Abstract
Objective
Young people moving from child and adolescent mental health services (CAMHS) to adult mental health services (AMHS) are faced with significant challenges. To improve this state of affairs, there needs to be a recognition of the problem and initiatives and an urgent requirement for appropriate tools for measuring readiness and outcomes at the transfer boundary (16–18 years of age in Europe). The objective of this study was to develop and validate the Transition Readiness and Appropriateness Measure (TRAM) for assessing a young person’s readiness for transition, and their outcomes at the transfer boundary.
Design
MILESTONE prospective study.
Setting
Eight European Union (EU) countries participating in the EU-funded MILESTONE study.
Participants
The first phase (MILESTONE validation study) involved 100 adolescents (pre-transition), young adults (post-transition), parents/carers and both CAMHS and AMHS clinicians. The second phase (MILESTONE cohort study and nested cluster randomised trial) involved over 1000 young people.
Results
The development of the TRAM began with a literature review on transitioning and a review of important items regarding transition by a panel of 34 mental health experts. A list of 64 items of potential importance were identified, which together comprised the TRAM. The psychometric properties of the different versions of the TRAM were evaluated and showed that the TRAM had good reliability for all versions and low-to-moderate correlations when compared with other established instruments and a well-defined factor structure. The main results of the cohort study with the nested cluster randomised trial are not reported.
Conclusion
The TRAM is a reliable instrument for assessing transition readiness and appropriateness. It highlighted the barriers to a successful transition and informed clinicians, identifying areas which clinicians on both sides of the transfer boundary can work on to ease the transition for the young person.
Trial registration number
ISRCTN83240263 (Registered 23 July 2015), NCT03013595 (Registered 6 January 2017); Pre-results.
Keywords: Transition Readiness and Appropriateness Measure (TRAM), validation, child and adolescent mental health services, adult mental health services, young persons
Strengths and limitations of this study.
The European Union-funded Managing the Link and Strengthening Transition from Child to Adult Mental Healthcare in Europe (MILESTONE) study provides a useful model to evaluate the readiness of transition for young people.
The MILESTONE study allowed the Transition Readiness and Appropriateness Measure (TRAM) to be holistic in its scope because it ensured that all the essential information to assist with transition from child and adolescent mental health services to adult mental health services was captured in its entirety, especially given the fact that the transition journey for young people is very difficult and often poorly managed.
The focus groups gathered extensive input from young people, their family members and mental health professionals with experience in transition within mental health.
The web-based aspect of the TRAM allowed it to be completed remotely using developmentally appropriate interfaces, which aided in its completion.
Transition is not static and further evaluation of the TRAM is warranted in young people to assess transition readiness longitudinally.
Introduction
Ensuring a smooth transition process from paediatric to adult healthcare services has been a significant challenge for healthcare providers in recent years. Young people with chronic somatic conditions usually undergo a review when they reach the service transfer boundary1; however, in the mental healthcare setting an assessment of transition readiness and appropriateness of young people to transfer has not been well developed. Transition in mental health services refers to the process of young people moving from child and adolescent mental health services (CAMHS) to adult mental health services (AMHS—specialist adult teams and community-based services),2 the boundary being the age at which they can no longer access care from CAMHS (16–18 years of age in Europe). Among countries in the European Union (EU), only Denmark and the UK have guidelines detailing how the process should be managed, and only 40% of member states have facilities for transition planning.3 Despite this, transition transfer across the CAMHS-AMHS boundary has received less research attention than transitions in other healthcare settings, such as for young people with chronic conditions4–7 or special healthcare needs.8
Transition in the mental health setting requires a multidimensional approach that covers a young person’s psychosocial, educational and vocational needs. Various assessments of improving transition outcomes have been developed.9–16 Some explore the readiness for transition, such as treatment engagement, medication use and housing,10 while others have focused specifically on the readiness for transition12 or assessing the quality of interaction in service user/practitioner relationships.13 The current evidence base does not suggest that one measure of transition might be more efficacious than another, however, in the mental healthcare setting it seems that certain components might be more useful than others. Some of the core components have been described and encompass measures that include the readiness, planning, transfer of care and transfer of completion.17 The Transitions of Care from Child and Adolescent Mental Health Services to Adult Mental Health Services (TRACK) study18 19 noted that youth reaching the CAMHS transition boundary have variable outcomes, including inadequate transition procedures and disengagement from services. These factors can have a significant health economic impact on young people and their families.20 Others have shown that transition should be both personalised and flexible, and crucially incorporate the perspectives of young people.21
A mapping survey of 28 EU countries showed that the characteristics of CAMHS to AMHS transition services varies in terms of distribution of services, funding and user access.22 This implies that not enough resources and funds have been allocated to prevent discontinuity of care at the transfer boundary and that disengagement from services may be a significant problem across the continent. Furthermore, while new national and international initiatives are clearly warranted, tools to inform decision-making at the transfer boundary and to enable reliable and consistent assessment of transition outcomes are also urgently needed. It is for this reason that a bespoke suite of measures, focusing on transition of young people from CAMHS to AHMS, was developed within the EU-funded Managing the Link and Strengthening Transition from Child to Adult Mental Healthcare in Europe (MILESTONE) study.23 The MILESTONE suite of measures comprises the Transition Readiness and Appropriateness Measure (TRAM), for assessing whether transition is appropriate for any young person who is approaching their transfer boundary in CAMHS, and whether they are ready for it, and the Transition Related Outcome Measure (TROM), which evaluates the outcomes of transition. The TRAM is currently being used within the MILESTONE cluster randomised controlled trial as one of the components of the MILESTONE study23 to inform ‘Managed transition’ in the intervention arm. The present paper presents the findings on the validation of the TRAM.
Methods
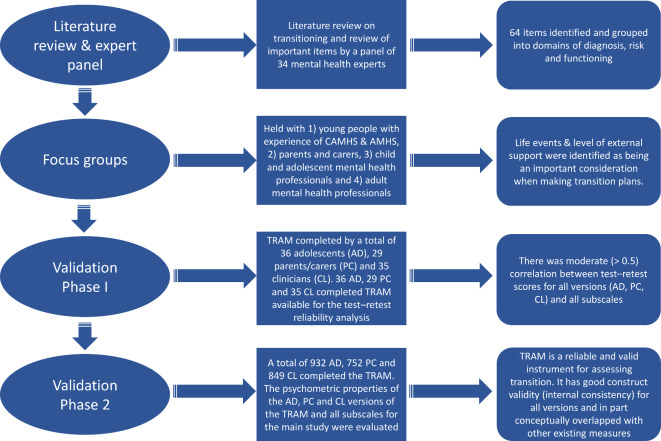
The methods linked with the development and validation of the TRAM have been described previously.24 Figure 1 summarises the main stages, methods and analyses; only the key points are mentioned here. The US FDA Guidance for Patient-reported Outcome Measures (PROM) was followed25 by beginning with a literature review on transitioning, which was followed by an expert panel review, a focus group phase measure consisting of development and translation (so that the testing of the web-based versions could take place in the eight EU countries participating in the MILESTONE study),23 and finally a two-phase process. The first phase (MILESTONE validation study) involved 100 adolescents (pre-transition), young adults (post-transition), parents/carers and both CAMHS and AMHS clinicians and assessed content validity, construct validity and test–retest reliability. Participants completed the TRAM plus other existing measures (figure 1). The second phase involved over 1000 young people as part of the MILESTONE cohort study and nested cluster randomised trial and assessed the responsiveness and interpretability of the TRAM and the psychometric properties (apart from test–retest). All study participants gave informed consent as per study guidelines. A complete list of all the ethics committees that provided ethical approval are provided in online supplementary information table 1. Data collection was part of the MILESTONE study, which has been described elsewhere alongside a detailed summary of the measures that were completed by participants and subsequently used in the validation of the TRAM.23
Figure 1.
