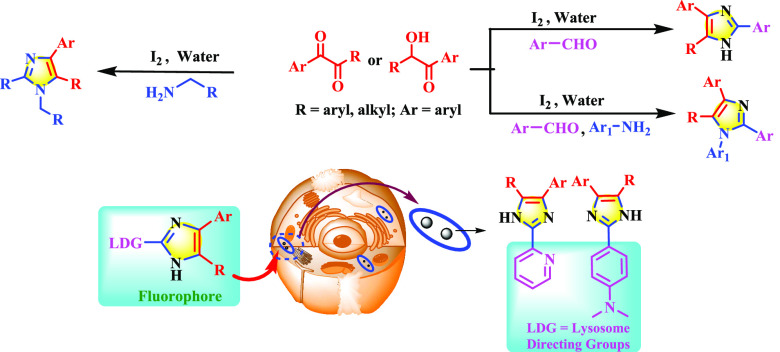
Abstract
An iodine-catalyzed, environmentally benign one-pot methodology has been developed for the synthesis of diverse substituted imidazoles. This transition-metal-free, aerobic, water-mediated cyclization reaction is operationally simple and works well with different amines or aldehydes by multiple C–N bond formations with satisfactory yield. The methodology is regioselective as well as scalable. These imidazole derivatives show excellent fluorescence properties both in the solid and solution phase, which is further extended to live-cell imaging. Due to the suitable fluorescence properties of these scaffolds, lysosome-directing groups are incorporated in two of these derivatized imidazoles to track intracellular lysosomes. Successfully, those molecules show bright blue fluorescence while detecting lysosomes in human or murine cells and can be considered to be rapid lysosome-staining probes.
Introduction
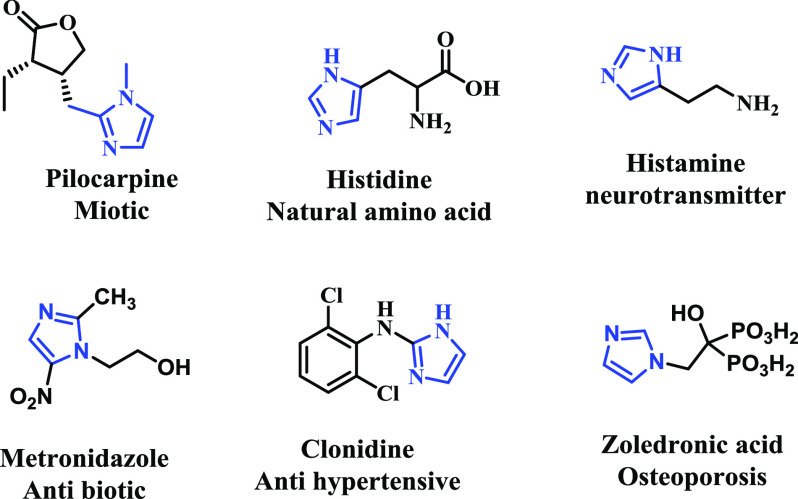
Imidazoles are the most important privileged nitrogen-containing heterocyclic scaffolds present in many natural products and pharmaceutical drugs (Figure 1).1−5 They are known to exhibit a broad range of biological activities, such as anticancer, antimicrobial, antihypertensive, and protein kinase inhibitor properties.6−10 Apart from these activities, imidazole-containing molecules are also reported to exhibit fluorescence properties. These properties are further utilized in metal sensing, biological imaging applications, and organic light-emitting diodes (OLEDs).11−15 A lysosome is an important organelle in eukaryotic cells that is involved in the degradation of foreign internalized particles. Lysosomes also play an active role in autophagy, cellular metabolism and recycling. Thus, it becomes an important candidate for immunological research, where the resolution of infection is often dependent on lysosome-mediated degradation of engulfed pathogens by phagocytic immune cells, such as macrophages and neutrophils. Lysosome-dependent processing of pathogens is also related to antigen display and antibody production.16 These organelles are involved in many cellular signaling functions, including intracellular transport, cell antigen processing, and the initiation of apoptosis.17,18 Lysosomes are acidic, membrane-bound organelles (pH ≤5) present in cells. Dysfunctions of lysosomes have been implicated in several diseases, such as tumour generation and neurodegenerative diseases.16 Selective probing of these organelles with small fluorescent molecules has been reported recently, and further, these probes are useful to reveal the underlying mechanism behind the cause of diseases.19
Figure 1.
Some of the important imidazole-containing natural products and drugs.
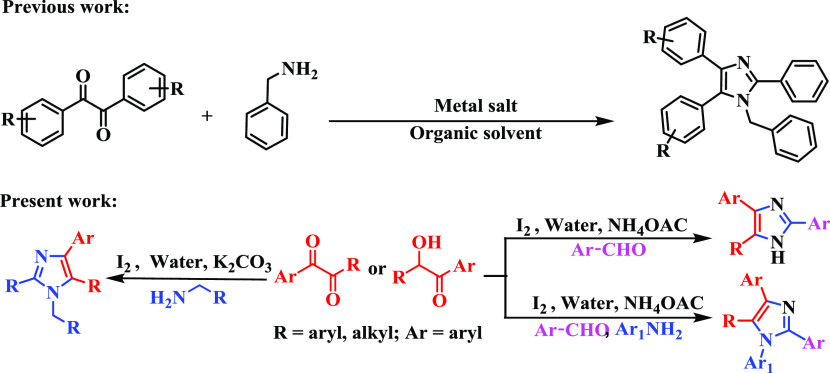
In the previous literature, these imidazole molecules were synthesized using transition-metal-catalyzed approaches, with transition metals such as copper, palladium, silver, etc., but these synthetic approaches practically have several drawbacks, such as moisture and air sensitivity, requirement of careful handling, hazardous and harsh conditions, heavy metal poisoning, and scale-up difficulties.20−25 These synthetic limitations have alerted chemists to find new environment-friendly pathways to construct small organic molecules. In view of the above considerations, metal-free organic reactions have gained much importance and have undergone alterations in both industry and academia. Studies revealed that molecular iodine has the ability to functionalize C–H bonds to form new C–C and also C–heteroatom bonds.25−31 Iodine has also gained much attention in synthetic organic methodologies due to its low toxicity, metal-like characteristics, environmentally benign nature, and ease of handling in the laboratory, making it more suitable as a catalyst.32,33 Accordingly, iodine serves as an alternative catalyst instead of transition metals in many organic reactions.34,35
In recent times, several organic reactions have been effectively scrutinized in water medium to avoid organic solvents due to their toxic nature.36 As water is a non-flammable, non-hazardous, non-volatile, and nontoxic solvent, nature drives all reactions in aqueous medium. In the face of severe environmental pollution due to various toxins, the “green chemistry” methodology is now a very popular approach. For this reason, synthetically more straightforward and convergent reactions taking water as the green reaction medium is of key interest at present. Therefore, because of the worldwide demand for environmentally benign organic syntheses, and our continuing research interest in developing strategies for iodine-catalyzed small heterocycle synthesis, herein we developed aerobic iodine-catalyzed oxidative Csp3–H functionalization from readily available starting materials to synthesize highly substituted imidazoles in the aqueous medium.37 This approach has a broad substrate scope, is regioselective in nature, and also tolerates gram-scale synthesis (Scheme 1).
Scheme 1. Synthetic Strategies for Imidazoles from 1,2-Diketone and α-Hydroxy Ketone.
Interestingly, all of these molecules show good fluorescence properties. We further utilized these properties to visualize lysosomes in live cells. Considering the acidic nature of lysosomes, two molecules bearing a weak basic group were selected from the series and studied for selective lysosome tracking. Observations revealed that these molecules could permeate into cells and selectively go into the targeted lysosome, giving blue fluorescence. Thus, it can be an added value for organelle-targetable fluorescent probes (OTFPs).
Results and Discussion
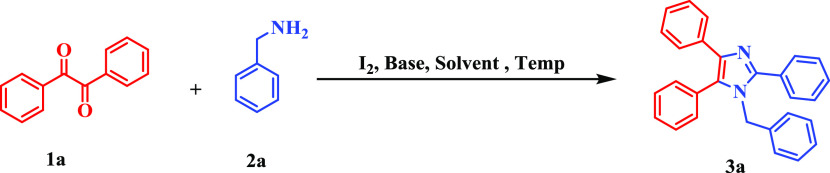
To identify the optimal reaction conditions, easily accessible benzil (1a) and benzylamine (2a) were selected as model substrates for the optimization of the reaction (Table 1). The initial screening reaction began with the treatment of 1a and 2a with 20 mol % iodine in water at room temperature (rt) under open-air conditions for 6 h, affording the desired product 1-benzyl-2,4,5-triphenyl-1H-imidazole (3a) in 32% yield. Increasing the amount of iodine to 30 mol % provided the desired product 3a in 38% yield. The yield did not change remarkably with a further increase in the amount of iodine to 40 mol % (entries 1–3). Temperature screening revealed that 70 °C was optimal for the desired transformation (entries 4–6) and further increasing the temperature resulted in no improvement of the yield (entry 7). Different bases were then scrutinized to improve the yield, and K2CO3 was found to be the best one with maximum yield of up to 94% (entries 8–14). There was no product formation observed in the presence of N2 atmosphere (entry 15) and without the catalyst (entry 16), respectively. Other iodine catalysts, such as tetrabutylammonium iodide (TBAI) and KI, were not efficient in this protocol (entries 17 and 18). From the studies, the reaction of 1a (1 equiv) with 2a (2 equiv) with 30 mol % iodine in water (2 mL) under open-air conditions at 70 °C for 6 h (entry 8) was established as the optimal reaction conditions. A previous literature study revealed that an α-hydroxy ketone (benzoin) could be oxidized to a diketone (benzil) in the presence of an oxidant, and hence, benzoin was also used in this methodology.38
Table 1. Optimization of the Reaction Conditionsa.
| entry | catalyst (mol %) | base (3 equiv) | solvent | temp (°C) | oxidant | yield (%) 3a |
|---|---|---|---|---|---|---|
| 1 | I2 (20) | H2O | rt | air | 32 | |
| 2 | I2 (30) | H2O | rt | air | 38 | |
| 3 | I2 (40) | H2O | rt | air | 39 | |
| 4 | I2 (30) | H2O | 50 | air | 62 | |
| 5 | I2 (30) | H2O | 60 | air | 65 | |
| 6 | I2 (30) | H2O | 70 | air | 71 | |
| 7 | I2 (30) | H2O | 80 | air | 71 | |
| 8 | I2 (30) | K2CO3 | H2O | 70 | air | 94 |
| 9 | I2 (30) | NaHCO3 | H2O | 70 | air | 69 |
| 10 | I2 (30) | Na2CO3 | H2O | 70 | air | 74 |
| 11 | I2 (30) | Cs2CO3 | H2O | 70 | air | trace |
| 12 | I2 (30) | TEA | H2O | 70 | air | trace |
| 13 | I2 (30) | DBU | H2O | 70 | air | trace |
| 14 | I2 (30) | pyridine | H2O | 70 | air | trace |
| 15 | I2 (30) | K2CO3 | H2O | 70 | N2-atm | 0 |
| 16 | K2CO3 | H2O | 70 | air | 0 | |
| 17 | TBAI | K2CO3 | H2O | 70 | air | trace |
| 18 | KI | K2CO3 | H2O | 70 | air | trace |
Reaction conditions: 1a (0.5 mmol, 1 equiv), 2a (1.0 mmol, 2 equiv), catalyst (0.15 mmol, 0.3 equiv) in solvent (2.0 mL) for 6 h; H2O = water, rt = room temperature.
