Abstract
Aux/IAA genes are early auxin-responsive genes and essential for auxin signaling transduction. There is little information about Aux/IAAs in the agriculturally important cereal, barley. Using in silico method, we identified and subsequently characterized 36 Aux/IAAs from the barley genome. Based on their genomic sequences and the phylogenic relationship with Arabidopsis and rice Aux/IAA, the 36 HvIAAs were categorized into two major groups and 14 subgroups. The indication of the presence or absence of these domains for the biological functions and acting mechanisms was discussed. The cis-element distributions in HvIAA promoters suggests that the HvIAAs expressions may not only regulated by auxin (the presence of AuxREs and TGA-element) but also by other hormones and developmental and environmental cues. We then studied the HvIAAs expression in response to NAA (1-Naphthaleneacetic acid) using quantitative real-time PCR (qRT-PCR). Like the promoter analysis, only 14 HvIAAs were upregulated by NAA over two-fold at 4 h. HvIAAs were clustered into three groups based on the spatiotemporal expression data. We confirmed by qRT-PCR that most HvIAAs, especially HvIAA3, HvIAA7, HvIAA8, HvIAA18, HvIAA24 and HvIAA34, are expressed in the developing barley spike compared within seedling, suggesting their roles in regulating spike development. Taken together, our data provide a foundation for further revealing the biological function of these HvIAAs.
Subject terms: Plant breeding, Plant development, Plant domestication, Plant genetics, Plant signalling
Introduction
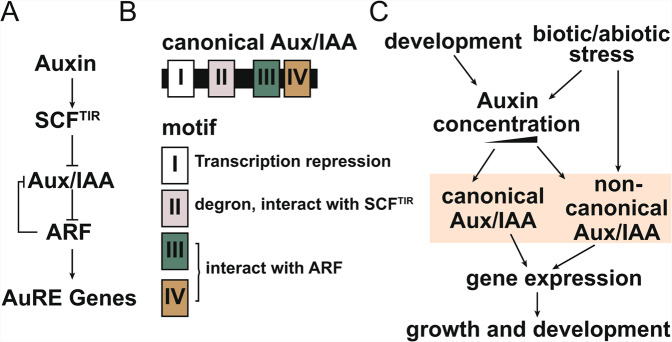
Auxin plays a vital regulatory role in plant growth and development processes. Aux/IAAs belong to primary/early auxin-response genes including GH3 (Gretchen Hagen 3) and SAUR (small auxin up RNA)1,2. The transcription of these genes responds to auxin treatment quickly and they play an important role at the early stage in auxin signal transduction. Aux/IAAs inhibit the function of the transcription factors ARFs (auxin response factor) by the physical interaction with ARFs3. The SCFTIR1 protein complex (Auxin Transport Inhibitor Response 1-SKP1-Cullin-F-box complex) can sense auxin and degrade the Aux/IAAs expression level through auxin concentration dependent ubiquitin-mediated pathway4. Therefore, the Aux/IAAs mediate the release of ARFs with auxin level to activate auxin response gene expression (Fig. 1A)5.
Figure 1.
Aux/IAAs participate in the auxin signaling pathway. (A) The canonical auxin pathway. (B) The structure of canonical Aux/IAAs. (C) Aux/IAAs integrate the development and environment cues to modulate growth and development. SCFTIR: Auxin Transport Inhibitor Response 1- SKP1-Cullin-F-box complex; ARF: Auxin Response Factor; AuRE genes: Auxin response gene; I, II, III and IV: the conserved domain of Aux/IAAs.
The canonical Aux/IAA proteins contain four highly conserved domains (domains I-IV), which underlie the functional properties of these proteins (Fig. 1B)6. The domain I of N-terminal acts as a transcriptional repressor, it has an epistatic effect on the transcriptional activation of ARF7. Domain II has a specific sequence containing 13 amino acids, forming a degron to regulate the stability of Aux/IAA protein through interaction with ubiquitination complex TIR18,9. The half-life of Aux/IAAs varies from 10 minutes to several hours depends majorly on the property of domain II10. Some Aux/IAA proteins carrying mutations in domain II have a longer half-life and are insensitive to auxin2,5. The C-terminal domains III and IV of Aux/IAA share homology with domains of ARF, which renders the polymerization of Aux/IAA and ARF thus inhibiting the ARF function11,12. These Aux/IAAs lacking at least one conserved domain are regarded as non-canonical Aux/IAAs10. The emergence of non-canonical Aux/IAAs seems to be an ancient evolution event and important for plant adaption to different environment, as evident that non-canonical Aux/IAAs are shown to be presented in the Aux/IAA gene family in various plants13–18. The mechanism of non-canonical Aux/IAA proteins function is gaining attention. Two independent groups showed that non-canonical Aux/IAAs, IAA32, IAA33 and IAA34, act on the high concentration of auxin, and in a separate pathway than the canonical SCFTIR pathway19,20. In rice, OsIAA26 with amino acids substitution in the degron in domain II works downstream of the canonical Aux/IAA OsIAA9 to integrate auxin and ethylene signaling21. It is also plausible that the non-canonical Aux/IAAs work together with the canonical Aux/IAAs to integrate the auxin pathway with multiple signaling cascades (Fig. 1C)20,21. Therefore, the domain composition may reveal the functions and downstream mechanisms of the Aux/IAAs.
Previous studies indicated that auxin is critical nearly in every aspect of plant development processes, including cell division, embryogenesis, lateral root initiation, vascular bundle extension, leaf extension, flowering patterning, fruit ripening, apical dominance, tropic growth and stress resistance15,22–25. The functional mechanism of Aux/IAA in various biological processes in plant growth and deployment has been well summarized carefully as in a canonical auxin pathway, especially in the Arabidopsis22,26,27. Apart from the role of trapping ARF, Aux/IAAs may also act as a hub to integrate other environmental cues28,29. For example, the screening for the suppressor of the phytochrome chromophore-deficient mutant hy2 isolated a dominant shy2/iaa3 mutant, suggesting the Aux/IAAs participating in the light signaling30. The mutation of AXR2/IAA7 caused increased susceptibility to the necrotrophic fungi Plectosphaaerella cucumerina and Botrytis cinerea31. Rice OsIAA10 was found to be hijacked by a Rice dwarf virus protein to enhance viral infection and pathogenesis32. Moreover, researches of Aux/IAAs in crops provided us new insights on the roles of Aux/IAAs in some species unique developmental processes which were largely neglected before, such as rice aerenchyma formation33 and maize tassels and ears formation34. In order to crack the complexity of elucidating the function of IAAs, their expression patterns are needed as a road map.
According to FAOSTAT (The Food and Agriculture Organization Corporate Statistical Database), barley is the fourth largest cereal crop (barley is grown in about 70 million hectares in the world) and is used for animal feed and malt. Genome-wide study of the barley Aux/IAA gene family has not yet been reported. We think that a comprehensive analyze of barley Aux/IAAs may help us analysis important agricultural traits associated with auxin in reverse genetics. As noticed by Youssef and Hansson35, auxin may play a major role in hormone crosstalk at the basal section of the spike. It would be interesting to test what are the roles of HvIAA in spikelet development.
The release of the barley genomic data36 enables us to isolate the Aux/IAA gene family in barley. We further performed a detailed analysis of sequence alignment, phylogenetic relationship, chromosome locations, gene structure, conserved domains, cis-acting elements, different expression patterns during 15 tissues based on the RNA-seq data, in response to NAA treatment condition and spike development. Our research revealed the expression of Aux/IAAs during barley spike development and the expressional response to exogenous auxins. These results provide clues for the functional characterization of HvIAA involved in the development of barley spike.
