Abstract
Somatic mutations have emerged as the likely cause of focal epilepsies associated with developmental malformations and epilepsy-associated glioneuronal tumors (GNT). Somatic BRAFV600E mutations in particular have been detected in the majority of low-grade neuroepithelial tumors (LNETS) and in neurons in focal cortical dysplasias adjacent to epilepsy-associated tumors. Furthermore, conditional expression of an activating BRAF mutation in neocortex causes seizures in mice. In this study we characterized the cellular electrophysiology of layer 2/3 neocortical pyramidal neurons induced to express BRAFV600E from neural progenitor stages. In utero electroporation of a piggyBac transposase plasmid system was used to introduce transgenes expressing BRAF wild type (BRAFwt), BRAFV600E, and/or enhanced green fluorescent protein (eGFP) and monomeric red fluorescent protein (mRFP) into radial glia progenitors in mouse embryonic cortex. Whole cell patch-clamp recordings of pyramidal neurons in slices prepared from both juvenile and adult mice showed that BRAFV600E resulted in neurons with a distinct hyperexcitable phenotype characterized by depolarized resting membrane potentials, increased input resistances, lowered action potential (AP) thresholds, and increased AP firing frequencies. Some of the BRAFV600E-expressing neurons normally destined for upper cortical layers by their birthdate were stalled in their migration and occupied lower cortical layers. BRAFV600E-expressing neurons also displayed increased hyperpolarization-induced inward currents (Ih) and decreased sustained potassium currents. Neurons adjacent to BRAFV600E transgene-expressing neurons, and neurons with TSC1 genetically deleted by CRISPR or those induced to carry PIK3CAE545K transgenes, did not show an excitability phenotype similar to that of BRAFV600E-expressing neurons. Together, these results indicate that BRAFV600E leads to a distinct hyperexcitable neuronal phenotype.
NEW & NOTEWORTHY This study is the first to report the cell autonomous effects of BRAFV600E mutations on the intrinsic neuronal excitability. We show that BRAFV600E alters multiple electrophysiological parameters in neocortical neurons. Similar excitability changes did not occur in cells neighboring BRAFV600E-expressing neurons, after overexpression of wild-type BRAF transgenes, or after introduction of mutations affecting the mammalian target of rapamycin (mTOR) or the catalytic subunit of phosphoinositide 3-kinase (PIK3CA). We conclude that BRAFV600E causes a distinct, cell autonomous, highly excitable neuronal phenotype when introduced somatically into neocortical neuronal progenitors.
Keywords: BRAFV600E, cortical dysplasia, epilepsy, ganglioglioma, hyperexcitability
INTRODUCTION
Somatic mutations arising spontaneously in the developing nervous system have gained particular interest as possible causes of disorders of nervous system function (McConnell et al. 2017). For example, mutations in neuroglial progenitors in genes in the mammalian target of rapamycin (mTOR) pathway are known to cause focal cortical dysplasias and hemimegaloencephaly (D’Gama and Walsh 2018; Jamuar and Walsh 2014; Lim and Crino 2013). Such cortical pathologies lead to epilepsy even though as few as 3–10% of neurons within the malformations may contain somatic mutations. This suggests that even a relatively small set of neurons may acquire a somatic mutation and yet have a large impact on function. The highly interconnected neural networks of cortex and the possibility that some mutations may have large effects on a neuron’s excitability indicate a possible way in which even mutations in scattered neurons could result in pathophysiological states. Specifying the physiological effects of different somatic mutations is an important step to understanding how such mutations may lead to disorders of function.
Activating mutations in BRAF, a serine/threonine kinase in the Ras/MAPK signaling pathway, have been identified in both a genetic syndrome and in tumors associated with seizures and epilepsy. The most common mutations in the autosomal dominant genetic disorder cardiofaciocutaneous syndrome (CFC) are activating mutations (Niihori et al. 2006; Yoon et al. 2007; reviewed in Tidyman and Rauen 2009), and more than 50% of individuals with CFC have seizures. In addition, the activating BRAF mutation V600E is the most common somatic mutation identified in tissue resected from surgery of intractable focal epilepsy patients with low-grade tumors (Niestroj et al. 2019; Sim et al. 2019; reviewed in Barkovich et al. 2015; Blümcke et al. 1999; Guerrini et al. 2015; Thom et al. 2012). Somatic activating mutations in BRAF have been identified in ~50–70% of low-grade neuroepithelial tumors (LNETs) (Dodgshun et al. 2016; Kakkar et al. 2016; Koelsche et al. 2013; Prabowo et al. 2014; Schindler et al. 2011), and BRAFV600E mutations have also been identified in neurons in focal cortical dysplasias frequently found adjacent to epilepsy-associated tumors including ganglioglioma (Blumcke et al. 2014; Marucci et al. 2014). A clear causal relationship between BRAFV600E mutations and development of seizures was recently shown by Koh et al. (2018), who demonstrated that BRAF somatic mutations induced in a focal patch of neocortex by in utero electroporation of Cre recombinase in mice developed recurrent seizures, and moreover, these seizures could be rescued by expression of REST. Koh et al. (2018) used EEG recordings and extracellular microelectrode array recordings in slices to show that cortical tissue with BRAFV637E mutations (the homologous mutation in mouse to BRAFV600E in humans) shows an increased spiking rate and electrographic seizures.
Previous studies have shown that activating BRAF mutations and manipulations of signaling proteins downstream of BRAF in the MAPK/ERK pathway can have effects on various cellular electrophysiological properties of neurons. For example, constitutively active BRAF mutants (T599E/S602D and V600E) introduced into hippocampal neurons in slices by viruses increase α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) synaptic responses through activation of N-methyl-d-aspartate (NMDA) receptors, without changing intrinsic neuronal properties or GABA-mediated synaptic responses (Lim et al. 2017a). In a related finding, Yeh et al. (2018) in a CFC patient with another BRAF activating mutation (Q257R) in inhibitory postsynaptic current (iPSC)-derived neuronal cultures, and Zhang et al. (2010) in acute spinal cord slices reported that TNFα treatment activating MAPK/ERK pathways increased spontaneous excitatory postsynaptic currents (sEPSCs); however, in spinal cord this increase is mediated by inhibition of GABAergic neurons, or disinhibition of the network (Zhang et al. 2010), compared with an NMDA-dependent mechanism of AMPA response increase in hippocampal slices reported by Lim et al. (2017a), and this was blocked by a p38 MAPK inhibitor, suggesting that TNFα may activate MAPK/ERK pathway in a different way, comparative to IL-1β (Binshtok et al. 2008), or there might be a subtle difference in the pathway activation in spinal cord compared with hippocampal neurons in slices. Activation of p38 MAPK, a molecule downstream to BRAF in MAPK/ERK pathway, has also been shown to decrease action potential (AP) firing rate, possibly through hyperpolarization-activated cyclic nucleotide-gated (HCN) channel activation, in a pilocarpine-induced seizure model (Jung et al. 2010; Poolos et al. 2006), or through a direct effect on Nav1.6 current density (Gasser et al. 2010; Wittmack et al. 2005).
In the current study we sought to determine whether BRAFV600E mutations introduced into cortical progenitors alter intrinsic neuronal excitability in neocortical neurons in acute ex vivo mouse slices. We used in utero electroporation to introduce BRAFV600E transgenes into two radial glia progenitor populations that generate either primarily pyramidal neurons or a combination of pyramidal neurons and astrocytes (Siddiqi et al. 2014). By using a NESTIN promoter we delivered transgene integration into progenitors that gave rise to almost exclusively pyramidal neurons and not astrocytes, and using a GLAST promoter we directed transgene integration into progenitors that gave rise to both astrocytes and pyramidal neurons. BRAFV600E transgenes in either progenitor resulted in neurons that had hyperexcitable intrinsic properties characterized most notably by a threefold increase in action potential firing rates, low thresholds to firing action potentials, depolarized membrane potentials, elevated hyperpolarization-activated inward currents, and decreased sustained potassium currents. We also found that increased expression of BRAF wild type (BRAFwt) and mutations in genes in the mTOR pathways, TSC1 knockout (KO) by CRISPR or expression of PIK3CAE545K, did not recapitulate the same hyperexcitable phenotype caused by BRAFV600E, suggesting that different somatic mutations associated with seizures can have distinct effects on neuronal physiology.
MATERIALS AND METHODS
Plasmid vectors.
We used a nonviral binary piggyBac transposase system to deliver integrated transgenes into the genomes of neocortical glial neuronal progenitors. The system consists of a transposase-expressing plasmid and a donor plasmid containing the transgene to be genomically integrated. The transposase helper plasmids pGlast-PBase and pNestin-PBase were as previously described (Siddiqi et al. 2014). The transgene donor plasmids used were pPBCAG-monomeric red fluorescent protein (mRFP) and pPBCAG-enhanced green fluorescent protein (EGFP), constructed as previously described (Chen and LoTurco 2012). Human BRAFV600E (pBABEbleo-Flag-BRAFV600E) was donated by Dr. Christopher Counter and obtained from Addgene (plasmid no. 53156) (Brady et al. 2014), and human PIK3CAE545K (pBabe-puro-HA-PIK3CAE545K) was donated by Dr. Jean Zhao (Zhao et al. 2005) and also obtained from Addgene (plasmid no. 12525). The BRAFV600E and PIK3CAE545K inserts were amplified with standard PCR and cloned into pPBCAG-EGFP, replacing the EGFP sequence using the EcoRI and NotI sites. Hemagglutinin (HA), a 27-nucleotide epitope tag (5′-AGCGTAATCTGGAACATCGTATGGGTA-3′) was inserted into pPBase-BRAFV600E after the BRAFV600E sequence and before the NotI site. pPBase-BRAFwt was generated with the QuikChange II XL single-nucleotide site-directed mutagenesis kit from Agilent according to the manufacturer’s protocol, to change E (a glutamic amino acid) back to V (valine) at position 600, restoring the mutated sequence back to its wild type. Guide RNA (gRNA) CRISPR TSC1 (T4–5′-CCATGCTGGATCCTCCACACTG-3′) was chosen based on Lim et al. (2017a). These sequences were cloned into pX330 vector (Addgene; plasmid no. 42230) (Cong et al. 2013). The experimental conditions used to direct transgenes into GLAST-prog. progenitor populations combined the transposase helper plasmid pGlast-PBase with either BRAFV600E (pBCAG-Flag-BRAFV600E) and fluorescent marker (pPBCAG-EGFP) or BRAFwt (pBCAG-Flag-BRAFwt) and fluorescent marker, or either of two fluorescent markers as a control condition (pPBCAG-EGFP or pPBCAG-mRFP). For the experimental conditions targeting NESTIN-prog. progenitor populations, the pNestin-PBase transposase helper plasmid substituted the pGlast-PBase transposase helper plasmid.
Animals.
Pregnant CD1 mice were obtained from Charles River Laboratories (Wilmington, MA). PV-Cre:Ai14 and SST-Cre:A14 mice were generated by crossing PV-Cre [B6;129P2-Pvalbtm1(cre)Arbr/J; The Jackson Laboratory] or SST-Cre [Ssttm2.1(cre)Zjh/J; The Jackson Laboratory] and Ai14 [B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; The Jackson Laboratory], and mice were maintained at the University of Connecticut vivarium. Animal gestational ages were determined via palpation before surgery and confirmed during the surgery based on crown-ramp length (Mu et al. 2008). Female and male mice were used for cortical transgene delivery with in utero electroporation and for all electrophysiological recordings. Male and female mice postnatal day 15–70 (P15–P70; average P36.05, mode = 36, median = 35) were used in all brain slice electrophysiology experiments. No mice in this study, with the unilateral transfections described below, showed spontaneous seizures observed in video EEG, video alone, or daily observations. All procedures and experimental approaches were approved by the University of Connecticut Institutional Animal Care and Use Committee.
In utero electroporation.
In utero electroporation was performed as previously described (Chen and LoTurco 2012). Briefly, mice were anesthetized with a mixture of ketamine and xylazine (100 and 10 mg/kg ip). Metacam analgesic was administered daily at a dosage of 1 mg/kg sc for 2 days following surgery. To visualize the plasmid during electroporation, plasmids were mixed with 2 mg/mL Fast Green (MilliporeSigma, catalog no. F7252). In all conditions, pPBCAG-EGFP, pPBCAG-mRFP, pPB-BRAFV600E, pPB-BRAFwt, pPB-PIK3CAE545K, pGlast-PBase, and pNestin-PBase were used at the final concentration of 1.0 µg/µL. Electroporation was performed at embryonic day 14 or 15 (E14–E15). During surgery, the uterine horns were exposed and one lateral ventricle of each embryo was pressure injected with 1–2 µL of plasmid DNA. Injections were made through the uterine wall and embryonic membranes by inserting pulled glass microelectrodes (Drummond Scientific) into the lateral ventricle and injecting by pressure pulses delivered with Picospritzer II (General Valve). Electroporation was accomplished with a BTX 8300 pulse generator (BTX Harvard Apparatus) and BTX tweezertrodes. A voltage of 35–45 V was used for electroporation, and transfections were delivered unilaterally to the somatosensory cortex.
