ketone esters or salts as drinks over a 24-hour period can deliver 24 g of BHB as effectively as an infusion (Stubbs et al., 2017).
There is also a suggestion that ketone ester or salt drinks can be modified to produce a relatively low level of KB supplementation and increase the ratio of carbohydrates and proteins in the mix (Choi et al., 2018). This method has produced therapeutic levels of BHB following drinks containing a KB ester to carbohydrate to protein ratio of 1:7:1, which approaches the composition of a standard diet and may further improve tolerability and compliance (Choi et al., 2018). The use of ketone ester drinks also induces levels of BHB in plasma, which does not appear to be significantly affected by food intake, and thus the use of KB drinks could avoid the rigors of dietary restriction. This would appear to be another potential benefit in terms of a long-term therapeutic intervention for patients with neuroprogressive disorders (Stubbs et al., 2017; Kovács et al., 2019).
In this comprehensive review, it has been suggested that induced ketosis or protracted KB ingestion may reduce oxidative stress, improve cellular bioenergetics, and upregulate the activity of PPAR, SIRT-1, and AMPK as well as brain NAD+ levels. These changes speak to the potential therapeutic value of this dietary change for neuroprogressive disorders such as SZ, BPD, and MDD and suggest that clinical trials of ketogenic dietary strategies focusing on the use of ketone esters in these disorders are timely.
Acknowledgments
MB is supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (APP1059660 and APP1156072).
The authors received no financial support for the research, authorship, and/or publication of this article.
Statement of Interest
None.
References
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105:14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alageel A, Tomasi J, Tersigni C, Brietzke E, Zuckerman H, Subramaniapillai M, Lee Y, Iacobucci M, Rosenblat JD, Mansur RB, McIntyre RS (2018) Evidence supporting a mechanistic role of sirtuins in mood and metabolic disorders. Prog Neuropsychopharmacol Biol Psychiatry 86:95–101. [DOI] [PubMed] [Google Scholar]
- Aquilano K, Baldelli S, Pagliei B, Cannata SM, Rotilio G, Ciriolo MR (2013) p53 orchestrates the PGC-1α-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxid Redox Signal 18:386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach AC, Ingenbleek Y, Frey A (1996) The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy? J Lipid Res 37:708–726. [PubMed] [Google Scholar]
- Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E (2009) A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab 297:E1197–E1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balietti M, Giorgetti B, Di Stefano G, Casoli T, Platano D, Solazzi M, Bertoni-Freddari C, Aicardi G, Lattanzio F, Fattoretti P (2010) A ketogenic diet increases succinic dehydrogenase (SDH) activity and recovers age-related decrease in numeric density of SDH-positive mitochondria in cerebellar Purkinje cells of late-adult rats. Micron 41:143–148. [DOI] [PubMed] [Google Scholar]
- Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohé R (2005) The GI-GPx gene is a target for Nrf2. Mol Cell Biol 25:4914–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini D, Commodi J, Piroddi M, Incipini L, Sancineto L, Santi C, Galli F (2015) Glutathione S-transferase pi expression regulates the Nrf2-dependent response to hormetic diselenides. Free Radic Biol Med 88:466–480. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yücel M, Gama CS, Dodd S, Dean B, Magalhães PV, Amminger P, McGorry P, Malhi GS (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35:804–817. [DOI] [PubMed] [Google Scholar]
- Blacker TS, Duchen MR (2016) Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic Biol Med 100:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L (2019) Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients 11:962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock ECS, Kirkby KC, Taylor BVM (2017a) The current status of the ketogenic diet in psychiatry. Front Psychiatry 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock ECS, Kirkby KC, Taylor BVM (2017b) The current status of the ketogenic diet in psychiatry. Front Psychiatry 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N (2004) Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37:755–767. [DOI] [PubMed] [Google Scholar]
- Brietzke E, Mansur RB, Subramaniapillai M, Balanzá-Martínez V, Vinberg M, González-Pinto A, Rosenblat JD, Ho R, McIntyre RS (2018) Ketogenic diet as a metabolic therapy for mood disorders: evidence and developments. Neurosci Biobehav Rev 94:11–16. [DOI] [PubMed] [Google Scholar]
- Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM (2009) Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr 90:23–32. [DOI] [PubMed] [Google Scholar]
- Cai QY, Zhou ZJ, Luo R, Gan J, Li SP, Mu DZ, Wan CM (2017) Safety and tolerability of the ketogenic diet used for the treatment of refractory childhood epilepsy: a systematic review of published prospective studies. World J Pediatr 13:528–536. [DOI] [PubMed] [Google Scholar]
- Camberos-Luna L, Gerónimo-Olvera C, Montiel T, Rincon-Heredia R, Massieu L (2016) The ketone body, β-hydroxybutyrate stimulates the autophagic flux and prevents neuronal death induced by glucose deprivation in cortical cultured neurons. Neurochem Res 41:600–609. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebula M, Schmidt EE, Arnér ES (2015) TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid Redox Signal 23:823–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai D, Zhang L, Xi S, Cheng Y, Jiang H, Hu R (2018) Nrf2 activation induced by Sirt1 ameliorates acute lung injury after intestinal ischemia/reperfusion through NOX4-mediated gene regulation. Cell Physiol Biochem 46:781–792. [DOI] [PubMed] [Google Scholar]
- Chen LY, Wang Y, Terkeltaub R, Liu-Bryan R (2018) Activation of AMPK-SIRT3 signaling is chondroprotective by preserving mitochondrial DNA integrity and function. Osteoarthritis Cartilage 26:1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry AD, Suliman HB, Bartz RR, Piantadosi CA (2014) Peroxisome proliferator-activated receptor γ co-activator 1-α as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J Biol Chem 289:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinwong S, Chinwong D, Mangklabruks A (2017) Daily consumption of virgin coconut oil increases high-density lipoprotein cholesterol levels in healthy volunteers: a randomized crossover trial. Evid Based Complement Alternat Med 2017:7251562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HI, Kim HJ, Park JS, Kim IJ, Bae EH, Ma SK, Kim SW (2017) PGC-1α attenuates hydrogen peroxide-induced apoptotic cell death by upregulating Nrf-2 via GSK3β inactivation mediated by activated p38 in HK-2 Cells. Sci Rep 7:4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-R, Kim J, Lim H, Park YK (2018) Two-week exclusive supplementation of modified ketogenic nutrition drink reserves lean body mass and improves blood lipid profile in obese adults: a randomized clinical trial. Nutrients 10:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury PS, Chamoto K, Kumar A, Honjo T (2018) PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8+ T cells and facilitates anti-PD-1 therapy. Cancer Immunol Res 6:1375–1387. [DOI] [PubMed] [Google Scholar]
- Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC (2010) Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 49:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, Ho M, Roberts A, Robertson J, Vanitallie TB, Veech RL (2012) Kinetics, safety and tolerability of ®-3-hydroxybutyl ®-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 63:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona JC, Duchen MR (2015) PPARγ and PGC-1α as therapeutic targets in Parkinson’s. Neurochem Res 40:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona JC, Duchen MR (2016) PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic Biol Med 100:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne-Loyer A, Croteau E, Castellano CA, St-Pierre V, Hennebelle M, Cunnane SC (2017a) Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab 37:2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne-Loyer A, Lowry CM, St-Pierre V, Vandenberghe C, Fortier M, Castellano CA, Wagner JR, Cunnane SC (2017b) Emulsification increases the acute ketogenic effect and bioavailability of medium-chain triglycerides in humans: protein, carbohydrate, and fat metabolism. Curr Dev Nutr 1:e000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullingford T. (2008) Peroxisome proliferator-activated receptor alpha and the ketogenic diet. Epilepsia 49 Suppl 8:70–72. [DOI] [PubMed] [Google Scholar]
- Dabrowska A, Venero JL, Iwasawa R, Hankir MK, Rahman S, Boobis A, Hajji N (2015) PGC-1α controls mitochondrial biogenesis and dynamics in lead-induced neurotoxicity. Aging (Albany NY) 7:629–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti HM, Al-Zaid NS, Mathew TC, Al-Mousawi M, Talib H, Asfar SK, Behbahani AI (2006) Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol Cell Biochem 286:1–9. [DOI] [PubMed] [Google Scholar]
- Davis J, Moylan S, Harvey BH, Maes M, Berk M (2014) Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry 48:512–529. [DOI] [PubMed] [Google Scholar]
- Dehghan M, et al. ; Prospective Urban Rural Epidemiology (PURE) Study Investigators (2017) Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 390:2050–2062. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittenhafer-Reed Kristin E, Richards Alicia L, Fan J, Smallegan Michael J, Fotuhi Siahpirani A, Kemmerer Zachary A, Prolla Tomas A, Roy S, Coon Joshua J, Denu John M (2015) SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab 21:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Zhang LF, Bao SL (2018) AMPK regulates energy metabolism through the SIRT1 signaling pathway to improve myocardial hypertrophy. Eur Rev Med Pharmacol Sci 22:2757–2766. [DOI] [PubMed] [Google Scholar]
- Echtay KS. (2007) Mitochondrial uncoupling proteins–what is their physiological role? Free Radic Biol Med 43:1351–1371. [DOI] [PubMed] [Google Scholar]
- Elamin M, Ruskin DN, Masino SA, Sacchetti P (2017) Ketone-based metabolic therapy: is increased NAD+ a primary mechanism? Front Mol Neurosci 10:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin M, Ruskin DN, Masino SA, Sacchetti P (2018a) Ketogenic diet modulates NAD+-dependent enzymes and reduces DNA damage in hippocampus. Front Cell Neurosci 12:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin M, Ruskin DN, Masino SA, Sacchetti P (2018b) Ketogenic diet modulates NAD+-dependent enzymes and reduces DNA damage in hippocampus. Front Cell Neurosci 12:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhanati S, Ben-Hamo R, Kanfi Y, Varvak A, Glazz R, Lerrer B, Efroni S, Cohen HY (2016) Reciprocal regulation between SIRT6 and miR-122 controls liver metabolism and predicts hepatocarcinoma prognosis. Cell Rep 14:234–242. [DOI] [PubMed] [Google Scholar]
- Evans M, Cogan KE, Egan B (2017) Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol 595:2857–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrazzo M. (2018) Impaired glucose metabolism in bipolar patients and response to mood stabilizer treatments. J Affect Disord 245:174–179. [DOI] [PubMed] [Google Scholar]
- Fan W, Evans R (2015) PPARs and ERRs: molecular mediators of mitochondrial metabolism. Curr Opin Cell Biol 33:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber EC, Peck B, Delpuech O, Bell GP, East P, Schulze A (2012) FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ 19:968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, Haigis MC (2011) Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One 6:e23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Haigis MC (2012) Metabolic regulation by SIRT3: implications for tumorigenesis. Trends Mol Med 18:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisler JS, Warden CH (2006) Uncoupling proteins, dietary fat and the metabolic syndrome. Nutr Metab (Lond) 3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S (2003) A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 348:2082–2090. [DOI] [PubMed] [Google Scholar]
- Frey S, Geffroy G, Desquiret-Dumas V, Gueguen N, Bris C, Belal S, Amati-Bonneau P, Chevrollier A, Barth M, Henrion D, Lenaers G, Bonneau D, Reynier P, Procaccio V (2017) The addition of ketone bodies alleviates mitochondrial dysfunction by restoring complex I assembly in a MELAS cellular model. Biochim Biophys Acta Mol Basis Dis 1863:284–291. [DOI] [PubMed] [Google Scholar]
- Fu SP, Li SN, Wang JF, Li Y, Xie SS, Xue WJ, Liu HM, Huang BX, Lv QK, Lei LC, Liu GW, Wang W, Liu JX (2014) BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-κB activation. Mediators Inflamm 2014:983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL, Li SN, Huang BX, Lv QK, Wang W, Liu JX (2015) Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Yamashita T (2018) Sirtuins in neuroendocrine regulation and neurological diseases. Front Neurosci 12:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14:661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano LB, Patel M, Rho JM (2014) Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res 55:2211–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bueno B, Bioque M, Mac-Dowell KS, Barcones MF, Martínez-Cengotitabengoa M, Pina-Camacho L, Rodríguez-Jiménez R, Sáiz PA, Castro C, Lafuente A, Santabárbara J, González-Pinto A, Parellada M, Rubio G, García-Portilla MP, Micó JA, Bernardo M, Leza JC (2014) Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull 40:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc K, Genc S (2009) Oxidative stress and dysregulated Nrf2 activation in the pathogenesis of schizophrenia. Bioscience Hypotheses 2:16–18. [Google Scholar]
