Abstract
While there is no proven treatment available for coronavirus disease 2019 (COVID-19), convalescent plasma (CP) may provide therapeutic relief as the number of cases escalate steeply world-wide. At the time of writing this review, vaccines, monoclonal antibodies or drugs are still lacking for the recent large COVID-19 outbreak, which restores the interest in CP as an empirical life-saving treatment. However, formal proof of efficacy is needed. The purpose of this review is to summarize all historical clinical trials on COVID-19 infected patients treated with CP to provide precise evidence for the efficacy and effectiveness of CP therapy in severe COVID-19 patients. Although there are many clinical trials in progress, high-quality clinical evidence is still lacking to analyze the existing problems. Meanwhile, based on the previous successful outcomes, we recommend healthcare systems to use CP therapy cautiously in critically ill COVID-19 patients.
Keywords: corona virus, COVID-19, pathogen inactivation, convalescent plasma, serology, Clinical trials
Statement of Significance: COVID-19 requires urgent development of effective treatment modality. This review first summarize all historical clinical trials of COVID-19 patients treated with convalescent plasma (CP) to provide precise evidence with a specific focus on potential applications and use of CP may be the first possible option to consider during this pandemic.
INTRODUCTION
World populations are currently facing an unprecedented health crisis caused by the spread of an infectious virus, the coronavirus-induced pneumonia, known as the Novel Coronaviruses disease (COVID-19), which has seriously affected human health worldwide [1]. Patients infected with this virus suffer from potential damage to vital organs especially the lungs, heart, liver and kidney [2, 3]. Furthermore, this infection poses a considerable risk to patients due to the high frequency of pneumonia, fever and dry cough [4].
At present, a limited number of antiviral treatments are available to treat this infection, most of which display very limited efficacy in combating COVID-19. Unfortunately, newly emerging viruses rarely provide time for the development of potent and effective vaccines that can be rapidly implemented in therapeutic settings. Prior to COVID-19, a number of infections have affected world populations, including the plague pandemic of 1855, which started in China and spread into India [5], the Spanish flu (1918–1920), which infected 500 million people around the world [6], the cholera pandemic (1817–1824), which spread across India [7], the Swine Flu (2009–2010), the AnH1N1 and A(H5N1), which caused significant mortality rates [8], the severe acute respiratory syndrome (SARS)-coronavirus (SARS-CoV) (2003), the Middle East respiratory syndrome (MERS) MERS-CoV (2012) [9] and SARS-CoV2/COVID-19 (2019) [10]. Antivirals drugs are currently available for all of these viral families excluding COVID-19. Additionally, many vaccines are often not affordable in developing countries, and their production is hard to scale up in short times, which further contributes to the severity of the pandemic.
A few treatment trials have been conducted since the emergence of COVID-19, in an effort to contain and restrain its effects, with limited success so far. For instance, a recent trial (ChiCTR2000029308) investigating lopinavir/ritonavir showed no significant benefit for SARS-CoV-2 infection [11]. Earlier, it was proven that transfusion of convalescent blood products (CBP), especially convalescent plasma transfusion (CPT), might be useful against a variety of pandemic outbreaks, including influenza, Ebola virus, SARS, MERS by reducing the hospital stays and improving patient survival [12].
The latest pilot study on CP therapy shows a potential emergent therapeutic effect and low risk in COVID-19 patients [13]. As we know, since COVID-19 is a novel infectious disease, scientists and doctors still lack the in-depth understanding of its mechanisms of transmission, infection and action that would permit the development of effective vaccines or therapies. To date, not a single clinical intervention trial of CP in COVID-19 has been completed, highlighting the necessity for conducting thorough research and meta-analyses to uncover novel treatments that will allow disease control and prevention. In order to palliate the lack of updated clinical trial reviews assessing the existing challenge and its potential clinical solutions, we have endeavored to summarize all clinical trials of COVID-19 patients treated with CP in an effort to provide updated evidence for the effectiveness and efficacy of this strategy in treating COVID-19.
