Abstract
The development of scalable deposition methods for perovskite solar cell materials is critical to enable the commercialization of this nascent technology. Herein, we investigate the use and processing of nanoparticle SnO2 films as electron transport layers in perovskite solar cells and develop deposition methods for ultrasonic spray coating and slot-die coating, leading to photovoltaic device efficiencies over 19%. The effects of postprocessing treatments (thermal annealing, UV ozone, and O2 plasma) are then probed using structural and spectroscopic techniques to characterize the nature of the np-SnO2/perovskite interface. We show that a brief “hot air flow” method can be used to replace extended thermal annealing, confirming that this approach is compatible with high-throughput processing. Our results highlight the importance of interface management to minimize nonradiative losses and provide a deeper understanding of the processing requirements for large-area deposition of nanoparticle metal oxides.
Keywords: tin oxide, perovskite solar cells, scalable processing, spray-coating, SnO2, interfaces
Introduction
Organic–inorganic hybrid perovskite materials have generated excitement and extensive research interest in the photovoltaic community since their demonstration in 2009, with the record single-junction power conversion efficiency (PCE) now reaching above 25%.1,2 This has been made possible by a distinctive set of characteristics in this family of materials, including high optical absorption, long charge-carrier lifetimes enabled by low nonradiative recombination rates, and extensive possibilities for compositional tuning.3,4 Typically, high efficiency n–i–p cell architectures have relied on a compact and mesoporous TiO2 electron transport layer (ETL) architecture.5 However, a primary concern with TiO2 is the inherent instability caused by UV light interacting with molecular O2 adsorbed at surface defect sites. This process may then lead to decomposition of the organic component of the active layer, with many stability studies on devices utilizing TiO2 typically making use of UV filters to negate such effects.6,7 TiO2-based systems also commonly require processing steps at temperatures above 450 °C.5 This temperature is, however, incompatible with many roll-to-roll (R2R) or sheet-to-sheet substrates such as polyethylene terephthalate (PET) and will also limit their use in tandem devices that may have other temperature-sensitive layers.
One approach to mitigate ETL UV instability and reduce the processing temperature is to replace TiO2 (band gap ∼3.3 eV) with a wider band gap metal oxide such as SnO2 (3.6–4.2 eV), with the wider band gap also reducing parasitic absorption.8,9 Compared to TiO2, crystalline SnO2 exhibits nearly 2 orders of magnitude higher electron mobility;10 a property that suggests it should act as a highly effective ETL. Atomic layer deposition (ALD) has been used to deposit amorphous SnO2, and it is thought that its conduction band is well aligned for barrier-free electron transfer from various perovskites.11 Various routes have been used to deposit planar SnO2 including chemical bath deposition (CBD), sol–gel conversion,12 chemical vapor deposition (CVD),13 plasma-enhanced ALD,14 electron-beam evaporation,15 thermal evaporation,16 sputtering,17 spin-coated sol–gel precursor in combination with CBD,18 nanoparticle routes,19,20 and mesoporous SnO2.21,22 Importantly, mesoporous SnO2 has also been demonstrated to have improved UV stability relative to mesoporous TiO2, although it has so far been processed at high temperature, preventing the use of fluorine–SnO2 (FTO) layers because of fluorine migration.21,22 Two key papers on planar SnO2-only ETL deposition routes have reported efficiencies of over 20%,18,19 with the work by Jiang et al. using an off-the-shelf nanoparticle SnO2 (np-SnO2) product subsequently leading to a record-breaking planar n–i–p device PCE of 23.3%.23 This np-SnO2 system has the advantage of not undergoing temperature-sensitive phase formation during annealing, which can impact the reproducibility of other SnO2 ETL deposition processes.
In this paper, we utilize an np-SnO2 system with a triple cation, mixed-halide perovskite5 with solution composition Cs0.05FA0.79MA0.16PbI2.45Br0.55 and demonstrate highly reproducible stabilized power output (SPO) efficiencies of up to 19.7% and good batch-to-batch reproducibility. We explore two scalable np-SnO2-coating methods (spray-coating and slot-die coating) and achieve peak PCEs of over 18% SPO. To demonstrate a rapid process compatible with R2R manufacture, we investigate both annealing-free and hot air flow (HAF) flash drying processes (120 °C for 1 min) combined with other low-temperature post-treatments (ultraviolet-ozone (UVO) and O2 plasma) to replace or reduce the commonly used annealing step (10–30 min at 150 °C).19,24 Using such techniques, we develop a rapid process that combines spray-coating, HAF at 120 °C, and UVO treatment to achieve 18.7% SPO using a fully scalable ETL deposition process. These results demonstrate the benefit of ex situ-crystallized nanoparticle metal oxides for achieving efficient, rapidly processed photovoltaic devices.
Results and Discussion
Electron Transport Layer Deposition
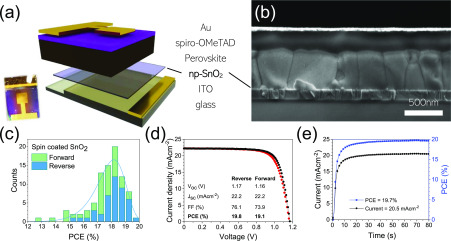
To establish a stable baseline for experiments using more scalable techniques, we explored the fabrication of perovskite solar cell (PSC) devices in which the ETL was deposited by spin-coating np-SnO2 from a diluted commercially available dispersion. Full details of the techniques used are given in Experimental Methods. Figure 1 shows the n–i–p configuration of the device, together with a scanning electron micrograph (SEM) cross-section of a completed device. Here, the full device structure is as follows: indium tin oxide (ITO), np-SnO2 ETL, triple cation perovskite Cs0.05FA0.79MA0.16PbI2.45Br0.55 absorbing layer, doped spiro-OMeTAD hole transport layer (HTL), and Au top contact.
Figure 1.
np-SnO2 device structure and performance. (a) Illustration of the n–i–p layer architecture with a photograph of a completed device inset. (b) Cross-sectional SEM image of a completed device showing densely packed perovskite grains and ultrathin np-SnO2 layer. (c) Histogram of all spin-coated device efficiencies (forward and reverse sweep), showing excellent reproducibility. Champion cell performance is illustrated by (d) a current–voltage sweep and (e) stabilized device performance at the J–V determined MPP.
We achieved a narrow distribution of device efficiencies on ITO for large 0.16 cm2 cells (see Figure 1c), with a champion device PCE of 19.8% reverse sweep (VOC to JSC) efficiency (Figure 1d) with the stabilized power output (SPO) closely matching the reverse sweep PCE at 19.7% (see Figure 1e). The batch-to-batch reproducibility and device metrics are promising; multiple devices were realized with a VOC of 1.17 V, corresponding to a voltage loss of 0.45 eV from the 1.62 eV band gap in the best cells (Figure S1). Comparable efficiencies of up to 18.8% were demonstrated for smaller devices fabricated on fluorine-doped tin oxide (FTO) substrates (see Figure S2). For comparison, we also show data in Figure S3 for small cells fabricated using the SnCl4·5H2O spin-coating method proposed by Ke et al. and developed by Anaraki et al.(8,18) Here, we found similar performance in some devices; however, we find the process to have low reproducibility (a broader distribution compared to np-SnO2 devices in Figure S2), with full cell performance parameters given in Table S1. The process also requires a drying step at 100 °C followed by a longer annealing time at higher temperature (180 °C for 60 min).18 Taken together, we believe that the low reproducibility, and temperature requirements to convert to SnO2, makes the SnCl4·5H2O conversion process unsuitable for use in scalable device architectures, with nanoparticle metal oxides being an attractive solution for rapid processing.
Slot-Die Coating
Two scalable deposition methods were investigated to deposit the ETL layer. Slot-die coating is widely used in industrial R2R processes and has the key benefit of minimal material wastage during coating.25,26 To deposit a range of thicknesses, we used a set solution concentration and flow speed and then explored a range of head speeds. Surface wetting was enhanced by UVO treating the ITO prior to deposition, with a 3 wt % np-SnO2 solution prepared by diluting with H2O. Improved wetting for contact coating methods can also be promoted by mixing the primary solvent with ethanol by dropwise addition.26 We note that when diluting using only H2O (which has a high surface tension of >70 mN m–1 at RT), a meniscus forms around the slot die head and at the substrate edges. Here, we mitigated this effect by placing the target substrate between two other substrates to ensure a uniform flow across the intended device area (see Figure S4), with the meniscus defining the thickness of the deposited layer (found to be ∼38 nm see Figure S5). This is confirmed by process optimization results, showing device efficiencies that are comparable across a range of head speeds from 3 to 15 mm s–1 (see Figure S6a). Champion and average device performance metrics are shown in Table S1 for all devices in both sweep directions, with a maximum PCE of 18.5% and only mild hysteresis for the reverse sweep direction (Figure S6b).
