Abstract
Background and Objectives:
The preoperative work up for bariatric surgery is variable and not all centers perform a preoperative upper gastrointestinal endoscopy. A study was undertaken to determine the frequency of clinically significant gross endoscopic and pathological diagnoses in a large sample of patients with obesity undergoing work-up for bariatric surgery.
Methods:
Routine endoscopy was performed on all preoperative bariatric patients. A retrospective chart review of 1000 consecutive patients was performed. Patients were divided into three groups: Group A (no endoscopic findings), Group B (clinically insignificant findings), Group C (clinically significant findings).
Results:
Patients had a mean body mass index (BMI) of 49 kg/m2 and 79% were female. In this sample one finding was found on preoperative EGD in 95.2% of patients, 33.9% had at least two diagnoses, and 29.9% had three or more diagnoses. Group A (no findings) consisted of 4.8% of patient, 52.5% in Group B (clinically insignificant findings), and 42.7% were in Group C (clinically significant findings). Clinically significant findings included hiatal hernia 23.5%, esophagitis 9.5%, H. pylori 7.1%, gastric erosions 5.7%, duodenitis 3.7%, Barrett's esophagus 3.1%, and Schatzki ring 1.2%. There was no significant correlation between preoperative BMI and any endoscopic findings (all p-value 0.05). Patients in Group C were statistically older than Groups A and B.
Conclusion:
Upper gastrointestinal pathology is highly common in patients with obesity. There is a significant rate of clinically significant endoscopy findings and all bariatric surgery patients should undergo preoperative endoscopy.
Keywords: Morbid obesity, Bariatric surgery, Preoperative endoscopy, Pathology findings
INTRODUCTION
Morbid obesity is a global health epidemic with increasing prevalence. Bariatric surgery is an effective treatment option for patients suffering from obesity with sustainable long-term weight loss and comorbidity resolution. Debate about the necessity of preoperative esophagogastroduodenoscopy (EGD) in bariatric patients is ongoing. It is known that patients with morbid obesity are at increased risk for upper gastrointestinal (UGI) diseases with two to three times more patients suffering from gastroesophageal reflux disease (GERD), erosive esophagitis, Barrett's esophagus, esophageal dysmotility, gastritis and Helicobacter pylori (H. pylori) infection, than their normal weight counterparts.1–3 Several studies suggest that there is a wide variation in the presence of UGI symptoms in patients with morbid obesity ranging from 10–87%,4–8 and that asymptomatic patients often have pathology noted on EGD.4,8–13 It is unclear if age and BMI correlate with endoscopic findings. Identification of UGI pathology preoperatively allows for treatment and proper preparation, which can help avoid perioperative pitfalls. The two most commonly performed bariatric procedures worldwide are the laparoscopic roux en y gastric bypass (LRYGB) and the laparoscopic sleeve gastrectomy (SG), and each have their own indications for preoperative EGD. The LRYGB excludes a portion of the GI tract rendering it inaccessible to future surveillance. The SG can lead to worsening reflux pathology so a baseline understanding of a patient's reflux disease can help tailor procedure choice. The opposing argument suggests symptoms do have a strong correlation with pathology,14 and that the clinical relevance of most endoscopic findings is low and unworthy of the cost and invasiveness of EGD in a high-risk patient population.14–16 This study was undertaken to determine the frequency of gross endoscopic and pathologic diagnoses in a large sample of patients with morbid obesity who are undergoing work-up for bariatric surgery, the rate of clinically significant endoscopic findings, as well as to determine the association of such diagnoses with body mass index (BMI) and age.
MATERIALS AND METHODS
An internal review board approved study was conducted at a university-affiliated tertiary care center with a high-volume bariatric surgery program participating in the American Society for Metabolic and Bariatric Surgery (ASMBS) Center of Excellence (COE) and subsequently the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). This is a retrospective chart review of 1000 consecutive patients from May 2007 to November 2012.