METHODS
Search strategy and selection criteria
All registered clinical trials studies that were published from 1 January 2020 to 14 April 2020 were identified from Chinese Clinical Trial Registry (chiCTR), European union clinical trials database (EUCTD), Clinicatrial.gov, Pubmed, National Library of Medicine (NLM), using the keywords CP and novel coronavirus 2019, CP and COVID-19, comorbidities, clinical manifestation, immunotherapy, vaccine and SARS CoV-2. Eligible studies were extracted by electronic searching of databases and an additional search was performed using the International Clinical Trials Registry Platform (WHO ICTRP) to identify ongoing trials. The last analysis was executed on 14 April 2020.
Literature inclusion and exclusion criteria
Two investigators selected the eligible studies independently. Inclusion criteria were: (1) case-control with COVID-19 patients, (2) clinical trials with a CP protocol, (3) published full-text with maximum detail on COVID-19 therapy, (4) any type of study design (interventional and observational), (5) studies involving the treatment of COVID-19 with CP and (6) studies written in English were preferred. Exclusion criteria were: (1) studies with duplicate data; (2) studies with no specific control group and (3) unclear theoretical research, and unregistered clinical trials.
Study quality assessment
The quality evaluation of all registered clinical studies and data extraction of each literature was critically appraised and discrepancy between investigators was resolved by dialogue. All studies were tabulated and summarized narratively and grouped by the treatment strategy. We categorized the table depending on trial numbers. Furthermore, we classified results as: type of study, title of study, sponsor, recruitment status, intervention, age, phase, date enrollment, study type, country and the number of patients included in that study.
RESULTS
Trial search outcomes
Searches were performed until 14 April 2020, using EUCTD, ChiCTR and NIH (Clinical Trails.gov), and 25 registered clinical trials of COVID-19 were retrieved with active and recruiting status. Subsequent screening of headings and abstracts allowed us to exclude withdrawn clinical trials. Overall, 22 studies used CP alone, one study used PC with combinations (Hydroxychloroquine, Azithromycin), one trial used umbilical cord blood plasma and one investigation used IgG Antibody Testing Kit for clinical trials. Summaries of detailed studies were presented in Table 1. Most of the registered trials have cleared ethical statements. Some of the studies are still in the recruiting stage, 10 trials have started to the recruitment of patients. Finally, remaining trials showed an active status and it will start recruiting patients upcoming days.
Table 1.
Updated all historical ongoing clinical trials of COVID-19 infected patients treated with CP patients listed in World Health Organization International Clinical Trial Registry Platform (ICTRP) database
| Trial ID | Scientific title | Sponsor | Recruitment status | Interventions | Inclusion age | Phase | Date enrollment | Study type | Enrollment | Countries |
|---|---|---|---|---|---|---|---|---|---|---|
| ChiCTR 2000029850 | Efficacy and safety of convalescent plasma treatment for severe patients with novel coronavirus pneumonia (COVID-19): a prospective cohort study | Zhejiang University School of Medicine | Recruiting | Convalescent plasma | 18 Years and older | N/A | 15-02-2020 | Interventional | 20 | China |
| ChiCTR 2000030179 | Experimental study of novel coronavirus pneumonia rehabilitation plasma therapy severe novel coronavirus pneumonia (COVID-19) | Hospital of Nanchang University | Recruiting | Convalescent plasma | 18 Years and older | N/A | 24-02-2020 | Interventional | 100 | China |
| ChiCTR 2000030046 | A single-arm trial to evaluate the efficacy and safety of anti-2019-nCoV inactivated convalescent plasma in the treatment of novel coronavirus pneumonia patient (COVID-19) | Hospital of Jiangxia District, Wuhan (Union JiangnanHospital) | Recruiting | Convalescent plasma | 18 Years and older | N/A | 07-02-2020 | Interventional | 10 | China |
