Abstract
Pancreatic adenocarcinoma (PDAC) is associated with poor clinical outcomes and incomplete responses to conventional therapy. Therefore, there is an unmet clinical need to better understand the predisposing factors for pancreatic cancer in hopes of providing early screening to high-risk patients. While select risk factors such as age, race, and family history, or predisposing syndromes are unavoidable, there are several new and established risk factors that allow for intervention, namely by counseling patients to make the appropriate lifestyle modifications. Here, we discuss the best-studied risk factors for PDAC such as tobacco use and chronic pancreatitis, as well as newly emerging risk factors including select nutritional deficits, bacterial infections, and psychosocial factors. As several of these risk factors appear additive or synergistic, by understanding their relationships and offering coordinated, multidisciplinary care to high-risk patients, it may be possible to reduce pancreatic cancer incidence and improve clinical outcomes through early detection.
1 -. INTRODUCTION
Pancreatic cancer is projected to be the second leading cause of cancer related death in the United States by 2030 (1). Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic cancer histotype, and typically presents at late clinical stages (2). As a result, most PDAC patients have poor responses to conventional therapy and median survival remains a dismal 6–12 months (2). Given the limited therapeutic options for pancreatic cancer patients and lack of adequate screening modalities, it is imperative to better understand the clinically identifiable risk factors for PDAC in hopes of identifying high-risk patients in the primary care setting, and providing early imaging and appropriate counseling in order to reduce the likelihood of developing this largely incurable malignancy.
2.1 -. UNAVOIDABLE RISK FACTORS
While many of the known risk factors for pancreatic cancer are actionable through direct patient counseling, there are several that do not provide the opportunity for intervention. Still, these risk factors are nonetheless informative as they may provide guidance regarding screening and differential diagnosis. While PDAC has a slight male predominance, this may be partially explained by lifestyle differences between men and women, and outcomes do not significantly differ between sexes (2). However, such lifestyle differences cannot fully explain the difference in disease incidence, particularly in light of recently identified sex differences in driving genes and biomarkers in a variety of cancer types (3). In fact, when evaluating the TCGA genomic databases of pancreatic cancer patients (N=185), we found that while male and female patients had no significant difference in clinical outcomes, the presence of any specific mutation, or copy number alteration, there were several highly significant differences in mRNA expression between the sexes (Figure 1A,B and Table 1). While the clinical relevance of these findings are not clear at this time, interestingly most genes comparatively overexpressed in women were found on the X chromosome, whereas men only had significantly increased expression of one gene located on chromosome 12 (Table 1).
Figure 1. Differences in gene expression by sex and race in the TCGA cohort of pancreatic cancer patients.
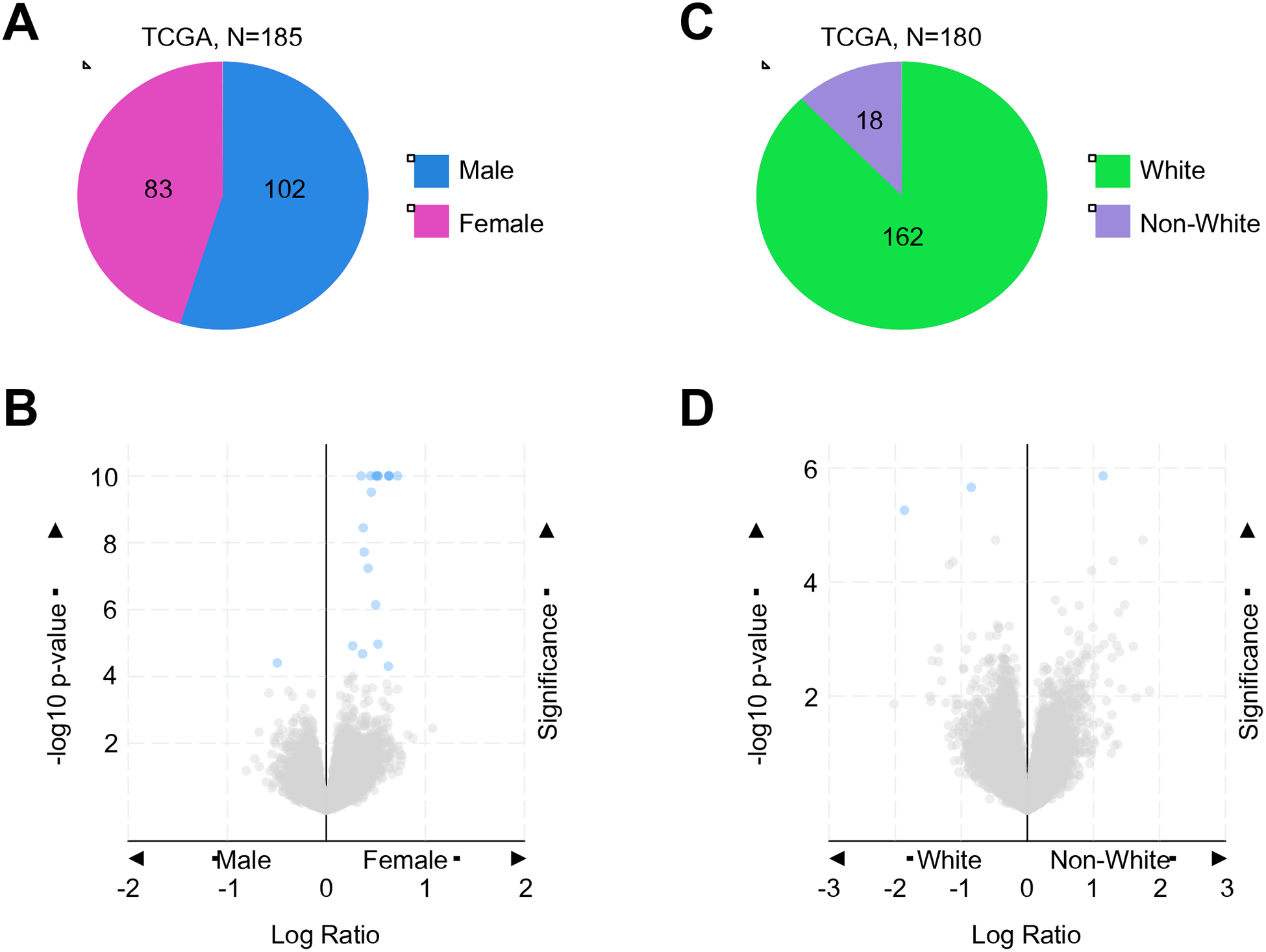
(A,B) Using the TCGA genomic databases of pancreatic cancer patients (N=185), we evaluated differences in gene expression between male (N=102) and female (N=83) patients. Differences in mRNA expression were visualized via volcano plot, and genes with significant (FDR adjusted p-value < 0.05) differences between groups colored blue. For a complete list of these genes see Table 1. (C,D) In the same patient cohort, we evaluated differences in gene expression based on race. Of the 180 patients for which ethnicity was documented, 162 were white, and the remaining 18 African American or Asian (non-white). Differences in mRNA expression were visualized via volcano plot, and genes with significant (FDR adjusted p-value < 0.05) differences between groups colored blue. For a complete list of these genes see Table 1.
Table 1.
Genes differentially expressed between males and females, or white and racial minority patients from the TCGA pancreatic cancer cohort
| Gene | Cytoband | p-Value | q-Value | Higher Expression |
|---|---|---|---|---|
| EIF1AX | Xp22.12 | 1.9*10−21 | 2.68*10−17 | Female |
| KDM5C | Xp11.22 | 2.66*10−18 | 1.88*10−14 | Female |
| KDM6A | Xp11.3 | 3.13*10−17 | 1.47*10−13 | Female |
| JPX | Xq13.2 | 9.15*10−17 | 3.23*10−13 | Female |
| ZRSR2 | Xp22.2 | 1.49*10−13 | 4.21*10−10 | Female |
| PNPLA4 | Xp22.31 | 1.31*10−12 | 3.09*10−9 | Female |
| PUDP | Xp22.31 | 3.7*10−12 | 7.46*10−9 | Female |
| SYAP1 | Xp22.2 | 1.44*10−11 | 2.27*10−8 | Female |
| ZFX | Xp22.11 | 1.45*10−11 | 2.27*10−8 | Female |
| DDX3X | Xp11.4 | 1.62*10−11 | 2.29*10−8 | Female |
| SMC1A | Xp11.22 | 3.07*10−10 | 3.94*10−7 | Female |
| CA5BP1 | Xp22.2 | 3.59*10−09 | 4.222*10−6 | Female |
| FUNDC1 | Xp11.3 | 1.9*10−08 | 2.064*10−5 | Female |
| RPS4X | Xq13.1 | 5.81*10−08 | 5.86*10−5 | Female |
| ARSD | Xp22.33 | 7.17*10−07 | 6.747*10−4 | Female |
| STS | Xp22.31 | 1.086*10−05 | 9.58*10−3 | Female |
| TRAPPC2 | Xp22.2 | 1.221*10−05 | 0.0101 | Female |
| CA5B | Xp22.2 | 2.114*10−05 | 0.0166 | Female |
| GYG2 | Xp22.33 | 4.977*10−05 | 0.0351 | Female |
| DDIT3 | 12q13.3 V | 3.913*10−05 | 0.0291 | Male |
| TMC5 | 16p12.3 | 1.381*10−06 | 0.0155 | Non-White |
| LRRC37A2 | 17q21.31 | 2.196*10−06 | 0.0155 | White |
| USP32P1 | 17p11.2 | 5.541*10−06 | 0.0261 | White |
q = FDR adjusted p-value
In addition to these sex differences, ethnicity also appears to play a role in PDAC. Though African Americans have the highest rates of PDAC and worst outcomes compared to other ethnic groups (4), the reasons for this are poorly understood and may implicate a variety of social factors discussed later in this article (5). As such, the associations between gender/ethnicity with PDAC may be avoidable to a degree. However, it is also highly likely that these factors may be compounded by an increased genetic susceptibility in certain ethnic groups. Using the previously mentioned TCGA cohort, we found that for the 180 patients where ethnicity was documented, 162 were listed as white, with the remaining 18 reported as either African American or Asian (Figure 1C). Despite the poor representation of minority groups in this sample set, we again identified significant differences in the mRNA expression of three genes between whites and non-whites (Figure 1D and Table 1). These data suggest that there may be an underlying genetic or epigenetic component to the differences in PDAC incidence and outcomes among racial groups. However, while the exact reasons select social and ethnic groups have higher rates of PDAC than others warrant further study, it is clear that certain communities are disproportionately affected by the disease, and may benefit from a greater emphasis on chemoprevention or early screening.
