Abstract
Safe and efficient drugs to combat the current COVID-19 pandemic are urgently needed. In this context, we have analyzed the anti-coronavirus potential of the natural product glycyrrhizic acid (GLR), a drug used to treat liver diseases (including viral hepatitis) and specific cutaneous inflammation (such as atopic dermatitis) in some countries. The properties of GLR and its primary active metabolite glycyrrhetinic acid are presented and discussed. GLR has shown activities against different viruses, including SARS-associated Human and animal coronaviruses. GLR is a non-hemolytic saponin and a potent immuno-active anti-inflammatory agent which displays both cytoplasmic and membrane effects. At the membrane level, GLR induces cholesterol-dependent disorganization of lipid rafts which are important for the entry of coronavirus into cells. At the intracellular and circulating levels, GLR can trap the high mobility group box 1 protein and thus blocks the alarmin functions of HMGB1. We used molecular docking to characterize further and discuss both the cholesterol- and HMG box-binding functions of GLR. The membrane and cytoplasmic effects of GLR, coupled with its long-established medical use as a relatively safe drug, make GLR a good candidate to be tested against the SARS-CoV-2 coronavirus, alone and in combination with other drugs. The rational supporting combinations with (hydroxy)chloroquine and tenofovir (two drugs active against SARS-CoV-2) is also discussed. Based on this analysis, we conclude that GLR should be further considered and rapidly evaluated for the treatment of patients with COVID-19.
Keywords: Glycyrrhizin, COVID-19, Coronavirus, HMGB1, Cholesterol, Natural product
Abbreviations: CQ, chloroquine; GLR, glycyrrhizin or glycyrrhizinic acid; HCQ, hydroxychloroquine; HMGB1, High-Mobility Group protein B1; LPS, lipopolysaccharide; SARS, Severe Acute Respiratory Syndrome; TLR4, Toll-Like Receptor 4.
1. Introduction
Effective medications are urgently needed to combat the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). At present, no treatment is available, apart from symptomatic interventions, but many vaccine- and drug-based approaches are being investigated worldwide. Drugs such as arbidol, remdesivir, favipiravir and the antibiotic teicoplanin are currently undergoing clinical trials in different countries (Baron, Devaux, Colson, Raoult, & Rolain, 2020; Dong, Hu, & Gao, 2020). The drugs tested also include chloroquine (CQ) and its analog hydroxychloroquine (HCQ) found to be efficient on SARS-CoV-2. A study with a small cohort of patients has shown that a treatment with HCQ can markedly reduce, if not eliminate, the viral load in patients with coronavirus disease 2019 (COVID-19) (Gautret et al., 2020). This first clinical observation corroborated preclinical data suggesting that HCQ was effective against the SARS-CoV-2 virus-infected cells in vitro (Liu et al., 2020). CQ is also active, although less efficacious than HCQ (Gao, Tian, & Yang, 2020; Yao et al., 2020). HCQ, known for decades for treatment of malaria, is a good antiviral agent active against most coronaviruses, including SARS-CoV-1 (Devaux, Rolain, Colson, & Raoult, 2020) but definitive proofs of its clinical efficacy to treat SARS-CoV-2 patients are awaited. At present, its safety/efficacy ratio is controversial (Roustit, Guilhaumou, Molimard, Drici, & Laporte, 2020; Sun et al., 2020).
Other drugs active against SARS-CoV-2 are actively studied, such as protease inhibitors. But drugs with distinct and varied mechanisms of action are needed to propose efficient drug combinations. Here we propose an innovative approach based on the use of the saponin drug glycyrrhizin, which we believe can be useful to combat the virus infection and to treat the associated respiratory symptoms. We searched for a drug that could not only inhibit viral replication but also interfere with the entry of the virus into cells. On the one hand, we reasoned that an amphiphilic compound like a saponin could interfere with the virus entry into cells, owing to the well-known membrane effects of this class of compounds. On the other hand, we considered that a target associated to “danger-signals” in cells, like HMGB1, could be a useful to trigger an immune response. A deep analysis of the literature was performed to identify this drug which, we think, should be considered for the treatment of COVID-19, for the reasons detailed below.
2. Glycyrrhizin (GLR), a natural product
Glycyrrhizin, also called glycyrrhizic acid (GLR), is a triterpenoid saponin mainly isolated from the roots (Glycyrrhizae Radix) of the plants Glycyrrhiza glabra (typically cultivated in Europe, henceforth called European licorice) and G. uralensis Fisch and G. inflata Bat (used in the Chinese Pharmacopoeia) (Pastorino, Cornara, Soares, Rodrigues, & Oliveira, 2018; Hayashi et al., 2019; Wang, Chen et al., 2020). G. glabra contains more than 10 GLR-related saponins (Schmid, Dawid, Peters, & Hofmann, 2018). The product has been found also in other Glycyrrhiza species such as G. triphylla (Shakeri et al., 2018) and in an edible marine alga Hizikia fusiformis (Harvey) Okamura, a brown seaweed (Seong et al., 2019; Wagle et al., 2018). The name derives from the Greek word ‘glykosrhiza’ or sweet root. GLR is commonly isolated from Glycyrrhizae Radix in the form of a monoammonium salt (C42H61O16_NH4), but occasionally studies are performed with the magnesium salt of 18β-glycyrrhizic acid (magnesium isoglycyrrhizinate) (Cao et al., 2019; Tan et al., 2018; Zou et al., 2018). Specific methods have been developed to monitor the extraction process and for GLR dosages in biological media (Wang, Shan, & Lü, 2020; Yu et al., 2017; Zeng et al., 2020).
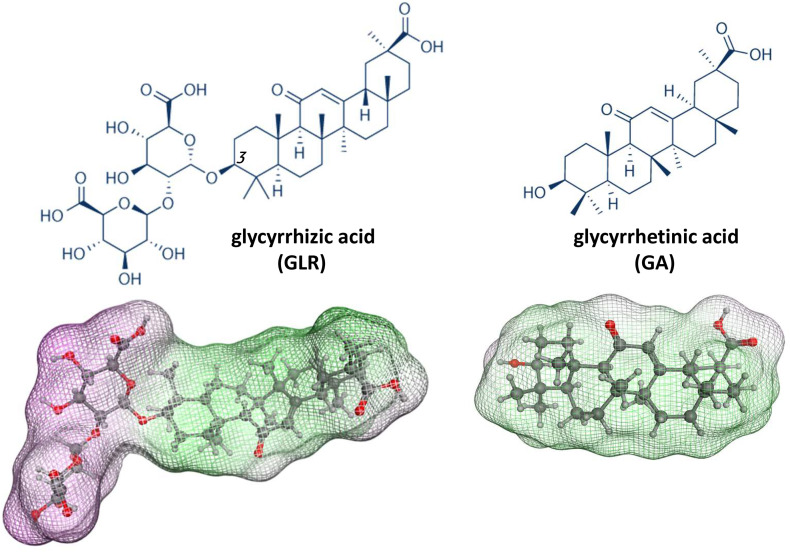
GLR is a glycoside of glycyrrhetinic acid (GA, the sapogenin moiety) with two residues of glucuronic acid (Fig. 1 ). GA is the major metabolite of GLR, together with 3-O-mono-β-D-glucuronyl-glycyrrhetinic acid (3MGA) and a minor sulfated metabolite (Ishiuchi et al., 2019; Morinaga et al., 2018). Orally administered GLR is metabolized into GA by intestinal bacteria and absorbed via the intestine. Nevertheless, GLR is detected in human plasma after oral administration of a clinical dose of GLR (Suzuki, Tsukahara, Akasaka, & Inoue, 2017). Both oral and intravenous formulations of GLR are used in Human.
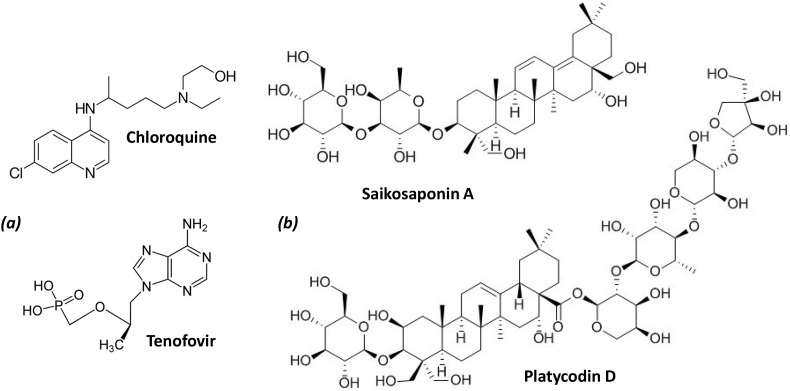
Fig. 1.
(a) Structures of glycyrrhizic acid (GLR) and glycyrrhetinic acid (GA). GLR is composed of a central saponin core flanked by a carbohydrate side chain at C-3. Below, the molecular models of GLR and GA show the accessible surface and hydrophilic (purple) and lipophilic (green) sites. For GLR, the central triterpenoid aglycone, largely hydrophobic, is bound to a hydrophilic sugar chain. The model of GA (aglycone) is shown for comparison. Models were built with Discovery Studio 2020 Client, Dassault Systemes Biovia Corp..
The natural product exists as two epimers 18α-glycyrrhizic acid (18α-GLR) and 18β-glycyrrhizic acid (18β-GLR), both active although the 18β-isomer seems to be more potent than the 18α-isomer. Indeed, 18β-glycyrrhetinic acid (IC50 = 8.9 μM) was found to be about 2-fold more active than GLR (IC50 value of 20.1 μM) and 11-fold more potent than 18α-glycyrrhetinic acid (IC50 = 104.3 μM) in an enzyme BACE1 inhibition assay (butyrylcholinesterase and β-site amyloid precursor protein cleaving enzyme 1) (Wagle et al., 2018). Bioproduction methods using metabolically engineered Saccharomyces cerevisiae (yeast cell factories) are being developed as an alternative “greener” approach to produce GLR (Wang et al., 2019). β-glucuronidase enzymes can be used to bio-transform GLR into GA (Li, Jiang, Liu, Feng, & Li, 2019). The product is often used as a natural emulsifier and gel-forming agent in foodstuffs, beverages and cosmetics. GLR is approved for use as an additive in foods since 1985 in the US and has the Generally Recognized as Safe (GRAS) status. In biology and medicine, GLR has been extensively studied for its very diverse pharmacological properties, including anti-inflammatory, antioxidative, anti-allergenic, antimicrobial, antiviral, antiparasite, anticancer properties. The properties and applications of GLR and GLR-containing extracts have been reviewed previously (Chen, Yang, Shen, & Zhu, 2019; El-Saber Batiha, Magdy Beshbishy, El-Mleeh, Abdel-Daim, & Prasad, 2020; Kwon, Son, Chung, & Lee, 2020; Ming & Yin, 2013). Nevertheless, in view of the increasing scientific interest in this product and the current COVID-19 pandemia, we decided to write a review essentially focus on the antiviral properties of the drug, to analyze its potential use for the treatment of coronavirus infections.
3. GLR as a drug used in human
In Japan, GLR has been used for more than 40 years as treatment for liver diseases, in particular to treat chronic hepatitis (Li, Cao, Liu, Cheng, & Sun, 2014). Intravenous GLR therapy is generally well tolerated and considered effective in different forms of hepatitis (Tandon, Tandon, & Bhujwala, 2002). It is an efficient hepatoprotective medication in patients with chronic hepatitis C (Tanaka et al., 2009) and more broadly to protect from a variety of hepatic diseases such as chronic viral hepatitis, drug- or chemical-induced liver injury, nonalcoholic fatty liver disease, autoimmune hepatitis and hepatocellular carcinoma (Li, Sun, & Liu, 2019). Most of these effects can be attributed to the drug inhibitory activity on inflammatory cytokines in the liver and activation of CD8+ T cells and Tregs proliferation (Gao et al., 2019). One of the main GLR-containing preparation is intravenous Stronger Neo-Minophagen C (SNMC, from Minophagen Pharmaceutical Co. Ltd., Tokyo) used for suppression of hepatitis activity and prevention of disease progression in patients with hepatitis B virus- and HCV-induced chronic hepatitis. SNMC is currently investigated for the treatment of acute hepatitis post transarterial chemoembolization (TACE) therapy in patients with hepatocellular carcinoma (NCT04015245).
GLR is also used to treat different forms of cutaneous inflammation (Kowalska & Kalinowska-Lis, 2019). For example, combined with methotrexate, GLR has been used successfully to treat erythrodermic psoriasis (Si et al., 2014; Yu et al., 2017; Yu, Jin, et al., 2017). But one of the most frequent inflammatory skin diseases treated with GLR (at least in Japan and Korea) is atopic dermatitis (AD). Recently, it was reported that GLR inhibited the release of cytokines IL-4 and IL-13 in a murine model of AD (Lee et al., 2019) and another study showed that, through inhibition of the protein HMGB1 (High-Mobility Group protein B1), GLR can ameliorate the symptoms of AD in a mouse model with the drug injected daily intraperitoneally (Wang, Zhang, Peng, & Han, 2018). By sequestering HMGB1 (see mechanism below), GLR modulates the production of inflammatory cytokines such as IL-18, to prevent contact dermatitis induced by contact allergens (Galbiati, Papale, Galli, Marinovich, & Corsini, 2014). The activity of GLR against cutaneous inflammation should be kept in mind considering the cutaneous manifestations in COVID-19-positive patients (Torres & Puig, 2020).
The drug is considered as having a good safety and economical profile. Its clinical use is increasing. In recent years, we noted the development of different clinical trials to investigate the benefit of GLR in multiple pathologies such as depression (Cao et al., 2020), Parkinson's disease (Petramfar, Hajari, Yousefi, Azadi, & Hamedi, 2020) and different cancers, such as hepatocellular carcinoma and pancreatic cancer (Kwon et al., 2020).
4. GLR antiviral activities
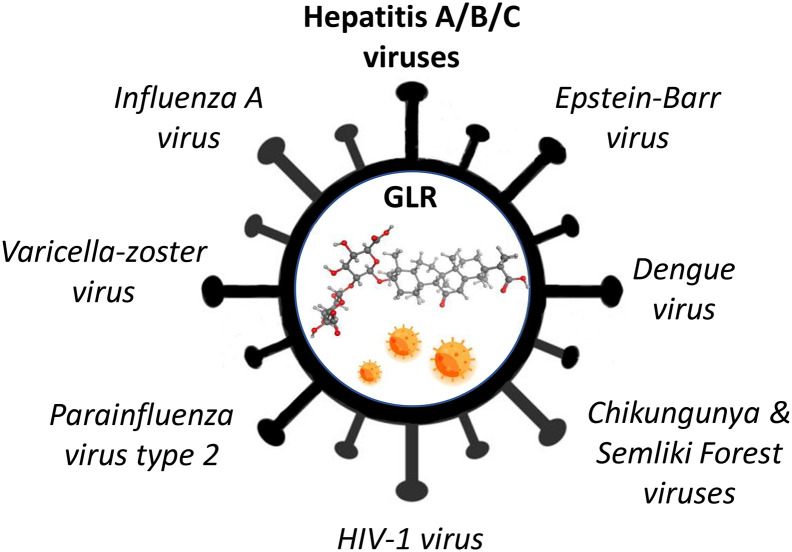
Over the past thirty years, the effects of GLR against a variety of human viruses have been reported (Fig. 2 ). The anti-HIV1 activity of GLR is well documented (Ashfaq, Masoud, Nawaz, & Riazuddin, 2011; Baba & Shigeta, 1987; Baltina et al., 2019; Bentz et al., 2019; Briolant, Garin, Scaramozzino, Jouan, & Crance, 2004; Crance et al., 1994; Crance, Biziagos, Passagot, van Cuyck-Gandré, & Deloince, 1990; Lin, 2003; Lin et al., 2008; Matsumoto et al., 2013; Michaelis et al., 2010; Michaelis et al., 2011; Sakai-Sugino et al., 2017; Sun, Zhao, Lu, Yang, & Zhu, 2019; Wolkerstorfer, Kurz, Bachhofner, & Szolar, 2009). GLR dose-dependently inhibits virus-cell binding and the replication of HIV-1 in human cells (Ito et al., 1988). The drug has been tested in AIDS patients, at a dose of 400–1600 mg/day given i.v., and the viral antigen was no longer detected at the end of the treatment course, suggesting a marked inhibition of HIV-1 replication (Hattori et al., 1989). GLR effectively inhibited HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients (Sasaki, Takei, Kobayashi, Pollard, & Suzuki, 2002–2003).
Fig. 2.
Antiviral activities of GLR. The drug is active against aa number of viruses in vitro. In vivo activities have been reported with a few viruses, notably HIV-1. But the main antiviral activity is against the hepatitis viruses A, B and C. The drug is used in Human to treat liver diseases, notably chronic viral hepatitis.