Summary of the main stages, methods and analyses of the TRAM. AD, adolescent; AMHS, adult mental health services; CAMHS, child and adolescent mental health services; CL, clinician; PC, parent/carer; TRAM, Transition Readiness and Appropriateness Measure.
bmjopen-2019-033324supp001.pdf (85.3KB, pdf)
Internal consistency of the TRAM was calculated by means of the Cronbach’s alpha (α). The Pearson’s product moment correlation between three existing measures (Health of the Nation Outcome Scale for Children and Adolescents (HoNOSCA)26 27 scale, Clinical Global Impression Severity (CGI-S)28 scale and Specific Levels of Functioning (SLOF) scale)29 and the new rating scales was calculated to assess whether the scores of the TRAM are related to scores on other instruments. Factor analysis was conducted to determine the underlying structure of the TRAM subscales and to identify patterns and characteristics of the factors. Results of the second phase of validation will also be used to inform modifications to the scale, in particular to improve the utility and accessibility of the measure and minimise completion burden.
To assess whether the demographic characteristics were related to the TRAM subscales, we estimated the deprivation index. The deprivation index was developed based on comparable variables present in the Jarman Index that is a widely used indicator for social deprivation.30 31 In the context of the present study, the variables of the deprivation index were captured using the sociodemographic variables in the Sociodemographic Interview for the Parent. The following variables were used to estimate the deprivation index: (a) employment of parents versus unemployed, (b) if the young person was attending school or not, (c) lone parent, (d) ethnic minority, (e) parental history of mental illness, (f) socioeconomic factors, that is, receiving state financial support and (g) level of parental education.
Patient and public involvement
Patients were involved in the development of the TRAM by taking part in focus groups and to discuss important themes to be assessed by the TRAM, and by piloting the initial versions of the measure.
Young project advisors were involved in the project to see how it could be implemented and how the changes could be adopted by current mental health transition services.
Results
Development of the TRAM
Literature review
The detailed review of current literature and measures on transition in both mental and physical health resulted in a list of 64 items of potential importance, grouped into three main domains—diagnosis, risk and functioning—forming the core structure of the TRAM.
Expert panel
It was decided that the TRAM should include questions on potential barriers to a successful transition (eg, young people not being able to act independently, not being motivated to manage their conditions or not understanding their conditions), as these reflect the young persons’ readiness for and functioning related to transition. Furthermore, it was hoped that addressing such issues within the TRAM would emphasise the need for CAMHS and AMHS to work together before and/or during transition. The TRAM also considered the young person’s desired level of parental involvement, the ease with which they formed clinical relationships and whether they were able to discuss their mental health history. These elements were also deemed relevant for services to understand to avoid difficult or tricky situations that may potentially derail transition.
Focus groups
Focus groups were held with young people with experience of CAMHS and transition to AMHS (if applicable), parents and carers, CAMHS professionals and AMHS professionals. During the focus group discussions, young people voiced that ‘life events’ should be taken into account when deciding about transition. A large number of recent life events was thought to suggest a greater need for AMHS. These raise some important points that need to be considered during transfer when developing readiness measures. Health transitions are only one of several life transitions during adolescence and young adulthood. Other factors also need to be taken into account during the transition period such as those relating to educational and social transitions including moving from parental home to independent living. Participants in the focus groups also identified the level of external support as being an important consideration when making transition plans, as those with less external support may have a greater need for continued statutory services. Young people, parents and CAMHS clinicians ranked social support and housing as important more often than the expert panel or AMHS clinicians. Poor engagement with tasks, lack of meaningful occupation and cognitive factors were considered the least important factors to consider when making a transition decision by all categories of participants. Questions relating to these issues were therefore removed from subsequent versions of the scale.
Organisation of items within subscales
Once the final list of items had been decided, the organisation of these items was discussed with further focus groups and the MILESTONE expert panel. Based on this feedback, the preliminary version of the TRAM included domains A–F, which capture the ‘appropriateness’ of transition, and G and H, which capture ‘readiness’ for transition, as follows:
(A) Symptoms
Frequency and severity of symptoms to include depression, mania, anxiety, post-traumatic stress, psychosis, personality disorder, antisocial personality disorder, attention deficit, social communication, eating difficulties and other mental health conditions.
(B) Overall illness
This considered severity, taking into account all symptoms across all existing conditions.
(C) Overall disruption
Effect of symptoms on functioning with respect to self-care, sleep, household chores, community, social, responsibility, relationships with family, friends/partner, peers/colleagues and education/work performance.
(D) Risk factors
Frequency and severity of stress, risk-taking behaviour, self-harm (no suicidal intent), suicidal thoughts, behaviours that risk harm to others and behaviours that risk harm from others.
(E) Factors affecting symptoms
Including need for ongoing treatment, inpatient admissions, relapse, side effects to medication, physical health comorbidities and drug and alcohol abuse.
(F) Health system factors
The health system factors that may affect a clinicians' transition decision include items such as financial implications of a transition to AMHS, the quality of the links between CAMHS and AMHS, the appropriateness of available statutory services, the availability of alternative services and the skills of local GPs with regard to mental health when treating a young person’s condition.
(G) Barriers to functioning
Including inability to act independently, poor understanding of condition, lack of knowledge on how to access services, lack of motivation, poor adherence to medication, lack of social support, not wanting carers to be involved, difficulty forming relationships with treatment team and difficulty repeating mental health history.
(H) Other life changes
Other life changes (positive or negative) relating to family relationships, relationships with friends and partner, moving home, school/college/work, illness/death, police involvement, pregnancy and other.
Both the frequency of symptoms and the severity of impairment (A (symptoms) and D (risk)) were assessed, as advised by focus groups participants. Again, following participant feedback, the severity of each symptom was recorded separately but a single assessment of impairment was made across all symptoms and conditions. Focus group participants also considered which options for assessing frequency and severity would be most appropriate. For frequency, the most popular choice was a 6-point ordinal scale (from not experienced in the past 6 months ranging to all of the time) and for severity, a 5-point ordinal scale (from very mild ranging to very severe). Unduly convoluted medical language was removed, and participants reported no major issues with completion of the scale. Experts in the field were asked to review the proposed scale. The agreed test version was translated into Croatian, Dutch, French, German and Italian using a back-translation process32 and, after final checks, uploaded on the HealthTracker system, a web-based portal for online measures.
