Abstract
Background:
Prior studies among women with impaired fecundity have consistently demonstrated a positive association between daily perceived stress and the ability to conceive. However, the effects of daily stress on time to pregnancy (TTP) among women with proven fertility is not known.
Materials and Methods:
One hundred and forty-three women ages 18–35, in a relationship of proven fertility, who desired to conceive were included in the analysis. Daily diaries recording perceived stress (scale 0–10) were completed for up to 7 menstrual cycles or until pregnancy. Cox proportional hazards regression models were used to estimate the association between time-varying perceived stress tertiles (high [ > 4.1–7.2], moderate [ > 2.7–4.1], and low [0.1–2.7]) and adjusted fecundability odds ratio (aFOR), 95% confidence intervals (CI), after taking into account age, parity, education, time-varying caffeine and alcohol intake, fertility awareness tracking, and cycle intent to conceive.
Results:
Among the 111 participants who completed daily diaries, 90 (81.1%) conceived. Women reporting high or moderate stress, versus low stress, had no difference in probability of achieving pregnancy (aFOR: 1.11 [95% CI: 0.58, 2.14]; and aFOR: 1.37 [0.71, 2.67]), respectively. Additional adjustment for intercourse frequency during narrow fertile window, or narrowing exposure focus to pre-ovulatory or pre-implantation stress did not appreciably alter the estimates.
Conclusion:
Daily perceived stress was not adversely associated with TTP among women with proven fertility. While a growing body of evidence supports adverse effects of more severe stressful life events on female reproductive function, moderate psychological stress, commonly referred to as eustress, among relatively healthy women with proven fertility does not appear to adversely impact TTP.
Keywords: Perceived stress, Psychological stress, Time to pregnancy, Fecundability, Fecundity
1. Introduction
Psychological stress is the set of physical or mental perceptions and emotional responses towards events or stimuli that tax or exceed an individual’s adaptive capacity (Catherino, 2011; Cohen et al., 2007). In recent years, the role of chronic and acute stress has been a critical concern among women of reproductive age (Lynch et al., 2014; Schliep et al., 2015). Previous studies have suggested that stress has a negative influence on sex steroid secretion, ovulation, and implantation in pre-menopausal women (Chrousos et al., 1998; Ferin, 1999; Schliep et al., 2015); however, the mechanism behind the association and populations most at risk have not been fully established.
Based on animal models, two biological pathways have been hypothesized to demonstrate the effects of stress on reproductive function in women (Lynch et al., 2014). Perceived stress may activate the hypothalamic-pituitary-adrenocortical (HPA) axis with consequent suppression of hypothalamic–pituitary gonadal (HPG) axis, which may lead to increased cortisol production, and interfere with a woman’s menstrual cycles by delaying or inhibiting the release of pre-requisite ovulatory hormones (Chrousos et al., 1998; Ferin, 1999). Alternatively, hyper-activated sympathetic adrenomedullary (SAM) system due to chronic perceived stress stimuli may release the norepinephrine into the bloodstream, increase the salivary alpha-amylase secretion by the parotid gland (Lynch et al., 2014), and produce adverse influences on conception by affecting the autoimmune conditions of the uterus (Makrigiannakis et al., 2001).
Derived from the proposed biological pathway, recent epidemiologic studies have been conducted with a preconceptional measurement of stress biomarkers, including salivary cortisol and alpha-amylase, to investigate a correlation with reduced fecundity in premenopausal women (Lynch et al., 2012, 2014). Results indicate no significant relationship between twice-measured preconception salivary cortisol concentration and fecundability while the preconception high salivary alpha-amylase tertile has exhibited a significant reduction in fecundity compared with the low tertile (Louis et al., 2011; Lynch et al., 2012, 2014). While these findings support the effects of stress on the SAM system, other recent studies have shown that daily perceived stress significantly reduces probability of conception (Akhter et al., 2016) and sex steroid synthesis, and leads to sporadic anovulation in regularly menstruating women (Schliep et al., 2015), lending credence to the potential effect of stress on the HPA axis.
Given the evidence to date, researchers propose that further investigation in preconception cohorts for which daily perceived stress data was captured will enable us to better understand critical windows of exposure and the temporal relationship between stress and human reproductive function (Bolger et al., 2003; Schliep et al., 2015; Wesselink et al., 2018). Additionally, populations most at risk for the adverse effects of stress on reproductive function should be clarified so that interventions are targeted appropriately. To address gaps in previous research, we evaluated the relationship between time-varying daily perceived stress for the overall menstrual cycle as well as restricted to the peri-ovulatory and peri-implantation windows, and TTP in a cohort of premenopausal women trying to conceive.
