Abstract
Acute respiratory distress syndrome (ARDS), replacing the clinical term acute lung injury, involves serious pathophysiological lung changes that arise from a variety of pulmonary and nonpulmonary injuries and currently has no pharmacological therapeutics. RNA interference (RNAi) has the potential to generate therapeutic effects that would increase patient survival rates from this condition. It is the purpose of this review to discuss potential targets in treating ARDS with RNAi strategies, as well as to outline the challenges of oligonucleotide delivery to the lung and tactics to circumvent these delivery barriers.
INTRODUCTION
Acute respiratory distress syndrome (ARDS) develops from direct or indirect injury to the lung, from conditions including sepsis, severe trauma, bacterial/viral pneumonia, and aspiration.1, 2 The disease occurs in approximately 80 out of 100,000 individuals per year in the United States, with a mortality rate of up to 40% when severe ARDS is developed.3, 4 Interestingly, studies have shown that ARDS survivors usually only demonstrate mild deficiencies in pulmonary functions and physical capacity following the first 3 months postrecovery.5, 6 Amazingly, patients that survive typically regain normal pulmonary function and capacity as early as 6-12 months postrecovery; unfortunately, long-term pulmonary structural changes are difficult to evaluate.7, 8, 9 This review will focus on the pathophysiological characteristics of ARDS, current approaches for treatment, methods for utilizing RNA interference (RNAi) therapeutics, and challenges for treating ARDS using these methods.
Molecular etiology of ARDS
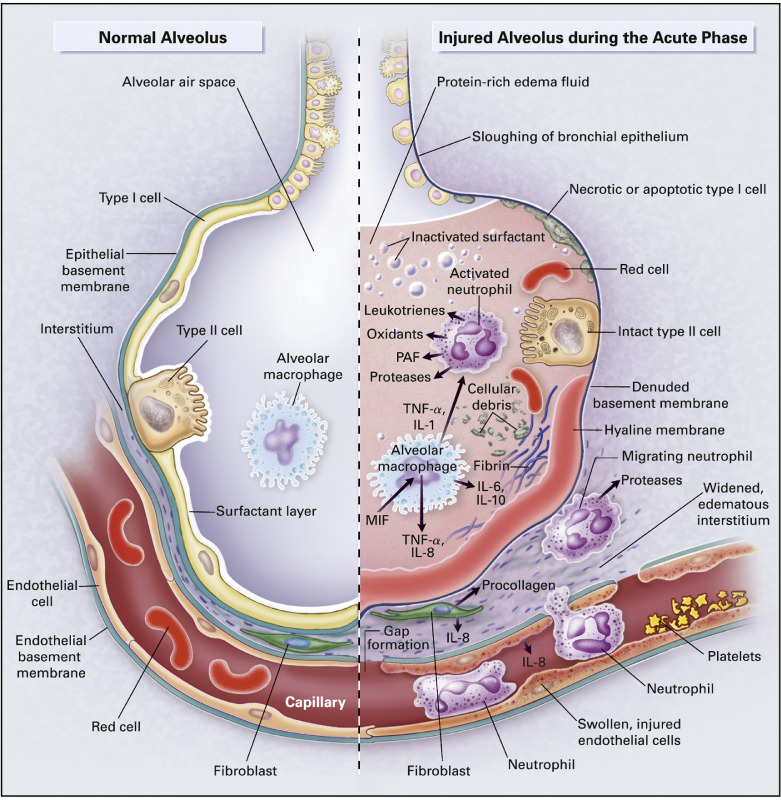
Clinical diagnosis of ARDS is characterized by an abrupt onset of severe hypoxia and accumulation of pulmonary edema.10 ARDS can result from direct injury to the lung (ARDSdirect) or extrapulmonary injury, nonpulmonary sepsis, and indirect trauma to the lung (ARDSindirect).11 Cellular characteristics of ARDS include loss of the alveolar-capillary membrane integrity, excessive transendothelial, and transalveolar neutrophil migration, and release of proinflammatory cytotoxic mediators, resulting in further disruption of the epithelial/endothelial barrier12, 13, 14 (Fig 1 ).
Fig 1.
Effects of ARDS on lung alveoli. This figure shows the molecular physiology of a healthy alveolus (left) compared to a patient with ARDS (right). The healthy alveolus is lined with pulmonary surfactant and free of pulmonary edema; however, ARDS patients experience impaired gas exchange due to pulmonary edema, resulting from massive influx of fluid and inflammatory cells secondary to loss of endothelial and epithelial barriers. The shedding of the surfactant layer triggers immune responses by macrophages and neutrophils, which further augments these processes. The figure illustrates some of the important regulatory signaling pathways activated by ARDS, which are discussed as potential therapeutic targets. From Ware and Matthay.14 (Color version of figure is available online.)
The alveolar-capillary unit is primarily composed of alveolar epithelial and pulmonary endothelial cells and which are both prone to injury in both ARDSdirect and ARDSindirect. Endothelial dysfunction is characterized by acquisition of proadhesive and proinflammatory phenotypes, and loss of endothelial barrier integrity, leading to inflammatory cell infiltration and plasma extravasation into the interstitium.15 Similar aberrant responses from activated epithelial cells, coupled with their diminished ability of fluid clearance, paves the way for a massive influx of inflammatory cells and protein-rich fluid from the interstitium into the alveolar space with severe consequences for pulmonary gas exchange.16, 17 These processes are further augmented by apoptosis and necrosis of these (endothelial and epithelial) cells, which promote further inflammation and activation of coagulation cascades on denuded surfaces in a continuous cycle that amplifies and exacerbates ARDS.15, 16, 18, 19
While the disease can be caused by a multitude of different factors, the molecular mechanisms have invited much investigation leading to two prevailing hypotheses to explain the etiology of ARDS: the neutrophil hypothesis and the epithelial/endothelial hypothesis.20 The neutrophil hypothesis states that neutrophils are activated in the bloodstream and in the lung by proinflammatory mediators and chemokines. Prolonged neutrophil activation causes rapid release of reactive oxygen species (ROS),21, 22, 23 preventing transition from a proinflammatory to an anti-inflammatory environment.24, 25 The epithelial/endothelial hypothesis states that the apoptosis of lung epithelial/endothelial cells results in loss of epithelial-endothelial barrier function.20, 26 Pathophysiological characteristics indicate that both hypotheses are implicated in ARDS development. Neutrophil recruitment and epithelial/endothelial cell death both play a key role in ARDSdirect, while epithelial/endothelial cell death is more prevalent in ARDSindirect.20, 25, 27, 28 While a higher degree of epithelial damage has been noted in lungs from septic patients compared to endothelial cells, it is attributed not to the lesser degree of damage, but to higher regenerative capacity of endothelial cells.29 Consistent with this, endothelial cells from endotoxemic mice have been shown to possess regenerative capacity, which relies on activation of Fox1M transcription factor.30
Endothelial barrier integrity is important because it restricts the movement of proteins and fluids into the interstitium. Endothelial cells form a continuous monolayer that creates an endothelial-capillary barrier via tight and adheren junctions.31, 32 Endothelial cells can cause the dysfunction of their junctions by secreting tumor necrosis factor α (TNF-α) to increase the influx of pulmonary edema, which acts by phosphorylating adheren junction proteins.33, 34 Interendothelial gaps can also result from cytoskeleton contraction due to phosphorylation of myosin light chain (MLC).35 Influx of Ca2+ has been shown to increase vascular permeability and allow for migration of neutrophils across the alveolocapillary barrier.36 The production of free radicals by neutrophils and other polymorphonuclear leukocytes contributes to vascular leakage.37, 38
Epithelial cell death is another mechanism in ARDS that greatly contributes to disease progression and severity.39 Lung epithelial cells are the cell type that mainly undergo apoptosis in ARDS, a process which has been detected as quickly as 6 hours in postinjury mice.39, 40 In healthy lung tissue, apoptosis is prevented by surfactant protein A released by type II pneumocytes.41, 42 Unfortunately, the bronchoalveolar (BAL) fluid of ARDS afflicted lung contains a reduced concentration of surfactant protein A, which greatly increases epithelial apoptosis propensity.43 Epithelial cell death can be triggered extrinsically or intrinsically. The intrinsic pathway is initiated by Bcl-2 proteins which increase the permeability of the outer mitochondrial membrane, resulting in the irreversible release of cytochrome c into the cytosol.44 The extrinsic pathway is initiated by ligation of cell-surface death receptors and their ligands.44 One of the most important death receptors is Fas, which interacts with soluble FasL or FasL expressed on lymphocytes to induce apoptosis. Onset of ARDS results in increased expression of Fas on alveolar epithelial cells and an increase in FasL concentration in BAL fluid.20 , 45, 46, 47 Fas silencing in lung epithelium potentially moderates ARDS by decreasing lung apoptosis and reducing the severity of pulmonary histological changes.20 The intrinsic and extrinsic pathways result in activation of caspases that cause irreversible DNA fragmentation and phosphatidylserine externalization.44 Epithelial apoptosis results in disruption of the alveolar barrier, allowing pulmonary edema to flood the alveolar spaces. This hypothesis has been supported by the detection of increased Fas expression in lung epithelium.26, 39, 48 Neutrophil accumulation in the lung during ARDS also increases epithelial cell death and inflammation.39, 48 Neutrophils release proinflammatory factors and ROS, triggering lung epithelial cell apoptosis. These molecular mechanisms provide many potential therapeutic targets that can be targeted with RNAi.
ARDS diagnosis is solely dependent on clinical criteria due to the impracticality of obtaining direct measurements from ailing patients.49 However, diagnosis is difficult due to its dependence on risk factors including, but not limited to; bacterial and viral respiratory lung infections,50, 51, 52 alcohol abuse,53, 54 cigarette smoking,55, 56 and air pollution.57, 58 Unfortunately, only a minority of patients with these risk factors develop ARDS, leading to poor clinical recognition: 51% in mild ARDS and 79% in severe ARDS.59, 60 Another challenge in proper diagnosis is the need to quickly differentiate ARDS from mimicking syndromes including, but not limited to, acute eosinophilic pneumonia, diffuse alveolar hemorrhage, acute interstitial pneumonia, and acute heart failure.49 Failure to differentiate these mimics from ARDS can contribute to the extremely rapid onset in disease progression. Therefore, effective therapeutics must not only mitigate symptoms of ARDS, but also treat the underlying risk factors.
Current treatment options for ARDS include mechanical ventilation, vasodilators, and corticosteroid administration.10 These treatment options are limited in their effectiveness because they focus on reducing further injury, rather than addressing the underlying molecular mechanisms impacting patient survival. Human trials have shown that corticosteroids do not decrease ARDS frequency and do not improve patient outcome with administration at early disease stages.61, 62, 63, 64 Extracorporeal membrane oxygenation uses an external machine to pump and oxygenate a patient's blood. This method in combination with carbon dioxide removal has not been shown to improve patient survival and actually results in substantial infection and bleeding risks.65, 66, 67, 68 Vasodilators, such as hydralazine and nitric oxide, have shown improved oxygenation and vascular resistance in ARDS patients; however, these improvements did not translate to improved clinical outcomes.69, 70, 71 While these interventions manage symptoms, ARDS is in need of new therapeutic strategies to reduce fatalities by treating underlying molecular mechanisms.
A promising therapeutic strategy is the use of noncoding RNAs to suppress expression of important genes involved in ARDS development and progression via RNAi. To date, there is only 1 FDA-approved RNAi-therapeutic; Patisiran, used to treat hereditary transthyretin-mediated amyloidosis.72 In addition, there are multiple RNAi therapeutics in phase III clinical trials, including therapeutics to treat hemophilia,73 hypercholesterolemia,74 acute hepatic porphyria,75 and primary hyperoxaluria type 1.76 A recent phase I clinical trial also tested the efficiency of an RNAi-based proprotein convertase subtilisin–kexin type 9 for lowering low-density lipoprotein cholesterol; a 100 mg dosage reduced cholesterol levels by 50.6%, while a 300 mg dosage saw a 74.5% reduction in cholesterol.77
RNA interference
RNAi is a cellular mechanism that induces gene silencing.78, 79, 80, 81, 82, 83, 84, 85 The RNAi pathway is initiated when noncoding double-stranded RNA (dsRNA) is processed into single-stranded RNA.86 There are 4 main categories of noncoding RNAs that participate in RNAi: long dsRNA and short-interfering RNA (siRNA), short hairpin RNA (shRNA), micro RNA (miRNA), and piwi-interacting RNA.