Validation of the TRAM
In the first phase, the three versions of the TRAM were completed by a total of 36 adolescents (AD), 29 parents/carers (PC) and 35 clinicians (CL), respectively.
In the main MILESTONE study (second phase), the TRAM was completed by a total of 932 AD, 752 PC and 849 CL.
First phase
Test–retest reliability
In order to assess test–retest reliability, Pearson’s correlation coefficients were calculated between responses per the first and subsequent completion (a maximum of 41 days after the first assessment) for each subscale and version (AD, PC, CL) of TRAM. There were 36 AD, 29 PC and 35 CL completed TRAM’s available for the test–retest reliability analysis. The results are summarised in table 1(A),(B). There was moderate (>0.5) correlation33 between test–retest scores for all versions (AD, PC, CL) and all subscales.
Table 1.
Test–retest reliability of the TRAM (n=100)
| Subscale | Adolescent | Parent/Carer | Clinician | |||
| (AD: n=36) | (PC: n=29) | (CL: n=35) | ||||
| (A) Pearson’s correlation coefficients | ||||||
| Symptoms | 0.928** | 0.936** | 0.773** | |||
| Overall disruption | 0.817** | 0.935** | 0.942** | |||
| Barriers to functioning | 0.813** | 0.908** | 0.824** | |||
| Risk factors | 0.897** | 0.864** | 0.914** | |||
| Factors affecting symptoms | 0.734** | 0.679** | 0.912** | |||
| (B) Mean and SD | ||||||
| Baseline | Test–retest | Baseline | Test–retest | Baseline | Test–retest | |
| Symptoms | ||||||
| Mean | 16.667 | 14.167 | 12.379 | 10.466 | 11.629 | 11.300 |
| SD | 9.789 | 9.667 | 8.548 | 8.757 | 5.945 | 6.253 |
| Overall disruption | ||||||
| Mean | 9.944 | 8.694 | 8.655 | 8.379 | 11.086 | 11.200 |
| SD | 6.697 | 5.956 | 8.784 | 8.954 | 7.625 | 8.554 |
| Barriers to functioning | ||||||
| Mean | 7.472 | 6.778 | 6.655 | 6.034 | 7.086 | 6.314 |
| SD | 3.501 | 3.743 | 4.685 | 3.530 | 3.673 | 3.636 |
| Risk factors | ||||||
| Mean | 6.847 | 6.167 | 4.621 | 4.379 | 5.829 | 6.014 |
| SD | 4.657 | 4.623 | 4.037 | 4.212 | 4.711 | 4.999 |
| Factors affecting symptoms | ||||||
| Mean | 2.167 | 1.889 | 1.759 | 1.276 | 2.171 | 2.029 |
| SD | 1.464 | 1.348 | 1.596 | 1.251 | 1.445 | 1.224 |
**p<0.01.
AD, adolescent; CL, clinician; PC, parent/carer; SD, standard deviation; TRAM, Transition Readiness and Appropriateness Measure.
Second phase
Demographics for the AD, PC and CL sample are presented in online supplementary tables 2,3. The psychometric properties of the AD, PC and CL versions of the TRAM and all subscales for the larger sample are described in the ‘Internal consistency (reliability)’ section.
Internal consistency (reliability)
Cronbach’s α was calculated for all versions of the TRAM (AD, PC and CL versions) for the following subscales: symptoms, overall disruption, barriers to functioning, risk factors and factors affecting symptoms. Alpha ≥0.70 is considered acceptable evidence of internal reliability.34 The consistency of responses between versions (AD, PC and CL) was assessed using Pearson’s correlation coefficient. The internal consistency of the symptoms subscale was shown to be high for the AD version (α=0.804), acceptable for the PC version (α=0.759) and moderate for the CL version (α=0.552). The AD version for symptoms moderately correlated with the PC and CL version of the symptom subscale (r=0.517, p<0.01 and r=0.396, p<0.01, respectively). Additionally, the PC version and CL version of the symptom subscale also revealed a moderate correlation (r=0.393, p<0.01). The overall disruption subscale demonstrated high levels of internal consistency for all versions of the scale (AD version, α=0.869; PC version, α=0.882, CL version, α=0.877). The AD version for overall disruptions correlated with the PC and CL version of the overall disruption subscale (r=0.420, p<0.01 and r=0.380, p<0.01, respectively). Furthermore, the PC version and CL version of the overall disruption subscale also revealed a moderate correlation (r=0.505, p<0.01).
The barriers to functioning subscale scored adequate reliability for the PC and CL versions (α=0.725 and 0.714, respectively) with the AD version demonstrating slightly lower consistency (α=0.616). The AD version for barriers to functioning subscale moderately correlated with the PC and CL version of the overall disruption subscale (r=0.327, p<0.01 and r=0.401, p<0.01, respectively). Furthermore, the PC and CL version of the barriers to functioning subscale also revealed a moderate correlation (r=0.380, p<0.01).
The risk factors subscale achieved adequate levels of internal consistency for the AD version (α=0.735), with the PC and CL versions revealing slightly lower consistency (α=0.654 and 0.684, respectively). Once again, the AD version for risk moderately correlated with the PC and CL version of the risk subscale (r=0.552, p<0.01 and r=0.557, p<0.01, respectively). Similarly, the PC version and CL version of the risk subscale also revealed a moderate correlation (r=0.529, p<0.01).
The factors affecting symptoms subscale did not exceed a Cronbach’s α of 0.70 for all versions (AD version α=0.554, PC version α=0.565, CL version α=0.522), with the AD version of the factors affecting symptoms subscale moderately correlating with the PC and CL version (r=0.610, p<0.01 and r=0.389, p<0.01, respectively). This relationship was also seen for the PC and CL versions of the factors affecting symptoms subscale (r=0.452, p<0.01).
The performance of the symptoms subscale for CL and the factors affecting symptoms subscale for AD, PC and CL fell below the minimum acceptable threshold. We therefore explored whether deletion of particular items might improve this and found that by removing the item relating to ‘attention deficit’ from the CL symptoms subscale, it would increase to 0.587. We also found that by removing the item about ‘medical comorbidity’ from the factors affecting symptoms subscale (AD, PC, CL), reliability would increase to 0.573 for the AD, 0.593 for the PC and 0.548 for the CL. Consequently, they were retained in the TRAM.
Correlations with other existing measures
To assess whether the TRAM could conceptually overlap with other existing instruments also completed by MILESTONE participants, the Pearson’s product moment correlation coefficient was calculated between each TRAM subscale and the gold standard HoNOSCA26 27 and the CGI-S28 (table 2). The Pearson’s product moment correlation coefficients for the TRAM subscales with HoNOSCA and CGI scales showed moderate correlations. Apart from the CGI (clinician version) for the symptoms and overall disruption, the correlation coefficients were all low (<0.500) suggesting a modest relationship between the TRAM subscale and HoNOSCA and CGI-S scores.
Table 2.
