Abstract
Background and Purpose:
Delayed neurological deficits are a devastating consequence of subarachnoid hemorrhage (SAH) which affects about 30% of surviving patients. Although a very serious concern, delayed deficits are understudied in experimental SAH models; it is not known if rodents recapitulate the delayed clinical decline seen in SAH patients. We hypothesized that mice with SAH develop delayed functional deficits and that microthrombi and infarction correlate with delayed decline.
Methods:
Adult C57BL/6J mice of both sexes were subjected to endovascular perforation to induce SAH. Mice were allowed to survive for up to one week post-ictus and behavioral performance was assessed daily. Post-mortem microthrombi, large artery diameters (to assess vasospasm), and infarct volume were measured. These measures were analyzed for differences between SAH mice which developed delayed deficits and SAH mice which did not get delayed deficits. Correlation analyses were performed to identify which measures correlated with delayed neurological deficits, sex, and infarction.
Results:
23% of males and 47% of females developed delayed deficits 3-6 days post-SAH. Female mice subjected to SAH had a significantly higher incidence of delayed deficits than male mice with SAH. Mice which developed delayed deficits had significantly more microthrombi and larger infarct volumes than SAH mice which did not get delayed deficits. Microthrombi positively correlated with infarct volume, and both microthrombi and infarction correlated with delayed functional deficits. Vasospasm did not correlate with either infarction delayed functional deficits.
Conclusions:
We discovered that delayed functional deficits occur in mice following SAH. Sex differences were seen in the prevalence of delayed deficits. The mechanism by which microthrombi cause delayed deficits may be via formation of infarcts.
Keywords: subarachnoid hemorrhage, functional deficits, delayed neurological deficits
Graphical Abstract
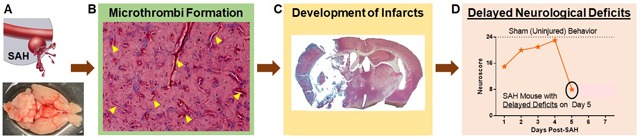
Subarachnoid hemorrhage (A) may lead to formation of small clots (microthrombi) that can occlude small vessels within the brain (B).Occluded vessels limits blood supply to the brain and prolonged blood flow cessation/reduction can lead to cell death(infraction C) which is caused delayed neurological deficits(D).
Introduction
Subarachnoid hemorrhage (SAH) affects about 30,000 individuals each year in the U.S. Furthermore, up to 30% of patients who survive the initial ictus develop delayed neurological deficits (DND) 4-10 days following SAH, which accounts for the most common cause of morbidity and mortality in SAH survivors.1 Multiple clinical studies indicate the cause of DND is multifactorial and may include microthrombi, vasospasm, cortical spreading depolarization, and/or inflammation.2
One concern with preclinical models of SAH is that the measurement of DND is not well-defined and may be difficult to quantify.3 In fact, while a number of studies have examined post-hemorrhage pathology (i.e. ischemia,4 vasospasm,5 and microthrombi5) and observed delayed mortality,6, 7 delayed functional deficits after SAH in rodents is understudied. Herein, we first provide evidence showing that SAH mice develop DND and then test the hypothesis that microthrombi count correlates with delayed functional decline.
Methods
Experiments were approved by the UTHealth Animal Welfare Committee, conducted according to the NIH Guidelines for the Use of Animals in Neuroscience Research, and are reported in compliance with the ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines. Data is available upon reasonable request. Detailed methods are in the Online Supplement (https://www.ahajournals.org/journal/str).
Adult C57BL/6J mice (4-6 months old) of both sexes were used. Based on our preliminary findings, sample size was calculated to be 38 male (8 sham, 30 SAH) and 28 female (8 sham, 20 SAH) mice. Mice were randomized into sham or SAH. The same individual performed all surgeries and investigators responsible for functional assessment, outcome measurement, and data analysis were blinded to sex and experimental groups.