Materials and methods
Identification of Aux/IAA gene family in barley
Arabidopsis AtIAAs and rice OsIAAs protein sequences were downloaded from The Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/)13 and Rice Genome Annotation Project (RGAP) (http://rice.plantbiology.msu.edu/)37, respectively. The barley protein and nucleotide sequences were obtained from the plant genomics database Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html)36. The HMM (Hidden Markov Model) file was constructed based on the multiple sequence alignment of rice Aux/IAA protein by HMMER 3.0 software, and Aux/IAAs were queried in barley protein database (E-value less than e-10)38,39. A total of 42 barley proteins identified in this initial search were analysed using the HMM profiles of the Aux/IAA protein family Pfam02309 (AUX/IAA Superfamily: cl03528) with E-value less than e-5. As some ARF protein family proteins share conserved C-terminals with Aux/IAA family proteins (discussed in the introduction), the initial found 42 barley proteins consist some ARF proteins with conserved B3 DNA binding domain (pfam02362) and AUX/RESP domain (pfam06507)40–42. We eliminated these proteins as well as the redundant proteins and retained 36 proteins for further study.
Primary sequence analysis
The isoelectric points, protein molecular weights and amino acids were obtained from ProtParam (https://web.expasy.org/protparam/)43, the Intro position was downloaded from Phytozome (http://www.phytozome.net)44. The Open Reading Frame (ORF) were obtained from the Sequence Manipulation Suite ORF Finder (http://www.bioinformatics.org/sms2/orf_find.html)45.
Phylogenetic analysis
The phylogenetic tree was constructed with the Aux/IAA proteins from barley, rice, and Arabidopsis by MEGA 7.0 using the Neighbor Joining (NJ) method with 1,000 bootstrap replicates46, and modified with iTOL (https://itol.embl.de/itol.cgi).
Analysis of gene structure and conserved motifs
For exon/intron structure of HvIAAs analysis, the CDS and protein sequences corresponding to each predicted gene were downloaded from Barley Genome Database Annotation on the Phytozome website. Multiple sequences alignments with barley (Hordeum vulgare L.) were conducted by Clustal W and Clustal X version 2.0 program of Jalview 2.11.0 software with Defaults47,48. The Multiple Expectation Maximization for motif Elicitation (MEME, http://meme-suite.org/tools/meme) tool was used to predict conserved motifs of HvIAA proteins49. The gene structure and conserved motifs were generated with TBtools software50.
Analysis of chromosome locations and cis-acting elements
Chromosome physical position of HvIAAs was obtained from Phytozome36, using MapGene2Chrom web v2 (http://mg2c.iask.in/mg2c_v2.0/) to draw chromosome physical map. The identified CDS sequence of the HvIAAs was downloaded from Phytozome, and translated into protein sequences, using Muscle program alignment, and then introduced into the DnaSP 6 software to calculate the Ka and Ks values among the sequences of paralogous genes51. Super Circos was generated using TBtools software. The cis-elements in the 2,000 bp sequences upstream of the coding sequences were analyzed by Plant CARE databases (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Expression analysis of barley Aux/IAA genes
Raw datasets were obtained from the BARLEX (Barley Genome Explorer)36 (https://apex.ipk-gatersleben.de/apex/f?p = 284:10:1811619314937)52. These data were applied to analyze HvIAAs expression profiles in different tissues. Heatmap was generated with the gplots package in R (https://www.r-project.org/). It showed the expression differences based on the FPKM values, which were normalized by log2(FPKM+1) transform. Hierarchical clustering algorithms was used for recognition of similar patterns in expression files53.
Plant growth, tissue collection and treatment
The spring barley cultivar of Gold Promise was grown in the greenhouse of Shanghai Jiao Tong University under 16 °C/14 °C day/night, 16 h/8 h light/dark, and 50% relative humidity. For NAA (1-Naphthaleneacetic acid) treatment, one-week-old seedling was sprayed with 5 nM NAA, and then the seedling was sampled at 0.5 h, 1 h, 2 h and 4 h after spraying. Seedlings were sprayed with DMSO (dimethyl sulfoxide) as a control (CK). To detect HvIAAs expression during the barley spike development, five stages were collected: two-week-old seedling (SD), the double ridge stage (DR), the lemma primordium stage (LP), the stamen primordium stage (SP), the awn primordium stage (AP) and the white anther stage (WA)54,55. The developmental stages were determined by dissection under a stereomicroscope. All samples were frozen in liquid nitrogen and stored at −80 °C until RNA extraction.
Quantitative RT-PCR analysis
Barley tissues total RNA were extracted with TRIZOL reagent (Invitrogen), then reverse transcription reaction was carried out using a PrimeScript RT reagent kit with gDNA eraser (Takara), according to the manufacturer’s instructions. SYBR Green SuperReal PreMix Plus (TIANGEN) kit was applied for quantitative RT-PCR(qRT-PCR) experiments using CFX96 Real-time PCR machine (Bio-Rad). HvACTIN (HORVU5Hr1G039850.3) was used as an internal control56. Three biological repeats with three technical repeats were performed (primers used are listed in Supplementary Table S5).
Results
Identification of the Aux/IAA gene family in barley genome
Supplementary Tablestified from the barley genome using Hidden Markov Model (HMM) methods38,39. HvIAAs were designated as HvIAA1-HvIAA36 according to their physical position on barley each chromosome. We provided the gene characteristics including physical position, ORF (Open Reading Frame) sequence size, amino acid length, molecular weight (Da), isoelectric points (PI) and intron numbers. The number of amino acid length of the predict HvIAA proteins ranged from 96 (HvIAA21) to 772 (HvIAA34). The molecular weight of the HvIAA proteins differed from 10819.43 (HvIAA21) to 86193.2 (HvIAA34) Da, and the PI of the HvIAA proteins varied from 4.5 (HvIAA21) to 9.56 (HvIAA23) (Supplementary Table S1).
Phylogenetic tree of the HvIAA proteins in barley
To examine the phylogenetic relationships among the Aux/IAAs from barley, rice and Arabidopsis (based on 31 Aux/IAA protein sequences in rice and 29 Aux/IAA protein sequences in Arabidopsis), phylogenetic evolutionary trees of 96 Aux/IAA protein sequences are constructed using MEGA7.0 software46. According to the classification of rice and Arabidopsis13,57, 96 Aux/IAAs are divided into A and B groups with the well-supported branch. Based on the evolutionary, the classification of Aux/IAAs in Arabidopsis and rice is consistent with previous reports. There are 17 HvIAAs distributed in group A, while another 19 are found in group B (Fig. 2). Group A and B could be further subdivided into 8 and 6 subgroups (A1-A8 and B1-B6), respectively, with varying degrees of bootstrap support.
Figure 2.

Phylogenetic tree of Aux/IAA proteins from barley, Arabidopsis and rice. The full-length amino acid sequences of 36 barley and 29 Arabidopsis Aux/IAA proteins combine with 31 Aux/IAA proteins from rice are aligned by ClustalW, and the neighbor-joining tree is constructed using MEGA7.0 with 1000 bootstrap replicates. Two groups A and B are the highlight in blue and green colors. Barley, rice and Arabidopsis are replaced by black, yellow and brown representing the Aux/IAAs, respectively.