Inclusion criteria: All included patients met the 1991 National Institutes for Health indication criteria for bariatric surgery with a preoperative BMI of 40kg/m2 or greater, or 35kg/m2 or greater with qualifying comorbidity. The study sample included 1000 consecutive patients enrolled for primary bariatric surgery who completed pre-operative EGD. EGD was routinely performed on all patients undergoing a preoperative evaluation for bariatric surgery, including laparoscopic adjustable gastric band (LAGB), LRYGB, and SG. Endoscopy was performed by three fellowship trained surgeons with extensive endoscopic experience. Routine antral gastric biopsy for H. pylori testing was obtained, however 42 patients who did not have a biopsy and no other EGD findings were included in the dataset. Additional biopsies were taken at the endoscopist's clinical discretion. There was no routine screening for small bowel pathology and patients underwent colon cancer screening according to current guidelines for indication. Data collected included age, sex, preoperative BMI, gross descriptive endoscopic diagnoses obtained from endoscopy reports, and microscopic pathological diagnoses. Excluded patients included patients presenting for revisional bariatric surgery and EGDs performed for follow up postoperative care. BMI was defined as BMI at time of program enrollment and was available for all 1000 patients. Age was defined as age at time of surgery if performed and was available for 822 patients.
Patients were divided into three groups based on their EGD findings. Group A patients had no gross or pathologic endoscopic findings and/or no biopsy performed. Group B patients had clinically insignificant gross or pathologic endoscopic findings. Group C patients had clinically significant findings. Clinically significant findings were defined as findings prompting change in medical treatment, need for repeat endoscopy prior to operation, or change in operation. Since the majority of patients had numerous endoscopic findings each patient was only placed into a single group based on the most severe diagnosis (i.e., any group C findings categorized the patient in group C). Patients without a biopsy (n = 42) also had no gross endoscopic findings and were included in Group A.
The data was collected in Microsoft Excel and analysis was done using Minitab 18. Age, race, BMI, and age at the time of surgery were tested against EGD/biopsy findings. A Chi-squared test was used to test for association between gender and the three groups: A, B, and C. The same test was performed to determine association between race (White, Non-Hispanic and Black, Non-Hispanic) and the three groups. The Anderson-Darling test was used for normality in the patient's age and BMI. Mood's Median test was used for differences in median BMI between the three groups since it is more robust to outliers. The Kruskal-Wallis test was used to test for differences in median age at time of surgery between the three groups. All p-values are two-tailed with alpha at 0.05.
RESULTS
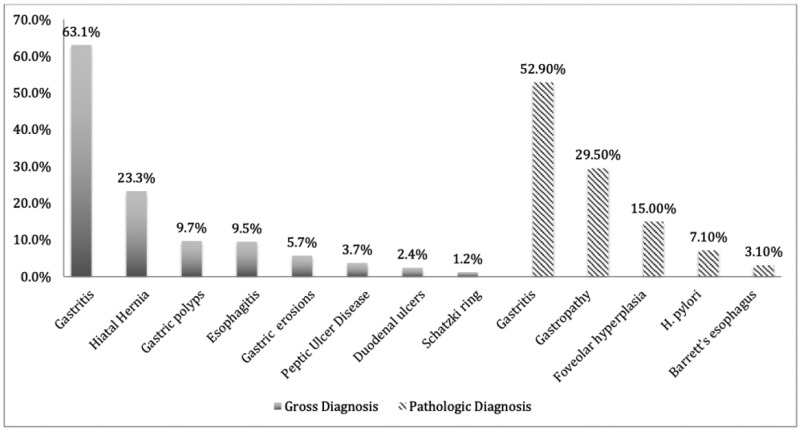
The study population mostly consists of White, Non-Hispanic (88%, n = 880) females (79%, n = 789) between the ages of 17 and 72. The mean age at the time of surgery is 47.21 and the mean BMI is 48.61. Patient demographics are summarized in Table 1. As shown in Table 2, of the 1000 patients, 48 had no gross or pathologic EGD findings and/or no biopsy performed, 525 patients had clinically insignificant findings and 427 patients had clinically significant findings. The most common EGD and or biopsy findings among the patients in the study are as follows: 828 patients (82.8%) were diagnosed with gastritis, 294 patients (29.4%) were diagnosed with gastropathy, 231 patients (23.1%) were diagnosed with hiatal hernia, 150 patients (15%) had foveolar hyperplasia, 71 patients (7.1%) had H.pylori infection, and 31 patients (3.1%) had Barrett's esophagus and are summarized in (Figure 1).
Table 1.