The drug is also active against some animal viruses. It functions as an immune-stimulant against duck hepatitis virus (DHV) (Okda, Yassein, Ahmed, Soufy, & Nasr, 2013; Soufy et al., 2012) and showed a marked direct antiviral activity, leading to complete inhibition of cell infection, against the avian infectious bronchitis virus (IBV) (Li, Yin, Sui, Li, & Ren, 2009). This antiviral activity coupled with a hepatoprotective effect (Li et al., 2019; Li, Li, Jiang, Li, & Yu, 2018) support the use of a licorice extract supplementation in poultry diets (Alagawany et al., 2019). A study with chicken HD11 macrophages indicated that GLR activates the immune regulatory functions, notably promoting the expression of cytokines IFN-γ, IL-6, and IL-10, and the production of nitric oxide (Wang et al., 2018; Wang et al., 2018). GLR is also effective against the porcine reproductive and respiratory syndrome virus (PRRSV, an enveloped single positive-strand RNA virus, Arterivirus), acting mainly at the virus penetration stage, not later steps of adsorption or release of PRRSV in its life cycle (Duan et al., 2015). The GLR derivative dipotassium glycyrrhetate inhibits PRRSV replication in vitro (Wang et al., 2013). Interestingly, a recent study revealed that the use of a polymeric form of GLR, to form monodisperse carbon dots (spherical carbon particles), leads to remarkable antiviral effects, with inhibition of PRRSV invasion and replication, inhibition of the accumulation of intracellular reactive oxygen species caused by PRRSV infection, and stimulation of antiviral innate immune responses. Moreover, this GLR-based product was also effective against the related nidovirus PEDV (porcine epidemic diarrhea virus) (Tong et al., 2020). In fact, GLR inhibits the entry and replication of PEDV (but not virus assembly and release), as well as the production of the proinflammatory cytokines IL-1β, IL-6, IL-8 and TNFα. More precisely, GLR inhibits the increase of these proinflammatory cytokines induced by PEDV infection (Huan et al., 2017).
5. GLR and SARS-associated coronaviruses
In 2003, it was reported that GLR effectively inhibited the replication of two clinical isolates of SARS-associated coronavirus (FFM-1 and FFM-2) in Vero cells. The drug was found to inhibit the cytopathic effect of the virus with an EC50 of 300 mg/ml, while being non cytotoxic to the host cells. GLR inhibited virus replication but also the adsorption and penetration of the virus into cells (Cinatl et al., 2003). The mechanism of action at the origin of this activity was not known at that time but a drug-induced production of nitrous oxide synthase was mentioned, suggesting that nitrous oxide could be responsible for the inhibition of virus replication (Cinatl et al., 2003). GLR also revealed active when it was tested against 10 clinical isolates of SARS coronavirus in infected Vero-E6 cells but the activity was limited in time. Apparently, the rapid metabolization of the drug limits the drug exposure, not permitting to reach the effective concentration. After an intravenous administration of GLR at a dose of 200 mg, the peak serum level was only 80 μg/ml, insufficient to induce the desired biological effects (EC50 > 400 μg/ml) (Chen et al., 2004). The modification of the GLR structures, notably to make amide derivatives and amino-acid conjugates can increase considerably the activity against SARS-CoV but it can be at the expense of an increased cytotoxicity (Hoever et al., 2005).
6. GLR and respiratory distress syndrome
Different elements suggest that GLR can be useful to treat respiratory infections and acute respiratory distress syndrome (ARDS). First, several medicinal plant preparations containing GLR are used for a long time to treat upper respiratory infections. This is the cases of the GLR-containing Siji-kangbingdu herbal mixture, Maxing Shigan decoction and Lianhua-Qingwen capsules, used in China to treat upper respiratory infections (Jia et al., 2015; Li et al., 2018; Yao et al., 2019). It is also the case for the GLR-containing traditional medicine Macmoondongtang used to treat pulmonary disease in Korea (Lee, Kang, Bok, Cho, & Park, 2019). Second, a clinical study performed in Japan in 2002 among military patients treated for upper respiratory tract infections (URTI) has shown that GLR therapy was associated with a shorter hospitalization, lower-grade fever and lower cost of therapy compared with controls. It was concluded that GLR could be useful to patients with URTI without acute bacterial infections (Yanagawa, Ogura, Fujimoto, Shono, & Okuda, 2004). Experimental studies have proposed also the use of GLR to treat respiratory distress syndrome on the basis of the capacity of the product to reduce the pulmonary accumulation of platelets (Yu et al., 2005) and to inhibit proinflammatory cytokines released from activated inflammatory cells in the initial phase of the syndrome (Lee, Lee, Kim, & Lee, 2019). The proinflammatory cytokine IL-33 is likely a major factor in ARDS and its expression is enhanced in the serum, bronchoalveolar fluid and lung tissue of mice with LPS-induced lung injury. A treatment with GLR was found to reduce the levels HMGB1 and IL-33 in the serum and bronchoalveolar fluid compared to the controls (Fu et al., 2016). This laboratory study supports the potentially beneficial effect of GLR to reduce ARDS. Other experimental studies using models of LPS-induced acute lung injury have reported beneficial effects of GLR, with evidences of drug-induced inactivation of the toll-like receptor (TLR) pathway (Kong, Wang, Tian, Liu, & Zhou, 2019).
7. Improved GLR formulation for in vivo administrations
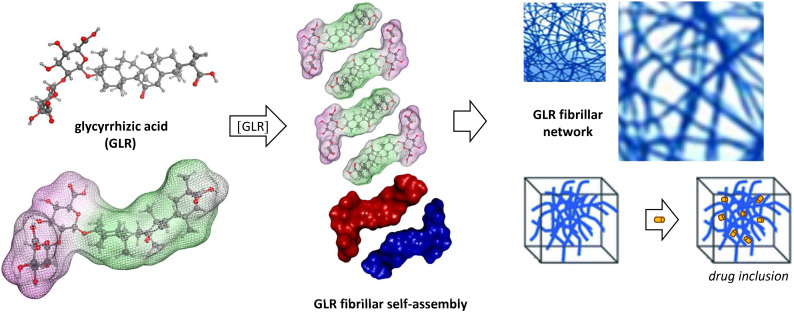
The amphiphilic and anisotropic structure of GLR led to the formation of rod-like micelles and fibrils that self-assembled into a fibrillary network (Saha, Adamcik, Bolisetty, Handschin, & Mezzenga, 2015). The hydrophobic aglycone moieties can interact on a lateral basis yielding a head-to-head configuration which exposes the hydrophilic di-glucuronic acid heads to the aqueous phase, as illustrated in Fig. 3 . The process is highly pH-dependent; the drug is practically insoluble at pH <4.5, rod-like micelles form at pH 5–6 and dissociate into monomers at pH 7 (Matsuoka, Miyajima, Ishida, Karasawa, & Yoshimura, 2016). The self-assembling of GLR into a fibrillar network leads to the formation of micro-structures (Fig. 4 ) with specific mechanical properties (Li et al., 2020; Wan et al., 2017; Wan et al., 2017; Wan et al., 2018). The self-assembly of supramolecular GLR nanofibrils can be exploited to form ultrastable, thermostimulable, and processable foams (Ma et al., 2019).
Fig. 3.
Illustration of the self-assembling of GLR to form a fibrillar network. Molecules of GLR associate in an anti-parallel orientation, with stacking of the hydrophobic sapogenin moieties to form fibrils. Depending on the conditions, GLR can also for micelles and other structures which can be used to entrap other small molecules, as a drug delivery system. The entrapment of molecules into a fibrillar network is illustrated.
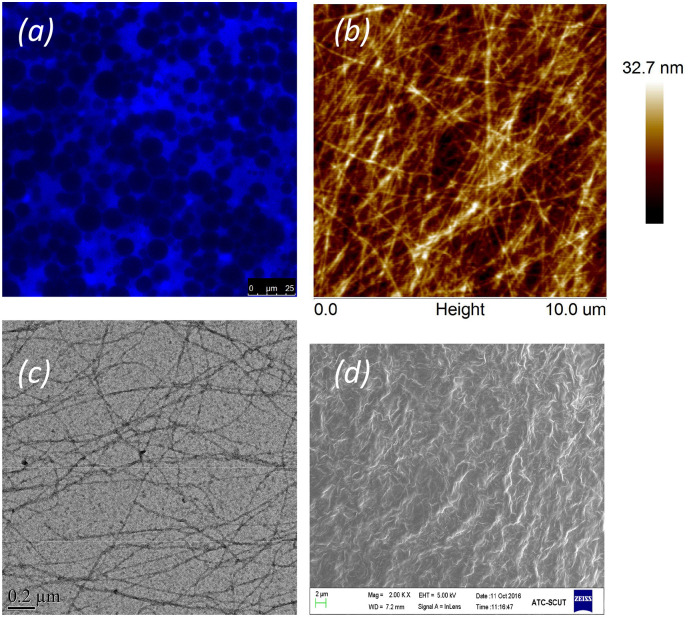
Fig. 4.
The fibrillar self-assembly of GLR. (a) Confocal laser scanning microscopy image of an emulsion gel stabilized by 4 wt% GLR nanofibrils (Thioflavin T fluorescence highlights GLR the fibrillar network). (b) 0.2 wt% GLR nanofibrils observed by atomic force microscopy. (c) 0.1 wt% GLR nanofibrils observed by transmission electron microscopy. (d) Scanning electron microscopy image of the aqueous phase network of an emulsion gel stabilized by 4 wt% GLR nanofibrils. (images kindly provided by Prof. Xiao-Quan Yang, Dpt of Food Science and Technology, South China University of Technology, Guangzhou, China).
Different drug delivery systems specifically adapted to GLR and/or GA have been elaborated (Cai et al., 2016). Notably, an encapsulation of GLR together with the flavonoid silibinin into nano-liposomes has been proposed for the treatment of hepatocellular carcinoma. Such a formulation reduces the half maximal inhibitory concentration (IC50 to reduce the growth of HepG2 cells in vitro) by about 10-fold for the encapsulated product versus the free drug (Ochi, Amoabediny, Rezayat, Akbarzadeh, & Ebrahimi, 2016). The preparation of GLR-based nano-liposomes has been optimized (Liu et al., 2018). The drug can be encapsulated as well as the liquorice extract, into liposomes and other types of vesicles, such as phospholipid-Na hyaluronate vesicles called hyalurosomes (Castangia et al., 2015). An alternative to conventional liposomes is the development of niosomes, as nonionic surfactant vesicles (NSV), which are prepared with Tween 20 and cholesterol, plus cholesteryl hemisuccinate to obtain stable but pH-sensitive vesicles which can release a payload at acidic pH. Such NSV loaded with GLR were found to display significant anti-inflammatory and antinociceptive effects in mice. Entrapment of GLR (1% w/v) into niosomes could be a convenient system to permit a prolonged release of the active principle (Marianecci et al., 2014). Improvements have been proposed to further ameliorate the stability, skin permeability and capacity of these niosomes to deliver the drug, with the use of gel-forming polysaccharides (Coviello et al., 2015).
GLR-containing vesicles can serve to transport and deliver GLR, but also to facilitate the transportation and delivery of co-loaded drugs. Indeed, GLR is viewed as a multifunctional carrier for a variety of hydrophobic molecules, by virtue of its amphiphilic properties (Fig. 1, Table 1 ). The Solvent Accessible Surface Area (SASA) for GLR is large, offering a significant potential to interact with both hydrophilic and hydrophobic molecules, and with multiple H-bond donor/acceptor sites (Table 1). GL complexes or micelles with many drugs have been reported, for examples with anticancer drugs such as paclitaxel, podophyllotoxin and camptothecin (Cai et al., 2019; Wang et al., 2016; Yang et al., 2015). Not only GLR can enhance the solubility of poorly soluble drugs but it can increase also their passive diffusion through cell membranes. The capacity of GLR to interact with phospholipids-based membrane models and to cause transient pore formation has been elegantly modeled. GLR was shown to embark a few water molecules within the membrane and to make the membrane thinner locally (Selyutina et al., 2016) but apparently it does not simply induce transient local pores in the membrane; the mechanism is more complex and seems to involve a drug-induced decrease in cholesterol within the lipid bilayer (Shelepova, Kim, Voloshin, & Medvedev, 2018). Therefore, the drug can be used as nonselective delivery vector for a variety of drugs (Selyutina & Polyakov, 2019; Su et al., 2017). A nice example has been reported recently with the GLR-mediated enhanced delivery of the anti-helminthic drug praziquantel, showing that the self-association of GLR molecules plays an important role in increasing the solubility and transport to the cell surface of praziquantel (Kim et al., 2019). A water-soluble preparation of disodium glycyrrhizinate micelles with inclusion of praziquantel proved to be a good anthelmintic agent in vitro and in vivo, owing to a significant improvement of the bioavailability of praziquantel (Avgustinovich et al., 2019; Meteleva et al., 2018).
Table 1.
Computed physico-chemical properties of GLR and GA.
| Product | GLR | GA |
|---|---|---|
| Molecular weight | 822.9 | 470.7 |
| Dipole moment (D) | 11.9 | 4.3 |
| Total solvent accessible surface area (SASA) (Å2)a | 1121.8 | 703.2 |
| Hydrophobic SASA | 631.5 | 551.8 |
| Hydrophilic SASA | 477.5 | 138.0 |
| Molecular volume (Å3) | 2258.2 | 1415.0 |
| Donor hydrogen bonds | 8 | 2 |
| Acceptor hydrogen bonds | 16 | 4 |
| log P (octanol/water) | 2.2 | 5.2 |
| log S (aqueous solubility) | −6.6 | −6.6 |
SASA calculated with a probe of 1.4 Å radius. Drug properties were calculated with the BOSS 4.9 software (Jorgensen & Tirado-Rives, 2005) according to published procedures (Lagant, Nolde, Stote, Vergoten, & Karplus, 2004; Vergoten, Mazur, Lagant, Michalski, & Zanetta, 2003).
8. Membrane effects of GLR: cholesterol-dependent disorganization of lipid rafts
The antiviral activity of GLR relies on both cytoplasmic and membrane effects. The drug inhibits hepatitis A virus penetration of the plasma membrane (Crance et al., 1994) and suppresses the secretion of viral antigens, such as the hepatitis B surface antigen. GLR inhibits the sialylation of the antigen and intracellular transport to induce its accumulation in cytoplasmic vacuoles in the Golgi apparatus (Sato et al., 1996; Takahara, Watanabe, & Shiraki, 1994). The saponin reduces the movement of molecules within the membrane and thus impedes the formation of fusion pores necessary for the entry of viruses. In fact, the drug lowers the membrane fluidity and thus suppresses infection by different viruses, such as HIV-1, HTLV-1, influenza A virus and vesicular stomatitis virus, but not by poliovirus (Harada, 2005). It is very important to underline that GLR is a non-lytic saponin. In contrast to other saponins like digitonin, GLR shows weak permeabilizing effects and thus has a very low hemolytic profile (Gilabert-Oriol et al., 2013). It does not destroy the whole membrane integrity and induces very little leakage from liposomes compared to other saponins (Malabed, Hanashima, Murata, & Sakurai, 2017). In the context of the Herpes simplex virus (HSV), it has been shown that GLR reduced adhesion force and stress between cerebral capillary vessel endothelial cells and polymorphonuclear leukocytes, thereby attenuating the inflammatory responses to HSV (Huang et al., 2012). In the context of HIV-1, the drug has been shown to bind to the conserved core sequence of V3 loop in the surface glycoprotein gp120 with a sub-micromolar affinity (Li, Zhao, Lin, Ye, & Ling, 2015).
At least a part of the membrane effects of GLR can be linked to an interaction with cholesterol (Fig. 5 ) and the reduction in cholesterol domain in membrane. The interaction of saponins with cholesterol is a main element of their mechanism of action, and their capacity to induce membrane disorganization and in some cases disruption. For example, cholesterol recognition by the anticancer saponin OSW-1 is essential for the subsequent membrane permeabilization (Malabed et al., 2017). Cholesterol is known as a very important modifier of the dynamics and structural properties of lipid membranes. Compared to other saponins like digitonin, OSW-1 and Quillaja saponin, GLR shows very weak membrane permeabilizing effects on membranes from lysosomes or red blood cells (Gilabert-Oriol et al., 2013). Nevertheless, GLR interacts with cholesterol and this interaction modifies the membrane permeability to ions and small molecules (Selyutina et al., 2016). A study using artificial membranes prepared at 1.5:1 cholesterol-to-phospholipid mole ratio has shown that GLR reduced cholesterol domain formation by 54.9% (Mason, Jacob, Shrivastava, Sherratt, & Chattopadhyay, 2016). Not only GLR decreases the level of cholesterol of lipid rafts but also inhibits the translocation of TLR-4 to lipid the rafts (Fu et al., 2014; Fu et al., 2014). This mode of action is distinct from that of conventional cholesterol-lowering statins. Consequently, combinations of GLR and the statins atorvastatin and simvastatin (leading to combo named atorvaglyzin and simvaglyzin) have been proposed to reinforce the cholesterol-lowering effect (Ragino et al., 2008; Stakhneva et al., 2013; Vavilin et al., 2008).