Brain slice preparation.
CD1 mice were deeply anesthetized with isoflurane and then decapitated. Brains were rapidly removed and immersed in ice-cold oxygenated (95% oxygen and 5% carbon dioxide) dissection buffer containing (in mM) 83 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 22 dextrose, 72 sucrose 0.5 CaCl2, and 3.3 MgCl2. Coronal slices (400 µm) were cut with a vibratome (VT1200S; Leica, Nussloch, Germany), incubated in dissection buffer for 40 min at 34°C, and then stored at room temperature for the remainder of the recording day. Voltage recordings were performed at 34°C (Warner Instruments); voltage-clamp recordings of potassium currents were made at room temperature to slow down the kinetics. Brain slices were visualized with inversion recovery differential interference microscopy (E600FN; Nikon, Tokyo, Japan) and a charge-coupled device camera (QICAM; QImaging, Surrey, British Columbia, Canada). Individual neurons were visualized with a ×40 Nikon Fluor water-immersion (0.8 N.A.) objective.
Electrophysiology.
Extracellular recording buffer was oxygenated (95% oxygen and 5% carbon dioxide) and contained (in mM) 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 25 dextrose, 1 MgCl2, and 1.3 CaCl2 (295–305 mosM). For potassium current recordings, the extracellular calcium was substituted with 1.3 CoCl2·6H2O (MilliporeSigma; CAS no. 7791-13-1). Patch pipettes were fabricated from borosilicate glass (N51A; King Precision Glass, Claremont, CA) to a resistance of 2–7 MΩ. Series resistance errors were minimized with bridge balance and capacitance compensation. Pipettes were filled with an internal solution containing (in mM) 125 K-gluconate, 10 HEPES, 4 Mg2-ATP, 3 Na-GTP, 0.1 EGTA, 10 Na-phosphocreatine, and 0.05% biocytin, adjusted to pH 7.3 with potassium hydroxide and to 275–285 mosM with double-deionized water (ddw). Signals were amplified with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), sampled at 20 kHz, digitized (ITC-18; HEKA instruments, Bellmore, NY), and filtered at 2 kHz with an 8-pole low-pass Bessel filter. Data were monitored, acquired, and in some cases analyzed with AxoGraph X software (Berkley, CA). Series resistance was monitored throughout the experiments by applying a small test voltage step and measuring the capacitive currents. Series resistance was 5 to ~25 MΩ, and only cells with <20% change in series resistance and holding current were included in the analysis.
In some experiments, d-aminophosphonovaleric acid (d-APV) dissolved in double-deionized water (ddw) to 50–100 mM stock (50 µM; Abcam, Cambridge, MA; catalog no. ab120003, lot no. GR205917) was used to block specifically NMDA receptor (NMDAR), and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX) dissolved in DMSO to 100 mM stock (10 µM; Abcam, Cambridge, MA; catalog no. ab120045, lot no. GR133243) was used to block AMPA receptors (AMPARs). SR-95531 dissolved in ddw to 25 mM stock (gabazine, 10 µM; Abcam, Cambridge, MA; catalog no. ab120042, lot no. GR69200) was used to block GABAA receptor (GABAAR); TTX-citrate dissolved in ddw to 10 mM (1 µM; Abcam, Cambridge, MA; catalog no. ab120055, lot no. GR246757) was used to block sodium currents; 4-aminopyridine (4-AP) dissolved in ddw to 200 mM (30 µM–3 mM; MilliporeSigma; Burlington, MA; catalog no. 275875) was used to block fast-activating, fast-inactivating potassium currents; tetraethylammonium chloride (TEA; 25–40 mM; Abcam, Cambridge, MA; catalog no. ab120275, lot no. GR69136) was used to block sustained potassium currents; ZD-7288 dissolved in ddw (50 µM; Cayman Chemicals, Ann Arbor, MI; catalog no. 1522820000040, batch no. 0476856-6) and ivabradine hydrochloride dissolved in ddw to 100 mM (10 µM; TOCRIS; catalog no. 6542) were used to block hyperpolarization-activated depolarizing currents (Ih). Ivabradine was washed into the recording bath for 15 min after baseline recordings, and the recordings were collected in drug after 15 min of incubation.
For action potential (AP) firing frequency and input resistance measurements, 1-s current steps were applied at 10-pA increments from −40 pA to 300 pA. For TSC1 CRISPR knockdown (KD) neurons, those steps increased to more than 300 pA. Input resistance was measured from the last 100 ms of 1-s hyperpolarizing and depolarizing subthreshold current steps, and the current-voltage relationship was fit with linear regression to estimate input resistance from both depolarizing and hyperpolarizing current steps. Resting membrane potential (RMP) was measured in the beginning of current-clamp protocols before application of current step pulses. Sag ratio was defined as , where VRMP is resting membrane potential, Vss is stable-state voltage in the last 100 ms of a 1-s, −40-pA pulse, and Vmin is minimal initial voltage deflection in response to a 1-s, −40-pA pulse. Rebound excitation was measured as an overshoot above RMP at the end of the −40-pA, 1-s current step, in some cells resulting in AP firing. AP voltage threshold was defined as the point at which the first derivative of voltage to time (dV/dt) crossed 50 V/s. Rheobase is the minimal current step required to elicit first AP firing. AP peak was measured from RMP. For the AP property measurements, the first AP at the rheobase was used for each and every cell.
To record potassium currents, neurons were held at −90 mV and 500-ms voltage steps proceeded with 10-mV increments from −100 to +20 mV. For sustained potassium currents, amplitude was measured at the last 100 ms of 500-ms voltage steps. The capacitive currents were canceled with internal Multiclamp 700B compensation circuit. Cell capacitance and input resistance in those experiments were monitored and measured before compensation from +5-mV, 150-ms voltage steps with an AxoGraph X built-in procedure designed to measure series resistance, membrane capacitance, and membrane resistance. To record hyperpolarization-activated depolarizing currents (Ih), two protocols were used. In the first, neurons were held at −50 mV and 1.5-s voltage steps proceeded with 5-mV increments from −120 mV to −35 mV without capacitance and series resistance compensation. The second protocol was used to increase stability of the recorded cells during drug wash in. In this protocol neurons were held at −70 mV and 1-s voltage steps proceeded with 5-mV increments from −100 mV to −45 mV. The peak measurement of Ih was done from the point at the beginning of the observed current to the stable state in the last 100 ms of the 1.5-s voltage step in the main protocol. Tail currents were measured after the voltage steps ceased. All measurements were done offline after offline leak current subtraction.
Histological preparation and image analysis.
Animals were deeply anesthetized with isoflurane and perfused transcardially with 4% paraformaldehyde-PBS (4% PFA). Samples were postfixed overnight in 4% PFA. For immunofluorescence, brains were sectioned at 50-µm thickness on a vibratome (Leica VT 1000S). Sections were processed as free floating and stained with Hoechst reagent. After blocking in PBS containing 5% normal goat serum (MilliporeSigma; catalog no. NS02L) and 0.5% Triton X-100 (MilliporeSigma) for 2 h at room temperature, tissue was washed three times in PBS with 2.5% normal goat serum and 0.2% Triton X-100 (washing solution), followed by incubation with primary antibodies overnight to 24 h at 4°C in the washing solution. Primary antibodies were rabbit anti-CDP/cut-like homeobox 1 (CUX1; Santa Cruz Biotechnology; catalog no. sc-13024, RRID:AB_2261231) or B-10 (Santa Cruz Biotechnology; catalog no. sc-514008) at 1:100 concentration and monoclonal rat anti-CTIP2 (clone 25B6; Abcam; catalog no. ab18465, RRID:AB_2064130) at 1:100 concentration. On the following day, tissue was washed again in washing solution and incubated with the appropriate secondary antibodies in washing solution (all Alexa Fluor at 1:1,000; Invitrogen) for 2 h at room temperature (Alexa Fluor 568 anti-mouse IgG, Alexa Fluor 647 anti-rabbit IgG, Alexa Fluor 568 anti-goat IgG). After 2 h, the tissue was washed again with washing solution once, stained with nuclear staining (Hoechst 33342, trihydrochloride, trihydrate, 10 mg/mL in water, 1:3,000 dilution; Molecular Probes by Life Technologies; catalog no. H3570), and washed again three times. Tissue was mounted on Fisherbrand ColorFrost Plus microscope slides (catalog no. 12-550-19) submerged in ProLong gold antifade (Life Technologies; catalog no. 36930), and coverslipped with Fisherfinest premium cover glasses (catalog nos. 12-548-5P, -5J, and -B, sizes 24 × 60, 24 × 40, and 22 × 22 mm, respectively). When ProLong gold antifade had cured, the coverslip edges were covered with transparent nail polish. Images were acquired on inverted Leica TSC SP8 confocal microscope with four photomultiplier tube (PMT) detectors and one HyD detector equipped with a 405-nm diode laser, argon (458/488/514 nm) laser, 561-nm diode-pumped solid-state (DPSS) laser, and 633-nm HeNe laser. Sets of images for all the experimental and control conditions in each group (GLAST-prog., NESTIN-prog.) were acquired on the same day with the same excitation power and gain settings. Some of the images were acquired using a Zeiss Axio Zoom.V16 with 405/488/568/647-nm filters and Lumencor’s SOLA SE 365 light engine with ~3.5-W white light output through a 3-mm-diameter liquid light guide (LLG) with a PlanNeoFluar Z ×2.3 objective with 0.57-N.A. lens. The Axio Zoom was equipped with a sCMOS pco.edge 4.2 camera with CIS2020A sensor. All the images were further processed in the ImageJ-Fiji package (version 1.51w, NIH; RRID:SRC_003070). For manual cell counting and distance-to-pia measurement, images were converted to black for EGFP and white background, pia was oriented as a horizontal plane, and cells were counted with the cell counter plugin in Fiji by R. U. Goz. Soma size was measured with a freehand selection tool and measured under the same brightness/contrast and color balance settings in all conditions. Balloon-like cells and aggregates were scanned and counted with Zeiss Axio Zoom.V16. Image processing for publication was done in Fiji and Corel Draw Graphics Suite X8 (Corel, Ottawa, Canada; RRID:SCR_002865).
To assess the possible reprogramming of pyramidal neuron progenitors to fast parvalbumin (PV) or somatostatin (SST) spiking interneuron identities, we transfected cells with BRAFV600E transgenes into PV-Cre:Ai14 or SST-Cre:Ai14 male mice crossed with CD1 females. In utero electroporation was applied to the embryos in these interneuron lines at E14–E15 with GLAST-PBase, BRAFV600E, and EGFP. The brain tissue from the offspring was dissected at P21–P22 after being perfuse-fixed with 4% PFA. The tissue was sectioned at 60-µm thickness and mounted on glass slides to be imaged with a Zeiss SP8 confocal microscope. A total of 120 EGFP+ neurons in PV-Cre:Ai14 tissue and 42 EGFP+ neurons in SST-Cre:Ai14 tissue were screened to test for EGFP-tdTomato colocalization.
Unsupervised hierarchical clustering analysis.
Hierarchical clustering was done with Gene Cluster 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm) (Eisen et al. 1998). We have used 20 recorded electrophysiological parameters, for which each observation was standardized by centering to the mean and dividing by standard deviation. The missing values were imputed by the Nonlinear Iterative Partial Least Squares (NIPALS) algorithm in the XLSTAT (https://www.xlstat.com/en/) Microsoft Excel (Microsoft, Redmond, WA) add-on (Wold 1973a; Wold 1973b). The heat map visualization was done in GenePattern (https://genepattern.broadinstitute.org/; RRID:SCR_003201; Reich et al. 2006) with HierarchicalClusteringViewer module v.11.3.
Statistical analysis.