Beyond sex and race, select non-O blood types have also been suggested to be associated with PDAC risk (6), though the clinical utility of these data are unclear. Age, however, is one of the most established risk factors for PDAC (2). Per the most recent SEER data, the median age at the time of diagnosis is 70 years, with most patients being diagnosed between 65 and 74 (2). Fewer than 10% of patients develop PDAC before the age of 50, and these are typically associated with a higher rate of predisposing genetic syndromes (7,8), discussed in detail below.
2.2 -. PREDISPOSING GENETIC SYNDROMES
As with many cancers, genetics appear to play a role in PDAC etiology. For example, patients with a first degree relative with a history of PDAC have a 1.5 to 3-fold increase in disease risk (9,10). To this end, though PDAC is seldom inherited, several predisposing genomic alterations have been described (11). For instance, autosomal recessive ataxiatelangiectasia is associated with an increased risk of several cancers, including PDAC (12). This syndrome is well characterized, involving an inherited mutation to the DNA response and repair gene ATM. This leads to increased genetic instability due to a loss of high-fidelity double-strand break homologous recombination and dysregulation of cell cycle checkpoints (13). Several other inherited defects in DNA repair also increase PDAC risk. These include hereditary breast and ovarian cancer syndrome, which is predominantly caused by deleterious mutations in homologous recombination genes BRCA1 and/or BRCA2 (14). Similarly, truncating mutations to the homologous recombination gene PALB2 also seem to increase risk of PDAC (15). Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer) is caused by a deficit in DNA mismatch repair caused by mutations to several genes including MLH1, MSH2, MSH6, PMS2, and EPCAM. Though classically associated with colon cancer, Lynch syndrome also increases the risk for several other tumor types including PDAC (16).
Beyond defects in DNA repair, several other syndromes increase the lifetime risk of PDAC. Familial adenomatous polyposis (FAP) also modestly predisposes to PDAC due to the presumptive loss of function of the APC tumor suppressor gene. However, like Lynch syndrome, FAP is better known for its strong association with colon cancer (17). Similarly, Peutz–Jeghers syndrome (PJS) is characterized by an autosomal dominant mutation in STK11 tumor suppressor gene, also known as LKB1. PJS is classically associated with benign hamartomatous polyps in the gastrointestinal tract, though these have low rates of malignant transformation (18). However, PJS also significantly increases the risk of developing several other cancers including PDAC (19,20). PDAC is also associated with familial atypical multiple mole melanoma, an autosomal dominant mutation in CDKN2A. As the name implies, this syndrome is strongly linked with multiple dysplastic nevi and high rates of melanoma due to impaired function in the tumor suppressors p16INK4a and p14ARF, both negative regulators of the cell cycle (21,22). Finally, hereditary pancreatitis is caused by autosomal dominant mutations to PRSS1, resulting in hyper-activation of its trypsinogen gene product. While the pancreatitis phenotype has high penetrance, patients suffering from hereditary pancreatitis also have an approximately 50% increase in their lifetime risk of PDAC (23,24).
While the above syndromes increase PDAC risk, very few influence the clinical management of PDAC once diagnosed. Though there is emerging evidence substantiating the use of PARP inhibitors in BRCA-mutated PDAC (25), no other pancreatic cancer associated syndrome is clinically actionable at this time. Hence, patients with a known or family history of these genetic events may benefit from early screening (26,27), and the precise contributions of these genomic alterations to the PDAC phenotype warrants further study.
2.3 -. GENETIC SUSCEPTIBILITY LOCI
In addition to the above syndromes, genome wide association studies (GWAS) have identified several susceptibility alleles for PDAC (28). These common germline variants predominantly single nucleotide polymorphisms (SNPs), conferring a modest risk increase of PDAC to carriers (29–34). For instance, in a 2009 GWAS, researchers genotyped 558,542 SNPs in 1,896 individuals with pancreatic cancer and 1,939 controls. The authors combined their results with an additional 2,457 pancreatic cancer patients and 2,654 controls from 8 additional studies, and found a significant association between a locus on 9q34 and disease incidence. This locus was marked by the SNP rs505922, mapping to the first intron of the ABO blood group gene (29). In 2010, a similar GWAS evaluating in 3,851 pancreatic cancer patients and 3,934 controls identified eight new cancer susceptibility loci. Five of these SNPs mapped to the gene NR5A2 on chromosome 1q32.1, including rs3790844. Two additional SNPs (rs9543325 and rs9564966) mapped to a non-genic region on chromosome 13q22.1. Finally, a single SNP (rs401681) mapped to the CLPTM1L-TERT locus on 5p15.33 (30).
A larger 2014 study examined 7,683 pancreatic cancer patients and 14,397 controls, and identified several new susceptibility loci. These include rs6971499 that maps to the LINC-PINT gene at 7q32.3, rs7190458 that maps to BCAR1/CTRB1/CTRB2 at 16q23.1, rs9581943 that maps to PDX1 at 13q12.2, and rs16986825 that maps to ZNRF3 at 22q12.1 The authors also identified a SNP (rs2736098) that mapped to exon 2 of TERT at 5p15.33, and another (rs1561927) mapping to PVT1 on 8q24.21 (31). In 2015, 3 new TERT variants were identified (rs2736100, rs4583925, and rs2735948) all of which had a significant association with pancreatic cancer risk (32). In 2018, these investigators conducted their largest GWAS to date, including 9,040 pancreatic cancer patients and 12,496 controls. In this study, they identified five new susceptibility loci for pancreatic cancer. These include rs78417682 at the TNS3 locus on 7p12, rs13303010 at the NOC2L locus on 1p36.33, rs2941471 at the HNF4G locus on 8q21.11, rs4795218 at the HNF1B locus on 17q12, and rs1517037 at the GRP locus on 18q21.32 (34). As additional susceptibility loci are beginning to emerge, it is becoming clear that these and other genetic variants may have important roles in guiding decisions regarding both pancreatic cancer screening and treatment (35–39).
2.4 -. ADDITIONAL GENOMIC ALTERATIONS
While pancreatic cancers typically have fewer mutations than most other tumor types (40), exome and copy number variation (CNV) studies have determined that PDAC tumors have a highly complicated mutational landscape, with variable alterations to several different cell processes (41,42). A 2015 study shed further light on the complex genetic landscape of pancreatic cancer by conducting whole-genome sequencing and CNV analysis on 100 PDAC patients. This work identified frequent chromosomal rearrangements leading to copy number alterations in several genes with known roles in pancreatic carcinogenesis including TP53, SMAD4, CDKN2A, ARID1A and ROBO2, as well as novel genes such as KDM6A and PREX2. Further, the authors used variation in chromosomal structure to group these PDAC patients into 4 subtypes: stable, locally rearranged, scattered, and unstable (43).
While these findings are likely to be most useful in managing advanced PDAC, other studies have suggested that copy number alterations in select genes may have relevance in determining pancreatic cancer risk. This includes copy number amplification of SKAP2/SCAP2, which may have an association with the development of PDAC (44). Additionally, a single study identified 93 non-redundant copy number variations associated with familial pancreatic cancer (45). However, others suggest that copy number variation may not have a significant role in the etiology of sporadic pancreatic cancer (46). It is important to note that the majority of copy number variation studies have been performed on advanced cancer specimens, where a certain degree of genetic instability is expected. Therefore, it is unclear which of these findings, if any, will have a role in helping to predict for pancreatic cancer in healthy populations. However, recent findings suggest that mitochondrial DNA copy number variations in peripheral blood leukocytes are associated with PDAC (47). Additionally, others report that leukocyte DNA from PDAC patients harbor as many as 431 copy number variations that may associate with disease incidence (48). Hence, this is an important area of research that warrants continued exploration.