Fig. 5.
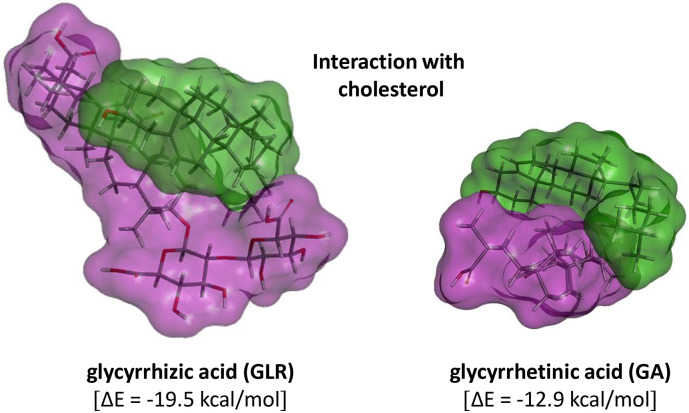
Molecular models of the interaction between GLR (purple) and cholesterol (green). The two different orientations show the stacking of the two hydrophobic parts of each molecule and the contacts made with the glycoside moiety of GLR which contribute to clamp the cholesterol unit.
We have used molecular modeling to compare the interaction with cholesterol of GLR and platycodin D, a bidesmosic saponin known to exhibit cholesterol-lowering effects (Zhao et al., 2006). As shown in Fig. 5, the modeling analysis shows that GLR can form stable complexes with cholesterol, mainly via hydrophobic interaction with the sapogenin moiety of GLR. The potential energy of interaction is lower for the complex of GLR with cholesterol (ΔE = −19.5 kcal/mol) compared to that measured for the platycodin D-cholesterol complex (ΔE = −24.3 kcal/mol) and this is in agreement with the very weak hemolytic capacity of GLR. However, the interaction is significant, stronger than with GA and sufficient to induce plasma membrane perturbations. It has been shown that the drug can move within the membrane, with exchange of GLR molecules from solution to the hydrophobic interior of the lipid bilayer (Selyutina et al., 2016). Normally, cholesterol makes the bilayer more rigid but the interaction with GLR reduces the rigidification of the membrane, and thus possibly increases membrane permeability. This interaction contributes to the regulation of the size of lipid raft domains observed in the presence of GLR (Sakamoto, Nakahara, Uto, Shoyama, & Shibata, 2013). GA was found to interact even more strongly with a raft monolayer model than GLR (Sakamoto et al., 2013; Sakamoto, Uto, & Shoyama, 2015). The disorganization of cholesterol-containing lipid rafts by GLR is an important element to consider in the light of a recent work showing that a ganglioside-binding domain of the spike (S) protein of SARS-CoV-2 likely plays a role in the surface attachment of the virus to lipid rafts. This highly conserved domain would facilitate the entry of the virus into respiratory cells. Chloroquine and hydroxychloroquine bind strongly to sialic acids and gangliosides, thereby limiting attachment of the virus via the spike to the gangliosides on the raft platforms (Fantini, Scala, Chahinian, & Yahi, 2020). A combination with GLR may further inhibit the entry of the virus, via a membrane destabilizing effect. Recently, inhibition of viral lipid-dependent attachment of the virus envelope of the SARS-COV-2 to host cell plasma membrane in vitro, with natural products such as cyclodextrin and sterols, has been proposed as a strategy for reducing SARS-COV-2 infectivity (Baglivo et al., 2020). Our proposal is totally in-line with this view.
Lipid rafts play an important role in the life cycle of SARS-coronavirus. The depletion of cholesterol (with methyl-β-cyclodextrin) inhibits the production of coronavirus particles released from infected cells (Li, Li, Yamate, Li, & Ikuta, 2007). Different studies suggest that lipid rafts serve as an entry port for SARS-CoV (Choi, Aizaki, & Lai, 2005; Lu, Liu, & Tam, 2008; Wang et al., 2008). Similarly, the depletion of plasma membrane cholesterol with methyl-β-cyclodextrin or a statin drug considerably reduces the infection by the coronavirus infectious bronchitis virus (IBV) in vitro, presumably impairing the attachment of the virus to the cell surface (Guo et al., 2017). PEDV and human metapneumovirus (hMPV) can also enter cells through lipid raft-mediated endocytosis (Chen et al., 2019; Wei, She, Wu, Xue, & Cao, 2020). In fact, lipid rafts play a key role in the endocytosis process of many viruses and, as a corollary, interfering with lipid raft organization is a mechanism to control virus infection. Moreover, this is probably the reason why the impairment of cellular cholesterol metabolism and lipid raft functionality have been evoked as a co-morbidity factor in some viral diseases, such as HIV infection (Sviridov, Mukhamedova, Makarov, Adzhubei, & Bukrinsky, 2019).
The cholesterol lowering effect of GLR can explain its antiviral effect against the porcine virus PRRSV, at least partially. Indeed, it has been demonstrated that cell membrane cholesterol is required for porcine nidovirus entry into cells and a drug able to induce cholesterol depletion dose-dependently suppressed the replication of the nidoviruses PRRSV and PEDV (Jeon & Lee, 2017; Sun et al., 2011). Similarly, GLR reduces membrane cholesterol and, as discussed above, it is active against those two porcine nidoviruses (Duan et al., 2015; Tong et al., 2020). Other viruses also partly depend on membrane cholesterol for entry into cells. This is the case of another coronavirus, the highly neurovirulent porcine hemagglutinating encephalomyelitis virus (PHEV). Here also, a perturbation of membrane cholesterol fluidity (by cholesterol depletion with methyl-β-cyclodextrin in vitro) inhibited the virus endocytic route as well as the viral genome replication and viral protein synthesis (Li et al., 2017). Alternatively, PRRSV infection can be inhibited with the use of 25-hydroxycholesterol (25HC) which is normally formed by oxidation of cholesterol by the interferon-α-induced enzyme cholesterol 25-hydroxylase (CH25H). 25HC, as a regulator of cellular cholesterol biosynthesis, is a natural antiviral agent to combat infections by porcine viruses such as PRRSV and TGEV (porcine transmissible gastroenteritis virus) (Song et al., 2017; Song et al., 2019; Zhang et al., 2019) and other viruses (Yu et al., 2019). It would be interesting to evaluate the antiviral activity of a combination of GLR and 25HC.
9. Trapping of HMGB1 by GLR
The pharmacological action of GLR is not limited to the plasma membrane. The drug displays a marked anti-inflammatory activity and modulates the immune system, via an action on multiple pathways such as MAPK and Toll-like receptors signaling pathways (Zhao et al., 2016). The signaling activities of GLR likely derive from its binding to high-mobility group proteins B1 (HMGB1 and possibly HMGB2), thereby inhibiting the DNA-binding and phosphorylation of the protein (Sakamoto, Okano, Takena, & Ohtsuki, 2001). Structural studies have revealed that GLR can physically bind to HMGB1 and a modeling study suggested that the drug can also bind to the nuclear HMGB1-DNA complex (Yamaguchi, Kidachi, Kamiie, Noshita, & Umetsu, 2012). GLR shows a modest affinity for HMGB1 (Kd ~ 150 μM, an abundant protein) via a binding on the shallow concave surface formed by the two arms of the HMGB protein, as represented in Fig. 6 . GLR derivatives bearing amino acid residues on the carbohydrate chain have been shown also to bind to HMGB1 and block its activity, but they were not significantly more potent than GLR itself (Du et al., 2013). GLR does not distort the protein structure upon binding but forms stable kissing complexes with the HMG protein. The stability of the drug-protein complex is mainly assured by favorable hydrophobic and electrostatic interactions between the triterpene scaffold of GLR and several key amino acid residues of the proteins (such as Y15, F37, A16 and V19) (Mollica et al., 2007).
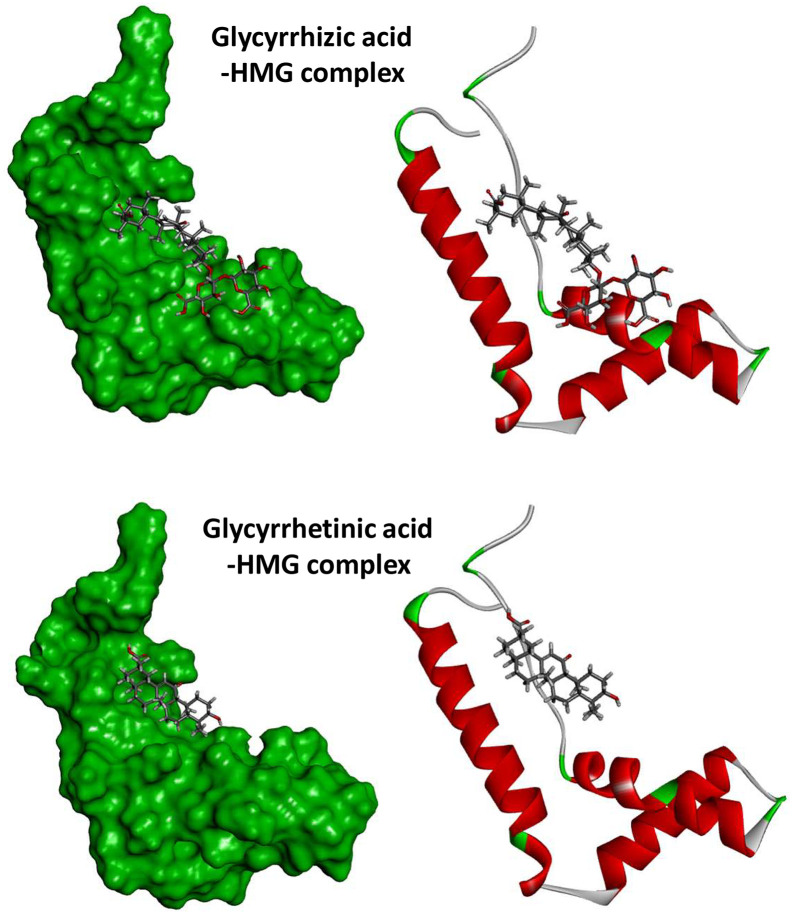
Fig. 6.
Global view of the complexes formed between GLR (top) or GA (bottom) with the HMG box motif of the B domain of HMG1 (PDB code: 1HME). The procedure used to construct the models is described Table 2.
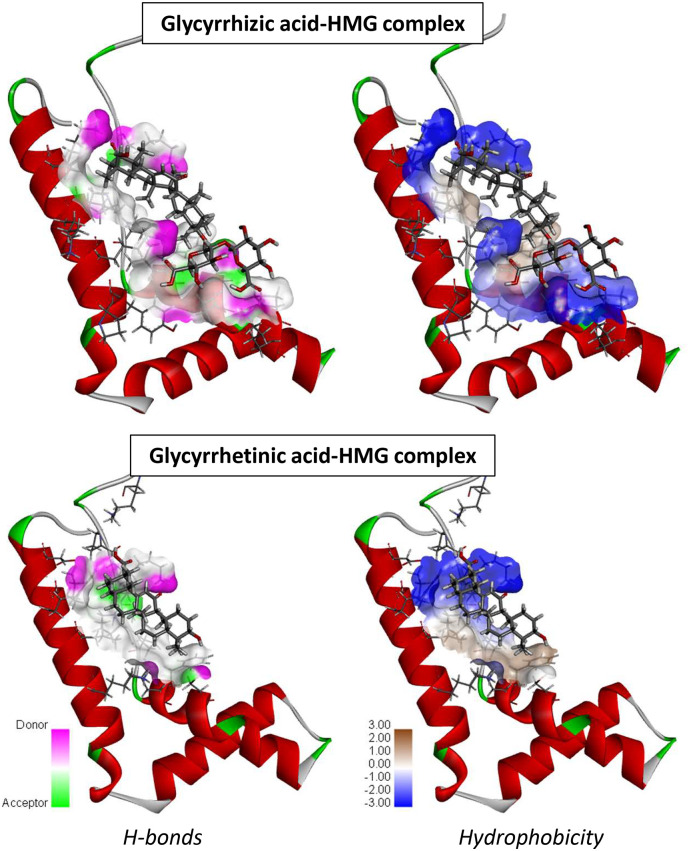
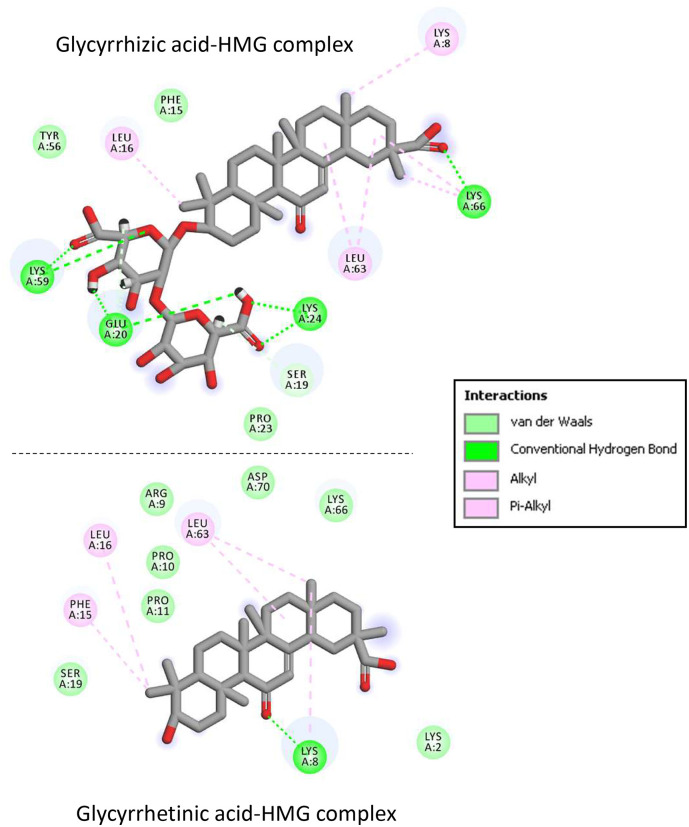
We have used molecular modeling to compare the overall binding of GLR and GA to HMGB1, on the basis of the crystallographic structure of the HMG box motif in the B domain of HMG1 (PDB code: 1HME) (Weir et al., 1993). It is an L-shaped small protein (77 amino acids) with three alpha-helices defining two arms (Fig. 6). Our molecular docking analysis indicates that GLR can form much more stable complexes with this HMG box domain than GA. The potential energy of interaction is 2.4 times better (more negative) with GLR than with GA. The Gibbs free energy of hydration (free enthalpy of hydration) is also much more favorable for GLR compared to GA (Table 2 ). The difference illustrates the major contribution of the carbohydrate moiety of GLR to the HMG interaction. A more detailed analysis of the binding shows the important contribution of both H-bonds and hydrophobic drug-protein interactions (Fig. 7 ). GLR, with a much higher number of H-bond donor/acceptor atoms (Table 1), is better adapted to fit into the concave side of the HMG box structure. The disaccharide motif of GLR sits on the L-structure, interacting with two alpha-helices. The contact map in Fig. 8 illustrates the higher number of interactions observed with the GLR-HMG structure, compared to the GA-HMG one, with in particular the major molecular contacts provided by the glycosyl moiety. In both cases, the aglycone hydrophobic core interacts about similarly with the protein (implicating residues Leu-16, Leu-63 and Lys-8, Lys-66 for both ligands) but in the case of GLR, the two glucuronic acid residues provide extra attachment sites to the protein (in particular with Lys-24, Lys-59 and Glu-20 residues). There is no doubt that GLR is a very well adapted HMG ligand. Our docking analysis is coherent with the published NMR structure of GLR bound to HMGB1 (Mollica et al., 2007) and highlights the contribution of the glycoside moiety of GLR to the protein interaction.
Table 2.
