Abstract
Silicon (Si) plays an important role in plant nutrient capture and absorption, and also promotes plant mechanical strength and light interception in alpine meadows. In this study, we conducted a field experiment to examine the effect of nitrogen (N) application, with (N + Si) and without Si (N-only), on the potential for soil nutrient and the growth of grass and legume plant functional types (PFTs) in an alpine meadow. It was found that N + Si resulted in higher soil nutrient contents, leaf N and P concentrations, abundance and biomass of legume and grass PFTs than N-only. The aboveground biomass of grass (598 g m−2) and legume (12.68 g m−2) PFTs under 600 kg ha−1 ammonium nitrate (NH4NO3) per year addition with Si was significantly higher than that under the same level of N addition without Si (515 and 8.68 g m−2, respectively). The grass:legume biomass ratio did not differ significantly between the N + Si and N-only. This demonstrates that Si enhances N fertilization with apparently little effect on grass:legume ratio and increases plant-available nutrients, indicating that Si is essential for the plant community in alpine meadows.
Subject terms: Grassland ecology, Conservation biology
Introduction
Nitrogen (N) is often the primary limiting nutrient for plant growth in terrestrial ecosystems, especially in alpine meadows, where the low temperatures and short growing season limit plant growth and nutrient cycling, and thus N fertilization is widely used in these environments1–3. Nitrogen fertilization has been reported to influence ecosystems in a variety of ways, including changes in species diversity, biomass production, nutrient availability and soil conditions1,4–7.
Silicon (Si) is important for nutrition and nutrient cycling in soil and plants, especially for grassland environments8–10. In natural ecosystems, Si fertilization can increase plant growth, plant N use efficiency7,11–13 and alleviate the loss of biodiversity induced by N addition6,14. The availability of Si during plant growth not only modifies the concentration of nutrient ions such as N and phosphorus (P) in soils15–17, but also strongly affects growth and abundance of grass and legume species in grasslands16,18–20. Hence, Si could play an important role in plant community composition such as plant abundance and biomass ratios of different plant functional types (PFTs), but has received little research attention21–23.
Legume species are an important PFT in grassland. Legumes not only increase soil N and plant biomass7,12, but also maintain a balance of dominance of grass and other non-legume species in plant community. Therefore, management involving mixing legumes with grasses provides economic and environmental benefits and is considered a sustainable intensification in grassland17,19,24. However, to our knowledge, no studies have examined the impacts of N + Si on soil nutrient ions and growth of grass and legume PFTs (plant abundance, aboveground biomass, leaf N and P concentration) and abundance and biomass grass:legume ratios. In this study, we tested the hypothesis that there are high differences in growth of grass and legume PFTs and soil N and P concentration in an alpine meadow under different levels of N addition without Si (N-only), Si addition only and N added with Si (N + Si), and N + Si has a more beneficial effect than N-only. To test this hypothesis, our objectives were to (1) evaluate the effect of Si or N addition only and the interactive effect of N + Si addition simultaneously on the concentrations of soil ammonium (NH4+-N), nitrate (NO3−-N) and available P, (2) identify whether there were any different responses of the growth of grass and legume PFTs under the different fertilizers and (3) determine whether or not there is a beneficial interaction effect of N and Si addition on grass:legume abundance and biomass ratios.
Results
Soil nutrient ions and pH
The fertilization of N-only and N + Si did not affect the soil organic carbon (C) and total N concentrations. Addition of 600 kg ha−1 ammonium nitrate (NH4NO3) per year significantly reduced soil pH by 0.35, but Si addition did not affect soil pH (Table S1). There were no significant interaction effects between Si and N on soil pH (two-way ANOVA).
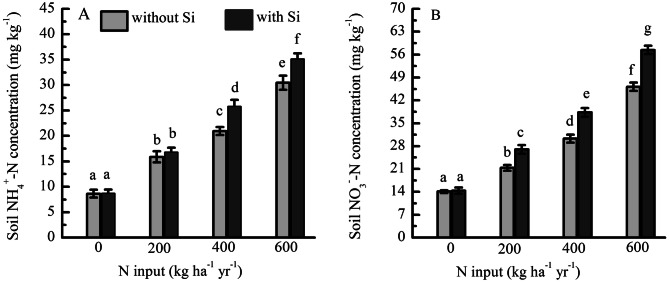
The fertilization of N-only and N + Si increased soil NH4+-N and NO3−-N concentrations (Fig. 1). The soil NH4+-N and NO3−-N concentrations increased by 84% and 52%, 142% and 115%, and 252% and 228% relative to the control (unfertilized) plot with the addition of 200, 400 and 600 kg ha−1 NH4NO3 only, respectively. The soil NH4+-N and NO3−-N concentrations increased by 93% and 88%, 196% and 167%, and 303% and 299% relative to the control plot with the addition of 200, 400 and 600 kg ha−1 NH4NO3 plus Si, respectively. The interaction between Si and N had significant effects on the soil NH4+-N (F = 36.52, P < 0.001) and NO3−-N (F = 110, P < 0.001) concentrations.