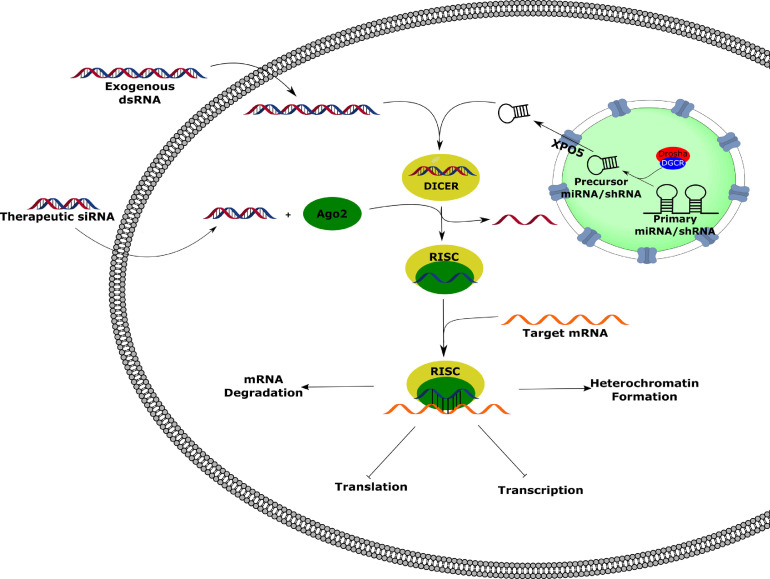
Exogenous dsRNA is processed into double-stranded siRNA by the endoribonuclease Dicer.87 The siRNA-Dicer complex is then loaded into the RNA-induced silencing complex (RISC), where the double-stranded siRNA is unwound, the passenger strand is degraded, and the remaining guide strand is used for target mRNA recognition through complementary binding; strand selection is dictated by thermodynamic stability of the 5′ terminus of the guide strand.83 (Fig 2 ) shRNA and miRNA are endogenous RNAs that operate by similar mechanisms. Both RNAs are transcribed in the nucleus, miRNAs by RNA polymerase II and shRNAs by either RNA polymerase II or RNA polymerase III, with their primary transcript containing similar stem-loop hairpin structures. The primary transcripts are processed by a ribonuclease III enzyme called Drosha and the heme-bound DGCR protein. The partially processed RNAs can then be transported to the cytoplasm by XPO5, where they are then cleaved and processed by Dicer and RISC in a similar manner as siRNA. Upon sequence recognition, the Argonaute-2 protein binds the mRNA to inhibit translational machinery or to signal for degradation, depending on siRNA-mRNA mismatching.80, 83 RNAi can also lead to heterochromatin formation, resulting in long-term or permanent gene silencing.81, 88
Fig 2.
RNA Interference. Upon cellular entry, exogenous double-stranded RNA (dsRNA) is processed by Dicer and Ago2 to form the RNA-induced silencing complex (RISC). The guide strand directs the RISC to the targeted mRNA to silence gene expression by heterochromatin formation, mRNA degradation, or inhibition of transcription or translation. Endogenous miRNAs and shRNAs transcribed in the nucleus are processed by Drosha/DGCR prior to nuclear export by exportin-5 (XPO5). Once in the cytosol, the processed miRNA/shRNA can be incorporated into the RISC to induce genetic silencing. Therapeutic siRNAs introduced to the cell do not need to be processed by Dicer prior to forming the RISC. (Color version of figure is available online.)
Gene silencing via RNAi is a promising therapeutic strategy for treatment of ALI/ARDS that has the potential to modify the underlying molecular mechanisms of these disorders. The introduction of appropriate siRNA, shRNA, or miRNA to lung tissue in ALI/ARDS states can be utilized to prevent epithelial-endothelial dysfunction and lung cell death by targeting critical proteins for disease progression. An RNAi-based therapeutic strategy is conceptually simple and is limited only by accurate understanding of the roles played by various proteins in the disease pathway. In principle, delivery of siRNA to appropriate lung cells will result in mRNA degradation or translational suppression of the target gene(s) by the RISC.87, 89 In fact, there are multiple examples of cell-specific targeting of siRNA. siRNA has been exclusively delivered to dendritic cells in mice by decorating siRNA-containing immunoliposomes with dendritic cell-specific DEC-205 mAb.90 Lymphocytes can also be targeted by using siRNA delivery vectors that can be recognized by RNAi-sensitive molecules such as retinoic acid-inducible gene-1 (RIG-1) and dsRNA-dependent protein kinase (PKR).91, 92 Similarly, lung endothelium has been targeted for cavelin-1 knockdown via IV injections of caveolin-1 siRNA/cationic liposomes and the depletion of caveolin-1 by this approach resulted in increased lung vascular permeability in mice. These studies prove to be encouraging examples that can guide future research for developing novel delivery vectors to specifically bind the various lung cells involved in ARDS. While this strategy is conceptually simple, the therapeutic application of RNAi has been impeded by technical challenges that include delivery.66, 87 , 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122
The major obstacle for in vivo RNAi therapy is efficient siRNA delivery to the cell in a form that is accessible to the RNAi machinery. The most common type of cellular entry observed for macromolecules is clathrin-dependent endocytosis.123 This results in the macromolecule being engulfed in an endosome, which then fuses with a lysosome, resulting in degradation of the endosome cargo.123, 124 Therefore, cellular uptake of therapeutic molecules via this mechanism requires a delivery system that enables early endosomal escape.82 , 99, 100, 101 , 109, 125 Oligonucleotides are poorly transported across the cell membrane and are subject to competitive degradation by endogenous nucleases. Therapeutic delivery of siRNA ideally requires a delivery mechanism that overcomes the poor cellular uptake of oligonucleotides and that stabilizes the siRNA to nucleolytic degradation prior to cellular uptake. In the next section, we will briefly outline common methods for drug delivery to the lung and the advantages and challenges of lung-specific siRNA delivery.
Pulmonary drug delivery
Pulmonary drug delivery is particularly amendable to tissue-specific delivery of siRNA. Drugs are commonly systemically administered either orally or intravenously, which requires that the therapeutic agent be sufficiently stable to survive circulation through various tissues and organs.126 Nucleic acids are highly sensitive to degradation by serum nucleases in the bloodstream, making intravenous delivery problematic for siRNA therapeutics. 87, 119, 127 In contrast, there are advantages to developing an siRNA delivery method that targets the lung. The surface area of the human lung is approximately 50 times the area of the skin.128 The human lung has approximately 480 million alveoli, with type I pneumocytes covering 95% of the alveolar space.129, 130 In total, the lung contains a vast capillary network spanning approximately 1000 km.128 Due to the high volume of alveoli, the cells are typically in very close contact, eliminating the need for connective tissue between them; pneumocytes communicate across an alveolar pore measuring 8–60 µm.130 Therefore, the architecture of the lungs independently confers a huge advantage for drug administration.
Although the lung architecture and vascularization provide significant advantages for drug delivery, the elaborate branched architecture of the lung also presents unique challenges.87 There are 2 types of naturally present fluids in the airways that provide an additional barrier to pulmonary lung delivery: mucus and pulmonary surfactant. Mucus strongly interacts with drug molecules via electrostatic, hydrophilic, and hydrophobic interactions.131 The continuous production and replacement of mucus in the airways efficiently traps and clears large particles.132, 133, 134 Pulmonary surfactant produced by lung cells can strongly interact with nonviral cationic lipid-based delivery systems, impeding their effectiveness.135, 136 As a result, effective delivery of therapeutics to the lung is heavily dependent on the mechanisms of particle deposition.137 The ideal drug formulation particle size for pulmonary delivery is 1–5 µm because larger particles would be trapped in the upper airways, while smaller particles would be easily exhaled.138, 139
The most commonly used methods for pulmonary drug administration are inhalation using metered dose inhalers (MDIs), dry powder inhalers (DPIs), or nebulizers.115 MDIs are the most common inhalation method, requiring a pressurized formulation of the drug. In order to drive aerosolization of the drug it must be dissolved in a propellant, such as chlorofluorocarbons or hydrofluoroalkanes. DPIs function by administering the drug as a cloud of dry particles, however, it can be challenging to create a stable drug powder, making this method less common. Lastly, nebulizers are typically used for drug molecules that cannot be administered with MDIs or DPIs. Nebulizers function by generating a liquid aerosol which allows for large dosage administration.115 Administration of a siRNA-based therapeutic would most likely use MDIs due to challenges that include formulation of biochemically stable siRNA-containing powders and an increased chance of siRNA degradation due to applied stress for DPI and nebulizers, respectively. An advantage of pulmonary delivery by these methods is that off-target effects observed by systemic delivery can be largely avoided since the bulk of the drug is retained in the lung.115
Unfortunately, treatment with mechanical ventilation for ARDS patients adds a unique challenge for delivery because drug particles cannot negotiate the ventilator circuits and endotracheal tubes.140 MDIs require actuator devices for drug delivery to connect the inhaler with the pressurized circuits of the ventilators.141 Drug delivery efficiency via this method varies with the design of the adaptor and the drugs/propellants used; in vitro studies have demonstrated extremely unpredictable efficiency ranging from 0.3% to 97.5%.140 , 142, 143, 144, 145, 146, 147, 148 Nebulizer efficiency is also dependent on the type of nebulizer used and the positioning of the inhaler in the ventilation circuitry; inhalers with greater residual volume will afford a lower concentration of nebulized drug particles.149, 150 Dry-powder drug particle administration can engage the ventilator's inspiratory air flow to generate the aerosol or the drug particles can be introduced to the ventilator's air flow.149
SIRNA DELIVERY SYSTEMS AND THERAPEUTICS
The efficacy of potential siRNA therapeutics is highly dependent on the delivery system used. Naked siRNA shows low immune and inflammatory responses in vivo but is highly susceptible to degradation and has poor cellular transduction. Naked siRNA transduction occurs effectively only with the use of electroporation, sonoporation, jet injection, or related membrane disruption methods.111 None of these methods have yet been tested in the lungs of patients, presenting an urgent need for development of improved methods for siRNA delivery. An ideal delivery system will be biodegradable, biocompatible, noncytotoxic, nonimmunogenic, and will adequately protect the payload from premature degradation.114, 119 In addition, the ideal delivery agent will facilitate translocation of siRNA cargo into cells without damaging the cell membrane or other cellular components and must bind siRNA reversibly to allow for selective release of the RNA from the carrier in the intracellular enviroment.114, 119 The different vectors that have been utilized for siRNA therapeutics are briefly discussed below, with their general pros and cons outlined in Table 1 .
Table 1.