Patient Demographics
| Characteristics | Total Population (n = 1000) | Group A (n = 48) | Group B (n = 525) | Group C (n = 427) | p-value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 211 (21.2%) | 13 (27.08%) | 121 (23.05%) | 77 (18.03%) | |
| Female | 789 (78.9%) | 35 (72.92%) | 404 (76.95%) | 350 (81.97%) | 0.098 |
| Race/Ethnicity | |||||
| White | 880 (88%) | 41 (85.42%) | 466 (88.76%) | 373 (87.35%) | |
| Black | 113 (11.3%) | 7 (14.58%) | 55 (10.48%) | 51 (11.94%) | 0.602 |
| Hispanic/Latino | 1 (0.1%) | 1 (0.19%) | |||
| Other | 2 (0.2%) | 2 (0.38%) | |||
| Unknown | 4 (0.4%) | 1 (0.19%) | 3 (0.7%) | ||
| BMI | |||||
| Mean (Std) | 48.61 (±8.40) | 48.66 (±8.65) | 48.72 (±8.64) | 48.47 (±8.1) | |
| Median (Range) | 46.65 (35.0, 107.1) | 47.15 (35.7, 66.6) | 46.6 (35.0, 84.7) | 46.5 (35.7, 107.1) | 0.957 |
| Age | (n = 822) | (n = 40) | (n = 431) | (n = 351) | |
| Mean (Std) | 47.21 (±11.74) | 43.98 (±10.07) | 46.77 (±11.96) | 48.12 (±11.58) | |
| Median (Range) | 48.0 (17.0, 72.0) | 41.0 (29.0, 64.0) | 47.0 (18.0, 72.0) | 49.0 (17.0, 70.0) | 0.047 |
BMI, body mass index; Std, standard deviation.
Table 2.
Esophagogastroduodenoscopy and Biopsy Findings of Patients by Group
| Group | No. of Patients, (%) | Findings | No. of Patients, (%) |
|---|---|---|---|
| A | 48 (4.8%) | Normal EGD with normal biopsy or normal EGD without biopsy results | 48 (4.8%) |
| B | 525 (52.5%) | Gastritis | 828 (82.8%) |
| Gastric polyps | 97 (9.7%) | ||
| Gastropathy | 294 (29.4) | ||
| Foveolar hyperplasia | 150 (15.0%) | ||
| C | 427 (42.7%) | Hiatal hernia | 231 (23.1%) |
| Esophagitis | 95 (9.5 %) | ||
| H. pylori | 71 (7.1%) | ||
| Gastric erosions | 58 (5.8%) | ||
| Duodenitis | 37 (3.7%) | ||
| Barrett's esophagus | 31 (3.1%) | ||
| Schatzki ring | 12 (1.2%) | ||
| Metastatic carcinoma | 1 (0.1%) | ||
| Ulcer | 24 (2.4%) |
EGD, esophagogastroduodenoscopy.
Figure 1.
Gross and Pathologic Diagnostic Findings.
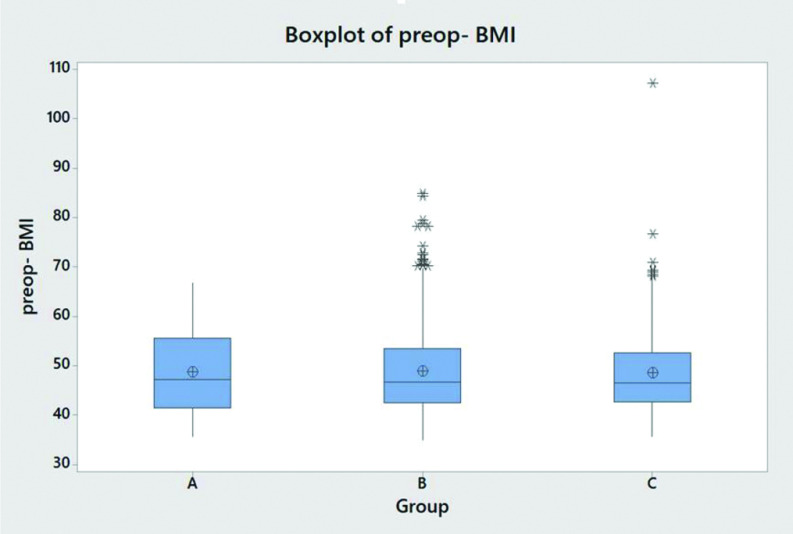
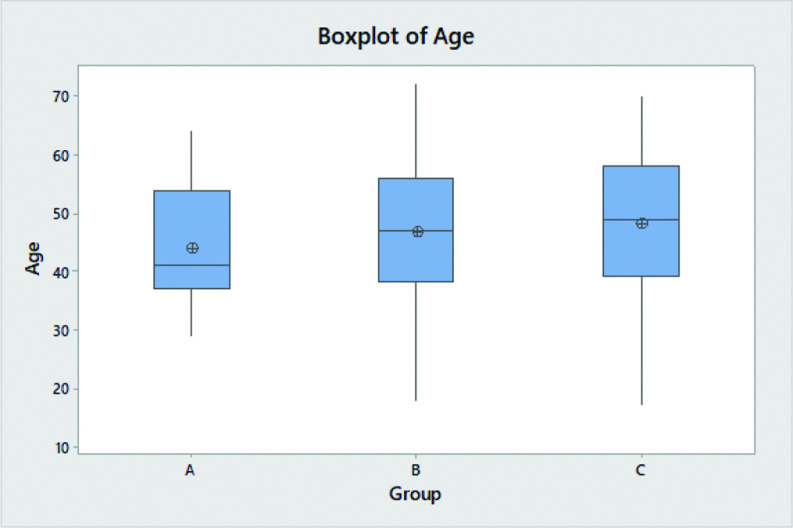