Calculated potential energy of interaction (ΔE) and free energy of hydration (ΔG) of the drug-HMG box complexes.a
| Compound | ΔE |
ΔG |
|---|---|---|
| (kcal/mol) | (kcal/mol) | |
| HMG box - glycyrrhizic acid (GLR) | −82.10 | −21.17 |
| HMG box - glycyrrhetinic acid (GA) | −33.62 | −14.77 |
In silico molecular docking procedure. The 3D structure of HMG box [Weir et al., 1993] was retrieved from the Protein Data Bank (www.rcsb.org) under the PDB code 1HME. Docking experiments were performed with the GOLD software (Cambridge Crystallographic Data Centre, Cambridge, UK). The drug-HMG structures have been optimized using a classical Monte Carlo conformational searching procedure as described in the BOSS software (Jorgensen & Tirado-Rives, 2005). Ligands are defined as flexible during the docking procedure. For each ligand, up to 30 poses that are energetically reasonable were kept while searching for the correct binding mode of the ligand. The decision to keep a trial pose is based on ranked poses, using the PLP fitness scoring function (which is the default in GOLD version 5.3 used here). In addition, an empirical potential energy of interaction ΔE for the ranked complexes is evaluated using the simple expression ΔE(interaction) = E(complex) - (E(protein) + E(ligand)). For that purpose, the Spectroscopic Empirical Potential Energy function SPASIBA and the corresponding parameters were used (Lagant et al., 2004; Vergoten et al., 2003). Molecular graphics and analysis were performed using the Discovery Studio 2020 Client software, Dassault Systemes Biovia Corp.
Fig. 7.
Views of the GLR- and GA-HMG complexes with a focus on the H-bond and hydrophobic surfaces exposed implicated in the protein complex formation. Note the specific contribution of the carbohydrate moiety of GLR to the protein interaction. In both cases, the specific color code is indicated.
Fig. 8.
Binding map contacts for GLR and GA bound to the HMG box.
The binding of GLR to HMGB1 is not extremely tight but sufficiently strong to perturb the various physiological activities of the protein, notably its interaction with other proteins such as the receptor for advanced glycation end products (RAGE), TLR2 and TLR4. The TLR4/HMGB1-dependent anti-inflammatory effects of GLR have been largely documented. In cases of viral hepatitis, GLR alleviates inflammation via a HMGB1-TLR4 signaling pathway (Shi et al., 2020). Inhibition of this axis also permits to reduce radiation-induced acute lung injury (Zheng et al., 2020) and to protect from ischemia/reperfusion injury (Yan et al., 2019). By decreasing TLRs activity, GLR can reduce the levels of inflammatory cytokines and the effects of different inflammatory mediators such as the TLR4 ligand nicotinate phosphoribosyltransferase (Managò et al., 2019). GLR has the capacity to sequester HMGB1, inhibiting its nuclear translocation and/or cellular release. It also inhibits the expression of the protein and downregulates the expression of inflammatory cytokines. Consequently, GLR interferes with several HMGB1-mediated pathological conditions, notably a variety of neurological disorders including traumatic brain injury, epileptic seizures, multiple sclerosis, and Alzheimer's and Parkinson diseases (Paudel et al., 2020). In fact, the successfully inhibition of HMGB1 by GLR translates into a variety of effects depending on the cell system and its environment. By modulating HMGB1 protein-binding, GLR (i) alters the epithelial-to-mesenchymal transition (Chang, Chen, Wu, Lu, & Yen, 2019), (ii) reduces cancer cell growth (Wu et al., 2018), (iii) reduces pain and inflammation in diabetic neuropathy conditions (Thakur, Sadanandan, & Chattopadhyay, 2020), (iv) attenuates hemorrhagic transformation in ischemic stroke (Chen et al., 2019), (v) inhibits the tendon's inflammatory reactions (Zhao et al., 2019), (vi) reduces the level of circulating, serum HMGB1 in models of lupus nephritis (Li, Yue, Zhu, & Xiong, 2015; Li, Zhao, et al., 2015) and atherosclerosis (Wang et al., 2018), (vii) attenuates chronic inflammatory pain (Sun et al., 2018). Given the central roles of HMGB1 both as a transcription regulator in the nucleus and as a circulating damage-associated molecular pattern (DAMP), the targeting of HMGB1 is considered a valid strategy in many diseases: cancer, autoimmune diseases, inflammatory heart diseases, neurological diseases, trauma (Musumeci, Roviello, & Montesarchio, 2014; Ugrinova & Pasheva, 2017; Venereau et al., 2016).
HMGB1 plays important roles in viral infections and the trapping of HMGB1 by GLR has multiple consequences in terms of viral pathogenicity. Different examples can be cited to underline the relationship between HMGB1-GLR binding and viral infection: (i) GLR reduces HMGB1 binding to DNA and thus inhibits influenza virus polymerase activity (Moisy et al., 2012); (ii) GLR inhibits HMGB1 upregulation in cells infected with the respiratory syncytial virus (RSV) and this effect is associated with significant reduction of viral replication (Manti, Harford, Salpietro, Rezaee, & Piedimonte, 2018); (iii) treatment of HBV-infected mice with GLR significantly decreases the intrahepatic recruitment of inflammatory cells (Sitia, Iannacone, Müller, Bianchi, & Guidotti, 2007), (iv) as mentioned above, inhibition of HMGB1 by GLR restricts the entry and replication of PEDV (Huan et al., 2017), (v) GLR blocks the release of HMGB1 by HSV-2 infected cells and thus abrogates HIV-1 reactivation (Borde et al., 2011), (vi) the drug can also favor the elimination of HIV-1-infected dendritic cells by this HMGB1 trapping mechanism (Melki, Saïdi, Dufour, Olivo-Marin, & Gougeon, 2010; Saïdi, Melki, & Gougeon, 2008). Moreover, HMGB1 inhibition attenuates the proinflammatory response engendered by the PEDV infection. Indeed, PEDV infection induces HMGB1 transcription and its subsequent release. GLR can be used to counteract this effect (Huan et al., 2016, Huan et al., 2017). Therefore, we could expect a similar advantage using GLR to alleviate the effects of COVID-19. A beneficial effect on the respiratory distress syndrome could be expected also because HMGB1 has a significant role in the development and progression of acute respiratory distress syndrome (ARDS), through the regulation of cytokines such as IL-33. As mentioned above, inhibition of HMGB1 release by GLR is associated with a decrease of the HMGB1-induced up-regulation of IL-33 expression in a mouse model of LPS-induced lung inflammation/injury (Fu et al., 2016).
A few other HMGB1 inhibitors have revealed interesting antiviral activities such as acteoside (a phenylpropanoid glycoside from Kuding Tea) which blocks HMGB1 release (Seo et al., 2013) and displays antiviral effects, presumably via its capacity to induce IFN-γ production (Song et al., 2016). Similarly, the non-narcotic alkaloid papaverine has been identified recently as a direct inhibitor of the HMGB1/RAGE interaction and a suppressor of the HMGB1-mediated production of pro-inflammatory cytokines (Tamada et al., 2019) and it is also active against influenza viruses and paramyxoviruses (Aggarwal, Leser, & Lamb, 2020). But in the present context, our favorite example is chloroquine which has been shown previously to inhibit HMGB1 release in different cell types (macrophages, monocytes, and endothelial cells) in a mouse model of endotoxemia or sepsis (Yang et al., 2013). Chloroquine downregulates the expression of HMGB1 and reduces the level of serum HMGB1 in a model of chemical-induced acute liver injury (Dai et al., 2018).
10. Other targets and mechanisms
In addition to HMG proteins, GLR has also been shown to bind to other proteins such as serum albumin (Wang et al., 2020; Wang et al., 2020; Wang, Shan, & Lü, 2020) and to recombinant HIV-1 reverse transcriptase to inhibit the protein phosphorylation (Harada et al., 1998). Binding of GA to the active site of lactate dehydrogenase from Plasmodium falciparum (malaria parasite) has been proposed based on molecular modeling, mimicking the binding of chloroquine to the same site and possibly explaining the modest antimalarial activity of GLR (Kalani et al., 2013). Another important enzyme target of GLR is 11β-hydroxysteroid dehydrogenase. 11β-HSD are enzymes involved in the regulation of the level of active glucocorticoids in different tissues. There are two isoforms, 11β-HSD1 and 11β-HSD2, with opposing functions. In Human, 11β-HSD2 catalyzes the reversible conversion of active cortisol to the inactive 11-keto derivative cortisone, whereas 11β-HSD1 can function both as a reductase and as a dehydrogenase to catalyze both the activation and deactivation of the glucocorticoid. GA is an efficient non-selective inhibitor of both enzymes (Kratschmar et al., 2011). This activity supports the use of GLR in the protection of bone against glucocorticoid-induced osteoporosis (Ramli, Suhaimi, Asri, Ahmad, & Soelaiman, 2013).
GLR is a multi-target compound and novel potential targets are regularly disclosed. For example, in silico molecular docking has predicted that GLR binds to the Nrf2 (Nuclear factor-E2 related factor 2) peptide binding site on Keap-1 (Kelch like ECH-associated protein 1) and thus can possibly function as a Nrf2 stimulator (Kamble et al., 2017). GLR exerts Nrf2-dependent activities (He et al., 2019; Mou et al., 2019) but thus far a direct, physical interaction with Keap-1 has not been evidenced. Another recent study revealed that GLR (as well as its analogues GA and carbenoxolone (18β-glycyrrhetinic acid 3β-O-hemisuccinate) are selective and competitive inhibitors of kynurenine aminotransferase 2 (KAT2), the enzyme which catalyzes the conversion of kynurenine to kynurenic acid (Yoshida et al., 2019). This inhibitory effect may contribute to the anti-Parkinson activity of GLR (Wang, Lian, et al., 2018). GLR can also bind very weakly to nucleic acids, both DNA and RNA (Nafisi, Bonsaii, Manouchehri, & Abdi, 2012; Nafisi, Manouchehri, & Bonsaii, 2012).
11. GLR and drug combinations: focus on chloroquine and tenofovir
GLR can be easily combined with many types of drugs, either to promote solubilization and bioavailability of the co-transported product as discussed above, or to complement its mechanism of action leading to synergistic effects in some cases (Liu, Huang, Li, Zheng, & Zhang, 2019; Zhang et al., 2017). We will not review here all combinations previously reported, but only focus on two combinations useful in the context of coronavirus infections, with the antiviral drugs chloroquine and tenofovir.
As mentioned above, chloroquine downregulates HMGB1 expression and reduces HMGB1 serum level in a model of acute liver injury (Dai et al., 2018). Given the therapeutic potential of chloroquine against SARS-CoV-2 (Cortegiani, Ingoglia, Ippolito, Giarratano, & Einav, 2020; Fantini et al., 2020), it could make sense to consider both GLR and chloroquine for the treatment of the current pandemia. Human viruses exploit the autophagy pathway to help viral propagation and escape immune response (Abdoli, Alirezaei, Mehrbod, & Forouzanfar, 2018). In particular, coronavirus infection has been demonstrated to induce autophagy (Cong, Verlhac, & Reggiori, 2017; Maier & Britton, 2012), notably through the membrane-associated papain-like protease PLP2 (PLP2-TM) acting as an autophagy-inducing protein, via a direct interaction with the key autophagy regulators LC3 and Beclin1 (Chen et al., 2014). For example, the porcine viruses PEDV and PHEV both induce autophagy to benefit their replication (Ding et al., 2017; Guo et al., 2017; Guo, Huang, et al., 2017). As another example, the mycotoxin ochratoxin A induces autophagy to promote porcine circovirus type 2 (PCV2) replication, whereas inhibitors of autophagy such as 3-methylademine and chloroquine significantly attenuate PCV2 replication (Qian et al., 2017). Consequently, interfering with the autophagy process could perturb coronavirus infection. Autophagy inhibition can be induced by chloroquine (or hydroxychloroquine), leading to inhibition of virus replication. For example, chloroquine-induced inhibition of autophagy suppresses the replication of the hepatitis C virus (Mizui et al., 2010). GLR has been shown also to modulate autophagy in different cell systems, although both inhibition (Jeon et al., 2019) and activation (Qu et al., 2019; Umar et al., 2019; Yang et al., 2018) of autophagy have been reported. However, it has been shown that HMGB1 translocation and release induce autophagy in lung macrophages and this process can be attenuated via the blockade of HMGB1 with GLR (Le et al., 2020). Moreover, the use of an autophagy inhibitor enhances the anticancer activity of GA (Chen et al., 2018; Shen et al., 2017). Thus, we believe that the combination of GLR and (hydroxy)chloroquine could be useful to inhibit coronavirus replication. Chloroquine is hydrophilic compound, relatively well absorbed orally and with good bioavailability. It exhibits linear absorption and clearance (Rainsford, Parke, Clifford-Rashotte, & Kean, 2015; Zhao, Tensfeldt, Chandra, & Mould, 2014). However, the entrapment of chloroquine into multilamellar vesicles has been shown to enhance drug delivery (Fotoran et al., 2019).
Tenofovir (Viread®, TDF, Fig. 9 ) is a nucleotide analog reverse-transcriptase inhibitor widely used as the first-line therapy to inhibit hepatitis B virus replication. It is also a recommended first-line drug for HIV treatment. The combination of tenofovir and GLR has been investigated in a pilot clinical study with a cohort of patients with severe acute exacerbation of chronic hepatitis B and showed that the early introduction of GLR can be safe and beneficial for those patients difficult to manage (Hung et al., 2017). The molecular basis of this benefit is not known but a hypothesis can be advanced because it was shown GA increases distribution in the cytoplasm and nucleus of liver cells of the related antiviral drug entecavir (Chen et al., 2017). On the other hand, a recent molecular modeling study has suggested that tenofovir binds tightly to the RNA-dependent RNA polymerase of the SARS-CoV-2 virus and thus could be a useful antiviral agent (Elfiky, 2020). A clinical trial including tenofovir and other antiviral agents is on-going in China (Zhai et al., 2020; Zhu, Wei, & Niu, 2020). The convergence of these pieces of information led us to consider also the combination of GLR and tenofovir as a potential anti-coronavirus approach.
Fig. 9.
Chemical structures of two saponins (platycodin D and saikosaponin A), and two drugs that could be combined with GLR (chloroquine and tenofovir), mentioned in this study.
12. Other saponins with activities against coronavirus
The membrane-perturbating effects of GLR have been also observed with other structurally related saponins such as platycodin D (Fig. 9) and β-escin (Bailly & Vergoten, 2020). These two natural products interact with cholesterol thereby modulating the organization of lipid rafts in membranes. This activity likely contributes to their biological activity, in particular to the antiviral effects reported with both products (Kim et al., 2017) and their adjuvant properties to increase immunogenicity of proteins and vaccines (Xie et al., 2009, Xie et al., 2010). Escin has been recently proposed as add-on therapy in acute lung injury related to COVID-19 infection (Gallelli, Zhang, Wang, & Fu, 2020). Platycodin D has been characterized as a potent inhibitor of PPRSV infection in vitro, directly inhibiting the virus replication and reducing the production of different virus-induced cytokines (Zhang et al., 2018). A recent modeling study indicated that this saponin derivative presents a high binding affinity to papain-like protease of SARS-CoV-2 (Wu et al., 2020). We must also mention the saikosaponins, structurally close to GLR, which have been characterized for their antiviral effects against coronavirus 229E. Saikosaponin B2 inhibited viral attachment and penetration, and virus infection (Cheng, Ng, Chiang, & Lin, 2006). This plant glycoside (a component of the traditional Chinese herbal medicine xiao-chai-hu-tang) is also active against the HBV and HCV viruses (Lee et al., 2019). Saikosaponin A (Fig. 9) was found to be active against different influenza A virus strains (including a pathogenic H5N1 strain) (Chen et al., 2015) and saikosaponins A and D showed interesting activities against porcine circovirus 2 (PCV2) (Yang, Chen, Jiang, He, & Hu, 2017), as well as PPRSV infections, enhancing the immune responses and decreasing the incidence and severity of PRRSV-induced immunopathological damages in vivo (Hu et al., 2020). We consider this information as indirect element to support the potential use of a saponin like GLR for the treatment of coronavirus infections. GLR is a relatively safe product, well tolerated, inducing limited undesirable effects and used for a long time in medicine, whereas saponins like platycodin D and saikosaponin A are only laboratory tools, not drugs. Moreover, the HMGB-1 binding activity is specific to GLR, therefore making this natural product a unique drug to be further considered.