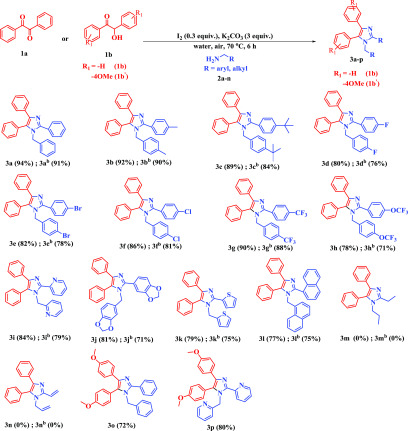
With the best reaction conditions in hand, the scope of this methodology was studied thoroughly with a diverse range of amines bearing electron-releasing and electron-deficient groups. As shown in Scheme 2, the reactions were very clean and the desired imidazoles were observed in satisfactory yield. Substitution of amine on the phenyl ring containing an electron-neutral (4-H), electron-donating (4-Me, 4-t-Bu), and electron-withdrawing (4-CF3) group successfully took place under the above reaction conditions, producing the desired product in good yield (Scheme 2, entries 3a/3ab–3c/3cb and 3g/3gb). Under the optimal reaction conditions, different halogen-substituted amines underwent a smooth conversion affording the corresponding imidazoles in moderate yields, which gave chances for further functionalization (Scheme 2, entries 3d/3db–3f/3fb). The scope of this methodology was again estimated using amines that contain trifluoromethoxy (−OCF3), naphthyl, and heterocyclic moieties (Scheme 2, entries 3h/3hb, 3l/3lb, and 3i/3ib–3k/3kb). The trifluoromethoxy amine offered the desired imidazoles 3h (78% yield) and 3hb (71% yield), and naphthyl amine provided 3l (77% yield) and 3lb (75% yield), respectively. Heterocyclic amines, including pyridyl, and other amines successfully underwent a smooth conversion, furnishing the corresponding imidazoles 3i/3ib–3k/3kb in good to moderate yields (Scheme 2). The versatility of the methodology was checked with the α-hydroxy ketone, 2-hydroxy-1,2-bis(4-methoxyphenyl)ethanone (1b′). The optimized reaction conditions facilitated smooth conversion with different amines, including heterocyclic amines with good responses (Scheme 2, entries 3o and 3p). All of the synthesized imidazoles were characterized by NMR spectroscopy and mass spectrometry. The exact structure of 3l was further confirmed by X-ray single-crystal analysis (see p S60 and S61, Supporting Information (SI)).
Scheme 2. Substrate Scope of Amines and α-Hydroxy Ketones.
Reaction conditions: 1aa/1bb(0.5 mmol, 1 equiv), 2a–n (1.0 mmol, 2 equiv), I2 (0.15 mmol, 0.3 equiv), K2CO3 (1.5 mmol, 3 equiv), H2O (2 mL) in open air at 70 °C.
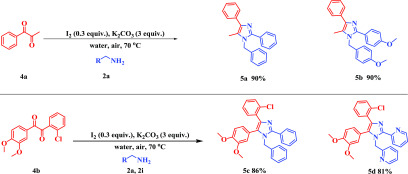
This methodology produced exclusively one regioisomer product in the case of unsymmetrical diketones, including aryl alkyl diketones with satisfactory yield (5a–d, Scheme 3). The structure of the unsymmetrical imidazole was further confirmed by a single-crystal X-ray diffraction study of compound 5a (see p S63 and S64, SI). Next, an aliphatic diketone was introduced in the reaction to test the feasibility of the protocol, but unfortunately, the reaction did not proceed (Scheme 4).
Scheme 3. Scope of 1,2-Diketone Derivatives with Amines.
Reaction conditions: 4a and 4b (0.5 mmol, 1 equiv), 2a and 2i (1.0 mmol, 2 equiv), I2 (0.15 mmol, 0.3 equiv), K2CO3 (1.5 mmol, 3 equiv), H2O (2 mL) in open air at 70 °C.
Scheme 4. Scope of Aliphatic Diketones.
Reaction conditions: 6a (0.5 mmol, 1 equiv), 2a (1.0 mmol, 2 equiv), I2 (0.15 mmol, 0.3 equiv), K2CO3 (1.5 mmol, 3 equiv), H2O (2 mL) in open air at 70 °C.
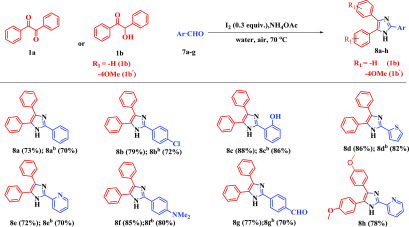
Encouraged by these results, we turned our attention to three-component reactions to extend the scope and practicability of the methodology. While surveying the previous literature, many reports were found based on three-component reactions for triaryl-substituted imidazole synthesis.39−41 In this work, the three-component reaction was examined using diketone/α-hydroxy ketone (1a/b), aromatic aldehydes (7a–g), and ammonium acetate. Here, aromatic aldehydes and ammonium acetate were used instead of benzylamine. Several aldehydes containing electron-donating and electron-withdrawing groups provided the desired triaryl-substituted imidazoles in moderate to good yield (8a–g, Scheme 5). Moreover, the reaction of 2-hydroxy-1,2-bis(4-methoxyphenyl)ethan-1-one (anisoin) (1b′) was examined by reacting with pyridine-2-carbaldehyde (7e) and ammonium acetate producing the desired 8h in 78% yield (Scheme 5).
Scheme 5. Scope of Triarylimidazoles from Aromatic Aldehydes.
Reaction conditions: 1a/b (0.5 mmol, 1 equiv), 7a–g (0.5 mmol, 1 equiv), NH4OAc (1.0 mmol, 2 equiv), I2 (0.15 mmol, 0.3 equiv), H2O (2 mL) in open air at 70 °C.
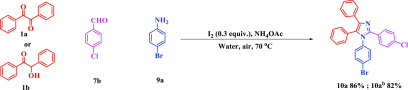
After successful achievement of the synthesis of triaryl-substituted imidazoles, the versatility of this one-pot reaction protocol was further tested for the synthesis of tetraaryl-substituted imidazoles. Thus, the diketone/α-hydroxy ketone (1a/1b), aromatic aldehyde (7b), aromatic amine (9a), and ammonium acetate successfully reacted with each other to furnish the corresponding product satisfactorily (Scheme 6, entry 10a).
Scheme 6. Scope of Tetraarylimidazoles from Aromatic Amines.
Reaction conditions: 1a/b (0.5 mmol, 1 equiv), 7b (0.5 mmol, 1 equiv), 9a (0.5 mmol, 1 equiv), NH4OAc (1.0 mmol, 2 equiv), I2 (0.15 mmol, 0.3 equiv), H2O (2 mL) in open air at 70 °C.
After examining the reaction scope, the span of the flexibility of this protocol was successfully extended to the gram-scale level for benzil (1a), benzoin (1b), anisoin (1b′), and aryl alkyl ketone (4a) with different amines, including heterocyclic ones. In each case, satisfactory results were observed (Table 2).
Table 2. Gram-Scale Synthesis.
| diketone | α-hydroxy ketone | amines/aldehyde | product from diketone | yield from diketone (%) | product from α-hydroxy ketone | yield from α-hydroxy ketone (%) |
|---|---|---|---|---|---|---|
| 1a | 2a | 3a | 85 | |||
| 1b′ | 2i | 3ib | 73 | |||
| 1b′ | 2i | 3p | 78 | |||
| 4a | 2a | 5a | 76 | |||
| 1a | 7e | 8e | 66 |
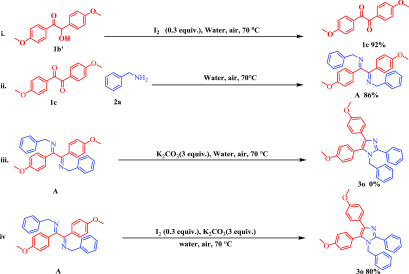
After a successful evaluation of the reaction scope, the mechanistic aspect of the methodology was next investigated. To establish the mechanism, some experiments were performed. Initially, benzil (1a) and benzylamine (2a) reacted with each other under the optimized reaction conditions in the presence of radical capture reagents, such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and 2,6-di-tert-butyl-4-methylphenol (BHT), which proceeded smoothly to form the product 3a in 93 and 92% yield, respectively, indicating the reaction may not proceed through a radical mechanism pathway (Scheme 7). Thereafter, some control experiments were performed (Scheme 8). α-Hydroxy ketone (1b′) was oxidized to the diketone product (1c) in the presence of iodine (Scheme 8i). The diketone product 1c then reacted with amine (2a) to give the addition product A (isolated and characterized), followed by cyclization to give the final product (3o) (Scheme 8).
Scheme 7. Reaction in the Presence of Radical Capture Reagents.
Scheme 8. Control Experiments.
(i) Formation of diketone (1c) from α-hydroxy ketone (1b′) by oxidation in the presence of iodine. (ii) Formation of the addition product A. (iii) Standard condition reaction in the absence of iodine. (iv) Standard condition reaction.