- Genzer Y, Dadon M, Burg C, Chapnik N, Froy O (2015) Ketogenic diet delays the phase of circadian rhythms and does not affect AMP-activated protein kinase (AMPK) in mouse liver. Mol Cell Endocrinol 417:124–130. [DOI] [PubMed] [Google Scholar]
- Gershuni VM, Yan SL, Medici V (2018) Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep 7:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibas MK, Gibas KJ (2017) Induced and controlled dietary ketosis as a regulator of obesity and metabolic syndrome pathologies. Diabetes Metab Syndr 11 Suppl 1:S385–S390. [DOI] [PubMed] [Google Scholar]
- Goday A, Bellido D, Sajoux I, Crujeiras AB, Burguera B, García-Luna PP, Oleaga A, Moreno B, Casanueva FF (2016) Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes 6:e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajulu M, Pinky PD, Bloemer J, Ghanei N, Suppiramaniam V, Amin R (2018) Signaling mechanisms of selective PPARγ modulators in Alzheimer’s disease. PPAR Res 2018:2010675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabacka M, Pierzchalska M, Dean M, Reiss K (2016a) Regulation of ketone body metabolism and the role of PPARalpha. Int J Mol Sci 17:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabacka M, Pierzchalska M, Dean M, Reiss K (2016b) Regulation of ketone body metabolism and the role of PPARα. Int J Mol Sci 17:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff EC, Fang H, Wanders D, Judd RL (2016) Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism 65:102–113. [DOI] [PubMed] [Google Scholar]
- Grav HJ, Tronstad KJ, Gudbrandsen OA, Berge K, Fladmark KE, Martinsen TC, Waldum H, Wergedahl H, Berge RK (2003) Changed energy state and increased mitochondrial β-Oxidation rate in liver of rats associated with lowered proton electrochemical potential and stimulated uncoupling protein 2 (UCP-2) expression: evidence for peroxisome proliferator-activated receptor-α independent induction of UCP-2 expression. J Biol Chem 278:30525–30533. [DOI] [PubMed] [Google Scholar]
- Greco T, Glenn TC, Hovda DA, Prins ML (2016) Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cereb Blood Flow Metab 36:1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A (2007) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282:30107–30119. [DOI] [PubMed] [Google Scholar]
- Groesbeck DK, Bluml RM, Kossoff EH (2006) Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol 48:978–981. [DOI] [PubMed] [Google Scholar]
- Guo M, Wang X, Zhao Y, Yang Q, Ding H, Dong Q, Chen X, Cui M (2018) Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing Drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front Mol Neurosci 11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta L, Khandelwal D, Kalra S, Gupta P, Dutta D, Aggarwal S (2017) Ketogenic diet in endocrine disorders: current perspectives. J Postgrad Med 63:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güzel O, Yılmaz U, Uysal U, Arslan N (2016) The effect of olive oil-based ketogenic diet on serum lipid levels in epileptic children. Neurol Sci 37:465–470. [DOI] [PubMed] [Google Scholar]
- Haces ML, Hernández-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L (2008) Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol 211:85–96. [DOI] [PubMed] [Google Scholar]
- Hagenbuchner J, Ausserlechner MJ (2013) Mitochondria and FOXO3: breath or die. Front Physiol 4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD, Chung ST (2018) Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care 21:308–312. [DOI] [PubMed] [Google Scholar]
- Hallows WC, Lee S, Denu JM (2006) Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A 103:10230–10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Miller AH (2017) Inflammation effects on glutamate as a pathway to neuroprogression in mood disorders. Mod Trends Pharmacopsychiatry 31:37–55. [DOI] [PubMed] [Google Scholar]
- Harun-Or-Rashid M, Inman DM (2018) Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. Journal of Neuroinflammation 15:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CJDC, Schofield GM, Williden M (2018) The use of nutritional supplements to induce ketosis and reduce symptoms associated with keto-induction: a narrative review. Peerj 6:e4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan-Olive MM, Lauritzen KH, Ali M, Rasmussen LJ, Storm-Mathisen J, Bergersen LH (2019) A ketogenic diet improves mitochondrial biogenesis and bioenergetics via the PGC1α-SIRT3-UCP2 axis. Neurochem Res 44:22–37. [DOI] [PubMed] [Google Scholar]
- Hashim SA, VanItallie TB (2014) Ketone body therapy: from the ketogenic diet to the oral administration of ketone ester. J Lipid Res 55:1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC (2009) Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E (2010a) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E (2010b) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmström KM, Kostov RV, Dinkova-Kostova AT (2016) The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol 1:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høstmark AT, Haug A (2013) Percentage oleic acid is inversely related to percentage arachidonic acid in total lipids of rat serum. Lipids Health Dis 12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Moreau F, Chadee K (2012) PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat Commun 3:1300. [DOI] [PubMed] [Google Scholar]
- Huang K, Gao X, Wei W (2017) The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp Cell Res 361:63–72. [DOI] [PubMed] [Google Scholar]
- Hughes SD, Kanabus M, Anderson G, Hargreaves IP, Rutherford T, O’Donnell M, Cross JH, Rahman S, Eaton S, Heales SJ (2014) The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem 129:426–433. [DOI] [PubMed] [Google Scholar]
- Hussain TA, Mathew TC, Dashti AA, Asfar S, Al-Zaid N, Dashti HM (2012) Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 28:1016–1021. [DOI] [PubMed] [Google Scholar]