13. Conclusion
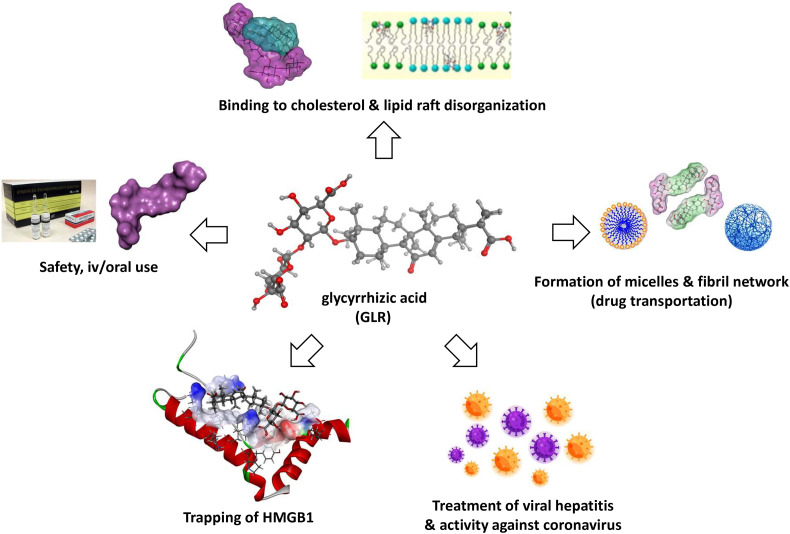
GLR is a well-established oriental phytomedicine used for a long time to treat hepatic disorders. The production of the drug is securitized, and drug products of good quality can be found easily. Our scientific analysis highlighted the following main characteristics (Fig. 10 ):
-
-
GLR is considered a safe natural product, with a long-established use in Human as a hepato-protecting agent. Adverse effects are relatively rare and manageable (Nazari, Rameshrad, & Hosseinzadeh, 2017).
-
-
GLR is used in Human for the treatment of chronic hepatitis (and other liver diseases) and has shown marked activity against some coronaviruses such as the porcine virus PEDV. It is also used to treat cutaneous inflammation.
-
-
The anti-inflammatory activity of GLR could be useful to alleviate the respiratory distress syndrome associated to the viral infection.
-
-
There is no obstacle to the combination of GLR with a variety of drugs and its combination with antiviral drugs such as chloroquine or tenofovir could be beneficial. GLR, which can be administered iv and orally, can serve as a codrug to increase the bioavailability of poorly soluble products. It makes sense also to combine GLR with other antiviral agents having different mechanisms to reinforce the antiviral response (combination of cytoplasmic and membrane effects).
-
-
GLR induces cholesterol-dependent disorganization of lipid rafts which are important for the entry of coronavirus into cells. These membrane effects, also observed with other amphiphilic saponins, likely play an important role in the antiviral activity. GLR is considered a non-hemolytic saponin.
-
-
GLR is an efficient binder of HMGB1. The glycoside moiety of GLR plays a major role in the interaction with the HMG box protein. Considering the multiple functions of HMGB1 in viral infections and replication, the trapping of HMGB1 by GLR could contribute significantly to a diminution of the virus-induced excessive inflammatory response and the viral replication.
Fig. 10.
Summary of the main characteristics and properties of GLR, supporting its potential activity against the SARS-CoV-2 coronavirus. The drug is safe, used for a long time to treat viral hepatitis (iv and oral formulations available). GLR binds to cholesterol, thereby affecting the organization of lipid rafts that are essential for the entry of the virus into cells. GLR forms stable complexes with HMGB1 protein, thereby blocking the propagation of the danger signals. GLR displays antiviral activities against multiple viruses, including hepatitis virus A-B-C and some coronavirus.
For all these reasons, we believe that GLR should be rapidly tested as an anti-SARS-CoV-2 agent, alone and in combination with other drugs (notably CQ/HCQ and tenofovir) to combat the current COVID-19 pandemic.
While this manuscript was reviewed, three groups also proposed the use of GLR, alone or in combination with other drugs, to treat coronavirus infections (Chen et al., 2020; Luo, Liu, & Li, 2020; Zhao et al., 2020). Notably, one study underlined the capacity of GLR to bind to the angiotensin converting enzyme 2 (ACE2) which represents a SARS-CoV-2 receptor. Therefore, the targeting of ACE2 could be very useful to inhibit the virus from diffusing out of infected cells and to enter new cells (Luo et al., 2020). Even more recently, a study reported interesting clinical data for a patient with severe COVID-19 who recovered upon treatment with diammonium glycyrrhizinate (Ding et al., 2020). These data are encouraging and support our proposal to clinically evaluate GLR as a drug to treat SARS-CoV-2 infections, considering notably that the drug and derivatives have shown activity against other SARS-coronavirus (Hoever et al., 2005). The risk of hypertension induced by GLR, due to pseudo-hyperaldosteronism, should not be neglected (Li, Li, et al., 2018; Li, Qi, et al., 2018) but in the current situation, without an efficient treatment, it is worth evaluating the antiviral benefit of this drug of natural origin.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
The authors thank Prof. Xiao-Quan Yang (Research and Development Centre of Food Proteins, Department of Food Science and Technology, South China University of Technology, Guangzhou 510640, People's Republic of China) who kindly provided the illustrations of GLR nanofibrils presented in Fig. 4.
References
- Abdoli A., Alirezaei M., Mehrbod P., Forouzanfar F. Autophagy: The multi-purpose bridge in viral infections and host cells. Reviews in Medical Virology. 2018;28 doi: 10.1002/rmv.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal M., Leser G.P., Lamb R.A. Repurposing papaverine as an antiviral agent against influenza viruses and paramyxoviruses. Journal of Virology. 2020;94 doi: 10.1128/JVI.01888-19. (e01888-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., Abd El-Hack M.E., Khafaga A.F., Taha A.E.…Dhama K. Use of licorice (Glycyrrhiza glabra) herb as a feed additive in poultry: Current knowledge and prospects. Animals (Basel) 2019;9 doi: 10.3390/ani9080536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq U.A., Masoud M.S., Nawaz Z., Riazuddin S. Glycyrrhizin as antiviral agent against Hepatitis C Virus. Journal of Translational Medicine. 2011;9:112. doi: 10.1186/1479-5876-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgustinovich D., Tsyganov M., Vishnivetskaya G., Kovner A., Sorokina I., Orlovskaya I.…Mordvinov V. Effects of supramolecular complexation of praziquantel with disodium glycyrrhizinate on the liver fluke Opisthorchis felineus: An in vitro and in vivo study. Acta Tropica. 2019;194:1–12. doi: 10.1016/j.actatropica.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Baba M., Shigeta S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antiviral Research. 1987;7:99–107. doi: 10.1016/0166-3542(87)90025-8. [DOI] [PubMed] [Google Scholar]
- Baglivo M., Baronio M., Natalini G., Beccari T., Chiurazzi P., Fulcheri E.…Bertelli M. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: a possible strategy for reducing SARS-COV-2 infectivity? Acta BioMedica. 2020;91:161–164. doi: 10.23750/abm.v91i1.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C., Vergoten G. Proposed mechanisms for the extracellular release of PD-L1 by the anticancer saponin platycodin D. International Immunopharmacology. 2020;85:106675. doi: 10.1016/j.intimp.2020.106675. (INTIMP_106675) [DOI] [PubMed] [Google Scholar]
- Baltina L.A., Tasi Y.T., Huang S.H., Lai H.C., Baltina L.A., Petrova S.F.…Lin C.W. Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorganic and Medicinal Chemistry Letters. 2019;29:126645. doi: 10.1016/j.bmcl.2019.126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S.A., Devaux C., Colson P., Raoult D., Rolain J.M. Teicoplanin: An alternative drug for the treatment of COVID-19? International Journal of Antimicrobial Agents. 2020;55:105944. doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz G.L., Lowrey A.J., Horne D.C., Nguyen V., Satterfield A.R., Ross T.D.…McKallip R.J. Using glycyrrhizic acid to target sumoylation processes during Epstein-Barr virus latency. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borde C., Barnay-Verdier S., Gaillard C., Hocini H., Maréchal V., Gozlan J. Stepwise release of biologically active HMGB1 during HSV-2 infection. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briolant S., Garin D., Scaramozzino N., Jouan A., Crance J.M. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antiviral Research. 2004;61:111–117. doi: 10.1016/j.antiviral.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Cai J., Luo S., Lv X., Deng Y., Huang H., Zhao B.…Li G. Formulation of injectable glycyrrhizic acid-hydroxycamptothecin micelles as new generation of DNA topoisomerase I inhibitor for enhanced antitumor activity. International Journal of Pharmacy. 2019;571:118693. doi: 10.1016/j.ijpharm.2019.118693. [DOI] [PubMed] [Google Scholar]
- Cai Y., Xu Y., Chan H.F., Fang X., He C., Chen M. Glycyrrhetinic acid mediated drug delivery carriers for hepatocellular carcinoma therapy. Molecular Pharmacology. 2016;13:699–709. doi: 10.1021/acs.molpharmaceut.5b00677. [DOI] [PubMed] [Google Scholar]
- Cao Y., Shi H., Sun Z., Wu J., Xia Y., Wang Y.…Lu Y. Protective effects of magnesium glycyrrhizinate on methotrexate-induced hepatotoxicity and intestinal toxicity may be by reducing COX-2. Frontiers in Pharmacology. 2019;10:119. doi: 10.3389/fphar.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z.Y., Liu Y.Z., Li J.M., Ruan Y.M., Yan W.J., Zhong S.Y.…Jiang C.L. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: A randomized placebo-controlled clinical trial. Journal of Affective Disorders. 2020;265:247–254. doi: 10.1016/j.jad.2020.01.048. [DOI] [PubMed] [Google Scholar]
- Castangia I., Caddeo C., Manca M.L., Casu L., Latorre A.C., Díez-Sales O.…Manconi M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydrate Polymers. 2015;134:657–663. doi: 10.1016/j.carbpol.2015.08.037. [DOI] [PubMed] [Google Scholar]
- Chang H.Y., Chen S.Y., Wu C.H., Lu C.C., Yen G.C. Glycyrrhizin attenuates the process of epithelial-to-mesenchymal transition by modulating hmgb1 initiated novel signaling pathway in prostate cancer cells. Journal of Agriculture and Food Chemistry. 2019;67:3323–3332. doi: 10.1021/acs.jafc.9b00251. [DOI] [PubMed] [Google Scholar]
- Chen F., Chan K.H., Jiang Y., Kao R.Y., Lu H.T., Fan K.W.…Yuen K.Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. Journal of Clinical Virology. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Guan B., Wang B., Pu H., Bai X., Chen X.…Shen J. Glycyrrhizin prevents hemorrhagic transformation and improves neurological outcome in ischemic stroke with delayed thrombolysis through targeting peroxynitrite-mediated hmgb1 signaling. Translational Stroke Research. 2019 doi: 10.1007/s12975-019-00772-1. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Chen J., Duan M., Zhao Y., Ling F., Xiao K., Li Q.…Chen W. Saikosaponin A inhibits influenza A virus replication and lung immunopathology. Oncotarget. 2015;6:42541–42556. doi: 10.18632/oncotarget.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zhang Z.Q., Jia Song J., Liu Q.M., Wang C., Huang Z.…Chen X.P. 18β-Glycyrrhetinic-acid-mediated unfolded protein response induces autophagy and apoptosis in hepatocellular carcinoma. Scientific Reports. 2018;8:9365. doi: 10.1038/s41598-018-27142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Yang R., Shen F., Zhu H.L. Advances in pharmacological activities and mechanisms of glycyrrhizic acid. Current Medicinal Chemistry. 2019 doi: 10.2174/0929867325666191011115407. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., Du J. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: A perspective from system biology analysis. Nutrients. 2020;12:1193. doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Chen H., Wang W., Liu J., Liu W., Ni P.…Zhang J. Glycyrrhetic acid, but not glycyrrhizic acid, strengthened entecavir activity by promoting its subcellular distribution in the liver via efflux inhibition. European Journal of Pharmaceutical Sciences. 2017;106:313–327. doi: 10.1016/j.ejps.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Chen S., He H., Yang H., Tan B., Liu E., Zhao X., Zhao Y. The role of lipid rafts in cell entry of human metapneumovirus. Journal of Medical Virology. 2019;91:949–957. doi: 10.1002/jmv.25414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wang K., Xing Y., Tu J., Yang X., Zhao Q.…Chen Z. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein & Cell. 2014;5:912–927. doi: 10.1007/s13238-014-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.W., Ng L.T., Chiang L.C., Lin C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clinical and Experimental Pharmacology and Physiology. 2006;33:612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.S., Aizaki H., Lai M.M. Murine coronavirus requires lipid rafts for virus entry and cell-cell fusion but not for virus release. Journal of Virology. 2005;79:9862–9871. doi: 10.1128/JVI.79.15.9862-9871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. The Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Verlhac P., Reggiori F. The interaction between nidovirales and autophagy components. Viruses. 2017;9:E182. doi: 10.3390/v9070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. Journal of Critical Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coviello T., Trotta A.M., Marianecci C., Carafa M., Di Marzio L., Rinaldi F.…Matricardi P. Gel-embedded niosomes: Preparation, characterization and release studies of a new system for topical drug delivery. Colloids and Surfaces B: Biointerfaces. 2015;125:291–299. doi: 10.1016/j.colsurfb.2014.10.060. [DOI] [PubMed] [Google Scholar]
- Crance J.M., Biziagos E., Passagot J., van Cuyck-Gandré H., Deloince R. Inhibition of hepatitis A virus replication in vitro by antiviral compounds. Journal of Medical Virology. 1990;31:155–160. doi: 10.1002/jmv.1890310214. [DOI] [PubMed] [Google Scholar]
- Crance J.M., Lévêque F., Biziagos E., van Cuyck-Gandré H., Jouan A., Deloince R. Studies on mechanism of action of glycyrrhizin against hepatitis A virus replication in vitro. Antiviral Research. 1994;23:63–76. doi: 10.1016/0166-3542(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Dai C., Xiao X., Li D., Tun S., Wang Y., Velkov T., Tang S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death & Disease. 2018;9:1164. doi: 10.1038/s41419-018-1136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? International Journal of Antimicrobial Agents. 2020;55:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Deng W., Ding L., Ye X., Yin S., Huang W. Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in nonhospitalized COVID-19 patients. Journal of Medical Virology. 2020 doi: 10.1002/jmv.26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N., Zhao K., Lan Y., Li Z., Lv X., Su J.…He W. Induction of atypical autophagy by porcine hemagglutinating encephalomyelitis virus contributes to viral replication. Frontiers in Cellular and Infection Microbiology. 2017;7:56. doi: 10.3389/fcimb.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discovery and Therapeutics. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Du D., Yan J., Ren J., Lv H., Li Y., Xu S.…Yu S. Synthesis, biological evaluation, and molecular modeling of glycyrrhizin derivatives as potent high-mobility group box-1 inhibitors with anti-heart-failure activity in vivo. Journal of Medicinal Chemistry. 2013;56:97–108. doi: 10.1021/jm301248y. [DOI] [PubMed] [Google Scholar]
- Duan E., Wang D., Fang L., Ma J., Luo J., Chen H.…Xiao S. Suppression of porcine reproductive and respiratory syndrome virus proliferation by glycyrrhizin. Antiviral Research. 2015;120:122–125. doi: 10.1016/j.antiviral.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sciences. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saber Batiha G., Magdy Beshbishy A., El-Mleeh A., Abdel-Daim M.M., Prasad D.H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae) Biomolecules. 2020;10:E352. doi: 10.3390/biom10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J., Scala C.D., Chahinian H., Yahi N. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. International Journal of Antimicrobial Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotoran W.L., Müntefering T., Kleiber N., Miranda B.N.M., Liebau E., Irvine D.J., Wunderlich G. A multilamellar nanoliposome stabilized by interlayer hydrogen bonds increases antimalarial drug efficacy. Nanomedicine. 2019;22 doi: 10.1016/j.nano.2019.102099. [DOI] [PubMed] [Google Scholar]
- Fu J., Lin S.H., Wang C.J., Li S.Y., Feng X.Y., Liu Q., Xu F. HMGB1 regulates IL-33 expression in acute respiratory distress syndrome. International Immunopharmacology. 2016;38:267–274. doi: 10.1016/j.intimp.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Fu Y., Zhou E., Wei Z., Liang D., Wang W., Wang T.…Yang Z. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS Journal. 2014;281:2543–2557. doi: 10.1111/febs.12801. [DOI] [PubMed] [Google Scholar]