| ChiCTR 2000030010 | A randomized, double-blind, parallel-controlled, trial to evaluate the efficacy and safety of anti-SARS-CoV-2 virus inactivated plasma in the treatment of severe novel coronavirus pneumonia patients (COVID-19) | Wuhan Jinyintan Hospital (Wuhan Infectious Diseases Hospital) | Not Recruiting | Convalescent plasma | 18 Years and older | N/A | 19-02-2020 | Interventional | 100 | China |
| ChiCTR 2000030039 | Clinical study for infusing convalescent plasma to treat patients with new coronavirus pneumonia (COVID-19) | Affiliated Hospital of Xuzhou Medical University | Recruiting | Convalescent plasma | 18 Years and older | N/A | 31-05-2020 | Interventional | 90 | China |
| ChiCTR 2000030627 | Study on the application of convalescent plasma therapy in severe COVID-19 | The First Affiliated Hospital of Zhengzhou University | Recruiting | Convalescent plasma | 18 Years and older | N/A | 01-02-2020 | Interventional | 30 | China |
| ChiCTR 2000029757 | Convalescent plasma for the treatment of severe and critical novel coronavirus pneumonia (COVID-19): a prospective randomized controlled trial | China-Japan friendship hospital | Recruiting | Convalescent plasma | 18 Years and older | N/A | 14-02-2020 | Interventional | 200 | China |
| NCT 04292340 | The Efficacy and Safety of Anti-SARS-CoV-2 Inactivated Convalescent Plasma in the Treatment of Novel Coronavirus Pneumonia Patient (COVID-19): An Observational Study | Shanghai Public Health Clinical Center | Recruiting | Convalescent plasma | 18 Years and older | Phase 2 Phase 3 | 01-02-2020 | Observational | 15 | China |
| ChiCTR 2000030312 | A single-center, open-label and single-arm trial to evaluate the efficacy and safety of anti-SARS-CoV-2 inactivated convalescent plasma in the treatment of novel coronavirus pneumonia (COVID-19) patient | First people’s hospital of Jiangxi district, Wuhan | Not Recruiting | Convalescent plasma | 18 Years and older | N/A | 29-02-2020 | Interventional | 24 | China |
| ChiCTR 2000030702 | Convalescent plasma for the treatment of common COVID-19: a prospective randomized controlled trial | China-Japan friendship hospital | Recruiting | Convalescent plasma | 18 Years and older | N/A | 15-02-2020 | Interventional | 50 | China |
| ChiCTR 2000030381 | A randomized, open-label, controlled and single-center trial to evaluate the efficacy and safety of anti-SARS-CoV-2 inactivated convalescent plasma in the treatment of novel coronavirus pneumonia (COVID-19) patient | First people’s hospital of Jiangxi district, Wuhan | Not Recruiting | Convalescent plasma | 18 Years and older | N/A | 29-02-2020 | Interventional | 40 | China |
| ChiCTR 2000029818 | Clinical Study for Umbilical Cord Blood Plasma in the Treatment of Acute Novel Coronavirus Pneumonia (COVID-19) | Guangzhou reborn health management consultation co., LTD | Not Recruiting | Umbilical Cord Blood Plasma | 18 Years and older | N/A | 20-02-2020 | Interventional | 60 | China |
| ChiCTR 2000030929 | A randomized, double-blind, parallel-controlled trial to evaluate the efficacy and safety of anti-SARS-CoV-2 virus inactivated plasma in the treatment of severe novel coronavirus pneumonia (COVID-19) | Renmin Hospital of Wuhan University | Not Recruiting | Convalescent plasma | 18 Years and older | N/A | 17-03-2020 | Interventional | 60 | China |
| NCT 04323800 | Convalescent Plasma to Stem Coronavirus: A Randomized, Blinded Phase 2 Study Comparing the Efficacy and Safety Human Coronavirus Immune Plasma (HCIP) vs. Control (SARS-CoV-2 Non-immune Plasma) Among Adults Exposed to COVID-19 | Johns Hopkins University | Not recruiting | Convalescent plasma | 18 Years and older | Phase 2 | 01-04-2020 | Interventional | N/A | United States |
| NCT 04321421 | Plasma From Donors Recovered From New Coronavirus 2019 As Therapy For Critical Patients With COVID-19 | Foundation IRCCS San Matteo Hospital | Not recruiting | Convalescent plasma | 18 Years and older | N/A | 17-03-2020 | Interventional | N/A | Italy |