3.1 -. CLINCIALLY ACTIONABLE RISK FACTORS
Though the above factors may only inform screening modalities, there are several lifestyle and social factors that are also associated with pancreatic cancer incidence, as well as other serious health conditions that are often comorbid with pancreatic cancer. While these risk factors may also inform early screening, many are potentially reversible through proper patient counseling and education, particularly in the primary care setting.
3.2 -. SMOKING
Smoking is one of the best-studied and most important avoidable risk factors for PDAC (49,50). Nearly 25% of pancreatic cancer deaths are linked to tobacco use (51,52), and a California-based study suggests that smoking 1 pack per day increases the lifetime risk of developing pancreatic cancer by 5–6 times (53). The exact risk increase seems to vary, as this is based largely on the duration and intensity of exposure (52,54). A META analysis evaluating 82 studies published between 1950 and 2007 determined that there was a 75% increase in the risk of pancreatic cancer in smokers compared to non-smokers, which persists for at least 10 years after smoking cessation (55). Interestingly, this pertains primarily to cigarette and cigar smokers, with no correlation between disease incidence and pipe or smokeless tobacco use (49,50). Cigarette smoking is also a strong predictor of pancreatic cancer mortality (5), further underscoring the importance of proper patient education.
Though this clinical phenomenon is well established, the cellular mechanisms linking smoking and PDAC are poorly understood. Several studies have sought to address this, providing unprecedented insight into the potential means through which tobacco smoke promotes pancreatic carcinogenesis. For instance, cigarette smoke contains a variety of aryl hydrocarbon receptor (AhR) ligands that have been implicated in smoke-induced induction of the cyclooxygenase (COX)/prostaglandin (PG) pathways, as well as activation of fibroblasts in the lung (56,57). Inflammation is a key driver of pancreatic cancer (58), which is closely linked to fibroblast activation and tumor-associated fibrosis (59). Accordingly, AhR agonists 2,3,7,8-tetrachlorodibenzo-p-dioxin or benzo[a]pyrene accelerated chronic pancreatitis in vivo, particularly through the upregulation of the inflammatory cytokine IL22 (60). Similarly, clinical data suggests that circulating IL22 is elevated in chronic pancreatitis, is associated with cigarette smoking, and therapeutic inhibition of IL22 reduces disease progression in vivo. As discussed later in this article, chronic pancreatitis is another key risk factor for pancreatic cancer (61), and IL22 appears to promote pancreatic tumorigenesis through a variety of mechanisms (62). However, the link between smoking and IL22 in PDAC is not clear, and warrants further study.
Additional evidence appears to implicate aberrations to the epigenome in smoking-associated pancreatic carcinogenesis. For instance, histone deacetylase 3 (HDAC3) is a key regulator of several normal and pathologic cell processes, with important roles in PDAC (63,64). Studies in murine models of early disease demonstrated that aerial exposure to cigarette smoke accelerated lesion development in an HDAC3-dependent manner, promoting stellate cell activation, IL6 biosynthesis, and epithelial to mesenchymal transition (65). These events were reversed using the HDAC inhibitor Saha, offering a potential therapeutic option for smoking-associated PDAC (65).
In vitro studies have also helped identify potential mechanisms to link smoking and PDAC, namely via aberrant AKT signaling (66). Incubating human pancreatic duct cells with either cigarette smoke extract or the smoking-associated compound 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone reduced apoptosis and enhanced AKT activation, Bcl-xL expression, and the early formation of autophagic vacuoles (66). Interestingly, prolonged exposure with these compounds further suppressed apoptosis and abolished autophagy (66). Given the established roles for AKT (67) and autophagy (68) in PDAC, this is another potential means through which smoking may promote or accelerate PDAC development.
3.3. DIET & OBESITY
Obesity is an epidemic in the United States, affecting 1 in 3 adults and 17% of adolescents (69). Beyond the well-studied link between obesity and several serious health conditions including hypertension, cardiovascular disease, and diabetes (70), obesity is associated with the incidence of several cancers including PDAC (71). Obesity has been suggested to increase the incidence of PDAC by roughly 50% (72). This has been corroborated through several META analyses, the first of which found only a weak association between PDAC and body mass index (BMI) (73). Subsequent META analyses have substantiated a more significant association between an obese BMI and PDAC incidence (74–76), particularly with respect to waist circumference and centralized fat distribution (74,77).
Additional studies have suggested that obesity is also associated with poor clinical outcomes or treatment related complications. In patients undergoing pancreaticoduodenectomy, those with a BMI ≥30 have longer operative times, more intraoperative blood loss, and an increased risk of developing pancreatic fistulas (78). Though this study did not find an association between obesity and post-operative survival (78), a large single institution study determined that obese patients have improved long-term survival independent of known clinical or pathologic factors (79). Paradoxically, another similar study determined that patients with a BMI ≥35 are more likely to have node-positive pancreatic cancer and decreased survival after surgical resection (80), and potentially poorer responses to cytotoxic chemotherapy (81). Though these conflicting results suggest that the predictive value for BMI in surgically resectable PDAC treatment is unclear, larger studies including inoperable disease suggest that an obese BMI is associated with poor overall survival in PDAC independent of additional factors such as diabetes and hyperglycemia (82).
Similarly, diet may also have a causative role in PDAC (83). In a META analysis of 11 prospective studies, increased consumption of red and processed meats was strongly associated with disease incidence in men (84). Deficiencies in several individual nutrients have also been suggested to correspond to pancreatic cancer risk, though these data are often contradictory (85). For example, studies have suggested that increased dietary intake of vitamin C is inversely associated with PDAC risk (86,87). However, a subsequent study suggested that the inverse association that had been observed in case-control studies may have been affected by recall and selection biases, and that additional prospective studies are required before substantiating vitamin C as a predictor of PDAC risk (88). Similarly, while case-control studies have suggested that low dietary folate (vitamin B9) may correspond to increased risk, others have contradicted these findings or suggest that supplemental folate does not provide any significant risk reduction (89–92).
Others have suggested potential protective roles of lipid soluble vitamins A, D, and E; however, none has produced conclusive evidence linking dietary deficiency with pancreatic cancer risk (86,93–101). Likewise, associations with PDAC and dietary lipids or selenium intake have only proven significant in case-control studies, and while low intake of omega-3 polyunsaturated fatty acids or high intake of cholesterol may have a positive association with overall risk, the predictive value of these factors also remains unclear (86,102–108).
Hence, studies evaluating single nutritional deficiencies have produced unclear and often contradictory results. Therefore, none has proven to be a clinically relevant predictor of pancreatic cancer risk. Recently, there is an increasing emphasis placed on overall dietary patterns rather than one specific nutritional deficiency (85). For example, a META analysis of 16 case-control and cohort studies determined that typical Western diets rich in animal products, starches, or fats are associated with a significant risk increase, whereas there was an inverse association between pancreatic cancer risk and diets high in fruits, vegetables, and fiber (109). Additional studies have suggested that a Western diet high in saturated fat confers an increased disease risk, and healthy/prudent or Mediterranean diets have favorable effects on disease incidence (110). Hence, rather than attempting to risk stratify or counseling patients to correct specific dietary deficiencies, all patients should be encouraged to make more sensible food choices and more closely adhere to a healthy diet, and care providers should be prepared to provide adequately resources to help patients make this transition.
3.4 -. DIABETES MELITUS
Diabetes is closely linked with diet/obesity, and is associated with an increased risk of pancreatic cancer. Further, up to 80% of pancreatic cancer patients will present with new-onset type 2 diabetes or hyperglycemia at the time of diagnosis (111,112). This is particularly noteworthy, particularly in light of recent molecular evidence suggesting that increased glucose concentrations trigger nucleotide imbalance through aberrant O-GlcNAcylation, inducing de novo KRAS mutations in pancreatic epithelial cells (113). However, there also is a growing body of evidence highlighting diabetes as an early paraneoplastic consequence of PDAC, leading to controversy over whether diabetes mellitus is an independent risk factor or early marker for pancreatic cancer (112). While new onset diabetes has been suggested as a potential screening tool for pancreatic cancer (111), additional studies have evaluated the relative risk for diabetic patients of developing pancreatic cancer, particularly with respect to the duration of their diabetes.
For instance, a META analysis evaluating 20 case-control and cohort studies between 1975 and 1994 determined that diabetic patients had a relative risk of 1.8–2.6 (114). When evaluating patients with a diabetes duration of at least 5 years, the relative risk was 2.0 (114). An updated META analysis examining 36 studies between 1966 and 2005 determined that patients with a <4 year history of diabetes had a 50% greater risk of pancreatic cancer compared to those with who had diabetes for ≤5 years (115). Though these results suggest a modest causal relationship between pancreatic cancer and diabetes, subsequent studies have identified a more significant link.
