Abstract
[18F]-labeled aryl fluorides are widely used as radiotracers for positron emission tomography (PET) imaging. Aryl halides (ArX) are particularly attractive precursors to these radiotracers, as they are readily available, inexpensive, and stable. However, to date, the direct preparation of [18F]-aryl fluorides from aryl halides remains limited to SNAr reactions between highly activated ArX substrates and K18F. This report describes an aryl halide radiofluorination reaction in which the C(sp2)–18F bond is formed via a copper-mediated pathway. Copper N-heterocyclic carbene complexes serve as mediators for this transformation, using aryl halide substrates with directing groups at the ortho position. This reaction is applied to the radiofluorination of electronically diverse aryl halide derivatives, including the bioactive molecules vismodegib and PH-089.
Graphical Abstract
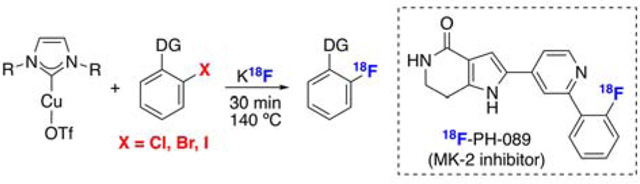
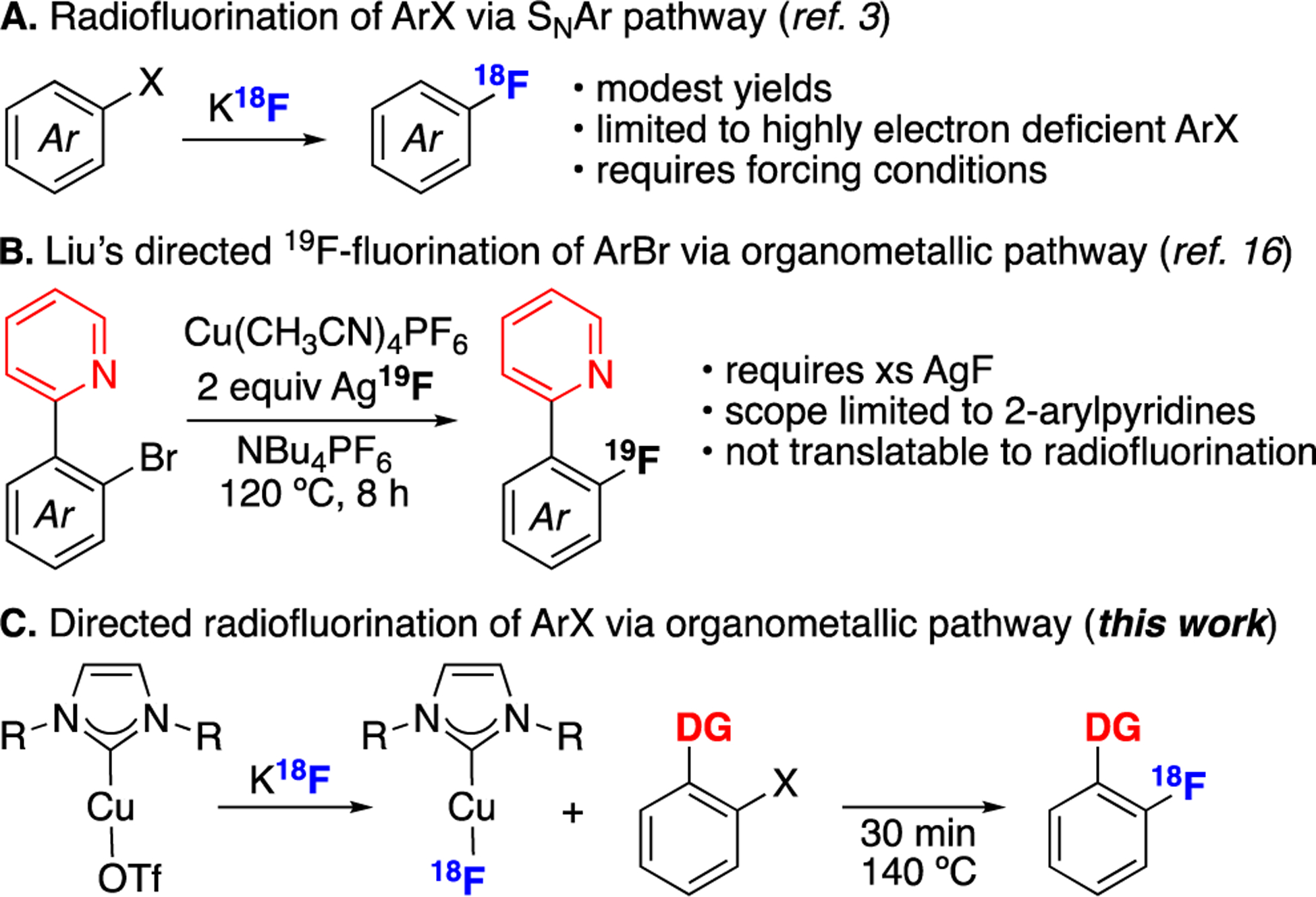
Late-stage methods for constructing 18F–(hetero)aryl bonds are highly valued for the synthesis of positron emission tomography (PET) radiotracers.1,2 Historically, 18F-labeled aromatic substrates have most commonly been prepared via SNAr reactions between electron deficient aryl halide precursors and K18F (Scheme 1A).3,4 Aryl halides are particularly attractive radiofluorination precursors because they are abundant, stable, and synthetically accessible. However, the substrate scope of SNAr (radio)fluorination reactions remains narrow, as resonance electron withdrawing substituents on the aromatic ring are required to stabilize Meisenheimer-type intermediates.1,5 Furthermore, even with such highly activated substrates, SNAr pathways often require long reaction times and forcing conditions, which renders them ill-suited for many late-stage radiofluorination applications.6,7 As such, a key objective for the field is to develop complementary methods for the radiofluorination of (hetero)aryl–halides and pseudohalides.8,9
Scheme 1.