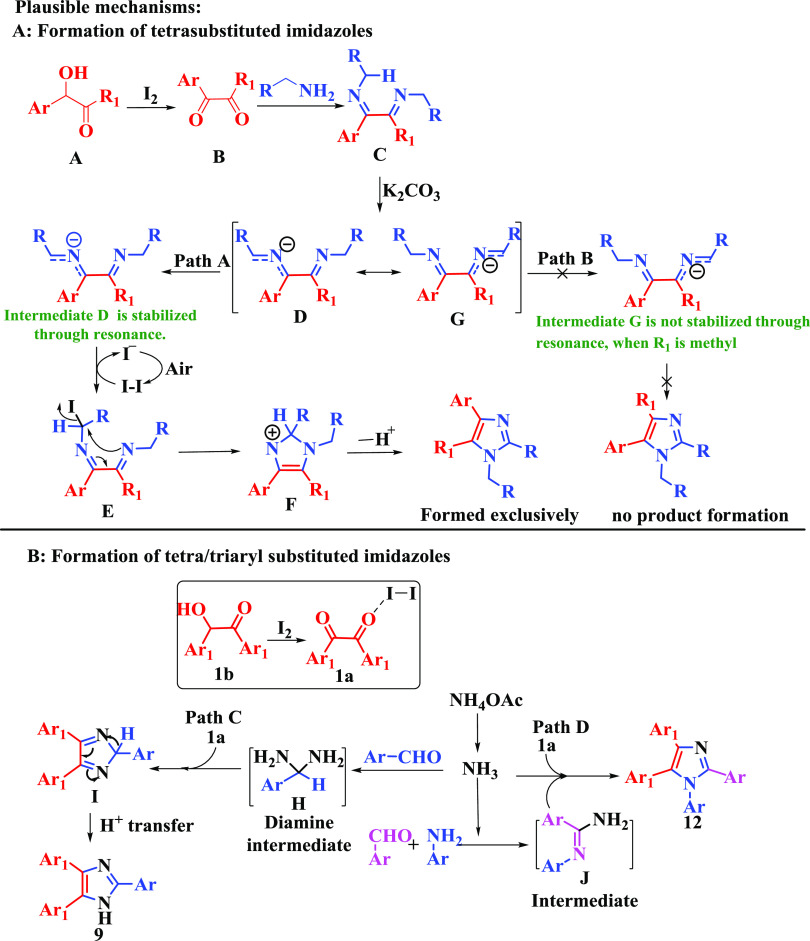
Based on the above control experiments and with supporting references, a plausible mechanism is proposed for the formation of both tri- and tetrasubstituted imidazoles in Scheme 9. For tetrasubstituted imidazole formation, first, the α-hydroxyl ketone (A) is oxidized to a diketone (B) in the presence of iodine.42 Next, the diketone (B) is converted to the addition product (C) via simple addition with amine (2 equiv) (2). Two equivalents of aryl/heteroaryl amine form the Schiff base on both carbonyls in dicarbonyl compounds (Scheme 9B), making the addition product (Scheme 9C), whereas 1 equiv of aryl/heteroaryl amine forms the Schiff base only at the selected carbonyl centre in dicarbonyl compounds that form the desired product, oxazole derivatives.37 This addition product forms two possible anionic intermediates (D and G) in the presence of base (K2CO3). Here, we expect that the formation of two regioisomers is possible, but only one regioisomer is formed in the reaction medium. It is suspected that the anionic stability of these intermediates limits the reaction to the formation of only one regioisomer in the case of the unsymmetrical diketone. The stable anionic intermediate (D) further undergoes iodine-catalyzed oxidative cyclization to yield the desired product.
Scheme 9. Plausible Mechanism.
For tri/tetraaryl-substituted imidazole formation, initially, the α-hydroxyl ketone (1b) is oxidized to a diketone (1a). Then, in situ generated ammonia from ammonium acetate43 will react via two pathways, i.e., path C and path D, to give the desired triaryl- and tetraaryl-substituted imidazoles, respectively. In path C, ammonia reacts with the aldehyde to form a diamine intermediate (H). This diamine intermediate (H) is added to 1a to give an imino intermediate (I),44 which upon proton transfer gives the triaryl-substituted imidazoles (9). In path D, the aldehyde, aromatic amine, and ammonia form intermediate J. Finally, this intermediate J reacts with 1a to form tetraaryl-substituted imidazoles (12).
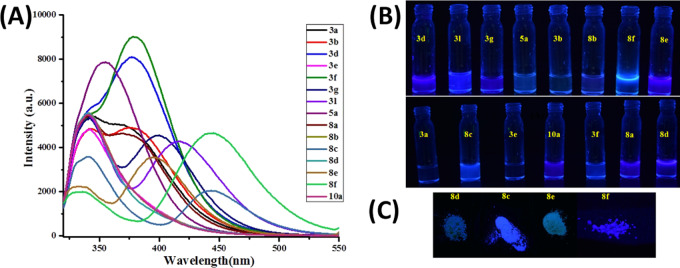
Next, the photophysical properties of tri- and tetrasubstituted imidazole derivatives were explored. Substituted imidazole derivatives showed good fluorescence responses in the blue range when irradiated at 365 nm. Fluorescence of some selected imidazole derivatives was recorded in dimethyl sulfoxide (DMSO) (Figure 2) and is reported in this paper. It was observed that the imidazole derivatives containing electron-donating groups (i.e., OH, NMe2) as well as highly conjugated groups (i.e., naphthyl) exhibited increased fluorescence responses (Figure 2: 3l, 8c, and 8f). Among them, 3l, 8c, and 8f showed the best fluorescence responses. Solid-state fluorescence of selected compounds was captured by irradiating at 365 nm.
Figure 2.
(A) Fluorescence spectra of some selected imidazole derivatives (500 nM) in DMSO (λex= 290–330 nm). (B) Fluorescence of 10 μM DMSO solution of selected compounds captured by irradiating at 365 nm. (C) Solid-state fluorescence of selected compounds captured by irradiating at 365 nm.
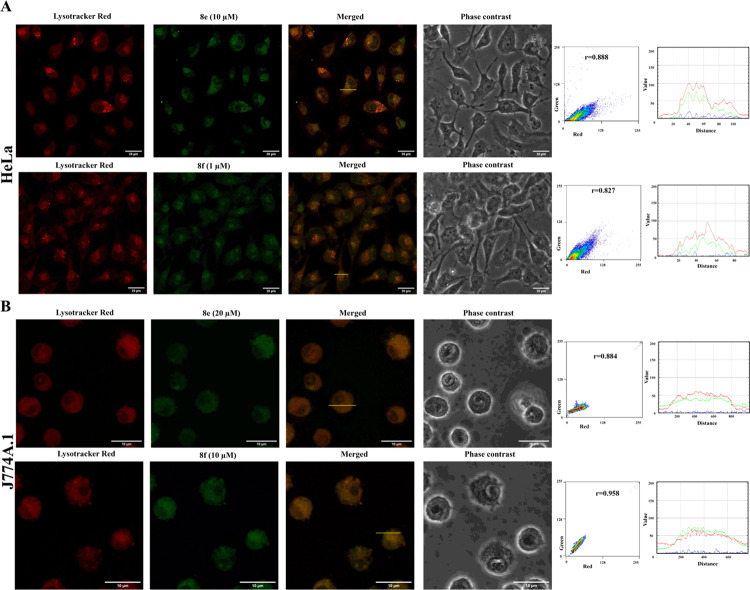
The bright blue fluorescence of these polysubstituted imidazoles encouraged us to explore their application as biological intracellular probes. Accordingly, some derivatives were preinstalled with lysosome-directing groups into the parent compound as a lysosome-detecting probe. We explored the possible applications of the fluorescence properties of two selected molecules for imaging lysosomes in a representative human cervical cancer cell line (HeLa) and a murine macrophage cell line (J774A.1). Two compounds, 8e and 8f, having strong fluorescence properties in the UV (4′,6-diamidino-2-phenylindole (DAPI)) channel were preinstalled with lysosome-directing groups.
Both HeLa and J774A.1 cells were mixed separately with these compounds along with a commercially available lysosome-specific stain, the LysoTracker Red DND-99 dye (100 nmol), and incubated for 30 min at 37 °C. Stained HeLa cells were subsequently fixed with paraformaldehyde, while murine macrophages (J774A.1) were observed without any fixation. In both cell lines, the compounds were found to be membrane-permeant and localized within the cytoplasm (Figure 3). Although the compounds fluoresced in the DAPI channel, signals from the blue channel were converted to a green color to easily observe their localization inside cells.
Figure 3.
HeLa cells (A) and J774A.1 cells (B) were stained with LysoTracker Red (100 nM) and compounds 8e and 8f were imaged via confocal microscopy to observe cellular localization of the synthesized dyes. Colocalization between a synthesized dye (green channel) and LysoTracker (red channel) was calculated from Pearson’s correlation coefficient from two-dimensional (2D) histograms. Intensity profiles of RGB channels along a linear region of interest (ROI), as indicated with a yellow line, were also analyzed.
Intracellular localization of our synthesized molecules and the LysoTracker Red DND-99 dye was compared in both cell lines. Confocal microscopy revealed that both compounds 8e and 8f were localized in the same compartment as LysoTracker Red (Figure 3) with high Pearson’s correlation coefficient values (greater than 0.5). Also, the macrophage cell morphology was not affected due to incubation with a higher concentration of compounds. These compounds were used to successfully observe lysosomes in living as well as fixed cells, and cellular distributions of the dyes were also not affected by paraformaldehyde-based fixation.
Conclusions
In summary, we have successfully developed a transition-metal-free, environmentally benign method for the synthesis of both tetra- and trisubstituted imidazoles from readily available starting materials via an iodine-catalyzed areal oxidative one-pot reaction in water for the first time. This method with no toxic byproducts has advantages such as peroxide- and organic-solvent-free reactions. The mild procedure is cost-effective, atom-economic, and scalable. Thus, it is more practically applicable. In addition, this synthetic methodology was successfully employed at the gram-scale level. Some of these imidazole derivatives also showed excellent fluorescence responses. Two such synthesized molecules 8e and 8f were modified with lysosome-directing groups and were found to be successfully colocalized with LysoTracker Red in cell-imaging studies having very high Pearson coefficient values, and hence, these two molecules are new additions to organelle-targetable fluorescent probes. Therefore, we believe that the current methodology produces some very useful polysubstituted imidazole derivatives by an environmentally benign method.
Experimental Section
General Information
All of the necessary chemicals and organic solvents that were utilized for the methodology were acquired from Sigma-Aldrich, Thermo Fischer Scientific, and TCI chemicals. These were used without additional purification unless otherwise noted. The melting point of the final imidazole derivatives was determined using a one side open capillary tube. Analytical thin-layer chromatography (TLC) was performed using silica gel 60 F254 aluminum TLC sheets. The developed chromatogram was visualized by UV absorbance. For purification of the crude imidazole mixture, silica gel (100–200 and 230–400 mesh) was utilized for column chromatographic separations. The structure of the tetra- and trisubstituted imidazoles was confirmed using 1H NMR, 13C NMR, electron ionization (EI)-mass, and electrospray ionization (ESI)-mass spectrometry studies. NMR spectra of the imidazole derivatives were recorded on a Bruker 600 MHz spectrometer and a JEOL RESONANCE 400 MHz spectrometer. Deuterated NMR solvents, CDCl3 and DMSO-d6, were utilized for recording NMR spectra and tetramethylsilane (TMS) was used as the internal standard. Chemical shifts (δ) are given in parts per million (ppm) relative to TMS (δ = 0.00). The coupling constants (J) of the NMR spectra are expressed in hertz (Hz). All copies of 1H and 13C NMR spectra are attached in the Supporting Information. High-resolution mass spectrometry (HRMS) (m/z) and ESI mass data analysis were performed using EI techniques (JEOL-JMS 700 spectrometer) and an LCQ-ORBITRAP-XL instrument, respectively. For solving the crystal structure of the selected imidazole derivatives (3l and 5a), a Bruker Kappa Apex II X-ray crystallography instrument was used. Singlet (s), doublet (d), double doublet (dd), triplet (t), and multiplet (m) were used for describing 1H NMR multiplicity patterns.