Subgroup A3 and A4 may specific to dicots, as they do not contain rice and barley IAAs. Subgroup A3 contains AtIAA5, AtIAA6, and AtIAA19. The Arabidopsis iaa5/6/19 triple mutants line has a minor defect in stomatal movement under drought stress58,59. The missing of A3 subgroup in monocot indicate that the dumbbell stomata apparatus in monocot does not require the A3 subgroup IAAs paralog. Subgroup A4 contains AtIAA8 and AtIAA9, which were reported to regulate vasculature formation, adventitious root formation, and lateral root elongation60–62. As IAAs are essential for regulating lateral roots and adventitious roots both in monocot and dicots63, the absence of subgroups A4 IAA function may be compensated by other IAAs in other subgroups. For instance, OsIAA11 and OsIAA13 from subgroup A5 were reported in regulating lateral roots in rice63,64. We also identified subgroup A7, A8 and B3 IAAs as monocot specific. In our phylogeny tree, the subgroup A7 contains only two members, HvIAA21 and OsIAA26, which are also the smallest proteins in the Aux/IAA family. OsIAA26 was shown to be important for rice root elongation and the protein abundance is indirectly regulated by TIRSCF1-IAA signaling21. There is no data directly deciphering the function of A8 groups IAAs. However, studies suggested that the rice A8 groups IAA genes OsIAA14 and OsIAA24 are regulated by root development regulators, Crown Rootless1 (CRL1), MADS- box transcription factor (OsMADS25), and Nonexpressor of Pathogenesis-Related Genes1 (OsNPR1), indicating that this group of IAAs may function specifically in root development65–67. Likewise, there is no direct data to infer the function of B3 IAAs. Interestingly, the subgroup B5 only contains barley and Arabidopsis IAAs. It is intriguing to ask whether this group of IAAs is related to the xeromorph of barley and Arabidopsis. All the three Arabidopsis members, IAA32, IAA33and IAA34 were shown to act on high auxin level19,20.
Chromosomal locations of HvIAA genes
The physical location of 35 HvIAAs determines the location on the chromosome. 35 HvIAAs (97.2%, 35/36) locate unevenly on 7 chromosomes, HvIAA36 was not able to be mapped to chromosome (Supplementary Table S1). ChrUn is composed of sequence fragments originating from BAC (bacterial artificial chromosome) overlap clusters not placed in the Hi-C (high-throughput/resolution chromosome conformation capture) map-, or gene-bearing fragments of BAC sequences and Morex WGS (whole genome shotgun) contigs selected in addition to the non-redundant sequence68. The nine HvIAAs are located on chromosome 5, two on chromosome 2 and 4, five are located on chromosome 1, eight on chromosome 3, three on chromosome 6, and six on chromosome 7, respectively (Supplementary Fig. S1).
Phylogenetic analyses can often be used to uncover the duplication events that led to the generation of large sets of tandemly duplicated genes69. In this study, 36 HvIAAs only formed 8 sister pairs (Supplementary Fig. S2) with strong bootstrap support (>97%), at least 11 paralogs of HvIAAs might have undergone gene duplication, which may be caused by segment replication and tandem replication events (Fig. 3; Supplementary Fig. S2)70. Segment duplication leads to many homologies of HvIAAs between the chromosomes.
Figure 3.
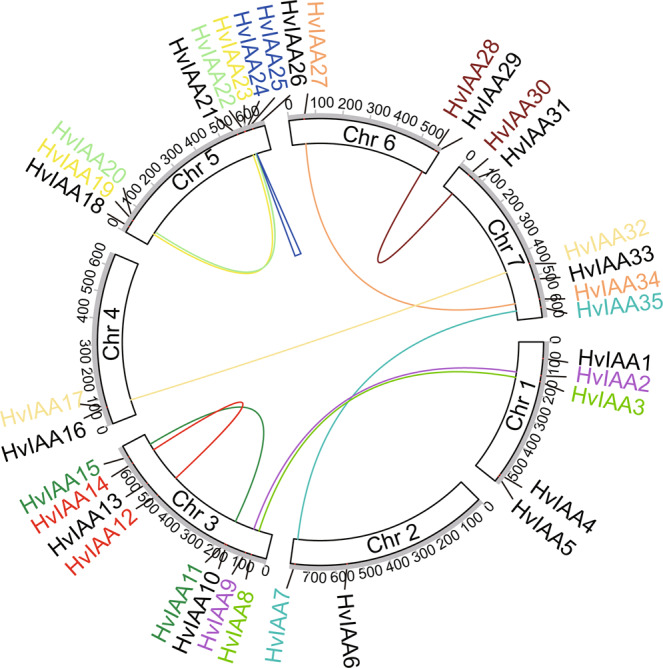
Chromosomal locations and duplication events of HvIAA members in barley genome Colorful lines and blue triangle represent gene duplications.
In order to explore which type of selective pressure determines the divergence process of HvIAA after replication. Ka/Ks is the ratio of nonsynonymous substitution (Ka) to synonymous substitution (Ks), the Ka/Ks substitution ratio is used to assess the coding sequences of 11 pairs of HvIAAs. A Ka/Ks ration greater than 1 represents positive selection, a ratio of 1 represents neutral evolution and a ratio less than1 represents purifying selection71. We found that nine out of the eleven Aux/IAA paralog pairs had the ratio of Ka/Ks among 11 pairs genes was less than 1(Supplementary Table S2), suggesting a purified selection that favors synonymous substitutions than nonsynonymous substitutions to prevents the change of an amino acid residues had occurred following the duplications.
Gene structure and conserved motif analysis of the barley Aux/IAA genes
In the previous research, gene structure diversity provides the main impetus for the evolution of polygenic families72–74. Therefore, the gene structural diversity of HvIAAs is further studied with exon/intron analysis. The result shows that the sequence length and the number of introns (exons) of 36 HvIAAs vary greatly. The number of introns ranges from 0 to 13, of which HvIAA25 has a complete lack of introns, and HvIAA35 has most introns, with 13 introns. Additionally, some HvIAAs in the same phylogenetic subgroup had the same number of exons such as HvIAA2 and HvIAA9, HvIAA34 and HvIAA27. Interestingly, these two pairs of HvIAAs have similar gene sequence length (Fig. 4).
Figure 4.
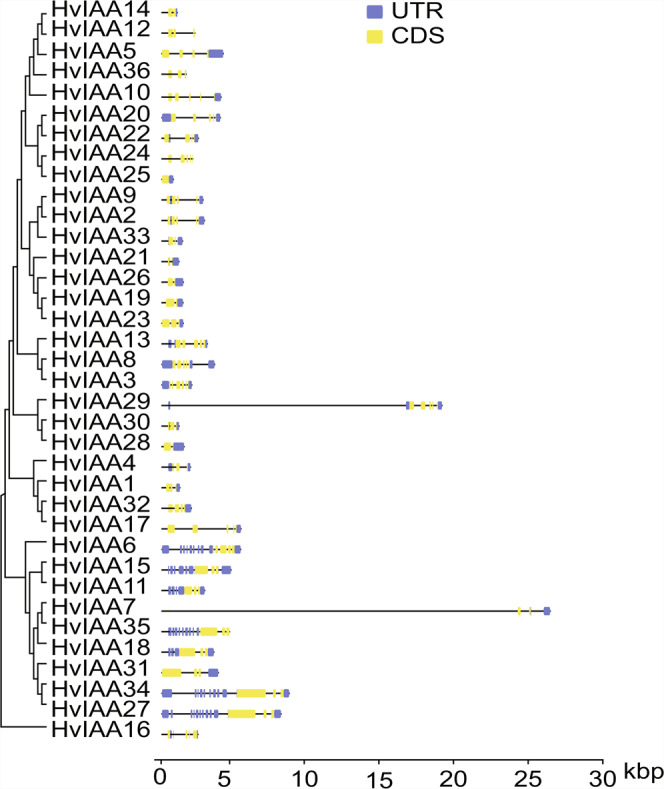
Gene structure analysis of barley HvIAAs according to a phylogenetic relationship. The UTR, exons, introns, are represented by blue boxes, yellow boxes and black lines, respectively.
The motif discovery (classic mode) of the MEME online tool49 is used to analysis motif distribution of barley Aux/IAA protein. 5 motifs are found in most of the HvIAA proteins. According to our MEME analysis, 4 conserved motifs of HvIAA are corresponding to the four conserved domains of Aux/IAA protein. Motif 1, 2, 3 and 5 are corresponding to domain IV, III, II, I, respectively. 18 HvIAA proteins have all the four conserved domains, while the rest 18 proteins lack at least one conserved domain, belonging to non-canonical Aux/IAAs. Some of the HvIAA protein sequences lack conserved motifs, such as 6 HvIAA proteins (16.7%, 6/36) do not have motif 1, 6 HvIAA proteins (16.7%, 6/36) do not have motif 2, 13 HvIAA proteins (36.1%, 13/36) do not have motif 3, and 23 HvIAA proteins (63.7%, 23/36) have motif 5. Peculiarly, HvIAA4, HvIAA7, HvIAA18, HvIAA27, HvIAA31, HvIAA34, HvIAA35 proteins contain only one motif (Fig. 5). Multiple alignment analysis of 36 HvIAA protein sequences in barley, two nuclear localization signals (NLSs), multiple phosphorylation sites and βαα motif are found in most of identified HvIAA proteins (Supplementary Fig. S3).