- Hutfles LJ, Wilkins HM, Koppel SJ, Weidling IW, Selfridge JE, Tan E, Thyfault JP, Slawson C, Fenton AW, Zhu H, Swerdlow RH (2017) A bioenergetics systems evaluation of ketogenic diet liver effects. Appl Physiol Nutr Metab 42:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt HW, Kephart WC, Holland AM, Mumford P, Mobley CB, Lowery RP, Roberts MD, Wilson JM, Kavazis AN (2016) A Ketogenic diet in rodents elicits improved mitochondrial adaptations in response to resistance exercise training compared to an isocaloric western diet. Front Physiol 7:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JY, Lee KW, Woo JM, Junn E, Mouradian MM (2012) DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet 21:3013–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Mann GE (2014) Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol 2:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska E, Gromadzinska J, Peplonska B, Fendler W, Reszka E, Krol MB, Wieczorek E, Bukowska A, Gresner P, Galicki M, Zambrano Quispe O, Morawiec Z, Wasowicz W (2015) Lipid peroxidation and glutathione peroxidase activity relationship in breast cancer depends on functional polymorphism of GPX1. BMC Cancer 15:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Milder JB, Liang LP, Patel M (2008) The ketogenic diet increases mitochondrial glutathione levels. J Neurochem 106:1044–1051. [DOI] [PubMed] [Google Scholar]
- Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS (2011) Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp Neurol 232:195–202. [DOI] [PubMed] [Google Scholar]
- Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG (2016) AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at Serine 550. Mol Cell Biol 36:1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julio-Amilpas A, Montiel T, Soto-Tinoco E, Gerónimo-Olvera C, Massieu L (2015) Protection of hypoglycemia-induced neuronal death by β-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J Cereb Blood Flow Metab 35:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanakis M, Liuba P, Odermarsky M, Lundgren J, Hallböök T (2014) Effects of ketogenic diet on vascular function. Eur J Paediatr Neurol 18:489–494. [DOI] [PubMed] [Google Scholar]
- Kemper MF, Srivastava S, Todd King M, Clarke K, Veech RL, Pawlosky RJ (2015) An ester of β-hydroxybutyrate regulates cholesterol biosynthesis in rats and a cholesterol biomarker in humans. Lipids 50:1185–1193. [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E (2007) A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 292:E1724–E1739. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Sharp SJ, Finikarides L, Afzal I, Lentjes M, Luben R, Forouhi NG (2018) Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open 8:e020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Vallejo J, Rho JM (2010) Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J Neurochem 114:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Abdelwahab MG, Lee SH, O’Neill D, Thompson RJ, Duff HJ, Sullivan PG, Rho JM (2015) Ketones prevent oxidative impairment of hippocampal synaptic integrity through KATP channels. PLoS One 10:e0119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Yang Q (2013) Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J Cardiol 5:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid B, Bossy-Wetzel E (2013) Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Fukuo Y, Kitajima T, Okochi T, Yamanouchi Y, Kinoshita Y, Kawashima K, Inada T, Kunugi H, Kato T, Yoshikawa T, Ujike H, Ozaki N, Iwata N (2011) SIRT1 gene, schizophrenia and bipolar disorder in the Japanese population: an association study. Genes Brain Behav 10:257–263. [DOI] [PubMed] [Google Scholar]
- Klein P, Tyrlikova I, Mathews GC (2014) Dietary treatment in adults with refractory epilepsy: a review. Neurology 83:1978–1985. [DOI] [PubMed] [Google Scholar]
- Klotz LO, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M (2015) Redox regulation of FoxO transcription factors. Redox Biol 6:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S, Budney S, Deodhar M, Matthews SA, Simeone KA, Simeone TA (2018) Ketogenic diet regulates the antioxidant catalase via the transcription factor PPARγ2. Epilepsy Res 147:71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Huang Z, Ji W, Wang X, Liu J, Wu X, Huang Z, Li R, Zhu Q (2017) The ketone metabolite β-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class I histone deacetylases. J Neurotrauma 34:2645–2655. [DOI] [PubMed] [Google Scholar]
- Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y (2010) Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5:e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitar-Jerala N. (2015) Innate immune response in brain, NF-kappa B signaling and cystatins. Front Mol Neurosci 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski C, Jornayvaz FR (2017) Effects of ketogenic diets on cardiovascular risk factors: evidence from animal and human studies. Nutrients 9:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, et al. ; Charlie Foundation; Matthew’s Friends; Practice Committee of the Child Neurology Society (2018) Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 3:175–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, Hartman AL (2012) Ketogenic diets: new advances for metabolism-based therapies. Curr Opin Neurol 25:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac S, Domijan AM, Walker MC, Abramov AY (2012) Prolonged seizure activity impairs mitochondrial bioenergetics and induces cell death. J Cell Sci 125:1796–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács Z, D’Agostino DP, Diamond D, Kindy MS, Rogers C, Ari C (2019) Therapeutic potential of exogenous ketone supplement induced ketosis in the treatment of psychiatric disorders: review of current literature. Front Psychiatry 10:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs JD, Bell D, Hall R, Parry-Strong A, Docherty PD, Clarke K, Chase JG (2013) Improvements in glucose metabolism and insulin sensitivity with a low-carbohydrate diet in obese patients with type 2 diabetes. J Am Coll Nutr 32:11–17. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ (2012) Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging 33:425.e19–425.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiterovich PO Jr, Vining EP, Pyzik P, Skolasky R Jr, Freeman JM (2003) Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA 290:912–920. [DOI] [PubMed] [Google Scholar]
- Laeger T, Metges CC, Kuhla B (2010) Role of beta-hydroxybutyric acid in the central regulation of energy balance. Appetite 54:450–455. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD (2004) Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Lange KM, Makulska-Gertruda E, Nakamura Y, Reissmann A, Kanaya S, Hauser J (2017) Ketogenic diets and Alzheimer’s disease. Food Sci Hum Wellness 6:1–9. [Google Scholar]
- LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, Asara JM, Kalluri R (2014) PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16:992–1003, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wall SB, Ren C, Velten M, Hill CL, Locy ML, Rogers LK, Tipple TE (2016) Thioredoxin reductase inhibition attenuates neonatal hyperoxic lung injury and enhances nuclear factor E2-related factor 2 activation. Am J Respir Cell Mol Biol 55:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, Coselli JS, Chen L, Wang XL, Zhang Y, Shen YH (2009) Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes 58:2246–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang Y, Wang Y, Tang H, Zhang F, Zhang Y, Zhao Y (2018) Ketogenic diet for treatment of intractable epilepsy in adults: a meta-analysis of observational studies. Epilepsia Open 3:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TF, Vachharajani VT, Yoza BK, McCall CE (2012) NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem 287:25758–25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YM, Lowe H, Zak MM, Kobayashi J, Chan VW, Donner EJ (2013) Can children with hyperlipidemia receive ketogenic diet for medication-resistant epilepsy? J Child Neurol 28:479–483. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Tishkoff DX, Bao J (2011) Mitochondrial sirtuins in the regulation of mitochondrial activity and metabolic adaptation. Handb Exp Pharmacol 206:163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D, Wu H, Tsang AW, Poole LB, Yoza BK, Wang X, Vachharajani V, Furdui CM, McCall CE (2017) The oxidative state of cysteine Thiol 144 regulates the SIRT6 glucose homeostat. Sci Rep 7:11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Li J, Zhang H, Zhao X, Yan LJ, Yang X (2018) Role and possible mechanisms of Sirt1 in depression. Oxid Med Cell Longev 2018:8596903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM (2008) Oxidative impairment of hippocampal long-term potentiation involves activation of protein phosphatase 2A and is prevented by ketone bodies. J Neurosci Res 86:3322–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM (2007) Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 145:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Leonard B, Fernandez A, Kubera M, Nowak G, Veerhuis R, Gardner A, Ruckoanich P, Geffard M, Altamura C, Galecki P, Berk M (2011) (Neuro)inflammation and neuroprogression as new pathways and drug targets in depression: from antioxidants to kinase inhibitors. Prog Neuropsychopharmacol Biol Psychiatry 35:659–663. [DOI] [PubMed] [Google Scholar]
- Mailloux RJ, Harper ME (2011) Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med 51:1106–1115. [DOI] [PubMed] [Google Scholar]
- Malingriaux EA, Rupprecht A, Gille L, Jovanovic O, Jezek P, Jaburek M, Pohl EE (2013) Fatty acids are key in 4-hydroxy-2-nonenal-mediated activation of uncoupling proteins 1 and 2. PLoS One 8:e77786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Hernández D, Caso JR, Javier Meana J, Callado LF, Madrigal JLM, García-Bueno B, Leza JC (2018) Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: effect of antidepressants. J Neuroinflammation 15:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massieu L, Haces ML, Montiel T, Hernández-Fonseca K (2003) Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience 120:365–378. [DOI] [PubMed] [Google Scholar]
- McCarty MF, DiNicolantonio JJ, O’Keefe JH (2015) Ketosis may promote brain macroautophagy by activating Sirt1 and hypoxia-inducible factor-1. Med Hypotheses 85:631–639. [DOI] [PubMed] [Google Scholar]
- McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD (2004) Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr 134:3499S–3506S. [DOI] [PubMed] [Google Scholar]
- Milder J, Patel M (2012) Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res 100:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milder JB, Liang LP, Patel M (2010) Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis 40:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VJ, Villamena FA, Volek JS (2018) Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab 2018:5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, Han X, Li J, Yang M, Wang Z, Wei D, Xiao H (2014) The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal 20:574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsalve FA, Pyarasani RD, Delgado-Lopez F, Moore-Carrasco R (2013) Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediators Inflamm 2013:549627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Anderson G, Berk M, Maes M (2013) Coenzyme Q10 depletion in medical and neuropsychiatric disorders: potential repercussions and therapeutic implications. Mol Neurobiol 48:883–903. [DOI] [PubMed] [Google Scholar]
- Morris G, Berk M (2015) The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med 13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK (2018) Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res 341:154–175. [DOI] [PubMed] [Google Scholar]
- Morris G, Berk M, Maes M, Carvalho AF, Puri BK (2019b) Socioeconomic deprivation, adverse childhood experiences and medical disorders in adulthood: mechanisms and associations. Mol Neurobiol 56:5866–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Berk M, Walder K, Maes M (2015a) Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med 13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Berk M, Walder K, Maes M (2015b) Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med 13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Maes M, Berk M, Puri BK (2019a) Myalgic encephalomyelitis or chronic fatigue syndrome: how could the illness develop? Metab Brain Dis 34:385–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Puri BK, Walker AJ, Berk M, Walder K, Bortolasci CC, Marx W, Carvalho AF, Maes M (2019d) The compensatory antioxidant response system with a focus on neuroprogressive disorders. Prog Neuropsychopharmacol Biol Psychiatry 95:109708. [DOI] [PubMed] [Google Scholar]
- Morris G, Puri BK, Walker AJ, Maes M, Carvalho AF, Bortolasci CC, Walder K, Berk M (2019c) Shared pathways for neuroprogression and somatoprogression in neuropsychiatric disorders. Neurosci Biobehav Rev 107:862–882. [DOI] [PubMed] [Google Scholar]
- Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, Berk M (2017) A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev 74:1–20. [DOI] [PubMed] [Google Scholar]
- Mumme K, Stonehouse W (2015) Effects of medium-chain triglycerides on weight loss and body composition: a meta-analysis of randomized controlled trials. J Acad Nutr Diet 115:249–263. [DOI] [PubMed] [Google Scholar]
- Murphy EA, Jenkins TJ (2019) A ketogenic diet for reducing obesity and maintaining capacity for physical activity: hype or hope? Curr Opin Clin Nutr Metab Care 22:314–319. [DOI] [PubMed] [Google Scholar]
- Nagao M, Toh R, Irino Y, Mori T, Nakajima H, Hara T, Honjo T, Satomi-Kobayashi S, Shinke T, Tanaka H, Ishida T, Hirata K (2016) β-Hydroxybutyrate elevation as a compensatory response against oxidative stress in cardiomyocytes. Biochem Biophys Res Commun 475:322–328. [DOI] [PubMed] [Google Scholar]