Figure 1.
(A) Soil NH4+-N and (B) NO3−-N concentrations under fertilization by N with Si (N + Si) and without Si (N-only) expressed as 3-year averages (2011–2013, N = 6).
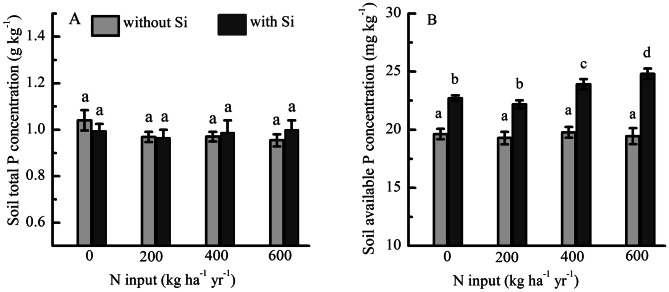
Experimental additions of N-only had no effect on soil total P concentration (F = 1.938, P = 0.156) (Fig. 2A) and soil available P (F = 0.159, P = 0.923) (Fig. 2B) relative to the control plot. The soil available P concentration increased significantly with increasing inputs of N + Si, with an increase of 15.70%, 20.83% and 27.51%, respectively, with addition of N + Si relative to the addition of N-only (Fig. 2B). There was a highly significant interaction between Si and N (F = 3.171, P = 0.034) in the soil available P concentration.
Figure 2.
(A) Soil total P and (B) available P concentrations under fertilization by N with Si (N + Si) and without Si (N-only) expressed as 3-year averages (2011–2013, N = 6).
Aboveground biomass, abundance of the grass and legume PFTs
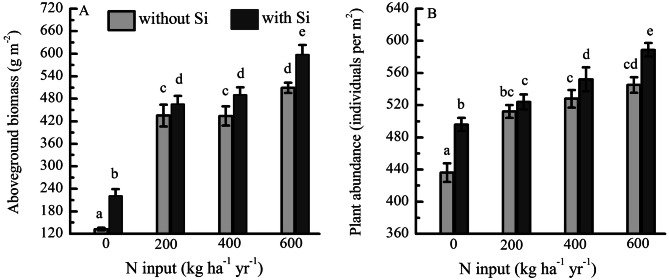
The aboveground biomass (from 133 to 509 g per m2) (Fig. 3A) and plant abundance (from 436 to 545 individuals per m2) (Fig. 3B) of the grass PFTs increased with increasing rates of N. The addition of N + Si led to a significantly higher aboveground biomass and abundance than addition N-only (Fig. 3). The aboveground biomass (589 g per m2) under N addition with 600 kg ha−1 NH4NO3 per year plus Si was significantly higher than that (509 g per m2) under the same level of N addition without Si. There were significant interaction effects between Si and N in the aboveground biomass and abundance (two-way ANOVA analysis).
Figure 3.
(A) Aboveground biomass and (B) abundance of grass PFTs under fertilization by N with Si (N + Si) and without Si (N-only) expressed as 3-year averages (2011–2013, N = 6).
The aboveground biomass (from 32.68 to 8.64 g per m2) (Fig. 4A) and abundance (from 350 to 78.52 per m2) (Fig. 4B) of the legume PFTs decreased with increasing rates of N application from control to N addition with 600 kg ha−1 NH4NO3. The aboveground biomass (12.68 g per m2) under N addition (600 kg ha−1 NH4NO3) with Si was significantly higher than that (8.68 g per m2) under the same level of N addition without Si. The interaction of N fertilization with Si had significant effects on plant abundance of the legume PFTs.
Figure 4.
(A) Aboveground biomass and (B) abundance of legume PFTs under fertilization by N with Si (N + Si) and without Si (N-only) expressed as 3-year averages (2011–2013, N = 6).
Leaf N and P concentration of grass and legume PFTs
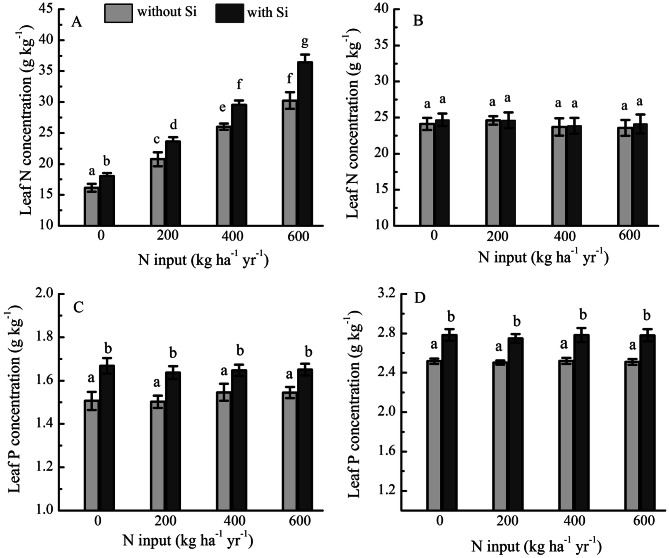
Leaf N (36.42 g kg−1) and P concentration (1.65 g kg−1) of grass and leaf P concentration (2.78 g kg−1) of legume PFTs under N addition (600 kg ha−1 NH4NO3) with Si was significantly higher than that under the same level of N addition only (leaf N and P concentration of grass PFTs is 30.25 g kg−1 and 1.54 g kg−1, and leaf P concentration of legume PFTs is 2.51 g kg−1 under 600 kg ha−1 NH4NO3 addition, respectively). Application of Si markedly increased leaf P concentration of both grass and legume PFTs (Fig. 5). There were significant interaction effects between Si and N fertilization in leaf N concentration (F = 4.641, P = 0.007) of grass PFTs (two-way ANOVA).