Subarachnoid hemorrhage was induced via endovascular perforation.8 Isoflurane anesthetized mice were placed supine, a vertical midline incision was made, and the external carotid artery was ligated, leaving a stump. A 5-0 monofilament nylon suture was inserted into the external carotid artery stump and advanced until vessel perforation (~11 mm after insertion). Perforation was confirmed by respiratory distress (Table I). The suture was withdrawn and the animal was allowed to recover. Sham animals underwent all surgical procedures except for vessel perforation. After recovery from anesthesia, mice were observed and only mice without hemiparesis were included in this study.
Mice were allowed to survive for up to 7 days post-SAH. Health status was assessed a minimum of three times per day. Mice which were deemed unlikely to survive overnight were euthanized and brains were collected for post-mortem analysis of vasospasm and microthrombi.
Daily behavioral performance was assessed 1-7 days post-SAH using an 8-test sensorimotor neuroscore which evaluates functional performance in exploration, climbing, forelimb and hindlimb use, whisker and side sensation, balance, and visual reflex (Table II).8
At the time of euthanasia or 7 days post-SAH, heparin was given and then mice (4-5/group) underwent perfusion of PBS, then DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), and 4% PFA at 80-100 mmHg.9 The circle of Willis was imaged using a Leica M205FA stereo-fluorescence microscope. The remaining mice did not receive DiI perfusion.
Brains were sectioned into 40 μm thick slices to obtain the cerebral (anterior (ACA) and middle (MCA)), internal carotid (ICA), and basilar arteries (BA).5 Vasospasm was evaluated by determining the smallest vessel diameter within each artery. Microthrombi were counted for the entire section at −2 from bregma in in Martius, Scarlet, and Blue (MSB) stained coronal sections (and confirmed using H&E staining (see Supplemental Material)). All mice used for vasospasm analysis were also used for microthrombi counts. Infarct volume was assessed in MSB-stained slices every 1 mm from +2 to −3 from Bregma using ImageJ.
Mice dying from early brain injury (0-48 hours post-SAH) were excluded from analysis (but mortality is reported). Vasospasm and microthrombi count were analyzed using one-way ANOVAs with Tukey post-hoc. Time to DND was analyzed by a log-rank test. Correlation was computed using Pearson or point-biserial correlation coefficients. GraphPad Prism 6 was all used for analysis.
Results
We performed an initial series of experiments to define DND in SAH mice; 4/24 males (17%) and 8/22 females (36%) developed delayed deficits 3-5 days post-SAH. Based on this cohort, we defined DND as a drop in 5 or more points within the motor subtests (spontaneous activity, climbing, balance, and forelimb and hindlimb use, Table III) after some recovery from day 1.
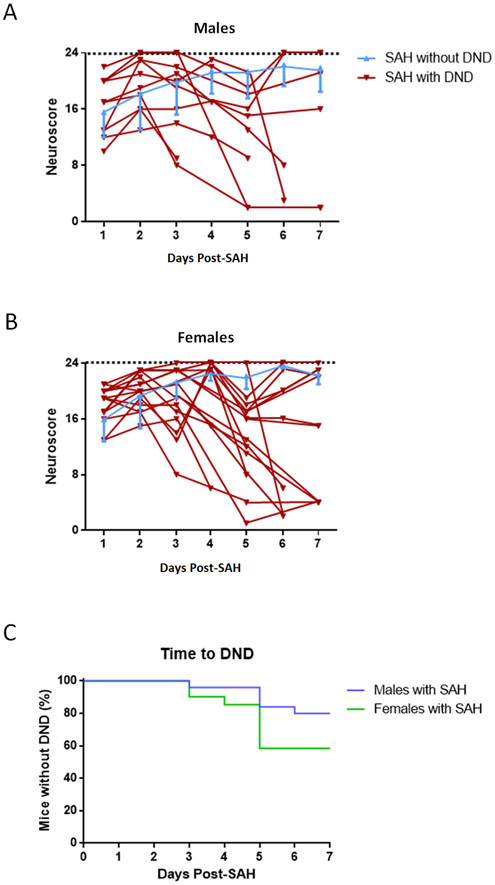
In this study, no sham mice died. Four males (one died immediately after perforation, three died overnight after surgery) and one female (died 6 hours post-SAH) were excluded. Six of twenty-six (23%) male SAH mice developed DND 3-6 days post-SAH (Figure 1A, Table IV). Nine of nineteen (47%) female SAH mice had DND 3-6 days after SAH (Figure 1B, Table V). Two female mice developed DND twice. Additionally, female SAH mice develop DND at a higher frequency compared to males (p=0.0014, odds ratio=2.05, Figure 1C).