Summary of the advantages and disadvantages of different viral vectors that have been utilized for siRNA delivery
| Delivery vector | Advantages | Disadvantages | |
|---|---|---|---|
| Viruses | Adenovirus | Highly efficient transduction profile111 | Immunological responses, nonspecific cell targetting,111 acute toxicity151 |
| AAV | Highly efficient transduction profile,111 reduce inflammatory and immune responses152, 153 | Requires helper virus,152 small cloning capacity154 | |
| Retrovirus | High gene transduction | Dependence of cell differentiated state,155 insertion into host genomes115 | |
| Lentivirus | High gene transduction, cell differentiated state independence156 | Insertion into host genomes115 | |
| Lipids | Cationic | Good transfection efficiency, electrostatic interactions with siRNA115 | Poor stability, cellular toxicity, immune response elicitation106, 157 |
| Neutral | Reduced cytotoxicity115 | Reduced ability to complex with siRNA115 | |
| Nanoparticles | Charge variable, complex formation prior to administration, neutralized at physiological pH, reduced toxicity and immunological responses, increased membrane destabilizing capacity, higher endosomolytic activity110, 115, 158 | Synthetic challenges | |
| Polymers | Polycations | Modifiable size115 | Weaker interactions with siRNA115 |
| Nanoparticles | Biocompatible, biodegradable, synthetic diversity115 | Large surface area causes aggregation159 | |
| Peptides | PLL | Electrostatic interactions with siRNA, synthetic diversity120 | Toxicity, nonspecific binding, poor endosomal escape102, 160, 161 |
| CPPs | Synthetic diversity, covalent and noncovalent complex formation120 | Membrane permeability challenges, poor endosomal escape120, 162 | |
Viral vectors
Adenovirus
Adenovirus is the most widely used viral vector in lung-targeted oligonucleotide therapeutics.111 For the virus to reach its receptor on the basolateral surface of lung epithelial cells in an ALI/ARDS lung, adenovirus must rely on transient barrier dysfunction.155 Clinical trials and animal models have shown that use of adenovirus clears transduced cells and limits the number of tolerated doses.151, 163 Adenovirus has been demonstrated to be efficient in the delivery of noncoding RNA in lung cancer and sepsis-induced lung injury. Adenovirus expressing matrix metalloproteinases-2 siRNA was shown to mitigate metastasis and caner-induced angiogenesis.164 The model studied consisted of A549 human lung carcinoma cells in severe combined immunodeficient mice. Treatment with adenovirus-expressing metalloproteinases-2 siRNA resulted in a 60% reduction in tumor volume and no metastatic foci in mouse lung tissues.164 C5aR-shRNA was delivered via an adenovirus vector in sepsis-induced mice.165 Significant knockdown of C5aR, which has been shown to be important in lung permeability, was achieved 4 days postadministration.165, 166
Adeno-associated virus
Adeno-associated virus (AAV) is a nonpathogenic vector that overcomes some of the deficiencies encountered with adenovirus as a vector for oligonucleotide delivery.155, 167 The AAV vector genomes are approximately 5 kilobases in length, greatly limiting the size of the gene encoding the noncoding RNA.154 siRNA has been successfully delivered to HeLa S3 cells via an AAV vector to silence p53 and caspase-8 expression.168 shRNA expressing AAV was used to target eotaxin-1 in an in vivo study conducted in mice to treat chronic asthma.169 Intratracheal injection of the AAV vector reduced levels of IL-4, IL-5, and IL-10 in the BAL fluid and reduced polymorphonuclear leukocyte infiltration.
Retroviruses
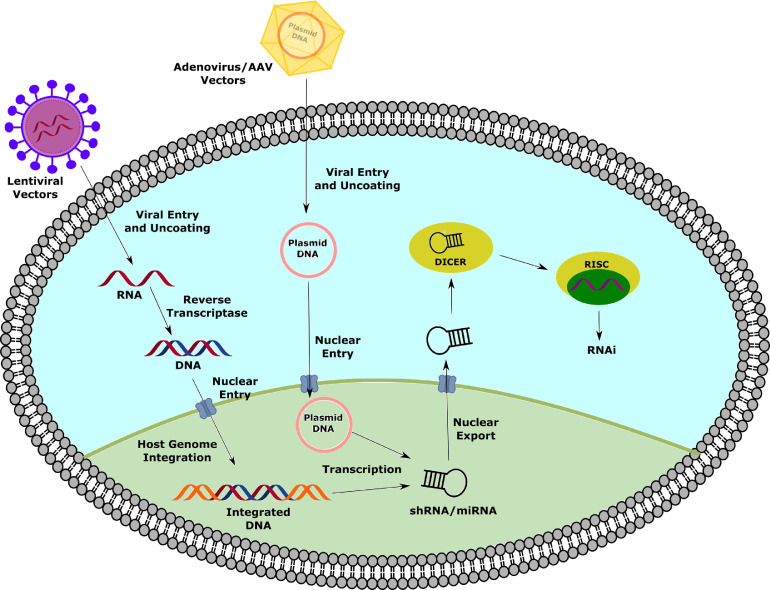
Retroviruses have also been utilized for drug delivery to the lung. In a cystic fibrosis study, a replication-deficient CFTR HIV viral vector demonstrated high gene transduction in a differentiated cystic fibrosis-derived human bronchial cell xenograph, while a fully functional CFTR HIV vector could not.170 In another study, a lentivirus was used to deliver p65-shRNA in mouse lungs, resulting in inhibition of NF-κB activity.171 Lentiviral livin-shRNA was delivered to lung adenocarcinoma xenografts in mice; gene silencing resulted in tumor cell apoptosis and reduced cellular proliferation and growth172 (Fig 3 ).
Fig 3.
Delivery of noncoding RNAs with different viral vectors. Viral vectors act by different mechanisms to produce siRNAs. Lentiviral vectors are uncoated upon cellular entry, which releases their single-stranded RNA (ssRNA) into the cytosol. Reverse transcriptase encoded by the virus allows the ssRNA to be transcribed to double-stranded DNA (dsDNA), which can integrate in the host genome upon nuclear entry. Adenovirus/AAV vectors carry a DNA plasmid encoding for the siRNA, which doesn't require extensive processing prior to nuclear entry. Both viral vectors use the host genome to transcribe the encoded siRNA, which can then be exported from the nucleus to enact gene silencing. (Color version of figure is available online.)
Nonviral vectors: lipids
Cationic lipids
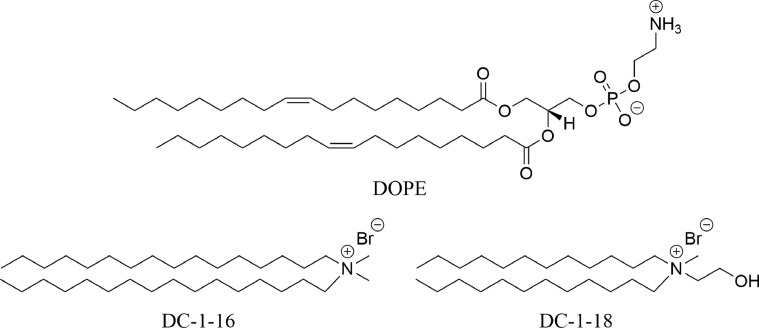
Cationic lipids can spontaneously form lipoplexes with negatively charged siRNA.115 A recent study tested 6 cationic cholesterol derivatives, 11 cationic glycerol-based derivatives, and 17 cationic liposomes to compare the efficiency of Tie2 siRNA delivery to lung tissue when injected intravenously in mice.173 The cholesterol derivates demonstrated very efficient gene silencing, while glycerol-derivatives with short-length alkyl chains and long linker arms decreased the efficiency of gene silencing. However, accumulation was not consistent, with some complexes accumulating in the liver. The authors also demonstrated that 3 glycerol-based derivatives could suppress tumor growth in mice with Lewis lung carcinoma (Fig 4 ).
Fig 4.
Examples of cationic lipids used for non-coding RNA delivery. DC-1-16 and DC-1-18 were used in conjugation with DOPE to treat Lewis lung cell carcinoma by suppressing tumor growth in mice.
Neutral lipids
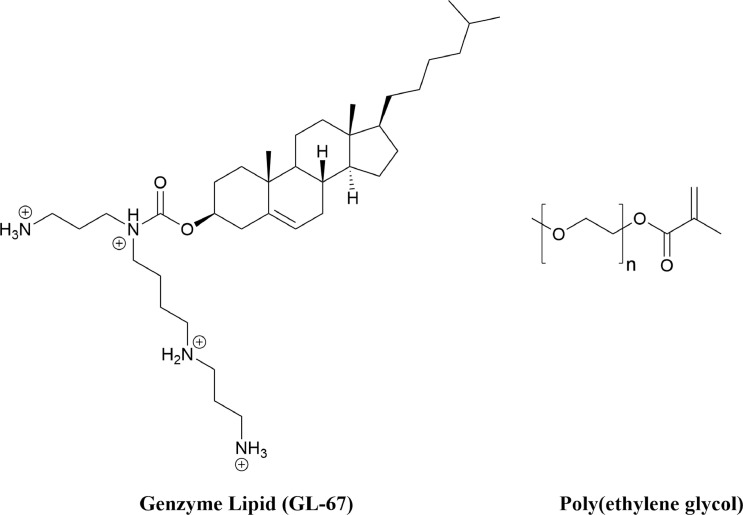
Neutral lipids are significantly less cytotoxic than cationic lipids.115 However, the loss of net charge significantly reduces their ability to effectively complex with negatively charged siRNA.115 Methods for chemically conjugating cholesterol-derived lipids to siRNA have been developed to overcome this disadvantage.82, 125 This conjugation reduced the inflammatory response and knockdown duration but did not improve knockdown.125 Cationic lipids modified with neutral polymer polyethylene glycol have been studied for siRNA delivery with the aim of reducing immunological responses and cellular toxicity.115 Genzyme lipid (GL-67) is an example of a neutral polyethylene glycolylated lipid that has been successfully utilized for plasmid DNA and siRNA delivery to lung cells.174, 175, 176 (Fig 5 ) β-galactosidase-siRNA was delivered to mouse lung via intranasal administration, resulting in 33% knockdown in alveolar cells.176
Fig 5.
Chemical structures of GL-67 and PEG. Poly(ethylene glycol) has been utilized with many different delivery vectors to enhance cellular uptake One such example, is the PEGylated Genzyme Lipid that was used to deliver antisense oligonucleotides and siRNA to mice lung epithelia.
Lipid nanoparticles
Ionizable, pH-sensitive amino lipids have been developed to encapsulate oligonucleotides in the form of lipid nanoparticles.158 The method was improved by developing stable nucleic acid lipid particles with 2 distinctive properties: the ability to induce nonbilayer phase structure with anionic lipids and the ability to tune the surface charge by modifying the pK a of the lipid constituents.110
Nonviral vectors: polymers
Polymer-based delivery systems have also been explored for siRNA delivery. Polycations and polymeric nanoparticles are two polymer types which have been utilized for siRNA delivery.115
Polycations
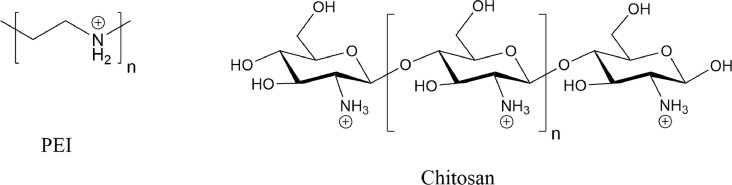
Polycations with a high charge density form electrostatic complexes with negatively charged siRNA.115 The size of polymer-siRNA polyplexes can be modified as a function of polymer molecular weight, polymer charge ratio, solution pH, and solution ionic strength. Although delivery with DNA has been highly successful, siRNA has proven more difficult due to the stiffer RNA structure, resulting in weaker interactions, making the siRNA more susceptible to degradation.115 Polyethylenimine (PEI) has demonstrated high intracellular efficiency for siRNA delivery. The high buffer capacity of PEI results in swelling of the complex in the endosome environment, which results in subsequent rupturing of endosomes. Unfortunately, the high toxicity and nonbiodegradability of PEI has limited its application as an effective delivery system.177 Chitosan is a natural cationic polysaccharide polymer that has advantages over PEI for siRNA delivery due to its biocompatibility, biodegradability, decreased cytotoxicity, and mucosal permeation properties.107, 178, 179 (Fig 6 ) Transgenic endogenous green fluorescent protein (EGFP) mice were used to study the efficiency of the pulmonary delivery by a chitosan-based siRNA vector.178 Intratracheal administration of chitosan-siRNA nanoparticles over a 5-day period demonstrated 43% knockdown of EGFP in bronchial epithelial cells.178 A more recent study used a nebulizing catheter for chitosan-siRNA delivery in transgenic EGFP mice, resulting in an improved 68% knockdown of EGFP in mice lungs.180
Fig 6.
Chemical structures of PEI and Chitosan. Polyethylenimine (PEI) has been used in conjugation with other delivery systems to enhance cellular delivery of siRNA. Chitosan, a linear polysaccharide, has been used as a biocompatible and biodegradable alternative to PEI.
Polymeric nanoparticles
Polymeric nanoparticles are primarily formed from hydrophobic polymers and act to encapsulate siRNA in the surrounding polymer matrix.115 Poly(D,L-lactic-co-glycolic acid) (PLGA) is a copolymer that has been used for gene therapy in the lung.115 PLGA is a copolymer derived from lactic and glycolic acid. Poly glycolic acid is not utilized because it is more rapidly degraded via hydrolysis in the body.181 Addition of lactic acid motifs with methyl side groups, increases the hydrophobicity of the polymer, decreasing the degradation rate of the polymer.181 (Fig 7 ) Many derivatives of this copolymer can be developed with tailored properties. For example, sustained payload release can be obtained by altering the block copolymer composition and molecular weight, which changes the degradation rate of the polymer.182
Fig 7.