There is no statistically significant association (p = 0.098) between gender and the three groups of EGD/biopsy findings (Table 1). There is no statistically significant association (p = 0.602) between White, Non-Hispanic patients and Black, Non-Hispanic patients and the three groups of EGD/biopsy findings. The Anderson-Darling test for age (p < 0.005) as well as for BMI (p < 0.005) does not follow a specified distribution. There is no statistically significant difference in median BMI (p = 0.957) between the three groups (Table 1 and Figure 2). There is statistically significant difference in median age at the time of surgery (p = 0.047) between the three groups, with increasing age correlating with increased likelihood of statistically significant EGD findings (Table 1 and Figure 3).
Figure 2.
Boxplot of Preop BMI. Group A: Patients had no gross or pathologic endoscopic findings and/or no biopsy performed. Group B: Patients had clinically insignificant gross or pathologic endoscopic findings. Group C: Patients had clinically significant findings.
Figure 3.
Boxplot of Age. Group A: Patients had no gross or pathologic endoscopic findings and/or no biopsy performed. Group B: Patients had clinically insignificant gross or pathologic endoscopic findings. Group C: Patients had clinically significant findings.
At least one gross or pathologic diagnosis was found on preoperative EGD in 95.2% of patients, 33.9% had at least two diagnoses, and 29.9% had three or more diagnoses.
DISCUSSION
There is ongoing debate over the necessity of preoperative EGD in patients suffering from obesity undergoing evaluation for bariatric surgery. The ASMBS currently recommends EGD or UGI series in patients with UGI symptoms prior to bariatric surgery.17 Guidelines from the European Association for Endoscopic Surgery are dated but recommend EGD or UGI series in all patients prior to bariatric surgery, and strongly recommend it in patients who are to undergo LRYGB.18These recommendations are based on mostly retrospective studies with small sample sizes.
This study represents a large review assessing preoperative EGD findings in patients suffering from morbid obesity. It highlights the prevalence of UGI disease in this patient population with 95.2% having at least one EGD diagnosis, and 29.9% having three or more diagnoses. Clinically significant findings were found in 42.7% of patients. Even though it is clear from other research that obesity correlates with increasing rates of UGI pathology, we found no direct correlation between BMI and any specific diagnosis.