| NCT 04325672 | Convalescent Plasma to Limit Coronavirus Associated Complications: An Open-Label, Phase 2A Study of High-Titer Anti-SARS-CoV-2 Plasma in Hospitalized Patients With COVID-19 | Mayo Clinic | Not recruiting | Convalescent plasma | 18 Years and older | Phase 2 | 01-04-2020 | Interventional | NA | United States |
| NCT 04342182 | Convalescent Plasma Therapy From Recovered Patients to Treat Severe SARS-CoV-2 Disease (CONCOVID Study) | Erasmus Medical Center| Maasstad Hospital | Recruiting | Convalescent plasma | 18 Years and older | Phase 2 Phase 3 | 01-04-2020 | Randomized | 426 | Netherlands |
| NCT 04334876 | Rapid SARS-CoV-2 IgG Antibody Testing in High Risk Healthcare Workers | Indiana University | Not yet recruiting | Diagnostic Test: SARS-CoV-2 IgG Antibody Testing Kit | 18 Years and older | N/A | 01-04-2020 | Observational | 340 | United States |
| NCT 04338360 | Expanded Access to Convalescent Plasma for the Treatment of Patients With COVID-19 | Mayo Clinic | Available | Convalescent plasma | 18 Years and older | N/A | N/A | N/A | N/A | United States |
| NCT 04333355 | Safety in Convalescent Plasma Transfusion to COVID-19 | Hospital San Jose Tec de Monterrey| Tecnologico de Monterrey | Not yet recruiting | Convalescent Plasma | 18 Years and older | Phase 1 | 15-04-2020 | Interventional | 20 | Mexico |
| NCT 04332835 | Convalescent Plasma for Patients With COVID-19: A Randomized, Open Label, Parallel, Controlled Clinical Study (CP-COVID-19) | Universidad del Rosario | Not yet recruiting | Plasma, Hydroxychloroquine, Azithromycin | 18 Years and older | Phase 2 Phase 3 | 01-04-2020 | Interventional | 80 | Universidad del Rosario |
| NCT 04325672 | Convalescent Plasma to Limit Coronavirus Associated Complications: An Open label, Phase 2A Study of High-Titer Anti-SARS-CoV-2 plasma in hospitalized patients with COVID-19 | Johns Hopkins via a national IND | Not yet recruiting (stop) | Convalescent plasma | 18 Years and older | 2A Open Label | 27-03-2020 | Interventional | 5 | United States |
| NCT 04323800 | Convalescent Plasma to Stem Coronavirus: A Randomized Controlled Double Blinded Phase 2 Study Comparing the Efficacy and Safety of Human Coronavirus Immune Plasma (HCIP) vs. control (SARS-CoV-2 non-immune plasma) among Adults Exposed to COVID-19 | Johns Hopkins University | NA | Convalescent plasma | 18 Years and older | Phase 2 | 01-04-2020 | Interventional | 150 | United States |
| NCT 04332380 | Convalescent Plasmav for Patients With COVID-19: A Pilot Study (CP-COVID-19) | Universidad del Rosario | Not yet recruiting | Convalescent plasma | 18 Years and older | Phase 2 | 01-04-2020 | Interventional | 10 | Spain |
| NCT 04327349 | Investigating Effect of Convalescent Plasma on COVID-19 Patients Outcome: A Clinical Trial | Mazandaran University of Medical Sciences | Enrolling by invitation | Convalescent Plasma | Age 30 to 70 years | N/A | 28-03-2020 | Interventional | 30 | Iran |
| NCT04373460 | Convalescent Plasma to Limit SARS-CoV-2 Associated Complications(CSSC-004) | Johns Hopkins University | Not yet recruiting | Convalescent Plasma | 18 Years and older | Phase 2 | 19-05-2020 | Interventional | 1344 | United States |
| NCT04353206 | Convalescent Plasma in ICU Patients With COVID-19-induced Respiratory Failure | Noah Merin | Recruiting | Convalescent plasma | 18 Years and older | Early Phase 1 | 14-05-2020 | Interventional | 60 | United States |
NA: not available
Significance of convalescent plasma therapy for infectious diseases