Spectroscopic Measurements
Spectroscopic grade DMSO solvent was used for preparing the solutions of imidazole derivatives. A Shimadzu UV-1800 spectrophotometer was used for recording the UV–vis absorption spectra of the selected imidazole derivatives. A Hitachi F-7000 fluorescence spectrophotometer was used for recording the fluorescence spectra of the mentioned imidazole derivatives. High-quality quartz cuvettes were utilized while recording the fluorescence spectra at room temperature.
Synthetic Procedures
General Experimental Procedure for the Preparation of 1,2,4,5-Tetrasubstituted Imidazoles (3a–p, 5a–d)
1,2,4,5-Tetrasubstituted imidazoles were prepared by a one-pot reaction using a mixture of benzil (1a)/benzoin (1b) (0.50 mmol, 1 equiv) and the corresponding amine (2a–n) (1 mmol, 2 equiv) in water (2 mL) medium. The reaction was carried out by taking iodine (0.15 mmol, 0.3 equiv, 38.08 mg) as a catalyst in open air at 70 °C for 6 h. The complete conversion of the reactants into desired products was checked by TLC, and the reaction mixture was allowed to cool to room temperature. Then, a solid appeared, which was treated with 10% aqueous sodium thiosulfate (Na2S2O3) solution to remove the excess iodine. The aqueous phase was then extracted with EtOAc. The combined organic phase was then dried with anhydrous Na2SO4 and filtered, followed by concentration using a rotary evaporator under reduced pressure. Column chromatography (silica gel, 100–200 and 230–400 mesh) was used to purify the crude mixture eluting with EtOAc and petroleum ether to afford the desired 1,2,4,5-tetrasubstituted imidazoles (3a–p, 5a–d).
General Experimental Procedure for the Preparation of Triaryl-Substituted Imidazoles (8a–h)
Triaryl-substituted imidazoles were prepared using a one-pot reaction methodology taking a mixture of readily available starting materials, benzil (1a)/benzoin (1b) (0.50 mmol, 1 equiv), different substituted aromatic aldehydes (7a–h)(0.5 mmol, 1 equiv), and ammonium acetate (1 mmol, 2 equiv, 154.17 mg) in water (2 mL) medium. Molecular iodine (0.15 mmol, 0.3 equiv, 38.08 mg) was utilized as a catalyst here under open-air conditions at 70 °C for 6 h. The complete conversion of the reactants was checked by TLC. The reaction mixture was then allowed to cool to room temperature. Subsequently, a solid appeared, which was treated with 10% aqueous sodium thiosulfate (Na2S2O3) solution to remove the excess iodine from the reaction mixture. The aqueous phase was then extracted with ethyl acetate (EtOAc). Anhydrous Na2SO4 was used to dry the combined organic layers and filtered, followed by concentration using a rotary evaporator under a low pressure. Column chromatography (silica gel, 100–200 and 230–400 mesh) was used to purify the crude mixture eluting with EtOAc and petroleum ether to afford the desired triaryl-substituted imidazoles (8a–h).
General Experimental Procedure for the Preparation of the Tetraaryl-Substituted Imidazole (10a)
The tetraaryl-substituted imidazole was prepared using a one-pot reaction methodology taking a mixture of readily available starting materials, benzil (1a)/benzoin (1b) (0.50 mmol, 1 equiv), aromatic aldehyde (7b) (0.5 mmol, 1 equiv), aromatic amine (9a) (0.5 mmol, 1 equiv), and ammonium acetate (1 mmol, 2 equiv, 154.17 mg) in water (2 mL) medium. In particular, molecular iodine (0.15 mmol, 0.3 equiv, 38.08 mg) was used as a catalyst in open air at 70 °C for 6 h. The complete conversion of the reactants was checked by TLC. The reaction mixture was then allowed to cool to room temperature. Subsequently, a solid appeared, which was treated with 10% aqueous sodium thiosulfate (Na2S2O3) solution to remove the excess iodine from the reaction mixture. Ethyl acetate (EtOAc) was used to extract the aqueous phase and anhydrous Na2SO4 was used to dry the combined organic phases. The combined organic phase was then filtered, followed by concentration using a rotary evaporator under a very low pressure. Column chromatography (silica gel, 100–200 mesh) was used to purify the crude mixture eluting with ethyl acetate (EtOAc) and petroleum ether to afford the desired tetraaryl-substituted imidazole product (10a).
General Experimental Procedure for Scaling Up to the Gram Level for Tetrasubstituted Imidazoles (3a, 3i, 3p, and 5a)
The gram-scale level reactions were carried out by taking a mixture of diketone (1a/4a)/α-hydroxy ketone (1b/b′) (5 mmol, 1 equiv) and the amine (2a and 2i) (10 mmol, 2 equiv) in H2O (20 mL) in open air at 70 °C for 6 h. Molecular iodine (1.5 mmol, 0.3 equiv, 380.8 mg) was used as a catalyst for this transformation. The complete consumption of the reactants was checked by TLC. After that the reaction mixture was allowed to cool to room temperature. Subsequently, a solid appeared. The solid was further treated with 10% aqueous sodium thiosulfate (Na2S2O3) to remove extra iodine from the reaction mixture. The aqueous phase was then extracted with ethyl acetate (EtOAc) and the combined organic phase was dried with anhydrous Na2SO4. The dried organic phase was then filtered and concentrated utilizing a rotary evaporator under a low pressure. The crude mixture was then purified by column chromatography (silica gel, 100–200 and 230–400 mesh) with EtOAc and petroleum ether to get the desired imidazole products 3a, 3i, 3p, and 5a.
General Experimental Procedure for Scaling Up to the Gram Level for the Triaryl-Substituted Imidazole (8e)
The triaryl-substituted imidazole was prepared by taking a mixture of benzil (1a) (5.0 mmol, 1 equiv), the aromatic aldehyde (7e) (5.0 mmol, 1 equiv), and ammonium acetate (1 mmol, 2 equiv, 1541.7 mg) in H2O (20 mL) in open air and heating the reaction mixture at 70 °C for 6 h. Molecular iodine (1.5 mmol, 0.3 equiv, 380.8 mg) was used as a catalyst. The end point of the reaction was determined by checking TLC. A solid appeared after allowing the reaction mixture to cool to room temperature. Then, 10% aqueous sodium thiosulfate (Na2S2O3) solution was used to wash the reaction mixture to remove the excess iodine. EtOAc was used to extract the aqueous phase. Then, the combined organic phase was dried with anhydrous Na2SO4. The dried organic part was filtered and concentrated using a rotary evaporator under reduced pressure. Subsequently, column chromatography (silica gel, 100–200 mesh) was used to purify the crude mixture utilizing EtOAc and petroleum ether as the eluent to afford the desired product (8e).
Cell Lines and Cell Culture
A human cervical cancer cell line (HeLa) was acquired from the National Centre for Cell Science, Pune, India, and was used in the study. Cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM), supplemented with 10% fetal calf serum and 1% antibiotic, antimycotic solution and maintained at 37 °C under 5% CO2 and 95% air.
Microscopy
HeLa cells (5000) were seeded on sterilized grease-free coverslips and incubated overnight at 37 °C. The synthesized molecules (8e and 8f) were added to the culture medium at the following concentrations. After 10 min, a commercially available lysosome-specific stain, the LysoTracker Red DND-99 dye (75 nM concentration), was also added and incubated further for 20 min at 37 °C. The cells were then washed twice with phosphate-buffered saline (PBS, 0.02 M, pH 7.2) and fixed with 2% paraformaldehyde for 5 min at 25 °C. The cells were then washed with PBS, mounted on slides, and sealed. The prepared slides were imaged via an Olympus Fluoview confocal microscope (Fv10i). ImageJ software (NIH) was used to analyze the acquired images, and the extent of colocalization between the two dyes was quantified by calculating Pearson’s correlation coefficient. Similarly, histograms and RGB profiles along the linear region of interest (ROI) were also generated using the same software.
1-Benzyl-2,4,5-triphenyl-1H-imidazole (3a)
White solid; yield (3a, 94% and 3ab, 91%); Rf = 0.48 [10% EtOAc/petroleum ether (60–80 °C)]; m.p. 160–162 °C; 1H NMR (CDCl3, 600 MHz): δ 7.69 (d, J = 7.8 Hz, 2H), 7.62 (d, J = 7.8 Hz, 2H), 7.41–7.40 (m, 3H), 7.36 (d, J = 7.2 Hz, 1H), 7.34–7.32 (m, 2H), 7.25–7.21 (m, 7H), 7.17–7.15 (m, 1H), 6.82 (d, J = 6.0 Hz, 2H), 5.13 (s, 2H). 13C{1H} NMR (CDCl3, 150 MHz): δ 148.1, 138.1, 137.6, 134.5, 131.1, 131.0, 131.0, 130.1, 129.1, 129.0, 128.9, 128.7, 128.7, 128.6, 128.1, 127.4, 126.9, 126.4, 126.1, 48.3; HRMS (EI+) m/z: calcd: C28H22N2 (M+): 386.1783, found: 386.1789.
1-(4-Methyl-benzyl)-4,5-diphenyl-2-p-tolyl-1H-imidazole (3b)
White solid; yield (3b 92% and 3bb 90%); Rf = 0.46 [10% EtOAc/petroleum ether (60–80 °C)]; m.p. 132–134 °C; 1H NMR (CDCl3, 600 MHz): δ 7.65–7.64 (m, 2H), 7.61 (d, J = 8.4 Hz, 2H), 7.37–7.32 (m, 3H), 7.27–7.23 (m, 6H), 7.18–7.15 (m, 1H), 7.04 (d, J = 8.4 Hz, 2H), 6.74 (d, J = 7.8 Hz, 2H), 5.10 (s, 2H), 2.39 (s, 3H), 2.31 (s, 3H). 13C{1H} NMR (CDCl3, 150 MHz): δ 148.2, 138.8, 138.0, 137.0, 134.7, 134.7, 131.2, 131.2, 130.0, 129.3,129.3, 129.0, 128.8, 128.6, 128.2, 128.1, 126.9, 126.3, 125.9, 48.1, 21.4, 21.1; HRMS (ESI+) m/z: calcd: C30H26N2 (M + H+): 415.2174, found: 415.2177.
1-(4-tert-Butyl-benzyl)-2-(4-tert-butyl-phenyl)-4,5-diphenyl-1H-imidazole (3c)
White solid; yield (3c 89% and 3cb 84%); Rf = 0.43 [10% EtOAc/petroleum ether (60–80 °C)]; m.p. 140–142 °C; 1H NMR (DMSO-d6, 600 MHz): δ 7.57 (s, 2H), 7.45 (s, 2H), 7.41–7.39 (m, 6H), 7.25–7.19 (m, 6H), 6.66 (s, 2H), 5.11 (s, 2H), 1.28 (s, 9H), 1.18 (s, 9H). 13C{1H} NMR (CDCl3, 150 MHz): δ 151.9, 150.2, 148.1, 137.8, 134.6, 131.2, 131.1, 129.8, 128.7, 128.6, 128.4, 128.0, 128.0, 126.7, 126.2, 125.9, 125.5, 125.3, 48.1, 34.7, 34.4, 31.2, 31.2; HRMS (ESI+)m/z: calcd: C36H38N2 (M + H+): 499.3113, found: 499.3112.