2. Materials and methods
2.1. Study design and eligibility
The Study of Time to Pregnancy in Normal Fertility, conducted in 2003–2006, was a parallel randomized trial that followed 143 women from Salt Lake City, Utah for up to 7 cycles. Detailed study methods and procedures have been described in a previous publication (Stanford et al., 2014). The study was originally designed to assess TTP in couples of proven fertility, comparing those who receive instructions for fertility awareness using the Creighton Model Fertility Care System™ (CrMS) (n = 71) versus those who receive general preconception counseling, including advice to have intercourse 2–3 times per week, but no instruction regarding the timing of ovulation (n = 72) (Stanford et al., 2014). The study size was based on the power to detect a difference between the groups in cumulative pregnancy rates, assuming a 5% of lost to follow-up (Stanford et al., 2014).
Eligibility included women ages 18–35, in a relationship of proven fertility who desired to conceive, but had not yet started trying, and had no history of subfecundity. Women were excluded if they reported 9 months or more TTP for their most recent pregnancy, had more than one menstrual cycle that was less than 24 days or more than 35 days long in the past year, had any experience with a method related to the observation of vaginal secretions from cervical fluid, or had used a fertility monitor (Stanford et al., 2014). The University of Utah Institutional Review Board (IRB) approved the original study protocol and each of the women provided written informed consent prior to enrollment. The trial was registered on clinicaltrials.gov, NCT00161395.
2.2. Perceived stress and covariate assessment
Data on baseline demographics and social-behavioral characteristics were collected via interview-based self-report. All women completed a baseline questionnaire capturing demographic characteristics, reproductive and medical history, and lifestyle information including smoking, alcohol, and caffeine intake. At the baseline visit women were instructed on the use of their daily diary, which included recording bleeding, intercourse, alcohol (yes/no), tobacco (yes/no/passive), caffeinated coffee (8-oz cups), caffeinated tea (8-oz cups), caffeinated soda (8-oz cups), medications, illness, and perceived stress (scale with 0 being none and 10 being maximum) (Sullivan and Artino, 2013). Participants were also asked to record their intention to conceive or not at the start of each menstrual cycle, for that specific cycle. Daily diaries were identical for the intervention and control group, with the exception of the intervention group additionally recording their cervical fluid observations as per CrMS protocol. Women completed diaries until pregnant or for 7 cycles of follow-up. All women completed daily urine testing from cycle day 2 up through the end of the cycle or day 31 with a blinded version of the ClearBlue® Fertility Monitor; from this, we obtained daily data for urine LH concentration (Stanford et al., 2014).
2.3. Pregnancy assessment
Our primary outcome of interest was fecundability as measured with TTP, assessed by counting the number of cycles until chemical recognition of pregnancy (Stanford et al., 2014). The pregnancy was recognized based on missed menstruation or clinical symptoms and defined as a positive result in urine human chorionic gonadotropin testing at a study visit, sensitive to a concentration of 25 mIU/mL or higher (Clearblue® pregnancy tests) (Stanford et al., 2014). To assure adequate quality of the daily data collection before participants actually started trying to achieve pregnancy, women were asked to continue to avoid pregnancy until they had been in the study for one full menstrual cycle. After the study enrollment, women were followed until pregnant, beginning of any medical fertility treatment, cessation of sexual inter-course, lost to follow-up, or up to 7 full cycles. Women who conceived during the study were followed until pregnancy outcome (Stanford et al., 2014).
2.4. Statistical analysis
Descriptive statistics were used to assess women’s characteristics by study-average perceived stress tertile (low; ≤2.7, moderate; > 2.7–4.1, and high; > 4.1). To assess perceived stress variation across the menstrual cycle and over the study period, we used linear regression models and calculated the least square means and 95% confidence intervals (CI).
For the primary analysis, discrete-time survival analysis was used to estimate the effect of cycle-average, time-varying perceived stress on TTP (Radin et al., 2015; Powell and Bagnell, 2012; Cox, 2018). We calculated fecundability odds ratios (FOR) and 95% CIs with Cox proportional hazards regression, accounting for right censoring, where FOR < 1 indicates reduced fecundability with longer TTP and > 1 indicates increased fecundability with shorter TTP. To test the proportional hazard assumption in the Cox model, we performed flexible assessment of time-by-covariate interactions by including interaction of the predictors and a survival time function in the model. (Crowther and Lambert, 2017; Heinzl and Kaider, 1997; Royston and Parmar, 2002).
In addition to modeling time-varying stress exposure across each menstrual cycle, we further modeled stress exposure during specific windows: preovulatory window, pre-implantation window, broad fertile window, and narrow fertile window. To define the specific windows of each cycle, two trained reviewers identified the peak day of LH by manually reviewing each woman’s LH level, detected from the fertility monitor, with a third reviewer adjudicating any discrepancies. Pre-ovulatory window was defined from cycle start date to the estimated day of ovulation (EDO; +1 day from the peak LH surge day) and pre-implantation window was defined as EDO to day +8. Furthermore, fertile windows for each menstrual cycle were defined to more accurately assess the effects of daily stress on reproductive function during windows when women were “at risk” for conception. Broad and narrow fertile windows were defined as −6 to +3 days (10 days total) and −3 to +1 days (5 days total) from the peak LH surge day, respectively (Lynch et al., 2006). For comparability, we used the study-average perceived stress tertile cut points (low; ≤2.7, moderate; > 2.7–4.1, and high; > 4.1) to categorize stress tertiles across each menstrual cycle and for the specific menstrual cycle windows (i.e., pre-ovulatory window, pre-implantation window, broad fertile window, and narrow fertile window).