Strategies for direct fluorination of aryl halides.
Our approach to this challenge has focused on developing Cu-mediated methods for C(sp2)–18F coupling reactions.1,2b Recent studies have shown that Cu salts such as Cu(OTf)2 and Cu(CH3CN)4PF6 mediate the nucleophilic radiofluorination of aryl stannane,10 aryl boron,11 diaryliodonium,12 and aryl C–H substrates13 with K18F. In these systems the key C(sp2)–18F bond is formed via reductive elimination from an organometallic Cu(aryl)(18F-fluoride) intermediate.11f,14 This organometallic pathway is mechanistically distinct from an SNAr reaction. As such, it enables the radiofluorination of a wide scope of electronically diverse aryl groups.
Despite this progress, analogous Cu mediators have proven ineffective at engaging aryl halide substrates in radiofluorination reactions. Two reports have documented the Cu-promoted nucleophilic 19F-fluorination of aryl halides (e.g., the work of Liu in Scheme 1B). However, both require superstoichiometric AgF as the fluoride source,15,16 and neither has proven translatable to radiolabeling with 18F– (vide infra). This report describes the use of N-heterocyclic carbene (NHC) Cu complexes as mediators for ligand-directed aryl halide radiofluorination (Scheme 1C). The discovery of this transformation in the context of 19F-fluorination and its subsequent translation to radiofluorination are described in detail.
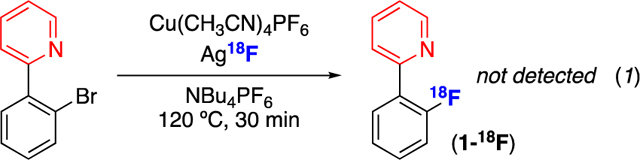
Our initial studies attempted to translate Liu’s 19F-fluorination of 2-(2-bromophenyl)pyridine (Scheme 1B) to a radiolabeling protocol. However, as shown in eq. 1, under the standard conditions (with CuI(CH3CN)4PF6, Ag18F, and NBu4PF6 in CH3CN at 120 °C), no trace of product 1-18F was detected by radio-TLC or radio-HPLC after 0.5 h. Furthermore, no improvement was observed upon variation of the 18F source, solvent, additives, or temperature (Table S7). We note that, in contrast to the 19F-fluorination, the radiofluorination reaction requires the use of Ag18F as the limiting reagent at sub-micromolar concentrations. We hypothesize that this renders CuI(CH3CN)4PF6-mediated radiofluorination prohibitively slow relative to the decay of the radionuclide (t1/2 ~110 min).
Literature reports suggest that aryl-bromide bond activation (via oxidative addition at CuI) is likely the slow step in this transformation.16,17 We reasoned that the introduction of a strongly electron donating NHC ligand at the CuI center would accelerate this key step.18,19 Furthermore, since (NHC)CuI(F) complexes can be generated directly from KF,20 this approach should eliminate the requirement for excess AgF. Finally, sterically bulky NHC ligands are known to stabilize CuI–fluoride complexes to dimerization or disproportionation,19,21 which are likely competing decomposition pathways for the Cu mediator.22
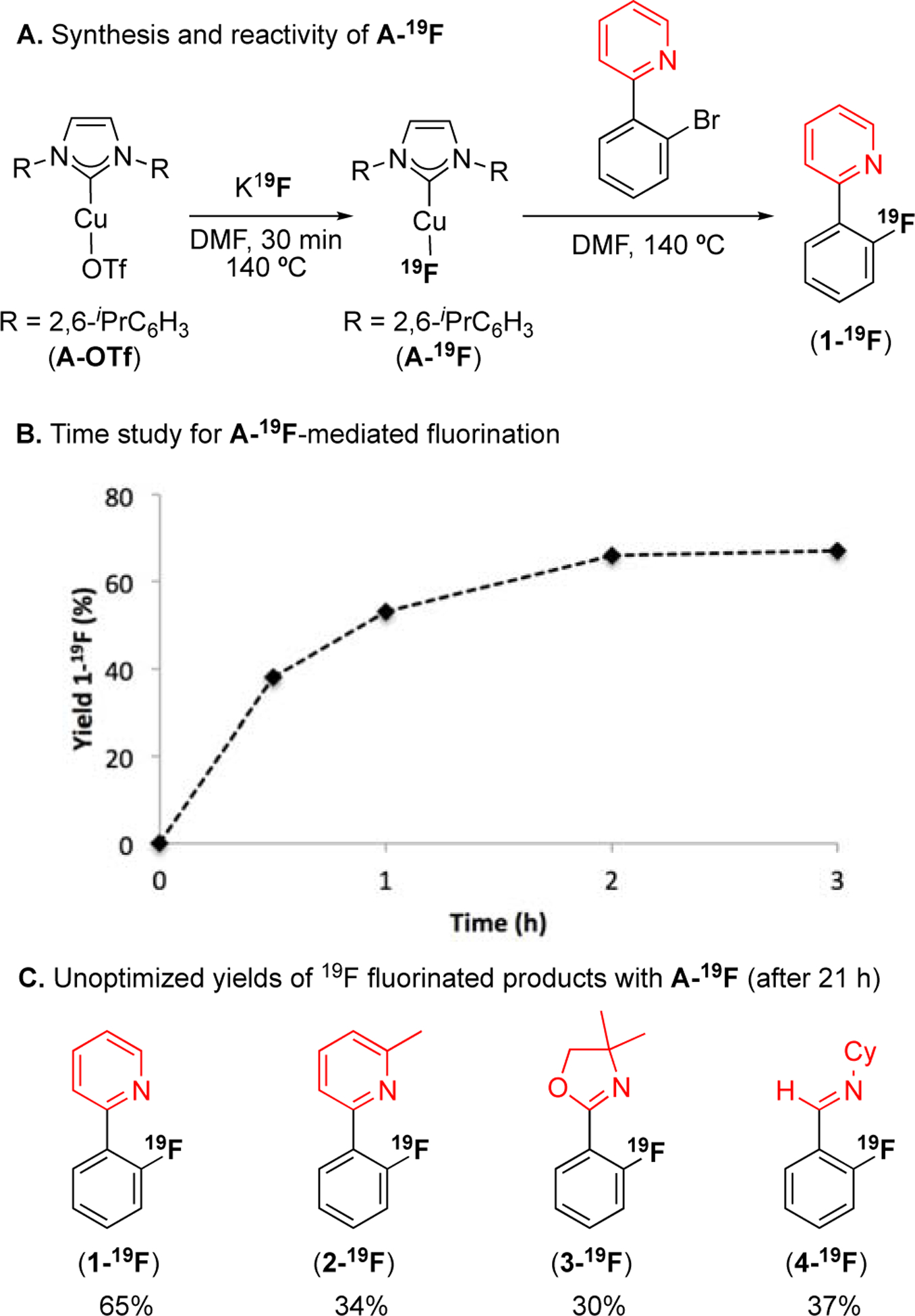
To test this hypothesis, we initially examined the reactivity of a series of (NHC)CuI(19F) complexes with 2-(2-bromophenyl)pyridine (Scheme 2A). As summarized in Table S3, the yield of fluorinated product 1-19F varied from 3–65% as a function of the structure of the NHC ligand,19,23 with 1,3-bis-(2,6-diisopropylphenyl)imidazol-2-ylidine (IPr) affording the optimal result. Notably, (IPr)CuI(19F) (A-19F) is available in nearly quantitative yield from the reaction of (IPr)CuI(OTf) (A-OTf) with K19F (Scheme 2A),20 thus precluding the requirement for Ag salts in this transformation. Importantly, control studies revealed that other group 11 metal salts including CuI(CH3CN)4PF6/KF, CuF2,24 or AgF afforded ≤3% of 1-19F under otherwise identical conditions (Table S4). Furthermore, no reaction was observed between the aryl bromide substrate and K19F under these conditions in the absence of copper.