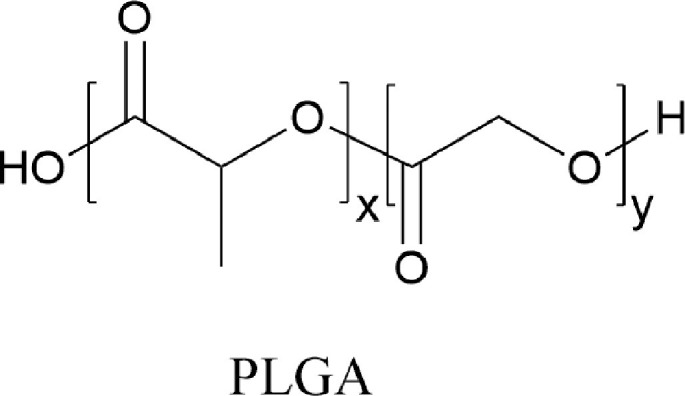
Chemical structure of PLGA copolymer. PLGA is a copolymer derived from lactic and glycolic acid for nucleic acid delivery. Hydrolysis in the body to yield lactic and glycolic acid greatly reduces its toxicity. By altering the number of monomers incorporated into PLGA, the degradation rate of the copolymer can be altered to alter payload release rates.
Nonviral vectors: peptides
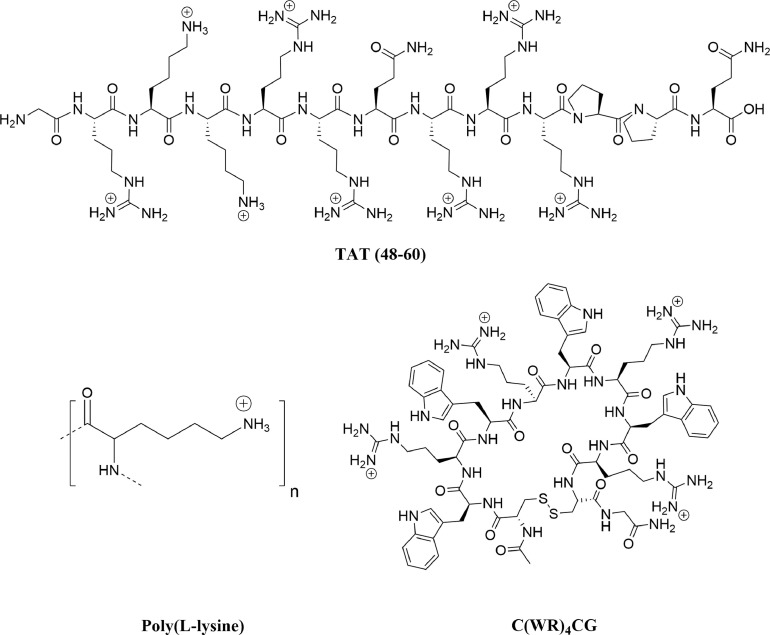
Poly(L-lysine) (PLL)
Poly(L-lysine) (PLL) is a polymer of the amino acid lysine that can interact with siRNA via electrostatic interactions between the negative phosphate backbone and positive lysine residues.120 (Fig 8 ) PLL is a versatile carrier as complex formation can be influenced by polymer molecular weight, pH, charge ratio, salt concentration, and mixing order.183
Fig 8.
Chemical structures of cell-penetrating peptides to deliver noncoding RNAs. HIV-1 TAT (48-60), cyclic C(WK)4CG, and poly(L-lysine) have been used for nucleic acid delivery. These peptides are cationic at physiological conditions, forming strong electrostatic interactions with nucleic acids. The structure of these peptides inhibits passive diffusion across the cell membrane, requiring an endocytosis-mediated mechanism for payload delivery.
Cell-penetrating peptides (CPPs)
A wide range of cell-penetrating peptides (CPPs) have been exploited for siRNA delivery. Some of the most commonly used CPPs for siRNA delivery include transportan,184 VP22,185 MAP,186 and synthetic arginine-rich peptides.187, 188 Cellular uptake of siRNA complexed with CPPs can occur via endocytic or nonendocytic pathways, depending on the particle size, peptide type, and siRNA loading method.120, 162 Covalent peptide complexes ensure that the conjugate remains intact, however, this may reduce the ability of the peptide to penetrate the cell membrane by neutralizing important side chains.120 Proteins and peptides containing dsRNA binding domains have also been complexed with siRNA for cellular delivery.120 HIV-1 trans-activator protein (TAT) has been used for delivery of p38 MAP kinase siRNA to the mouse lung via intratracheal administration.125 (Fig 8) Disulfide-linked conjugation of the siRNA with TAT(48–60) resulted in 20%–30% knockdown 6 hours after administration. TAT is a commonly used CPP, however, some studies have shown nonspecific cellular uptake with this vector.84 Other CPPS that can be used in siRNA delivery include: Protamine 1,189 Penetratin-1190 (PPR)n,191 (PRR)n,191 DPV peptides,192 MPG,193 POA,194 viral proteins,188, 195, 196 and others.197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213
Recently, our lab has developed a cyclic amphipathic peptide (CAP) delivery system for siRNA delivery to the lung.122 Our peptides consist of alternating hydrophobic and hydrophilic amino acids flanked by a cysteine residue at each terminus. The peptides are cyclized by oxidative intramolecular disulfide bond formation between the thiol groups of these flanking cysteines. (Fig 8) These peptides form noncovalent complexes with siRNA that effectively transport siRNA into the cytosol by a mechanism that we are currently working to elucidate. The cyclic peptides are resistant to degradation by proteases and protect the complexed siRNA from degradation by nucleases. Upon cellular entry, the peptide disulfide bonds are reduced in the cytosol and the linearized peptides are proteolytically degraded, resulting in release of the siRNA payload. Cyclic Ac-C(FKFE)2CG-NH2 and Ac-C(WK)4CG-NH2 peptides complexed with thyroid transcription factor-1 siRNA to form CAP-siRNA nanoparticles that were tested in vitro in human A549 lung epithelial cells and in vivo in mouse lung to show 80% knockdown of transcription factor-1 in each. These particles were delivered to mouse lung by aspiration. We are currently applying our CAPs for delivery of ALI-relevant siRNA to explore this as a strategy to intervene in the development of ALI.
Potential ALI/ARDS targets for RNAi intervention
With recent advancements for pulmonary delivery of siRNA, RNAi strategies have great potential as interventions for treatment of ARDS. The realization of this potential relies not only on the development of effective delivery modalities, but also on identification of appropriate ARDS gene targets for RNAi knockdown. Potential therapeutic targets for the treatment of ARDS include proteins affecting epithelial-endothelial dysfunction, neutrophil infiltration, and cell death. (Table 2 ) Much work has been done to find prospective siRNA targets for the modification of lung pathology and to reduce the mortality rate of ARDS. None of these potential targets are yet the subject of clinical trials as therapeutics, but current research is encouraging. In the remaining sections we will outline promising therapeutic targets for RNAi interventions and briefly discuss the data that illustrates the potential of these targets.
Table 2.
Summary of the potential therapeutic targets and their impact on the cellular signaling pathways in ARDS
| Target | Promotes | Inhibits | Epithelial apoptosis | Lung edema | Alveolar leakage | Barrier integrity | Inflammation | Inflamm. cell infiltration | Inflamm. mediators |
|---|---|---|---|---|---|---|---|---|---|
| Rip2 | NF-κB | - | - | P | P | I | P | P | - |
| RPS3 | NF-κB | - | - | P | P | I | P | P | - |
| NF-κB | KC, IL-17, Rip2 | - | - | - | - | I | P | P | - |
| Caspase-3 | KC, MIP-2 | - | P | P | P | - | P | P | - |
| S1P | IL-6, TNF-α | - | - | - | - | - | P | P | - |
| IL-17 | - | - | - | - | - | - | - | P | - |
| Fas | Caspase-3, TNF-α, IL-6, IL-12 | - | P | - | - | - | P | P | - |
| IL-6 | - | IL-10 | - | P | P | - | P | P | - |
| MTOR | - | NF-κB | - | - | - | P | I | I | P |
| KC | IL-6 | - | - | P | P | - | P | P | - |
| MIP-2 | - | - | - | - | - | - | P | - | - |
| IL-12 | - | - | - | - | - | - | P | - | - |
| IL-10 | - | - | - | I | I | - | I | - | - |
| TNF-α | - | - | - | - | - | - | P | - | - |
Abbreviations: I, inhibits; P, promotes.
Therapeutic targets for reducing vascular permeability
The claudin family of proteins is important for forming intercellular barriers at tight junctions.214 Claudin-4, specifically, is known to limit paracellular sodium transport and create chlorine-selective paracellular pores.215 Wray et al have used RNAi to demonstrate that claudin-4 knockdown resulted in leakier tight junctions at the endothelial-epithelial barrier, which results in decreased alveolar fluid clearance and increased pulmonary edema.214 In fact, claudin-4 expression is decreased in early stage ventilator-induced ARDS.214 Based on this data, claudin-4 itself is obviously not an appropriate target for ARDS RNAi intervention. However, additional research that identifies the upstream effectors of claudin-4 downregulation in ARDS progression may present novel valid targets for siRNA knockdown maintaining healthy pulmonary vascular permeability.
Epithelial-endothelial barrier dysfunction is also impacted by angiopoietin-2 (Ang-2).216 Ang-2 is a proinflammatory antagonist for the Tie-2 receptor found in high concentrations on lung endothelial cells, which acts by inhibiting the antiinflammatory angiopoietin-1 ligand from phosphorylating Tie-2.217 Inhibition of this phosphorylation also results in the upregulation of phosphorylated MLC, which increases endothelial barrier permeability.218, 219 A small clinical study found an increase in circulating Ang-2 levels in ARDS patients.216 Exposure of cultured endothelial cells to plasma from ARDS patients with high Ang-2 levels caused disruption of the endothelial barrier and this response was attenuated in the presence of angiopoietin-1, supporting the molecular mechanism of the role of Ang-2 in barrier dysfunction.216 Lung-specific down-regulation of Ang-2 via RNAi is thus a potential therapeutic target to improve the clinical outcomes of ARDS patients.
Therapeutic targets for reducing inflammation and cell death
Keratinocyte-derived chemokine (KC) and macrophage-inflammatory protein-2 (MIP-2) are 2 neutrophil-attracting chemokines that share a common affinity for CXC chemokine receptor 2.220 Upregulation of KC and MIP-2 was observed in a mouse model of ARDS.221, 222, 223 Naked KC and MIP-2 siRNA were administered via intratracheal administration 2 hours after inducing ARDS in order to assess the effects of downregulation of these proteins.221 Local MIP-2 levels were not reduced by KC nor MIP-2 silencing, indicating that circulating MIP-2 is more likely to be associated with the lung's inflammatory environment during ARDS. Decreased levels of interleukin-6 (IL-6) was 2 times lower with KC than with MIP-2 silencing. This study demonstrated a 40% suppression of chemokine expression. Based on this data, knockdown of KC in the lung may have therapeutic value for ARDS treatment, while systemic MIP-2 silencing, rather than lung-specific MIP-2 knockdown, may be necessary to modify ARDS.