Spray Coating
As a non-contact scalable deposition technique, spray-coating offers the benefit of higher throughput than is achievable using slot-die or other contact coating methods.27 However, care must be taken to ensure good wetting of the substrate, the formation of a uniform, leveled wet film and homogenous drying. In the case of perovskite films, solvent optimization is particularly critical as it is also necessary to control nucleation and crystallization behavior. This can be achieved through careful post-deposition treatments such as vacuum exposure,28 a technique that has also been used in inkjet printing.29 For the methods outlined here, we are using an ultrasonic spray coater, which atomizes the coating solution using a piezoelectric transducer into droplets from a moving head, which are directed with gas flow onto a static coating surface.27 Here, we have adopted a single-pass coating approach, operating in ambient conditions and using nontoxic solvents.
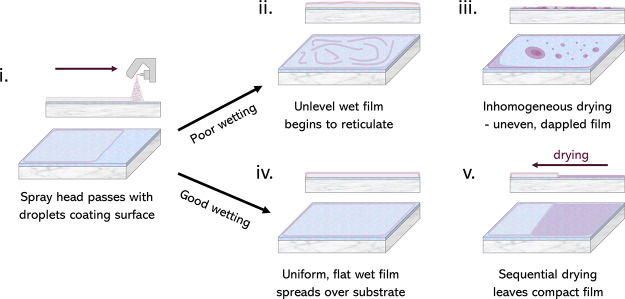
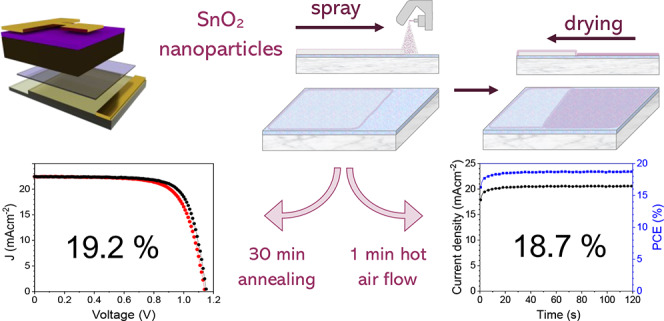
Typically, low surface tension and low boiling point solvents are used for spray-coating. When using H2O as a solvent to spray-coat SnO2, its high surface tension can lead to the formation of thick wet films despite its good wetting properties (indeed 30 s of UVO exposure is sufficient to encourage complete wetting, see Figure S7). The thickness of the wet film is also dictated by the volume of solution that lands on the substrate, the surface energy of the substrate, and meniscus effects at the edge of the substrate. We have optimized the spray-deposition process to deposit SnO2 films on ITO glass and find that the use of a low concentration solution (1:70 np-SnO2/H2O), together with a UVO treatment leads to the formation of a homogeneous wet film. From this wet film, drying proceeds over the substrate surface in around 60 s. We show this process schematically in Figure 2, with further images and uniform conformal coatings shown in Figure S8. We note that in an industrial process, drying could be further controlled by use of an air-blade24 or HAF across the surface, a process that we describe later.
Figure 2.
Scheme illustrating the optimized np-SnO2 drying process across the UVO-treated ITO surface: (i) spray-coating, (ii) fast reticulation, (iii) dry film with poor uniformity, (iv) ideal wet film, and (v) drying proceeds across the substrate.
Topography and Device Performance
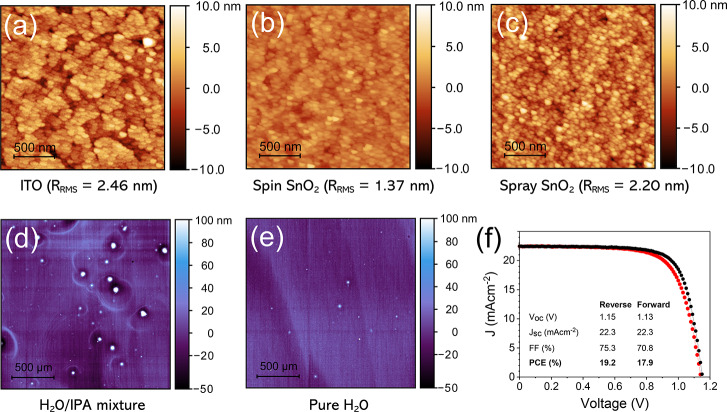
To investigate the uniformity and roughness of the deposited films, we performed atomic force microscopy (AFM) on spin- and spray-coated np-SnO2 (see Figure 3a–c). We found that spin coating significantly reduced the film root mean square surface roughness (RRMS) from 2.46 nm for the uncoated ITO to 1.37 nm, with the spray-coated film being slightly rougher (RRMS = 2.20 nm). Examining the topography of the coated surfaces, we find that the np-SnO2 film (prepared using both deposition techniques) significantly reduces the surface density of voids in the ITO. We have compared spin- and slot-die-coated surfaces and find that slot-die-coated np-SnO2 films exhibit comparable roughness to spin-coated films (1.37 nm) with annealing having little effect on film morphology (see Figure S9). We find that the spray-coated films have a reduced layer thicknesses, which is ∼17 nm (measured by spectroscopic ellipsometry), with this thickness being apparently insensitive to spray-coater head speed (see Figure S5). We speculate that this thin-sprayed np-SnO2 film dries conformally over the surface, with its greater roughness possibly reflecting the roughness of the underlying ITO (see Figure 2).
Figure 3.
AFM height maps for uniformity and roughness of (a) ITO, (b) spin-coated and (c) spray-coated np-SnO2 layers. Profilometric mapping of completed devices using spray-deposited np-SnO2 with (d) IPA/H2O mixed solvent and (e) H2O-only solution; here, np-SnO2 layer inhomogeneity in the IPA/H2O cast film leads to pinholes in subsequent layers. (f) J–V curve for the best-performing spray np-SnO2 device.
We have also performed surface profilometry mapping on completed devices (glass/ITO/np-SnO2/perovskite/spiro-OMeTAD/Au) to explore film morphology over larger length scales (2.5 mm × 2.5 mm). A surface map is shown in Figure 3d that was recorded from the surface of a device incorporating a spray-coated np-SnO2 layer deposited from an IPA/H2O/np-SnO2 solvent mixture, with similar mixtures having been used with slot-die coating to improve wetting.26 However, we found that de-mixing of this two-solvent system occurs during the atomization process in the ultrasonic spray head, as illustrated in Figure S10. Consequently, this poor uniformity bottom layer leads to a significant number of pinholes, with ring-like morphological defects resulting from undulations in the np-SnO2 layer apparently propagating through subsequent layers in the completed device. However, this behavior can be largely suppressed by judicious choice of the spray solvent. Here, Figure 3e shows a topographic image of a device surface in which the np-SnO2 was spray-coated with only H2O solvent, leading to a greatly reduced density of pinholes and no ring-like features evident. This result highlights the different challenges with solvent engineering for spray coating as compared to contact methods; further details on creating stable solvent mixtures for spray-coating are given in Supporting Information note 1.
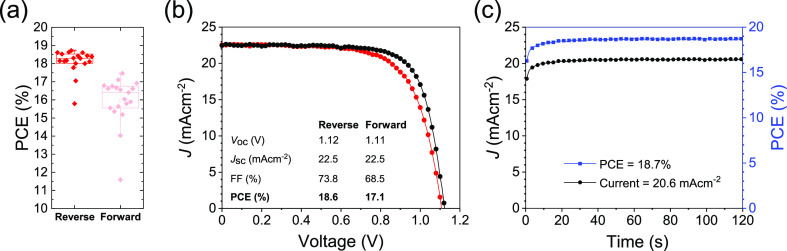
Device performance metrics for all devices are shown in Table 1. For optimized spray-coated np-SnO2 devices, we achieved a champion PCE of 19.2% with a SPO for the best-performing cell of 18.6% (see Figure S11). We find that devices fabricated using a spray-coated np-SnO2 layer exhibit increased J–V hysteresis, a result consistent with a reduced uniformity in layer thickness. Despite this, we find that other performance metrics for spray-coated and slot-die-fabricated devices closely match those of average spin-coated cells.
Table 1. Champion Device Performance Metrics for Spin- and Spray-Coated np-SnO2 Devices, with Average and Standard Deviation in Parenthesisa.
| coating | treatment | sweep | PCE (%) | JSC (mA cm–2) | VOC (V) | FF (%) | no. of cells |
|---|---|---|---|---|---|---|---|
| spin | annealed +15 min UVO | forward | 19.29 (17.21 ± 1.40) | 22.93 (22.13 ± 0.43) | 1.16 (1.12 ± 0.03) | 75.30 (69.23 ± 4.80) | 43 |
| reverse | 19.82 (18.17 ± 0.97) | 22.94 (22.15 ± 0.46) | 1.17 (1.13 ± 0.03) | 76.14 (72.58 ± 2.38) | |||
| spray | annealed +15 min UVO | forward | 17.86 (16.62 ± 1.75) | 22.26 (22.13 ± 0.26) | 1.13 (1.13 ± 0.01) | 70.76 (66.24 ± 6.13) | 13 |
| reverse | 19.22 (18.45 ± 1.18) | 22.29 (22.19 ± 0.20) | 1.15 (1.14 ± 0.01) | 75.27 (72.76 ± 3.75) | |||
| spray | annealed + delayed 15 min UVO | forward | 14.92 (11.32 ± 2.68) | 22.21 (22.01 ± 0.20) | 1.01 (0.94 ± 0.09) | 66.28 (53.76 ± 8.73) | 13 |
| reverse | 17.01 (15.08 ± 3.18) | 22.39 (22.11 ± 0.23) | 1.06 (1.00 ± 0.11) | 72.38 (67.09 ± 10.07) |
All devices use np-SnO2 layers which were thermally annealed (150 °C for 30 min) and post-treated with UVO. Performance for spray-coated cells with np-SnO2 layers left in air for 2 days following annealing is also shown, highlighting the issue of loss of performance resulting from surface contamination.