Figure 1.
Neurobehavioral Performance by Males (A) and Females (B). Individual performance is shown for SAH mice with DND (males n=6, females n=9). SAH mice without DND are plotted as mean and SD (males n=18-20/time-point, females n=10/time-point). Sham (n=8) performance is 23.5 (SD=0.70) for males and 23.8 (SD=0.30) for females. Time to delayed deficits after SAH in males (C) (with DND n=6, without DND n=20, p<0.0001) and females (D) (with DND n=9, without DND n=10, p=0.0413).
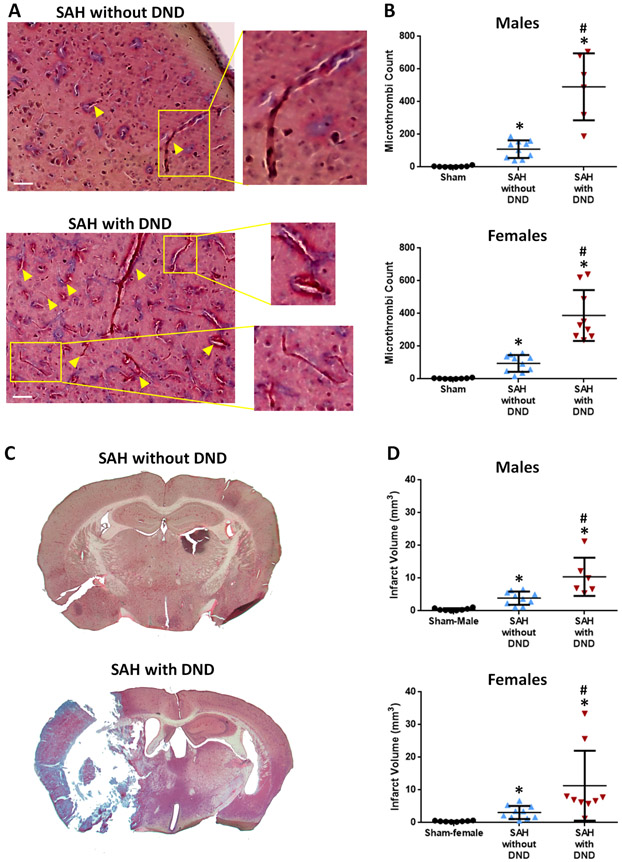
Microthrombi counts are significantly greater in mice with SAH (without DND: males=109.5 (SD=53.6), females=94.9 (SD=51.4), with DND: males=491.2 (SD=204.9), females=388.0 (SD=155.8)) compared to sham mice (males=3.4 (SD=4.27), females=3.0 (SD=3.02)). SAH mice which develop DND have more microthrombi than those without DND (males: p<0.0001, females: p=0.0276) (Figures 2A-B, Tables VI-VIII). Microthrombi count positively correlates with DND (p<0.0001) (Table IX).
Figure 2.
Microthrombi and Infarction Correlate with DND. A. Representative Images of Microthrombi (arrowheads) in MSB Staining. Bar=50 μm. B. Microthrombi Counts. C. Representative Images of Infarction after SAH. D. Infarct Volumes. Microthrombi counts and infarct volumes were assessed on Day 7 for Sham and SAH without DND; for SAH mice with DND, these measures were assessed on Day 5.7 (SD=1.37) for males and Day 6 (SD=1.05) for females. Sham n=8/sex, SAH without DND n=10/sex, SAH with DND n=6 males and n=9 females. * p<0.05 vs Sham, # p<0.05 vs SAH without DND.