- Namayandeh SM, Kaseb F, Lesan S (2013) Olive and sesame oil effect on lipid profile in hypercholesterolemic patients, which better? Int J Prev Med 4:1059–1062. [PMC free article] [PubMed] [Google Scholar]
- Ndisang JF. (2014) Cross-talk between heme oxygenase and peroxisome proliferator-activated receptors in the regulation of physiological functions. Front Biosci (Landmark Ed) 19:916–935. [DOI] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH (2008) The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 7:500–506. [DOI] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH (2009) A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 50:1109–1117. [DOI] [PubMed] [Google Scholar]
- Newman JC, Verdin E (2014) β-hydroxybutyrate: much more than a metabolite. Diabetes Res Clin Pract 106:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, Ghaznavi SA, Sande Mathias I, Ellard KK, Janos JA, Sylvia LG (2018) Peroxisome proliferator-activated receptor gamma coactivator-1 alpha as a novel target for bipolar disorder and other neuropsychiatric disorders. Biol Psychiatry 83:761–769. [DOI] [PubMed] [Google Scholar]
- Nivoli A, Porcelli S, Albani D, Forloni G, Fusco F, Colom F, Vieta E, Serretti A (2016) Association between Sirtuin 1 gene rs10997870 polymorphism and suicide behaviors in bipolar disorder. Neuropsychobiology 74:1–7. [DOI] [PubMed] [Google Scholar]
- Nizamuddin J, Turner Z, Rubenstein JE, Pyzik PL, Kossoff EH (2008) Management and risk factors for dyslipidemia with the ketogenic diet. J Child Neurol 23:758–761. [DOI] [PubMed] [Google Scholar]
- Nylen K, Velazquez JL, Sayed V, Gibson KM, Burnham WM, Snead OC 3rd (2009) The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1(-/-) mice. Biochim Biophys Acta 1790:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, Monsalve M (2009) Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem 284:14476–14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos Y, Sánchez-Gómez FJ, Wild B, García-Quintans N, Cabezudo S, Lamas S, Monsalve M (2013) SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid Redox Signal 19:1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H, Yoshida S, Ashida K, Nakamura K, Takahashi T, Kunugi H (2019) Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci Lett 690:232–236. [DOI] [PubMed] [Google Scholar]
- Paleologou E, Ismayilova N, Kinali M (2017) Use of the ketogenic diet to treat intractable epilepsy in mitochondrial disorders. J Clin Med 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli A. (2014) Ketogenic diet for obesity: friend or foe? Int J Environ Res Public Health 11:2092–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli A, Bosco G, Camporesi EM, Mangar D (2015) Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Lee KS, Kim SR, Min KH, Choe YH, Moon H, Chae HJ, Yoo WH, Lee YC (2009) Peroxisome proliferator-activated receptor gamma agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J Immunol 183:3259–3267. [DOI] [PubMed] [Google Scholar]
- Parodi-Rullán RM, Chapa-Dubocq XR, Javadov S (2018) Acetylation of mitochondrial proteins in the heart: the role of SIRT3. Front Physiol 9:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlosky RJ, Kemper MF, Kashiwaya Y, King MT, Mattson MP, Veech RL (2017) Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer’s disease. J Neurochem 141:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Yang L, Wang J, Ye F, Dan G, Zhao Y, Cai Y, Cui Z, Ao L, Liu J, Zou Z, Sai Y, Cao J (2017) The interaction of mitochondrial biogenesis and fission/fusion mediated by PGC-1α regulates rotenone-induced dopaminergic neurotoxicity. Mol Neurobiol 54:3783–3797. [DOI] [PubMed] [Google Scholar]
- Peserico A, Chiacchiera F, Grossi V, Matrone A, Latorre D, Simonatto M, Fusella A, Ryall JG, Finley LW, Haigis MC, Villani G, Puri PL, Sartorelli V, Simone C (2013) A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels. Cell Mol Life Sci 70:2015–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps JR, Siemers SV, El-Mallakh RS (2013) The ketogenic diet for type II bipolar disorder. Neurocase 19:423–426. [DOI] [PubMed] [Google Scholar]
- Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP (2018) Low-fat versus ketogenic diet in Parkinson’s disease: a pilot randomized controlled trial. Mov Disord 33:1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckaers PJ, Churchward-Venne TA, Bailey D, van Loon LJ (2017) Ketone bodies and exercise performance: the next magic bullet or merely hype? Sports Med 47:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvani S, Tarocchi M, Galli A (2012) PPARγ and oxidative stress: Con(β) catenating NRF2 and FOXO. PPAR Res 2012:641087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen Ll, Siersbæk M, Mandrup S (2012) PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol 23:631–639. [DOI] [PubMed] [Google Scholar]
- Price NL, et al. (2012a) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, et al. (2012b) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchalska P, Crawford PA (2017) Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 25:262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90. [DOI] [PubMed] [Google Scholar]
- Qu Q, Zeng F, Liu X, Wang QJ, Deng F (2016) Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis 7:e2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan P, Karthikeyan A, Lu J, Ling EA, Dheen ST (2015) Sirtuin 3 regulates Foxo3a-mediated antioxidant pathway in microglia. Neuroscience 311:398–414. [DOI] [PubMed] [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S (2004) Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging 25:311–314. [DOI] [PubMed] [Google Scholar]
- Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW, Schrauwen P, Schols AM (2009) PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab 297:E174–E183. [DOI] [PubMed] [Google Scholar]
- Roy M, Nugent S, Tremblay-Mercier J, Tremblay S, Courchesne-Loyer A, Beaudoin JF, Tremblay L, Descoteaux M, Lecomte R, Cunnane SC (2012) The ketogenic diet increases brain glucose and ketone uptake in aged rats: a dual tracer PET and volumetric MRI study. Brain Res 1488:14–23. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298:E751–E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L (2003) A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 348:2074–2081. [DOI] [PubMed] [Google Scholar]
- Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL (1995) Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 9:651–658. [DOI] [PubMed] [Google Scholar]