Several other studies specifically evaluating preoperative UGI symptomatology and pathology confirm this observation of a lack of correlation between symptoms and pathologic findings.4,8–13 The largest study to date by Abd Ellatif et al. is an Egyptian retrospective review of the pre- and postoperative role of EGD in 3,219 patients with obesity. This study argues the alternative showing a strong correlation between symptoms and EGD findings. They note only 6% of asymptomatic patients had abnormal findings on EGD, and only 25% overall had abnormal findings.14 This percentage of abnormal EGD findings is far lower than the reported range of 31–89.7%.8,10–11,13,15–16,19 The reason for this is not clear as the diagnostic findings considered abnormal in this study were consistent with other studies. However, in this study the EGD was performed by gastroenterologists, not bariatric surgeons as is the case in our study and the majority of comparative reviews and could account for some difference in test interpretation and reporting. They also only report H. pylori results for a small portion of the patients. Due to our findings and the abundance of studies suggesting a lack of correlation between symptoms and EGD findings, we do not believe symptomatology should be used as the sole criteria for preoperative EGD.
There are some studies that confirm the high rate of UGI diagnoses found in preoperative EGD but suggest the majority of these findings are of low clinical relevance and do not change clinical management.16 In studies including more than 400 patients both for and against routine EGD, the percentage of patients in which EGD findings changed medical or surgical management ranged from 0% to 63.8%.13–16,20–22 In a large study with about 1,500 patients Salama et al. found a 10% rate of clinically significant findings, which is less than our findings of 42.7% clinically significant pathology.22 However, Husein et al., in 2018 showed a 63.8% rate of clinically significant findings out of a cohort of 1278 patients.21 Findings cited to change medical or surgical management included mass lesions, severe esophagitis, gastritis or duodenitis, ulcers, Barrett's esophagus, hiatal hernia, H. pylori, polyps, stricture, esophageal diverticulum, arteriovenous malformations, cancer, and varices.8,10–11,13–16 We found almost all of these findings in our patients.
H. pylori infection is known to increase the risk of postoperative complications including marginal ulceration and perforation and is associated with increased risk of gastric cancer and peptic ulcer disease [21,22]. Routine preoperative testing and treatment of positive results is recommended by the ASMBS.17 In our study population, 7.1% of patients were positive for H. pylori infection, but the literature shows a large range of H. pylori prevalence. There seems to be some regional variation in H. pylori rates with one large study noting 52.5% of patients infected [10]. H. pylori testing is easily, quickly and accurately completed during routine preoperative EGD eliminating the need for additional testing.
In our study, gastritis was the most common EGD diagnosis (82.8%), which was consistent with other studies.10–11,13,15 In several studies this diagnosis is dismissed as benign or clinically irrelevant.15–16 Interestingly, a study by Loewen et al. noted a statistically significant correlation between the preoperative EGD finding of gastritis and postoperative anastomotic ulceration.15 This suggests preoperative knowledge and aggressive medical treatment prior to surgery may improve outcomes and supports the finding as clinically relevant. We chose to categorize gastritis as a Group B diagnosis, but viewed its more severe manifestations such as ulcers and duodenitis as a Group C diagnosis.
The diagnostic findings of esophagitis, gastric erosions, and duodenitis were identified in 21.3% of our patients. These findings are generally considered more significant and are typically treated with medical management, sometimes requiring a surgical delay to allow for healing and repeat EGD evaluation. These findings may also affect procedure selection by making SG a less attractive option for these patients. Saarin et al., evaluated 1,474 patients and determined that 23% of patients had findings significant for SG versus only 1.6% having findings significant for LRYGB. Therefore, some practices only perform EGD prior to SG.24–25
Our study showed a significant hiatal hernia was detected in 23.3% of patients. In the literature, significant hiatal hernia was identified in 2.4% to 40% of patients [8,10,13,14,23]. In a study of 1570 patients by Mohammed et al., EGD was noted to be an accurate tool for diagnosis of moderate and large sized hiatal hernias requiring surgical repair [23]. Preoperative knowledge of a hiatal hernia allows for operative planning for repair and may also affect procedure choice. Our practice prefers endoscopy to radiologic diagnosis of hiatal hernias as endoscopy allows for better identification of mucosal complications and Barrett's esophagus that may affect procedure choice. Some centers perform UGI radiographs to identify for hiatal hernias and H. pylori breath testing as a less invasive screening combination. This study does not include a cost analysis however this combination would miss most mucosal findings like gastritis, esophagitis, duodenitis, and Barrett's esophagus that we found in 25.8% of patients. A head-to-head comparative cost analysis would likely favor UGI with H. pylori testing, but it should be taken into account that many patients with that modality will need endoscopic evaluation to further work up and correlate radiologic findings prior to surgery.