Figure 5.
(A, B) Leaf N concentration and (C, D) leaf P concentration of grass (left) and legume (right) PFTs under fertilization by N with Si (N + Si) and without Si (N-only) expressed as 3-year averages (2011–2013, N = 6).
Grass:legume abundance and aboveground biomass ratios
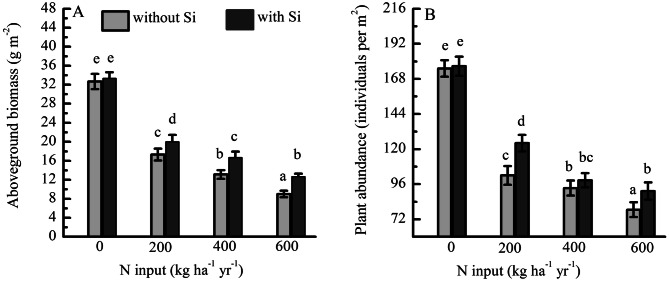
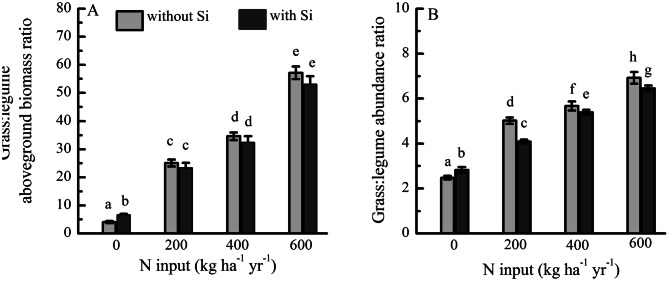
Grass:legume aboveground biomass ratio (4.31–57.17) and abundance ratio (2.48–6.92) increased significantly with increasing rates of N addition. Silicon addition resulted in a higher ratio of the aboveground biomass and plant abundance relative to the control plots. Compared with N addition, the addition of N + Si significantly decreased the plant abundance grass:legume ratio. There was no significant difference in the aboveground biomass grass:legume ratio between the addition of N-only and the addition of N + Si (Fig. 6).
Figure 6.
(A) Grass:legume ratio of aboveground biomass and (B) abundance under fertilization by N with Si (N + Si) and without Si (N-only) expressed as 3-year averages (2011–2013, N = 6).
Discussion
The addition of N did not affect the soil organic C (Table S1). This finding is consistent with several previous studies25–27, but contrasts with other reports28,29. The different findings may be attributed to (1) different amount and duration of N addition for the experimental duration. Sillen and Dieleman27 reported that moderate N additions promote belowground C decomposition processes, reducing the potential for increases soil C storage. Zhao et al.29 noted that 3-year optimization of N addition increase long-term C storage, for the experimental duration had a positive correlation with the response of soil organic carbon to N addition. (2) Different vegetation types and grassland degradation level for the response of soil C to N addition, which varied with vegetation types and the grassland degradation level25,29. For example, Zhao et al.29 noted that N addition increase soil C storage in degraded grasslands.
Silicon addition did not affect soil organic C (Table S1). This finding contrasts with recent reports23,30,31 that demonstrated that Si addition significantly increased soil total organic C and phytolith C sequestration. This could be because the short or long duration of the experiment for some studies found that Si plays an important role in regulating the global C balance and C turnover32,33. The processes of Si in regulating the C balance and C turnover involved in Si-enhanced soil organic C stability at a decadal scale include protection of soil organic C through amorphous Si33.
Our study also demonstrated that N fertilization did not affect soil total N. This finding was in line with a recent report28 but contrasted with other reports34. Several mechanisms could explain this discrepancy. Nitrogen addition results in N accumulation in aboveground biomass. The increasing aboveground biomass and N accumulation in aboveground biomass are often removed by livestock in vegetation types such as grasslands (alpine meadow, alpine steppe, and cultivated grassland)28, which is different from other vegetation types such as coniferous forest28. Gao et al. states that N addition promote soil total N accumulation of a cold-temperate coniferous forest34. Silicon addition did not affect soil total N (Table S1). This finding is inconsistent with another report that indicated that high Si addition considerably increased concentrations of N due to dissolved Si competing with other elements for binding sites at organic matter and mineral surfaces31. The different findings may be attributed to (1) different soil texture and (2) different Si fertilizer addition processes. Our study focused on Si-addition studies on the Qinghai–Tibetan Plateau, whereas Reithmaier et al.31 focused on influence of Si availability on the element concentrations in peatlands on northeastern Bavaria. Moreover, the Si was added directly to soil in our study, rather than through PVC tubes in Reithmaier et al.31.