- Satterstrom FK, Swindell WR, Laurent G, Vyas S, Bulyk ML, Haigis MC (2015) Nuclear respiratory factor 2 induces SIRT3 expression. Aging Cell 14:818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813:1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, et al. (2014) A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab 20:840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C (2008) Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol 382:790–801. [DOI] [PubMed] [Google Scholar]
- Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M (2015) Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 62:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Zhong L, Silberman M, Toiber D, Martinez B, Etchegaray J-P, Cosentino C, Giacosa S, Mostoslavsky R (2012) The histone deacetylase SIRT6, a critical modulator of metabolism and tumorigenesis. BMC Proceedings 6:O17. [Google Scholar]
- Seethalakshmi R, Parkar SR, Nair N, Adarkar SA, Pandit AG, Batra SA, Baghel NS, Moghe SH (2006) Regional brain metabolism in schizophrenia: an FDG-PET study. Indian J Psychiatry 48:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulic-Jablanovic M, Petkovic V, Wright MB, Kucharava K, Huerzeler N, Levano S, Brand Y, Leitmeyer K, Glutz A, Bausch A, Bodmer D (2017) Effects of peroxisome proliferator activated receptors (PPAR)-γ and -α agonists on cochlear protection from oxidative stress. PLoS One 12:e0188596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman MJ, Kraemer WJ, Love DM, Avery NG, Gómez AL, Scheett TP, Volek JS (2002) A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr 132:1879–1885. [DOI] [PubMed] [Google Scholar]
- Sheng S, Kang Y, Guo Y, Pu Q, Cai M, Tu Z (2015) Overexpression of Sirt3 inhibits lipid accumulation in macrophages through mitochondrial IDH2 deacetylation. Int J Clin Exp Pathol 8:9196–9201. [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E (2013) Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikder K, Shukla SK, Patel N, Singh H, Rafiq K (2018) High fat diet upregulates fatty acid oxidation and ketogenesis via intervention of PPAR-gamma. Cell Physiol Biochem 48:1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Matthews SA, Samson KK, Simeone KA (2017a) Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol 287:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Matthews SA, Samson KK, Simeone KA (2017b) Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol 287:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima E, Ohki T, Kurita Y, Yuan X, Tanaka K, Kakino S, Hara K, Nakayama H, Tajiri Y, Yamada K (2018) Protective effect of 3-hydroxybutyrate against endoplasmic reticulum stress-associated vascular endothelial cell damage induced by low glucose exposure. PLoS One 13:e0191147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SB, Jang SY, Kang HT, Wei B, Jeoun UW, Yoon GS, Hwang ES (2017) Modulation of mitochondrial membrane potential and ROS generation by nicotinamide in a manner independent of SIRT1 and mitophagy. Mol Cells 40:503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Baxa U, Niu G, Chen X, Veech RL (2013) A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 65:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge MP, Bosarge A, Goree LL, Darnell B (2008) Medium chain triglyceride oil consumption as part of a weight loss diet does not lead to an adverse metabolic profile when compared to olive oil. J Am Coll Nutr 27:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM (2003) Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem 278:26597–26603. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408. [DOI] [PubMed] [Google Scholar]
- St-Pierre V, Vandenberghe C, Lowry CM, Fortier M, Castellano CA, Wagner R, Cunnane SC (2019) Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day. Front Nutr 6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staverosky T. (2016) Ketogenic weight loss: the lowering of insulin levels is the sleeping giant in patient care. J Med Pract Manage 32:63–66. [PubMed] [Google Scholar]
- Steele ML, Fuller S, Patel M, Kersaitis C, Ooi L, Münch G (2013) Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol 1:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Imai S (2012) The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab 23:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams M, Gracely EJ, Samaha FF (2004) The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med 140:778–785. [DOI] [PubMed] [Google Scholar]
- Strom J, Xu B, Tian X, Chen QM (2016) Nrf2 protects mitochondrial decay by oxidative stress. FASEB J 30:66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor-Elliott S, Hiyama S, Stirling M, Clarke K (2017) On the metabolism of exogenous ketones in humans. Front Physiol 8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Cai Y, Xu Y, Dutt A, Shi S, Bramon E (2014) Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry 14:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueblinvong V, Mills ST, Neujahr DC, Go YM, Jones DP, Guidot DM (2016) Nuclear thioredoxin-1 overexpression attenuates alcohol-mediated Nrf2 signaling and lung fibrosis. Alcohol Clin Exp Res 40:1846–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM (2004) The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol 55:576–580. [DOI] [PubMed] [Google Scholar]
- Tan WQ, Wang K, Lv DY, Li PF (2008) Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem 283:29730–29739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanito M, Agbaga MP, Anderson RE (2007) Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med 42:1838–1850. [DOI] [PubMed] [Google Scholar]
- Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, Yancy WS Jr, Brinkworth GD (2014) A very low-carbohydrate, low-saturated fat diet for type 2 diabetes management: a randomized trial. Diabetes Care 37:2909–2918. [DOI] [PubMed] [Google Scholar]
- Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH (2018) Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimers Dement (N Y) 4:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut F. (2017) Neuroinflammation: new vistas for neuropsychiatric research. Dialogues Clin Neurosci 19:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholstrup T, Ehnholm C, Jauhiainen M, Petersen M, Høy CE, Lund P, Sandström B (2004) Effects of medium-chain fatty acids and oleic acid on blood lipids, lipoproteins, glucose, insulin, and lipid transfer protein activities. Am J Clin Nutr 79:564–569. [DOI] [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S (2003) D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta MC, Maisto R, Guida F, Boccella S, Luongo L, Balta C, D’Amico G, Herman H, Hermenean A, Bucolo C, D’Amico M (2019) The activation of retinal HCA2 receptors by systemic beta-hydroxybutyrate inhibits diabetic retinal damage through reduction of endoplasmic reticulum stress and the NLRP3 inflammasome. PLoS One 14:e0211005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Shieh SS, Wang DL (2013) SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med 63:222–234. [DOI] [PubMed] [Google Scholar]
- Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S (2011) The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res 2:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachharajani V, Liu T, McCall CE (2014) Epigenetic coordination of acute systemic inflammation: potential therapeutic targets. Expert Rev Clin Immunol 10:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M (2005) PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res 66:562–573. [DOI] [PubMed] [Google Scholar]
- Veech RL. (2004) The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids 70:309–319. [DOI] [PubMed] [Google Scholar]
- Veech RL. (2014) Ketone ester effects on metabolism and transcription. J Lipid Res 55:2004–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT (2017) Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 69:305–314. [DOI] [PubMed] [Google Scholar]
- Venditti P, Bari A, Di Stefano L, Cardone A, Della Ragione F, D’Esposito M, Di Meo S (2009) Involvement of PGC-1, NRF-1, and NRF-2 in metabolic response by rat liver to hormonal and environmental signals. Mol Cell Endocrinol 305:22–29. [DOI] [PubMed] [Google Scholar]
- Verdin E, Hirschey MD, Finley LW, Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci 35:669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volek JS, Sharman MJ, Forsythe CE (2005) Modification of lipoproteins by very low-carbohydrate diets. J Nutr 135:1339–1342. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ (2001) DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79:266–277. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Ruiter JP, IJLst L, Waterham HR, Houten SM (2010) The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis 33:479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Zhao XH, Chen W, Bo N, Wang XJ, Chi ZF, Wu W (2015) Sirtuin 1 activation enhances the PGC-1α/mitochondrial antioxidant system pathway in status epilepticus. Mol Med Rep 11:521–526. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu X, Liu Q, Kong G, Zhou J, Jiang J, Wu X, Huang Z, Su W, Zhu Q (2017) Ketogenic metabolism inhibits histone deacetylase (HDAC) and reduces oxidative stress after spinal cord injury in rats. Neuroscience 366:36–43. [DOI] [PubMed] [Google Scholar]
- Wang YX. (2010) PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res 20:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Tian W, Liu F, Xie G (2014) Protective effects of exogenous β-hydroxybutyrate on paraquat toxicity in rat kidney. Biochem Biophys Res Commun 447:666–671. [DOI] [PubMed] [Google Scholar]
- Westman EC, Yancy WS Jr, Mavropoulos JC, Marquart M, McDuffie JR (2008) The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond) 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Włodarczyk A, Wiglusz MS, Cubała WJ (2018) Ketogenic diet for schizophrenia: nutritional approach to antipsychotic treatment. Med Hypotheses 118:74–77. [DOI] [PubMed] [Google Scholar]
- Włodarek D. (2019) Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). Nutrients 11:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Wang RS, Handy DE, Loscalzo J (2018) NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid Redox Signal 28:251–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Ipek Ö, Beaumont M, Shevlyakova M, Christinat N, Masoodi M, Greenberg N, Gruetter R, Cuenoud B (2018) Nutritional ketosis increases NAD+/NADH ratio in healthy human brain: an in vivo study by 31P-MRS. Front Nutr 5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi T, Iwata M, Kamiya N, Tsunetomi K, Kajitani N, Wada N, Iitsuka T, Yamauchi T, Miura A, Pu S, Shirayama Y, Watanabe K, Duman RS, Kaneko K (2017) Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci Rep 7:7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Wang LH, Farrar WL (2008) A role for PPARgamma in the regulation of cytokines in immune cells and cancer. PPAR Res 2008:961753. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang Y, Sauve AA (2016) NAD(+) metabolism: bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta 1864:1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Li XJ, Jiang WL, Sun HB, Liu J (2015) Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis. J Clin Neurol 11:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Han P, Tang Z, Liu Q, Shi J (2015) Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab 35:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W. (2008) NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10:179–206. [DOI] [PubMed] [Google Scholar]
- Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD (2015) The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Dittenhafer-Reed KE, Denu JM (2012) SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 287:14078–14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SH, Han SH, Park JI (2018) Peroxisome proliferator-activated receptor γ and PGC-1α in cancer: dual actions as tumor promoter and suppressor. PPAR Res 2018:6727421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani GR, Mohammadi M, Ashrafi MR, Karimi P, Mahmoudi M, Badv RS, Tavassoli AR, Azizi Malamiri R (2016) The effects of classic ketogenic diet on serum lipid profile in children with refractory seizures. Acta Neurol Belg 116:529–534. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cao Q, Li S, Lu X, Zhao Y, Guan JS, Chen JC, Wu Q, Chen GQ (2013) 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 34:7552–7562. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Han M, Shirayama Y, Hashimoto K (2018) Keap1-Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci 268:865–870. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ren X, Zhang Q, Li Z, Ma S, Bao J, Li Z, Bai X, Zheng L, Zhang Z, Shang S, Zhang C, Wang C, Cao L, Wang Q, Ji J (2016) PGC-1α/ERRα-Sirt3 pathway regulates DAergic neuronal death by directly deacetylating SOD2 and ATP synthase β. Antioxid Redox Signal 24:312–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kuang Y, Xu K, Harris D, Lee Z, LaManna J, Puchowicz MA (2013) Ketosis proportionately spares glucose utilization in brain. J Cereb Blood Flow Metab 33:1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhang L, Chen R, Lu H, Sui M, Zhu Y, Zeng L (2018) SIRT3 protects against acute kidney injury via AMPK/mTOR-regulated autophagy. Front Physiol 9:1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li R, Liu C, Sun T, Htet Aung LH, Chen C, Gao J, Zhao Y, Wang K (2017) Foxo3a inhibits mitochondrial fission and protects against doxorubicin-induced cardiotoxicity by suppressing MIEF2. Free Radic Biol Med 104:360–370. [DOI] [PubMed] [Google Scholar]