Pearson’s product moment correlation coefficients for the TRAM subscales with HoNOSCA and CGI-S scales
| TRAM subscales | |||||
| Scales | Symptom | Overall disruption | Risk factors | Factors affecting symptoms | Barriers to functioning |
| HoNOSCA AD | 0.378** (n=914) | 0.345** (n=914) | 0.370** (n=914) | 0.306** (n=914) | 0.249** (n=577) |
| HoNOSCA PC | 0.369** (n=738) | 0.329** (n=738) | 0.374** (n=738) | 0.349** (n=738) | 0.151** (n=477) |
| HoNOSCA CL | 0.478** (n=845) | 0.437** (n=845) | 0.442** (n=845) | 0.357** (n=845) | 0.340** (n=502) |
| CGI-S AD | 0.242** (n=832) | 0.261** (n=832) | 0.210** (n=832) | 0.294** (n=832) | 0.149** (n=527) |
| CGI-S PC | 0.319** (n=684) | 0.285** (n=684) | 0.237** (n=684) | 0.338** (n=684) | 0.187** (n=444) |
| CGI-S CL | 0.548** (n=836) | 0.514** (n=836) | 0.373** (n=836) | 0.352** (n=182) | 0.307** (n=499) |
**p<0.01; null hypothesis is that the Pearson’s correlation coefficient equals zero.
AD, adolescent; CGI-S, Clinical Global Impression Severity; CL, clinician; HoNOSCA, Health of the Nation Outcome Scale for Children and Adolescents; PC, parent/carer; TRAM, Transition Readiness and Appropriateness Measure.
Pearson’s correlations were also determined between each TRAM subscale and the parent version of a behavioural rating scale: SLOF. The SLOF allows the capture of symptomatology using observable behavioural function in those with psychiatric illness.29 The subscale scores of the TRAM were analysed to see how well they correlate with the SLOF scale (AD, PC and CL) (table 3). The Pearson’s correlation between the TRAM subscales and SLOF subscales showed moderate associations. However, while the HoNOSCA and CGI showed significant correlations with the TRAM scales, albeit moderate relationships, the SLOF scale revealed poor relationships (non-significant correlations) between some constructs measured by the former two scales.
Table 3.
Summary of Pearson’s correlation of the TRAM subscales with SLOF subscales
| Sub-scale | Physical Functioning | Personal Care Skills | Interpersonal Relationships | Social Acceptability | Activities | Work Skills |
| Adolescent | ||||||
| Symptoms (n=732) | −0.068 | −0.098** | −0.159** | −0.302** | −0.054 | −0.085* |
| Overall disruption (n=732) | −0.107** | −0.157** | −0.265** | −0.286** | −0.181** | −0.138** |
| Risk Factors (n=732) | −0.026 | −0.022 | −0.106** | −0.299** | 0.006 | −0.004 |
| Factors Affecting Symptoms (n=732) | −0.062 | −0.033 | −0.087* | −0.217** | −0.042 | −0.025 |
| Barriers to Functioning (n=475) | −0.070 | −0.186** | −0.216** | −0.229** | −0.199** | −0.239** |
| Parent/Carer | ||||||
| Symptoms (n=744) | −0.180** | −0.275** | −0.411** | −0.569** | −0.306** | −0.223** |
| Overall disruption (n=744) | −0.234** | −0.552** | −0.571** | −0.494** | −0.575** | −0.436** |
| Risk Factors (n=744) | −0.170** | −0.228** | −0.300** | −0.597** | −0.223** | −0.125** |
| Factors Affecting Symptoms (n=744) | −0.067 | −0.141** | −0.201** | −0.394** | −0.126** | −0.091* |
| Barriers to Functioning (n=483) | −0.198** | −0.420** | −0.461** | −0.417** | −0.528** | −0.472** |
| Clinician | ||||||
| Symptoms (n=678) | −0.087* | −0.155** | −0.211** | −0.242** | −0.138** | −0.154** |
| Overall disruption (n=678) | −0.130** | −0.332** | −0.399** | −0.279** | −0.392** | −0.316** |
| Risk Factors (n=678) | −0.071 | −0.137** | −0.194** | −0.358** | −0.076* | −0.106** |
| Factors Affecting Symptoms (n=132) | 0.012 | −0.068 | −0.084 | −0.253** | −0.046 | −0.100 |
| Barriers to Functioning (n=414) | −0.048 | −0.240** | −0.189** | −0.275** | −0.256** | −0.322** |
*p<0.05, **p<0.01; null hypothesis is that the Pearson’s correlation coefficient equals zero.
SLOF, Specific Levels of Functioning; TRAM, Transition Readiness and Appropriateness Measure.
Exploratory factor analysis
Exploratory factor analysis (EFA) (principal axis, promax rotation) was undertaken to model the inter-relationships between the items in the TRAM and was performed on the adolescent version of the TRAM’s subscales. The TRAM was developed to assess whether transition is appropriate and whether the young person is ready for it. Based on this premise, the adolescent version of the scale was chosen as it was deemed to be the most relevant version clinically to explore the inter-relationship between the items.
The first set of EFAs was performed without a set number of factors, these analyses showed that for ‘symptoms’ and ‘factors affecting symptoms’ subscales a two factors model in line with the clinical knowledge can explain the relationship between the items of the subscales (see table 4 for the details of the factors). These analyses, however, did not produce meaningful models for the ‘overall disruption’, ‘risk factors’ and the ‘barriers to functioning’ subscales. Therefore, another set of EFAs were performed where the number of factors were set on two for the ‘overall disruption’, ‘risk factors’ and three for ‘barriers to functioning’ subscales. These EFAs returned for all the three subscales models which satisfy both statistical and clinical criteria. In the ‘barriers to functioning’ subscale, the three factors were identified based on clinical knowledge of barriers that might impede functioning such as ‘patient factors’, ‘family support’ and ‘treatment’. Table 4 summarises the results of EFAs for the TRAM subscales. For all the subscales and the items were clustered based on clinical relevance.
Table 4.