This includes a 2011 META analysis which, despite similar heterogeneity within the individual studies, found that diabetes was associated with increased pancreatic cancer risk independent of alcohol consumption, BMI, geographic location, sex, smoking status, and study design (116). Similar to other studies, this META analysis also found the highest risk of pancreatic cancer to be among patients with ≤1 year history of diabetes (116). A more recent analysis suggests the relative risk for pancreatic cancer is nearly 5-fold for patients with a diagnosis of diabetes within the last year, with a more modest 2-fold risk increase for patients diagnosed 1–4 years ago (117). This has been corroborated by additional studies, which in addition to finding an inverse association between PDAC risk and years with diabetes, found an increased risk for insulin users compared to non-users, which was restricted to insulin use of ≤3 years (118).
Combined, these studies strongly suggest that pancreatic-cancer associated diabetes is largely a paraneoplastic event. Further, it is well established that most pancreatic cancer patients will develop either glucose intolerance or diabetes prior to their cancer diagnosis (119–121). Additionally, longstanding diabetics tend to worsen in the months before their pancreatic cancer diagnosis (121–123), and diabetes tends to improve following surgical removal of pancreatic cancer (120–124). Accordingly, the Chari group has recently developed a novel model to determine risk of pancreatic cancer in individuals with new-onset diabetes, which they refer to as “enriching new-onset diabetes for pancreatic cancer” or END-PAC (125). By retrospectively evaluating data from 1,561 patients with new-onset diabetes, they created a model weighting the three factors most significant to predict for PDAC. These include change in weight, change in blood glucose, and age at onset of diabetes (125). This approach was highly effective in risk-stratifying patients for PDAC in an independent, population-based cohort of 1096 diabetics (125). Therefore, new-onset diabetes may be the most informative risk factor for early pancreatic cancer screening, and pancreatic cancer should be suspected in any patient meeting END-PAC criteria.
However, despite the well documented clinical association between pancreatic cancer and diabetes, as expertly reviewed by Sah and colleagues (121), the molecular mechanisms that underlie these events are only recently becoming clear. For instance, clinical evidence suggests that pancreatic cancer associated diabetes is at least in part due to a decline in β-cell function and increased insulin resistance (126). Accordingly, supernatants from PDAC cell lines promote insulin resistance in cultured hepatocytes and myoblasts in vitro, and intraperitoneal administration of MiaPaCa2-conditioned media induces β-cell dysfunction in vivo (127–130). While the soluble, tumor-derived factors that case these changes are not well characterized, a study examining pancreatic tumor tissue from patients with or without diabetes identified the 14 amino acid peptide from S100A8 as being differentially expressed in those with diabetes. Further, S100A8 impaired glucose catabolism by myoblasts in vitro, suggesting that it may very well have a causative role in pancreatic cancer-associated diabetes (131).
Other studies have also identified differences in proteins expression among PDAC patients with and without diabetes. For example, islets in PDAC patients with diabetes typically overexpress the gap junction protein Connexin-26 (132). Another study examined peripheral blood samples from either healthy controls, or patients diagnosed with pancreatic cancer and/or diabetes. This group found that blood from pancreatic cancer patients with diabetes had increased mRNA and protein expression of several factors, namely vanin-1 (VNN1) and matrix metalloproteinase 9 (MMP9) (133). Adrenomedullin is also upregulated in PDAC, particularly in those who develop diabetes. Additionally, adrenomedullin is carried in pancreatic cancer-associated exosomes, and also appears to cause insulin resistance in β-cells (134). Another study has identified pancreatic polypeptide (PP) as a potentially useful predictor of pancreatic cancer-associated diabetes. Though their sample size is small, the authors identified a blunted PP response in pancreatic cancer-associated diabetes compared to type 2 diabetics without a pancreatic cancer diagnosis. While this PP response may discriminate between type 2 diabetes and pancreatic cancer-associated diabetes, this phenomenon also appears to be exclusive to PDAC patients with tumors located in the head of the gland (135). Hence, by combining these observations with the END-PAC model, it may be possible to risk stratify newly diagnosed diabetics and identify those who would most benefit from early pancreatic cancer screening.
3.5 -. ALCOHOL & PANCREATITIS
Alcohol has long been suspect to confer an increased risk of pancreatic cancer. The carcinogenic properties of alcohol and its metabolites are well-documented (136). Accordingly, alcohol has been implicated in the development of a variety of solid tumors, and genetic variants of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) are associated with increased overall cancer risk (137). Select studies have identified a positive association between heavy alcohol consumption and PDAC, though this risk increase is not as strong as that caused by smoking (138–141). One case-control study suggested that alcohol consumption was not associated with pancreatic cancer risk overall, but that cigarette smoking modified the alcohol-cancer relationship, as heavy drinking is more frequently observed in smokers than non-smokers. In current smokers, light to moderate alcohol intake modestly increased pancreatic cancer risk, and heavy alcohol consumption significantly increased risk (142). Hence, it is difficult to establish alcohol as an independent risk factor for pancreatic cancer.
However, though the direct link between pancreatic cancer and alcohol remains unclear, the link between pancreatitis and pancreatic cancer has been known for decades. While gallstones are the most common cause of acute pancreatitis and the risk of future episodes can be eliminated through cholecystectomy, both alcohol and smoking also appear to be clinically significant risk factors (143). However, alcohol continues to be the most significant risk factor for chronic pancreatitis. While a diagnosis of acute pancreatitis appears to increase the long-term risk of pancreatic cancer (144), chronic pancreatitis is a much more significant risk factor (145), particularly in patients requiring surgery (146). Therefore, chronic pancreatitis is likely more informative when stratifying patients for early screening. In META analysis, chronic pancreatitis confers a nearly eight-fold increased risk of pancreatic cancer 5 years after diagnosis. However, this risk appeared to diminish with long-term follow up. The authors resultantly recommend close follow-up in the first years following a diagnosis of chronic pancreatitis (145), though aggressive surveillance of any patient with a history of pancreatitis and other known risk factors may be warranted.
4.1 -. NEWLY EMERGING RISK FACTORS
Recent evidence has illuminated several added risk factors that may also have utility when identifying high-risk patients. Interestingly, these appear to involve a number of infectious processes as well as a variety of underappreciated psychosocial factors. While the individual predictive values for several of these factors is still unclear, these may warrant clinical consideration particularly when patients also have a known history of more established risk factors.
4.2 -. INFECTION & THE MICROBIOME
Bacterial and viral species are of paramount importance to the etiology of several cancers (147). While the pancreas has long been considered a sterile organ, a recent study identified an abundance of select bacterial species in both human and murine pancreatic cancer specimens (148). The microbial signature of PDAC tumors also appears to have a prognostic value, as a signature rich for Pseudoxanthomonas, Streptomyces, Saccharopolyspora, and Bacillus clausii predicts long-term survival in PDAC patients (149) Further, ablation of intratumoral bacteria enhanced the efficacy of immunotherapy in vivo, which is particularly noteworthy as immunotherapy is beginning to show promise in PDAC (148,150–159). Interestingly, other roles for select bacterial species in PDAC are also emerging. For example, a recent study identified a potential role for intratumoral Gammaproteobacteria in the emergence of chemoresistance both in vitro and in vivo (160). However, a more causal role for select bacterial species has been suggested, particularly in light of clinical observations linking specific infections with pancreatic cancer risk.
For instance, a 1998 study identified a positive association between serum IgG antibodies against Helicobacter pylori (H. pylori), with 65% of PDAC patients testing positive compared to 45% of healthy controls (161). Subsequent studies have expanded on this relationship, including a larger 2001 study that found 82% of PDAC patients to be seropositive for H. pylori compared to 73% in controls. Additionally, compared to seronegative subjects, those seropositive for gene-A-positive H. plylori strains had a statistically significantly increased risk of pancreatic cancer (162).
A 2007 study evaluating gastric and duodenal ulcers suggested a 20% excess risk increase for unoperated gastric ulcer patients (163). This study also states that gastric ulcers are primarily associated with corpus colonization of H. pylori and the formation of N-nitrosamines, whereas duodenal ulcers are associated with antral colonization, hyperacidity and uninhibited secretin release. Hence, they suggest that N-nitrosamines may have a role in PDAC pathobiology (163), though this remains untested. While additional studies have contradicted these findings, a 2011 META analysis including 2,335 patients across 6 studies found a significant risk increase for H. pylori seropositivity (164). Hence, while H. pylori can colonize the pancreas and these infections may have a role in PDAC (165), its independent predictive value for H. pylori seropositivity is not clear, though this may warrant consideration in combination with other risk factors.
Beyond H. pylori, several other gut microbiota species also appear to be involved in PDAC. The microbial species that colonize the pancreas appear to have significant overlap with those of the gastrointestinal tract (149). Accordingly, microbes from the gastrointestinal tract, particularly the small intestine, are able to access the pancreas through both the biliary tract and the bloodstream (148,166,167). Depletion of the gut microbiome significantly reduced tumor burden in murine models of PDAC, however, this was not observed in mice lacking functional T and B cells (168). Further, human-into-mice fecal microbiota transplantation has been shown to differentially modulate the tumor microbiome in vivo, with significant effects on tumor growth and local immune responses (149). Hence, further investigation into the contributions of the gut microbiome into PDAC may lead to new insights into cancer treatment, prevention, and screening (169).