We note that it is imperative to use the np-SnO2 films directly after the application of annealing and UV ozone treatments. We observed that leaving annealed np-SnO2 films in ambient conditions, even if subject to a UVO treatment directly before coating with perovskite, leads to a substantial loss in VOC (see Table 1). This effect most likely results from the adsorption of organic species at the surface that cannot be effectively removed through the UVO treatment alone.
Scalable Processing
The methods outlined so far have utilized an annealing step of 150 °C for 30 min to dry, crystallize, and remove the solvent from the np-SnO2 layer. However, such an extended thermal treatment is incompatible with rapid R2R or continuous processing, where the duration of the longest process dictates the maximum web speed. Furthermore, our standard process also involves a UVO surface preparation treatment for 15 min to increase the surface energy for perovskite wetting and remove surface contaminants. In the following sections, we describe techniques that we have developed to reduce process time and temperature, while maintaining good device performance.
Effect of Thermal Annealing
To minimize the cost and duration of film processing, it is desirable to remove the transport layer thermal annealing step. Fortunately, as the SnO2 nanoparticle system is already composed of precrystallized nanoparticle domains, there is no phase change or oxidation process required to form the SnO2 phase. However, it is necessary to understand the effects played by any thermal treatments and UVO exposure on the transport layer and the SnO2/perovskite interface. Previous reports on the optical absorption of np-SnO2 suggest a band gap of 3.79–3.94 eV for annealed np-SnO2 films, but with only a limited wavelength range below the band gap, required for accurate fitting.19,30 Tauc-like plots from our transmission measurements indicate optical band gap values of 4.43 eV for non-annealed films and 4.39 eV for 150 °C annealed np-SnO2 films (Figure S12). These values are significantly higher than those typically expected for phase-pure SnO2, so we applied a band-fluctuations fitting model to spectroscopic ellipsometry data.31 This confirmed the apparent wide optical band gap of 4.48 and 4.45 eV for as-deposited and thermally annealed films, respectively (see Table S2); this model is discussed in Supporting Information note 2.
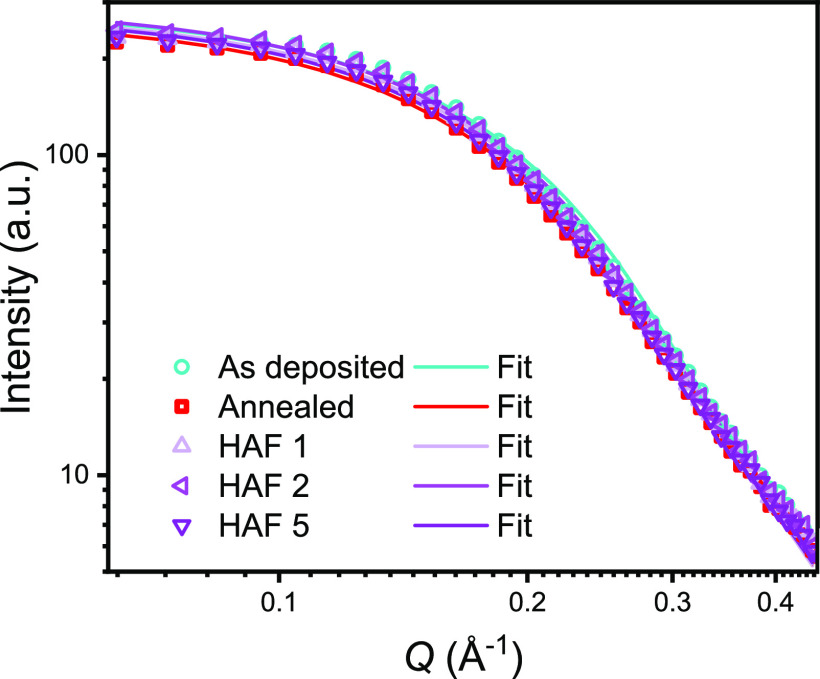
Grazing incidence small-angle X-ray scattering (GISAXS) techniques can provide a wealth of information about the thin-film material structure and has been used extensively for characterization of photovoltaic materials. Here, we collected 2D X-ray scattering patterns of np-SnO2 films (see Figure S13) and fitted an empirical Guinier–Porod model32 to in-plane cuts through this data (see Figure 4) to investigate changes that occur in the lateral structure during annealing. Using this approach, we determine typical correlation lengths for crystalline domains within our films between 1.1 and 1.2 nm (see Table 2), with such values agreeing with the manufacturer’s data. Here, GISAXS measurements were designed to preferentially probe the np-SnO2 layer averaging over the irradiated sample surface, where changes induced by annealing are most likely to be evident (our complete methodology is explained in Supporting Information note 3). From the fitted values shown in Table 2 (with further parameters presented in Table S3), thermal annealing increases the typical domain size for grains in the film from ∼1.17 to ∼1.22 nm, a result that confirms that thermal annealing increases the average size of the crystalline domains. We also determine a reduction in the Porod exponent d from ∼4 (corresponding to an ideal, smooth surface) to ∼3.6, a result that indicates the presence of less well-defined spherical boundaries between SnO2 domains following annealing. Therefore, we conclude that the annealed film can be considered as a densely packed layer of spheres that become fused, with the crystalline domaincontinuing to grow during thermal annealing.
Figure 4.
In-plane linecuts and Guinier–Porod fitting of GISAXS from np-SnO2 layers with different annealing conditions; as-deposited, 30 min 150 °C annealed, and HAF for 1, 2, and 5 min. 2D GISAXS patterns for all samples are shown in Figure S13.
Table 2. Guinier–Porod Fitting Parameters for GISAXS Profiles of np-SnO2 Filmsa.
| sample | Rg (Å–1) | d (Porod exponent) | fitted domain size (nm) |
|---|---|---|---|
| as deposited | 9.09 ± 0.05 | 3.984 ± 0.31 | 1.173 ± 0.006 |
| annealed | 9.43 ± 0.05 | 3.612 ± 0.24 | 1.218 ± 0.006 |
| HAF 1 min | 9.27 ± 0.05 | 3.829 ± 0.28 | 1.197 ± 0.006 |
| HAF 2 min | 9.31 ± 0.05 | 3.831 ± 0.27 | 1.202 ± 0.006 |
| HAF 5 min | 9.42 ± 0.05 | 3.661 ± 0.24 | 1.216 ± 0.006 |
Typical grain size is determined assuming spherical domains, with full details given in Supporting Information note 3.
Various rapid thermal processing techniques have been used to process perovskite layers, notably photonic curing, flash infra-red annealing, intense pulsed light (IPL), and rapid thermal processing.33−36 Such techniques have also been used to replace TiO2 sintering;37−39 however, the high transmissivity of thin np-SnO2 complicates their use for this material; for example, photonic curing or extended thermal annealing will instead cause damage to flexible plastic substrates like PET or PEN. To replace thermal annealing, we have explored the use of a rapid thermal HAF process at a temperature of 120 ± 10 °C for between 1 and 5 min, as has been used to process perovskite films.40 Here, a temperature-controlled heat gun was used to replicate the typical hot-plate thermal annealing process, representing a technique consistent with R2R processing in air. Our experimental setup is shown in Figure S14. Guinier–Porod fit parameters extracted from in-plane scatter measurements (Figure 4) from films processed using HAF show similar trends to those extracted following extended thermal annealing, with an increase in the grain radius and reduction of smoothness determined for increasing HAF process times (see Table 2). Indeed, both the Porod exponent and grain radius are found to closely match the extended annealing after only 5 min of HAF.