Summary of EFA for the adolescent version of the TRAM’s subscales
| Factors | |||
| Internalising symptoms | Externalising symptoms | ||
| Symptoms subscale | |||
| Anxiety | 0.896 | −0.222 | |
| Depression | 0.794 | 0.020 | |
| Borderline personality | 0.482 | 0.334 | |
| Post-traumatic stress | 0.358 | 0.197 | |
| Social communication difficulties | 0.356 | 0.196 | |
| Eating difficulties | 0.313 | 0.086 | |
| Other mental health | 0.186 | 0.107 | |
| Antisocial behaviour | −0.083 | 0.585 | |
| Mania | −0.019 | 0.570 | |
| Attention deficit | 0.139 | 0.380 | |
| Psychosis | 0.282 | 0.366 | |
| Relationships | Activities of daily living | ||
| Overall disruption subscale | |||
| Relationships with friends | 0.903 | −0.088 | |
| Relationships with peers/colleagues | 0.845 | −0.082 | |
| Social | 0.550 | 0.199 | |
| Relationships with family | 0.455 | 0.124 | |
| Education work performance | 0.406 | 0.256 | |
| Sleep | 0.360 | 0.244 | |
| Household chores | 0.017 | 0.721 | |
| Self-care | −0.059 | 0.708 | |
| Responsibility | 0.048 | 0.678 | |
| Community | 0.252 | 0.432 | |
| Patient factors | Family support | Treatment | |
| Barriers to functioning subscale | |||
| Knowledge of accessing service | 0.636 | −0.103 | −0.053 |
| Ability to act as independent | 0.591 | −0.232 | 0.031 |
| Understanding of mental health | 0.496 | 0.055 | −0.061 |
| Adolescent built trusting relationship | 0.420 | 0.225 | 0.011 |
| Ability to repeat history | 0.413 | 0.230 | −0.087 |
| Adolescent wants parent carer | −0.078 | 0.691 | −0.100 |
| Presence of support | 0.031 | 0.527 | 0.141 |
| Taking medication as prescribed | −0.149 | −0.052 | 0.529 |
| Motivation to manage condition | 0.172 | 0.081 | 0.496 |
| Internal risk | External risk | ||
| Risk factors subscale | |||
| Suicidal thoughts behaviours | 0.848 | 0.001 | |
| Self-harming behaviours | 0.788 | −0.056 | |
| Stress | 0.397 | 0.130 | |
| Risk to others | −0.073 | 0.649 | |
| Risk to self | 0.145 | 0.529 | |
| Risk from others | 0.234 | 0.269 | |
| Relapse of illness factor | Somatic illness factor | ||
| Factors affecting symptoms subscale | |||
| Inpatient hospital stays | 0.594 | −0.088 | |
| Service use in times of crisis | 0.569 | −0.037 | |
| Relapse likelihood | 0.477 | 0.145 | |
| Ongoing treatment need | 0.365 | 0.146 | |
| Drug alcohol misuse | 0.363 | −0.110 | |
| Presence of side effects | −0.041 | 0.461 | |
| Medical comorbidity | −0.039 | 0.335 | |
The values in bold represent the highest loading in a factor for each item of the subscale. The threshold of acceptance was set at >0.150.
EFA, exploratory factor analysis; TRAM, Transition Readiness and Appropriateness Measure.
Deprivation index
An approximate measure of deprivation was estimated by creating the deprivation index. The deprivation index correlations with the overall TRAM subscale scores in the AD, PC and CL version are shown in table 5. The deprivation index correlated significantly with the AD, PC and CL versions of the overall disruption subscale. Pearson’s correlations were significant for the PC and CL version but not the AD version of the 'symptoms' and 'risk factors' subscale.
Table 5.
Summary of deprivation index correlations with TRAM subscales
| Deprivation index correlations | |||
| AD | PC | CL | |
| Symptoms subscale | |||
| Pearson’s correlation | 0.027 | 0.116** | 0.089* |
| Sig. (two-tailed) | 0.454 | 0.002 | 0.017 |
| N | 768 | 732 | 719 |
| Risk factors subscale | |||
| Pearson’s correlation | 0.052 | 0.175** | 0.118** |
| Sig. (two-tailed) | 0.148 | 0.000 | 0.001 |
| N | 768 | 732 | 719 |
| Overall disruption subscale | |||
| Pearson’s correlation | 0.083* | 0.162** | 0.127** |
| Sig. (two-tailed) | 0.021 | 0.000 | 0.001 |
| N | 768 | 732 | 719 |
| Factors affecting symptoms subscale | |||
| Pearson’s correlation | 0.031 | 0.111** | 0.085 |
| Sig. (two-tailed) | 0.396 | 0.003 | 0.298 |
| N | 768 | 732 | 151 |
| Barriers to functioning subscale | |||
| Pearson’s correlation | −0.046 | 0.061 | −0.019 |
| Sig. (two-tailed) | 0.301 | 0.181 | 0.699 |
| N | 500 | 477 | 435 |
*p<0.05, **p<0.01; null hypothesis is that the Pearson’s correlation coefficient equals zero.
AD, adolescent; CL, clinician; PC, parent/carer; TRAM, Transition Readiness and Appropriateness Measure.
Discussion
This current manuscript reports on the development and validation of the TRAM. The TRAM was designed and worded specifically so that it can be completed online, optimising both completion time and accessibility; thus, increasing its potential applicability in an adolescent/young adult population. The benefit of following the rigorous FDA process while developing TRAM was that feedback on potential items was gained early on from end users. Importantly, items such as diagnosis, risk and functioning were identified as important items in the transition decision-making process. The psychometric analyses revealed that the TRAM is a reliable and valid instrument for assessing transition. The TRAM had good reliability for all versions and showed moderate-to-low correlations when pitted with other established instruments. This finding supports the use of TRAM to assess transition readiness, as higher correlations would imply that the TRAM was not adding anything new when compared with existing measures such as HoNOSCA. The goal of the TRAM to assess readiness and appropriateness were met because the TRAM was holistic in its scope to explore the key items that captured the overarching themes relating to transition readiness and appropriateness.
When looking more closely at the correlations of the TRAM, there were conceptual differences between the TRAM subscales and existing instruments. Regarding the HoNOSCA, the Pearson’s correlations were all below 0.500 for the different versions suggesting a modest relationship between the HoNOSCA total score and TRAM subscale scores. Previous studies that have assessed the correlations between the HoNOSCA total score and other instruments such as the parent and clinician rated Children's Global Assessment Scale35 and the Global Assessment of Psychosocial Disability36 have reported moderate correlations ranging from 0.4 to 0.6. The present study also reported modest correlations between the TRAM subscale and HoNOSCA total scores suggesting that conceptually the instruments measure different elements of transition. A similar reasoning can be put forward when examining the TRAMs performance with the CGI. The CGI considers aspects of three different global measures (i) severity of illness (CGI-S), (ii) global improvement and (iii) efficacy index.28 In the context of this study, the CGI-S was considered and embodies all the aspects regarding the overall severity of symptoms of the young person into a single score. In comparison, the TRAM subscales are more specific. The subscales of the SLOF make it conceptually closer to the TRAM in terms of looking at the functioning aspects of transition when compared with the HoNOSCA or CGI-S. Although there was not a complete overlap, it was easier to classify individual correlations based on their meaningfulness. As expected, there were poor correlations (not significant) that can be explained by a conceptual difference between the construct measured by SLOF (that does not specifically focus on transition readiness) and TRAM subscales.