Scheme 2.
NHC-Cu-mediated 19F-fluorination of aryl bromides
(A) Conditions: A-OTf (0.006 mmol, 1 equiv), KF (1.5 equiv), DMF (0.01 M), 140 °C for 30 min, then aryl bromide (0.006 mmol), 140 °C for 21 h. (B) Conditions: A-19F (0.01 mmol, 1 equiv), aryl bromide (1 equiv), DMF (0.015 M), 140 °C for 21 h. (C) Conditions: A-19F (0.006 mmol, 1 equiv), aryl bromide (1 equiv), DMF (0.01 M), 140 °C for 21 h. Yields determined by 19F NMR spectroscopic analysis of crude reaction mixtures.
A time study with A-19F shows that the fluorination reaction is complete within 2 h at 140 °C and affords 40% yield after just 30 min (Scheme 2B). This suggests the feasibility of achieving radiofluorination with this system. Finally, a preliminary survey of substrates revealed that A-19F-mediated fluorination has a significantly enhanced scope versus that of Liu’s CuI(CH3CN)4PF6/Ag19F system (Scheme 1B). For instance, the sterically hindered pyridine substrate 2-(2-(bromo)phenyl)-6-methylpyridine was unreactive under Liu’s conditions, but affords 2-19F in 34% yield with A-19F as the Cu mediator (Scheme 2C). Similarly, the oxazoline and imine substrates were unreactive under Liu’s conditions, but afford 30% and 37% yield of 3-19F and 4-19F, respectively, using A-19F.25
We next focused on translating these preliminary results to radiofluorination. The reaction of (IPr)CuI(OTf) (A-OTf) with 2-(2-bromophenyl)pyridine and K18F for 30 min at 140 °C in DMF afforded 1-18F in 10% radiochemical conversion (RCC) as determined by radio-TLC and radio-HPLC (Table 1, entry 1).26,27 The reaction was optimized by exploring additives that have been shown to enhance yields in other Cu-mediated C(sp2)–18F coupling reactions (e.g., phase transfer reagents, nitrogen heterocycles, Table 1, entries 2–5).1,11a,13,28 Of the surveyed additives, 1 equiv of 4-dimethylaminopyridine (DMAP) relative to the aryl bromide precursor proved optimal, affording 1-18F in 65% RCC.
Table 1.
Cu-mediated radiofluorination of aryl halides.
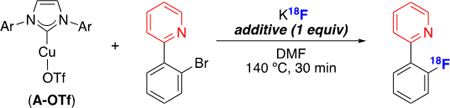 | ||
|---|---|---|
| entry | Additive | RCC (%) |
| 1 | none | 10 |
| 2 | Kryptofix | 26 |
| 3 | pyridine | 23 |
| 4 | DBU | 30 |
| 5 | DMAP | 65 |
Conditions: aryl bromide (0.005 mmol, 1 equiv), A-OTf(1 equiv), additive (1 equiv), K18F, DMF (0.015 M), N2 atmosphere, 140 °C, 30 min.27 RCC determined by radio-TLC (n ≥ 2).
With these optimized conditions in hand, we next explored the scope of the A-OTf-mediated radiofluorination of aryl halides. As shown in Figure 1, the chloro-, bromo-, and iodo-2-phenylpyridine precursors all reacted to afford 1-18F in RCCs ranging from 10–65%. In contrast, no 19F/18F exchange was detected with 1-19F under these conditions. It is currently unclear why 1-I affords lower yield than 1-Br; however, this observation is in line with Liu’s results for the Cu-catalyzed [19F]-fluorination of halophenylpyridines.16 Substitution on either the pyridine or aryl ring was tolerated to afford products such as 2-18F, 6-18F, and 7-18F. Other nitrogen-donors, including oxazoline, pyrazole, cyclohexyl imine, and mesityl imine, served as effective directing groups, affording 3-18F, 8-18F, 4-18F, and 9-18F, respectively. The scope of cyclohexyl imine derivatives was most thoroughly explored, as this directing group is straightforward to install and remove starting from readily available benzyaldehyde derivatives. Various substitution patterns on the (hetero)arene ring were well tolerated, affording compounds 10–16-18F in RCCs ranging from 16–74%. An intramolecular competition reaction between an ortho-chloride and bromide resulted in selective radiofluorination of the bromide to form 13-18F. This selectivity is consistent with that expected for a metal-mediated activation of a C(sp2)–X bond.29
Figure 1.