Post-Deposition Treatments
Various options exist to clean/process layers for R2R fabrication, including exposure to plasma. We note that the UVO system used here did not include an O2 gas feed, so we expect that process times could be significantly reduced by including an oxygen feed or by using more intense UV light sources. UVO has been widely used as a surface treatment for SnO2 and has been reported to enhance carrier extraction.41 The UVO process is believed to enhance surface hydroxylation42 (increasing the surface energy) and to reduce the surface-density of oxygen vacancies. Again, our objective is to explore an industrially applicable process, so we also investigated a 5 min O2 plasma treatment because of its extensive use in the coating industry and explore its impact on the np-SnO2 surface, and the SnO2/perovskite interface. The O2 plasma treatment combines UV cleaning under vacuum with cleaning by various ionized oxygen species. In optoelectronic devices, this has been used to modify surface electronic properties for enhanced carrier injection or extraction.43,44
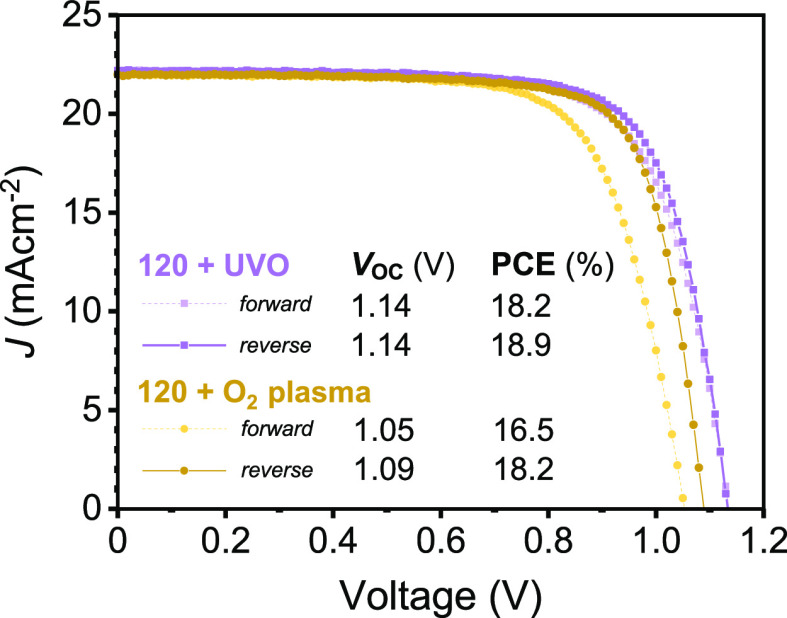
We first investigated the effect of removing the thermal annealing process on device performance. Here, PV devices were prepared by either drying np-SnO2 in air or by using a one-minute annealing step at 120 °C to match the fastest HAF process that would be compatible with processing on a flexible substrate. In each case, a further 15 min UVO (here termed “120 + UVO”) or O2 plasma (termed “120 + O2”) post-treatment was applied to the np-SnO2 ETL to understand its effect on cell performance. Table S4 shows device efficiencies following each treatment, with optimum device performance achieved using the 120 + UVO process, where a maximum reverse sweep PCE of 18.9% was recorded (champion device shown in Figure 5). This encouraging result suggests that high performance can be achieved using both reduced processing time and relatively low annealing temperatures, with even brief annealing having performance benefit. Table S4 also indicates a reduction in VOC by around 80 mV for devices treated using O2 plasma compared with those treated using UVO (average ∼1.05 V compared with ∼1.13 V, respectively). This reduction is accompanied by an increase in hysteresis during scanning, where the J–V sweep for the best-performing 120 + O2 device (see Figure 5) shows a significant difference in the forward and reverse sweep PCE, VOC, and FF compared with that processed using the 120 + UVO np-SnO2.
Figure 5.
J–V curves for the best-performing cells using np-SnO2 treated with 1 min 120 °C drying and either 15 min UVO or 5 min O2 plasma prior to perovskite deposition. Key sweep parameters are inset (full parameters in Table S4), with the O2 plasma-treated np-SnO2 device exhibiting lower VOC and increased hysteresis.
To first confirm whether these changes in device performance result from changes in the perovskite deposited on the treated surfaces, we performed white light absorption and steady-state photoluminescence (SSPL) measurements on bilayer np-SnO2/perovskite samples, with np-SnO2 exposed to various processing conditions. Figure S15 shows the optical density for np-SnO2/perovskite samples and corresponding Tauc-like plots where we assume a direct band gap. In all cases, films were found to have a similar optical band gap of ∼1.62 eV, corresponding well with PL emission (see Figure S16) at ∼1.63 eV in all the samples (Table S5). To further confirm whether changes observed in device performance are due to changes in the perovskite layer, we recorded grazing-incidence wide-angle X-ray scattering (GIWAXS) patterns for samples using np-SnO2 substrates with either the 120 + UVO or 120 + O2 plasma treatment (Figure S17). Here, we found no substrate-dependent effects on the perovskite crystallinity or orientation. From these observations, we conclude that the perovskite bulk composition and structure is comparable in both cases, irrespective of the np-SnO2 layer treatment, a result that suggests that the observed changes in device performance result from modification at the SnO2/perovskite interface.
Photoelectron Spectroscopy
To investigate the effect of UVO and O2 plasma treatments on the band structure and composition of the np-SnO2, we performed ultraviolet (UPS) and X-ray (XPS) photoelectron spectroscopy measurements. These highly surface sensitive techniques give a wealth of information on the electronic nature of surfaces and interfaces, with many reports investigating doped and undoped SnOx for many applications, from TCOs to gas sensing.45−48 Here, we prepared np-SnO2 films on ITO at the same thickness as used in completed devices. XPS and UPS measurements will also include the effect of surface contaminants and adsorbates, which will be heavily dependent on the SnO2 surface.49 Samples might typically be prepared by thorough cleaning, followed by Ar+ sputtering to remove adventitious carbon or other detectable species, and remain under high vacuum after preparation.50 Here, however, cleaning/sputtering of the SnO2 will affect its surface composition,51 so following UVO or O2 treatments in air, samples were sealed under vacuum and then rapidly transferred to high vacuum for the measurements.
By measuring XPS spectra across an extended binding energy range, we investigate the surface elemental composition of the np-SnO2 layers. We note that the np-SnO2 suspension used here is stabilized using KOH (with a solution pH of ∼11.5, see Figure S18). From survey (wide) scan spectra shown in Figure S19, we find that the O2 plasma-treated samples are characterized by reduced emission from K 2p core levels and weakly detectable emission from In 3d levels. This indicates that the O2 plasma partially removes KOH from the np-SnO2 surface, and also etches the np-SnO2 layer, allowing indium in the ITO substrate to be detected. We also find significantly increased F 1s intensity which we attribute to fluorine contamination arising from degraded PTFE coatings within the plasma reactor.52 Indeed, if fluorine is incorporated into the SnO2 surface, it may increase the optical band gap.53 We also compared the effect of each processing step on the adventitious carbon with C 1s core-level spectra (see Figure S20). As expected, we found that annealing does not remove carbon contaminants, whereas UVO and O2 plasma both significantly reduce carbon species present at the surface.
Various other stable adsorbates are expected to be present at SnO2 surfaces, notably O2, H2O, and hydroxide species, with their concentration heavily dependent on the SnO2 surface and stoichiometry.49,54,55 We probed the O 1s core-level XPS emission to understand changes in surface oxygen species, with spectra and fits for 120 + UVO and 120 + O2 plasma shown in Figure S21. Two components were fitted (in most cases) using a lower energy feature having a binding energy of 531.1–531.3 eV together with a higher energy shoulder at 532.4–532.6 eV (fitting methodology is described in the experimental methods). The peak at 531.2 eV is ascribed to lattice oxygen (O–Sn bonds) with the second broader feature originating from adsorbed species such as −OH groups and carboxides.55,56 In the O2 plasma-treated samples, a distinct third peak was detected at 533.3 eV which we suspect is related to an additional adsorbate species induced by fluorine modifying the surface.57 In Table S6, we compare the relative areas of O 1s and Sn 3d and find that annealed and 120 + UVO samples have the highest area ratio for both [O 1s]/[Sn 3d] and Sn–O/[Sn 3d], which is reduced following an O2 plasma treatment. While these values should be treated with caution because of the effect of adsorbates, our results suggest that 5 min of O2 plasma treatment can significantly modify the surface species, apparently leading to an Sn-rich, O-poor surface.58 A reduced O/Sn ratio has previously been observed following an O2 plasma treatment,59 but the reverse has also been reported for reduced SnO2 surfaces.60 In summary, the SnO2 surface following the treatment will be impacted by the initial stoichiometry and crystallinity of the surface, the proportion of different ionized oxygen species in the plasma, the nature of fluorine contamination, and the process duration.49,53
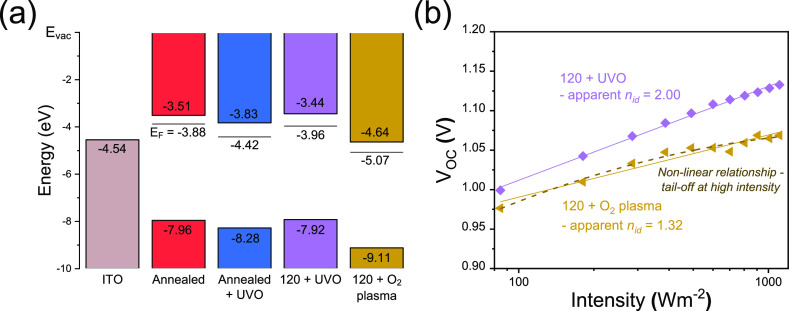
To analyze the electronic structure from UPS spectra, the secondary electron edge (or Ecutoff) was used to determine the work function (WF) for each layer, with the onset energy (Eonset) used to find the valence band maximum (VBM) with respect to the Fermi level (EF) (see Figure S22). To confirm the VBM positions (ionisation energy, IE) for all samples, we also extracted Eonset values from XPS valence spectra (see Figure S23). These values agreed with those determined from UPS measurements (see Tables S5 and S6). As noted above, the energy-level determination will be affected by extrinsic band bending because of adsorbates, with adsorbed moisture expected to lead to the formation of an accumulation layer at the SnO2/perovskite interface.54 Noting this, we show an apparent energy level diagram for ITO and key np-SnO2 samples in Figure 6a, illustrating the position of the Fermi level, the VBM and estimated conduction band minimum (CBM) using our previously determined optical band gap values for np-SnO2 (Figure S24 illustrates the energy level determination). Here, it is immediately apparent that the O2 plasma treatment has caused a significant shift of the Fermi level and ionization energy. Changes to the chemical species present, together with a reduced layer thickness and modified oxidation state have significantly altered the surface of the 120 + O2 sample surface, a conclusion confirmed by the “as-deposited + O2” sample exhibiting similarly shifted energy levels (see Table S7). We note that this is consistent with literature reports on TCOs treated with O2 plasma, a process that has been shown to downshift the Fermi level in FTO and ITO surfaces by 0.5–0.6 eV.43,49 We also note that the energy levels determined for the annealed sample are significantly modified following the UVO treatment, an observation that may partly result from removal of carbon species, as identified in Figure S20. For completeness, we give typical triple cation perovskite and spiro-OMeTAD energy levels (predominantly measured by UPS at the top surface combined with optical bandgap) in Table S9, with the triple-cation perovskite CBM typically in the range −3.79 to −4.46 eV.