Effect of N fertilization on pH decreased with N fertilization rate (Table S1), indicating that N fertilization induced soil acidification. This was probably because when an NH4+ ion is absorbed by plant roots, an H+ ion will be released into soil solution and cause soil acidification26,33–36. In contrast, NO3− anions lead to the loss of metal cations through their leaching based on the charge balance in soil solution37. Silicon addition did not affect soil pH since soil pH is usually very stable in soil38,39.
The soil NH4+-N and NO3−-N concentrations increased with the N addition rate (Fig. 1). These results are similar to previously reported N-addition experiments performed in this region and at other sites40,41. However, our findings contrasted with those of some other studies. For example, Gao et al.42 and Song et al.43 found that there were no significant effects on soil NH4+-N concentrations among control and N addition plots in an alpine meadow and a Korean pine plantation. The different findings may be caused by the different environmental conditions30,44. The present study showed that fertilization with N + Si increased the soil NH4+-N and NO3−-N concentrations. Reithmaier et al.31 similarly reported that the addition of Si increased the soil available N for Si can compete with other elements for binding sites at organic matter and mineral surfaces.
The study showed that N + Si increased the amount of soil available P. This may result from the following mechanisms. First, Si can directly increase the availability of P and decrease the P retention capacity of soil by mobilizing P from unavailable phases15,38,45. For example, Schaller et al.39 found that Si is positively related to P availability and is important for mobilizing P from previously unavailable phases. Second, Si availability mobilizes P from binding sites of soil minerals31,39. For example, some studies found that Si strongly competes with P for binding sites in Fe minerals, with a slightly lower binding affinity of silicic acid compared with P39.
The increases in the aboveground biomass and abundance of grass PFTs with increasing rates of N application confirm that they are limited or co-limited by the availability of N and P in alpine meadows (Fig. 3). This has been consistently observed in other studies of alpine meadows, which found that N addition generally increased aboveground biomass of grasses and sedges on the Qinghai–Tibetan Plateau2,6,46. Nitrogen plus Si application increases the aboveground biomass and abundance of grass PFTs. This was probably because Si helps to increase the erectness of leaves, which, in turn, reduced self-shading and increased the net photosynthetic rate, eventually leading to an increase in the abovegroundbiomass6,47. Some studies have shown that Si application increases the net photosynthesis rate and water use efficiency, while the expression of some key genes related to photosynthesis is increased with Si addition even under unstressed conditions13,30.
Higher N fertilization rates resulted in a significant reduction in abundance and aboveground biomass of legume PFTs (Fig. 4). This was because leguminous plants can increase N availability in soils by fixing atmospheric N via symbiotic rhizobia in an available form. Therefore, leguminous plants are adapted to low-N conditions48. This leads to a lower abundance of leguminous plants in N-fertilized soils1,6, which has been consistently observed in other studies of alpine meadows2,6,46. The application of N + Si had a higher plant abundance and aboveground biomass than N-only. Many studies have reported that Si addition can stimulate higher rates of photo-assimilate translocation, consequently enhancing C sink strength13,49. Furthermore, some studies have reported that plants that accumulate Si can increase their competitiveness in herbaceous communities under nutrient-enriched soil, leading to increased abundance of leguminous plants in the community20,48,50.
The leaf N and P concentrations of grass and the leaf P concentration of legume PFTs were higher with Si than without Si fertilization (Fig. 5). As suggested by the growth-rate hypothesis, higher N and P concentrations in plant leaves can achieve rapid growth, and thus the growth response of the grass and legume PFTs to Si fertilization observed here could be at least partially mediated by the effect of Si on the N use efficiency and the availability of P 13,39. This finding agrees with a previous report examining the role of Si in growth and leaf N and P concentrations of Phragmites australis16.
Our study found that fertilization Si only increased grass:legume abundance and biomass ratios compared with the control plot (Fig. 6), which may suggest that Si can promote the growth of both grass and legumes. Our results also confirm that the addition of N + Si not only increased aboveground biomass of grass and legume PFTs, but also maintained a constant grass:legume ratio of aboveground biomass. Johnson et al.18 and Mali et al.51 observed that Si addition was beneficial not only for nodule growth but also for plant growth in terms of relative yield of root and shoot, and the increasing in root yield lead to an enhancement of root nodulation18. Furthermore, Si and N addition can affect grass and legume biomass production, nutrient content, and the relationship between legumes and grasses in grassland, leading to changes in plant species coexistence in the community15–17,51. These results suggest that application of N + Si alleviates the shift in the species composition, and promotes coexistence of grass and legume species, especially at high levels of N addition.