Oral microbiota also appear to have important roles in PDAC etiology, and may have a place in PDAC screening given the ease at which saliva samples can be obtained in the primary care setting. Periodontal disease has also been linked to PDAC risk, particularly those caused by Porphyromonas gingivalis (P. gingivalis). For example, an early study found a positive association between tooth loss and PDAC risk (170), with similar observations in periodontal disease (171). Several subsequent studies have affirmed this relationship (172), including a European study examining the relationship between serum antibodies to 25 different oral bacteria and pancreatic cancer risk. This identified found a near two-fold risk increase in patients with a high P. gingivalis antibody titer (173). Though the mechanisms underlying this association warrant investigation, P. gingivalis antibody titers may be informative in the appropriate clinical context.
There is emerging evidence that the oral microbiome may also have a role in helping to select patients for pancreatic screening. A large nested case–control study examining 361 pancreatic cancer patients and 371 matched controls found that the presence of P. gingivalis and Aggregatibacter actinomycetemcomitans were associated with higher risk of pancreatic cancer, whereas the presence of phylum Fusobacteria and its genus Leptotrichia were associated with a decreased disease risk (174). Additionally, Neisseria elongata (N. elongata) and Streptococcus mitis (S. mitis) were found to be significantly lower in the saliva of PDAC patients than that of healthy controls (175). As the authors conclude that these two bacterial species has strong predictive value for PDAC, the relative absence of N. elongata and S. mitis warrant consideration in risk stratifying patients for early imaging (175). Additional studies have identified several other bacterial species that seem to be differentially present in the saliva of healthy controls and PDAC patients. These include bacteria belonging to the genera Corynebacterium and Aggregatibacter that are frequently reduced in PDAC patients, and Bacteroides and Granulicatella adiacens that are more represented in the saliva of PDAC patients (167). Hence, while our understanding of this topic is evolving, it is clear that the microbiome is an important and long underappreciated aspect of PDAC pathobiology that may have clinical utility in disease detection, prevention, and therapy.
4.3 -. FAMILY, EDUCATION, INCOME, AND ACCESS TO CARE
As discussed, while several studies have implicated race as a risk factor for pancreatic cancer, recent evidence has also identified a variety of additional social factors that may in part explain some of these associations. A study evaluating nearly diagnosed patients found that while only 13% eventually undergo pancreatic resection, this was largely influenced by residential instability and material deprivation (176). Specifically, patients living in rural areas or urban patients with lower incomes are less likely to undergo surgical intervention, however, socio-demographic marginalization was not a predictive factor of outcomes in patients who did undergo surgical resection (176). Hence, there may be significant barriers to care that not only dictate the treatment of pancreatic cancer, but may also limit the availability to intervene with the many actionable risk factors listed above.
Finally, mental health is emerging as a potential factor in PDAC risk. Though the importance of providing appropriate mental health support has been emphasized in patients already diagnosed with PDAC, others have suggested that psychosocial distress may even play a more causative role in PDAC etiology. For instance, a 2013 study of 16,522 cases and 82,107 controls found that the loss of a child was associated with a risk increase for PDAC, particularly within the first 5 years or if losing a child to suicide (177). Hence, extreme psychosocial stress may be implicated in PDAC risk, through this warrants additional investigation, as other factors such as maladaptive coping strategies e.g. alcohol and tobacco use may confound these observations.
5 -. PERSPECTIVE & FUTURE DIRECTION
In recent years, due to advances in both early detection and therapy, survival has improved for nearly all solid malignancies. However, despite significant improvement in our understanding of pancreatic cancer pathobiology, there remain extremely limited therapeutic options for patients with advanced disease. It is now more important than ever to both identify high-risk patients for who would benefit from early imaging, as well as counsel patients to avoid key risk factors in hopes of reducing disease incidence. As the preventable risk factors for PDAC are shared with any number of other conditions, all patients can and should be counseled to make these lifestyle modifications by their primary care physicians. However, screening poses a more significant challenge, particularly as there is a lack of consensus regarding which asymptomatic patients should be evaluated for PDAC. Unlike other cancers, there is no practical test that can be used to inform these decisions. Early detection for PDAC is based almost entirely on imaging studies, which simply cannot be offered to all patients and must be reserved for those showing one or more early warning signs for PDAC.
For example, as discussed a new diagnosis of diabetes is one of the most telling early signs of disease. However, it is important to note that very few cases of diabetes will be due to an underlying etiology of PDAC. Therefore, while it is easy to recommend that all newly diagnosed diabetics be screened for PDAC, this may not be practical or feasible given the expense, particularly in low resource settings. Therefore, it is imperative to better develop a multivariate “risk signature” in order to identify the patients that would most benefit from early imaging. As demonstrated, patients who both smoke and have a family history of PDAC have a staggering 12.8 fold risk increase (Table 2). Similarly, current smokers with a diagnosis of diabetes mellitus had a 9.3 fold risk increase (Table 2). These and the many other studies described in this review clearly show that certain risk factors can synergize with others to increase the likelihood of developing PDAC. However, there is a lack of consensus regarding the combinations that are the most informative. As a result, who is screened for PDAC is left solely to the discretion of care providers, and the patients sent for imaging will vary based on provider experience, expertise, and local resources. Through more careful and rigorous analyses of the interactions between the risk factors described above, it may be possible to reach consensus regarding which patients should be screened, thereby improving outcomes through earlier detection.
Table 2.
Abbreviated list of key clinical risk factors for PDAC
| Risk Factor | OR | 95% CI | Reference |
|---|---|---|---|
| Smoking | |||
| Current Smoker (overall) | 1.77 | 1.38–2.26 | (54) |
| Current Smoker ≥30 cigarettes/day | 1.75 | 1.27–2.42 | - |
| Current Smoker ≥40 cigarettes/day | 1.78 | 1.35–2.34 | - |
| Current Smoker v. Never Smoker | 2.20 | 1.70–2.80 | (49) |
| Former Smoker v. Never Smoker | 1.20 | 1.00–1.30 | - |
| Alcohol | |||
| 1–3 drinks/week | 0.78 | 0.58–1.05 | (142) |
| 4–20 drinks/week | 0.86 | 0.63–1.17 | - |
| ≥21 drinks/week | 1.35 | 0.81–2.27 | - |
| Medical Histor | |||
| Diabetes mellitus (≤2 years after diagnosis) | 2.90 | 2.10–3.90 | (118) |
| Diabetes mellitus (3–5 years after diagnosis) | 1.90 | 1.30–2.60 | - |
| Diabetes mellitus (6–10 years after diagnosis) | 1.60 | 1.20–2.30 | - |
| Diabetes mellitus (11–15 years after diagnosis) | 1.30 | 0.90–2.00 | - |
| Diabetes mellitus (>15 years after diagnosis) | 1.40 | 1.00–2.00 | - |
| Chronic pancreatitis | 2.23 | 1.43–3.49 | (178) |
| All pancreatitis | 3.42 | 1.98–5.91 | - |
| Gastric ulcer disease | 1.20 | 1.00–1.40 | (163) |
| H. pylori seropositive | 1.38 | 1.08–.75 | (164) |
| P. gingivalis antibody ≥200ng/ml | 2.38 | 1.16–4.90 | (173) |
| Family history of PDAC | 1.76 | 1.19–2.61 | (179) |
| Multivariate Risk Factors | |||
| Non-smoker, ≥21 drinks/week | 2.01 | 1.50–8.18 | (142) |
| Current Smoker, ≥21 drinks/week | 4.04 | 1.58–10.37 | - |
| Current smoker, diabetes mellitus | 9.30 | 2.00–44.1 | (180) |
| Current smoker, family history of PDAC | 12.8 | 1.60–108.9 | - |
OR = Odds Ration, CI = Confidence Interval
6 -. SUMMARY
Given the significant clinical challenge of treating late stage PDAC, it is imperative that high-risk patients be identified as early as possible in order to provide early and aggressive screening, as well as allow for appropriate intervention to support the appropriate lifestyle modifications. While many of the factors described in this review appear to have independent predictive value for PDAC risk, pancreatic cancer etiology is highly complex and involves a variety of cell types and processes. Through understanding the interactions between these many risk factors, it may be possible to better identify those with the highest risk, and improve outcomes in what is generally considered an incurable disease.