Figure 6.
Understanding the effect of UVO and O2 plasma treatments. (a) Electronic structure at the np-SnO2 surface with the Fermi level (EF) from UPS measurements, valence band from UPS and XPS and estimated conduction band from the optical band gap. (b) Stabilized light-VOC measurements for 120 + UVO and 120 + O2 devices, showing reduced VOC for the plasma treatment. Apparent nid from linear fits are shown, and in the O2 plasma case, behavior indicates increased nonradiative recombination at the ETL interface.64,67
Device Physics
Previous work has shown that the illumination intensity-dependent variation of VOC can provide information about the dominant recombination mechanisms in photovoltaic devices. Classically, the light ideality factor (nid) extracted using this approach determines whether recombination is primarily bimolecular (band-to-band) or monomolecular (trap-assisted), in cases with comparable electron and hole densities.61,62 With varied carrier densities, trap energies and trap locations (bulk or interface), nid can take a range of values (nid ≈ 1–2); analysis is complicated in hybrid perovskite devices by the influence of mobile ions, which lead to transient modification of interfaces, and therefore carrier extraction behavior.62,63 Adopting the approach modeled by Tress et al., we recorded stabilized VOC measurements after a set illumination period and found that the 120 + O2 sample exhibits behavior consistent with poor charge selectivity (see Figure 6b).64 This result suggests increased interface recombination in this case despite a lower apparent nid extracted from a linear fit (1.32 compared to 2.00 in the 120 + UVO sample). Transient photovoltage measurements recorded at 1 sun are shown in Figure S25 and show slower voltage stabilization for O2 plasma-treated devices. Over tens of seconds, these changes in VOC are likely to result from dynamic processes at the ETL interface caused by the interaction of both mobile ions and charge carriers, modifying carrier extraction behaviour.63,65
Therefore, we conclude that the observed VOC loss shown in Figure 5 and Table S4 for devices utilizing the 120 + O2 ETL results from a significant modification of the surface chemistry. This leads to an apparent downshift of the VBM, Fermi level, and CBM of the SnO2, resulting in a loss of electron selectivity, with reduced quasi-Fermi level splitting in the device. While the observed energy shifts are significant, they are affected by adsorbates which also affect the extrinsic electron density at the interface and may even evolve dynamically under operation.54,55 However, it is clear that both misalignment and modified doping density will change the charge and ionic screening behavior of the interface, a process that most likely causes the increased hysteresis observed in O2 plasma-treated samples.66 However, it is unclear whether band alignment and doping effects can be independently modified with O2 plasma.30,67 It has also been reported that ALD SnO2 with different oxidants (such as O2 plasma or ozone) can lead to changes in the electronic properties of the ETL layer, as well as modifying the subsequent perovskite growth,68 a process that may also be influenced by the observed reduction in KOH.26 It is clear that further research is needed to characterize chemical reactivity between different substrate transport materials and the perovskite layer during film formation.9,68
Overall, it is evident that O2 plasma can have a detrimental impact on the SnO2/perovskite interface if not properly controlled; however, promising results using shorter treatment times have been achieved.69 Surface preparation equipment (both UVO and O2 plasma) used in research contexts varies greatly in device performance, power and process control, making exact methods difficult to reproduce between laboratories. As such, further efforts must be made to replicate industrially relevant plasma cleaning approaches used in interface preparation.
Rapid Processing
Combining the optimized spray coating, 1 min HAF, and UVO treatment, PV devices were prepared using this series of processes that have the potential to be fully transferrable to R2R or other low-cost processing. In Figure 7, we show a histogram of all device efficiencies, as well as the current–voltage performance and SPO for the champion device with a stabilized PCE of 18.7%. To accompany this, average and champion performance parameters are shown in Table 3, showing comparable metrics to annealed spin- and spray-coated devices presented earlier, although with increased hysteresis and slightly reduced average VOC and FF. The total processing time here is around 1 min (mainly determined by the annealing step), with spatial atomic layer deposition (SALD) being the only comparably rapid, low-temperature deposition technique that operates in an ambient atmosphere able to create functional SnOx layers for applications in perovskite solar cells. We note that Hoffmann et al. fabricated p–i–n devices incorporating a SnOx ETL (that also acted as an impermeable barrier layer), with devices with over 12% PCE. Here, devices were fabricated using a substrate processing speed and temperature of 20–80 mm s–1 and 80 °C, respectively (compared with 25 °C and up to 180 mm s–1 achieved in our process), but with no further annealing required.70
Figure 7.
Photovoltaic performance for devices prepared using fast processing: spray coating, 1 min HAF, and UVO treatment. (a) Reverse and forward sweep efficiencies for 19 operational cells, (b) J–V sweep, and (c) SPO of the best-performing cell.
Table 3. Fast-Processed Device Performance Using Sprayed np-SnO2, 1 min HAF and UVO Treatment.
| coating | treatment | sweep | PCE (%) | JSC (mA cm–2) | VOC | FF | no. of cells |
|---|---|---|---|---|---|---|---|
| spray | HAF 1 min + UVO | forward | 17.11 (15.95 ± 1.29) | 22.55 (22.54 ± 0.18) | 1.11 (1.09 ± 0.03) | 68.52 (65.08 ± 3.62) | 19 |
| reverse | 18.62 (18.11 ± 0.66) | 22.45 (22.55 ± 0.17) | 1.12 (1.11 ± 0.01) | 73.81 (72.32 ± 1.93) |
Conclusions
We have explored the deposition of SnO2 nanoparticle layers using two scalable deposition processes (spray coating and slot-die coating) and fabricated perovskite devices with performance and morphology comparable to those of spin-coating. The effect of annealing is investigated using GISAXS, where we quantify the fusing of nanoparticles into a densely packed ETL. The effect of annealing, UV ozone, and O2 plasma post-treatments were also investigated using UPS, XPS, optical, and electrical measurements. We observed significant modification to adsorbed species, concomitant with shifts of apparent np-SnO2 energy levels. Using an O2 plasma treatment, a significant reduction in the Fermi level led to a loss of VOC and increased current–voltage hysteresis. Finally, we demonstrated an optimized fast deposition technique involving annealing the SnO2 layer in hot air to create photovoltaic devices yielding stabilized power conversion efficiencies close to 19%. This work outlines the future design requirements for rapid processing of functional metal oxide nanoparticle layers, deposited at high speed and under ambient conditions—conditions compatible with R2R processing.
Acknowledgments
We thank the EPSRC for PhD studentships through the Centre for Doctoral Training in New and Sustainable PV, EP/L01551X/1 (J.A.S., E.L.K.S., C.G.) and via the University of Sheffield DTG account (J.E.B., R.C.K.). T.I.A. thanks the Saudi Government for funding via a PhD studentship. We also acknowledge funding from EPSRC to support this work via grants “Hybrid Polaritonics” (EP/M025330/1), “High-resolution mapping of performance and degradation mechanisms in printable photovoltaic devices” (EP/M025020/1), “The integration of photovoltaic devices with carbon-fibre composites” (EP/S009213/1) and from the Global Challenges Research Fund (GCRF) through Science and Technology Facilities Council (STFC), grant number ST/R002754/1 “Synchrotron Techniques for African Research and Technology (START)”. J.A.S. also thanks the Erasmus + exchange program for support. We also thank Thomas Featherstone for assistance with XPS data analysis, Carolin Rehermann for helping with UV–Vis measurements, as well as Selina Olthof and Aboma Merdasa for useful discussions. The XPS instrument belongs to the Sheffield Surface Analysis Centre, a facility run from the Department of Chemistry, University of Sheffield, and is led by Professor Graham Leggett. We thank the company Xenocs for their help and ongoing support in the X-ray scattering user program at Sheffield, and we thank the EPSRC for funding the purchase of this instrument.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsaem.0c00525.