Grass and legume mixtures in a community can give a considerable advantage in terms of both productivity and resource utilization and widely used in grassland ecosystems25,52. For example, Xu et al.53 reported that when the grass was mixed with the legume species, the capture and absorption of N and P by the grass were greatly improved. Our study suggests that the addition of N + Si increases the amount of available P, NH4+-N and NO3−-N in the soil and the aboveground biomass of grasses and legumes relative to addition N only. This not only helps the legume to maintain its advantage in the plant community, but also improves the capture and absorption of N and P in grass and P in legume species. These results together show that Si enhances N fertilization with apparently few effects on grass:legume ratios and benefits availability of nutrients to plants, suggesting that Si is essential for the plant community in alpine meadows.
Materials and methods
Study site
This study was conducted in an alpine meadow at an elevation of 3,500 m above sea level on average in the Research Station of Alpine Meadow and Wetland Ecosystems of Lanzhou University, which is located on the eastern Qinghai–Tibetan Plateau (33°58′N, 101°53′E)6. The mean annual temperature is 1.2 °C and the mean annual precipitation is 620 mm on average from 1969 to 2005, mainly falling during the short and cool summer in this area. The plant community in this region is a typical alpine meadow with an alpine meadow soil. Soil organic matter contents, total nitrogen, total phosphorus and pH values are 70.52 g kg−1, 3.72 g kg−1, 0.98 g kg−1 and 6.33, respectively.
Experimental design
In this study, the experiment was laid out inside a fence to exclude disturbance by grazing (yak and Tibetan sheep) during the plant growth season and laid out in a completely randomized block design in early May 2011. Six 16 m × 30 m blocks were selected and eight 5 m × 5 m plots were established in each block. All plots were separated by a 2 m buffer of unfertilized strips (48 plots in total, with six blocks per site). The experimental design included four different levels of N fertilization (0, 200, 400 and 600 kg ha−1 ammonium nitrate (NH4NO3) per year), comprising simultaneous fertilization in the plots without Si (N-only) (0 kg ha−1 H4SiO4 per year) or with Si (N + Si) (40 kg ha−1 hydrated silica (H4SiO4) per year). The fertilization took place on rainy days in early May in each year when rainfall was abundant in this region.
Soil sampling collection and analyses
Soil samples were collected in August of 2011, 2012 and 2013 for soil nutrient analysis. The topsoil samples (0–20 cm) were collected using a bucket auger (diameter 3.8 cm, depth 20 cm) from three sites chosen at random within each plot and mixed to give a single sample. The soil samples were air-dried and any visible roots and stones removed, and then passed through a 1-mm mesh sieve. The Walkley–Black dichromate oxidation method was used to determine the soil organic C concentration. The concentrations of soil total P and available P were measured using an inductively coupled plasma spectrometer (ICP; SPECTRO ARCOS EOP, Germany). Soil total N, NH4+-N and NO3−-N concentrations were determined following the same approach as Han et al.26. Soil pH was measured using glass electrode in the supernatant by homogeneously mixing 5 g of soil and 25 ml of water.
The growth of grass and legume PFTs
Plant samples were investigated from one 0.5 m × 0.5 m quadrat in every 5 m × 5 m plot when peak biomass was reached, including plant abundance and aboveground biomass. The number of all grass PFTs or of all legume PFTs was counted as grass abundance or legume abundance, respectively. All plants in each quadrat were clipped at the surface of the soil and plants from the two PFTs were selected. The dry aboveground biomass of the two PFTs in every quadrat was weighed after oven-drying at 70 °C for 48 h. The grass:legume biomass ratio was calculated as the aboveground biomass of grass PFTs/aboveground biomass of legume PFTs and the grass:legume abundance ratio was calculated as abundance of grass PFTs/abundance of legume PFTs.
Statistical analysis
Before taking the averages of the 3 years of data (2011–2013), two-way ANOVAs (year, fertilization treatment, and interaction) were performed to determine whether there was an interaction effect of year and it was found that year had no effect on the results. Therefore, all data were presented as mean ± standard deviations of 3-year averages with six replicates in the figures. We used fertilizer amounts of N as one variable, and + or − Si as the second variable. Two-way ANOVAs (N amounts, Si, and interaction) were used to obtain an interaction effect between level of N and Si. In these cases, we then proceeded with multiple comparison tests to compare differences among means using LSD test at P < 0.05. Statistical analysis was conducted using SPSS 18.0 for windows (SPSS Inc., Chicago, IL, USA).
Supplementary information
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2016YFC0501906) and the National Natural Sciences Foundation of China (Nos. 31860176 and 30900171). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
X.D.H. and G.T.P. conceived the study and wrote most of the manuscript, B.H.Y, L.Q.X and W.X.N did the soil and plant sampling, F.X.W and Z.R.Y did the measurements and wrote the part of the manuscript. All co-authors contributed to and commented the manuscript.
Competing interests
The authors declare no competing interests (include financial and non-financial interests).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67333-7.