Potential confounders were determined via directed acyclic graphs. Potential covariates that we considered included age (continuously), race/ethnicity (categorically), BMI (categorically), parity (one prior pregnancy vs more than one prior pregnancy), education (≥college graduate vs < college graduate), income (≥$40,000/year vs < $40,000/year), prior use of oral contraceptives (yes/no), time-varying cycle-average caffeine/alcohol intake (continuous), prior his-tory of smoking (yes/no), CrMS treatment (yes/no), and cycle intent to conceive (yes/no). Age, prior pregnancy history, education level, CrMS intervention, and cycle intent to conceive were included in our final multivariate hazard ratios estimation.
To test the robustness of our findings, we conducted several sensitivity analyses. Given that time-varying intercourse frequency may additionally affect the relationship between perceived stress and fecundability, as has been assessed by others (Louis et al., 2011; Lynch et al., 2014), we additionally checked to see if adding this potential confounding factor altered our estimates. To address potential selection bias due to missing data (i.e., women with higher perceived stress may be less likely to complete their daily diaries and may also have lower fecundability), we imputed missing stress values using multiple imputation. To assure that our inferences were not changed due to the imputed stress values, we compared estimated FORs with only observed stress data to the estimated FORs with full stress data, after imputing missing stress values. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina).
3. Results
Of the 143 women randomized, 3 participants randomized to the CrMS group were found subsequently to be ineligible. Of the remaining 140 women, 6 became pregnant in cycle 0, and 1 in cycle 5 with no daily diary data while the remaining 12 chose to withdraw (n = 6), needed medical treatment (n = 1), chose to not get pregnant (n = 4), or were lost to follow up (n = 1), also with no daily diary data. Additionally, 6 women were excluded from the analysis because they became pregnant at cycle 1 (n = 5), considered as protocol violation, or chose to not get pregnant (n = 1). For the analysis pre-ovulatory/pre-implantation stress exposure, we further eliminated 4 women with no EDO data because they had no luteinizing hormone (LH) data (n = 1) or had no detected peak day of LH (n = 3). The final analytical sample included in our primary analyses is 111 (see Supplementary data, Fig. S1).
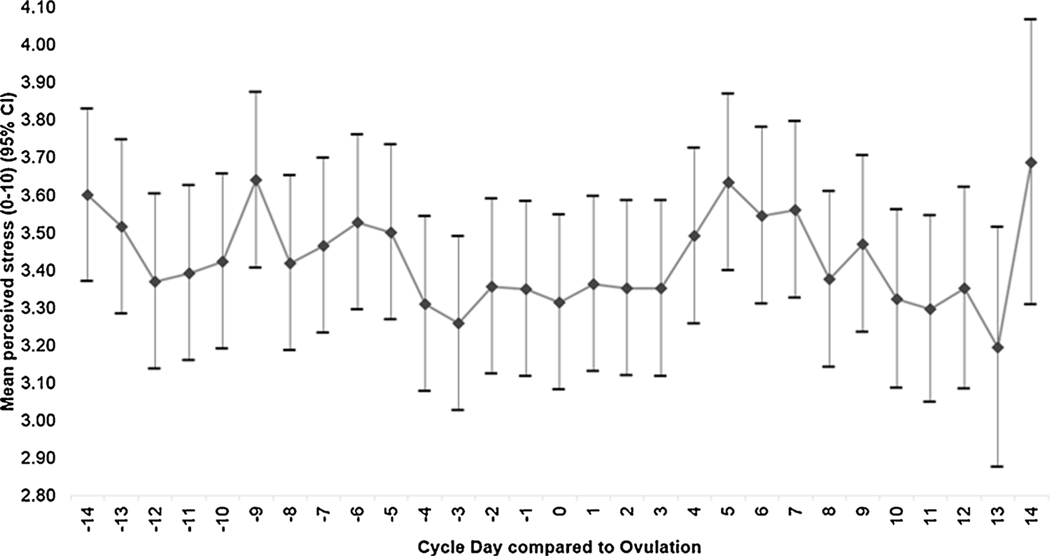
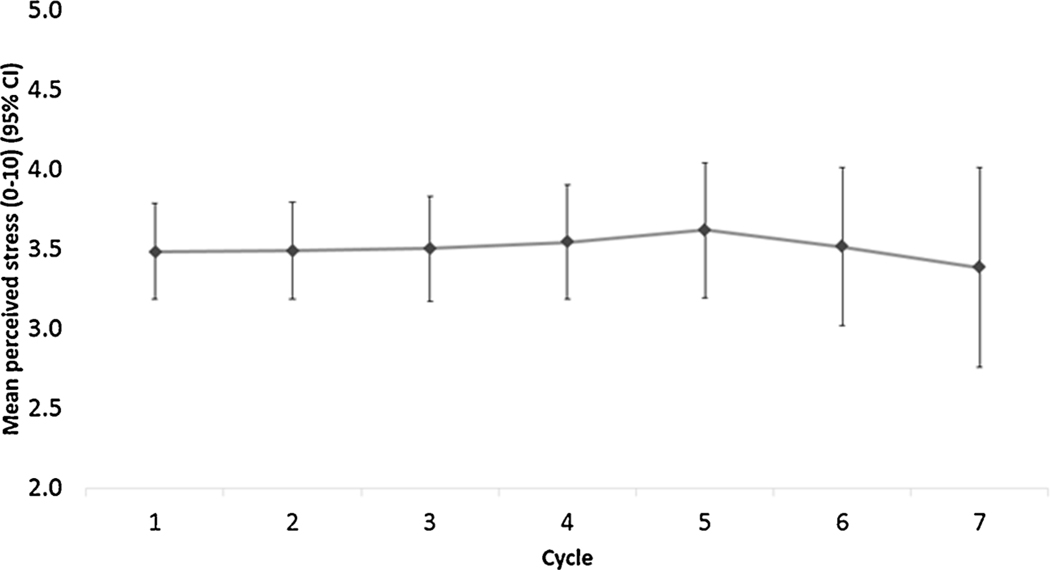