Figure 5.

The conserved motifs of HvIAAs according to a phylogenetic relationship. All motifs are identified by MEME with the complete amino acid sequences of HvIAAs. Different motifs are represented by different box colors. The bits indicate amino acid conservation in each position. The conserved sequences are highlighted with black boxes on each domain. Red, green, blue, yellow and purple boxes represent motif 5, motif 3, motif 2, motif 1 and motif 4, respectively.
Analysis of cis-elements in HvIAA genes
In order to identify the potential regulatory elements response to auxin. We used the Plant Care database to identify the cis-acting elements existing in the promoter region of HvIAAs. The detailed information of cis-acting elements at the promoter regions was listed in (Supplementary Table S3), except for one gene (HvIAA6, no clear promoter sequence). According to the previous study, we classify them into six major functional categories; development/tissue specificity, promoter/enhancer element, light response, circadian control, stress and hormone response75. The result reveals that two types of cis-acting elements are involved in hormone response and related to auxin response, including AuxRRs and TGA-element (Marked in red color in Supplementary Table S3). It can be inferred that HvIAAs can response to exogenous auxin76. Moreover, CAAT-box and TATA-box are ubiquitously presented in the promoter/enhancer element of HvIAAs (Supplementary Fig. S4), it is one of the binding sites for RNA polymerase and it also determines the initiation of gene transcription and its efficiency. Therefore, CAAT-box and TATA-box may play an important role in the initiation of controlling HvIAAs expression time and degree.
Expression analysis of HvIAA genes in different tissues
Analysis of gene family expression patterns provides information for studying their functions77,78. The results of expression analysis show that 26 HvIAAs have different expression patterns in 15 different developmental stages based on RNA-seq data, and the other 10 HvIAAs have no data (Fig. 6; Supplementary Table S4). Hierarchical clustering was used to cluster the genes with similar expression patterns, which divided the HvIAA into three clusters. The HvIAAs in Cluster I (including eleven members) display low expression levels in most tissues, whereas HvIAA16 and HvIAA17 have high expression in the specific tissues compared with other genes in Cluster I. In Cluster II (including three members) genes are general highly expressed in different tissues, for example, HvIAA20 shows a strong expression level in developing tillers (NOD) compared with other 14 tissues. HvIAA transcripts are less abundant in developing grain (CAR 15) and senescing leaves (SEN). In Cluster III (including twelve members), genes are expressed at a moderate level.
Figure 6.
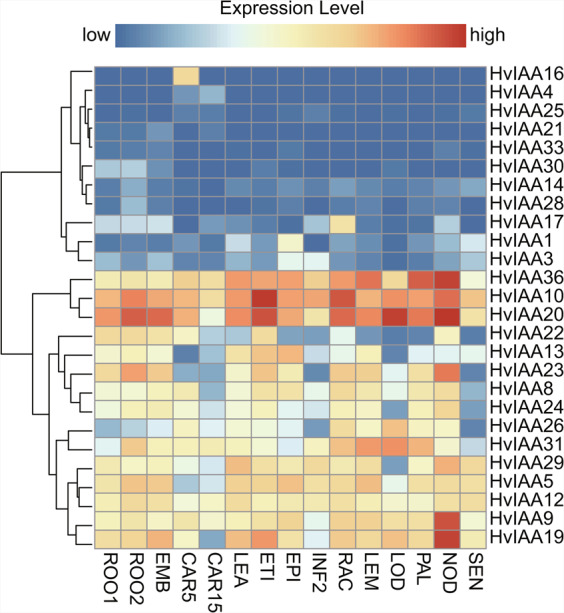
Hierarchical clustering of HvIAAs expression in various tissue. FPKM values were normalized by log2(FPKM+1) transform to represent color scores. ROO1, roots from seedlings (10 cm shoot stage); ROO2, roots (28 DAP); EMB, 4 day embryos; CAR5, developing grain (5 DAP); CAR15, developing grain (15 DAP); LEA, shoots from seedlings (10 cm shoot stage); ETI, etiolated seedling, dark cond. (10 DAP); EPI, epidermal strips (28 DAP); INF2, developing inflorescences (1–1.5 cm); RAC, inflorescences, rachis (35 DAP); LEM, inflorescences, lemma (42 DAP); LOD, inflorescences, lodicule (42 DAP); PAL, dissected inflorescences, palea (42 DAP); NOD, developing tillers, 3rd internode (42 DAP); SEN, senescing leaves (56 DAP).
HvIAAs in Cluster I is merely mildly expressed, suggesting that these genes might be less required in those tissues. Remarkably, HvIAA16 is only expressed in developing grain (CAR 5). The expression of genes in Cluster II, in etiolated seedling (ETI), inflorescences, rachis (RAC) and developing tillers (NOD) was higher than other genes in this group. The expression of genes in 15 different tissues of Cluster III shows a fluctuating trend. For instance, the expression of HvIAA19 is highest in developing tillers (NOD), but it is the lowest in developing grain (CAR 15). HvIAA19 may play a key role in development tillers (NOD) in the barley development. These results suggest that these genes have specific functions in different tissue and development processes.
Expression of HvIAAs genes in the spike development and response to NAA treatment
To investigate whether HvIAAs respond to auxin, the expression levels of HvIAAs are evaluated in the barley one-week seedling by qRT-PCR under NAA treatment. According to expression pattern after NAA treatment, then clustered using hierarchical clustering algorithms, these genes are divided into C1- C8. The data showed that the transcript levels of group C2, 4, 5, and 6 of the HvIAAs are upregulated at 4 h after NAA treatment. Especially, HvIAA30 is upregulated over ten-fold at 4 h, HvIAA2 and HvIAA30 are upregulated over two-fold at 2 and 4 h (HvIAA4, HvIAA16 and HvIAA21 are not detectable). Interestingly, HvIAA33 is decreased at 0.5 h after NAA treatment (Fig. 7).
Figure 7.
Expression profiles of the HvIAAs in response to NAA treatment. qRT-PCR analyses are used to assess HvIAAs transcript levels in the one-week seedlings sampled at 0.5, 1, 2 and 4 h after spraying 5 nM NAA. The number on x-axis indicate hours after NAA treatment. These genes are divided into C 1– C 8. The abscissas 1, 2, 3, and 4 represent 0.5 h, 1 h, 2 h and 4 h, respectively.
To investigate the expression of HvIAAs associated with barley spike development, the transcript levels of each HvIAA is monitored in the spike development and their expression patterns are analyzed using qRT-PCR. The result shows that most of the HvIAAs are detected in the barley spike development compare with two-week-old seedling (SD) (HvIAA1, HvIAA4, HvIAA16 and HvIAA30 are not detect data), such as double ridge stage (DR), lemma primordium stage (LP), stamen primordium stage (SP), awn primordium stage (AP) and white anther stage (WA) (Supplementary Fig. S5). However, some HvIAAs genes display a high expression during barley spike development. For example, HvIAA3, HvIAA7, HvIAA8 and HvIAA18 are highly expressed in the DR, LP, SP, AP and WA. Interestingly, HvIAA24 shows the highest expression level in WA. HvIAA34 displays a higher expression level in the DR, LP and SP than in the other stages (Fig. 8).
Figure 8.
Expression profiles of the HvIAAs during barley spike five developmental stages. qRT-PCR analyses are performed using RNA generate from barley spike developmental stages. SD: two-week-old seedling; DR: double ridge stage; LP: lemma primordium stage; SP: stamen primordium stage; AP: awn primordium stage; WA: white anther stage.