During the pre-antibiotic era, infectious diseases had high morbidity and mortality. Plasma antibody was developed against a variety of infectious diseases because there were no alternative therapeutic options. Plasma antibody uses a potential therapeutic strategy initially obtained from immune survivors of an infectious disease that was formally introduced in 1910 for poliomyelitis in animal model [14]. Subsequently, plasma therapy was tested in human patients with acute poliomyelitis treated with CP from polio survivors and showed promising results [15]. CP was later introduced as a primary drug against several infectious diseases including influenza pneumonia, chicken pox, measles and mums [16, 17] Transfusion of CP has been shown to reduce viral load, serum cytokine response and mortality in severe H1N1 influenza patients [18]. Recently, Mair-Jenkins et al. have conducted a systematic review and meta-analysis to assess the effectiveness of CP, serum or hyperimmune immunoglobulin for the treatment of SARS coronavirus infection, severe influenza (H1N1, H5N1) and Ebola virus disease (EVD). Their results revealed that CP therapy significantly reduced mortality without causing any severe adverse effects [19, 20].
Convalescent plasma therapy for COVID-19
The US Food and Drug Administration (FDA) have recently approved the use of plasma therapy from recovered COVID-19 patients to treat critically ill patients. As per FDA recommendations, the plasma must be collected from a donor who showed no symptoms for the last 14 days and had negative recent COVID-19 results [21, 22]. The first pilot CP treatment study was conducted in three participating hospitals for 10 severe COVID-19 patients using a single dose of 200 ml CP and revealed that patients significantly increased or maintained the neutralizing antibodies at a high level while clinical symptoms rapidly improved within 3 days [13]. After COVID-19 was declared a global pandemic, many scientists suggested that CP could be used as a potential therapeutic strategy to alleviate the infection’s symptoms [23–25]. A China-based study demonstrated that the inflammatory cytokine IL-6 levels were significantly elevated in critically ill COVID-19 patients, indicating that the viral load was strongly associated with a cytokine storm and can be used to predict poor COVID-19 prognosis [26]. Another case report study in five patients reported that maximal supportive care and administration of antiviral agents with CP transfusion (neutralizing antibody titers 1:640) could potentially improve clinical outcomes without severe adverse effects [27] Furthermore, Zhang et al. showed that after 11 days of CP infusion, patients did not require mechanical ventilation and were moved to the general ward with better outcomes [28]. Additionally, six more confirmed COVID-19 patients showed better improvement after treatment with CP in Wuhan, China [29].
Based on these preliminary results, USA-based John Hopkins University is currently leading a randomized trial (Phase 2) on 150 older participants undergoing CP treatment with a titer of neutralizing antibody > 1:64 for post-exposure prevention [30]. A Mayo Clinic-sponsored phase 2 trial investigating CP treatment with a titer > 1:64 is also currently recruiting [31]. Another study analyzing results from 173 patients traced the dynamics of antibody responses during disease progression. Periodic antibody detection revealed that the appearance of antibodies was <40% among patients in the first week of COVID-19 infection, then rapidly increased to 100% Ab, 79.8% IgG and 94.3% IgM, respectively, since 2nd week after infection onset, highlighting the importance of routine testing in the context of COVID-19 infections [32]. Furthermore, it was noticed that the average IgG antibody level was higher in female patients than in male patients, particularly in severe cases, which could account for the differences in COVID-19 outcomes between genders [33]. Previous studies on the duration of the serological response profile in patients infected with earlier strains of the SARS coronavirus revealed that IgM was still detectable after 7 months of postinfection. Hence, a suitable donor could donate 200 × 3 times single dose of plasma during a period of 6 months [34]. Based on these findings, many pharma companies such as Israeli company Kamada are collecting plasma in different facilities from people who have recovered from this viral disease [35]. As the various results summarized in this section indicate, CP administration seems to reduce viral load and is a safe treatment strategy with minimal side effects. A CP collections workflow and protocol are presented in Fig. 1.