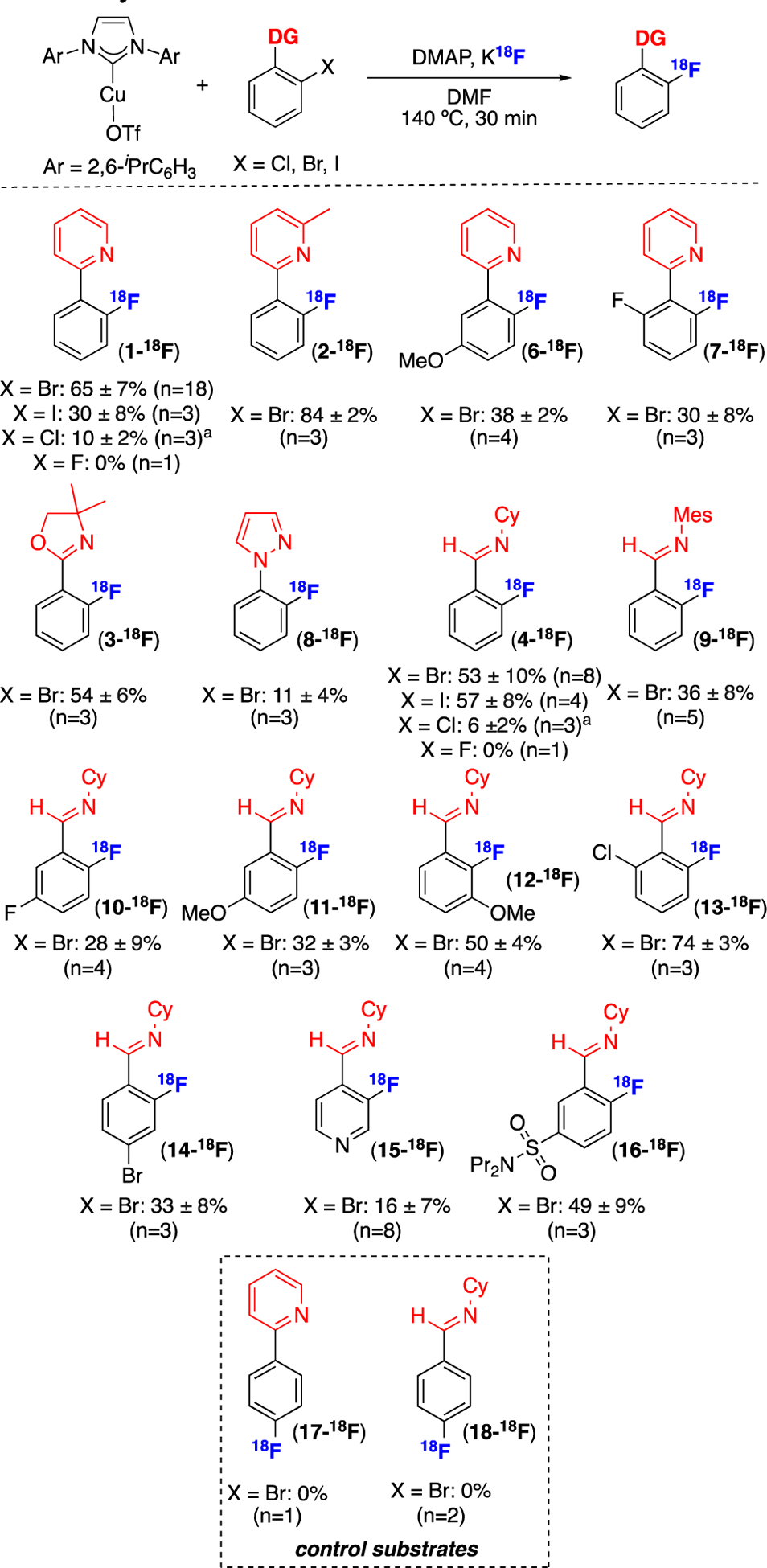
Substrate scope of Cu-mediated radiofluorination of aryl halides.
Conditions: aryl halide (0.005 mmol, 1 equiv), A-OTf (1 equiv), DMAP (1 equiv), K18F, DMF (0.015 M), N2 atmosphere, 140 °C for 30 min.27 RCC determined by radio-TLC (n ≥ 3). aReaction conducted at 160 °C.
Importantly, a variety of control reactions were conducted in these systems. First, the 4-substituted aryl bromides in the pyridine and cyclohexyl imine series were subjected to the reaction conditions. These are electronically similar, but do not benefit from the directing effect. As shown in Figure 1, these substrates did not afford detectable 17-18F or 18-18F under the optimized conditions.30 In addition, all of these reactions were conducted in the absence of Cu to test for background SNAr reactivity. As shown in Table S12, ≤1% of compounds 1–16-18F were detected under these conditions. Finally, substituting simple CuI or CuII salts for (IPr)CuI(OTf) afforded yields of ≤5% for representative substrates (Table S11), underscoring the central role of the NHC ligand in these transformations.
18F-analogues of several bioactive molecules could also be accessed using this approach. In a first example, the bromide analogue of vismodegib (19-Br), a basal cell carcinoma treatment,31 underwent radiofluorination to afford 19-18F (Scheme 3). In a second example, 18F-labeled PH-089 (20-18F in Scheme 3), an MK-2 inhibitor,32 was synthesized in 5% RCC from the chloride precursor.