1-(4-Fluoro-benzyl)-2-(4-fluoro-phenyl)-4,5-diphenyl-1H-imidazole (3d)
White solid; yield (3d 80% and 3db 76%); Rf = 0.49 [15% EtOAc/petroleum ether (60–80 °C)]; m.p. 144–146 °C; 1H NMR (DMSO-d6, 600 MHz): δ 7.68 (s, 2H), 7.45–7.41 (m, 5H), 7.30 (s, 4H), 7.19 (s, 2H), 7.12 (s, 1H), 6.99 (t, J = 8.4 Hz, 2H), 6.75 (s, 2H), 5.11 (s, 2H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 163.6, 162.4, 162.0, 160.8, 146.6, 137.3, 134.8, 133.8, 133.7, 131.3, 131.3, 130.9, 130.6, 129.5, 129.4, 128.6, 128.3, 128.2, 127.7, 127.7, 126.8, 126.5, 116.2, 116.1, 115.9, 115.8, 47.5; HRMS (ESI+) m/z: calcd: C28H20F2N2 (M + H+): 423.1673, found: 423.1676.
1-(4-Bromo-benzyl)-2-(4-bromo-phenyl)-4,5-diphenyl-1H-imidazole (3e)
Off-white solid; yield (3e 82% and 3eb 78%); Rf = 0.37 [10% EtOAc/petroleum ether (60–80 °C)]; m.p. 128–130 °C; 1H NMR (CDCl3, 600 MHz): δ 7.57–7.54 (m, 4H), 7.52–7.50 (m, 2H), 7.38 (d, J = 7.2 Hz, 1H), 7.36–7.34 (m, 4H), 7.23–7.21 (m, 4H), 7.17–7.15 (m, 1H), 6.67 (d, J = 8.4 Hz, 2H), 5.03 (s, 2H). 13C{1H} NMR (CDCl3, 150 MHz): δ 146.8, 138.5, 136.3, 134.0, 132.0, 131.9, 131.0, 130.6, 130.5, 130.3, 129.6, 129.1, 129.0, 128.2, 127.6, 126.8, 126.7, 123.5, 121.5, 47.8; HRMS (ESI+) m/z: calcd: C28H20Br2N2 (M + H+): 543.0071, found: 543.0075.
1-(4-Chloro-benzyl)-2-(4-chloro-phenyl)-4,5-diphenyl-1H-imidazole (3f)
White solid; yield (3f 86% and 3fb 81%); Rf = 0.46 [15% EtOAc/petroleum ether (60–80 °C)]; m.p. 160–162 °C; 1H NMR (CDCl3, 600 MHz): δ 7.58–7.55 (m, 4H), 7.39–7.38 (m, 3H), 7.36–7.34 (m, 2H), 7.23–7.19 (m, 6H), 7.17–7.15 (m, 1H), 6.73 (d, J = 8.4 Hz, 2H), 5.06 (s, 2H). 13C{1H} NMR (CDCl3, 150 MHz): δ 146.8, 138.4, 135.7, 135.2, 134.0, 133.4, 131.0, 130.6, 130.2, 129.2, 129.0, 129.0, 128.9, 128.2, 127.3, 126.8, 126.7, 47.7; HRMS (EI+) m/z: calcd: C28H20Cl2N2 (M+): 454.1004, found: 454.1015.
4,5-Diphenyl-1-(4-trifluoromethyl-benzyl)-2-(4-trifluoromethyl-phenyl)-1H-imidazole (3g)
White solid; yield (3g 90% and 3gb 88%); Rf = 0.44 [15% EtOAc/petroleum ether (60–80 °C)]; m.p. 158–160 °C; 1H NMR (CDCl3, 600 MHz): δ 7.77 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 8.4 Hz, 2H), 7.57 (d, J = 7.2 Hz, 2H), 7.49 (d, J = 8.4 Hz, 2H), 7.41–7.39 (m, 1H), 7.37–7.34 (m, 2H), 7.24–7.21 (m, 4H), 7.19–7.17 (m, 1H), 6.93 (d, J = 8.4 Hz, 2H), 5.18 (s, 2H). 13C{1H} NMR (CDCl3, 150 MHz): δ 146.5, 141.1, 138.9, 134.1, 133.9, 131.1, 130.9, 130.8, 130.7, 130.3, 130.2, 129.9, 129.1, 129.1, 128.3, 126.8, 126.8, 126.6, 126.5, 126.2, 125.8, 125.8, 125.8, 125.7, 124.8, 124.7, 123.0, 122.9, 121.2, 121.1, 48.1; HRMS (EI+) m/z: calcd: C30H20F6N2 (M+): 522.1531, found: 522.1535.
4,5-Diphenyl-1-(4-trifluoromethoxy-benzyl)-2-(4-trifluoromethoxy-phenyl)-1H-imidazole (3h)
White solid; yield (3h 78% and 3hb 71%); Rf = 0.53 [10% EtOAc/petroleum ether (60–80 °C)]; m.p. 154–156 °C; 1H NMR (CDCl3, 600 MHz): δ 7.67 (d, J = 8.4 Hz, 2H), 7.56 (d, J = 7.8 Hz, 2H), 7.40–7.38 (m, 1H), 7.36–7.34 (m, 2H), 7.28 (s, 2H), 7.23–7.21 (m, 4H), 7.17–7.15 (m, 1H), 7.05 (d, J = 8.4 Hz, 2H), 6.79 (d, J = 9.0 Hz, 2H), 5.10 (s, 2H). 13C{1H} NMR (CDCl3, 150 MHz): δ 149.7, 148.5, 146.7, 138.4, 135.8, 134.0, 130.8, 130.7, 130.6, 130.2, 130.1, 130.0, 129.5, 129.0, 129.0, 128.7, 128.3, 128.2, 127.6, 127.1, 126.7, 126.7, 121.2, 121.2, 119.6, 119.5, 47.8; HRMS (EI+) m/z: calcd: C30H20F6N2O2 (M+): 554.1429, found: 554.1428.
2-(4,5-Diphenyl-1-(pyridin-2-ylmethyl)-1H-imidazol-2-yl)pyridine (3i)
Light yellow solid; yield (3i 84% and 3ib 79%); Rf = 0.41 [30% EtOAc/petroleum ether (60–80 °C)]; m.p. 164–166 °C; 1H NMR (CDCl3, 600 MHz): δ 8.43 (d, J = 4.2 Hz, 1H), 8.40–8.39 (m, 2H), 7.73 (td, J1 = 7.8 Hz, J2 = 1.8 Hz, 1H), 7.61–7.60 (m, 2H), 7.49 (td, J1 = 7.8 Hz, J2 = 1.8 Hz, 1H), 7.37–7.35 (m, 1H), 7.33–7.31 (m, 2H), 7.24–7.21 (m, 4H), 7.17–7.15 (m, 1H), 7.14–7.12 (m, 1H), 7.06–7.04 (m, 1H), 6.80 (d, J = 7.8 Hz, 1H), 5.92 (s, 2H). 13C{1H} NMR (CDCl3, 150 MHz): δ 158.3, 150.4, 148.8, 148.3, 144.6, 138.2, 136.7, 136.6, 134.3, 132.3, 131.1, 130.4, 129.9, 129.0, 128.2, 126.9, 126.6, 123.2, 122.6, 121.8, 120.4, 51.0; HRMS (EI+) m/z: calcd: C26H20N4 (M+): 388.1688, found: 388.1691.
2-Benzo[1,3]dioxol-5-yl-1-benzo[1,3]dioxol-5-ylmethyl-4,5-diphenyl-1H-imidazole (3j)
White solid; yield (3j 81% and 3jb 71%); Rf = 0.52 [20% EtOAc/petroleum ether (60–80 °C)]; m.p. 174–176 °C; 1H NMR (DMSO-d6, 600 MHz): δ 7.43–7.41 (m, 5H), 7.28 (m, 2H), 7.20–7.18 (m, 3H), 7.14–7.11 (m, 2H), 6.99 (d, J = 7.8 Hz, 1H), 6.71 (d, J = 7.8 Hz, 1H), 6.27 (s, 1H), 6.15 (d, J = 9.0 Hz, 1H), 6.07 (s, 2H), 5.92 (s, 2H), 5.02 (s, 2H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 148.2, 147.8, 147.2, 146.7, 137.0, 134.9, 131.5, 131.2, 131.1, 130.3, 129.4, 129.3, 129.0, 128.5, 126.6, 126.5, 124.9, 123.1, 119.3, 109.3, 108.9, 108.7, 106.6, 101.8, 101.5, 47.8; HRMS (ESI+) m/z: calcd: C30H22N2O4 (M + H+): 475.1658, found: 475.1657.
4,5-Diphenyl-2-thiophen-2-yl-1-thiophen-2-ylmethyl-1H-imidazole (3k)
Light yellow solid; yield (3k 79% and 3kb 75%); Rf = 0.49 [20% EtOAc/petroleum ether (60–80 °C)]; m.p. 168–170 °C; 1H NMR (CDCl3, 600 MHz): δ 7.54 (d, J = 8.4 Hz, 2H), 7.43–7.39 (m, 4H), 7.32–7.30 (m, 3H), 7.22–7.15 (m, 4H), 7.08–7.07 (m, 1H), 6.86 (dd, J1 = 4.8 Hz, J2 = 3.6 Hz, 1H), 6.58 (m, 1H), 5.31 (s, 2H). 13C{1H} NMR (CDCl3, 150 MHz): δ 141.7, 139.6, 138.3, 134.1, 132.5, 131.1, 130.5, 130.1, 129.0, 128.9, 128.0, 127.5, 127.2, 126.9, 126.8, 126.6, 126.5, 125.4, 125.1, 44.0; HRMS (EI+) m/z: calcd: C24H18N2S2 (M+): 398.0911, found: 398.0903.
2-Naphthalen-1-yl-1-naphthalen-1-ylmethyl-4,5-diphenyl-1H-imidazole (3l)
Light yellow solid; yield (3l 77% and 3lb 75%); Rf = 0.40 [15% EtOAc/petroleum ether (60–80 °C)]; m.p. 246–248 °C; 1H NMR (CDCl3, 600 MHz): δ 8.03 (d, J = 8.4 Hz, 1H), 7.82 (dd, J1 = 15.6 Hz, J2 = 8.4 Hz, 2H), 7.74 (d, J = 7.8 Hz, 1H), 7.69 (d, J = 7.8 Hz, 2H), 7.64 (d, J = 7.8 Hz, 1H), 7.56–7.49 (m, 3H), 7.41 (d, J = 8.4 Hz, 1H), 7.39–7.35 (m, 3H), 7.33–7.25 (m, 8H), 7.20–7.18 (m, 1H), 6.84 (d, J = 7.2 Hz, 1H), 5.37 (s, 2H). 13C{1H} NMR (CDCl3, 150 MHz): δ 146.9, 138.2, 134.6, 133.7, 133.2, 133.0, 132.6, 131.0, 130.9, 129.8, 129.7, 128.9, 128.7, 128.7, 128.6, 128.3, 128.2, 128.2, 127.9, 127.0, 126. 9, 126.5, 126.2, 126.1, 125.7, 125.6, 125.3, 125.0, 123.7, 122.0, 46.1; HRMS (EI+) m/z: calcd: C36H26N2 (M+): 486.2096, found: 486.2098.