References
- 1.Craine JM, Froehle J, Tilman DG, Wedin DA, Chapin FS. The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos. 2001;93:274–285. doi: 10.1034/j.1600-0706.2001.930210.x. [DOI] [Google Scholar]
- 2.Song MH, Yu FH, Ouyang H, Cao GM, Xu XL, Cornelissen JHC. Different responses to availability and form of nitrogen in space and time explain species coexistence in an alpine meadow community after release from grazing. Glob. Change Biol. 2012;18:3100–3111. doi: 10.1111/j.1365-2486.2012.02738.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhou XM. Alpine Kobresia meadows in China. Beijing: Science Press; 2001. pp. 51–62. [Google Scholar]
- 4.Fujita Y, Robroek BJM, de Ruiter PC, Heil GW, Wassen MJ. Increased N affects P uptake of eight grassland species: The role of root surface phosphatase activity. Oikos. 2010;119:1665–1673. doi: 10.1111/j.1600-0706.2010.18427.x. [DOI] [Google Scholar]
- 5.Güsewell S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004;164:43–266. doi: 10.1111/j.1469-8137.2004.01192.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu DH, Gao XG, Gao TP, Mou J, Li JH, Bu HY, Zhang RY, Li QX. Interactive effects of nitrogen and silicon addition on growth of five common plant species and structure of plant community in alpine meadow. CATENA. 2018;169:80–89. doi: 10.1016/j.catena.2018.05.017. [DOI] [Google Scholar]
- 7.Dhamala NR, Rasmussen J, Carlsson G, Søegaard K, Eriksen J. N transfer in three-species grass-clover mixtures with chicory, ribwort plantain or caraway. Plant Soil. 2017;413:217–230. doi: 10.1007/s11104-016-3088-6. [DOI] [Google Scholar]
- 8.Schaller J, Struyf E. Silicon controls microbial decay and nutrient release of grass litter during aquatic decomposition. Hydrobiologia. 2013;709:201–212. doi: 10.1007/s10750-013-1449-1. [DOI] [Google Scholar]
- 9.Schaller J, Hines J, Brackhage C, Baucker E, Gessner MO. Silica decouples fungal growth and litter decomposition without changing responses to climate warming and N enrichment. Ecology. 2014;95:3181–3189. doi: 10.1890/13-2104.1. [DOI] [Google Scholar]
- 10.Marxen A, et al. Interaction between silicon cycling and straw decomposition in a silicon deficient rice production system. Plant Soil. 2016;398:153–163. doi: 10.1007/s11104-015-2645-8. [DOI] [Google Scholar]
- 11.Sommer M, Kaczoek D, Kuzyakov Y, Breuer J. Silicon pools and fluxes in soils and landscapes—a review. J. Plant Nutr. Soil Sci. 2006;169:310–329. doi: 10.1002/jpln.200521981. [DOI] [Google Scholar]
- 12.Bruning B, Rozema J. Symbiotic nitrogen fixation in legumes: Perspectives for saline agriculture. Environ. Exp. Bot. 2013;92:134–143. doi: 10.1016/j.envexpbot.2012.09.001. [DOI] [Google Scholar]
- 13.Detmann KC, Araujo WL, Martins SCV, Sanglard LMVP, Reis JV, Detmann E, Rodrigues FÁ, Nunes-Nesi A, Fernie AR, DaMatta FM. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012;196:752–762. doi: 10.1111/j.1469-8137.2012.04299.x. [DOI] [PubMed] [Google Scholar]
- 14.Xu DH, Fang XW, Zhang RY, Gao TP, Bu HY, Du GZ. Influences of nitrogen, phosphorus and silicon addition on plant productivity and species richness in an alpine meadow. AoB Plants. 2015;7:plv125. doi: 10.1093/aobpla/plv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neu S, Schaller J. Dudel EG (2017) Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.) Sci. Rep. 2017;7:40829. doi: 10.1038/srep40829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaller J, Brackhage C, Gessner MO, Bäker E, Dudel EG. Silicon supply modifies C:N:P stoichiometry and growth of Phragmites australis. Plant Biol. 2012;14:392–396. doi: 10.1111/j.1438-8677.2011.00537.x. [DOI] [PubMed] [Google Scholar]
- 17.Schaller J, Roscher C, Hillebrand H, Weigelt A, Oelmann Y, Wilcke W, Ebeling A, Wolfgang WW. Plant diversity and functional groups affect Si and Ca pools in aboveground biomass of grassland systems. Oecologia. 2016;182:277–286. doi: 10.1007/s00442-016-3647-9. [DOI] [PubMed] [Google Scholar]
- 18.Johnson SN, Hartley SE, Ryalls JMW, Frew A, DeGabriel JL, Duncan M, Gherlenda AN. Silicon-induced root nodulation and synthesis of essential amino acids in a legume is associated with higher herbivore abundance. Funct. Ecol. 2017;31:1903–1909. doi: 10.1111/1365-2435.12893. [DOI] [Google Scholar]