Figure 1.
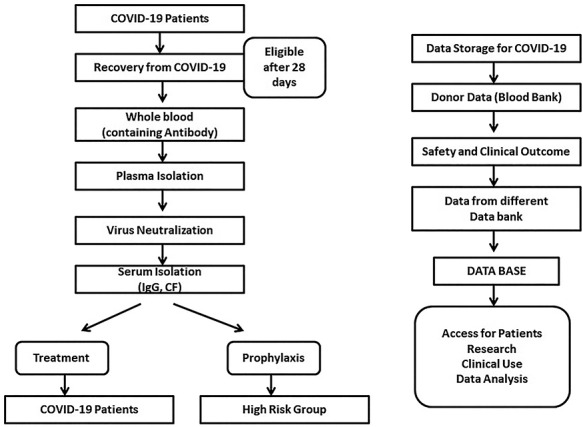
Flow for possible CP therapy and data storage of COVID-19 patients samples.
DISCUSSION
In this paper, we have summarized the current registered clinical trials on CP initiated following the onset of the COVID-19 pandemic outbreak. Despite the potential utility of CP treatments, there have been few concerted efforts to use them as initial therapies against pandemic. The main contraindications to CP therapy are an allergic reaction to plasma protein. As in many other trials examining clinical–pathological symptoms observed during viral or bacterial infections, thrombosis, multiple organ failure, as well as pregnant or lactation schedules are also contraindications.
The advantages and disadvantages of human plasma therapies are often related to conventional antiviral drugs. However, Abs are specific in their modes of action in diverse therapeutic classes. Anti-COVID-19 plasma differs from regular human plasma only by the presence of antibodies against the coronavirus. Donor selection criteria are the same as outlined by FDA guidelines. Therefore, the risks to transfusion receivers are likely to be same as those of standard plasma therapy.
One of the major challenges and concerns with plasma therapies is ensuring the prevention of transfusion–transmitted infections (TTI). Recent pathogen inactivation (PI) technologies, combined with nucleic acid testing (NAT) of an individual donor may represent a good option to reduce the risk of additional TTIs. At present, due to globalization, regulatory systems may require further tests to ensure additional transfusion safety. For instance, one UK based study suggests that blood donors are not screened for HEV infections although this infection is widespread in English populations. In fact, it was reported that after CP transfusion, 10 recipients developed prolonged or persistent hepatitis E virus infection [36]. Although this does not preclude COVID-19 with CP from being therapeutically utilized in the UK, these risks should be considered in clinical trials at the individual patient level.
There are also some non-infectious risks linked with CPT, such as allergic transfusion reactions and transfusion associated circulatory overload (TACO). Another major concern is transfusion-related acute lung injury (TRALI), an immune-mediated transfusion reaction that can cause severe complications or even death. Previous reports have shown that female donors with a history of pregnancy have higher risk of TRALI [37, 38]. TRALI is particularly challenging in patients presenting with severe COVID-19 symptoms given the potential priming of the pulmonary endothelium. Male donors are the first choice to further decrease the risk of transfusing human leukocyte antigen (HLA) antibody from parous women. Recent COVID-19 data show that female patients display higher IgG levels [39]. In certain cases, the presence of specific antibodies may account for antibody-dependent enhancement (ADE) before this phenomenon is noticed for dengue virus [40]. This virus-specific antibody enhancement could theoretically increase COVID-19 virus entry in monocytes or macrophages cells and increase its severity. In this context, ADE infection could pose major concerns during vaccination. However, the identification and characterization of viral epitopes are important before CPT for COVID-19 [41].