Among the 111 participants who were included for analysis, a total of 90 (81.1%) conceived and 76 (68.5%) achieved a live birth during the average follow-up time of 4.01 cycles (standard deviation [SD]: 1.79). Over the study period via the daily diaries, women reported an average stress level of 3.5 (SD: 1.5), and mean intercourse frequency of 3.3 (SD: 1.3) during broad (10 days) and 2.1 (SD: 0.8) during narrow (5 days) fertile windows, respectively. Additionally, over the study period via the daily diaries, 16% of women reported consuming coffee (median = 0.36 cups, IQR = 0.10, 0.84 among consumers) while 5% reported consuming alcohol (median = 0.14 cups, IQR = 0.05, 0.27 among consumers). When assessing the mean (95% CI) variation of stress captured in the daily diary, there were no significant differences in daily perceived stress level over the menstrual cycle (Fig. 1a) or over the study period (Fig. 1b).
Fig. 1.
Mean (95% CI) variation in daily diary reported perceived stress over the menstrual cycle. The variation of repeatedly measured stress level in daily diary was assessed, both across the menstrual cycle and between menstrual cycles. Pairwise comparisons of self-reported stress exposures were made.
Women who reported higher versus lower stress levels across the study period were more likely to be in the control (non-CrMS intervention) group (non-CrMS intervention), obese at baseline, and have higher family income (Table 1). Women with higher stress levels were also less likely to have intercourse during narrow fertile window compared to women with low stress levels. No significant differences were observed in the distribution of age, parity, education, prior oral contraceptive use, coffee/alcohol consumption, cycle intent to conceive or smoking history by stress tertiles.
Table 1.
Characteristics of women trying to conceive by stress tertiles (n = 111).
| Total n = 111 | Stress tertiles | |||
|---|---|---|---|---|
| Low (≤2.7) n = 36 | Moderate (> 2.7–4.1) n = 37 | High (> 4.1) n = 38 | ||
| Age (mean ± SD) | 28.5 ± 3.1 | 28.2 ± 2.7 | 28.9 ± 3.0 | 28.5 ± 3.5 |
| Race, n (%) | ||||
| White non-Hispanic | 105 (94.6) | 34 (94.4) | 37 (100.0) | 34 (89.5) |
| White Hispanic | 3 (2.7) | 0 | 0 | 3 (7.9) |
| Non-white non-Hispanic | 3 (2.7) | 2 (5.6) | 0 | 1 (2.6) |
| Partner’s race, n (%) | ||||
| White non-Hispanic | 107 (96.4) | 35 (97.2) | 36 (97.3) | 36 (94.7) |
| White Hispanic | 1 (0.9) | 0 | 0 | 1 (2.6) |
| Non-white non-Hispanic | 3 (2.7) | 1 (2.8) | 1 (2.7) | 1 (2.6) |
| Pregnancy history, n (%) | ||||
| 1 | 50 (45.1) | 18 (50.0) | 17 (45.9) | 15 (39.5) |
| 2+ | 61 (55.9) | 18 (50.0) | 20 (54.1) | 23 (60.5) |
| Education, n (%) | ||||
| < College graduate | 51 (46.0) | 19 (52.8) | 16 (43.2) | 16 (42.1) |
| ≥ College graduate | 60 (54.0) | 17 (47.2) | 21 (56.8) | 22 (57.9) |
| Family income, n (%) | ||||
| < $ 40,000 per year | 39 (35.8) | 18 (50.0) | 9 (25.0) | 12 (32.4) |
| ≥ $ 40,000 per year | 70 (64.2) | 18 (50.0) | 27 (75.0) | 25 (67.6) |
| missing | 2 | 0 | 1 | 1 |
| Body Mass Index, n (%) | ||||
| < 18.5 kg/m2 | 10 (9.0) | 4 (11.1) | 3 (8.1) | 3 (7.9) |
| 18.5–24.9 kg/m2 | 68 (61.3) | 22 (61.1) | 23 (62.2) | 23 (60.5) |
| 25–29.9 kg/m2 | 19 (17.1) | 5 (13.9) | 8 (21.6) | 6 (15.8) |
| 30+ kg/m2 | 14 (12.6) | 5 (13.9) | 3 (8.1) | 6 (15.8) |