Mice subjected to SAH had larger infarctions than sham mice (males: sham=0.43 (SD=0.371), SAH without DND=3.9 (SD=2.00), SAH with DND=10.42 (SD=5.88); females: sham=0.41 (SD=0.181), SAH without DND=3.12 (SD=2.00), SAH with DND=11.32 (SD=10.69)). SAH mice which developed DND had larger infarctions than mice without DND (males: p=0.0018, females: p=0.0240) (Figures 2C-D, Table X); no difference was observed between males and females (SAH without DND: p=0.3957, SAH with DND: p=0.8546). Infarct volume correlates with DND (p<0.01) and microthrombi counts (p<0.01) (Tables IX and XI).
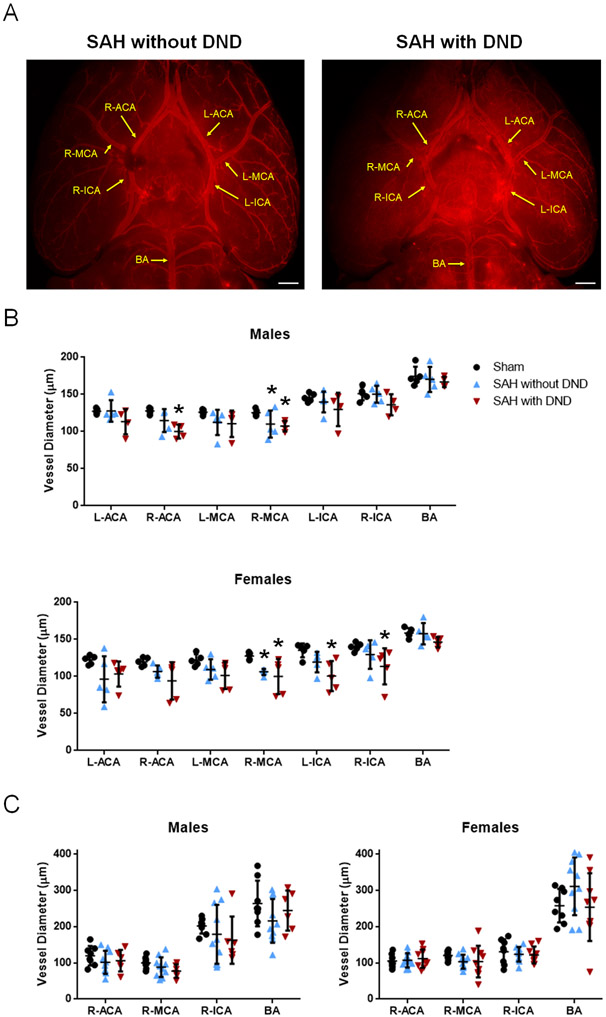
Large artery vasospasm was not significantly different between SAH mice with DND and those without DND for either sex (Figure 3, Tables XII-XV). For all large arteries, no correlations were observed between vasospasm and DND or infarct volume (p>0.05) (Tables IX and XI).
Figure 3.
Large Artery Vasospasm Does Not Correlate with DND. A. Representative Images of Brain Vasculature. Bar=0.5 mm. B. Vessel Diameters from Vessel Painted Brains. Sham n=5/sex, SAH without DND n=5/sex, SAH with DND n=4 males and n=5 females. C. Vessel Diameters from H&E Slices. Sham n=8/sex, SAH without DND=10/sex, SAH with DND n=6 males and n=9 females. Vessel diameters were assessed on Day 7 for Sham and SAH without DND; for SAH mice with DND, it was measured on Day Day 5.7 (SD=1.37) for males and Day 6 (SD=1.05) for females. * p<0.05 vs Sham.