1-Benzyl-4,5-bis-(4-methoxy-phenyl)-2-phenyl-1H-imidazole (3o)
White solid; yield (3o 72%); Rf = 0.60 [20% EtOAc/petroleum ether (60–80 °C)]; m.p. 128–130 °C; 1H NMR (DMSO-d6, 600 MHz): δ 7.62–7.61 (m, 2H), 7.41–7.37 (m, 5H), 7.20–7.17 (m, 4H), 7.15–7.12 (m, 1H), 6.93 (d, J = 9.0 Hz, 2H), 6.79–6.75 (m, 4H), 5.11 (s, 2H), 3.74 (s, 3H), 3.67 (s, 3H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 159.8, 158.2, 147.0, 138.03, 137.3, 132.7, 131.4, 129.4, 129.1, 129.0, 128.9, 127.8, 127.7, 127.6, 126.0, 123.0, 114.8, 114.0, 55.5, 55.4, 48.0; HRMS (ESI+) m/z: calcd: C30H26N2O2 (M + H+): 447.2073, found: 447.2076.
2-(4,5-Bis(4-methoxyphenyl)-1-(pyridin-2-ylmethyl)-1H-imidazol-2-yl)pyridine (3p)
Light yellow solid; yield (3p 80%); Rf = 0.38 [30% EtOAc/petroleum ether (60–80 °C)]; m.p. 186–188°C; 1H NMR (DMSO-d6, 600 MHz): δ 8.40 (m, 1H), 8.35–8.34 (m, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.85 (t, J = 7.8 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 8.4 Hz, 2H), 7.27–7.25 (m, 1H), 7.17 (d, J = 7.8 Hz, 2H), 7.13–7.11 (m, 1H), 6.94 (d, J = 8.4 Hz, 2H), 6.81 (d, J = 8.4 Hz, 3H), 5.79 (s, 2H), 3.75 (s, 3H), 3.69 (s, 3H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 160.0, 158.4, 157.9, 150.7, 149.3, 148.7, 143.7, 138.1, 137.5, 137.1, 132.7, 131.5, 127.7, 127.5, 123.2, 123.0, 122.6, 122.5, 120.8, 114.9, 114.1, 55.6, 55.4, 50.5; HRMS (ESI+) m/z: calcd: C28H24N4O2 (M + H+): 449.1978, found: 449.1971.
1-Benzyl-5-methyl-2,4-diphenyl-1H-imidazole (5a)
White solid; yield (5a 90%); Rf = 0.51 [15% EtOAc/petroleum ether (60–80 °C)]; m.p. 112–114 °C; δ 7.77 (d, J = 7.2 Hz, 2H), 7.59–7.57 (m, 2H), 7.43 (t, J = 7.8 Hz, 2H), 7.38–7.36 (m, 5H), 7.33–7.28 (m, 2H), 7.07 (d, J = 7.2 Hz, 2H), 5.24 (s, 2H), 2.32 (s, 3H). 13C{1H} NMR (CDCl3, 150 MHz): δ 147.6, 137.9, 137.1, 135.3, 130.9, 129.1, 128.9, 128.8, 128.6, 128.4, 127.6, 127.4, 126.4, 125.7, 124.9, 48.1, 10.6; HRMS (ESI+) m/z: calcd: C23H20N2 (M + H+): 325.1705, found: 325.1720.
1-(4-Methoxy-benzyl)-2-(4-methoxy-phenyl)-5-methyl-4-phenyl-1H-imidazole (5b)
White solid; yield (5b 82%); Rf = 0.54 [20% EtOAc/petroleum ether (60–80 °C)]; m.p. 144–146 °C; 1H NMR (CDCl3, 600 MHz): δ 7.75 (d, J = 7.8 Hz, 2H), 7.50 (d, J = 9.0 Hz, 2H), 7.42 (t, J = 7.8 Hz, 2H), 7.28–7.25 (m, 1H), 6.97 (d, J = 8.4 Hz, 2H), 6.89 (t, J = 9.0 Hz, 4H), 5.13 (s, 2H), 3.79 (s, 3H), 3.79 (s, 3H), 2.30 (s, 3H). 13C{1H} NMR (CDCl3, 150 MHz): δ 160.0, 159.0, 147.5, 137.5, 135.4, 130.3, 129.1, 128.4, 127.4, 126.9, 126.3, 124.6, 123.4, 114.4, 114.0, 55.3, 47.5, 10.6; HRMS (ESI+) m/z: calcd: C25H24N2O2 (M + H+): 385.1916, found: 385.1911.
1-Benzyl-4-(2-chloro-phenyl)-5-(3,4-dimethoxy-phenyl)-2-phenyl-1H-imidazole (5c)
White solid; yield (5c 86%); Rf = 0.43 [20% EtOAc/petroleum ether (60–80 °C)]; m.p. 164–166 °C; 1H NMR (DMSO-d6, 600 MHz): δ 7.64–7.63 (m, 2H), 7.46–7.39 (m, 5H), 7.31–7.27 (m, 4H), 7.20 (t, J = 7.2 Hz, 1H), 6.87 (d, J = 7.8 Hz, 2H), 6.81 (d, J = 8.4 Hz, 1H), 6.67 (m, 1H), 6.63 (dd, J1 = 8.4 Hz, J2 = 1.8 Hz, 1H), 5.31 (s, 2H), 3.66 (s, 3H), 3.37 (s, 3H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 149.0, 148.6, 147.3, 139.6, 138.5, 136.8, 134.8, 133.7, 133.1, 132.5, 131.2, 129.8, 129.6, 129.2, 129.1, 128.8, 127.7, 127.2, 125.9, 122.9, 122.3, 113.6, 111.9, 55.7, 55.4, 48.6; HRMS (ESI+) m/z: calcd: C30H25N2O2Cl (M + H+): 481.1683, found: 481.1685.
2-(4-(2-Chlorophenyl)-5-(3,4-dimethoxyphenyl)-1-(pyridin-2-ylmethyl)-1H-imidazol-2-yl)pyridine (5d)
Light yellow solid; yield (5d 81%); Rf = 0.48 [40% EtOAc/petroleum ether (60–80 °C)]; m.p. 178–180 °C; 1H NMR (DMSO-d6, 600 MHz): δ 8.43–8.41 (m, 1H), 8.16 (d, J = 8.4 Hz, 1H), 7.84 (td, J1 = 7.8 Hz, J2 = 1.8 Hz, 1H), 7.64 (td, J1 = 7.8 Hz, J2 = 1.8 Hz, 1H), 7.43–7.42 (m, 2H), 7.32–7.26 (m, 4H), 7.19–7.17 (m, 1H), 6.89 (d, J = 8.4 Hz, 1H), 6.82 (d, J = 8.4 Hz, 1H), 6.72–6.71 (m, 1H), 6.65 (dd, J1 = 8.4 Hz, J2 = 1.8 Hz, 1H), 5.95 (s, 2H), 3.67 (s, 3H), 3.37 (s, 3H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 158.2, 150.5, 149.4, 149.2, 148.7, 148.6, 143.8, 137.5, 137.2, 136.8, 134.5, 134.3, 133.7, 133.1, 129.8, 129.7, 127.2, 123.3, 123.0, 123.0, 122.5, 121.7, 120.8, 113.8, 111.9, 55.7, 55.4, 51.1; HRMS (ESI+) m/z: calcd: C28H24N4O2Cl (M + H+): 483.1588, found: 483.1591.
2,4,5-Triphenyl-1H-imidazole (8a)
White solid; yield (8a 73% and 8ab70%); Rf = 0.48 [30% EtOAc/petroleum ether (60–80 °C)]; m.p. 272–274 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.77 (s, 1H), 8.09 (d, J = 8.4 Hz, 2H), 7.55–7.53 (m, 5H), 7.50–7.48 (m, 2H), 7.44 (t, J = 7.8 Hz, 2H), 7.38 (t, J = 7.2 Hz, 1H), 7.29 (t, J = 7.8 Hz, 2H), 7.22 (t, J = 7.2 Hz, 1H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 144.8, 137.7, 135.4, 133.2, 131.3, 129.6, 129.2, 129.1, 129.0, 128.9, 128.6, 128.3, 127.5, 127.3, 127.0; HRMS (ESI+) m/z:calcd: C21H16N2 (M + H+): 297.1392, found: 297.1392.
2-(4-Chloro-phenyl)-4,5-diphenyl-1H-imidazole (8b)
White solid; yield (8b 79% and 8bb 72%); Rf = 0.44 [30% EtOAc/petroleum ether (60–80 °C)]; m.p. 260–262 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.68 (s, 1H), 8.07 (d, J = 7.8 Hz, 2H), 7.54 (d, J = 7.2 Hz, 2H), 7.50–7.48 (m, 3H), 7.47–7.44 (m, 3H), 7.37 (t, J = 7.2 Hz, 2H), 7.29 (t, J = 7.2 Hz, 2H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 145.9, 135.6, 133.3, 130.8, 129.7, 129.1, 129.0, 128.9, 128.7, 128.6, 128.2, 127.5, 126.9, 125.6; HRMS (ESI+) m/z: calcd: C21H15N2Cl (M + H+): 331.1002, found: 331.1004.
2-(4,5-Diphenyl-1H-imidazol-2-yl)-phenol (8c)
White solid; yield (8c 88% and 8cb 86%); Rf = 0.42 [40% EtOAc/petroleum ether (60–80 °C)]; m.p. 206–208 °C; 1H NMR (DMSO-d6, 600 MHz): δ 13.03 (s, 1H), 12.94 (s, 1H), 8.03–8.02 (m, 1H), 7.53–7.52 (m, 2H), 7.50–7.46 (m, 4H), 7.42 (t, J = 7.2 Hz, 1H), 7.35–7.32 (m, 2H), 7.28–7.25 (m, 2H), 6.97 (d, J = 8.4 Hz, 1H), 6.95–6.92 (m, 1H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 157.1, 146.3, 134.5, 134.0, 130.7, 130.5, 129.2, 129.0, 128.8, 127.7, 127.5, 127.2, 125.4, 119.3, 117.3, 113.3; HRMS (ESI+) m/z: calcd: C21H16N2O (M + H+): 313.1341, found: 313.1342.
4,5-Diphenyl-2-thiophen-2-yl-1H-imidazole (8d)
Light yellow solid; yield (8d 86% and 8db 82%); Rf = 0.41 [30% EtOAc/petroleum ether (60–80 °C)]; m.p. 258–260 °C; 1H NMR (DMSO-d6, 600 MHz): δ 12.77 (s, 1H), 7.68–7.67 (m, 1H), 7.54 (d, J = 4.8 Hz, 1H), 7.50–7.47 (m, 4H), 7.44–7.42 (m, 2H), 7.38–7.35 (m, 1H), 7.29 (t, J = 7.8 Hz, 2H), 7.22–7.20 (m, 1H), 7.14–7.13 (m, 1H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 142.0, 137.2, 135.2, 134.4, 131.3, 129.2, 128.7, 128.7, 128.4, 128.3, 128.1, 127.5, 127.0, 126.7, 124.7; HRMS (ESI+) m/z: calcd: C19H14N2S (M + H+): 303.0956, found: 303.0961.