- 19.Schaller J, Hodson MJ, Struyf E. Is relative Si/Ca availability crucial to the performance of grassland ecosystems? Ecosphere. 2017;8:e01726. doi: 10.1002/ecs2.1726. [DOI] [Google Scholar]
- 20.Schoelynck J, Müller F, Vandevenne F, Bal K, Barão L, Smis A, Opdekamp W, Meire P, Struyf E. Silicon–vegetation interaction in multiple ecosystems: A review. J. Veg. Sci. 2014;25:301–313. doi: 10.1111/jvs.12055. [DOI] [Google Scholar]
- 21.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002;16:545–556. doi: 10.1046/j.1365-2435.2002.00664.x. [DOI] [Google Scholar]
- 22.Seyfferth AL, Fendorf S. Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.) Environ. Sci. Technol. 2012;46:13176–13183. doi: 10.1021/es3025337. [DOI] [PubMed] [Google Scholar]
- 23.Song Z, Liu H, Zhao F, Xu C. Ecological stoichiometry of N:P:Si in China’s grasslands. Plant Soil. 2014;380:165–179. doi: 10.1007/s11104-014-2084-y. [DOI] [Google Scholar]
- 24.Suter M, Connolly J, Finn JA, Loges R, Kirwan L, Sebastià MT, Lüscher A. Nitrogen yield advantage from grass-legume mixtures is robust over a wide range of legume proportions and environmental conditions. Glob. Change Biol. 2015;21:2424–2438. doi: 10.1111/gcb.12880. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Dong S, Zhao Z, Sha W, Li S, Shen H, Xiao J, Zhang J, Wu X, Jiang X, Zhao J, Liu S, Dong Q, Zhou H, Jane CY. Response of soil nutrients and stoichiometry to elevated nitrogen deposition in alpine grassland on the Qinghai–Tibetan Plateau. Geoderma. 2019;343:263–268. doi: 10.1016/j.geoderma.2018.12.050. [DOI] [Google Scholar]
- 26.Fu G, Shen ZX. Response of alpine soils to nitrogen addition on the Tibetan Plateau: A meta-analysis. Appl. Soil Ecol. 2017;114:99–104. doi: 10.1016/j.apsoil.2017.03.008. [DOI] [Google Scholar]
- 27.Sillen WMA, Dieleman WIJ. Effects of elevated CO2 and N fertilization on plant and soil carbon pools of managed grasslands: A meta-analysis. Biogeosciences. 2012;9:2247–2258. doi: 10.5194/bg-9-2247-2012. [DOI] [Google Scholar]
- 28.Malhi SS, Harapiak JT, Nyborg M, Gill KS, Monreal CM, Gregorich EG. Total and light fraction organic C in a thin Black Chernozemic grassland soil as affected by 27 annual applications of six rates of fertilizer N. Nutr. Cycl. Agroecosyst. 2003;66:33–41. doi: 10.1023/a:1023376905096. [DOI] [Google Scholar]
- 29.Zhao Y, Song Z, Xu X, Liu H, Wu X, Li Z, Guo F, Pan W. Nitrogen application increases phytolith carbon sequestration in degraded grasslands of North China. Ecol. Res. 2016;31:117–123. doi: 10.1007/s11284-015-1320-0. [DOI] [Google Scholar]
- 30.Ji Z, Yang X, Song Z, Liu H, Liu X, Qiu S, Li J, Guo F, Wu Y, Zhang X. Silicon distribution in meadow steppe and typical steppe of northern China and its implications for phytolith carbon sequestration. Grass Forage Sci. 2018;73:482–492. doi: 10.1111/gfs.12316. [DOI] [Google Scholar]
- 31.Reithmaier GMS, Knorr KH, Arnhold S, Planer-Friedrich B, Schaller J. Enhanced silicon availability leads to increased methane production, nutrient and toxicant mobility in peatlands. Sci. Rep. 2017 doi: 10.1038/s41598-017-09130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu M, Yang YQ, Fang CM, Zhou XH, Chen JK, Yang X, Li B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2011;189:1040–1050. doi: 10.1111/j.1469-8137.2010.03563.x. [DOI] [PubMed] [Google Scholar]
- 33.Song Z, Liu C, Müller K, Yang X, Wu Y, Wang H. Silicon regulation of soil organic carbon stabilization and its potential to mitigate climate change. Earth Sci. Rev. 2018;185:463–475. doi: 10.1016/j.earscirev.2018.06.020. [DOI] [Google Scholar]
- 34.Gao WL, Zhao W, Yang H, Yang HJ, Chen GQ, Luo YC, Fang HJ, Li SG. Effects of nitrogen addition on soil inorganic N content and soil N mineralization of a cold-temperate coniferous forest in Great Xing'an Mountains. Acta Ecol. Sin. 2015;35:130–136. doi: 10.1016/j.chnaes.2015.07.003. [DOI] [Google Scholar]
- 35.Finzi AC, Canham CD, Van Breemen N. Canopy tree–soil interactions within temperate forests: Species effects on pH and cations. Ecol. Appl. 1998;8:447. doi: 10.2307/2641083. [DOI] [Google Scholar]
- 36.Tian D, Niu S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015;10:024019. doi: 10.1088/1748-9326/10/2/024019. [DOI] [Google Scholar]