We identified Schatzki ring in 1.2% of our patients. Schatzki ring or stricture was noted in 0.5% to 3.0% of patients in the literature.8,13,24 Strictures may have more serious implications and should be evaluated prior to surgery to rule out underlying malignancy. It can also be seen as a marker of severity of reflux prompting avoidance of SG. The strictures or rings may also create difficulty passing instruments down the esophagus during the operation. It is helpful to prepare for these procedural modifications in advance to prevent complications.
Barrett's esophagus or intestinal metaplasia was noted in 3.1% of our patients with a prevalence of 0.2% to 3.1% in the literature.8,10,14,24 Although this diagnosis is not prominent, it may lead to a delay in surgery, medical treatment, repeat EGD procedures, or change in procedure choice. At the most recent international consensus conference on sleeve gastrectomy, 94.5% of experts agreed that Barrett's esophagus is a major contraindication to the performance of SG.25 Failure to identify Barrett's esophagus preoperatively could lead to the performance of a potentially harmful procedure. We also identified one esophageal carcinoma based on preoperative EGD. The patient's bariatric procedure was canceled, and he was referred for oncologic care.
Gastric polyposis was a prominent finding in our study, present in 9.7% of patients, all benign by histopathology. Gastric polyps are often a benign finding as a result of treatment of UGI pathology with proton pump inhibitors and often dismissed as insignificant. However, gastric mucosal lesions, including polyps, can be precursors to premalignant lesions and gastric cancers. In one study examining the role of preoperative EGD in 626 patients suffering from morbid obesity, 10 patients were found to have lesions with histopathology demonstrating gastric intestinal metaplasia or dysplasia, premalignant gastric lesions, and one patient had early gastric cancer. These preoperative findings led to a change in surgical approach with performance of LRYGB with complete resection of the gastric remnant.10 A few other studies have reported cases of early gastric cancers or gastrointestinal stromal tumors diagnosed on preoperative bariatric surgery EGD assessment.24,26 There are several case studies detailing the diagnosis of gastric pouch or remnant cancers after LRYGB.27–29 The majority of these cancers seem to occur in the excluded stomach leading to delays in diagnosis and treatment.28–29 Two of these case studies describe cancer in the remnant noted shortly after LRYGB suggesting they could have been identified on preoperative evaluation.28
Gastrointestinal endoscopy has been proved safe with serious cardiorespiratory complications and death occurring at exceeding low rates. Patients suffering from morbid obesity are at higher risk for cardiorespiratory complications with conscious sedation due to increased rates of obstructive sleep apnea, hypertension, and obesity hypoventilation syndrome. In the study by Abd Ellatif, et al., seven of the 3,219 patients undergoing EGD had critical hypoxemic events requiring oxygen supplementation.14 In a study evaluating the safety of endoscopy in preoperative bariatric patients, two of 69 patients had critical hypoxemic events requiring intubation.9 This study and others determined that routine preoperative EGD is safe in bariatric patients, but that anesthesia preparation and support, as well as careful monitoring, should be available during conscious sedation of these patients.9
Limitations of our study include the retrospective nature and completion at a single institution. Our study had high rates of endoscopic findings (95.2%), however, other studies have found rates as high as 89%.21 The high rates of overall findings could be due to aggressive definitions of gastritis by surgeons as opposed to gastroenterologists. There could also be administrative pressure to record a diagnosis leading to over reporting. However, clinically significant findings were present in 42.7% of patients, which is consistent with other studies. A limitation in the database is that it only included age at time of surgery and not at endoscopic procedure. For that reason, only 822 patients could be analyzed for age. Also, the study includes only patients suffering from morbid obesity who meet clinical and insurance criteria for bariatric surgery, effectively eliminating a significant proportion of patients with obesity. There is no cost analysis included on expense of EGD for all patients as opposed to UGI series and H. pylori testing.