All data measurements were kept in Excel (Microsoft, Redmond, WA) and in Origin (OriginLab, Northampton, MA; RRID:SCR_002815). All the data were analyzed in SPSS v.24 (RRID:SCR_002865; IBM Corporation 2016) for large samples, one-way analysis of variance (ANOVA) with Tukey post hoc correction was used, and when the samples had nonhomogeneous variance (significant Levene’s test for equality of variance), Welch’s test with Games–Howell post hoc correction was used. For small samples from different observations, independent-samples two-tailed Student’s t test was used, and depending on Levene’s test significance, the t statistics for equal or unequal variance are reported. For measurements coming from the same neurons before and after treatment, paired-samples two-tailed Student’s t test was used. Graphical visualization of data was prepared in Origin and exported to Corel Draw Graphic Suite X8 for further processing. Arithmetical averages and SEs are reported for all results unless otherwise specified.
RESULTS
BRAFV600E increases firing frequencies in pyramidal neurons.
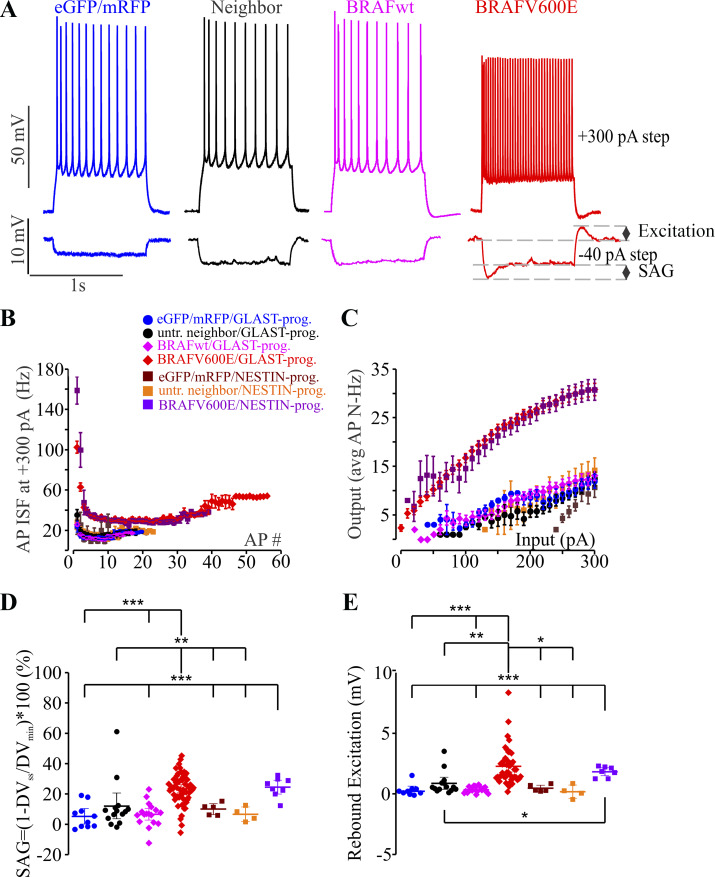
To test whether BRAFV600E is sufficient to alter cortical pyramidal neuron physiology, we introduced a BRAFV600E transgene or fluorescent protein-transfected control (control-FP) transgenes (mRFP) into populations of neocortical progenitors using the binary piggyBac transposon system (Cary et al. 1989; Ding et al. 2005; Siddiqi et al. 2014; Wu et al. 2006). We directed transgene integration into either a population of GLAST-prog. neural progenitors that generates both pyramidal neurons and astrocytes (Anthony and Heintz 2008; Siddiqi et al. 2014) or into the NESTIN-prog. population that generates primarily pyramidal neurons by expressing piggyBac transpose in either of those two respective populations (reviewed in Götz 2003; Kriegstein and Götz 2003; Pinto and Götz 2007). We performed whole cell patch-clamp recording from pyramidal neurons in upper layers 2/3. We recorded from neurons in all five separate transgene conditions (GLAST-prog. BRAFV600E, GLAST-prog. BRAFwt, GLAST-prog. control-FP, NESTIN-prog. BRAFV600E, and NESTIN-prog. control-FP) and in both transfected and neighboring neurons not positive for fluorescent markers of transgenesis. In current-clamp recordings, we found that BRAFV600E neurons displayed significantly higher action potential (AP) firing frequencies to 1-s depolarizing current pulses (Fig. 1A, top, and Fig. 1C; P < 0.001 for current steps from 20 to 300 pA; Supplemental Table S1; all Supplemental material is available at http://doi.org/10.17605/OSF.IO/NRWT2). This significantly increased firing rate was true for neurons from both the NESTIN+ and GLAST+ progenitor populations. Neither BRAFwt nor neighboring untransfected neurons in BRAFV600E conditions showed elevated firing frequencies above fluorescent protein-transfected controls (control-FP). Instantaneous AP frequency (ISF) measured at +300-pA, 1-s current step was significantly higher in BRAFV600E neurons (Fig. 1B). In addition, in 4 of 59 GLAST-prog. BRAFV600E neurons and in 1 of 9 NESTIN-prog. BRAFV600E neurons, we observed an unusual bursting pattern and post-action potential depolarization waves that were not observed in any of the non-BRAFV600E conditions.
Fig. 1.
BRAFV600E-expressing pyramidal neurons are hyperexcitable. A: representative traces of voltage recordings from 4 pyramidal neurons in parietal somatosensory cortex in 4 different conditions. Each neuron is shown responding to 300-pA depolarizing current injection and −40-pA, 1-s hyperpolarizing current injection. Representative traces show the representative phenotypic differences between neurons expressing monomeric red fluorescent protein (mRFP) and/or enhanced green fluorescent protein (eGFP) alone (blue), untransfected cells neighboring BRAFV600E-transfected cells (neighbor; black), a cell expressing wild-type BRAF (BRAFwt; magenta), and BRAFV600E-transfected neurons (red). B: instantaneous frequency (ISF) of action potentials (APs) in the train at +300-pA, 1-s depolarizing current steps was significantly higher in GLAST-prog. BRAFV600E-transfected neurons compared with all other conditions. One-way ANOVA (F6,93–104 = 7.7–28.06, P = 1.0122E-19 to 0.045424; see Supplemental Table S1 at http://doi.org/10.17605/OSF.IO/NRWT2 for more extended statistical comparisons) with Tukey post hoc correction, Welch’s test with Games–Howell post hoc correction, or independent-samples equal variance 2-tailed t tests were used. For instance, the ISF was significantly higher in GLAST-prog. (n = 64) and NESTIN-prog. (n = 8) BRAFV600E neurons compared with untransfected neighbors (n = 7) for the first 3 action potentials in the train (Welch’s test: t6,16.779–18.594 = 14.113–33.123, P = 5E-06 to 2.11E-08 with Games–Howell post hoc correction, P = 1E-06 to 2.01E-07). C: input-output curve shows more than 2 times higher AP firing frequency in GLAST-prog. BRAFV600E-transfected neurons (n = 48) and NESTIN-prog. BRAFV600E transfected neurons (n = 7) compared with all other conditions: GLAST-prog. untransfected neighbor (n = 8), GLAST-prog. eGFP/mRFP (n = 11), GLAST-prog. BRAFwt (n = 13), NESTIN-prog. eGFP/mRFP (n = 3), and NESTIN-prog. untransfected neighbor (n = 6). One-way ANOVA (F6,89 = 33.064–40.874, P = 9.49E-24 to 4.94E-02) with Tukey post hoc correction was used (see Supplemental Table S1 for more extended statistical comparisons). D: sag ratio (SAG) to hyperpolarization is significantly larger in BRAFV600E neurons (n = 57). One-way ANOVA with Tukey’s post hoc tests (F6,108 = 11.46, P = 6.93E-10) was used. **P < 0.01; ***P < 0.001, Tukey post hoc tests (see Supplemental Table S1 for more extended statistical comparisons). E: rebound excitation was significantly larger in BRAFV600E neurons (n = 42). Welch’s test (t6,18.867 = 18.345, P = 6.01E-7) was used to test for significance overall with Games–Howell post hoc correction, and independent-samples 2-tailed t tests were used for statistical comparisons between groups with small sample size. *P < 0.05; **P < 0.01; ***P < 0.001, Tukey or Games–Howell post hoc tests or independent-samples 2-tailed t test. Extended statistical information and comparisons are provided in Supplemental Table S1. AP#, no. of action potentials; AP N, action potential firing frequency; DVmin, minimal initial voltage deflection; DVss, change in stable-state voltage; untr., untransfected.
BRAFV600E increases hyperpolarization induced “sag.”
In response to hyperpolarizing current pulses in whole cell current-clamp mode, BRAFV600E neurons in either GLAST-prog. or NESTIN-prog. condition displayed an initial deflection, or sag, that was absent in untransfected neighbor neurons, control-FP neurons, and BRAFwt neurons (Fig. 1A, bottom, and Fig. 1D; Supplemental Table S1). Sag ratio was calculated as , where VRMP is resting membrane potential, Vss is stable-state voltage in the last 100 ms of a 1-s, −40-pA pulse, and Vmin is minimal initial voltage deflection in response to a 1-s, −40-pA pulse. GLAST-prog. BRAFV600E neurons (n = 57) had average SAG of 23.41 ± 1.33% that was significantly larger than in their untransfected neighbor neurons (4.46 ± 1.48%, n = 20, P = 0.004584), in GLAST-prog. control-FP neurons (6.82 ± 1.86%, n = 15, P = 0.000016), and in GLAST-prog. BRAFwt neurons (6.59 ± 1.94%, n = 17, P = 5.2408E-07; Fig. 1D; Supplemental Table S1). In NESTIN-prog. BRAFV600E neurons, average SAG was 24.48 ± 2.27% (n = 8), and it was significantly increased compared with all non-BRAFV600E conditions (Fig. 1D; Supplemental Table S1); in NESTIN-prog. control-FP neurons, the average SAG was 10.03 ± 1.77% (n = 5, independent-samples equal variance 2-tailed t11 = 4.48, P = 0.000931), and in NESTIN-prog. untransfected neighbor neurons, the average SAG was 6.59 ± 2.41% (n = 4, independent-samples equal variance 2-tailed t10 = 4.873, P = 0.000649). Average rebound excitation measured as an overshoot above RMP at the end of 1-s, −40-pA current steps was also larger in GLAST-prog. BRAFV600E neurons (2.28 ± 0.24 mV, n = 42) compared with that in their untransfected neighbor neurons (0.69 ± 0.18 mV, n = 22, P = 0.003797), in GLAST-prog. control-FP neurons (0.37 ± 0.11 mV, n = 14, P = 5.2781E-07), in GLAST-prog. BRAFwt neurons (0.34 ± 0.06 mV, n = 17, P = 8.8697E-09), in NESTIN-prog. untransfected neighbor neurons (0.18 ± 0.27 mV, n = 4, independent-samples equal variance 2-tailed t44 = 2.685, P = 0.010185), and in NESTIN-prog. control-FP neurons (0.47 ± 0.13 mV, n = 5, independent-samples equal variance 2-tailed t45 = 2.596, P = 0.012697). In NESTIN-prog. BRAFV600E, rebound excitation was increased (1.82 ± 0.17 mV, n = 7) compared with non-BRAFV600E conditions (GLAST-prog. BRAFwt: n = 17, independent-samples equal variance 2-tailed t22 = 10.448, P = 5.41E-10; GLAST-prog. untransfected neighbor: n = 14, independent-samples equal variance 2-tailed t19 = 2.543, P = 0.019831; GLAST-prog. control-FP: n = 9, independent-samples equal variance 2-tailed t14 = 6.439, P = 0.000016; NESTIN-prog. untransfected neighbor: n = 4, independent-samples equal variance 2-tailed t9 = 5.514, P = 0.000373; NESTIN-prog. control-FP: n = 5, independent-samples equal variance 2-tailed t10 = 6.035, P = 0.000126; Fig. 1E and Supplemental Table S1). In 20.34% of GLAST-prog. BRAFV600E neurons (12/59) and in 11.11% of NESTIN-prog. BRAFV600E neurons (1/9), rebound excitation resulted in AP firing. The BRAFV600E neurons that fired rebound action potentials were excluded from rebound depolarization statistical comparisons shown in Fig. 1E.
BRAFV600E delays neuronal migration to the appropriate layers and does not respecify neurons to fast-spiking interneurons.