Grant Support:
This work was supported by Veterans Affairs Merit Award BX002703 and Career Scientist Award BX004855 to A. Rana, and by NIH F30CA236031 to D.R Principe.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: The authors have no conflicts to disclose.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Li CH, Haider S, Shiah YJ, Thai K, Boutros PC. Sex Differences in Cancer Driver Genes and Biomarkers. Cancer Res 2018;78(19):5527–37. [DOI] [PubMed] [Google Scholar]
- 4.Khawja SN, Mohammed S, Silberfein EJ, Musher BL, Fisher WE, Van Buren G 2nd. Pancreatic cancer disparities in African Americans. Pancreas 2015;44(4):522–7. [DOI] [PubMed] [Google Scholar]
- 5.Arnold LD, Patel AV, Yan Y, Jacobs EJ, Thun MJ, Calle EE, et al. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev 2009;18(9):2397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst 2009;101(6):424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raimondi S, Maisonneuve P, Lohr JM, Lowenfels AB. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev 2007;16(9):1894–7. [DOI] [PubMed] [Google Scholar]
- 8.Pandol S, Gukovskaya A, Edderkaoui M, Dawson D, Eibl G, Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol 2012;27 Suppl 2:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenk M, Schwartz AG, O’Neal E, Kinnard M, Greenson JK, Fryzek JP, et al. Familial risk of pancreatic cancer. J Natl Cancer Inst 2001;93(8):640–4. [DOI] [PubMed] [Google Scholar]
- 10.McWilliams RR, Rabe KG, Olswold C, De Andrade M, Petersen GM. Risk of malignancy in first-degree relatives of patients with pancreatic carcinoma. Cancer 2005;104(2):388–94. [DOI] [PubMed] [Google Scholar]
- 11.Carrera S, Sancho A, Azkona E, Azkuna J, Lopez-Vivanco G. Hereditary pancreatic cancer: related syndromes and clinical perspective. Hered Cancer Clin Pract 2017;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2012;2(1):41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 2008;9(10):759–69. [DOI] [PubMed] [Google Scholar]
- 14.Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J Clin Oncol 2015;33(28):3124–9. [DOI] [PubMed] [Google Scholar]
- 15.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 2009;324(5924):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302(16):1790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993;34(10):1394–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010;59(7):975–86. [DOI] [PubMed] [Google Scholar]
- 19.Korsse SE, Harinck F, van Lier MG, Biermann K, Offerhaus GJ, Krak N, et al. Pancreatic cancer risk in Peutz-Jeghers syndrome patients: a large cohort study and implications for surveillance. J Med Genet 2013;50(1):59–64. [DOI] [PubMed] [Google Scholar]
- 20.Latchford A, Greenhalf W, Vitone LJ, Neoptolemos JP, Lancaster GA, Phillips RK. Peutz-Jeghers syndrome and screening for pancreatic cancer. Br J Surg 2006;93(12):1446–55. [DOI] [PubMed] [Google Scholar]
- 21.Vasen HF, Gruis NA, Frants RR, van Der Velden PA, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer 2000;87(6):809–11. [PubMed] [Google Scholar]
- 22.Parker JF, Florell SR, Alexander A, DiSario JA, Shami PJ, Leachman SA. Pancreatic carcinoma surveillance in patients with familial melanoma. Arch Dermatol 2003;139(8):1019–25. [DOI] [PubMed] [Google Scholar]
- 23.Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK Jr., Perrault J, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst 1997;89(6):442–6. [DOI] [PubMed] [Google Scholar]
- 24.Weiss FU. Pancreatic cancer risk in hereditary pancreatitis. Front Physiol 2014;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog 2012;51(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med 1999;131(4):247–55. [DOI] [PubMed] [Google Scholar]
- 28.Amundadottir LT. Pancreatic Cancer Genetics. Int J Biol Sci 2016;12(3):314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet 2009;41(9):986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen GM, Amundadottir L, Fuchs CS, Kraft P, Stolzenberg-Solomon RZ, Jacobs KB, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet 2010;42(3):224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolpin BM, Rizzato C, Kraft P, Kooperberg C, Petersen GM, Wang Z, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet 2014;46(9):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campa D, Rizzato C, Stolzenberg-Solomon R, Pacetti P, Vodicka P, Cleary SP, et al. TERT gene harbors multiple variants associated with pancreatic cancer susceptibility. Int J Cancer 2015;137(9):2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Childs EJ, Mocci E, Campa D, Bracci PM, Gallinger S, Goggins M, et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat Genet 2015;47(8):911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein AP, Wolpin BM, Risch HA, Stolzenberg-Solomon RZ, Mocci E, Zhang M, et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat Commun 2018;9(1):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimitrakopoulos C, Vrugt B, Flury R, Schraml P, Knippschild U, Wild P, et al. Identification and Validation of a Biomarker Signature in Patients With Resectable Pancreatic Cancer via Genome-Wide Screening for Functional Genetic Variants. JAMA Surg 2019;154(6):e190484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzato C, Campa D, Giese N, Werner J, Rachakonda PS, Kumar R, et al. Pancreatic cancer susceptibility loci and their role in survival. PLoS One 2011;6(11):e27921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueno M, Ohkawa S, Morimoto M, Ishii H, Matsuyama M, Kuruma S, et al. Genome-wide association study-identified SNPs (rs3790844, rs3790843) in the NR5A2 gene and risk of pancreatic cancer in Japanese. Sci Rep 2015;5:17018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willis JA, Olson SH, Orlow I, Mukherjee S, McWilliams RR, Kurtz RC, et al. A replication study and genome-wide scan of single-nucleotide polymorphisms associated with pancreatic cancer risk and overall survival. Clin Cancer Res 2012;18(14):3942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Wei P, Chang P, Li Y, Yan D, Liu C, et al. Genetic polymorphisms associated with pancreatic cancer survival: a genome-wide association study. Int J Cancer 2017;141(4):678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science 2007;318(5853):1108–13. [DOI] [PubMed] [Google Scholar]
- 41.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321(5897):1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491(7424):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518(7540):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harada T, Chelala C, Bhakta V, Chaplin T, Caulee K, Baril P, et al. Genome-wide DNA copy number analysis in pancreatic cancer using high-density single nucleotide polymorphism arrays. Oncogene 2008;27(13):1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Sukhni W, Joe S, Lionel AC, Zwingerman N, Zogopoulos G, Marshall CR, et al. Identification of germline genomic copy number variation in familial pancreatic cancer. Hum Genet 2012;131(9):1481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willis JA, Mukherjee S, Orlow I, Viale A, Offit K, Kurtz RC, et al. Genome-wide analysis of the role of copy-number variation in pancreatic cancer risk. Front Genet 2014;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gentiluomo M, Katzke VA, Kaaks R, Tjonneland A, Severi G, Perduca V, et al. Mitochondrial DNA Copy-Number Variation and Pancreatic Cancer Risk in the Prospective EPIC Cohort. Cancer Epidemiol Biomarkers Prev 2020;29(3):681–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fanale D, Iovanna JL, Calvo EL, Berthezene P, Belleau P, Dagorn JC, et al. Analysis of germline gene copy number variants of patients with sporadic pancreatic adenocarcinoma reveals specific variations. Oncology 2013;85(5):306–11. [DOI] [PubMed] [Google Scholar]
- 49.Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol 2012;23(7):1880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertuccio P, La Vecchia C, Silverman DT, Petersen GM, Bracci PM, Negri E, et al. Cigar and pipe smoking, smokeless tobacco use and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2011;22(6):1420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis 2010;28(4–5):645–56. [DOI] [PubMed] [Google Scholar]
- 52.Pandol SJ, Apte MV, Wilson JS, Gukovskaya AS, Edderkaoui M. The burning question: why is smoking a risk factor for pancreatic cancer? Pancreatology 2012;12(4):344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mack TM, Yu MC, Hanisch R, Henderson BE. Pancreas cancer and smoking, beverage consumption, and past medical history. J Natl Cancer Inst 1986;76(1):49–60. [PubMed] [Google Scholar]
- 54.Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol 2009;170(4):403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008;393(4):535–45. [DOI] [PubMed] [Google Scholar]
- 56.Stedman RL. The chemical composition of tobacco and tobacco smoke. Chem Rev 1968;68(2):153–207. [DOI] [PubMed] [Google Scholar]
- 57.Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 2005;289(3):L391–9. [DOI] [PubMed] [Google Scholar]
- 58.Hausmann S, Kong B, Michalski C, Erkan M, Friess H. The role of inflammation in pancreatic cancer. Adv Exp Med Biol 2014;816:129–51. [DOI] [PubMed] [Google Scholar]
- 59.Phillips P Pancreatic stellate cells and fibrosis In: Grippo PJ, Munshi HG, editors. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India)2012. [Google Scholar]
- 60.Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, et al. Aryl Hydrocarbon Receptor Ligands in Cigarette Smoke Induce Production of Interleukin-22 to Promote Pancreatic Fibrosis in Models of Chronic Pancreatitis. Gastroenterology 2016;151(6):1206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhar P, Kalghatgi S, Saraf V. Pancreatic cancer in chronic pancreatitis. Indian J Surg Oncol 2015;6(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curd LM, Favors SE, Gregg RK. Pro-tumour activity of interleukin-22 in HPAFII human pancreatic cancer cells. Clin Exp Immunol 2012;168(2):192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001;1(3):194–202. [DOI] [PubMed] [Google Scholar]