CONCLUSION
In the currently available retrospective studies addressing the topic of preoperative EGD evaluation in bariatric patients, similar results are interpreted subjectively with recommendations for and against routine preoperative EGD based on similar data. This will likely be the case until a large randomized prospective trial is undertaken. This study is one of the larger retrospective studies to date examining preoperative EGD findings in patients with morbid obesity. It shows the overwhelming presence of UGI pathology in this patient population. Despite numerous gross and pathologic EGD findings, there was no direct correlation between these diagnoses and BMI. Patients with clinically significant EGD findings were statistically older than patients with insignificant findings. The shear prevalence of UGI pathology, the non-correlation of pathology and symptoms, the resultant change in management associated with many of these diagnoses, and the number of diagnoses that carry high medical legal implications, all advocate for routine preoperative EGD in all preoperative bariatric patients, preferably performed by a bariatric surgeon.
Contributor Information
Kristine Makiewicz, John H. Stroger Jr. Hospital of Cook County, Chicago, IL, USA..
Lindsay Berbiglia, Lee Health, Cape Coral, FL, USA..
Deborah Douglas, Summa Health, Akron City Hospital, Akron, OH, USA..
Ashley Bohon, Summa Health, Akron City Hospital, Akron, OH, USA..
John Zografakis, Summa Health, Akron City Hospital, Akron, OH, USA..
Adrian Dan, Summa Health, Akron City Hospital, Akron, OH, USA..
References:
- 1. National Institute of Health. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults—The Evidence Report. 1998. NIH Publication No 98–4083.
- 2. Koppman JS, Poggi L, Szomstein S, Ukleja A, Botoman A, Rosenthal R. Esophageal motility disorders in the morbidly obese population. Surg Endosc. 2007;21:761–764. [DOI] [PubMed] [Google Scholar]
- 3. Renshaw AA, Rabaza JR, Gonzalez AM, Verdeja JC. Helicobacter pylori infection in patients undergoing gastric bypass surgery for morbid obesity. Obes Surg. 2001;11:281–283. [DOI] [PubMed] [Google Scholar]
- 4. Frigg A, Peterli R, Zynammon A, Lang C, Tondelli P. Radiologic and endoscopic evaluation for laparoscopic adjustable gastric banding: preoperative and follow-up. Obes Surg. 2001;11:594–599. [DOI] [PubMed] [Google Scholar]
- 5. Korenkov M, Kohler L, Yucel N, et al. Esophageal motility and reflux symptoms before and after bariatric surgery. Obes Surg. 2002;12:72–76. [DOI] [PubMed] [Google Scholar]
- 6. Frezza EE, Ikramuddin S, Gourash W, et al. Symptomatic improvement in gastroesophageal reflux disease (GERD) following laparoscopic roux-en-y gastric bypass. Surg Endosc. 2002;16:1027–1031. [DOI] [PubMed] [Google Scholar]
- 7. Kral JG. Morbidity of severe obesity. Surg Clin North Am. 2001;81:1039–1061. [DOI] [PubMed] [Google Scholar]
- 8. Sharaf RN, Weinshel EH, Bini EJ, Rosenberg J, Sherman A, Ren CJ. Endoscopy plays an important preoperative role in bariatric surgery. Obes Surg. 2004;12:1367–1372. [DOI] [PubMed] [Google Scholar]
- 9. Kuper MA, Kratt T, Kramer KM, et al. Effort, safety and findings of routine preoperative endoscopic evaluation of morbidly obese patients undergoing bariatric surgery. Surg Endosc. 2001;24:1996–2001. [DOI] [PubMed] [Google Scholar]
- 10. Munoz R, Ibanez L, Salinas J, et al. Importance of routine preoperative upper GI endoscopy: why all patients should be evaluated? Obes Surg. 2009;19:427–431. [DOI] [PubMed] [Google Scholar]
- 11. Csendes A, Burgos AM, Smok G, Beltran M. Endoscopic and histologic findings in the foregut in 426 patients with morbid obesity. Obes Surg. 2007;17:28–34. [DOI] [PubMed] [Google Scholar]
- 12. deMoura Almeida A, Cotrim HP, et al. Preoperative upper gastrointestinal endoscopy in obese patients undergoing bariatric surgery: is it necessary? Surg Obes Relat Dis. 2008;4:144–1449. [DOI] [PubMed] [Google Scholar]