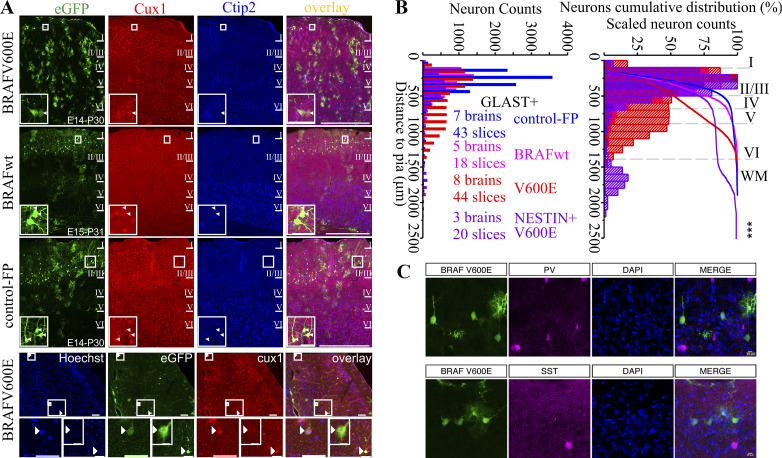
Given the large differences in cellular physiology between unaffected pyramidal neurons and BRAFV600E-expressing pyramidal neurons, we also examined the position and fates of neurons expressing BRAFV600E. We imaged and quantified the position of transfected neurons in each condition (Fig. 2, A and B; Supplementary Table S1), and we found that BRAFV600E expression resulted in a significant displacement of neuronal position, significantly shifting approximately half of neurons to deeper cortical layers (Fig. 2B). We next tested whether the neurons in upper layers, in the position we recorded neurons from in this study, continued to express appropriate upper layer cortical markers. We found that BRAFV600E-expressing neurons expressed the upper layer pyramidal neuron marker Cux1 and failed to express the deeper layer marker Ctip2 (Ferrere et al. 2006) (Fig. 2A). In addition, BRAFV600E, but not BRAFwt, caused an increase in astrocytes in GLAST+ progenitors (Fig. 2A). Astrocyte number increases were not observed in the NESTIN+ progenitor population. Given the high action potential firing rates of the BRAFV600E neurons, we investigated whether there was a possible respecification to interneuron fates in BRAFV600E-expressing neurons. We crossed PV-Cre:Ai14 and SST-Cre:Ai14 interneuron-specific reporter lines with CD1 female mice and targeted radial glia cells of the offspring with BRAFV600E-carrying vectors. Of 120 BRAFV600E-transfected neurons in PV-Cre:Ai14xCD1 tissue and 42 transfected neurons in SST-Cre:Ai14xCD1 tissue, we detected no colocalization of tdTomato signal (Fig. 2C). Taken together with the Cux1 positivity results, we conclude the high firing rates induced by BRAFV600E are not due to a respecification to fast-firing interneuron identities but that BRAFV600E does cause a partial disruption in the migration of pyramidal neurons destined for upper layers (Fig. 2B).
Fig. 2.
Most BRAFV600E neurons migrate to appropriate superficial cortical layers, express pyramidal neuron makers, and do not express interneuron markers. A: images of representative somatosensory cortices containing cells transfected with BRAFV600E, wild-type BRAF (BRAFwt), and enhanced green fluorescent protein (eGFP) transgenes (control-FP). Cux1, an upper cortical layer marker, is expressed by cells in all transfected conditions in superficial cells and in cells ectopically located in deeper layers (bottom row), while Ctip2, a deeper layer marker, is not expressed. Scale bars for A: 500 µm, 50 µm, and 10 µm. B: total neuron counts plotted by their distance from pia (left) and as a percentage of total neurons from the pia (right) indicate a significant proportion of BRAFV600E transgene-containing neurons become positioned abnormally in deeper cortical layers relative to control transfections and BRAFwt. Both GLAST-prog. and NESTIN-prog. progenitor-generated neurons are positioned at a greater distance from the pia than eGFP- or BRAFwt-expressing neurons (Welch’s test: t3,9803.913 = 970.974, P = 0.00E+00). There was also a significant difference in neuronal distance to pia between GLAST and NESTIN transgene conditions such that the population of neurons places in the NESTIN transgene condition (purple lines and bars) were positioned deeper in the cortical lamina, more distant from pia, than the displaced GLAST population (red lines and bars). Extended statistical information and comparisons are provided in Supplemental Table S1 (see http://doi.org/10.17605/OSF.IO/NRWT2). C: images of td-Tomato-labeled interneurons and eGFP-transfected neurons in PV-Cre:Ai14 and SST-Cre:Ai14 mouse lines with BRAFV600E neurons labeled with eGFP. Note that none of the eGFP-positive neurons are positive for either parvalbumin (PV) or somatostatin (SST) reporters. No td-Tomato signal was observed in any of 120 BRAFV600E-transfected neurons in PV-Cre:Ai14xCD1 tissue and none in 42 BRAFV600E-transfected neurons in SST-Cre:Ai14xCD1 tissue.
BRAFV600E alters single-action potential properties, resting membrane potential, and input resistance.
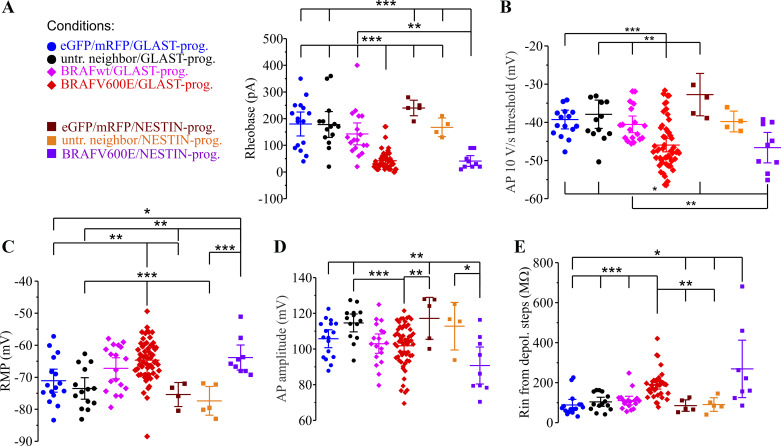
In addition to a significantly higher AP frequency, BRAFV600E neurons also had a lower rheobase, the current threshold to AP firing or the minimal depolarizing current step required to elicit first AP (n = 49; Fig. 3A, Supplemental Table S1), and a lower voltage threshold to fire action potentials measured at the voltage at which voltage change with respect to time (dV/dt) increased to either 10 V/s or 50 V/s (Fig. 3B; Supplemental Table S1). Differences were also observed in AP amplitude (Fig. 3D; Supplemental Table S1). Passive membrane properties were also significantly different in BRAFV600E neurons, with the resting membrane potential (RMP) more depolarized in BRAFV600E neurons (n = 62, −64.91 ± 0.76 mV) compared with untransfected neighbor neurons (n = 14, −73.33 ± 1.55 mV, P = 0.000139; Fig. 3C; Supplemental Table S1). Input resistances measured to either hyperpolarizing or subthreshold depolarizing current steps were also significantly increased in BRAFV600E neurons (n = 38) compared with all other conditions (Fig. 3E; Supplemental Table S1). The same pattern of changes was observed when BRAFV600E was introduced in either GLAST+ progenitors or NESTIN+ progenitors. The significantly elevated resting membrane potential in BRAFV600E neurons could not explain the increased firing rates, since untransfected neighboring neurons (n = 5) depolarized to −60 mV did not achieve AP firing frequencies similar to BRAFV600E neurons, and hyperpolarization of BRAFV600E neurons to more negative resting membrane potentials did not eliminate the high firing frequencies they achieved without injecting a background hyperpolarizing current (n = 14, average RMP = −70.66 ± 0.58 mV).
Fig. 3.
Action potential and membrane properties are significantly altered by BRAFV600E. A: the minimal depolarizing current required to induce an action potential (rheobase) is decreased by BRAFV600E. Both NESTIN progenitor (dark purple symbols)- and GLAST progenitor (red symbols)-induced BRAFV600E transgenes caused decreased rheobase in neurons. Due to nonhomogeneous variance, Levene’s test (W6,108 = 4.95, P = 0.000164) and Welch’s test (t6,20.31 = 36.75, P = 7.16E-10) with Games–Howell post hoc correction were used to compare rheobase between experimental conditions. **P < 0.01; ***P < 0.001, Tukey post hoc or independent-samples 2-tailed t test). Extended statistical information and comparisons are provided in Supplemental Table S1 (see http://doi.org/10.17605/OSF.IO/NRWT2). B: voltage threshold for action potentials is decreased by BRAFV600E at the 10 V/s and 50 V/s voltage inflection point. One-way ANOVA for 10V/s (F6,108 = 6.532, P = 7.00E-06) with Tukey post hoc correction was used for statistical comparison. *P < 0.05; **P < 0.01; ***P < 0.001, Tukey post hoc or independent-samples t test. Extended statistical information and comparisons are provided in Supplemental Table S1. C: resting membrane potential (RMP) is elevated in GLAST BRAFV600E and NESTIN BRAFV600E progenitors. One-way ANOVA (F6,121 = 8.51, P = 1.03E-07) with Tukey post hoc correction test was used for statistical comparison. **P < 0.01; ***P < 0.001, Tukey post hoc or independent-samples 2-tailed t test. Extended statistical information and comparisons are provided in Supplemental Table S1. D: AP amplitude measured from RMP was smaller in BRAFV600E neurons (n = 59) relative to untransfected neighboring neurons (n = 14) or to BRAFwt-expressing neurons (n = 18). One-way ANOVA (F6,119 = 5.64, P = 3.50E-05) was used. *P < 0.05;**P < 0.01; ***P < 0.001, Tukey post hoc or independent-samples 2-tailed t test. Extended statistical information and comparisons are provided in Supplemental Table S1. E: input resistance (Rin) was elevated in BRAFV600E neurons (red and purple symbols). Due to nonhomogeneous variance, Levene’s test (W6,97 = 6.49, P = 0.000009) and Welch’s test (t6,23.20 = 8.999, P = 3.90E-05) with Games–Howell post hoc correction were used for input resistance comparisons. *P < 0.05; **P < 0.01; ***P < 0.001, Games–Howell post hoc or independent-samples 2-tailed t test. Error bars are ±2 SE. Extended statistical information and comparisons are provided in Supplemental Table S1. depol., Depolarization; eGFP, enhanced green fluorescent protein; mRFP, monomeric red fluorescent protein; prog., progenitor; untr., untransfected.
BRAFV600E decreases peak delayed rectifier potassium currents but not current density.
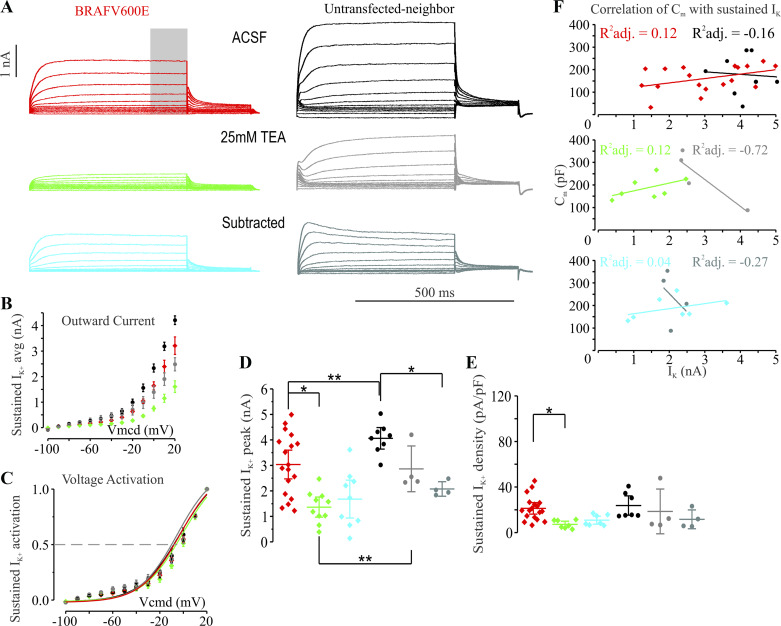
The significant decrease in action potential accommodation and the ability of BRAFV600E neurons to sustain high firing frequencies across 1 s of depolarizing current stimulation suggested the possibility that delayed rectifier potassium currents may be decreased in BRAFV600E-expressing neurons. We therefore performed whole cell voltage clamp in GLAST-prog. BRAFV600E neurons and their untransfected neighbors in conditions that isolate potassium currents from Na+ and Ca2+ currents (Fig. 4A). Potassium currents were recorded in the absence and presence of 25 mM TEA to determine the contribution of TEA-sensitive currents. Peak currents were measured in the last 100 ms of 500-ms depolarizing voltage pulses across a range of voltage steps. The voltage dependence of activation for the sustained current was not significantly different between BRAFV600E and neighboring neurons (Fig. 4C; Supplemental Table S1). BRAFV600E neurons (n = 18) did show significantly reduced peak sustained outward currents (n = 8, independent-samples unequal variance 2-tailed t23.504 = 2.918, P = 0.007637; Fig. 4, B and D; Supplemental Table S1); however, current density was not significantly different compared with untransfected neighboring pyramidal neurons (Fig. 4E; Supplemental Table S1). As the current density measurement is a normalization of currents to capacitance, and capacitance is an estimate of cell surface area, often inaccurate for cells with complex morphologies, we tested for whether within each group there was a significant correlation between peak current and capacitance (Fig. 4F; Supplemental Table S1). For the current density normalization to be a correct assumption for adjusting for cell size, there should be within each group a correlation between capacitance and peak sustained current. In contrast, the correlation of capacitance with peak sustained potassium currents was weak and statistically not significant for cells within each groups and for cells across groups, suggesting that the normalization for current density may not be an accurate normalization for cell size for sustained potassium currents in the pyramidal neurons we recorded from (Fig. 4F; Supplemental Table S1). If BRAFV600E neurons were variably increased or decreased in soma or dendrite surface area and had decreased potassium channel function in a low-surface area region of the cell such as the axon initial segment, then this could explain a significant decrease in current amplitude without a decrease in apparent current density. To test whether an increase in delayed rectifier type currents could slow action potential firing frequencies in BRAFV600E neurons, we treated several BRAFV600E neurons with 10 µM retigabine, an allosteric potentiator of KCNQ2 potassium channels. Consistent with known effects on KCNQ2 channels (Gu et al. 2005; Lawrence et al. 2006), retigabine decreased action potential firing frequencies by 30% in BRAFV600E neurons (n = 6). Retigabine, however, failed to fully return BRAFV600E neurons to firing frequencies approaching that of control pyramidal neurons, suggesting that changes in conductances may also contribute to the increased firing frequency of BRAFV600E neurons. We also found that the 25 mM TEA-sensitive component (difference current with and without TEA) of the delayed potassium current was not significantly decreased in BRAFV600E neurons relative to controls (Fig. 4D; Supplemental Table S1), suggesting further that a TEA-insensitive conductance may be responsible for the decreased outward current amplitude in BRAFV600E neurons.