Discussion
Herein, we designed a mouse study to evaluate the development of DND during the first week after SAH. In an initial series of experiments, 17% of males and 36% of females develop delayed deficits and/or death 3-5 days post-SAH. These initial findings were validated in this study. Herein, we observed 23% of male and 47% of female mice experience decline in neurological performance 3-6 days post-ictus after recovering from early brain injury deficits. Interestingly, our sex difference in DND incidence follows the trend observed in humans.10 We further found a correlation between the number of microthrombi and the development of DND; specifically, mice which develop DND have higher microthrombi counts than mice which do not develop DND. Infarction also positively correlated with DND, as well as microthrombi number. We did not observe a correlation between large artery vasospasm and either DND or infarction.
Doubts exist about the utility of SAH rodent models when it comes to DND. A recent review performed a detailed literature search for evidence of delayed cerebral ischemia and DND.3 There is an abundance of preclinical evidence of vasospasm, microthrombi, microvascular constriction, cerebral perfusion, and infarction in SAH rodents, but the evidence for delayed deficits in rodents is limited. To date, only one mouse and two male rat studies have reported delayed functional deficits and delayed mortality.6, 7, 11 However, the incidence ratios of DND are unclear; our findings provide more evidence of DND in rodents after SAH.
One obstacle for detecting DND in SAH rodents is that when all SAH mice are grouped together (i.e. SAH mice without DND in the same group as SAH mice with DND), the mean/SD do not indicate a presence of DND (see Figure III). Another reason DND is difficult to assess is that individual functional performance is not monitored daily, and it is typical to exclude mice which die before the study endpoints. Thus, studies investigating DND should have strict inclusion/exclusion criteria.
A limitation of this study is that microthrombi, vasospasm, and infarction were not measured at exactly the same time for all mice; some mice were euthanized before day 7. Another limitation is that endovascular perforation may be caused different SAH severities in rodents resulting in differences between animals with respect to hemorrhage volume, intracranial pressure, and cerebral blood flow. It is possible that differences in SAH severity affect microthrombi formation, infarction, and DND. None of these measures (hemorrhage volume, intracranial pressure, nor cerebral blood flow) were measured, so this is a limitation of this study. In future studies we will monitor cerebral blood flow and intracranial pressure to be sure that all animals are receiving similar SAH injury/severity. A third limitation is that only microthrombi, vasospasm, and infarction were examined in relation to DND. Inflammation has also been implicated in the pathogenesis of DND, but we did not investigate inflammation in this study. In the future, we will examine the involvement of inflammation in DND, as well as determine if microthrombi are a cause or consequence of DND. If microthrombi are found to be mechanistically important to DND development, strategies to prevent their formation is crucial. Finally, we did not assess the estrous stages of female mice and sex-specific factors have been implicated in aneurysm development/rupture.10
The findings of this study suggest that microthrombi may be a critical contributor to DND, but clinical trials using therapeutics aimed at preventing microthrombi have not been successful. Aspirin is unable prevent DND after SAH. Although aspirin can cause irreversible inhibition of platelets through the COX pathway, even in the presence of aspirin, platelets can still become activated via other signaling pathways.12 Until now, it is not known which signaling pathway(s) are involved in microthrombi formation, and thus would be treatment targets. An anticoagulant heparin has also been unsuccessful in clinical trials. Heparin may have failed because, while it can inhibit Factor Xa, it can also induce platelet activation13 which may promote microthrombi.
While aspirin and heparin have failed to prevent DND post-SAH, nimodipine, the only FDA-approved treatment for SAH, has been successful. Historically thought to be as a vasodilator, nimodipine’s fibrinolytic activity may be responsible for the observed benefits on DND and delayed injury following SAH.14 If microthrombi is a key contributor to DND pathogenesis, as suggested by nimodipine’s clinical success and the findings of this study, then the next step is determine what is/are the therapeutic targets to prevent microthrombi formation which can further reduce the incidence of DND following SAH.