Experimental methods, notes on solvent mixtures, band-fluctuation modeling and GISAXS modeling; extended device performance data, process images and diagrams, ellipsometry, contact angle measurements, additional AFM, PL, absorption, Tauc plots, GIWAXS, XPS spectra, UPS spectra, energy-level determination, and photovoltage measurements; further device metrics, Guinier–Porod fitting, XPS fits, electronic structure determination, and energy levels (PDF)
The authors declare the following competing financial interest(s): D.G.L. is a co-director of the company Ossila that retail materials and equipment used in perovskite photovoltaic device research and development.
Supplementary Material
References
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- NREL . Best Research-Cell Efficiencies. https://www.nrel.gov/pv/cell-efficiency.html (accessed Dec 10, 2019).
- Stranks S. D. Nonradiative Losses in Metal Halide Perovskites. ACS Energy Lett. 2017, 2, 1515–1525. 10.1021/acsenergylett.7b00239. [DOI] [Google Scholar]
- Egger D. A.; Bera A.; Cahen D.; Hodes G.; Kirchartz T.; Kronik L.; Lovrincic R.; Rappe A. M.; Reichman D. R.; Yaffe O. What Remains Unexplained about the Properties of Halide Perovskites?. Adv. Mater. 2018, 30, 1800691. 10.1002/adma.201800691. [DOI] [PubMed] [Google Scholar]
- Saliba M.; Matsui T.; Seo J.-Y.; Domanski K.; Correa-Baena J.-P.; Nazeeruddin M. K.; Zakeeruddin S. M.; Tress W.; Abate A.; Hagfeldt A.; Grätzel M. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. 10.1039/C5EE03874J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe T. A.; Su W.-N.; Chen C.-H.; Pan C.-J.; Cheng J.-H.; Chen H.-M.; Tsai M.-C.; Chen L.-Y.; Dubale A. A.; Hwang B.-J. Organometal Halide Perovskite Solar Cells: Degradation and Stability. Energy Environ. Sci. 2016, 9, 323–356. 10.1039/C5EE02733K. [DOI] [Google Scholar]
- Leijtens T.; Eperon G. E.; Pathak S.; Abate A.; Lee M. M.; Snaith H. J. Overcoming Ultraviolet Light Instability of Sensitized TiO2 with Meso-Superstructured Organometal Tri-Halide Perovskite Solar Cells. Nat. Commun. 2013, 4, 2885. 10.1038/ncomms3885. [DOI] [PubMed] [Google Scholar]
- Ke W.; Fang G.; Liu Q.; Xiong L.; Qin P.; Tao H.; Wang J.; Lei H.; Li B.; Wan J.; Yang G.; Yan Y. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. 10.1021/jacs.5b01994. [DOI] [PubMed] [Google Scholar]
- Christians J. A.; Schulz P.; Tinkham J. S.; Schloemer T. H.; Harvey S. P.; Tremolet de Villers B. J.; Sellinger A.; Berry J. J.; Luther J. M. Tailored Interfaces of Unencapsulated Perovskite Solar Cells for >1,000 Hour Operational Stability. Nat. Energy 2018, 3, 68–74. 10.1038/s41560-017-0067-y. [DOI] [Google Scholar]
- Tiwana P.; Docampo P.; Johnston M. B.; Snaith H. J.; Herz L. M. Electron Mobility and Injection Dynamics in Mesoporous ZnO, SnO2, and TiO2 Films Used in Dye-Sensitized Solar Cells. ACS Nano 2011, 5, 5158–5166. 10.1021/nn201243y. [DOI] [PubMed] [Google Scholar]
- Baena J. P. C.; Steier L.; Tress W.; Saliba M.; Neutzner S.; Matsui T.; Giordano F.; Jacobsson T. J.; Kandada A. R. S.; Zakeeruddin S. M.; Petrozza A.; Abate A.; Nazeeruddin M. K.; Grätzel M.; Hagfeldt A. Highly Efficient Planar Perovskite Solar Cells through Band Alignment Engineering. Energy Environ. Sci. 2015, 8, 2928–2934. 10.1039/C5EE02608C. [DOI] [Google Scholar]
- Dong Q.; Shi Y.; Wang K.; Li Y.; Wang S.; Zhang H.; Xing Y.; Du Y.; Bai X.; Ma T. Insight into Perovskite Solar Cells Based on SnO2 Compact Electron-Selective Layer. J. Phys. Chem. C 2015, 119, 10212–10217. 10.1021/acs.jpcc.5b00541. [DOI] [Google Scholar]
- Bush K. A.; Palmstrom A. F.; Yu Z.; Boccard M.; Cheacharoen R.; Mailoa J. P.; McMeekin D. P.; Hoye R. L. Z.; Bailie C. D.; Leijtens T.; Peters I. M.; Minichetti M. C.; Rolston N.; Prasanna R.; Sofia S. E.; Harwood D.; Ma W.; Moghadam F.; Snaith H. J.; Buonassisi T.; Holman Z. C.; Bent S. F.; McGehee M. D. 23.6%-Efficient Monolithic Perovskite/Silicon Tandem Solar Cells with Improved Stability. Nat. Energy 2017, 2, 17009. 10.1038/nenergy.2017.9. [DOI] [Google Scholar]
- Wang C.; Zhao D.; Grice C. R.; Liao W.; Yu Y.; Cimaroli A.; Shrestha N.; Roland P. J.; Chen J.; Yu Z.; Liu P.; Cheng N.; Ellingson R. J.; Zhao X.; Yan Y. Low-Temperature Plasma-Enhanced Atomic Layer Deposition of Tin Oxide Electron Selective Layers for Highly Efficient Planar Perovskite Solar Cells. J. Mater. Chem. A 2016, 4, 12080–12087. 10.1039/C6TA04503K. [DOI] [Google Scholar]
- Ma J.; Zheng X.; Lei H.; Ke W.; Chen C.; Chen Z.; Yang G.; Fang G. Highly Efficient and Stable Planar Perovskite Solar Cells With Large-Scale Manufacture of E-Beam Evaporated SnO2 Toward Commercialization. Sol. RRL 2017, 1, 1700118. 10.1002/solr.201700118. [DOI] [Google Scholar]
- Guo Y.; Yin X.; Liu J.; Chen W.; Wen S.; Que M.; Xie H.; Yang Y.; Que W.; Gao B. Vacuum Thermal-Evaporated SnO2 as Uniform Electron Transport Layer and Novel Management of Perovskite Intermediates for Efficient and Stable Planar Perovskite Solar Cells. Org. Electron. 2019, 65, 207–214. 10.1016/j.orgel.2018.11.021. [DOI] [Google Scholar]
- Qiu L.; Liu Z.; Ono L. K.; Jiang Y.; Son D. Y.; Hawash Z.; He S.; Qi Y. Scalable Fabrication of Stable High Efficiency Perovskite Solar Cells and Modules Utilizing Room Temperature Sputtered SnO2 Electron Transport Layer. Adv. Funct. Mater. 2019, 29, 1806779. 10.1002/adfm.201806779. [DOI] [Google Scholar]
- Anaraki E. H.; Kermanpur A.; Steier L.; Domanski K.; Matsui T.; Tress W.; Saliba M.; Abate A.; Grätzel M.; Hagfeldt A.; Correa-Baena J.-P. Highly Efficient and Stable Planar Perovskite Solar Cells by Solution-Processed Tin Oxide. Energy Environ. Sci. 2016, 9, 3128–3134. 10.1039/C6EE02390H. [DOI] [Google Scholar]
- Jiang Q.; Zhang L.; Wang H.; Yang X.; Meng J.; Liu H.; Yin Z.; Wu J.; Zhang X.; You J. Enhanced Electron Extraction Using SnO2 for High-Efficiency Planar-Structure HC(NH2)2PbI3-Based Perovskite Solar Cells. Nat. Energy 2017, 2, 16177. 10.1038/nenergy.2016.177. [DOI] [Google Scholar]
- Duan J.; Xiong Q.; Feng B.; Xu Y.; Zhang J.; Wang H. Low-Temperature Processed SnO2 Compact Layer for Efficient Mesostructure Perovskite Solar Cells. Appl. Surf. Sci. 2017, 391, 677–683. 10.1016/j.apsusc.2016.06.187. [DOI] [Google Scholar]
- Roose B.; Baena J.-P. C.; Gödel K. C.; Graetzel M.; Hagfeldt A.; Steiner U.; Abate A. Mesoporous SnO2 Electron Selective Contact Enables UV-Stable Perovskite Solar Cells. Nano Energy 2016, 30, 517–522. 10.1016/j.nanoen.2016.10.055. [DOI] [Google Scholar]