A recent study of six COVID-19 patients with respiratory failure that received CPT after 21.5 days of first viral detection indicated that they all tested negative for COVID-19 by 3 days after infusion, and five died in due course. These findings suggest that CP treatment may reduce COVID-19 RNA shedding but cannot decrease mortality rates in critical patients [42]. Deploying CP therapies against the COVID-19 patients provides an unprecedented opportunity to perform clinical research and gather evidence of the effectiveness of this treatment against viral infection. At present, both academic researchers and industry groups have started to investigate the effectiveness and clinical usefulness of CP therapies for COVID-19. If clinical trials results clearly establish the effectiveness and potential benefit of CP, the USA and other countries that have exponential growth of coronavirus deaths, may consider a national campaign to provide plasma treatment. Although a logistical challenge may be one an evidence-based approach to protect particularly high-risk populations.
LIMITATIONS
Based on the current study and available clinical trial results, we unable to make a definitive conclusion on CPT as a treatment option for COVID-19. Due to some limitations, first, insufficient clinical trial evidence. Most of these trials are still ongoing and their result has not been published yet. Second, it is unclear if patients would have improved with CP or other medication because at present, it is not clear that the administration timing of CP after admission. Third, whether CP treatment strategy would reduce mortality rates of COVID-19 patients is still unknown.
CONCLUSIONS
Plasma therapy from recovered COVID-19 patients is anticipated to be safe and potentially useful for treatment, based on the available data. In the current situation, COVID-19 requires urgent development of effective treatment modalities. The use of CP may be the first potential option to consider during this pandemic while antiviral drugs are being tested. Developing successful therapies will require the engagement and coordination of several different entities, such as blood banking specialists, virologists, hematologists and other health care workers, to ensure the proper interpretation of disease severity. It also requires ethical statements and controlled hygiene to ensure optimal safety to both donors and recipients. In the context of the public health emergency posed by COVID-19, the FDA has issued a special guidance (24 March 2020) on exploring the use of CP treatment against COVID-19. The released guidelines introduce measures for assessing the safety and efficacy of CP in clinical trials to monitor the success rates of the therapy and evaluate its safety and suitability in the clinical setting. As most countries are beginning to recover from the outbreak and ease lockdown measures, we recommend that, while the medical community still awaits the results of current randomized controlled trials of CP, hospitals can nonetheless consider the emergency use of CP to treat critically ill COVID-19 patients under the current challenging circumstances posed by the pandemic.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
All the authors have read the manuscript and have approved this submission.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
Not applicable.
CONTRIBUTION OF THE AUTHORS
All authors contributed equally.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1. Yang, Y, Shen, C, Li, Jet al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol 2020; S0091-6749: 30576–5. [published online ahead of print, 2020 Apr 29]. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valizadeh, R, Baradaran, A, Mirzazadeh, Aet al. Coronavirus-nephropathy; renal involvement in COVID-19. J Renal Inj Prev 2020; 9: e18. [Google Scholar]
- 3. Yalameha, B, Roshan, B, Bhaskar, LVKSet al. Perspectives on the relationship of renal disease and coronavirus disease 2019. J Nephropharmacol 2020; 9: e22. [Google Scholar]
- 4. Qiu, H, Wu, J, Hong, Let al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020; 20: 689–96. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bramanti, B, Dean, KR, Walloe, Let al. The third plague pandemic in Europe. Proc Biol Sci 2019; 286: 20182429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taubenberger, JK, Morens, DM. 1918 influenza: the mother of all pandemics. Emerg Infect Dis 2006; 12: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chowdhury, FR, Nur, Z, Hassan, Net al. Pandemics, pathogenicity and changing molecular epidemiology of cholera in the era of global warming. Ann Clin Microbiol Antimicrob 2017; 16: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korteweg, C, Gu, J. Pandemic influenza A (H1N1) virus infection and avian influenza A (H5N1) virus infection: a comparative analysis. Biochem Cell Biol 2010; 88: 575–87. [DOI] [PubMed] [Google Scholar]