Scheme 3.
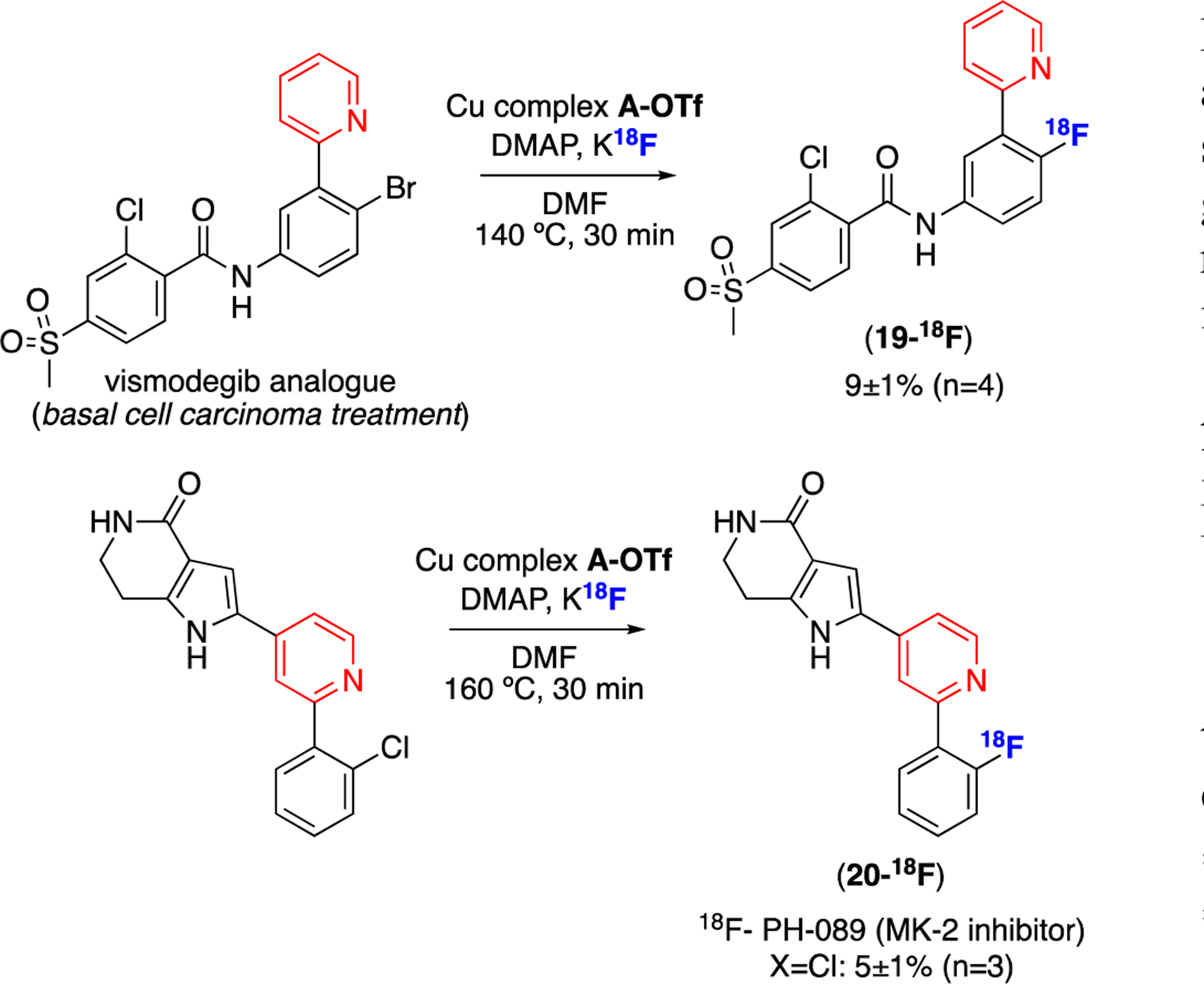
Radiofluorination of bioactive molecules.
A final set of studies focused on automating the radiosynthesis of 1-18F using a TRACERLab FXFN synthesis module. Initial automated studies using 241.1 mCi (8.93 × 109 Bq) of K18F gave 57 ± 8 % radiochemical yield (RCY; n = 2), demonstrating the compatibility of the method with automation. Further investigations coupled automated synthesis with semi-preparative HPLC purification to afford 1-18F in 14.3 ± 3.2% RCY (decay-corrected; 119.9 mCi ± 28; n = 2) with good molar activity (1614 ± 353 Ci/mmol; n = 2) and radiochemical purity. While unoptimized, this result demonstrates the potential of this method for PET applications.
In conclusion, we have developed a Cu-mediated protocol for the 19F- and 18F-fluorination of diverse aryl halide substrates. Strategic design of the Cu mediator was necessary to achieve the reaction rates/yields required for efficient radiofluorination, and an NHC-ligated Cu complex ultimately proved optimal in this system. A wide scope of nitrogen-containing directing groups and substituted aryl halide derivatives underwent 18F-fluorination, and the reaction proved effective for the synthesis of biologically relevant molecules such as 19-18F and 20-18F. More broadly, this work demonstrates that NHC-type ligands enable new C(sp2)–F coupling reactions at Cu. As such, this work opens up opportunities for designing next-generation Cu mediators for the radiofluorination of currently inert substrates (e.g., aryl halides that lack a directing group).
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the NIH [award number F32GM136022 (LSS) and award number R01EB021155 (MSS and PJHS)]. We acknowledge Dr. Devin M. Ferguson, Isaac M. Blythe, Dr. Yiyang See, Dr. Sean M. Preshlock, Dr. Jay S. Wright and Pronay Roy for helpful discussions. We also acknowledge Dr. Rachel S. Plumb for assistance with data processing.
Footnotes
Supporting Information. A listing of the contents of each file supplied as Supporting Information should be included.