- Fu Y., Zhou E., Wei Z., Song X., Liu Z., Wang T.…Yang Z. Glycyrrhizin inhibits lipopolysaccharide-induced inflammatory response by reducing TLR4 recruitment into lipid rafts in RAW264.7 cells. Biochimica et Biophysica Acta. 2014;1840:1755–1764. doi: 10.1016/j.bbagen.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Galbiati V., Papale A., Galli C.L., Marinovich M., Corsini E. Role of ROS and HMGB1 in contact allergen-induced IL-18 production in human keratinocytes. The Journal of Investigative Dermatology. 2014;134:2719–2727. doi: 10.1038/jid.2014.203. [DOI] [PubMed] [Google Scholar]
- Gallelli L., Zhang L., Wang T., Fu F. Severe acute lung injury related to COVID-19 infection: A review and the possible role for Escin. Journal of Clinical Pharmacology. 2020 doi: 10.1002/jcph.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Bioscience Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gao M., Li X., He L., Yang J., Ye X., Xiao F., Wei H. Diammonium glycyrrhizinate mitigates liver injury via inhibiting proliferation of NKT cells and promoting proliferation of tregs. Drug Design and Development Therapeutics. 2019;13:3579–3589. doi: 10.2147/DDDT.S220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M.…Raoult D. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gilabert-Oriol R., Mergel K., Thakur M., von Mallinckrodt B., Melzig M.F., Fuchs H., Weng A. Real-time analysis of membrane permeabilizing effects of oleanane saponins. Bioorganic and Medicinal Chemistry. 2013;21:2387–2395. doi: 10.1016/j.bmc.2013.01.061. [DOI] [PubMed] [Google Scholar]
- Guo H., Huang M., Yuan Q., Wei Y., Gao Y., Mao L.…Sun S. The important role of lipid raft-mediated attachment in the infection of cultured cells by coronavirus infectious bronchitis virus beaudette strain. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Zhang M., Zhang X., Tan X., Guo H., Zeng W.…He Q. Porcine epidemic diarrhea virus induces autophagy to benefit its replication. Viruses. 2017;9 doi: 10.3390/v9030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochemical Journal. 2005;392:191–199. doi: 10.1042/BJ20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Maekawa T., Haneda E., Morikawa Y., Nagata N., Ohtsuki K. Biochemical characterization of recombinant HIV-1 reverse transcriptase (rRT) as a glycyrrhizin-binding protein and the CK-II-mediated stimulation of rRT activity potently inhibited by glycyrrhetinic acid derivative. Biological and Pharmaceutical Bulletin. 1998;21:1282–1285. doi: 10.1248/bpb.21.1282. [DOI] [PubMed] [Google Scholar]
- Hattori T., Ikematsu S., Koito A., Matsushita S., Maeda Y., Hada M.…Takatsuki K. Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antiviral Research. 1989;11:255–261. doi: 10.1016/0166-3542(89)90035-1. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Yokoshima K., Chiba R., Fujii I., Fattokhov I., Saidov M. Field survey of glycyrrhiza plants in Central Asia (5). Chemical characterization of G. bucharica collected in Tajikistan. Chemical and Pharmaceutical Bulletin (Tokyo) 2019;67:534–539. doi: 10.1248/cpb.c18-00881. [DOI] [PubMed] [Google Scholar]
- He H., Wei D., Liu H., Zhu C., Lu Y., Ke Z.…Huang J. Glycyrrhizin protects against sodium iodate-induced RPE and retinal injury though activation of AKT and Nrf2/HO-1 pathway. Journal of Cellular and Molecular Medicine. 2019;23:3495–3504. doi: 10.1111/jcmm.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A.…Cinatl J., Jr. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. Journal of Medicinal Chemistry. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang B., Wang W., Zhou J., Li B., He K. Therapeutic effects of saponin components on porcine reproductive and respiratory syndrome virus-infected piglets. Journal of Animal Physiology and Animal Nutrition (Berl) 2020;104:637–644. doi: 10.1111/jpn.13302. [DOI] [PubMed] [Google Scholar]
- Huan C.C., Wang H.X., Sheng X.X., Wang R., Wang X., Liao Y.…Mao X. Porcine epidemic diarrhea virus nucleoprotein contributes to HMGB1 transcription and release by interacting with C/EBP-β. Oncotarget. 2016;7:75064–75080. doi: 10.18632/oncotarget.11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan C.C., Wang H.X., Sheng X.X., Wang R., Wang X., Mao X. Glycyrrhizin inhibits porcine epidemic diarrhea virus infection and attenuates the proinflammatory responses by inhibition of high mobility group box-1 protein. Archives of Virology. 2017;162:1467–1476. doi: 10.1007/s00705-017-3259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Chen X., Li Q., Li P., Zhao G., Xu M., Xie P. Inhibition of intercellular adhesion in herpex simplex virus infection by glycyrrhizin. Cell Biochemistry and Biophysics. 2012;62:137–140. doi: 10.1007/s12013-011-9271-8. [DOI] [PubMed] [Google Scholar]
- Hung C.H., Kee K.M., Chen C.H., Tseng P.L., Tsai M.C., Chen C.H.…Lu S.N. A randomized controlled trial of glycyrrhizin plus tenofovir vs. tenofovir in chronic hepatitis B with severe acute exacerbation. Clinical and Translational Gastroenterology. 2017;8 doi: 10.1038/ctg.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiuchi K., Morinaga O., Ohkita T., Tian C., Hirasawa A., Mitamura M.…Makino T. 18β-glycyrrhetyl-3-O-sulfate would be a causative agent of licorice-induced pseudoaldosteronism. Scientific Reports. 2019;9:1587. doi: 10.1038/s41598-018-38182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., Baba M.…Yamamoto N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV) Antiviral Research. 1988;10:289–298. doi: 10.1016/0166-3542(88)90047-2. [DOI] [PubMed] [Google Scholar]
- Jeon J.H., Lee C. Cellular cholesterol is required for porcine nidovirus infection. Archives of Virology. 2017;162:3753–3767. doi: 10.1007/s00705-017-3545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y.R., Roh H., Jung J.H., Ahn H.M., Lee J.H., Yun C.O., Lee W.J. Antifibrotic effects of high-mobility group box 1 protein inhibitor (Glycyrrhizin) on keloid fibroblasts and keloid spheroids through reduction of autophagy and induction of apoptosis. International Journal of Molecular Sciences. 2019;20 doi: 10.3390/ijms20174134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Wang C., Wang Y., Pan G., Jiang M., Li Z., Zhu Y. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. Scientific World Journal. 2015;2015:731765. doi: 10.1155/2015/731765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen W.L., Tirado-Rives J. Molecular modeling of organic and biomolecular systems using BOSS and MCPRO. Journal of Computer Chemistry. 2005;26:1689–1700. doi: 10.1002/jcc.20297. [DOI] [PubMed] [Google Scholar]
- Kalani K., Agarwal J., Alam S., Khan F., Pal A., Srivastava S.K. In silico and in vivo anti-malarial studies of 18β glycyrrhetinic acid from Glycyrrhiza glabra. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble S.M., Patel H.M., Goyal S.N., Noolvi M.N., Mahajan U.B., Ojha S., Patil C.R. In silico Evidence for binding of pentacyclic triterpenoids to Keap1-Nrf2 protein-protein binding site. Combination Chemistry and High Throughput Screening. 2017;20:215–234. doi: 10.2174/1386207319666161214111822. [DOI] [PubMed] [Google Scholar]
- Kim A.V., Shelepova E.A., Selyutina O.Y., Meteleva E.S., Dushkin A.V., Medvedev N.N.…Lyakhov N.Z. Glycyrrhizin-assisted transport of praziquantel anthelmintic drug through the lipid membrane: An experiment and MD simulation. Molecular Pharmocology. 2019;16:3188–3198. doi: 10.1021/acs.molpharmaceut.9b00390. [DOI] [PubMed] [Google Scholar]
- Kim J.W., Ha T.K., Cho H., Kim E., Shim S.H., Yang J.L., Oh W.K. Antiviral escin derivatives from the seeds of Aesculus turbinata Blume (Japanese horse chestnut) Bioorganic and Medicinal Chemistry Letters. 2017;27:3019–3025. doi: 10.1016/j.bmcl.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D., Wang Z., Tian J., Liu T., Zhou H. Glycyrrhizin inactivates toll-like receptor (TLR) signaling pathway to reduce lipopolysaccharide-induced acute lung injury by inhibiting TLR2. Journal of Cellular Physiology. 2019;234:4597–4607. doi: 10.1002/jcp.27242. [DOI] [PubMed] [Google Scholar]
- Kowalska A., Kalinowska-Lis U. 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. International Journal of Cosmetic Sciences. 2019;41:325–331. doi: 10.1111/ics.12548. [DOI] [PubMed] [Google Scholar]
- Kratschmar D.V., Vuorinen A., Da Cunha T., Wolber G., Classen-Houben D., Doblhoff O.…Odermatt A. Characterization of activity and binding mode of glycyrrhetinic acid derivatives inhibiting 11β-hydroxysteroid dehydrogenase type 2. Journal of Steroid Biochemistry and Molecular Biology. 2011;125:129–142. doi: 10.1016/j.jsbmb.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Kwon Y.J., Son D.H., Chung T.H., Lee Y.J. A review of the pharmacological efficacy and safety of licorice root from corroborative clinical trial findings. Journal of Medical Food. 2020;23:12–20. doi: 10.1089/jmf.2019.4459. [DOI] [PubMed] [Google Scholar]
- Lagant P., Nolde D., Stote R., Vergoten G., Karplus M. Increasing normal modes analysis accuracy: The SPASIBA spectroscopic force field introduced into the CHARMM program. Journal of Physical Chemistry A. 2004;108:4019–4029. [Google Scholar]
- Le Y., Wang Y., Zhou L., Xiong J., Tian J., Yang X.…Sun Y. Cigarette smoke-induced HMGB1 translocation and release contribute to migration and NF-κB activation through inducing autophagy in lung macrophages. Journal of Cellular and Molecular Medicine. 2020;24:1319–1331. doi: 10.1111/jcmm.14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.A., Lee S.H., Kim J.Y., Lee W.S. Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model. Journal of Thoracic Diseases. 2019;11:1287–1302. doi: 10.21037/jtd.2019.04.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Bae I.H., Choi H., Choi H.W., Oh S., Marinho P.A.…Lee J. Ameliorating effect of dipotassium glycyrrhizinate on an IL-4- and IL-13-induced atopic dermatitis-like skin-equivalent model. Archives of Dermatology Research. 2019;311:131–140. doi: 10.1007/s00403-018-1883-z. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Kang B., Bok S.H., Cho S.S., Park D.H. Macmoondongtang modulates Th1-/Th2-related cytokines and alleviates asthma in a murine model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.P., Lan K.L., Liao S.X., Huang Y.H., Hou M.C., Lan K.H. Antiviral effect of saikosaponin B2 in combination with daclatasvir on NS5A resistance-associated substitutions of hepatitis C virus. Journal of Chinese Medical Association. 2019;82:368–374. doi: 10.1097/JCMA.0000000000000095. [DOI] [PubMed] [Google Scholar]
- Li D.M., Qi R.H., Zhang H.C., Liao X., Xie Y.M., Zhang J.H., Zhang B.L. Clinical application evaluation and revision suggestions of clinical practice guideline on traditional Chinese medicine therapy alone or combined with antibiotics for community acquired pneumonia. Zhongguo Zhong Yao Za Zhi. 2018;43:4759–4764. doi: 10.19540/j.cnki.cjcmm.20181009.006. [DOI] [PubMed] [Google Scholar]
- Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes and Infection. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yin J., Sui X., Li G., Ren X. Comparative analysis of the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian Pathology. 2009;38:215–221. doi: 10.1080/03079450902912184. [DOI] [PubMed] [Google Scholar]
- Li J.Y., Cao H.Y., Liu P., Cheng G.H., Sun M.Y. Glycyrrhizic acid in the treatment of liver diseases: Literature review. Biomedical Research International. 2014;2014:872139. doi: 10.1155/2014/872139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., He Q., Xu M., Li J., Liu X., Wan Z., Yang X. Food-grade emulsions and emulsion gels prepared by soy protein-pectin complex nanoparticles and glycyrrhizic acid nanofibrils. Journal of Agriculture and Food Chemistry. 2020;68:1051–1063. doi: 10.1021/acs.jafc.9b04957. [DOI] [PubMed] [Google Scholar]
- Li Q., Jiang T., Liu R., Feng X., Li C. Tuning the pH profile of β-glucuronidase by rational site-directed mutagenesis for efficient transformation of glycyrrhizin. Applied Microbiology and Biotechnology. 2019;103:4813–4823. doi: 10.1007/s00253-019-09790-3. [DOI] [PubMed] [Google Scholar]
- Li W., Guo F., Jiang X., Li Y., Li X., Yu Z. Compound ammonium glycyrrhizin protects hepatocytes from injury induced by lipopolysaccharide/florfenicol through oxidative stress and a MAPK pathway. Comparative Biochemistry and Physiology - Part C: Toxicology and Pharmacology. 2019;225:108585. doi: 10.1016/j.cbpc.2019.108585. [DOI] [PubMed] [Google Scholar]
- Li W., Li Y., Jiang X., Li X., Yu Z. Compound ammonium glycyrrhizin protects hepatocytes from injury induced by lipopolysaccharide/florfenicol through a mitochondrial pathway. Molecules. 2018;23 doi: 10.3390/molecules23092378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Sun R., Liu R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacology Research. 2019;144:210–226. doi: 10.1016/j.phrs.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Li X., Yue Y., Zhu Y., Xiong S. Extracellular, but not intracellular HMGB1, facilitates self-DNA induced macrophage activation via promoting DNA accumulation in endosomes and contributes to the pathogenesis of lupus nephritis. Molecular Immunology. 2015;65:177–188. doi: 10.1016/j.molimm.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Li Z., Zhao K., Lan Y., Lv X., Hu S., Guan J.…He W. Porcine hemagglutinating encephalomyelitis virus enters neuro-2a cells via clathrin-mediated endocytosis in a Rab5-, Cholesterol-, and pH-dependent manner. Journal of Virology. 2017;91 doi: 10.1128/JVI.01083-17. (e01083-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhao Y., Lin W., Ye M., Ling X. Rapid screening and identification of active ingredients in licorice extract interacting with V3 loop region of HIV-1 gp120 using ACE and CE-MS. Journal of Pharmaceutical and Biomedical Analysis. 2015;111:28–35. doi: 10.1016/j.jpba.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Lin J.C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antiviral Research. 2003;59:41–47. doi: 10.1016/s0166-3542(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Lin J.C., Cherng J.M., Hung M.S., Baltina L.A., Baltina L., Kondratenko R. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: Structure-activity relationships. Antiviral Research. 2008;79:6–11. doi: 10.1016/j.antiviral.2008.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H.…Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhu W., Han C., Sui X., Liu C., Ma X., Dong Y. Preparation of glycyrrhetinic acid liposomes using lyophilization monophase solution method: Preformulation, optimization, and in vitro evaluation. Nanoscale Research Letters. 2018;13:324. doi: 10.1186/s11671-018-2737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Huang S., Li Y., Zheng X., Zhang K. Synergistic effect of tolfenamic acid and glycyrrhizic acid on TPA-induced skin inflammation in mice. Medicinal Chemistry Communication. 2019;10:1819–1827. doi: 10.1039/c9md00345b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Liu D.X., Tam J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochemical and Biophysical Research Communications. 2008;369:344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu D., Li J. Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. International Journal of Antimicrobial Agents. 2020;55:105995. doi: 10.1016/j.ijantimicag.2020.105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Li Q., Du Z., Su E., Liu X., Wan Z., Yang X. A natural supramolecular saponin hydrogelator for creation of ultrastable and thermostimulable food‐grade foams. Advances in Material Interface. 2019;6 [Google Scholar]
- Maier H.J., Britton P. Involvement of autophagy in coronavirus replication. Viruses. 2012;4:3440–3451. doi: 10.3390/v4123440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malabed R., Hanashima S., Murata M., Sakurai K. Sterol-recognition ability and membrane-disrupting activity of Ornithogalum saponin OSW-1 and usual 3-O-glycosyl saponins. Biochimica Biophysica Acta Biomembrane. 2017;1859:2516–2525. doi: 10.1016/j.bbamem.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Managò A., Audrito V., Mazzola F., Sorci L., Gaudino F., Gizzi K.…Deaglio S. Extracellular nicotinate phosphoribosyltransferase binds Toll like receptor 4 and mediates inflammation. Nature Communications. 2019;10:4116. doi: 10.1038/s41467-019-12055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manti S., Harford T.J., Salpietro C., Rezaee F., Piedimonte G. Induction of high-mobility group Box-1 in vitro and in vivo by respiratory syncytial virus. Pediatric Research. 2018;83:1049–1056. doi: 10.1038/pr.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianecci C., Rinaldi F., Di Marzio L., Mastriota M., Pieretti S., Celia C.…Carafa M. Ammonium glycyrrhizinate-loaded niosomes as a potential nanotherapeutic system for anti-inflammatory activity in murine models. International Journal of Nanomedicine. 2014;9:635–651. doi: 10.2147/IJN.S55066. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mason R.P., Jacob R.F., Shrivastava S., Sherratt S.C.R., Chattopadhyay A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochimica et Biophysica Acta. 2016;1858:3131–3140. doi: 10.1016/j.bbamem.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuura T., Aoyagi H., Matsuda M., Hmwe S.S., Date T.…Aizaki H. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Miyajima R., Ishida Y., Karasawa S., Yoshimura T. Aggregate formation of glycyrrhizic acid. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2016;500:112–117. [Google Scholar]