- 9. Luk, HKH, Li, X, Fung, Jet al. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect Genet Evol 2019; 71: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai, CC, Shih, TP, Ko, WCet al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020; 55: 105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao, B, Wang, Y, Wen, Det al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382: 1787–99. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marano, G, Vaglio, S, Pupella, Set al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus 2016; 14: 152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duan, K, Liu, B, Li, Cet al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci 2020; 117: 9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flexner, S, Lewis, PA. Experimental epidemic poliomyelitis in monkeys. J Exp Med 1910; 12: 227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amoss, HL, Chesney, AM. A report on the serum treatment of twenty-six cases of epidemic poliomyelitis. J Exp Med 1917; 25: 581–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGuire, LW, Redden, WR. The use of convalescent human serum in influenza pneumonia-a preliminary report. Am J Public Health 1918; 8: 741–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gunn, W. Convalescent serum in prophylaxis of measles, chicken-pox, and mumps. Br Med J 1932; 1: 183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung, IF, To, KK, Lee, CKet al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011; 52: 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mair-Jenkins, J, Saavedra-Campos, M, Baillie, JKet al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015; 211: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gutfraind, A, Meyers, LA. Evaluating large-scale blood transfusion therapy for the current Ebola epidemic in Liberia. J Infect Dis 2015; 211: 1262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanne, JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ 2020; 368: m1256. [DOI] [PubMed] [Google Scholar]
- 22. Center for Biologics Evaluation and Research INDs., C.I.C.-C.P.-E . Recommendations for Investigational COVID-19 Convalescent Plasma, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/investigational-covid-19-convalescent-plasma.
- 23. Casadevall, A, Pirofski, LA. The convalescent sera option for containing COVID-19. J Clin Invest 2020; 130: 1545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen, L, Xiong, J, Bao, Let al. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 2020; 20: 398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen, C, Wang, Z, Zhao, Fet al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323: 1582–9. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen, X, Zhao, B, Qu, Yet al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv 2020; 2020.02.29.20029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duan, K, Liu, B, Li, Cet al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv 2020; 10.1101/2020.03.16.20036145. [DOI] [Google Scholar]
- 28. Zhang, L, Pang, R, Xue, Xet al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging 2020; 12: 6536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ye, M, Fu, D, Ren, Yet al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China [published online ahead of print, 2020 Apr 15]. J Med Virol 2020. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. NCT04323800 . (2020) Efficacy and Safety Human Coronavirus Immune Plasma (HCIP) vs. Control (SARS-CoV-2 Non-immune Plasma) Among Adults Exposed to COVID-19 (CSSC-001). https://clinicaltrials.gov/ct2/show/NCT04323800.
- 31. NCT04325672 . (2020) Convalescent Plasma to Limit Coronavirus Associated Complications. https://clinicaltrials.gov/ct2/show/NCT04325672.
- 32. Zhao, J, Yuan, Q, Wang, Het al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. medRxiv 10.1101/2020.03.02.20030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeng, F, Dai, C, Cai, Pet al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between gender. medRxiv 2020. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan, KH, Cheng, VC, Woo, PCet al. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin Diagn Lab Immunol 2005; 12: 1317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamada . (2020) Israeli Company Kamada Working on ‘Passive Vaccine’ for Coronavirus. https://www.jpost.com/health-science/israeli-company-kamada-working-on-passive-vaccine-for-coronavirus-621333.
- 36. Hewitt, PE, Ijaz, S, Brailsford, SRet al. Hepatitis E virus in blood components: a prevalence and transmission study in Southeast England. Lancet 2014; 384: 1766–73. [DOI] [PubMed] [Google Scholar]
- 37. Hendrickson, JE, Hillyer, CD. Noninfectious serious hazards of transfusion. Anesth Analg 2009; 108: 759–69. [DOI] [PubMed] [Google Scholar]
- 38. Triulzi, DJ, Kleinman, S, Kakaiya, RMet al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion 2009; 49: 1825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thevarajan, I, Nguyen, THO, Koutsakos, Met al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med 2020; 26: 453–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katzelnick, LC, Gresh, L, Halloran, MEet al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017; 358: 929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fleming, AB, Raabe, V. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibody-dependent enhancement. J Clin Virol 2020; 127: 104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng, Q-L, Yu, Z-J, Gou, J-Jet al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis 2020; 29: jiaa228. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