- Li Y.; Zhu J.; Huang Y.; Liu F.; Lv M.; Chen S.; Hu L.; Tang J.; Yao J.; Dai S. Mesoporous SnO2 Nanoparticle Films as Electron-Transporting Material in Perovskite Solar Cells. RSC Adv. 2015, 5, 28424–28429. 10.1039/c5ra01540e. [DOI] [Google Scholar]
- Jiang Q.; Zhao Y.; Zhang X.; Yang X.; Chen Y.; Chu Z.; Ye Q.; Li X.; Yin Z.; You J. Surface Passivation of Perovskite Film for Efficient Solar Cells. Nat. Photonics 2019, 13, 460–466. 10.1038/s41566-019-0398-2. [DOI] [Google Scholar]
- Ding J.; Han Q.; Ge Q.-Q.; Xue D.; Ma J.-Y.; Zhao B.; Chen Y.-X.; Liu J.; Mitzi D. B.; Hu J.-S. Fully Air-Bladed High-Efficiency Perovskite Photovoltaics. Joule 2019, 3, 402. 10.1016/j.joule.2018.10.025. [DOI] [Google Scholar]
- Dou B.; Whitaker J. B.; Bruening K.; Moore D. T.; Wheeler L. M.; Ryter J.; Breslin N. J.; Berry J. J.; Garner S. M.; Barnes F. S.; Shaheen S. E.; Tassone C. J.; Zhu K.; van Hest M. F. A. M.; Sean E.; Tassone C. J.; Zhu K.; Hest M. F. A. M. Van. Roll-to-Roll Printing of Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 2558–2565. 10.1021/acsenergylett.8b01556. [DOI] [Google Scholar]
- Bu T.; Li J.; Zheng F.; Chen W.; Wen X.; Ku Z.; Peng Y.; Zhong J.; Cheng Y.-B.; Huang F. Universal Passivation Strategy to Slot-Die Printed SnO2 for Hysteresis-Free Efficient Flexible Perovskite Solar Module. Nat. Commun. 2018, 9, 4609. 10.1038/s41467-018-07099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. E.; Routledge T. J.; Lidzey D. G. Advances in Spray-Cast Perovskite Solar Cells. J. Phys. Chem. Lett. 2018, 9, 1977–1984. 10.1021/acs.jpclett.8b00311. [DOI] [PubMed] [Google Scholar]
- Bishop J. E.; Smith J. A.; Greenland C.; Kumar V.; Vaenas N.; Game O. S.; Routledge T. J.; Wong-Stringer M.; Rodenburg C.; Lidzey D. G. High-Efficiency Spray-Coated Perovskite Solar Cells Utilizing Vacuum-Assisted Solution Processing. ACS Appl. Mater. Interfaces 2018, 10, 39428–39434. 10.1021/acsami.8b14859. [DOI] [PubMed] [Google Scholar]
- Mathies F.; Eggers H.; Richards B. S.; Hernandez-Sosa G.; Lemmer U.; Paetzold U. W. Inkjet-Printed Triple Cation Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 1834–1839. 10.1021/acsaem.8b00222. [DOI] [Google Scholar]
- Yun A. J.; Kim J.; Hwang T.; Park B. Origins of Efficient Perovskite Solar Cells with Low-Temperature Processed SnO2 Electron Transport Layer. ACS Appl. Energy Mater. 2019, 2, 3554–3560. 10.1021/acsaem.9b00293. [DOI] [Google Scholar]
- Guerra J. A.; Tejada A.; Töfflinger J. A.; Grieseler R.; Korte L. Band-Fluctuations Model for the Fundamental Absorption of Crystalline and Amorphous Semiconductors: A Dimensionless Joint Density of States Analysis. J. Phys. D: Appl. Phys. 2019, 52, 105303. 10.1088/1361-6463/aaf963. [DOI] [Google Scholar]
- Hammouda B. A New Guinier-Porod Model. J. Appl. Crystallogr. 2010, 43, 716–719. 10.1107/S0021889810015773. [DOI] [Google Scholar]
- Troughton J.; Carnie M. J.; Davies M. L.; Charbonneau C.; Jewell E. H.; Worsley D. A.; Watson T. M. Photonic Flash-Annealing of Lead Halide Perovskite Solar Cells in 1 ms. J. Mater. Chem. A 2016, 4, 3471–3476. 10.1039/c5ta09431c. [DOI] [Google Scholar]
- Sanchez S.; Hua X.; Phung N.; Steiner U.; Abate A. Flash Infrared Annealing for Antisolvent-Free Highly Efficient Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1702915. 10.1002/aenm.201702915. [DOI] [Google Scholar]
- Ghahremani A. H.; Martin B.; Gupta A.; Bahadur J.; Ankireddy K.; Druffel T. Rapid Fabrication of Perovskite Solar Cells through Intense Pulse Light Annealing of SnO2 and Triple Cation Perovskite Thin Films. Mater. Des. 2020, 185, 108237. 10.1016/j.matdes.2019.108237. [DOI] [Google Scholar]
- Ouyang Z.; Yang M.; Whitaker J. B.; Li D.; van Hest M. F. A. M. Towards Scalable Perovskite Solar Modules Using Blade-Coating and Rapid Thermal Processing. ACS Appl. Energy Mater. 2020, 3, 3714. 10.1021/acsaem.0c00180. [DOI] [Google Scholar]
- Charbonneau C.; Holliman P. J.; Davies M. L.; Watson T. M.; Worsley D. A. Facile Self-Assembly and Stabilization of Metal Oxide Nanoparticles. J. Colloid Interface Sci. 2015, 442, 110–119. 10.1016/j.jcis.2014.11.042. [DOI] [PubMed] [Google Scholar]
- Das S.; Gu G.; Joshi P. C.; Yang B.; Aytug T.; Rouleau C. M.; Geohegan D. B.; Xiao K. Low Thermal Budget, Photonic-Cured Compact TiO2 Layers for High-Efficiency Perovskite Solar Cells. J. Mater. Chem. A 2016, 4, 9685–9690. 10.1039/c6ta02105k. [DOI] [Google Scholar]
- Feleki B.; Bex G.; Andriessen R.; Galagan Y.; Di Giacomo F. Rapid and Low Temperature Processing of Mesoporous TiO2 for Perovskite Solar Cells on Flexible and Rigid Substrates. Mater. Today Commun. 2017, 13, 232–240. 10.1016/j.mtcomm.2017.09.007. [DOI] [Google Scholar]
- Liu Y.; Shin I.; Hwang I.-W.; Lee J.; Kim S.; Lee D. Y.; Lee S.-H.; Jang J.-W.; Jung Y. K.; Jeong J. H.; Park S. H.; Kim K. H. Effective Hot-Air Annealing for Improving the Performance of Perovskite Solar Cells. Sol. Energy 2017, 146, 359–367. 10.1016/j.solener.2017.03.005. [DOI] [Google Scholar]
- Dong Q.; Shi Y.; Zhang C.; Wu Y.; Wang L. Energetically Favored Formation of SnO2 Nanocrystals as Electron Transfer Layer in Perovskite Solar Cells with High Efficiency Exceeding 19%. Nano Energy 2017, 40, 336–344. 10.1016/j.nanoen.2017.08.041. [DOI] [Google Scholar]
- Méndez P. F.; Muhammed S. K. M.; Barea E. M.; Masi S.; Mora-Seró I. Analysis of the UV–Ozone-Treated SnO2 Electron Transporting Layer in Planar Perovskite Solar Cells for High Performance and Reduced Hysteresis. Sol. RRL 2019, 3, 1900191. 10.1002/solr.201900191. [DOI] [Google Scholar]
- Milliron D. J.; Hill I. G.; Shen C.; Kahn A.; Schwartz J. Surface Oxidation Activates Indium Tin Oxide for Hole Injection. J. Appl. Phys. 2000, 87, 572–576. 10.1063/1.371901. [DOI] [Google Scholar]
- Lee K. H.; Jang H. W.; Kim K.-B.; Tak Y.-H.; Lee J.-L. Mechanism for the Increase of Indium-Tin-Oxide Work Function by O2 Inductively Coupled Plasma Treatment. J. Appl. Phys. 2004, 95, 586. 10.1063/1.1633351. [DOI] [Google Scholar]
- Themlin J. M.; Sporken R.; Darville J.; Caudano R.; Gilles J. M.; Johnson R. L. Resonant-Photoemission Study of SnO2: Cationic Origin of the Defect Band-Gap States. Phys. Rev. B: Condens. Matter Mater. Phys. 1990, 42, 11914–11925. 10.1103/PhysRevB.42.11914. [DOI] [PubMed] [Google Scholar]
- McGuinness C.; Stagarescu C. B.; Ryan P. J.; Downes J. E.; Fu D.; Smith K. E.; Egdell G. Influence of Shallow Core-Level Hybridization on the Electronic Structure of Post-Transition-Metal Oxides Studied Using Soft X-Ray Emission and Absorption. Phys. Rev. B: Condens. Matter Mater. Phys. 2003, 68, 165104. 10.1103/PhysRevB.68.165104. [DOI] [Google Scholar]