REFERENCES
- 1.Preshlock S; Tredwell M; Gouverneur V 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev 2016, 116, 719–766. [DOI] [PubMed] [Google Scholar]
- 2.(a) Miller PW; Long NJ; Vilar R; Gee AD Synthesis of 11C, 18F, 15O, and 13N Radiolabels for Positron Emission Tomography. Angew. Chem. Int. Ed 2008, 47, 8998–9033. [DOI] [PubMed] [Google Scholar]; (b) Brooks AF; Topczewski JJ; Ichiishi N; Sanford MS; Scott PJH Late-Stage [18F]Fluorination: New Solutions to Old Problems. Chem. Sci 2014, 5, 4545–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Coenen HH; Ermert J 18F-labelling Innovations and Their Potential for Clinical Application. Clin. Transl. Imaging 2018, 6, 169–193. [Google Scholar]; (d) Chen W; Huang Z; Tay NES; Giglio B; Wang M; Wang H; Wu Z; Nicewicz DA; Li Z Direct Arene C-H Fluorination with 18F- via Organic Photoredox Catalysis. Science 2019, 364, 1170–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Xu P; Zhao D; Berger F; Hamad A; Rickmeier J; Petzold R; Kondratiuk M; Bohdan KJ; Ritter T Site-Selective Late-Stage Aromatic [18F]Fluorination via Aryl Sulfonium Salts. Angew. Chem. Int. Ed 2020, 59, 1956–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. For examples of the SNAr radiofluorination of aryl halides, see:; (a) Lemaire C; Guillaume M; Christiaens L; Palmer AJ; Cantineau R A New Route for the Synthesis of [18F]Fluoroaromatic Substituted Amino Acids: No Carrier Added L-p-[18F]-Fluorophenylalanine. Appl. Radiat. Isot 1987, 38, 1033–1038. [DOI] [PubMed] [Google Scholar]; (b) Pascali C; Luthra SK; Pike VW; Price GW; Ahier RG; Hume SP; Mers R; Manjil L; Cremer JE The Radiosynthesis of [18F]PK 14105 as an Alternative Radioligand for Peripheral Type Benzodiazepine Binding Sites. Appl. Radiat. Isot 1990, 41, 477–482. [DOI] [PubMed] [Google Scholar]; (c) Hashizume K; Tamakawa H; Hashimoto N; Miyake Y Single-step Synthesis of [18F]Haloperidol from the Chloro-precursor and its Application in PET Imaging of a Cat’s Brain. Appl. Radiat. Isot 1997, 48, 1179–1185. [DOI] [PubMed] [Google Scholar]; (d) Shen B; Loffler D; Zeller K-P; Ubele M; Reischl G; Machulla H-J Decarbonylation of Multi-substituted [18F]- Benzaldehydes for Modelling Synthesis of 18F-Labelled Aromatic Amino Acids. Appl. Radiat. Isot 2007, 65, 1227–1231. [DOI] [PubMed] [Google Scholar]
- 4. For examples of the SNAr radiofluorination of halopyridines, see:; (a) Beer H-F; Haeberli M; Ametamey S; Schubiger PA Comparison of Two Synthetic Methods to Obtain [18F] N-(2-aminoethyl)-5-fluoropyridine-2-carboxamide, a Potential Mao-B Imaging Tracer for PET. J. Labelled Compd. Radiopharm 1995, 36, 933–945. [Google Scholar]; (b) Karramkam M; Hinnen F; Vaufrey F; Dollé, F. 2-, 3- and 4- [18F]Fluoropyridine by No-Carrier-Added Nucleophilic Aromatic Substitution with K[18F]F-K222 − A Comparative Study. J. Labelled Compd. Radiopharm 2003, 46, 979–992. [Google Scholar]; (c) Malik N; Voelter W; Machulla H-J; Solbach C Radiofluorination of 2-Fluoropyridines by Isotopic Exchange with [18F]Fluoride. J. Radioanal. Nucl. Chem 2011, 287, 287–292. [Google Scholar]
- 5.(a) Terrier F Modern Nucleophilic Aromatic Substitution; Wiley-VCH, Weinheim, 2013, p. 236–268. [Google Scholar]; (b) Cole EL; Stewart MN; Littich R; Hoareau R; Scott PJH Radiosyntheses using Fluorine-18: the Art and Science of Late Stage Fluorination. Curr. Top. Med. Chem 2014, 14, 875–900. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jacobson O; Kiesewetter DO; Chen X Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem 2015, 26, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. In many cases, radiofluorination of simple building blocks followed by subsequent synthetic steps is required. See:; (a) Krüll J; Heinrich MR [18F]Fluorine-Labeled Pharmaceuticals: Direct Aromatic Fluorination Compared to Multi-Step Strategies. Asian J. Org. Chem 2019, 8, 576–590. [Google Scholar]; (b) van der Born D; Pees A; Poot AJ; Orru RVA; Windhorst AD; Vugts DJ Fluorine-18 Labelled Building Blocks for PET Tracer Synthesis. Chem. Soc. Rev 2017, 46, 4709–4773. [DOI] [PubMed] [Google Scholar]
- 7.(a) Jacobson O; Chen X PET Designated Flouride-18 Production and Chemistry. Curr. Top. Med. Chem 2010, 10, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sun H; DiMagno S G Competitive Demethylation and Substitution in N,N,N-Trimethylanilinium Fluorides. J. Fluor. Chem 2007, 128, 806–812. [Google Scholar]; (c) Ermert J; Hocke C; Ludwig T; Gail R; Coenen HH Comparison of Pathways to the Versatile Synthon of No-Carrier-Added 1-Bromo-4-[18F]fluorobenzene. J. Labelled Compd. Radiopharm 2004, 47, 429–441. [Google Scholar]
- 8.(a) Grushin VV The Organometallic Fluorine Chemistry of Palladium and Rhodium: Studies toward Aromatic Fluorination. Acc. Chem. Res 2010, 43, 160–171. [DOI] [PubMed] [Google Scholar]; (b) Campbell MG; Ritter T Modern Carbon−Fluorine Bond Forming Reactions for Aryl Fluoride Synthesis Chem. Rev 2015, 115, 612–633. [DOI] [PubMed] [Google Scholar]