From a clinical viewpoint, the EFA for the adolescent version showed that a two factors model was the most suitable for the ‘symptoms’, ‘overall disruption’, ‘risk factors’ and ‘factors affecting symptoms’ TRAM subscale. The items were grouped together based on clinical judgement, for example, in the ‘symptoms’ subscale the items anxiety and depression were grouped together in factor one while antisocial behaviour and mania were categorised together in factor 2. In some instances, however, some items had lower loading values. The item ‘other mental health’ had a loading score of 0.186 in the ‘symptoms’ subscale suggesting a weaker association in comparison to the other items in this subscale. There is, however, no rule of thumb regarding the optimal strength of factor loadings and thresholds. Indeed, one meta-analysis of the variance in factor loading has shown that there is no agreement to what constituents a high or low factor loading.37 The items anxiety and depression clustered together with factor loading scores >0.7 reflecting a higher degree of impact these items have when a young person prepares for transition. Similarly, in the ‘risk factors’ subscale the items ‘suicidal thoughts behaviours’ and ‘self-harming behaviours’ had the highest factor loading scores in comparison to the other items indicating that when it comes to risk and how it impacts on the preparedness of when a young person’s transition, suicidal thoughts and self-harming behaviours are two elements that can have a significant impact on how a young person navigates transition. The barriers to functioning subscale revealed a three-factor model. On closer examination, while five items clustered together in factor 1, the item ‘knowledge of accessing services’ had the highest factor loading score in this factor. Interestingly, this score was higher than the ‘ability to act independently’ score. This observation suggests that when it comes to examine the barriers of transition, knowledge of accessing services are more important than whether the young person has the ability to act independently or understands the degree of how the severity of their mental illness will impact on the transition process. This point is echoed in the literature and supports the overarching theme voiced by young people and others that transition from CAMHS to AMHS should be individualised and be flexible enough to manage the obstacles encountered during the transition process.17 21 22 38 Despite this, the ability to act independently should not be understated. Young people will have different developmental milestones during their transition journey. This is particularly important during the latter stages of transition which often takes place in young adulthood as the brain is still developing. From a neurodevelopmental perspective, this point should not be taken lightly by services who sometimes forget that even at this stage of the transition process they are dealing with developing young people. Overall, these findings showed that the items could be mapped onto readiness and appropriateness. This will form the basis of a transition passport that will assist in the identification of high-risk cases or those who can be appropriately discharged or transitioned to another community service. The transition passport will be described elsewhere.
The study was able to estimate a deprivation index based on sociodemographic variables captured as part of the MILESTONE study and showed a significant relationship with the ‘overall disruption subscale’ in all versions of the scale, and the parent and clinician version of the ‘symptoms’ and ‘risk factors’ subscale. This is not surprising as these subscales have items grouped according to relationships, internalising/externalising symptoms and risk and these factors would be related to the sociodemographic aspects assessed using the deprivation index. While there are several indices that can be used for outcome services, the Jarman Index can be used as a proxy for deprivation and while some evidence has shown that it might not be entirely suited to the planning of healthcare outcomes,39 we have used elements of it to estimate a deprivation index that showed significant inter-relationships with TRAM subscales.
Strengths and limitations
The TRAM has a dual purpose: to identify who should be transitioned to adult mental health services and to pinpoint areas which should be considered or addressed to ensure that the transition process is smooth. The barriers to a successful transition are areas which clinicians on both sides of the transfer boundary can work on to improve the ease of transition. These barriers include young people not being ready to act as an independent adult; young people not understanding their mental health condition or not being motivated to manage their condition; not having social support, not easily building therapeutic relationships and not easily being able to repeat history. The TRAM score summary report contains the TRAM responses of the AD, PC and CL, presented in visually attractive graphs and tables and serves as a clinician decision support tool and communication aid. Yellow highlights help clinicians focus on items requiring attention. If a referral to adult services seems appropriate for the young person but barriers are highlighted, the clinician can add these to the care plan and address them in a timely fashion to help smoothen the transition process. Moving forward, based on the TRAM validation study findings, a MILESTONE Transition Predictor will be developed on the HealthTracker platform, to be used in association with the TROM. As transition is dependent on symptoms clusters, the transition predictor will be able to provide a personalised transition approach depending on symptom profiling. This will involve using a traffic light scoring system to a modified TRAM score summary report to predict the outcome of transition based on symptom profiling. Together with the TROM, these clinical decision-making tools will be valuable in identifying cases who need to transition based on symptomatology and then to assess the outcomes of the transition process. Young person’s undergoing transition present with complex psychopathology and as such those participants who were the most severely ill or less engaged with the transition process are least likely to have responded. Notwithstanding these concerns, the measure is still likely to be useful in these high-risk groups and would be beneficial for healthcare practitioners. Despite the focus groups not having patients who were very ill, the validation was done in a mixed group of patients with multiple disorders of varying complexity and hence shows that the TRAM can be used in complex psychopathology. The present study was also unable to assess transition readiness and how it can evolve over across time. This would be important given that young people are likely to have several transitions during their transition journey and although the TRAM did not capture transition from other services ie, within social care, it could still be used as a foundation to develop similar measures for other services. Future work would need to explore transition readiness in young people during their entire transition journey and the usefulness of TRAM across other age-based services.
Conclusion
The current study suggests that the TRAM is a viable instrument for determining the readiness of a young person and the appropriateness for transition from CAMHS. It is holistic in its scope to ensure that the young person is seen as more than a list of symptoms and assessment involves not only clinicians but also young people and their parents/carers. Being web-based allows the measure to be used across countries by end users and enriches the transition process from CAMHS to AMHS. This means that the TRAM has the potential to be used worldwide by end users, thereby contributing to a smoother transition process and allowing for personalised mental healthcare. Ultimately, this will have added value in informing the transition process from CAMHS to AMHS. The TRAM is designed to work in conjunction with an instrument that examines the outcome of transition.