- Melki M.T., Saïdi H., Dufour A., Olivo-Marin J.C., Gougeon M.L. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLoS Pathogens. 2010;6 doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteleva E.S., Chistyachenko Y.S., Suntsova L.P., Tsyganov M.A., Vishnivetskaya G.B., Avgustinovich D.F.…Lyakhov N.Z. Physicochemical properties and anti-opisthorchosis effect of mechanochemically synthesized solid compositions of praziquantel with glycyrrhizic acid disodium salt. Doklady Biochemistry and Biophysics. 2018;481:228–231. doi: 10.1134/S1607672918040142. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Geiler J., Naczk P., Sithisarn P., Leutz A., Doerr H.W., Cinatl J., Jr. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M., Geiler J., Naczk P., Sithisarn P., Ogbomo H., Altenbrandt B.…Cinatl J., Jr. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Medical Microbiology and Immunology. 2010;199:291–297. doi: 10.1007/s00430-010-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming L.J., Yin A.C. Therapeutic effects of glycyrrhizic acid. Natural Product Communications. 2013;8:415–418. [PubMed] [Google Scholar]
- Mizui T., Yamashina S., Tanida I., Takei Y., Ueno T., Sakamoto N.…Watanabe S. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. Journal of Gastroenterology. 2010;45:195–203. doi: 10.1007/s00535-009-0132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisy D., Avilov S.V., Jacob Y., Laoide B.M., Ge X., Baudin F.…Jestin J.L. HMGB1 protein binds to influenza virus nucleoprotein and promotes viral replication. Journal of Virology. 2012;86:9122–9133. doi: 10.1128/JVI.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica L., De Marchis F., Spitaleri A., Dallacosta C., Pennacchini D., Zamai M.…Bianchi M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chemistry and Biology. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Morinaga O., Ishiuchi K., Ohkita T., Tian C., Hirasawa A., Mitamura M.…Makino T. Isolation of a novel glycyrrhizin metabolite as a causal candidate compound for pseudoaldosteronism. Scientific Reports. 2018;8:15568. doi: 10.1038/s41598-018-33834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou K., Pan W., Han D., Wen X., Cao F., Miao Y., Li P. Glycyrrhizin protects human melanocytes from H2O2‑induced oxidative damage via the Nrf2‑dependent induction of HO‑1. International Journal of Molecular Medicine. 2019;44:253–261. doi: 10.3892/ijmm.2019.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci D., Roviello G.N., Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacology and Therapeutics. 2014;141:347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Nafisi S., Bonsaii M., Manouchehri F., Abdi K. Interaction of glycyrrhizin and glycyrrhetinic acid with DNA. DNA and Cell Biology. 2012;31:114–121. doi: 10.1089/dna.2011.1287. [DOI] [PubMed] [Google Scholar]
- Nafisi S., Manouchehri F., Bonsaii M. Study on the interaction of glycyrrhizin and glycyrrhetinic acid with RNA. Journal of Photochemistry and Photobiology B. 2012;111:27–34. doi: 10.1016/j.jphotobiol.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Nazari S., Rameshrad M., Hosseinzadeh H. Toxicological effects of Glycyrrhiza glabra (Licorice): A review. Phytotherapy Research. 2017;31:1635–1650. doi: 10.1002/ptr.5893. [DOI] [PubMed] [Google Scholar]
- Ochi M.M., Amoabediny G., Rezayat S.M., Akbarzadeh A., Ebrahimi B. In vitro co-delivery evaluation of novel pegylated nano-liposomal herbal drugs of silibinin and glycyrrhizic acid (nano-phytosome) to hepatocellular carcinoma cells. Cell Journal. 2016;18:135–148. doi: 10.22074/cellj.2016.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okda F.A., Yassein S., Ahmed A.R., Soufy H., Nasr S.M. Some haematological and biochemical investigations on duck virus hepatitis following administration of glycyrrhizin. ISRN Pharmacology. 2013;2013:849412. doi: 10.1155/2013/849412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytotherapy Research. 2018;32:2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel Y.N., Angelopoulou E., Semple B., Piperi C., Othman I., Shaikh M.F. Potential neuroprotective effect of the HMGB1 inhibitor glycyrrhizin in neurological disorders. ACS Chemical Neuroscience. 2020;11:485–500. doi: 10.1021/acschemneuro.9b00640. [DOI] [PubMed] [Google Scholar]
- Petramfar P., Hajari F., Yousefi G., Azadi S., Hamedi A. Efficacy of oral administration of licorice as an adjunct therapy on improving the symptoms of patients with Parkinson’s disease, A randomized double blinded clinical trial. Journal of Ethnopharmacology. 2020;247:112226. doi: 10.1016/j.jep.2019.112226. [DOI] [PubMed] [Google Scholar]
- Qian G., Liu D., Hu J., Gan F., Hou L., Chen X., Huang K. Ochratoxin A-induced autophagy in vitro and in vivo promotes porcine circovirus type 2 replication. Cell Death and Disease. 2017;8 doi: 10.1038/cddis.2017.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L., Chen C., He W., Chen Y., Li Y., Wen Y.…Shen L. Glycyrrhizic acid ameliorates LPS-induced acute lung injury by regulating autophagy through the PI3K/AKT/mTOR pathway. American Journal of Translational Research. 2019;11:2042–2055. [PMC free article] [PubMed] [Google Scholar]
- Ragino Y.I., Vavilin V.A., Salakhutdinov N.F., Makarova S.I., Stakhneva E.M., Safronova O.G.…Tolstikov G.A. Antioxidant and endothelium-stabilizing effects of simvaglyzin on rabbits with experimental hypercholesterolemia. Bulletin of Experimental Biology and Medicine. 2008;146:206–209. doi: 10.1007/s10517-008-0252-x. [DOI] [PubMed] [Google Scholar]
- Rainsford K.D., Parke A.L., Clifford-Rashotte M., Kean W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23:231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- Ramli E.S., Suhaimi F., Asri S.F., Ahmad F., Soelaiman I.N. Glycyrrhizic acid (GCA) as 11β-hydroxysteroid dehydrogenase inhibitor exerts protective effect against glucocorticoid-induced osteoporosis. Journal of Bone and Mineral Metabolism. 2013;31:262–273. doi: 10.1007/s00774-012-0413-x. [DOI] [PubMed] [Google Scholar]
- Roustit M., Guilhaumou R., Molimard M., Drici M.D., Laporte S. Montastruc JL; French Society of Pharmacology and Therapeutics (SFPT). (2020). Chloroquine and hydroxychloroquine in the management of COVID-19: Much kerfuffle but little evidence. Therapie. 2020 doi: 10.1016/j.therap.2020.05.010. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A., Adamcik J., Bolisetty S., Handschin S., Mezzenga R. Fibrillar networks of glycyrrhizic acid for hybrid nanomaterials with catalytic features. Angewandte Chemie International Edition in English. 2015;54:5408–5412. doi: 10.1002/anie.201411875. [DOI] [PubMed] [Google Scholar]
- Saïdi H., Melki M.T., Gougeon M.L. HMGB1-dependent triggering of HIV-1 replication and persistence in dendritic cells as a consequence of NK-DC cross-talk. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai-Sugino K., Uematsu J., Kamada M., Taniguchi H., Suzuki S., Yoshimi Y.…Komada H. Glycyrrhizin inhibits human parainfluenza virus type 2 replication by the inhibition of genome RNA, mRNA and protein syntheses. Drug Discoveries and Therapeutics. 2017;11:246–252. doi: 10.5582/ddt.2017.01048. [DOI] [PubMed] [Google Scholar]
- Sakamoto R., Okano M., Takena H., Ohtsuki K. Inhibitory effect of glycyrrhizin on the phosphorylation and DNA-binding abilities of high mobility group proteins 1 and 2 in vitro. Biological and Pharmaceutical Bulletin. 2001;24:906–911. doi: 10.1248/bpb.24.906. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Nakahara H., Uto T., Shoyama Y., Shibata O. Investigation of interfacial behavior of glycyrrhizin with a lipid raft model via a Langmuir monolayer study. Biochimica et Biophysica Acta. 2013;1828:1271–1283. doi: 10.1016/j.bbamem.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S., Uto T., Shoyama Y. Effect of glycyrrhetinic acid on lipid raft model at the air/water interface. Biochimica et Biophysica Acta. 2015;1848:434–443. doi: 10.1016/j.bbamem.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Takei M., Kobayashi M., Pollard R.B., Suzuki F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology. 2002–2003;70:229–236. doi: 10.1159/000069334. [DOI] [PubMed] [Google Scholar]
- Sato H., Goto W., Yamamura J., Kurokawa M., Kageyama S., Takahara T.…Shiraki K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Research. 1996;30:171–177. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- Schmid C., Dawid C., Peters V., Hofmann T. Saponins from European licorice roots (Glycyrrhiza glabra) Journal of Natural Products. 2018;81:1734–1744. doi: 10.1021/acs.jnatprod.8b00022. [DOI] [PubMed] [Google Scholar]
- Selyutina O.Y., Apanasenko I.E., Kim A.V., Shelepova E.A., Khalikov S.S., Polyakov N.E. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloids Surface B – Biointerfaces. 2016;147:459–466. doi: 10.1016/j.colsurfb.2016.08.037. [DOI] [PubMed] [Google Scholar]
- Selyutina O.Y., Polyakov N.E. Glycyrrhizic acid as a multifunctional drug carrier - From physicochemical properties to biomedical applications: A modern insight on the ancient drug. International Journal of Pharmacy. 2019;559:271–279. doi: 10.1016/j.ijpharm.2019.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E.S., Oh B.K., Pak J.H., Yim S.H., Gurunathan S., Kim Y.P., Lee K.J. Acteoside improves survival in cecal ligation and puncture-induced septic mice via blocking of high mobility group box 1 release. Molecules and Cells. 2013;35:348–354. doi: 10.1007/s10059-013-0021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong S.H., Nguyen D.H., Wagle A., Woo M.H., Jung H.A., Choi J.S. Experimental and computational study to reveal the potential of non-polar constituents from Hizikia fusiformis as dual protein tyrosine phosphatase 1B and α-glucosidase inhibitors. Marine Drugs. 2019;17 doi: 10.3390/md17050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri A., Masullo M., D’Urso G., Iranshahi M., Montoro P., Pizza C., Piacente S. In depth chemical investigation of Glycyrrhiza triphylla Fisch roots guided by a preliminary HPLC-ESIMSn profiling. Food Chemistry. 2018;248:128–136. doi: 10.1016/j.foodchem.2017.12.031. [DOI] [PubMed] [Google Scholar]
- Shelepova E.A., Kim A.V., Voloshin V.P., Medvedev N.N. Intermolecular voids in lipid bilayers in the presence of glycyrrhizic acid. Journal of Physical Chemistry B. 2018;122:9938–9946. doi: 10.1021/acs.jpcb.8b07989. [DOI] [PubMed] [Google Scholar]
- Shen S., Zhou M., Huang K., Wu Y., Ma Y., Wang J.…Fan S. Blocking autophagy enhances the apoptotic effect of 18β-glycyrrhetinic acid on human sarcoma cells via endoplasmic reticulum stress and JNK activation. Cell Death and Diseases. 2017;8 doi: 10.1038/cddis.2017.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Yu L., Zhang Y., Liu Z., Zhang H., Zhang Y.…Du P. Glycyrrhetinic acid alleviates hepatic inflammation injury in viral hepatitis disease via a HMGB1-TLR4 signaling pathway. International Immunopharmacology. 2020;84:106578. doi: 10.1016/j.intimp.2020.106578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X., Ge L., Xin H., Cao W., Sun X., Li W. Erythrodermic psoriasis with bullous pemphigoid: Combination treatment with methotrexate and compound glycyrrhizin. Diagnostic Pathology. 2014;9:102. doi: 10.1186/1746-1596-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia G., Iannacone M., Müller S., Bianchi M.E., Guidotti L.G. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. Journal of Leukocyte Biology. 2007;81:100–107. doi: 10.1189/jlb.0306173. [DOI] [PubMed] [Google Scholar]
- Song X., He J., Xu H., Hu X.P., Wu X.L., Wu H.Q.…He Z.D. The antiviral effects of acteoside and the underlying IFN-γ-inducing action. Food & Function. 2016;7:3017–3030. doi: 10.1039/c6fo00335d. [DOI] [PubMed] [Google Scholar]
- Song Z., Bai J., Nauwynck H., Lin L., Liu X., Yu J., Jiang P. 25-Hydroxycholesterol provides antiviral protection against highly pathogenic porcine reproductive and respiratory syndrome virus in swine. Veterinary Microbiology. 2019;231:63–70. doi: 10.1016/j.vetmic.2019.02.035. [DOI] [PubMed] [Google Scholar]
- Song Z., Zhang Q., Liu X., Bai J., Zhao Y., Wang X., Jiang P. Cholesterol 25-hydroxylase is an interferon-inducible factor that protects against porcine reproductive and respiratory syndrome virus infection. Veterinary Microbiology. 2017;210:153–161. doi: 10.1016/j.vetmic.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Soufy H., Yassein S., Ahmed A.R., Khodier M.H., Kutkat M.A., Nasr S.M., Okda F.A. Antiviral and immune stimulant activities of glycyrrhizin against duck hepatitis virus. African Journal of Traditional, Complementary and Alternative Medicines. 2012;9:389–395. doi: 10.4314/ajtcam.v9i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakhneva E.M., Vavilin V.A., Ragino Y.I., Safronova O.G., Shintyapina A.B., Ivanova M.V. Effects of simvaglyzin and atorvaglyzin on the expression of 3-hydroxy-3-methyl-glutaryl-CoA reductase in rat liver. Bulletin of Experimental and Biological Medicine. 2013;156:63–65. doi: 10.1007/s10517-013-2278-y. [DOI] [PubMed] [Google Scholar]
- Su X., Wu L., Hu M., Dong W., Xu M., Zhang P. Glycyrrhizic acid: A promising carrier material for anticancer therapy. Biomedicine & Pharmacotherapy. 2017;95:670–678. doi: 10.1016/j.biopha.2017.08.123. [DOI] [PubMed] [Google Scholar]
- Sun J., Chen Y., Fan X., Wang X., Han Q., Liu Z. Advances in the use of chloroquine and hydroxychloroquine for the treatment of COVID-19. Postgraduate Medicine. 2020 doi: 10.1080/00325481.2020.1778982. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zeng H., Wang Q., Yu Q., Wu J., Feng Y.…Zhang H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Experimental Cell Research. 2018;369:112–119. doi: 10.1016/j.yexcr.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Sun Y., Xiao S., Wang D., Luo R., Li B., Chen H., Fang L. Cellular membrane cholesterol is required for porcine reproductive and respiratory syndrome virus entry and release in MARC-145 cells. Science China Life Sciences. 2011;54:1011–1018. doi: 10.1007/s11427-011-4236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.G., Zhao T.T., Lu N., Yang Y.A., Zhu H.L. Research progress of glycyrrhizic acid on antiviral activity. Mini Review in Medicinal Chemistry. 2019;19:826–832. doi: 10.2174/1389557519666190119111125. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Tsukahara M., Akasaka Y., Inoue H. A highly sensitive LC-MS/MS method for simultaneous determination of glycyrrhizin and its active metabolite glycyrrhetinic acid: Application to a human pharmacokinetic study after oral administration. Biomedical Chromatography. 2017;31:4032. doi: 10.1002/bmc.4032. [DOI] [PubMed] [Google Scholar]
- Sviridov D., Mukhamedova N., Makarov A.A., Adzhubei A., Bukrinsky M. Co-morbidities of HIV infection: Role of Nef-induced impairment of cholesterol metabolism and lipid raft functionality. AIDS. 2019;34:1–13. doi: 10.1097/QAD.0000000000002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara T., Watanabe A., Shiraki K. Effects of glycyrrhizin on hepatitis B surface antigen: A biochemical and morphological study. Journal of Hepatology. 1994;21:601–609. doi: 10.1016/s0168-8278(94)80108-8. [DOI] [PubMed] [Google Scholar]
- Tamada K., Nakajima S., Ogawa N., Inada M., Shibasaki H., Sato A.…Tanuma S. Papaverine identified as an inhibitor of high mobility group box 1/receptor for advanced glycation end-products interaction suppresses high mobility group box 1-mediated inflammatory responses. Biochemical and Biophysical Research Communications. 2019;511:665–670. doi: 10.1016/j.bbrc.2019.01.136. [DOI] [PubMed] [Google Scholar]