- 9. For an example of indirect radiofluorination of aryl bromides via isolated NiII-aryl intermediates, see:; Lee E; Hooker JM; Ritter T Nickel-Mediated Oxidative Fluorination for PET with Aqueous [18F] Fluoride. J. Am. Chem. Soc 2012, 134, 17456–17458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Makaravage KJ; Brooks AF; Mossine AV; Sanford MS; Scott PJH Copper-Mediated Radiofluorination of Arylstannanes with [18F]KF. Org. Lett 2016, 18, 5440–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gamache RF; Waldmann C; Murphy JM Copper-Mediated Oxidative Fluorination of Aryl Stannanes with Fluoride. Org. Lett 2016, 18, 4522–4525. [DOI] [PubMed] [Google Scholar]; (c) Zarrad F; Zlatopolskiy BD; Krapf P; Zischler J; Neumaier B A Practical Method for the Preparation of 18F-Labeled Aromatic Amino Acids from Nucleophilic [18F]Fluoride and Stannyl Precursors for Electrophilic Radiohalogenation. Molecules. 2017, 22, 2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Mossine AV; Brooks AF; Makaravage KJ; Miller JM; Ichiishi N; Sanford MS; Scott PJH Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett 2015, 17, 5780–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tredwell M; Preshlock SM; Taylor NJ; Gruber S; Huiban M; Passchier J; Mercier J; Génicot C; Gouverneur V A General Copper-Mediated Nucleophilic 18F Fluorination of Arenes Angew. Chem. Int. Ed 2014, 53, 7751–7755. [DOI] [PubMed] [Google Scholar]; (c) Zlatopolskiy BD Zischler J; Krapf P; Zarrad F; Urusova EA; Kordys E; Endepols H; Neumaier B Copper-mediated Aromatic Radiofluorination Revisited: Efficient Production of PET Tracers on a Preparative Scale. Chem. Eur. J 2015, 21, 5972–5979. [DOI] [PubMed] [Google Scholar]; (d) Preshlock S; Calderwood S; Verhoog S; Tredwell M; Huiban M; Hienzsch A; Gruber S; Wilson TC; Taylor NJ; Cailly T; Schedler M; Collier TL; Passchier J; Smits R; Mollitor J; Hoepping A; Mueller M; Genicot C; Mercier J; Gouverneur V Enhanced Copper-Mediated 18F-Fluorination of Aryl Boronic Esters provides Eight Radiotracers for PET Applications. Chem. Commun 2016, 52, 8361–8364. [DOI] [PubMed] [Google Scholar]; (e) Mossine AV; Brooks AF; Bernard-Gauthier V; Bailey JJ; Ichiishi N; Schirrmacher R; Sanford MS; Scott PJH Automated Synthesis of PET Radiotracers by Copper-mediated 18F-Fluorination of Organoborons: Importance of the Order of Addition and Competing Protodeborylation. J. Label. Compd. Radiopharm 2018, 61, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Taylor NJ; Emer E; Preshlock S; Schedler M; Tredwell M; Verhoog S; Mercier J; Genicot C; Gouverneur V Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc 2017, 139, 8267–8276. [DOI] [PubMed] [Google Scholar]; (g) Zischler J; Kolks N; Modemann D; Neumaier B; Zlatopolskiy BD Alcohol-enhanced Cu-mediated Radiofluorination. Chem. Eur. J 2017, 23, 3251–3256. [DOI] [PubMed] [Google Scholar]; (h) Wilson TC; Cailly T; Gouverneur V Boron Reagents for Divergent Radiochemistry. Chem Soc Rev 2018, 47, 6990–7005. [DOI] [PubMed] [Google Scholar]
- 12.Ichiishi N; Brooks AF; Topczewski JJ; Rodnick ME; Sanford MS; Scott PJH Copper- Catalyzed [18F]Fluorination of (Mesityl)(aryl)iodonium Salts. Org. Lett 2014, 16, 3224–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ; Makaravage KJ; Brooks AF; Sanford MS; Scott PJH Cu-Mediated Aminoquinoline-Directed Radiofluorination of Aromatic C–H Bonds with KF. Angew. Chem. Int. Ed 2019, 58, 3119–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Casitas A; Canta M; Sola M; Costas M; Ribas X Nucleophilic Aryl Fluorination and Aryl Halide Exchange Mediated by a CuI/CuIII Catalytic Cycle. J. Am. Chem. Soc 2011, 133, 19386–19392. [DOI] [PubMed] [Google Scholar]; (b) Ichiishi N; Canty AJ; Yates BF; Sanford MS Mechanistic Investigations of Cu-Catalyzed Fluorination of Diaryliodonium Salts: Elaborating the CuI/CuIII Manifold for Copper Catalysis. Organometallics 2014, 33, 5525–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ye Y; Schimler SD; Hanley PS; Sanford MS Cu(OTf)2-Mediated Fluorination of Aryltrifluoroborates with Potassium Fluoride. J. Am. Chem. Soc 2013, 135, 16292–16295. [DOI] [PubMed] [Google Scholar]; (d) Yao B,; Wang Z-L; Zhang H; Wang D-X; Zhao L; Wang M-X Cu(ClO4)2-Mediated Arene C–H Bond Halogenations of Azacalixaromatics Using Alkali Metal Halides as Halogen Sources. J. Org. Chem 2012, 77, 3336–3340. [DOI] [PubMed] [Google Scholar]
- 15.Fier PS; Hartwig JF Copper-Mediated Fluorination of Aryl Iodides. J. Am. Chem. Soc 2012, 134, 10795–10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu X; Zhang H; Chen P; Liu G Copper-Catalyzed Fluorination of 2-Pyridyl Aryl Bromides. Chem. Sci 2014, 5, 275–280. [Google Scholar]