Supplementary Material
Footnotes
Collaborators: The MILESTONE consortium members and partner institutions: Swaran Singh, Helena Tuomainen, Jason Madan, Jane Warwick, Cathy Street, Dieter Wolke, Moli Paul, Claire Daffern, Priya Tah, Rebecca Appleton, Alastair Canaway, James Griffin, Philip Wells, Rose-Marie Lomax (University of Warwick, UK), Giovanni de Girolamo, Giulia Signorini, Alessandro Ferrari, Cecilia Ferrari, Laura Rivolta, Flavia Levi, Lidia Manenti, Eliza Gheza, Adriana Pastore, Maria Cataldo, Giorgia Morini, Cecilia Toselli, Pamela Stagni, Pamela Varvara (IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy), Giovanni Allibrio (ASST of Spedali Civili, Brescia, Italy), Ottaviano Martinelli (ASST Lecco, Italy), Patrizia Conti (ASST Lariana), Emiliano Monzani (Niguarda Metropolitan Great Hospital, Italy), Francesco Rinaldi (ASST of Valcamonica, Italy), Francesco Margari, Lucia Margari (University of Bari, Italy), Paola Stagi, Fabrizio Starace (Modena, Italy), Giuseppe Carrà (San Gerardo Hospital, Italy), Renata Nacinovic (ASST Monza, Italy), Paolo Scocco, Michela Gatta (DISM, Italy), Fabio Vanni, Sabrina Ferrari (AUSL of Parma, Italy), Marco Armando (Child Neuropsychiatric Unit, Department of Neuroscience, Bambino Gesù Children's Hospital, IRCCS, Rome, Italy; Department of Psychiatry of the University of Geneva, Switzerland), Stefano Vicari (Child Neuropsychiatric Unit, Department of Neuroscience, Bambino Gesù Children's Hospital, IRCCS, Rome, Italy), Angelo Bertani (ASST Santi Paolo e Carlo, Italy), Edda Zanetti (ASST Spedali Civili, Brescia, Italy), Paramala Santosh, Leighton McFadden, Natalie Heaney, Mathilde Mastroianni, Federico Fiori, Jatinder Singh, Ilyas Sagar-Ouriaghli, Cassandra Deane (Kings College London, UK), Laura Adams (Plymouth University, UK), Diane Purper-Ouakil, Frédérick Russet, Virginie Maurice, Véronique Humbertclaude (Hôpital Saint Eloi, France), Renaud Jardri, Aesa Parenti (Centre Hospitalier Universitaire de Lille, France), David Da Fonseca, Isabelle Charvin (Centre Hospitalier Universitaire de Marseille, France), Frédérique Bonnet-Brilhault, Chrystèle Bodier, Catherine Prigent (Centre Hospitalier Universitaire de Tours, France), Mario Speranza, Hélène Lida-Pulik (CH Versailles, France), Athanasios Maras, Larissa van Bodegom, Mathilde Overbeek, Esther Kooymans, Iris Link (Yulius Academie, the Netherlands), Ulrike Schulze, Melanie Saam, Ulrike Breuninger, Jörg Fegert, Sonja Aslan, Sarah Miller (University of Ulm, Germany), Renate Schepker, Beata Williams, Anne Sartor, Elena Tanase (ZfP Südwürttemberg, Germany), Nichele Noterdaeme, Vehbi Sakar, Carolin von Bentzel (Josefinum Augsburg, Germany), Sabine Tremmery, Gaëlle Hendrickx, Veronique De Roeck (KU Leuven, Belgium), Fiona McNicholas, Lesley O’Hara, Rachael McKenna, Aleksandra Gronostaj, Ingrid Holme (University College Dublin, Ireland), Tomislav Franić, Nikolina Davidović (University Hospital Split, Croatia), Vlatka Kovač, Katarina Dodig-Ćurković (University of Osijek, Croatia), Frank Verhulst, Gwen Dieleman, Suzanne Gerritsen (Erasmus MC, The Netherlands), Kate Lievesley, Helen Furse, Paulina Nikolova, Todor Mutafov, Ivo Viliev, Vasil Dimov (HealthTracker, UK), Amanda Tuffrey, Anna Wilson, Charlotte Gatherer, Leanne Walker, Sarah Buttle, Caoimhe Kelly, Meghan Killilea, James Kirwan, Courtney Smyth (young project advisors affiliated with University of Warwick), Andrea Wohner (concentris research management GmbH, Germany), Nigel Jeffery, Suhas Hydros (Croydon CAMHS, UK), Richard Church (Lambeth CAMHS, UK), Omer Moghraby (Lewisham CAMHS, UK); Partha Banerjea (Southwark CAMHS, UK), Genevieve Riley, Pablo Ronzini, Rosemary Richards (2Gether NHS Foundation Trust, UK), Jo Berriman (South Staffordshire and Shropshire Healthcare NHS Foundation Trust SSSFT, UK), Vinuthna Pemmaraju (The Black Country Partnership Foundation NHS Trust, UK), Michael Slowik (Dudley and Walsall Mental Health Partnership NHS Trust, UK), Ben Rogers, Alan Farmer (Worcestershire Health and Care NHS Trust, UK), Ashley Liew, Tanveer Sandhu (Birmingham Children's Hospital, UK).
Contributors: PS is the Principal Investigator; JS, HT and JW wrote the manuscript and alongside SG, ND and KD-Ć assisted in revising the subsequent versions. NH, IS-O, MM, AP, EG, LM, GS, GA, FM, FR, VS and RA recruited subjects and were involved in data collection/management. LA, KL, HT and PT were involved in recruitment and the focus groups. FF was responsible for the data management component and subsequent analyses for the validation. SPS is the Chief Investigator of the MILESTONE project and obtained funding together with AM, CS, DP-O, DW, FV, FMc, GD, GdG, JM, MP, PS, ST, TF and US. AT, AW and CG served as young project advisors and helped with the development of the TRAM. All authors critically reviewed the protocol and the manuscript and gave approval for the publication.
Funding: Funding for the MILESTONE project was given by the EU Seventh Framework Programme for research, technological development and demonstration (grant number: 602442).
Disclaimer: This paper reflects only the authors' views and the European Union is not liable for any use that may be made of the information contained therein.
Competing interests: PS is the co-inventor of the HealthTracker and is the Chief Executive Officer and shareholder in HealthTracker. FF is a Chief Technical Officer and KL is a Project Manager employed by HealthTracker. FV is the Dutch distributor of ASEBA from which he receives remuneration. SPS is part-funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care WM (NIHR CLAHRC WM).
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the 'Methods' section for further details.
Patient consent for publication: Not required.
Ethics approval: The MILESTONE study protocol was approved by the UK National Research Ethics Service (15/WM/0052). Ethics approval was also granted by the ethics boards in the different MILESTONE participating centres; for London, this was the NRES Committee London—Camberwell St Giles (reference: 14/LO/1049). A complete list of all the ethics committees that provided ethical approval are provided in online supplementary information table 1.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Reasonable requests from individuals who wish to access the data can be done so by contacting the corresponding author.