Discussion
Here, we identified 36 Aux/IAAs from the barley genome, each of them has at least one conserved Aux/IAA domain. Intriguingly, barley (5.1 GB) has a large difference in genome size compare to rice (430 MB) and Arabidopsis (135 MB), but the numbers of Aux/IAA members are very close13,58. In contrast to the higher plants, Marchantia and Physcomitrella have only one and three Aux/IAAs, respectively79–81.
This implies that Aux/IAA gene families are expanded when plants start to conquer the land during evolution and the current number for higher plants is essential for their well-being. Nevertheless, only a few of the Aux/IAAs loss-of-function mutants in Arabidopsis have only mild phenotypes82. Investigation of additional species outside of Arabidopsis is recommended for a better understand of the true biological functions of Aux/IAAs82.Consistent with the finding in Arabidopsis, there is no premature stop-codon found in barley Aux/IAA genes, suggested that they are not likely pseudogenes57.
The 36 barley, 29 Arabidopsis, and 31 rice Aux/IAA proteins sequences were used to construct evolutionary tree by using Neighbor-Joining method.The 36 HvIAAs can be classified into two groups, Group A (17 members) and B (19 members), which is consistent with the classification in rice13 and Arabidopsis57. We found that most of the HvIAAs are located at the distal ends of the chromosome. Similar findings on other barley gene families were also reported53,83,84. This may due to the fact that there are overall more abundant genes at both ends than in the middle of the chromosome in barley36. It is the meiotic homologous chromosome recombination mainly confined to the distal regions of all chromosomes that renders the uneven density of genes on cereal chromosomes and most of the genes concentrated in the distal regions85. Gene duplication events accelerate the rapid expansion and evolution of gene families. In this study, at least 11 pairs of HvIAAs undergone gene duplication (Fig. 3; Supplementary Fig. S2), including segment duplication and tandem duplication events. For instance HvIAA2 and HvIAA9 may be the products of genomic segment replication. HvIAA24 and HvIAA25 are the products of genomic tandem replication. Generally, this kind of genes arrangement is difficult to segregate via hybridization in breeding or research. These members belonging to the same clades usually share similar conserved motif and exon/intron, and they may have similar functions. Nine out of eleven pairs of the paralogous genes are Ka/Ks less than 1, indicating they have undergone purifying selection.
The domain I containing the LxLxLx motif (L, leucine, x represents any amino acid residue) is important for the IAAs as active transcriptional repressors. It couples the TOPLESS protein scaffold (TPL) and thereafter recruiting histone deacetylase to the ARF transcription factor. The Lego bricks structure transforms the ARF to a transcriptional repressor7,86,87. Nevertheless, there are abundant numbers of IAA lacking domain I in various species and they are also able to suppress the expression of auxin response genes. IAAs lacking domain I oligomerize with ARF to prevent the activation activity of ARF87. In this case, Aux/IAA is an indirect transcription activator acting through modulating the free ARFs level. Besides, IAAs were shown to repress ARF activity without dimerizing with ARF88. In our study, we identified HvIAA25 from A5 subgroup, HvIAA21 from A7 subgroup, HvIAA4 from A8 subgroup, HvIAA13 from B2 subgroup, HvIAA16 from B3 subgroup and HvIAA6, HvIAA7, HvIAA11, HvIAA15, HvIAA18, HvIAA27, HvIAA34 and HvIAA35 from B5 subgroup are lacking domain I (Fig. 2 and Fig. 5). However, how different in the transcription repression activity of the barely IAAs with/without domain I need to be further checked.
The core sequence of Domain II with GWPPV/I is the target site for ubiquitination degradation of Aux/IAA protein by interaction with TIR1 of SCFTIR1 complex. The absence of domain II in AtIAA20 and AtIAA30 leads to the longer half-life of the proteins comparing to the canonical Aux/IAA proteins, and overexpression of either of them disturbs auxin physiology and causes auxin-related aberrant phenotypes89. Previous studies showed non canonical IAA proteins lacking domain II are not subject to the degradation by the SCFTIR1 protein complex and stabilized by the phosphorylation of IAAs on high auxin level19,20. In our study, we identified HvIAA21 from A7 subgroup, HvIAA4 from A8 subgroup, HvIAA6, HvIAA7, HvIAA11, HvIAA15, HvIAA18, HvIAA27, HvIAA31, HvIAA34 and HvIAA35 from B5 subgroup, and HvIAA1 from B6 subgroup and HvIAA16 from B3 subgroup are lacking domain II (Fig. 2 and Fig. 5). They may share the common function mechanism as these reported non canonical IAAs. Similar with lacking domain II, mutations in the conserved motif GWPPV/I motif in the domain II, such as AtIAA31 in Arabidopsis and OsIAA4 and OsIAA10 in rice, and the Arabidopsis mutants shy2–2 and shy2–3 (IAA3), iaa18-1 (IAA18), arx2-1 (IAA7), arx3-1 and arx3-3 (IAA17), also stabilize the protein and the overexpression of these genes exhibits the typical auxin-related aberrant phenotypes such as dwarfism, increased tiller angles, reduced gravity response89–92. In our study, similar dominant mutation in IAA domain II if it has domain II was not found in the barley genome (Supplementary Fig. S3).
Domain III and IV are conserved between the Aux/IAA and ARF protein families, they are mainly responsible for the homo- and hetero-dimerization among the Aux/IAA proteins and ARFs to inhibit the transcription of auxin-responsive genes11. Additionally, the βαα motif in domain III composed of a β-sheet and two α-helices (α 1 and α 2) is critical for the dimerization of Aux/IAA protein7. Most of the HvIAA proteins have two putative nuclear localization signals (NLS), the first one is composed of a bipartite structure, conserved double KR between domain I and domain II and basic amino acids in domain II, while the second one includes SV40-type NLS located in domain III93, assuming that HvIAA proteins may play a role in the nucleus. Furthermore, multiple phosphorylation sites are discovered in the HvIAA proteins, these different phosphorylation sites may regulate nuclear transportation (Supplementary Fig. S3)94. Domain III and domain IV in the C-terminal region, has well-known Phox and Bem 1 (PB1) protein-protein interaction domain that mediates homo- and heterodimerization95. In our study, we identified HvIAA7, HvIAA18, HvIAA27, HvIAA31, HvIAA34 and HvIAA35 from subgroup B5, are lacking domain III. HvIAA14 and HvIAA36 from subgroup A1, HvIAA25 from subgroup A5, HvIAA28 and HvIAA30 from subgroup B4, and HvIAA1 from subgroup B6, which are lacking domain IV. Genetic and biochemical data on their function are needed to further elucidate these non-canonical IAAs.
Gene expression gives hints for the function of the gene. In order to characterize the expression regulation of HvIAAs, we check cis-elements in the promoter regions of the HvIAAs. We found that the promoter regions of HvIAAs contain cis-elements related to development/tissue specificity, promoter/enhancer element, light response, circadian control rhythm, stress and hormone response (Supplementary Table S3), indicating that expression of HvIAAs is tailored to adapt the multiple functions in various biological processes. The result shows that 18 HvIAAs contains one or two auxin response elements, which may be regulated by auxin. HvIAA8, HvIAA15, HvIAA20, HvIAA27 have two auxin response elements, and the other 18 HvIAAs do not have any auxin response elements (Supplementary Table S3). The presence of these cis-acting elements suggests that the HvIAAs play important roles in the early response of auxin in barley. Previous work shows that auxin and light, brassinolide and abiotic stress signal are mutually regulated96–98. OsIAA1 and HvIAA9, OsIAA6 and HvIAA13 are in the same clades, and cis-acting elements analysis suggesting that canonical HvIAA9 and HvIAA13 contain TGA-element in their promoters, it can respond to exogenous auxin. Undoubtedly, several of cis-acting elements in the promoter regions of HvIAAs coordinate the regulate expression of the HvIAAs to facilitate their functions in barley development. It is helpful to validate the expressional pattern of HvIAAs experimentally to reveal the gene function of HvIAAs.