The effects of direct caspase-3 knockdown have also been interrogated.40 The effects of 4 different caspase-3-siRNA duplexes were compared following intratracheal administration of these siRNAs in mice following hemorrhagic shock. Transfection of caspase-3-siRNA resulted in over 90% knockdown of caspase-3 mRNA, protecting epithelial cells from caspase-dependent apoptosis.40 Caspase-3 knockdown suppressed TNF-α, IL-6, and KC levels within 24 hours, resulting in a reduction in neutrophil infiltration. Systematic inflammation was reduced following a decrease in local and plasma TNF-α, IL-6, KC, and MIP-2 levels. The suppression of caspase-3 also resulted in improved alveolar architecture and reduced alveolar leakage. Mice that received the caspase-3-siRNA had a 10-day greater survival rate than the control group, indicating the promise of caspase-3 as an RNAi target for potential ARDS intervention.40
IL-6 is also a potential target for siRNA-based therapies. A lentiviral vector was recently used to deliver IL-6 shRNA to rat lung following intestinal ischemia-reperfusion (II/R).224 Intestinal II/R involves reoxygenation of the intestines following vascular blockage for a prolonged period of time.225 This type of injury activates the immune system due to the production of ROS, cytokines, and chemokines, leading to distal organ dysfunction, particularly in the lung.224, 225 II/R significantly increased IL-6 expression, with the highest concentration recorded 8 hours postinjury. The shRNA-lentivirus complex was delivered by direct injection to lung tissue. IL-6 levels and red blood cell concentration was significantly decreased in pulmonary alveoli 16 hours postinjury. Silencing of IL-6 increased IL-10 expression and reduced lung edema and alveolar leakage, indicating that IL-6 is a candidate for RNAi intervention in ARDS.
Homeodomain-interacting protein kinase 1 (HIPK1) is an important pro-oncogene involved in regulating apoptosis and DNA-damage repair.226, 227, 228 Lipopolysaccharide-induced ARDS mice exhibited elevated levels of HIPK1. Lipofectamine-delivered HIPK1 siRNA significantly reduced HIPK1 mRNA expression and reduced IL-6 and TNF-α levels. This indicates that HIPK1 may also be an appropriate ARDS target for RNAi intervention.
Another potential target is sphingosine-1-phosphate (S1P), which reduces acute pulmonary inflammation by blocking NF-κB signaling in macrophages.229 Inhibition of NF-κB impacts barrier integrity, cytokine release, and neutrophil infiltration.229 S1P lyase (S1Plyase) is an essential enzyme for S1P activity; silencing of this enzyme inhibits S1P, thereby, reducing inflammation and neutrophil infiltration.229 Oh and Lee silenced S1Plyase activity by intratracheally coadministering S1Plyase siRNA with high mobility group box-1 peptide (HMGB1A). High mobility group box-1 (HMGB1) induces ARDS by releasing inflammatory cytokines; HMGB1A functions as an antagonist of HMGB1.230, 231, 232 Nanoparticles were formed by using R3V6 peptide as an S1Plyase siRNA carrier and administration of these particles reduced S1Plyase, IL-6, and TNF-α concentrations in BAL fluid. The authors also compared siRNA delivery by these nanoparticles to delivery with PEI and Lipofectamine carriers. The R3V6 peptide demonstrated higher delivery efficiency and was found to be less cytotoxic than the PEI and Lipofectamine nanoparticles. HMGB1A has also been linked to lung-epithelial binding peptide (LEBP).233 LEBP linkage resulted in epithelial-specific transfection, with an improved transfection efficiency over PLL or the non-LEBP HMGB1A conjugate. The use of this linker was shown to be noncytotoxic.
Receptor-interacting protein 2 (Rip2) positively regulates the NF-κB pathway and is upregulated by NF-κB expression.234 Patients in late stage ARDS demonstrate significant upregulation of Rip2, making it an important potential target for silencing.235 Lipofectamine-delivered Rip2 siRNA was administered intratracheally to mice for 3 consecutive days following extensive cigarette smoke (CS) exposure for 5 weeks.236 Rip2 knockdown reduced neutrophil and lymphocyte infiltration, suppressed proinflammatory mediators in the BAL fluid, and inhibited expression of TNF-α and KC, indicating the potential of Rip2 as a target for RNAi intervention.
NF-κB can also be directly targeted to modify ARDS progression. Lipofectamine was used to deliver NF-κB siRNA to sepsis-induced ARDS in mice. Sepsis induced by cecal ligation and puncture was observed 3 hours after surgery.237 IL-17 and IL-6 count increased 20-fold in the BAL fluid 6 hours and 12 hours postsurgery, respectively. Administration of NF-κB siRNA reduced inflammatory cell infiltration and TNF-α expression after 2 hours. Lung histology was improved and IL-6 and IL-17 levels decreased 12 hours after treatment. However, IL-6 and IL-17 levels reached their minimum at 6 hours.237
Another study targeted NF-κB by targeting ribosomal protein S3 (RPS3) in a CS induced ARDS model.238, 239, 240 RPS3 is an integral part of NF-κB, and is therefore required for correct function. CS exposure increased macrophage, neutrophil, IL-6, KC, and TNF-α levels in the BAL fluid. Administration of RPS3 siRNA reduced levels of all proinflammatory factors and neutrophils. CS exposure correlated with an increase in epithelial wall thickness; RPS3 siRNA knockdown reduced this thickening. The NF-κB p65 subunit and RPS3 nuclear concentrations were both reduced following siRNA treatment.238
Recently, it has been reported that mechanistic target of rapamycin (MTOR) has a complex involvement in ARDS pathology.241, 242 While some studies have shown that MTOR promotes lung inflammation and injury, others have reported an inhibitory role of MTOR in inflammatory lung injury.241, 242, 243 The differential effects of MTOR signaling on ARDS can be ascribed to a cell-specific role of MTOR. We and others have shown that it functions as an anti-inflammatory molecule to limit endothelial cell inflammation, whereas it serves a proinflammatory function to promote inflammation in epithelial cells.243, 244 Similar proinflammatory and anti-inflammatory roles for MTOR have also been reported in neutrophils and monocytes/macrophages, respectively.242, 245 This data demonstrates a significant need for therapeutics with high cell specificity, since it appears that downregulation of MTOR in the lung epithelium and coincident upregulation in the lung endothelium is optimal for ARDS intervention. We have initiated studies to exploit disulfide-constrained CAPs in complex with MTOR siRNA to understand the selectivity of MTOR knockdown in epithelial and endothelial cells in the lung. The etiology of ARDS is increasingly understood to be highly nuanced, and greater understanding of disease-modifying proteins is necessary to validate efficacious protein targets for siRNA-based interventions.
Therapeutics for Active inflammation resolution and pulmonary repair
Although prevention of ARDS progression has been the focus of therapeutic development, therapeutics that activate beneficial innate immunity pathways would also serve to benefit ARDS patients. Regulatory T-cell activation and clearance of apoptotic/necrotic cells by phagocytic cells has been shown to be important for lung repair in ARDS patients.246, 247 One way in which T-cells promote active repair is by secreting IL-10, suggesting that upregulation of T-cell activation by RNAi can be a useful therapeutic tool.247, 248, 249 Regulatory T-cell activation and differentiation is dependent on CD4 T cell coreceptor, CD25,250, 251 and Forkhead box P3 (FoxP3)252, making these additional targets for RNAi therapy.
CONCLUSIONS
ARDS is a serious medical condition with a very high mortality rate, due to difficulty in adequately diagnosing the disease. Current methods of treatment focus on preventing further tissue damage without addressing the underlying mechanisms involved. RNAi is an attractive choice for therapeutic development because it can both prevent further pulmonary damage and reverse current pulmonary damage before serious disease progression occurs. Although there are many advantages to pulmonary delivery, there are multiple lung-specific barriers that need to be overcome. In addition, siRNA requires a delivery system which is biodegradable, biocompatible, noncytotoxic, nonimmunogenic, and that adequately protects the payload from degradation and binds siRNA reversibly to allow for efficient release of the drug. Although different viral and nonviral vectors have been developed, none are ideal and are in need of further development. While the architecture of the lung and the possibility for isolated delivery to the lung is advantageous for selective siRNA delivery, challenges presented by mucous and pulmonary surfactant A need to be overcome when designing effective delivery vectors. Potential targets for the treatment of ARDS are extensive, with new targets still being discovered, making RNAi a promising therapeutic strategy. Overcoming significant deficits with understanding of ARDS progression as well as the development of next-generation delivery vectors are both necessary in order to realize the potential of RNAi intervention as a treatment modality for ARDS.
Acknowledgments
Declaration of competing interest: All the authors have read the journal's policy regarding disclosure of potential conflicts of interests and declare that there are no conflicts of interest.
Preparation of this manuscript was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL138538 and R01HL120521).
Author contribution: The journal's authorship agreement has been reviewed and the manuscript has been reviewed and approved by all authors involved.
We also thank Jade Welch for editorial suggestions during the preparation of the manuscript.
REFERENCES
- 1.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Ragaller M, Richter T. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock. 2010;3:43–51. doi: 10.4103/0974-2700.58663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan C, Liu L, Xie JF, Qiu HB. Acute respiratory distress syndrome: challenge for diagnosis and therapy. Chin Med J. 2018;131:1220–1224. doi: 10.4103/0366-6999.228765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 7.Puybasset L, Cluzel P, Chao N, et al. A computed tomography scan assessment of regional lung volume in acute lung injury. Am J Respir Crit Care Med. 1998;158:1644–1655. doi: 10.1164/ajrccm.158.5.9802003. [DOI] [PubMed] [Google Scholar]
- 8.Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210:29–35. doi: 10.1148/radiology.210.1.r99ja2629. [DOI] [PubMed] [Google Scholar]
- 9.Desai SR, Wells AU, Suntharalingam G, et al. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary injury: a comparative CT study. Radiology. 2001;218:689–693. doi: 10.1148/radiology.218.3.r01mr31689. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 11.Perl M, Chung C-S, Lomas-Neira J, et al. Silencing of fas, but not Caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson LD, Steinberg KP. Epidemiology of acute lung injury and ARDS. Chest. 1999;116:74S–82S. doi: 10.1378/chest.116.suppl_1.74s-a. [DOI] [PubMed] [Google Scholar]
- 14.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 15.Orfanos SE, Mavrommati I, Korovesi I, Roussos C. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med. 2004;30:1702–1714. doi: 10.1007/s00134-004-2370-x. [DOI] [PubMed] [Google Scholar]
- 16.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 18.Calfee CS, Eisner MD, Ware LB, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders*. Crit Care Med. 2007;35:2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glas GJ, Van Der Sluijs KF, Schultz MJ, et al. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J Thromb Haemost. 2013;11:17–25. doi: 10.1111/jth.12047. [DOI] [PubMed] [Google Scholar]
- 20.Perl M, Chung CS, Perl U, et al. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am J Respir Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayala A, Chung C-S, Lomas JL, et al. Shock-induced neutrophil mediated priming for acute lung injury in mice. Am J Pathol. 2002;161:2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matute-Bello G, Liles WC, Radella F, Steinberg KP, Ruzinski JT, Hudson LD, MArtin TR. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal A, Baker CS, evans TW, Haslam PL. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J. 2000;15:895–901. doi: 10.1034/j.1399-3003.2000.15e14.x. [DOI] [PubMed] [Google Scholar]
- 24.Teder P, Vandivier RW, Jiang D, et al. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 25.Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura Y, Hashimoto S, Mizuta N, et al. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med. 2001;163:762–769. doi: 10.1164/ajrccm.163.3.2003065. [DOI] [PubMed] [Google Scholar]