- 37.Rothwell JJ, Futter M, Nand Dise NB. A classification and regression tree model of controls on dissolved inorganic nitrogen leaching from European forests. Environ. Pollut. 2008;156:544–552. doi: 10.1016/j.envpol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Kostic L, Nikolic N, Bosnic D, Samardzic J, Nikolic M. Silicon increases phosphorus (P) uptake by wheat under low P acid soil conditions. Plant Soil. 2017;419:447–455. doi: 10.1007/s11104-017-3364-0. [DOI] [Google Scholar]
- 39.Schaller J, Faucherre S, Joss H, Obst M, Goeckede M, Planer-Friedrich B, Peiffer S, Gilfedder B, Elberling B. Silicon increases the phosphorus availability of Arctic soils. Sci. Rep. 2019;9:449. doi: 10.1038/s41598-018-37104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawar K, Zaman M, Rowarth JS, Blennerhassett J, Turnbull MH. Urease inhibitor reduces N losses and improves plant-bioavailability of urea applied in fine particle and granular forms under field conditions. Agric. Ecosyst. Environ. 2011;144:41–50. doi: 10.1016/j.agee.2011.08.007. [DOI] [Google Scholar]
- 41.Zhang J, Yan X, Su F, Li Z, Wang Y, Wei Y, Ji Y, Zhou X, Guo H, Hu S. Long-term N and P additions alter the scaling of plant nitrogen to phosphorus in a Tibetan alpine meadow. Sci. Total Environ. 2018;625:440–448. doi: 10.1016/j.scitotenv.2017.12.292. [DOI] [PubMed] [Google Scholar]
- 42.Gao Y, Ma X, Cooper DJ. Short-term effect of nitrogen addition on nitric oxide emissions from an alpine meadow in the Tibetan plateau. Environ. Sci. Pollut. Res. 2016;23:12474–12479. doi: 10.1007/s11356-016-6763-5. [DOI] [PubMed] [Google Scholar]
- 43.Song L, Tian P, Zhang J, Jin G. Effects of three years of simulated nitrogen deposition on soil nitrogen dynamics and greenhouse gas emissions in a Korean pine plantation of northeast China. Sci. Total Environ. 2017;609:1303–1311. doi: 10.1016/j.scitotenv.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Tian L, Zhao L, Wu X, Fang H, Zhao Y, Yue G, Liu G, Chen H. Vertical patterns and controls of soil nutrients in alpine grassland: Implications for nutrient uptake. Sci. Total Environ. 2017;607–608:855–864. doi: 10.1016/j.scitotenv.2017.07.080. [DOI] [PubMed] [Google Scholar]
- 45.Ma JF, Takahashi E. Effect of silicate on phosphate availability for rice in a P-deficient soil. Plant Soil. 1991;133:151–155. doi: 10.1007/BF00009187. [DOI] [Google Scholar]
- 46.Xu XL, Wanek W, Zhou CP, Richter A, Song MH, Cao GM, Kuzyakov Y. Nutrient limitation of alpine plants: Implications from leaf N:P stoichiometry and leaf δ15N. J. Plant Nutr. Soil Sci. 2014;177:378–387. doi: 10.1002/jpln.201200061. [DOI] [Google Scholar]
- 47.Ma JF, Mitani N, Nagao S, Konishi S, Tamai K, Iwashita T, Yano M. Characterization of the silicon uptake system and molecular mapping of the silicon transporter gene in rice. Plant Physiol. 2004;136:3284–3289. doi: 10.1104/pp.104.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang BJ, Qiao N, Xu XL, Ouyang H. Symbiotic nitrogen fixation by legumes in two Chinese grasslands estimated by 15N dilution technique. Nutr. Cycl. Agroecosyst. 2011;91:91–98. doi: 10.1007/s10705-011-9448-y. [DOI] [Google Scholar]
- 49.Li ZC, Song ZL, Yang XM, Song AL, Yu CX, Wang T, Xia SP, Liang YC. Impacts of silicon on biogeochemical cycles of carbon and nutrients in croplands. J. Integr. Agric. 2018;17:2182–2195. doi: 10.1016/S2095-3119(18)62018-0. [DOI] [Google Scholar]
- 50.Epstein E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009;155:155–160. doi: 10.1111/j.1744-7348.2009.00343.x. [DOI] [Google Scholar]
- 51.Mali M, Aery NC. Silicon effects on nodule growth, dry-matter production, and mineral nutrition of cowpea (Vigna unguiculata) J. Plant Nutr. Soil Sci. 2008;171:835–840. doi: 10.1002/jpln.200700362. [DOI] [Google Scholar]
- 52.Liu ZP, Shao MA, Wang YQ. Spatial patterns of soil total nitrogen and soil total phosphorus across the entire loess plateau region of China. Geoderma. 2013;197–198:67–78. doi: 10.1016/j.geoderma.2012.12.011. [DOI] [Google Scholar]
- 53.Xu BC, Gao ZJ, Wang J, Xu WZ, Palta JA, Chen YL. N:P ratio of the grass Bothriochloa ischaemum mixed with the legume Lespedeza davurica under varying water and fertilizer supplies. Plant Soil. 2016;400:67–79. doi: 10.1007/s11104-015-2714-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.