- Batzill M. Surface Science Studies of Gas Sensing Materials: SnO2. Sensors 2006, 6, 1345–1366. 10.3390/s6101345. [DOI] [Google Scholar]
- Das S.; Jayaraman V. SnO2: A Comprehensive Review on Structures and Gas Sensors. Prog. Mater. Sci. 2014, 66, 112–255. 10.1016/j.pmatsci.2014.06.003. [DOI] [Google Scholar]
- Weidner M.Fermi Level Determination in Tin Oxide by Photoelectron Spectroscopy. Ph.D. Thesis, TU Darmstadt, 2015.Thesiss [Google Scholar]
- Helander M. G.; Greiner M. T.; Wang Z. B.; Lu Z. H. Pitfalls in Measuring Work Function Using Photoelectron Spectroscopy. Appl. Surf. Sci. 2010, 256, 2602–2605. 10.1016/j.apsusc.2009.11.002. [DOI] [Google Scholar]
- Egdell R. G.; Eriksen S.; Flavell W. R. A Spectroscopic Study of Electron and Ion Beam Reduction of SnO2(110). Surf. Sci. 1987, 192, 265–274. 10.1016/S0039-6028(87)81175-5. [DOI] [Google Scholar]
- Karulkar P. C. XPS/AES Investigation of Cross Contamination in a Plasma Etcher. J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct. 1985, 3, 889. 10.1116/1.583077. [DOI] [Google Scholar]
- Swallow J. E. N.; Williamson B. A. D.; Whittles T. J.; Birkett M.; Featherstone T. J.; Peng N.; Abbott A.; Farnworth M.; Cheetham K. J.; Warren P.; Scanlon D. O.; Dhanak V. R.; Veal T. D. Self-Compensation in Transparent Conducting F-Doped SnO2. Adv. Funct. Mater. 2018, 28, 1701900. 10.1002/adfm.201701900. [DOI] [Google Scholar]
- Trost S.; Becker T.; Polywka A.; Görrn P.; Oszajca M. F.; Luechinger N. A.; Rogalla D.; Weidner M.; Reckers P.; Mayer T.; Riedl T. Avoiding Photoinduced Shunts in Organic Solar Cells by the Use of Tin Oxide (SnOx) as Electron Extraction Material Instead of ZnO. Adv. Energy Mater. 2016, 6, 1600347. 10.1002/aenm.201600347. [DOI] [Google Scholar]
- Roose B.; Friend R. H. Extrinsic Electron Concentration in SnO2 Electron Extracting Contact in Lead Halide Perovskite Solar Cells. Adv. Mater. Interfaces 2019, 6, 1801788. 10.1002/admi.201801788. [DOI] [Google Scholar]
- Chistiakova G.; Mews M.; Wilks R. G.; Bär M.; Korte L. In-System Photoelectron Spectroscopy Study of Tin Oxide Layers Produced from Tetrakis(Dimethylamino)Tin by Plasma Enhanced Atomic Layer Deposition. J. Vac. Sci. Technol., A 2018, 36, 02D401. 10.1116/1.5015967. [DOI] [Google Scholar]
- Noonuruk R.; Mekprasart W.; Vittayakorn N.; Sritharathikhun J.; Pecharapa W. Physical, Electrical and Optical Properties of F/Sb Codoped SnO2 Synthesized Via Sonochemical Process. Ferroelectrics 2016, 490, 136–148. 10.1080/00150193.2015.1072694. [DOI] [Google Scholar]
- Jiang J. C.; Lian K.; Meletis E. I. Influence of Oxygen Plasma Treatment on the Microstructure of SnOx Thin Films. Thin Solid Films 2002, 411, 203–210. 10.1016/s0040-6090(02)00288-2. [DOI] [Google Scholar]
- Mathur S.; Ganesan R.; Grobelsek I.; Shen H.; Ruegamer T.; Barth S. Plasma-Assisted Modulation of Morphology and Composition in Tin Oxide Nanostructures for Sensing Applications. Adv. Eng. Mater. 2007, 9, 658–663. 10.1002/adem.200700086. [DOI] [Google Scholar]
- Cavicchi R.; Tarlov M.; Semancik S. Preparation of Well-ordered, Oxygen-rich SnO2 (110) Surfaces via Oxygen Plasma Treatment. J. Vac. Sci. Technol., A 1990, 8, 2347–2352. 10.1116/1.576696. [DOI] [Google Scholar]
- Wolff C. M.; Caprioglio P.; Stolterfoht M.; Neher D. Nonradiative Recombination in Perovskite Solar Cells: The Role of Interfaces. Adv. Mater. 2019, 31, 1902762. 10.1002/adma.201902762. [DOI] [PubMed] [Google Scholar]
- Calado P.; Burkitt D.; Yao J.; Troughton J.; Watson T. M.; Carnie M. J.; Telford A. M.; Regan B. C. O.; Nelson J.; Barnes P. R. F. Identifying Dominant Recombination Mechanisms in Perovskite Solar Cells by Measuring the Transient Ideality Factor. Phys. Rev. Appl. 2019, 11, 044005. 10.1103/PhysRevApplied.11.044005. [DOI] [Google Scholar]
- Moia D.; Gelmetti I.; Calado P.; Fisher W.; Stringer M.; Game O.; Hu Y.; Docampo P.; Lidzey D.; Palomares E.; Nelson J.; Barnes P. R. F. Ionic-to-Electronic Current Amplification in Hybrid Perovskite Solar Cells: Ionically Gated Transistor-Interface Circuit Model Explains Hysteresis and Impedance of Mixed Conducting Devices. Energy Environ. Sci. 2019, 12, 1296–1308. 10.1039/C8EE02362J. [DOI] [Google Scholar]
- Tress W.; Yavari M.; Domanski K.; Yadav P.; Niesen B.; Correa Baena J. P.; Hagfeldt A.; Graetzel M. Interpretation and Evolution of Open-Circuit Voltage, Recombination, Ideality Factor and Subgap Defect States during Reversible Light-Soaking and Irreversible Degradation of Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 151–165. 10.1039/c7ee02415k. [DOI] [Google Scholar]
- Walter D.; Fell A.; Wu Y.; Duong T.; Barugkin C.; Wu N.; White T.; Weber K. Transient Photovoltage in Perovskite Solar Cells: Interaction of Trap-Mediated Recombination and Migration of Multiple Ionic Species. J. Phys. Chem. C 2018, 122, 11270–11281. 10.1021/acs.jpcc.8b02529. [DOI] [Google Scholar]
- Courtier N. E.; Cave J. M.; Foster J. M.; Walker A. B.; Richardson G. How Transport Layer Properties Affect Perovskite Solar Cell Performance: Insights from a Coupled Charge Transport/Ion Migration Model. Energy Environ. Sci. 2019, 12, 396–409. 10.1039/C8EE01576G. [DOI] [Google Scholar]
- Aygüler M. F.; Hufnagel A. G.; Rieder P.; Wussler M.; Jaegermann W.; Bein T.; Dyakonov V.; Petrus M. L.; Baumann A.; Docampo P. Influence of Fermi Level Alignment with Tin Oxide on the Hysteresis of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 11414–11419. 10.1021/acsami.8b00990. [DOI] [PubMed] [Google Scholar]
- Hu T.; Becker T.; Pourdavoud N.; Zhao J.; Brinkmann K. O.; Heiderhoff R.; Gahlmann T.; Huang Z.; Olthof S.; Meerholz K.; Többens D.; Cheng B.; Chen Y.; Riedl T.; Heiderhoff R.; Gahlmann T.; Huang Z.; Olthof S.; Meerholz K.; Többens D.; Cheng B.; Chen Y.; Riedl T. Indium-Free Perovskite Solar Cells Enabled by Impermeable Tin-Oxide Electron Extraction Layers. Adv. Mater. 2017, 29, 1606656. 10.1002/adma.201606656. [DOI] [PubMed] [Google Scholar]
- Luan Y.; Yi X.; Mao P.; Wei Y.; Zhuang J.; Chen N.; Lin T.; Li C.; Wang J. High-Performance Planar Perovskite Solar Cells with Negligible Hysteresis Using 2,2,2-Trifluoroethanol-Incorporated SnO2. iScience 2019, 16, 433–441. 10.1016/j.isci.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L.; Brinkmann K. O.; Malerczyk J.; Rogalla D.; Becker T.; Theirich D.; Shutsko I.; Görrn P.; Riedl T. Spatial Atmospheric Pressure Atomic Layer Deposition of Tin Oxide as an Impermeable Electron Extraction Layer for Perovskite Solar Cells with Enhanced Thermal Stability. ACS Appl. Mater. Interfaces 2018, 10, 6006–6013. 10.1021/acsami.7b17701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.