Fig. 4.
Sustained potassium currents are decreased in GLAST-prog. BRAFV600E neurons compared with their untransfected neighbors. A, left: representative traces of potassium currents in GLAST-prog. BRAFV600E neuron recorded in the presence of 3 mM 4-aminopyridine, 1 µM TTX, 10 µM 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX), 50 µM d-aminophosphonovaleric acid, 10 µM SR-95531, 50 µM ZD-7288, and 1 mM Co2+ substitution for Ca2+ (5 min in, holding voltage is −80 mV, holding current −32.41 pA) with whole cell capacitance compensated (top; ACSF with inhibitor cocktail without TEA; red), where gray bar indicates the region where the measurement was made in all conditions; middle shows traces of the same neuron 9 min after application of 25 mM tetraethylammonium chloride (TEA; green) with previous inhibitor cocktail (holding current −40.25 pA); bottom shows subtracted traces before and after 25 mM TEA with voltage-step protocol (subtracted; cyan). Right: representative traces of potassium currents in GLAST-prog. untransfected neighbors recorded in the presence of the same inhibitor cocktail as at left (6 min in, holding voltage is −80 mV, holding current is −48.04 pA) with whole cell capacitance compensated (top); middle shows traces from the same neuron 9 min after application of 25 mM TEA with previous inhibitor cocktail (holding current −71.67 pA); bottom shows subtracted traces before and after 25 mM TEA. B: average sustained potassium current (IK) activation curve showing decreased peak at all tested voltages in BRAFV600E neurons relative to untransfected neighbors. C: activation curves for the delayed sustained outward currents did not differ for BRAFV600E and untransfected neighboring neurons. D: comparison of maximal sustained outward currents measured at +20-mV voltage step show significant decreases compared with their untransfected neighbors, while the TEA subtracted currents do not show a significant decrease. *P < 0.05; **P < 0.01, either paired-samples or independent-samples 2-tailed t test. Extended statistical information and comparisons are provided in Supplemental Table S1 (see http://doi.org/10.17605/OSF.IO/NRWT2). E: current density was not significantly different between BRAFV600E neurons (n = 18) and untransfected neighbors (n = 7; t23 = 0.509, P = 0.615643). Extended statistical information and comparisons are provided in Supplemental Table S1. F: correlation plots of capacitance (Cm) with sustained potassium currents measured before and after application of 25 mM TEA show no significant correlation of the two values in any of the transfection conditions. R2 adjusted for the variance in each group (reflect R2 divided by degrees of freedom). One-way ANOVA for residuals of the adjusted R2 (R2adj.) for GLAST-prog. BRAFV600E: F = 3.21, P = 0.09; for GLAST-prog. BRAFV600E after 25 mM TEA: F = 1.8, P = 0.24; for GLAST-prog. BRAFV600E subtracted: F = 1.22, P = 0.32; for GLAST-prog. untransfected neighbor: F = 0.03, P = 0.86; for GLAST-prog. untransfected neighbor after 25 mM TEA: F = 8.58, P = 0.1; for GLAST-prog. untransfected neighbor subtracted: F = 0.36, P = 0.61. Extended statistical information and comparisons are provided in Supplemental Table S1. ACSF, artificial cerebrospinal fluid.
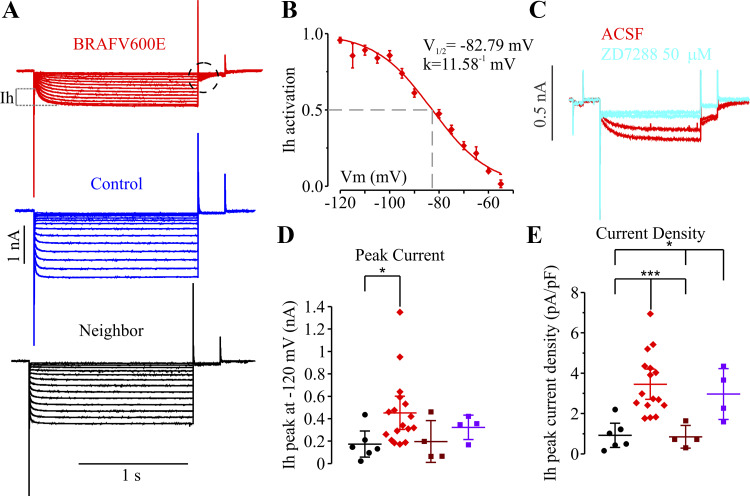
Elevated Ih in BRAFV600E neurons.
Increases in SAG ratio and rebound excitation have been previously shown to be associated with increases in the hyperpolarization-activated current Ih (Guan et al. 2015; Hawkins et al. 2015; Jung et al. 2010; Schridde et al. 2006; Timofeev et al. 2002). To test whether BRAFV600E-expressing neurons have increased Ih, we recorded cells in whole cell voltage-clamp configuration to measure currents during hyperpolarizing steps in neurons with BRAFV600E transgenes, BRAFwt, control neurons expressing only fluorescent proteins, or untransfected neighboring neurons (Fig. 5A). We found that BRAFV600E neurons had prominent Ih. Ih in control layer 2/3 pyramidal neurons, in contrast, were small or undetectable. The currents in BRAFV600E were blocked by application of 50 µM ZD-7288, an Ih inhibitor (Fig. 5C; Supplemental Table S1), and ivabradine (10 μM), and the currents showed voltage-dependent properties consistent with Ih with a half-activation voltage (V1/2) of −82.79 mV and a slope factor k = 11.58−1 mV (Fig. 5B). Both Ih peak currents and Ih current densities were significantly increased in BRAFV600E neurons (n = 17) compared with neighboring untransfected neurons, neurons expressing fluorescent proteins alone, or neurons expressing BRAF wildtype transgenes (Fig. 5, D and E; Supplemental Table S1). We next tested whether the increase in Ih was causally related to the increased firing frequency by treating BRAFV600E neurons with either of two blockers of Ih, ZD-7288 or ivabradine(10 µM). We found that both ZD-7288 (50 µM) and ivabradine (10 µM) blocked the Ih-mediated voltage SAG responses to hyperpolarizing currents, but failed to decrease elevated AP firing frequencies in BRAFV600E neurons (ivabradine: SAG ratio without drug, 32 ± 2.7%, n = 7, and with ivabradine, 10 ± 1.7%, n = 7, P < 0.0001, t test; AP frequency at 300-pA current step: without ivabradine, 26 ± 2.7 Hz, n = 7, and with ivabradine, 23 ± 1.5 Hz, n = 7, P = 0.27; ZD7288: SAG ratio without drug, 28.35 ± 4.94%, n = 5, and with ZD-7288, 5.45 ± 1.9%, n = 5, paired-samples t4 = 4.89, P = 0.008103); AP frequency at 300-pA current step: without ZD-7288, 30.6 ± 2.1, n = 5, and with ZD-7288, 33.0 ± 3.0, n = 2, P = 0.56). The BRAFV600E-induced increase in Ih therefore cannot easily explain the increased AP firing frequency and suggests at least two mechanistically independent electrophysiological changes.
Fig. 5.
Hyperpolarization-activated depolarizing current (Ih) recorded in whole cell voltage-clamp configuration is increased in BRAFV600E-expressing cortical neurons. A: representative traces of currents in response to hyperpolarizing voltage-step protocol. B: Ih activation curve from the voltage-step protocol shown in A (tail currents, dashed circle) with maximal activation around −120 mV, half-activation voltage (V1/2) of −82.79 mV, and slope factor k of 11.58−1 mV, which are averaged and fit with Boltzmann curve (n = 18). C: representative traces from a single cell in whole cell voltage-clamp configuration. Application of 50 µM ZD-7288, a known Ih inhibitor, in perfusion system for 5 min blocked Ih. D: Ih peak current measured as shown in A. The significant increase was only found in GLAST-prog. BRAFV600E neurons (n = 17) compared with their untransfected neighbors (n = 6; t = 2.117, P = 0.046). E: Ih peak current density was increased in GLAST-prog. BRAFV600E neurons (n = 16) compared with their untransfected neighbors (n = 6; t20 = 3.918, P = 0.000852) and NESTIN-prog. eGFP/mRFP (n = 4; t14.08 = 5.546, P = 0.000071); it was also significantly increased in NESTIN-prog. BRAFV600E neurons (n = 4) compared with GLAST-prog. untransfected neighbors (n = 6; t8 = 3.275, P = 0.011276) and NESTIN-prog. ; enhanced green fluorescent protein/monomeric red fluorescent protein (eGFP/mRFP; n = 4; t4.16 = 3.066, P = 0.035546). *P < 0.05; **P < 0.01; ***P < 0.001, independent-samples 2-tailed t test. Extended statistical information and comparisons are provided in Supplemental Table S1 (see http://doi.org/10.17605/OSF.IO/NRWT2). Error bars are ±2 SE. ACSF, artificial cerebrospinal fluid; Vm, membrane potential.
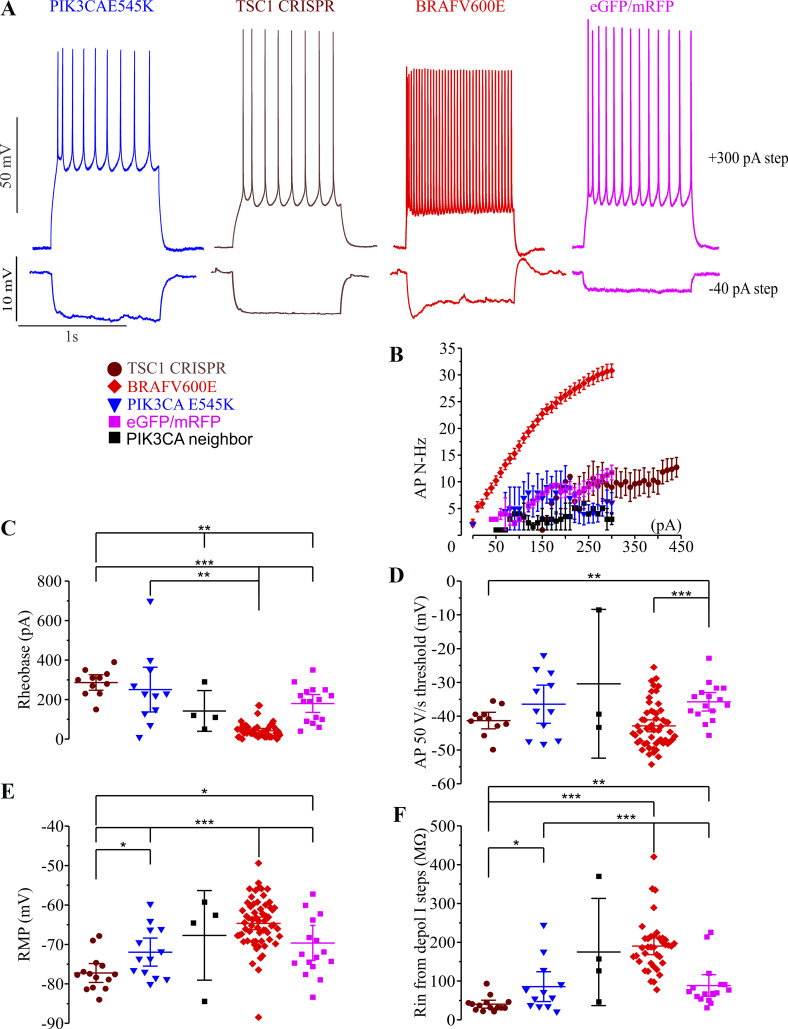
Somatic mutations associated with focal cortical dysplasia confer a different neurophysiological phenotype than BRAFV600E.