| Prior use of oral contraceptives, n (%) | ||||
| No | 5 (4.5) | 3 (8.3) | 1 (2.7) | 1 (2.6) |
| Yes | 106 (95.5) | 33 (91.7) | 36 (97.3) | 37 (97.4) |
| Consumed coffee in the past month, n (%) | ||||
| No | 101 (91.0) | 34 (94.4) | 34 (91.9) | 33 (86.8) |
| Yes | 10 (9.0) | 2 (5.6) | 3 (8.1) | 5 (13.2) |
| Consumed alcohol in the past month, n (%) | ||||
| No | 96 (86.5) | 33 (91.7) | 31 (83.8) | 32 (84.2) |
| Yes | 15 (13.5) | 3 (8.3) | 6 (16.2) | 6 (15.8) |
| Any prior history of smoking, n (%) | ||||
| No | 93 (83.8) | 31 (86.1) | 30 (81.1) | 32 (84.2) |
| Yes | 18 (16.2) | 5 (13.9) | 7 (18.9) | 6 (15.8) |
| CrMS group, n (%) | ||||
| Intervention | 59 (53.2) | 25 (69.4) | 16 (43.2) | 18 (47.4) |
| Control | 52 (46.8) | 11 (30.6) | 21 (56.8) | 20 (52.6) |
| Perceived stress (mean ± SD) | 3.5 ± 1.6 | 1.7 ± 0.7 | 3.4 ± 0.4 | 5.2 ± 0.9 |
| Pregnancy outcome, n (%) | ||||
| Miscarriage | 14 (12.6) | 3 (8.3) | 5 (13.5) | 6 (15.8) |
| Live birth | 76 (68.5) | 24 (66.7) | 24 (64.9) | 28 (73.7) |
| Not pregnant | 21 (18.9) | 9 (25.0) | 8 (21.6) | 4 (10.5) |
| Intent to conceive, n (%) | ||||
| Strong | 99 (90.0) | 34 (94.4) | 32 (86.5) | 33 (89.2) |
| Moderate | 6 (5.4) | 2 (5.6) | 2 (5.4) | 2 (5.4) |
| Weak | 5 (4.6) | 0 | 3 (8.1) | 2 (5.4) |
| missing | 1 | 0 | 0 | 1 |
| Intercourse frequency (mean ± SD) | ||||
| Broad fertile window | 3.3 ± 1.3 | 3.6 ± 1.3 | 3.1 ± 1.5 | 3.0 ± 1.1 |
| Narrow fertile window | 2.1 ± 0.8 | 2.3 ± 0.8 | 2.0 ± 0.8 | 2.0 ± 0.7 |
Perceived stress captured from daily diaries (scale with 0 being none and 10 being maximum) for up to 7 menstrual cycles. Broad fertile window=−6 to +3 days from the LH peak, Narrow fertile window=−3 to +1 days from the LH peak. SD = Standard deviation, n=Number of participants, CrMS = The Creighton Model Fertility Care System™.
Overall, women with moderate or high levels of perceived stress, compared to low-stress levels, had no differences in ability to achieve pregnancy (FOR: 1.37, 95% CI: 0.71–2.67 and FOR: 1.11, 95% CI: 0.58–2.14, respectively) after adjusting for age, parity, education, caffeine/alcohol intake, CrMS intervention, and cycle intent to conceive (Table 2). There was no appreciable difference in FORs after additionally adjusting for intercourse frequency during fertile window. When assessing stress during specific time period of each cycle, high versus low levels of pre-ovulatory stress and pre-implantation stress again showed a null association (FOR: 1.79, 95% CI: 0.90–3.56 and FOR: 1.08, 95% CI: 0.56–2.10). Further adjustment of intercourse frequency during fertile window did not greatly impact FOR. We did not observe any significant association between broad/narrow fertile window stress and TTP (Supplementary data, Table S1). In sensitivity analysis comparing the estimated FORs with only observed stress data to the estimated FORs with imputed stress data, we did not observe any notable changes in the estimates. (Fig. 2).