The diversity of the expression profiles of HvIAAs in tissues and developmental stages indicates that HvIAAs regulate multiple developmental processes. The expression pattern of HvIAA provides hints to further investigation of their biological function. For example, the high expression of HvIAA20 in inflorescence (LOD) implies that HvIAA20 may regulate inflorescence development. HvIAA16 displays expression in developing grain (CAR5), which suggests that HvIAA16 may be related to grain development. Furthermore, HvIAA9, HvIAA19, HvIAA20 and HvIAA36 have a high expression level in developing tillers (NOD), indicating that these four genes may together involve in developing tillers development (Fig. 6). In previous studies, OsIAA9 promotes lateral root formation in rice, and mediates geotropic of deletion is associated with starch granule synthesis in root tips99. According to expression analysis and phylogenetic relation of OsIAA9 with HvIAA28 and HvIAA30, HvIAA28 and HvIAA30 may influence the root growth. OsIAA11 inhibits the development of lateral roots and influences inflorescence genes in rice100. The expression profile and phylogenetic tree indicate that the subcluster including OsIAA11, HvIAA20 and HvIAA22 may probably participate in the root and inflorescence growth. The mutation of AtIAA12 in Arabidopsis specifically affects embryonic development101.
Although Aux/IAA transcription was initially thought to be primary auxin responsive, different expression patterns in response to auxin were found among the gene family in various species13,102,103. In C 2, 4, 5 and 6 group, the expression levels are significantly upregulated at 4 h after NAA treatment. Especially, HvIAA30 is upregulated over ten-fold at 4 h, HvIAA2 and HvIAA30 are upregulated over two-fold at 2 and 4 h (Fig. 7). Our promotor analysis identified two auxin signaling transduction-related cis-elements presenting in the promoter regions of the 18 HvIAAs (Supplementary Table S3). The diversity of the numbers and locations of these cis-elements may partly explain the different expression patterns of HvIAAs under NAA treatment. However, HvIAA2, HvIAA5, HvIAA10, HvIAA14, HvIAA19 and HvIAA28 are not found the auxin-responsive elements in the promoter regions, the relative mRNA levels of these 6 genes increased at 4 h after the NAA treatment (Fig. 7). Similar results have been reported in previous research104. The transcript levels of the OsIAA9, OsIAA19, OsIAA20 and OsIAA31 are prominent upregulated under auxin treatment13, HvIAA28, HvIAA5, HvIAA30 and HvIAA19 are homologs of these four OsIAA genes, respectively (Fig. 2), and detect the expression of upregulated after NAA treatment.
Traits of barley spikelet largely contribute to the yield. Therefore, the investigation of genes regulating spikelet development may serve for the barley breeding aimed for improving yield. There are accumulating evidence that Aux/IAA genes involve in regulating inflorescence structure and thus spikelet in various species34,35,105. In the previous study, overexpression of IAA1 in Arabidopsis significantly reduced cell length and cell number in inflorescences and leaves, and affected cell shape105. BIF1 (BARREN INFLORESCENCE1) and BIF4 (BARREN INFLORESCENCE4) encoded the Aux/IAA protein regulate the early steps required for inflorescence formation34. In our study, the expression of HvIAA3, HvIAA7, HvIAA8, HvIAA18, HvIAA15 and HvIAA34 are increased during the barley spike developmental process, suggesting that these 6 genes might play a key role in the development of barley spike. Interestingly, HvIAA24 displays the highest expression level in the WA (floral organ differentiation was completed), implying that this gene may act in awn development. HvIAA34 exhibits higher expression in the DR, LP and SP than in the other tissues, indicating that this gene may function specifically in DR, LP and SP stages during barley spike development (Fig. 8). Again, there is not likely IAA pseudogenes in barley as all of them are found expressed at least in one condition.
As in other plants, the Aux/IAAs could play important roles in the growth and development of barley. However, the biological function of the barley Aux/IAAs remains to be elucidated in detail. Our data on the phylogeny, gene structure, conserved motifs, chromosome locations, cis-acting elements and expression profiles provide essential clues for exploring the biological functions of the 36 Aux/IAAs in barley.
Conclusion
In this study, we identified 36 Aux/IAAs in barley, similar number as that in rice and Arabidopsis. Half of them are canonical Aux/IAAs containing the typical four conserved IAA domains and the rest are non-canonical Aux/IAAs. We identified 14 expressional auxins responding HvIAAs and numbers of HvIAAs that may regulate spike development. Our findings would facilitate the functional study of Aux/IAA genes and molecular breeding of barley.
Supplementary information
Acknowledgements
This work was supported by Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF, CX(18)1001). W.C. is supported by China Postdoctoral Science Foundation (Grant 2017M621451).
Author contributions
Conceived by: D.Z. and W.C. Q.S. did the major analysis and experiments. S.Q., D.Z. and W.C. wrote the manuscript. Y.Z., V.T. and J.S. helped with the analysis and comment on the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dabing Zhang, Email: zhangdb@sjtu.edu.cn.
Wenguo Cai, Email: wenguo.cai@sjtu.edu.cn.
Supplementary information
is available for this paper at 10.1038/s41598-020-66860-7.
References
- 1.Abel S, Theologis A. Early genes and auxin action. Plant Physiol. 1996;111:9–17. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos JA, Zenser N, Leyser O, Callis J. Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell. 2001;13:2349–2360. doi: 10.1105/tpc.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman EJ, Estelle M. Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 2009;43:265–285. doi: 10.1146/annurev-genet-102108-134148. [DOI] [PubMed] [Google Scholar]
- 4.Calderón Villalobos LI, et al. A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat. Chem. Biol. 2016;8:477–485. doi: 10.1038/nchembio.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- 6.Hagen G, Guilfoyle T. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 2002;49:373–385. [PubMed] [Google Scholar]
- 7.Tiwari SB, Gretchen H, Guilfoyle TJ. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell. 2004;16:533–543. doi: 10.1105/tpc.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefan K, Ottoline L. Auxin-induced SCFTIR1-Aux/IAA interaction involves stable modification of the SCFTIR1 complex. Proc. Natl. Acad. Sci. USA. 2004;101:12381–12386. doi: 10.1073/pnas.0402868101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 10.Dreher KA, Brown J, Saw RE, Callis J. The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell. 2006;18:699–714. doi: 10.1105/tpc.105.039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maraschin FDS, Memelink J, Offringa R, Auxin-induced SCF. -mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J. 2009;59:10. doi: 10.1111/j.1365-313X.2009.03854.x. [DOI] [PubMed] [Google Scholar]
- 12.Piya S, Shrestha SK, Binder BSC, Jr, Hewezi T. Protein-protein interaction and gene co-expression maps of ARFs and Aux/IAAs in Arabidopsis. Front. Plant Sci. 2014;5:744. doi: 10.3389/fpls.2014.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain M, et al. Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa) Funct. Integr. Genomics. 2006;6:47–59. doi: 10.1007/s10142-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 14.Kalluri, U. C., DiFazio, S. P., Brunner, A. M. & Tuskan, G. A. Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biology7, 10.1186/1471-2229-7-59 (2007). [DOI] [PMC free article] [PubMed]
- 15.Wang Y, Deng D, Bian Y, Lv Y, Qin X. Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays. L.) Mol. Biol. Rep. 2010;37:3991–4001. doi: 10.1007/s11033-010-0058-6. [DOI] [PubMed] [Google Scholar]
- 16.Gan D, Zhuang D, Ding F, Zhenzhou YU, Zhao Y. Identification and expression analysis of primary auxin-responsive Aux/IAA gene family in cucumber (Cucumis sativus) Journal of Genetics. 2013;92:513–521. doi: 10.1007/s12041-013-0306-3. [DOI] [PubMed] [Google Scholar]
- 17.Qiao L, et al. A genome-wide analysis of the auxin/indole-3-acetic acid gene family in hexaploid bread wheat (Triticum aestivum L.) Front. Plant Sci. 2015;6:770. doi: 10.3389/fpls.2015.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, et al. Genome-wide analysis of the Auxin / Indoleacetic acid (Aux/IAA) gene family in allotetraploid rapeseed (Brassica napus L.) BMC Plant Biol. 2017;17:204. doi: 10.1186/s12870-017-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao M, et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature. 2019;568:240–243. doi: 10.1038/s41586-019-1069-7. [DOI] [PubMed] [Google Scholar]