References
- 1.While A, Forbes A, Ullman R, et al. Good practices that address continuity during transition from child to adult care: synthesis of the evidence. Child Care Health Dev 2004;30:439–52. 10.1111/j.1365-2214.2004.00440.x [DOI] [PubMed] [Google Scholar]
- 2.Department of Health Future in mind: promoting, protecting and improving our children and young people’s mental health and wellbeing. London: Department of Health Children and Young People’s Mental Health Taskforce, 2015. [Google Scholar]
- 3.Signorini G, Singh SP, Marsanic VB, et al. The interface between child/adolescent and adult mental health services: results from a European 28-country survey. Eur Child Adolesc Psychiatry 2018;27:501–11. 10.1007/s00787-018-1112-5 [DOI] [PubMed] [Google Scholar]
- 4.Steinkamp G, Ullrich G, Müller C, et al. Transition of adult patients with cystic fibrosis from paediatric to adult care--the patients' perspective before and after start-up of an adult clinic. Eur J Med Res 2001;6:85–92. [PubMed] [Google Scholar]
- 5.Shaw KL, Southwood TR, McDonagh JE, et al. User perspectives of transitional care for adolescents with juvenile idiopathic arthritis. Rheumatology 2004;43:770–8. 10.1093/rheumatology/keh175 [DOI] [PubMed] [Google Scholar]
- 6.McDonagh JE, Shaw KL, Southwood TR. Growing up and moving on in rheumatology: development and preliminary evaluation of a transitional care programme for a multicentre cohort of adolescents with juvenile idiopathic arthritis. J Child Health Care 2006;10:22–42. 10.1177/1367493506060203 [DOI] [PubMed] [Google Scholar]
- 7.Zhang LF, Ho JSW, Kennedy SE. A systematic review of the psychometric properties of transition readiness assessment tools in adolescents with chronic disease. BMC Pediatr 2014;14:4. 10.1186/1471-2431-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotstein DS, McPherson M, Strickland B, et al. Transition planning for youth with special health care needs: results from the National survey of children with special health care needs. Pediatrics 2005;115:1562–8. 10.1542/peds.2004-1262 [DOI] [PubMed] [Google Scholar]
- 9.Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ-Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011;36:160–71. 10.1093/jpepsy/jsp128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donahue SA, Manuel JI, Herman DB, et al. Development and use of a transition readiness scale to help manage ACT team capacity. Psychiatr Serv 2012;63:223–9. 10.1176/appi.ps.201100041 [DOI] [PubMed] [Google Scholar]
- 11.Ferris ME, Harward DH, Bickford K, et al. A clinical tool to measure the components of health-care transition from pediatric care to adult care: the UNC TR(x)ANSITION scale. Ren Fail 2012;34:744–53. 10.3109/0886022X.2012.678171 [DOI] [PubMed] [Google Scholar]
- 12.van Staa A, van der Stege HA, Jedeloo S, et al. Readiness to transfer to adult care of adolescents with chronic conditions: exploration of associated factors. J Adolesc Health 2011;48:295–302. 10.1016/j.jadohealth.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 13.Ware NC, Dickey B, Tugenberg T, et al. Connect: a measure of continuity of care in mental health services. Ment Health Serv Res 2003;5:209–21. 10.1023/A:1026276918081 [DOI] [PubMed] [Google Scholar]
- 14.Hadjistavropoulos H, Biem H, Sharpe D, et al. Patient perceptions of hospital discharge: reliability and validity of a patient continuity of care questionnaire. Int J Qual Health Care 2008;20:314–23. 10.1093/intqhc/mzn030 [DOI] [PubMed] [Google Scholar]
- 15.Durbin J, Goering P, Streiner DL, et al. Continuity of care: validation of a new self-report measure for individuals using mental health services. J Behav Health Serv Res 2004;31:279–96. 10.1007/BF02287291 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz LA, Daniel LC, Brumley LD, et al. Measures of readiness to transition to adult health care for youth with chronic physical health conditions: a systematic review and recommendations for measurement testing and development. J Pediatr Psychol 2014;39:588–601. 10.1093/jpepsy/jsu028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleverley K, Rowland E, Bennett K, et al. Identifying core components and indicators of successful transitions from child to adult mental health services: a scoping review. Eur Child Adolesc Psychiatry 2020;29:107–21. 10.1007/s00787-018-1213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh SP, Paul M, Ford T, et al. Transitions of care from child and adolescent mental health services to adult mental health services (TRACK study): a study of protocols in greater London. BMC Health Serv Res 2008;8:1–7. 10.1186/1472-6963-8-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam Z, Ford T, Kramer T, et al. Mind how you cross the gap! outcomes for young people who failed to make the transition from child to adult services: the TRACK study. BJPsych Bull 2016;40:142–8. 10.1192/pb.bp.115.050690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr NG, Longo CJ, Embrett MG, et al. The transition from youth to adult mental health services and the economic impact on youth and their families. Healthc Manage Forum 2017;30:283–8. 10.1177/0840470417709579 [DOI] [PubMed] [Google Scholar]
- 21.Broad KL, Sandhu VK, Sunderji N, et al. Youth experiences of transition from child mental health services to adult mental health services: a qualitative thematic synthesis. BMC Psychiatry 2017;17:380. 10.1186/s12888-017-1538-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Signorini G, Singh SP, Boricevic-Marsanic V, et al. Architecture and functioning of child and adolescent mental health services: a 28-country survey in Europe. Lancet Psychiatry 2017;4:715–24. 10.1016/S2215-0366(17)30127-X [DOI] [PubMed] [Google Scholar]
- 23.Singh SP, Tuomainen H, Girolamo Gde, et al. Protocol for a cohort study of adolescent mental health service users with a nested cluster randomised controlled trial to assess the clinical and cost-effectiveness of managed transition in improving transitions from child to adult mental health services (the MILESTONE study). BMJ Open 2017;7:e016055. 10.1136/bmjopen-2017-016055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santosh P, Adams L, Fiori F, et al. Protocol for the development and validation procedure of the managing the link and strengthening transition from child to adult mental health care (MILESTONE) suite of measures. BMC Pediatr 2020;20:167. 10.1186/s12887-020-02079-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Department of Health and Human Services Food and Drug Administration Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claimsSecondary guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims, 2009. [Google Scholar]
- 26.Gowers SG, Harrington RC, Whitton A, et al. Brief scale for measuring the outcomes of emotional and behavioural disorders in children. health of the nation outcome scales for children and adolescents (HoNOSCA). Br J Psychiatry 1999;174:413–6. 10.1192/bjp.174.5.413 [DOI] [PubMed] [Google Scholar]
- 27.Garralda ME, Yates P, Higginson I. Child and adolescent mental health service use. HoNOSCA as an outcome measure. Br J Psychiatry 2000;177:52–8. 10.1192/bjp.177.1.52 [DOI] [PubMed] [Google Scholar]
- 28.Guy W.Rush AJ, Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; Clinical Global Impressions Scale (CGI), 2000: 100–2. [Google Scholar]
- 29.Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr 1983;19:9–21. 10.1093/swra/19.3.9 [DOI] [PubMed] [Google Scholar]
- 30.Jarman B. Identification of underprivileged areas. Br Med J 1983;286:1705–9. 10.1136/bmj.286.6379.1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarman B. Underprivileged areas: validation and distribution of scores. Br Med J 1984;289:1587–92. 10.1136/bmj.289.6458.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (pro) measures: report of the ISPOR Task force for translation and cultural adaptation. Value Health 2005;8:94–104. 10.1111/j.1524-4733.2005.04054.x [DOI] [PubMed] [Google Scholar]
- 33.Sullivan GM, Feinn R. Using effect Size-or why the P value is not enough. J Grad Med Educ 2012;4:279–82. 10.4300/JGME-D-12-00156.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 3rd ed Upper Saddle River, N.J: Pearson/Prentice Hall, 2009. [Google Scholar]
- 35.Yates P, Garralda ME, Higginson I. Paddington complexity scale and health of the nation outcome scales for children and adolescents. Br J Psychiatry 1999;174:417–23. 10.1192/bjp.174.5.417 [DOI] [PubMed] [Google Scholar]
- 36.Bilenberg N. Health of the Nation Outcome Scales for Children and Adolescents (HoNOSCA)--results of a Danish field trial. Eur Child Adolesc Psychiatry 2003;12:298–302. 10.1007/s00787-003-0343-1 [DOI] [PubMed] [Google Scholar]
- 37.Peterson RA. A meta-analysis of variance accounted for and factor loadings in exploratory factor analysis. Mark Lett 2000;11:261–75. 10.1023/A:1008191211004 [DOI] [Google Scholar]
- 38.Schraeder KE, Reid GJ. Who should transition? defining a target population of youth with depression and anxiety that will require adult mental health care. J Behav Health Serv Res 2017;44:316–30. 10.1007/s11414-015-9495-2 [DOI] [PubMed] [Google Scholar]
- 39.Talbot RJ. Underprivileged areas and health care planning: implications of use of Jarman indicators of urban deprivation. BMJ 1991;302:383–6. 10.1136/bmj.302.6773.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033324supp001.pdf (85.3KB, pdf)