- 64.Lee HS, Park SB, Kim SA, Kwon SK, Cha H, Lee DY, et al. A novel HDAC inhibitor, CG200745, inhibits pancreatic cancer cell growth and overcomes gemcitabine resistance. Sci Rep 2017;7:41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edderkaoui M, Xu S, Chheda C, Morvaridi S, Hu RW, Grippo PJ, et al. HDAC3 mediates smoking-induced pancreatic cancer. Oncotarget 2016;7(7):7747–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park CH, Lee IS, Grippo P, Pandol SJ, Gukovskaya AS, Edderkaoui M. Akt kinase mediates the prosurvival effect of smoking compounds in pancreatic ductal cells. Pancreas 2013;42(4):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baer R, Cintas C, Therville N, Guillermet-Guibert J. Implication of PI3K/Akt pathway in pancreatic cancer: When PI3K isoforms matter? Adv Biol Regul 2015;59:19–35. [DOI] [PubMed] [Google Scholar]
- 68.Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med 2019;25(4):628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panuganti KK, Gossman WG. Obesity. StatPearls; Treasure Island (FL)2019. [Google Scholar]
- 70.Hruby A, Manson JE, Qi L, Malik VS, Rimm EB, Sun Q, et al. Determinants and Consequences of Obesity. Am J Public Health 2016;106(9):1656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013;2013:291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ 2012;344:e2476. [DOI] [PubMed] [Google Scholar]
- 73.Berrington de Gonzalez A, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer 2003;89(3):519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170(9):791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer 2007;120(9):1993–8. [DOI] [PubMed] [Google Scholar]
- 76.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371(9612):569–78. [DOI] [PubMed] [Google Scholar]
- 77.Berrington de Gonzalez A, Spencer EA, Bueno-de-Mesquita HB, Roddam A, Stolzenberg-Solomon R, Halkjaer J, et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 2006;15(5):879–85. [DOI] [PubMed] [Google Scholar]
- 78.Shamali A, Shelat V, Jaber B, Wardak A, Ahmed M, Fontana M, et al. Impact of obesity on short and long term results following a pancreatico-duodenectomy. Int J Surg 2017;42:191–96. [DOI] [PubMed] [Google Scholar]
- 79.Tsai S, Choti MA, Assumpcao L, Cameron JL, Gleisner AL, Herman JM, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg 2010;14(7):1143–50. [DOI] [PubMed] [Google Scholar]
- 80.Fleming JB, Gonzalez RJ, Petzel MQ, Lin E, Morris JS, Gomez H, et al. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg 2009;144(3):216–21. [DOI] [PubMed] [Google Scholar]
- 81.Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov 2016;6(8):852–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McWilliams RR, Matsumoto ME, Burch PA, Kim GP, Halfdanarson TR, de Andrade M, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer 2010;116(21):5054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371(11):1039–49. [DOI] [PubMed] [Google Scholar]
- 84.Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer 2012;106(3):603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salem AA, Mackenzie GG. Pancreatic cancer: A critical review of dietary risk. Nutr Res 2018;52:1–13. [DOI] [PubMed] [Google Scholar]
- 86.Chen J, Jiang W, Shao L, Zhong D, Wu Y, Cai J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. Int J Food Sci Nutr 2016;67(7):744–53. [DOI] [PubMed] [Google Scholar]
- 87.Fan H, Kou J, Han D, Li P, Zhang D, Wu Q, et al. Association between vitamin C intake and the risk of pancreatic cancer: a meta-analysis of observational studies. Sci Rep 2015;5:13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hua YF, Wang GQ, Jiang W, Huang J, Chen GC, Lu CD. Vitamin C Intake and Pancreatic Cancer Risk: A Meta-Analysis of Published Case-Control and Cohort Studies. PLoS One 2016;11(2):e0148816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chuang SC, Stolzenberg-Solomon R, Ueland PM, Vollset SE, Midttun O, Olsen A, et al. A U-shaped relationship between plasma folate and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Eur J Cancer 2011;47(12):1808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang JY, Butler LM, Wang R, Jin A, Koh WP, Yuan JM. Dietary Intake of One-Carbon Metabolism-Related Nutrients and Pancreatic Cancer Risk: The Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev 2016;25(2):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin HL, An QZ, Wang QZ, Liu CX. Folate intake and pancreatic cancer risk: an overall and dose-response meta-analysis. Public Health 2013;127(7):607–13. [DOI] [PubMed] [Google Scholar]
- 92.Bao Y, Michaud DS, Spiegelman D, Albanes D, Anderson KE, Bernstein L, et al. Folate intake and risk of pancreatic cancer: pooled analysis of prospective cohort studies. J Natl Cancer Inst 2011;103(24):1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeurnink SM, Ros MM, Leenders M, van Duijnhoven FJ, Siersema PD, Jansen EH, et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: a nested case-control study: plasma micronutrients and pancreatic cancer risk. Int J Cancer 2015;136(6):E665–76. [DOI] [PubMed] [Google Scholar]
- 94.Peng L, Liu X, Lu Q, Tang T, Yang Z. Vitamin E intake and pancreatic cancer risk: a meta-analysis of observational studies. Med Sci Monit 2015;21:1249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang T, Chen H, Qin S, Wang M, Wang X, Zhang X, et al. The association between dietary vitamin A intake and pancreatic cancer risk: a meta-analysis of 11 studies. Biosci Rep 2016;36(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang X, Gao Y, Zhi X, Ta N, Jiang H, Zheng J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci Rep 2016;6:38936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu SL, Zhao YP, Dai MH, You L, Wen Z, Xu JW. Vitamin D status and the risk of pancreatic cancer: a meta-analysis. Chin Med J (Engl) 2013;126(17):3356–9. [PubMed] [Google Scholar]
- 98.Waterhouse M, Risch HA, Bosetti C, Anderson KE, Petersen GM, Bamlet WR, et al. Vitamin D and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Case-Control Consortium. Ann Oncol 2015;26(8):1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, Qi D, Patel AV, Helzlsouer KJ, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010;172(1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolpin BM, Ng K, Bao Y, Kraft P, Stampfer MJ, Michaud DS, et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2012;21(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bao Y, Ng K, Wolpin BM, Michaud DS, Giovannucci E, Fuchs CS. Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer 2010;102(9):1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Banim PJ, Luben R, McTaggart A, Welch A, Wareham N, Khaw KT, et al. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut 2013;62(10):1489–96. [DOI] [PubMed] [Google Scholar]
- 103.Wang L, Wang J, Liu X, Liu Q, Zhang G, Liang L. Association between selenium intake and the risk of pancreatic cancer: a meta-analysis of observational studies. Biosci Rep 2016;36(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shen QW, Yao QY. Total fat consumption and pancreatic cancer risk: a meta-analysis of epidemiologic studies. Eur J Cancer Prev 2015;24(4):278–85. [DOI] [PubMed] [Google Scholar]
- 105.Yao X, Tian Z. Saturated, Monounsaturated and Polyunsaturated Fatty Acids Intake and Risk of Pancreatic Cancer: Evidence from Observational Studies. PLoS One 2015;10(6):e0130870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin B, Xun P, He K. Fish or long-chain (n-3) PUFA intake is not associated with pancreatic cancer risk in a meta-analysis and systematic review. J Nutr 2012;142(6):1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hidaka A, Shimazu T, Sawada N, Yamaji T, Iwasaki M, Sasazuki S, et al. Fish, n-3 PUFA consumption, and pancreatic cancer risk in Japanese: a large, population-based, prospective cohort study. Am J Clin Nutr 2015;102(6):1490–7. [DOI] [PubMed] [Google Scholar]
- 108.Wang J, Wang WJ, Zhai L, Zhang DF. Association of cholesterol with risk of pancreatic cancer: a meta-analysis. World J Gastroenterol 2015;21(12):3711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng J, Guinter MA, Merchant AT, Wirth MD, Zhang J, Stolzenberg-Solomon RZ, et al. Dietary patterns and risk of pancreatic cancer: a systematic review. Nutr Rev 2017;75(11):883–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu PY, Shu L, Shen SS, Chen XJ, Zhang XY. Dietary Patterns and Pancreatic Cancer Risk: A Meta-Analysis. Nutrients 2017;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Souza A, Irfan K, Masud F, Saif MW. Diabetes Type 2 and Pancreatic Cancer: A History Unfolding. JOP 2016;17(2):144–48. [PMC free article] [PubMed] [Google Scholar]
- 113.Hu CM, Tien SC, Hsieh PK, Jeng YM, Chang MC, Chang YT, et al. High Glucose Triggers Nucleotide Imbalance through O-GlcNAcylation of Key Enzymes and Induces KRAS Mutation in Pancreatic Cells. Cell Metab 2019;29(6):1334–49 e10. [DOI] [PubMed] [Google Scholar]
- 114.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995;273(20):1605–9. [PubMed] [Google Scholar]
- 115.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92(11):2076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 2011;47(13):1928–37. [DOI] [PubMed] [Google Scholar]
- 117.Muniraj T, Chari ST. Diabetes and pancreatic cancer. Minerva Gastroenterol Dietol 2012;58(4):331–45. [PMC free article] [PubMed] [Google Scholar]
- 118.Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control 2011;22(2):189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008;134(4):981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 2013;10(7):423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Girelli CM, Reguzzoni G, Limido E, Savastano A, Rocca F. Pancreatic carcinoma: differences between patients with or without diabetes mellitus. Recenti Prog Med 1995;86(4):143–6. [PubMed] [Google Scholar]