- 27.Menezes SL, Bozza PT, Neto HC, et al. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol (1985) 2005;98:1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 28.Maniatis NA, Kotanidou A, Catravas JD, Orfanos SE. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol. 2008;49:119–133. doi: 10.1016/j.vph.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachofen H, Bachofen M, Weibel ER. Ultrastructural aspects of pulmonary edema. J Thorac Imaging. 1988;3:1–7. doi: 10.1097/00005382-198807000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Zhao YY, Gao XP, Zhao YD, et al. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–2343. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 32.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 33.Georgieva GS, Kurata S, Ikeda S, et al. Nonischemic lung injury by mediators from unilateral ischemic reperfused lung: ameliorating effect of tumor necrosis factor-alpha-converting enzyme inhibitor. Shock. 2007;27:84–90. doi: 10.1097/01.shk.0000235131.89986.45. [DOI] [PubMed] [Google Scholar]
- 34.Angelini DJ, Hyun SW, Grigoryev DN, et al. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1232–L1245. doi: 10.1152/ajplung.00109.2006. [DOI] [PubMed] [Google Scholar]
- 35.Reutershan J, Stockton R, Zarbock A, et al. Blocking p21-activated kinase reduces lipopolysaccharide-induced acute lung injury by preventing polymorphonuclear leukocyte infiltration. Am J Respir Crit Care Med. 2007;175:1027–1035. doi: 10.1164/rccm.200612-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez DF, King JA, Weber D, et al. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res. 2006;99:988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maniatis NA, Orfanos SE. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14:22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 38.Gao XP, Zhu X, Fu J, et al. Blockade of class IA phosphoinositide 3-kinase in neutrophils prevents NADPH oxidase activation- and adhesion-dependent inflammation. J Biol Chem. 2007;282:6116–6125. doi: 10.1074/jbc.M610248200. [DOI] [PubMed] [Google Scholar]
- 39.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31:S184–S188. doi: 10.1097/01.CCM.0000057841.33876.B1. [DOI] [PubMed] [Google Scholar]
- 40.Perl M, Chung CS, Perl U, et al. Therapeutic accessibility of caspase-mediated cell death as a key pathomechanism in indirect acute lung injury. Crit Care Med. 2010;38:1179–1186. doi: 10.1097/CCM.0b013e3181d4563f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez de Lara L, Becerril C, Montano M, et al. Surfactant components modulate fibroblast apoptosis and type I collagen and collagenase-1 expression. Am J Physiol Lung Cell Mol Physiol. 2000;279:L950–L957. doi: 10.1152/ajplung.2000.279.5.L950. [DOI] [PubMed] [Google Scholar]
- 42.White MK, Baireddy V, Strayer DS. Natural protection from apoptosis by surfactant protein A in type II pneumocytes. Exp Cell Res. 2001;263:183–192. doi: 10.1006/excr.2000.5120. [DOI] [PubMed] [Google Scholar]
- 43.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 44.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol. 2008;294:L632–L641. doi: 10.1152/ajplung.00262.2007. [DOI] [PubMed] [Google Scholar]
- 45.Fine A, Anderson NL, Rothstein TL, Williams MC, Gochuico BR. Fas expression in pulmonary alveolar type II cells. Am J Physiol. 1997;273:L64–L71. doi: 10.1152/ajplung.1997.273.1.L64. [DOI] [PubMed] [Google Scholar]
- 46.Hamann KJ, Dorscheid DR, Ko FD, et al. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19:537–542. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]
- 47.Wen LPM, Madani K, Fahrni JA, Duncan SR, Rosen GD. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Am J Physiol. 1997;273:L921–L9L9. doi: 10.1152/ajplung.1997.273.5.L921. [DOI] [PubMed] [Google Scholar]
- 48.Martin TR, Hagimoto N, Nakamura M, Matute-Bello G. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc. 2005;2:214–220. doi: 10.1513/pats.200504-031AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nature Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187:736–742. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levitt JE, Calfee CS, Goldstein BA, Vojnik R, Matthay MA. Early acute lung injury: criteria for identifying lung injury prior to the need for positive pressure ventilation*. Crit Care Med. 2013;41:1929–1937. doi: 10.1097/CCM.0b013e31828a3d99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moss M. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- 54.Moss M, Burnham EL. Chronic alcohol abuse, acute respiratory distress syndrome, and multiple organ dysfunction. Crit Care Med. 2003;31:S207–SS12. doi: 10.1097/01.CCM.0000057845.77458.25. [DOI] [PubMed] [Google Scholar]
- 55.Calfee CS, Matthay MA, Eisner MD, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med. 2011;183:1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calfee CS, Matthay MA, Kangelaris KN, et al. Cigarette smoke exposure and the acute respiratory distress syndrome*. Crit Care Med. 2015;43:1790–1797. doi: 10.1097/CCM.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ware LB, Zhao Z, Koyama T, et al. Long-term ozone exposure increases the risk of developing the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;193:1143–1150. doi: 10.1164/rccm.201507-1418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reilly JP, Zhao Z, Shashaty MGS, et al. Low to moderate air pollutant exposure and acute respiratory distress syndrome after severe trauma. Am J Respir Crit Care Med. 2019;199:62–70. doi: 10.1164/rccm.201803-0435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Critical Care. 2007;11:R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cortegiani A, Madotto F, Gregoretti C, et al. Immunocompromised patients with acute respiratory distress syndrome: secondary analysis of the LUNG SAFE database. Crit Care. 2018;22:157. doi: 10.1186/s13054-018-2079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137–1143. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- 62.Weigelt JA. Early steroid therapy for respiratory failure. Arch Surg. 1985;120:536–540. doi: 10.1001/archsurg.1985.01390290018003. [DOI] [PubMed] [Google Scholar]
- 63.Bone RC, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest. 1987;92:1032–1036. doi: 10.1378/chest.92.6.1032. [DOI] [PubMed] [Google Scholar]
- 64.Luce JM, Montgomery AB, Marks JD, et al. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138:62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 65.Zapol WM. Extracorporeal membrane oxygenation in severe acute respiratory failure. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 66.Gattinoni L. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256:881–886. [PubMed] [Google Scholar]
- 67.Brunet F, Belghith M, Mira J-P, et al. Extracorporeal carbon dioxide removal and low-frequency positive-pressure ventilation. Chest. 1993;104:889–898. doi: 10.1378/chest.104.3.889. [DOI] [PubMed] [Google Scholar]
- 68.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- 69.Adhikari N, Granton JT. Inhaled nitric oxide for acute lung injury: no place for NO? JAMA. 2004;291:1629–1631. doi: 10.1001/jama.291.13.1629. [DOI] [PubMed] [Google Scholar]
- 70.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 71.Troncy E, Collet JP, Shapiro S, et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. 1998;157:1483–1488. doi: 10.1164/ajrccm.157.5.9707090. [DOI] [PubMed] [Google Scholar]
- 72.Kristen AV, Ajroud-Driss S, Conceição I, et al. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegenerative Dis Manag. 2019;9:5–23. doi: 10.2217/nmt-2018-0033. [DOI] [PubMed] [Google Scholar]
- 73.Ragni MV, Georgiev P, Mant T, et al. Fitusiran, an investigational RNAi therapeutic targeting antithrombin for the treatment of Hemophilia: updated results from a phase 1 and phase 1/2 extension study in patients without inhibitors. Blood. 2016;128:2572. [Google Scholar]
- 74.Kosmas CE, Muñoz Estrella A, Sourlas A, et al. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases. 2018;6:63. doi: 10.3390/diseases6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balwani M, Gouya L, Rees D, et al. GS-14-ENVISION, a phase 3 study to evaluate efficacy and safety of givosiran, an investigational RNAi therapeutic targeting aminolevulinic acid synthase 1, in acute hepatic porphyria patients. J Hepatol. 2019;70:e81–ee2. [Google Scholar]
- 76.Frishberg Y, Deschenes G, Cochat P, et al. Safety and efficacy study of lumasiran, an investigational rna interference (RNAi) therapeutic, in adult and pediatric patients with primary hyperoxaluria type 1 (PH1) J Urol. 2019;201:e174–e389. [Google Scholar]
- 77.Fitzgerald K, White S, Borodovsky A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51. doi: 10.1056/NEJMoa1609243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 79.Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 81.Lippman Z, Martienssen R. The role of RNA interference in heterochromatic silencing. Nature. 2004;431:364–370. doi: 10.1038/nature02875. [DOI] [PubMed] [Google Scholar]
- 82.Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 83.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geoghegan JC, Gilmore BL, Davidson BL. Gene silencing mediated by siRNA-binding fusion proteins is attenuated by double-stranded RNA-binding domain structure. Mol Ther Nucleic Acids. 2012;1:e53. doi: 10.1038/mtna.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dana H, Chalbatani GM, Mahmoodzadeh H, et al. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. 2017;13:48–57. [PMC free article] [PubMed] [Google Scholar]
- 87.Qiu Y, Lam JK, Leung SW, Liang W. Delivery of RNAi therapeutics to the airways-from bench to bedside. Molecules. 2016;21:1249. doi: 10.3390/molecules21091249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sugiyama T, Cam H, Verdel A, Moazed D, Grewal SI. RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc Natl Acad Sci U S A. 2005;102:152–157. doi: 10.1073/pnas.0407641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orban TI, Izaurralde E. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. RNA. 2005;11:459–469. doi: 10.1261/rna.7231505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng X, Vladau C, Zhang X, et al. A novel in vivo siRNA delivery system specifically targeting dendritic cells and silencing CD40 genes for immunomodulation. Blood. 2009;113:2646. doi: 10.1182/blood-2008-04-151191. [DOI] [PubMed] [Google Scholar]
- 91.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Petterson T, Månsson A, Riesbeck K, Cardell LO. Nucleotide-binding and oligomerization domain-like receptors and retinoic acid inducible gene-like receptors in human tonsillar T lymphocytes. Immunology. 2011;133:84–93. doi: 10.1111/j.1365-2567.2011.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiss B, Davidkova G, Zhou LW. Antisense RNA gene therapy for studying and modulating biological processes. Cell Mol Life Sci. 1999;55:334–358. doi: 10.1007/s000180050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernandez-Carneado J, Kogan MJ, Pujals S, Giralt E. Amphipathic peptides and drug delivery. Biopolymers. 2004;76:196–203. doi: 10.1002/bip.10585. [DOI] [PubMed] [Google Scholar]
- 95.Reynolds A, Leake D, Boese Q, et al. Rational siRNA design for RNA interference. Nature Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 96.Oliveira S, Storm G, Schiffelers RM. Targeted delivery of siRNA. J Biomed Biotechnol. 2006;2006:63675. doi: 10.1155/JBB/2006/63675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li CX, Parker A, Menocal E, et al. Delivery of RNA interference. Cell Cycle. 2006;5:2103–2109. doi: 10.4161/cc.5.18.3192. [DOI] [PubMed] [Google Scholar]
- 98.Lundberg P, El-Andaloussi S, Sutlu T, Johansson H, Langel U. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB J. 2007;21:2664–2671. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- 99.Thomas M, Lu JJ, Chen J, Klibanov AM. Non-viral siRNA delivery to the lung. Adv Drug Deliv Rev. 2007;59:124–133. doi: 10.1016/j.addr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moschos SA, Williams AE, Lindsay MA. Cell-penetrating-peptide-mediated siRNA lung delivery. Biochem Soc Trans. 2007;35:807–810. doi: 10.1042/BST0350807. [DOI] [PubMed] [Google Scholar]
- 101.Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123:1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 102.Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J. 2007;9:E18–E29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Joanne Lomas-Neira C-SC, Ayala Alfred. RNA interference as a potential therapeutic treatment for inflammation associated lung injury. International J Clin Exp Med. 2008;1:154–160. [PMC free article] [PubMed] [Google Scholar]