Table 2.
Unadjusted and adjusted FORs for overall, pre-ovulatory, and pre-implantation stress among women trying to conceive (n = 111).
| Unadjusted FOR | Adjusted FORa | Adjusted FORb | ||||
|---|---|---|---|---|---|---|
| FOR | 95% CI | FOR | 95% CI | FOR | 95% CI | |
| Overall cycle stress | ||||||
| Low (≤2.7) | 1.0 | 1.0 | 1.0 | |||
| Moderate (> 2.7–4.1) | 1.35 (0.74, 2.46) | 1.37 (0.71, 2.67) | 1.37 (0.71, 2.67) | |||
| High (> 4.1) | 0.93 (0.52, 1.66) | 1.11 (0.58, 2.14) | 1.12 (0.58, 2.15) | |||
| Pre-ovulatory stress | ||||||
| Low (≤2.7) | 1.0 | 1.0 | 1.0 | |||
| Moderate (> 2.7–4.1) | 1.61 (0.88, 2.97) | 1.88 (0.93, 3.78) | 1.69 (0.83, 3.48) | |||
| High (> 4.1) | 1.28 (0.71, 2.28) | 1.79 (0.90, 3.56) | 1.55 (0.75, 3.21) | |||
| Pre-implantation stress | ||||||
| Low (≤2.7) | 1.0 | 1.0 | 1.0 | |||
| Moderate (> 2.7–4.1) | 1.05 (0.56, 1.99) | 0.93 (0.44, 1.96) | 0.87 (0.40, 1.89) | |||
| High (> 4.1) | 0.98 (0.56, 1.72) | 1.08 (0.56, 2.10) | 1.10 (0.55, 2.20) | |||
FOR = Fecundability Odds Ratio, CI = Confidence Interval. FOR < 1 indicates reduced fecundability with longer TTP and FOR > 1 indicates improved fecundability with shorter TTP. Pre-ovulatory stress is the stress level from day 0 to EDO of each cycle and pre-implantation stress is the stress level from EDO to day +8 of each cycle.
Adjusted for age, parity, education, caffeine/alcohol intake, CrMS intervention, and cycle intent to conceive.
Additionally adjusted for intercourse frequency during narrow fertile window.
Fig. 2.
Mean (95% CI) variation in daily diary reported perceived stress over the study period. The variation of repeatedly measured stress level in daily diary was assessed and pairwise comparisons of self-reported stress exposures were made.
4. Discussion
To our knowledge, this is one of few cohort studies to examine the hypothesized association between preconception stress and fecund-ability prospectively among women trying to conceive (Louis et al., 2011; Lynch et al., 2012, 2014), and one of only two to measure daily self-reported preconception perceived in relation to fecundability (Akhter et al., 2016). After adjustment of confounding factors, we found a higher but non-significant association between preconception daily perceived stress and fecundability among women with proven fertility. These findings held true regardless of whether perceived stress was assessed during the pre-ovulatory, pre-implantation, or specific fertile window. Our findings are consistent with one prior epidemiologic study that captured perceived stress at each preconception cycle (up to 6) via the Cohen’s Perceived Stress Scale (PSS) (Cohen et al., 1983) and found no significant association (Lynch et al., 2012).
While the PSS (Cohen et al., 1983) has been widely used around the world to study the role of chronic psychological stress in the etiology of adverse health outcomes, several researchers have been questioning its appropriateness for studies assessing women’s menstrual cycle and fecundability (Lynch et al., 2012; Nakamura et al., 2008; Schliep et al., 2015). Given that reproductive outcomes are known to be sensitive to critical windows of exposure, measuring daily perceived stress over the course of the menstrual cycle may more precisely capture the physical responses to daily hassles in women compared to measuring a single baseline stress (Akhter et al., 2016; Bolger et al., 2003; Schliep et al., 2015). Indeed, one recent study, conducted among 259 premenopausal women found a significant inverse relationship between daily perceived stress and total estradiol, luteal progesterone, and ovulatory function, whereas there was no association when using the baseline PSS-14 or the cycle PSS-4 (Schliep et al., 2015). Furthermore, in another recent study assessing the effect of daily perceived stress on fecundability among 400 women from the US (Akhter et al., 2016), higher stress after ovulation was associated with increased probability of conception (FOR:1.63, 95% CI:1.07–2.50) but there was a significantly reduced fecundability among women who had higher stress during the estimated ovulatory window. While we did not find similar opposing direction of effects as Akhter et al in our study, we did find a stronger magnitude of effect of daily stress in the pre-ovulatory versus pre-implantation window, highlighting the need to capture stress during critical windows of exposure.