- Tan Q.Y., Hu Q., Zhu S.N., Jia L.L., Xiao J., Su H.Z.…Jin J. Licorice root extract and magnesium isoglycyrrhizinate protect against triptolide-induced hepatotoxicity via up-regulation of the Nrf2 pathway. Drug Delivery. 2018;25:1213–1223. doi: 10.1080/10717544.2018.1472676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Horiuchi A., Yamaura T., Komatsu M., Yokoyama T., Okaniwa S.…Tanaka E. Efficacy and safety of addition of minor bloodletting (petit phlebotomy) in hepatitis C virus-infected patients receiving regular glycyrrhizin injections. Journal of Gastroenterology. 2009;44:577–582. doi: 10.1007/s00535-009-0034-x. [DOI] [PubMed] [Google Scholar]
- Tandon A., Tandon B.N., Bhujwala R.A. Clinical spectrum of acute sporadic hepatitis E and possible benefit of glycyrrhizin therapy. Hepatology Research. 2002;23:55–61. doi: 10.1016/s1386-6346(01)00155-3. [DOI] [PubMed] [Google Scholar]
- Thakur V., Sadanandan J., Chattopadhyay M. High-mobility group box 1 protein signaling in painful diabetic neuropathy. International Journal of Molecular Sciences. 2020;21 doi: 10.3390/ijms21030881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T., Hu H., Zhou J., Deng S., Zhang X., Tang W.…Liang J. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16 doi: 10.1002/smll.201906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres T., Puig L. Managing cutaneous immune-mediated diseases during the COVID-19 pandemic. American Journal of Clinical Dermatology. 2020;21:307–311. doi: 10.1007/s40257-020-00514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugrinova I., Pasheva E. HMGB1 protein: A therapeutic target inside and outside the cell. Advances in Protein Chemistry and Structural Biology. 2017;107:37–76. doi: 10.1016/bs.apcsb.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Umar S.A., Tanveer M.A., Nazir L.A., Divya G., Vishwakarma R.A., Tasduq S.A. Glycyrrhizic acid prevents oxidative stress mediated DNA damage response through modulation of autophagy in ultraviolet-B-irradiated human primary dermal fibroblasts. Cellular Physiology and Biochemistry. 2019;53:242–257. doi: 10.33594/000000133. [DOI] [PubMed] [Google Scholar]
- Vavilin V.A., Salakhutdinov N.F., Ragino I.I., Poliakov N.E., Taraban M.B., Leshina T.V.…Tolstikov G.A. Cholesterol lowering properties of a complex compound simvastatin with glycyrrhizic acid (simvaglyzin) in experimental models. Biomeditsinskaya Khimiya. 2008;54:301–313. [PubMed] [Google Scholar]
- Venereau E., De Leo F., Mezzapelle R., Careccia G., Musco G., Bianchi M.E. HMGB1 as biomarker and drug target. Pharmacological Research. 2016;111:534–544. doi: 10.1016/j.phrs.2016.06.031. [DOI] [PubMed] [Google Scholar]
- Vergoten G., Mazur I., Lagant P., Michalski J.C., Zanetta J.P. The SPASIBA force field as an essential tool for studying the structure and dynamics of saccharides. Biochimie. 2003;85:65–73. doi: 10.1016/s0300-9084(03)00052-x. [DOI] [PubMed] [Google Scholar]
- Wagle A., Seong S.H., Zhao B.T., Woo M.H., Jung H.A., Choi J.S. Comparative study of selective in vitro and in silico BACE1 inhibitory potential of glycyrrhizin together with its metabolites, 18α- and 18β-glycyrrhetinic acid, isolated from Hizikia fusiformis. Archives of Pharmaceutical Research. 2018;41:409–418. doi: 10.1007/s12272-018-1018-2. [DOI] [PubMed] [Google Scholar]
- Wan Z., Sun Y., Ma L., Guo J., Wang J., Yin S., Yang X. Thermoresponsive structured emulsions based on the fibrillar self-assembly of natural saponin glycyrrhizic acid. Food and Function. 2017;8:75–85. doi: 10.1039/c6fo01485b. [DOI] [PubMed] [Google Scholar]
- Wan Z., Sun Y., Ma L., Yang X., Guo J., Yin S. Responsive emulsion gels with tunable properties formed by self-assembled nanofibrils of natural saponin glycyrrhizic acid for oil structuring. Journal of Agriculture and Food Chemistry. 2017;65:2394–2405. doi: 10.1021/acs.jafc.6b05242. [DOI] [PubMed] [Google Scholar]
- Wan Z., Sun Y., Ma L., Zhou F., Guo J., Hu S., Yang X. Long-lived and thermoresponsive emulsion foams stabilized by self-assembled saponin nanofibrils and fibrillar network. Langmuir. 2018;34:3971–3980. doi: 10.1021/acs.langmuir.8b00128. [DOI] [PubMed] [Google Scholar]
- Wang B., Lian Y.J., Dong X., Peng W., Liu L.L., Su W.J.…Wang Y.X. Glycyrrhizic acid ameliorates the kynurenine pathway in association with its antidepressant effect. Behavioural Brain Research. 2018;353:250–257. doi: 10.1016/j.bbr.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Wang B.K., Mao Y.L., Gong L., Xu X., Jiang S.Q., Wang Y.B., Li W.F. Glycyrrhizic acid activates chicken macrophages and enhances their Salmonella-killing capacity in vitro. Journal of Zhejiang University - Science B. 2018;19:785–795. doi: 10.1631/jzus.B1700506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chen L., Xu C., Shi J., Chen S., Tan M.…Liu X. A comprehensive review for phytochemical, pharmacological, and biosynthesis studies on Glycyrrhiza spp. American Journal of Chinese Medicine. 2020;48:17–45. doi: 10.1142/S0192415X20500020. [DOI] [PubMed] [Google Scholar]
- Wang C., Shi D., Zhang F., Yu X., Lin G., Zhou Z. Characterization of binding interaction between magnesium isoglycyrrhizinate and human serum albumin. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2020;234:118245. doi: 10.1016/j.saa.2020.118245. [DOI] [PubMed] [Google Scholar]
- Wang C., Su X., Sun M., Zhang M., Wu J., Xing J.…Chen S. Efficient production of glycyrrhetinic acid in metabolically engineered Saccharomyces cerevisiae via an integrated strategy. Microbial Cell Factories. 2019;18:95. doi: 10.1186/s12934-019-1138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Shan H., Lü H. Preparative separation of liquiritigenin and glycyrrhetic acid from Glycyrrhiza uralensis Fisch using hydrolytic extraction combined with high-speed countercurrent chromatography. Biomedical Chromatography. 2020;34 doi: 10.1002/bmc.4788. [DOI] [PubMed] [Google Scholar]
- Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Research. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Wu W., Li W., Huang S., Li Z., Liu R.…Wang S. Activation of NLRP3 inflammasome promotes foam cell formation in vascular smooth muscle cells and atherogenesis via HMGB1. Journal of American Heart Association. 2018;7 doi: 10.1161/JAHA.118.008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Y., Peng G., Han X. Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1. International Immunopharmacology. 2018;60:9–17. doi: 10.1016/j.intimp.2018.04.029. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao B., Wang S., Liang Q., Cai Y., Yang F., Li G. Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Delivery. 2016;23:1623–1635. doi: 10.3109/10717544.2015.1135489. [DOI] [PubMed] [Google Scholar]
- Wang Z.W., Sun N., Wu C.H., Jiang J.B., Bai Y.S., Li H.Q. In vitro antiviral activity and underlying molecular mechanisms of dipotassium glycyrrhetate against porcine reproductive and respiratory syndrome virus. Antiviral Therapy. 2013;18:997–1004. doi: 10.3851/IMP2662. [DOI] [PubMed] [Google Scholar]
- Wei X., She G., Wu T., Xue C., Cao Y. PEDV enters cells through clathrin-, caveolae-, and lipid raft-mediated endocytosis and traffics via the endo-/lysosome pathway. Veterinary Research. 2020;51:10. doi: 10.1186/s13567-020-0739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir H.M., Kraulis P.J., Hill C.S., Raine A.R., Laue E.D., Thomas J.O. Structure of the HMG box motif in the B-domain of HMG1. EMBO Journal. 1993;12:1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkerstorfer A., Kurz H., Bachhofner N., Szolar O.H. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Research. 2009;83:171–178. doi: 10.1016/j.antiviral.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y.…Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Wang W., Chen Y., Liu X., Wang J., Qin X.…E Y, Pei D. Glycyrrhizin suppresses the growth of human NSCLC cell line HCC827 by downregulating HMGB1 level. Biomedical Research International. 2018;2018:6916797. doi: 10.1155/2018/6916797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Sun H.X., Li D. Platycodin D is a potent adjuvant of specific cellular and humoral immune responses against recombinant hepatitis B antigen. Vaccine. 2009;27:757–764. doi: 10.1016/j.vaccine.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Xie Y., Sun H.X., Li D. Platycodin D improves the immunogenicity of newcastle disease virus-based recombinant avian influenza vaccine in mice. Chemical Biodiversity. 2010;7:677–689. doi: 10.1002/cbdv.200900183. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kidachi Y., Kamiie K., Noshita T., Umetsu H. Structural insight into the ligand-receptor interaction between glycyrrhetinic acid (GA) and the high-mobility group protein B1 (HMGB1)-DNA complex. Bioinformation. 2012;8:1147–1153. doi: 10.6026/97320630081147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Fang C., Cao L., Wang L., Du J., Sun Y.…Wu X. Protective effect of glycyrrhizic acid on cerebral ischemia/reperfusion injury via inhibiting HMGB1-mediated TLR4/NF-κB pathway. Biotechnology and Applied Biochemistry. 2019;66:1024–1030. doi: 10.1002/bab.1825. [DOI] [PubMed] [Google Scholar]
- Yanagawa Y., Ogura M., Fujimoto E., Shono S., Okuda E. Effects and cost of glycyrrhizin in the treatment of upper respiratory tract infections in members of the Japanese maritime self-defense force: Preliminary report of a prospective, randomized, double-blind, controlled, parallel-group, alternate-day treatment assignment clinical trial. Current Therapeutic Research, Clinical and Experimental. 2004;65:26–33. doi: 10.1016/S0011-393X(04)90002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.H., Zhang Q., Liang Q.Y., Wang S.Q., Zhao B.X., Wang Y.T.…Li G.F. Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: Paclitaxel-loaded glycyrrhizic acid micelles. Molecules. 2015;20:4337–4356. doi: 10.3390/molecules20034337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Li J., Cai Y., Yang Z., Li R., Fu W. Glycyrrhizic acid alleviates 6-hydroxydopamine and corticosterone-induced neurotoxicity in SH-SY5Y cells through modulating autophagy. Neurochemistry Research. 2018;43:1914–1926. doi: 10.1007/s11064-018-2609-5. [DOI] [PubMed] [Google Scholar]
- Yang H., Chen X., Jiang C., He K., Hu Y. Antiviral and immunoregulatory role against PCV2 in vivo of Chinese herbal medicinal ingredients. Journal of Veterinary Research. 2017;61:405–410. doi: 10.1515/jvetres-2017-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Cao L., Xie M., Yu Y., Kang R., Yang L.…Tang D. Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochemical Pharmacology. 2013;86:410–418. doi: 10.1016/j.bcp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P.…Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa237. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Yu J., Tang Z., Liu H., Ruan K., Song Z.…Ma H. Multi-evaluating strategy for Siji-kangbingdu mixture: Chemical profiling, fingerprint characterization, and quantitative analysis. Molecules. 2019;24 doi: 10.3390/molecules24193545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Fujigaki H., Kato K., Yamazaki K., Fujigaki S., Kunisawa K.…Saito K. Selective and competitive inhibition of kynurenine aminotransferase 2 by glycyrrhizic acid and its analogues. Scientific Reports. 2019;9:10243. doi: 10.1038/s41598-019-46666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.J., Zhang C.S., Coyle M.E., Du Y., Zhang A.L., Guo X.…Lu C. Compound glycyrrhizin plus conventional therapy for psoriasis vulgaris: A systematic review and meta-analysis of randomized controlled trials. Current Medical Research and Opinion. 2017;33:279–287. doi: 10.1080/03007995.2016.1254605. [DOI] [PubMed] [Google Scholar]
- Yu S., Yin C., Song K., Li S., Zheng G.L., Li L.F.…Qiu H.J. Engagement of cellular cholesterol in the life cycle of classical swine fever virus: Its potential as an antiviral target. Journal of General Virology. 2019;100:156–165. doi: 10.1099/jgv.0.001178. [DOI] [PubMed] [Google Scholar]
- Yu W., Jin H., Shen A., Deng L., Shi J., Xue X.…Liang X. Purification of high-purity glycyrrhizin from licorice using hydrophilic interaction solid phase extraction coupled with preparative reversed-phase liquid chromatography. Journal of Chromatography B Analytical Technology in Biomedical Life Sciences. 2017;1040:47–52. doi: 10.1016/j.jchromb.2016.11.031. [DOI] [PubMed] [Google Scholar]
- Yu Z., Ohtaki Y., Kai K., Sasano T., Shimauchi H., Yokochi T.…Endo Y. Critical roles of platelets in lipopolysaccharide-induced lethality: Effects of glycyrrhizin and possible strategy for acute respiratory distress syndrome. International Immunopharmacology. 2005;5:571–580. doi: 10.1016/j.intimp.2004.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Zhou Z., Liao Y., Ma L., Huang X., Zhang J.…Wu Z. System optimisation quantitative model of on-line NIR: A case of Glycyrrhiza uralensis Fisch extraction process. Phytochemical Analysis. 2020 doi: 10.1002/pca.2919. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. International Journal of Antimicrobial Agents. 2020;55:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Liu B., Cao B., Wei F., Yu X., Li G.F.…Wang P.L. Synergistic protection of Schizandrin B and Glycyrrhizic acid against bleomycin-induced pulmonary fibrosis by inhibiting TGF-β1/Smad2 pathways and overexpression of NOX4. International Immunopharmacology. 2017;48:67–75. doi: 10.1016/j.intimp.2017.04.024. [DOI] [PubMed] [Google Scholar]
- Zhang M., Du T., Long F., Yang X., Sun Y., Duan M.…Chen J. Platycodin D suppresses type 2 porcine reproductive and respiratory syndrome virus in primary and established cell lines. Viruses. 2018;10 doi: 10.3390/v10110657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Song Z., Wang M., Lan M., Zhang K., Jiang P.…Wang X. Cholesterol 25-hydroxylase negatively regulates porcine intestinal coronavirus replication by the production of 25-hydroxycholesterol. Veterinary Microbiology. 2019;231:129–138. doi: 10.1016/j.vetmic.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Zhang J., Nie D., Zhou Y., Li F., Onishi K.…Wang J.H. HMGB1 mediates the development of tendinopathy due to mechanical overloading. PLoS One. 2019;14 doi: 10.1371/journal.pone.0222369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.L., Cho K.H., Ha Y.W., Jeong T.S., Lee W.S., Kim Y.S. Cholesterol-lowering effect of platycodin D in hypercholesterolemic ICR mice. European Journal of Pharmacology. 2006;537:166–173. doi: 10.1016/j.ejphar.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Tensfeldt T.G., Chandra R., Mould D.R. Population pharmacokinetics of azithromycin and chloroquine in healthy adults and paediatric malaria subjects following oral administration of fixed-dose azithromycin and chloroquine combination tablets. Malaria Journal. 2014;13:36. doi: 10.1186/1475-2875-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Jiang Y., Zhao Y., Xi H., Liu C., Qu F., Feng X. Analysis of the susceptibility to COVID-19 in pregnancy and recommendations on potential drug screening. European Journal of Clinical Microbiology and Infectious Diseases. 2020 doi: 10.1007/s10096-020-03897-6. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.K., Li L., Liu X., Cheng S.P., Guo Q.Q., Fan D.P.…Lv A.P. Explore pharmacological mechanism of glycyrrhizin based on systems pharmacology. Zhongguo Zhong Yao Za Zhi. 2016;41:1916–1920. doi: 10.4268/cjcmm20161026. [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhu Q., Xu C., Li M., Li H., Yi P.Q.…Chen J.Y. Glycyrrhizin mitigates radiation-induced acute lung injury by inhibiting the HMGB1/TLR4 signalling pathway. Journal of Cellular and Molecular Medicine. 2020;24:214–226. doi: 10.1111/jcmm.14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Wei L., Niu P. The novel coronavirus outbreak in Wuhan, China. Global Health Research Policy. 2020;5:6. doi: 10.1186/s41256-020-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Wang Y., Peng C., Wang B., Niu Z., Li Z., Niu J. Magnesium isoglycyrrhizinate has hepatoprotective effects in an oxaliplatin‑induced model of liver injury. International Journal of Molecular Medicine. 2018;42:2020–2030. doi: 10.3892/ijmm.2018.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]