2-(4,5-Diphenyl-1H-imidazol-2-yl)-pyridine (8e)
White solid; (8e 72% and 8eb 70%); Rf = 0.39 [40% EtOAc/petroleum ether (60–80 °C)]; m.p. 186–188 °C; 1H NMR (DMSO-d6, 600 MHz): δ 13.15 (s, 1H), 8.63 (d, J = 4.8 Hz, 1H), 8.14 (d, J = 7.8 Hz, 1H), 7.91 (td, J1 = 7.8 Hz, J2 = 1.8 Hz, 1H), 7.51 (s, 4H), 7.39–7.37 (m, 3H), 7.30–7.23 (m, 4H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 149.4, 149.2, 145.9, 138.3, 137.6, 135.5, 131.2, 129.6, 129.0, 128.9, 128.6, 128.2, 127.8, 127.2, 123.6, 120.4; HRMS (ESI+) m/z: calcd: C20H15N3 (M + H+): 298.1344, found: 298.1345.
[4-(4,5-Diphenyl-1H-imidazol-2-yl)-phenyl]-dimethyl-amine (8f)
White solid; (8f 85% and 8fb 80%); Rf = 0.42 [40% EtOAc/petroleum ether (60–80 °C)]; m.p. 192–194 °C; 1H NMR (DMSO-d6, 400 MHz): δ 12.47 (s, 1H), 7.87 (d, J = 8.0 Hz, 2H), 7.47 (d, J = 7.6 Hz, 4H), 7.31(t, J1 = 7.6 Hz, 4H), 7.25–7.22 (m, 2H), 6.75 (d, J = 8.8 Hz, 2H), 2.92 (s, 6H). 13C{1H} NMR (DMSO-d6, 100 MHz): δ 150.9, 146.9, 129.8, 129.1, 128.9, 128.3, 127.5, 126.9, 118.4, 112.4, 40.5; HRMS (ESI+) m/z: calcd: C23H21N3 (M + H+): 340.1814, found: 340.1812.
4-(4,5-Diphenyl-1H-imidazol-2-yl)-benzaldehyde (8g)
White solid; (8g 77% and 8gb 70%); Rf = 0.44 [40% EtOAc/petroleum ether (60–80 °C)]; m.p. 188–190 °C; 1H NMR (DMSO-d6, 600 MHz): δ 13.00 (s, 1H), 10.02 (s, 1H), 8.29 (d, J = 8.4 Hz, 2H), 8.00 (d, J = 7.8 Hz, 2H), 7.55 (d, J = 7.2 Hz, 2H), 7.51 (d, J = 7.8 Hz, 2H), 7.46 (t, J1 =7.8 Hz, 2H), 7.40 (t, J = 7.2 Hz, 2H), 7.31 (m, 2H), 7.24 (m, 2H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 192.9, 144.6, 138.5, 135.9, 135.8, 135.2, 131.1, 130.5, 130.0, 129.2, 129.0, 128.7, 128.5, 127.5, 127.2, 125.8; HRMS (ESI+) m/z: calcd: C22H16N2O (M + H+): 325.1341, found: 325.1341.
2-[4,5-Bis-(4-methoxy-phenyl)-1H-imidazol-2-yl]-pyridine (8h)
Light yellow solid; yield (8h 78%); Rf = 0.59 [40% EtOAc/petroleum ether (60–80 °C)]; m.p. 246–248 °C; 1H NMR (DMSO-d6, 400 MHz): δ 12.93 (s, 1H), 8.57 (d, J = 4.4 Hz, 1H), 8.08 (d, J = 4.4 Hz 1H), 7.84 (td, J1 = 7.6 Hz, J2 = 1.6 Hz, 1H), 7.39 (d, J = 8.0 Hz, 4H), 7.33–7.30 (m, 1H), 6.87 (s, 4H), 3.72 (s, 6H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 149.5, 145.3, 137.6, 130.3, 129.0, 123.5, 120.3, 114.3, 113.9, 55.6; HRMS (ESI+) m/z: calcd: C22H19N3O2 (M + H+): 358.1556, found: 358.1552.
1-(4-Bromo-phenyl)-2-(4-chloro-phenyl)-4,5-diphenyl-1H-imidazole (10a)
White solid; yield (10a 86% and 10ab 82%); Rf = 0.58 [15% EtOAc/petroleum ether (60–80 °C)]; m.p. 244–246 °C; 1H NMR (DMSO-d6, 600 MHz): δ 7.53 (d, J = 8.4 Hz, 2H), 7.48–7.46 (m, 2H), 7.41–7.37 (m, 4H), 7.33–7.32 (m, 3H), 7.25–7.22 (m, 6H), 7.18 (t, J = 7.2 Hz, 1H). 13C{1H} NMR (DMSO-d6, 150 MHz): δ 145.4, 137.5, 136.2, 134.5, 133.7, 132.7, 131.8, 131.6, 131.2, 130.5, 129.4, 129.1, 129.1, 128.9, 128.6, 127.1, 126.7, 122.4; HRMS (ESI+) m/z: calcd: C27H18BrClN2 (M + H+): 485.0420, found: 485.0415.
1,2-Bis(4-methoxyphenyl)ethane-1,2-dione (1c)
Yellow solid; yield (1c 92%); Rf = 0.52 [10% EtOAc/petroleum ether (60–80 °C)]; m.p. 140–142 °C; 1H NMR (CDCl3, 600 MHz): δ 7.93 (d, J = 9.0 Hz, 4H), 6.95 (d, J = 9.0 Hz, 4H), 3.86 (s, 6H). 13C{1H} NMR (CDCl3, 150 MHz): δ 193.5, 164.8, 132.3, 126.2, 114.3, 55.6; HRMS (ESI+) m/z: calcd: C16H14O4 (M + Na+): 293.0790, found: 293.0801.
Benzyl-[2-benzylimino-1,2-bis-(4-methoxy-phenyl)-ethylidene]-amine (A)
Yellow solid; yield (A 86%); Rf = 0.56 [15% EtOAc/petroleum ether (60–80 °C)]; m.p. 246–248 °C; 1H NMR (CDCl3, 600 MHz): δ 7.77 (d, J = 9.0 Hz, 4H), 7.35 (d, J = 4.2 Hz, 8H), 7.30–7.26 (m, 2H), 6.91 (d, J = 9.0 Hz, 4H), 4.63 (d, J = 5.4 Hz, 4H), 3.84 (s, 6H); 13C{1H} NMR (CDCl3, 150 MHz): δ 167.0, 162.2, 138.4, 128.8, 128.8, 127.9, 127.6, 126.6, 113.8, 55.4, 44.1; HRMS (ESI+) m/z: calcd: C30H28N2O2 (M + H+): 449.2229, found: 449.2241.
Acknowledgments
The authors thank CSIR. S.A., K.M., and L.M. want to thank UGC, India, for a Senior Research Fellowship. S.P. and R.S. want to thank CSIR, India, and NIPER, India, respectively. The authors thank Sandip Kundu, CSIR-IICB, for recording X-ray data, E. Padmanaban for recording the NMR, Sandip Chowdhury for recording the EI mass spectra, and Soumik Laha and Diptendu Bhattacharyya for recording the ESI HRMS data. C.M. sincerely acknowledges the financial support from the J.C. Bose National Fellowship and DBT-Distinguished Biotechnology Research Professorship.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00934.
The authors declare no competing financial interest.