One of the strongest confounding factors that arose from our analysis was the impact of cycle intent to conceive. When examining the effect of modifiable risk factors on women’s fecundability, many researchers assume that couples enrolled in preconception studies will always intend to conceive and consequently engage in intercourse over the entire study period. However, as we observed in our study, participant’s intent to conceive can vary by cycle and impact the couple’s fecundability (Stanford et al., 2014). While one previous study adjusted for an overall intent to conceive (Akhter et al., 2016), in our statistical models, we adjusted for cycle intent to conceive, which had a sub-stantial impact on the results of the model towards a stronger positive association.
Our study had many strengths including prospective design, repeated measures of stress exposure, assessment of many important confounders, and a rigorous application in estimating the effect of various critical windows of perceived stress exposure on fertility outcome. Despite our limited sample size, our use of the daily diary for our stress exposure for up to 6 cycles assisted with precision in our effect estimates. Additionally, our sensitivity analyses accounting for missing information on perceived stress and covariates allowed us to minimize selection bias. In addition, by recruiting only couples of proven fertility, we were able to minimize reverse causality whereby subfertility causes the stress versus the stress leading to subfertility. Unlike previous studies that simply used preconception average stress levels, our study could evaluate the impact of time-varying stress across cycles on time to pregnancy.
Our study has some limitations. First, given that examining the association between stress and fecundability was not the primary objective of the study, we may be underpowered to detect statistically significant differences. However, given our overall pattern of improved fecundability with high versus low perceived stress among women with proven fertility (FORs all > 1.0), future studies among a larger cohort with similar characteristics should be carried out prior to drawing definitive conclusions. Additionally, we did not have data on baseline chronic stress, life stressors, nor on stress biomarkers, which would have given us a more detailed picture of how various forms of stress may impact fecundability and on the potential mechanism of action. However, epidemiologic research to date suggests that the HPA axis and SAM pathway may show an inconsistent reaction to different types of stressors (Louis et al., 2011; Lynch et al., 2012, 2014). Further, there is a poor correlation between salivary cortisol levels and scores on the PSS questionnaires (Lynch et al., 2012; van Eck et al., 1996), and perceived stress is not the only element that can influence salivary alpha-amylase levels (Lynch et al., 2014; Rohleder and Nater, 2009; Stegmann, 2011). Hence, further work is warranted looking into an interplay between perceived stress, responses to stress, stress biomarkers, and reproductive health.
Our study may have limited generalizability given that our study included healthy women in a relationship of proven fertility who were predominantly white, college-educated, with higher family income. Additionally, women from our study tended to consume less coffee and alcohol compared to other US adults of similar age (Loftfield et al., 2016; Substance Abuse and Mental Health Services Administration (SAMHSA, 2015; Daniels et al., 2004). One possible explanation of why we have failed to confirm our hypothesis of a negative impact of perceived stress on fecundability is that high stress among our sample may be relatively modest in comparison to other populations who may have higher daily stress due to adverse circumstances including poverty, disadvantage, and/or racism (Harville et al., 2009; Lynch et al., 2012). Aschbacher et al. reported that manageable stress, eustress exposure, may lead to beneficial or resilient health effects among individuals who experience episodic stress or lower intensity stress compared to the national average (Aschbacher et al., 2013). Thus, albeit non-significant, our findings of high/moderate stress being associated with increased fecundability among a cohort consisting of mostly white women of higher socioeconomic status is plausible. In addition, chronic stress accumulated in a woman’s life, in addition to cycle average stress, may be one of the major factors affecting the reproductive system that we were not able to measure (Lynch et al., 2012). Thus, future research studying the effects of stress on women’s reproductive health should be expanded to include women of less privileged backgrounds who may experience more severe or alternative forms of psychosocial stress.
5. Conclusions
In summary, among this cohort of predominantly healthy white, college-educated women, we found that perceived stress is not adversely associated with reduced fecundability. After adjusting for confounding variables, we found a positive but non-significant association between cycles with the highest tertile of stress and fecundability compared to cycles with the lowest tertiles of stress, most consistently for the pre-ovulatory window. Our findings highlight that applying cycle-varying covariates in the multivariable-adjusted models is important to assess the association of perceived stress and TTP. Finally, taking into consideration if women are actually intending to get pregnant for each cycle is critical when estimating the effect of modifiable risk factors on women’s fecundity.
Supplementary Material
Acknowledgements
The authors thank all women who participated in the Study of Time to Pregnancy in Normal Fertility. The authors also acknowledge Sydney Willis for her contribution to this work.
Funding
This work was supported by 1K23 HD0147901–01A1from the Eunice Kennedy Shriver National Institute for Child Health and Human Development, and by the Health Studies Fund, Department of Family and Preventive Medicine, University of Utah.
Footnotes
Declaration of Competing Interest
All authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.psyneuen.2019.104446.