- 13. Azagury D, Dumonceau JM, Morel P, Chassot G, Huber O. Preoperative work-up in asymptomatic patients undergoing Roux-en-y gastric bypass: is endoscopy mandatory? Obes Surg. 2006;16:1304–1311. [DOI] [PubMed] [Google Scholar]
- 14. Abd Ellatif ME, Alfalah H, Asker WA, et al. Place of upper endoscopy before and after bariatric surgery: a multicenter experience with 3,219 patients. World J Gastrointest Endosc. 2016;8(10):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loewen M, Giovanni J, Barba C. Screening endoscopy before bariatric surgery: a series of 448 patients. Surg Obes Relat Dis. 2008;4:709–712. [DOI] [PubMed] [Google Scholar]
- 16. Schigt A, Coblijn U, Lagarde S, Kuiken S, Scholten P, van Wagensveld B. Is esophagogastroduodenoscopy before Roux-en-Y gastric bypass or sleeve gastrectomy mandatory? Surg Obes Relat Dis. 2014;10(3):411–417. [DOI] [PubMed] [Google Scholar]
- 17. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient – 2013 update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic and Bariatric Surgery. Surg Obes Relat Dis. 2013;9:159–191. [DOI] [PubMed] [Google Scholar]
- 18. Sauerland S, Angrisani L, Belachew M, et al. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc. 2005;19(2):200–221. [DOI] [PubMed] [Google Scholar]
- 19. Wolter S, Dupree A, Miro J, et al. Upper gastrointestinal endoscopy prior to bariatric surgery- mandatory or expendable? an analysis of 801 cases. Obes Surg. 2017;27:1938–1943. [DOI] [PubMed] [Google Scholar]
- 20. Endo Y, Ohta M, Tada K, et al. Clinical significance of upper gastrointestinal endoscopy before laparoscopic bariatric procedures in Japanese patients. Surg Today. 2019;49:27–31. [DOI] [PubMed] [Google Scholar]
- 21. Husein BA, Khammas A, Shokr M, et al. Role of routine upper endoscopy before bariatric surgery in the Middle East population: a review of 1278 patients. Endosc Int Open. 2018;6(10):E1171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salma A, Saafan T, El Ansari W, Karam M, Bashah M. Is routine preoperative esophagogastroduodenoscopy screening necessary prior to laparoscopic sleeve gastrectomy? review of 1555 cases and comparison with current literature. Obes Surg. 2018;28:52–60. [DOI] [PubMed] [Google Scholar]
- 23. Mohammed R, Fei P, Phu J, Asai M, Antanavicius G. Efficiency of preoperative esophagogastroduodenoscopy in identifying operable hiatal hernia for bariatric surgery in patients. Surg Obes Relat Dis. 2017;13(2):287–290. [DOI] [PubMed] [Google Scholar]
- 24. Saarinen T, Kettunen U, Pietilainen KH, Juuti A. Is preoperative gastroscopy necessary before sleeve gastrectomy and roux-en-y gastric bypass? Surg Obes Relat Dis. 2018;14:757–763. [DOI] [PubMed] [Google Scholar]
- 25. Schnider R, Lazaridis I, Kraljevic M, Belinger C, Wolnerhanssen B, Peterli R. The impact of preoperative investigations on the management of bariatric patients; results of a cohort of more than 1200 cases. Surg Obes Relat Dis. 2018;14:693–699. [DOI] [PubMed] [Google Scholar]
- 26. Boru C, Silecchia G, Pecchia A, et al. Prevalence of cancer in Italian obese patients referred for bariatric surgery. Obes Surg. 2005;15:1171–1176. [DOI] [PubMed] [Google Scholar]
- 27. Trincado MT, del Olmo JC, Garcia Castano J, et al. Gastric pouch carcinoma after gastric bypass for morbid obesity. Obes Surg. 2005;15:1215–1217. [DOI] [PubMed] [Google Scholar]
- 28. Harper JL, Beech D, Tichansky DS, Madan AK. Cancer in the bypassed stomach presenting early after gastric bypass. Obes Surg. 2007;17:1268–1271. [DOI] [PubMed] [Google Scholar]
- 29. Magge D, Holtzman MP. Gastric adenocarcinoma in patients with roux-en-y gastric bypass: a case series. Surg Obes Relat Dis. 2015;11(5):35–38. [DOI] [PubMed] [Google Scholar]