To compare the effects of BRAFV600E with those of somatic mutations identified in focal cortical dysplasia and shown in mice to result in spontaneous seizures, we either introduced PIK3CAE545K transgenes (Roy et al. 2015) into cortical progenitors or created somatic TSC1 cellular knockouts by CRISPR-Cas9 of TSC1 as done previously (Lim et al. 2015). GLAST+ progenitors were transfected with PIK3CAE545K and piggyBac transposase plasmids at E14–E15 in the same way as BRAFV600E was introduced, and we used the CRISPR-Cas9 TSC1 gRNA described previously by Lim et al. 2017. We assessed and compared the major cellular phenotypes we previously identified for BRAFV600E between the three conditions (Fig. 6A). AP instantaneous frequency to depolarizing stimuli, AP firing frequencies (Fig. 6B; Supplemental Table S1), AP thresholds (Fig. 6D; Supplemental Table S1), resting membrane potential (Fig. 6E; Supplemental Table S1), and input resistance (Fig. 6F; Supplemental Table S1) were all significantly different between BRAFV600E and either TSC1 CRISPR KD or PIK3CAE545K. These results indicate that alterations in neuronal electrophysiological properties in neocortical pyramidal neurons are affected differently by pathological mutations in the mTOR pathway compared with BRAFV600E, which primarily activates the MAPK/ERK pathway.
Fig. 6.
Mammalian target of rapamycin (mTOR) pathway mutations result in different pyramidal neuron phenotypes than BRAFV600E. A: representative traces from BRAFV600E (red), PIK3CAE545K (blue), CRISPR knockdown (KD) of TSC1 (brown), and control fluorescent protein-transfected neurons (eGFP/mRFP, enhanced green fluorescent protein/monomeric red fluorescent protein; light purple) showing action potential (AP) firing at +300 pA (upper panel), and response to membrane hyperpolarization by −40 pA. B: average AP firing frequency in all 3 conditions, GLAST-prog. BRAFV600E (n = 49), CRISPR TSC1 KD (n = 11), and GLAST-prog. PIK3CAE545K (n = 11) and in GLAST-prog. eGFP/mRFP (n = 16) and GLAST-prog. PIK3CA E545K untransfected neighbors (n = 4). C: rheobase for all 3 conditions was compared using Welch’s test (t5,17.44 = 36.896, P = 1.11E-08) due to nonhomogeneous variance (Levene’s test: W5,96 = 7.68, P = 4E-06) together with Games–Howell post hoc correction. Rheobase was lower in GLAST-prog. BRAFV600E (n = 49) compared with CRISPR TSC1 KD (n = 11, independent-samples unequal variance 2-tailed t = 11.89, P = 8.3037E-08), GLAST-prog. PIK3CAE545K (n = 11, independent-samples unequal variance 2-tailed t = 3.68, P = 0.004157), and GLAST-prog. eGFP/mRFP (n = 16, independent-samples unequal variance 2-tailed t = 5.95, P = 0.000017). It was significantly higher in CRISPR TSC1 KD (n = 11) compared with GLAST-prog. eGFP/mRFP (n = 16, independent-samples equal variance 2-tailed t = 3.34, P = 0.002604); it was also higher in CRISPR TSC1 KD (n = 11) compared with GLAST-prog. PIK3CA neighbor (n = 3, independent-samples equal variance 2-tailed t = 3.24, P = 0.006437). Extended statistical information and comparisons are provided in Supplemental Table S1 (see http://doi.org/10.17605/OSF.IO/NRWT2). D: AP 50 V/s threshold was not different when compared using Welch’s test (t5,15.16 = 7.551, P = 0.000976) together with Games–Howell post hoc correction due to nonhomogeneous variance (Levene’s test: W5,100 = 7.003, P = 0.000012): GLAST-prog. BRAFV600E (n = 54) compared with CRISPR TSC1 KD (n = 11, independent-samples equal variance 2-tailed t63 = 0.749, P = 0.456642), GLAST-prog. BRAFV600E compared with GLAST-prog. PIK3CAE545K (n = 11, P = 0.12; independent-samples 2-tailed unequal variance Student’s t12.088 = 2.17, P = 0.050665). It was lower in CRISPR TSC1 (n = 11) compared with GLAST-prog. eGFP/mRFP (n = 16, independent-samples equal variance 2-tailed Student’s t25 = 2.871, P = 0.008222). It was significantly lower in GLAST-prog. BRAFV600E (n = 54) compared with GLAST-prog. eGFP/mRFP (n = 16, independent-samples equal variance 2-tailed Student’s t68 = 3.9392, P = 0.000195). Extended statistical information and comparisons are provided in Supplemental Table S1. E: resting membrane potential (RMP; recorded before application of current steps) was compared using Student’s t test. GLAST-prog. BRAFV600E (n = 62) had more depolarized RMP compared with CRISPR TSC1 KD (n = 14, independent-samples equal variance 2-tailed t74 = 7.134, P = 5.5272E-10) and with GLAST-prog. PIK3CAE545K (n = 13, independent-samples equal variance 2-tailed t73 = 3.833, P = 0.000266). RMP was significantly more hyperpolarized in CRISPR TSC1 KD (n = 14) compared with GLAST-prog. PIK3CAE545K (n = 13, independent-samples equal variance 2-tailed t25 = 2.487, P = 0.019922). It was significantly more hyperpolarized in CRISPR TSC1 KD (n = 14) compared with GLAST-prog. eGFP/mRFP (n = 16, independent- sample equal variance 2-tailed t28 = 2.763, P = 0.010002). It was significantly more depolarized in GLAST-prog. BRAFV600E (n = 62) compared with GLAST-prog. eGFP/mRFP (n = 16, independent-samples equal variance 2-tailed t76 = 3.56, P = 0.000643). Extended statistical information and comparisons are provided in Supplemental Table S1. F: input resistance (Rin) from depolarizing current (depol. I) steps (due to hyperpolarization-activated depolarizing current activation in BRAFV600E) was compared using Student’s t test. GLAST-prog. BRAFV600E (n = 38) had higher Rin compared with CRISPR TSC1 KD (n = 14, independent-samples unequal variance 2-tailed t48.252 = 12.33, P = 1.6158E-16) and with GLAST-prog. PIK3CAE545K (n = 12, independent-samples equal variance 2-tailed t48 = 4.66, P = 0.000025). Input resistance was higher in GLAST-prog. BRAFV600E cells (n = 38) compared with GLAT-prog. eGFP/mRFP (n = 16, independent-samples equal variance 2-tailed t52 = 5.28, P = 0.000003). CRISPR TSC1 KD (n = 14) had lower input resistance compared with GLAST-prog. PIK3CAE545K (n = 12, independent-samples unequal variance 2-tailed t12.547 = 2.273, P = 0.041326). Input resistance was significantly lower in CRISPR TSC1 KD (n = 14) compared with GLAST-prog. eGFP/mRFP (n = 16, independent-samples unequal variance 2-tailed t18.953 = 3.259, P = 0.004138). *P < 0.05; **P < 0.01; ***P < 0.001. Extended statistical information and comparisons are provided in Supplemental Table S1.
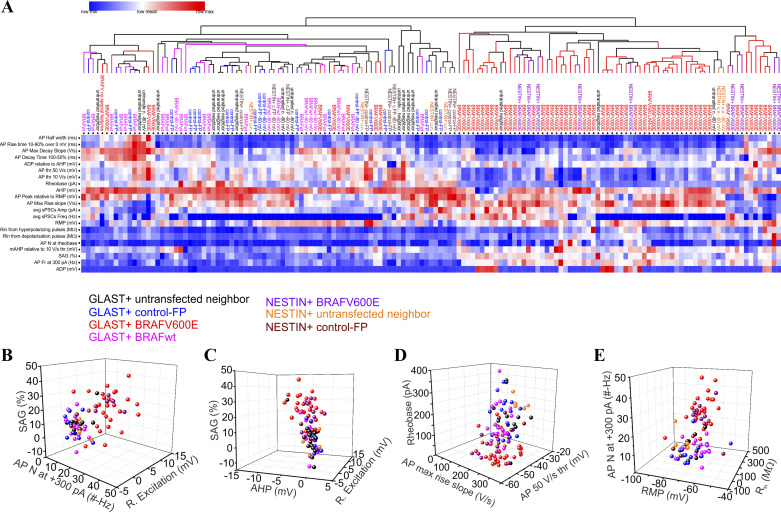
Hierarchical clustering indicates induction of a distinct electrophysiological type of pyramidal neuron.
We used unsupervised hierarchical clustering analysis (HCA) using 20 electrophysiological parameters we assessed in our current-clamp recordings to test whether BRAFV600E induced a distinct and unique physiological type of neuronal physiology. Measurements for each parameter were centered to the mean and divided by the standard deviation. HCA (Gene Pattern Broad Institute, MIT) showed that BRAFV600E-transfected neurons segregated into a major cluster according the electrophysiological properties recorded (Fig. 7A). Principal component analysis (PCA) indicated that the first two principal components accounted for 48.88% of the variability across electrophysiological parameters. The most highly weighted parameters contributing to the first two principle components were AP frequency during 300-pA, 1-s pulses, rheobase AP threshold, AP voltage threshold, hyperpolarization SAG, and maximal rising AP voltage slope (Fig. 7, B–D). The unbiased hierarchical cluster analysis supports the hypothesis that BRAFV600E results in the acquisition of a unique pyramidal neuron phenotype with multiple cellular properties contributing to a hyperexcitable functional state.
Fig. 7.
BRAFV600E causes development of a distinct class of pyramidal neuron physiology. A: unsupervised hierarchical cluster analysis was performed on 20 recorded electrophysiological parameters, showing that most of the BRAFV600E neurons segregate together by electrophysiological parameters recorded. The parameters are action potential (AP) width at 50% height from resting membrane potential (RMP) in ms, AP maximal decay slope in V/s, AP decay time from 100% to 50% height in ms, AP rise time from 10% to 90% height in ms, AP 50 V/s voltage threshold (thr) in mV, AP 10 V/s voltage threshold in mV, rheobase in pA, afterhyperpolarization (AHP) measured at the end of +300-pA, 1-s current step in mV, AP peak relative to RMP in mV, AP maximal rise slope in V/s, average spontaneous postsynaptic current (sPSC) amplitude (Amp) in pA, average sPSC instantaneous frequency (Freq) in Hz, RMP in mV, input resistance (Rin) from hyperpolarizing pulses in MΩ, Rin from depolarizing pulses in MΩ, number of APs (AP N) at rheobase, medium AHP (mAHP) measured relative to AP 10 V/s voltage threshold for rheobase APs in mV, sag ratio (SAG) in %, AP frequency (AP Fr) at +300-pA, 1-s current step in Hz, and rebound excitation measured as an overshoot above RMP (ADP) in mV. B–E: most contributing electrophysiological parameters to the variability in principal component analysis (PCA) shown in 3-dimensional plots. B: SAG is on the z-axis, AP number at +300-pA, 1-s pulse is on the x-axis, and rebound excitation is on the y-axis. C: SAG is on the z-axis, AHP at the end of +300-pA, 1-s pulse is on the x-axis, and rebound excitation is on the y-axis. D: rheobase is on the z-axis, AP maximal rise slope is on the x-axis, and AP 50 V/s voltage threshold is on the y-axis. E: AP number at +300-pA, 1-s pulse is on the z-axis, resting membrane potential (RMP) is on the x-axis, and input resistance from depolarizing current pulses (Rin) is on the y-axis.