References
- Akhter S, Marcus M, Kerber RA, Kong M, Taylor KC, 2016. The impact of periconceptional maternal stress on fecundability. Ann. Epidemiol. 26 (710–716), e717. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E, 2013. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38, 1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger N, Davis A, Rafaeli E, 2003. Diary methods: capturing life as it is lived. Annu. Rev. Psychol. 54, 579–616. [DOI] [PubMed] [Google Scholar]
- Catherino WH, 2011. Stress relief to augment fertility: the pressure mounts. Fertil. Steril. 95, 2462–2463. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW, 1998. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann. Intern. Med. 129, 229–240. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE, 2007. Psychological stress and disease. JAMA 298, 1685–1687. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. [PubMed] [Google Scholar]
- Cox DR, 2018. Analysis of Survival Data. Chapman & Hall, New York. [Google Scholar]
- Crowther MJ, Lambert PC, 2017. Parametric multistate survival models: flexible modelling allowing transition-specific distributions with application to estimating clinically useful measures of effect differences. Stat. Med. 36, 4719–4742. [DOI] [PubMed] [Google Scholar]
- Daniels M, Merrill RM, Lyon JL, Stanford JB, White GL Jr., 2004. Associations between breast cancer risk factors and religious practices in Utah. Prev. Med. 38 (1), 28–38. [DOI] [PubMed] [Google Scholar]
- Ferin M, 1999. Clinical review 105: stress and the reproductive cycle. J. Clin. Endocrinol. Metab. 84, 1768–1774. [DOI] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM, 2009. Stress questionnaires and stress biomarkers during pregnancy. J. Womens Health (Larchmt) 18, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzl H, Kaider A, 1997. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput. Methods Programs Biomed. 54, 201–208. [DOI] [PubMed] [Google Scholar]
- Loftfield E, Freedman ND, Dodd KW, Vogtmann E, Xiao Q, Sinha R, Graubard BI, 2016. Coffee drinking is widespread in the United States, but usual intake varies by key demographic and lifestyle factors. J. Nutr. 146, 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GM, Lum KJ, Sundaram R, Chen Z, Kim S, Lynch CD, Schisterman EF, Pyper C, 2011. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil. Steril. 95, 2184–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Jackson LW, Buck Louis GM, 2006. Estimation of the day-specific probabilities of conception: current state of the knowledge and the relevance for epidemiological research. Paediatr. Perinat. Epidemiol. 20 (Suppl 1), 3–12. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Sundaram R, Buck Louis GM, Lum KJ, Pyper C, 2012. Are increased levels of self-reported psychosocial stress, anxiety, and depression associated with fecundity? Fertil. Steril. 98, 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Sundaram R, Maisog JM, Sweeney AM, Buck Louis GM, 2014. Preconception stress increases the risk of infertility: results from a couple-based prospective cohort study–the LIFE study. Hum. Reprod. 29, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, Rice KC, Gravanis A, Chrousos GP, 2001. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat. Immunol. 2, 1018–1024. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sheps S, Arck PC, 2008. Stress and reproductive failure: past notions, present insights and future directions. J. Assist. Reprod. Genet. 25, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TM, Bagnell ME, 2012. Your “survival” Guide to Using Time-dependent Covariates SAS Global Forum. [Google Scholar]
- Radin RG, Rothman KJ, Hatch EE, Mikkelsen EM, Sorensen HT, Riis AH, Fox MP, Wise LA, 2015. Maternal recall error in retrospectively reported time-to-Pregnancy: an assessment and Bias analysis. Paediatr. Perinat. Epidemiol. 29, 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Nater UM, 2009. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology 34, 469–485. [DOI] [PubMed] [Google Scholar]
- Royston P, Parmar MK, 2002. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat. Med. 21, 2175–2197. [DOI] [PubMed] [Google Scholar]
- Schliep KC, Mumford SL, Vladutiu CJ, Ahrens KA, Perkins NJ, Sjaarda LA, Kissell KA, Prasad A, Wactawski-Wende J, Schisterman EF, 2015. Perceived stress, reproductive hormones, and ovulatory function: a prospective cohort study. Epidemiology 26, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JB, Smith KR, Varner MW, 2014. Impact of instruction in the Creighton model fertilitycare system on time to pregnancy in couples of proven fecundity: results of a randomised trial. Paediatr. Perinat. Epidemiol. 28, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann BJ, 2011. Other nonstress influences can alter salivary α-amylase activity. Fertil. Steril. 95, 2190–2191. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2015. National Survey on Drug Use and Health (NSDUH). [Google Scholar]
- Sullivan GM, Artino AR Jr., 2013. Analyzing and interpreting data from likert-type scales. J. Grad. Med. Educ. 5, 541–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J, 1996. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom. Med. 58, 447–458. [DOI] [PubMed] [Google Scholar]
- Wesselink AK, Hatch EE, Rothman KJ, Weuve JL, Aschengrau A, Song RJ, Wise LA, 2018. Perceived stress and fecundability: a preconception cohort study of north american couples. Am. J. Epidemiol. 187, 2662–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.