- 20.Lv B, et al. Non-canonical AUX/IAA protein IAA33 competes with canonical AUX/IAA repressor IAA5 to negatively regulate auxin signaling. EMBO J. 2019;0:e101515. doi: 10.15252/embj.2019101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, et al. E3 ubiquitin ligase SOR1 regulates ethylene response in rice root by modulating stability of Aux/IAA protein. Proc. Natl. Acad. Sci. USA. 2018;115:4513–4518. doi: 10.1073/pnas.1719387115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed JW. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 2001;6:420–425. doi: 10.1016/s1360-1385(01)02042-8. [DOI] [PubMed] [Google Scholar]
- 23.Smet ID, Jürgens G. Patterning the axis in plants – auxin in control. Curr. Opin. Genet. Dev. 2007;17:337–343. doi: 10.1016/j.gde.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Nesrine, S., Rayda, B. A., Mustapha, G. & Ahmed, R. Prediction of auxin response elements based on data fusion in Arabidopsis thaliana. Mol. Biol. Rep. 45 (2018). [DOI] [PubMed]
- 25.Woodward AW, Bartel B. Auxin: Regulation, action, and interaction. Annu. Rev. Plant Biol. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liscum E, Reed JW. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002;49:387. [PubMed] [Google Scholar]
- 27.Weijers D, Wagner D. Transcriptional responses to the auxin hormone. Annu. Rev. Plant Biol. 2016;67:539–574. doi: 10.1146/annurev-arplant-043015-112122. [DOI] [PubMed] [Google Scholar]
- 28.Halliday KJ, Martínez-García JF, Josse E-M. Integration of light and auxin signaling. Cold Spring Harb. Perspect. Biol. 2009;1:a001586–a001586. doi: 10.1101/cshperspect.a001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waters MT, Gutjahr C, Bennett T, Nelson DC. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017;68:291. doi: 10.1146/annurev-arplant-042916-040925. [DOI] [PubMed] [Google Scholar]
- 30.Tian Q, Uhlir NJ, Reed JW. Arabidopsis SHY2/IAA3 Inhibits Auxin-Regulated Gene Expression. Plant Cell. 2002;14:301–319. doi: 10.1105/tpc.010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llorente F, et al. Repression of the auxin response pathway increases Arabidopsis susceptibility to necrotrophic fungi. Mol Plant. 2008;1:496–509. doi: 10.1093/mp/ssn025. [DOI] [PubMed] [Google Scholar]
- 32.Lian J, et al. Rice Dwarf Virus P2 Protein hijacks auxin signaling by directly targeting the rice OsIAA10 protein, enhancing viral infection and disease development. PLoS Pathog. 2016;12:e1005847-. doi: 10.1371/journal.ppat.1005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaki, Y. et al. Fine control of aerenchyma and lateral root development through AUX/IAA- and ARF-dependent auxin signaling. Proc. Natl. Acad. Sci. USA116 (2019). [DOI] [PMC free article] [PubMed]
- 34.Galli M, et al. Auxin signaling modules regulate maize inflorescence architecture. Proc. Natl. Acad. Sci. USA. 2015;112:13372–13377. doi: 10.1073/pnas.1516473112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youssef HM, Hansson M. Crosstalk among hormones in barley spike contributes to the yield. Plant Cell Rep. 2019;38:1013–1016. doi: 10.1007/s00299-019-02430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer KFX, et al. A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012;491:711–716. doi: 10.1038/nature11543. [DOI] [PubMed] [Google Scholar]
- 37.Kawahara Y, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6:1–10. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eddy SR. Accelerated profile HMM searches. PLoS Comput. Biol. 2011;7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finn RD, Jody C, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:29–37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finn RD, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchlerbauer A, et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45:D200–D203. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finn RD, et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panu A, et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40:W597–603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodstein DM, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques. 2000;28:1102–1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larkin M, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 48.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey T, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol. 1994;2:28–36. [PubMed] [Google Scholar]
- 50.Chen, C., Chen, H., He, Y. & Xia, R. TBtools, a Toolkit for Biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv, 289660, 10.1101/289660 (2018).
- 51.Rozas, J. et al. DnaSP 6: DNA Sequence Polymorphism analysis of large datasets. Mol. Biol. Evol. 34 (2017). [DOI] [PubMed]
- 52.Colmsee C, et al. BARLEX-the barley draft genome explorer. Mol. Plant. 2015;8:964–966. doi: 10.1016/j.molp.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M, et al. Genome-wide analysis of the barley non-specific lipid transfer protein gene family. Crop J. 2019;7:65–76. [Google Scholar]
- 54.Sreenivasulu N, Schnurbusch T. A genetic playground for enhancing grain number in cereals. Trends Plant Sci. 2012;17:91–101. doi: 10.1016/j.tplants.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Waddington SR, Cartwright PM, Wall PC. A quantitative scale of spike initial and pistil development in barley and wheat. Ann. Bot. 1983;51:119–130. [Google Scholar]
- 56.Komatsuda T, et al. Six-rowed barley originated from a mutation in a homeodomain-leucine zipper I-class homeobox gene. Proc. Natl. Acad. Sci. USA. 2007;104:1424. doi: 10.1073/pnas.0608580104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Remington DL, Vision TJ, Guilfoyle TJ, Reed JW. Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol. 2004;135:1738–1752. doi: 10.1104/pp.104.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Overvoorde PJ, et al. Functional Genomic Analysis of the AUXIN/INDOLE-3-ACETIC ACID Gene Family Members in Arabidopsis thaliana. Plant Cell. 2005;17:3282–3300. doi: 10.1105/tpc.105.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salehin M, et al. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019;10:4021. doi: 10.1038/s41467-019-12002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Groover AT, Pattishall A, Jones AM. IAA8 expression during vascular cell differentiation. Plant Mol. Biol. 2003;51:427–435. doi: 10.1023/A:1022039815537. [DOI] [PubMed] [Google Scholar]
- 61.Lakehal A, et al. A molecular framework for the control of adventitious rooting by TIR1/AFB2-Aux/IAA-dependent auxin signaling in Arabidopsis. Mol Plant. 2019;12:1499–1514. doi: 10.1016/j.molp.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Arase F, et al. IAA8 involved in lateral root formation interacts with the TIR1 auxin receptor and ARF transcription factors in. Arabidopsis. PloS One. 2012;7:e43414. doi: 10.1371/journal.pone.0043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellini C, Pacurar DI, Perrone I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014;65:639–666. doi: 10.1146/annurev-arplant-050213-035645. [DOI] [PubMed] [Google Scholar]
- 64.Kitomi Y, Inahashi H, Takehisa H, Sato Y, Inukai Y. OsIAA13-mediated auxin signaling is involved in lateral root initiation in rice. Plant Sci. 2012;190:116–122. doi: 10.1016/j.plantsci.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Li X, et al. The systemic acquired resistance regulator OsNPR1 attenuates growth by repressing auxin signaling through promoting IAA-amido synthase expression. Plant Physiol. 2016;172:546–558. doi: 10.1104/pp.16.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang G, Xu N, Chen H, Wang G, Huang J. OsMADS25 regulates root system development via auxin signalling in rice. Plant J. 2018;95:1004–1022. doi: 10.1111/tpj.14007. [DOI] [PubMed] [Google Scholar]
- 67.Coudert Y, et al. Transcript profiling of crown rootless1 mutant stem base reveals new elements associated with crown root development in rice. BMCGenom. 2011;12:387. doi: 10.1186/1471-2164-12-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beier S, et al. Construction of a map-based reference genome sequence for barley, Hordeum vulgare L. Sci Data. 2017;4:170044. doi: 10.1038/sdata.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor, J. S. & Raes, J. The evolution of the genome (ed T. Ryan Gregory) 289-327 (Academic Press (2005).