Supplementary Material
References
- Cui B.; Zheng B. L.; He K.; Zheng Q. Y. Imidazole alkaloids from lepidium meyenii. J. Nat. Prod. 2003, 66, 1101–1103. 10.1021/np030031i. [DOI] [PubMed] [Google Scholar]
- Weinreb S. M. Some recent advances in the synthesis of polycyclic imidazole-containing marine natural products. Nat. Prod. Rep. 2007, 24, 931–948. 10.1039/b700206h. [DOI] [PubMed] [Google Scholar]
- Girish K. G.; Kumar V.; Kaur K. Imidazole containing natural products as antimicrobial agents: a review. J. Nat. Prod. 2014, 4, 73–81. 10.2174/221031550402141009095725. [DOI] [Google Scholar]
- Zhang L.; Peng X. M.; Damu G. L. V.; Geng R. X.; Zhou C. H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. 10.1002/med.21290. [DOI] [PubMed] [Google Scholar]
- Wittine K.; Babić M. S.; Makuc D.; Plavec J.; Pavelić S. K.; Sedić M.; Pavelić K.; Leyssen P.; Neyts J.; Balzarini J.; Mintas M. Novel 1, 2, 4-triazole and imidazole derivatives of L-ascorbic and imino-ascorbic acid: Synthesis, anti-HCV and antitumor activity evaluations. Bioorg. Med. Chem. 2012, 20, 3675–3685. 10.1016/j.bmc.2012.01.054. [DOI] [PubMed] [Google Scholar]
- Yang F.; Nickols N. G.; Li B. C.; Marinov G. K.; Said J. W.; Dervan P. B. Antitumor activity of a pyrrole-imidazole polyamide. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 1863–1868. 10.1073/pnas.1222035110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K. I.; Bando T.; Sasaki S.; Sakakibara Y.; Minoshima M.; Sugiyama H. Antitumor activity of sequence-specific alkylating agents: Pyrolle-imidazole CBI conjugates with indole linker. Cancer Sci. 2006, 97, 219–225. 10.1111/j.1349-7006.2006.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo R.; Tafi A.; Costi R.; Botta M.; Artico M.; Corelli F.; Forte M.; Caporuscio F.; Angiolella L.; Palamara A. T. Antifungal agents. 11. N-substituted derivatives of 1-[(aryl)(4-aryl-1 H-pyrrol-3-yl) methyl]-1 H-imidazole: synthesis, anti-candida activity, and QSAR studies. J. Med. Chem. 2005, 48, 5140–5153. 10.1021/jm048997u. [DOI] [PubMed] [Google Scholar]
- Fan-Havard P.; Capano D.; Smith S. M.; Mangia A.; Eng R. H. Development of resistance in candida isolates from patients receiving prolonged antifungal therapy. Antimicrob. Agents Chemother. 1991, 35, 2302–2305. 10.1128/AAC.35.11.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. R.; McLaughlin M. M.; Kumar S.; Kassis S.; Doyle M. L.; McNulty D.; Gallagher T. F.; Fisher S.; McDonnell P. C.; Carr S. A.; Huddleston M. J.; et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J. Biol. Chem. 1997, 272, 12116–12121. 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. P.; Tonzola C. J.; Babel A.; Jenekhe S. A. Electron transport materials for organic light-emitting diodes. Chem. Mater. 2004, 16, 4556–4573. 10.1021/cm049473l. [DOI] [Google Scholar]
- Fabbrizzi L.; Foti F.; Patroni S.; Pallavicini P.; Taglietti A. A Sleeping Host Awoken by Its Guest: Recognition and Sensing of Imidazole-Containing Molecules Based on Double Cu2+ Translocation inside a Polyaza Macrocycle. Angew. Chem., Int. Ed. 2004, 43, 5073–5077. 10.1002/anie.200460568. [DOI] [PubMed] [Google Scholar]
- Li Z. A.; Lou X.; Yu H.; Li Z.; Qin J. An imidazole-functionalized polyfluorene derivative as sensitive fluorescent probe for metal ions and cyanide. Macromolecules 2008, 41, 7433–7439. 10.1021/ma8013096. [DOI] [Google Scholar]
- Zeng Q.; Cai P.; Li Z.; Qin J.; Tang B. Z. An imidazole-functionalized polyacetylene: convenient synthesis and selective chemosensor for metal ions and cyanide. Chem. Commun. 2008, 1094–1096. 10.1039/b717764j. [DOI] [PubMed] [Google Scholar]
- Song Z.; Zhang W.; Jiang M.; Sung H. H.; Kwok R. T.; Nie H.; Williams I. D.; Liu B.; Tang B. Z. Synthesis of Imidazole-Based AIEgens with Wide Color Tunability and Exploration of their Biological Applications. Adv. Funct. Mater. 2016, 26, 824–832. 10.1002/adfm.201503788. [DOI] [Google Scholar]
- Ning P.; Wang W.; Chen M.; Feng Y.; Meng X. Recent advances in mitochondria-and lysosomes-targeted small-molecule two-photon fluorescent probes. Chin. Chem. Lett. 2017, 28, 1943–1951. 10.1016/j.cclet.2017.09.026. [DOI] [Google Scholar]
- Wu D.; Shen Y.; Chen J.; Liu G.; Chen H.; Yin J. Naphthalimide-modified near-infrared cyanine dye with a large stokes shift and its application in bioimaging. Chin. Chem. Lett. 2017, 28, 1979–1982. 10.1016/j.cclet.2017.07.004. [DOI] [Google Scholar]
- Wang W.; Ning P.; Wang Q.; Zhang W.; Jiang J.; Feng Y.; Meng X. pH-Independent two-photon fluorescent lysotrackers for real-time monitoring autophagy. J. Mater. Chem. B 2018, 6, 1764–1770. 10.1039/C8TB00229K. [DOI] [PubMed] [Google Scholar]
- Fan F.; Nie S.; Yang D.; Luo M.; Shi H.; Zhang Y. H. Labeling lysosomes and tracking lysosome-dependent apoptosis with a cell-permeable activity-based probe. Bioconjugate Chem. 2012, 23, 1309–1317. 10.1021/bc300143p. [DOI] [PubMed] [Google Scholar]
- Li J.; Neuville L. Copper-catalyzed oxidative diamination of terminal alkynes by amidines: synthesis of 1, 2, 4-trisubstituted imidazoles. Org. Lett. 2013, 15, 1752–1755. 10.1021/ol400560m. [DOI] [PubMed] [Google Scholar]
- Kanazawa C.; Kamijo S.; Yamamoto Y. Synthesis of imidazoles through the copper-catalyzed cross-cycloaddition between two different isocyanides. J. Am. Chem. Soc. 2006, 128, 10662–10663. 10.1021/ja0617439. [DOI] [PubMed] [Google Scholar]
- Siamaki A. R.; Arndtsen B. A. A direct, one step synthesis of imidazoles from imines and acid chlorides: a palladium catalyzed multicomponent coupling approach. J. Am. Chem. Soc. 2006, 128, 6050–6051. 10.1021/ja060705m. [DOI] [PubMed] [Google Scholar]
- Zaman S.; Mitsuru K.; Abell A. D. Synthesis of trisubstituted imidazoles by palladium-catalyzed cyclization of O-pentafluorobenzoylamidoximes: Application to amino acid mimetics with a C-terminal imidazole. Org. Lett. 2005, 7, 609–611. 10.1021/ol047628p. [DOI] [PubMed] [Google Scholar]
- Sarkar R.; Mukhopadhyay C. Silver-Mediated Cα(sp3)–H Functionalization of Primary Amines: An Oxidative C–N Coupling Strategy for the Synthesis of Two Different Types of 1, 2, 4, 5-Tetrasubstituted Imidazoles. Eur. J. Org. Chem. 2015, 1246–1256. 10.1002/ejoc.201403465. [DOI] [Google Scholar]
- Bhattacharjee P.; Bora U. Molecular Iodine-Catalyzed Selective C-3 Benzylation of Indoles with Benzylic Alcohols: A Greener Approach toward Benzylated Indoles. ACS Omega 2019, 4, 11770–11776. 10.1021/acsomega.9b01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C.; Muniz K. An Iodine-Catalyzed Hofmann–Löffler Reaction. Angew. Chem., Int. Ed. 2015, 54, 8287–8291. 10.1002/anie.201501122. [DOI] [PubMed] [Google Scholar]
- Zeng L. Y.; Cai C. Iodine catalyzed one-pot multicomponent synthesis of a library of compounds containing tetrazolo [1, 5-a] pyrimidine core. J. Comb. Chem. 2010, 12, 35–40. 10.1021/cc9000983. [DOI] [PubMed] [Google Scholar]
- von der Heiden D.; Bozkus S.; Klussmann M.; Breugst M. Reaction mechanism of iodine-catalyzed michael additions. J. Org. Chem. 2017, 82, 4037–4043. 10.1021/acs.joc.7b00445. [DOI] [PubMed] [Google Scholar]
- Parvatkar P. T.; Parameswaran P. S.; Tilve S. G. Recent developments in the synthesis of five-and six-membered heterocycles using molecular iodine. Chem. – Eur. J. 2012, 18, 5460–5489. 10.1002/chem.201100324. [DOI] [PubMed] [Google Scholar]
- Duhamel T.; Martínez M. D.; Sideri I. K.; Muñiz K. 1, 3-Diamine Formation from an Interrupted Hofmann–Löffler Reaction: Iodine Catalyst Turnover through Ritter-Type Amination. ACS Catal. 2019, 9, 7741–7745. 10.1021/acscatal.9b01566. [DOI] [Google Scholar]
- Xie J.; Jiang H.; Cheng Y.; Zhu C. Metal-free, organocatalytic cascade formation of C–N and C–O bonds through dual sp3 C–H activation: oxidative synthesis of oxazole derivatives. Chem. Commun. 2012, 48, 979–981. 10.1039/C2CC15813B. [DOI] [PubMed] [Google Scholar]
- Ren Y. M.; Cai C.; Yang R. C. Molecular iodine-catalyzed multicomponent reactions: an efficient catalyst for organic synthesis. RSC Adv. 2013, 3, 7182–7204. 10.1039/c3ra23461d. [DOI] [Google Scholar]
- Sashidhara K. V.; Palnati G. R.; Singh L. R.; Upadhyay A.; Avula S. R.; Kumar A.; Kant R. Molecular iodine catalysed one-pot synthesis of chromeno [4, 3-b] quinolin-6-ones under microwave irradiation. Green Chem. 2015, 17, 3766–3770. 10.1039/C5GC00756A. [DOI] [Google Scholar]
- Jayram J.; Jeena V. An iodine/DMSO-catalyzed sequential one-pot approach to 2, 4, 5-trisubstituted-1 H-imidazoles from α-methylene ketones. RSC Adv. 2018, 8, 37557–37563. 10.1039/C8RA07238H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwai M.; Bansal V.; Mothsra P.; Saxena S.; Somvanshi R. K.; Dey S.; Singh T. P. Molecular iodine: A versatile catalyst for the synthesis of bis (4-hydroxycoumarin) methanes in water. J. Mol. Catal. A: Chem. 2007, 268, 76–81. 10.1016/j.molcata.2006.11.054. [DOI] [Google Scholar]
- Vasuki G.; Kumaravel K. Rapid four-component reactions in water: synthesis of pyranopyrazoles. Tetrahedron Lett. 2008, 49, 5636–5638. 10.1016/j.tetlet.2008.07.055. [DOI] [Google Scholar]
- Banerji B.; Adhikary S.; Majumder L.; Ghosh S. A Green Synthetic Approach towards Polyarylated Oxazoles via Iodine-Catalyzed One-Pot sp3 C–H Functionalization in Water: From Natural Product Synthesis To Photophysical Studies. Asian J. Org. Chem. 2019, 8, 514–525. 10.1002/ajoc.201800742. [DOI] [Google Scholar]
- Xue W. J.; Li Q.; Zhu Y. P.; Wang J. G.; Wu A. X. Convergent integration of two self-labor domino sequences: a novel method for the synthesis of oxazole derivatives from methyl ketones and benzoins. Chem. Commun. 2012, 48, 3485–3487. 10.1039/c2cc18077d. [DOI] [PubMed] [Google Scholar]
- Kidwai M.; Mothsra P.; Bansal V.; Goyal R. Efficient Elemental Iodine Catalyzed One-Pot Synthesis of 2,4,5-Triarylimidazoles. Monatsh. Chem. 2006, 137, 1189–1194. 10.1007/s00706-006-0518-9. [DOI] [Google Scholar]
- Kidwai M.; Mothsra P. A one-pot synthesis of 1,2,4,5-tetraarylimidazoles using molecular iodine as an efficient catalyst. Tetrahedron Lett. 2006, 47, 5029–5031. 10.1016/j.tetlet.2006.05.097. [DOI] [Google Scholar]
- Kidwai M.; Mothsra P.; Bansal V.; Somvanshi R. K.; Ethayathulla A. S.; Dey S.; Singh T. P. One-pot synthesis of highly substituted imidazoles using molecular iodine: A versatile catalyst. J. Mol. Catal. A: Chem. 2007, 265, 177–182. 10.1016/j.molcata.2006.10.009. [DOI] [Google Scholar]
- Wang J.; Zhang F. D.; Tang D.; Wu P.; Zhang X. G.; Chen B. H. I2/TBPB mediated oxidative reaction of aryl acetaldehydes with amidines: synthesis of 1, 2, 5-triaryl-1 H-imidazoles. RSC Adv. 2017, 7, 24594–24597. 10.1039/C7RA01966A. [DOI] [Google Scholar]
- Samai S.; Nandi G. C.; Singh P.; Singh M. S. l-Proline: an efficient catalyst for the one-pot synthesis of 2, 4, 5-trisubstituted and 1, 2, 4, 5-tetrasubstituted imidazoles. Tetrahedron 2009, 65, 10155–10161. 10.1016/j.tet.2009.10.019. [DOI] [Google Scholar]
- Chen C. Y.; Hu W. P.; Yan P. C.; Senadi G. C.; Wang J. J. Metal-free, acid-promoted synthesis of imidazole derivatives via a multicomponent reaction. Org. Lett. 2013, 15, 6116–6119. 10.1021/ol402892z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.