Supplementary Material
Acknowledgments
Funding Sources
Funding support was provided by two Brain Aneurysm Foundation grants (DWM), an NIH K23 grant (NS106054-01A1, SLB), and an American Heart Association fellowship (19POST34380074, S-HH).
Footnotes
Disclosures
None.
References
- 1.Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vasospasm. Br J Anaesth. 2012;109:315–329 [DOI] [PubMed] [Google Scholar]
- 2.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58 [DOI] [PubMed] [Google Scholar]
- 3.Oka F, Chung DY, Suzuki M, Ayata C. Delayed cerebral ischemia after subarachnoid hemorrhage: Experimental-clinical disconnect and the unmet need. Neurocritical Care. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutoh T, Mutoh T, Sasaki K, Nakamura K, Tatewaki Y, Ishikawa T, et al. Neurocardiac protection with milrinone for restoring acute cerebral hypoperfusion and delayed ischemic injury after experimental subarachnoid hemorrhage. Neurosci lett. 2017;640:70–75 [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Chen G, Zhu WW, Bian JY, Shen XO, Zhou D. Influence of simvastatin on microthrombosis in the brain after subarachnoid hemorrhage in rats: A preliminary study. Ann Clin Lab Sci. 2010;40:32–42 [PubMed] [Google Scholar]
- 6.Guresir E, Raabe A, Jaiimsin A, Dias S, Raab P, Seifert V, et al. Histological evidence of delayed ischemic brain tissue damage in the rat double-hemorrhage model. J Neurol Cci. 2010;293:18–22 [DOI] [PubMed] [Google Scholar]
- 7.Guresir E, Vasiliadis N, Dias S, Raab P, Seifert V, Vatter H. The effect of common carotid artery occlusion on delayed brain tissue damage in the rat double subarachnoid hemorrhage model. Acta Neurochir (Wien). 2012;154:11–19 [DOI] [PubMed] [Google Scholar]
- 8.Matsumura K, Kumar TP, Guddanti T, Yan Y, Blackburn SL, McBride DW. Neurobehavioral deficits after subarachnoid hemorrhage in mice: Sensitivity analysis and development of a new composite score. J Am Heart Assoc. 2019;8:e011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes S, Dashkin O, Defazio RA. Vessel painting technique for visualizing the cerebral vascular architecture of the mouse. Methods in Molecular Biology (Clifton, N.J.). 2014;1135:127–138 [DOI] [PubMed] [Google Scholar]
- 10.Germans MR, Jaja BNR, de Oliviera Manoel AL, Cohen AH, Macdonald RL. Sex differences in delayed cerebral ischemia after subarachnoid hemorrhage. J Neurosurg. 2018;129:458–464 [DOI] [PubMed] [Google Scholar]
- 11.El Amki M, Dubois M, Lefevre-Scelles A, Magne N, Roussel M, Clavier T, et al. Long-lasting cerebral vasospasm, microthrombosis, apoptosis and paravascular alterations associated with neurological deficits in a mouse model of subarachnoid hemorrhage. Mol Neurobiol. 2018;55:2763–2779 [DOI] [PubMed] [Google Scholar]
- 12.Bates SM, Weitz JI. Prevention of activation of blood coagulation during acute coronary ischemic syndromes: Beyond aspirin and heparin. Cardiovas Res. 1999;41:418–432 [DOI] [PubMed] [Google Scholar]
- 13.Gao C, Boylan B, Fang J, Wilcox DA, Newman DK, Newman PJ. Heparin promotes platelet responsiveness by potentiating αiibβ3-mediated outside-in signaling. Blood. 2011;117:4946–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergouwen MD, Vermeulen M, de Haan RJ, Levi M, Roos YB. Dihydropyridine calcium antagonists increase fibrinolytic activity: A systematic review. J Cereb Blood Flow Metab. 2007;27:1293–1308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.