- 17.Le C; Chen TQ; Liang T; Zhang P; MacMillan DWC A Radical Approach to the Copper Oxidative Addition Problem: Trifluoromethylation of Bromoarenes. Science, 2018, 360, 1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Hopkinson MN; Richter C; Schedler M; Glorius F An Overview of N-Heterocyclic Carbenes. Nature 2014, 510, 485–496. [DOI] [PubMed] [Google Scholar]; (b) Danopoulos AA; Simler T; Braunstein P N-Heterocyclic Carbene Complexes of Copper, Nickel, and Cobalt. Chem. Rev 2019, 119, 3730–3961. [DOI] [PubMed] [Google Scholar]; (c) Díez-Gonzalez S; Marion N; Nolan SṔ N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev 2009, 109, 3612–3676. [DOI] [PubMed] [Google Scholar]; (d) Jurkauskas V; Sadighi JP; Buchwald SL Conjugate Reduction of α,β-Unsaturated Carbonyl Compounds Catalyzed by a Copper Carbene Complex. Org. Lett 2003, 5, 2417–2420. [DOI] [PubMed] [Google Scholar]
- 19.The reaction of the (NHC)Cu(19F) complex (sICy)Cu(19F) with p-NO2PhI at 100 °C was reported to afford p-NO2Ph19F in 16% yield. However, this result was not followed up in further detail. Laitar, D. S. Synthetic and Catalytic Studies of Group 11 N-Heterocyclic Carbene Complexes. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, 2006. [Google Scholar]
- 20.Dang H; Mailig M; Lalic G Mild Copper-Catalyzed Fluorination of Alkyl Triflates with Potassium Fluoride. Angew. Chem. Int. Ed 2014, 53, 6473–6476. [DOI] [PubMed] [Google Scholar]
- 21.(a) Herron JR; Ball ZT Synthesis and Reactivity of Functionalized Arylcopper Compounds by Transmetalation of Organosilanes. J. Am. Chem. Soc 2008, 130, 16486–16487. [DOI] [PubMed] [Google Scholar]; (b) Fujihara T; Xu T; Semba K; Terao J; Tsuji Y Copper-Catalyzed Hydrocarboxylation of Alkynes Using Carbon Dioxide and Hydrosilanes. Angew. Chem. Int. Ed 2011, 50, 523–527. [DOI] [PubMed] [Google Scholar]
- 22.Waddington TC The Lattice Energies and Thermodynamic Properties of the Hypothetical Compounds AuF and CuF. Trans. Faraday Soc 1959, 55, 1531–1535. [Google Scholar]
- 23. With many of these complexes, a side product resulting from the C–C coupling of the NHC ligand and 2-(2-bromophenyl)pyridine was detected by high resolution mass spectrometry (see SI, section 3.6 for compete details). Notably, both NHC-aryl and NHC-halide coupling products have been observed in other reactions of (NHC)Cu complexes.; (a) Williams TJ; Bray JTW; Lake BRM; Willans CE; Rajabi NA; Ariafard A; Manzini C; Bellina F; Whitwood AC; Fairlamb IJS Mechanistic Elucidation of the Arylation of Non-Spectator N‐Heterocyclic Carbenes at Copper Using a Combined Experimental and Computational Approach. Organometallics 2015, 34, 3497–3507. [Google Scholar]; (b) Grandbois A; Mayer M-E; Bedard M; Collins SK; Michel T Synthesis of C1-Symmetric BINOLs Employing N-Heterocyclic Carbene– Copper Complexes Organometallics 2010, 29, 3683–3685. [DOI] [PubMed] [Google Scholar]; (c) Cheng J; Wang L; Wang P; Deng L High-Oxidation-State 3d Metal (Ti−Cu) Complexes with N‐Heterocyclic Carbene Ligation. Chem. Rev 2018, 118, 9930–9987. [DOI] [PubMed] [Google Scholar]
- 24.The following patent reported the stoichiometric fluorination of aryl halides with CuF2 at 180 °C. However, minimal details are provided. Grushin, V. V. (DuPont & Co., USA) Process for Preparing Fluoroarenes from Haloarenes. U.S. Patent 7,202,388, May 10, 2007. [Google Scholar]
- 25. In contrast, aryl halides without directing groups (e.g., 4-bromobiphenyl) afforded no detectable fluorinated products under these conditions (see SI, section 3.3 for complete details).
- 26. Control reactions (in the absence of Cu or using various other Cu precursors) result in ≤1% RCC under analogous conditions (Tables S11 and S12).
- 27. Procedure for radiofluorination involves initial combination of aryl halide, A-OTf, DMAP and DMF in a N2 glove box. The reaction mixture is removed from the glovebox and a DMF solution of K18F is added to the reaction vial through a septa cap prior to heating. See SI, section 5.3 for full details.
- 28.Mossine AV; Brooks AF; Ichiishi N; Makaravage KJ; Sanford MS; Scott PJH Development of Customized [18F]Fluoride Elution Techniques for the Enhancement of Copper-Mediated Late-Stage Radiofluorination. Sci. Rep 2017, 7, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomashenko OA; Grushin VV Aromatic Trifluoromethylation with Metal Complexes. Chem. Rev 2011, 111, 4475–4521. [DOI] [PubMed] [Google Scholar]
- 30. Similarly, other aryl halides lacking directing groups were not effective substrates (SI, Table S13)
- 31.Dlugosz A; Agrawal S; Kirkpatrick P Vismodegib. Nat. Rev. Drug Discovery 2012, 11, 437–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The chloride substrate was used (rather than the bromide) based on the availability of a straightforward literature prep for this compound. Anderson DR; Meyers MJ; Vernier WF; Mahoney MW; Kurumbail RG; Caspers N; Poda GI; Schindler JF; Reitz DB; Mourey RJ Pyrrolopyridine Inhibitors of Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 (MK-2). J. Med. Chem 2007, 50, 2647–2654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