- 123.Pannala R, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol 2009;104(9):2318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg 1993;80(8):1047–50. [DOI] [PubMed] [Google Scholar]
- 125.Sharma A, Kandlakunta H, Nagpal SJS, Feng Z, Hoos W, Petersen GM, et al. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018;155(3):730–39 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chari ST, Zapiach M, Yadav D, Rizza RA. Beta-cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology 2005;5(2–3):229–33. [DOI] [PubMed] [Google Scholar]
- 127.Basso D, Brigato L, Veronesi A, Panozzo MP, Amadori A, Plebani M. The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res 1995;15(6B):2585–8. [PubMed] [Google Scholar]
- 128.Basso D, Valerio A, Brigato L, Panozzo MP, Miola M, Lucca T, et al. An unidentified pancreatic cancer cell product alters some intracellular pathways of glucose metabolism in isolated rat hepatocytes. Pancreas 1997;15(2):132–8. [DOI] [PubMed] [Google Scholar]
- 129.Valerio A, Basso D, Brigato L, Ceolotto G, Baldo G, Tiengo A, et al. Glucose metabolic alterations in isolated and perfused rat hepatocytes induced by pancreatic cancer conditioned medium: a low molecular weight factor possibly involved. Biochem Biophys Res Commun 1999;257(2):622–8. [DOI] [PubMed] [Google Scholar]
- 130.Basso D, Millino C, Greco E, Romualdi C, Fogar P, Valerio A, et al. Altered glucose metabolism and proteolysis in pancreatic cancer cell conditioned myoblasts: searching for a gene expression pattern with a microarray analysis of 5000 skeletal muscle genes. Gut 2004;53(8):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Basso D, Greco E, Fogar P, Pucci P, Flagiello A, Baldo G, et al. Pancreatic cancer-derived S-100A8 N-terminal peptide: a diabetes cause? Clin Chim Acta 2006;372(1–2):120–8. [DOI] [PubMed] [Google Scholar]
- 132.Pfeffer F, Koczan D, Adam U, Benz S, von Dobschuetz E, Prall F, et al. Expression of connexin26 in islets of Langerhans is associated with impaired glucose tolerance in patients with pancreatic adenocarcinoma. Pancreas 2004;29(4):284–90. [DOI] [PubMed] [Google Scholar]
- 133.Huang H, Dong X, Kang MX, Xu B, Chen Y, Zhang B, et al. Novel blood biomarkers of pancreatic cancer-associated diabetes mellitus identified by peripheral blood-based gene expression profiles. Am J Gastroenterol 2010;105(7):1661–9. [DOI] [PubMed] [Google Scholar]
- 134.Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee GG, et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in beta cells and mice. Gastroenterology 2012;143(6):1510–17 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hart PA, Baichoo E, Bi Y, Hinton A, Kudva YC, Chari ST. Pancreatic polypeptide response to a mixed meal is blunted in pancreatic head cancer associated with diabetes mellitus. Pancreatology 2015;15(2):162–6. [DOI] [PubMed] [Google Scholar]
- 136.Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, et al. A review of human carcinogens--Part F: chemical agents and related occupations. Lancet Oncol 2009;10(12):1143–4. [DOI] [PubMed] [Google Scholar]
- 137.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health 2007;30(1):38–41, 44–7. [PMC free article] [PubMed] [Google Scholar]
- 138.Bouchardy C, Clavel F, La Vecchia C, Raymond L, Boyle P. Alcohol, beer and cancer of the pancreas. Int J Cancer 1990;45(5):842–6. [DOI] [PubMed] [Google Scholar]
- 139.Gupta S, Wang F, Holly EA, Bracci PM. Risk of pancreatic cancer by alcohol dose, duration, and pattern of consumption, including binge drinking: a population-based study. Cancer Causes Control 2010;21(7):1047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tramacere I, Scotti L, Jenab M, Bagnardi V, Bellocco R, Rota M, et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer 2010;126(6):1474–86. [DOI] [PubMed] [Google Scholar]
- 141.Wang YT, Gou YW, Jin WW, Xiao M, Fang HY. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer 2016;16:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rahman F, Cotterchio M, Cleary SP, Gallinger S. Association between alcohol consumption and pancreatic cancer risk: a case-control study. PLoS One 2015;10(4):e0124489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144(6):1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology 2018;154(6):1729–36. [DOI] [PubMed] [Google Scholar]
- 145.Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol 2017;112(9):1366–72. [DOI] [PubMed] [Google Scholar]
- 146.Zheng Z, Chen Y, Tan C, Ke N, Du B, Liu X. Risk of pancreatic cancer in patients undergoing surgery for chronic pancreatitis. BMC Surg 2019;19(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018;33(4):570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov 2018;8(4):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019;178(4):795–806 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110(50):20212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vonderheide RH, Bajor DL, Winograd R, Evans RA, Bayne LJ, Beatty GL. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother 2013;62(5):949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 2015;38(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res 2015;3(4):399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW, et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett 2018;417:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018;67(2):320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Burrack AL, Spartz EJ, Raynor JF, Wang I, Olson M, Stromnes IM. Combination PD-1 and PD-L1 Blockade Promotes Durable Neoantigen-Specific T Cell-Mediated Immunity in Pancreatic Ductal Adenocarcinoma. Cell Rep 2019;28(8):2140–55 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Principe DR, Park A, Dorman MJ, Kumar S, Viswakarma N, Rubin J, et al. TGFbeta Blockade Augments PD-1 Inhibition to Promote T-Cell-Mediated Regression of Pancreatic Cancer. Mol Cancer Ther 2019;18(3):613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ma Y, Li J, Wang H, Chiu Y, Kingsley CV, Fry D, et al. Combination of PD1 Inhibitor and OX40 Agonist Induces Tumor Rejection and Immune Memory in Mouse Models of Pancreatic Cancer. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Principe DR, Narbutis M, Kumar S, Park A, Viswakarma N, Dorman MJ, et al. Long-Term Gemcitabine Treatment Reshapes the Pancreatic Tumor Microenvironment and Sensitizes Murine Carcinoma to Combination Immunotherapy. Cancer Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357(6356):1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Raderer M, Wrba F, Kornek G, Maca T, Koller DY, Weinlaender G, et al. Association between Helicobacter pylori infection and pancreatic cancer. Oncology 1998;55(1):16–9. [DOI] [PubMed] [Google Scholar]
- 162.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, Perez-Perez G, Taylor PR, Virtamo J, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst 2001;93(12):937–41. [DOI] [PubMed] [Google Scholar]
- 163.Luo J, Nordenvall C, Nyren O, Adami HO, Permert J, Ye W. The risk of pancreatic cancer in patients with gastric or duodenal ulcer disease. Int J Cancer 2007;120(2):368–72. [DOI] [PubMed] [Google Scholar]
- 164.Trikudanathan G, Philip A, Dasanu CA, Baker WL. Association between Helicobacter pylori infection and pancreatic cancer. A cumulative meta-analysis. JOP 2011;12(1):26–31. [PubMed] [Google Scholar]
- 165.Nilsson HO, Stenram U, Ihse I, Wadstrom T. Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. World J Gastroenterol 2006;12(19):3038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fritz S, Hackert T, Hartwig W, Rossmanith F, Strobel O, Schneider L, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg 2010;200(1):111–7. [DOI] [PubMed] [Google Scholar]
- 167.Wei MY, Shi S, Liang C, Meng QC, Hua J, Zhang YY, et al. The microbiota and microbiome in pancreatic cancer: more influential than expected. Mol Cancer 2019;18(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018;155(1):33–37 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Archibugi L, Signoretti M, Capurso G. The Microbiome and Pancreatic Cancer: An Evidence-based Association? J Clin Gastroenterol 2018;52 Suppl 1, Proceedings from the 9th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition & Human and Microbiota Health Meeting, held in Rome, Italy from September 10 to 12, 2017:S82–S85. [DOI] [PubMed] [Google Scholar]
- 170.Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, Virtamo J, Taylor PR, Albanes D. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am J Clin Nutr 2003;78(1):176–81. [DOI] [PubMed] [Google Scholar]
- 171.Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst 2007;99(2):171–5. [DOI] [PubMed] [Google Scholar]
- 172.Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis 2013;34(10):2193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjonneland A, et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013;62(12):1764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 2018;67(1):120–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012;61(4):582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kagedan DJ, Abraham L, Goyert N, Li Q, Paszat LF, Kiss A, et al. Beyond the dollar: Influence of sociodemographic marginalization on surgical resection, adjuvant therapy, and survival in patients with pancreatic cancer. Cancer 2016;122(20):3175–82. [DOI] [PubMed] [Google Scholar]
- 177.Huang J, Valdimarsdottir U, Fall K, Ye W, Fang F. Pancreatic cancer risk after loss of a child: a register-based study in Sweden during 1991–2009. Am J Epidemiol 2013;178(4):582–9. [DOI] [PubMed] [Google Scholar]
- 178.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 2010;24(3):349–58. [DOI] [PubMed] [Google Scholar]
- 179.Jacobs EJ, Chanock SJ, Fuchs CS, Lacroix A, McWilliams RR, Steplowski E, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer 2010;127(6):1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol 2007;102(12):2696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]