- 104.de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19:125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- 105.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61:850–862. doi: 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 107.Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009;11:639–652. doi: 10.1208/s12248-009-9140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Semple SC, Akinc A, Chen J, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 111.Lin X, Dean DA. Gene therapy for ALI/ARDS. Crit Care Clin. 2011;27:705–718. doi: 10.1016/j.ccc.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krebs MD, Alsberg E. Localized, targeted, and sustained siRNA delivery. Chemistry. 2011;17:3054–3062. doi: 10.1002/chem.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gunther M, Lipka J, Malek A, et al. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm. 2011;77:438–449. doi: 10.1016/j.ejpb.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Merkel OM, Kissel T. Nonviral pulmonary delivery of siRNA. Acc Chem Res. 2012;45:961–970. doi: 10.1021/ar200110p. [DOI] [PubMed] [Google Scholar]
- 115.Lam JK, Liang W, Chan HK. Pulmonary delivery of therapeutic siRNA. Adv Drug Deliv Rev. 2012;64:1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamasaki T, Suzuki H, Shirohzu H, et al. Efficacy of a novel class of RNA interference therapeutic agents. PLoS One. 2012;7:e42655. doi: 10.1371/journal.pone.0042655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujita Y, Takeshita F, Kuwano K, Ochiya T. RNAi therapeutic platforms for lung diseases. Pharmaceuticals. 2013;6:223–250. doi: 10.3390/ph6020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171:349–357. doi: 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Merkel OM, Rubinstein I, Kissel T. siRNA delivery to the lung: what's new? Adv Drug Deliv Rev. 2014;75:112–128. doi: 10.1016/j.addr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shukla RS, Qin B, Cheng K. Peptides used in the delivery of small noncoding RNA. Mol Pharm. 2014;11:3395–3408. doi: 10.1021/mp500426r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H, Tsui TY, Ma W. Intracellular delivery of molecular cargo using cell-penetrating peptides and the combination strategies. Int J Mol Sci. 2015;16:19518–19536. doi: 10.3390/ijms160819518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Welch JJ, Swanekamp RJ, King C, Dean DA, Nilsson BL. Functional delivery of siRNA by disulfide-constrained cyclic amphipathic peptides. ACS Med Chem Lett. 2016;7:584–589. doi: 10.1021/acsmedchemlett.6b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zaki NM, Tirelli N. Gateways for the intracellular access of nanocarriers: a review of receptor-mediated endocytosis mechanisms and of strategies in receptor targeting. Expert Opin Drug Deliv. 2010;7:895–913. doi: 10.1517/17425247.2010.501792. [DOI] [PubMed] [Google Scholar]
- 124.Gilleron J, Querbes W, Zeigerer A, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 125.Moschos SA, Jones SW, Perry MM, et al. Lung delivery studies using siRNA conjugated to TAT(48-60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug Chem. 2007;18:1450–1459. doi: 10.1021/bc070077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patrick GL. 6 ed. United States of America: Oxford University Press; New York, NY: 2007. An Introduction to Medicinal Chemistry; p. 832. 10016. [Google Scholar]
- 127.Zheng M, Librizzi D, Kilic A, et al. Enhancing in vivo circulation and siRNA delivery with biodegradable polyethylenimine-graft-polycaprolactone-block-poly(ethylene glycol) copolymers. Biomaterials. 2012;33:6551–6558. doi: 10.1016/j.biomaterials.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 128.Emeritus CPHJ, Keen SL, Larson A, et al. McGraw-Hill Education; 2013. Integrated principles of zoology. [Google Scholar]
- 129.Ochs M, Nyengaard JR, Jung A, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–124. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 130.Gartner LP, Hiatt JL. Saunders Elsevier; Philadelphia: 2007. Color textbook of histology. [Google Scholar]
- 131.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 132.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 134.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duncan JE, Whitsett JA, Horowitz AD. Pulmonary surfactant inhibits cationic liposome-mediated gene delivery to respiratory epithelial cells in vitro. Hum Gene Ther. 1997;8:431–438. doi: 10.1089/hum.1997.8.4-431. [DOI] [PubMed] [Google Scholar]
- 136.De Backer L, Braeckmans K, Demeester J, De Smedt SC, Raemdonck K. The influence of natural pulmonary surfactant on the efficacy of siRNA-loaded dextran nanogels. Nanomedicine. 2013;8:1625–1638. doi: 10.2217/nnm.12.203. [DOI] [PubMed] [Google Scholar]
- 137.Paranjpe M, Muller-Goymann CC. Nanoparticle-mediated pulmonary drug delivery: a review. Int J Mol Sci. 2014;15:5852–5873. doi: 10.3390/ijms15045852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58:1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 139.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 140.Macintyre NR, Silver RM, Miller CW, Schuler F, Coleman RE. Aerosol delivery in intubated, mechanically ventilated patients. Crit Care Med. 1985;13:81–84. doi: 10.1097/00003246-198502000-00005. [DOI] [PubMed] [Google Scholar]
- 141.Dhand R. Inhalation therapy with metered-dose inhalers and dry powder inhalers in mechanically ventilated patients. Respir Care. 2005;50:1331–1334. [PubMed] [Google Scholar]
- 142.Fink JB, Dhand R, Duarte AG, Jenne JW, Tobin MJ. Aerosol delivery from a metered-dose inhaler during mechanical ventilation. An in vitro model. Am J Respir Crit Care Med. 1996;154:382–387. doi: 10.1164/ajrccm.154.2.8756810. [DOI] [PubMed] [Google Scholar]
- 143.Fink JB, Dhand R, Grychowski J, Fahey PJ, Tobin MJ. Reconciling in vitro and in vivo measurements of aerosol delivery from a metered-dose inhaler during mechanical ventilation and defining efficiency-enhancing factors. Am J Respir Crit Care Med. 1999;159:63–68. doi: 10.1164/ajrccm.159.1.9803119. [DOI] [PubMed] [Google Scholar]
- 144.Rau JL, Dunlevy CL, Hill RL. A comparison of inline MDI actuators for delivery of a beta agonist and a corticosteroid with a mechanically ventilated lung model. Respir Care. 1998;43:705–712. [Google Scholar]
- 145.Ran JL, Harwood RJ, Groff JL. Evaluation of a reservoir device for metered-dose bronchodilator delivery to intubated adults: an in Vitro study. Chest. 1992;102:924–930. doi: 10.1378/chest.102.3.924. [DOI] [PubMed] [Google Scholar]
- 146.Taylor RH, Lerman J, Chambers C, Dolovich M. Dosing efficiency and particle-size characteristics of pressurized metered-dose inhaler aerosols in narrow catheters. Chest. 1993;103:920–924. doi: 10.1378/chest.103.3.920. [DOI] [PubMed] [Google Scholar]
- 147.Diot P, Morra L, Smaldone G. Albuterol delivery in a model of mechanical ventilation Comparison of metered-dose inhaler and nebulizer efficiency1995;152: 1391–4. [DOI] [PubMed]
- 148.Thomas SHL, O'Doherty MJ, Page CJ, Treacher DF, Nunan TO. Delivery of ultrasonic nebulized aerosols to a lung model during mechanical ventilation. Am Rev Respir Dis. 1993;148:872–877. doi: 10.1164/ajrccm/148.4_Pt_1.872. [DOI] [PubMed] [Google Scholar]
- 149.Dhand R. How should aerosols be delivered during invasive mechanical ventilation? Respir Care. 2017;62:1343–1367. doi: 10.4187/respcare.05803. [DOI] [PubMed] [Google Scholar]
- 150.Ari A, Fink JB, Dhand R. Inhalation therapy in patients receiving mechanical ventilation: an update. J Aerosol Med Pulm Drug Deliv. 2012;25:319–332. doi: 10.1089/jamp.2011.0936. [DOI] [PubMed] [Google Scholar]
- 151.Schaack J. Adenovirus vectors deleted for genes essential for viral DNA replication. Front Biosci. 2005;10:1146–1155. doi: 10.2741/1607. [DOI] [PubMed] [Google Scholar]
- 152.Tal J. Adeno-associated virus-based vectors in gene therapy. J Biomed Sci. 2000;7:279–291. doi: 10.1007/BF02253246. [DOI] [PubMed] [Google Scholar]
- 153.Moss RB, Rodman D, Spencer LT, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis. Chest. 2004;125:509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- 154.Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kushwah R, Cao H, Hu J. Potential of helper-dependent adenoviral vectors in modulating airway innate immunity. Cell Mol Immunol. 2007;4:81–89. [PubMed] [Google Scholar]
- 156.Engelhardt JF, Yankaskas JR, Wilson JM. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Invest. 1992;90:2598–2607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dokka S, Toledo D, Shi X, Castranova V, Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17:521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 158.Semple SC, Klimuk SK, Harasym TO, et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochimica et Biophysica Acta. 2001;1510:152–166. doi: 10.1016/s0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 159.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 160.Patil ML, Zhang M, Minko T. Multifunctional triblock Nanocarrier (PAMAM-PEG-PLL) for the efficient intracellular siRNA delivery and gene silencing. ACS Nano. 2011;5:1877–1887. doi: 10.1021/nn102711d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dash PR, Read ML, Fisher KD, et al. Decreased binding to proteins and cells of polymeric gene delivery vectors surface modified with a multivalent hydrophilic polymer and retargeting through attachment of transferrin. J Biol Chem. 2000;275:3793–3802. doi: 10.1074/jbc.275.6.3793. [DOI] [PubMed] [Google Scholar]
- 162.Tai W, Gao X. Functional peptides for siRNA delivery. Adv Drug Deliv Rev. 2017;110–111:157–168. doi: 10.1016/j.addr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Joseph PM, O'Sullivan BP, Lapey A, et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Hum Gene Ther. 2001;12:1369–1382. doi: 10.1089/104303401750298535. [DOI] [PubMed] [Google Scholar]
- 164.Chetty C, Bhoopathi P, Joseph P, et al. Adenovirus-mediated small interfering RNA against matrix metalloproteinase-2 suppresses tumor growth and lung metastasis in mice. Mol Cancer Ther. 2006;5:2289–2299. doi: 10.1158/1535-7163.MCT-06-0169. [DOI] [PubMed] [Google Scholar]
- 165.Sun L, Gao H, Sarma VJ, Guo RF, Ward PA. Adenovirus-mediated in vivo silencing of anaphylatoxin receptor C5aR. J Biomed Biotechnol. 2006;2006:28945. doi: 10.1155/JBB/2006/28945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Huber-Lang MS, Riedeman NC, Sarma JV, et al. Protection of innate immunity by C5aR antagonist in septic mice. FASEB J. 2002;16:1567–1574. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- 167.Conway JE, Zolotukhin S, Muzyczka N, Howard GS, Byrne BJ. Recombinant adeno-associated virus type 2 replication and packagin is entirely support by a herpes simplex virus type 1 amplicon expressing rep and cap. J Virol. 1997;71:8780–8789. doi: 10.1128/jvi.71.11.8780-8789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tomar RS, Matta H, Chaudhary PM. Use of adeno-associated viral vector for delivery of small interfering RNA. Oncogene. 2003;22:5712–5715. doi: 10.1038/sj.onc.1206733. [DOI] [PubMed] [Google Scholar]
- 169.Wu CJ, Huang WC, Chen LC, Shen CR, Kuo ML. Pseudotyped adeno-associated virus 2/9-delivered CCL11 shRNA alleviates lung inflammation in an allergen-sensitized mouse model. Hum Gene Ther. 2012;23:1156–1165. doi: 10.1089/hum.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Goldman MJ, Lee PS, Yang JS, Wilson JM. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 171.Wilson AA, Kwok LW, Porter EL, et al. Lentiviral delivery of RNAi for in vivo lineage-specific modulation of gene expression in mouse lung macrophages. Mol Ther. 2013;21:825–833. doi: 10.1038/mt.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen YS, Li HR, Miao Y, et al. Local injection of lentivirus-delivered livinshRNA suppresses lung adenocarcinoma growth by inducing a G0/G1 phase cell cycle arrest. Int J Clin Exp Pathol. 2012;5:796–805. [PMC free article] [PubMed] [Google Scholar]
- 173.Hattori Y, Nakamura M, Takeuchi N, et al. Effect of cationic lipid in cationic liposomes on siRNA delivery into the lung by intravenous injection of cationic lipoplex. J Drug Target. 2019;27:217–227. doi: 10.1080/1061186X.2018.1502775. [DOI] [PubMed] [Google Scholar]
- 174.Alton E, Stern M, Farley R, et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353:947–954. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- 175.Ruiz FE, Clancy JP, Perricone MA, et al. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum Gene Ther. 2001;12:751–761. doi: 10.1089/104303401750148667. [DOI] [PubMed] [Google Scholar]
- 176.Griesenbach U, Kitson C, Escudero Garcia S, et al. Inefficient cationic lipid-mediated siRNA and antisense oligonucleotide transfer to airway epithelial cells in vivo. Respir Res. 2006;7:26. doi: 10.1186/1465-9921-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Boussif O, Lezoualc'h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Howard KA, Rahbek UL, Liu X, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 179.Liu X, Howard KA, Dong M, et al. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 2007;28:1280–1288. doi: 10.1016/j.biomaterials.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 180.Nielsen EJ, Nielsen JM, Becker D, et al. Pulmonary gene silencing in transgenic EGFP mice using aerosolised chitosan/siRNA nanoparticles. Pharm Res. 2010;27:2520–2527. doi: 10.1007/s11095-010-0255-y. [DOI] [PubMed] [Google Scholar]