DISCUSSION
We have shown that introduction of human BRAFV600E transgenes into either of two types of radial glia progenitors in embryonic mouse neocortex results in the development of pyramidal neurons with a highly hyperexcitable phenotype. Multiple electrophysiological parameters were altered by BRAFV600E, leading to a distinct physiological type. The most prominent of these features was a fast-spiking phenotype that allowed BRAFV600E neurons to reach firing frequencies nearly three times higher than typical layer 2/3 pyramidal neurons. Given this highly unusual spiking phenotype, we assessed whether the BRAFV600E might have changed the fates of pyramidal neuron progenitors to interneuron types. Inconsistent with this possibility, we did not find expression of the interneuron marker GAD1, PV, or SST, but we did observe expression of the layer 2/3 pyramidal neuron transcription factor Cux1 in BRAFV600E neurons. Future experiments will be required to determine when and mechanistically how this coordinated set of electrophysiological properties is established in cortical progenitors and neurons expressing BRAFV600E; however, our current results do indicate that the mechanism is cell autonomous as neighboring neurons are not affected, and it cannot be explained by overactivation of the MTOR pathway, as TSC1 KO and PIK3CAE545K fail to produce the BRAFV600E phenotype.
Our observation of a significant increase in the hyperpolarization-activated inward current (Ih) in BRAFV600E neurons may explain the finding of more depolarized resting membrane potentials in these neurons. Indeed, Ih inhibition with ZD-7288 hyperpolarized membrane potentials in BRAFV600E neurons (data not shown). Elevated Ih currents have generally been associated with decreases in neuronal excitability in layer 5 cortical neurons because of a resulting reduction in input resistance in dendrites, which can lead to a decrease in amplitudes of propagating excitatory postsynaptic potentials (EPSPs) and dampened temporal summation (Magee 1998, 1999; reviewed in Magee 2000). In BRAFV600E layer 2/3 neurons, however, we observed an overall increase in input resistance in addition to an increase in Ih. In this cellular context, Ih is likely to further enhance excitability both by increasing resting membrane potential and by resulting in elevated rebound excitation following hyperpolarizing events, such as large synchronized inhibitory postsynaptic potentials (IPSPs), which can occur during epileptiform activity in circuits (Chang et al. 2018; reviewed in Avoli et al. 2005), to ictal events through postinhibitory rebound excitation.
In addition to increased Ih, BRAFV600E neurons displayed decreased sustained potassium currents, which contribute to action potential adaptation (Gamkrelidze et al. 1998; Mo et al. 2002; Zaika et al. 2006). At this point we do not know the identity of the specific voltage-gated potassium channel(s) that might be decreased in function in BRAFV600E neurons; however, we did test whether enhancing activation of KCNQ-type potassium channels Kv7.2, Kv7.3, or Kv7.4 with retigabine would decrease the frequency of BRAFV600E action potentials by applying retigabine.
We found that BRAFV600E transforms pyramidal neuron physiologies differently from that somatic mutations altering signaling in the mTOR pathway. We also found that rapamycin treatment of slices did not change the hyperexcitability phenotype of BRAFV600E neurons (data not shown). Although mTOR and MAPK/ERK pathways do exhibit some cross talk (Carracedo et al. 2008; Guan et al. 2000; Ma et al. 2005), it is not unexpected that constitutive activation of BRAF would result in a different electrophysiological phenotype given that BRAFV600E activates the MAPK/ERK pathway while mTOR does not. Moreover, it has been shown that activation of MAPK/ERK in neurons can have multiple effects on neuronal physiology (Binshtok et al. 2008; Black et al. 2008; Chen et al. 2015; Fitzgerald 2000; Gasser et al. 2010; Jung et al. 2010; Lim et al. 2017a; Poolos et al. 2006; Wittmack et al. 2005; Yeh et al. 2018; Zhang et al. 2010) that do not occur with mTOR pathway activation. In addition, previous studies on neurons recorded from resected tissue from patients with tuberous sclerosis complex (TSC) (Cepeda et al. 2003, 2007, 2010, 2012, 2014) did not show significant increases in action potential firing frequencies, and similarly, in a mouse conditional TSC1 KO (Wang et al. 2007), there was no appreciable increase in pyramidal neuron firing frequency (Baek et al. 2015; Lim et al. 2015, 2017b; Roy et al. 2015).
We found that initiating BRAFV600E expression and transgene integration in neural progenitors that give rise to either primarily neurons or a combination of neurons and astrocytes results in hyperexcitable pyramidal neurons. We have not determined when the development and differentiation of pyramidal neurons has its transformative effect on excitability. We performed experiments in which we treated slices with the BRAFV600E-specific inhibitor vemurafenib (10–50 µM) (Bollag et al. 2010; Lee et al. 2010) for 1–5 h to determine whether acutely blocking BRAFV600E activity could rescue the hyperexcitability phenotype. We found no significant differences in any of the major parameters affected by BRAFV600E relative to vehicle control (data not shown). This suggests that BRAFV600E may exert its effects earlier in development and may require changes in gene expression. This possibility is consistent with the recent observations by Koh et al. (2018) that dominant negative REST introduced in utero at the same time that BRAF somatic mutations were conditionally expressed could rescue the epileptogenic effects and neural gene expression, including several ion channel genes, caused by activating somatic BRAF mutations. In future studies it will be important to determine whether preventing gene expression changes induced by BRAFV600E can prevent the development of the hyperexcitable neuronal phenotype.
GRANTS
The current work was supported by National Institute of Neurological Disorders and Stroke Grant R01NS104999 (to J.J.L.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.U.G. and J.J.L. conceived and designed research; R.U.G. and G.A. performed experiments; R.U.G. and G.A. analyzed data; R.U.G. and J.J.L. interpreted results of experiments; R.U.G. and J.J.L. prepared figures; R.U.G. and J.J.L. drafted manuscript; R.U.G. and J.J.L. edited and revised manuscript; R.U.G., G.A., and J.J.L. approved final version of manuscript.
REFERENCES
- Anthony TE, Heintz N. Genetic lineage tracing defines distinct neurogenic and gliogenic stages of ventral telencephalic radial glial development. Neural Dev 3: 30, 2008. doi: 10.1186/1749-8104-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Pumain R, Köhling R. Cellular and molecular mechanisms of epilepsy in the human brain. Prog Neurobiol 77: 166–200, 2005. doi: 10.1016/j.pneurobio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Baek ST, Copeland B, Yun EJ, Kwon SK, Guemez-Gamboa A, Schaffer AE, Kim S, Kang HC, Song S, Mathern GW, Gleeson JG. An AKT3-FOXG1-reelin network underlies defective migration in human focal malformations of cortical development. Nat Med 21: 1445–1454, 2015. doi: 10.1038/nm.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Dobyns WB, Guerrini R. Malformations of cortical development and epilepsy. Cold Spring Harb Perspect Med 5: a022392, 2015. doi: 10.1101/cshperspect.a022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci 28: 14062–14073, 2008. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol 64: 644–653, 2008. doi: 10.1002/ana.21527. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Aronica E, Urbach H, Alexopoulos A, Gonzalez-Martinez JA. A neuropathology-based approach to epilepsy surgery in brain tumors and proposal for a new terminology use for long-term epilepsy-associated brain tumors. Acta Neuropathol 128: 39–54, 2014. doi: 10.1007/s00401-014-1288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I, Beck H, Lie AA, Wiestler OD. Molecular neuropathology of human mesial temporal lobe epilepsy. Epilepsy Res 36: 205–223, 1999. doi: 10.1016/S0920-1211(99)00052-2. [DOI] [PubMed] [Google Scholar]
- Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton EA, Wong B, Tsang G, West BL, Powell B, Shellooe R, Marimuthu A, Nguyen H, Zhang KY, Artis DR, Schlessinger J, Su F, Higgins B, Iyer R, D’Andrea K, Koehler A, Stumm M, Lin PS, Lee RJ, Grippo J, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, Chapman PB, Flaherty KT, Xu X, Nathanson KL, Nolop K. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467: 596–599, 2010. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ, Counter CM. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509: 492–496, 2014. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118: 3065–3074, 2008. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LC, Goebel M, Corsaro BG, Wang HG, Rosen E, Fraser MJ. Transposon mutagenesis of baculoviruses: analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology 172: 156–169, 1989. doi: 10.1016/0042-6822(89)90117-7. [DOI] [PubMed] [Google Scholar]
- Cepeda C, André VM, Hauptman JS, Yamazaki I, Huynh MN, Chang JW, Chen JY, Fisher RS, Vinters HV, Levine MS, Mathern GW. Enhanced GABAergic network and receptor function in pediatric cortical dysplasia type IIB compared with tuberous sclerosis complex. Neurobiol Dis 45: 310–321, 2012. doi: 10.1016/j.nbd.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, André VM, Wu N, Yamazaki I, Uzgil B, Vinters HV, Levine MS, Mathern GW. Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia. Epilepsia 48, Suppl 5: 79–85, 2007. doi: 10.1111/j.1528-1167.2007.01293.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, André VM, Yamazaki I, Hauptman JS, Chen JY, Vinters HV, Mathern GW, Levine MS. Comparative study of cellular and synaptic abnormalities in brain tissue samples from pediatric tuberous sclerosis complex and cortical dysplasia type II. Epilepsia 51, Suppl 3: 160–165, 2010. doi: 10.1111/j.1528-1167.2010.02633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Chen JY, Wu JY, Fisher RS, Vinters HV, Mathern GW, Levine MS. Pacemaker GABA synaptic activity may contribute to network synchronization in pediatric cortical dysplasia. Neurobiol Dis 62: 208–217, 2014. doi: 10.1016/j.nbd.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Flores-Hernández J, Hernández-Echeagaray E, Klapstein GJ, Boylan MK, Calvert CR, Jocoy EL, Nguyen OK, André VM, Vinters HV, Ariano MA, Levine MS, Mathern GW. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res 72: 472–486, 2003. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- Chang M, Dian JA, Dufour S, Wang L, Moradi Chameh H, Ramani M, Zhang L, Carlen PL, Womelsdorf T, Valiante TA. Brief activation of GABAergic interneurons initiates the transition to ictal events through post-inhibitory rebound excitation. Neurobiol Dis 109: 102–116, 2018. doi: 10.1016/j.nbd.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Chen F, LoTurco J. A method for stable transgenesis of radial glia lineage in rat neocortex by piggyBac mediated transposition. J Neurosci Methods 207: 172–180, 2012. doi: 10.1016/j.jneumeth.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Sheng J, Guo J, Gao F, Zhao X, Dai J, Wang G, Li K. Tumor necrosis factor-α enhances voltage-gated Na+ currents in primary culture of mouse cortical neurons. J Neuroinflammation 12: 126, 2015. doi: 10.1186/s12974-015-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823, 2013. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Gama AM, Walsh CA. Somatic mosaicism and neurodevelopmental disease. Nat Neurosci 21: 1504–1514, 2018. doi: 10.1038/s41593-018-0257-3. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu X, Li G, Han M, Zhuang Y, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122: 473–483, 2005. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Dodgshun AJ, SantaCruz N, Hwang J, Ramkissoon SH, Malkin H, Bergthold G, Manley P, Chi S, MacGregor D, Goumnerova L, Sullivan M, Ligon K, Beroukhim R, Herrington B, Kieran MW, Hansford JR, Bandopadhayay P. Disseminated glioneuronal tumors occurring in childhood: treatment outcomes and BRAF alterations including V600E mutation. J Neurooncol 128: 293–302, 2016. doi: 10.1007/s11060-016-2109-x. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868, 1998. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrere A, Vitalis T, Gingras H, Gaspar P, Cases O. Expression of Cux-1 and Cux-2 in the developing somatosensory cortex of normal and barrel-defective mice. Anat Rec A Discov Mol Cell Evol Biol 288: 158–165, 2006. doi: 10.1002/ar.a.20284. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EM. Regulation of voltage-dependent calcium channels in rat sensory neurones involves a Ras-mitogen-activated protein kinase pathway. J Physiol 527: 433–444, 2000. doi: 10.1111/j.1469-7793.2000.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamkrelidze G, Giaume C, Peusner KD. The differential expression of low-threshold sustained potassium current contributes to the distinct firing patterns in embryonic central vestibular neurons. J Neurosci 18: 1449–1464, 1998. doi: 10.1523/JNEUROSCI.18-04-01449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser A, Cheng X, Gilmore ES, Tyrrell L, Waxman SG, Dib-Hajj SD. Two Nedd4-binding motifs underlie modulation of sodium channel Nav1.6 by p38 MAPK. J Biol Chem 285: 26149–26161, 2010. doi: 10.1074/jbc.M109.098681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M. Glial cells generate neurons–master control within CNS regions: developmental perspectives on neural stem cells. Neuroscientist 9: 379–397, 2003. doi: 10.1177/1073858403257138. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol 566: 689–715, 2005. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, Armstrong WE, Foehring RC. Electrophysiological properties of genetically identified subtypes of layer 5 neocortical pyramidal neurons: Ca2+ dependence and differential modulation by norepinephrine. J Neurophysiol 113: 2014–2032, 2015. doi: 10.1152/jn.00524.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem 275: 27354–27359, 2000. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Duchowny M, Jayakar P, Krsek P, Kahane P, Tassi L, Melani F, Polster T, Andre VM, Cepeda C, Krueger DA, Cross JH, Spreafico R, Cosottini M, Gotman J, Chassoux F, Ryvlin P, Bartolomei F, Bernasconi A, Stefan H, Miller I, Devaux B, Najm I, Giordano F, Vonck K, Barba C, Blumcke I. Diagnostic methods and treatment options for focal cortical dysplasia. Epilepsia 56: 1669–1686, 2015. doi: 10.1111/epi.13200. [DOI] [PubMed] [Google Scholar]
- Hawkins VE, Hawryluk JM, Takakura AC, Tzingounis AV, Moreira TS, Mulkey DK. HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. J Neurophysiol 113: 1195–1205, 2015. doi: 10.1152/jn.00487.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corporation IBM SPSS Statistics for Windows, Version 24.0 2016.