- 70.Hurles M. Gene duplication: the genomic trade in spare parts. PLoS Biol. 2004;2:E206. doi: 10.1371/journal.pbio.0020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akhunov ED, et al. Comparative analysis of syntenic genes in grass genomes reveals accelerated rates of gene structure and coding sequence evolution in polyploid wheat. Plant Physiol. 2013;161:252–265. doi: 10.1104/pp.112.205161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh, V. K. & Jain, M. Genome-wide survey and comprehensive expression profiling of Aux/IAA gene family in chickpea and soybean. Front. Plant Sci6, 10.3389/fpls.2015.00918 (2015). [DOI] [PMC free article] [PubMed]
- 73.Luo S, et al. Genome-wide identification, classification, and expression of phytocyanins in Populus trichocarpa. Planta. 2018;247:1133–1148. doi: 10.1007/s00425-018-2849-2. [DOI] [PubMed] [Google Scholar]
- 74.Kong W, et al. Evolutionary analysis of GH3 genes in six oryza species/subspecies and their expression under salinity stress in Oryza sativa ssp. Japonica. Plants. 2019;8:30. doi: 10.3390/plants8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tombuloglu H. Genome-wide analysis of the Auxin Response Factors (ARF) gene family in barley (Hordeum vulgare L.) J. Plant Biochem. Biotechnol. 2019;28:14–24. [Google Scholar]
- 76.Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 77.Mao DH, Chen CY. Colinearity and similar expression pattern of rice DREB1s reveal their functional conservation in the cold-responsive pathway. PloS One. 2012;7:e47275. doi: 10.1371/journal.pone.0047275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao J, et al. Genome-wide survey of Aux/IAA gene family members in potato (Solanum tuberosum): Identification, expression analysis, and evaluation of their roles in tuber development. Biochem. Biophys. Res. Commun. 2016;471:320–327. doi: 10.1016/j.bbrc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Flores-Sandoval E, Eklund DM, Bowman JL. A simple auxin transcriptional response system regulates multiple morphogenetic processes in the liverwort Marchantia polymorpha. PLoS Genet. 2015;11:e1005207–e1005207. doi: 10.1371/journal.pgen.1005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kato H, et al. Auxin-mediated transcriptional system with a minimal set of components is critical for morphogenesis through the life cycle in Marchantia polymorpha. PLoS Genet. 2015;11:e1005084–e1005084. doi: 10.1371/journal.pgen.1005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lavy M, et al. Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. eLife. 2016;5:e13325. doi: 10.7554/eLife.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ori N. Dissecting the biological functions of ARF and Aux/IAA genes. Plant Cell. 2019;31:1210–1211. doi: 10.1105/tpc.19.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pourabed, E., Golmohamadi, F. G., Monfared, P. S., Razavi, S. M. & Shobbar, Z.-S. Basic Leucine Zipper family in barley: Genome-wide characterization of members and expression analysis. Mol. Biotechnol. 57 (2015). [DOI] [PubMed]
- 84.Guo B, et al. Genome-wide analysis of APETALA2/Ethylene-Responsive Factor (AP2/ERF) gene family in barley (Hordeum vulgare L.) PloS One. 2016;11:e0161322. doi: 10.1371/journal.pone.0161322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Higgins JD, et al. Spatiotemporal asymmetry of the meiotic program underlies the predominantly distal distribution of meiotic crossovers in barley. Plant Cell. 2012;24:4096–4109. doi: 10.1105/tpc.112.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szemenyei H, Hannon M, Long JA. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science. 2008;319:1384–1386. doi: 10.1126/science.1151461. [DOI] [PubMed] [Google Scholar]
- 87.Leyser O. Auxin signaling. Plant Physiol. 2018;176:465–479. doi: 10.1104/pp.17.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pierre-Jerome E, Moss BL, Lanctot A, Hageman A, Nemhauser JL. Functional analysis of molecular interactions in synthetic auxin response circuits. Proc. Natl. Acad. Sci. USA. 2016;113:11354–11359. doi: 10.1073/pnas.1604379113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sato A, Yamamoto KT. Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol. Plant. 2010;133:397–405. doi: 10.1111/j.1399-3054.2008.01055.x. [DOI] [PubMed] [Google Scholar]
- 90.Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- 91.Ploense SE, Wu M-F, Nagpal P, Reed JW. A gain-of-function mutation in IAA18 alters Arabidopsis embryonic apical patterning. Development. 2009;136:1509–1517. doi: 10.1242/dev.025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ouellet F, Overvoorde PJ, Theologis A. IAA17/AXR3: biochemical insight into an auxin mutant phenotype. Plant Cell. 2001;13:829. doi: 10.1105/tpc.13.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim J, Harter K, Theologis A. Protein-protein interactions among the Aux/IAA proteins. Proc. Natl. Acad. Sci. USA. 1997;94:11786–11791. doi: 10.1073/pnas.94.22.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jans DA. The regulation of protein transport to the nucleus by phosphorylation. Biochem. J. 1995;311(Pt 3):705–716. doi: 10.1042/bj3110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guilfoyle TJ. The PB1 domain in auxin response factor and Aux/IAA proteins: a versatile protein interaction module in the auxin response. Plant Cell. 2015;27:33–43. doi: 10.1105/tpc.114.132753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Colón-Carmona A, Chen DL, Yeh KC, Abel S. Aux/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol. 2000;124:1728–1738. doi: 10.1104/pp.124.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jain M, Khurana JP. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J. 2009;276:3148–3162. doi: 10.1111/j.1742-4658.2009.07033.x. [DOI] [PubMed] [Google Scholar]
- 98.Song YL, You J, Xiong LZ. Characterization of OsIAA1 gene, a member of rice Aux/IAA family involved in auxin and brassinosteroid hormone responses and plant morphogenesis. Plant Mol. Biol. 2009;70:297–309. doi: 10.1007/s11103-009-9474-1. [DOI] [PubMed] [Google Scholar]
- 99.Luo, S. et al. Constitutive expression of OsIAA9 affects starch granules accumulation and root gravitropic response in Arabidopsis. Front. Plant Sci6##2015). [DOI] [PMC free article] [PubMed]
- 100.Zhu ZX, et al. A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol Plant. 2012;5:154–161. doi: 10.1093/mp/ssr074. [DOI] [PubMed] [Google Scholar]
- 101.Ive, D. S. et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA107 (2010). [DOI] [PMC free article] [PubMed]
- 102.Abel S, Nguyen MD, Theologis A. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J. Mol. Biol. 1995;251:533–549. doi: 10.1006/jmbi.1995.0454. [DOI] [PubMed] [Google Scholar]
- 103.Ainley WM, Walker JC, Nagao RT, Key JL. Sequence and characterization of two auxin-regulated genes from soybean. J. Biol. Chem. 1988;263:10658–10666. [PubMed] [Google Scholar]
- 104.Liu K, et al. Genome-wide analysis and characterization of Aux/IAA family genes related to fruit ripening in papaya (Carica papaya L.) BMC Genom. 2017;18:351. doi: 10.1186/s12864-017-3722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ku SJ, Park JY, Ha SB, Kim J. Overexpression of IAA1 with domain II mutation impairs cell elongation and cell division in inflorescences and leaves of Arabidopsis. J. Plant Physiol. 2009;166:548–553. doi: 10.1016/j.jplph.2008.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.