- 181.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 183.Zheng C, Niu L, Yan J, et al. Structure and stability of the complex formed by oligonucleotides. Phys Chem Chem Phys. 2012;14:7352–7359. doi: 10.1039/c2cp24086f. [DOI] [PubMed] [Google Scholar]
- 184.Pooga M, Hallbrink M, Zorko M, Langel U. Cell penetration by transportin. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 185.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 186.Oehlke J, Scheller A, Wiesner B, et al. Cellular uptake of an α-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochimica Biophysica Acta. 1998;1414:127–139. doi: 10.1016/s0005-2736(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 187.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 188.Futaki S, Suzuki T, Ohashi W., Yagami T., Tanaka S., Ueda K., Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- 189.Reynolds F, Weissleder R, Josephson L. Protamine as an efficient membrane-translocating peptide. Bioconjug Chem. 2005;16:1240–1245. doi: 10.1021/bc0501451. [DOI] [PubMed] [Google Scholar]
- 190.Davidson TJ, Harel S, Arboleda VA, et al. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Daniels DS, Schepartz A. Intrinsically cell-permeable miniature proteins based on a minimal cationic PPII motif. J Am Chem Soc. 2007;129:14578–14579. doi: 10.1021/ja0772445. [DOI] [PubMed] [Google Scholar]
- 192.De Coupade C, Fittipaldi A, Chagnas V, et al. Novel human-derived cell-penetrating peptides for specific subcellular delivery of therapeutic biomolecules. Biochem J. 2005;390:407–418. doi: 10.1042/BJ20050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acid Res. 2003;31:2717–2724. doi: 10.1093/nar/gkg385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Unnamalai N, Kang BG, Lee WS. Cationic oligopeptide-mediated delivery of dsRNA for post-transcriptional gene silencing in plant cells. FEBS Lett. 2004;566:307–310. doi: 10.1016/j.febslet.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 195.Nakase I, Hirose H, Tanaka G, et al. Cell-surface accumulation of flock house virus-derived peptide leads to efficient internalization via macropinocytosis. Mol Ther. 2009;17:1868–1876. doi: 10.1038/mt.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Godet AN, Guergnon J, Croset A, et al. PP2A1 binding, cell transducing and apoptotic properties of Vpr(77-92): a new functional domain of HIV-1 Vpr proteins. PLoS One. 2010;5:e13760. doi: 10.1371/journal.pone.0013760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Noguchi H, Matsushita M, Matsumoto S, et al. Mechanism of PDX-1 protein transduction. Biochem Biophys Res Commun. 2005;332:68–74. doi: 10.1016/j.bbrc.2005.04.092. [DOI] [PubMed] [Google Scholar]
- 198.Langedijk JPM, Olijhoek T, Meloen RH. Application, efficiency and cargo-dependence of transport peptides. Int Congress Ser. 2005;1277:95–107. [Google Scholar]
- 199.Wang YF, Xu X, Fan X, et al. A cell-penetrating peptide suppresses inflammation by inhibiting NF-kappaB signaling. Mol Ther. 2011;19:1849–1857. doi: 10.1038/mt.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Johansson HJ, El-Andaloussi S, Holm T, et al. Characterization of a novel cytotoxic cell‐penetrating peptide derived from p14ARF protein. Mol Ther. 2008;16:115–123. doi: 10.1038/sj.mt.6300346. [DOI] [PubMed] [Google Scholar]
- 201.El-Andaloussi S, Johansson HJ, Holm T, Langel U. A novel cell-penetrating peptide, M918, for efficient delivery of proteins and peptide nucleic acids. Mol Ther. 2007;15:1820–1826. doi: 10.1038/sj.mt.6300255. [DOI] [PubMed] [Google Scholar]
- 202.Elmquist A, Lindgren M, Bartfai T, Langel U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp Cell Res. 2001;269:237–244. doi: 10.1006/excr.2001.5316. [DOI] [PubMed] [Google Scholar]
- 203.Nakayama F, Yasuda T, Umeda S, et al. Fibroblast growth factor-12 (FGF12) translocation into intestinal epithelial cells is dependent on a novel cell-penetrating peptide domain: involvement of internalization in the in vivo role of exogenous FGF12. J Biol Chem. 2011;286:25823–25834. doi: 10.1074/jbc.M110.198267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang W, Song J, Zhang B, et al. Design of acid-activated cell penetrating peptide for delivery of active molecules into cancer cells. Bioconjug Chem. 2011;22:1410–1415. doi: 10.1021/bc200138d. [DOI] [PubMed] [Google Scholar]
- 205.Delaroche D, Aussedat B, Aubry S, et al. Tracking a new cell-penetrating (W/R) nonapeptide, through an enzyme-stable mass spectrometry reporter tag. Anal Chem. 2007;79:1932–1938. doi: 10.1021/ac061108l. [DOI] [PubMed] [Google Scholar]
- 206.Oehlke J, Krause E, Wiesner B, Beyermann M, Bienert M. Extensive cellular uptake into endothelial cells of an amphipathic β-sheet forming peptide. FEBS Lett. 1997;415:196–199. doi: 10.1016/s0014-5793(97)01123-x. [DOI] [PubMed] [Google Scholar]
- 207.Sadler K, Eom KD, Yang J-L, Dimitrova Y, Tam JP. Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7†. Biochemistry. 2002;41:14150–14157. doi: 10.1021/bi026661l. [DOI] [PubMed] [Google Scholar]
- 208.Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 209.El-Sayed A, Masuda T, Khalil I, Akita H, Harashima H. Enhanced gene expression by a novel stearylated INF7 peptide derivative through fusion independent endosomal escape. J Control Release. 2009;138:160–167. doi: 10.1016/j.jconrel.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 210.Konate K, Crombez L, Deshayes S, et al. Insight into the cellular uptake mechanism of a secondary amphipathic cell-penetrating peptide for siRNA delivery. Biochemistry. 2010;49:3393–3402. doi: 10.1021/bi901791x. [DOI] [PubMed] [Google Scholar]
- 211.Rhee M, Davis P. Mechanism of uptake of C105Y, a novel cell-penetrating peptide. J Biol Chem. 2006;281:1233–1240. doi: 10.1074/jbc.M509813200. [DOI] [PubMed] [Google Scholar]
- 212.Gao C, Mao S, Ditzel HJ, et al. A cell-penetrating peptide from a novel pVII–pIX phage-displayed random peptide library. Bioorg Med Chem. 2002;10:4057–4065. doi: 10.1016/s0968-0896(02)00340-1. [DOI] [PubMed] [Google Scholar]
- 213.Smaldone G, Falanga A, Capasso D, et al. gH625 is a viral derived peptide for effective delivery of intrinsically disordered proteins. Int J Nanomedicine. 2013;8:2555–2565. doi: 10.2147/IJN.S44186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wray C, Mao Y, Pan J, et al. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–L227. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29:656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 218.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tinsley JH, Teasdale NR, Yuan SY. Myosin light chain phosphorylation and pulmonary endothelial cell hyperpermeability in burns. Am J Physiol Lung Cell Mol Physiol. 2004;286:L841–L847. doi: 10.1152/ajplung.00341.2003. [DOI] [PubMed] [Google Scholar]
- 220.Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- 221.Lomas-Neira JL, Chung CS, Wesche DE, Perl M, Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukoc Biol. 2005;77:846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 222.Lomas JL, Chung CS, Grutkoski PS, LeBlanc BW, Lavigne L, Reichner J, Gregory SH, Doughty LA, Cioffi WG, Ayala A. Differential effects of macrophage inflammatory chemokine-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cells transfer in mice. Shock. 2003;19:358–365. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 223.Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76:58–64. doi: 10.1189/jlb.1103541. [DOI] [PubMed] [Google Scholar]
- 224.Yuan B, Xiong LL, Wen MD, et al. Interleukin6 RNA knockdown ameliorates acute lung injury induced by intestinal ischemia reperfusion in rats by upregulating interleukin10 expression. Mol Med Rep. 2017;16:2529–2537. doi: 10.3892/mmr.2017.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gubernatorova EO, Perez-Chanona E, Koroleva EP, Jobin C, Tumanov AV. Murine model of intestinal ischemia-reperfusion injury. J Vis Exp. 2016;111:53881. doi: 10.3791/53881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rey C, Soubeyran I, Mahouche I, et al. HIPK1 drives p53 activation to limit colorectal cancer cell growth. Cell Cycle. 2013;12:1879–1891. doi: 10.4161/cc.24927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Choi CY, Kim YH, Kim YO, et al. Phosphorylation by the DHIPK2 protein kinase modulates the corepressor activity of Groucho. J Biol Chem. 2005;280:21427–21436. doi: 10.1074/jbc.M500496200. [DOI] [PubMed] [Google Scholar]
- 228.Kim YH, Choi CY, Lee SJ, Conti MA, Kim Y. Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J Biol Chem. 1998;273:25875–25879. doi: 10.1074/jbc.273.40.25875. [DOI] [PubMed] [Google Scholar]
- 229.Oh B, Lee M. Combined delivery of HMGB-1 box A peptide and S1PLyase siRNA in animal models of acute lung injury. J Control Release. 2014;175:25–35. doi: 10.1016/j.jconrel.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 230.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 231.Ueno H, Matsuda T, Hashimoto S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 232.Lin X, Yang H, Sakuragi T, et al. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L583–L590. doi: 10.1152/ajplung.00091.2005. [DOI] [PubMed] [Google Scholar]
- 233.Kim HA, Park JH, Cho SH, Lee M. Lung epithelial binding peptide-linked high mobility group box-1 A box for lung epithelial cell-specific delivery of DNA. J Drug Target. 2011;19:589–596. doi: 10.3109/1061186X.2010.547584. [DOI] [PubMed] [Google Scholar]
- 234.Yin X, Krikorian P, Logan T, Csizmadia V. Induction of RIP-2 kinase by proinflammatory cytokines is mediated via NF-kappaB signaling pathways and involves a novel feed-forward regulatory mechanism. Mol Cell Biochem. 2010;333:251–259. doi: 10.1007/s11010-009-0226-y. [DOI] [PubMed] [Google Scholar]
- 235.Singh D, Fox SM, Tal-Singer R, et al. Induced sputum genes associated with spirometric and radiological disease severity in COPD ex-smokers. Thorax. 2011;66:489–495. doi: 10.1136/thx.2010.153767. [DOI] [PubMed] [Google Scholar]
- 236.Dong J, Liao W, Tan LH, et al. Gene silencing of receptor-interacting protein 2 protects against cigarette smoke-induced acute lung injury. Pharmacol Res. 2019;139:560–568. doi: 10.1016/j.phrs.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 237.Jin LY, Li CF, Zhu GF, et al. Effect of siRNA against NF-kappaB on sepsisinduced acute lung injury in a mouse model. Mol Med Rep. 2014;10:631–637. doi: 10.3892/mmr.2014.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dong J, Liao W, Peh HY, et al. Ribosomal protein S3 gene silencing protects against cigarette smoke-induced acute lung injury. Mol Ther Nucleic Acids. 2018;12:370–380. doi: 10.1016/j.omtn.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wan F, Anderson DE, Barnitz RA, et al. Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell. 2007;131:927–939. doi: 10.1016/j.cell.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 240.Wan F, Lenardo MJ. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20:24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nadon AM, Perez MJ, Hernandez-Saavedra D, et al. Rtp801 suppression of epithelial mTORC1 augments endotoxin-induced lung inflammation. Am J Pathol. 2014;184:2382–2389. doi: 10.1016/j.ajpath.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fielhaber JA, Carroll SF, Dydensborg AB, et al. Inhibition of mammalian target of rapamycin augments lipopolysaccharide-induced lung injury and apoptosis. J Immunol. 2012;188:4535–4542. doi: 10.4049/jimmunol.1003655. [DOI] [PubMed] [Google Scholar]
- 243.Hu Y, Lou J, Mao YY, et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy. 2016;12:2286–2299. doi: 10.1080/15548627.2016.1230584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dan HC, Cooper MJ, Cogswell PC, et al. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 246.Schmidt EP, Tuder RM. Role of apoptosis in amplifying inflammatory responses in lung diseases. J Cell Death. 2010;2010:41–53. doi: 10.4137/JCD.S5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.D'Alessio FR, Tsushima K, Aggarwal NR, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Weirather J, Hofmann UDW, Beyersdorf N, et al. Foxp3+CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 249.Arpaia N, Green JA, Moltedo B, et al. A distinct function of regulatory T Cells in tissue protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 251.Ng WF, Duggan PJ, Ponchel F, et al. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 252.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]