- Jamuar SS, Walsh CA. Somatic mutations in cerebral cortical malformations. N Engl J Med 371: 2038, 2014. doi: 10.1056/NEJMoa1314432. [DOI] [PubMed] [Google Scholar]
- Jung S, Bullis JB, Lau IH, Jones TD, Warner LN, Poolos NP. Downregulation of dendritic HCN channel gating in epilepsy is mediated by altered phosphorylation signaling. J Neurosci 30: 6678–6688, 2010. doi: 10.1523/JNEUROSCI.1290-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar A, Majumdar A, Kumar A, Tripathi M, Pathak P, Sharma MC, Suri V, Tandon V, Chandra SP, Sarkar C. Alterations in BRAF gene, and enhanced mTOR and MAPK signaling in dysembryoplastic neuroepithelial tumors (DNTs). Epilepsy Res 127: 141–151, 2016. doi: 10.1016/j.eplepsyres.2016.08.028. [DOI] [PubMed] [Google Scholar]
- Koelsche C, Wöhrer A, Jeibmann A, Schittenhelm J, Schindler G, Preusser M, Lasitschka F, von Deimling A, Capper D. Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol 125: 891–900, 2013. doi: 10.1007/s00401-013-1100-2. [DOI] [PubMed] [Google Scholar]
- Koh HY, Kim SH, Jang J, Kim H, Han S, Lim JS, Son G, Choi J, Park BO, Heo WD, Han J, Lee HJ, Lee D, Kang HC, Shong M, Paik SB, Kim DS, Lee JH. BRAF somatic mutation contributes to intrinsic epileptogenicity in pediatric brain tumors. Nat Med 24: 1662–1668, 2018. doi: 10.1038/s41591-018-0172-x. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Götz M. Radial glia diversity: a matter of cell fate. Glia 43: 37–43, 2003. doi: 10.1002/glia.10250. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, Saraga F, Churchill JF, Statland JM, Travis KE, Skinner FK, McBain CJ. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci 26: 12325–12338, 2006. doi: 10.1523/JNEUROSCI.3521-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Li L, Brafford PA, van den Eijnden M, Halloran MB, Sproesser K, Haass NK, Smalley KS, Tsai J, Bollag G, Herlyn M. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res 23: 820–827, 2010. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Kang X, Mirabella V, Zhang H, Bu Q, Araki Y, Hoang ET, Wang S, Shen Y, Choi S, Kaang BK, Chang Q, Pang ZP, Huganir RL, Zhu JJ. BRaf signaling principles unveiled by large-scale human mutation analysis with a rapid lentivirus-based gene replacement method. Genes Dev 31: 537–552, 2017a. doi: 10.1101/gad.294413.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JS, Gopalappa R, Kim SH, Ramakrishna S, Lee M, Kim WI, Kim J, Park SM, Lee J, Oh JH, Kim HD, Park CH, Lee JS, Kim S, Kim DS, Han JM, Kang HC, Kim HH, Lee JH. Somatic mutations in TSC1 and TSC2 CAUSE FOCAL CORTICAL DYSPLASIA. Am J Hum Genet 100: 454–472, 2017b. doi: 10.1016/j.ajhg.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JS, Kim WI, Kang HC, Kim SH, Park AH, Park EK, Cho YW, Kim S, Kim HM, Kim JA, Kim J, Rhee H, Kang SG, Kim HD, Kim D, Kim DS, Lee JH. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med 21: 395–400, 2015. doi: 10.1038/nm.3824. [DOI] [PubMed] [Google Scholar]
- Lim KC, Crino PB. Focal malformations of cortical development: new vistas for molecular pathogenesis. Neuroscience 252: 262–276, 2013. doi: 10.1016/j.neuroscience.2013.07.037. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 2: 508–514, 1999. [Erratum in Nat Neurosci 2: 848, 1999]. 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci 1: 181–190, 2000. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Marucci G, de Biase D, Visani M, Giulioni M, Martinoni M, Volpi L, Riguzzi P, Rubboli G, Michelucci R, Tallini G. Mutant BRAF in low-grade epilepsy-associated tumors and focal cortical dysplasia. Ann Clin Transl Neurol 1: 130–134, 2014. doi: 10.1002/acn3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell MJ, Moran JV, Abyzov A, Akbarian S, Bae T, Cortes-Ciriano I, Erwin JA, Fasching L, Flasch DA, Freed D, Ganz J, Jaffe AE, Kwan KY, Kwon M, Lodato MA, Mills RE, Paquola ACM, Rodin RE, Rosenbluh C, Sestan N, Sherman MA, Shin JH, Song S, Straub RE, Thorpe J, Weinberger DR, Urban AE, Zhou B, Gage FH, Lehner T, Senthil G, Walsh CA, Chess A, Courchesne E, Gleeson JG, Kidd JM, Park PJ, Pevsner J, Vaccarino FM; Brain Somatic Mosaicism Network . Intersection of diverse neuronal genomes and neuropsychiatric disease: The Brain Somatic Mosaicism Network. Science 356: eaal1641, 2017. doi: 10.1126/science.aal1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo ZL, Adamson CL, Davis RL. Dendrotoxin-sensitive K+ currents contribute to accommodation in murine spiral ganglion neurons. J Physiol 542: 763–778, 2002. doi: 10.1113/jphysiol.2002.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Slevin JC, Qu D, McCormick S, Adamson SL. In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound. Reprod Biol Endocrinol 6: 34, 2008. doi: 10.1186/1477-7827-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niestroj LM, May P, Artomov M, Kobow K, Coras R, Pérez-Palma E, Altmüller J, Thiele H, Nürnberg P, Leu C, Palotie A, Daly MJ, Klein KM, Beschorner R, Weber YG, Blümcke I, Lal D. Assessment of genetic variant burden in epilepsy-associated brain lesions. Eur J Hum Genet 27: 1738–1744, 2019. doi: 10.1038/s41431-019-0484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niihori T, Aoki Y, Narumi Y, Neri G, Cavé H, Verloes A, Okamoto N, Hennekam RC, Gillessen-Kaesbach G, Wieczorek D, Kavamura MI, Kurosawa K, Ohashi H, Wilson L, Heron D, Bonneau D, Corona G, Kaname T, Naritomi K, Baumann C, Matsumoto N, Kato K, Kure S, Matsubara Y. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet 38: 294–296, 2006. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- Pinto L, Götz M. Radial glial cell heterogeneity–the source of diverse progeny in the CNS. Prog Neurobiol 83: 2–23, 2007. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Bullis JB, Roth MK. Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase. J Neurosci 26: 7995–8003, 2006. doi: 10.1523/JNEUROSCI.2069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabowo AS, Iyer AM, Veersema TJ, Anink JJ, Schouten-van Meeteren AY, Spliet WG, van Rijen PC, Ferrier CH, Capper D, Thom M, Aronica E. BRAF V600E mutation is associated with mTOR signaling activation in glioneuronal tumors. Brain Pathol 24: 52–66, 2014. doi: 10.1111/bpa.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet 38: 500–501, 2006. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- Roy A, Skibo J, Kalume F, Ni J, Rankin S, Lu Y, Dobyns WB, Mills GB, Zhao JJ, Baker SJ, Millen KJ. Mouse models of human PIK3CA-related brain overgrowth have acutely treatable epilepsy. eLife 4: e12703, 2015. doi: 10.7554/eLife.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, Louis DN, Korshunov A, Pfister S, Hartmann C, Paulus W, Reifenberger G, von Deimling A. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol 121: 397–405, 2011. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- Schridde U, Strauss U, Bräuer AU, van Luijtelaar G. Environmental manipulations early in development alter seizure activity, Ih and HCN1 protein expression later in life. Eur J Neurosci 23: 3346–3358, 2006. doi: 10.1111/j.1460-9568.2006.04865.x. [DOI] [PubMed] [Google Scholar]
- Siddiqi F, Chen F, Aron AW, Fiondella CG, Patel K, LoTurco JJ. Fate mapping by piggyBac transposase reveals that neocortical GLAST+ progenitors generate more astrocytes than Nestin+ progenitors in rat neocortex. Cereb Cortex 24: 508–520, 2014. doi: 10.1093/cercor/bhs332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim NS, Ko A, Kim WK, Kim SH, Kim JS, Shim KW, Aronica E, Mijnsbergen C, Spliet WGM, Koh HY, Kim HD, Lee JS, Kim DS, Kang HC, Lee JH. Precise detection of low-level somatic mutation in resected epilepsy brain tissue. Acta Neuropathol 138: 901–912, 2019. doi: 10.1007/s00401-019-02052-6. [DOI] [PubMed] [Google Scholar]
- Thom M, Blümcke I, Aronica E. Long-term epilepsy-associated tumors. Brain Pathol 22: 350–379, 2012. doi: 10.1111/j.1750-3639.2012.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev 19: 230–236, 2009. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Bazhenov M, Sejnowski T, Steriade M. Cortical hyperpolarization-activated depolarizing current takes part in the generation of focal paroxysmal activities. Proc Natl Acad Sci USA 99: 9533–9537, 2002. doi: 10.1073/pnas.132259899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Greenwood JS, Calcagnotto ME, Kirsch HE, Barbaro NM, Baraban SC. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol 61: 139–152, 2007. doi: 10.1002/ana.21058. [DOI] [PubMed] [Google Scholar]
- Wittmack EK, Rush AM, Hudmon A, Waxman SG, Dib-Hajj SD. Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase. J Neurosci 25: 6621–6630, 2005. doi: 10.1523/JNEUROSCI.0541-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold H. Nonlinear iterative partial least squares (NIPALS) modeling: some current developments. In Multivariate Analysis III. Proceedings of the 3rd International Symposium on Multivariate Analysis, edited by Krishnaiah PR. New York: Academic Press, 1973a, p. 383–407. [Google Scholar]
- Wold H. Aspects opératoires des modèles économétriques et sociologiques. Développement actuel de l’estimation ‘F.P.’ (Point fixe) et de la modélisation NIPALS (linéarisation par itération de moindres carrés partiels). Econ Appl 2–4: 389–421, 1973b. [Google Scholar]
- Wu SC, Meir YJ, Coates CJ, Handler AM, Pelczar P, Moisyadi S, Kaminski JM. piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and Mos1 in mammalian cells. Proc Natl Acad Sci USA 103: 15008–15013, 2006. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Dao DQ, Wu ZY, Kandalam SM, Camacho FM, Tom C, Zhang W, Krencik R, Rauen KA, Ullian EM, Weiss LA. Patient-derived iPSCs show premature neural differentiation and neuron type-specific phenotypes relevant to neurodevelopment. Mol Psychiatry 23: 1687–1698, 2018. doi: 10.1038/mp.2017.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G, Rosenberg J, Blaser S, Rauen KA. Neurological complications of cardio-facio-cutaneous syndrome. Dev Med Child Neurol 49: 894–899, 2007. doi: 10.1111/j.1469-8749.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- Zaika O, Lara LS, Gamper N, Hilgemann DW, Jaffe DB, Shapiro MS. Angiotensin II regulates neuronal excitability via phosphatidylinositol 4,5-bisphosphate-dependent modulation of Kv7 (M-type) K+ channels. J Physiol 575: 49–67, 2006. doi: 10.1113/jphysiol.2006.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J Neurosci 30: 12844–12855, 2010. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110α and p110β phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci USA 102: 18443–18448, 2005. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]